- 1Department of Pharmacy, Mindong Hospital Affiliated to Fujian Medical University, Ningde, Fujian, China
- 2Department of Pathology, Mindong Hospital Affiliated to Fujian Medical University, Ningde, Fujian, China
- 3Department of Emergency Medicine, Mindong Hospital Affiliated to Fujian Medical University, Ningde, Fujian, China
Background: The efficacy and safety of enfortumab vedotin combined with pembrolizumab (EV-PEMB) was investigated as a first-line treatment for advanced urothelial carcinoma (UC) in a phase III clinical trial (EV-302). The trial findings indicated significant prolonged progression-free survival (PFS) and overall survival (OS) compared to chemotherapy with a favorable safety profile. However, EV-PEMB is costly and it is unknown whether it is cost-effective compared to chemotherapy. This study aimed to conduct a cost-effectiveness analysis of EV-PEMB versus chemotherapy as a first-line treatment for advanced UC from the perspective of the Chinese healthcare system.
Methods: A Markov model with three distinct health states was developed to assess the cost-effectiveness of EV-PEMB as a first-line treatment for advanced UC versus chemotherapy based on the EV-302 trial. Drug costs were obtained from national tender prices. Other expenses and utility values were sourced from the literature or expert advice. The findings of the study included total costs, quality-adjusted life years (QALYs), and incremental cost-effectiveness ratios (ICERs). We conducted a one-way sensitivity analysis and probabilistic sensitivity analysis to ensure the model’s robustness.
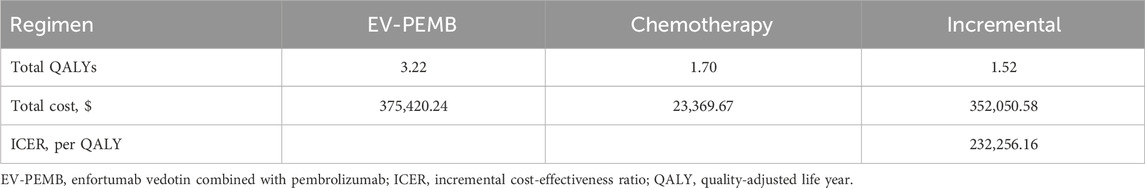
Results: The EV-PEMB regimen demonstrated a gain of 3.22 QALYs at $375,420.24, compared to the chemotherapy regimen with 1.70 QALYs at $23,369.67. ICER for EV-PEMB compared to chemotherapy was at $232,256.16 per QALY gained. In China, at a willingness-to-pay threshold of $38,133 per QALY, EV-PEMB has a 0% probability of being cost-effective as a first-line treatment for advanced UC compared to chemotherapy.
Conclusion: From the perspective of the Chinese healthcare system, EV-PEMB is unlikely to be a cost-effective first-line treatment option for advanced UC compared to chemotherapy.
1 Introduction
Urothelial carcinoma (UC) is an epithelial cancer caused due to a malignant transformation of the overlying epithelium of the urinary tract (NCCN, 2022). UC has a non-negligible morbidity and mortality rate worldwide (Wong et al., 2018). UC can occur in any part of the urinary system, and more than 90% of the cases involve the bladder (Cathomas et al., 2022). In 2020, more than 573,000 new cases of bladder cancer were reported, with approximately 213,000 associated deaths, making it the 10th most malignant tumor globally (Sung et al., 2021). Early-stage UC is curable; however, more than 5% of patients with UC are first diagnosed with locally advanced or metastatic disease and typically show a poor prognosis (Lopez-Beltran et al., 2021), with 5-year survival rates of only 34% and 5.4%, respectively (National Cancer Institute, 2023). Platinum-based chemotherapy is the first-line treatment for advanced UC; however, treatment outcomes remain unsatisfactory (Powles et al., 2024). For instance, patients receiving cisplatin-based chemotherapy have a median progression-free survival (PFS) of only 7–9 months and a median overall survival (OS) of approximately 16–18 months (El Rassy et al., 2019; Sorce et al., 2022; van der Heijden et al., 2023; Powles et al., 2024). Immune checkpoint inhibitors (ICIs), such as nivolumab and pembrolizumab, also failed to provide significant clinical benefit in advanced UC (van der Heijden et al., 2023; Flaig et al., 2022; Powles et al., 2021). Therefore, there is a need for a new treatment option for patients with advanced UC.
Enfortumab vedotin (EV) is an antibody-drug conjugate (ADC) known to induce cell cycle arrest and cell death (O'Donnell et al., 2023). It confers survival advantages to patients with locally advanced or metastatic UC who have received prior therapy (Yu et al., 2021). Preclinical data suggest that EV, in combination with pembrolizumab, can augment antitumor activity synergistically, leveraging their respective mechanisms of action to achieve complementary efficacy (O'Donnell et al., 2023). Recently, in a phase III clinical trial (EV-302) (Powles et al., 2024) involving previously untreated patients with advanced UC, EV combined with pembrolizumab (EV-PEMB) demonstrated enhanced clinical benefits and a manageable safety profile compared to chemotherapy. EV-302, a global randomized controlled trial, demonstrated that EV-PEMB, as the first-line treatment option, improved the median PFS (12.5 months vs. 6.3 months) and median OS (31.5 months vs. 16.1 months) in patients with advanced UC.
Although the clinical efficacy of EV-PEMB in treating advanced UC is superior to chemotherapy, the associated increase in healthcare costs must be considered, especially in countries with less affluent healthcare resources, such as China. Therefore, an economic evaluation of EV-PEMB is necessary to comprehensively assess its cost-effectiveness, weighing clinical advantages and potential economic implications as a first-line therapeutic option for advanced UC. The cost-effectiveness of EV-PEMB as a first-line treatment for advanced UC has not yet been evaluated. Therefore, we aim to fill this gap by evaluating the economic feasibility of EV-PEMB compared to chemotherapy in the context of the Chinese healthcare system based on the results of the EV-302 trial. Our study adheres to the Comprehensive Health Economic Evaluation and Reporting Standards (CHEERS) guidelines, ensuring transparency and methodological rigor (Husereau et al., 2022).
2 Methods
2.1 Clinical information
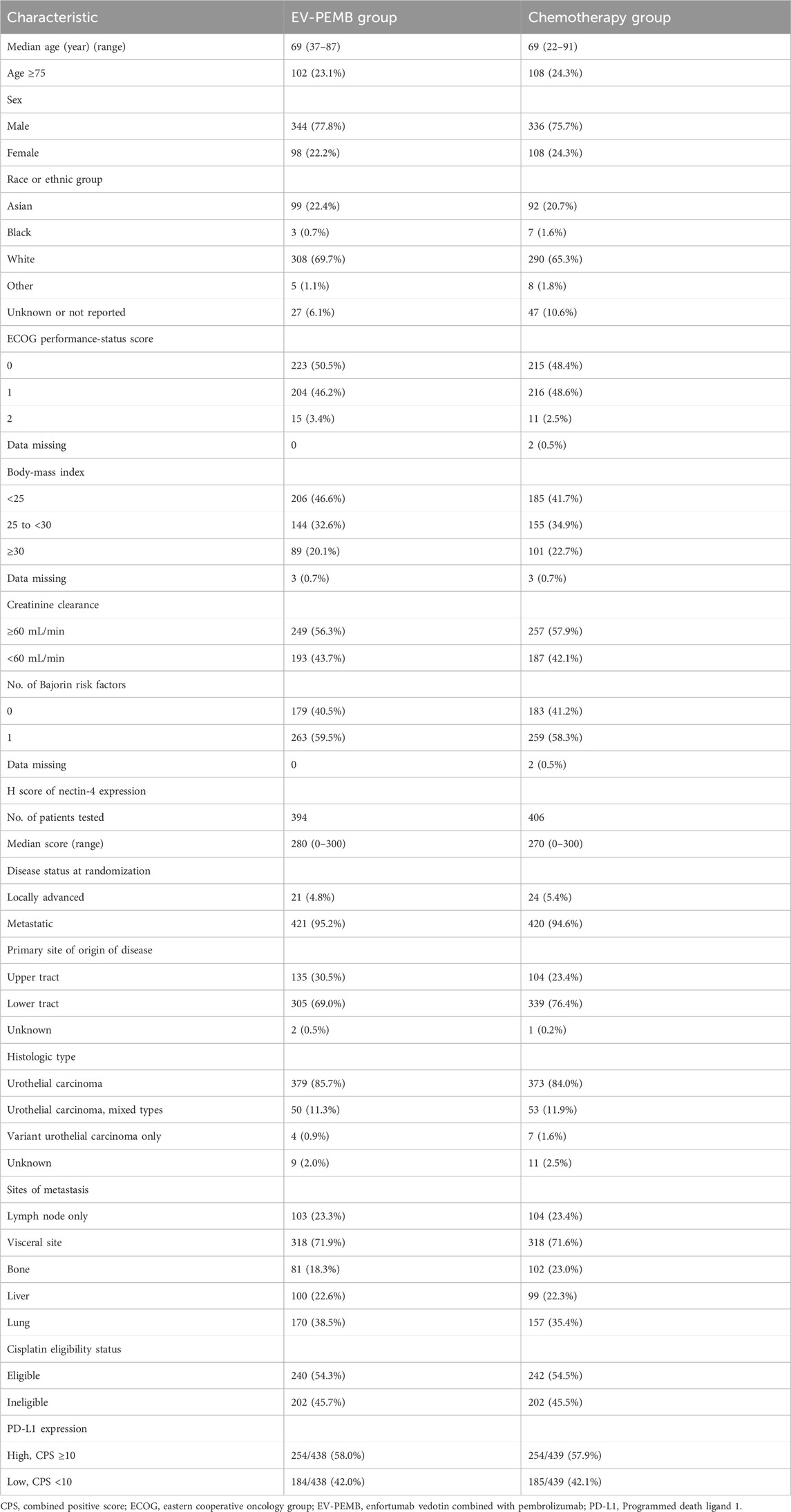
The clinical data used in this study were derived from the EV-302 trial (Powles et al., 2024). Specifically, the data included probabilities related to transitions in the patient health state over the course of the disease and the probability of adverse drug reactions. The enrolled population was consistent with the target group of the EV-302 trial. Inclusion criteria were adulthood, histologically unresectable or metastatic UC, and no previous systemic treatment. The main exclusion criteria included prior treatment with programmed death 1 or programmed death ligand 1 (PD-L1) inhibitors or other systemic therapies (except for neoadjuvant or adjuvant chemotherapy with recurrence occurring more than 12 months after completion of treatment), uncontrolled diabetes, persistent grade 2 or higher sensory or motor neuropathy, and a history of autoimmune diseases with prior systemic therapy within the past 2 years. Patients were randomly assigned to receive either EV-PEMB or chemotherapy, with both groups receiving treatment in 3-week cycles. Patients in the EV-PEMB group received an intravenous EV infusion (1.25 mg/kg of body weight, maximum dose 125 mg) on the first and eighth day of each cycle, and 200 mg pembrolizumab was intravenously administered after EV administration on the first day of each cycle. Patients in the chemotherapy group received gemcitabine in combination with cisplatin or carboplatin. Specifically, 1,000 mg/m2 body surface area gemcitabine was intravenously infused on the first and eighth day of each cycle. Cisplatin was intravenously infused at a dose of 70 mg/m2 body surface area on the first day of each cycle. Carboplatin was intravenously infused with an area under the curve of 5 mg/ml/min and the dosage was calculated using the Calvert formula. Treatment was continued until disease progression, development of unacceptable toxic effects, or completion of the maximum number of treatment cycles (chemotherapy, six cycles; pembrolizumab, 35 cycles; EV, no maximum). Patients included in the EV-PEMB and chemotherapy groups had similar baseline characteristics (Table 1). Based on the results of the EV-302 trial, the median duration of treatment for EV, pembrolizumab, and chemotherapy duration were 7, 8.5, and 4.1 months, respectively. The EV-302 trial did not provide comprehensive treatment data on disease progression. Therefore, all patients were presumed to receive the best supportive care (BSC) following disease progression or unacceptable toxicity occurs, which consists of palliative radiotherapy, symptom control, nutritional and psychological support (NCCN Guidelines, 2024).
2.2 Constructing the model
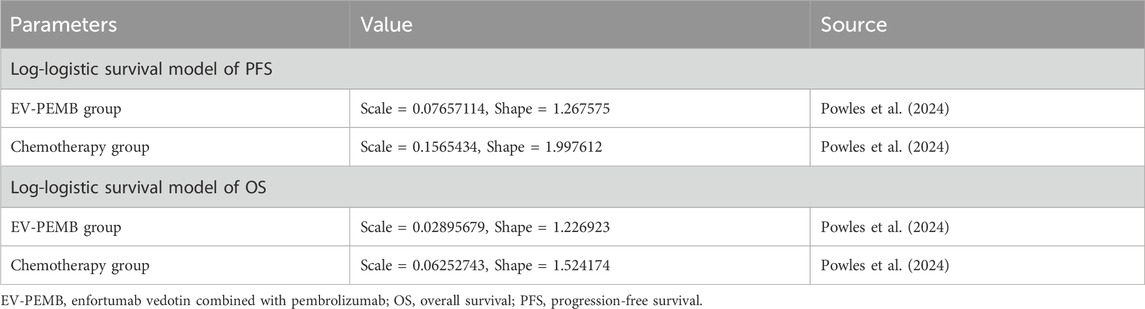
We evaluated the cost-effectiveness of EV-PEMB or chemotherapy as a first-line treatment option for advanced UC using Markov models. Kaplan-Meier survival curves for PFS and OS from the EV-302 trial (Powles et al., 2024) were digitized into data points using GetData Graph Digitizer (version 1.2). Subsequently, these data points were fitted to various survival distributions using the “survival,” “survHE,” and “survminer” packages in R software, following the methodology described by Hoyle et al. (Hoyle and Henley, 2011). The best-fit survival distributions were chosen based on the Akaike information criterion (AIC) and Bayesian information criterion (BIC); lower values indicated a better fit (Williams et al., 2017). AIC and BIC values for various survival distributions of PFS and OS curves are detailed in Supplementary Table B. Ultimately, the log-logistic distribution (S(t) = (1 + (λt)^γ)) offered the best fit for the PFS and OS data (Supplementary Figure A). It was used to calculate the transition probabilities between different health states for patients during the execution of the model. The estimated shape parameter (γ) and size parameter (λ) are presented in Table 2. The model considered the background mortality rate in China (Compiled by the National Bureau of Statistics of China, 2024).
Our model delineated three distinct and mutually exclusive health states based on tumor progression: PFS, progressive disease (PD), and death (Figure 1). We assumed that all patients entered the model in the PFS state (Shen et al., 2022). Individuals either remained in their current health state or progressed to a different one throughout the simulation, with no option to regress to the previous state. Each cycle within the model spanned a fixed duration of 21 days. The simulation extended over 15 years, during which over 90% of patients died. In the model, the survival probabilities for each cycle for the two patient groups are detailed in Supplementary Data Sheet S2. Outcomes of the model encompassed total costs, quality-adjusted life years (QALYs), and the incremental cost-effectiveness ratios (ICERs) associated with the two treatment regimens. Following the China Pharmacoeconomic Evaluation Guidelines (Yue et al., 2021), three times the per capita GDP of China in 2023 was considered the willingness-to-pay (WTP) threshold ($38,133). A treatment regimen was considered cost-effective if its ICER value was below this pre-determined WTP threshold.
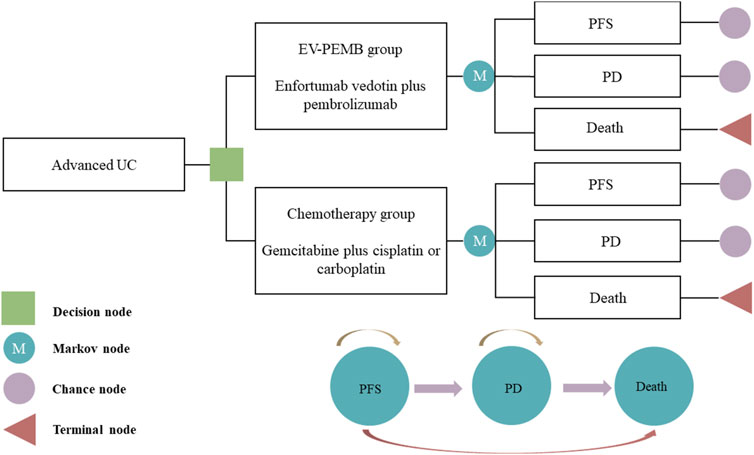
Figure 1. The Markov model simulating outcomes for the EV-302 trial. All patients started with PFS state and received treatment with EV-PEMB or chemotherapy. EV-PEMB, enfortumab vedotin combined with pembrolizumab; PD, progressive disease; PFS, progression-free survival; UC, urothelial carcinoma.
2.3 Costs and utility
This study exclusively incorporated direct medical costs, comprising drugs, tests, routine follow-up, BSC, end-of-life care, and management of grade 3 and higher adverse reactions with an incidence exceeding 5% (Table 3). Drug prices were determined from the national drug tender prices. The price of sacituzumab govitecan (an ADC approved by the FDA for UC) in China was used as the reference for EVs because EV is not yet available in China. Other data on costs were sourced from literature or expert advice and adjusted to values in 2023 based on the China Bureau of Statistics Medical Price Index (Compiled by National Bureau of Statistics of China, 2024). All costs are presented in USD, converted at the average 2023 exchange rate (1 USD = 7.03 CNY). Utility values are indicators that assess a patient’s social functioning and overall health, including physical, mental, and disease-related aspects, on a scale from 0 to 1, where 0 represents death and 1 represents optimal health. The utility values of this study assigned to PFS and PD were obtained from literature in China because of the absence of quality-of-life data in the EV-302 trial (Table 3). The detrimental utility impact of grade 3 or higher adverse reactions with an incidence exceeding 5% was considered. Costs and utilities in this study were discounted at a rate of 5% (Yue et al., 2021).
2.4 Sensitivity analysis
We conducted a sensitivity analysis to assess the robustness of the results from the model. One-way sensitivity analyses were performed by adjusting parameters within specified ranges to identify those that affected the ICER. These results are represented in a tornado diagram. All parameters were varied within their 95% confidence intervals derived from the literature, using benchmark values of ±20% in the absence of data. The discount rate varied between 0% and 8% (Table 3). Probabilistic sensitivity analyses through 1,000 Monte Carlo simulations were performed to evaluate how simultaneous alterations in multiple parameters affected the model outcomes. All parameters followed pre-determined distributions, and the results from these probabilistic sensitivity analyses are presented as scatter plots (Table 3). Furthermore, the calculation of the ICER for EV-PEMB compared to chemotherapy was repeated by continuously decreasing the price of EV and pembrolizumab to determine the price of EV and pembrolizumab at which EV-PEMB could be cost-effective.
2.5 Subgroup analysis
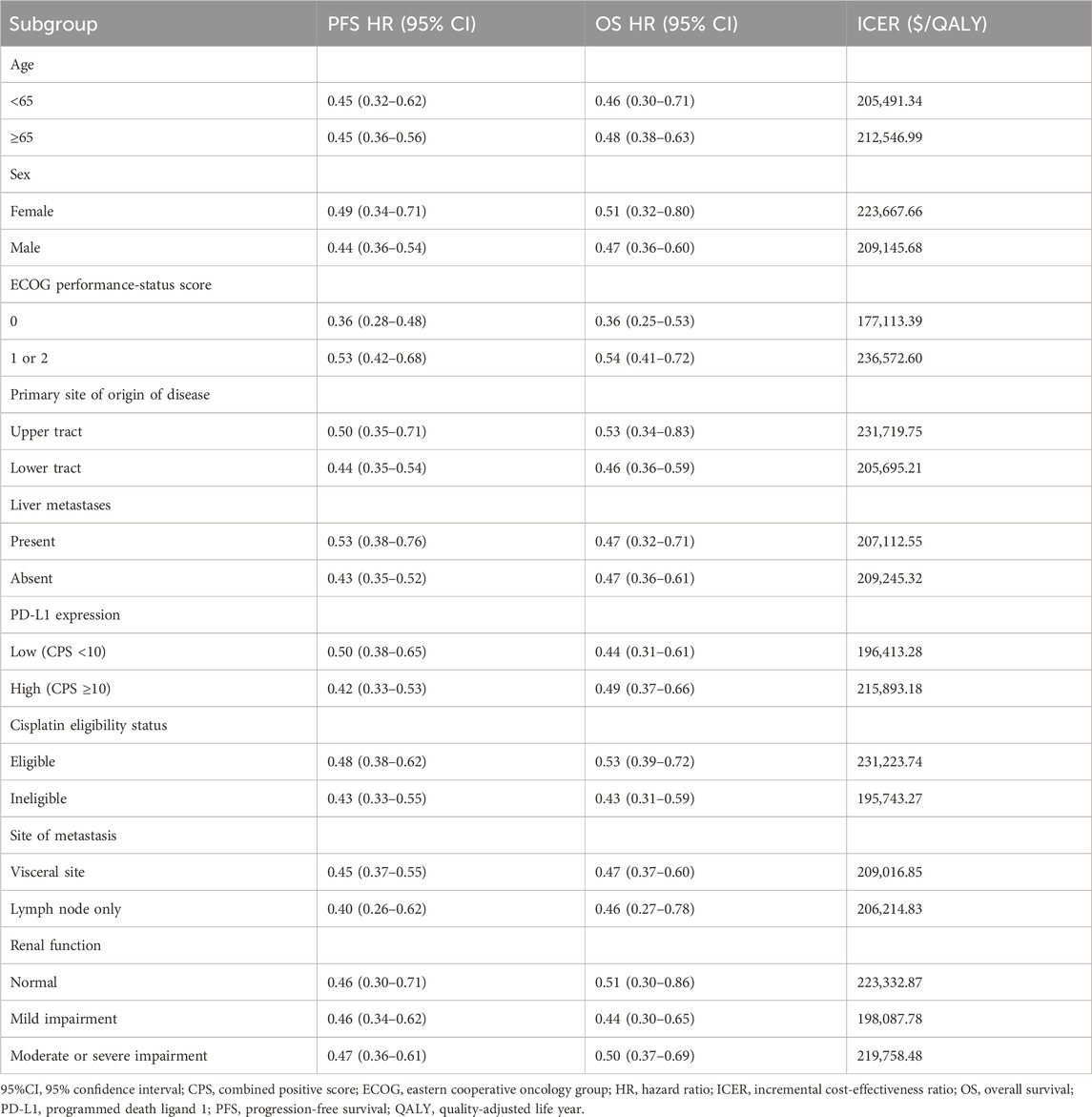
Exploratory subgroup analyses were conducted to evaluate the potential effect of subgroups with varying baseline characteristics on model outcomes. Subgroups were defined based on age, sex, Eastern Cooperative Oncology Group (ECOG) performance-status score, primary site of origin of disease, PD-L1 expression, cisplatin eligibility status, site of metastasis, and renal function (Table 4). We assumed the same survival curves for all subgroups in the chemotherapy arm of the trial based on the methodology provided by Hoyle et al. (2010) as the EV-302 trial did not provide survival curves for individual subgroups and calculated ICERs and acceptable probabilities of cost-effectiveness for each subgroup using the subgroup-specific risk ratios from the EV-302 trial.
2.6 Scenario analysis
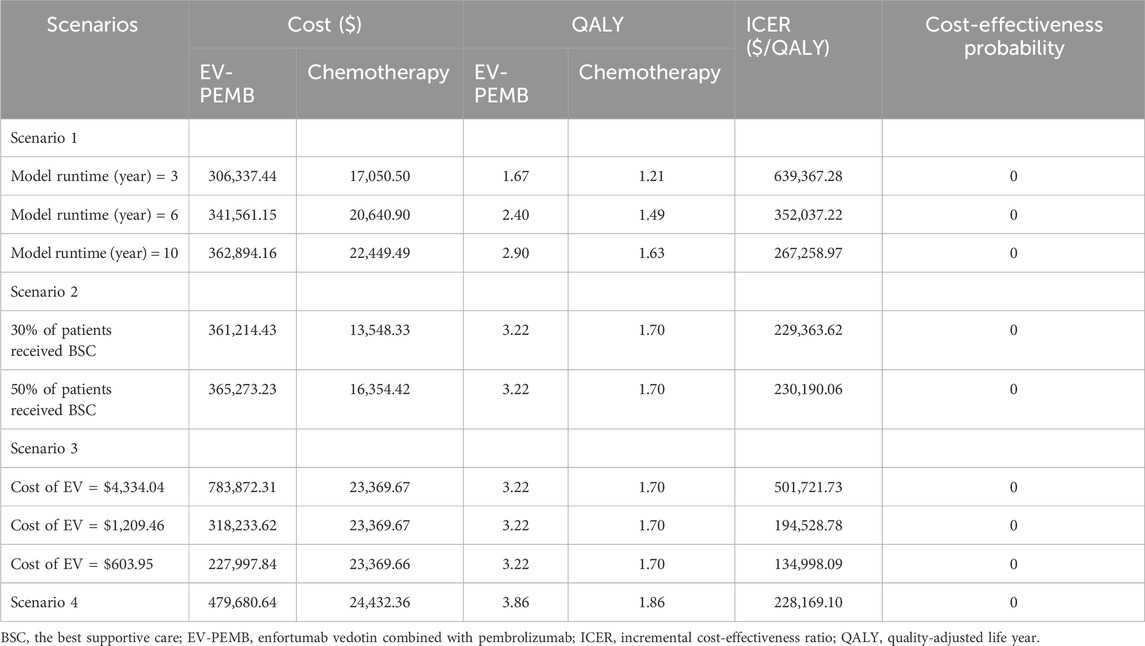
A scenario analysis was conducted to improve the applicability of the results of this study. In scenario 1, with no changes in treatment, model run times were adjusted to 3, 6, and 10 years to assess their effects on the model results. In scenario 2, we assumed that only 30% or 50% of patients received BSC after disease progression to model discontinuation of treatment in clinical practice by some patients due to several reasons. Scenario 3, due to unavailability of pricing for EV in China, we used prices from the US, Norway, and Japan as reference points for conducting cost-effectiveness analyses. Scenario 4, given the higher complete remission rate of EV-PEMB, we hypothesize that patients in the EV-302 trial who do not show progress by the final data collection point (8 August 2023) have achieved cure. They discontinue the aforementioned treatment but continue periodic follow-ups and undergo one examination per cycle. Adjusting patients’ survival probabilities using the background mortality rate in China, we ultimately calculate cost-effectiveness to examine potential biases in our analyses that did not account for the impact of permanent cure rates.
3 Results
3.1 Base case analysis
The results of this study are expressed in terms of total cost, QALYs, and ICER (Table 5). The EV-PEMB group achieved 3.22 QALYs at $375,420.24. In the chemotherapy group, the effectiveness was 1.70 QALYs at $23,369.67. The mean incremental effectiveness and cost in the EV-PEMB group were 1.52 QALYs and $352,050.58, respectively, relative to the chemotherapy group. The ICER for EV-PEMB versus chemotherapy was $232,256.16 per QALY gained. In China, EV-PEMB is unlikely to be cost-effective for treating advanced UC compared to chemotherapy when the WTP threshold is $38,133 per QALY.
3.2 Sensitivity analysis
The results of the one-way sensitivity analysis are shown as a tornado diagram (Figure 2). The parameters with the most significant effect on the model were the discount rate, the patient’s weight, the price of EV, and the price of pembrolizumab. When the values of these parameters were allowed to vary within a given range, the ICER was always higher than our pre-determined WTP threshold, implying that variations in the model input parameters did not affect the model results, indicating that the results are robust. The results of the probabilistic sensitivity analysis are presented in a scatter plot (Figure 3). The probability that the EV-PEMB group was cost-effective compared to the chemotherapy group was 0 at the WTP threshold of $38,133/QALY. EV-PEMB had the probability of being a cost-effective regimen for the treatment of advanced UC compared to chemotherapy only when the prices of EV and pembrolizumab simultaneously decreased to 13.1% of the original, i.e., $208.7 and $333.9 for EV and pembrolizumab, respectively.

Figure 2. One-way sensitivity analyses of EV-PEMB in comparison to chemotherapy. EV-PEMB, enfortumab vedotin combined with pembrolizumab; ICER, incremental cost-effectiveness ratio; PD, progressive disease; PFS, progression-free survival; WTP, willingness-to-pay.
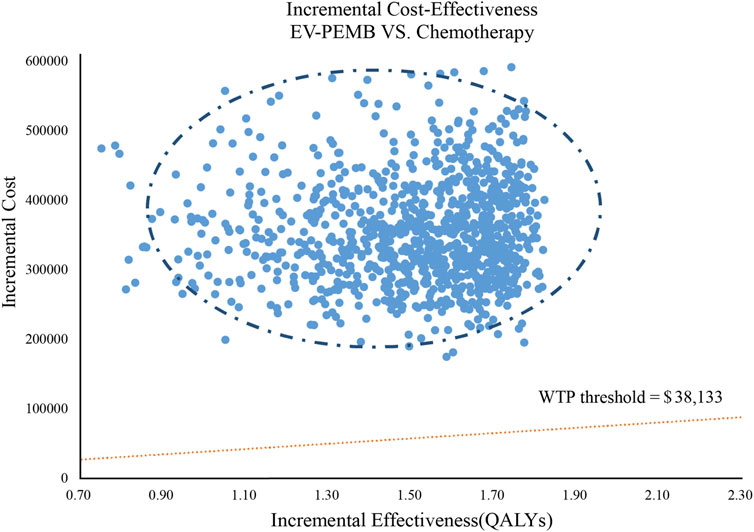
Figure 3. A probabilistic scatter plot of the ICER between the EV-PEMB group and the chemotherapy group. Each point means the ICER for 1 simulation. Ellipses are used to indicate 95% confidence intervals. Points that lie below the ICER threshold represent cost-effective simulations. EV-PEMB, enfortumab vedotin combined with pembrolizumab; WTP, willingness-to-pay.
3.3 Subgroup analysis
For all subgroups of the population showing different characteristics, the ICER in the EV-PEMB group was above the WTP threshold of $38,133. All subgroups had a probability of 0 for being cost-effective compared to the chemotherapy group (Table 4). The ICER was relatively low in the ECOG = 0, low PD-L1 expression, not applicable to cisplatin chemotherapy, and renal function with mild impairment populations. These results should be interpreted with caution owing to the small enrollment of the subgroups.
3.4 Scenario analysis
The results of the scenario analysis are shown in Table 6. In scenario 1, the ICER value of EV-PEMB gradually decreased compared to chemotherapy as the model run time increased, tending towards being more economical. In scenario 2, changes in the proportion of patients receiving BSC appeared to have little effect on the ICER value of EV-PEMB after disease progression. In Scenario 3, the EV prices were analyzed for cost-effectiveness with reference to US, Norwegian and Japanese prices, respectively, and the results show that EV-PBCM is not cost-effective. In Scenario 4, although the ICER values from the analysis are lower than the base case analysis, EV-PBCM is still not cost-effective.
4 Discussion
Chemotherapy or ICIs are the standard first-line therapeutic options for locally advanced or metastatic UC according to the guidelines for diagnosing and treating UC (2023) introduced by the Chinese Society of Clinical Oncology. However, their therapeutic efficacies are unsatisfactory (Nadal et al., 2024). ADCs (including EV) may represent a new therapeutic option. For untreated locally advanced or metastatic UC, the EV-302 trial showed that treatment with EV-PEMB was more effective than chemotherapy, with a lower incidence of grade 3 or higher adverse events than the latter (55.9% vs. 69.5%). The results of the EV-302 trial are expected to promote the use of EV-PEMB for treating advanced UC, leading to an increase in the economic burden on society and patients. This will play out as an important issue for healthcare policymakers. Therefore, an evaluation of the cost-effectiveness of EV-PEMB for treating advanced UC is necessary.
The results of our study showed that EV-PEMB cost an additional $232,256.16 per QALY compared to chemotherapy, much higher than our pre-determined WTP ($38,133/QALY). In China, EV-PEMB is not cost-effective as a first-line treatment for advanced UC, possibly due to the longer median treatment durations of EV and pembrolizumab in the EV-302 trial (7 and 8.5 months respectively) compared to chemotherapy (4.1 months). This, coupled with the fact that EV and pembrolizumab are inherently more expensive than chemotherapeutic agents, results in an EV-PEMB regimen that is far more costly to treat than chemotherapy without delivering sufficient incremental survival benefit. One-way sensitivity analyses also confirmed that the high price of EV and pembrolizumab were important influences on the lack of cost-effectiveness of EV-PEM. Therefore, addressing the high price of EV and pembrolizumab is key to making EV-PEMB cost-effective. We adjusted the prices of EV and pembrolizumab to assess cost-effectiveness. EV-PEMB was cost-effective only when the prices of both EV and pembrolizumab were reduced to 13.1% of their original prices, i.e., $208.7 and $333.9, respectively. The Chinese government has implemented an important policy since 2016, the National Drug Declaration List Negotiation, to improve drug accessibility (Zhu et al., 2022). By adopting the price negotiation policy, the state has reduced the prices of drugs, including anticancer drugs, especially innovative ones, to reduce the burden on the state, health insurance, and patients. Neither EV nor pembrolizumab has yet been included in the drug negotiation list, thereby providing great scope for the price reduction of these two drugs. Our results provide an important economic reference for the health insurance department in price negotiations.
We performed an economic evaluation of the nine subgroup populations defined in the EV-302 trial to gain insights into the cost-effectiveness of EV-PEMB in specific patient populations. Some populations, such as those with ECOG = 0, low PD-L1 expression, not applicable to cisplatin chemotherapy, and renal function with mild impairment, showed better economics than others. Physicians, patients, and policymakers may benefit from economic information for these subgroups. We also conducted a scenario analysis, which enhanced the applicability of our findings. As shown in scenario analysis 1, more than 80% of the cost-incurring years were the first 3 years. Although the cost-effectiveness probability of EV-PEMB was always 0%, which means that it was not economically superior to chemotherapy, the ICER value of EV-PEMB gradually decreased with increasing treatment time and approached the WTP value that we set. This suggests that EV-PEMB has relatively improved economics in the long term. In scenario 2, there was little change in ICER for EV-PEMB compared to chemotherapy when the proportion of patients treated with BSC after disease progression increased, suggesting that the cost outlay for BSC does not reduce the ICER of EV-PEMB. This result seems to encourage patients in the EV-PEMB group to receive possibly BSC after disease progression, consistent with scenario 1. In Scenario 3, when using prices from other countries as references for EV, the EV-PBCM remains cost-ineffective. Scenario 4’s findings indicate that not accounting for patients’ permanent cure rates does not affect the model’s outcomes, further validating the robustness of our results.
To date, only four economic evaluations of ICIs or ADCs for advanced UC from the perspective of the Chinese healthcare system have been conducted. Zhang et al. (Chen et al., 2024) and Liu S. et al. (2022) showed that atezolizumab in combination with chemotherapy as first-line treatment for metastatic UC was not cost-effective, with the price of atezolizumab having the greatest effect. Xie et al. (2022) concluded that nivolumab was not cost-effective as a maintenance treatment of advanced or metastatic UC in the total and PD-L1-positive populations at a WTP threshold of $30,447.09. Wu et al. (2022) demonstrated that EV for treating previously treated advanced UC had a 0% probability of being cost-effective. These results are consistent with our findings.
Our study has several advantages. First, this is the first assessment of the cost-effectiveness of EV-PEMB as a first-line treatment for advanced UC. This pioneering analysis is expected to guide the development of health insurance policies and clinical decisions in China and other countries. Second, the EV-302 trial directly compared EV-PEMB to chemotherapy, and our study used the most recent survival data available from the EV-302 trial for a cost-effectiveness analysis. Third, 21.6% of the participants in the EV-302 trial were from Asia, so the findings largely apply to the Chinese context. Undeniably, there are some limitations in our study, first, due to the lack of survival data after the follow-up period, our long-term survival data were simulated by survival modeling, which may introduce some bias in the model results. We plan to refine the cost-effectiveness analysis when long-term survival data are available. Second, due to the lack of detailed treatment data after patients’ disease progression, we assumed that all patients received BSC as a second-line treatment regimen, which may be biased from clinical practice. However, such an assumption would not change the model results when the one-way sensitivity results suggest otherwise. Third, our analysis only considered grade ≥3 adverse reactions with an incidence of more than 5%. However, sensitivity analyses confirmed that changes in the probability of occurrence of these adverse reactions would not affect the results.
5 Conclusion
In summary, from the perspective of the Chinese healthcare system, EV-PEMB is not cost-effective as a first-line treatment strategy for advanced UC compared to chemotherapy. Substantial reductions in the price of EV and pembrolizumab are necessary to make EV-PEMB cost-effective. These findings provide essential economic evidence for healthcare providers and patients to assess the suitability of this treatment option. Furthermore, our research supports the healthcare insurance sector in China with crucial economic analysis for pricing strategies following the introduction of EV. From a behavioral standpoint, these results may influence healthcare providers’ and patients’ attitudes towards treatment choices, encouraging greater consideration of cost-effectiveness and long-term outcomes.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author contributions
MY: Writing–review and editing. QZ: Writing–review and editing. YH: Writing–original draft, Writing–review and editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study was supported in part by grants from Natural Science Foundation of Ningde (Grant number: 2022J29). This study was not supported by any pharmaceutical company.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2024.1412292/full#supplementary-material
References
Cathomas, R., Lorch, A., Bruins, H. M., Compérat, E. M., Cowan, N. C., Efstathiou, J. A., et al. (2022). The 2021 updated European association of urology guidelines on metastatic urothelial carcinoma. Eur. Urol. 81, 95–103. doi:10.1016/j.eururo.2021.09.026
Chen, Q., Sun, Q., Zhang, J., Li, B., Feng, Q., and Liu, J. (2024). Cost-effectiveness analysis of Tislelizumab vs Sorafenib as the first-line treatment of unresectable hepatocellular carcinoma. PLoS ONE 19, e0295090. doi:10.1371/journal.pone.0295090
Compiled by National Bureau of Statistics of China (2024). Compiled by national Bureau of Statistics of China. Available at: https://www.stats.gov.cn/sj/ndsj/2023/indexch.htm (Accessed March 20, 2024).
El Rassy, E., Assi, T., Bakouny, Z., Pavlidis, N., and Kattan, J. (2019). Beyond first-line systemic treatment for metastatic urothelial carcinoma of the bladder. Clin. Transl. Oncol. 21, 280–288. doi:10.1007/s12094-018-1935-z
Flaig, T. W., Spiess, P. E., Abern, M., Agarwal, N., Bangs, R., Boorjian, S. A., et al. (2022). NCCN Guidelines® insights: bladder cancer, version 2.2022. Natl. Compr. Canc Netw. 20 (8), 866–878. doi:10.6004/jnccn.2022.0041
Hoyle, M., Green, C., Thompson-Coon, J., Liu, Z., Welch, K., Moxham, T., et al. (2010). Cost-effectiveness of temsirolimus for first line treatment of advanced renal cell carcinoma. Value Health 13, 61–68. doi:10.1111/j.1524-4733.2009.00617.x
Hoyle, M. W., and Henley, W. (2011). Improved curve fits to summary survival data: application to economic evaluation of health technologies. BMC Med. Res. Methodol. 11, 139. doi:10.1186/1471-2288-11-139
Husereau, D., Drummond, M., Augustovski, F., de Bekker-Grob, E., Briggs, A. H., Carswell, C., et al. (2022). Consolidated health economic evaluation reporting standards 2022 (CHEERS 2022) statement: updated reporting guidance for health economic evaluations. Value Health 25, 3–9. doi:10.1016/j.jval.2021.11.1351
Liu, L., Wang, L., Chen, L., Ding, Y., Zhang, Q., and Shu, Y. (2023). Cost-effectiveness of sintilimab plus chemotherapy versus chemotherapy alone as first-line treatment of locally advanced or metastatic oesophageal squamous cell carcinoma. Front. Immunol. 14, 1092385. doi:10.3389/fimmu.2023.1092385
Liu, Q., Tan, C., Yi, L., Wan, X., Peng, L., Li, J., et al. (2021). Cost-effectiveness analysis of pembrolizumab plus chemotherapy as first-line therapy for extensive-stage small-cell lung cancer. PLoS ONE 16, e0258605. doi:10.1371/journal.pone.0258605
Liu, S., Dou, L., Wang, K., Shi, Z., Wang, R., Zhu, X., et al. (2022a). Cost-effectiveness analysis of nivolumab combination therapy in the first-line treatment for advanced esophageal squamous-cell carcinoma. Front. Oncol. 12, 899966. doi:10.3389/fonc.2022.899966
Liu, X., Lang, Y., Chai, Q., Lin, Y., Liao, Y., and Zhu, Y. (2022b). Atezolizumab plus platinum-based chemotherapy as first-line therapy for metastatic urothelial cancer: a cost-effectiveness analysis. Front. Pharmacol. 13, 872196. doi:10.3389/fphar.2022.872196
Lopez-Beltran, A., Cimadamore, A., Blanca, A., Massari, F., Vau, N., Scarpelli, M., et al. (2021). Immune checkpoint inhibitors for the treatment of bladder cancer. Cancers (Basel) 13, 131. doi:10.3390/cancers13010131
National Cancer Institute (2023). SEER cancer statistics fact sheets: bladder cancer. Surveillance, Epidemiology, and End Results (SEER) Program. Available at: https://seer.cancer.gov/statfacts/html/bladder.html (Accessed March 20, 2024).
Nadal, R., Valderrama, B. P., and Bellmunt, J. (2024). Progress in systemic therapy for advanced-stage urothelial carcinoma. Nat. Rev. Clin. Oncol. 21, 8–27. doi:10.1038/s41571-023-00826-2
NCCN (2022). NCCN clinical practice guidelines in Oncology: bladder cancer, version 2. 2022. Natl. Compr. Cancer Netw. 2022.
NCCN Guidelines (2024). NCCN clinical practice guidelines in Oncology. Available at: https://www.nccn.org/guidelines/category_3 (Accessed July 12, 2024).
O'Donnell, P. H., Milowsky, M. I., Petrylak, D. P., Hoimes, C. J., Flaig, T. W., Mar, N., et al. (2023). Enfortumab vedotin with or without pembrolizumab in cisplatin-ineligible patients with previously untreated locally advanced or metastatic urothelial cancer. J. Clin. Oncol. 41, 4107–4117. doi:10.1200/JCO.22.02887
Powles, T., Csőszi, T., Özgüroğlu, M., Matsubara, N., Géczi, L., Cheng, S. Y., et al. (2021). Pembrolizumab alone or combined with chemotherapy versus chemotherapy as first-line therapy for advanced urothelial carcinoma (KEYNOTE-361): a randomised, open-label, phase 3 trial. Lancet Oncol. 22, 931–945. doi:10.1016/S1470-2045(21)00152-2
Powles, T., Valderrama, B. P., Gupta, S., Bedke, J., Kikuchi, E., Hoffman-Censits, J., et al. (2024). Enfortumab vedotin and pembrolizumab in untreated advanced urothelial cancer. N. Engl. J. Med. 390, 875–888. doi:10.1056/NEJMoa2312117
Shen, J., Du, Y., Shao, R., and Jiang, R. (2022). First-line sintilimab plus chemotherapy in locally advanced or metastatic esophageal squamous cell carcinoma: a cost-effectiveness analysis from China. Front. Pharmacol. 13, 967182. doi:10.3389/fphar.2022.967182
Sorce, G., Chierigo, F., Flammia, R. S., Hoeh, B., Hohenhorst, L., Tian, Z., et al. (2022). Survival trends in chemotherapy exposed metastatic bladder cancer patients and chemotherapy effect across different age, sex, and race/ethnicity. Urol. Oncol. 40, 380.e19–380.e27. doi:10.1016/j.urolonc.2022.03.014
Sung, H., Ferlay, J., Siegel, R. L., Laversanne, M., Soerjomataram, I., Jemal, A., et al. (2021). Global cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 71, 209–249. doi:10.3322/caac.21660
van der Heijden, M. S., Sonpavde, G., Powles, T., Necchi, A., Burotto, M., Schenker, M., et al. (2023). Nivolumab plus gemcitabine-cisplatin in advanced urothelial carcinoma. N. Engl. J. Med. 389, 1778–1789. doi:10.1056/NEJMoa2309863
Williams, C., Lewsey, J. D., Mackay, D. F., and Briggs, A. H. (2017). Estimation of survival probabilities for use in cost-effectiveness analyses: a comparison of a multi-state modeling survival analysis approach with partitioned survival and Markov decision-analytic modeling. Med. Decis. Mak. 37, 427–439. doi:10.1177/0272989X16670617
Wong, M., Fung, F., Leung, C., Cheung, W., Goggins, W. B., and Ng, C. F. (2018). The global epidemiology of bladder cancer: a joinpoint regression analysis of its incidence and mortality trends and projection. Sci. Rep. 8, 1129. doi:10.1038/s41598-018-19199-z
Wu, Q., Qin, Y., Liao, W., Zhang, M., Yang, Y., Zhang, P., et al. (2022). Cost-effectiveness of enfortumab vedotin in previously treated advanced urothelial carcinoma. Ther. Adv. Med. Oncol. 14, 17588359211068733. doi:10.1177/17588359211068733
Xie, Q., Zheng, H., Chen, Y., and Peng, X. (2022). Corrigendum: cost-effectiveness of avelumab maintenance therapy plus best supportive care vs. best supportive care alone for advanced or metastatic urothelial carcinoma. Front. Public Health 10, 965798. doi:10.3389/fpubh.2022.965798
Yaozh (2024). The big data service platform for China’s health industry: information Query of Drug Bid Winning. Available at: https://data.yaozh.com (Accessed March 20, 2024).
Yu, E. Y., Petrylak, D. P., O'Donnell, P. H., Lee, J. L., van der Heijden, M. S., Loriot, Y., et al. (2021). Enfortumab vedotin after PD-1 or PD-L1 inhibitors in cisplatin-ineligible patients with advanced urothelial carcinoma (EV-201): a multicentre, single-arm, phase 2 trial. Lancet Oncol. 22, 872–882. doi:10.1016/S1470-2045(21)00094-2
Yue, X., Li, Y., Wu, J., and Guo, J. J. (2021). Current development and practice of pharmacoeconomic evaluation guidelines for universal health coverage in China. Value Health Reg. Issues 24, 1–5. doi:10.1016/j.vhri.2020.07.580
Zhang, Q., Wu, P., He, X., Ding, Y., and Shu, Y. (2021). Cost-effectiveness analysis of camrelizumab vs. Placebo added to chemotherapy as first-line therapy for advanced or metastatic esophageal squamous cell carcinoma in China. Front. Oncol. 11, 790373. doi:10.3389/fonc.2021.790373
Zhao, Z., Chen, T., Zhou, Z., Guo, R., and Liu, Q. (2023). Cost-effectiveness of camrelizumab combined with chemotherapy in the first-line treatment of recurrent or metastatic nasopharyngeal carcinoma in China. BMJ Open 13, e071832. doi:10.1136/bmjopen-2023-071832
Zheng, Z., Chen, H., and Cai, H. (2023). Cost-effectiveness analysis of serplulimab combination therapy versus chemotherapy alone for patients with extensive-stage small cell lung cancer. Front. Oncol. 13, 1259574. doi:10.3389/fonc.2023.1259574
Keywords: cost-effectiveness, enfortumab vedotin, pembrolizumab, urothelial carcinoma, first-line treatment
Citation: You M, Zheng Q and He Y (2024) Enfortumab vedotin plus pembrolizumab as a first-line treatment for advanced urothelial carcinoma: a cost-effectiveness analysis from China based on the EV-302 trial. Front. Pharmacol. 15:1412292. doi: 10.3389/fphar.2024.1412292
Received: 04 April 2024; Accepted: 05 September 2024;
Published: 26 September 2024.
Edited by:
Yifei Liu, University of Missouri–Kansas City, United StatesReviewed by:
Guru P. Sonpavde, Advent Health Orlando, United StatesTerence Friedlander, University of California, San Francisco, United States
Ana Sabo, University of Novi Sad, Serbia
Copyright © 2024 You, Zheng and He. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ying He, aGV5aW5nMjQ3OEAxNjMuY29t
†These authors have contributed equally to this work
 Maojin You
Maojin You Qiaoyan Zheng2†
Qiaoyan Zheng2†