- 1Agri-Food and Health Laboratory (AFHL), École Supérieure Normale, Hassan First University, Settat, Morocco
- 2Laboratoire d’Amélioration des Productions Agricoles, Biotechnologie et Environnement (LAPABE), Faculté des Sciences, Université Mohammed Premier, Oujda, Morocco
- 3Laboratory of Bioressources, Biotechnology, Ethnopharmacology and Health, Faculty of Sciences, Mohammed First University, Oujda, Morocco
- 4Laboratory of Biological Engineering, Team of Functional and Pathological Biology, University Sultan Moulay Slimane Faculty of Sciences and Technology Beni Mellal, Meknes, Morocco
- 5Department of Pharmacognosy, College of Pharmacy, King Saud University, Riyadh, Saudi Arabia
- 6Laboratories TBC, Laboratory of Pharmacology, Pharmacokinetics and Clinical Pharmacy, Faculty of Pharmacy, University of Lille, Lille, France
Introduction: Liver diseases represent a significant global health challenge, with primary causes including excessive alcohol consumption, infections, chemotherapy, and autoimmune disorders. Medicinal plants, due to their natural bioactive compounds, hold promise for developing effective treatments and preventive measures against liver ailments. This study aimed to document the use of herbal remedies in northeastern Morocco for liver diseases and correlate these uses with scientific evidence through a bibliometric analysis.
Methods: An ethnobotanical survey was conducted in remote communities of northeastern Morocco from October 2020 to January 2022. A total of 189 informants were interviewed using semi-structured questionnaires to gather information on local medicinal plants used for liver ailments. The data were analyzed using four ethnobotanical quantitative indices: use value (UV), familial use value (FUV), informant consensus factor (ICF), and fidelity level (FL). Additionally, a bibliometric analysis was performed to evaluate the scientific support for the ethnopharmacological uses documented.
Results: The survey identified 45 plant species from 26 different families used in the treatment of liver diseases. The most frequently utilized species were Cuminum cyminum L. (UV = 0.1065), Allium sativum L. (UV = 0.1015), Salvia officinalis L. (UV = 0.0761), Asparagus officinalis L. (UV = 0.0558), and Ziziphus lotus (L.) Lam. (UV = 0.0457). The Apiaceae family showed the highest familial use value (FUV = 0.1066), followed by Alliaceae (FUV = 0.1015). Liver congestion had the highest informant consensus factor (ICF = 0.83), followed by hepatic colic (ICF = 0.80). Bibliometric analysis revealed that 61% of the plants identified had documented pharmacological effects related to liver health.
Discussion: The study demonstrates that traditional knowledge in northeastern Morocco encompasses a rich diversity of medicinal plants used to treat liver diseases. The high ICF values indicate a strong consensus among informants on the efficacy of these remedies. The correlation between ethnopharmacological use and scientific validation for a significant portion of these plants suggests their potential as reliable therapeutic agents for liver conditions. However, further scientific investigations are necessary to confirm their efficacy and safety in clinical settings. This research contributes valuable information for future studies on the therapeutic potential of these plants.
Conclusion: This ethnobotanical survey provides a comprehensive database of medicinal plants used in northeastern Morocco for liver diseases. The findings highlight the potential of these plants in developing novel treatments for hepatic conditions, although further research is essential to substantiate their therapeutic claims.
1 Introduction
The liver is one of the most critical organs in the human body, playing a pivotal role in several physiological functions, including the regulation of metabolic processes, maintenance of blood sugar levels, bile production, and detoxification of foods, water, drugs, and xenobiotics (Marcellin and Kutala, 2018). These functions are vital for sustaining overall health, as the liver processes everything that enters the body, ensuring that nutrients are metabolized correctly and harmful substances are neutralized. Given its extensive involvement in maintaining homeostasis, the liver is susceptible to various diseases, which can manifest as serious clinical syndromes such as jaundice, hepatitis, hepatocarcinoma, and cirrhosis. Because of its essential functions, the liver is often considered a reflection of an individual’s overall health (Rinder et al., 2011).
Liver dysfunction is a significant global health problem, with a variety of causes that contribute to its widespread prevalence. These causes include excessive alcohol consumption, infections (notably viral hepatitis), the use of chemotherapeutic agents, exposure to toxic chemicals, and autoimmune disorders (Wei et al., 2022). The impact of liver diseases is profound, with global mortality rates reaching approximately 2 million deaths annually. Of these, 1 million deaths are attributed to complications arising from viral hepatitis and hepatocellular carcinoma, while another 1 million result from cirrhosis (Asrani et al., 2019). The growing burden of liver diseases has underscored the urgent need for effective therapeutic strategies, particularly in regions where access to conventional medical treatments is limited.
Medicinal plants have long been recognized as a valuable source of therapeutic agents, offering potential remedies for a wide array of health conditions, including liver diseases (Bhagawan et al., 2023a). The use of plants in traditional medicine is deeply rooted in human history, with ethnobotanical practices providing insights into natural remedies that have been utilized for centuries (Bhagawan et al., 2022; 2023b; 2024). In Morocco, traditional herbal medicine remains a cornerstone of healthcare, especially in rural and underserved areas. Recent ethnobotanical research indicates that a significant proportion of the Moroccan population—ranging from 60% to 80%—relies on medicinal plants to meet their healthcare needs (Jamila and Mostafa, 2014; Labiad et al., 2020; Alami Merrouni et al., 2021; Fakchich and Elachouri, 2021; Bencheikh et al., 2022; 2023). This reliance is driven by several factors, including the high cost of conventional medications, limited access to adequate healthcare facilities, and socio-economic challenges, particularly in remote and underdeveloped regions (Bencheikh et al., 2021e; Fakchich and Elachouri, 2021).
The cultural heritage of North-Eastern Morocco, like that of other regions in the country, is steeped in a rich tradition of herbal medicine that dates back to the Arab influence in the 7th century. Over centuries, the indigenous population has developed and maintained extensive knowledge of medicinal plants, which forms the foundation of the region’s traditional medical system. This knowledge is passed down orally from one generation to the next, ensuring the continuity of these traditional practices. However, this oral transmission is also a source of vulnerability. The absence of formal documentation and the lack of ethnobotanical archives pose significant threats to the preservation of this cultural heritage. As modern influences encroach and younger generations turn to contemporary medicine, there is a real risk that this indigenous medicinal knowledge, along with the phytogenetic resources it depends on, could be lost (Eddouks et al., 2017).
In Morocco, despite the widespread use of traditional medicine, there is a notable gap in ethnobotanical documentation, particularly concerning medicinal plants used for treating liver diseases. This lack of documented evidence limits the potential for scientific validation and integration of these traditional practices into modern healthcare systems. To address this gap, we propose a study aimed at documenting and analyzing the traditional knowledge related to medicinal plants used in rural areas of North-East Morocco for the treatment of liver diseases. The study will also seek to correlate these traditional uses with scientific evidence through a bibliometric review, thereby providing a comprehensive understanding of the therapeutic potential of these plants and contributing to the preservation of Morocco’s ethnobotanical heritage.
2 Materials and methods
2.1 Study area
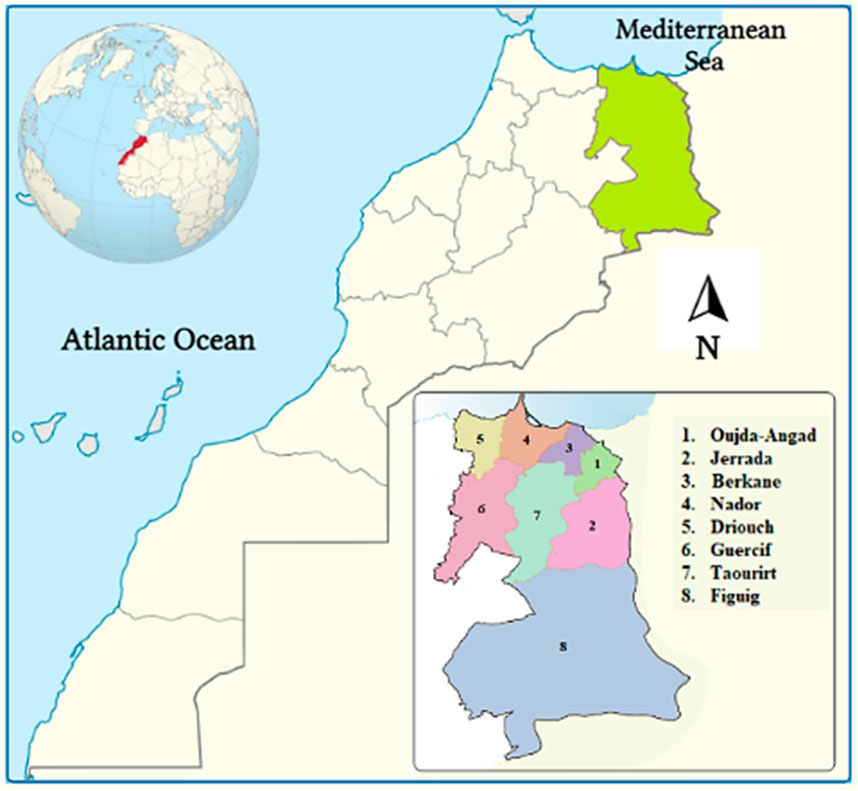
The Eastern region of Morocco, covers 90,130 km2, or 12% of the country’s total size (Figure 1). This region is limited to the West by the provinces of Al Hoceima, Taza, Boulmane, and Errachidia, to the North by the Mediterranean, to the East, and to the South by the Morocco-Algerian border. The population of this region reached 2,314,346 people (6.8% of the total population), with a density of 26 people per square kilometer, according to the national census report issued in 2014 (RGPH, 2014). According to the High Commission for Planning’s survey, the dialect of Arabic was spoken here the most frequently, followed by Berber or Tamazight, which is split into two tiny dialects: Tarifit in the north and tachelhit in the south. The territory’s southern zone is characterized by the vast Highlands and Sahara, while the mountainous areas of Beni Snassen, Rif, and Horst reach 1800 m, 1,500 m, and 1,100 m, respectively, elevations. The region also has 200 km of Mediterranean coastline. With hot, dry summers and cooler, humid winters, the region has a Mediterranean climate zone, with average annual rainfall ranging from 100 mm in the south to 400 mm in the north. Additionally, the area has a number of protected areas and sites of biological and ecological interest, including Al Hoceima National Park, Benisnassen, Jbel Gorougou, Cap des Trois Fourches, Chekhar, Lalla Chafia, and Lalla Mimouna. In fact, these places had already been chosen because of their biological and ecological characteristics as well as their indigenous flora (Fennane, 2004; Fakchich and Elachouri, 2021).
2.2 Ethnobotanical data collection
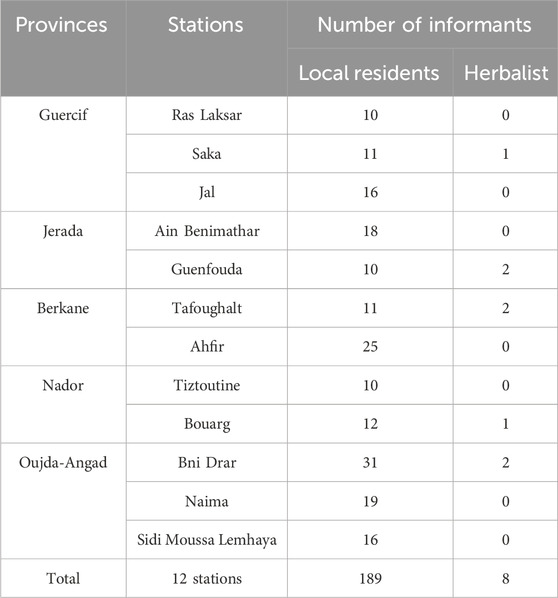
The collection of ethnobotanical data on liver diseases was conducted between October 2020 and January 2022 across twelve rural communes located in five provinces of northeastern Morocco. Traditional knowledge was randomly selected from twelve stations studied through structured and semi-structured interviews using a questionnaire sheet with 189 local residents and 8 traditional herbalists participated. Verbal informed consent was gained from informants following verbal explanation of the study aims. The established best practice for ethnobotanical investigations, the International Society of Ethnobiology’s Code of Ethics, was followed when conducting interviews (International Society of Ethnobiology, 2006). The questionnaire sheet utilized in this study has two sections: the first lists the respondents’ demographic information, and the second lists their floristic and ethnic backgrounds.
2.3 Identification of specimens
We were able to transform the common names of plants identified during our ethnobotanical survey into their botanical names using some relevant references (Bellakhdar et al., 1991; Jamila and Mostafa, 2014). Subsequently, plant samples were collected from various vegetation sites across the northeastern region of Morocco. After the harvest, the botanical identification of the samples was carried out in the Laboratory of Bioresources, Biotechnologies, Ethnopharmacology and Health of the Faculty of Sciences of Mohammed first University, Oujda, Morocco, with the help of available herbaria and a number of essential references such as the catalogue of Moroccan plants and the practical flora of Morocco (Jahandiez and Maire, 1931; 1932; 1934; Fennane et al., 1999; 2007; 2014). After the samples were identified, specimens were placed in the Mohammed First University Herbarium in Oujda, Morocco. Using the World Flora Online (WFO) Plants database (https://wfoplantlist.org/), all scientific names were reviewed once more. Additionally, a group of flowering plants (angiosperms) known as Angiosperm Phylogeny Group III - 2009 has been given credit for naming all plant families (A.P.G III, 2009).
2.4 Quantitative data analysis
To quantify the ethnobotanical information, we adopted a quantitative analysis using ethnobotanical indices such as the Medicinal Use Value (UV), the Family Use Value (FUV), Informant Consensus Factor (ICF), and the Fidelity Level (FL).
• Medicinal use value (UV)
We analyzed the medicinal use value of each plant species to identify the relative relevance of each plant species that is locally recognized to be utilized in herbal treatments. This index is calculated using the formula below (Tabuti et al., 2003):
Where;
UV: medicinal use value, U: number of citations per species, N: number of informants. The UV value will be larger if a plant has a high utilization ratio, indicating that the plant is significant, however if there are few utilization ratios, it will be near to zero.
• Botanical Family Use Value (FUV)
We used the family use value index to analyze the association between botanical families and users of taxa that correspond to these families. This index is equal to the mean total use value of each species in the family (Hoffman and Gallaher, 2007).
Where;
FUV is the family use value, UV is the utility value of the family's species, and N is the number 173 of species in the family.
• Informant Consensus Factor (ICF).
The ICF demonstrates the uniformity of traditional knowledge exchange amongst informants regarding the usage of plants to cure different types of diseases. The following formula was used to determine ICF (Bencheikh et al., 2021e).
Where;
Nur denotes the number of use-reports for an ailment category and Nt denotes the total number of plants used by all informants for that illnesses category. The ICF values range between 0 and 1, with values close to 0 indicating that the herbs were picked at random or that there was no exchange of information about plant usage within the population. Furthermore, ICF values close to 1 indicate a clear selection of medical species and information sharing about their use in the population.
• Fidelity Level (FL).
The level of fidelity (FL) identifies a plant species’ ability to effectively combat a certain disease. FL was determined using the formula below (Sreekeesoon and Mahomoodally, 2014).
Where;
Lu denotes the total number of interviewers who cited all uses of the particular species for the therapies of all liver pathologies, and Ip represents the number of individuals who used a particular species for a specific type of liver disease.
2.5 Pharmacological validation
A bibliographic search was conducted to identify the biological activities of identified plants against liver disease, by the mean of the following databases: PubMed, Science Direct, Google Scholar, Scopus and Web of Science with keywords like “liver disease,” “liver disease,” “Liver failure,” “hepatitis,” “Jaundice,” and “Hepatoprotective” combined with the scientific name of each plant.
3 Results and discussions
3.1 Informants’ sociodemographic profile
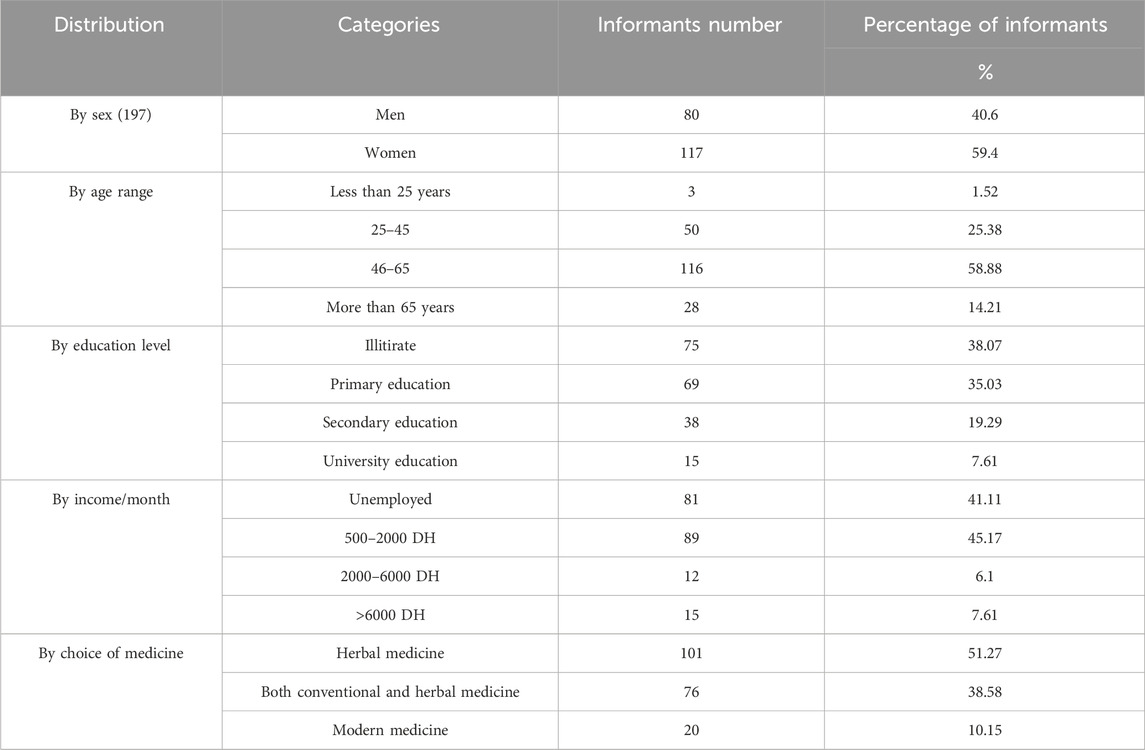
A total of 197 informants, including 189 non-specialists and 8 herbalists interviewed for this study. These interviewees are spread over twelve rural stations in five provinces of North-East Morocco (Table 1). The socio-demographic profile of the participants in this study (The variable comprising age, sex, education level, income and attitude towards drugs) were grouped in the Table 2. Analysis of the data presented in Table 2 shows that Women had the highest share of participants (59%), followed by men (40.6%). The use of medicinal plants for the treatment of liver disease in the study areas is widespread in all age groups. The 46–65 age group is the most represented in this study with a frequency of 58.88%, followed by the 25–45 age group with a percentage of 25.38%, the over-65 age group with 14.21%, and the under-25 age group with a percentage of 1.52%. The results of numerous research have consistently shown that older people had more traditional knowledge on how to use medicinal herbs than did younger people (Alami Merrouni et al., 2021; Bencheikh et al., 2021e; Hachlafi et al., 2022). The discomfort of the younger generation, which tends not to accept popular medicine due to the effect of exotic culture, and the influence of lifestyle modernization can be used to explain the gradual loss of traditional knowledge about medicinal plants (Sargin et al., 2015). The fact that there were fewer informants over the age of 65 (14.21%) is a reflection of the depth of traditional knowledge being lost as rural elders pass away.
In terms of educational attainment, the findings revealed that 38.07% of the informants are illiterate, followed by the categories of secondary and primary education, with percentages, respectively 35.03% and 19.29%, and lastly the university level, with a percentage of 7.61%. These findings are consistent with those of other ethnobotanical studies conducted in various regions of Morocco (Khouchlaa et al., 2017b; Bencheikh et al., 2021e; Hachlafi et al., 2022). The study area’s rising illiteracy rate may be caused by the fact that poverty is still pervasive in the rural areas examined. This is indicated in our results, where the majority of respondents had a low socio-economic level (41.11% unemployed, and 45.17% between 500 and 2000 DH/month).
There are differences in how the people in this area feel about treating liver illness. The results shown in Table 2 demonstrate the extreme variety of usage patterns. In fact, the majority of interviews indicated that traditional medicine was their first choice of treatment when they were ill, with a percentage of 51.27%, followed by the use of conventional and herbal medicine in second place, with a percentage of 38.58%, and exclusively modern medicine in third place, with a percentage of 10.15%. Access to modern medication is hampered by a lack of health facilities and trained medical personnel, a lack of infrastructure, particularly paved roads, a lack of transportation options, a lack of logistical support, and the high expense of treating liver disease with modern medicine (El Hassani et al., 2013; Eddouks et al., 2017). The aforementioned factors all strongly encourage rural populations to switch to traditional healthcare, especially the usage of medicinal herbs.
3.2 Diversity of plant species used to treat liver diseases
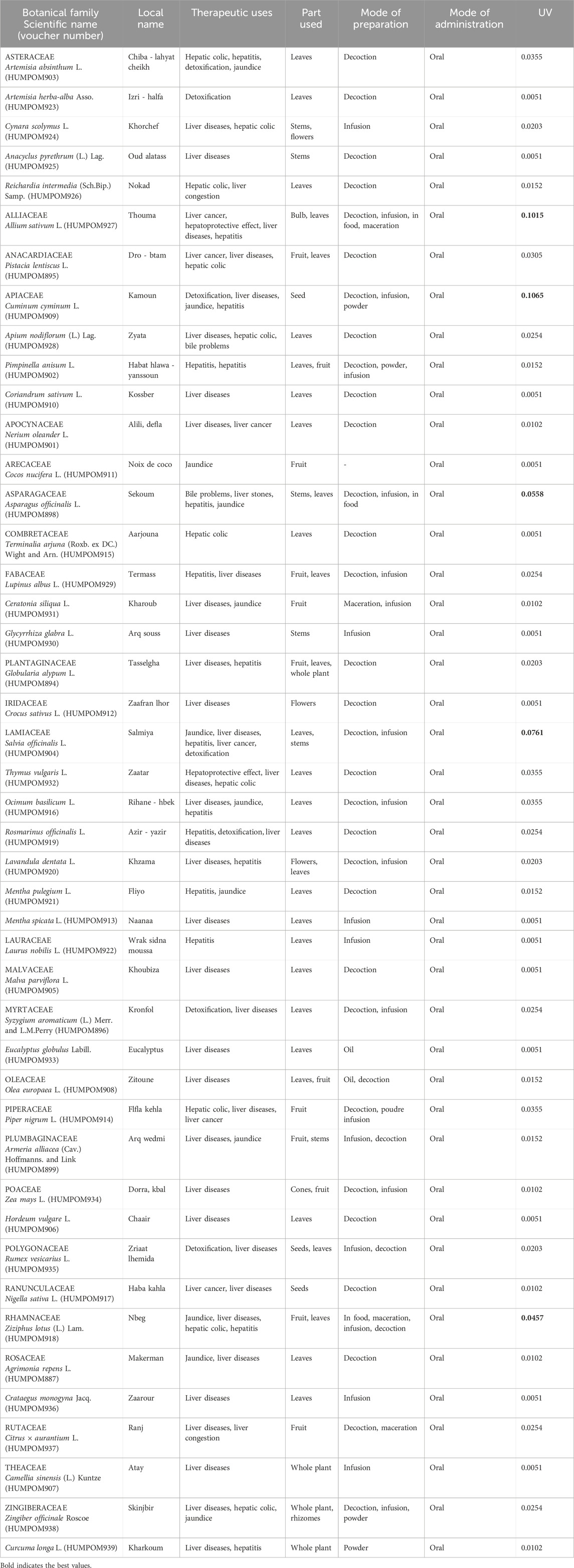
This study recorded the use of 45 medicinal plants, spread across 26 families and 43 genera, for the treatment of liver disease in the study area. Traditional information on the applications of these plants has been developed (Table 3), including the use value, scientific name, botanical family, popular names, traditional uses, parts utilized, preparation procedure, and mode of administration for each medicinal species.
3.2.1 Frequency of families and their use value
As indicated in Table 4, a total of 26 botanical families were used in rural areas of North-East Morocco for the treatment of liver pathologies. However, the families most used are Lamiaceae (7 species; 6 genera) in the first position, followed by Asteraceae (5 species; 4 genera), Apiaceae (4 species; 4 genera), Fabaceae (3 species; 3 genera), Myrtaceae, Poaceae, Rosaceae and Zingiberaceae with (3 species; 3 genera) for each. There are only one species and one genus for the other families. Similarly, the Lamiaceae, Asteraceae, and Apiaceae botanical families are the ones that are most prevalent in Mediterranean countries (Benítez et al., 2010; Savo et al., 2011). The predominance of the Asteraceae family in the traditional treatment of liver disease has already been confirmed by an ethnobotanical study carried out in the Maritime region of Togo (Kpodar et al., 2016).
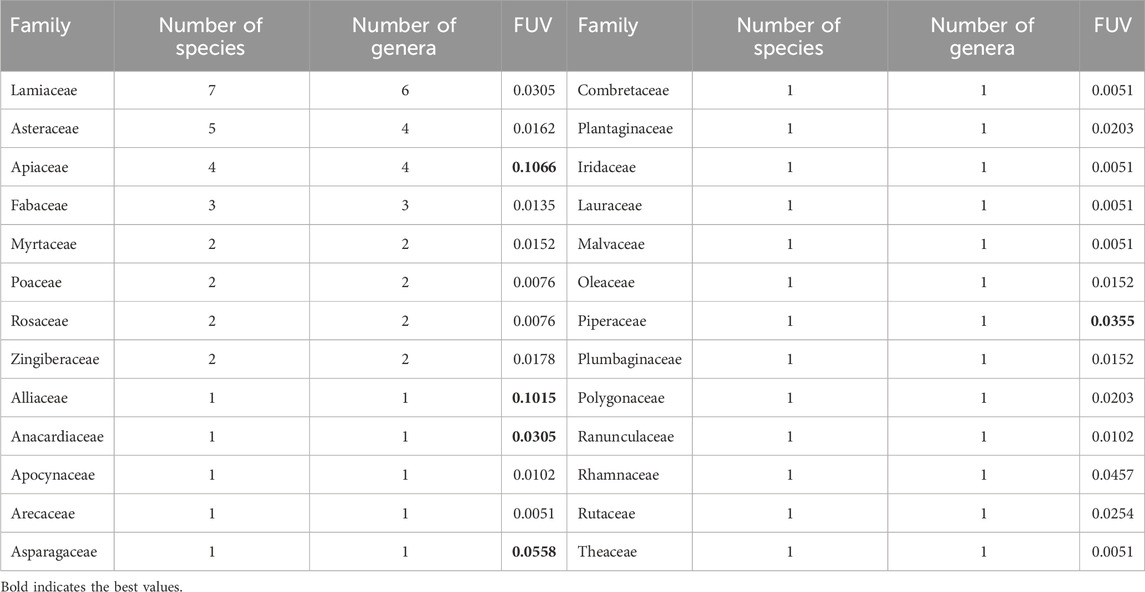
Table 4. Distribution of botanical medicinal families according to species and genera. FUV: Family Use Value.
Families with high FUV are Apiaceae (0.1066), Alliaceae (0.1015), Asparagaceae (0.0558), and Piperaceae (0.0355) (Table 4). However, there aren't many species in these groups to represent them. It appears that the value of using ethnobotanical families is not dependent on their particular wealth but rather on the significance and value of the use of the individual species (Najem et al., 2019). Additionally, these families’ significant FUV would be mostly dependent on their abundance of bioactive compounds, which would confer multiple benefits such as antimicrobial, anti-allergic, anti-oxidant, and anti-inflammatory properties (Bencheikh et al., 2022).
3.2.2 Most used plants species to treat liver diseases according to use value index
In this work, we inventoried 45 different medicinal plants that are utilized to treat liver ailments in rural areas of North Eastern Morocco. Nevertheless, the most widely used plants for the treatment of liver diseases are Cuminum cyminum L. (UV = 0.1065), followed by Allium sativum L. (UV = 0.1015), Salvia officinalis L. (UV = 0.0761), Asparagus officinalis L. (UV = 0.0558), and Ziziphus lotus (L.) Lam. (UV = 0.0457) (Table 3). These five species made up 27.84% of all use ratios, while the other 40 species only made up 72.16% of all use ratios. Similar studies conducted in other nations have shown that high utilization values have been attained for plants other than those in the current study (Kotoky and Das, 2008; Kpodar et al., 2016). This difference in species similarity could be explained by the difference in bioclimate between countries, which will favor the difference in the abundance of certain plant species from one country to another. In addition, geographic distance between countries has a direct impact on the traditional cultures of indigenous peoples, as evidenced by Alami Merrouni et al. (2021), in which they demonstrated that the increase in distance between countries is accompanied by the increase in the difference in the cultures of these countries and vice versa. Thus, all these factors can lead to differences between countries in the use of plant species to treat a particular health condition.
These five medicinal plants were frequently utilized in traditional Moroccan medicine to cure a wide range of illnesses:
Cuminum cyminum L.: This Apiaceae family medicinal plant was one of the first plants grown in Asia, Africa, and Europe (Al-snafi, 2017). Since antiquity, C. cyminum seeds have persisted in popularity as culinary seasonings and are widely utilized in folk therapy across a variety of geographic regions. This plant, called in Morocco as “Kammun”, is frequently used conventionally to treat digestive system issues, including diarrhea (Jamila and Mostafa, 2014). According to the analysis of the data collected during our investigation, C. cyminum is the most widely used to treat liver pathologies in the North-Eastern Moroccan population with a usage value of 0.1065. Indeed, the seeds of C. cyminum, in decoction or infusion, are used by the study population as treatment of jaundice, and hepatitis, and thus for liver detoxification. In Ayurveda (former Indian therapeutic system), seeds of C. cyminum are traditionally used against jaundice and to improve liver function (Andallu and Ramya, 2007; Johri, 2011).
Allium sativum L.: This plant, called locally as “Thouma” in Morocco, is one of the earliest known to have been cultivated (Thomson and Ali, 2003). Traditional Moroccan medicine makes extensive use of garlic to cure and prevent a wide range of illnesses, including cancer, lung disease, hypertension, diabetes, microbiological infections, infertility, and problems with the kidneys (Fakchich and Elachouri, 2021). According to the results of our investigation, this plant is classified according to its use value in the second position as the plant most used to treat liver diseases in the northeast of Morocco. Indeed, the leaves and bulb of this plant in decoction or infusion are widely recommended by the inhabitants of the study area to fight against liver cancer and hepatitis, and thus declared that it has hepatoprotective effects. Furthermore, it has been reported that portions of this plant are commonly used to heal liver problems in Togo’s Maritime region (Kpodar et al., 2016). The bulb of plant is often used to treat jaundice in the southern region of Algeria (Bendaif et al., 2021).
Salvia officinalis L.: This round perennial shrub belongs to the Lamiaceae family and is called to as “Salmiya” in the Oriental area of Morocco. It is indigenous to the Middle East and the Mediterranean, although it has since become naturalized everywhere (Ghorbani and Esmaeilizadeh, 2017). In Morocco, the aerial part of S. officinalis is used to handle gastrointestinal problems, metabolic disorders, and renal ailment (Bencheikh et al., 2021e; Fakchich and Elachouri, 2021). Based on the findings of the current investigation, this plant is classified among the three most used medicinal species in the study area for the treatment of liver diseases. Indeed, the leaves and stems of S. officinalis, in decoction or infusion are widely used in rural areas of north-eastern Morocco to prevent and treat jaundice, hepatitis, and liver cancer, and thus to detoxify the liver. In the middle Oum Rbia region of Morocco, leaves and whole plant decocted were used for liver problems (Ben Akka et al., 2019). In addition to these local uses, in traditional South-West Algerian medicine, the flowers of this plant were also used to treat liver symptoms (Benarba, 2016).
Asparagus officinalis L.: Since ancient times, asparagus, a perennial herbaceous plant of the Asparagaceae family, has been utilized extensively in food and medicinal. This plant is called « Sekoum» in Morocco, is used to treat various ailments such as respiratory diseases, digestive problems, kidney diseases, liver diseases and diabetes (Alami Merrouni and Elachouri, 2021; Fakchich and Elachouri, 2021; Bencheikh et al., 2022). In our study, asparagus is ranked fourth among the most cited plants for the treatment of liver patients. In fact, this plant’s leaves and stems are frequently used to treat biliary issues, liver stones, hepatitis, and jaundice.
Ziziphus lotus (L.) Lam.: The majority of Africa, numerous Asian nations, including China, Iran, and South Korea, as well as several European nations, including Cyprus, Spain, and Greece, are all home to this medicinal plant (Adeli and Samavati, 2014; Bencheikh et al., 2021d). In Morocco, Z. lotus is locally known as “Sedra,” and “Nbeg” for its fruits, and is widely found in arid and semi-arid areas (Bencheikh et al., 2019). Plant parts were traditionally used to combat various health problems such as sedation, anxiety, urinary problems, diabetes, skin infections, scarring, and bronchitis (Khouchlaa et al., 2017a; Bencheikh et al., 2021e; Fakchich and Elachouri, 2021). As per the findings of our survey, Z. lotus in rural parts of North-East Morocco, is one of the top five plants used to treat liver disorders such as jaundice, hepatic colic, and hepatitis. Furthermore, the fruits of this medicinal plant are traditionally used to treat lung diseases, jaundice, and as an emollient in El Hammadia, Algeria (Bendaif et al., 2021).
3.3 Ethnic medicinal characteristics
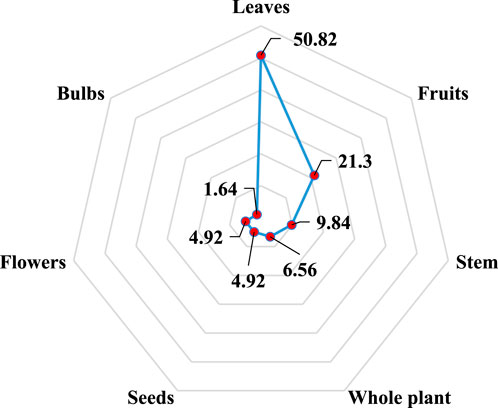
In this study, different parts of plants are used as medicines to treat liver problems in rural areas of North-East Morocco (Figure 2). Thus, on the basis of calculating the percentage of use of each part (%), the leaves (50.82) appear to be the most commonly utilized for the treatment of liver illnesses in the study area, followed by fruits (21.3), stems (9.84), whole plants (6.56), the seeds, and the flowers (4.92) for each, and finally the bulbs (1.64). The leaves are both a source of photochemical reactions and a repository of organic stuff created from them, which explains why they are used so frequently (Bencheikh et al., 2021e). In addition, it is important to avoid pulling out the entire plant or picking up the roots of the plants, as this will promote deforestation and put the species at risk (Kadir et al., 2013). On the contrary, the use of leaves contributes to the conservation and sustainable use of the plant.
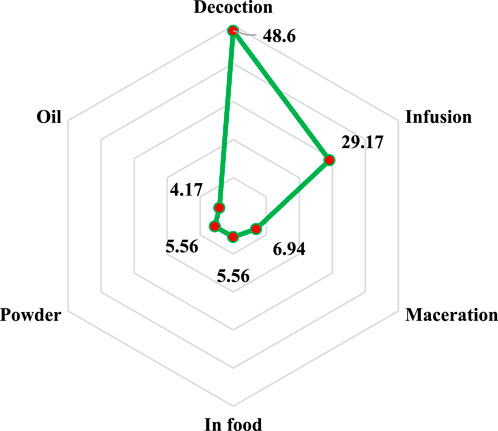
As seen in Figure 3, various techniques are used in rural North-East Morocco to make alternative therapies for treating liver disease. Nevertheless, with a percentage of 48.61%, decoction remains the most commonly employed method of preparation, followed by infusion (29.17%), maceration (6.94%), powder and preparation in the diet (5.56% for each), and finally oils with 4.17%. The preparation technique is frequently correlated with the type of use (external or internal); typically, external usage involves the use of a mask, massage, or suppositories, while internal use involves the use of decoction, infusion, maceration, and other techniques (Eddouks et al., 2017). Decoction’s supremacy may thus be explained by the fact that it allows for the capture of the greatest amount of bioactive molecules and reduces or eliminates the toxic effects of some recipes (Noureddine et al., 2022).
3.4 Hepatic ailments categories and their informant consensus factor (ICF) values
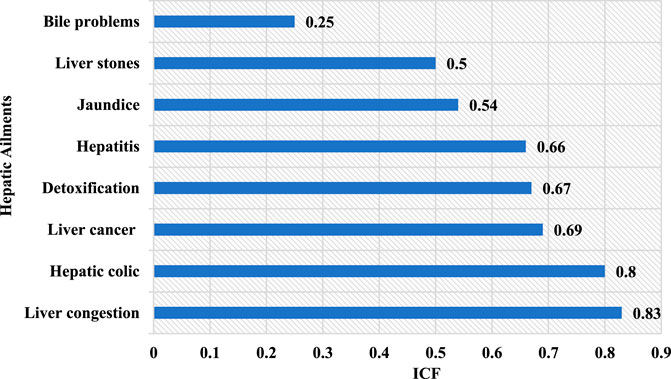
In this study, we identified eight liver pathologies that were treated with medicinal plants in rural areas of North-East Morocco (Figure 4). The ICF values of the plant species cataloged in this investigation extended from a minimum of 0.25 to a maximum of 0.83. (Figure 5). This index has the highest value for liver congestion (ICF = 0.83), followed by hepatic colic (ICF = 0.80), liver cancer (ICF = 0.69), liver detoxification (ICF = 0.67), hepatitis (ICF = 0.66), jaundice (ICF = 0.54), liver stone (ICF = 0.50), and bile problems with ICF = 0.25. High values (around 1) of this index for liver congestion, hepatic colic, and liver cancer suggest that a small number of species were employed by many informants, reflecting a high level of consensus on the use of plants in the management of these illnesses. The low accord between both interviews was witnessed for biliary problems. This could be attributed to a lack of interaction and knowledge exchange among individuals (Al-Qura’n, 2005).
3.5 Fidelity level (FL)
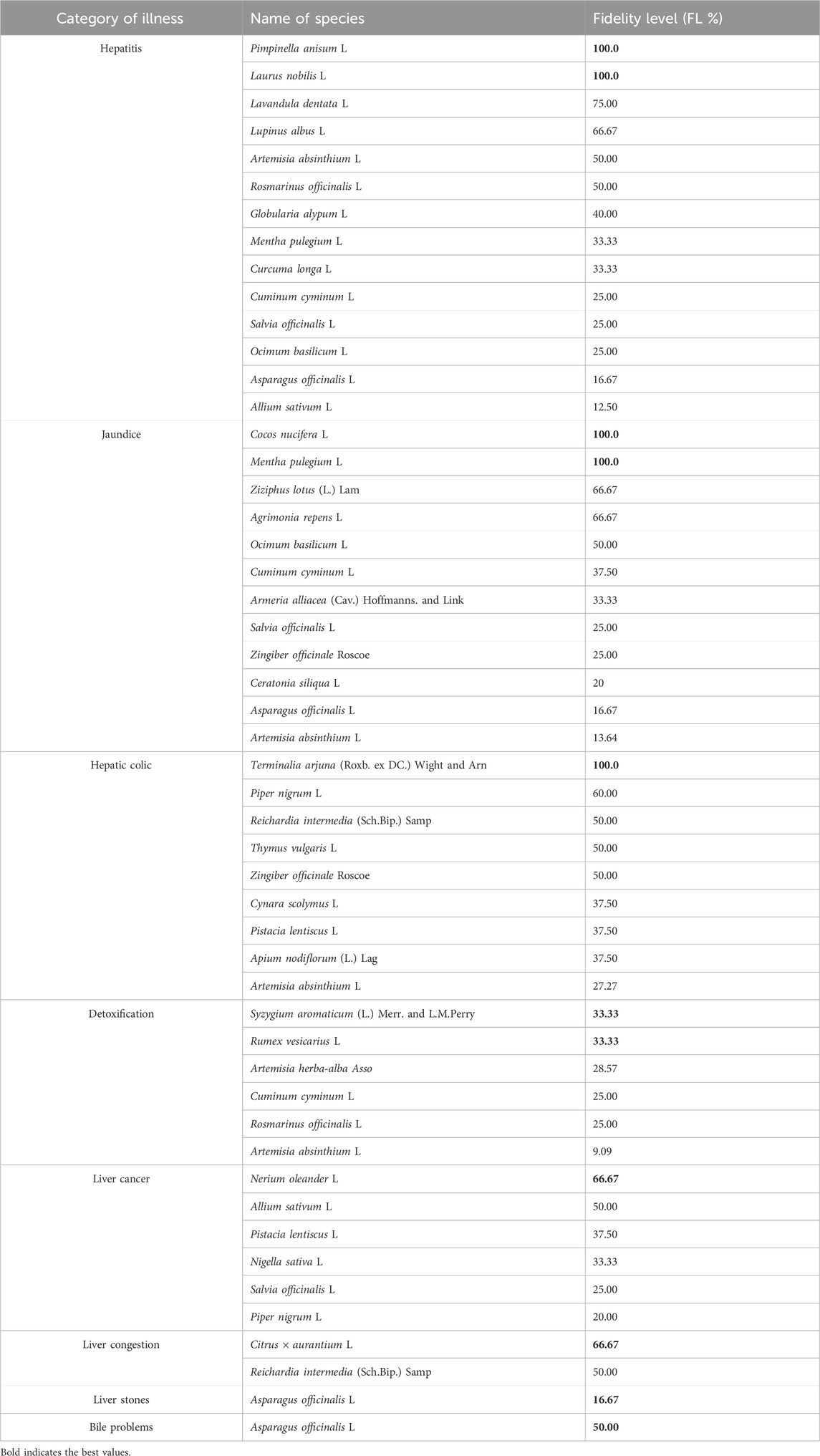
According to the corresponding level of fidelity, we categorized the medicinal plants used to treat liver illness in Table 5. According to our findings, the level of fidelity of plant species for a particular liver condition ranged between 9.09% and 100%. Concerning hepatitis problems, the most important species according to the level of fidelity were Pimpinella anisum L. (FL = 100%), Laurus nobilis L. (FL = 100%), Lavandula dentata L. (FL = 75%), and Lupinus albus L. (FL = 66.67%). For the jaundice, Cocos nucifera L. (FL = 100%), Mentha pulegium L. (FL = 100%), Z. lotus (L.) Lam. (FL = 66.67%), and Agrimonia repens L. (FL = 66.67%) were the most important. The most widely known species in the hepatic colic group were Terminalia arjuna (Roxb. ex DC.) Wight & Arn. (FL = 100%), and Piper nigrum L. (FL = 60%). For liver detoxification, plants with the highest FL were Syzygium aromaticum (L.) Merr. & L.M. Perry (FL = 33.33%), Rumex vesicarius L. (FL = 33.33%), and Artemisia herba-alba Asso (FL = 28.57%). Nerium oleander L. (FL = 66.67%), and A. sativum L. (FL = 50%) were species with the highest fidelity level. For liver congestion, Citrus × aurantium L. (Fl = 66.67%) was the most important. In the end, Asparagus officinalis L. is the most important for liver stone and bile problems. The importance of these plants for the treatment of liver diseases in the study area could be due to their wide use in traditional Moroccan medicine to treat various diseases (Fakchich and Elachouri, 2021).
3.6 Pharmacological confirmation data of the medicinal plants
The current ethnobotanical fieldwork confirmed that the inhabitants of northeastern Morocco has extensive ethnobotanical information concerning the use of herbal remedies in the treatment of liver conditions. These conventional data, which detailed a wide variety of quantitative factors, were particularly intriguing for the goal of bioprospecting to identify novel drugs to cure liver pathological conditions. It could be worthwhile to look up these plants’ pharmacological properties in the literature. According to the results of our bibliographic survey, of the 46 plant species registered for the treatment of liver diseases in the study area, 28 plant species from 20 botanical families have already been pharmacologically validated for liver diseases (Table 6). It can be concluded that the majority of them significantly reduce the risk of liver disorders. These findings demonstrated the potential of ethnobotanical knowledge as a preferable traditional database for plant species with beneficial therapeutic effects connected to liver illnesses. The pharmacological data collected for the plants selected during our survey were grouped in the Table 6.
According to the results of our ethnobotanical survey, C. cyminum L. (UV = 0.1065), A. sativum L. (UV = 0.1015), S. officinalis L. (UV = 0.0761), Asparagus officinalis L. (UV = 0.0558), and Z. lotus (L.) Lam. (UV = 0.0457) are the medical species commonly used in Northeastern Morocco for the treatment or prevention of liver problems. To support the use of these plants in conventional medicine, it may be interesting to further explore and discuss their pharmacological properties related to liver problems. To this goal, we shall explore the pharmacological potential of these herbs in the following paragraphs to validate their benefits against liver diseases:
Allium sativum L. is ranked as the second most used species (UV = 0.1015) for the treatment of liver diseases. According to ethnobotanical findings, this plant is widely used in rural areas of northeastern Morocco for its hepatoprotective effect, against liver cancer, and viral infections (hepatitis). The leaves and bulb of A. sativum have demonstrated antioxidant and hepatoprotective effects against ethanol-induced hepatotoxicity in rats (Nencini et al., 2010). Indeed, the administration of an extract of the leaves or bulbs of A. sativum At a dose of 250 mg/kg, the Glutathion reductase (GR), catalase (CAT), and superoxide dismutase (SOD) activities were restored, and the levels of malondialdehyde, ascorbic acid, and glutathion were reduced and oxidized in the liver tissue of rats exposed to ethanol (Nencini et al., 2010). A study also discovered that A. sativum has a cytoprotective impact in HepG2 cells submitted to mycotoxines, specifically Beauvericin, α-Zearalenol and β-Zearalenol (Juan-García et al., 2021b). The presence of antioxidant compounds, according to the authors, is responsible for this cytoprotective effect, which involves the activation of defensive pathways as an enzymatic defence mechanism from within cells, the control of the cell cycle, and cell death, all of which can be provoked by these mycotoxines (Juan-García et al., 2021b). Another research revealed that an aqueous extract of garlic bulbs reduces alloxane elevation of biological parameters of liver and kidney functions in rats (Aprioku and Amah-Tariah, 2017b). According to prior study, garlic includes a number of bioactive components such as organosulfur compounds, saponins, and phenolic compounds (Bradley et al., 2016; Diretto et al., 2017). Organosulfur compounds such as diallyl thiosulfonate (allicin), diallyl sulfide, diallyl disulfide, diallyl trisulfide, E/Z-ajoene, S-allyl-cysteine, and S-allyl-cysteine sulfoxide have been claimed to be the principal active phytochemicals found in garlic (Figure 5) (Yoo D. Y. et al., 2014; Yoo et al., 2014 M.; Kodera et al., 2017; Mansingh et al., 2018). These compounds were discovered to be related to the plant’s powerful antioxidant and antitumor ability (Bagul et al., 2015).
Asparagus officinalis L. this plant is ranked third among the plants most commonly used for the treatment of liver diseases by the Moroccan population. It is frequently used to treat biliary problems, hepatic stones, hepatitis, and jaundice, as shown in Table 3. It has been reported that the aqueous and ethanolic extract of A. officinalis have hypolipidemic and hepatoprotective effects in mice fed a high-fat diet (Zhu et al., 2010). According to the findings of this study, daily treatment of 200 mg/kg of either ethanolic or aqueous extract for 8 weeks enhanced lipid parameters, transaminase (Alanine and Aspartate) activity, superoxide dismutase (SOD) and antioxidant capacity, and hepatic malondialdehyde levels. In addition, an in vivo research indicate that the aqueous extract of A. officinalis roots has protective properties on cadmium chloride-induced liver injury in rats (Abedi et al., 2018). This investigation found that treatment by aqueous extract of A. officinalis roots at 200 and 400 mg/kg for 28 days significantly restored liver biomarkers in cadmium chloride poisoned rats. Several phytochemical investigations have revealed that the main bioactive compounds found in asparagus include phenolic compounds, sterols, and saponins (Jang et al., 2004; Fuentes-Alventosa et al., 2013). Asparanin A, Asparoffin C, Asparoffin D, Asparenyol, Gobicusin B, Protodioscin and 1-methoxy-2-hydroxy-4-[5-(4-hydroxyphenoxy)-3-penten1-ynyl] phenol are the main phytochemicals found in asparagus, with Rutin (Quercetin 3-rutinoside) as the major compound (Figure 5) (Fan et al., 2015). These compounds’ antioxidant action is well-known (Sun et al., 2007; Solana et al., 2015; Slatnar et al., 2018), that may contribute in the hepatoprotective effects of the plant.
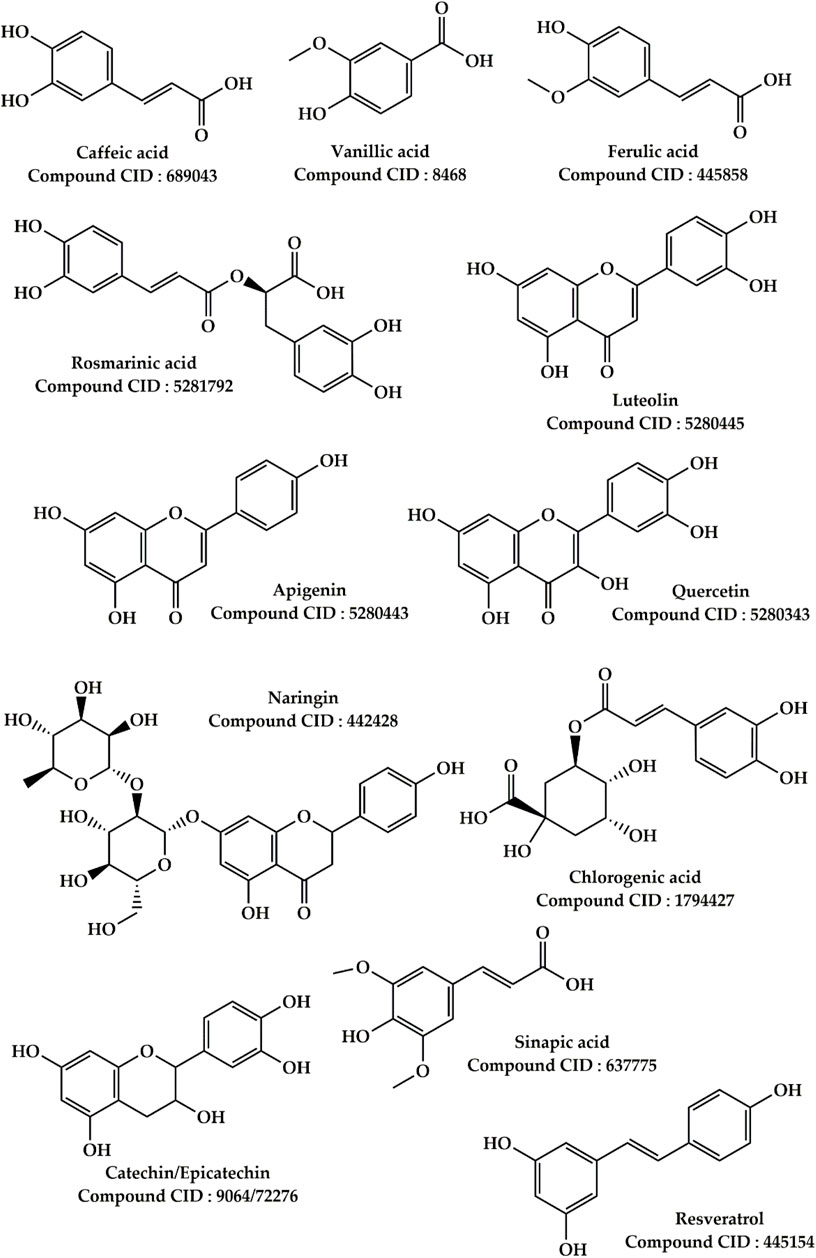
Salvia officinalis L. This plant is widely used in folk medicine in North East Morocco to treat liver failure. It is ranked fourth of the most commonly used plants, with a use value of 0.0761. According to our findings, various parts of this plant have traditionally been used to treat jaundice, hepatitis, and liver cancer in the study site. Several preclinical investigations on plant parts were carried out to examine its medicinal qualities for liver failure. In fact, 8 weeks of daily administration of S. officinalis essential oil at 4 mg/kg enhanced hyperlipidemia, hepatic, and renal lesions in mice fed a high-fat diet (Koubaa-Ghorbel et al., 2020b). This effect of S. officinalis essential oil was more effective than that of simvastatin (standard drug for this purpose). In addition, daily intake of 15 mg/kg of the essential oil of S. officinalis showed a protective effect against vanadium-induced hepatotoxicity in Wistar rats (Koubaa et al., 2021b). The treatment of rats with 200 mg/kg of S. officinalis aqueous extract for 15 days showed protective effects against ethanol-induced hepatotoxicity (Jedidi et al., 2022b). According to the same authors, this effect is reflected in the improvement of plasma transaminase activity and the restoration of hepatocyte structure in rats poisoned with ethanol. Besides, it was shown that administering a hydroalcoholic extract of S. officinalis at a dose of 250 mg/kg protected rats from isoniazid-induced hepatotoxicity (Shahrzad et al., 2014b). Furthermore, a previous investigation showed that an ethanolic extract of S. officinalis leaves protects human HepG2 cells from hydrogen peroxide and 2,3-dimethoxy-1,4-naphthoquinone-induced DNA damage (Kozics et al., 2013b). As shown in Figure 6, common sage contains a variety of biologically active compounds, primarily two types of relatively abundant phenolic components: phenolic acids (caffeic, vanillic, ferulic, and rosmarinic acids) and flavonoids (luteolin, apigenin, and quercetin) (Lu and Foo, 2002; Roby et al., 2013). These phenolic components are well-known as hepatoprotective agents (Kiokias and Oreopoulou, 2021; Venmathi Maran et al., 2022).
Ziziphus lotus (L.) Lam. According to our ethnobotanical study conducted in several parts of the Moroccan North-East, this plant ranks sixth among the most commonly utilized herbs to cure liver disorders. Indeed, the leaves and fruits of this plant were utilized to treat jaundice, hepatic colic, and hepatitis in the research region. Previous pharmacological work has demonstrated that Z. lotus extracts exert hepatoprotective effects at the preclinical stage. In a rat investigation, an aqueous extract of Z. lotus fruits was found to have hepatoprotective properties against CCl4-induced liver damage (Bencheikh et al., 2019). The findings of this study indicate that administration of aqueous extract of Z. lotus fruits at doses of 200 and 400 mg/kg restored the biochemical parameters (liver biomarkers) altered during hepatotoxicity induced by CCl4 injections in rats. Similarly, it has been reported that treatment of rats with the aqueous extract of Z. lotus fruit at doses of 200 and 400 mg/kg protects the liver and kidney from gentamicin poisoning (Bencheikh et al., 2021b). In the literature, it has been well demonstrated that the hepatotoxicity caused by the agent CCl4 and gentamicin is related to the oxidative stress caused by these chemical compounds (Lin and Huang, 2000; Achuthan et al., 2003). In this context, several authors confirm that the use of natural antioxidants to fight against the oxidative stress caused by CCl4 and gentamicin is the best strategy to prevent hepatotoxicity produced by these hepatotoxic substances (Bencheikh et al., 2021a; Bouhrim et al., 2021; Ouahhoud et al., 2021b). Extracts of Z. lotus fruits are high in phenolic compounds such as Rutin, Naringin, Chlorogenic acid, Rosmarinic acid, Quercetin, Cat-echin, Epicatechin, Sinapic acid, Resveratrol, and Caffeic acid, according to phytochemical research (Figure 6) (Marmouzi et al., 2019; Bencheikh et al., 2021c; 2021d). These photochemical compounds thanks to their antioxidant powers could be responsible for the hypatoprotective effects.
4 Conclusion
This ethnobotanical study reveals that locals in remote areas of northern Morocco possess extensive traditional knowledge about using medicinal plants to treat liver diseases, reflecting the region’s floristic richness. The findings demonstrate the potential of these herbs in addressing liver-related health issues within these communities. However, caution is necessary when using these remedies. The study is limited by its small sample size and lack of a control group, which may affect the robustness of the conclusions.
Further research is essential to evaluate the pharmacological benefits and phytochemical components of these plants, identify active ingredients, and confirm their clinical efficacy. Additionally, safety data are needed to standardize dosages and ensure safe use. Addressing these limitations will help in the development of effective medications derived from these medicinal plants for liver disease treatment.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Author contributions
NB: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing–original draft, Writing–review and editing. AE: Formal Analysis, Investigation, Software, Validation, Writing–original draft, Writing–review and editing. AB: Formal Analysis, Investigation, Software, Writing–original draft, Writing–review and editing. MB: Data curation, Investigation, Methodology, Software, Writing–review and editing. AA: Formal Analysis, Validation, Writing–review and editing. MA: Formal Analysis, Resources, Validation, Visualization, Writing–review and editing. RM: Funding acquisition, Resources, Validation, Writing–review and editing. HA-Y: Funding acquisition, Resources, Writing–review and editing. BE: Investigation, Validation, Writing–review and editing. ME: Formal Analysis, Resources, Validation, Visualization, Writing–review and editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. Researchers Supporting Project number (RSP 2024R119), King Saud University, Riyadh, Saudi Arabia.
Acknowledgments
The authors extend their appreciation to Researchers Supporting Project number (RSP 2024R119), King Saud University, Riyadh, Saudi Arabia for funding this work.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abdulkarimi, R., Daneshyar, M., and Aghazadeh, A. (2011). Thyme (Thymus vulgaris) extract consumption darkens liver, lowers blood cholesterol, proportional liver and abdominal fat weights in broiler chickens. Ital. J. Anim. Sci. 10, e20. doi:10.4081/ijas.2011.e20
Abedi, H. A., Jahromi, H. K., Sadeghi, N., Amjadi, S. P., and Jahromi, Z. K. (2018). Evaluating the effect of aqueous extract of the roots of valuating the effect of aqueous extract of the roots of native edible asparagus in Iran (asparagus officinalis L.) on the ative edible asparagus in Iran (Asparagus officinalis L) on the concentratio. J. Fundam. Appl. Sci. 4, 9–10. doi:10.4314/jfas.v8i2s.161
Abosaleh, S., Salama, M. F., and Hassan, M. Z. (2019). The possible ameliorative effect of nigella sativa on aflatoxin-induced liver damage in chicken. Alex J. Vet. Sci. 63, 113–120. doi:10.5455/ajvs.73879
Abou-Seif, H. S., Hozayen, W. G., and Hashem, K. S. (2019). Thymus vulgaris extract modulates dexamethasone induced liver injury and restores the hepatic antioxidant redox system. Beni Suef Univ. J. Basic Appl. Sci. 8, 21–29. doi:10.1186/s43088-019-0021-0
Abozid, M. M., and Farid, H. E. A. (2018). Protective role and antioxidant activity of aqueous extract of rosmarinus officinalis against trichloroacetate-induced toxicity in liver of male rats. Asian J. Pharm. Clin. Res. 11, 420–424. doi:10.22159/ajpcr.2018.v11i6.25353
Abulnaja, K. O., and Rabey, H. A.El (2015). The efficiency of barley (hordeum vulgare) bran in ameliorating blood and treating fatty heart and liver of male rats. Evidence-Based Complementary Altern. Med. 16, 13. doi:10.1155/2015/740716
Achuthan, C. R., Babu, B. H., and Padikkala, J. (2003). Antioxidant and hepatoprotective effects of Rosa damascena. Pharm. Biol. 41, 357–361. doi:10.1076/phbi.41.5.357.15945
Adeli, M., and Samavati, V. (2014). Studies on the steady shear flow behavior and chemical properties of water-soluble polysaccharide from Ziziphus lotus fruit. Int. J. Biol. Macromol. 72, 580–587. doi:10.1016/j.ijbiomac.2014.08.047
Ahmadi, A., Heidarian, E., and Ghatreh-Samani, K. (2019). Modulatory effects of artichoke (Cynara scolymus L.) leaf extract against oxidative stress and hepatic TNF- α gene expression in acute diazinon-induced liver injury in rats. J. Basic Clin. Physiol. Pharmacol. 2019, 1–10. doi:10.1515/jbcpp-2018-0180
Alami Merrouni, I., and Elachouri, M. (2021). Anticancer medicinal plants used by Moroccan people: ethnobotanical, preclinical, phytochemical and clinical evidence. J. Ethnopharmacol. 266, 113435. doi:10.1016/j.jep.2020.113435
Alami Merrouni, I., Kharchoufa, L., Bencheikh, N., and Elachouri, M. (2021). Ethnobotanical profile of medicinal plants used by people of North-eastern Morocco: cross-cultural and historical approach (part I). Ethnobot. Res. Appl. 21, 1–45. doi:10.32859/ERA.21.34.1-45
Al-azzawi, A. F. S., and Baraaj, A. H. (2016). Histological and biochemical study of nigella sativa seeds effects on liver of male albino rats treated with rifampicin. Iraqi J. Sci. 57, 2829–2839.
Aleem, B., Hingoro, M. A., Awan, H., Aleem, F., Mastoi, A. G., and Mastor, S. M. (2020). Effects of nigella sativa extract against carbon tetrachloride induced liver injury in Swiss albino male mice. JKCD 45, 1–6.
Al-ghamdi, M. (2015). Protective effect of Nigella sativa seeds against carbon tetrachloride-induced liver damage. Am. J. Chin. Med. Gard. City N Y 31, 721–728. doi:10.1142/S0192415X03001399
Alhussaini, M. S. (2015a). Protective role of nigella sativa oil on ochratoxin A toxicity in liver and kidney of male albino rats: histological and histochemical studies. J. klagenfrt Austria 21, 59–77.
Alhussaini, M. S. (2015b). Protective role of nigella sativa oil on ochratoxin A toxicity in liver and kidney of male albino rats: histological and histochemical studies. J. klagenfrt Austria 21, 59–77.
Almakhatreh, M., Hafez, E., Tousson, E., and Masoud, A. (2019). Biochemical and molecular studies on the role of rosemary (rosmarinus officinalis) extract in reducing liver and kidney toxicity due to etoposide in male rats. Asian J. Res. Med. Pharm. Sci. 7, 1–11. doi:10.9734/ajrimps/2019/v7i430126
Almalki, D. A. (2022). Hepatoprotective Effect of Lavandula dentata leaves extracts on Thioacetamide- Induced hepatic fibrosis in male albino mice. Curr. Sci. Int. 11, 217–223. doi:10.36632/csi/2022.11.2.16
Al-Olayan, E. M., El-Khadragy, M. F., Alajmi, R. A., Othman, M. S., Bauomy, A. A., Ibrahim, S. R., et al. (2016). Ceratonia siliqua pod extract ameliorates Schistosoma mansoni-induced liver fibrosis and oxidative stress. BMC Complement. Altern. Med. 16, 434–511. doi:10.1186/s12906-016-1389-1
Al-Qura’n, S. (2005). Ethnobotanical survey of folk toxic plants in southern part of Jordan. Toxicon 46, 119–129. doi:10.1016/j.toxicon.2005.04.010
Al-Razzuqi, R. A., Al-Jawad, F. H., and Al- Hussaini, A., (2012). Hepatoprotective effect of Glycyrrhiza glabra in carbon tetrachloride-induced model of acute liver injury. J. Phys. Pharm. Adv. 7, 259–263.
Al-razzuqi, R. A. M., Al-hussaini, J. A., and Al-jeboori, A. A. (2011). Protective effect of Nigella sativa against carbon tetrachlorideinduced acute liver injury in experimental rabbit models. Int. J. Green Pharm. 41, 198–200. doi:10.4103/0973-8258.91227
Al-snafi, A. E. (2017). The pharmacological activities of Cuminum cyminum - a review. IOSR J. Pharm. 6, 46–65.
Amat, N., Upur, H., and Blažeković, B. (2010). In vivo hepatoprotective activity of the aqueous extract of Artemisia absinthium L. against chemically and immunologically induced liver injuries in mice. J. Ethnopharmacol. 131, 478–484. doi:10.1016/j.jep.2010.07.023
Anbalagan, N., Mallika, M., Kuruvilla, S., Prasad, M. V. V., Patra, A., and Balakrishna, K. (2007). Antioxidant and hepatoprotective activities of alcoholic extract of Terminalia arjuna. Nat. Product. Sci. 13, 105–109.
Andallu, B., and Ramya, V. (2007). Anti-hyperglycemic, cholesterol-lowering and HDL–raising effects of cumin (Cuminum cyminum) seeds in type 2 diabetes. J. Nat. Remedies 7, 142–149. doi:10.18311/jnr/2007/207
Antiya, M. C., Eteng, O. E., Alimi, M. A., Adeyi, O., Adeyi, E. O., Okolo, I., et al. (2021). Hepatoprotective effect of ethyl acetate extract of Curcuma longa on alcohol-induced liver damage in female Wistar rats. Biokemistri 33, 97–104.
Antonio, S. G. W., Silva-Correa Carmen, R., Villarreal-La Torre Víctor, E., Cruzado-Razco José, L., Calderón-Peña Abhel, A., Aspajo-Villalaz Cinthya, L., et al. (2020). Hepatoprotective and nephroprotective activity of artemisia absinthium l. On diclofenac-induced toxicity in rats. Pharmacogn. J. 12, 1032–1041. doi:10.5530/PJ.2020.12.146
Aouad, R. F., Boufadi, M. Y., Adli, D. E. H., Moulai-Hacene, F., Kahloula, K., and Slimani, M. (2021). Chemical composition and protective effect of rosmarinus officinalis on alcohol-induced serum hepatic changes and liver injury in male rats. Pharmacogn. J. 13, 1205–1215. doi:10.5530/pj.2021.13.154
A.P.G III (2009). An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG III. Botanical J. Linn. Soc. 161, 105–121. doi:10.1111/j.1095-8339.2009.00996.x
Aprioku, J., and Amah-Tariah, F. (2017a). Garlic (Allium sativum L.) protects hepatic and renal toxicity of alloxan in rats. J. Pharm. Res. Int. 17, 1–7. doi:10.9734/jpri/2017/34909
Aprioku, J., and Amah-Tariah, F. (2017b). Garlic (Allium sativum L.) protects hepatic and renal toxicity of alloxan in rats. J. Pharm. Res. Int. 17, 1–7. doi:10.9734/jpri/2017/34909
Article, O., Khazdair, M. R., Mohebbati, R., Karimi, S., and Abbasnezhad, A. (2024). The protective effects of Curcuma longa extract on oxidative stress markers in the liver induced by Adriamycin in rats. Physiology Pharmacol. 20, 31–37.
Asala, T. M., Abatan, M. O., Salami, S. A., Oluwatosin, O., Akanbi, O. B., Rowaiye, A. B., et al. (2021). The ameliorative effect of the solvent extracts of Ocimum basilicum against acetaminophen-induced liver damage in albino rats. J. Phytomedicine Ther. 20, 615–623. doi:10.4314/jopat.v20i1.4
Asrani, S. K., Devarbhavi, H., Eaton, J., and Kamath, P. S. (2019). Burden of liver diseases in the world. J. Hepatol. 70, 151–171. doi:10.1016/j.jhep.2018.09.014
Azeem, E. M. A.El, Alaa, B., and Zakaria, Z. (2016). Anti-obesity and anti-fatty liver effects of cynara scolymus L. Leaf extract in mice under diet-induced obesity. Int. J. Biochem. Res. Rev. 11, 1–11. doi:10.9734/IJBCRR/2016/23807
Azizi, M., Abbasi, N., Mohamadpour, M., Bakhtiyari, S., Asadi, S., Shirzadpour, E., et al. (2019). Investigating the effect of Crocus sativus L. Petal hydroalcoholic extract on inflammatory and enzymatic indices resulting from alcohol use in kidney and liver of male rats. J. Inflamm. Res. 12, 269–283. doi:10.2147/JIR.S216125
Baghdadi, H. H., El-Demerdash, F. M., Hussein, S., and Radwan, E. H. (2016). The protective effect of Coriandrum sativum L. oil against liver toxicity induced by Ibuprofen in rats. J. Biosci. Appl. Res. 2, 197–202. doi:10.21608/jbaar.2016.106944
Bagul, M., Kakumanu, S., and Wilson, T. A. (2015). Crude garlic extract inhibits cell proliferation and induces cell cycle arrest and apoptosis of cancer cells in vitro. J. Med. Food 18, 731–737. doi:10.1089/jmf.2014.0064
Bandegi, A. R., Rashidy-pour, A., Vafaei, A. A., and Ghadrdoost, B. (2014). Protective effects of crocus sativus L. Extract and crocin against chronic-stress induced oxidative damage of brain, liver and kidneys in rats. Adv. Pharm. Bull. 4, 493–499. doi:10.5681/apb.2014.073
Bayomy, M. F. F., Sakr, S. A., and M, G. S. E. (2016). Biochemical and histological studies on the possible protective impact of the herb basil (Ocimum basilicum) on adriamycin induced toxicity in rats. I. Influence on the liver. J. Biosci. Appl. Res. 2, 634–640. doi:10.21608/jbaar.2016.109005
Bellakhdar, J., Claisse, R., Fleurentin, J., and Younos, C. (1991). Repertory of standard herbal drugs in the Moroccan pharmacopoeia. J. Ethnopharmacol. 35, 123–143. doi:10.1016/0378-8741(91)90064-k
Ben Akka, F., Salhi, S., Benkhnigue, O., Dahmani, J., Douira, A., and Zidane, L. (2019). Ethnobotanical study of medicinal plants used in the region of Middle Oum Rbia (Morocco). Plant Arch. 19, 2005–2017.
Benarba, B. (2016). Medicinal plants used by traditional healers from South-West Algeria: an ethnobotanical study. J. Intercult. Ethnopharmacol. 5, 320–330. doi:10.5455/jice.20160814115725
Bencheikh, N., Bouhrim, M., Kharchoufa, L., Al Kamaly, O. M., Mechchate, H., Es-safi, I., et al. (2021a). The nephroprotective effect of zizyphus lotus L. (desf.) fruits in a gentamicin-induced acute kidney injury model in rats: a biochemical and histopathological investigation. Molecules 26, 4806. doi:10.3390/molecules26164806
Bencheikh, N., Bouhrim, M., Kharchoufa, L., Choukri, M., Bnouham, M., and Elachouri, M. (2019). Protective effect of Zizyphus lotus L.(Desf.) fruit against CCl4-induced acute liver injury in rat. Evidence-based Complementary Altern. Med. 2019, 6161593. doi:10.1155/2019/6161593
Bencheikh, N., Bouhrim, M., Kharchoufa, L., Kamaly, O., Al, M., Mechchate, H., et al. (2021b). The nephroprotective effect of Zizyphus lotus L. (desf.) fruits in a gentamicin-induced acute kidney injury model in rats: a biochemical and histopathological investigation. Molecules 26, 4806. doi:10.3390/molecules26164806
Bencheikh, N., Bouhrim, M., Kharchoufa, L., Kamaly, O., Al, M., Mechchate, H., et al. (2021c). The nephroprotective effect of Zizyphus lotus L. (desf.) fruits in a gentamicin-induced acute kidney injury model in rats: a biochemical and histopathological investigation. Molecules 26, 4806. doi:10.3390/molecules26164806
Bencheikh, N., Bouhrim, M., Merrouni, I. A., Boutahiri, S., Legssyer, A., Elachouri, M., et al. (2021d). Antihyperlipidemic and antioxidant activities of flavonoid-rich extract of Ziziphus lotus (L.) Lam. Fruits. Appl. Sci. 11, 7788. doi:10.3390/app11177788
Bencheikh, N., Elachouri, M., and Subhash, C. M. (2022). Ethnobotanical, pharmacological, phytochemical, and clinical investigations on Moroccan medicinal plants traditionally used for the management of renal dysfunctions. J. Ethnopharmacol. 292, 115178. doi:10.1016/j.jep.2022.115178
Bencheikh, N., Elbouzidi, A., Kharchoufa, L., Ouassou, H., Merrouni, I. A., Mechchate, H., et al. (2021e). Inventory of medicinal plants used traditionally to manage kidney diseases in north-eastern Morocco: ethnobotanical fieldwork and pharmacological evidence. Plants 10, 1966. doi:10.3390/plants10091966
Bencheikh, N., Radi, F. Z., Fakchich, J., Elbouzidi, A., and Ouahhoud, S. (2023). Pharmacological properties of Ziziphus lotus (L.) Lam.: a comprehensive review. Pharmaceuticals 16, 36. doi:10.3390/ph16040575
Bendaif, H., Harir, M., Yahiaoui, M., Souilah, N., Hechaichi, F., Zohra Djamel Miara, M., et al. (2021). Ethnobotanical survey of herbal remedies traditionally used in El Hammadia (Southern region of the province of Bordj Bou Arreridj, Algeria). Algerian J. Biosci. 02, 6–15.
Benítez, G., González-Tejero, M. R., and Molero-Mesa, J. (2010). Pharmaceutical ethnobotany in the western part of Granada province (southern Spain): ethnopharmacological synthesis. J. Ethnopharmacol. 129, 87–105. doi:10.1016/j.jep.2010.02.016
Ben Saad, A., Rjeibi, I., Alimi, H., Ncib, S., Bouhamda, T., and Zouari, N. (2018). Protective effects of Mentha Spicata against nicotine-induced toxicity in liver and erythrocytes of Wistar rats. Appl. Physiology, Nutr. Metabolism 43, 77–83. doi:10.1139/apnm-2017-0144
Bhagawan, W. S., Ekasari, W., and Agil, M. (2023a). Ethnopharmacology of medicinal plants used by the tenggerese community in bromo tengger semeru national Park, Indonesia. Biodiversitas 24. doi:10.13057/biodiv/d241028
Bhagawan, W. S., Ekasari, W., and Agil, M. (2023b). Ethnopharmacology of medicinal plants used by the tenggerese community in bromo tengger semeru national Park, Indonesia. Biodiversitas J. Biol. Divers. 24. doi:10.13057/biodiv/d241028
Bhagawan, W. S., Ekasari, W., and Agil, M. (2024). Ethnobotanical survey of herbal steam baths among the tenggerese community in bromo tengger semeru national Park, Indonesia, IOP Conf. Ser. Earth Environ. Sci. 1352, 12103.doi:10.1088/1755-1315/1352/1/012103
Bhagawan, W. S., Suproborini, A., Putri, D. L. P., Nurfatma, A., and Putra, R. T. (2022). Ethnomedicinal study, phytochemical characterization, and pharmacological confirmation of selected medicinal plant on the northern slope of Mount Wilis, East Java, Indonesia. Biodiversitas J. Biol. Divers. 23. doi:10.13057/biodiv/d230855
Bouasla, I., Bouasla, A., Boumendjel, A., Messarah, M., Abdennour, C., Boulakoud, M. S., et al. (2014). Nigella sativa oil reduces aluminium chloride-induced oxidative injury in liver and erythrocytes of rats. Biol. Trace Elem. Res. 43, 252–261. doi:10.1007/s12011-014-0114-5
Bouhrim, M., Bencheikh, N., Imtara, H., Daoudi, N. E., Mechchate, H., Ouassou, H., et al. (2021). Protective effect of opuntia dillenii (ker gawl.) haw. Seed oil on gentamicin-induced nephrotoxicity: a biochemical and histological analysis. Sci. World J. 7, 2173012. doi:10.1155/2021/2173012
Boutlelis, D. A., Mounia, B., Salah, B., and Bordjiba, O. (2020). Antioxidant and hepatoprotective Potential of Coriandrum sativum L. against hepatic injury by Lambda-cyhalothrin insecticide. J. Drug Deliv. Ther. 10, 182–188. doi:10.22270/jddt.v10i3-s.4186
Bradley, J. M., Organ, C. L., and Lefer, D. J. (2016). Garlic-derived organic polysulfides and myocardial protection. J. Nutr. 146, 403S-409S–409S. doi:10.3945/jn.114.208066
Ch, N., Vardhan, A., and Reddy, D. (2015). Protective effect of aqueous bark extract of Terminalia arjunaa against alcohol-induced hepato and nephrotoxicity in rats. Intern Natl. J. Phytomedicine 7, 142–153.
Chester, K., Zahiruddin, S., Ahmad, A., Khan, W., Paliwal, S., and Ahmad, S. (2017). Antioxidant effect of Terminalia arjuna extract against acetaminophen-induced hepatotoxicity via the regulation of cytochrome P450 2E1, phosphatidylinositol-3-kinase/protein kinase B. Pharmacogn. Mag. 13 (Suppl. l), 179–188. doi:10.4103/pm.pm
Chithra, M. A., Ijinu, T. P., Kharkwal, H., Sharma, R. K., Janardhanan, K. K., Pushpangadan, P., et al. (2020). Cocos nucifera l. Inflorescence extract: an effective hepatoprotective agent. Indian J. Traditional Knowl. 19, 128–136.
Cikman, O., Ozkan, A., Alkis, H., and Taysi, S. (2014). Radioprotective effects of nigella sativa oil against oxidative stress in liver tissue of rats exposed to total head irradiation. J. of Investigative Surg. 27, 262–266. doi:10.3109/08941939.2014.898811
Coban, S., Yildiz, F., Terzi, A., Al, B., Aksoy, N., Bitiren, M., et al. (2010). The effects of Nigella sativa on bile duct ligation induced-liver injury in rats. Cell. Biochem. Funct. 28, 83–88. doi:10.1002/cbf.1624
Colak, E., Ustuner, M. C., Tekin, N., Colak, E., Burukoglu, D., Degirmenci, I., et al. (2016). The hepatocurative effects of Cynara scolymus L. leaf extract on carbon tetrachloride-induced oxidative stress and hepatic injury in rats. Springerplus 5, 216–219. doi:10.1186/s40064-016-1894-1
Corsi, L., Avallone, R., Cosenza, F., Farina, F., Baraldi, C., and Baraldi, M. (2002). Antiproliferative effects of Ceratonia siliqua L. on mouse hepatocellular carcinoma cell line. Fitoterapia 73, 674–684. doi:10.1016/s0367-326x(02)00227-7
Danladi, J., Abdulsalam, A., Timbuak, J. A., Ahmed, S. A., Mairiga, A. A., and Dahiru, A. U. (2013). Hepatoprotective effect of black seed (nigella sativa) oil on carbon tetrachloride (CCl4) induced liver toxicity in adult wistar rats hepatoprotective effect of black seed (nigella sativa) oil on carbon tetrachloride (CCl 4) induced liver toxicity in. J. Dent. Med. Sci. (IOSR-JDMS) 4, 56–62. doi:10.9790/0853-0435662
Develi, S., Evran, B., Kalaz, E. B., Koçak-toker, N., and Erata, G. Ö. (2014). Protective effect of Nigella sativa oil against binge ethanol-induced oxidative stress and liver injury in rats. Chin. J. Nat. Med. 12, 495–499. doi:10.1016/S1875-5364(14)60077-7
Dinakar, A., Swarnalatha, D., Kumar, R. P., Alekya, K., Kumar, S. P., Kumar, D. V., et al. (2010). Inhibition of thioacetamide – induced liver fibrosis by piper nigrum linn. J. Glob. Trends Pharm. Sci. 1, 1–8.
Diretto, G., Rubio-Moraga, A., Argandoña, J., Castillo, P., Gómez-Gómez, L., and Ahrazem, O. (2017). Tissue-specific accumulation of sulfur compounds and saponins in different parts of garlic cloves from purple and white ecotypes. Molecules 22, 1359. doi:10.3390/molecules22081359
Doorika, P., and Ananthi, T. (2002). Antioxidant and hepatoprotective properties of Terminalia arjuna bark on isoniazed induced toxicity in albino rats. Asian J. Pharm. Technol. 2, 15–18.
Dutta, M., Chattopadhyay, A., Bose, G., Ghosh, A., Banerjee, A., Ghosh, A., et al. (2014). Aqueous bark extract of Terminalia arjuna protects against high fat diet aggravated arsenic-induced oxidative stress in rat heart and liver, involvement of antioxidant mechanisms. J. Pharm. Res. 8, 1285–1302.
Eddouks, M., Ajebli, M., and Hebi, M. (2017). Ethnopharmacological survey of medicinal plants used in Daraa-Tafilalet region (Province of Errachidia), Morocco. J. Ethnopharmacol. 198, 516–530. doi:10.1016/j.jep.2016.12.017
El Hassani, M., Douiri, E. M., Bammi, J., Zidane, L., Badoc, A., and Douira, A. (2013). Plantes médicinales de la Moyenne Moulouya (Nord-Est du Maroc). Ethnopharmacologia 50, 39.
El-Mesallamy, A. M. D., Abdel-Hamid, N., Srour, L., and Hussein, S. A. M. (2020). Identification of polyphenolic compounds and hepatoprotective activity of artichoke (Cynara scolymus L.) edible part extracts in rats. Egypt J. Chem. 63, 2273–2285. doi:10.21608/ejchem.2020.22707.2348
El Shahat, A. N., El-Shennawy, H. M., and Abd El-Megid, M. H. M. (2017). Studying the protective effect of gamma-irradiated basil (Ocimum basilicum L.) against methotrexate-induced liver and renal toxicity in rats. Indian J. Anim. Res. 51, 135–140. doi:10.18805/ijar.9631
Erisgin, Z., Atasever, M., Cetinkaya, K., Özen, S., Dizakar, A., Omeroglu, S., et al. (2019). Protective effects of Nigella sativa oil against carboplatin-induced liver damage in rats. Biomed. and Pharmacother. 110, 742–747. doi:10.1016/j.biopha.2018.12.037
Fakchich, J., and Elachouri, M. (2021). An overview on ethnobotanico-pharmacological studies carried out in Morocco, from 1991 to 2015: systematic review (part 1). J. Ethnopharmacol. 267, 113200–200. doi:10.1016/j.jep.2020.113200
Fan, R., Yuan, F., Wang, N., Gao, Y., and Huang, Y. (2015). Extraction and analysis of antioxidant compounds from the residues of Asparagus officinalis L. J. Food Sci. Technol. 52, 2690–2700. doi:10.1007/s13197-014-1360-4
Farooqui, Z., Afsar, M., Rizwan, S., Ahmed, A., and Khan, F. (2016a). Oral administration of Nigella sativa oil ameliorates the effect of cisplatin on membrane enzymes, carbohydrate metabolism and oxidative damage in rat liver. Toxicol. Rep. 3, 328–335. doi:10.1016/j.toxrep.2016.02.004
Farooqui, Z., Afsar, M., Rizwan, S., Ahmed, A., and Khan, F. (2016b). Oral administration of Nigella sativa oil ameliorates the effect of cisplatin on membrane enzymes, carbohydrate metabolism and oxidative damage in rat liver. Toxicol. Rep. 3, 328–335. doi:10.1016/j.toxrep.2016.02.004
Fennane, M., Ibn Tattou, M., Mathez, J., Ouyahya, A., and El Oualidi, J. (1999). Flore Pratique du Maroc. Pteridophyta, Gymnospermae, Angiospermae (LauraceaeNeuradaceae) Man. détermination plantes vasculaires.Travaux l’Institut Sci. série Bot. n°36. Rabat 1.
Fennane, M., Ibn Tattou, M., Ouyahya, A., and El Oualidi, J. (2007). Flore Pratique du Maroc. Angiospermae (Leguuminosae – Lentibulariaceae) Man. détermination plantes vasculaires.Travaux l’Institut Sci. série Bot. n°38. Rabat 2.
Fennane, M., Ibn Tattou, M., Ouyahya, A., and El Oualidi, J. (2014). Manuel de Détermination des plantes vasculaires.Travaux de l’Institut Scientifique, série botanique n°40. Rabat. Dicotyledones (P.P), Monocotyledones 3.
Fuentes-Alventosa, J. M., Jaramillo-Carmona, S., Rodríguez-Gutiérrez, G., Guillén-Bejarano, R., Jiménez-Araujo, A., Fernández-Bolaños, J., et al. (2013). Preparation of bioactive extracts from asparagus by-product. Food Bioprod. Process. 91, 74–82. doi:10.1016/j.fbp.2012.12.004
Ganaie, M. A., Khan, T. H., Siddiqui, N. A., and Ansari, M. N. (2015). Ameliorative effect of methanol extract of Rumex vesicarius on CCl4-induced liver damage in Wistar albino rats. Pharm. Biol. 53, 1163–1167. doi:10.3109/13880209.2014.967782
Gasparyan, G., Tiratsuyan, S., Sh, K., and Vardapetyan, H. (2015). Effect of Laurus nobilis extract on the functioning of liver against Ccl4 induced toxicity. J. Exp. Biol. Agric. Sci. 3, 174–183. doi:10.18006/2015.3(2).174.183
Gebhardt, R. (1998). Inhibition of cholesterol biosynthesis in primary cultured rat hepatocytes by artichoke (cynara scolymus L.) extracts. J. Pharmacol. Exp. Ther. 286, 1122–1128.
Gholami-ahangaran, M., Rangsaz, N., and Azizi, S. (2016). Evaluation of turmeric (Curcuma longa) effect on biochemical and pathological parameters of liver and kidney in chicken aflatoxicosis. Pharm. Biol. 209, 780–787. doi:10.3109/13880209.2015.1080731
Ghorbani, A., and Esmaeilizadeh, M. (2017). Pharmacological properties of Salvia officinalis and its components. J. Tradit. Complement. Med. 7, 433–440. doi:10.1016/j.jtcme.2016.12.014
Ghosh, J., Das, J., Manna, P., and Sil, P. C. (2010a). Protective effect of the fruits of Terminalia arjuna against cadmium-induced oxidant stress and hepatic cell injury via MAPK activation and mitochondria dependent pathway. Food Chem. 123, 1062–1075. doi:10.1016/j.foodchem.2010.05.062
Ghosh, J., Das, J., Manna, P., and Sil, P. C. (2010b). Protective effect of the fruits of Terminalia arjuna against cadmium-induced oxidant stress and hepatic cell injury via MAPK activation and mitochondria dependent pathway. Food Chem. 123, 1062–1075. doi:10.1016/j.foodchem.2010.05.062
Hachlafi, N.El, Benkhaira, N., Ferioun, M., Kandsi, F., Jeddi, M., Chebat, A., et al. (2022). Moroccan medicinal plants used to treat cancer: ethnomedicinal study and insights into pharmacological evidence. Evidence-Based Complementary Altern. Med. 19. doi:10.1155/2022/1645265
Hamza, R. Z., and Al-harbi, M. S. (2015). Amelioration of paracetamol hepatotoxicity and oxidative stress on mice liver with silymarin and Nigella sativa extract supplements. Asian Pac J. Trop. Biomed. 5, 521–531. doi:10.1016/j.apjtb.2015.03.011
Harach, T., Aprikian, O., Monnard, I., Moulin, J., Membrez, M., Béolor, J. C., et al. (2010). Rosemary (Rosmarinus officinalis L.) Leaf extract limits weight gain and liver steatosis in mice fed a high-fat diet. Planta Med. 76, 566–571. doi:10.1055/s-0029-1240612
Hoffman, B., and Gallaher, T. (2007). Importance indices in ethnobotany. Ethnobot. Res. Appl. 5, 201–218. doi:10.17348/era.5.0.201-218
Hoshyar, R., Sebzari, A., Balforoush, M., Valavi, M., and Hosseini, M. (2019). The impact of Crocus sativus stigma against methotrexate-induced liver toxicity in rats. J. ofComplementary IntegrativeMedicine 13, 1–9. doi:10.1515/jcim-2019-0201
Hsouna, A. B., Saoudi, M., Trigui, M., Jamoussi, K., Boudawara, T., Jaoua, S., et al. (2011). Characterization of bioactive compounds and ameliorative effects of Ceratonia siliqua leaf extract against CCl₄ induced hepatic oxidative damage and renal failure in rats. Food Chem. Toxicol. 49, 3183–3191. doi:10.1016/j.fct.2011.09.034
Huseini, F. H., Mahmoudabady, Z. M., Ziai, S. A., Mehrazma, M., Alavian, S. M., Mehdizadeh, M., et al. (2011). The effects of cynara scolymus L. Leaf and cichorium intybus L. Root extracts on carbon tetrachloride induced liver toxicity in rats fallah. J. Med. Plants 10, 33–40.
Husna, M., and Sajjad, S. (2017). The effect of crude nigella sativa oil against the acute toxicity of diclofenac sodium and ibuprofen on the liver of albino mice. Slov. Vet. Res. 54, 20–27.
Hussain, M., Tunio, A. G., Akhtar, L., and Shaikh, G. S. (2017). Effects of nigella sativa on various parameters in patients of non-alcoholic fatty liver disease. J. Ayub Med. Coll. Abbottabad 29, 403–407.
International Society of Ethnobiology (2006). ISE Code of Ethics (with 2008 additions). J. Am. Med. Assoc. doi:10.1001/jama.1893.02420400024007
Iqbal, M., Butt, M., Shehzad, A., and Asghar, M. (2018). Evaluating therapeutic potential of coriander seeds and leaves (Coriandrum sativum L.) to mitigate carbon tetrachloride-induced hepatotoxicity in rabbits. Asian Pac J. Trop. Med. 11, 209–213. doi:10.4103/1995-7645.228435
Jamila, F., and Mostafa, E. (2014). Ethnobotanical survey of medicinal plants used by people in Oriental Morocco to manage various ailments. J. Ethnopharmacol. 154, 76–87. doi:10.1016/j.jep.2014.03.016
Jang, D. S., Cuendet, M., Fong, H. H. S., Pezzuto, J. M., and Kinghorn, A. D. (2004). Constituents of Asparagus officinalis evaluated for inhibitory activity against cyclooxygenase-2. J. Agric. Food Chem. 52, 2218–2222. doi:10.1021/jf0305229
Jebur, A. B., El-Sayed, R. A., and El-Demerdash, F. M. (2022). Ocimum basilicum essential oil modulates hematotoxicity, oxidative stress, DNA damage, and cell cycle arrest induced by β-cyfluthrin in rat liver. Front. Pharmacol. 12, 784281–784314. doi:10.3389/fphar.2021.784281
Jedidi, S., Aloui, F., Selmi, S., Selmi, H., Sammari, H., Ayari, A., et al. (2022a). Antioxidant properties of Salvia officinalis decoction extract and mechanism of its protective effects on ethanol-induced liver and kidney injuries. J. Med. Food 25, 546–556. doi:10.1089/jmf.2021.0134
Jedidi, S., Aloui, F., Selmi, S., Selmi, H., Sammari, H., Ayari, A., et al. (2022b). Antioxidant properties of Salvia officinalis decoction extract and mechanism of its protective effects on ethanol-induced liver and kidney injuries. J. Med. Food 25, 546–556. doi:10.1089/jmf.2021.0134
Johri, R. K. (2011). Cuminum cyminum and Carum carvi: an update. Pharmacogn. Rev. 5, 63–72. doi:10.4103/0973-7847.79101
Juan-García, A., Agahi, F., Drakonaki, M., Tedeschi, P., Font, G., and Juan, C. (2021a). Cytoprotection assessment against mycotoxins on HepG2 cells by extracts from Allium sativum L. Food Chem. Toxicol. 151, 112129. doi:10.1016/j.fct.2021.112129
Juan-García, A., Agahi, F., Drakonaki, M., Tedeschi, P., Font, G., and Juan, C. (2021b). Cytoprotection assessment against mycotoxins on HepG2 cells by extracts from Allium sativum L. Food Chem. Toxicol. 151, 112129. doi:10.1016/j.fct.2021.112129
Kadir, M. F., Bin Sayeed, M. S., and Mia, M. M. K. (2013). Ethnopharmacological survey of medicinal plants used by traditional healers in Bangladesh for gastrointestinal disorders. J. Ethnopharmacol. 147, 148–156. doi:10.1016/j.jep.2013.02.023
Kannappan, S. G. P., Raghunath, G., Sivanesan, S., and Vijayaraghavan, R. (2020). A study on the inhibition of oxidative stress, inflammation and apoptosis by Terminalia arjuna against acetaminophen-induced hepatotoxicity in wistar albino rats. Indian J. Biochem. Biophys. 57, 51–57. doi:10.56042/ijbb.v57i1.26107
Kanter, M., Coskun, O., and Budancamanak, M. (2005). Hepatoprotective effects of Nigella sativa L and Urtica dioica L on lipid peroxidation, antioxidant enzyme systems and liver enzymes in carbon tetrachloride-treated rats. World J. Gastroenterology ISSN 11, 6684–6688. doi:10.3748/wjg.v11.i42.6684
Khouchlaa, A., Talbaoui, A., El Yahyaoui El Idrissi, A., Bouyahya, A., Ait Lahsen, S., Kahouadji, A., et al. (2017a). Détermination des composés phénoliques et évaluation de l’activité litholytique in vitro sur la lithiase urinaire d’extrait de Zizyphus lotus L. d’origine marocaine. Phytotherapie, 1–6. doi:10.1007/s10298-017-1106-3
Khouchlaa, A., Tijane, M., Chebat, A., Hseini, S., and Kahouadji, A. (2017b). Ethnopharmacology study of medicinal plants used in the treatment of urolithiasis (Morocco). Phytotherapie 15, 274–287. doi:10.1007/s10298-016-1073-4
Khouja, H. (2017). Turmeric (curcuma longa) protection against the liver toxicity caused by aluminum chloride (AlCl3) in adult male rats. Int. J. Pharm. Res. and Allied Sci. 6, 110–127.
Kim, M. S., Koppula, S., Sung, S. J., Lee, S. R., Park, Y. D., Lee, K. A., et al. (2014). Olea europaea Linn (Oleaceae) fruit pulp exhibits hypocholesterolemic and hepatoprotective effects via regulation of peroxisome proliferation-activated receptor alpha in high-fat diet-fed rats. Trop. J. Pharm. Res. 13, 31–39. doi:10.4314/tjpr.v13i1.5
Kingsley, U. I. (2020). Ameliorative effect of hydroalcoholic extracts of Nigella sativa seed against CCl4-induced acute liver injury in rats. J. Drug Deliv. Ther. 10, 164–169. doi:10.22270/jddt.v10i3.4006
Kiokias, S., and Oreopoulou, V. (2021). A review of the health protective effects of phenolic acids against a range of severe pathologic conditions (including coronavirus-based infections). Molecules 26, 5405. doi:10.3390/molecules26175405
Kodera, Y., Ushijima, M., Amano, H., Suzuki, J., and Matsutomo, T. (2017). Chemical and biological properties of S-1-propenyl-l-cysteine in aged garlic extract. Molecules 22, 570. doi:10.3390/molecules22040570
Kotoky, J., and Das, P. N. (2008). Medicinal plants used for liver diseases in some parts of Kamrup district of Assam, a North Eastern State of India. Fitoterapia 79, 384–387. doi:10.1016/j.fitote.2008.02.003
Koubaa, F. G., Chaâbane, M., Turki, M., Ayadi, F. M., and El Feki, A. (2021a). Antioxidant and hepatoprotective effects of Salvia officinalis essential oil against vanadium-induced oxidative stress and histological changes in the rat liver. Environ. Sci. Pollut. Res. 28, 11001–11015. doi:10.1007/s11356-020-11303-z
Koubaa, F. G., Chaâbane, M., Turki, M., Ayadi, F. M., and El Feki, A. (2021b). Antioxidant and hepatoprotective effects of Salvia officinalis essential oil against vanadium-induced oxidative stress and histological changes in the rat liver. Environ. Sci. Pollut. Res. 28, 11001–11015. doi:10.1007/s11356-020-11303-z
Koubaa-Ghorbel, F., Chaâbane, M., Turki, M., Makni-Ayadi, F., and El Feki, A. (2020a). The protective effects of Salvia officinalis essential oil compared to simvastatin against hyperlipidemia, liver, and kidney injuries in mice submitted to a high-fat diet. J. Food Biochem. 44, 131600–e132. doi:10.1111/jfbc.13160
Koubaa-Ghorbel, F., Chaâbane, M., Turki, M., Makni-Ayadi, F., and El Feki, A. (2020b). The protective effects of Salvia officinalis essential oil compared to simvastatin against hyperlipidemia, liver, and kidney injuries in mice submitted to a high-fat diet. J. Food Biochem. 44, 131600–e132. doi:10.1111/jfbc.13160
Kozics, K., Klusová, V., Srančíková, A., Mučaji, P., Slameňová, D., Hunáková, Ľ., et al. (2013a). Effects of Salvia officinalis and Thymus vulgaris on oxidant-induced DNA damage and antioxidant status in HepG2 cells. Food Chem. 141, 2198–2206. doi:10.1016/j.foodchem.2013.04.089
Kozics, K., Klusová, V., Srančíková, A., Mučaji, P., Slameňová, D., Hunáková, Ľ., et al. (2013b). Effects of Salvia officinalis and Thymus vulgaris on oxidant-induced DNA damage and antioxidant status in HepG2 cells. Food Chem. 141, 2198–2206. doi:10.1016/j.foodchem.2013.04.089
Kpodar, M. S., Karou, S. D., Katawa, G., Anani, K., Gbekley, H. E., Adjrah, Y., et al. (2016). An ethnobotanical study of plants used to treat liver diseases in the Maritime region of Togo. J. Ethnopharmacol. 181, 263–273. doi:10.1016/j.jep.2015.12.051
Labiad, H., Et-tahir, A., Ghanmi, M., Satrani, B., Aljaiyash, A., Chaouch, A., et al. (2020). Ethnopharmacological survey of aromatic and medicinal plants of the pharmacopoeia of northern Morocco. Ethnobot. Res. Appl. 19, 1–16. doi:10.32859/era.19.45.1-16
Laylani, L. A.-A.-S. S. (2016). Hepatoprotective effect of Glycyrrhiza glabra L. Extracts against carbon tetrachloride-induced acute liver damage in rats. Int. J. Veterinary Sci. Med. and Res. 1, 1–8.
Lin, C. C., and Huang, P. C. (2000). Antioxidant and hepatoprotective effects of Acathopanax senticosus. Phytotherapy Res. 14, 489–494. doi:10.1002/1099-1573(200011)14:7<489::AID-PTR656>3.0.CO;2-G
Löhr, G., Deters, A., and Hensel, A. (2009). In vitro investigations of Cynara scolymus L. extract on cell physiology of HepG2 liver cells. Braz. J. Pharm. Sci. 45, 201–208. doi:10.1590/s1984-82502009000200003
Lu, Y., and Foo, L. Y. (2002). Polyphenolics of salvia—a review. Phytochemistry 59, 117–140. doi:10.1016/s0031-9422(01)00415-0
Lucarini, R., Bernardes, W. A., Tozatti, M. G., Filho, A. A. da S., Silva, M. L. A., Momo, C., et al. (2014). Hepatoprotective effect of Rosmarinus officinalis and rosmarinic acid on acetaminophen-induced liver damage. Emir J. Food Agric. 26, 878–884. doi:10.9755/ejfa.v26i10.17836
Maameri, Z., Djerrou, Z., Halmi, S., Djaalab, H., Riachi, F., and Hamdipacha, Y. (2015). Evaluation of hepatoprotective effect of Pistacia lentiscus L. Fatty oil in rats intoxicated by carbon tetrachloride. Int. J. Pharmacogn. Phytochemical Res. 7, 251–254.
Mahdi, S., Turfi, A., Mohammed, A., and Chasib Jabal, B. (2022). Evaluation of the effect of alcoholic extract of Laurus nobilis leaves on blood biochemical parameters and histological changes in the liver and kidney among female wistar rats treated with depakene (sodium valproate). Arch. Razi Inst. 77, 981–989. doi:10.22092/ARI.2022.357272.2011
Mahmoud, M. R., El-abhar, H. S., and Saleh, S. (2002). The effect of Nigella sativa oil against the liver damage induced by Schistosoma mansoni infection in mice. J. Ethnopharmacol. 79, 1–11. doi:10.1016/s0378-8741(01)00310-5
Mallhi, T. H., Abbas, K., Ali, M., Qadir, M. I., Saleem, M., and Khan, Y. H. (2014). Hepatoprotective activity of methanolic extract of Malva parviflora against paracetamol-induced hepatotoxicity in mice. Bangladesh J. Pharmacol. 9, 342–346. doi:10.3329/bjp.v9i3.19105
Manna, P., Sinha, M., and Sil, P. C. (2006). Aqueous extract of Terminalia arjuna prevents carbon tetrachloride induced hepatic and renal disorders. BMC Complement. Altern. Med. 6, 33–10. doi:10.1186/1472-6882-6-33
Mansingh, D. P., Dalpati, N., Sali, V. K., and Vasanthi, A. H. R. (2018). Alliin the precursor of allicin in garlic extract mitigates proliferation of gastric adenocarcinoma cells by modulating apoptosis. Pharmacogn. Mag. 14, 84. doi:10.4103/pm.pm_342_17
Marcellin, P., and Kutala, B. K. (2018). Liver diseases: a major, neglected global public health problem requiring urgent actions and large-scale screening. Liver Int. 38, 2–6. doi:10.1111/liv.13682
Marmouzi, I., Kharbach, M., El Jemli, M., Bouyahya, A., Cherrah, Y., Bouklouze, A., et al. (2019). Antidiabetic, dermatoprotective, antioxidant and chemical functionalities in Zizyphus lotus leaves and fruits. Ind. Crops Prod. 132, 134–139. doi:10.1016/j.indcrop.2019.02.007
Meera, R., Devi, P., Kameswari, B., Madhumitha, B., and Merlin, N. J. (2009). Antioxidant and hepatoprotective activities of ocimum basilicum linn. And trigonella foenum-graecum linn. against H2O2 and CCl 4 induced hepatotoxicity in goat liver. Indian J. Exp. Biol. 47, 584–590.
Miccadei, S., Venere, D.Di, Cardinali, A., Romano, F., Durazzo, A., Foddai, M. S., et al. (2008). Antioxidative and apoptotic properties of polyphenolic extracts from edible part of artichoke (Cynara scolymus L.) on cultured rat hepatocytes and on human hepatoma cells. Nutr. Cancer 60, 276–283. doi:10.1080/01635580801891583
Mohajeri, D., Abbasi, M. M., Delazar, A., Doustar, Y., and Nouri, M. H. K. (2007). Subacute toxicity of crocus sativus L. (Saffron) stigma ethanolic extract in rats. Am. J. Pharmacol. Toxicol. 2 (2), 189–193. doi:10.3844/ajptsp.2007.189.193
Mohamed, H. A., El-Sayed, I. H., and Moawad, M. (2011). Protective effect of nigella sativa seeds against dimethylaminoazobenzene (dab) induced liver carcinogenesis. Cancer Biol. 1, 13–19.
Mohammadian, A., Moradkhani, S., Ataei, S., Shayesteh, T. H., Sedaghat, M., Kheiripour, N., et al. (2016). Antioxidative and hepatoprotective effects of hydroalcoholic extract of Artemisia absinthium L. in rat. J. HerbMed Pharmacol. 5, 29–32.
Mohammed, H. H., Rabeh, N. M., and Haggag, M. H. (2020). Efficacy of coconut oil (Cocos nucifera L.) fortification on liver functions rats with induced hypothyroidism. Curr. Sci. Int. 9, 240–250. doi:10.36632/csi/2020.9.2.20
Mokrane, N., Omar, K., Fatima, Z. T., Akila, G., and Abdelkader, A. (2020). The effect of Thymus vulgaris L. on renal and liver toxicity in wistar rats exposed to aluminum. J. Med. Plants Res. 14, 13–23. doi:10.5897/jmpr2019.6819
Mostafa, M. E. A., Al-Amri, M., and Kamel, A. M. F. (2018). Hepatoprotective potential of Rumex vesicarius against malathion hepatotoxicity in adult albino rats. Eur. J. Anat. 22, 449–459.
Mushtaq, A., Aslam, B., Muhammad, F., and Khan, J. A. (2021). Hepatoprotective activity of nigella sativa and piper nigrum against concanavalin a-induced acute liver injury in mouse model. Pak Vet. J. 41, 78–84. doi:10.29261/pakvetj/2020.076
Najem, M., Ibijbijen, J., and Nassiri, L. (2019). Quantitative ethnobotanical study of poisonous medicinal plants used in the traditional pharmacopoeia of the central middle atlas region: Morocco. Ethnobot. Res. Appl. 18, 1–17. doi:10.32859/era.18.36.1-17
Nehar, S., and Kumari, M. (2013). Ameliorating effect of nigella sativa oil in thioacetamide-induced liver cirrhosis in albino rats. Indian J. Pharm. Educ. Res. 47, 135–139.
Nencini, C., Franchi, G. G., Cavallo, F., and Micheli, L. (2010). Protective effect of Allium neapolitanum Cyr. versus Allium sativum L. on acute ethanol-induced oxidative stress in rat liver. J. Med. Food 13, 329–335. doi:10.1089/jmf.2008.0180
Noureddine, B., Mostafa, E., and Mandal, S. C. (2022). Ethnobotanical, pharmacological, phytochemical, and clinical investigations on Moroccan medicinal plants traditionally used for the management of renal dysfunctions. J. Ethnopharmacol. 292, 115178. doi:10.1016/j.jep.2022.115178
Nutrition, A., Medi-, V., Nutrition, A., and Medi-, V. (2017). The effect of thyme essential oil (thymus vulgaris) added to quail diets on performance, some blood parameters, and the antioxidative metabolism of the serum and liver tissues. Braz. hournal Poult. Sci. 19, 297–304. doi:10.1590/1806-9061-2016-0403
Nwozo, S. O., Osunmadewa, D. A., and Oyinloye, B. E. (2014). Anti-fatty liver effects of oils from Zingiber officinale and Curcuma longa on ethanol-induced fatty liver in rats. J. Integr. Med. 12, 59–65. doi:10.1016/S2095-4964(14)60006-6
Omar, T. Y., and Omar, T. Y. (2014). Protective efficacy of Glycyrrhiza glabra on Ccl4 induced liver injury in rabbits. World J. Pharm. Res. 3, 3627–3638.
Omidi, A., Riahinia, N., Bagher, M., and Torbati, M. (2014). Hepatoprotective effect of Crocus sativus (saffron) petals extract against acetaminophen toxicity in male Wistar rats. Avicenna J. Phytomed 4, 330–336.
Osman, N. N., Ghazwani, A. H., and Balamash, K. S. (2020). Evaluation of the effect of gamma-irradiated basil (ocimum basilicum L.) on liver toxicity induced by arsenic in rats. J. Radiat. Res. Appl. Sci. 13, 552–559. doi:10.1080/16878507.2020.1777656
Ouahhoud, S., Touiss, I., Khoulati, A., Lahmass, I., Mamri, S., Meziane, M., et al. (2021a). Hepatoprotective effects of hydroethanolic extracts of Crocus sativus tepals, stigmas and leaves on carbon tetrachloride induced acute liver injury in rats. Physiology Pharmacol. 25, 178–188. doi:10.32598/ppj.25.2.30
Ouahhoud, S., Touiss, I., Khoulati, A., Lahmass, I., Mamri, S., Meziane, M., et al. (2021b). Hepatoprotective effects of hydroethanolic extracts of Crocus sativus tepals, stigmas and leaves on carbon tetrachloride induced acute liver injury in rats. Physiology Pharmacol. 25, 178–188. doi:10.32598/ppj.25.2.30
Pa, S., My, P., Vt, T., and Tr, G. (2010). Protective effect of hordeum vulgare linn. on acetaminophen- induced liver damage. Pharmacology 1, 336–340. doi:10.4103/0975-1483.59324
Panezai, A. J., Khan, M., Ishaque, S. M., Ullah, R., Khan, A. A., and Rahat, N. (2022). Variable doses of Nigella sativa in isoniazid induced liver toxicity in rabbits. Pak Euro J. Med. Life Sci. 5 (2), 187–194. doi:10.31580/pjmls.v5i2.2405
Parsai, A., Eidi, M., and Sadeghipour, A. (2015). Hepatoprotective effect of sage (Salvia officinalis L.) leaves hydro-methanolic extract against Aspergillus parasiticus aflatoxin-induced liver damage in male rats. 4th Natl. Congr. Med. Plants 12, 704.
Pereira, R., Jacociunas, L. V., De, R. F., Regina, B., Abreu, R.De, Lehmann, M., et al. (2017). Genotoxic and chemopreventive assessment of Cynara scolymus L. aqueous extract in a human-derived liver cell line aqueous extract in a human-derived liver cell line. Drug Chem. Toxicol. 0545, 1525–6014. doi:10.1080/01480545.2017.1279625
Prajapati, H. A., and Verma, R. J. (2022). Protective effect of Nigella sativa against diethyl phthalate-induced changes in mitochondrial enzymatic activities in liver of mice. J. Sci. Healthc. Explor. 4, 6–10. doi:10.2019/JSHE/202206002
Prasad, R., Ali, S., and Khan, L. A. (2010). Hepatoprotective effect of Syzygium aromaticum extract on acute liver injury induced by thioacetamide. Int. J. Pharm. Clin. Res. 2, 68–71.
Radwan, R. R., and Mohamed, H. A. (2018). Nigella sativa oil modulates the therapeutic efficacy of mesenchymal stem cells against liver injury in irradiated rats. J. Photochem Photobiol. 178, 447–456. doi:10.1016/j.jphotobiol.2017.11.037
Rats, L., Beheshti, F., Norouzi, F., Abareshi, A., Khazaei, M., Alikhani, V., et al. (2018). Nigella sativa prevented liver and renal tissue damage in lipopolysaccharide-treated rats. Saudi J. Kidney Dis. Transplant. 29, 554–566. doi:10.4103/1319-2442.235184
Ravindran, C. A., Murugaiyah, V. A. L., and Khiang, P. E. H. K. O. K. (2013). Hepatoprotective activity of leaf of methanol extract of Laurus nobilis.L against paracetamol induced hepatotoxicity in rats. Asian J. Pharm. Clin. Res. 6, 7–11.
Riaz, H., Saleem, N., Ahmad, M., and Mehmood, Y. (2016). Hepatoprotective effect of crocus sativus on amiodarone-induced liver toxicity hepatoprotective effect of crocus sativus on amiodarone-induced liver toxicity. Br. J. Pharm. Res. 12, 1–11. doi:10.9734/BJPR/2016/27219
Roby, M. H. H., Sarhan, M. A., Selim, K. A.-H., and Khalel, K. I. (2013). Evaluation of antioxidant activity, total phenols and phenolic compounds in thyme (Thymus vulgaris L.), sage (Salvia officinalis L.), and marjoram (Origanum majorana L.) extracts. Ind. Crops Prod. 43, 827–831. doi:10.1016/j.indcrop.2012.08.029
Rtibi, K., Selmi, S., Jabri, M. A., El-Benna, J., Amri, M., Marzouki, L., et al. (2016). Protective effect of ceratonia siliqua L. Against a dextran sulfate sodium-induced alterations in liver and kidney in rat. J. Med. Food 19, 882–889. doi:10.1089/jmf.2016.0020
Sadeghi, H., Azarmehr, N., Mansourian, M., Khalvati, B., Kokhdan, E. P., Salehpour, Z., et al. (2020). The hydroalcoholic extract of Rosmarinus officinalis attenuates liver damage after bile-duct ligation in rats. J. Anim. Plant Sci. 31, 432–440. doi:10.36899/JAPS.2021.2.0232
Saira, K., Faiza, N., Imran, S., Sarmad, I., Faiza, A., Sana, S., et al. (2020). Hepatoprotective role of fruit extract of Terminalia arjuna in acetaminophen intoxicated mice. Adv. Life Sci. 8, 63–67.
Salama, S. M., Abdulla, M. A., Alrashdi, A. S., Ismail, S., Alkiyumi, S. S., and Golbabapour, S. (2013). Hepatoprotective effect of ethanolic extract of Curcuma longa on thioacetamide induced liver cirrhosis in rats. BMC Complement. Altern. Med. 13, 56–17. doi:10.1186/1472-6882-13-56
Salahshoor, M. R., Roshankhah, S., and Jalili, C. (2019). Antioxidative Properties of Thymus vulgaris on Liver Rats Induced by Paclitaxel. Pharmacogn. Res. 11. doi:10.4103/pr.pr_45_19
Salem, A. M., Mahdy, K. A., Hassan, N. S., El-saeed, G. S. M., Razik, A., Farrag, H., et al. (2017). Nigella sativa seed reduced galectin-3 level and liver fibrosis in thioacetamide-induced liver injury in rats. J. Arab Soc. Med. Res. 12, 46–55. doi:10.4103/jasmr.jasmr_8_17
Salem, M. B., Ksouda, K., Dhouibi, R., Charfi, S., Turki, M., Hammami, S., et al. (2019). LC-MS/MS analysis and hepatoprotective activity of artichoke (cynara scolymus L.) leaves extract against high fat diet-induced obesity in rats. Biomed. Res. Int. 12. doi:10.1155/2019/4851279
Samarghandian, S., Asadi-samani, M., and Farkhondeh, T. (2016). Assessment the effect of saffron ethanolic extract (Crocus sativus L.) on oxidative damages in aged male rat liver. Scholars Res. Libr. Der 8, 283–290.
Samojlik, I., Lakic, N., Mimica-Dukic, N., Akovic-Svajcer, K., and Bozin, B. (2010). Antioxidant and hepatoprotective potential of essential oils of coriander (coriandrum sativum L.) and caraway (carum carvi L.) (Apiaceae). Agric. Food Chem. 58, 8848–8853. doi:10.1021/jf101645n
Sangamithira, S. P., Revathy, J., Sheik Abdullah, S., and Sampath Kumar, P. (2011). The hepatoprotective effect of ethanolic bark extract of Terminalia arjuna on paracetamol induced liver damage. Biosci. Biotechnol. Res. Asia 8, 777–781. doi:10.13005/bbra/934
Sargin, S. A., Selvi, S., and Büyükcengiz, M. (2015). Ethnomedicinal plants of aydincik district of mersin, Turkey. J. Ethnopharmacol. 174, 200–216. doi:10.1016/j.jep.2015.08.008
Sasaki, K., Wada, K., Tanaka, Y., Yoshimura, T., Matuoka, K., and Anno, T. (2005). Thyme (Thymus vulgaris L.) leaves and its constituents increase the activities of xenobiotic-metabolizing enzymes in mouse liver. J. Med. Food 8, 184–189. doi:10.1089/jmf.2005.8.184
Savo, V., Caneva, G., Maria, G. P., and David, R. (2011). Folk phytotherapy of the amalfi coast (campania, southern Italy). J. Ethnopharmacol. 135, 376–392. doi:10.1016/j.jep.2011.03.027
Shah, P. V., Parmar, M., Thakkar, V., and Tejal, G. (2009). Hepatoprotective activity of Hordeum Vulgare linn. Seeds against ethanol-induced liver damage in rats. Pharmacologyonline 2, 538–545.
Shahrzad, K., Mahya, N., Fatemeh, T. B., Maryam, K., Mohammadreza, F. B., and Jahromy, M. H. (2014a). Hepatoprotective and antioxidant effects of Salvia officinalis L. Hydroalcoholic extract in male rats. Chin. Med. 05, 130–136. doi:10.4236/cm.2014.52016
Shahrzad, K., Mahya, N., Fatemeh, T. B., Maryam, K., Mohammadreza, F. B., and Jahromy, M. H. (2014b). Hepatoprotective and antioxidant effects of Salvia officinalis L. Hydroalcoholic extract in male rats. Chin. Med. 05, 130–136. doi:10.4236/cm.2014.52016
Shalizar-jalali, A., Hasanzadeh, S., and Karimi, A. (2013). Crataegus monogyna fruit aqueous extract as an attenuator of doxorubicin-induced hepatotoxicity in rat model. Toxicology 2013, 15–17.
Sharma, V., and Agrawal, R. (2014). In vivo antioxidant and hepatoprotective potential of Glycyrrhiza glabra extract on carbon tetra chloride (CCl4) induced oxidative-stress mediated hepatotoxicity. Int. J. Res. Med. Sci. 2, 314. doi:10.5455/2320-6012.ijrms20140260
Shinde, D. B., Koratkar, S., and Shitole, A. (2016). Antioxidant activity and antiproliferative action of methanolic extract of liquorice (Glycyrrhiza glabra) in Hepg2 cell line. Int. J. Pharm. Pharm. Sci. 8, 293–298. doi:10.22159/ijpps.2016v8i9.11954
Shivananjappa, M. M., and Joshi, M. K. (2012). Influence of Emblica officinalis aqueous extract on growth and antioxidant defense system of human hepatoma cell line (HepG2). Pharm. Biol. 50, 497–505. doi:10.3109/13880209.2011.618501
Shivananjappa, M. M., Mhasavade, D., and Joshi, M. K. (2013). Aqueous extract of Terminalia arjuna attenuates tert-butyl hydroperoxide-induced oxidative stress in HepG2 cell model. Cell. Biochem. Funct. 31, 129–135. doi:10.1002/cbf.2867
Sivalokanathan, S., Vijayababu, M. R., and Balasubramanian, M. P. (2006). Effects of Terminalia arjuna bark extract on apoptosis of human hepatoma cell line HepG2. World J. Gastroenterol. 12, 1018–1024. doi:10.3748/wjg.v12.i7.1018
Slatnar, A., Petkovsek, M. M., Stampar, F., Veberic, R., Horvat, J., Jakse, M., et al. (2018). Game of tones: sugars, organic acids, and phenolics in green and purple asparagus (Asparagus officinalis L.) cultivars. Turkish J. Agric. For. 42, 55–66. doi:10.3906/tar-1707-44
Sneha, K., Reddy, A. G., Rani, M. U., Ramya, B., Kumar, P. S., and Kumar, B. A. (2021). Evaluation of Terminalia arjuna in comparison to taurine against experimental nephrotoxicity due to cisplatin in rats. Indian J. Anim. Res. 11, 367–373. doi:10.18805/ijar.b-4466
Solana, M., Boschiero, I., Dall’Acqua, S., and Bertucco, A. (2015). A comparison between supercritical fluid and pressurized liquid extraction methods for obtaining phenolic compounds from Asparagus officinalis L. J. Supercrit. Fluids 100, 201–208. doi:10.1016/j.supflu.2015.02.014
Sreekeesoon, D. P., and Mahomoodally, M. F. (2014). Ethnopharmacological analysis of medicinal plants and animals used in the treatment and management of pain in Mauritius. J. Ethnopharmacol. 157, 181–200. doi:10.1016/j.jep.2014.09.030
Sun, T., Powers, J. R., and Tang, J. (2007). Evaluation of the antioxidant activity of asparagus, broccoli and their juices. Food Chem. 105, 101–106. doi:10.1016/j.foodchem.2007.03.048
Tabuti, J. R. S., Dhillion, S. S., and Lye, K. A. (2003). Traditional medicine in Bulamogi county, Uganda: its practitioners, users and viability. J. Ethnopharmacol. 85, 119–129. doi:10.1016/S0378-8741(02)00378-1
Tajua, G., Majeeda, S. A., Nambia, K. S. N., and Jayanthib, M. (2011). Hepatoprotective and antioxidant effects of Glycyrrhiza glabra extract against paracetamol induced liver damage in rats. J. Pharm. Res. 4, 3798–3802.
Tanbek, K., Ozerol, E., Bilgic, S., Iraz, M., Sahin, N., and Colak, C. (2017). Protective effect of Nigella sativa oil against thioacetamide-induced liver injury in rats. Med. Sci. | Int. Med. J. 6, 1–103. doi:10.5455/medscience.2016.05.8531
Temiz, M. A., Temur, A., and Çelik, İ. (2015). Antioxidant role and hepatoprotective effects of carob (ceratonia siliqua L.) seeds against ethanol-induced oxidative stress in rats. J. Food Nutr. Res. 3, 57–61. doi:10.12691/jfnr-3-1-10
Thomson, M., and Ali, M. (2003). Garlic [Allium sativum]: a review of its potential use as an anti-cancer agent. Curr. Cancer Drug Targets 3, 67–81. doi:10.2174/1568009033333736
Tu, Z., Moss-Pierce, T., Ford, P., and Jiang, T. A. (2013). Rosemary (Rosmarinus officinalis L.) extract regulates glucose and lipid metabolism by activating AMPK and PPAR pathways in HepG2 cells. J. Agric. Food Chem. 61, 2803–2810. doi:10.1021/jf400298c
Uchio, R., Higashi, Y., Kohama, Y., Kawasaki, K., Hirao, T., Murosaki, K. M. S., et al. (2017). A hot water extract of turmeric (Curcuma longa) suppresses acute ethanol-induced liver injury in mice by inhibiting hepatic oxidative stress and inflammatory cytokine production. J. Nutr. Sci. 6, e3–e9. doi:10.1017/jns.2016.43
Usia, S. T., Kadota, S., and Tezuka, Y. (2005). Alkamides from piper nigrum L. And their inhibitory activity against human liver microsomal cytochrome P450 2D6 (CYP2D6). Nat. Prod. Commun. 1, 1–7. doi:10.1055/s-2006-931558
Ustuner, D., Colak, E., Dincer, M., Tekin, N., Burukoglu Donmez, D., Akyuz, F., et al. (2018). Posttreatment effects of olea europaea L. Leaf extract on carbon tetrachloride-induced liver injury and oxidative stress in rats. J. Med. Food 21, 899–904. doi:10.1089/jmf.2017.0143
Varghese, A., Savai, J., Pandita, N., and Gaud, R. (2015). In vitro modulatory effects of Terminalia arjuna, arjunic acid, arjunetin and arjungenin on CYP3A4, CYP2D6 and CYP2C9 enzyme activity in human liver microsomes. Toxicol. Rep. 2, 806–816. doi:10.1016/j.toxrep.2015.02.008
Venmathi Maran, B. A., Iqbal, M., Gangadaran, P., Ahn, B.-C., Rao, P. V., and Shah, M. D. (2022). Hepatoprotective potential of Malaysian medicinal plants: a review on phytochemicals, oxidative stress, and antioxidant mechanisms. Molecules 27, 1533. doi:10.3390/molecules27051533
Vidičević, S., Tošić, J., Stanojević, Ž., Isaković, A., Mitić, D., Ristić, D., et al. (2020). Standardized Olea europaea L. leaf extract exhibits protective activity in carbon tetrachloride-induced acute liver injury in rats: the insight into potential mechanisms. Arch. Physiol. Biochem. 126, 399–407. doi:10.1080/13813455.2018.1550095
Wei, C., Qiu, J., Wu, Y., Chen, Z., Yu, Z., Huang, Z., et al. (2022). Promising traditional Chinese medicine for the treatment of cholestatic liver disease process (cholestasis, hepatitis, liver fibrosis, liver cirrhosis). J. Ethnopharmacol. 297, 115550. doi:10.1016/j.jep.2022.115550
Wei, X., Xia, L., Ziyayiding, D., Chen, Q., Liu, R., Xu, X., et al. (2019). The Extracts of Artemisia absinthium L. Suppress the growth of hepatocellular carcinoma cells through induction of apoptosis via endoplasmic reticulum stress and mitochondrial-dependent pathway. Molecules 24, 913–917. doi:10.3390/molecules24050913
Yacout, G., Nihal, M. E., and Eman, F. E. A. (2012). Hepatoprotective effect of basil (Ocimum basilicum L.) on CCl4-induced liver fibrosis in rats. Afr. J. Biotechnol. 11, 15702–15711. doi:10.5897/ajb12.2048
Yin, G., Fisheries, F., Jeney, G., Hingary, S., Nakao, M., and Lu, C. (2011). Hepatoprotective and antioxidant effects of Glycyrrhiza glabra extract against carbon tetrachloride (CCl4)-induced hepatocyte damage in common carp (Cyprinus carpio). Fish. Physiol. Biochem. 37, 209–216. doi:10.1007/s10695-010-9436-1
Yoo, D. Y., Kim, W., Nam, S. M., Yoo, M., Lee, S., Yoon, Y. S., et al. (2014a). Neuroprotective effects of Z-ajoene, an organosulfur compound derived from oil-macerated garlic, in the gerbil hippocampal CA1 region after transient forebrain ischemia. Food Chem. Toxicol. 72, 1–7. doi:10.1016/j.fct.2014.06.023
Yoo, M., Lee, S., Kim, S., Hwang, J.-B., Choe, J., and Shin, D. (2014b). Composition of organosulfur compounds from cool-and warm-type garlic (Allium sativum L.) in Korea. Food Sci. Biotechnol. 23, 337–344. doi:10.1007/s10068-014-0047-y
Youdim, K. A., and Deans, S. G. (1999). Dietary supplementation of thyme (Thymus vulgaris L.) essential oil during the lifetime of the rat: its effects on the antioxidant status in liver, kidney and heart tissues. Mech. Ageing Dev. 109, 163–175. doi:10.1016/S0047-6374(99)00033-0
Zhang, C., Zhao, J., Famous, E., Pan, S., Peng, X., and Tian, J. (2021). Antioxidant, hepatoprotective and antifungal activities of black pepper (Piper nigrum L.) essential oil. Food Chem. 346, 128845. doi:10.1016/j.foodchem.2020.128845
Keywords: ethnobotany, ethnopharmacology, traditional medicine, medicinal plants, liver diseases
Citation: Bencheikh N, Elbouzidi A, Baraich A, Bouhrim M, Azeroual A, Addi M, Mothana RA, Al-Yousef HM, Eto B and Elachouri M (2024) Ethnobotanical survey and scientific validation of liver-healing plants in northeastern Morocco. Front. Pharmacol. 15:1414190. doi: 10.3389/fphar.2024.1414190
Received: 08 April 2024; Accepted: 26 August 2024;
Published: 10 September 2024.
Edited by:
Da-Cheng Hao, Dalian Jiaotong University, ChinaReviewed by:
Nilufer ORHAN, Independent Researcher, Austin, United StatesShibani Mohapatra, Siksha “O” Anusandhan University, India
Weka Sidha Bhagawan, University of PGRI Madiun, Indonesia
Copyright © 2024 Bencheikh, Elbouzidi, Baraich, Bouhrim, Azeroual, Addi, Mothana, Al-Yousef, Eto and Elachouri. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Amine Elbouzidi, YW1pbmUuZWxib3V6aWRpQHVtcC5hYy5tYQ==; Noureddine Bencheikh, YmVuY2hlaWtoX25vdXJlZGRpbmUxNzE4QHVtcC5hYy5tYQ==; Mohamed Bouhrim, bW9oYW1lZC5ib3VocmltQGdtYWlsLmNvbQ==
 Noureddine Bencheikh
Noureddine Bencheikh Amine Elbouzidi
Amine Elbouzidi Abdellah Baraich
Abdellah Baraich Mohamed Bouhrim
Mohamed Bouhrim Abdelhamid Azeroual
Abdelhamid Azeroual Mohamed Addi
Mohamed Addi Ramzi A. Mothana5
Ramzi A. Mothana5 Hanan M. Al-Yousef
Hanan M. Al-Yousef