- 1Center for Experimental and Biomedical Imaging in Örebro (CEBIO), Faculty of Medicine and Health, Örebro University, Örebro, Sweden
- 2Department of Radiology and Medical Physics, Faculty of Medicine and Health, Örebro University, Örebro, Sweden
- 3Department of Psychiatry, School of Medical Sciences, Faculty of Medicine and Health, Örebro University, Örebro, Sweden
- 4Centre for Clinical Research and Education, Central Hospital, Karlstad, Sweden
- 5School of Medical Sciences, Faculty of Medicine and Health, Örebro University, Örebro, Sweden
- 6Department of Clinical Neuroscience, Karolinska Institutet, Stockholm, Sweden
Psychiatric disorders are categorized on the basis of presence and absence of diagnostic criteria using classification systems such as the international classification of diseases (ICD) and the diagnostic and statistical manual for mental disorders (DSM). The research domain criteria (RDoC) initiative provides an alternative dimensional framework for conceptualizing mental disorders. In the present paper, we studied neural and behavioral effects of central stimulant (CS) medication in adults with attention deficit hyperactivity disorder (ADHD) and healthy controls using categorical and dimensional stratifications. AX-Continuous Performance Task (AX-CPT) was utilized for the later purpose, and participants were classified as “reactive” or “proactive” based on their baseline proactive behavioral index (PBI). Out of the 65 individuals who participated (33 healthy controls and 32 patients with ADHD), 53 were included in the final analysis that consisted of 31 healthy controls and 22 ADHD patients. For the dimensional stratification, a median split of PBI scores divided participants into “reactive” and “proactive” groups irrespective of whether they had ADHD or not. Participants performed AX-CPT in conjunction with functional magnetic resonance imaging (fMRI) before and after CS medication. We found no significant within or between group CS effect when participants were categorically assigned as healthy controls and ADHD patients. For the dimensional stratification, however, CS selectively increased activation in frontoparietal cognitive areas and induced a shift towards proactive control mode in the reactive group, without significantly affecting the proactive group. In conclusion, the neural and behavioral effects of CS were more clear-cut when participants were stratified into dimensional groups rather than diagnostic categories.
1 Introduction
Psychiatric disorders, as a rule complex syndromes with substantial heterogeneity, are categorized into distinct diagnostic groups on the basis of presence and absence of characteristic criteria using classification systems such as the diagnostic and statistical manual for mental disorders (DSM) (American Psychiatric Association, 2013) and the international classification of disease (ICD). The research domain criteria initiative (RDoC) takes a different approach and utilizes neurobiologically informed domains of function to provide a dimensional framework for conceptualizing mental disorders (Cuthbert, 2022). In clinical settings, DSM/ICD classifications are used exclusively. However, these classification systems lead to widely heterogeneous groups within the same diagnostic category and their utility for research purposes has been questioned (Widiger and Samuel, 2005; Wardenaar and de Jonge, 2013; Borgogna, Owen, and Aita, 2023). In the present study, we compared effects of stimulant medication in healthy individuals and adults with attention deficit hyperactivity disorder (ADHD) using categorical (presence and absence of ADHD diagnosis according to DSM 5) and dimensional (RDoC cognitive control construct) stratification methods.
ADHD is a neurodevelopmental disorder, with impaired cognitive control and impulsivity/hyperactivity as core symptoms (Faraone et al., 2021). Although not part of the diagnostic criteria, there is also evidence indicating that emotional dysregulation may be a core impairment in ADHD, making ADHD an even more heterogeneous syndrome than currently clinically conceptualized (Retz et al., 2012; Shaw et al., 2014). Central stimulants (CS), which act by blocking dopamine (DA) and noradrenaline (NA) reuptake, are the mainstay of pharmacological treatment (Volkow et al., 2001; Arnsten and Dudley, 2005; Berridge et al., 2006; Faraone and Glatt, 2010), and their effect in alleviating core ADHD symptoms has been shown to be robust in the clinical setting (Faraone and Glatt, 2010; Cortese et al., 2018). Roughly 70%–80% of ADHD patients treated with CS are clinically deemed to be treatment responders (Spencer et al., 2005). CS is also used by segments of the healthy population wanting to improve cognitive performance, but the beneficial effects of CS in normal functioning adults is less clear (Repantis et al., 2010; Smith and Farah, 2011). Meta-analyses in healthy individuals showed small but significant effects of methylphenidate (MPH) on working memory, inhibitory control and processing speed (Smith and Farah, 2011; Marraccini et al., 2016). A meta-analysis that compared the effect of three cognitive enhancers in healthy individuals, MPH, modafinil and dexamphetamine, found that MPH had the strongest effect of the three, with small improvements on recall, attention and inhibitory control (Roberts et al., 2020). A recent study that evaluated the effects of CS in everyday complex tasks in healthy subjects showed that CS increased effort but reduced quality of effort, and performance across participants was reversed by CS such that above average performers ended up being below average after CS and vice versa for below average performers (Bowman et al., 2023). Effects of CS have also been shown to vary with baseline cognitive capacity (Mattay et al., 2000; Cools et al., 2008; Rostami Kandroodi et al., 2021) and baseline DA and NA levels (Cools and D'Esposito, 2011). Thus, not only effects of CS may differ between healthy subjects and ADHD patients, but they may also differ within the same group on the bases of baseline behavioral and neurochemical factors.
Cognitive control, also known as top-down control or executive control is a neuropsychological construct pertaining to the flexible regulation of goal-directed behaviour and is generally associated with the functions of lateral prefrontal cortex and posterior parietal cortex (Miller and Cohen, 2001; Friedman and Robbins, 2022). Cognitive control subconstructs such as goal selection, updating, representation, and maintenance can be studied using AX-Continuous Performance Task (AX-CPT), a paradigm that is often used to assess cognitive control (Lopez-Garcia et al., 2016). In AX-CPT, single letters (A, B, X, Y) are displayed on a screen and participants are instructed to make a target response when presented with the letter X, but only if it is preceded by the letter A. For all other letter combinations, participants are instructed to make a non-target response. To create expectancy, AX trials are generally made to occur more frequently than AY, BX and BY trials (Barch et al., 2003). Cognitive control mode during AX-CPT is assessed by the proactive behavioral index (PBI), where a proactive cognitive control mode indicates better performance on BX trials than AY trials, and the opposite being the case for reactive control mode.
In a study that made use of the dual system theory of decision-making that contrasts quick heuristic mode of decision making with a slower deliberative mode, Yechiam and Zeif found that MPH improved performance by selectively enhancing the slower deliberative mode of decision-making compared to the quicker heuristic mode (Yechiam and Zeif, 2022). A conceptually comparable dual mechanism framework (DMC) has been suggested for cognitive control, contrasting proactive and reactive control modes (Braver et al., 2009; Braver, 2012). The proactive control mode has been shown to dominate in healthy adults, while patients with psychotic (Barch et al., 2003; MacDonald and Carter, 2003) and anxiety disorders (Schmid, Kleiman, and Amodio, 2015) are more prone to employ a more reactive control mode.
In the present paper, we studied behavioral and neural effects of CS in healthy controls and individuals with ADHD using two stratification strategies, (i) categorical stratification on the bases of presence and absence of ADHD diagnosis according to DSM 5, and (ii) dimensional stratification on the basis of proactive and reactive cognitive control mode. We hypothesized that the behavioral and neural effects of CS in the categorical and dimensional groups may not always parallel each other, and that the neurobehavioral homogeneity created by dimensional stratification using RDoC cognitive control domain might reveal CS effects masked by heterogeneity in the categorical stratification using diagnosis classes.
2 Materials and methods
The study was approved by the Swedish Ethical Review Authority (Dnr 2020-02278; 2020-05590) and written consent was obtained from all participants in the study.
2.1 Participants and study outline
Thirty-three healthy controls and 32 adults with ADHD were included in the study that was conducted between December 2020 and December 2023. The ADHD group was a well-characterized clinical cohort, who had clinically responded to CS medication and are recruited from the Neuropsychiatric Outpatient Clinic at Örebro University Hospital (Rode et al., 2023). Inclusion criteria for the ADHD cohort were (i) ADHD diagnosis after extensive neuropsychiatric evaluation by a dedicated team of psychologists and senior consultants in psychiatry according to Swedish guidelines, (ii) no ongoing psychosis, bipolar, depressive, substance use or sever autism spectrum disorder, (iii) no suicidal or aggressive tendency and (iv) no contraindication for MRI investigation. Choice of medication and treatment optimization was carried out by the treating physician and followed Swedish guidelines for the pharmacological treatment of ADHD (Läkemedelsverket, 2016). Patients had to have a minimum of 4–6 weeks of stable medication before they could be enrolled in the study. Clinical response was determined by a score of 1 or 2 using clinician and patient-rated Clinical Global Impression–Improvement (CGI-I). The control group was recruited by advertising at a university campus and hospital area. Exclusion criteria for the healthy controls were (i) current or previous psychiatric and neurological ailment including substance use, (ii) ongoing psychoactive medication use, (iii) narrow-angle glaucoma, and (iv) incompatibility with magnetic resonance imaging (MRI). The control group was also assessed for potential allergic reaction to MPH or any of its ingredients. The CS medication used by the ADHD patients was either methylphenidate (MPH) or lisdexamfetamine (LDX) as selected and dose-optimized by the treating physician.
All participants underwent MRI examinations before and after CS using the same MRI scanner and protocol settings. The first session was performed in the absence of CS and the second 1–2 h after ingestion of CS, which constituted of 30 mg short-acting MPH for the healthy controls and MPH or LDX for the ADHD group as selected and dose-optimized by the treating physician. ADHD patients were instructed to abstain from CS medication for 24 h before the start of the first MRI session. CS was ingested directly after the end of the first session, and the second session started 1–2 h later to synchronize MRI examination with peak CS concentration in brain tissue. Each MRI session included a functional MRI (fMRI) acquisition with the participants performing an AX-CPT task that lasted for 14 min and 22 s.
2.2 Cognitive control task (AX-CPT)
AX-CPT was implemented using E-Prime (Psychology Software Tools version 3.0, Pittsburgh, PA, United States) and started with a rest period of 30 s displaying a fixation cross at the center of the screen, followed by 160 AX-CPT trials. An AX-CPT trial consisted of two stimuli; a cue letter (“A” or “B”) followed by a probe letter (“X” or “Y”) with a blank inter-stimulus interval (ISI) in between, followed by an inter-trial interval (ITI) where a fixation cross was displayed. Participants used pistol-grips held in each hand and target-responses were made by pressing a button on the right grip and on the left grip for non-target responses. Reaction time (RT) and response were recorded from the onset of the probe to, at maximum, the end of the ITI. Duration of cue and probe was 500 msec. ISI was jittered between 900–1,100 msec with average duration of 1,000 msec and ITI was jittered between 1,500–2,500 msec with average duration of 2,000 msec. The order of the trial types was randomized, with 70% being target trials (AX) and 30% divided equally between non-target trials (AY, BX, BY). Participants got instructions about AX-CPT and practiced prior MRI until they felt sure they understood the task and could perform it with ease.
RT and responses were analyzed separately for each trial type (AX, AY, BX and AY). The proactive behavioral index (PBI) was calculated using the RTs for AY and BX according to the formula (AY-BX)/(AY + BX) (Braver et al., 2009; Gonthier et al., 2016). High PBI was interpreted as a dominance of a proactive cognitive control mode and low PBI as dominance of reactive control mode. The calculation of error rate for the different trial types was based on recorded responses (correct/incorrect) while trials without any responses were omitted.
2.3 Categorical and dimensional stratification
Participants were stratified into categorical and dimensional groups on the basis of presence and absence of ADHD diagnosis according to DSM 5 (categorical stratification), or on the basis of RDoC cognitive control domain (dimensional stratification). For the categorical stratification, the two groups thus consisted of healthy controls and ADHD patients, and for the dimensional stratification of reactive and proactive individuals irrespective of their DSM 5 diagnosis status. The two dimensional groups (reactive vs. proactive) were created by a median split of baseline PBI scores (PBIpre). Participants with PBIpre score less than the median value formed the reactive group and those with PBIpre score equal to or greater than the median value formed the proactive group.
2.4 MRI and preprocessing of fMRI data
A 3.0T MR system (Signa Premier, GE Medical Systems, WI) and a 48-channel head coil were used for all MR examinations, which included a structural image of the brain and an fMRI acquisition during the AX-CPT task. Parameters for the structural scan (3D T1w IR-prepared fast spoiled gradient recalled echo, “BRAVO”) were: TR/TE = 7.3/3.0 msec, acquired voxel size 0.9 × 0.9 × 1.2 mm, parallel imaging acceleration (ARC) factor of 2. The fMRI acquisition used a gradient echo EPI pulse sequence with TR/TE = 2000/35 msec, slice thickness 2.5 mm, acquired pixel size 2.5 × 2.5 mm, no slice gap, ARC factor of 2 and a hyperband factor of 2. Acquired images were converted to nifti-format using dcm2niix (https://github.com/rordenlab/dcm2niix).
Preprocessing of fMRI data (slice-time correction, realigning, unwarping, normalizing to MNI template, smoothing) was performed using CONN (Whitfield-Gabrieli and Nieto-Castanon, 2012). Default settings were applied except for a smoothing kernel of 6 mm instead of 8 mm. The final preprocessing step included anatomical component-based noise correction (aCompCor) based on white matter and cerebrospinal fluid (CSF), scrubbing and bandpass filtering. Further details regarding the preprocessing are provided in the supplement.
2.5 fMRI analysis
First-level analysis of each AX-CPT acquisition (pre and post CS) was performed using a general linear model (GLM) consisting of 4 regressors (AX, AY, BX and BY trials) where the onset and duration for each trial started with the presentation of the cue and lasted until the end of ITI. Group (second-level) analysis was carried out by setting up a full factorial model including beta values (i.e., BOLD signals) from the four different trials (AX, AY, BX and BY), before and after the administration of CS.
Correlations were tested between baseline PBI (PBIpre) and differences in brain activity pre and post CS for the trial types (AY, BX). This was achieved by calculating a difference image (BOLD image post—BOLD image pre) for each trial type and then correlating it using PBIpre as covariate.
Exploratory whole-brain analysis was performed and to correct for multiple comparisons, a pixelwise significance threshold level of p < 0.001 and cluster size threshold of FWE<0.05 were applied. All fMRI analyses were performed using SPM12 running on Matlab R2019b.
Labels in the Harvard-Oxford cortical structural atlas, available in FSLeyes (version 0.34.2, https://git.fmrib.ox.ac.uk/fsl/fsleyes/fsleyes), were used to provide anatomical information about significant clusters.
2.6 Statistical analysis
Behavioral data were analyzed using SPSS (IBM SPSS Statistics version 28.0), and Matlab (R2019b) was used for graphical plots. Participants with PBIpre outside the range [Q1-1.5xIQR, Q3+1.5xIQR] were considered as outliers and their experimental protocol was reviewed for any outstanding issues that may explain the extreme values. (Q1 = 25th percentile, Q3 = 75th percentile, interquartile range (IQR) = Q3-Q1). Parametric tests were applied if the data could be adequately modelled according to the normal distribution, otherwise non-parametric tests were used. Two-sided p-values are reported in all cases.
3 Results
3.1 Study groups and participant data
Of the 65 initial participants, 53 were included in the final analysis, which consisted of 31 healthy controls and 22 patients with ADHD (Figure 1). Five outliers were identified, and their experimental protocol carefully reviewed for any outstanding issue; in all cases circumstances could be identified that explained the extreme values and justified the exclusion of the participants. For the dimensional stratification, there were 26 individuals in the reactive group consisting of 14 healthy controls and 12 ADHD patients and 27 individuals in the proactive group consisting of 17 healthy controls and 10 ADHD patients. There was no significant difference in the number of healthy controls and ADHD patients clustering to the reactive and proactive groups (Pearson chi-square, χ(1) = 0.45, p = 0.50). Baseline and behavioral data for all participants are shown in Table 1.
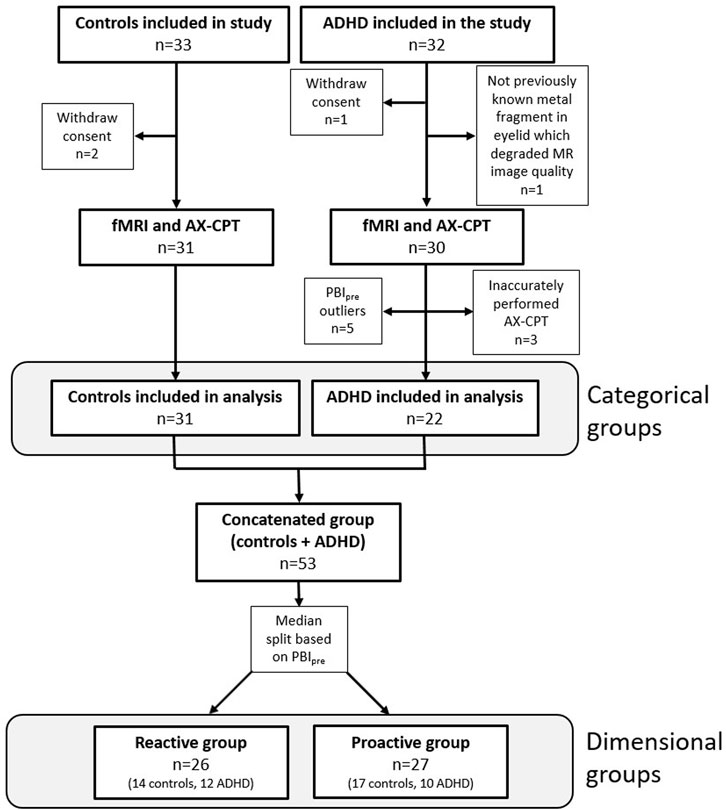
Figure 1. Flowchart showing inclusion and exclusion of participants in the study and the number of participants in the categorical and dimensional groups. Three participants from the ADHD group were excluded since PBI could not be calculated because they inaccurately performed the AX-CPT.
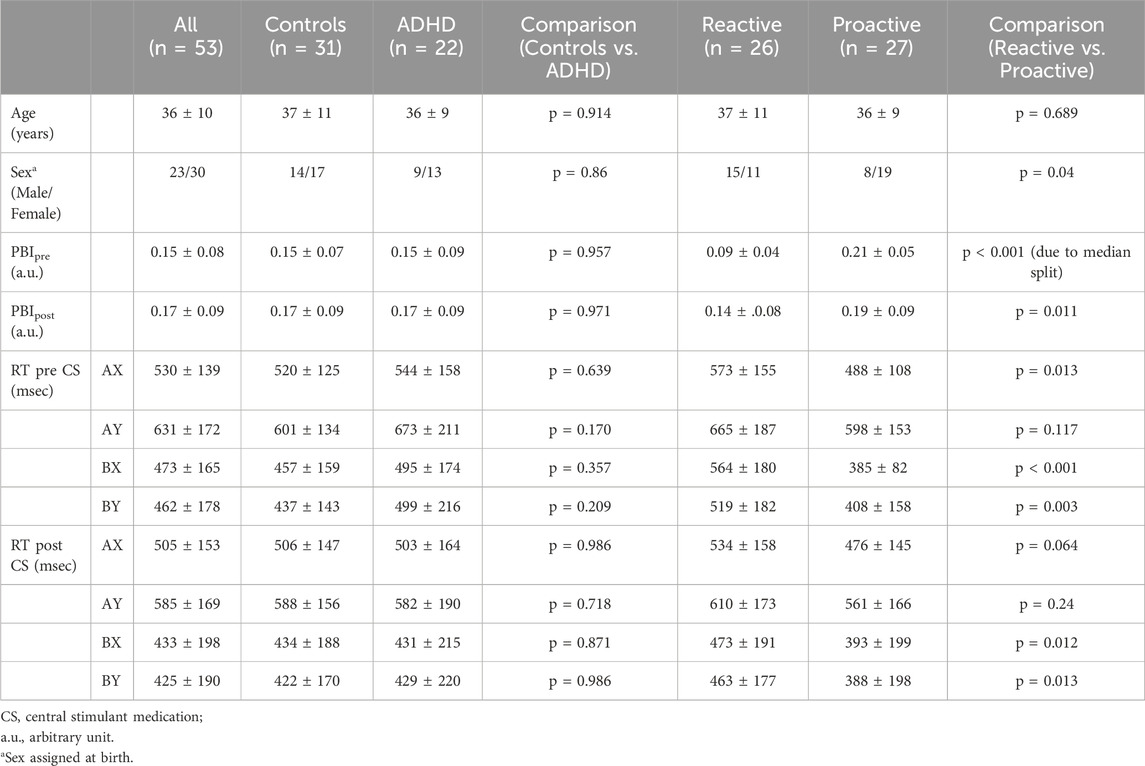
Table 1. Baseline characteristics of study participants in the two stratification groups. Values are presented as mean ± 1 standard deviation. PBI is calculated using RT for AY and BX as PBI = (AY-BX)/(AX + BX).
3.2 Cognitive control paradigm (AX-CPT)–behavioral data
The AX-CPT used in the current study resulted in the expected behavioral features, and as previously reported in the literature AY trials showed the longest reaction time and highest error rate (Table 1; Supplementary Figure S1). For the behavioral data, we analyzed potential differences in reaction time (RT) and proactive behavioral index (PBI) in the categorical and dimensional groups before and after administration of CS by evaluating interaction effects. For the categorical stratification, we found no significant interaction regarding RT or PBI between healthy controls and ADHD patients before and after CS. For the dimensional stratification, however, a 2 × 2 repeated measure ANOVA showed a significant (F (1,51) = 8.777, p = 0.005) interaction for PBI where the reactive group significantly increased its mean PBI (p = 0.006) compared to the proactive group, which did not show any significant change in PBI, although there was a slight decrease in this (Figure 2A). Also, there was a significant pre-post CS interaction in RT for BX [F (1,51) = 4.516, p = 0.038], where the reactive (p = 0.004) but not proactive group significantly decreased its RT for the BX trial (Figure 2B).
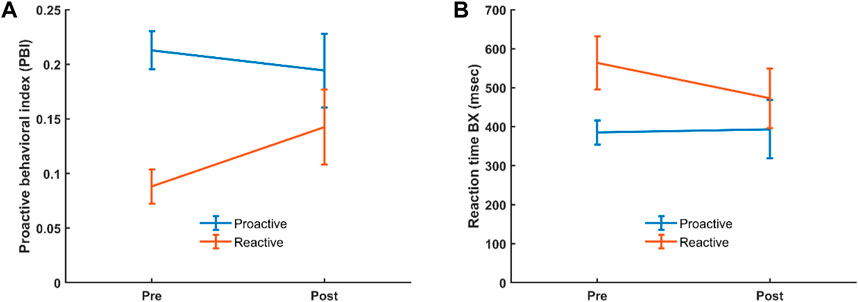
Figure 2. Interaction for PBI (A) and BX RT (B) in the reactive and proactive cognitive control mode groups, before and after administration of CS. Error bars show 95% confidence interval.
3.3 Functional brain activation data
Similar to the behavioral data, we analyzed the fMRI data for interaction effects in order to assess potential brain activation differences between groups in the categorical and dimensional stratifications before and after administration of CS. The AY and BX trials were used as regressors, as they are the basis for calculating PBI and determining reactive and proactive cognitive control mode groups.
There were no significant interaction effects between healthy controls and patients with ADHD before and after administration of CS in the categorical stratification. For the dimensional stratification, however, there was a significant interaction effect for BX. This interaction was noticed in a cluster located in the superior frontal and paracingulate gyri, showing that in these areas the reactive group increased its brain activation more after CS compared to the proactive group. The cluster consisted of 167 voxels (voxel size = 2 × 2 × 2 mm3) with a peak t-statistic coordinate at (−4, 42, 40 mm) in MNI-space (Figure 3).
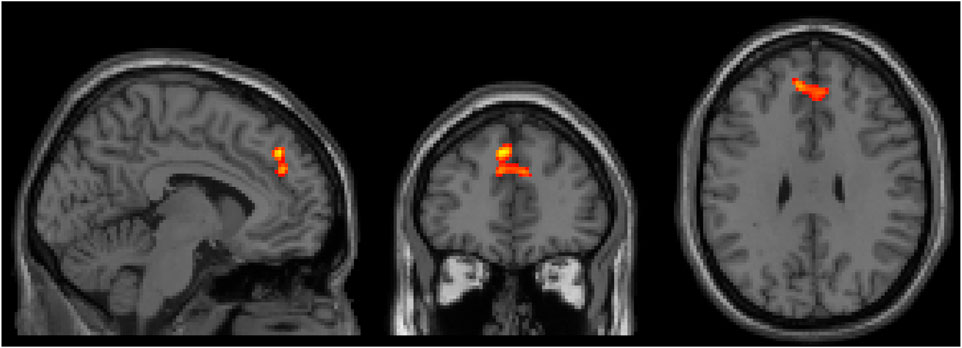
Figure 3. Three orthogonal slices (sagittal, coronal and axial) showing brain area where there was a significant increase of brain activity after CS for the reactive group compared to the proactive group. Images are shown using neurological orientation, i.e., subject left to the left in image.
Correlation between baseline PBI (PBIpre) and changes in brain activation for the AY and BX trials after CS was also evaluated considering all participants as one group (n = 53). There were four significant clusters showing a negative correlation between PBIpre and changes in brain activation after CS for the BX trial (BX1-BX4), and one cluster (BX5) showing a positive correlation (Figure 4). We saw no significant correlations between PBIpre and changes in brain activation after CS for the AY trial. A summary of all displayed clusters shown in Figure 4 is provided in Supplementary Table S1 together with anatomical information about clusters.
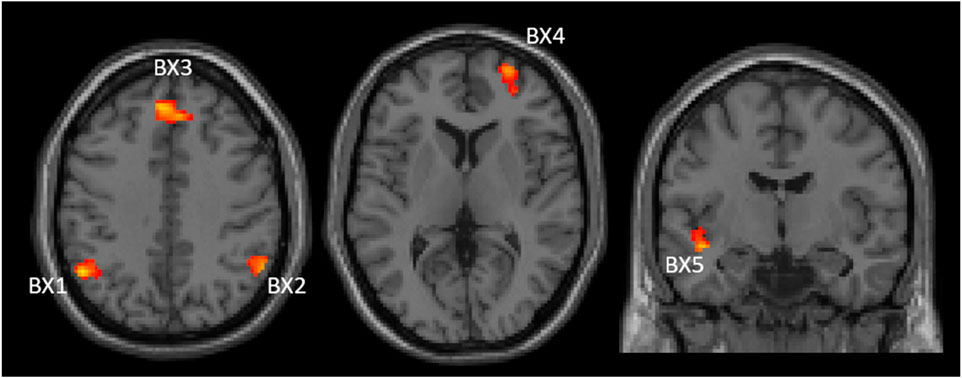
Figure 4. Two axial (left and middle) and one coronal (right) images showing significant clusters correlations between changes in brain activities (post-pre administration of CS) and PBIpre during BX trials. The clusters are labelled (BX1-BX5) to be used for identification in text and Supplementary table S1 Images are shown using neurological orientation.
Figure 5 shows the correlations between PBIpre and BX1-BX5 activation clusters. BX3 is virtually the same cluster obtained when comparing CS effects between the reactive and proactive groups in the dimensional stratification (Figure 3). The negative correlation indicates that reactive individuals increase brain activation in these areas after CS compared to proactive individuals, who show a decrease in brain activation after CS. For illustrative purposes, the individuals with largest increase in PBI after CS (defined as greater than the 75th percentile) are indicated in Figure 5. The majority of these individuals (11 out of 13) belong to the reactive group and most have an increase in brain activation after CS in clusters BX1-BX4 (BX1: 8/13, BX2: 8/13, BX3: 7/13, BX4: 10/13). In cluster BX5 the majority (8/13) decreased brain activity after CS. The regression lines in Figure 5 cross the x-axis at PBIpre equal to 0.13/0.15/0.14/0.16/0.13 for clusters BX1-BX5, respectively.
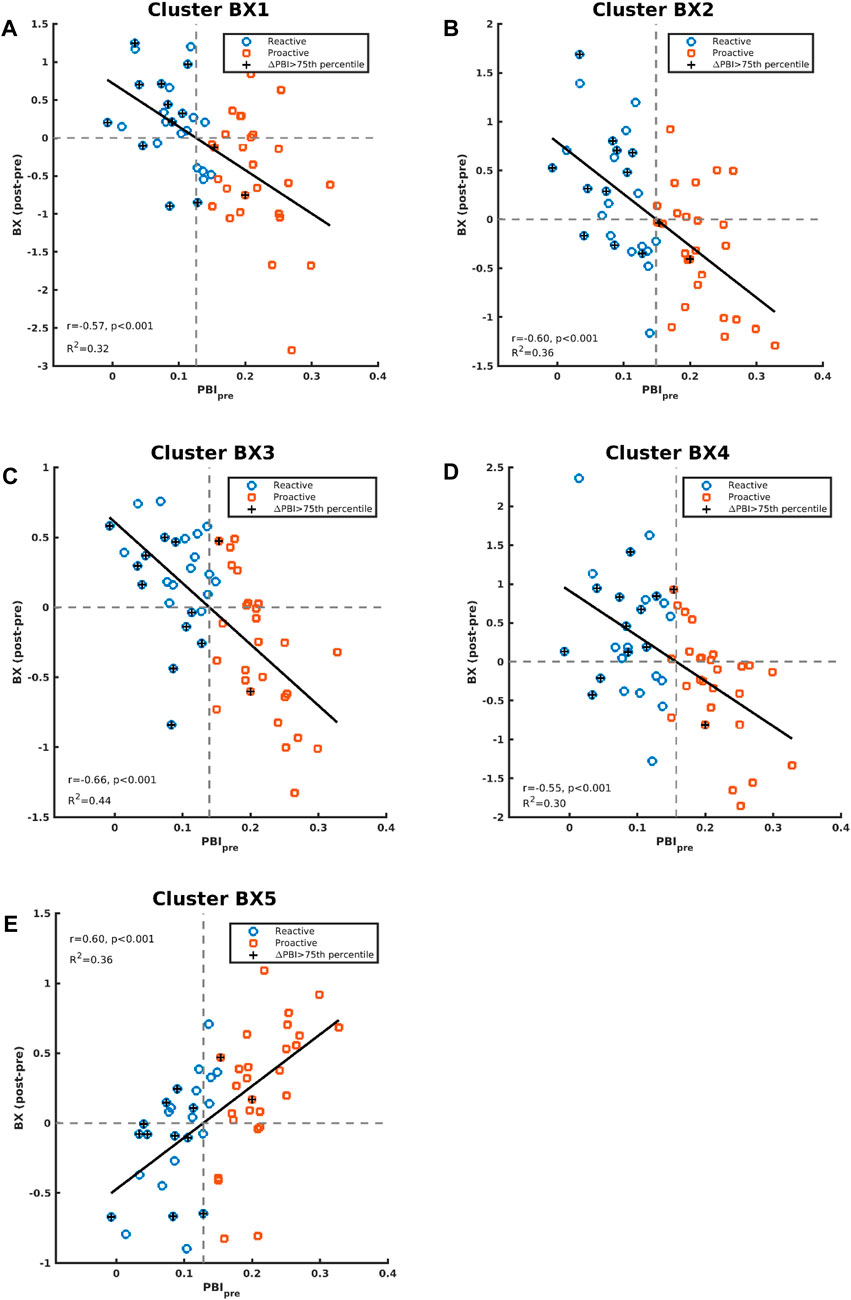
Figure 5. Correlations between contrast differences for BX (post-pre administration of CS) and PBIpre are shown in panels (A–E) for cluster BX1-BX5, respectively. The individuals in the whole study who had an increase of PBI (PBIpost vs PBIpre) greater than the 75th percentile are indicated with “+”.
4 Discussion
Psychiatric disorders are in almost all instances complex syndromes with as yet not fully established etiopathology (Insel, 2010). Diagnostic classification in clinical settings is guided by the presence and absence of characteristic phenomenological criteria that in themselves remain agnostic as to the neurobiological underpinning of the disorders (American Psychiatric Association, 2013). Lately, concern has been raised pertaining to a putative lack of reproducibility in the psychiatry/psychology research fields (Open Science Collaboration, 2015). Diagnostic heterogeneity has been postulated as one possible reason behind this (Borgogna et al., 2023). The utility of these classification systems for research purposes has thus been questioned, among other things, due to the substantial within group heterogeneity between group overlap the current categorical classification systems lead to (Widiger and Samuel, 2005; Wardenaar and de Jonge, 2013; Borgogna et al., 2023). The research domain criteria initiative (RDoC), on the other hand, although not a diagnostic tool per se, provides a neurobiologically informed dimensional framework for conceptualizing psychiatric disorders by utilizing neurobehavioral domains of function (Insel et al., 2010). Thus, dimensional stratification by reducing within group heterogeneity (Borgogna et al., 2023) may contribute to the standardization of a study population in a such a way that intervention, control and outcome (PICO) may become reproducible when similar PICOs are studied in different research facilities. The RDoC domains that are thought to be most relevant for ADHD are cognitive control and positive valence domains, pertaining to inattention and hyperactivity/impulsivity aspects of the disorder, respectively. Paradigms that evaluate these domains include the AX Continuous Performance Task (AX-CPT) for cognitive control domains such as goal selection, goal maintenance and updating (Lopez-Garcia et al., 2016), the Go NoGo task for response selection and response inhibition (Boucher et al., 2007), and various incentive delay tasks for positive valence domains such as reward anticipation (Knutson et al., 2000).
In this study, neural and behavioral effects of CS medication during a cognitive control task (AX-CPT) were investigated using two classification strategies to stratify study participants, (i) categorical stratification using DSM 5 diagnosis and (ii) dimensional stratification using RDoC cognitive control domain irrespective of diagnosis status. Median split of proactive behavioral index (PBI) calculated from AY and BX trials of the AX-CPT (Gonthier et al., 2016) was used to divided participants into reactive and proactive groups for the dimensional stratification without paying attention to diagnosis. Based on previous studies that found the effect of CS on cognitive control to vary with baseline cognitive capacity (Mattay et al., 2000; van der Schaaf et al., 2013; Rostami Kandroodi et al., 2021), we hypothesized that dimensional stratification may be superior to categorical stratification in revealing neural and behavioral effects of CS. Also, in the categorical stratification, we expected the effects of CS to be more prominent in ADHD patients compared to healthy controls, based on previous findings that showed a robust clinical effect of CS in ADHD patients (Faraone and Glatt, 2010; Cortese et al., 2018) and a less clear-cut cognitive enhancing effects in the non-clinical population (Repantis et al., 2010; Smith and Farah, 2011).
There was, of course, the possibility that the two stratification methods would result in largely similar individuals clustering in the same groups, in a way that would undermine our study design, i.e., that most ADHD patients would cluster into the reactive group and most healthy controls into the proactive group and that there would be no meaningful difference between the two stratification methods. Contrary to our initial apprehension, however, we found an almost 50/50 clustering of patients and healthy controls into the two dimensional groups, with the median split resulting in 14 healthy controls (45.2%) and 12 ADHD patients (54.5%) clustering in the reactive group and 17 healthy individuals (54.8%) and 10 ADHD patients (45.5%) clustering in the proactive group, with no significant difference in this between healthy controls and ADHD patients. The fact that cognitive control mode cuts across diagnosis indicates that ADHD patients may not markedly differ from healthy controls in this respect, unlike, for example, psychosis patients who have been shown to employ a markedly reactive cognitive control mode compared to healthy individuals (MacDonald, 2008).
Once it was ascertained that the stratification method gave variable clustering of individuals into the categorical and dimensional groups, we went ahead to systematically evaluate the effects of CS on the groups within the two stratification strategies. When participants were categorically assigned as healthy controls and ADHD patients, on the basis of DSM 5 diagnosis, we found no significant behavioral or brain activation effects of CS in these two groups. On the other hand, when study participants were stratified along dimensional factors into reactive and proactive cognitive control mode groups ignoring diagnosis status, CS selectively increased activation in paracingulate and superior frontal gyri in the reactive group compared to the proactive group and induced a shift towards proactive control mode in the reactive group without significantly affecting the proactive group. The shift towards proactive control mode in the reactive group was mediated by a decrease in reaction time for BX trials, without significant concomitant effects on AY trials. Besides these direct and selective effects of CS on brain activation and proactive behavioral index (PBI), we also found a significant correlation between baseline PBI and the neural effects of CS. In 4 out of the 5 active frontoparietal clusters for the BX contrast, lower baseline PBI was associated with greater post CS increase in brain activation. Clusters in frontal (frontal pole, bilateral paracingulate, right superior frontal gyrus) and parietal (bilateral angular and left supramarginal gyri) brain areas all showed increased brain activation after CS that was negatively correlated with baseline PBI scores. One left-lateralized temporo-insular cluster consisting of left insular cortex and left planum polare showed CS induced activation changes that were positively correlated to baseline PBI, i.e., the post-CS increase in these two areas was greater the higher baseline PBI score was.
Our results show that the neural and behavioral effects of CS were more clear-cut when participants were stratified along dimensional factors than diagnostic categories. In a recent study that included ADHD patients who responded and did not respond to CS treatment, we reported that saliency (“wanting”) and hedonic experience (“liking”), pertaining to the positive valence RDoC domain, could predict response to CS treatment and that that the scores for “wanting” were positively correlated to resting state connectivity increase in the ventral striatum (Rode et al., 2023). Although all included patients in the present study were clinical responders to CS treatment as evaluated by clinician- and patient-rated clinical global impression–improvement (CGI-I), our results from this study indicate that cognitive control mode might also be a potential predictor of CS response in clinical the setting. In a more recent paper Hung et al., 2024 reported that striatal structural connectivity and higher pre-treatment working memory scores were correlated with greater response to CS medication in patients with ADHD (Hung et al., 2024).
As mentioned above, the effect of CS on behavioral or neural outcomes varies with baseline cognitive capacity (Mattay et al., 2000; van der Schaaf et al., 2013; Rostami Kandroodi et al., 2021), baseline DA and NA levels (Cools and D'Esposito, 2011) and rate of behavioral, physical or electrical stimulation (Sanger and Blackman, 1976). Because the proactive group had substantially lower mean RT scores for, among other things, BX and BY trials, if the proactive group were to be allowed to respond at a higher rate, this could potentially affect the results due to the rate dependency of CS effect (Sanger and Blackman, 1976). However, in the present paper, the rate of stimulus (interstimulus interval) across all subjects and conditions was constant. Thus, even though a participant had a shorter RT, this was not allowed to alter the rate of response. Furthermore, due to the low mean RT scores in the proactive group, their response to CS as far as RT is concerned could be restricted due to ceiling effects. However, when we looked at baseline RT scores across all participants, we found that both proactive and reactive individuals had baseline RT scores that ranged from low to high and did not cluster around any particular value in a way that could have imposed significant ceiling effects.
A plausible clinical implication based on the results in the current study might be that ADHD patients employing reactive cognitive control mode, as well as healthy controls who might use CS off-label, would respond better to CS than those employing a more proactive control mode. This assumes that a transition from reactive to proactive control mode is equated to being a responder of CS. Further studies are needed to elucidate this and should include groups of both CS responding and CS non-responding ADHD patients.
The lack of effect of CS in unstratified ADHD patients and unstratified healthy controls, we suspect, might be due to substantial within group heterogeneity and the known inverted U-form dose-effect curve of the signal substances dopamine and noradrenaline whose peri-synaptic levels are enhanced by CS medication. Similar lack of effect was previously reported in unstratified study participants (Cools et al., 2008), and the CS effects could be revealed when participants were stratified along baseline cognitive capacity (Cools et al., 2008) or baseline dopamine synthesis capacity (Westbrook et al., 2020).
Our implementation of the AX-CPT paradigm in this study corresponds to a low load task (Mäki-Marttunen, Hagen, and Espeseth, 2019), since it only consisted of two cue letters (“A” and “B”) and two probes (“X” and “Y”) and that the intra-trial interval was short. The behavioral and neural effects of the task can be expected to be larger when using a more challenging version with more cues and probes and longer intra-trial interval. However, we found such versions too difficult for some of the ADHD patients that could have led to an even greater loss of included ADHD participants, which occurred even when using this easier version (see Figure 1).
4.1 Limitations
In this study we included only ADHD patients that have clinically been judged to be responders to CS treatment. It is theoretically possible that the results may have been different had we also included ADHD patients who were CS non-responders. Another intriguing question is why ADHD patients who have clinically responded to CS medication become “non-responders” when tested with a cognitive control paradigm, if they happen to employ proactive cognitive control mode. One possible explanation for this is suggested by the recent finding of (Bowman et al., 2023), who in healthy subjects found that CS increased motivation/effort but reduced quality of effort, which suggests that CS responding ADHD patients might improve on certain but not all aspects of their impairment.
5 Conclusion
We can draw several conclusions from the current study, (i) cognitive control mode cuts across diagnostic categories and there is equal likelihood for ADHD patients to employ reactive and proactive cognitive control mode as healthy controls, (ii) dimensional stratification under our experimental condition seems to be superior to categorical stratification in revealing neural and behavioral effects of CS, and (iii) baseline cognitive control mode might potentially be a predictor of CS treatment effect in the clinical setting.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the Swedish Ethical Review Authority (EPM). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
PT: Conceptualization, Data curation, Formal Analysis, Methodology, Project administration, Software, Writing–original draft, Writing–review and editing. MR: Formal Analysis, Methodology, Writing–original draft, Writing–review and editing. JR: Formal Analysis, Methodology, Writing–original draft, Writing–review and editing. MM: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Validation, Writing–original draft, Writing–review and editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. Nyckelfonden, Örebro, Sweden (OLL 935421), ALF Grants, Region Örebro län, Sweden (OLL 973230).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2024.1412178/full#supplementary-material
References
American Psychiatric Association (2013). Diagnostic and statistical manual of mental disorders. 5th ed. American Psychiatric Association. doi:10.1176/appi.books.9780890425596
Arnsten, A. F., and Dudley, A. G. (2005). 'Methylphenidate improves prefrontal cortical cognitive function through alpha2 adrenoceptor and dopamine D1 receptor actions: relevance to therapeutic effects in Attention Deficit Hyperactivity Disorder. Behav. Brain Funct. 1, 2. doi:10.1186/1744-9081-1-2
Barch, D. M., Carter, C. S., MacDonald, A. W., Braver, T. S., and Cohen, J. D. (2003). 'Context-processing deficits in schizophrenia: diagnostic specificity, 4-week course, and relationships to clinical symptoms. J. Abnorm Psychol. 112, 132–143. doi:10.1037/0021-843x.112.1.132
Berridge, C. W., Devilbiss, D. M., Andrzejewski, M. E., Arnsten, A. F., Kelley, A. E., Schmeichel, B., et al. (2006). Methylphenidate preferentially increases catecholamine neurotransmission within the prefrontal cortex at low doses that enhance cognitive function. Biol. Psychiatry 60, 1111–1120. doi:10.1016/j.biopsych.2006.04.022
Borgogna, N. C., Owen, T., and Aita, S. L. (2023). The absurdity of the latent disease model in mental health: 10,130,814 ways to have a DSM-5-TR psychological disorder. J. Ment. Health, 1–9. doi:10.1080/09638237.2023.2278107
Boucher, L., Palmeri, T. J., Logan, G. D., and Schall, J. D. (2007). Inhibitory control in mind and brain: an interactive race model of countermanding saccades. Psychol. Rev. 114, 376–397. doi:10.1037/0033-295X.114.2.376
Bowman, E., Coghill, D., Murawski, C., and Bossaerts, P. (2023). 'Not so smart? "Smart" drugs increase the level but decrease the quality of cognitive effort. Sci. Adv. 9, eadd4165. doi:10.1126/sciadv.add4165
Braver, T. S. (2012). The variable nature of cognitive control: a dual mechanisms framework. Trends Cogn. Sci. 16, 106–113. doi:10.1016/j.tics.2011.12.010
Braver, T. S., Paxton, J. L., Locke, H. S., and Barch, D. M. (2009). Flexible neural mechanisms of cognitive control within human prefrontal cortex. Proc. Natl. Acad. Sci. U. S. A. 106, 7351–7356. doi:10.1073/pnas.0808187106
Cools, R., and D'Esposito, M. (2011). Inverted-U-shaped dopamine actions on human working memory and cognitive control. Biol. Psychiatry 69, e113–e125. doi:10.1016/j.biopsych.2011.03.028
Cools, R., Gibbs, S. E., Miyakawa, A., Jagust, W., and D'Esposito, M. (2008). 'Working memory capacity predicts dopamine synthesis capacity in the human striatum. J. Neurosci. 28, 1208–1212. doi:10.1523/JNEUROSCI.4475-07.2008
Cortese, S., Adamo, N., Giovane, C. D., Mohr-Jensen, C., Hayes, A. J., Carucci, S., et al. (2018). Comparative efficacy and tolerability of medications for attention-deficit hyperactivity disorder in children, adolescents, and adults: a systematic review and network meta-analysis. Lancet. Psychiatry 5, 727–738. doi:10.1016/S2215-0366(18)30269-4
Cuthbert, B. N. (2022). Research domain criteria (RDoC): progress and potential. Curr. Dir. Psychol. Sci. 31, 107–114. doi:10.1177/09637214211051363
Faraone, S. V., Banaschewski, T., Coghill, D., Zheng, Y., Biederman, J., Bellgrove, M. A., et al. (2021). The world federation of ADHD international consensus statement: 208 evidence-based conclusions about the disorder. Neurosci. Biobehav. Rev. 128, 789–818. doi:10.1016/j.neubiorev.2021.01.022
Faraone, S. V., and Glatt, S. J. (2010). A comparison of the efficacy of medications for adult attention-deficit/hyperactivity disorder using meta-analysis of effect sizes. J. Clin. Psychiatry 71, 754–763. doi:10.4088/JCP.08m04902pur
Friedman, N. P., and Robbins, T. W. (2022). The role of prefrontal cortex in cognitive control and executive function. Neuropsychopharmacology 47, 72–89. doi:10.1038/s41386-021-01132-0
Gonthier, C., Macnamara, B. N., Chow, M., Conway, A. R., and Braver, T. S. (2016). 'Inducing proactive control shifts in the AX-CPT. Front. Psychol. 7, 1822. doi:10.3389/fpsyg.2016.01822
Hung, Y., Green, A., Kelberman, C., Gaillard, S., Capella, J., Rudberg, N., et al. (2024). Neural and cognitive predictors of stimulant treatment efficacy in medication-naive ADHD adults: a pilot diffusion tensor imaging study. J. Atten. Disord. 28, 936–944. doi:10.1177/10870547231222261
Insel, T., Cuthbert, B., Garvey, M., Heinssen, R., Pine, D. S., Quinn, K., et al. (2010). Research domain criteria (RDoC): toward a new classification framework for research on mental disorders. Am. J. Psychiatry 167, 748–751. doi:10.1176/appi.ajp.2010.09091379
Knutson, B., Westdorp, A., Kaiser, E., and Hommer, D. (2000). FMRI visualization of brain activity during a monetary incentive delay task. Neuroimage 12, 20–27. doi:10.1006/nimg.2000.0593
Läkemedelsverket (2016). Läkemedel vid adhd - behandlingsrekommendation. Inf. från Läkemedelsv. 27, 13–24.
Lopez-Garcia, P., Lesh, T. A., Salo, T., Barch, D. M., MacDonald, A. W., Gold, J. M., et al. (2016). The neural circuitry supporting goal maintenance during cognitive control: a comparison of expectancy AX-CPT and dot probe expectancy paradigms. Cogn. Affect Behav. Neurosci. 16, 164–175. doi:10.3758/s13415-015-0384-1
MacDonald, A. W. (2008). Building a clinically relevant cognitive task: case study of the AX paradigm. Schizophr. Bull. 34, 619–628. doi:10.1093/schbul/sbn038
MacDonald, A. W., and Carter, C. S. (2003). Event-related FMRI study of context processing in dorsolateral prefrontal cortex of patients with schizophrenia. J. Abnorm Psychol. 112, 689–697. doi:10.1037/0021-843X.112.4.689
Mäki-Marttunen, V., Hagen, T., and Espeseth, T. (2019). Task context load induces reactive cognitive control: an fMRI study on cortical and brain stem activity. Cogn. Affect Behav. Neurosci. 19, 945–965. doi:10.3758/s13415-019-00691-6
Marraccini, M. E., Weyandt, L. L., Rossi, J. S., and Gudmundsdottir, B. G. (2016). 'Neurocognitive enhancement or impairment? A systematic meta-analysis of prescription stimulant effects on processing speed, decision-making, planning, and cognitive perseveration. Exp. Clin. Psychopharmacol. 24, 269–284. doi:10.1037/pha0000079
Mattay, V. S., Callicott, J. H., Bertolino, A., Heaton, I., Frank, J. A., Coppola, R., et al. (2000). Effects of dextroamphetamine on cognitive performance and cortical activation. Neuroimage 12, 268–275. doi:10.1006/nimg.2000.0610
Miller, E. K., and Cohen, J. D. (2001). 'An integrative theory of prefrontal cortex function. Annu. Rev. Neurosci. 24, 167–202. doi:10.1146/annurev.neuro.24.1.167
Open Science Collaboration (2015). PSYCHOLOGY. Estimating the reproducibility of psychological science. Science 349 (6251), aac4716. PMID: 26315443. doi:10.1126/science.aac4716
Repantis, D., Schlattmann, P., Laisney, O., and Heuser, I. (2010). Modafinil and methylphenidate for neuroenhancement in healthy individuals: a systematic review. Pharmacol. Res. 62, 187–206. doi:10.1016/j.phrs.2010.04.002
Retz, W., Stieglitz, R. D., Corbisiero, S., Retz-Junginger, P., and Rosler, M. (2012). Emotional dysregulation in adult ADHD: what is the empirical evidence? Expert Rev. Neurother. 12, 1241–1251. doi:10.1586/ern.12.109
Roberts, C. A., Jones, A., Sumnall, H., Gage, S. H., and Montgomery, C. (2020). How effective are pharmaceuticals for cognitive enhancement in healthy adults? A series of meta-analyses of cognitive performance during acute administration of modafinil, methylphenidate and D-amphetamine. Eur. Neuropsychopharmacol. 38, 40–62. doi:10.1016/j.euroneuro.2020.07.002
Rode, J., Runnamo, R., Thunberg, P., and Msghina, M. (2023). Salience and hedonic experience as predictors of central stimulant treatment response in ADHD - a resting state fMRI study. J. Psychiatric Res. 163, 378–385. doi:10.1016/j.jpsychires.2023.05.073
Rostami Kandroodi, M., Cook, J. L., Swart, J. C., Frobose, M. I., Geurts, D. E. M., Vahabie, A. H., et al. (2021). Effects of methylphenidate on reinforcement learning depend on working memory capacity. Psychopharmacol. Berl. 238, 3569–3584. doi:10.1007/s00213-021-05974-w
Sanger, D. J., and Blackman, D. E. (1976). 'Rate-dependent effects of drugs: a review of the literature. Pharmacol. Biochem. Behav. 4, 73–83. doi:10.1016/0091-3057(76)90178-7
Schmid, P. C., Kleiman, T., and Amodio, D. M. (2015). Neural mechanisms of proactive and reactive cognitive control in social anxiety. Cortex 70, 137–145. doi:10.1016/j.cortex.2015.05.030
Shaw, P., Stringaris, A., Nigg, J., and Leibenluft, E. (2014). Emotion dysregulation in attention deficit hyperactivity disorder. Am. J. Psychiatry 171, 276–293. doi:10.1176/appi.ajp.2013.13070966
Smith, M. E., and Farah, M. J. (2011). Are prescription stimulants "smart pills"? The epidemiology and cognitive neuroscience of prescription stimulant use by normal healthy individuals. Psychol. Bull. 137, 717–741. doi:10.1037/a0023825
Spencer, T., Biederman, J., Wilens, T., Doyle, R., Craig, S., Prince, J., et al. (2005). 'A large, double-blind, randomized clinical trial of methylphenidate in the treatment of adults with attention-deficit/hyperactivity disorder. Biol. Psychiatry 57, 456–463. doi:10.1016/j.biopsych.2004.11.043
van der Schaaf, M. E., Fallon, S. J., Ter Huurne, N., Buitelaar, J., and Cools, R. (2013). 'Working memory capacity predicts effects of methylphenidate on reversal learning. Neuropsychopharmacology 38, 2011–2018. doi:10.1038/npp.2013.100
Volkow, N. D., Wang, G., Fowler, J. S., Logan, J., Gerasimov, M., Maynard, L., et al. (2001). Therapeutic doses of oral methylphenidate significantly increase extracellular dopamine in the human brain. J. Neurosci. 21, RC121. doi:10.1523/JNEUROSCI.21-02-j0001.2001
Wardenaar, K. J., and de Jonge, P. (2013). Diagnostic heterogeneity in psychiatry: towards an empirical solution. BMC Med. 11, 201. doi:10.1186/1741-7015-11-201
Westbrook, A., van den Bosch, R., Maatta, J. I., Hofmans, L., Papadopetraki, D., Cools, R., et al. (2020). 'Dopamine promotes cognitive effort by biasing the benefits versus costs of cognitive work. Science 367, 1362–1366. doi:10.1126/science.aaz5891
Whitfield-Gabrieli, S., and Nieto-Castanon, A. (2012). Conn: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect. 2, 125–141. doi:10.1089/brain.2012.0073
Widiger, T. A., and Samuel, D. B. (2005). Diagnostic categories or dimensions? A question for the diagnostic and statistical manual of mental disorders--fifth edition. J. Abnorm Psychol. 114, 494–504. doi:10.1037/0021-843X.114.4.494
Keywords: cognitive control, central stimulants, ADHD, AX-CPT, proactive behavioral index
Citation: Thunberg P, Reingardt M, Rode J and Msghina M (2024) Categorical and dimensional aspects of stimulant medication effects in adult patients with ADHD and healthy controls. Front. Pharmacol. 15:1412178. doi: 10.3389/fphar.2024.1412178
Received: 04 April 2024; Accepted: 24 June 2024;
Published: 10 July 2024.
Edited by:
Christina Dalla, National and Kapodistrian University of Athens, GreeceReviewed by:
Pilar Flores, University of Almeria, SpainStefania Schiavone, University of Foggia, Italy
Copyright © 2024 Thunberg, Reingardt, Rode and Msghina. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mussie Msghina, bXVzc2llLm1zZ2hpbmFAb3J1LnNl
 Per Thunberg
Per Thunberg Maria Reingardt
Maria Reingardt Julia Rode
Julia Rode Mussie Msghina
Mussie Msghina