- 1Center of Encephalopathy, The First Affiliated Hospital of Henan University of Chinese Medicine, Zhengzhou, Henan, China
- 2First Clinical Medical College, Henan University of Chinese Medicine, Zhengzhou, Henan, China
Background: Intracerebral haemorrhage (ICH) is the deadliest subtype of stroke. Surgery remains a vital measure for life-saving in emergency situations, however, the recovery of post-operative patients is not optimistic. This study aimed to evaluate the evidence of the efficacy and safety of Xingnaojing injection (XNJ) for post-operative patients of ICH.
Methods: From inception to 31 January 2024, we searched eight representative databases for randomized controlled trials on post-operative patients of ICH treated with XNJ. A meta-analysis was conducted using R4.2.2, and the quality of the evidence was evaluated by GRADE criteria.
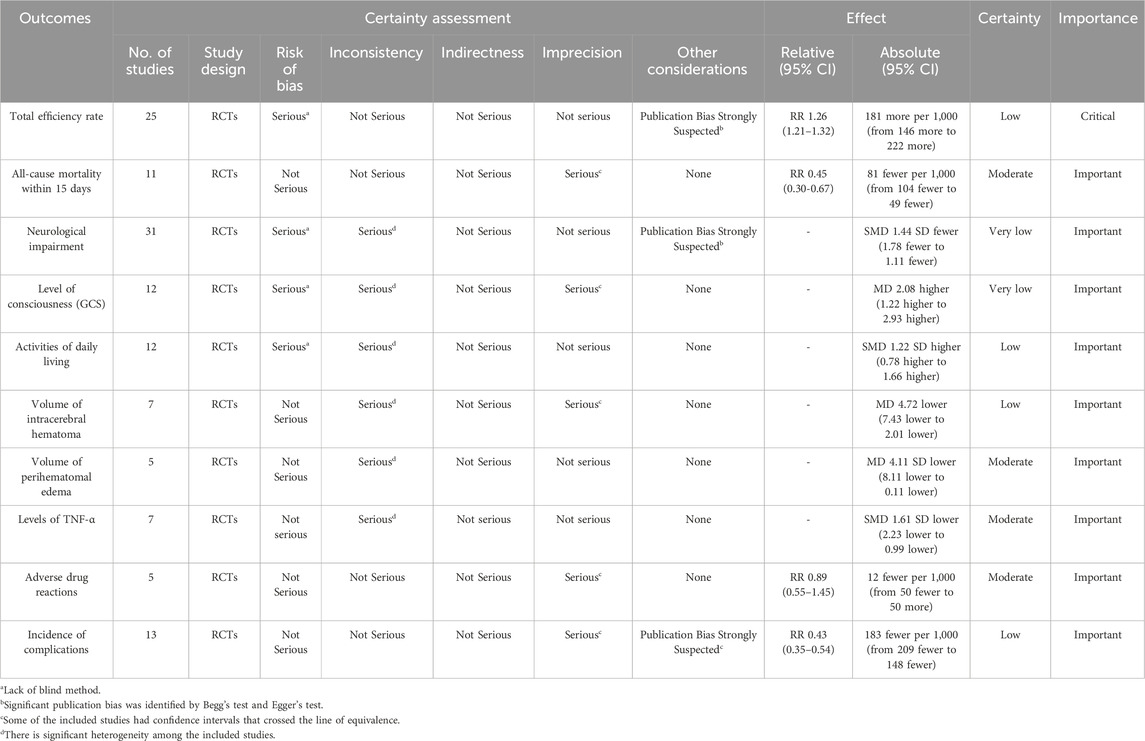
Results: The results indicated that the combination of XNJ with conventional western medicine therapy improved the total efficiency rate (RR = 1.26; 95% CI [1.21 to 1.32]; p < 0.0001), reduced the all-cause mortality within 15 days (RR = 0.45; 95% CI [0.30 to 0.67]; p < 0.0001), decreased the volume of hematoma (MD = −4.72; 95% CI [-7.43 to −2.01]; p = 0.0006) and perihematomal edema (MD = −4.11; 95% CI [-8.11 to −0.11]; p = 0.0441), reduced the TNF-α levels (SMD = −1.61, 95% CI [−2.23 to −0.99], p < 0.0001), decreased neurological impairment (SMD = −1.44; 95% CI [-1.78 to −1.11]; p < 0.0001), improved the activities of daily living (SMD = 1.22; 95% CI [0.78 to 1.66]; p < 0.0001), and enhanced the consciousness level (MD = 2.08, 95% CI [1.22 to 2.93], p < 0.0001). In addition, the complications of the combination therapy group were lower (RR = 0.43; 95% CI [0.35 to 0.54]; p < 0.0001) and the adverse drug reactions were comparable to the control group (RR = 0.89; 95% CI [0.55 to 1.45]; p = 0.6521). The trial sequential analysis results showed that the sample size is sufficient.
Conclusion: Current evidence indicates that XNJ can enhance the efficiency, reduce mortality, and lower the incidence of complications, while demonstrating good tolerability of post-operative patients of ICH. However, the level of evidence from existing studies is relatively weak, and only prove short-term effects, and high-quality RCTs are needed to further verify the accuracy of these conclusions.Systematic Review Registration: identifier (PROSPERO 2024 CRD42024503006). https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42024503006, Identifier CRD42024503006.
1 Introduction
Intracerebral haemorrhage (ICH) refers to non-traumatic spontaneous intracerebral haemorrhage (NBCMA and CDGNBCMA, 2019), which falls under the category of hemorrhagic stroke in Traditional Chinese Medicine (TCM) (Wang and Gao, 2008). China has the highest number of stroke cases in the world (Wang L. D. et al., 2022). Data from the Global burden of disease study (GBD, 2019 Diseases and Injuries Collaborators, 2020) indicates that stroke is the leading cause of death and disability among adults in China, with an ICH prevalence rate of 306 per 100,000 in 2019. Data from 1,672 public tertiary hospitals in the hospital quality monitoring system showed that ICH cases accounted for 14.2% of all stroke cases admitted for treatment in China in 2019 (Wang Y. J. et al., 2022). It is evident that ICH poses a serious threat to public health and impacts economic and social development. Currently, the primary treatment for ICH still focuses on symptomatic relief (Tang et al., 2018). Surgery may improve neurological recovery after ICH, but the presence of perihematomal edema post-surgery and potential secondary damage caused by the operation may limit its therapeutic effectiveness (Thompson et al., 2015).
Excitingly, TCM has accumulated a wealth of clinical experience in the treatment of ICH. With the approval of the National Medical Products Administration of China, Xingnaojing injection (XNJ), a derivative of Angong Niuhuang pill which have been used clinically for over 200 years, is a representative injectable drug in TCM used for the treatment of stroke, and it possesses the effects of clearing heat and detoxifying, cooling the blood and promoting circulation, as well as consciousness-restoring (Deng et al., 2010; Yue et al., 2019), Its formulation of Chinese botanical drugs comprises Dryobalanops aromatica C.F.Gaertn [Dipterocarpaceae; Borneolum], Curcuma aromatica Salisb. [Zingiberaceae; Curcumae Radix], Gardenia jasminoides J. Ellis [Rubiaceae; Gardeniae Fructus], Moschus berezovskii Flerov, M. sifanicus Przewalski, or M. moschiferus Linnaeus [Cervidae; Moschus], and it is produced using steam distillation to extract the water-soluble or volatile metabolites from the botanical drugs conveniently and effectively, resulting in an intravenous injection (Deng et al., 2010; Yue et al., 2019).
In China, XNJ is produced by three pharmaceutical companies (Wuxi Jiyu Shanhe Pharmaceutical Co., Ltd, Henan Tiandi Pharmaceutical Co., Ltd, and Dali Pharmaceutical Co., Ltd). The production processes and quality standards of all the three companies adhere to the National Drug Standards WS3-B-3353-98-2003 of China. In the preparation process, 30 g of Curcumae Radix and 30 g of Gardeniae Fructus are initially distilled with 1,500 mL of water, yielding 1,000 mL of distillate; subsequently, 7.5 g of Moschus and 250 mL of distilled water are introduced to the aforementioned distillate, followed by the collection of another 1,000 mL of distillate for later use. Next, 1 g of Borneolum and 8 g of polysorbate 80 are pulverized and combined with the distillate. Finally, 8 g of sodium chloride is incorporated, and the mixture is stirred, blended, left to settle overnight in a refrigerated environment, filtered, transferred to containers, and sterilized. Regarding the identified active components, borneol, which is traditionally utilized to monitor the quality of XNJ, should meet a minimum concentration of 0.7 g/L as stipulated by the drug standards set forth by the National Medical Products Administration of China (Pharmacopoeia Commission of the Ministry of Public Health of the People’s Republic of China, 1998; China Food and Drug Administration, 2003). Moreover, by using gas chromatography–mass spectrometry (GC-MS), high performance liquid chromatography (HPLC), network pharmacology, and molecular docking technology, researchers recently found that the representative active metabolites of XNJ also include muscone, camphor, eucarvone, isophorone, 4-methylene-isophorone, curcumenone, curcumenol, curdione, curzerenone, furanodienone, curcumol, germacrone, geniposide, etc. (Yang et al., 2016; Fang et al., 2017; Huang et al., 2017; Wu et al., 2021). A previous study has analyzed the 27 possible metabolites of XNJ, and found that among them, the camphor, borneol, and muscone account for more than 85% of the peak area of GC-MS (Zhang et al., 2004).
Numerous systematic reviews and meta-analyses have demonstrated the efficacy and safety of XNJ in the treatment of acute ICH (Peng et al., 2014; Wu et al., 2016; Yu et al., 2016; Xu et al., 2018; Ma et al., 2020; Wang et al., 2021). Many guidelines and consensus in China also recommend the use of XNJ for the emergency treatment of ICH (Gao, 2016; Ni et al., 2020; Gao and Zhao, 2023), but did not specify the recommendations and treatment advantages of XNJ application after the surgery of ICH. The results of one meta-analysis showed that for patients after ICH surgery, the addition of proprietary Chinese patent medicine (Naoxueshu oral liquid) had better clinical efficacy (Yu et al., 2023). Another network meta-analysis (Ren et al., 2022) found that compared with ICH patients who underwent surgery plus conventional western medicine (CWM) treatment, the addition of Chinese herbal injections on this basis could increase the total efficiency rate, lower National Institutes of Health Stroke Scale (NIHSS) scores, and improve Glasgow Coma Scale (GCS) scores, with good safety, and XNJ was ranked first in lowering NIHSS scores. Sadly, this study did not report mortality, perihematomal edema volume, or activities of daily living (ADL) ability, and did not specifically analyze and report the results of the traditional meta-analysis. Thus, there is currently a lack of systematic reviews and meta-analyses for the use of XNJ treatment in post-operative patients of ICH. Consequently, we used R 4.2.2 to invoke the meta package to perform a meta-analysis on the efficacy and safety of XNJ treatment after surgery of ICH, to provide evidence-based support for the application of it in this field.
2 Materials and methods
We performed this meta-analysis in strict accordance with the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) 2020 statement (Page et al., 2021). The protocol was already registered in PROSPERO (CRD42024503006).
2.1 Search strategy
Comprehensively searched the published RCTs included in PubMed, Cochrane Library, Embase, Web of Science, China National Knowledge Infrastructure (CNKI), VIP database, Wanfang Database, and SinoMed. Search period: from the inception of the databases to 31 January 2024. Languages: Chinese and English. Search method: using both Medical Subject Headings (MeSH) terms and free-text keywords. The search strategy was appropriately adjusted according to the individual features of each database. The detailed search strategies are provided in the Supplementary Material S1.
2.2 Eligibility criteria
2.2.1 Inclusion criteria
(1) Study type: Randomized Controlled Trials (RCTs).
(2) Study subjects: Post-operative adult patients of ICH (as diagnosed by a clinician, or using any recognized diagnostic criteria). And surgical treatments including soft/hard channel puncture hematoma aspiration/fragmentation and drainage surgery, ventricular drainage surgery, neuroendoscopic hematoma evacuation surgery, craniotomy for hematoma removal, etc.
(3) Interventions: Both groups received CWM treatments (including hemostasis, dehydration, intracranial pressure reduction, blood pressure reduction, neural nutrition, cerebral cell activation, hyperbaric oxygen therapy, anti-infection, gastric acid suppression, etc.) as the foundation; the experimental group received additional intravenous injections of XNJ, with no restrictions on dosage or course of treatment.
(4) Outcome indicators: The study included at least one of the following outcomes: total efficiency rate; all-cause mortality; neurological impairment, assessed by NIHSS, European Stroke Scale (ESS), Chinese Stroke Scale (CSS), etc.; ability of ADL, assessed by Barthel Index (BI), modified Barthel Index (mBI), etc.; level of consciousness, assessed by GCS, etc.; volume of intracerebral hematoma; volume of perihematomal edema; levels of inflammatory indicator TNF- α, and safety indicators (including adverse drug reactions and incidence of complications).
2.2.2 Exclusion criteria
(1) Study that was grouped by incorrect random methods such as the order of admission or treatment method.
(2) Intervention measures include other therapies, in addition to XNJ (intravenous injection) and CWM treatment.
(3) Study with statistical errors where the data cannot be aggregated.
(4) For the same literature published repeatedly, one with complete data was reserved, and the rest were excluded.
(5) For studies with completely duplicated data but different authors, the latest published studies were excluded.
2.3 Study selection and data extraction
Two researchers independently screened and extracted information from the literature according to the inclusion and exclusion criteria set forth in the study. In cases where there was disagreement, a discussion was initiated; if disagreements persisted, a third party would consider the different viewpoints and make a decision. The extracted information by preformulated data collection form included the first author, publication year, sample size, gender, age, interventions, duration of treatment, outcome indicators, and their respective data.
2.4 Assessment of risk of bias
The quality of the studies was assessed using the Risk of Bias assessment tool (ROB2.0) in the Cochrane Handbook (v6.4) (Higgins et al., 2023). The assessment was cross-checked by two researchers, and any discrepancies were resolved through discussion. If consensus could not be reached, a third researcher was consulted to make a decision. The assessment covered the following domains: randomization process, deviations from intended interventions, missing outcome data, measurement of the outcome, selection of the reported result, and overall risk of bias. Each domain encompassed one to seven specific questions. The results were classified as “low risk of bias”, “some concerns” and “high risk of bias".
2.5 Statistical analysis
Meta-analysis was carried out using R 4.2.2. The effect size for dichotomous variables was expressed as relative risks (RR) with their 95% confidence intervals (CI), and a continuity correction of 0.5 was added to each side when there were studies with 0 cells.
For continuous variables, when the outcomes were in the same unit, the weighted mean difference (WMD) with its 95% CI was calculated using the noStandard method; when the units varied or the scales different for the same outcome in different studies, the standardized mean difference (SMD) with its 95% CI was computed using Hedges’ method.
Before combining effect sizes, an assessment (
2.6 Trial sequential analysis (TSA) and quality of evidence
Trial sequential analysis (TSA) was performed using TSA 0.9.5.10 Beta (Thorlund et al., 2017). And calculated the Required Information Size (RIS) to determine the possibility of false negative results. The quality of evidence was graded using the web-based development tool of GRADEpro GDT (https://www.gradepro.org/). Based on the GRADE methodology applied in systematic reviews (Guyatt et al., 2011), three upgrading factors (potential confounders, dose-response relationship, large magnitude of effect) and five downgrading factors (publication bias, indirectness, heterogeneity, imprecision, and risk of bias) were thoroughly considered to classify the quality of evidence into four levels: very low, low, moderate, and high. If more than one downgrading factor was present, the quality of evidence would be downgraded, resulting in the formation of an evidence profile.
3 Results
3.1 Literature search
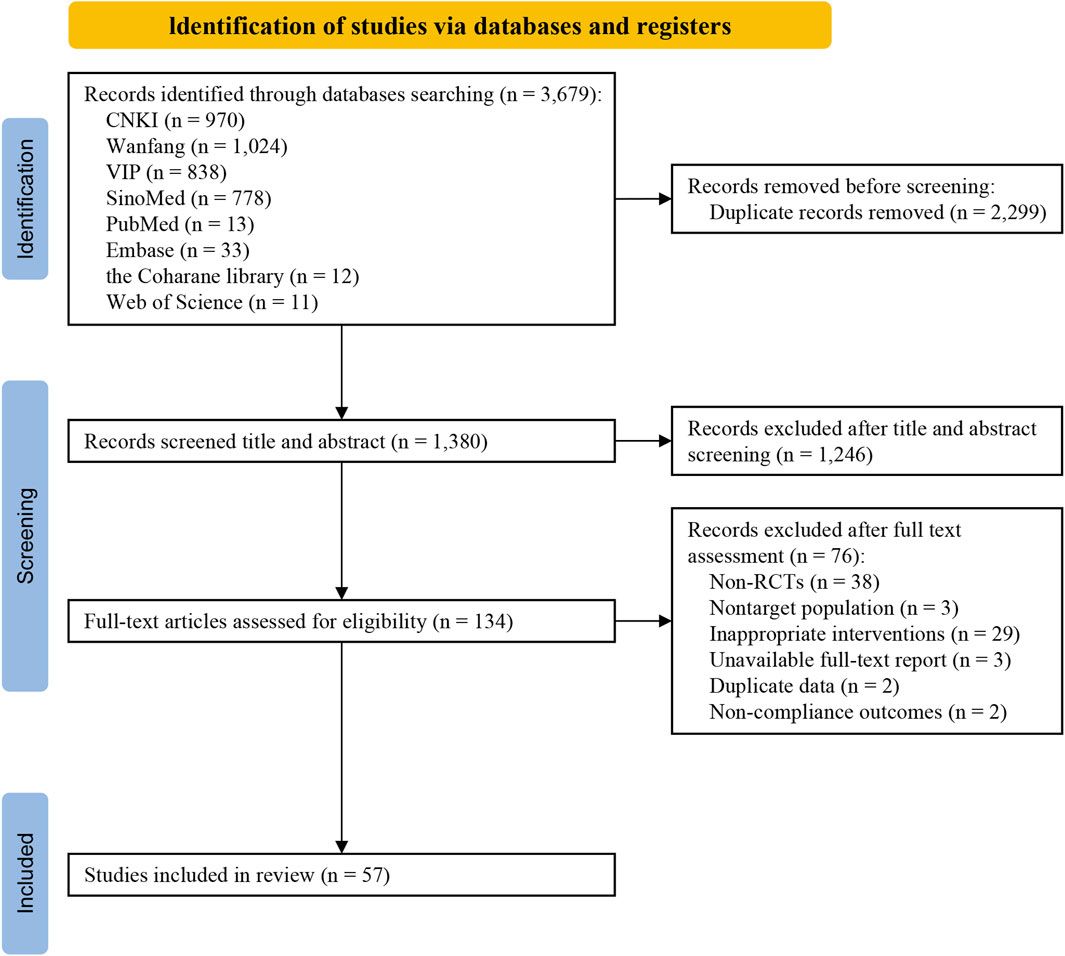
In this study, a total of 3,679 studies were retrieved, including 3,610 Chinese studies and 69 English studies. The retrieved study citations were imported into NoteExpress v3.5, and a total of 57 studies (Wang et al., 2001; Lin et al., 2003; Wu and Han, 2004; Huang, 2005; Tong et al., 2006; Lin, 2009; Nie, 2010; Li et al., 2011; Lu and Tang, 2011; Wu et al., 2011; Yang et al., 2011; Zhao, 2011; Li, 2012; Li et al., 2012; Li and Zhou, 2012; Jin, 2013; Shi et al., 2013; Sun and Zhong, 2013; Tao et al., 2013; Xin, 2013; Guo and Wen, 2014; Huang and Guo, 2014; Zhou, 2014; Chen and Li, 2015; Cheng, 2015; Dai et al., 2015; Guo, 2015; He et al., 2015; Tang, 2015; Xu and Lv, 2015; Zhang et al., 2015; Zhou, 2015; Lian, 2016; Liu et al., 2016; Ren, 2016; Tong et al., 2016; Xia et al., 2016; Zhang et al., 2016; Gu and Zhang, 2017; Shuang et al., 2017; Zhou and Sun, 2017; Cheng, 2018; Jiang and Xiao, 2018; Jin and Wang, 2018; Li et al., 2018; Li, 2019; Wang, 2019; You, 2019; Zhang, 2019; Liang and Qin, 2020; Shu, 2020; Xu, 2020; Chen, 2021; Deng et al., 2021; Xiao and Wu, 2021; Sun et al., 2022; Hao et al., 2024) were ultimately included after screening (Figure 1).
3.2 Characteristics of the included studies
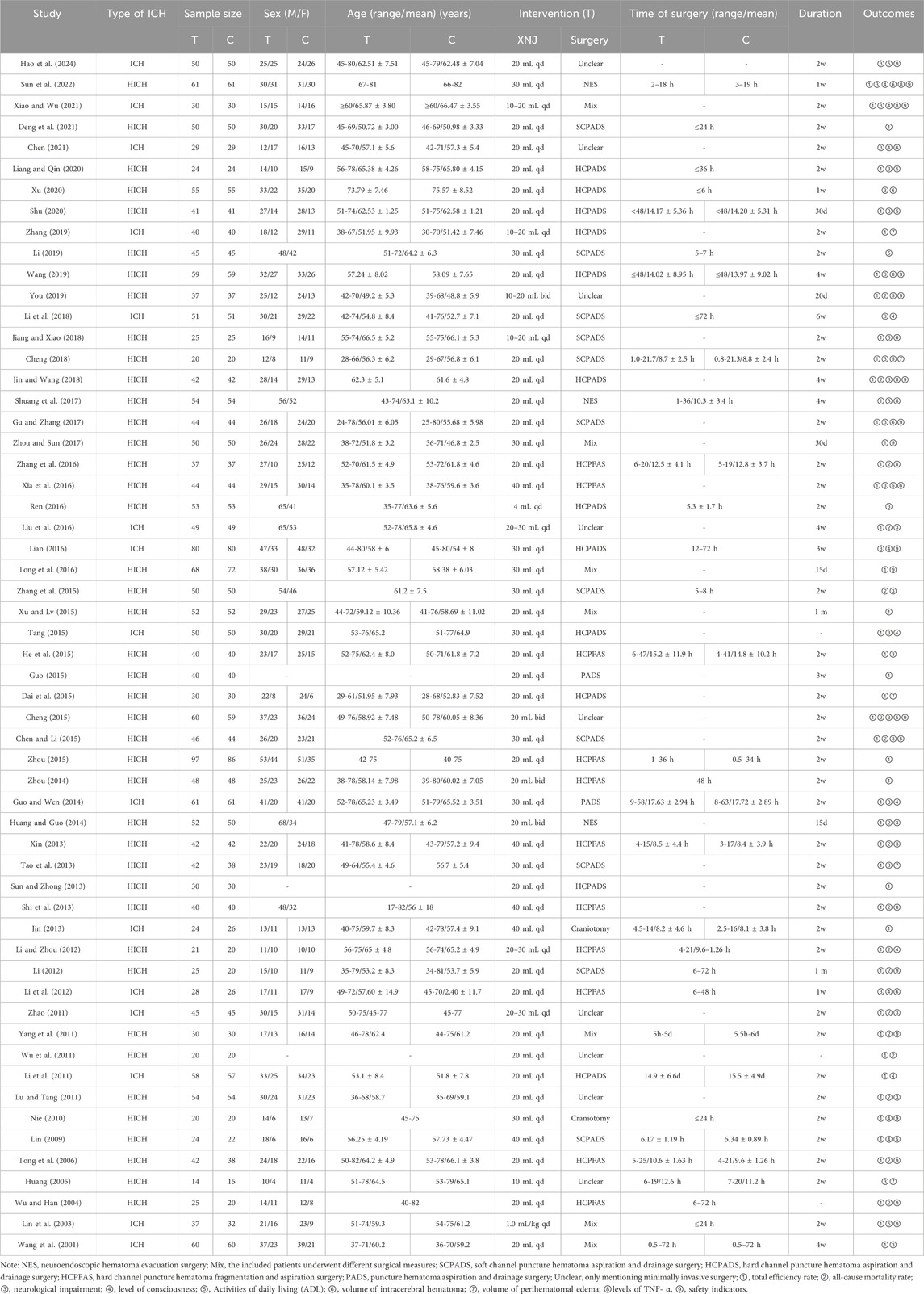
This study incorporated a total of 57 RCTs, involving 4,852 patients, with 2,445 in the integration of XNJ and CWM therapy group (experimental group) and 2,407 in the CWM treatment group (control group). All studies were conducted in China and were single-center RCTs. The sample sizes ranged from 29 to 183. All RCTs were based on surgery and CWM treatment, with one group receiving additional XNJ (Table 1).
3.3 Risk of bias assessment
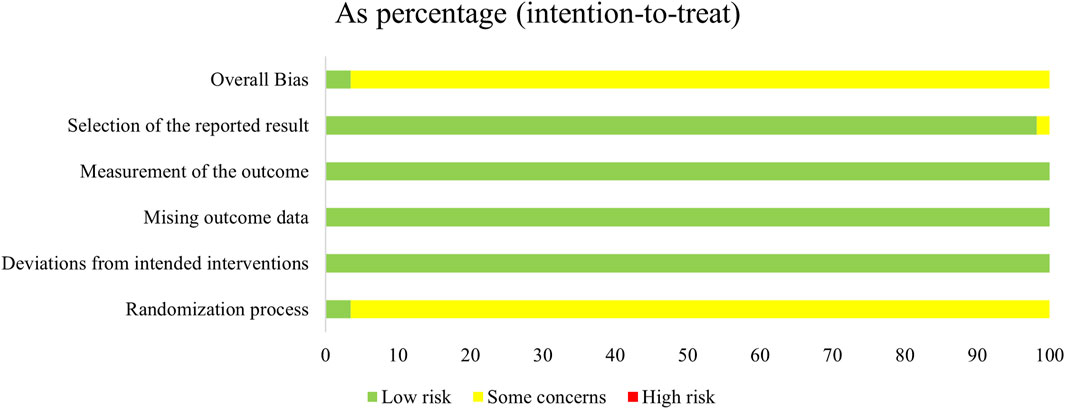
Regarding the “randomization process”, all studies reported comparability of baseline data between the two groups, and with 24 studies (Lin et al., 2003; Lin, 2009; Li, 2012; Tao et al., 2013; Guo and Wen, 2014; Chen and Li, 2015; Dai et al., 2015; Xu and Lv, 2015; Lian, 2016; Ren, 2016; Xia et al., 2016; Zhang et al., 2016; Gu and Zhang, 2017; Cheng, 2018; Jiang and Xiao, 2018; Jin and Wang, 2018; Li et al., 2018; Li, 2019; Wang, 2019; You, 2019; Liang and Qin, 2020; Chen, 2021; Hao et al., 2024) reported specific and correct randomization methods. But only two studies (Lin, 2009; Nie, 2010) of them assessed as “low risk of bias” because they explicitly mentioned the use of opaque envelopes to conceal allocations. In contrast, the remaining studies were assessed as “some concerns” due to the absence of specific randomization strategies or conceal allocations mentioned. For the “deviations from the intended interventions”, we assessed it as “low risk of bias”. Although only one study (Liu et al., 2016) used a double-blind design, and two studies (Jin, 2013; Tong et al., 2016) administered a placebo treatment (intravenous saline injection) to the control group. However, 14 studies (Lin, 2009; Nie, 2010; Li et al., 2011; Li et al., 2012; Li and Zhou, 2012; Guo and Wen, 2014; He et al., 2015; Tang, 2015; Lian, 2016; Li et al., 2018; Zhang, 2019; Chen, 2021; Xiao and Wu, 2021; Sun et al., 2022) mentioned that all included patients had varying degrees of consciousness impairment, we expect these patients likely did not know which treatment measures they were receiving. There were no instances of patients switching groups due to awareness or unawareness of their treatment modalities. All studies used an intention-to-treat analysis (ITT) to estimate the effects of allocated intervention measures, and were assessed to be at low risk. In addition, we assessed the “missing outcome data” as “low risk” due to no loss to follow-up was reported, or negligible losses to follow-up was founded. The “outcome measurements” were assessed as “low risk” because the criteria for evaluating the outcome indicators between the two groups were reasonable and consistent in all studies. Although they did not mention whether blinding was implemented for the outcome assessors, it is still unclear whether this would affect the judgment of the results, as the outcome assessors’ potential preference bias towards the two treatment measures is unknown. Apart from one study (Huang, 2005) (assessed as 'some concerns’ due to the safety situation reported only for the experimental group), we assessed all remaining studies as having a “low risk of bias” in the case of “selective reporting” because all of them had clear outcome indicators and comprehensively reported results whether they were statistically significant or not (Figure 2; Supplementary Figure S1).
3.4 Primary outcomes
3.4.1 Total efficiency rate
A total of 47 studies (Wang et al., 2001; Lin et al., 2003; Wu and Han, 2004; Tong et al., 2006; Lin, 2009; Nie, 2010; Li et al., 2011; Lu and Tang, 2011; Wu et al., 2011; Yang et al., 2011; Zhao, 2011; Li, 2012; Li and Zhou, 2012; Jin, 2013; Shi et al., 2013; Sun and Zhong, 2013; Xin, 2013; Guo and Wen, 2014; Huang and Guo, 2014; Zhou, 2014; Chen and Li, 2015; Cheng, 2015; Dai et al., 2015; Guo, 2015; He et al., 2015; Tang, 2015; Xu and Lv, 2015; Zhou, 2015; Liu et al., 2016; Tong et al., 2016; Xia et al., 2016; Zhang et al., 2016; Gu and Zhang, 2017; Shuang et al., 2017; Zhou and Sun, 2017; Cheng, 2018; Jiang and Xiao, 2018; Jin and Wang, 2018; Wang, 2019; You, 2019; Zhang, 2019; Liang and Qin, 2020; Shu, 2020; Deng et al., 2021; Xiao and Wu, 2021; Sun et al., 2022) comprising 3,943 participants reported the total efficiency rate. We first conducted a meta-analysis on 25 of the studies (Wang et al., 2001; Tong et al., 2006; Nie, 2010; Li et al., 2011; Lu and Tang, 2011; Yang et al., 2011; Zhao, 2011; Li and Zhou, 2012; Jin, 2013; Shi et al., 2013; Sun and Zhong, 2013; Tao et al., 2013; Xin, 2013; Guo and Wen, 2014; Huang and Guo, 2014; Zhou, 2014; Chen and Li, 2015; Cheng, 2015; Tang, 2015; Liu et al., 2016; Jiang and Xiao, 2018; Jin and Wang, 2018; Wang, 2019; Shu, 2020; Deng et al., 2021) which used “18% reduction in post-treatment neurological impairment scales scores” as the criterion for total efficiency rate. A fixed-effect model was used due to the low heterogeneity (I2 = 0%, p = 0.52), and the pooled data showed that XNJ significantly improved the total efficiency rate (RR = 1.26; 95% CI [1.21 to 1.32]; p < 0.0001) (Figure 3). Sensitivity analysis clarified that the combined effect size was stable (Supplementary Figure S2).
Considering that different studies have adopted various methods of evaluating therapeutic efficacy, further analyses were then performed, respectively, by different efficacy evaluation criteria. The results indicated that there were no significant differences in treatment efficacy among them (p = 0.58) (Supplementary Figure S12).
3.5 Secondary outcomes
3.5.1 All-cause mortality
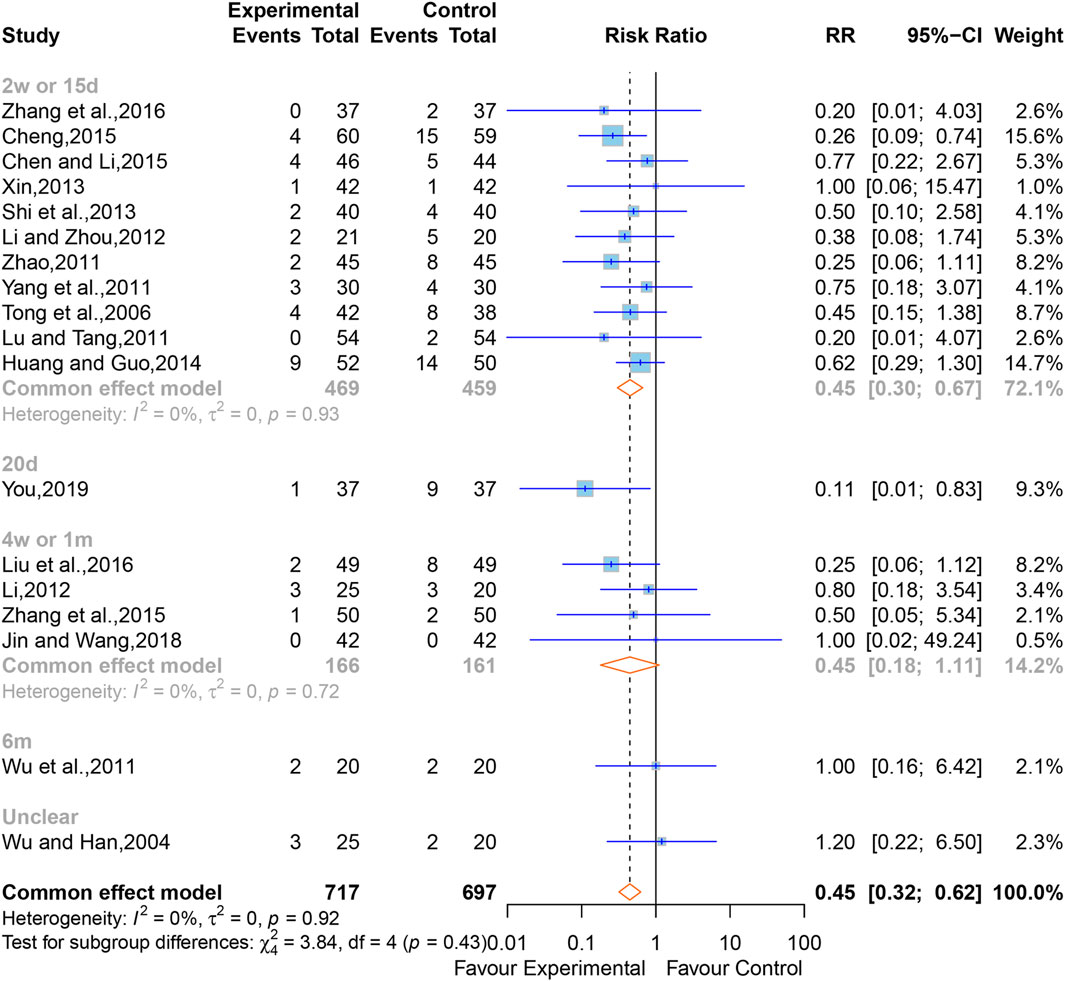
In all, 18 studies (Wu and Han, 2004; Tong et al., 2006; Lu and Tang, 2011; Wu et al., 2011; Yang et al., 2011; Zhao, 2011; Li, 2012; Li and Zhou, 2012; Shi et al., 2013; Xin, 2013; Huang and Guo, 2014; Chen and Li, 2015; Cheng, 2015; Zhang et al., 2015; Liu et al., 2016; Zhang et al., 2016; Jin and Wang, 2018; You, 2019) containing 1,414 cases reported the all-cause mortality. The overall effect of meta-analysis indicated that XNJ reduced the all-cause mortality (RR = 0.45; 95% CI [0.32 to 0.62]; p < 0.0001), and a fixed-effects model was applied due to the low heterogeneity (I2 = 0%, p = 0.92) (Figure 4). Sensitivity analysis indicated that the combined effect size was stable (Supplementary Figure S3).
Subgroup analyses were conducted according to the time point of observation. The outcomes clarified that, compared to the control group, XNJ significantly reduced all-cause mortality after 2 weeks or 15 days of starting treatment. However, at the periods of 4 weeks or 1 month, 6 months, and unclear time for observing the all-cause mortality, the result of the XNJ group was no significant compared with that of the control group. Additionally, for the 20 days, 6 months, and unclear groups, there was only one study each (2w or 15d, RR = 0.45; 95% CI [0.30 to 0.67]; 20d, RR = 0.11; 95% CI [0.01 to 0.83]; 4w or 1m, RR = 0.45; 95% CI [0.18 to 1.11]; 6m, RR = 1.00; 95% CI [0.16 to 6.42]; Unclear, RR = 1.20; 95% CI [0.22 to 6.50]) (Figure 4).
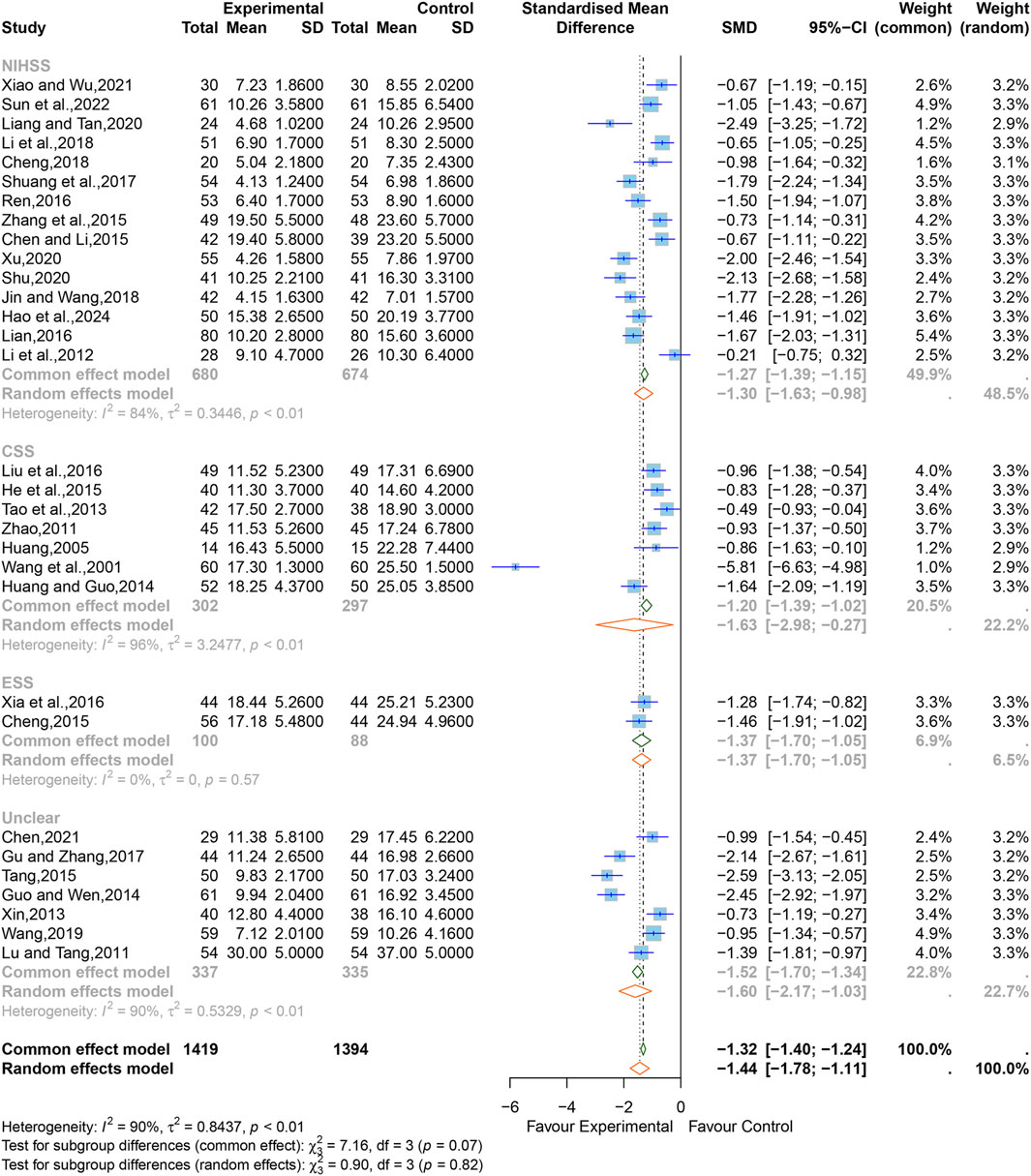
3.5.2 Neurological impairment
Regarding neurological impairment, 15 studies (Li et al., 2012; Chen and Li, 2015; Zhang et al., 2015; Lian, 2016; Ren, 2016; Shuang et al., 2017; Cheng, 2018; Jin and Wang, 2018; Li et al., 2018; Liang and Qin, 2020; Shu, 2020; Xu, 2020; Xiao and Wu, 2021; Sun et al., 2022; Hao et al., 2024) containing 1,414 cases reported the grading by NIHSS, seven studies (Wang et al., 2001; Huang, 2005; Zhao, 2011; Li, 2012; Tao et al., 2013; Huang and Guo, 2014; He et al., 2015; Liu et al., 2016) containing 1,414 cases reported the grading by CSS, two studies (Cheng, 2015; Xia et al., 2016) containing 1,414 cases reported the grading by ESS, and seven studies (Lu and Tang, 2011; Xin, 2013; Guo and Wen, 2014; Tang, 2015; Gu and Zhang, 2017; Wang, 2019; Chen, 2021) did not specify the evaluation scale used.
The SMD was chosen to standardize the effect sizes across studies to counteract the total score differences caused by the use of different scales among the studies. Due to significant heterogeneity among them (I2 = 90%, p < 0.01), a random-effects model was applied. The results indicated that XNJ significantly improved neurological impairment (SMD = −1.44; 95% CI [−1.78 to −1.11]; p < 0.0001) (Figure 5). Sensitivity analysis showed that the results of the combined effect size are stable (Supplementary Figure S4).
Based on the subgroup analyses of different evaluation scales, it can be seen that the heterogeneity of the ESS group was significantly reduced (I2 = 0%, p = 0.57), but the CSS, the NIHSS, and the unclear group were relatively high (NIHSS, I2 = 84%, p < 0.01; CSS, I2 = 96%, p < 0.01; unclear, I2 = 90%, p < 0.01) (Figure 5). The rest of the subgroup analyses are listed in Supplementary Table S1. The overall effect combined by MD showed that there was still statistical significance in each subgroup, which further verified the reliability of the results (NIHSS, MD = −3.56; 95% CI [−4.40 to −2.72]; CSS, MD = −5.26; 95% CI [−7.18 to −3.35]; ESS, MD = −7.30; 95% CI [−8.80 to −5.80]; unclear, MD = −5.65; 95% CI [−6.99 to −4.32]) (Supplementary Figure S13).
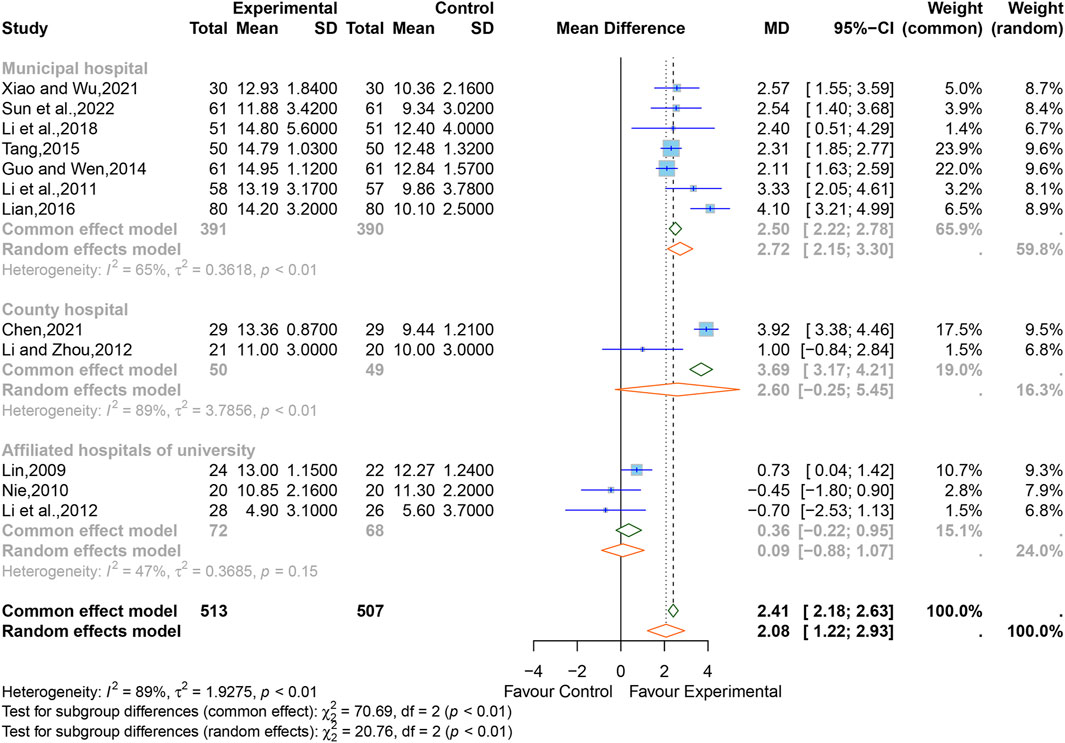
3.5.3 Consciousness
Of all studies, 12 studies (Lin, 2009; Nie, 2010; Li et al., 2011; Li et al., 2012; Li and Zhou, 2012; Guo and Wen, 2014; Tang, 2015; Lian, 2016; Li et al., 2018; Chen, 2021; Xiao and Wu, 2021; Sun et al., 2022) comprising 1,020 participants reported the state of consciousness of patients after treatment, all evaluated using the GCS. In view of the significant heterogeneity between the studies (I2 = 89%, p < 0.01), thus, a random-effects model was used. The result showed that XNJ significantly improved the GCS scores (MD = 2.08, 95% CI [1.22 to 2.93], p < 0.0001) (Figure 6). Sensitivity analysis indicated that the result was stable (Supplementary Figure S5).
The results of the subgroup analyses based on “hospital” demonstrated that the heterogeneity of the “affiliated hospital of university” group was significantly reduced (I2 = 47%, p = 0.15), the heterogeneity within the other subgroups still remained high (county hospital, I2 = 89%, p < 0.01; municipal hospital, I2 = 65%, p < 0.01), and the results for both the “affiliated hospital of university” and “county hospital” groups indicated that the GCS scores of the XNJ group was non-significant compared with that of the control group (affiliated hospital of university, MD = 0.36, 95% CI [-0.22 to 0.95]; county hospital, MD = 2.60, 95% CI [-0.25 to 5.45]). However, the results from the “municipal hospital” group showed that there is a statistically significant difference between the two groups (MD = 2.72, 95% CI [2.15 to 3.30]). The rest of the subgroup analyses are listed in Supplementary Table S2.
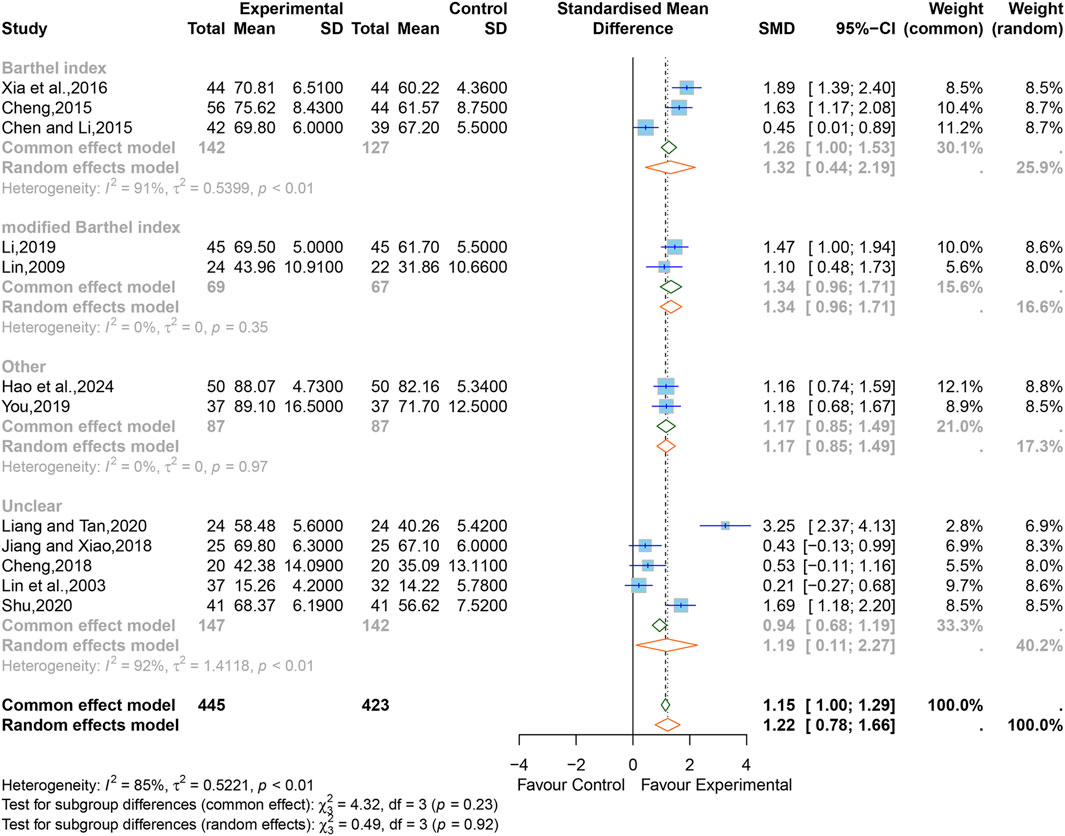
3.5.4 Activities of daily living
After treatment, three studies (Chen and Li, 2015; Cheng, 2015; Xia et al., 2016) containing 269 cases used BI scores to evaluate ADL, two studies (Lin, 2009; Li, 2019) containing 136 cases used mBI, two studies (You, 2019; Hao et al., 2024) containing 174 cases used other scales (SS-QOL, QLQ-C30), and five studies (Lin et al., 2003; Cheng, 2018; Jiang and Xiao, 2018; Liang and Qin, 2020; Shu, 2020) containing 289 cases did not specify the type of scale used.
There was high heterogeneity between the studies (I2 = 85%, p < 0.01), therefore a random-effects model was used with SMD as a summary statistic. The results showed that the difference was statistically significant (SMD = 1.22; 95% CI [0.78 to 1.66]; p < 0.0001) (Figure 7). Sensitivity analysis showed that the overall effect was stable (Supplementary Figure S6).
We performed subgroup analyses, respectively, by the different evaluation scales, and the results indicated that the heterogeneity of the “mBI” and “other scales" groups were significantly reduced (mBI, I2 = 0%, p = 0.35; other scales, I2 = 0%, p = 0.97), the heterogeneity within the other subgroups still remained high (BI, I2 = 91%, p < 0.01; Unclear, I2 = 92%, p < 0.01). The rest of the subgroup analyses are listed in Supplementary Table S3. The results of the combined effect size by MD showed that there was still statistical significance in each subgroup, which further verified the reliability of the results (BI, MD = 9.02; 95% CI [2.39 to 15.64]; mBI, MD = 8.27; 95% CI [6.21 to 10.32]; other, MD = 11.20; 95% CI [-0.03 to 22.42]; unclear, MD = 8.23; 95% CI [1.79 to 14.67]) (Supplementary Figure S14).
3.5.5 Volume of intracerebral hematoma (mL)
A total of seven studies (Li et al., 2012; Xia et al., 2016; Gu and Zhang, 2017; Jiang and Xiao, 2018; Xu, 2020; Chen, 2021; Sun et al., 2022) comprising 570 participants reported the intracerebral hematoma volume. Considering that high heterogeneity (I2 = 92%, p < 0.01), a random-effects model was adopted, and the pooled data showed that XNJ reduced the hematoma volume (MD = −4.72; 95% CI [-7.43 to −2.01]; p = 0.0006] (Figure 8A). The subgroup analyses could not explain the source of the heterogeneity (Supplementary Table S4). Sensitivity analysis indicated that the combined effect size was stable (Supplementary Figure S7).
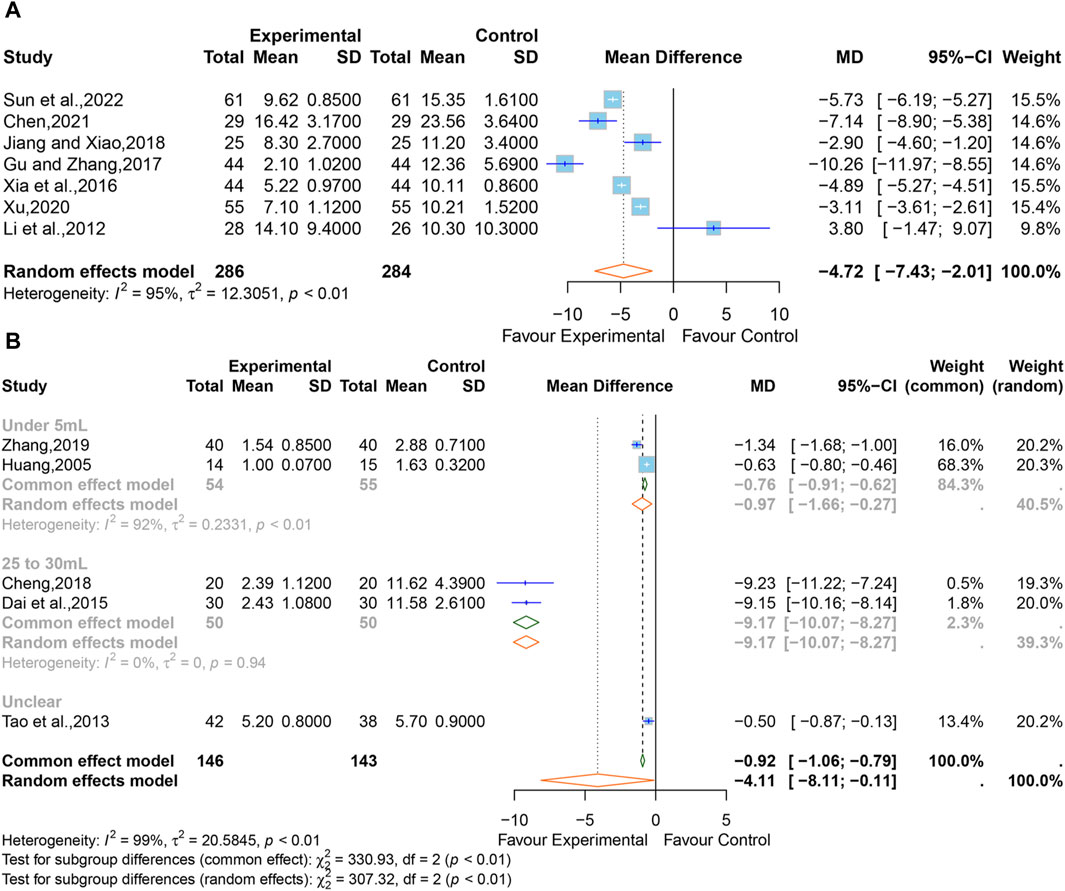
Figure 8. (A) Forest plot for the effect of Xingnaojing on the volume of intracerebral hematoma. (B) Forest plot for the effect of Xingnaojing on the volume of perihematomal edema.
3.5.6 Volume of perihematomal edema (mL)
Five studies (Huang, 2005; Tao et al., 2013; Dai et al., 2015; Cheng, 2018; Zhang, 2019) reported the volume of perihematomal edema. The pooled results indicated statistically significant differences (MD = −4.11; 95% CI [-8.11 to −0.11]; p = 0.0441) between the XNJ group and the control group and showed large heterogeneity (I2 = 99%, p < 0.01) (Figure 8B).
The subgroup analyses based on “the volume of perihematomal edema before treatment” are shown in Figure 9. It can be seen that the heterogeneity in the group with 25–30 mL of perihematomal edema volume before treatment was significantly reduced (I2 = 0%, p = 0.94), whereas there was considerable heterogeneity in the group with less than 5 mL of perihematomal edema volume before treatment (I2 = 92%, p < 0.01), and one study did not specify the volume of perihematomal edema before treatment. The rest of the subgroup analyses are listed in Supplementary Table S5. Sensitivity analysis indicated that the combined effect size was stable (Supplementary Figure S8).
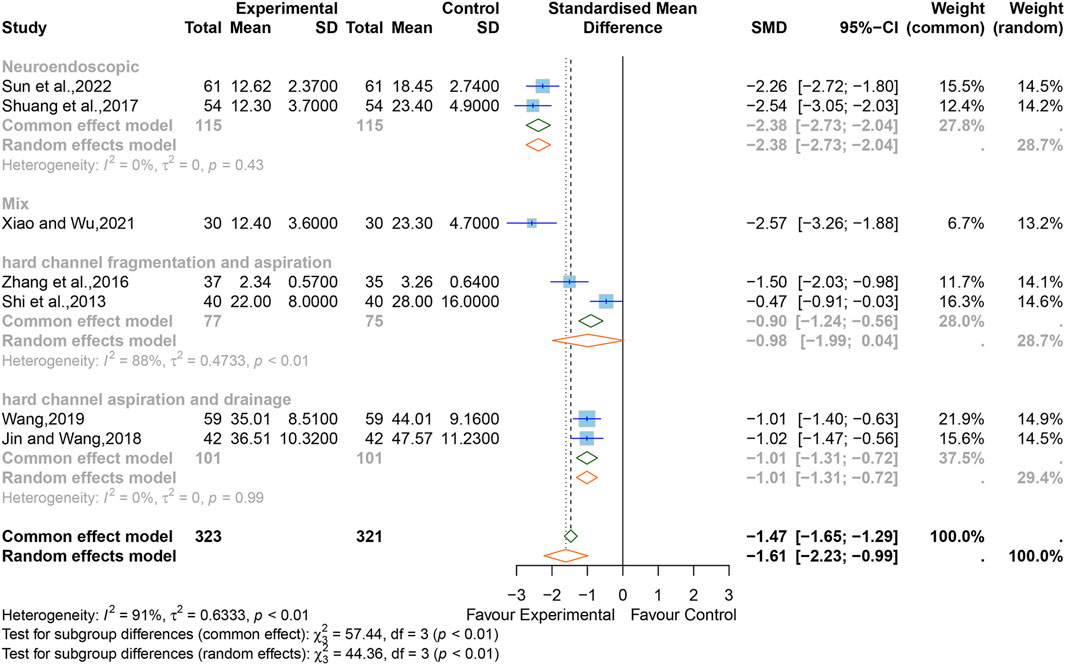
3.5.7 Levels of TNF-α
Seven studies (Shi et al., 2013; Zhang et al., 2016; Shuang et al., 2017; Jiang and Xiao, 2018; Wang, 2019; Xiao and Wu, 2021; Sun et al., 2022) reported the levels of TNF-α. The SMD was used as a summary statistic due to the consistency of the units in these studies being unclear. The outcome showed statistically significant differences (SMD = −1.61, 95% CI [−2.23 to −0.99], p < 0.0001) (Figure 9) between the XNJ and the control group and indicated large heterogeneity (I2 = 91%, p < 0.01). Sensitivity analysis confirmed that the combined effect size was stable (Supplementary Figure S9).
Subgroup analyses based on different surgical techniques revealed that the heterogeneity was significantly reduced in the “neuroendoscopic hematoma evacuation surgery (NES)” group and the “hard channel puncture hematoma aspiration and drainage surgery (HCPADS)” group, while it was still high in the “hard channel puncture hematoma fragmentation and aspiration surgery (HCPFAS)” group (NES, I2 = 0%, p = 0.99; HCPADS, I2 = 0%, p = 0.43; HCPFAS, I2 = 88%, p < 0.01). The “mixed surgery” group (which included patients who underwent different surgical techniques) incorporated only one study. The rest of the subgroup analyses are listed in Supplementary Figure S6.
3.5.8 Safety outcomes (adverse drug reactions, incidence of complications)
In all, six studies reported adverse drug reactions following treatment. However, one study (Huang, 2005) merely stated that no adverse reactions or complications were observed in the XNJ group. We were unable to synthesize this study. The pooled results of the other five studies (Cheng, 2015; Lian, 2016; Jin and Wang, 2018; Xiao and Wu, 2021; Hao et al., 2024) clarified that there was no significant difference between the XNJ group and the control group (RR = 0.89; 95% CI [0.55 to 1.45]; p = 0.6521) (Figure 10A). Meanwhile, No heterogeneity was found (I2 = 22%, p = 0.27); thus, a fixed-effects model was adopted. Sensitivity analysis indicated that the overall effect was stable (Supplementary Figure S10).
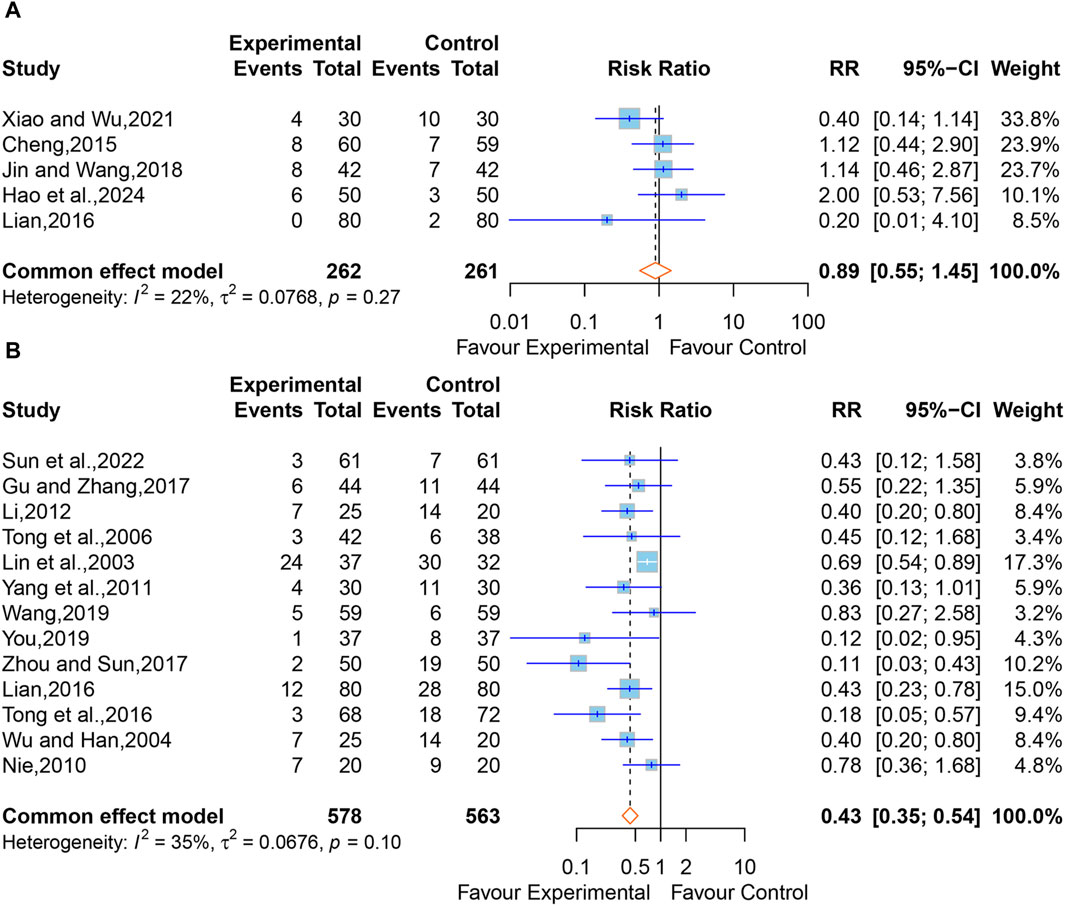
Figure 10. (A) Forest plot for the effect of Xingnaojing on adverse drug reactions. (B) Forest plot for the effect of Xingnaojing on incidence of complications.
Apart from one study (Huang, 2005) that only mentioned no complications in the XNJ group, 15 studies (Lin et al., 2003; Wu and Han, 2004; Tong et al., 2006; Nie, 2010; Yang et al., 2011; Li, 2012; Lian, 2016; Tong et al., 2016; Gu and Zhang, 2017; Zhou and Sun, 2017; Wang, 2019; You, 2019; Sun et al., 2022) reported the postoperative complications in two groups of post-operative patients of ICH. But two of them (Guo, 2015; Xu and Lv, 2015) reported data on the person-time of the outcome, we were unable to synthesize this study. Using the fixed-effect model (I2 = 35%, p = 0.10) for the meta-analysis of the remaining 13 studies, and the results demonstrated that XNJ significantly reduced the incidence of postoperative complications in post-operative patients of ICH (RR = 0.43; 95% CI [0.35 to 0.54]; p < 0.0001) (Figure 10B). Sensitivity analysis indicated that the overall effect was stable (Supplementary Figure S11). Specific adverse drug reactions and incidence of complications mentioned in the studies are listed in Supplementary Tables S7-S8.
3.6 Publication bias
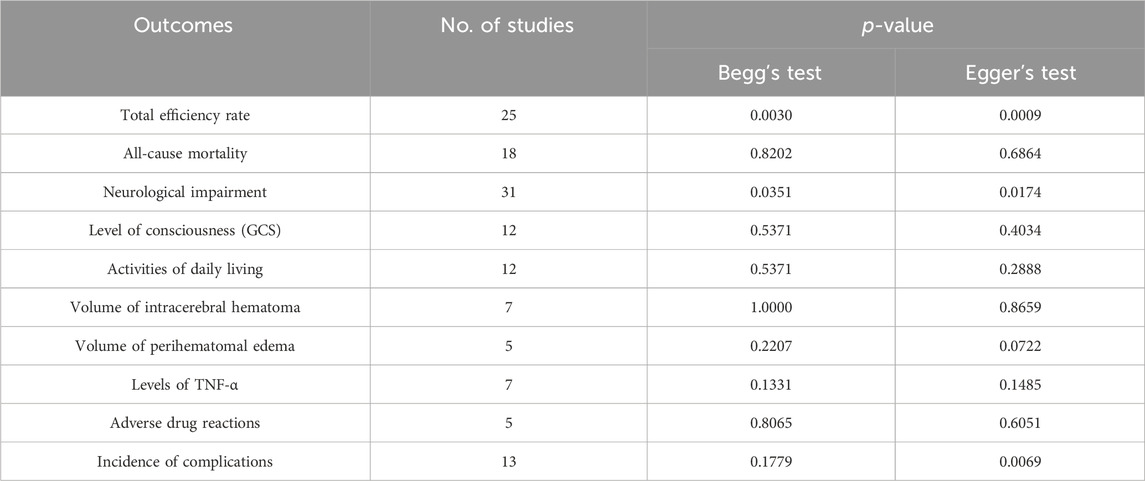
The statistical test showed that no obvious publication bias was found in included trials regarding the all-cause mortality (Begg’s test, p = 0.8202; Egger’s test, p = 0.6864), the GCS score (Begg’s test, p = 0.5371; Egger’s test, p = 0.4034), the ADL (Begg’s test, p = 0.5371; Egger’s test, p = 0.2888), the hematoma volume (Begg’s test, p = 1.0000; Egger’s test, p = 0.8659), the volume of perihematomal edema (Begg’s test, p = 0.2207; Egger’s test, p = 0.0722), the TNF-α (Begg’s test, p = 0.1331; Egger’s test, p = 0.1485), and adverse drug reactions (Begg’s test, p = 0.8065; Egger’s test, p = 0.6051). However, a publication bias risk was present for the total efficiency rate (Begg’s test, p = 0.0030; Egger’s test, p = 0.0009), the neurological impairment (Begg’s test, p = 0.0351; Egger’s test, p = 0.0174), and the incidence of complications (Begg’s test, p = 0.1779; Egger’s test, p = 0.0069) (Table 2).
3.7 Results of TSA
In this study, TSA analysis was performed on 25 studies that reported total effective rates which used “18% reduction in post-treatment neurological impairment scales scores” as the criterion, and the parameters were set according to the user manual for TSA (Thorlund et al., 2017), the type of boundary value was set as two-sided, type I error was defined as α = 0.05, statistical efficacy 1-β = 0.8. The results showed that the cumulative Z-value crossed the traditional boundary value (Z = 1.96) when included in study 1 (Wang et al., 2001), crossed the TSA boundary value when included in study 5 (Lu and Tang, 2011), and reached the RIS when included in study 17 (Chen and Li, 2015). The penalised Z-curve also crossed the traditional boundary value after the inclusion of study 2 (Tong et al., 2006), crossed the TSA boundary value when included study 6 (Yang et al., 2011) and reached the RIS when included in study 17 (Chen and Li, 2015) (Figure 11).
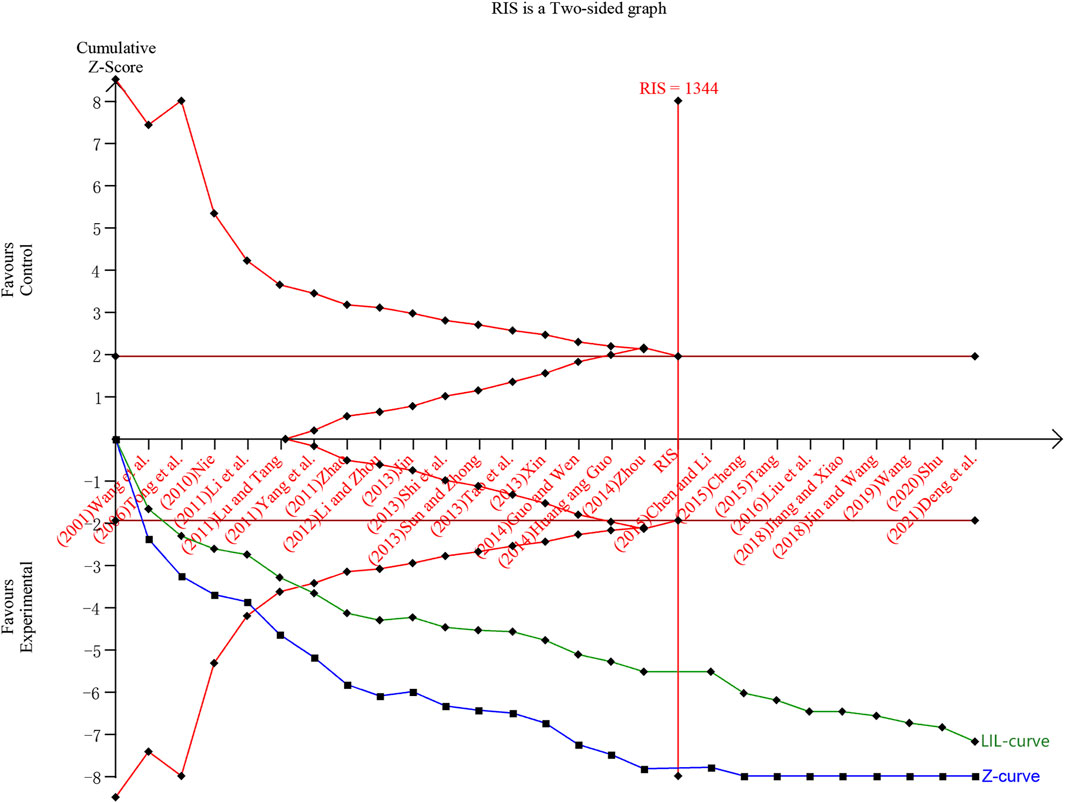
Figure 11. Trial sequential analysis and penalty statistics analysis of total efficiency rate. Note: The blue curve is the cumulative Z curve, the green curve (LIL-curve) is the penalized Z curve, the red horizontal line is the traditional threshold, the red curve is the TSA threshold, and the red vertical line is the RIS.
3.8 Quality of evidence
The certainty of the evidence of XNJ on all-cause mortality within 15 days, volume of perihematomal edema, levels of TNF-α, and adverse drug reactions was rated as “moderate”; that on total efficiency rate, ADL, intracerebral hematoma volume, and incidence of complications was “low”; and that on neurological impairment and the GCS score was “very low” (Table 3). We judged the quality of evidence as moderate to very low, mainly due to the high risk of bias, imprecision and the severe heterogeneity.
4 Discussion
4.1 Research significance
Intracerebral haemorrhage (ICH) is the most difficult to treat, the most disabling, and the deadliest type of stroke subtype (Tapia-Pérez et al., 2014; Wilkinson et al., 2018). The mechanisms of pathological damage after ICH primarily involve: the mass effect and mechanical rupture caused by the initial or ongoing bleeding and the expansion of the hematoma, which raise the overall pressure (intracranial pressure) and directly lead to primary brain injury; the physiological response to the hematoma (primarily edema and inflammation), the metabolic effects of thrombotic components, and secondary brain injury caused by toxic biochemicals (Wilkinson et al., 2018). One-third of ICH patients die within a month, and a large number of survivors are left with permanent disabilities (Steiner et al., 2011). Symptomatic treatment has been the primary treatment strategy for ICH to date (Tang et al., 2018). Surgery remains a vital measure for saving lives in emergency situations; however, the most common sites for ICH are deep brain structures, such as the basal ganglia and thalamus. Surgery requires passage through portions of brain tissue, which can lead to iatrogenic injury of healthy brain tissue. In addition, the presence of perihematomal edema after surgery may limit the therapeutic effect (Thompson et al., 2015; de Oliveira Manoel, 2020). Therefore, treatment targeting residual hematoma and cerebral edema post-operatively is a crucial aspect of care (Murthy et al., 2015; Chen et al., 2016).
Results from a systematic pharmacology study (Chen et al., 2018) suggest that XNJ might exert an anti-stroke effect by responding to oxidative stress, regulating blood pressure, calcium signaling pathways, and cell apoptosis among other biological processes and pathways, and Akt1, HIF1a, and ITGB2 may play key roles in the occurrence and regulation of stroke. 1,7-Diphenyl-3-acetoxy-6(E)-hepten, oxycurcumenol and beta-sitosterol may be essential compounds in XNJ and have been reported as effective ingredients for the treatment of stroke. The study also experimentally demonstrated that the oxycurcumenol has a protective effect on PC12 cells against oxidative stress-induced cellular damage. This mechanism does not involve cell cycle-dependent processes but may function through the regulation of autophagy, preliminarily unveiling the potential mechanisms by which XNJ treats stroke systematically. Our research further clarified the clinical efficacy and safety of XNJ in treating post-operative patients with ICH through an evidence-based evaluation, providing support for the clinical application of XNJ from an evidence-based perspective.
4.2 Summary of the main results
This meta-analysis included a total of 57 studies involving post-operative patients with ICH. It encompassed 2,445 cases that received a combination of XNJ with CWM treatment and 2,407 cases that received only CWM treatment. This study indicated that in comparison to other outcome indicators, authors of previous studies appeared to prefer to use the total efficiency rate rather than all-cause mortality as an endpoint indicator. And a few studies hold a dialectical perspective toward this phenomenon (Shi et al., 2023). This is due to the fact that the total efficiency rate, as a composite indicator, still lacks a universally accepted standardized evaluation method, and it is an insufficient strategy to evaluate a composite endpoint as if it were a sole primary endpoint (McCoy, 2018). However, we hold a conservative view on this because for patients, efficacy as a positive outcome may be more acceptable than mortality. And the “Clinical neurological impairment scoring standards for stroke patients” (NACCDC, 1996) formed at the fourth Chinese conference on cerebrovascular diseases in 1995 unified the criteria for assessing the “effectiveness” of stroke patient treatment, which was defined as a reduction in neurological impairment score of ≥18% after treatment. The results of this study showed that most of the previous studies used the aforementioned assessment method to evaluate the total efficiency rate. However, many studies also used different efficacy assessment criteria, and we found through subgroup analyses that there was no significant difference between results using different criteria, and there was low heterogeneity in the overall effect of the meta-analysis.
As for the outcome indicators of neurological impairment and ADL, there were similar issues, especially regarding the assessment of ADL. Some studies only mentioned the use of ADL scales but did not specify the names and criteria of the scales used. In fact, there were many scales commonly used to assess ADL, such as the BI and mBI, etc. We merged the effect sizes of all the studies included in the outcome indicators of neurological impairment and ADL through SMD and compared them with results obtained by merging effect sizes using MD. The results showed that XNJ could significantly reduce neurological impairment and improve ADL after treatment. In contrast, regarding the consciousness state, all studies used GCS for evaluation, and results showed that XNJ significantly improved GCS scores after treatment, but there was also obvious heterogeneity between studies.
Compared with the above subjective outcome indicators, this study also included some objective outcome indicators, and the results showed that XNJ significantly reduced all-cause mortality, hematoma volume, perihematomal edema, and the inflammatory marker TNF-α after treatment. However, subgroup analyses indicated that XNJ had a significant effect on reducing all-cause mortality at 2 weeks or 15 days after starting treatment, but could not reduce all-cause mortality at 4 weeks or 1 month, and even longer time points by pooling a few corresponding data, although there is still a lack of sufficient research to prove its therapeutic effect on 6-month mortality. Despite this, the outcome is still encouraging, as so far, no intervention has demonstrated improved outcomes. Additionally, we conducted specific analyses on safety indicators. Although some studies mentioned adverse reactions, we found that they include two situations: drug adverse reactions and postoperative complications. Our specific analysis showed that XNJ could significantly reduce the incidence of postoperative complications after the surgery of ICH without increasing drug adverse reactions.
Due to the influence of many confounding factors such as surgical methods, geographical regions, age, and methods of outcome evaluation, significant heterogeneity existed among studies included for outcome indicators other than the overall efficacy rate, all-cause mortality, and safety metrics. Despite conducting subgroup and sensitivity analyses, we still cannot completely rule out the impact of confounding factors on the results. Subgroup analyses revealed that the heterogeneity was significantly reduced in the ESS group for the outcome of neurological impairment and in the mBI group for the outcome of ADL. The Subgroup analyses regarding GCS found lower heterogeneity among studies conducted in major affiliated hospitals of the university but yielded negative results, which may be due to the fact that the included patients were those with complex or more severe conditions due to the higher hospital level, thus limiting the treatment effect. The subgroup analyses on perihematomal edema volume showed higher heterogeneity in the group with postoperative edema volume of less than 5 mL, which may be related to the larger measurement errors associated with lower edema volumes. The subgroup analyses targeting TNF-α found that the NES group and the HCPADS group had lower heterogeneity and that the NES group achieved better therapeutic effects. The sources of heterogeneity in the remaining subgroup analyses could not be well explained. Moreover, the results of TSA showed that the cumulative Z-value and the penalised Z-curve crossed both the traditional boundaries and the TSA boundaries, reached the RIS, led to a positive conclusion and excluding the possibility of false positives. Unfortunately, due to the high level of heterogeneity and the absence of blinding in subjective outcome indicators, the level of evidence for the study results is generally low, and our findings according to the current studies should be considered carefully in the clinic.
4.3 Strengths and limitations
Compared to the previous network meta-analysis concerning post-operative patients with ICH (Ren et al., 2022), this meta-analysis included the latest RCTs. Past network meta-analyses only focused on the total efficiency rate, NIHSS, and intracerebral hematoma volume. However, we attempted to investigate whether XNJ could reduce all-cause mortality, perihematomal edema volume, TNF-α, and improve the ADL, which are more objective and important for post-operative patients with ICH. We comprehensively collected and assessed existing research data for each outcome indicator, despite that they adopted different evaluation methods for the same outcome indicator. Moreover, we conducted subgroup analyses for different evaluation methods and displayed the results for the convenience of clinical specialists and other researchers’ access. In addition, this study performed more comprehensive subgroup analyses for outcome indicators with high heterogeneity, to interpret sources of the heterogeneity and the efficacy results, and explored the stability of the results through sensitivity analyses, etc. Previously, there have been no conventional meta-analysis studies published that specifically involve the use of TCM injections in post-operative patients with ICH.
This study also has certain limitations: the included 57 studies were mostly single-center and small-sample research; some studies only mentioned random allocation without specifying the exact methods; the surgical methods, efficacy evaluation criteria, and outcome indicators varied among the studies; the studies reported only short-term mortality rates, lacking long-term prognosis follow-up, etc. At the same time, the presence of significant publication bias might also affect the reliability of the results.
In view of the above limitations, future research should strengthen the integrity of experimental designs, pay special attention to the accurate application of random methods, allocation concealment, and blinding, clearly define long-term efficacy and safety, and to the extent possible choose widely recognized, unified outcome indicators (Liu et al., 2018), etc. Considering these limitations, the results of this study still await further high-quality RCT research to provide more reliable evidence-based support.
5 Conclusion
In conclusion, the present meta-analysis and systematic review of 57 RCTs indicates that the administration of XNJ for post-operative patients with ICH is associated with favorable short-term outcomes (within 1 moth). And it can improve total efficiency rate, level of consciousness, and activities of daily living; alleviate neurological impairment; reduce all-cause mortality, volume of cerebral hematoma, volume of perihematomal edema, levels of TNF- α, incidence of complications, and has good tolerability. However, The current evidence base is insufficient and requires substantiation from further high-quality studies. Methodological shortcomings and a substantial risk of bias have curtailed the positive effects, undermining confidence in the synthesis of evidence. Given the preliminary nature of the evidence and that XNJ has enormous potential as a therapeutic agent for ICH, it is imperative to conduct more stringent RCTs to validate the efficacy of XNJ in post-operative patients with ICH.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Author contributions
YS: Data curation, Formal Analysis, Methodology, Project administration, Software, Visualization, Writing–original draft, Writing–review and editing. FX: Data curation, Project administration, Software, Visualization, Writing–original draft, Writing–review and editing. SL: Data curation, Software, Visualization, Writing–review and editing. YS: Conceptualization, Funding acquisition, Supervision, Validation, Writing–review and editing. XW: Conceptualization, Funding acquisition, Methodology, Supervision, Validation, Writing–review and editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Chinese Medicine Heritage and Innovation “Hundred-Thousand-Ten Thousand” Talent Project-National Chinese Medicine Leading Talent Support Program (No. (2018) 284), Special Project on Traditional Chinese Medicine Scientific Research for the Establishment of “Double First-Class” Disciplines in Henan Province (HSRP-DFCTCM-2023-5-07), and the Henan University of Chinese Medicine 2023 Graduate Student Research and Innovation Program (2023KYCX035).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2024.1411026/full#supplementary-material
References
Chen, G. F., Ping, L., Zhou, S. K., Liu, W. W., Liu, L. J., Zhang, D. M., et al. (2016). Early prediction of death in acute hypertensive intracerebral hemorrhage. Exp. Ther. Med. 11, 83–88. doi:10.3892/etm.2015.2892
Chen, T. (2021). Analysis of the wake-up promoting effect of Xingnaojing on postoperative coma in patients with cerebral hemorrhage. Chin. Sci. Technol. J. Db. Med. 12, 74–75.
Chen, Y. H., and Li, X. X. (2015). Xingnaojing injection assisted soft channel puncture drainage for the treatment of 46 cases of hypertensive cerebral hemorrhage. China. Pharm. 24, 105–106.
Chen, Y. H., Sun, Y., Li, W. D., Wei, H., Long, T. L., Li, H., et al. (2018). Systems pharmacology dissection of the anti-stroke mechanism for the Chinese traditional medicine Xing-Nao-Jing. J. Pharmacol. Sci. 136, 16–25. doi:10.1016/j.jphs.2017.11.005
Cheng, L. (2015). The therapeutic effect of naloxone combined with Xingnaojing on hypertensive intracerebral hemorrhage after minimally invasive hematoma removal surgery and its impact on neurological function. Chin. J. Pract. Nerv. Dis. 18, 102–103. doi:10.3969/j.issn.1673-5110.2015.11.070
Cheng, X. J. (2018). Observation on the therapeutic effect of puncture drainage combined with Xingnaojing injection on patients with hypertensive basal ganglia hemorrhage. Med. J. Chin. People's Health 30, 31–32. doi:10.3969/j.issn.1672-0369.2018.18.013
China Food and Drug Administration (2003) National drug standards WS3-B-3353-98-2003. Beijing, China: China Food and Drug Administration.
Dai, X. J., Dong, S. J., Jia, Y., Liu, J. J., and Chen, L. N. (2015). The effect of Xingnaojing injection on brain edema and inflammatory mediators in patients with hypertensive basal ganglia hemorrhage after minimally invasive puncture drainage surgery. Chin. J. Pract. Nerv. Dis. 18, 79–80. doi:10.3969/j.issn.1673-5110.2015.22.053
Deng, L. L., Tian, L., and Wang, H. C. (2010). Research progress on clinical application of Angong niuhuangwan and its derivative prescription. Chin. J. Exp. Tradit. Med. Formulae. 16, 215–219. doi:10.13422/j.cnki.syfjx.2010.12.053
Deng, Z. F., Yu, Y. X., Huang, W. H., Chen, W. S., and Jiang, J. Y. (2021). Clinical effect analysis of minimally invasive intracranial hematoma removal surgery combined with Xingnaojing injection in the treatment of hypertensive intracerebral hemorrhage. J. Front. Med. 11, 113–114.
de Oliveira Manoel, A. L. (2020). Surgery for spontaneous intracerebral hemorrhage. Crit. Care. 24, 45. doi:10.1186/s13054-020-2749-2
Fang, J. Y., Yang, B., Ge, Z. W., Bai, X., and Yan, B. J. (2017). Single standard substance for the determination of nine volatile components in the distillate of Fructus Gardeniae and Radix Curcumae (an intermediate of Xingnaojing Injection). J. Sep. Sci. 40, 3946–3957. doi:10.1002/jssc.201700593
Gao, L. (2016). Expert consensus on hypertensive intracerebral hemorrhage in acute stage in diagnosis and treatment combining traditional Chinese medicine and Western medicine. Chin. Gen. Pract. 19, 3641–3648. doi:10.3969/j.issn.1007-9572.2016.30.001
Gao, Y., and Zhao, X. Q. (2023) Guideline for the diagnosis and treatment of intracerebral hemorrhage with integrated traditional Chinese and western medicine. Beijing, China: Chinese Society of Integrated Traditional Chinese and Western Medicine.
GBD 2019 Diseases and Injuries Collaborators (2020). Global burden of 369 diseases and injuries in 204 countries and territories, 1990-2019: a systematic analysis for the global burden of disease study 2019. Lancet 396, 1204–1222. doi:10.1016/s0140-6736(20)30925-9
Gu, H. Q., and Zhang, G. B. (2017). Analysis of the effect of Xingnaojing combined with minimally invasive puncture and drainage surgery on moderate to equal volume hypertensive basal ganglia hemorrhage. Mod. Diagn. Treat. 28, 1136–1137. doi:10.3969/j.issn.1001-8174.2017.06.096
Guo, C. H., and Wen, F. (2014). Clinical observation of removal of intracranial hematoma combined with xingnaojing injection on cerebral hemorrhage. J. Hubei Univ. Chin. Med. 16, 68–69. doi:10.3969/j.issn.1008-987x.2014.02.25
Guo, L. (2015). Observation on the clinical efficacy of Xingnaojing combined with head hypothermia in the treatment of cerebral hemorrhage after stereotactic hematoma puncture and aspiration surgery. World Chin. Med. 10, 2.
Guyatt, G., Oxman, A. D., Akl, E. A., Kunz, R., Vist, G., Brozek, J., et al. (2011). GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J. Clin. Epidemiol. 64, 383–394. doi:10.1016/j.jclinepi.2010.04.026
Hao, S. G., Wang, J. X., Wu, W. X., and Yang, B. G. (2024). Effect of Xingnaojing injection combined with nimodipine injection on intracranial blood flow and rehabilitation effect in patients with cerebral hemorrhage after operation. Chin. J. Clin. Ration. Drug Use 17, 11–14. doi:10.15887/j.cnki.13-1389/r.2024.02.004
He, W. G., Pan, S. Q., Zhang, B., Hu, J., Zhou, J. Q., and Jiang, H. Q. (2015). The effect of Xingnaojing injection combined with Western medicine on postoperative neurological function recovery in patients with hypertensive intracerebral hemorrhage. New Chin. Med. 47, 37–39. doi:10.13457/j.cnki.jncm.2015.10.017
Higgins, J. P. T., Thomas, J., Chandler, J., Cumpston, M., Li, T., Page, M. J., et al. (2023). Cochrane Handbook for Systematic Reviews of Interventions version 6.4 (updated August 2023) (Cochrane).
Huang, B. F. (2005). Xingnaojing injection assisted minimally invasive clearing surgery for 29 cases of intracranial hematoma. Chin. J. Integr. Tradit. West. Med. Intensive Crit. Care 12, 253–254. doi:10.3321/j.issn:1008-9691.2005.04.022
Huang, K. Y., Wei, D. N., Fang, J. Y., Li, X. Y., and Yan, B. J. (2017). Rapid determination of nine components in the first extraction process of Xingnaojing injection by using ultraviolet spectroscopy. China J. Chin. Mater. Med. 42, 3755–3760. doi:10.19540/j.cnki.cjcmm.20170907.015
Huang, S. M., and Guo, S. Q. (2014). Safety evaluation of xingnaojing combined with naloxone in treatment of hypertensive cerebral hemorrhage. China Mod. doct. 52, 115–117.
Jiang, Y. M., and Xiao, W. X. (2018). Observation on efficacy of xingnaojing injection combined with soft-channel minimally invasive hematoma drainage in treatment of hypertensive cerebral hemorrhage. Eval. Anal. Drug-use Hosp. Chin. 18, 1495–1496. doi:10.14009/j.issn.1672-2124.2018.11.022
Jin, D., and Wang, J. (2018). Clinical study on Xingnaojing Injection combined with urokinase in treatment of hypertensive cerebral hemorrhage. Drugs Clin. 33, 1342–1346. doi:10.7501/j.issn.1674-5515.2018.06.011
Jin, X. G. (2013). Analysis of the therapeutic effect of craniotomy hematoma removal combined with Xingnaojing in the treatment of cerebral hemorrhage. J. Med. Inf. 26, 669–670. doi:10.3969/j.issn.1006-1959.2013.12.973
Li, C. L., and Zhou, Z. S. (2012). Observation on the therapeutic effect of Xingnaojing injection combined with minimally invasive removal of intracranial hematoma in the treatment of hypertensive intracerebral hemorrhage. Clin. J. Tradit. Chin. Med. 24, 933–934. doi:10.16448/j.cjtcm.2012.10.075
Li, C. Y., Zhang, G. H., and Wang, X. L. (2012). Observation on the therapeutic effect of Xingnaojing on 54 cases of cerebral hemorrhage with consciousness disorders after minimally invasive intracranial hematoma aspiration surgery. Int. J. Trad. Chin. Med. 34, 1115–1116. doi:10.3760/cma.j.issn.1673-4246.2012.12.020
Li, Q. P., Zhang, H., Song, L., and Yang, S. (2018). The effect of Xingnaojing injection on the levels of IFN-γand TIM-3 in patients with basal ganglia hemorrhage underwent super early minimally invasive surgery. J. Guangxi Med. Univ. 35, 502–505. doi:10.16190/j.cnki.45-1211/r.2018.04.020
Li, X. G. (2012) Clinical observation on the treatment of severe hypertensive intracerebral hemorrhage with directional drainage of hematoma and Xingnaojing. Changchun.
Li, X. Y., Li, R., and Zhou, L. M. (2011). Observation on the therapeutic effect of minimally invasive debridement combined with Xingnaojing in the treatment of cerebral hemorrhage. Mod. J. Integr. Tradit. Chin. West. Med. 20, 29–30. doi:10.3969/j.issn.1008-8849.2011.01.014
Li, Y. C. (2019). Therapeutic effect of Xingnaojing injection assisted soft-channel puncture drainage on hypertensive intracerebral hemorrhage. Psychol. Mag. 14, 120–121. doi:10.19738/j.cnki.psy.2019.02.081
Lian, S. M. (2016). Analysis on the curative effect of Xingnaojing injection in the treatment of secondary brain injury followed by intracerebral hemorrhage. China Mod. doct. 54, 81–83.
Liang, L. Z., and Qin, Y. A. (2020). The impact of xingnaojing injection combined with minimally invasive hematoma evacuation on serum S-100β and NSE levels in patients with intracerebral hemorrhage. Mod. Med. Health Res. Electron. Ed. 4, 62–63.
Lin, J., Zhang, N., Zhou, W. H., and Lin, L. Y. (2003). Evaluation of the therapeutic effect of Xingnaojing injection on postoperative hypertensive intracerebral hemorrhage. Chin. J. Integr. Tradit. West. Med. 23, 389–390. doi:10.3321/j.issn:1003-5370.2003.05.025
Lin, Z. S. (2009) A clinical research of Xingnaojing injection after Stereotactic operation in the Treatment of Hypertensive intracerebral hemorrhage. Guangzhou.
Liu, H. R., Wu, H. Q., Xu, L. M., Xin, X. W., and Li, X. J. (2016). Effect of xingnaojing injection on postoperative patients with minimally invasive conical hematoma removal in cerebral hemorrhage. Strait. Pharm. J. 28, 181–182. doi:10.3969/j.issn.1006-3765.2016.05.101
Liu, M., Zhang, S. H., and Zhu, Y. C. (2018). Consensus on clinical research standards for acute stroke in China 2018. Chin. J. Neurol. 51, 247–255. doi:10.3760/cma.j.issn.1006-7876.2018.04.003
Lu, J., and Tang, H. Q. (2011). Observation on the therapeutic effect of Xingnaojing injection after surgery for hypertensive intracerebral hemorrhage. Shanxi Med. J. 40, 1025–1026. doi:10.3969/j.issn.0253-9926.2011.10.042
Ma, X., Wang, T., Wen, J. X., Wang, J., Zeng, N., Zou, W. J., et al. (2020). Role of Xingnaojing Injection in treating acute cerebral hemorrhage: a systematic review and meta-analysis. Med. Baltim. 99, e19648. doi:10.1097/MD.0000000000019648
McCoy, C. E. (2018). Understanding the use of composite endpoints in clinical trials. West. J. Emerg. Med. 19, 631–634. doi:10.5811/westjem.2018.4.38383
Murthy, S. B., Moradiya, Y., Dawson, J., Lees, K. R., Hanley, D. F., Ziai, W. C., et al. (2015). Perihematomal edema and functional outcomes in intracerebral hemorrhage: influence of hematoma volume and location. Stroke 46, 3088–3092. doi:10.1161/STROKEAHA.115.010054
NACCDC (1996). Scoring criteria for clinical neurological impairment in stroke patients (1995). Chin. J. Neurol. 29, 381–383.
NBCMA and CDGNBCMA (2019). Chinese guidelines for diagnosis and treatment of acute intracerebral hemorrhage 2019. Chin. J. Neurol. 52, 994–1005. doi:10.3760/cma.j.issn.1006-7876.2019.12.003
Ni, X. J., Chen, Y. L., and Cai, Y. F. (2020). Evidence-based practice guideline on integrative medicine for stroke 2019. Chin. J. Evid-Based. Med. 20, 901–912. doi:10.7507/1672-2531.202001075
Nie, J. (2010) Microinvasive surgey within 24 hours combined with western medicine and Xingnaojing Injection for Hypertensive intracerebral hemorrhage. Shanghai.
Page, M. J., McKenzie, J. E., Bossuyt, P. M., Boutron, I., Hoffmann, T. C., Mulrow, C. D., et al. (2021). The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 372, n71. doi:10.1136/bmj.n71
Peng, W. J., Yang, J. J., Wang, Y., Wang, W. H., Xu, J. X., Wang, L. X., et al. (2014). Systematic review and meta-analysis of randomized controlled trials of xingnaojing treatment for stroke. Evid-Based Compl. Alt. 2014, 210851. doi:10.1155/2014/210851
Pharmacopoeia Commission of the Ministry of Public Health of the People’s Republic of China (1998) Drug standard of Ministry of public health of the People’s Republic of China - the 17th volume of Chinese patent drugs (WS3-B-3353-98). Beijing, China: China Medical Science Press.
Ren, P., Cao, L., Zhao, X. K., Zhu, B. B., and Liu, K. (2022). Network Meta-analysis of Chinese medicine injections in treatment of hypertensive intracerebral hemorrhage. China J. Chin. Mater. Med. 47, 3637–3647. doi:10.19540/j.cnki.cjcmm.20220214.501
Ren, X. (2016). Observation on efficacy of xingnaojing combined with minimally invasive operation in treatment of hypertensive cerebral hemorrhage. Eval. Anal. Drug-use Hosp. China 16, 463–465. doi:10.14009/j.issn.1672-2124.2016.04.013
Shi, D. J., Cheng, Z. P., and Zeng, L. W. (2013). Effect of xingnaojing injection on serum cytokines after minimally invasive surgery crashing and aspirating hematoma in hypertensive cerebral hemorrhage. China Pharm. 22, 5–6. doi:10.3969/j.issn.1006-4931.2013.13.002
Shi, X. Y., Feng, L. D., Li, Y. X., Qin, M. Z., Li, T. T., Cheng, Z. X., et al. (2023). Efficacy and safety of Panax notoginseng saponins (Xuesaitong) for patients with acute ischemic stroke: a systematic review and meta-analysis of randomized controlled trials. Front. Pharmacol. 14, 1280559. doi:10.3389/fphar.2023.1280559
Shu, W. H. (2020). The effect of piracetam combined with Xingnaojing on the neurological function and daily living ability of patients with hypertensive intracerebral hemorrhage. Jiangxi Med. J. 55, 1629–1630. doi:10.3969/j.issn.1006-2238.2020.11.026
Shuang, Y. H., Mao, Z. L., and Yu, L. (2017). Observation on the application effect of xingnaojing injection in postoperative rehabilitation of minimally invasive hematoma removal in patients with hypertensive cerebral hemorrhage. Shandong Med. J. 57, 93–95. doi:10.3969/j.issn.1002-266X.2017.12.032
Steiner, T., Petersson, J., Al-Shahi Salman, R., Christensen, H., Cordonnier, C., Csiba, L., et al. (2011). European research priorities for intracerebral haemorrhage. Cerebrovasc. Dis. 32, 409–419. doi:10.1159/000330653
Sun, G. L., and Zhong, B. (2013). Xingnaojing injection combined with stereotactic hematoma puncture aspiration for the treatment of 30 cases of hypertensive intracerebral hemorrhage. Chin. Med. Mod. Distance Educ. China 11, 44–45. doi:10.3969/j.issn.1672-2779.2013.15.032
Sun, Y. H., Liu, H. R., Zhang, S. N., and Liu, C. L. (2022). Clinical efficacy of neuroendoscopic hematoma removal combined with Xingnaojing injection in the treatment of hypertensive intracerebral hemorrhage. Chin. Sci. Technol. J. Db. Cit. Ed. Med. Health 13, 139–142.
Tang, T. D. (2015). Evaluation of the value of intracranial hematoma removal surgery combined with Xingnaojing injection in the treatment of cerebral hemorrhage. Chin. J. Mod. Drug. Appl. 9, 172–173. doi:10.14164/j.cnki.cn11-5581/r.2015.21.128
Tang, Y. P., Yin, F. Q., Fu, D. L., Gao, X. H., Lv, Z. C., and Li, X. T. (2018). Efficacy and safety of minimal invasive surgery treatment in hypertensive intracerebral hemorrhage: a systematic review and meta-analysis. Bmc. Neurol. 18, 136. doi:10.1186/s12883-018-1138-9
Tao, Y. Q., Li, Z. Y., Xu, F., Wen, L., Zhang, Y. Q., and Jiang, W. (2013). Clinical efficacy of stereotactic aspiration combined with xingnaojing injection in treatment of hypertensive cerebral hemorrhage study. J. Liaoning Univ. Tradit. Chin. Med. 15, 215–217. doi:10.13194/j.issn.1673-842x.2013.11.101
Tapia-Pérez, J. H., Gehring, S., Zilke, R., and Schneider, T. (2014). Effect of increased glucose levels on short-term outcome in hypertensive spontaneous intracerebral hemorrhage. Clin. Neurol. Neurosurg. 118, 37–43. doi:10.1016/j.clineuro.2013.12.018
Thompson, B. G., Brown, R. D., Amin-Hanjani, S., Broderick, J. P., Cockroft, K. M., Connolly, E. S., et al. (2015). Guidelines for the management of patients with unruptured intracranial aneurysms: a guideline for healthcare professionals from the American heart association/American stroke association. Stroke 46, 2368–2400. doi:10.1161/STR.0000000000000070
Thorlund, K., Engstrøm, J., Wetterslev, J., Brok, J., Imberger, J., and Gluud, C. (2017) User manual for trial sequential analysis (TSA). 2nd ed. Copenhagen: Copenhagen Trial Unit, 1–119.
Tong, H. G., Ke, J. Q., Gan, S. X., Lin, P. M., and Zhang, Z. F. (2006). Clinical observation of Xingnaojing injection assisted with hematoma clearance surgery in the treatment of hypertensive intracerebral hemorrhage. Zhejiang Pract. Med. 11, 389–390. doi:10.3969/j.issn.1007-3299.2006.06.005
Tong, M. F., Liu, J. H., and Dai, H. B. (2016). Effect of Xingnaojing Injection on nitric oxide,insulin-like growth factor-1 and macrophage inhibitory factor of hypertensive patients with intracerebral hemorrhage. Chin. J. Clin. Pharmacol. 32, 399–401. doi:10.13699/j.cnki.1001-6821.2016.05.005
Wang, L. D., Peng, B., Zhang, H. Q., Wang, Y. L., Liu, M., Shan, C. L., et al. (2022a). Brief report on stroke prevention and treatment in China, 2020. Chin. J. Cerebrovasc. Dis. 19, 136–144. doi:10.3969/j.issn.1672-5921.2022.02.011
Wang, L. X., Liu, G. H., Wang, G. S., Yan, R. P., and Cao, J. W. (2001). The effect of early combining Xingnao Jing on nervous function and lipoprotein of patients with acute cerebral haemorrhage. Chin. Gen. Pract. 4, 189–190. doi:10.3969/j.issn.1007-9572.2001.03.012
Wang, M., Jia, M., Du, W. Q., Zhang, X. Y., Jiao, W. W., Chen, Q., et al. (2021). Overview of systematic reviews/Meta-analysis of Xingnaojing Injection in treatment of intracerebral hemorrhage. China J. Chin. Mater. Med. 46, 4633–4643. doi:10.19540/j.cnki.cjcmm.20210622.501
Wang, Y. J., Li, Z. X., Gu, H. Q., Zhai, Y., Zhou, Q., Jiang, Y., et al. (2022b). China stroke statistics: an update on the 2019 report from the national center for healthcare quality management in neurological diseases, China national clinical research center for neurological diseases, the Chinese stroke association, national center for chronic and non-communicable disease control and prevention, Chinese center for disease control and prevention and institute for global neuroscience and stroke collaborations. Stroke Vasc. Neurol. 7, 415–450. doi:10.1136/svn-2021-001374
Wang, Y. L. (2019). Effects of Xingnaojing injection on inflammatory factors and nerve function in patients with hypertensive intracerebral hemorrhage. Hebei Med. J. 41, 3291–3294. doi:10.3969/j.issn.1002-7386.2019.21.022
Wang, Y. Y., and Gao, Y. (2008) Diagnosis and treatment guidelines for common diseases in traditional Chinese medicine: stroke. Beijing, China: China Press of Traditional Chinese Medicine, 56–62.
Wilkinson, D. A., Pandey, A. S., Thompson, B. G., Keep, R. F., Hua, Y., and Xi, G. (2018). Injury mechanisms in acute intracerebral hemorrhage. Neuropharmacology 134, 240–248. doi:10.1016/j.neuropharm.2017.09.033
Wu, C. J., and Han, J. G. (2004). Minimally invasive puncture and intracranial hematoma pulverization combined with Xingnaojing injection for the treatment of hypertensive intracerebral hemorrhage. Chin. J. Clin. 32, 31–33. doi:10.3969/j.issn.1008-1089.2004.03.022
Wu, J. N., Liu, R. M., Xu, D. N., Li, Y. X., Chang, Z. M., Hao, J. X., et al. (2021). Anti-cerebral ischemia mechanisms of brain absorption components of Xingnaojing Injection based on GC-MS and network pharmacology. Chin. Tradit. Herb. Drugs 52, 808–820. doi:10.7501/j.issn.0253-2670.2021.03.024
Wu, L. J., Zhang, H., Xing, Y. W., Gao, Y. H., Li, Y. D., Ren, X. M., et al. (2016). Meta-analysis of the effects of xingnaojing injection on consciousness disturbance. Med. Baltim. 95, e2875. doi:10.1097/MD.0000000000002875
Wu, R., Li, R. H., and Shi, H. P. (2011). Clinical application of Xingnaojing injection combined with local mild hypothermia in the early postoperative stage of hypertensive intracerebral hemorrhage. Chin. J. Pract. Nerv. Dis. 14, 78–79. doi:10.3969/j.issn.1673-5110.2011.09.048
Xia, Z. Q., Du, Y. T., Du, G. Y., Gao, L., Zhang, W., Li, T., et al. (2016). Observation on the therapeutic effect of Xingnaojing injection in the treatment of hypertensive intracerebral hemorrhage after puncture aspiration surgery. J. Clin. Res. 33, 2051–2053. doi:10.3969/j.issn.1671-7171.2016.10.062
Xiao, F., and Wu, J. B. (2021). The effect of Xingnao Jingdong Diphosphate Choline on postoperative efficacy in patients with cerebral hemorrhage. Chin. J. Gerontol. 41, 5508–5510. doi:10.3969/j.issn.1005-9202.2021.24.008
Xin, H. B. (2013). Clinical experience of Xingnaojing combined with intracranial hematoma removal surgery in the treatment of cerebral hemorrhage. China Pract. Med. 8, 155–156. doi:10.3969/j.issn.1673-7555.2013.06.119
Xu, S. B. (2020). Clinical value analysis of Xingnaojing injection combined with urokinase in the treatment of hypertensive intracerebral hemorrhage. J. Hubei Univ. Sci. Technol. Med. Sci. 34, 18–20. doi:10.16751/j.cnki.2095-4646.2020.01.0018
Xu, W. B., and Lv, J. G. (2015). Observation on the short-term therapeutic effect of Xingnaojing injection after surgery for hypertensive intracerebral hemorrhage. Med. Forum. 19, 47–48.
Xu, Y. M., Wang, X. C., Zhang, S. J., Xu, T. T., Li, H. Y., Hei, S. Y., et al. (2018). Role of Xingnaojing combined with naloxone in treating intracerebral haemorrhage: a systematic review and meta-analysis of randomized controlled trials. Med. Baltim. 97, e12967. doi:10.1097/MD.0000000000012967
Yang, D. J., Liu, Y. F., Ye, D. J., and Wu, Y. (2011). Craniopuncture scavenging technique and xingnaojing to treat hypertensive intracerebral hemorrhage. Chin. J. Hemorheol. 21, 611–613. doi:10.3969/j.issn.1009-881X.2011.04.015
Yang, L. X., Feng, W. H., Xia, B. H., Lin, L. M., Liu, W. W., Miao, W. Q., et al. (2016). HPLC specific chromatograms of Xingnaojing injection. China J. Chin. Mater. Med. 41, 1640–1645. doi:10.4268/cjcmm20160912
You, H. F. (2019). Effect of Xingnaojing combined with fasudil Hydrochloride on postoperative hypertensive intracerebral Hemorrhage. Contemp. Med. 25, 55–57. doi:10.3969/j.issn.1009-4393.2019.13.019
Yu, J. G., Li, R., Liang, W., Zhu, L. J., and Yao, Y. S. (2016). Effects of Xingnaojing on hypertensive cerebral hemorrhage: a Meta-analysis. Chin. J. Clin. Pharmacol. Ther. 21, 417–424.
Yu, M., Zhang, L. N., Ding, W., and Zhang, G. M. (2023). The effect and safety of minimally invasive surgery combined with Naoxueshu oral liquid on spontaneous cerebral hemorrhage: meta-analysis and quality evaluation. J. Hainan Med. Univ. 2023, 1–16. doi:10.13210/j.cnki.jhmu.20230728.003
Yue, M. X., Li, L., Lu, C. Z., and Jiang, L. Y. (2019). Expert consensus on the clinical application of Xingnaojing injection in the treatment of acute and critical diseases (symptom). Chin. J. Hyg. Rescue (Electron. Ed.) 5, 65–70. doi:10.3877/cma.j.issn.2095-9133.2019.02.001
Zhang, J. L., Wang, L. P., Yuan, S. S., and Liu, Y. M. (2004). Headspace solid-phase microextraction gas chromatography-mass spectrometry study on the fingerprint of Xingnaojing injection. China J. Chin. Mater. Med. 29, 86–87. doi:10.3321/j.issn:1001-5302.2004.05.027
Zhang, K. W., Liu, Y. P., Liu, C. Q., Bai, J. L., Lu, L. J., and Zhou, J. F. (2015). Observation on the therapeutic effect of soft channel puncture and drainage surgery on hypertensive intracerebral hemorrhage. Health Prot. promot. B, 146. doi:10.3969/j.issn.1671-0223.2015.06.133
Zhang, S. C. (2019). Clinical efficacy of Xingnaojing injection combined with puncture and drainage in the treatment of moderate cerebral hemorrhage in the basal ganglia region. Chin. J. Integr. Med. Cardio-Cerebrovasc. Dis. 17, 639–640. doi:10.12102/j.issn.1672-1349.2019.04.050
Zhang, S. P., Gao, Y. S., Hu, C. X., Luo, X. M., Guo, H. W., and Chai, C. (2016). Observation on the therapeutic effect of Xingnaojing injection combined with puncture aspiration for the treatment of hypertensive intracerebral hemorrhage. Chin. J. Pract. Nerv. Dis. 19, 121–122. doi:10.3969/j.issn.1673-5110.2016.13.079
Zhao, X. P. (2011). Effect of Xingnaojing injection on patients with cerebral hemorrhage minimally invasive cranial hematoma. Hebei J. Tradit. Chin. Med. 33, 1845–1847. doi:10.3969/j.issn.1002-2619.2011.12.047
Zhou, L. L., and Sun, M. F. (2017). Analysis on the effect of Xingnaojing injection on clinical indexes of patients with hypertensive intracerebral hemorrhage. China Mod. doct. 55, 116–118.
Zhou, R. Z. (2014). Observation on the therapeutic effect of minimally invasive intracranial hematoma removal surgery combined with Xingnaojing injection in the treatment of hypertensive intracerebral hemorrhage. Med. Inf. 27, 95. doi:10.3969/j.issn.1006-1959.2014.13.104
Keywords: Xingnaojing injection, traditional Chinese medicine, Chinese patent medicine, injection, intracerebral haemorrhage, post-operative patients, meta-analysis
Citation: Song Y, Xu F, Li S, Sun Y and Wang X (2024) Efficacy and safety of Xingnaojing injection for post-operative patients of intracerebral haemorrhage: a meta-analysis and systematic review. Front. Pharmacol. 15:1411026. doi: 10.3389/fphar.2024.1411026
Received: 02 April 2024; Accepted: 08 May 2024;
Published: 05 June 2024.
Edited by:
Junfeng Wang, Massachusetts General Hospital and Harvard Medical School, United StatesReviewed by:
Luo Qiang, Children’s Hospital of Chongqing Medical University, ChinaYinhui Yao, Affiliated Hospital of Chengde Medical University, China
Copyright © 2024 Song, Xu, Li, Sun and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yongkang Sun, c3VueW9uZ2thbmcwMEAxMjYuY29t; Xinzhi Wang, em5xem5xQDE2My5jb20=
†These authors have contributed equally to this work and share first authorship
 Yanbo Song
Yanbo Song Fangbiao Xu
Fangbiao Xu Shuliang Li1,2
Shuliang Li1,2 Yongkang Sun
Yongkang Sun