- 1College of Korean Medicine, Daejeon University, Daejeon, Republic of Korea
- 2Department of Korean Rehabilitation Medicine, College of Korean Medicine, Daejeon University, Daejeon, Republic of Korea
- 3Institute of Bioscience and Integrative Medicine, Department of Korean Medicine, Daejeon University, Daejeon, Republic of Korea
- 4Department of Herbal Formula, College of Korean Medicine, Daejeon University, Daejeon, Republic of Korea
Background: Osteoporosis (OP) is a significant medical issue associated with population aging. Recent research on herbal medicines (HMs) for OP has been increasing, with these therapies sometimes used in conjunction with bisphosphonates (BPs), the standard treatment for OP. We conducted a systematic review and meta-analysis to evaluate the effects of combining HMs with BPs on improving bone mineral density (BMD) in patients with primary OP.
Methods: We searched nine databases—PubMed, Embase, Cochrane Library, China National Knowledge Infrastructure Wanfang, KISS, Kmbase, Science On, and Oasis—up to 31 August 2023. We selected randomized controlled trials (RCTs) comparing BMD between HMs plus BPs and BPs alone in primary OP. A meta-analysis with BMD as the primary outcome was performed using RevMan version 5.4. Study quality and evidence certainty were assessed through Cochrane’s risk of bias2 and GRADE.
Results: Out of 43 RCTs involving 4,470 participants (mean age 65.8 ± 6.6 years), 35 RCTs with 3,693 participants were included in the meta-analysis. The combination of HMs and BPs was found to be more effective in improving BMD compared to BPs alone, with improvements of 0.10 g/cm2 at the lumbar spine (33 RCTs, 95% CI: 0.07–0.12, p < 0.001, I2 = 93%) and 0.08 g/cm2 at the femoral neck (20 RCTs, 95% CI: 0.05–0.12, p < 0.001, I2 = 94%), though this result was associated with high heterogeneity, high risk of bias, and very low certainty of evidence.
Conclusion: Our data suggest the possibility that combining HMs with BPs may improve BMD in primary OP more effectively than using BPs alone. However, the results should be interpreted with caution due to the high heterogeneity and low quality of the studies included in the review. Therefore, further well-designed RCTs are needed to confirm these findings.
Systematic Review Registration: https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42023392139.
1 Introduction
Osteoporosis (OP) is a chronic progressive disorder characterized by a decline in bone mineral density (BMD) and an increase in bone fragility (Raisz, 2005; Seeman and Delmas, 2006). The prevalence rate of OP worldwide is 18.3%, with 23.1% and 11.7% prevalence rates among women and men, respectively (Salari et al., 2021). The 1-year fatality rate of osteoporotic fractures is as high as 36.7% (van Staa et al., 2001; Clynes et al., 2020). Moreover, OP incurs substantial socioeconomic costs; in the United States, the cost of OP is projected to exceed $95 billion by 2040 (Lewiecki et al., 2019). Therefore, there is an urgent need to manage patients with OP to reduce patient suffering and economic burden.
OP can be classified into primary and secondary types, with primary OP further divided into postmenopausal OP (PMOP) and senile OP (Jolly et al., 2018). PMOP occurs in women shortly after menopause due to estrogen deficiency, which activates osteoclast differentiation and increases osteoblast apoptosis. This leads to a rate of bone resorption that exceeds the rate of bone formation (Iseme et al., 2017). On the other hand, senile OP arises from aging-related increases in reactive oxygen species (ROS), which activate osteoclasts. Additionally, decreased renal function leads to reduced vitamin D synthesis, and impaired osteoblast function results in bone formation that cannot keep pace with bone resorption (McClung et al., 2013).
The International Osteoporosis Foundation (IOF) has recommended pharmacologic treatments for OP, including bisphosphonates, selective estrogen receptor modulators (SERMs), hormone replacement therapy (HRT), anabolic agents, and receptor activator of nuclear factors κB ligand (RANKL) inhibitors (Tu et al., 2018). These treatments address OP by either slowing bone resorption or promoting bone formation (Khosla and Hofbauer, 2017). However, SERMs can increase the risk of deep vein thrombosis (Artero et al., 2012), HRT is associated with a heightened risk of breast and uterine cancers (Sjögren et al., 2016; Lupo et al., 2015), anabolic agents like teriparatide can cause hypercalcemia (Minisola et al., 2019), and RANKL inhibitors like denosumab may result in hypocalcemia or an increased risk of infections (Tsvetov et al., 2020).
Among them, the most widely recognized standard treatment for OP is BPs. While BPs effectively inhibit excessive bone resorption, this inhibition can lead to side effects and a decline in bone quality through coupling events (Ensrud and Crandall, 2017; Park and Ko, 2022). This reduction in bone quality is associated with an increased risk of atypical fractures (Tella and Gallagher, 2014; Ensrud and Crandall, 2017). As a result, a “drug holiday” has been considered for patients undergoing BP therapy to reduce these risks (McClung et al., 2013). Nevertheless, it is worth noting that in patients at high risk within the osteoporosis group, drug holiday was associated with an elevated risk of clinical fractures (Camacho et al., 2020). Therefore, there is a need for studies to identify alternatives that can safely and effectively compensate for BPs by increasing BMD in a shorter period.
Herbal medicines (HMs), a rich source of diverse bioactive compounds awaiting discovery (Słupski et al., 2021), have already been reported in various studies for their potential effects on OP (Liu et al., 2014; Lu et al., 2022). In clinical settings across Asia, where HMs are frequently prescribed, case series indicate that the concurrent use of HMs and BPs has sometimes potential benefits over using BPs alone in improving BMD in patients with OP (Yu et al., 2012; Li et al., 2020). Nevertheless, no previous studies have analyzed the overall effect of combined HMs and BPs in patients with primary OP.
Therefore, this study aims to evaluate the effects of concurrent use of HMs and BPs in patients with primary OP, identify which specific HMs and botanical drugs are predominantly utilized, assess the BMD improvement effects of frequently used herbal remedies, and provide relevant research insights.
2 Methods
This systematic review and meta-analysis was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) and A Measurement Tool to Assess Systematic Reviews (AMSTAR2) guidelines. This review was registered in the Prospective Register of Systematic Reviews (PROSPERO) [registration number CRD42023392139].
2.1 Search strategy
We conducted a comprehensive search for randomized controlled trials (RCTs) across 9 databases, including PubMed, Embase, Cochrane Library, China National Knowledge Infrastructure (CNKI), Wanfang, Koreanstudies Information Service System (KISS), Kmbase, Science On, and Oasis, up to 31 August 2023. We identified additional RCTs through a review of the bibliographies of relevant articles and a manual search on Google Scholar. No language restrictions were imposed. The search strategy included the following keywords: osteoporosis, herbs, decoctions, plant extracts, traditional medicine, and Chinese medicine. The combination of keywords was tailored to each database, and the corresponding details are presented in Supplementary Table S1A–S1C.
2.2 Study selection and eligibility criteria
Two authors (M-G Kim and Y-S Yoo) separately checked the titles and abstracts of relevant articles and subsequently assessed the full text against the eligibility criteria for final inclusion. Any disagreements regarding the selection of studies were resolved through discussion with a third researcher (E-J Lee). The eligibility criteria applied for study selection are discussed in the following sections.
2.2.1 Participants
We included studies involving patients diagnosed with primary OP, including PMOP and senile OP, regardless of sex. According to the World Health Organization (WHO) diagnostic criteria, only patients with OP with a T-score of −2.5 or lower were considered for inclusion.
2.2.2 Intervention types
In our study, we included research in which the intervention group involved the concurrent treatment of HMs and BPs. There were no restrictions on the types of HMs and BPs. Additionally, there were no constraints on supplements other than HMs and BPs.
2.2.3 Control types
We included studies that used BP administration as the control group without imposing restrictions on the types of BPs or the use of supplements. Additionally, to facilitate the comparison between the combined treatment of HMs plus BPs and BPs monotherapy, we only included studies where treatments other than HM were identically used in the control group.
2.2.4 Outcome measures
We included studies that specifically involved BMD values as outcome measures using dual-energy X-ray absorptiometry (DXA) for at least one of the following sites: lumbar spine, femoral neck, or total hip. Additionally, studies that presented only T-scores without BMD values or did not provide post-treatment BMD results were excluded.
2.2.5 Study design
We included only RCTs in this review. Studies using the term 'randomization' without providing details were included. However, quasi-RCTs with inappropriate random sequence generation were excluded. Additionally, RCTs with Jadad scores (Jadad et al., 1996) below two were excluded to ensure quality control in the review.
2.3 Data extraction
Two authors (M-G Kim and Y-S Yoo) extracted data using a standardized data collection form (Excel 2007; Microsoft, Redmond, WA, United States), followed by cross-checking. The extracted information included: title, first author, published year, Digital Object Identifier (DOI), setting of study, type of OP, inclusion of fracture, number of arms, number of participants, age of participants, duration of OP, duration of menopause, intervention/control (types of BPs, types of HMs, the botanical drugs constituting HM, type of formula, pattern identification of HMs, dose, and treatment period), site of BMD measurement (e.g., lumbar spine, femoral neck, and total hip), BMD values (mean and standard deviation of baseline and endpoint and evaluation period), bone marker values (mean and standard deviation of baseline and endpoint and evaluation period), statistical significance of the outcome, adverse effects (frequency and type), withdrawal (number and reason), study institution and country of the corresponding research. The type of OP was classified based on the criteria outlined in the referenced RCTs.
In particular, following the Consensus statement on the Phytochemical Characterisation of Medicinal Plant extracts (ConPhyMP) guidelines (Heinrich et al., 2022), data were extracted on the reporting of the pharmaceutical producer, extraction process, quality control reports, and chemical analysis reports for the HMs used in RCTs included in the review. As per the clinical practice guidelines of Korean medicine for OP (Xie et al., 2011), HMs used in this study were classified into three types: kidney yang deficiency pattern, liver-kidney yin deficiency pattern, and syndrome of qi stagnation and blood stasis. In addition, we summarized the botanical drugs used in more than 10 RCTs. However, the courier botanical drugs that harmonize the drug, such as Ziziphus jujuba Mill [Rhamnaceae; Jujubae Fructus] and Glycyrrhiza uralensis Fisch. ex DC [Fabaceae; Glycyrrhizae Radix et Rhizoma], were not counted as frequently used botanical drugs. Furthermore, all botanical drugs have been presented with their scientific names, including authorities, and their respective families.
Any disagreements were resolved via a discussion until a consensus was reached or by consulting a third author (E-J Lee). If additional information was required, we contacted the corresponding author of the relevant study via email.
2.4 Quality assessment
Two authors (M-G Kim and Y-S Yoo) individually assessed the risk of bias in the included studies and evaluated the quality of evidence for each key finding. Any discrepancies between the two authors were resolved through discussion with a third author (E-J Lee) until a consensus was reached.
The risk of bias in the included studies was assessed using Cochrane’s Risk of Bias 2 (RoB2) tool, evaluating five different domains: randomization process, deviations from intended interventions, missing outcome data, measurement of the outcome, and selection of the reported result. Each domain was categorized into one of three groups: “low risk,” “some concern,” or “high risk.” The overall risk of bias assessment for each study was determined as follows: if all domains were classified as low risk, the overall risk was considered low; if there were some concerns in one or more domains, the overall risk was categorized as some concerns; and if any domain was classified as high risk, the overall risk was assessed as high risk. To evaluate publication bias, both graphical presentations (Funnel plot) and statistical tests (Egger’s regression test) were carried out by the “meta” package (by Guido Schwarzer, R Foundation for Statistical Computing, Vienna, Austria) in R version 4.3.2. When potential publication bias was suspected by Egger’s test, we corrected the effect size using the trim-and-fill method.
To assess the quality of evidence for the meta-analysis results, the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) approach was employed. Using the online GRADEpro Guideline Development Tool (https://gdt.gradepro.org/), we evaluated the certainty of evidence for meta-analytic results including three or more RCTs as high, moderate, low, or very low. This assessment took into consideration various factors, including study design, risk of bias, inconsistency, indirectness, imprecision, publication bias, effect size, and potential confounding.
2.5 Statistical and meta-analysis
To present the mean and standard deviation (SD) of age, treatment duration, OP and menopausal periods, and BMD, we utilized the weighted mean and SD functions, considering the sample size. In cases where data such as age, OP duration, menopausal period, and BMD values were not available, we contacted the corresponding authors of the relevant studies via email to obtain the necessary information. In instances where this was not possible, we synthesized the remaining data excluding missing values. We analyzed the p-values of mean age, initial BMD, treatment duration, and duration of OP and confidence intervals (CI) of the BMD change rate. Statistical significance was set at p < 0.05, and the CI was set at 95%.
We conducted a meta-analysis using Review Manager software version 5.4.1 (Cochrane, London, United Kingdom) to determine if combining HM with BP is more effective compared to BP alone in improving BMD and bone markers in patients with primary OP. Meta-analyses were performed for studies using the same interventions, comparisons, and outcome measurements. Studies incorporating a combination of calcitonin and estrogen, which can considerably affect BMD values, as well as those with disparate treatment and assessment durations, were excluded from the meta-analysis.
When the necessary data were accessible, subgroup analyses were conducted based on OP types (PMOP, Senile OP), treatment duration (3, 6, and 12 months), and frequently used HM to inspect heterogeneity or evaluate treatment effects among subgroups. BMD with the same unit of measurement was pooled using mean difference (MD) with a 95% CI, while bone markers with different units were pooled using standardized mean difference (SMD). Heterogeneity among studies was assessed using the chi-squared (χ2) test and the I-squared (I2) statistic. We had planned to use a random-effects model for high heterogeneity (I2 ≥ 50%) and apply a fixed-effects model for cases of low heterogeneity. However, due to the diverse HMs used in the studies included in our review, leading to potential heterogeneity, we applied a random-effects model throughout the analysis. Furthermore, to confirm the robustness of the meta-analysis results, sensitivity analyses were conducted by iteratively excluding one study at a time or by excluding studies by the group from each meta-analysis.
3 Results
3.1 Basic characteristics of included RCTs
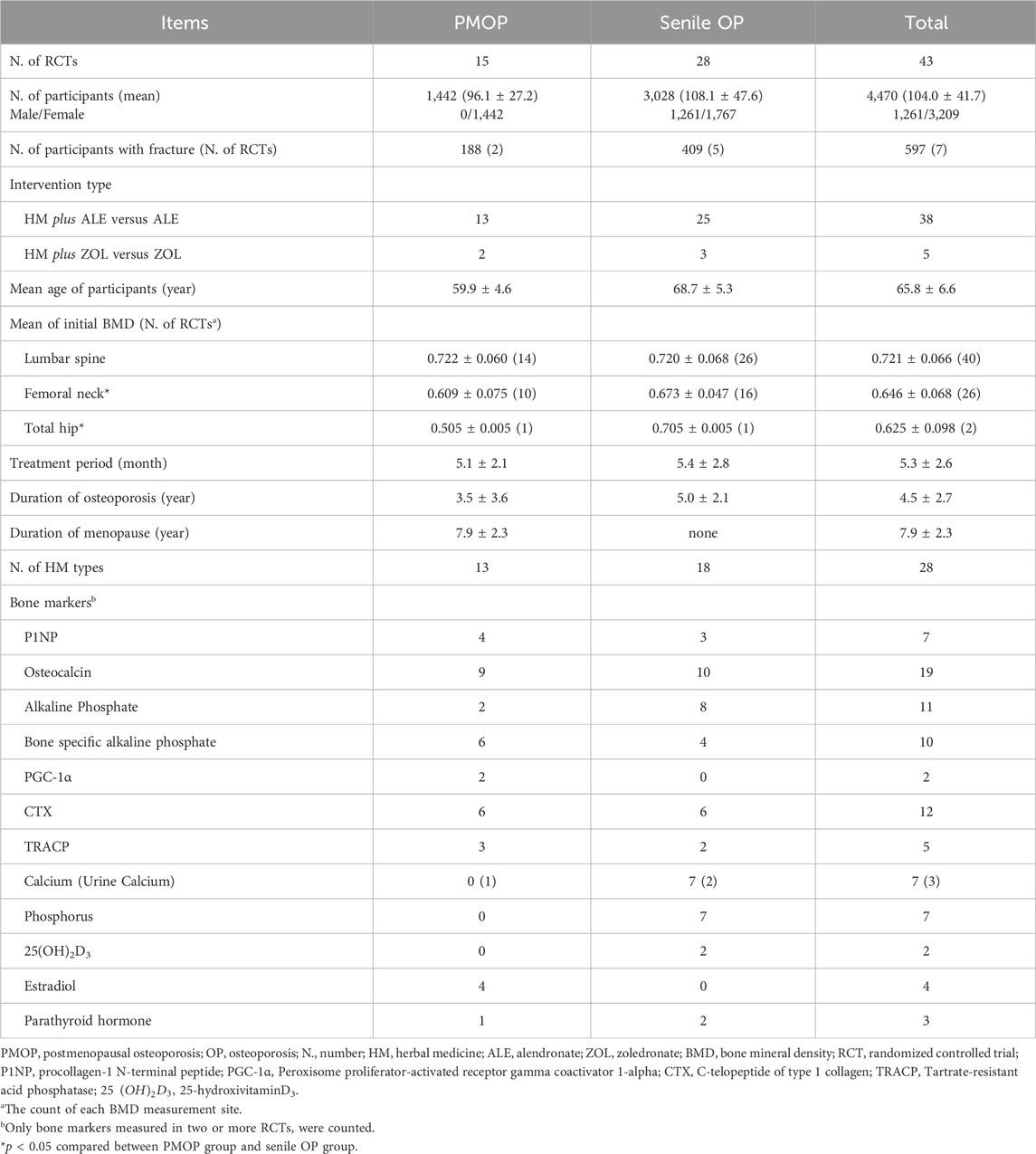
Of the 13,540 studies identified during the initial screening, 43 met the eligibility criteria (Figure 1). In total, 4,470 individuals (1,261 males and 3,209 females) participated in the study, including 1,442 participants with PMOP from 15 RCTs and 3,028 participants with senile OP from 28 RCTs. Among the included participants, 188 in two RCTs on PMOP and 409 in five RCTs on senile OP experienced fractures.
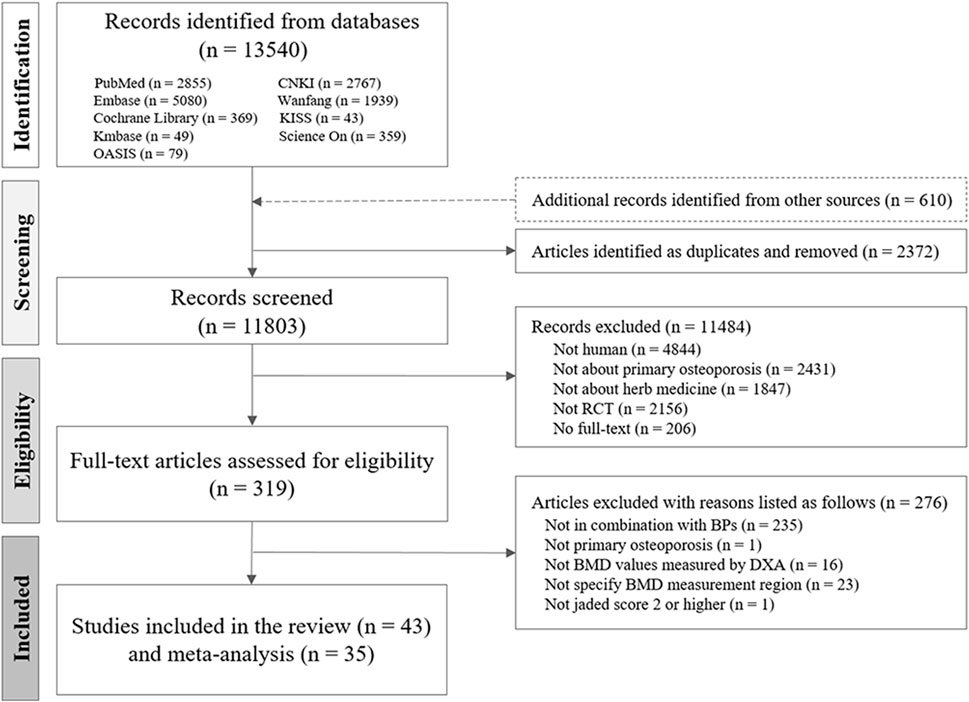
Figure 1. Flow chart of the study selection process. CNKI, China National Knowledge Infrastructure; RCT, randomized controlled trial; HM, herbal medicine; BMD, bone mineral density; OP, osteoporosis; BP, bisphosphonate.
The mean age of the participants was 65.8 ± 6.6 years, the average duration of OP was 4.5 ± 2.7 years, the average duration of menopause was 7.9 ± 2.3 years, and the average treatment period was 5.3 ± 2.6 months. The mean initial BMD of participants was 0.721 ± 0.066 g/cm2 at the lumbar spine, 0.646 ± 0.068 g/cm2 at the femoral neck, and 0.625 ± 0.098 g/cm2 at the total hip. In each RCT, there were no significant differences in the basic characteristics between the intervention and control groups (Table 1).
3.2 Characteristics of intervention and control
The control groups received BPs without restrictions on supplements (vitamin D or calcium), and the intervention groups received HMs in addition to the BPs received by the control groups. In 38 RCTs (4,052 participants), the control groups received alendronate (ALE), and in five RCTs (418 participants), the control groups received zoledronate (ZOL). Ten RCTs used ALE or ZOL alone, and the remaining 33 RCTs used ALE or ZOL in combination with supplements (calcium or vitamin D in 27 RCTs, calcitonin in 5 RCTs, and estrogen in one RCT) (Tables 1 and 2).
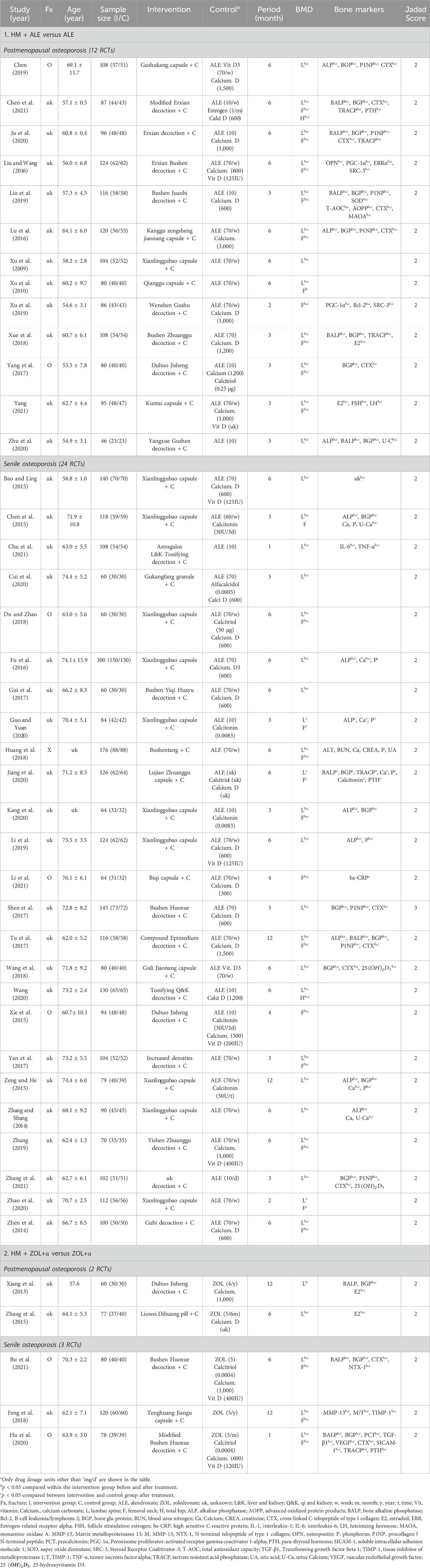
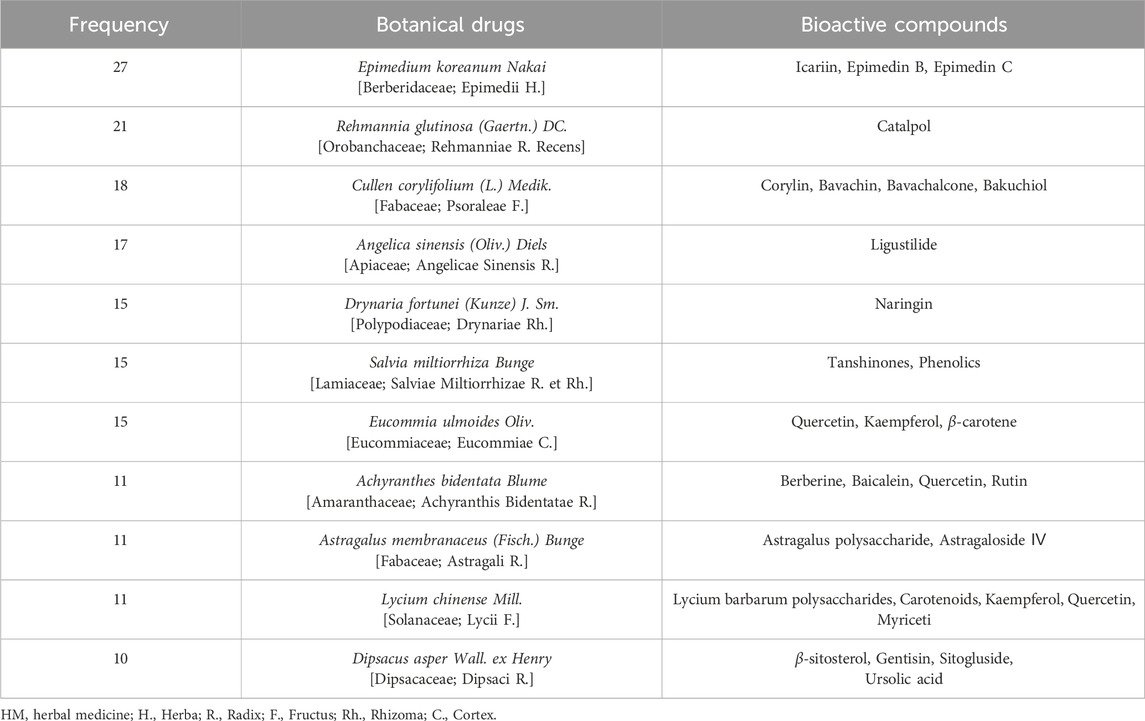
The most frequently used HM was Xianlinggubao capsule (XLGB) in 11 RCTs, followed by Bushen Huoxue (three RCTs), Duhuo Jisheng (three RCTs), and Erxian (two RCTs) decoctions. HMs were prescribed mainly by applying three pattern identifications: kidney yang deficiency (26 RCTs), syndrome of qi stagnation and blood stasis (six RCTs), and liver-kidney yin deficiency (two RCTs) (Table 1; Supplementary Table S2). Seventy botanical drugs were used in this review, 11 of which were used at least 10 times. The botanical drugs used frequently included Epimedium koreanum Nakai [Berberidaceae; Epimedii Herba] (27 RCTs), Rehmannia glutinosa (Gaertn.) DC [Orobanchaceae; Rehmanniae Radix Recens] (21 RCTs), Cullen corylifolium (L.) Medik [Fabaceae; Psoraleae Fructus] (18 RCTs), and Angelica sinensis (Oliv.) Diels [Apiaceae; Angelicae Sinensis Radix] (17 RCTs) (Table 3). Detailed information on the HMs, including composition, dosage, pharmaceutical producer, extraction process, quality control reports, and chemical analysis reports, is provided in Supplementary Table S2.
In total, 28 types of HMs each consisting of an average of seven botanical drugs prepared using traditional methods, were prescribed in the RCTs included in our review. Among these, 17 were traditional decoctions, while 11 were modernized formulations, including 9 capsules, 1 granule, and 1 pill. Of the modernized formulations, 7 types of HMs with active compounds reported in prior studies are specified, and the bone metabolism-related functions of each compound are detailed in Supplementary Table S3.
3.3 Characteristics of outcome measurement
All 43 RCTs established BMD as the primary outcome measured using DXA, and BMD was measured at the lumbar spine (40 RCTs), femoral neck (26 RCTs), and total hip (two RCTs). A total of 34 types of bone markers were used as secondary outcomes in 32 RCTs. Among these RCTs, the following five bone formation markers were employed in two or more studies: procollagen-1 N-terminal peptide (P1NP) in seven RCTs, osteocalcin (OC) in 19 RCTs, alkaline phosphate (ALP) in 11 RCTs, bone-specific alkaline phosphate (BALP) in 10 RCTs, and peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC-1α) in two RCTs. Additionally, two bone resorption markers, C-telopeptide of type 1 collagen (CTX) in 12 RCTs and tartrate-resistant acid phosphatase (TRACP) in five RCTs, were used, along with six mineral/hormone indicators: calcium (Ca) in seven RCTs, urine calcium (U-Ca) in three RCTs, phosphorus (P) in seven RCTs, 25-hydroxyvitamin D3 (25(OH)2D3) in two RCTs, estradiol in four RCTs, and parathyroid hormone (PTH) in three RCTs (Table 1).
3.4 Combined effect of HMs plus BPs
A meta-analysis was conducted on 35 out of the total 43 RCTs, excluding eight RCTs. Exclusions encompassed six RCTs (Chen et al., 2015; Xie et al., 2015; Zeng and He, 2015; Guo and Yuan, 2020; Kang et al., 2020; Chen et al., 2021) that included the use of calcitonin and estrogen and two RCTs (Shen et al., 2017; Yang, 2021) that included disparate treatment and evaluation periods.
3.4.1 HMs plus BPs versus BPs
In total, 35 RCTs (HM plus ALE versus ALE in 30 RCTs and HM plus ZOL versus ZOL in 5 RCTs) were included in the meta-analysis to investigate the BMD improvement effect of HM plus BPs compared with that of BPs alone.
Compared to the BPs alone group, the HM plus BPs group showed improved BMD score by 0.10 g/cm2 (33 RCTs, 95% CI: 0.07–0.12, p < 0.001, I2 = 93%) at the lumbar spine over 5.6 months and by 0.08 g/cm2 (20 RCTs, 95% CI: 0.05–0.12, p < 0.001, I2 = 94%) at the femoral neck over 5.4 months (Figures 2, 3). In the subgroup analysis according to OP type, there was no statistically significant difference in the improvement of BMD between the PMOP and Senile OP types (Figures 2, 3).
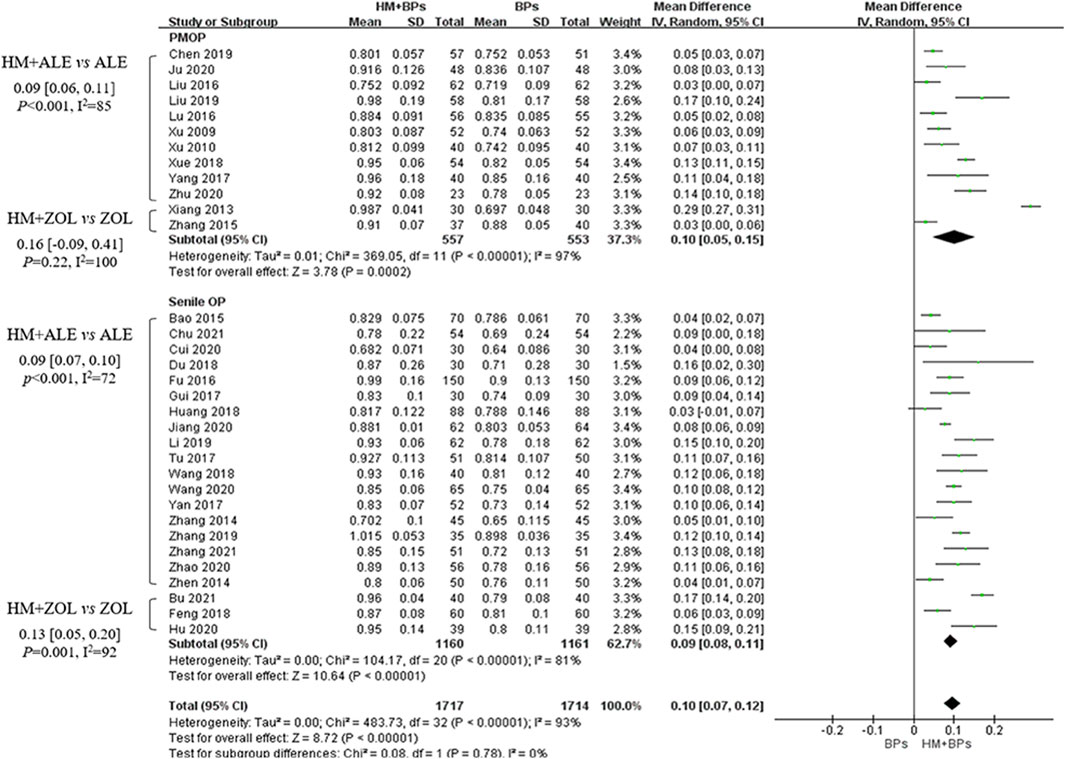
Figure 2. BMD improvement effects of the combined therapy of HM and BPs at the lumbar spine. BMD, bone mineral density; BP, bisphosphonate; HM, herbal medicine; ALE, alendronate; ZOL, zoledronate; PMOP, postmenopausal osteoporosis; SD, standard deviation; CI, confidence interval.
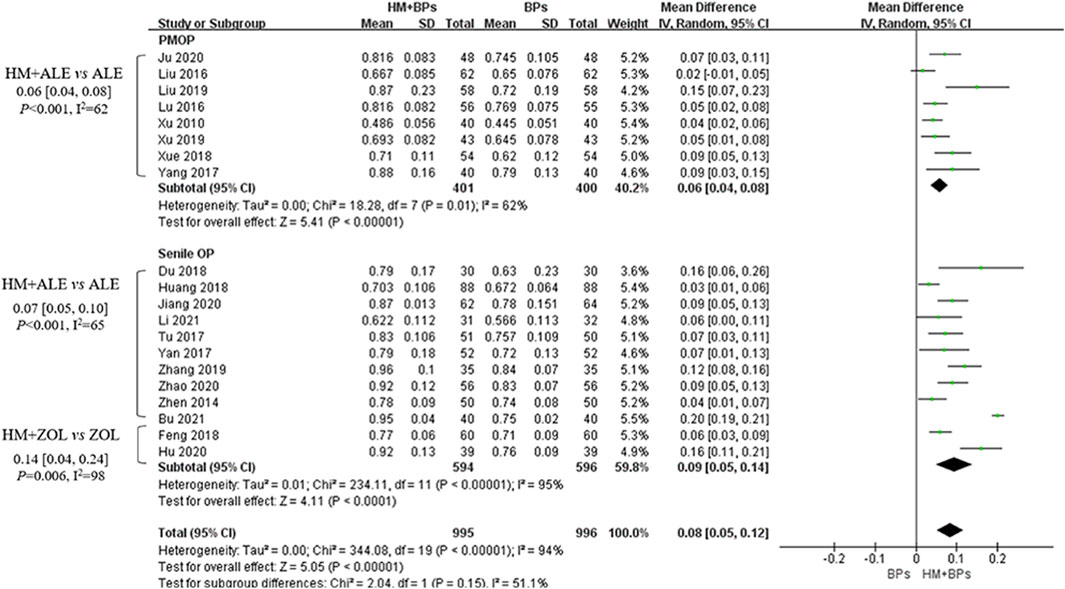
Figure 3. BMD improvement effects of the combined therapy of HM and BPs at the femoral neck. BMD, bone mineral density; BP, bisphosphonate; HM, herbal medicine; ALE, alendronate; ZOL, zoledronate; PMOP, postmenopausal osteoporosis; SD, standard deviation; CI, confidence interval.
In the subgroup analysis based on the types of concomitant BPs, BMD improvement in the HMs plus ZOL group was approximately 1.5–2 times higher than that in the HMs plus ALE group (Figures 2–4; Supplementary Figures S1–S3). However, the number of studies investigating the combination of HMs plus ZOL was relatively small compared to those examining combinations with ALE, and heterogeneity was higher. In sensitivity analysis, heterogeneity decreased upon excluding RCTs involving HMs plus ZOL. Specifically, in the analysis of lumbar and femoral neck BMD in senile OP, excluding RCTs on HMs plus ZOL resulted in an upward shift in the certainty of evidence by one grade to ‘Low’ due to decreased heterogeneity (Table 4; Supplementary Table S4).
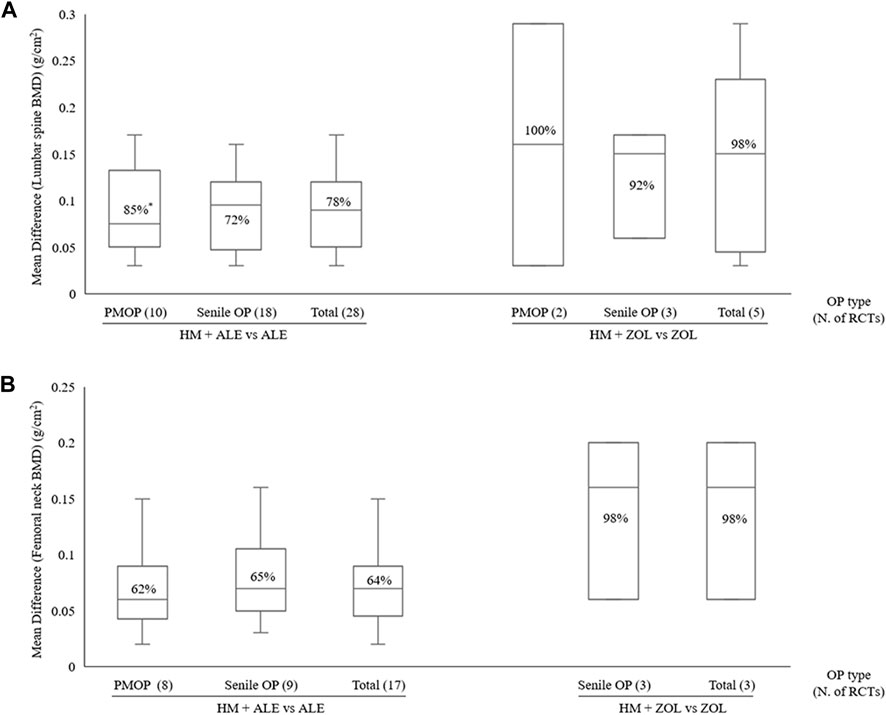
Figure 4. BMD improvement effects according to OP type with combined HM and BPs therapy (A) Lumbar spine (B) Femoral neck. BMD, bone mineral density; BP, bisphosphonate; HM, herbal medicine; M, month; PMOP, postmenopausal osteoporosis; OP, osteoporosis; N, number; RCT; randomized controlled trial; *, I2.
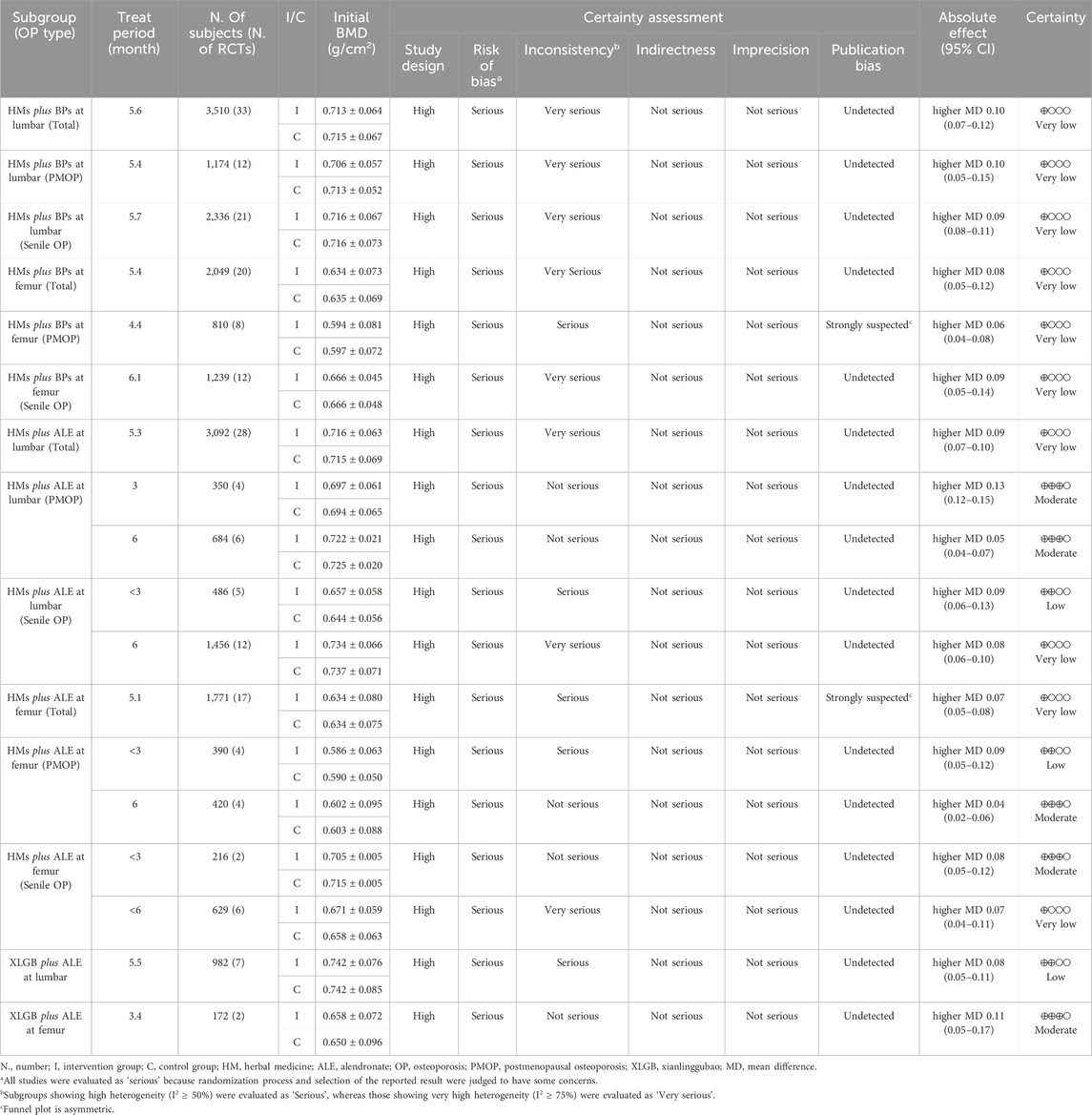
Table 4. Characteristics of 35 randomized controlled trials included in the meta-analysis and GRADE evidence profiles.
3.4.2 HMs plus ALE versus ALE
HMs plus ALE group showed improved BMD score by 0.09 g/cm2 (28 RCTs, 95% CI: 0.07–0.10, p < 0.001, I2 = 78%) at the lumbar spine with very low certainty of evidence (Supplementary Figure S1; Table 4) and by 0.07 g/cm2 (17 RCTs, 95% CI: 0.05–0.08, p < 0.001, I2 = 64%) at the femoral neck with very low certainty of evidence (Supplementary Figure S2; Table 4). There was no significant difference in BMD improvement between PMOP and senile OP.
Subgroup analysis based on treatment duration within OP type revealed that in PMOP, taking HMs with ALE for ≤3 months resulted in greater improvements in BMD compared to ALE alone, with a moderate certainty at the lumbar spine of 0.13 g/cm2 (4 RCTs, 95% CI: 0.12–0.15, p < 0.001, I2 = 0%) and low certainty at the femoral neck of 0.09 g/cm2 (4 RCTs, 95% CI: 0.05–0.12, p < 0.001, I2 = 56%) (Table 4; Figure 5; Supplementary Figures S4, S5). This was of greater magnitude compared to administering HMs plus ALE for 6 months. In senile OP, taking HMs with ALE for less than 3 months resulted in greater improvements in BMD compared to ALE alone, with a low certainty at the lumbar spine of 0.09 g/cm2 (5 RCTs, 95% CI: 0.06–0.13, p < 0.001, I2 = 52%) and moderate certainty at the femoral neck of 0.08 g/cm2 (2 RCTs, 95% CI: 0.05–0.12, p < 0.001, I2 = 0%), with no difference observed in the improvement when taken for 6 months (Table 4; Figure 5; Supplementary Figures S4, S5). Furthermore, sensitivity analysis revealed that excluding studies reporting outlier results (Xu et al., 2019; Liu and Wang, 2016) in the meta-analysis of HM plus ALE treated for less than 3 months and 6 months in the femoral neck of PMOP, respectively, and Cui et al. (2020) in the meta-analysis of HM plus ALE treated for less than 3 months in the lumbar spine of senile OP) led to a reduction in heterogeneity approaching '0′and an upward adjustment in the level of evidence (Supplementary Figures S6, S7).
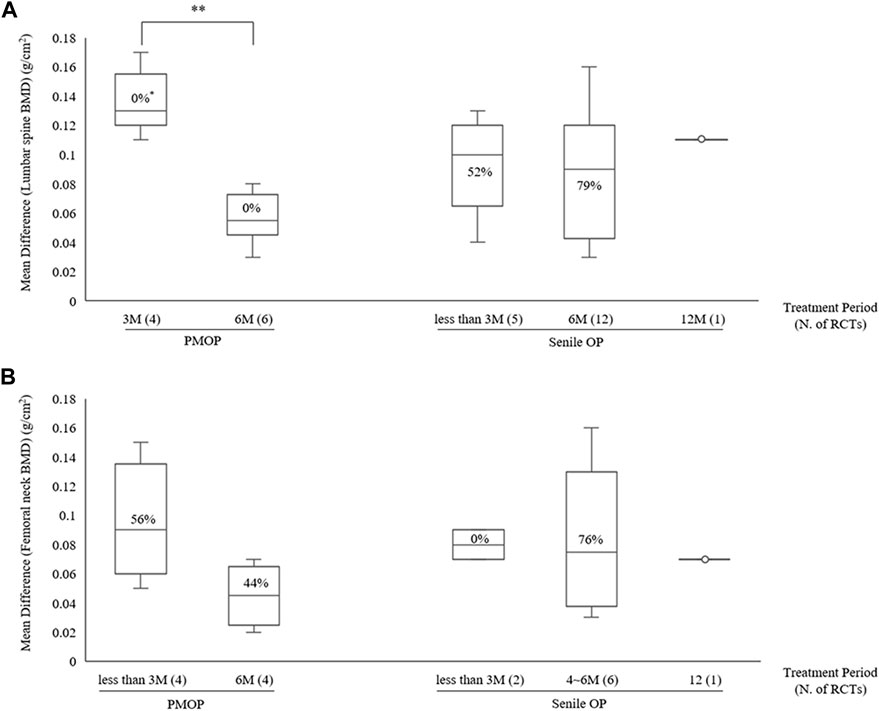
Figure 5. BMD improvement effects according to treatment period with combined HM and ALE therapy (A) Lumbar spine (B) Femoral neck. BMD, bone mineral density; HM, herbal medicine; ALE, Alendronate; M, month; PMOP, postmenopausal osteoporosis; OP, osteoporosis; N, number; RCT; randomized controlled trial; *, I2.
3.4.3 XLGB plus ALE versus ALE
Compared to the ALE alone group, the combination group of the most commonly used herbal medicine XLGB with ALE demonstrated a significant improvement in BMD at the lumbar spine (7 RCT, 0.08 g/cm2, 95% CI: 0.05–0.11, p < 0.001, I2 = 73%) with low certainty and the femoral neck (2 RCT, 0.11 g/cm2, 95% CI: 0.05–0.17, p < 0.001, I2 = 37%) with moderate certainty of evidence (Figure 6; Table 4). Interestingly, upon excluding two studies, one with a duration of 3 months (Zhao et al., 2020) and another that presented an outlier result (Li et al., 2019), heterogeneity decreased from 73% to 47%, adjusting the level of evidence for the combined effect of XLGB and ALE at the lumbar spine (5 RCT, 0.06 g/cm2, 95% CI: 0.04–0.09, p < 0.001, I2 = 47%) to moderate (Supplementary Figure S8).
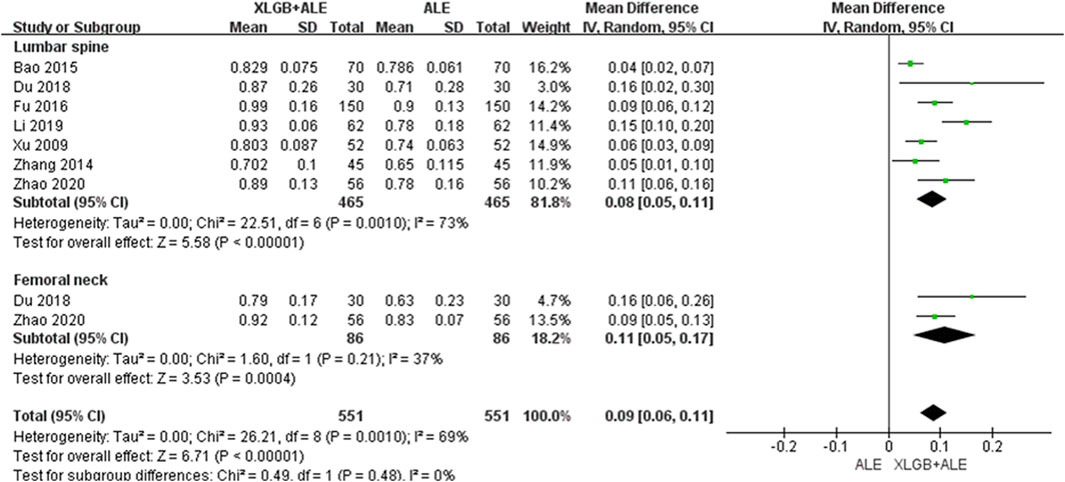
Figure 6. BMD improvement effect of the combined use of XLGB and ALE in primary OP. BMD, bone mineral density; XLGB, Xianlinggubao; ALE, Alendronate; OP, osteoporosis; SD, standard deviation; CI, confidence interval.
3.4.4 Bone marker analysis of HMs plus ALE versus ALE
Seventeen RCTs (PMOP in 9 RCTs; Senile OP in 8 RCTs) were included in the meta-analysis of bone markers (16 RCTs on bone formation markers, 10 RCTs on bone resorption markers, and seven RCTs on bone mineral markers), and 10 types of bone markers were analyzed. In these RCTs, the HMs plus ALE group, compared to the ALE alone, demonstrated a significant increase in PGC-1α (SMD: 2.36, 95% CI: 0.94–3.79, p < 0.01, I2 = 93%) as a bone formation marker and a significant decrease in P1NP (SMD: −0.9, 95% CI: −1.15 to −0.65, p < 0.001, I2 = 56%) as a bone formation marker and CTX (SMD: −0.83, 95% CI: −0.95 to −0.65, p < 0.001, I2 = 0%) as a bone resorption marker (Figure 7A). There were 10 RCTs that measured both bone formation and bone resorption markers, and a decrease in bone resorption markers was observed in the HMs plus ALE group compared to those in the ALE group, except for those observed in the study by Jiang et al., 2020 (Figure 7B). Notably, in PMOP participants taking HMs plus ALE, a concurrent reduction in both bone resorption and formation markers was observed, which was more pronounced compared to the trend seen in senile OP participants.
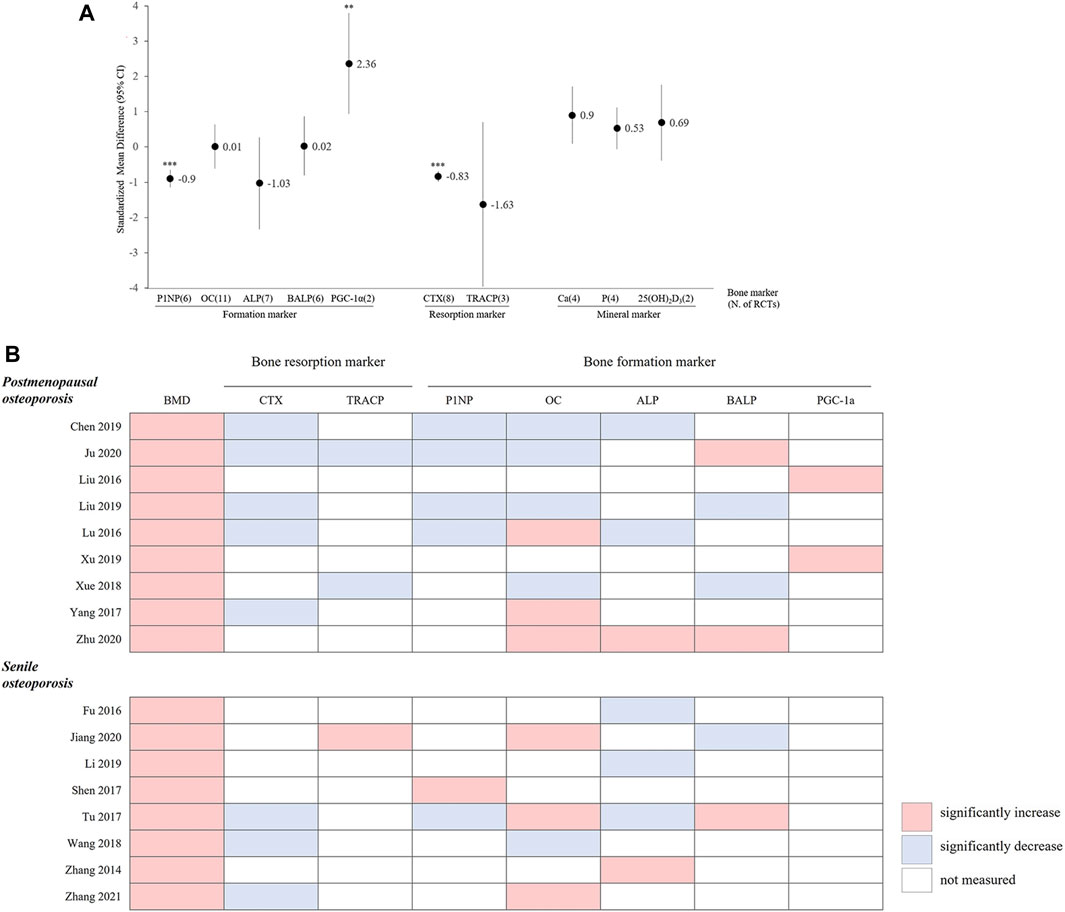
Figure 7. (A) Standardized mean difference in bone markers between HM plus ALE and ALE. P1NP, procollagen-1 N-terminal peptide; HM, herbal medicine; ALE, alendronate; OC, osteocalcin; ALP, alkaline phosphate; BALP, bone specific alkaline phosphate; SRC-3, Steroid Receptor Coabtivator-3; PGC-1α, Peroxi-some proliferator-activated receptor gamma coactivator 1-alpha; CTX, C-telopeptide of type 1 collagen; TRACP, Tartrate-resistant acid phosphatase; Ca, calcium; P, phosphorus; 25(OH)2D3, 25-hydroxivitaminD3; *: p < 0.05 in overall effect, **: p < 0.01 in overall effect, ***: p < 0.001 in overall effect. (B) The pattern of changes in BMD and bone markers in the HM plus ALE group. BMD, bone mineral density; CTX, C-telopeptide of type 1 collagen; TRACP, Tartrate-resistant acid phosphatase; P1NP, procollagen-1 N-terminal peptide; OC, osteocalcin; ALP, alkaline phosphate; BALP, bone specific alkaline phosphate; PGC-1α, Peroxi-some proliferator-activated receptor gamma coactivator 1-alpha.
3.5 Adverse events
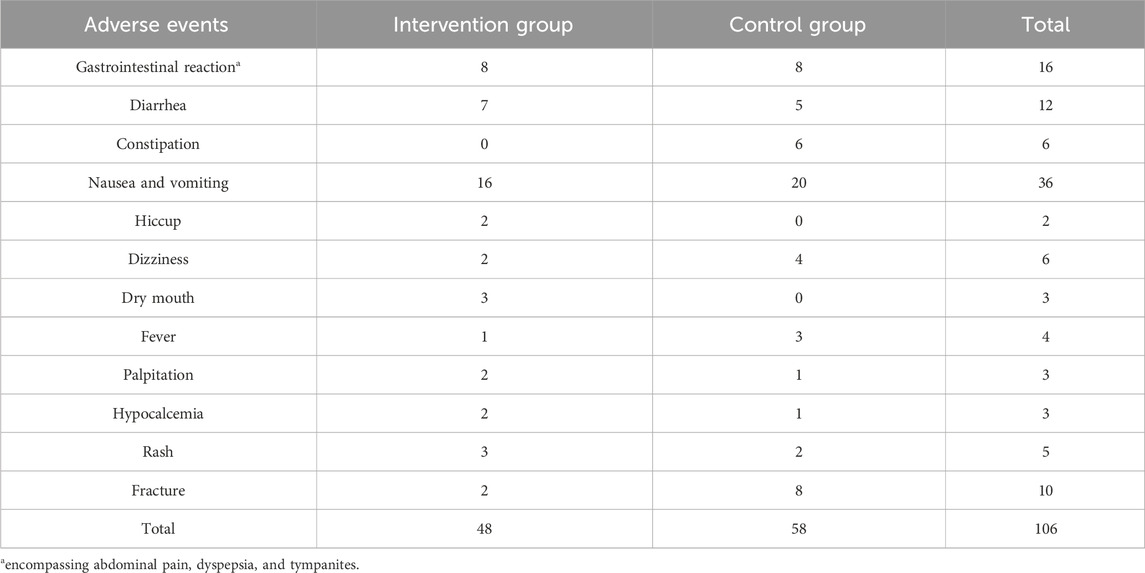
Out of the 43 RCTs, only 21 reported adverse events. Among these, 15 RCTs documented a total of 106 adverse events (48 in the intervention group and 58 in the control group), while 6 RCTs reported no adverse events. The most frequently occurring adverse events were gastrointestinal issues, with nausea and vomiting occurring in 36 patients, gastrointestinal reactions in 16, and diarrhea in 12 (Table 5). In each study, there was no significant difference between the intervention and control groups (p > 0.05).
3.6 Quality assessment
3.6.1 Risk of bias
The overall risk of bias of every RCT included in our review was assessed to have “some concerns.”
All RCTs were judged to be of some concern as there was no information on whether there was allocation concealment during the randomization process. Not all studies blinded participants and assessors; however, as outcome measures such as BMD measured by DXA and bone markers measured by blood tests were not influenced by intended interventions, these studies were evaluated to have a “low risk” due to intended interventions. Additionally, none of the studies included in our review had previously published a study protocol, leading to some concerns regarding the selection of the reported results (Supplementary Figure S6).
3.6.2 Publication bias
The presence of publication bias was confirmed in studies comparing the effectiveness of HMs combined with BPs to BPs alone in the femoral neck of patients with PMOP (p-value for bias: 0.008), as well as in studies comparing the effectiveness of HMs combined with ALE to ALE monotherapy in the femoral neck (p-value for bias: 0.001), as indicated by the funnel plot and Egger’s test. However, after adjusting for effect size using the trim-and-fill method, the estimated effects were reduced from 0.06 to 0.043 for studies on the HM + BP group in the femoral neck of patients with PMOP and from 0.07 to 0.049 for studies on the HM + ALE group in the femoral neck, while maintaining statistical significance. No evidence of publication bias was observed in other effect analyses (Supplementary Figure S7; Supplementary Tables S9A, S9B).
3.6.3 Quality of evidence
In the comparison of HMs plus BPs with BPs, the quality of evidence ranged from “Very low” to “Low”. Meanwhile, the quality of evidence ranged from “Very low” to “Moderate” in the comparison of HMs plus ALE with ALE (Table 4). The quality of the evidence was not high primarily due to the high risk of bias and heterogeneity observed in the RCTs included in each meta-analysis, leading to a downgrade. Therefore, in the subgroup analysis divided by OP type and treatment duration, there was a tendency for heterogeneity to decrease while the grade of evidence increased.
4 Discussion
OP occurs when bone resorption exceeds bone formation in the bone remodeling process due to estrogen deficiency and aging (Nuti et al., 2019; Föger-Samwald et al., 2022). Because OP consequently increases the risk of fractures (Cosman et al., 2014), which in turn reduce quality of life and increase mortality in older people (Johnston and Dagar, 2020; Lee and Nam, 2021), prompt OP treatment to prevent fractures is crucial (Kanis et al., 2019).
BPs are powerful bone resorption inhibitors recommended as first-line agents for the treatment of OP (Rogers, 2003; Fuggle et al., 2019). In contrast, HMs have the advantage of promoting bone formation while simultaneously inhibiting bone resorption through multi-targeting (Leung and Siu, 2013; Jin et al., 2017). Given the characteristics of these two medications, a systematic review and meta-analysis were conducted to evaluate the combined effects of HMs and BPs on improving BMD in patients with primary OP.
As a result, the addition of HMs showed a potential for improving BMD compared to the use of BPs alone, regardless of the type of OP or the BMD measurement site. During the same treatment period, the addition of HMs led to approximately a 2.3 times increase (MD: 0.10 g/cm2) in BMD in the lumbar spine and approximately a 2.1 times increase (MD: 0.08 g/cm2) in BMD in the femoral neck compared to the use of BPs alone (Table 4; Figures 2, 3). However, the risk of bias and heterogeneity among the RCTs included in this review was very high, resulting in an overall certainty of evidence rated as “very low” to “low” (Table 4). Accurate assessment of intervention effects requires appropriate blinding and allocation concealment, and these processes should be reported in detail in the studies. Although the RCTs included in our review employed random sampling, specific details on blinding and allocation concealment were not provided. Despite the likely minimal impact of blinding deficiencies on outcome measures such as BMD assessments using DXA and blood tests, this represents a significant limitation in the interpretation of our review results.
In total, 4,470 participants (1,261 males and 3,209 females) were included in the present study, with a male-to-female ratio of 1:2.5. Of these, 597 had fractures. The mean age of patients with senile OP was approximately 8 years higher than that of patients with PMOP (68.7 ± 5.3 years for senile OP vs 59.9 ± 4.6 years for PMOP), and the duration of OP was approximately 1.5 years longer. Since we classified the OP types according to the definitions provided in each RCT, there was no overlap among the participants in the 43 RCTs. When referencing prior literature that distinguishes between PMOP and senile OP around the age of 70 (Aibar-Almazán et al., 2022; Sözen et al., 2017), the average ages for the OP types provided in Table 1 appear logical. However, due to the lack of detailed age-related distribution information in each RCT, it is not possible to exclude the possibility that some female participants of the same age might be classified under both PMOP and senile OP.
The initial BMD was 0.721 ± 0.066 g/cm2 in the lumbar spine, 0.646 ± 0.068 g/cm2 in the femoral neck, and 0.625 ± 0.098 g/cm2 in the total hip (Table 1). These BMD values were lower than those of the general population of the same age but similar to the BMD values of patients with OP reported in previous studies (Zhang and Shang, 2014; Zhao et al., 2021). As is well known, risk factors for OP include age, sex, diet, physical activity, weight, smoking, alcohol consumption, and genetic factors (Pouresmaeili et al., 2018). All participants in this review were Chinese, with no identifiable information available regarding OP risk factors other than age and sex.
Observing the BMD change pattern at the lumbar spine of the general Chinese population (Zeng and He, 2015), peak bone mass (1.077 g/cm2) is reached in the 20s and 30s age groups; BMD decreases by approximately 5% (0.045 g/cm2) on average every 10 years. After their 50s, men and women show different bone loss patterns. Due to these characteristic differences, primary OP can be classified into two types: PMOP and senile OP. In their 50s–60s, patients undergo a rapid decline in BMD, particularly in women who show a rapid decrease of 8%–13% due to menopause. Furthermore, the BMD of both men and women reaches approximately 0.902 g/cm2 when they reach their 60s. Considering the pattern of BMD changes among these Chinese individuals, our data suggests that the combination of HMs and BPs may improve the BMD of patients with primary OP, making their BMD levels similar to those of individuals approximately 10–15 years younger. However, the certainty of the evidence for the combined effects of HMs and BPs in our study was generally rated as “very low” to “low” due to high heterogeneity and risk of bias (Table 4).
The high heterogeneity is presumed to be due to the diversity in OP types, duration of treatment, and types of HMs used. Subgroup analysis based on OP type and treatment duration substantially reduced heterogeneity, indicating a moderate certainty of BMD improvement. Specifically, when HMs were combined with ALE, compared to ALE alone, for 6 months in patients with PMOP, a greater increase of 0.05 g/cm2 (6 RCTs, 95% CI: 0.04–0.07, p < 0.001, I2 = 0%) in the lumbar spine and 0.04 g/cm2 (4 RCTs, 95% CI: 0.02–0.06, p < 0.001, I2 = 44%) in the femoral neck was observed. Additionally, in patients with Senile OP, a greater increase of 0.08 g/cm2 (2 RCTs, 95% CI: 0.05–0.12, p < 0.001, I2 = 0%) in the femoral neck was observed after approximately 3 months of treatment compared to ALE alone (Supplementary Figure S4, S5; Table 4).
The notable finding from the subgroup analysis of HMs plus ALE is that, for senile OP, treatment duration did not significantly affect the Mean Difference (MD). However, in PMOP, adding HMs led to a greater MD improvement at 3 months compared to ALE alone, although this difference decreased at 6 months (Figure 5). This pattern in PMOP, which includes only women, may suggest a gender-based difference in response to BPs and that HMs might initially improve BMD. Nonetheless, due to the lack of individual data for men and women, the exact cause of this effect remains unclear.
In the RCTs included in our review, a total of 28 types of HMs were administered. Among these, 17 were traditional decoctions, while 11 were modernized formulations in the form of capsules, granules, or pills. Of the modernized formulations, 7 types with reported active compounds from previous studies included Xianlinggubao capsule (XLGB), Gushukang capsule, Qianggu capsule, Kuntai capsule, Tenghuang Jiangu capsules, Biqi capsule, and Liuwei Dihuang pill (Bao et al., 2020; Chai et al., 2019; Jiannong et al., 2015; Liu and Sun, 2023; Li et al., 2023; Jia et al., 2024; Wang et al., 2013). These HMs contained active compounds that promote osteoblast activity and inhibit osteoclast activity, including quercetin, icariin, naringin, and others (Supplementary Table S3). Classifying HMs based on these chemical compounds and evaluating their effects on BMD proved challenging due to the wide variety of compounds present in traditional HMs.
Nevertheless, we attempted to classify the HMs according to three pattern identifications (kidney yang deficiency, syndrome of qi stagnation and blood stasis, and liver-kidney yin deficiency) and conducted a subgroup analysis to assess BMD improvement effects. However, no significant differences were found between the groups (Supplementary Figure S8, S9).
Meanwhile, a meta-analysis of the BMD improvement effects of XLGB, which was frequently prescribed in 11 RCTs in our review, revealed a reduction in heterogeneity and demonstrated a similar degree of overall BMD improvement as the concurrent use of HMs and BPs. The combination of XLGB and ALE showed a tendency for more BMD improvement compared to ALE alone, with results observed in the lumbar spine (7 RCTs, 0.08 g/cm2, 95% CI: 0.05–0.11, p < 0.001, I2 = 73%) and femoral neck (2 RCTs, 0.11 g/cm2, 95% CI: 0.05–0.17, p < 0.001, I2 = 37%). Consequently, the level of evidence for this combination was upgraded to ‘moderate’ (Figures 2, 3, 6; Table 4).
XLGB is one of the most recommended traditional Chinese medicines for OP treatment (Xing et al., 2013). It comprises E. koreanum Nakai [Berberidaceae; Epimedii Herba], Dipsacus asper Wall. ex Henry [Dipsacaceae; Dipsaci Radix], C. corylifolium (L.) Medik [Fabaceae; Psoraleae Fructus], R. glutinosa (Gaertn.) DC [Orobanchaceae; Rehmanniae Radix], Salvia miltiorrhiza Bunge [Lamiaceae; Salviae Miltiorrhizae Radix et Rhizoma], and Anemarrhena asphodeloides Bunge [Liliaceae; Anemarrhenae Rhizoma], which are also among the most frequently used botanical drugs in the RCTs included in this study (Table 3; Supplementary Table S2). Furthermore, XLGB contains 146 major compounds, including key active compounds related to bone metabolism such as quercetin (Satué et al., 2013), luteolin (Kim et al., 2011; Nash et al., 2015), kaempferol (Wong et al., 2019), anhydroicaritin (Zheng et al., 2017), and diosgenin (Alcantara et al., 2011; Zhang et al., 2017). These compounds have been shown to promote osteoblast activity while inhibiting osteoclast activity.
The efficacy of XLGB is not attributable to a single mechanism of action. It involves various anti-osteoporotic mechanisms, including the PI3K-Akt-mTOR signaling pathway (Bao et al., 2020), inhibition of glycogen synthase kinase-3β and cathepsin K (Qiu et al., 2021), the cAMP signaling pathway, and the calcium signaling pathway (Zeng et al., 2024). Similar characteristics are also found in other HMs with reported bioactive compounds.
In contrast, BPs are derivatives of inorganic pyrophosphates and form a P-C-P bond, which gives them a strong affinity for calcium phosphate, thereby inhibiting both normal and ectopic mineralization (Fleisch et al., 1969). The mechanism of action of BPs in inhibiting bone resorption involves the suppression of farnesyl pyrophosphate (FPP) synthase and/or isopentenyl pyrophosphate isomerase. This inhibition disrupts the mevalonate pathway, which is crucial for the prenylation of small GTPase signaling proteins, ultimately leading to the inhibition of osteoclast activity and, consequently, bone resorption (Dunford et al., 2001; Van Beek et al., 2002).
We found that naringin, the active compound of Drynaria fortunei (Kunze) J. Sm [Polypodiaceae; Drynariae Rhizoma] often used in the included RCTs, inhibits the mevalonate pathway. This mechanism is similar to that of BPs (Hirata et al., 2009; Wu et al., 2021). However, it is unclear whether the improvement in bone mineral density by Gushukang and Qianggu capsules, both containing naringin, is due to this same pathway inhibition. Unlike BPs, which target specific pathways, HMs contain multiple active compounds. This makes it difficult to confirm if their primary mechanism for improving BMD is similar to that of BPs.
BTMs are a useful adjunct for the diagnosis and therapeutic monitoring of bone metabolic disorders (Greenblatt et al., 2017) The bone resorption marker CTX was significantly decreased in the HMs plus ALE group compared to the ALE group. However, the increase in bone formation markers was not consistent (Figure 7A).
BPs are known to strongly inhibit bone resorption. Due to their mechanism of maintaining the balance between resorption and formation during bone remodeling, they typically result in a reduction in bone formation rates as well (Jensen et al., 2021). In particular, PMOP patients often exhibit an abnormal increase in both bone resorption and formation markers approximately 5 years post-menopause. This increase then normalizes with BP treatment, leading to a concurrent decrease in both markers and a reduction in the rate of bone formation (Kuo and Chen, 2017). In our review, among the 10 RCTs that observed both resorption and formation markers, a trend was noted where both markers decreased and BMD improved, particularly in PMOP participants compared to those with senile OP (Figure 7B). Thus, when analyzing BTMs, it is crucial to assess not only their simple increases or decreases but also the balance between bone formation and resorption and the rate of normal bone remodeling (Hu et al., 2013). Additionally, bone markers are sensitive indicators affected by factors such as age, sex, and circulation. Therefore, it is important to consider the timing and conditions under which the tests are performed. Nevertheless, few studies have accurately specified the conditions and test procedures in the RCTs included in this review. Future studies need to establish accurate test protocols and report findings clearly for the scientific evaluation of bone markers.
In our study, the incidence of adverse events in the HM plus BPs group was 4.3%, with gastrointestinal reactions being the most commonly observed type. These reactions are consistent with those typically associated with both BPs and HMs. The common adverse effects of BPs include gastrointestinal reactions (28%–91%) (Tadrous et al., 2014) and musculoskeletal reactions (20.1%–25.0%) (Pazianas and Abrahamsen, 2011). Similarly, adverse events reported for HMs used in OP predominantly involve gastrointestinal issues such as diarrhea, constipation, nausea, and vomiting, with incidence rates ranging from 8.69% to 29.4% (Jia et al., 2022; Shi et al., 2017; Li et al., 2017). Similar to our study, the adverse events of HMs combined with conventional drugs such as BPs for OP also showed similar findings, with gastrointestinal reactions such as nausea, vomiting, constipation, and diarrhea occurring at rates of 5.72%–5.76% (Chen et al., 2021; Kwon et al., 2023). Consequently, the adverse reactions associated with the combination of HMs and BPs in our study were comparable to those reported in the existing literature, with no serious adverse effects observed.
However, among the 43 RCTs included in our review, it remains unclear whether 20 of these trials specified cardiovascular diseases or breast cancer - conditions potentially influenced by BPs - as exclusion criteria. Additionally, the interactions between BPs and HMs are not yet well understood. Therefore, the safety of combining HMs and BPs remains an unresolved issue, and further research is needed to elucidate the interactions and potential risks associated with these two treatments.
Our study has several limitations. Firstly, the included RCTs are characterized by generally low quality, high risk of bias, and significant heterogeneity, resulting in a low level of evidence. Secondly, the lack of separate information on participant sex and age, coupled with difficulties in verifying the diagnostic criteria for PMOP and senile OP in each RCT, hampers clear identification of characteristics based on OP types. Thirdly, the absence of data on factors influencing OP in each RCT prevents the exclusion of potential confounders that could affect BMD improvements. Fourthly, there is insufficient information regarding the quality control, extraction processes, and chemical analysis of HMs, as well as a lack of safety data on the combination of HMs and BPs. Additionally, our review did not include grey literature and conference abstracts, which may contribute to publication bias and limit the perspective on various approaches.
Despite these limitations, our study is significant as it represents the first systematic review to assess the BMD improvement effects of combination therapy with HMs and BPs in patients with primary OP and to evaluate the certainty of the evidence. Additionally, the number of included RCTs and the overall sample size were sufficiently large for analysis. Based on our findings, to investigate the effects of HMs and BPs in OP patients, future studies should employ RCTs with low risk of bias and utilize a double-blind design incorporating placebo controls for HMs. These studies should include standardized protocols for HMs, detailed information on quality control, and safety data. Additionally, it is crucial to control for confounding factors that may impact BMD as thoroughly as possible.
In the future, to provide scientific evidence for the effectiveness of HM combination therapy in patients with primary OP, a well-designed, large-scale clinical study that compensates for the limitations of previous research is needed.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author contributions
Y-SY: Data curation, Formal Analysis, Methodology, Software, Visualization, Writing–original draft. M-GK: Data curation, Formal Analysis, Methodology, Software, Visualization, Writing–original draft. H-JP: Data curation, Formal Analysis, Methodology, Software, Visualization, Writing–original draft. M-YC: Writing–original draft, Data curation, Formal analysis. Y-JC: Data curation, Formal Analysis, Methodology, Software, Visualization, Writing–original draft. C-KO: Data curation, Formal Analysis, Visualization, Writing–original draft. C-GS: Investigation, Supervision, Validation, Writing–review and editing. E-JL: Conceptualization, Funding acquisition, Investigation, Resources, Supervision, Validation, Writing–review and editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT) (No. NRF-2021R1A2C2013483).
Acknowledgments
We acknowledge all colleagues who participated in this study and all the authors of the contributing studies.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2024.1413515/full#supplementary-material
References
Aibar-Almazán, A., Voltes-Martínez, A., Castellote-Caballero, Y., Afanador-Restrepo, D. F., Carcelén-Fraile, M. D. C., and López-Ruiz, E. (2022). Current status of the diagnosis and management of osteoporosis. Int. J. Mol. Sci. 23 (16), 9465. doi:10.3390/ijms23169465
Alcantara, E. H., Shin, M. Y., Sohn, H. Y., Park, Y. M., Kim, T., Lim, J. H., et al. (2011). Diosgenin stimulates osteogenic activity by increasing bone matrix protein synthesis and bone-specific transcription factor Runx2 in osteoblastic MC3T3-E1 cells. J. Nutr. Biochem. 22 (11), 1055–1063. doi:10.1016/j.jnutbio.2010.09.003
Artero, A., Tarín, J. J., and Cano, A. (2012). The adverse effects of estrogen and selective estrogen receptor modulators on hemostasis and thrombosis. Seminars thrombosis hemostasis 38 (08), 797–807. doi:10.1055/s-0032-1328883
Bao, D., and Ling, S. (2015). Clinical observation of Xianling Gubao capsule combined with alendronate sodium enteric dissolved tablets in treatment of osteoporosis. New Chin. Med. 47, 133–134. [in Chinese]. doi:10.13457/j.cnki.jncm.2015.01.063
Bao, H., Guo, H., Feng, Z., and Li, X. (2020). Deciphering the underlying mechanism of Xianlinggubao capsule against osteoporosis by network pharmacology. BMC Complement. Med. Ther. 20, 208. doi:10.1186/s12906-020-03007-1
Bu, B., Bu, L.-l., and Wang, Y. (2021). Effects of zoledronic acid combined with Bushen Huoxue decoction on bone mineral density and biochemical markers of bone metabolism in patients with OVCF during recovery period. Chinese Journal of Integrated Traditional Chinese and Western Medicine Surgery. 4, 233-236. doi:10.3969/j.issn.1007-6948.2021.02.013
Camacho, P. M., Petak, S. M., Binkley, N., Diab, D. L., Eldeiry, L. S., Farooki, A., et al. (2020). AMERICAN association of clinical endocrinologists/AMERICAN College of endocrinology clinical practice guidelines for the diagnosis and treatment of postmenopausal osteoporosis-2020 update. Endocr. Pract. Am. Assoc. Clin. ENDOCRINOLOGISTS/AMERICAN. 26 (Suppl. 1), 1–46. doi:10.4158/GL-2020-0524SUPPL
Chai, S., Wan, L., Wang, J. L., Huang, J. C., and Huang, H. X. (2019). Gushukang inhibits osteocyte apoptosis and enhances BMP-2/Smads signaling pathway in ovariectomized rats. Phytomedicine 64, 153063. doi:10.1016/j.phymed.2019.153063
Chen, J., Zheng, J., Chen, M., Lin, S., and Lin, Z. (2021). The efficacy and safety of Chinese herbal medicine Xianling Gubao capsule combined with alendronate in the treatment of primary osteoporosis: a systematic review and meta-analysis of 20 randomized controlled trials. Front. Pharmacol. 12, 695832. [in Chinese]. doi:10.3389/fphar.2021.695832
Chen, T., Jiao, J., and Xue, J. (2015). Curative observation of combining Xianling Gubao capsule with western medicine to treat osteoporosis. J. Sichuan Trad. Chin. Med. 33, 171–173. [In Chinese, English Abstract].
Chen, Y. (2019). The effect of Gushukang capsule treatment on osteoporotic. Chin. J. Osteoporos./Zhongguo Guzhi Shusong Zazhi 25 (11). [In Chinese, English Abstract]. doi:10.3969/j.issn.1006.7108.2019.11.013
Chu, Y.-j., Meng, W., and Qiu, X. (2021). Clinical efficacy of astragalus liver·-and kidney-·Tonifying decoction combined with alendronate sodium tablets on senile osteoporosis and its influence on inflammatory factors and bone density. Henan Trad. Chin. Med. 41. [In Chinese, English Abstract]. doi:10.16367/j.issn.1003-5028.2021.04.0133
Clynes, M. A., Harvey, N. C., Curtis, E. M., Fuggle, N. R., Dennison, E. M., and Cooper, C. (2020). The epidemiology of osteoporosis. Br. Med. Bull. 133, 105–117. doi:10.1093/bmb/ldaa005
Cosman, F., de Beur, S. J., LeBoff, M. S., Lewiecki, E. M., Tanner, B., Randall, S., et al. (2014). Clinician’s guide to prevention and treatment of osteoporosis. Osteoporos. Int. 25, 2359–2381. doi:10.1007/s00198-014-2794-2
Cui, B., Chen, M., Wu, J., and Liu, Y. (2020). Effect of Gukangfang Granules in the treatment of senile osteoporosis. Chin. Mod. Med. 27, 101–104. [In Chinese, English Abstract].
Du, X., and Zhao, X. (2018). Clinical observation of radio frequency acupotomy combined with Xianling Gubao capsule for the treatment of senile osteoporotic vertebral compression fracture PVP postoperative pain. J. Tianjin Univ. Tradit. Chin. Med. 35, 590. [in Chinese]. doi:10.11656/j.issn.1672-1519.2018.08.09
Dunford, J. E., Thompson, K., Coxon, F. P., Luckman, S. P., Hahn, F. M., Poulter, C. D., et al. (2001). Structure-activity relationships for inhibition of farnesyl diphosphate synthase in vitro and inhibition of bone resorption in vivo by nitrogen-containing bisphosphonates. J. Pharmacol. Exp. Ther. 296 (2), 235–242.
Ensrud, K. E., and Crandall, C. J. (2017). Osteoporosis. Ann. Intern. Med. 167, ITC17-ITC32–ITC32. doi:10.7326/AITC201708010
Feng, X.-b., Peng, Y., Wu, T.-h., and Yang, K. (2018). Clinical study on Tenghuang Jiangu Capsules combined with zoledronic acid in treatment of senile primary osteoporosis. Drugs Clin. 33, 1788–1792. [In Chinese, English Abstract]. doi:10.7501/j.issn.1674-5515.2018.07.052
Fleisch, H., Graham, R., Russell, G., and Francis, M. D. (1969). Diphosphonates inhibit hydroxyapatite dissolution in vitro and bone resorption in tissue culture and in vivo. Science 165 (3899), 1262–1264. doi:10.1126/science.165.3899.1264
Föger-Samwald, U., Kerschan-Schindl, K., Butylina, M., and Pietschmann, P. (2022). Age related osteoporosis: targeting cellular senescence. Int. J. Mol. Sci. 23, 2701. doi:10.3390/ijms23052701
Fu, C., Zhang, T., and Dong, Q. (2016). Calcic D Xianling Gubao capsules and alendronate tablets in the treatment of osteoporosis in the elderly clinical effect and safety analysis. Zhejiang Clin. Med. 18, 2193–2195. [In Chinese, English Abstract].
Fuggle, N., Al-Daghri, N., Bock, O., Branco, J., Bruyère, O., Casado, E., et al. (2019). Rehmannia glutinosa Libosch extracts prevent bone loss and architectural deterioration and enhance osteoblastic bone formation by regulating the IGF-1/PI3K/mTOR pathway in streptozotocin-induced diabetic rats. Int. J. Mol. Sci. 20, 3964. doi:10.3390/ijms20163964
Greenblatt, M. B., Tsai, J. N., and Wein, M. N. (2017). Bone turnover markers in the diagnosis and monitoring of metabolic bone disease. Clin. Chem. 63 (2), 464–474. doi:10.1373/clinchem.2016.259085
Gui, X., Gong, G.-x., and Huang, F.-m. (2017). Clinical observation of 60 cases of primary osteoporosis treated with Bushen Yiqi Huayu decoction. Drugs Clin. 14, 18–20. [In Chinese, English Abstract].
Guo, H., and Yuan, L. (2020). Effect of xianling gubao capsule combined with salmon calcitonin and alendronate on the treatment of senile osteoporosis. J Med Theor and Prac. [in Chinese]. doi:10.19381/j.issn.1001-7585.2020.21.030
Heinrich, M., Jalil, B., Abdel-Tawab, M., Echeverria, J., Kulić, Ž., McGaw, L. J., et al. (2022). Best practice in the chemical characterisation of extracts used in pharmacological and toxicological research—the ConPhyMP—guidelines. Front. Pharmacol. 13, 953205. doi:10.3389/fphar.2022.953205
Hirata, M., Matsumoto, C., Takita, M., Miyaura, C., and Inada, M. (2009). Naringin suppresses osteoclast formation and enhances bone mass in mice. J. Health Sci. 55 (3), 463–467. doi:10.1248/jhs.55.463
Hu, J., Sun, X., and Zhang, B. (2020). Effects modified Bushen Huoxue decoction on promoting postoperative bone healing in patients with osteoporotic vertebral compression fracture. World Chin. Med. 15, 2602–2607.
Hu, W. W., Zhang, Z., He, J. W., Fu, W. Z., Wang, C., Zhang, H., et al. (2013). Establishing reference intervals for bone turnover markers in the healthy shanghai population and the relationship with bone mineral density in postmenopausal women. Int. J. Endocrinol. 2013 (1), 513925. doi:10.1155/2013/513925
Huang, Y., Shao, M., Xu, S., Jiang, T., Chen, Q., and Wang, Q. (2018). Clinical observation on Bushen tang combined with alendronate sodium tablets for primary osteoporosis with kidney deficiency type. New Chin. Med. 50, 102–105. [In Chinese, English Abstract]. doi:10.13457/j.cnki.jncm.2018.09.029
Iseme, R. A., Mcevoy, M., Kelly, B., Agnew, L., Walker, F. R., and Attia, J. (2017). Is osteoporosis an autoimmune mediated disorder? Bone Rep. 7, 121–131. doi:10.1016/j.bonr.2017.10.003
Jadad, A. R., Moore, R. A., Carroll, D., Jenkinson, C., Reynolds, D. J., Gavaghan, D. J., et al. (1996). Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control. Clin. Trials 17, 1–12. doi:10.1016/0197-2456(95)00134-4
Jensen, P. R., Andersen, T. L., Chavassieux, P., Roux, J. P., and Delaisse, J. M. (2021). Bisphosphonates impair the onset of bone formation at remodeling sites. Bone 145, 115850. doi:10.1016/j.bone.2021.115850
Jia, Y., Sun, J., Zhao, Y., Tang, K., Zhu, R., Zhao, W., et al. (2022). Chinese patent medicine for osteoporosis: a systematic review and meta-analysis. Bioengineered 13 (3), 5581–5597. doi:10.1080/21655979.2022.2038941
Jia, Z., Zhang, J., Yang, X., Chen, H., Wang, Y., Francis, O. B., et al. (2024). Bioactive components and potential mechanisms of Biqi Capsule in the treatment of osteoarthritis: based on chondroprotective and anti-inflammatory activity. Front. Pharmacol. 15, 1347970. doi:10.3389/fphar.2024.1347970
Jiang, Y., Chen, Q., Hu, J., and Liang, Z. (2020). Clinical study of deerhorn zhuanggu capsule in treatment of renyang deficient senile osteoporosis. Orient. Medicat. Diet., 2020. [in Chinese].
Jiannong, W., Junjie, J., Yanming, X., Xu, W., Jianpeng, L., Jingli, D., et al. (2015). Effect of naringenin in Qianggu capsule on population pharmacokinetics in Chinese women with primary osteoporosis. J. Traditional Chin. Med. 35 (2), 141–153. doi:10.1016/S0254-6272(15)30021-2
Jin, Y. X., Wu, P., Mao, Y. F., Wang, B., Zhang, J. F., Chen, W. L., et al. (2017). Chinese herbal medicine for osteoporosis: a meta-analysis of randomized controlled trials. J. Clin. Densitom. 20, 516–525. doi:10.1016/j.jocd.2017.07.003
Johnston, C. B., and Dagar, M. (2020). Osteoporosis in older adults. Med. Clin. North Am. 104, 873–884. doi:10.1016/j.mcna.2020.06.004
Jolly, J. J., Chin, K. Y., Alias, E., Chua, K. H., and Soelaiman, I. N. (2018). Protective effects of selected botanical agents on bone. Int. J. Environ. Res. public health 15 (5), 963. doi:10.3390/ijerph15050963
Ju, Y., Wu, B., and Qu, N. (2020). Clinical observation of erxian decoction in the treatment of postmenopausal osteoporosis. J. Liaoning Univ. TCM. 22, 199–202. [In Chinese, English Abstract]. doi:10.13194/j.issn.1673-842x.2020.03.052
Kang, L., Li, W., Lan, H., and Deng, W. (2020). Effects of Xianling Guzhi capsule on bone mineral density and bone metabolism in aged osteoporosis patients. Med. Innov. China. 17, 037–040. [In Chinese, English Abstract]. doi:10.3969/j.issn.1674-4985.2020.22.010
Kanis, J. A., Cooper, C., Rizzoli, R., and Reginster, J. Y.Scientific Advisory Board of the European Society for Clinical and Economic Aspects of Osteoporosis ESCEO and the Committees of Scientific Advisors and National Societies of the International Osteoporosis Foundation IOF (2019). European guidance for the diagnosis and management of osteoporosis in postmenopausal women. Osteoporos. Int. 30 (1), 3–44. doi:10.1007/s00198-018-4704-5
Khosla, S., and Hofbauer, L. C. (2017). Osteoporosis treatment: recent developments and ongoing challenges. lancet Diabetes and Endocrinol. 5 (11), 898–907. doi:10.1016/S2213-8587(17)30188-2
Kim, T. H., Jung, J. W., Ha, B. G., Hong, J. M., Park, E. K., Kim, H. J., et al. (2011). The effects of luteolin on osteoclast differentiation, function in vitro and ovariectomy-induced bone loss. J. Nutr. Biochem. 22 (1), 8–15. doi:10.1016/j.jnutbio.2009.11.002
Kuo, T. R., and Chen, C. H. (2017). Bone biomarker for the clinical assessment of osteoporosis: recent developments and future perspectives. Biomark. Res. 5, 18–19. doi:10.1186/s40364-017-0097-4
Kwon, D. Y., Gu, J. H., Oh, M., and Lee, E. J. (2023). Combination effects of herbal and western medicines on osteoporosis in rheumatoid arthritis: systematic review and meta-analysis. Front. Pharmacol. 14, 1164898. doi:10.3389/fphar.2023.1164898
Lee, E. W., and Nam, J. Y. (2021). Sex difference in the socioeconomic burden of osteoporosis among south koreans. Healthc. (Basel) 9, 1304. doi:10.3390/healthcare9101304
Leung, P. C., and Siu, W. S. (2013). Herbal treatment for osteoporosis: a current review. J. Tradit. Complement. Med. 3, 82–87. doi:10.4103/2225-4110.110407
Lewiecki, E. M., Ortendahl, J. D., Vanderpuye-Orgle, J., Grauer, A., Arellano, J., Lemay, J., et al. (2019). Healthcare policy changes in osteoporosis can improve outcomes and reduce costs in the United States. JBMR Plus 3, e10192. doi:10.1002/jbm4.10192
Li, J., Wang, W., Feng, G., Du, J., Kang, S., Li, Z., et al. (2020). Efficacy and safety of Duhuo Jisheng decoction for postmenopausal osteoporosis: a systematic review and meta-analysis. Evid. Based Complement. Altern. Med. 2020, 6957825. doi:10.1155/2020/6957825
Li, J. Y., Jia, Y. S., Chai, L. M., Mu, X. H., Ma, S., Xu, L., et al. (2017). Effects of Chinese herbal formula Erxian decoction for treating osteoporosis: a systematic review. Clin. Interventions Aging 12, 45–53. doi:10.2147/CIA.S117597
Li, M., Jiang, Y., Feng, L., Ping, S., and Liu, Y. (2021). Treatment of femoral neck fracture in elderly patients after artificial joint replacement by integrative medical treatment. CJITWM 41, 806–811. [In Chinese, English Abstract]. doi:10.7661/j.cjim.20210120.128
Li, M., Ni, J., Hu, M., and Qiu, X. (2019). Comparison of the efficacy of Xianling Gubao Capsule combined with Calcium D 600 and alendronate tablet in the treatment of senile osteoporosis. Shanxi Med. J. 48. [In Chinese, English Abstract]. doi:10.3969/j.issn.0253-9926.2019.03.007
Li, M., Tang, H., Hu, Y., Li, S., Kang, P., Chen, B., et al. (2023). Integrating network pharmacology and experimental verification strategies to reveal the active ingredients and molecular mechanism of Tenghuang Jiangu Capsule against osteoporosis. Heliyon 9 (9), e19812. doi:10.1016/j.heliyon.2023.e19812
Liu, F. X., and Sun, Y. (2023). Identification of the active ingredients and pharmacological effects of Kuntai capsules in the treatment of primary ovarian insufficiency: a review. Medicine 102 (21), e33884. doi:10.1097/MD.0000000000033884
Liu, Y., Liu, J. P., and Xia, Y. (2014). Chinese herbal medicines for treating osteoporosis. Cochrane Database Syst. Rev. 2014, CD005467. [In Chinese, English Abstract]. doi:10.1002/14651858.CD005467.pub2
Liu, Y., and Wang, C.-w. (2016). Clinical effect of Erxian Bushen decoction combined with warm acupuncture in treating post-menopause osteoporosis with syndrome of kidney-Yang deficiency. Chin. J. Exp. Trad. Med. Formulae. 22. [In Chinese, English Abstract]. doi:10.13422/j.cnki.syfjx.2016090162
Liu, Y., Xu, H., Long, S., Xing, M., Xu, L., Lu, L., et al. (2019). Effect of Bushen Juanbi decoction combined with western medicine on bone metabolism and levels of oxidative stress products in postmenopausal osteoporosis. Hebei J. TCM. 41, 827–832. doi:10.3969/j.issn.1002-2619.2019.06.006
Lu, L., Wang, Z., Zhang, H., Liu, T., and Fang, H. (2022). Drynaria fortunei improves lipid profiles of elderly patients with postmenopausal osteoporosis via regulation of Notch1-NLRP3 inflammasome-mediated inflammation. Gynecol. Endocrinol. 38, 176–180. doi:10.1080/09513590.2021.2015760
Lu, Z., Wu, Q., and Zhou, Z. (2016). Evaluation of the effect of anti-bone hyperplasia capsules in comprehensive postmenopausal osteoporosis intervention program. Chongqing Med. 45, 3557–3560. [in Chinese]. doi:10.3969/j.issn.1671-8348.2016.25.035
Lupo, M., Dains, J. E., and Madsen, L. T. (2015). Hormone replacement therapy: an increased risk of recurrence and mortality for breast cancer patients? J. Adv. Pract. Oncol. 6 (4), 322–330. doi:10.6004/jadpro.2015.6.4.3
McClung, M., Harris, S. T., Miller, P. D., Bauer, D. C., Davison, K. S., Dian, L., et al. (2013). Bisphosphonate therapy for osteoporosis: benefits, risks, and drug holiday. Am. J. Med. 126, 13–20. doi:10.1016/j.amjmed.2012.06.023
Minisola, S., Cipriani, C., Grotta, G. D., Colangelo, L., Occhiuto, M., Biondi, P., et al. (2019). Update on the safety and efficacy of teriparatide in the treatment of osteoporosis. Ther. Adv. Musculoskelet. Dis. 11, 1759720X19877994. doi:10.1177/1759720X19877994
Nash, L. A., Sullivan, P. J., Peters, S. J., and Ward, W. E. (2015). Rooibos flavonoids, orientin and luteolin, stimulate mineralization in human osteoblasts through the Wnt pathway. Mol. Nutr. and food Res. 59 (3), 443–453. doi:10.1002/mnfr.201400592
Nuti, R., Brandi, M. L., Checchia, G., Di Munno, O., Dominguez, L., Falaschi, P., et al. (2019). Guidelines for the management of osteoporosis and fragility fractures. Intern. Emerg. Med. 14, 85–102. doi:10.1007/s11739-018-1874-2
Park, J. H., and Ko, H. J. (2022). The association between treatment with bisphosphonates and the risk of atrial fibrillation: a meta-analysis of observational studies. Korean J. Fam. Med. 43 (1), 69–76. doi:10.4082/kjfm.21.0110
Pazianas, M., and Abrahamsen, B. O. (2011). Safety of bisphosphonates. Bone 49 (1), 103–110. doi:10.1016/j.bone.2011.01.003
Pouresmaeili, F., Kamalidehghan, B., Kamarehei, M., and Goh, Y. M. (2018). A comprehensive overview on osteoporosis and its risk factors. Ther. Clin. Risk Manag. 14, 2029–2049. doi:10.2147/TCRM.S138000
Qiu, Z. C., Tang, X. Y., Wu, Q. C., Tang, Z. L., Wong, M. S., Chen, J. X., et al. (2021). A new strategy for discovering effective substances and mechanisms of traditional Chinese medicine based on standardized drug containing plasma and the absorbed ingredients composition, a case study of Xian-Ling-Gu-Bao capsules. J. Ethnopharmacol. 279, 114396. doi:10.1016/j.jep.2021.114396
Raisz, L. G. (2005). Pathogenesis of osteoporosis: concepts, conflicts, and prospects. J. Clin. Invest. 115, 3318–3325. doi:10.1172/JCI27071
Rogers, M. J. (2003). New insights into the molecular mechanisms of action of bisphosphonates. Curr. Pharm. Des. 9, 2643–2658. doi:10.2174/1381612033453640
Salari, N., Ghasemi, H., Mohammadi, L., Behzadi, M. H., Rabieenia, E., Shohaimi, S., et al. (2021). The global prevalence of osteoporosis in the world: a comprehensive systematic review and meta-analysis. J. Orthop. Surg. Res. 16, 609. doi:10.1186/s13018-021-02772-0
Satué, M., del Mar Arriero, M., Monjo, M., and Ramis, J. M. (2013). Quercitrin and taxifolin stimulate osteoblast differentiation in MC3T3-E1 cells and inhibit osteoclastogenesis in RAW 264.7 cells. Biochem. Pharmacol. 86 (10), 1476–1486. doi:10.1016/j.bcp.2013.09.009
Seeman, E., and Delmas, P. D. (2006). Bone quality—the material and structural basis of bone strength and fragility. N. Engl. J. Med. 354, 2250–2261. doi:10.1056/NEJMra053077
Shen, W., Yang, W., Zhang, G.-y., and Li, W.-l. (2017). Clinical observation on the treatment of senile osteoporosis with Bushen Huoxue tang combining calcium supplement. Rheum. Arthritis. 6, 22–25. [In Chinese, English Abstract].
Shi, Z. Y., Zhang, X. G., Li, C. W., Liu, K., Liang, B. C., and Shi, X. L. (2017). Effect of traditional Chinese medicine product, QiangGuYin, on bone mineral density and bone turnover in Chinese postmenopausal osteoporosis. Evidence-Based Complementary Altern. Med. 2017 (1), 6062707. doi:10.1155/2017/6062707
Sjögren, L. L., Mørch, L. S., and Løkkegaard, E. (2016). Hormone replacement therapy and the risk of endometrial cancer: a systematic review. Maturitas 91, 25–35. doi:10.1016/j.maturitas.2016.05.013
Słupski, W., Jawień, P., and Nowak, B. (2021). Botanicals in postmenopausal osteoporosis. Nutrients 13, 1609. doi:10.3390/nu13051609
Sözen, T., Özışık, L., and Başaran, N. Ç. (2017). An overview and management of osteoporosis. Eur. J. rheumatology 4 (1), 46–56. doi:10.5152/eurjrheum.2016.048
Tadrous, M., Wong, L., Mamdani, M. M., Juurlink, D. N., Krahn, M. D., Lévesque, L. E., et al. (2014). Comparative gastrointestinal safety of bisphosphonates in primary osteoporosis: a network meta-analysis. Osteoporos. Int. 25, 1225–1235. doi:10.1007/s00198-013-2576-2
Tella, S. H., and Gallagher, J. C. (2014). Prevention and treatment of postmenopausal osteoporosis. J. Steroid Biochem. Mol. Biol. 142, 155–170. doi:10.1016/j.jsbmb.2013.09.008
Tsvetov, G., Amitai, O., Shochat, T., Shimon, I., Akirov, A., and Diker-Cohen, T. (2020). Denosumab-induced hypocalcemia in patients with osteoporosis: can you know who will get low? Osteoporos. Int. 31, 655–665. doi:10.1007/s00198-019-05261-7
Tu, K. N., Lie, J. D., Wan, C. K. V., Cameron, M., Austel, A. G., Nguyen, J. K., et al. (2018). Osteoporosis: a review of treatment options. Pharm. Ther. 43 (2), 92–104.
Tu, Y., Xiong, L., Liu, X., Shen, Y., and Qin, Y. (2017). Clinical research on compound Epimedium Oral liquid treatment of primary osteoporosis. Acta Chin. Med. 32, 1981–1984. [In Chinese, English Abstract]. doi:10.16368/j.issn.1674-8999.2017.10.520
Van Beek, E. R., Löwik, C. W. G. M., and Papapoulos, S. E. (2002). Bisphosphonates suppress bone resorption by a direct effect on early osteoclast precursors without affecting the osteoclastogenic capacity of osteogenic cells: the role of protein geranylgeranylation in the action of nitrogencontaining bisphosphonates on osteoclast precursors. Bone 30 (1), 64–70. doi:10.1016/S8756-3282(01)00655-X
van Staa, T. P., Dennison, E. M., Leufkens, H. G., and Cooper, C. (2001). Epidemiology of fractures in england and wales. Bone 29, 517–522. doi:10.1016/s8756-3282(01)00614-7
Wang, B., Shen, L., Cong, W., Lin, X., Feng, Y., Zhu, Y., et al. (2013). A simple HPLC method for simultaneous analysis of 7 bioactive constituents for Liuwei Dihuang Pill and its application in quality consistency evaluation. Anal. Methods 5 (9), 2384–2390. doi:10.1039/C3AY40125A
Wang, J. (2020). Clinical observation of 65 cases of primary osteoporosis treated with Qi-boosting Shen decoction. Hunan J. Trad. Chin. Med. [in Chinese]. doi:10.16808/j.cnki.issn1003-7705.2020.02.019
Wang, K., Yang, J., Zhang, H., Huang, W., Zheng, X., and Wan, M. (2018). Efficacy and effect of bone metabolism markers and bone strength in the treatment of senile osteoporosis. Chin. J. Gerontol. 38, 864–866. [in Chinese]. doi:10.3969/j.issn.1005-9202.2018.04.041
Wong, S. K., Chin, K. Y., and Ima-Nirwana, S. (2019). The osteoprotective effects of kaempferol: the evidence from in vivo and in vitro studies. Drug Des. Dev. Ther. 13, 3497–3514. doi:10.2147/DDDT.S227738
Wu, G. J., Chen, K. Y., Yang, J. D., Liu, S. H., and Chen, R. M. (2021). Naringin improves osteoblast mineralization and bone healing and strength through regulating estrogen receptor alpha-dependent alkaline phosphatase gene expression. J. Agric. Food Chem. 69 (44), 13020–13033. doi:10.1021/acs.jafc.1c04353
Xiang, K., Zhuang, Q., Huang, Y., Zheng, X., Zeng, J., and Lai, H. (2013). A clinical study on the treatment of postmenopausal hepatic and renal deficiency osteoporosis with zoledonic acid. J. Guiyang Coll. Trad. Chin. Med. 35, 27–30. [in Chinese]. doi:10.3969/j.issn.1002-1108.2013.04.0013
Xie, Y.-j., Zhang, S.-d., and Wu, B. (2015). Clinical observation of Duhuo Jisheng decoction in staging treatment for 48 cases with senile osteoporotic simple thoracolumbar compression fracture. Chin. J. Exp. Trad. Med. Formulae. 21, 176–179. [In Chinese, English Abstract]. doi:10.13422/j.cnki.syfjx.2015150176
Xie, Y. M., Yuwen, Y., Dong, F. H., Sun, S. C., Wang, H. M., Liu, Q. S., et al. (2011). Clinical practice guideline of traditional medicine for primary osteoporosis. Chin. J. Integr. Med. 17, 52–63. doi:10.1007/s11655-011-0613-6
Xing, Y., Bi, H. Y., and Zhang, Q. N. (2013). Introduction of common Chinese patent medicines for the treatment of osteoporosis. Chin. J. Osteoporos. 19 (1), 83–85.
Xu, H., Ren, D., Liang, Z., and Wang, J. (2010). Clinical observation of Qianggu capsule plus alendronate on postmenopausal osteoporosis. J. Zhejiang Univ. Trad. Chin. Med. 34, 503–504. [In Chinese, English Abstract]. doi:10.16466/j.issn1005-5509.2010.04.072
Xu, M., Liu, B.-x., Huang, C.-j., Tang, F.-y., Lou, Y.-m., Liang, Z., et al. (2009). Clinical observation of Xianlinggubao plus alendronate on postmenopausal osteoporosis. J. Liaoning Univ. TCM. 11, 94–95. [In Chinese, English Abstract]. doi:10.13194/j.jlunivtcm.2009.01.96.xum.106
Xu, R., Chen, M., Lin, Y., Huang, J., and Zhou, L. (2019). Effect Wenshen Gushu recipe on the expression of steroid receptor coactivator 3, transcription element col-gnactivator protein, B – cell lymphoma gene – 2 and the Notch signaling pathway of bone marrow mesenchymal stem cells in postmenopausal osteoporosis patients with kidney yang deficiency type. Hebei J. TCM. 41, 1470–1474. [In Chinese, English Abstract]. doi:10.3969/j.issn.1002∼2619.2019.10.006
Xue, J., Xue, L., and Chen, H. (2018). Clinical observation on the efficacy and safety of integrated traditional Chinese and western medicine in treatment of postmenopausal osteoporosis. Med. Innov. China. 15, 11–15. [In Chinese, English Abstract]. doi:10.3969/j.issn.1674-4985.2018.14.003
Yan, L., Yan, B., and Shen, W. (2017). The therapeutic effect of Zengdian decoction combined with alendronate Tablets on osteoporosis in the elderly was observed. Chin. Trad. Med. 24, 226–227. [in Chinese].
Yang, X. (2021). Clinical observation of Kuntai capsule for postmenopausal osteoporosis. J. Pract. Trad. Chin. Med. 37. [in Chinese].
Yang, Y., Zhang, Y., and Li, X. (2017). Effects of angelicae pubescentis and Loranthi decoction on bone mineral density and bone metabolism in postmenopausal osteoporosis (liver-kidney yin deficiency) patients. Med. Innov. China. 14, 061–064. [In Chinese, English Abstract].
Yu, S.-J., Ahn, H.-B., Kim, J.-Y., and Lee, D.-N. (2012). A comparative study of BMD between osteoporosis patients taking Fosamax and taking Fosamax and Samgieumgamibang. J. Korean Obstet. Gynecol. 25, 11–19.
Zeng, J., Li, C., and Gu, Z. (2024). A network pharmacological study to unveil the mechanisms of xianlinggubao capsule in the treatment of osteoarthritis and osteoporosis. Archives Med. Sci. AMS 20 (2), 557–566. doi:10.5114/aoms.2020.92931
Zeng, W., and He, W. (2015). The efficacy of Xianlinggubao capsule combined with alendronate sodium tablets and salmon calcitonin in the treatment for the patients with osteoporosis. Shanghai Pharma. 36 (17), 27–30. [In Chinese, English Abstract].
Zhang, C., Peng, R., Zhao, J., Deng, Q., Li, Z., Guan, X., et al. (2017). YiShen Dihuang tang combined with risedronate sodium in the treatment for 45 cases of postmenopausal osteoporosis of kidney yang deficiency type. West. J. Trad. Chin. Med. 30.
Zhang, H. (2019). Effect of Yishen Zhuanggu decoction combined with alendronate sodium and calcium carbonate D3 on osteoporosis. Med. J. Chin. Peoples Health. 31, 99–101. [in Chinese]. doi:10.3969/j.issn.1672-0369.2019.15.043
Zhang, X., Li, H., Zhang, X., and Wang, Y. (2021). Clinical observation on the treatment of primary osteoporosis by traditional Chinese medicine combined with pulse magnetic field therapy instrument. J. Pract. Trad. Chin. Med. 37. [in Chinese].
Zhang, Y., Zeng, Y., Liao, M., Jiang, Y., Luo, Y., Liu, Q., et al. (2015). Clinical study on addition treatment of liver – kidney yin deficiency and postmenopausal osteoporosis with Liuwei Dihuang pill. Chin. J. Prim. Med. Pharm. 12, 1797–1801. [In Chinese, English Abstract]. doi:10.3760/cma.j.issn.1008-6706.2015.12.013
Zhang, Z., and Shang, L. (2014). Clinical efficacy and safety evaluation on integrative treatment of osteoporosis. Chin. Arch. Trad. Chin. Med. 32, 3061–3063. [In Chinese, English Abstract]. doi:10.13193/j.issn.1673-7717.2014.12.079
Zhang, Z. Q., Ho, S. C., Chen, Z. Q., Zhang, C. X., and Chen, Y. M. (2014). Reference values of bone mineral density and prevalence of osteoporosis in Chinese adults. Osteoporos. Int. 25, 497–507. doi:10.1007/s00198-013-2418-2
Zhao, J., Zeng, L., Wu, M., Huang, H., Liang, G., Yang, W., et al. (2021). Efficacy of Chinese patent medicine for primary osteoporosis: a network meta-analysis. Complement. Ther. Clin. Pract. 44, 101419. doi:10.1016/j.ctcp.2021.101419
Zhao, L., Li, W., Lan, H., and Deng, W. (2020). Effect of Xianling Gubao capsule on bone density and bone metabolism level in senile osteoporosis patients. Med. Innov. China. [In Chinese, English Abstract].
Zhen, W., Pan, J., and Fang, J. (2014). Clinical observation of 50 cases of osteoporosis treated with bone bi decoction and western medicine. New Chin. Med. 46, 103–105. [in Chinese]. doi:10.13457/j.cnki.jncm.2014.05.040
Zheng, Z. G., Zhang, X., Zhou, Y. P., Lu, C., Thu, P. M., Qian, C., et al. (2017). Anhydroicaritin, a SREBPs inhibitor, inhibits RANKL-induced osteoclastic differentiation and improves diabetic osteoporosis in STZ-induced mice. Eur. J. Pharmacol. 809, 156–162. doi:10.1016/j.ejphar.2017.05.017
Zhu, C., Chen, S., and Wang, Y. (2020). Clinical observation on Yangxue Gushen decoction in the treatment of postmenopausal osteoporosis. CJGMCM 35. [In Chinese, English Abstract]. doi:10.3969/j.issn.1003-8914.2020.19.028
Glossary
Keywords: primary osteoporosis, herbal medicines, bisphosphonates, combination therapy, bone mineral density
Citation: Yoo Y-S, Kim M-G, Park H-J, Chae M-Y, Choi Y-J, Oh C-K, Son C-G and Lee E-J (2024) Additional effects of herbal medicine combined with bisphosphonates for primary osteoporosis: a systematic review and meta-analysis. Front. Pharmacol. 15:1413515. doi: 10.3389/fphar.2024.1413515
Received: 07 April 2024; Accepted: 26 August 2024;
Published: 13 September 2024.
Edited by:
Wei Peng, Chengdu University of Traditional Chinese Medicine, ChinaReviewed by:
Amirala Bakhshian Nik, Medical College of Wisconsin, United StatesPeng Zhang, Guangzhou University of Chinese Medicine, China
Copyright © 2024 Yoo, Kim, Park, Chae, Choi, Oh, Son and Lee. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Eun-Jung Lee, anVuZ2thaG5AZGp1Lmty
†These authors have contributed equally to this work and share first authorship
 Young-Seo Yoo
Young-Seo Yoo Min-Gyeong Kim
Min-Gyeong Kim Hee-Joo Park1
Hee-Joo Park1 Eun-Jung Lee
Eun-Jung Lee