- 1Dongzhimen Hospital, Beijing University of Chinese Medicine, Beijing, China
- 2Liver Diseases Academy of Traditional Chinese Medicine, Beijing University of Chinese Medicine, Beijing, China
- 3Xiyuan Hospital of China Academy of Chinese Medical Sciences, Beijing, China
- 4First Affiliated Hospital of Hebei University of Chinese Medicine, Shijiazhuang, China
- 5Dongfang Hospital, Beijing University of Chinese Medicine, Beijing, China
- 6Key Laboratory of Chinese Internal Medicine of Ministry of Education and Beijing, Dongzhimen Hospital, Beijing University of Chinese Medicine, Beijing, China
With the general improvement in living standards in recent years, people’s living habits, including their dietary habits, have changed. More people around the world do not follow a healthy diet, leading to an increase in morbidity and even mortality due to digestive system diseases, which shows an increasing trend every year. The advantage of traditional Chinese medicine (TCM) in treating digestive system diseases is evident. Consequently, the mechanisms of action of single Chinese herbs and compound Chinese medicines have become the focus of research. The research method of the network pharmacology system was highly consistent with the holistic concept of TCM, and provided a new perspective and theoretical basis for basic research on digestive system diseases. This article summarizes the common databases currently used in research on TCM. It also briefly introduces the basic methods and technologies of network pharmacology studies. It also summarizes the advancements of network pharmacology technology through a comprehensive literature search on PubMed. Based on this analysis, we further explored the role of TCM in treating digestive system diseases, including chronic gastritis, gastric cancer, ulcerative colitis, and liver cirrhosis. This study provides new ideas and references for treating digestive system diseases with TCM in the future and serves as a reference for relevant researchers.
Introduction
Network pharmacology as an emerging discipline was first proposed by Hopkins, a British pharmacologist, in Nature Biotechnology in 2007 (Hopkins, 2007). Under the premise of the rapid development of network databases, network pharmacology is based on the theories of pharmacology, bioinformatics, and other disciplines. Further use of visualization technology, high-throughput technology, network analysis, and other methods could explore the effective mechanisms of single medicines or compounds of TCM in treating diseases. From a macro perspective, these methods elucidated the interaction mechanisms between single herbs or compounds in TCM and diseases. Furthermore, this comprehensive approach highlighted the characteristics of multiple compounds, targets, and pathways (Hopkins, 2007; Li and Zhang, 2013; Wu, et al., 2016; Zhang, et al., 2019a).
Network pharmacology is connected with systemic biomedical technology. Through computer software and the TCM database websites, the unique advantages of TCM in treating diseases have been combined with single medicines or compound medicines used in TCM. Until now, numerous active compounds or compound medicines used in TCM have been analyzed by the network pharmacology method, and the key action mechanisms for treating diseases have been discussed. These studies of network pharmacology provided a better reference for treating diseases and for the production of new clinical medicines. Thus, the theory of “medicine–compound–target–disease” in network pharmacology coincided with the holistic concept proposed by TCM (Hopkins, 2008). Nowadays, the application scope of network pharmacology is increasing, which effectively explores the pharmacological role and mechanism of TCM in the treatment of diseases, studies the application scope of TCM in the treatment of diseases, and effectively analyzes the theory of TCM (Liu, et al., 2017; Zhan and Su, 2018; Guo, et al., 2019; Han, et al., 2019). This paper used network pharmacology to review the research results and status quo of TCM in the treatment of digestive system diseases, to identify problems in this research field, and to provide new ideas and methods for the prevention and treatment of digestive system diseases in the future.
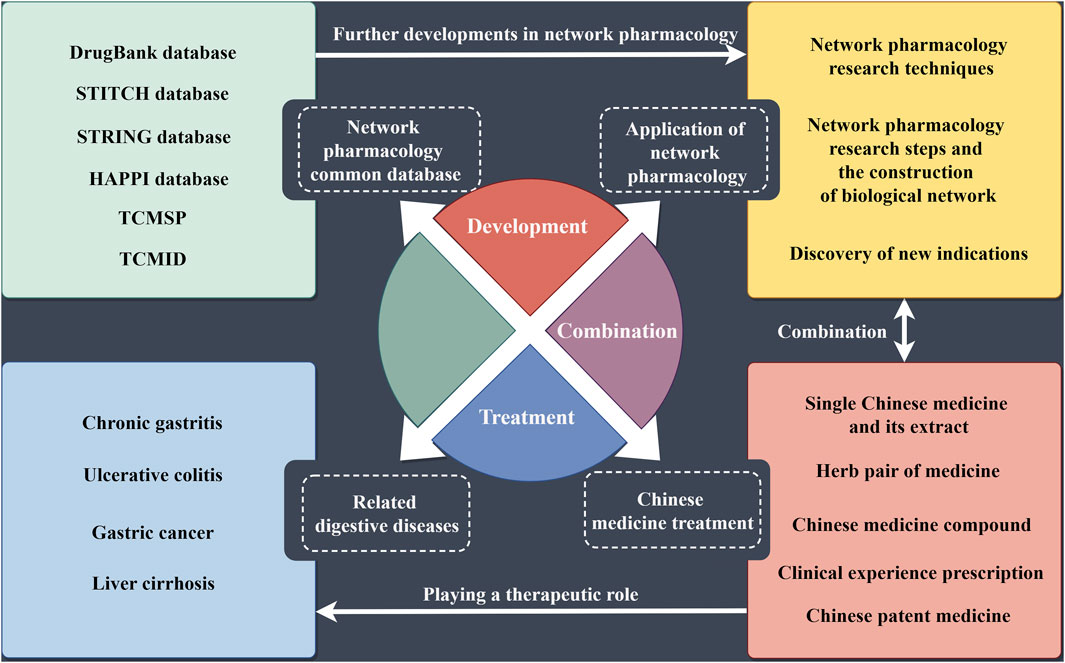
Digestive system diseases belong to the field of internal medicine and include conditions affecting the digestive tract, digestive glands, mesentery, and peritoneal organs. The digestive tract is composed of the mouth, esophagus, throat, stomach, small intestine, large intestine, and anus (Zhu, et al., 2017). Common diseases in this category mainly include chronic gastritis, gastric cancer, liver cirrhosis, and ulcerative colitis. China is recognized as a high-risk country for esophageal cancer (Liu, et al., 2022). In China, malignant tumors of the digestive system are the most prevalent type of cancer, whereas in Western countries, inflammatory bowel diseases (IBDs) are more common (Park and Cheon, 2021). The burden of digestive system diseases is significant, with high morbidity and mortality rates. For instance, liver cancer and stomach cancer are among the leading causes of cancer-related deaths worldwide. Chronic conditions such as gastroesophageal reflux disease (GERD) and ulcerative colitis severely impact the quality of life and require long-term management. Current treatment methods for digestive system diseases include medication, lifestyle changes, and surgical interventions. However, these treatments have limitations. For example, medications for GERD often need to be taken long term and could have side effects. Surgical treatments for cancers of the digestive system, while potentially curative, come with risks and potential complications. Furthermore, treatments for chronic conditions such as IBD often involve immunosuppressive drugs that have significant adverse effects and may not be effective for all patients (Feng, et al., 2022). The symptoms of digestive system diseases are diverse and can often be accompanied by symptoms of other systemic diseases. Therefore, it is crucial to fully understand the characteristics of these symptoms, the accompanying symptoms, and the appropriate medication for accurate diagnosis and treatment. Studies found that sleep quality could also affect the pathogenesis and symptoms of digestive system diseases (Orr, et al., 2020). This highlighted the close relationship between digestive system diseases and other conditions, emphasizing the importance of these diseases for public health (Figure 1). In conclusion, digestive system diseases pose a significant health burden due to their high prevalence, complex symptoms, and the limitations of current treatment options. A comprehensive understanding of these diseases and ongoing research into more effective treatments are essential to improving patient outcomes and quality of life.
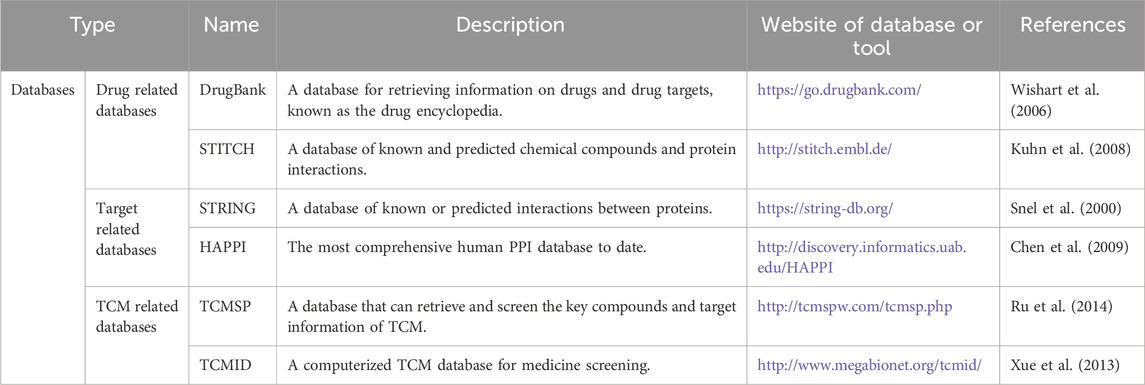
Common network pharmacology databases
DrugBank database
The DrugBank (https://go.drugbank.com/) database (Wishart, et al., 2006) is a comprehensive, freely accessible database that serves as a unique chemoinformatics and bioinformatics tool for retrieving information about drugs and drug targets. It combines comprehensive drug target information with detailed drug data, functioning like a drug encyclopedia. The majority of the drugs listed in Wikipedia are included in the DrugBank database, which has facilitated the discovery and repurposing of existing drugs to treat new or rare diseases more quickly. In network pharmacology, the DrugBank database is primarily used to identify disease target genes. The researchers then took the intersection of these target genes with the genes of the TCM compounds for further analysis. This process helps understand the molecular mechanisms of TCM treatments and validate their efficacy through scientific data. For example, a recent study used network pharmacology to analyze chronic atrophic gastritis, utilizing the DrugBank database to obtain target genes associated with the condition. Subsequent analysis and validation explored the mechanisms of action of TCM molecules on the disease and their interactions with disease targets (Weng, et al., 2023). Another study combined network pharmacology and experimental validation to investigate the molecular mechanisms of the anti-colorectal cancer activity of Curcuma aromatica Salisb. In this research, scholars used the DrugBank database to search for FDA-approved anti-colorectal cancer drugs and their targets for subsequent analysis (Yin, et al., 2024).
STITCH database
The STITCH (search tool for interactions of chemicals, http://stitch.embl.de/) database (Kuhn, et al., 2008) contains known and predicted chemical compounds and protein interactions, including functional and physical interactions. The STITCH database is very informative, consisting of information on 2.6 million proteins and 390,000 small-molecule interactions. The researchers can determine chemical compounds with similar molecular structures by inputting the chemical molecular structures of the ingredients. The targets of these structurally similar chemical compounds could be considered putative targets of the chemical ingredient to be determined. The data sources of the STITCH database include knowledge transfer between species, computer prediction, text mining, and integration of other databases. The biggest advantage of this database is its extensive data and structural similarity. Regarding the application of the STITCH database in network pharmacology, researchers primarily use it to screen for the corresponding targets of active ingredients in TCM. The STITCH database includes both known interactions and predicted targets of active compounds in TCM based on the chemical similarity and pharmacophore model (Cao, et al., 2021; Chen, et al., 2023). For example, in a study comparing the molecular mechanisms of Fuzi Lizhong pills and Huangqin decoction in treating cold and heat syndromes of ulcerative colitis, researchers used the STITCH database to identify targets of active compounds for subsequent analysis (Hu, et al., 2023). Additionally, in a study on the compounds and mechanisms of Solanum nigrum in the treatment of colorectal cancer, the authors used the STITCH database to screen target genes of active compounds based on chemical similarity and pharmacophore models, providing a foundation for further steps (Chen, et al., 2023).
STRING database
The STRING (https://string-db.org/) database (Snel, et al., 2000) is a source of known or predicted interactions between proteins, providing a comprehensive overview of the many interactions and functional synergies occurring between proteins as well as a background for systems biology. The STRING database aims to provide an integrated and critical assessment of protein–protein interactions (PPIs), including functional and physical associations. STRING offers a broader perspective on multispecies interactions, with a wide range of network visualizations and functional analysis tools. In network pharmacology, the STRING database played a crucial role in constructing PPI networks from TCM active molecules to disease targets. By utilizing the PPI networks generated from the STRING database, researchers were able to identify and filter key targets for subsequent analyses. This process aided in understanding the mechanisms of action of TCM and identifying potential therapeutic targets for various diseases. One study used network pharmacology and molecular docking to investigate the mechanism by which curcumin inhibits esophageal squamous cell carcinoma. After constructing a PPI network using the STRING database, the researchers identified the key targets of curcumin in inhibiting esophageal squamous cell carcinoma. Subsequent validation work was based on these identified targets (Wang, et al., 2024). Additionally, a network pharmacology study on the anti-gastric cancer effects of Bidentis bipinnata Herba also employed the STRING database to construct relevant PPI networks. Hub genes were identified through this process, followed by subsequent validation work based on these findings (Guo, et al., 2024).
HAPPI database
The HAPPI (http://discovery.informatics.uab.edu/HAPPI) database (Chen, et al., 2009) is the most comprehensive database for human PPI data to date, containing 2,922,202 unique PPIs. The HAPPI database collects 23,060 human proteins, including functional and physical interactions. It is favored by many scholars in the field of bioinformatics and provides important support for the study of network pharmacology in TCM. While the use of the HAPPI database is not as widespread as that of STRING, it focuses specifically on human protein interactions, ensuring detailed and rigorous data quality. In network pharmacology studies, the HAPPI database plays a crucial role in constructing relevant PPI networks. For instance, in a study investigating the treatment of colitis with dragon’s blood tablets, researchers used the HAPPI database to collect PPI network data, which provided a foundational basis for subsequent studies on the medicine’s mechanism of action in treating the disease (Xu, et al., 2014).
TCMSP database
TCMSP (traditional Chinese medicine systems pharmacology database and analysis platform, http://tcmspw.com/tcmsp.php) (Ru, et al., 2014) uses the HIT database prediction algorithm, through which the specific relationship between medicine targets can be obtained, and the disease information in the database comes from the PharmGKB database and TTD database. The key compounds and target information of TCM can be retrieved and screened in the TCMSP database to match the target of the disease. The database contains 499 kinds of TCM drugs, 837 related diseases, 29,384 kinds of compounds, and 3,311 targets. It is a common tool for screening ingredients and targets of TCM in network pharmacology. TCMSP focuses more on the pharmacological properties of TCM ingredients and systems pharmacology research, making it suitable for analyzing the multitarget mechanisms of these ingredients. TCMSP is widely used in network pharmacology research (Wang, et al., 2022a; Cao, et al., 2022). Its primary role is to screen the active ingredients of TCM and subsequently identify the corresponding targets of these active ingredients. For example, in the study by Hu et al., in which network pharmacology was used to explore the treatment of non-alcoholic fatty liver disease with Xiaochaihu decoction, the TCMSP database was used to collect data on active compounds and related targets of seven TCM drugs. Hu and his team then conducted further analysis based on these data (Hu, et al., 2020). Another network pharmacology analysis related to the treatment of ulcerative colitis using Fructus mume and Rhizoma coptidis similarly utilized TCMSP to screen the active ingredients of these TCM drugs and identify the corresponding targets. These data served as the basis for subsequent research steps (Yang, et al., 2023).
TCMID database
TCMID (traditional Chinese medicine integrated database, http://www.megabionet.org/tcmid/) (Xue, et al., 2013) is the world’s largest computerized TCM database for medicine screening, containing 3,791 diseases, 47,000 prescriptions, 8,159 TCM drugs, 6,828 medicines, 25,210 compounds, and 17,521 target genes. It provides a Java-based, web-based analysis tool. The TCMID database records important TCM-related information collected from various sources. Users can use text mining to correlate the information in TCMID with the OMIM, DrugBank, and PubChem databases. TCMID is frequently used in network pharmacology analysis and is recognized by many scholars. The TCMID database serves a similar role as the TCMSP database, primarily screening for active ingredients of TCM drugs and identifying the corresponding targets of these active compounds. For instance, in a study investigating the appetite regulation mechanism of the Chinese medicine jujube using network pharmacology, the authors used both the TCMSP and TCMID databases to obtain data on active compounds and conduct further analysis (Zhu, et al., 2022). Additionally, in a network pharmacology study on the treatment of constipation with TCM, the authors utilized both the TCMSP and TCMID databases to acquire data on active compounds and targets, forming the basis for subsequent analysis (Xu, et al., 2022) (Table 1).
Application of network pharmacology in traditional Chinese medicine research
Network pharmacology research techniques
Supported by emerging technologies such as molecular interaction technology, high-throughput technology, and omics technology, network pharmacology further improved and validated a constructed “medicine–compound–target–disease” biological network and enhanced the accuracy of prediction models. Molecular interaction technology, as one of the important emerging technologies, includes biofilm interference technology, plasma resonance technology, and nanoliquid chromatography–mass spectrometry analysis technology (Hsieh and Korfmacher, 2006; Wartchow, et al., 2011; Guo, 2012). High-throughput technology allows for automatic operation and precise processing of cell experiments, quickly obtaining much useful information. Some studies confirmed that network pharmacology was more effective in revealing the principle of small-molecule regulation by using high-throughput technology when constructing a “complex protein–disease” network (Zhang, et al., 2019a). Omics technology, including genomics, proteomics, and metabolomics, more intuitively observes the different regulatory effects of single or compound Chinese medicines at different levels. As an emerging scientific technique, proteomics effectively reveals potential target proteins or protein biomarkers, transforming the promises of TCM into powerful modern therapies (Suo, et al., 2016). These emerging technologies play a strong supporting role in the construction of a biological network, transforming the complex connections and information within the network into a more intuitive visual network, reducing the difficulty of text information, and allowing for further study and research.
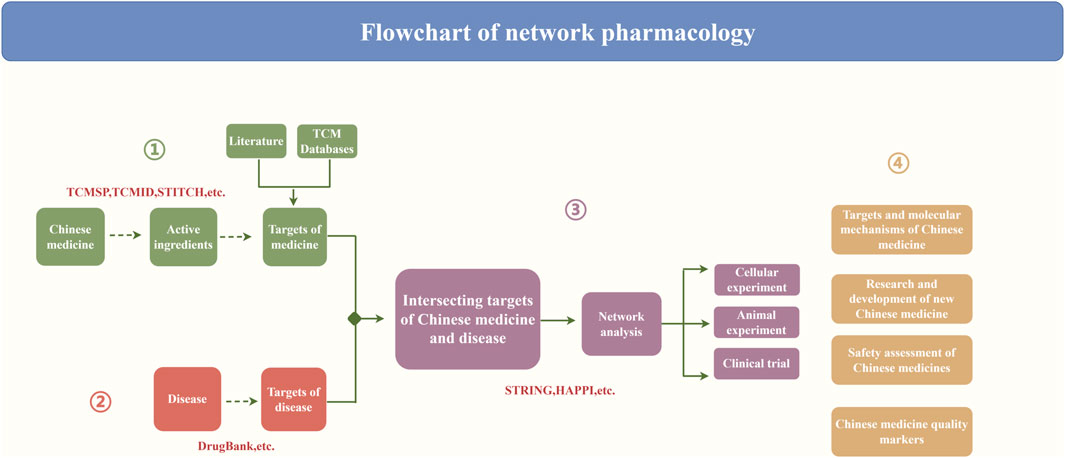
Network pharmacology research steps and the construction of a biological network
The key problem of pharmacologic analysis of the TCM network was evaluating the synergistic effect of a single medicine or multiple target genes of TCM compounds on disease-related molecular networks (Cao, et al., 2023). Current TCM network pharmacology research methods are divided into several steps. First, the key active ingredients of TCM are screened, followed by further screening of the active ingredient of the target gene. Second, the disease-related targets are screened out. Finally, the “medicine–compound–target–disease” network is constructed, and the intersecting targets are subjected to network analysis and relevant experimental validation. Ultimately, it will be applied to the research and development of new Chinese medicines, the safety assessment of Chinese medicines, and so on (Figure 2). In addition to constructing the “medicine–compound–target–disease” network, the interactions between medicines and targets and those between the targets themselves are also crucial. Constructing these relationships further enriches the connotation and significance of biological networks.
Discovery of new indications
Through the construction of a biological network, network pharmacology can obtain the compounds, targets, and related signaling pathways of TCM and explore and speculate on new indications for possible TCM treatment based on “unfamiliar” signaling pathways. The characteristics of TCM in selecting appropriate treatment methods according to pathogenesis are consistent with the idea of precision treatment in modern medicine. This provides a reference for the exploration of new treatment methods and new medicine research and development, in line with the development direction of modern medicine.
Limitations of network pharmacology
Network pharmacology has emerged as a promising approach to the study of TCM, offering a novel perspective on the multi-compound and multi-target nature of TCM drugs. However, its application has significant limitations. First, network pharmacology and molecular docking experiments, as bioinformatics methods, cannot directly reflect the dose–effect relationship, which hinders the accurate evaluation of therapeutic effects (Li, et al., 2022). Second, the reliance on large datasets means that underreported critical targets may leave essential pathways undetected, and key targets and pathways often lack experimental validation. Third, TCM-specific databases, while relevant, are limited by redundancy and incomplete data, further complicated by the alias problem of medicinal herbs. Despite these challenges, broader databases should be considered in future studies. Fourth, network pharmacology predictions require further experimental validation to confirm the mechanisms of TCM in diseases. Fifth, the incompleteness of data on medicines, genes, and proteins, coupled with insufficient computational software, restricts network pharmacology from making reasonable predictions that need rigorous verification. Finally, determining whether herb–target correlations are direct or indirect and whether the interactions are positive or negative remains difficult, necessitating further experimental investigation. Thus, while network pharmacology offers valuable insights, integrating extensive data resources and rigorous experimental validation is essential to enhancing the reliability and depth of TCM research.
Application of network pharmacology in digestive diseases
Application of Chinese medicine in the treatment of chronic gastritis
Chronic gastritis, which is divided into chronic atrophic gastritis and chronic non-atrophic gastritis, is characterized by inflammatory changes in the gastric mucosa due to one or more risk factors. Common symptoms include mucosa atrophy and a rough appearance under the gastroscope. The incidence and detection rates increase with age and are common in clinical settings. It is conservatively estimated that more than half of the world’s population suffers from this disease to some degree (Sipponen and Maaroos, 2015). Chronic atrophic gastritis and chronic non-atrophic gastritis are two different forms and phenotypes of chronic gastritis at different stages of the same disease (Schindler, 1966; Correa, 1988; Price, 1991; Siurala, 1991; Dixon, et al., 1996). There is evidence that the eradication of Helicobacter pylori can restore normal gastric mucosa and play a significant role in the cure of chronic gastritis (Valle, et al., 1991; Hojo, et al., 2002; Arkkila, et al., 2006). Chronic gastritis, the most common disease of the digestive system, is often treated with Chinese medicines, with good results in clinical practice.
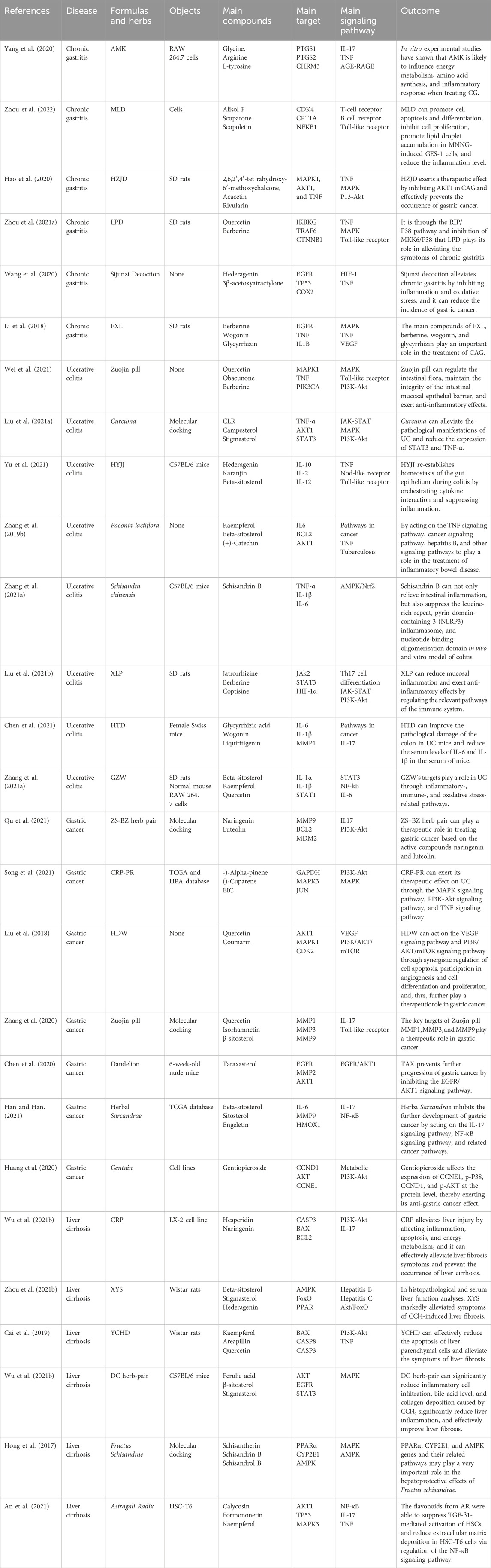
Yang et al. identified that Atractylodes macrocephala Koidz (AMK), a Chinese medicine, had a significant effect on treating chronic gastritis. Using network pharmacology analysis, 27 effective compounds were selected for the treatment of chronic gastritis, forming a network of “medicine–compound–gene–disease”. They identified 26 related signaling pathways, and the reliability of these active ingredients and targets was finally confirmed through in vitro experiments. AMK plays a role in the treatment of chronic gastritis by affecting energy metabolism, inflammatory response, and amino acid synthesis (Yang, et al., 2020). Zhou found that network pharmacology, such as the proprietary Chinese medicine Moluodan (MLD) treatment of chronic atrophic gastritis in cell differentiation, apoptosis, cell proliferation, etc., and with the body’s immune response, is associated with inflammation, and it can effectively suppress the GES–1 cell proliferation induced by MNNG, further promote cell differentiation and apoptosis, eventually reduce the inflammation of the stomach, and relieve chronic gastritis symptoms (Zhou, et al., 2022). The clinical empirical formula of Huazhuojiedu decoction, used to treat chronic atrophic gastritis, had 20 key targets that play crucial roles in the treatment, which are closely related to tumor-related pathways and cell proliferation. Animal experiments confirmed that the level of AKT1 increased in model rats. The Huazhuojiedu formulas significantly inhibited AKT1, confirming their therapeutic effect in chronic atrophic gastritis and preventing gastric cancer (Hao et al., 2020). Zhou et al. confirmed that the commonly used formula Lianpu Drink, primarily composed of Coptis chinensis and Magnolia officinalis, alleviated chronic gastritis symptoms by constructing the “compound–target–disease” network and screening common targets. They confirmed through animal experiments that Lianpu Drink played its role through the RIP/P38 pathway and inhibition of MKK6/P38 (Zhou, et al., 2021a).
To reduce the incidence of gastric cancer developed from chronic gastritis, Wang et al. conducted a network pharmacology study on the traditional Sijunzi formula and found that it mainly treated chronic gastritis by acting on 18 target genes. GO analysis showed that these targets are mainly enriched in the reactions of cells to medicines, the nitric oxide biosynthesis process, the MAPK cascade, angiogenesis, etc. Finally, it is concluded that the Sijunzi formulae reduce the incidence of gastric cancer by improving the inflammation of peripheral blood white blood cells and local inflammation of the stomach (Wang, et al., 2020). Based on the network pharmacology research method for blood absorption ingredients, Li et al. (2018) found that the main ingredients of the Fufang-Xialian capsule, berberine, wogonin, and glycyrrhizin, played an important role in the treatment of chronic atrophic gastritis, and this new method is more straightforward than the traditional network pharmacology approach. These studies confirmed that TCM played an important role in the treatment of chronic gastritis. The research and development of medicines for the treatment of chronic gastritis could fully utilize the network pharmacology research platform, combining the characteristics and advantages of TCM with the latest advancements in science and technology. The specific mechanisms and material basis of TCM and its compound prescriptions in treating chronic gastritis have turned the disadvantages of complex chemical compounds and unclear pharmacological mechanisms of TCM compounds into advantages.
Application of Chinese medicine in the treatment of ulcerative colitis
Ulcerative colitis (UC), another common digestive system disease, often has a poor prognosis. UC is an inflammatory bowel disease characterized by abdominal pain, diarrhea, mucus pus, and bloody stool as the main clinical manifestations. Studies confirmed that there is no significant difference in the incidence of ulcerative colitis in men and women, but it mainly occurs in young or middle-aged individuals (Cosnes, et al., 2011; Molodecky, et al., 2012). Modern medicine believes that the etiology and pathological mechanisms of UC include environmental factors, impaired immune response, epithelial barrier defects, or microbial interference (Ungaro, et al., 2017; Ramos and Papadakis, 2019). The prevalence and incidence of UC are on the rise, the treatment is more difficult, and the age at onset is less, causing significant inconvenience to patients’ work and lives (Ng, et al., 2017). Commonly used medicines for the treatment of UC in modern medicine mainly include immunosuppressants and aminosalicylic acids such as mesalazine, but their long-term use will cause adverse side effects and damage to the patient’s body (Wan, et al., 2014). Recently, Chinese medicine has played a significant role in treating UC. Academics have increasingly researched the treatment of such inflammatory bowel diseases from the perspective of TCM, achieving greater gains.
The classic prescription Zuojin pill has been known to have the potential to prevent UC since ancient times, but its effective mechanism of action is unclear. The network pharmacology analysis of the Zuojin pill found that the 26 key targets for treating the disease were closely related to the inflammatory response, apoptotic process, signaling, and immune response of UC. By acting on the MAPK signaling pathways, PI3KAkt signaling pathway, prolactin signaling pathways, and toll-like receptor signaling pathways involved in the treatment of UC, it effectively plays an anti-inflammatory role, maintains the integrity of the intestinal mucosa epithelial barrier, and better regulates the intestinal flora. The study reflects the multiple compounds, multiple targets, multiple pathways, and the characteristics of comprehensive control, and it will better guide clinical practice in the future (Wei, et al., 2021). Turmeric, widely used in treating UC, colon cancer, malaria, and Crohn’s disease, was found to have 54 overlapping targets in the treatment of UC. These targets played a therapeutic effect by acting on the MAPK signaling pathway, PI3K-Akt signaling pathway, and JAK-STAT signaling pathway, which are highly correlated with inflammatory bowel disease. Experiments confirmed that turmeric could effectively relieve UC pathological manifestations, reduce the expression of STAT3 and TNF-α, and relieve intestinal inflammation by inactivating epithelial cell signal transduction in the Helicobacter pylori infection pathway, TNF pathway, and inflammatory bowel disease pathway (Liu, et al., 2021b).
In China, Huiyangjiuji formulas (HYJJD) were widely used in the treatment of inflammation, including UC, but their potential therapeutic mechanism was not completely clear. Some scholars identified the active ingredients of HYJJD through network pharmacology analysis. KEGG pathway analysis revealed that the target genes were enriched in the inflammatory pathway, pathogen-induced infection pathway, and tumor-related pathway. In vitro experiments also confirmed that HYJJD had significant inhibitory effects on interleukins such as IL-10, IL-2, and IL-12. Moreover, it promoted intestinal mucosal healing, effectively repaired the damaged IO function of TNF-α, re-established intestinal epithelial homeostasis, and further played an anti-inflammatory role (Yu, et al., 2021). Paeonia lactiflora, a common Chinese medicine used to treat dysentery, was found through network pharmacology studies to have 70 common target genes with UC, involving biological functions such as reaction to medicines, reaction to lipopolysaccharides, positive regulation of nitric oxide biosynthesis, and reaction to estradiol. By acting on the TNF signaling pathway, cancer signaling pathway, hepatitis B, tuberculosis, and other signaling pathways, it played a role in treating inflammatory bowel disease and relieving UC symptoms (Zhang, et al., 2019b). Experiments by Zhang et al. (2021b) found that Schisandrin B, the main ingredient of Schisandra chinensis, could not only relieve intestinal inflammation but also suppress the leucine-rich repeat, pyrin domain-containing 3 (NLRP3) inflammasome, and nucleotide-binding oligomerization domain in in vivo and in vitro models of colitis. A new study found that there are 50 crossover genes between nine compounds in Xianglian pill (XLP) and UC, which mainly reduced mucosal inflammation and exerted anti-inflammatory effects by regulating the relevant pathways of the immune system (Liu, et al., 2021a). The study by Chen et al. on Huangtu decoction (HTD) found that HTD could significantly improve the pathological damage in the colon in UC mice, reduce the serum levels of IL-6 and IL-1β in mice, and finally relieve their symptoms (Chen, et al., 2021). Studies have shown that there are 26 active compounds and 148 key targets in Guchang Zhixie Wan (GZW), which are closely related to UC, and GZW was able to exert a therapeutic effect on UC by acting on inflammation, immune and oxidative stress-related pathways (Zhang, et al., 2021a). According to the above network pharmacology study on single or compound Chinese medicines, the mechanism of TCM in preventing and treating UC might be achieved by regulating the expression of inflammatory factors, changing the quantity and abundance of intestinal microbiota, and regulating the intestinal immune balance. The clinical efficacy was clear, but there are problems with complex ingredients and synergistic effects. Currently, its mechanism of action is not completely clear, and further exploration is still required.
Application of Chinese medicine in the treatment of gastric cancer
As the most common disease of the digestive system, chronic gastritis could progress to intestinal metaplasia or intraepithelial neoplasia in the epithelium and the glands of the gastric mucosa under the action of H. pylori infection, adverse environmental conditions, and an unhealthy diet and eventually develop into gastric cancer. Gastric cancer refers to a malignant tumor arising in the gastric mucus membrane, and clinical symptoms include epigastric ache, emaciation, anorexia, anemia, and an apparent bump that can be touched in the epigastric region when terminal. Helicobacter pylori infection and the inclusion of preserved foods in the diet are the main causes of gastric cancer (Johnston and Beckman, 2019). Gastric cancer ranks first among digestive system cancers and third in cancer fatality rates. It predominantly affects the elderly, and the ratio of its incidence in men and women is approximately 2:1. In recent years, the diagnosis rate of gastric cancer in East Asia and other regions with inadequate medical equipment accounts for approximately half of the global diagnosis rate of gastric cancer, while the incidence in Western countries is relatively low. Therefore, regular screening plays a crucial role in gastric cancer prevention. Modern medicine generally treats gastric cancer with surgery, and some doctors use endoscopic treatment, which has the advantages of fewer adverse reactions and good effects. Additionally, chemotherapy could alleviate the symptoms of gastric cancer. TCM also has unique advantages in the treatment of gastric cancer, significantly improving the patient’s quality of life, prolonging survival, and causing fewer side effects. The application of network pharmacology to analyze the mechanism of TCM in the prevention or treatment of gastric cancer could provide new ideas for the future prevention and alleviation of gastric cancer symptoms.
Qu et al. found through TCMSP and other databases that the ZhiShi–BaiZhu herb pair mainly treated gastric cancer through 27 key active compounds and 120 targets, which exerted therapeutic effects on the disease by acting on the IL17 signaling pathway and the PI3K-Akt signaling pathway; the main targets include MMP9, BCL2, MDM2, AKT1, and MOTR; and the final molecular docking showed that the two key ingredients of naringin and luteolin could stably bind to the target (Qu, et al., 2021). Studies have found that the main compounds of Citri reticulatae pericarpium pinelliae rhizoma (CRP-PR) include naringin and stigmasterol. The main targets of CRP-PR obtained from the STRING database are JUN, GSK3B, SMAD2, GAPDH, and STAT3, involving 118 cellular components, 540 biological processes, and 171 molecular functions. It was predicted that CRP-PR could exert a therapeutic effect through the MAPK signaling pathway, the PI3K-Akt signaling pathway, and the TNF signaling pathway and exhibited a multi-compound, multi-target, and multi-pathway therapeutic effect of TCM in treating diseases by using the network pharmacology analysis method (Song, et al., 2021). In the study of Hedyotis diffusa Willd (HDW), Liu et al. screened 32 active ingredients and 353 key targets. Through network analysis, AKT1, MAPK1, CDK2, PIK3CA, and VEGFA were found to be the key targets of HDW in the treatment of gastric cancer. GO enrichment analysis and KEGG pathway enrichment analysis showed that HDW might act on the VEGF signaling pathway and PI3K/AKT/mTOR signaling pathway through synergistic regulation of cell apoptosis, angiogenesis, and cell differentiation and proliferation, thereby playing a therapeutic role in gastric cancer (Liu, et al., 2018). As a TCM prescription for chronic gastritis, Zuojin pill also had a therapeutic effect on gastric cancer. Network pharmacological analysis showed that Zuojin pill contained 47 key compounds, including quercetin, isorhamnetin, and β-sitosterol, and 48 targets related to gastric cancer. Protein–protein interaction analysis revealed that MMP1, MMP9, and MMP3 played key roles in the anti-gastric cancer mechanism, furthering our understanding of the mechanism of TCM compounds in treating gastric cancer (Zhang, et al., 2020).
Taraxasterol (TAX) (Chen, et al., 2020), with strong antitumor activity, was found to have five common targets with gastric cancer, namely, EGFR, MMP2, AKT1, BRAF, and FGFR2, through network pharmacology methods and animal experiments. TAX prevented further deterioration of gastric cancer by inhibiting the EGFR/AKT1 signaling pathway. Han and Han (2021) found that there are six medicinal compounds and 29 target genes related to gastric cancer in Herba Sarcandrae, which ultimately inhibit further development of gastric cancer by acting on the IL-17 signaling pathway, NF-κB signaling pathway, and related cancer pathways. This provides a new basis for future research on Herba Sarcandrae’s anti-tumor properties. Huang et al. (2020) found that gentiopicroside also plays an important role in the treatment of gastric cancer, and its anticancer activity may involve 53 related targets, confirming that key compounds affect the expression of CCNE1, p-P38, CCND1, and p-AKT at the protein level. The above studies have proven that the mechanism of action of TCM in treating gastric cancer or alleviating adverse symptoms has certain scientific significance, and the application of network pharmacology can better demonstrate the multi-compound, multi-target, and multi-pathway characteristics of TCM in treating diseases, providing a guarantee for future research and development of anti-cancer medicines.
Application of Chinese medicine in the treatment of liver cirrhosis
As an essential functional organ of the digestive system, the liver undertakes important functions such as metabolism and detoxification. Under the influence of various pathogenic factors, the liver experiences chronic inflammation, steatosis, hepatocyte reduction, and diffuse fibrosis, which can gradually develop into cirrhosis. Cirrhosis is a chronic liver disease characterized by diffuse fibrosis, regenerated nodules, and pseudolobule formation. The main clinical manifestations are portal hypertension and liver dysfunction, with many complications and a poor prognosis. Previous studies found that cirrhosis is the ultimate common pathologic pathway of liver damage caused by multiple chronic liver diseases (Melato and Mucli, 1989; Qua and Goh, 2011; Asrani, et al., 2013). In Western countries, common causes of cirrhosis include viral hepatitis infection, alcoholism, and non-alcoholic fatty liver disease, and improper care can easily transform cirrhosis into liver cancer (Di Bisceglie, 2000; Innes, et al., 2013). Modern treatment focuses on addressing the underlying causes to prevent further liver damage, using anti-liver fibrosis and hepatocyte protection medicines, and actively managing complications such as ascites. Given the serious complications and high mortality associated with cirrhosis, treating complications such as gastrointestinal bleeding is crucial. Network pharmacology analysis shows that TCM treatment of liver cirrhosis also has significant effects and can effectively inhibit its progression to liver cancer.
Citri Reticulatae Pericarpium (CRP), a Chinese medicine commonly used for treating liver diseases, contains a variety of flavonoids, including cinnamon and hesperidin. Previous studies found that CRP had anti-tumor and antibacterial effects, reduced cholesterol, and prevented fatty liver disease. Network pharmacology studies revealed that CRP played a significant role in preventing liver injury in 117 targets; GO enrichment analysis includes 139 molecular functions, 1,525 biological processes, and 55 cellular components and KEGG pathway enrichment analysis screened out 49 related pathways, including the PI3K-Akt signaling pathway and IL-17 signaling pathway. CRP alleviates liver injury by affecting inflammation, apoptosis, and energy metabolism, effectively reducing liver fibrosis and preventing cirrhosis (Wu, et al., 2021b). Zhou et al. constructed a “TCM–compound–target” network for Xiaoyaosan decoction (XYS) in treating liver fibrosis, including 8 TCM drugs, 108 active compounds, and 42 key targets. Enrichment analysis showed that AMPK, FoxO, PPAR, TGFβ, MAPK, and other key targets, as well as the hepatitis B and C pathways and the Akt/FoxO signaling pathway, participated in the anti-fibrotic effects of XYS, effectively preventing cirrhosis (Zhou, et al., 2021b).
In the analysis of Yinchenhao decoction (YCHD), Cai et al. (2019) found that it has 45 active compounds and 296 potential targets, which showed an anti-liver fibrosis effect by regulating the PI3K-Akt signaling pathway and the TNF signaling pathway, and animal experiments confirmed that YCHD can effectively reduce the apoptosis of liver parenchymal cells and alleviate the symptoms of liver fibrosis. Network pharmacology studies by Wu et al. confirmed that the Angelicae Sinensis Radix (Danggui) and Ligusticum Chuanxiong Rhizoma (Chuan Xiong) (DC) herb-pair was commonly used for pain relief and vascular disease prevention, and it had 13 key compounds and 46 anti-fibrosis targets. By acting on the MAPK signaling pathway, it can significantly reduce inflammatory cell infiltration, bile acid level, and collagen deposition caused by CCl4, significantly reduce liver inflammation, and effectively alleviate symptoms of liver fibrosis (Wu, et al., 2021a).
As a common Chinese herbal medicine, Fructus schisandrae has been found to have significant hepato-protective effects in clinical applications. A previous study showed that schisantherin, schisandrin B, schisandrol B, kadsurin, etc., had significant effects on treating liver disease, especially fatty liver disease, while PPARα, CYP2E1, and AMPK genes and related pathways might play a very important role in the hepatoprotective effects of F. schisandrae (Hong, et al., 2017). Astragalus flavonoids, present in Astragali Radix (AR), have a significant inhibitory effect on liver fibrosis (An, et al., 2021). An et al. found that AR flavonoids could inhibit transforming growth factor-beta 1 (TGF-β1)-mediated activation of hepatic stellate cells (HSCs) and reduce extracellular matrix deposition in HSC-T6 cells by regulating the NF-κB signaling pathway (An, et al., 2021). In addition to the above mechanisms, there are various factors and signaling pathways that are being studied and have not yet been discovered (Chen, et al., 2013). Faced with the complex situation of the current treatment strategies for liver cirrhosis, it is necessary to effectively use TCM to identify syndromes to treat the disease. Combining the advantages of TCM in treating liver cirrhosis with network pharmacology technology provides new solutions and ideas for future treatment approaches (Table 2).
Conclusion and future perspectives
In conclusion, TCM has played an irreplaceable role in treating digestive system diseases, and it is closely related to the holistic concepts of TCM. Despite its significant advantages, TCM faces limitations and challenges in clinical practice. The individualized nature of TCM treatments results in poor reproducibility, with the complex composition and lack of standardization of herbal medicines hindering widespread adoption. The theoretical differences between TCM and modern medicine further complicate research efforts. Future progress depends on the modernization and internationalization of TCM, which requires enhanced basic research, modern technological applications to elucidate mechanisms of action, large-scale clinical trials to confirm efficacy and safety, and robust quality standards for herbal medicines. Additionally, the application of TCM in treating digestive diseases is controversial, with skepticism from some in the Western medical community due to the perceived lack of scientific evidence, and cultural and philosophical differences contributing to the debate. Addressing these concerns through scientific methods and data is essential for fostering integration between TCM and Western medicine, ultimately improving patient care.
Network pharmacology technology has significant advantages in the field of TCM research, such as discovering the basis of pharmacodynamic substances, developing new indications, studying the mechanism of medical action, and studying the compatibility of TCM. The information network provides a new research direction for basic research on digestive system diseases. Under the guidance of the network pharmacology evaluation method guidance, the development of network pharmacology is expected to become increasingly healthy and standardized, and the network targets will help produce more high-quality research results for TCM and effectively promote the internationalization and modernization of TCM (Wang, et al., 2022b). However, the results of network pharmacology research are still affected by analysis methods, screening conditions, and database information. The system uses the common intersection of targets between medicines, and there are limitations in research aimed at using medicine synergy. In addition, network pharmacology ignores the influence of the amount of each compounds in TCM, and it is urgent to design a corresponding network algorithm to quantitatively evaluate how medicines work. These are challenges in the discovery of TCM combinations and new medicines, which require further research.
Importantly, future research must integrate the analysis results with traditional TCM theories to reveal the mechanism of TCM in treating diseases from multiple angles. Combining network data analysis with fundamental medical research can provide more opportunities for the modernization of TCM. In the new era, it is crucial to leverage the precious resource of network pharmacology, systematically combining TCM with the emerging network pharmacology technology. This approach will better explore and utilize TCM’s characteristic theories and rich experience. Integrating bioinformatics technology into specific medical life science research and fostering multidisciplinary cooperation will further advance the research process in diagnosing and treating digestive system diseases.
Author contributions
SZ: Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing–original draft, Writing–review and editing. YL: Formal Analysis, Supervision, Visualization, Conceptualization, Investigation, Writing–review and editing. TX: Data curation, Formal Analysis, Investigation, Writing–original draft, Writing–review and editing. WW: Conceptualization, Data curation, Supervision, Writing–review and editing. SL: Data curation, Supervision, Writing–review and editing. PZ: Supervision, Writing–review and editing. XL: Methodology, Writing–review and editing. XC: Data curation, Writing–review and editing. QL: Data curation, Methodology, Writing–review and editing. WQ: Data curation, Writing–review and editing. YY: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Project administration, Resources, Software, Supervision, Validation, Writing–review and editing. XZ: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing–review and editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study was supported by the Horizontal Project of Dongzhimen Hospital (HX-DZM-202343) and the National Postdoctoral Researcher Program (GZC20233132). The material in all the pictures of this article was provided by Figdraw.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
An, L., Lin, Y., Li, L., Kong, M., Lou, Y., Wu, J., et al. (2021). Integrating network pharmacology and experimental validation to investigate the effects and mechanism of Astragalus flavonoids against hepatic fibrosis. Front. Pharmacol. 11, 618262. doi:10.3389/fphar.2020.618262
Arkkila, P. E., Seppälä, K., Färkkilä, M. A., Veijola, L., and Sipponen, P. (2006). Helicobacter pylori eradication in the healing of atrophic gastritis: a one-year prospective study. Scand. J. Gastroenterol. 41, 782–790. doi:10.1080/00365520500463175
Asrani, S. K., Larson, J. J., Yawn, B., Therneau, T. M., and Kim, W. R. (2013). Underestimation of liver-related mortality in the United States. Gastroenterology 145 (2), 375–382. doi:10.1053/j.gastro.2013.04.005
Cai, F. F., Bian, Y. Q., Wu, R., Sun, Y., Chen, X. L., Yang, M. D., et al. (2019). Yinchenhao decoction suppresses rat liver fibrosis involved in an apoptosis regulation mechanism based on network pharmacology and transcriptomic analysis. Biomed. Pharmacother. 114, 108863. doi:10.1016/j.biopha.2019.108863
Cao, X., Liang, Y., Liu, R., Zao, X., Zhang, J., Chen, G., et al. (2022). Uncovering the pharmacological mechanisms of gexia-zhuyu formula (GXZY) in treating liver cirrhosis by an integrative pharmacology strategy. Front. Pharmacol. 13, 793888. doi:10.3389/fphar.2022.793888
Cao, X., Zao, X., Xue, B., Chen, H., Zhang, J., Li, S., et al. (2021). The mechanism of TiaoGanYiPi formula for treating chronic hepatitis B by network pharmacology and molecular docking verification. Sci. Rep. 11 (1), 8402. doi:10.1038/s41598-021-87812-9
Cao, X., Zhang, N., Chen, H., Wang, W., Liang, Y., Zhang, J., et al. (2023). Exploring the mechanism of JiGuCao capsule formula on treating hepatitis B virus infection via network pharmacology analysis and in vivo/vitro experiment verification. Front. Pharmacol. 14, 1159094. doi:10.3389/fphar.2023.1159094
Chen, J. F., Wu, S. W., Shi, Z. M., Qu, Y. J., Ding, M. R., and Hu, B. (2023). Exploring the components and mechanism of Solanum nigrum L. for colon cancer treatment based on network pharmacology and molecular docking. Front. Oncol. 13, 1111799. doi:10.3389/fonc.2023.1111799
Chen, J. Y., Mamidipalli, S., and Huan, T. (2009). HAPPI: an online database of comprehensive human annotated and predicted protein interactions. BMC Genomics 10, S16. doi:10.1186/1471-2164-10-S1-S16
Chen, S. L., Zheng, M. H., Shi, K. Q., Yang, T., and Chen, Y. P. (2013). A new strategy for treatment of liver fibrosis: letting MicroRNAs do the job. BioDrugs 27, 25–34. doi:10.1007/s40259-012-0005-2
Chen, W., He, L., Zhong, L., Sun, J., Zhang, L., Wei, D., et al. (2021). Identification of active compounds and mechanism of Huangtu decoction for the treatment of ulcerative colitis by network pharmacology combined with experimental verification. Drug Des. Dev. Ther. 15, 4125–4140. doi:10.2147/DDDT.S328333
Chen, W., Li, J., Li, C., Fan, H. N., Zhang, J., and Zhu, J. S. (2020). Network pharmacology-based identification of the antitumor effects of taraxasterol in gastric cancer. Int. J. Immunopathol. Pharmacol. 34, 2058738420933107. doi:10.1177/2058738420933107
Correa, P. (1988). Chronic gastritis: a clinico-pathological classification. Am. J. Gastroenterol. 83, 504–509.
Cosnes, J., Gower-Rousseau, C., Seksik, P., and Cortot, A. (2011). Epidemiology and natural history of inflammatory bowel diseases. Gastroenterol. 140, 1785–1794. doi:10.1053/j.gastro.2011.01.055
Di Bisceglie, A. M. (2000). Natural history of hepatitis C: its impact on clinical management. Hepatology 31, 1014–1018. doi:10.1053/he.2000.5762
Dixon, M. F., Genta, R. M., Yardley, J. H., and Correa, P. (1996). Classification and grading of gastritis. The updated sydney system. International workshop on the histopathology of gastritis, Houston 1994. Am. J. Surg. Pathol. 20, 1161–1181. doi:10.1097/00000478-199610000-00001
Feng, Y., Li, D., Ma, C., Tian, M., Hu, X., and Chen, F. (2022). Barley leaf ameliorates Citrobacter rodentium-induced colitis through preventive effects. Nutrients 14 (18), 3833. doi:10.3390/nu14183833
Guo, L., Zhou, Y., and Ma, R. (2024). Exploring the anti-gastric cancer mechanism of action of Bidentis Bipinnatae Herba based on network pharmacology, molecular docking, and cellular experimental validation. Naunyn Schmiedeb. Arch. Pharmacol. doi:10.1007/s00210-024-03169-6
Guo, S. Y., Wu, G. R., Zhou, W., Jia, S. S., Zhang, J. Y., Meng, Z. Q., et al. (2019). Study on mechanism of safflower ruyi pills in treatment of gynecological diseases based on network pharmacology. Eval. Analysis Drug-Use Hosp. China 19, 257–264.
Guo, X. (2012). Surface plasmon resonance based biosensor technique: a review. J. Biophot. 5, 483–501. doi:10.1002/jbio.201200015
Han, L., and Han, Y. (2021). Network pharmacology-based study on the active component and mechanism of the anti-gastric-cancer effect of Herba Sarcandrae. J. Healthc. Eng. 2021, 3001131. doi:10.1155/2021/3001131
Han, S., Lv, A. P., Li, J., and Jiang, M. (2019). Application review of network pharmacology in the study of properties theory of traditional Chinese medicine. J. Basic Chin. Med. 25, 127–130.
Hao, X., Liu, Y., Zhou, P., Jiang, Q., Yang, Z., Xu, M., et al. (2020). Integrating network pharmacology and experimental validation to investigate the mechanisms of Huazhuojiedu decoction to treat chronic atrophic gastritis. Evidence-Based Complement. Altern. Med. 2020, 2638362. doi:10.1155/2020/2638362
Hojo, M., Miwa, H., Ohkusa, T., Ohkura, R., Kurosawa, A., and Sato, N. (2002). Alteration of histological gastritis after cure of Helicobacter pylori infection. Aliment. Pharmacol. Ther. 16, 1923–1932. doi:10.1046/j.1365-2036.2002.01346.x
Hong, M., Zhang, Y., Li, S., Tan, H. Y., Wang, N., Mu, S., et al. (2017). A network pharmacology-based study on the hepatoprotective effect of Fructus schisandrae. Molecules 22, 1617. doi:10.3390/molecules22101617
Hopkins, A. L. (2007). Network pharmacology. Nat. Biotechnol. 25, 1110–1111. doi:10.1038/nbt1007-1110
Hopkins, A. L. (2008). Network pharmacology: the next paradigm in drug discovery. Nat. Chem. Biol. 4, 682–690. doi:10.1038/nchembio.118
Hsieh, Y., and Korfmacher, W. A. (2006). Increasing speed and throughput when using HPLC-MS/MS systems for drug metabolism and pharmacokinetic screening. Curr. Drug Metab. 7, 479–489. doi:10.2174/138920006777697963
Hu, Q., Wei, S., Wen, J., Zhang, W., Jiang, Y., Qu, C., et al. (2020). Network pharmacology reveals the multiple mechanisms of Xiaochaihu decoction in the treatment of non-alcoholic fatty liver disease. BioData Min. 13, 11. doi:10.1186/s13040-020-00224-9
Hu, X., Liu, W., He, M., Qiu, Q., Zhou, B., Liu, R., et al. (2023). Comparison of the molecular mechanisms of Fuzi Lizhong Pill and Huangqin decoction in the treatment of the cold and heat syndromes of ulcerative colitis based on network pharmacology. Comput. Biol. Med. 159, 106870. doi:10.1016/j.compbiomed.2023.106870
Huang, Y., Lin, J., Yi, W., Liu, Q., Cao, L., Yan, Y., et al. (2020). Research on the potential mechanism of Gentiopicroside against gastric cancer based on network pharmacology. Drug Des. Dev. Ther. 14, 5109–5118. doi:10.2147/DDDT.S270757
Innes, H. A., Hutchinson, S. J., Barclay, S., Cadzow, E., Dillon, J. F., Fraser, A., et al. (2013). Quantifying the fraction of cirrhosis attributable to alcohol among chronic hepatitis C virus patients: implications for treatment cost-effectiveness. Hepatology 57, 451–460. doi:10.1002/hep.26051
Johnston, F. M., and Beckman, M. (2019). Updates on management of gastric cancer. Curr. Oncol. Rep. 21, 67. doi:10.1007/s11912-019-0820-4
Kuhn, M., von Mering, C., Campillos, M., Jensen, L. J., and Bork, P. (2008). STITCH: interaction networks of chemicals and proteins. Nucleic Acids Res. 36, D684–D688. doi:10.1093/nar/gkm795
Li, S., Xu, T., Liu, S., Liu, Z., Pi, Z., Song, F., et al. (2018). Exploring the potential pharmacodynamic material basis and pharmacologic mechanism of the Fufang-Xialian-Capsule in chronic atrophic gastritis by network pharmacology approach based on the components absorbed into the blood. R. Soc. Open Sci. 5, 171806. doi:10.1098/rsos.171806
Li, S., and Zhang, B. (2013). Traditional Chinese medicine network pharmacology: theory, methodology and application. Chin. J. Nat. Med. 11, 110–120. doi:10.1016/S1875-5364(13)60037-0
Li, Y., Zhang, C., Ma, X., Yang, L., and Ren, H. (2022). Identification of the potential mechanism of Radix pueraria in colon cancer based on network pharmacology. Sci. Rep. 12 (1), 3765. doi:10.1038/s41598-022-07815-y
Liu, C. S., Xia, T., Luo, Z. Y., Wu, Y. Y., Hu, Y. N., Chen, F. L., et al. (2021a). Network pharmacology and pharmacokinetics integrated strategy to investigate the pharmacological mechanism of Xianglian pill on ulcerative colitis. Phytomedicine 82, 153458. doi:10.1016/j.phymed.2020.153458
Liu, S., Li, Q., Liu, F., Cao, H., Liu, J., Shan, J., et al. (2021b). Uncovering the mechanism of Curcuma in the treatment of ulcerative colitis based on network pharmacology, molecular docking technology, and experiment verification. Evidence-Based Complement. Altern. Med. 2021, 6629761. doi:10.1155/2021/6629761
Liu, X., Gao, K., Xing, W., Wang, Z., Sun, H., and Zheng, Y. (2022). Relationship between postoperative complications of esophageal cancer surgery and season: a retrospective study. Ann. Transl. Med. 10 (8), 438. doi:10.21037/atm-21-5050
Liu, X., Wu, J., Zhang, D., Wang, K., Duan, X., Meng, Z., et al. (2018). Network pharmacology-based approach to investigate the mechanisms of Hedyotis diffusa Willd. In the treatment of gastric cancer. Treat. Gastric Cancer. J. Evidence-Based Complementary Altern. Med. 2018, 7802639. doi:10.1155/2018/7802639
Liu, X. K., Wu, G. R., Lin, M. J., and Zhang, X. M. (2017). Mechanism of Si Junzitang based on network pharmacology. Chin. J. Exp. Tradit. Med. Formulae 23, 194–202.
Melato, M., and Mucli, E. (1989). Something new in liver cirrhosis epidemiology. Lancet 2, 395–396. doi:10.1016/s0140-6736(89)90578-3
Molodecky, N. A., Soon, I. S., Rabi, D. M., Ghali, W. A., Ferris, M., Chernoff, G., et al. (2012). Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterol. 142, 46–54. doi:10.1053/j.gastro.2011.10.001
Ng, S. C., Shi, H. Y., Hamidi, N., Underwood, F. E., Tang, W., Benchimol, E. I., et al. (2017). Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet 390, 2769–2778. doi:10.1016/S0140-6736(17)32448-0
Orr, W. C., Fass, R., Sundaram, S. S., and Scheimann, A. O. (2020). The effect of sleep on gastrointestinal functioning in common digestive diseases. Lancet Gastroenterol. Hepatol. 5, 616–624. doi:10.1016/S2468-1253(19)30412-1
Park, J., and Cheon, J. H. (2021). Incidence and prevalence of inflammatory bowel disease across Asia. Yonsei Med. J. 62 (2), 99–108. doi:10.3349/ymj.2021.62.2.99
Price, A. B. (1991). The Sydney System: histological division. J. Gastroenterol. Hepatol. 6, 209–222. doi:10.1111/j.1440-1746.1991.tb01468.x
Qu, Y., Yang, X., Li, J., Zhang, S., Li, S., Wang, M., et al. (2021). Network pharmacology and molecular docking study of zhishi-baizhu herb pair in the treatment of gastric cancer. J. Evidence-Based Complement. Altern. Med. 2021, 2311486. doi:10.1155/2021/2311486
Qua, C. S., and Goh, K. L. (2011). Liver cirrhosis in Malaysia: peculiar epidemiology in a multiracial Asian country. J. Gastroenterol. Hepatol. 26, 1333–1337. doi:10.1111/j.1440-1746.2011.06732.x
Ramos, G. P., and Papadakis, K. A. (2019). Mechanisms of disease: inflammatory bowel diseases. Mayo Clin. Proc. 94, 155–165. doi:10.1016/j.mayocp.2018.09.013
Ru, J., Li, P., Wang, J., Zhou, W., Li, B., Huang, C., et al. (2014). TCMSP: a database of systems pharmacology for drug discovery from herbal medicines. J. Cheminf. 6, 13. doi:10.1186/1758-2946-6-13
Sipponen, P., and Maaroos, H. I. (2015). Chronic gastritis. Scand. J. Gastroenterol. 50, 657–667. doi:10.3109/00365521.2015.1019918
Siurala, M. (1991). The story of gastritis. Scand. J. Gastroenterol. Suppl. 186, 1–3. doi:10.3109/00365529109103978
Snel, B., Lehmann, G., Bork, P., and Huynen, M. A. (2000). STRING: a web-server to retrieve and display the repeatedly occurring neighbourhood of a gene. Nucleic Acids Res. 28, 3442–3444. doi:10.1093/nar/28.18.3442
Song, S., Huang, W., Lu, X., Liu, J., Zhou, J., Li, Y., et al. (2021). A network pharmacology study based on the mechanism of Citri Reticulatae pericarpium-pinelliae rhizoma in the treatment of gastric cancer. J. Evidence-Based Complement. Altern. Med. 2021, 6667560. doi:10.1155/2021/6667560
Suo, T., Wang, H., and Li, Z. (2016). Application of proteomics in research on traditional Chinese medicine. Expert Rev. Proteomics 13, 873–881. doi:10.1080/14789450.2016.1220837
Ungaro, R., Mehandru, S., Allen, P. B., Peyrin-Biroulet, L., and Colombel, J. F. (2017). Ulcerative colitis. Lancet 389, 1756–1770. doi:10.1016/S0140-6736(16)32126-2
Valle, J., Seppälä, K., Sipponen, P., and Kosunen, T. (1991). Disappearance of gastritis after eradication of Helicobacter pylori. A morphometric study. Scand. J. Gastroenterol. 26, 1057–1065. doi:10.3109/00365529109003956
Wan, P., Chen, H., Guo, Y., and Bai, A. P. (2014). Advances in treatment of ulcerative colitis with herbs: from bench to bedside. World J. Gastroenterol. 20, 14099–14104. doi:10.3748/wjg.v20.i39.14099
Wang, J., Zhang, Z., Li, Q., Hu, Z., Chen, Y., Chen, H., et al. (2024). Network pharmacology and molecular docking reveal the mechanisms of curcumin activity against esophageal squamous cell carcinoma. Front. Pharmacol. 15, 1282361. doi:10.3389/fphar.2024.1282361
Wang, M., Li, J., Yin, Y., Liu, L., Wang, Y., Qu, Y., et al. (2022a). Network pharmacology and in vivo experiment-based strategy to investigate mechanisms of JingFangFuZiLiZhong formula for ulcerative colitis. Ann. Med. 54 (1), 3219–3233. doi:10.1080/07853890.2022.2095665
Wang, T., Feng, Y., Wang, H., Huo, G., Cai, Y., Wang, L., et al. (2020). The mechanisms of Sijunzi decoction in the treatment of chronic gastritis revealed by network pharmacology. Evidence-Based Complement. Altern. Med. 2020, 8850259. doi:10.1155/2020/8850259
Wang, Z. Y., Wang, X., Zhang, D. Y., Hu, Y. J., and Li, S. (2022b). Traditional Chinese medicine network pharmacology: development in new era under guidance of network pharmacology evaluation method guidance. Zhongguo Zhong Yao Za Zhi 47, 7–17. doi:10.19540/j.cnki.cjcmm.20210914.702
Wartchow, C. A., Podlaski, F., Li, S., Rowan, K., Zhang, X., Mark, D., et al. (2011). Biosensor-based small molecule fragment screening with biolayer interferometry. J. Comput.-Aided Mol. Des. 25, 669–676. doi:10.1007/s10822-011-9439-8
Wei, Y., Ren, S., Wang, R., Jing, M., Liu, H., Wang, M., et al. (2021). Based on network pharmacology to explore the potential bioactive compounds and mechanisms of Zuojin pill for the treatment of ulcerative colitis. Evidence-Based Complement. Altern. Med. 2021, 7567025. doi:10.1155/2021/7567025
Weng, J., Wu, X. F., Shao, P., Liu, X. P., and Wang, C. X. (2023). Medicine for chronic atrophic gastritis: a systematic review, meta- and network pharmacology analysis. Ann. Med. 55 (2), 2299352. doi:10.1080/07853890.2023.2299352
Wishart, D. S., Knox, C., Guo, A. C., Shrivastava, S., Hassanali, M., Stothard, P., et al. (2006). DrugBank: a comprehensive resource for in silico drug discovery and exploration. Nucleic Acids Res. 34, D668–D672. doi:10.1093/nar/gkj067
Wu, C. W., Lu, L., Liang, S. W., Chen, C., and Wang, S. M. (2016). Application of drug-target prediction technology in network pharmacology of traditional Chinese medicine. Zhongguo Zhongyao Zazhi 41, 377–382. doi:10.4268/cjcmm20160303
Wu, J., Ye, X., Yang, S., Yu, H., Zhong, L., and Gong, Q. (2021b). Systems pharmacology study of the anti-liver injury mechanism of Citri Reticulatae Pericarpium. Front. Pharmacol. 12, 618846. doi:10.3389/fphar.2021.618846
Wu, J. Z., Li, Y. J., Huang, G. R., Xu, B., Zhou, F., Liu, R. P., et al. (2021a). Mechanisms exploration of Angelicae Sinensis Radix and Ligusticum Chuanxiong Rhizoma herb-pair for liver fibrosis prevention based on network pharmacology and experimental pharmacologylogy. Chin. J. Nat. Med. 19, 241–254. doi:10.1016/S1875-5364(21)60026-2
Xu, H., Zhang, Y., Lei, Y., Gao, X., Zhai, H., Lin, N., et al. (2014). A systems biology-based approach to uncovering the molecular mechanisms underlying the effects of dragon's blood tablet in colitis, involving the integration of chemical analysis, ADME prediction, and network pharmacology. PLoS One 9 (7), e101432. doi:10.1371/journal.pone.0101432
Xu, S. M., Bai, L. P., Lu, J. G., Dong, Q. J., and Cao, B. (2022). Study on medication rules of traditional Chinese medicine in treating constipation through data mining and network pharmacology. Biomed. Res. Int. 2022, 6733851. doi:10.1155/2022/6733851
Xue, R., Fang, Z., Zhang, M., Yi, Z., Wen, C., and Shi, T. (2013). TCMID: traditional Chinese Medicine integrative database for herb molecular mechanism analysis. Nucleic Acids Res. 41, D1089–D1095. doi:10.1093/nar/gks1100
Yang, S., Zhang, J., Yan, Y., Yang, M., Li, C., Li, J., et al. (2020). Network pharmacology-based strategy to investigate the pharmacologic mechanisms of Atractylodes macrocephala Koidz. For the treatment of chronic gastritis. Front. Pharmacol. 10, 1629. doi:10.3389/fphar.2019.01629
Yang, Y., Qian, C., Wu, R., Wang, R., Ou, J., and Liu, S. (2023). Exploring the mechanism of the Fructus Mume and Rhizoma Coptidis herb pair intervention in Ulcerative Colitis from the perspective of inflammation and immunity based on systemic pharmacology. BMC Complement. Med. Ther. 23 (1), 11. doi:10.1186/s12906-022-03823-7
Yin, Z. H., Tan, W. H., and Jiang, Y. L. (2024). Exploration of the molecular mechanism of Curcuma aromatica Salisb's anticolorectal cancer activity via the integrative approach of network pharmacology and experimental validation. ACS Omega 9 (19), 21426–21439. doi:10.1021/acsomega.4c01759
Yu, W., Cheng, H., Zhu, B., and Yan, J. (2021). Network pharmacology-based validation of the efficacy of Huiyangjiuji decoction in the treatment of experimental colitis. Front. Pharmacol. 12, 666432. doi:10.3389/fphar.2021.666432
Zhan, L. L., and Su, H. H. (2018). Application of network pharmacology in the pharmacodynamics of traditional Chinese medicine. J. Clin. Med. 5, 197. doi:10.16281/j.cnki.jocml.2018.99.149
Zhang, R., Zhu, X., Bai, H., and Ning, K. (2019a). Network pharmacology databases for traditional Chinese medicine: review and assessment. Front. Pharmacol. 10, 123. doi:10.3389/fphar.2019.00123
Zhang, W., Chao, X., Wu, J. Q., Ma, X. B., Yang, Y. L., Wu, Y., et al. (2021a). Exploring the potential mechanism of Guchang Zhixie wan for treating ulcerative colitis by comprehensive network pharmacological approaches and molecular docking validation as well as cell experiments. Chem. Biodivers. 18, e2000810. doi:10.1002/cbdv.202000810
Zhang, W., Wang, W., Shen, C., Wang, X., Pu, Z., and Yin, Q. (2021b). Network pharmacology for systematic understanding of Schisandrin B reduces the epithelial cells injury of colitis through regulating pyroptosis by AMPK/Nrf2/NLRP3 inflammasome. Aging 13, 23193–23209. doi:10.18632/aging.203611
Zhang, Y., Li, X., Xu, X., and Yang, N. (2019b). Mechanisms of paeonia lactiflora in treatment of ulcerative colitis: a network pharmacological study. Med. Sci. Monit. 25, 7574–7580. doi:10.12659/MSM.917695
Zhang, Z., Li, B., Huang, J., Huang, S., He, D., Peng, W., et al. (2020). A network pharmacology analysis of the active components of the traditional Chinese medicine zuojinwan in patients with gastric cancer. Med. Sci. Monit. 26, e923327. doi:10.12659/MSM.923327
Zhou, S., Xu, X., Zeng, J., Liu, Z., Wang, M., and Lv, W. (2021a). Unveiling the mechanism of principal drugs of Lianpu Drink on chronic gastritis by network pharmacology. Evidence-Based Complement. Altern. Med. 2021, 5518750. doi:10.1155/2021/5518750
Zhou, W., Zhang, H., Wang, X., Kang, J., Guo, W., Zhou, L., et al. (2022). Network pharmacology to unveil the mechanism of Moluodan in the treatment of chronic atrophic gastritis. Phytomedicine 95, 153837. doi:10.1016/j.phymed.2021.153837
Zhou, Y., Wu, R., Cai, F. F., Zhou, W. J., Lu, Y. Y., Zhang, H., et al. (2021b). Xiaoyaosan decoction alleviated rat liver fibrosis via the TGFβ/Smad and Akt/FoxO3 signaling pathways based on network pharmacology analysis. J. Ethnopharmacol. 264, 113021. doi:10.1016/j.jep.2020.113021
Zhu, S., Ran, J., Yang, B., and Mei, Z. (2017). Aquaporins in digestive system. Adv. Exp. Med. Biol. 969, 123–130. doi:10.1007/978-94-024-1057-0_8
Keywords: network pharmacology, digestive system diseases, traditional Chinese medicine, compounds, targets
Citation: Zheng S, Liang Y, Xue T, Wang W, Li S, Zhang P, Li X, Cao X, Liu Q, Qi W, Ye Y and Zao X (2024) Application of network pharmacology in traditional Chinese medicine for the treatment of digestive system diseases. Front. Pharmacol. 15:1412997. doi: 10.3389/fphar.2024.1412997
Received: 06 April 2024; Accepted: 19 June 2024;
Published: 17 July 2024.
Edited by:
Zhe-Sheng Chen, St. John’s University, United StatesReviewed by:
Yi Yu, Wuhan University, ChinaYulin Dai, Changchun University of Chinese Medicine, China
Copyright © 2024 Zheng, Liang, Xue, Wang, Li, Zhang, Li, Cao, Liu, Qi, Ye and Zao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaobin Zao, QTM0MTdAYnVjbS5lZHUuY24=; Yongan Ye, eWV5b25nYW5AdmlwLjE2My5jb20=
†These authors have contributed equally to this work
 Shihao Zheng
Shihao Zheng Yijun Liang3†
Yijun Liang3†