- 1Department of Rehabilitation, Guangdong Provincial Hospital of Chinese Medicine, The Second Affiliated Hospital of Guangzhou University of Chinese Medicine, Guangzhou, China
- 2The Second Clinical College of Guangzhou University of Chinese Medicine, Guangzhou, China
- 3Medical College of Acu-Moxi and Rehabilitation, Guangzhou University of Chinese Medicine, Guangzhou, China
Background: Using Shuxuening injection (SXNI) for acute ischemic stroke (AIS) is popular in China, but its efficacy and safety remain controversial.
Purpose: This study aims to assess the efficacy and safety of SXNI as an add-on therapy for AIS.
Study design: Systematic review and meta-analysis.
Methods: We searched for randomized controlled trials (RCTs) on SXNI for AIS in seven databases and two clinical trial registration platforms from their inception to January 2023. We used the Cochrane risk of bias tool to assess the methodological quality of the included studies and performed the meta-analysis with R software. The primary outcome was clinical efficacy, assessed by the clinical effective rate (CER). The secondary outcomes were neurological function, activities of daily living (ADL), and adverse events (AEs).
Results: In total, 116 studies with 12,401 participants were included in this review. Fifteen (12.9%) studies were judged to be of moderate to high quality. SXNI plus conventional treatments (CTs) improved the CER compared with CTs alone (risk ratio [RR]: 1.21, 95% confidence interval [CI]: 1.17–1.25, p < 0.0001) or CTs plus other injections (RR: 1.18, 95% CI: 1.15–1.21, p < 0.0001). SXNI plus CTs reduced the National Institute of Health Stroke Scale score compared with CTs alone (mean difference [MD]: −4.00, 95% CI: −5.22 to −2.78, p < 0.0001) or CTs plus other injections (MD: −2.28, 95% CI: −3.41 to −1.16, p < 0.0001). SXNI plus CTs also decreased the Chinese Stroke Scale score compared with CTs alone (MD:
−5.01, 95% CI: −7.38 to −2.65, p < 0.0001) or CTs plus other injections (MD: −4.31, 95% CI: −5.75 to −2.88, p < 0.0001). SXNI plus CTs was superior for increasing the Barthel index score compared with CTs alone (MD: 11.58, 95% CI: 8.27–14.90, p < 0.0001) or CTs plus other injections (MD: 5.43, 95% CI: 0.48–10.39, p = 0.0317). The level of evidence for each outcome was assessed as low to very low. The most common AEs of SXNI were cardiovascular system events, and all these AEs were mild.
Conclusion: SXNI combined with CTs maybe better than CTs alone or CTs plus other injections for improving the clinical efficacy, neurological function, and ADL of AIS patients, with relatively reliable safety. However, due to the low quality of the included studies, more rigorously designed RCTs with large sample sizes should be conducted in the future.
Systematic Review Registration:: www.crd.york.ac.uk, identifier (CRD42023418565).
1 Introduction
Stroke is the third leading cause of death and disability globally, especially in low- and middle-income countries (GBD, 2019 Stroke Collaborators, 2021). From 1990 to 2019, the absolute number of global stroke events increased by 70.0%, and stroke mortality increased by 43.0% worldwide (GBD, 2019 Stroke Collaborators, 2021). Stroke patients often have motor dysfunction, loss of activities of daily living (ADL) (Jørgensen et al., 1999), and a significant decline in quality of life, which prevents them from returning to work, thereby resulting in economic losses for the country’s productivity (Rochmah et al., 2021). According to the American Heart Association, the total cost of stroke, including direct and indirect expenditures, is projected to increase from $105.2 billion in $2012 to $240.7 billion in 2030 (Ovbiagele et al., 2013).
Acute ischemic stroke (AIS) accounts for most of all strokes. The appropriate treatment for AIS is significantly associated with prognosis (Hasan et al., 2021; Rosa et al., 2022). The primary therapeutic goal of AIS is to restore perfusion of ischemic brain tissue, and the main treatments include intravenous thrombolysis (IVT) and/or endovascular thrombectomy (EVT) (Hasan et al., 2021). However, 3%–8% of patients receiving IVT experience symptomatic cerebral hemorrhage (Gumbinger et al., 2012). Due to the narrow time window, contraindications, and the relatively low recanalization rate of large artery occlusions, the number of AIS patients who benefit from IVT is not as large as expected (Bhatia et al., 2010). EVT can significantly improve the prognosis of stroke; however, most interventional neuroradiologists work in urban areas, while half of the world’s population lives in rural areas, resulting in limited access to EVT (Wassélius et al., 2022). Therefore, it is necessary to find an effective and safe therapy with a longer time window to improve the prognosis of stroke and address its burden.
Shuxuening injection (SXNI) extracted from Ginkgo biloba is a commercial Chinese polyherbal preparation that is widely used for stroke in China (Cui et al., 2020; Li et al., 2023). SXNI can dilate blood vessels and improve blood circulation, and it is mainly used for ischemia cardio-cerebrovascular diseases, such as cerebral infarction, vasospasm and coronary heart disease. The pharmacologically active metabolites of SXNI are flavonol glycosides and ginkgolide (Smith et al., 1996; Shi et al., 2009). Numerous in vitro and in vivo studies have confirmed the neuroprotective effect of G. biloba extract (GBE) (Oyama et al., 1996; Guidetti et al., 2001; Ahlemeyer and Krieglstein, 2003). GBE can improve blood circulation, strengthen capillary walls, prevent thrombosis, and protect nerve cells from damage during hypoxia (Singh et al., 2019). Two studies (Oskouei et al., 2013; Li et al., 2017) showed that GBE was better than placebo or aspirin in improving stroke patients’ neurological function, as assessed by the National Institutes of Health Stroke Scale (NIHSS). More interestingly, GBE can work within 1–3 h (Diamond et al., 2000). Compared with IVT or EVT, SXNI not only has a longer treatment time window and cheaper costs with fewer side effects (Li et al., 2023) but can also be implemented in urban and rural medical places without specially higher professional requirements for doctors. However, due to the complexity of the metabolites of SXNI, it requires more careful monitoring for patients to prevent adverse events (AEs) while administering the drug.
Previous systematic reviews published in 2011, 2012, and 2016 confirmed the efficacy of SXNI for treating AIS in terms of the clinical efficacy assessed by the clinical effective rate (CER), with a very low level of evidence. However, some outcomes that are conducive to assessing the overall status of stroke patients, such as neurological function assessed by internationally recognized tools (e.g., the NIHSS), activities of daily living (ADL), and AEs, were not reported in these reviews. In addition, numerous RCTs on SXNI for AIS have been published since 2016, and it is necessary to update the evidence. We aimed to comprehensively assess the efficacy and safety of SXNI as an add-on therapy for AIS in terms of clinical efficacy, neurological function, ADL, and AEs.
2 Methods
This study was conducted strictly following the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines (Page et al., 2021). The protocol was registered at PROSPERO (www.crd.york.ac.uk), and the registration number was CRD42023418565.
2.1 Types of studies
All RCTs about SXNI for AIS were eligible for inclusion. The language of publication was not limited. We excluded case-control studies, retrospective studies, case reports, animal experiments, cell experiments, reviews, meta-analyses, clinical experiences, commentaries, and conference abstracts.
2.2 Participants
Patients who were older than 18 years and diagnosed with AIS according to the recognized guidelines or criteria and confirmed by computed tomography (CT) or magnetic resonance imaging (MRI) were included without limitations on sex, culture, nationality, or race. The duration of the stroke should have been less than 14 days. We excluded patients with hypoxic-ischemic encephalopathy and postpartum apoplexy.
2.3 Types of interventions
The SXNI group received SXNI combined with conventional treatments (CTs), and the non-SXNI group received CTs alone or CTs combined with other injections. According to the Chinese guidelines for the diagnosis and treatment of AIS 2018; Chinese Society of Neurology Chinese Stroke Society, 2018), CTs include IVT, EVT, antiplatelet drugs, neuroprotective agents, and symptomatic supportive treatment, such as the management of blood pressure and blood lipid and sugar levels. Other injections were defined as any monodrug of non-Ginkgo biloba extract, such as compound Danshen injection, Shuxuetong injection, and so on. The duration of treatment had to be between 14 and 30 days. There were no restrictions on daily dose or frequency.
2.4 Outcome measures
The primary outcome was clinical efficacy assessed by the CER. According to the scoring criteria for clinical neurological deficit (CND) in stroke patients (Fourth Session of Chinese National Cerebrovascular Conference, 1996), improvements in CND were classified into five categories: a) recovery: 90%–100% reduction in CND with a disability level of 0; b) significant improvement: 46%–89% reduction in CND with a disability level of 1–3; c) progress: 18%–45% reduction in CND; d) inefficacy: less than 17% reduction in CND; e) deterioration: greater increase in CND. CER = (a + b + c) cases/total cases × 100% (National Administration of Traditional Chinese Medicine, 1994; Zheng, 2002).
The secondary outcomes included a) neurological function assessed by the Chinese Stroke Scale (CSS) and the NIHSS; b) ADL assessed by the Barthel Index (BI); c) AEs. The CSS consists of eight dimensions with a total score of 45. A higher CSS score indicates that a patient’s stroke is more severe (Fourth Session of Chinese National Cerebrovascular Conference, 1996). The NHISS consists of 15 items with a total score of 42 and is a validated tool for assessing the severity of acute stroke. The greater the NIHSS score is, the more severe the neurological deficit (Brott et al., 1989; Kwah and Diong, 2014). The highest BI score is 100, and the higher the BI score is, the better the individual’s ability to perform ADLs (Mahoney and Barthel, 1965). The common AEs related to SXNI are headache, flushing, and so on.
2.5 Data sources and searches
Seven databases were searched from their inception to January 2023, including PubMed, the Cochrane Library, Embase, the China National Knowledge Infrastructure (CNKI), the WanFang Database, the Chinese Scientific Journal Database (VIP), and the Chinese Biological Medicine Database (CBM). We also searched the Chinese Clinical Trial Registry (http://www.chictr.org.cn/) and the U.S. Clinical Trial Registry (https://clinicaltrials.gov/). We screened the reference lists of all included studies to identify more eligible studies. The search terms included (“stroke” OR “palsy” OR “apoplexy” OR “apoplexia” OR “cerebrovascular disorders” OR “cerebral infarction” OR “infarct of brain” OR “cerebral hemorrhage” OR “intracerebral hemorrhage” OR “brain hemorrhage”) AND (“shuxuening” OR “shuxuening injection” OR “Ginkgo Leaf” OR “Folium Ginkgo” OR “Chinese patent medicine”). The detailed search strategy is described in Supplementary Appendix 1.
2.6 Selection of studies
We not only removed reduplicated articles using NoteExpress 3.6 but also performed manual de-duplication. Then, two reviewers independently screened the literature based on predefined eligibility criteria. We first excluded irrelevant literature by reading the titles and abstracts and then reading the full texts to select eligible studies. When two or more studies reported the same RCT, we selected the study with the largest sample size, appropriate outcomes, and the earliest publication. We consulted a third reviewer to resolve any disagreements.
2.7 Data extraction and management
We developed standardized data extraction forms that included study information (e.g., author, publication year, city, and country), characteristics of participants (e.g., age, sex, and course of stroke), study design (e.g., sample size, interventions, administration methods, duration of intervention, and SXNI manufacturing company), and outcomes (e.g., CER, NIHSS, CSS, BI, and AEs). Two reviewers extracted the data independently and cross-checked the extracted data. We contacted the authors via email when the information in the article was unclear or insufficient. If there were disagreements on data extraction, a third reviewer was consulted.
2.8 Quality assessment
The quality of the included studies was evaluated by two reviewers using the Cochrane risk of bias (ROB) assessment tool (Higgins and Green, 2011). The ROB tool includes seven items: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other biases. Each item can be judged as “low risk”, “high risk” or “unclear risk”. If a study had a “low risk” of more than four domains, its overall quality was judged as high. Any disagreements between the two reviewers were resolved by a third reviewer.
2.9 Data synthesis
We used R software (version 4.1.2, https://cran.r-project.org/) and Review Manager software (version 5.4, Cochrane Collaboration, Copenhagen, Denmark) to analyze the data. Dichotomous data are expressed as risk ratios (RRs) and 95% confidence intervals (CIs). Continuous data assessed using the same scale are expressed as weighted mean differences (WMDs) and 95% CIs, whereas standardized mean differences (SMDs) and 95% CIs were used for the different scales. Statistical heterogeneity was assessed by I2 and p values from the Cochrane Q test. I2 < 50% and p ≥ 0.05 indicated no heterogeneity among studies. A random effects model was used if heterogeneity existed; otherwise, the fixed effects model was used. p < 0.05 was considered to indicate a statistically significant difference.
2.10 Sensitivity analysis
We tested the stability of the meta-analysis results by alternating the random and fixed effects models. Moreover, we excluded each study in turn to test the robustness of the results.
2.11 Subgroup analysis
We performed a subgroup analysis based on the duration of intervention, daily dose, and different injections used in the control group. We also conducted mixed-effects meta-regression analysis using sample size (n ≤ 107 vs. n > 107), quality of studies (high vs. low), publication year, mean age (≤60 years old vs. > 60 years old vs. mixed), and total dose of SXNI (≤280 mL vs. > 280 mL) as covariates to search for sources of heterogeneity.
2.12 Publication bias
Publication bias was assessed with “funnel” and Egger’s tests when the number of included studies was more than 10.
2.13 Quality of evidence
We evaluated the quality of evidence for each outcome using the Grading of Recommendation, Assessment, Development, and Evaluation (GRADE) tool (https://www.gradepro.org/). The GRADE included five domains: a) risk of bias, b) inconsistency, c) indirectness, d) imprecision, and e) other considerations, such as publication bias and number of included studies. The overall level of evidence for every outcome was judged as high, moderate, low, or very low. Any disagreement regarding the level of evidence was resolved through discussion and consultation.
3 Results
3.1 Study selection
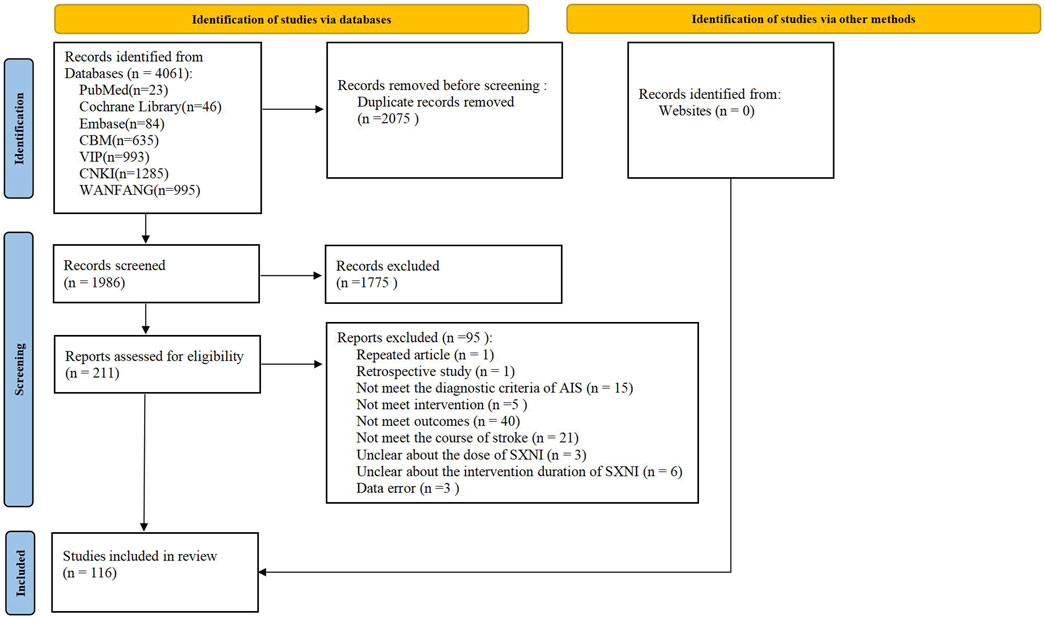
A total of 4,061 records were retrieved, 2075 of which were duplicate publications. After removing 1,775 records that did not meet the criteria by screening the titles and abstracts, we read the full texts of 211 articles. Finally, 116 eligible studies were included in the meta-analysis. The details of the study selection process are displayed in Figure 1. The full citation details of the included and excluded studies are described in Supplementary Appendix 2.
3.2 Characteristics of the included studies
All included studies were conducted in China and published in Chinese, with publication years distributed between 2002 and 2023. The most frequent provinces of author affiliation were Henan, Shandong, Guangdong, and Hebei. The sample sizes were between 40 and 438, and the duration of AIS ranged from less than 12 h to 14 days. The intervention duration ranged from 14 to 30 days. The daily dose of SXNI varied from 2 to 30 mL. The total dose of SXNI was between 28 and 900 mL. Thirty-six studies with 3,568 participants compared the efficacy of SXNI plus CTs vs. CTs alone, and eight studies with 8,833 participants compared the efficacy of SXNI plus CTs vs. CTs plus other injections. Of the 116 studies, 102 reported the CER, 14 reported the BI, 44 reported the CSS, and 16 reported the NIHSS. The characteristics of the included studies are described in Table 1.
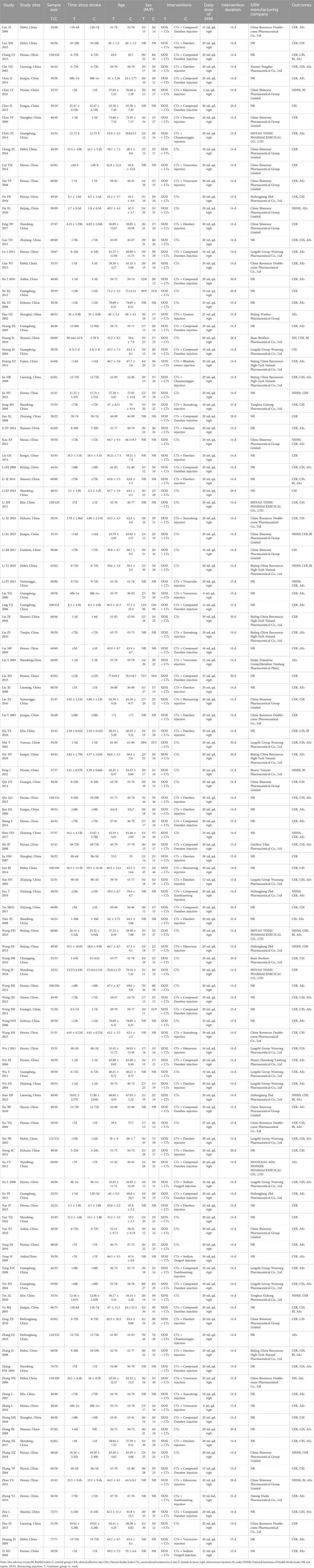
Table 1. Characteristics of the included RCTs. (Note. AEs, adverse events; BI, Barthel index; C, control group; CER, clinical effective rate; CSS, Chinese Stroke Scale; CTs, conventional treatments; d, day; F, female; h, hour; IVGTT, intravenous injection; M, male; NIHSS, National Institutes of Health Stroke Scale; NR, not reported; SXNI, shuxuening injection; T, treatment group; w, week.)
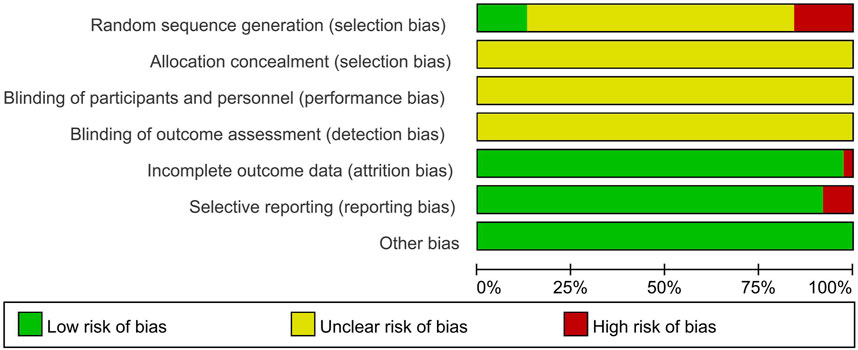
3.3 The quality of the included studies
Fifteen (12.9%) studies were judged to be of moderate to high quality (Table 2; Figure 2). Of 116 studies, 110 (94.8%) mentioned the word “randomization”, 15 (12.9%) used random number table methods, and the remaining studies used incorrect or undetailed randomization methods. Five (4.3%) studies mentioned the word “blinding” without a detailed description of the specific methods. None of the studies reported allocation concealment. Three (2.6%) studies were judged as high risk because they did not conduct intention-to-treat analysis and did not specify the reason for case dropout. Nine (7.8%) studies were assessed as high risk for selective reporting based on the description of the methods in the article. All included studies were free of other sources of ROB.
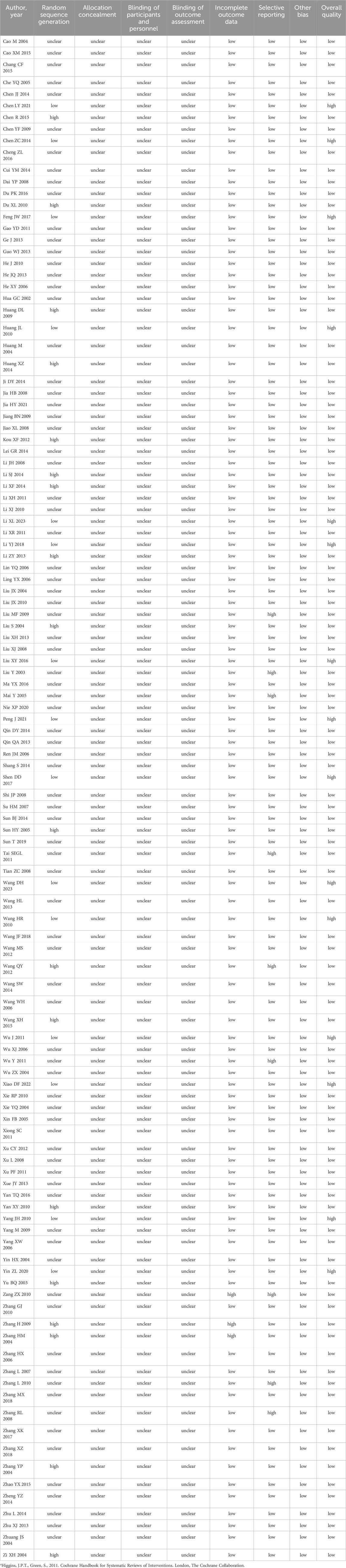
Table 2. Quality assessment of the included studies using the Cochrane risk of bias (ROB) assessment toola.
3.4 Primary outcome
3.4.1 Clinical efficacy
3.4.1.1 SXNI plus CTs vs. CTs alone
Thirty-two studies with 3,056 participants reported the CER. We used a fixed effects model due to the low heterogeneity (I2 = 0%, p = 0.68). The meta-analysis results showed that SXNI plus CTs was superior to CTs alone in terms of the CER (RR: 1.21, 95% CI: 1.17–1.25, Z = 11.22, p < 0.05) (Figure 3A). Sensitivity analysis was conducted by adjusting the random and fixed effects models (Supplementary Table S1) or excluding each study in turn (Supplementary Figure S1), which demonstrated that the results were robust. Subgroup analysis revealed that the effects of different daily doses and different intervention durations of SXNI on CER were not significantly different between SXNI plus CTs and CTs alone (Supplementary Figures S2, S3). Mixed-effect meta-regression analysis further revealed that the sample size, mean age, total dose of SXNI, publication year, and quality of studies were not the main sources of heterogeneity (Supplementary Table S2). Egger’s test (t = 4.80, p < 0.05) and funnel plots revealed significant publication bias (Figure 4A).
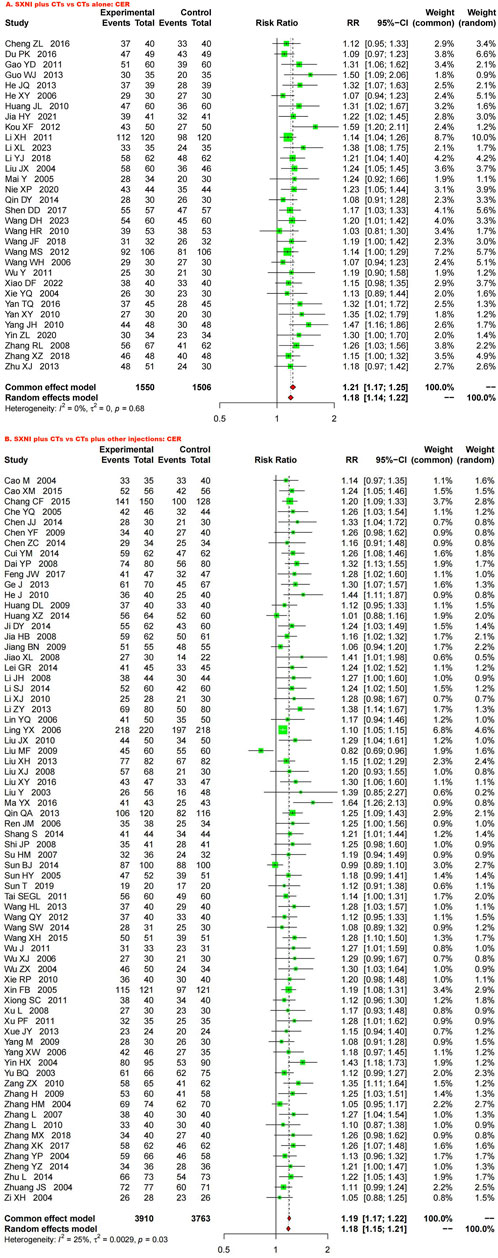
Figure 3. Forest plot and meta-analysis of clinical efficacy. (Note: CER, clinical effective rate; CTs, conventional treatments; SXNI, shuxuening injection).
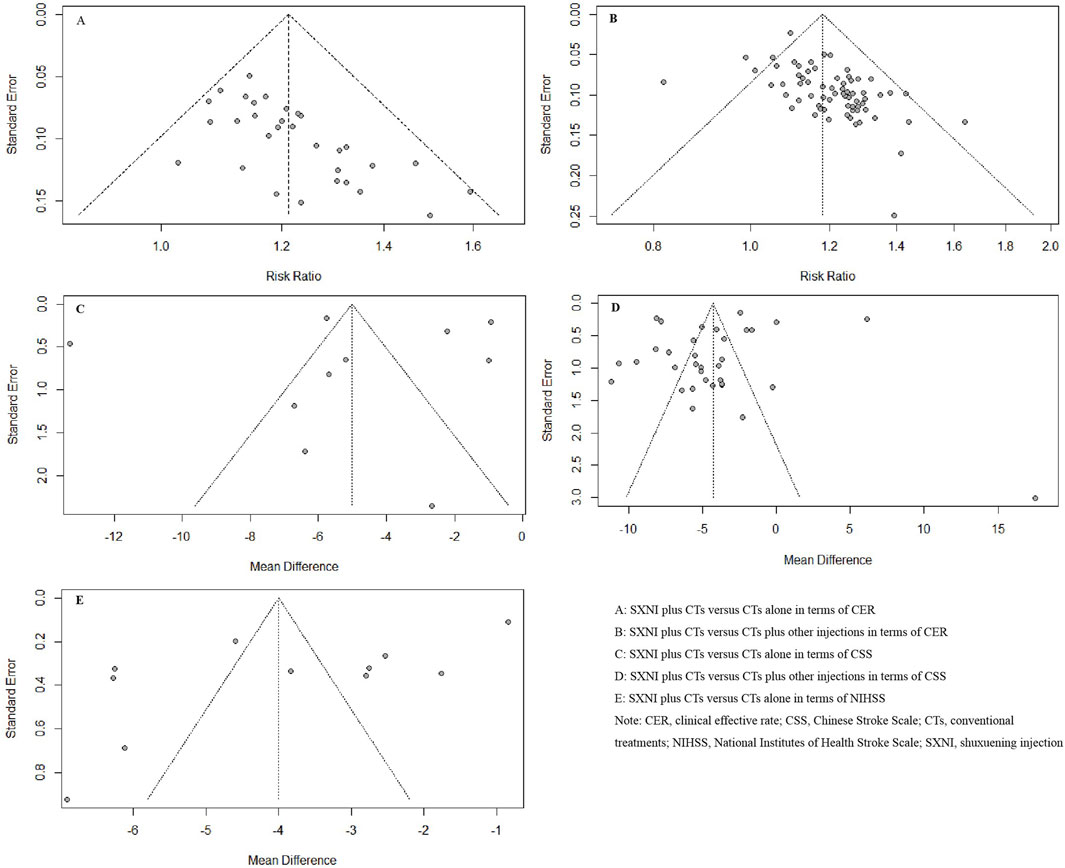
Figure 4. Funnel plots based on the CER, NIHSS, and CSS scores. (Note. CER, clinical effective rate; CSS, Chinese Stroke Scale; CTs, conventional treatments; NIHSS, National Institutes of Health Stroke Scale; SXNI, shuxuening injection).
3.4.1.2 SXNI plus CTs vs. CTs plus other injections
Seventy studies with 7,673 participants reported CER. A random effects model was used because of statistical heterogeneity (I2 = 25%, p < 0.05). The results of the meta-analysis showed that SXNI plus CTs was superior to CTs plus other injections in improving the CER (RR: 1.18, 95% CI: 1.15–1.21, Z = 13.26, p < 0.05) (Figure 3B). By adjusting the analysis models (Supplementary Table S1) or removing each study in turn (Supplementary Figure S4), sensitivity analysis indicated that the results were robust. Subgroup analysis revealed that the effects of different daily doses, different intervention durations, and different injections on CER were not significantly different between SXNI plus CTs and CTs plus other injections (Supplementary Figures S5–7). Mixed-effect meta-regression models did not reveal sample size, mean age, total dose of SXNI, publication year, or quality of studies as sources of heterogeneity (Supplementary Table S2). The results of Egger’s test (t = 5.25, p < 0.05) and funnel plots showed publication bias (Figure 4B).
3.5 Secondary outcomes
3.5.1 Neurological function
3.5.1.1 SXNI plus CTs vs. CTs alone
Eleven studies, including 1,126 participants, assessed neurological function using the NIHSS. We used a random effects model due to the significant heterogeneity (I2 = 98%, p < 0.05). SXNI plus CTs was superior for decreasing the NIHSS score compared with CTs alone (MD: −4.00, 95% CI: −5.22 to −2.78, Z = −6.42, p < 0.05) (Figure 5A). Sensitivity analysis was conducted by adjusting the random and fixed effects models (Supplementary Table S1) or deleting each study in turn (Supplementary Figure S8), which demonstrated that the meta-analysis results were robust. Mixed-effect meta-regression analysis revealed that the sample size, mean age, total dose of SXNI, publication year, and quality of studies did not contribute to the heterogeneity (Supplementary Table S2). Egger’s test (t = −2.99, p < 0.05) and funnel plots revealed publication bias (Figure 4E).
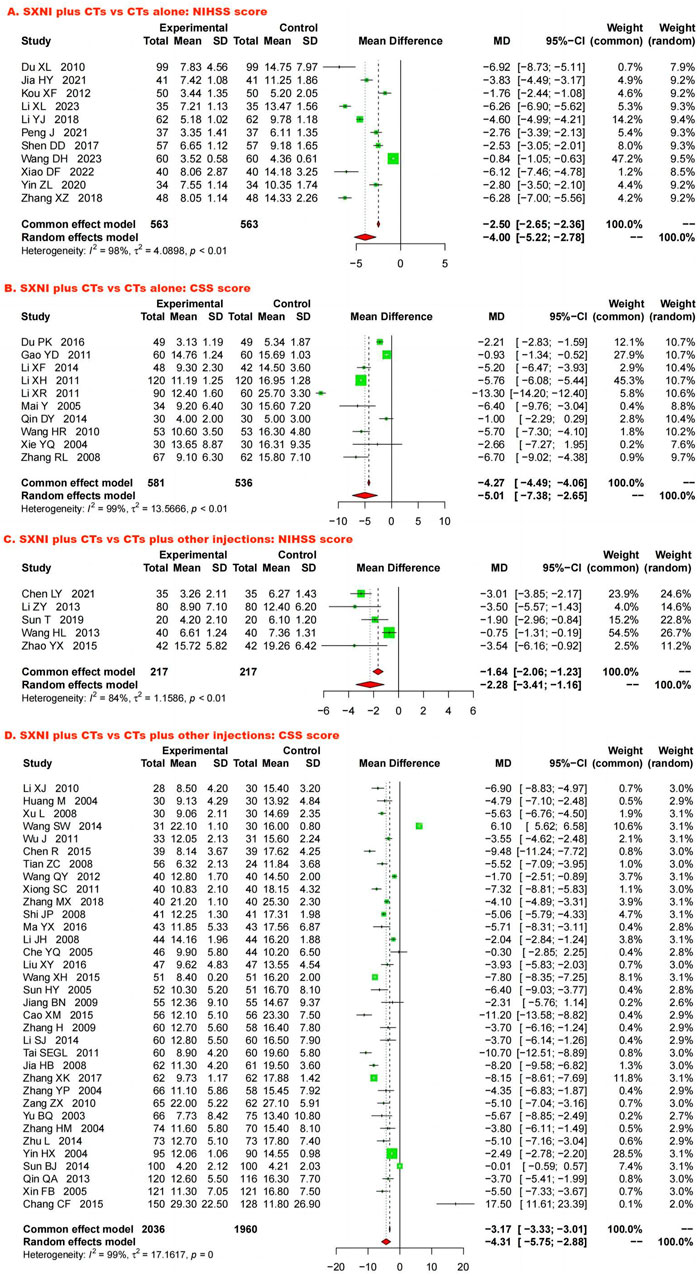
Figure 5. Forest plot and meta-analysis of neurological function. (Note. CSS, Chinese Stroke Scale; CTs, conventional treatments; NIHSS, National Institutes of Health Stroke Scale; SXNI, shuxuening injection).
Ten studies, including 1,117 participants, evaluated neurological function using CSS. Because of the significant heterogeneity (I2 = 99%, p < 0.05), we used a random effects model to pool the data. SXNI plus CTs was superior to CTs alone for decreasing the CSS (MD: −5.01, 95% CI: −7.38 to −2.65, Z = −4.15; p < 0.05) (Figure 5B). By adjusting the analysis models (Supplementary Table S1) or removing each study in turn (Supplementary Figure S9), sensitivity analysis indicated that the results were robust. Mixed-effect meta-regression analysis based on sample size, mean age, total dose of SXNI, publication year, and quality of studies did not reveal sources of heterogeneity (Supplementary Table S2). Egger’s test (t = −0.36, p > 0.05) and funnel plots revealed no publication bias (Figure 4C).
3.5.1.2 SXNI plus CTs vs. CTs plus other injections
Five studies with 434 participants assessed neurological function via the NIHSS. Because of significant heterogeneity (I2 = 84%, p < 0.05), we used a random effects model. The meta-analysis indicated that SXNI plus CTs improved the NIHSS score better than CTs plus other injections did (MD: −2.28, 95% CI: −3.41 to −1.16, Z = −3.97, p < 0.05) (Figure 5C). Sensitivity analysis was conducted by adjusting the random and fixed effects models (Supplementary Table S1) or deleting each study in turn (Supplementary Figure S10), which demonstrated that the meta-analysis results were robust. No source of heterogeneity was found in mixed-effect meta-regression models based on sample size, mean age, total dose of SXNI, publication year, or quality of studies (Supplementary Table S2).
Thirty-four studies involving 3,996 participants assessed neurological function using the CSS. A random effects model was used to pool the data due to the significant heterogeneity (I2 = 99%, p < 0.05). The results of the meta-analysis showed that SXNI plus CTs decreased the CSS score in AIS patients more than CTs plus other injections did (MD: −4.31, 95% CI: −5.75 to −2.88, Z = −5.89, p < 0.05) (Figure 5D). By adjusting the analysis models (Supplementary Table S1) or removing each study in turn (Supplementary Figure S11), sensitivity analysis indicated that the results were robust. Subgroup analysis indicated that the effects of different daily doses, different intervention durations, and different injections on decreasing the CSS score were not significantly different between SXNI plus CTs and CTs plus other injections (Supplementary Figures S12–14). Mixed-effect meta-regression models revealed that sample size, mean age, total dose of SXNI, publication year, and quality of studies were not the main sources of heterogeneity (Supplementary Table S2). Egger’s test (t = −1.20, p > 0.05) and funnel plots revealed no publication bias (Figure 4D).
3.5.2 Activities of daily living
3.5.2.1 SXNI plus CTs vs. CTs alone
Seven studies with 648 participants assessed ADLs using the BI. We used a random effects model to pool the data because of the substantial heterogeneity (I2 = 96%, p < 0.05). The meta-analysis showed that SXNI plus CTs was better at improving the BI score than CTs alone (MD: 11.58, 95% CI: 8.27–14.90, Z = 6.84, p < 0.05) (Figure 6A). By adjusting the analysis models (Supplementary Table S1) or removing each study in turn (Supplementary Figure S15), sensitivity analysis demonstrated the reliability of the results. Mixed-effect meta-regression analysis revealed that sample size, mean age, total dose of SXNI, publication year, and quality of studies were not the sources of heterogeneity (Supplementary Table S2).
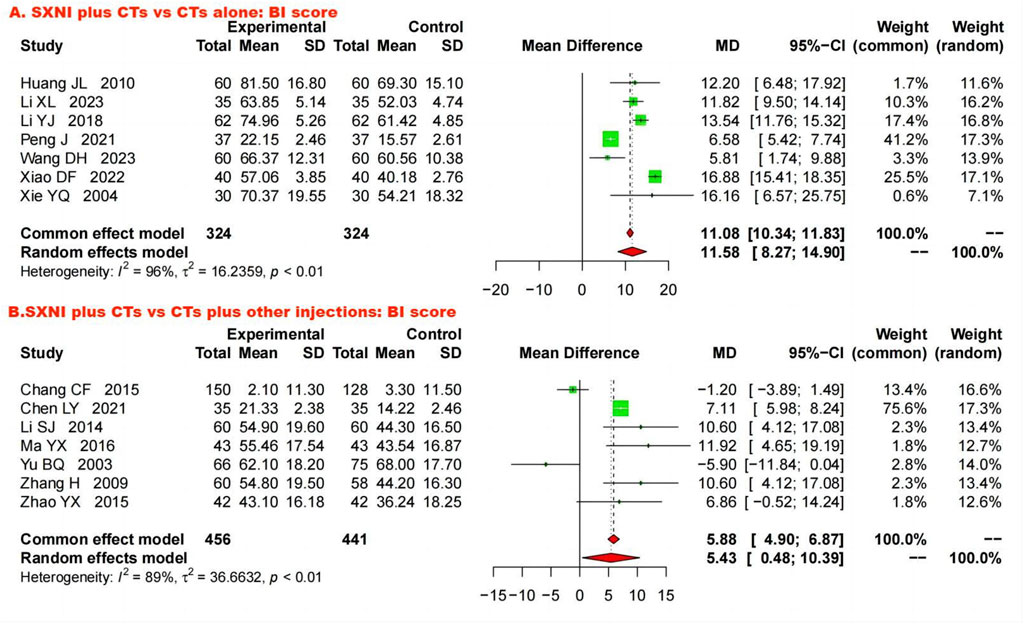
Figure 6. Forest plot and meta-analysis of activities of daily living. (Note. BI, Barthel index; CTs, conventional treatments; SXNI, shuxuening injection).
3.5.2.2 SXNI plus CTs vs. CTs plus other injections
Seven studies involving 897 participants evaluated ADL by the BI. Due to significant heterogeneity (I2 = 89%, p < 0.05), a random effects model was used. The results showed that SXNI plus CTs was superior to CTs plus other injections for increasing BI scores (MD: 5.43, 95% CI: 0.48–10.39, Z = 2.15, p < 0.05) (Figure 6B). Sensitivity analysis by adjusting the statistical models showed that the meta-analysis results were robust (Supplementary Table S1). However, the difference in the BI scores between SXNI plus CTs and CTs plus other injections was not significant when five studies were removed one at a time (Chen, 2021; Li, 2014; Ma, 2016; Zhang et al., 2009; Zhao, 2015) (Supplementary Figure S16). Mixed-effect meta-regression did not reveal sample size, mean age, total dose of SXNI, publication year, or quality of studies as sources of heterogeneity (Supplementary Table S1).
3.6 AEs
Seventy-one studies reported AEs, and 49 reported no AEs. Twenty-two studies reported AEs related to the SXNI group or non-SXNI group, five of which did not report the number of AEs in the SXNI group, and two did not report the number of AEs in the non-SXNI group. SXNI plus CTs (1.53%, 61/3,994) was similarly safe to CTs alone or CTs plus other injections (1.32%, 50/3,797). The most common AEs related to SXNI were cardiovascular system events, and the five most common symptoms were dizziness, flushing, palpitations, nausea, and headache. The most common AEs related to the non-SXNI group were digestive system events, and the five most common symptoms were palpitations, nausea, dizziness, flushing, and vomiting. Moreover, all these symptoms in both the SXNI group and the non-SXNI group were mild and disappeared after discontinuation of the drug and symptomatic treatment. The symptoms of AEs are detailed in Supplementary Table S3.
3.7 Quality of evidence
We used the GRADE tool to systematically evaluate the quality of each outcome. The levels of evidence for CER and BI scores were low in SXNI plus CTs compared with CTs alone for AIS, while the levels of evidence for the NIHSS score and CSS score were very low (Table 3). Additionally, the level of evidence for the CER, NIHSS, CSS, and BI scores were all very low for SXNI plus CTs compared with CTs plus other injections for AIS (Table 3). The grade was decreased mainly because of the low methodological quality of the included studies, high heterogeneity among the included studies, and potential publication bias.
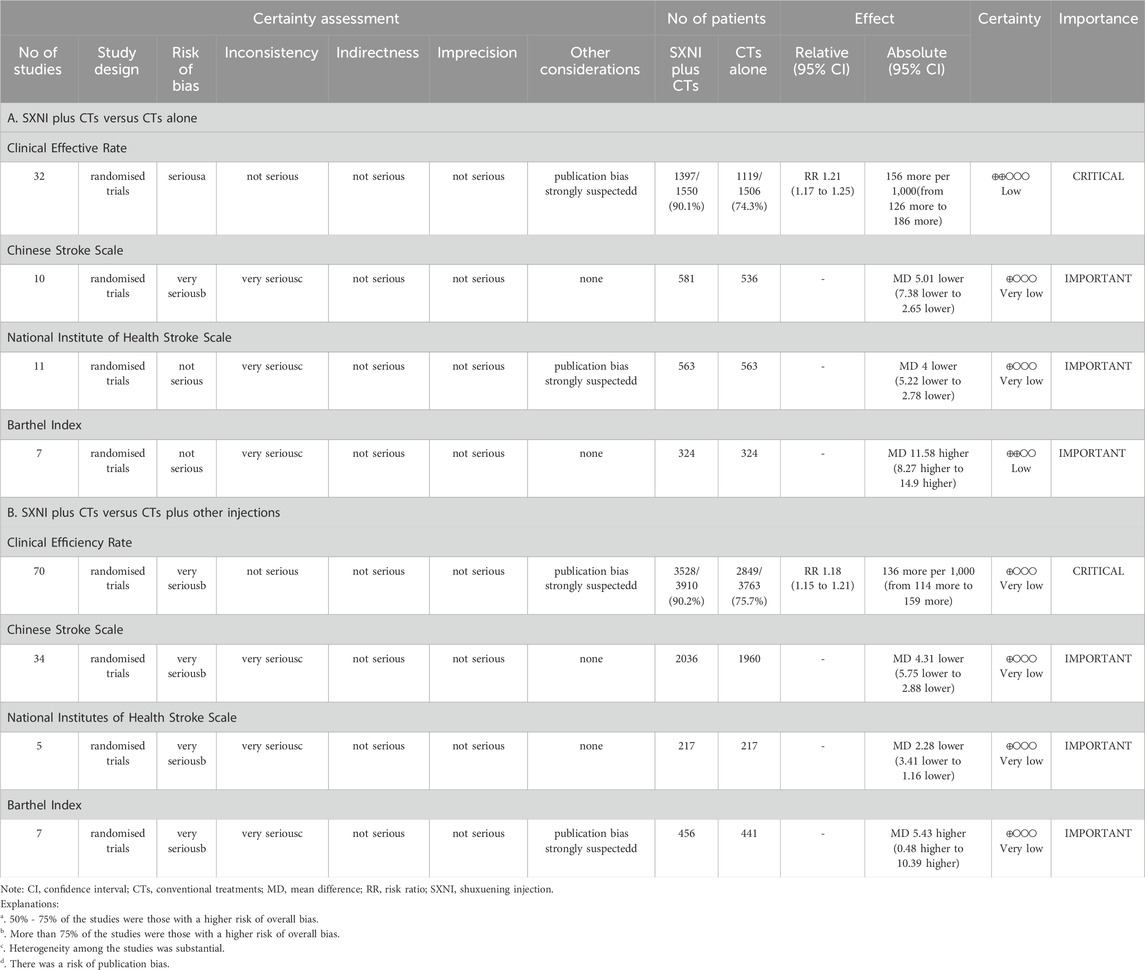
Table 3. Summary of findings according to the Grading Recommendations Assessment, Development, and Evaluations (GRADE) tool. (Note. CI, confidence interval; CTs, conventional treatments; MD, mean difference; RR, risk ratio; SXNI, shuxuening injection).
4 Discussion
4.1 Summary of the evidence
A total of 116 studies involving 12,401 participants were included in this review to assess the efficacy and safety of SXNI as an add-on therapy for patients with AIS. The meta-analysis results showed that SXNI plus CTs was superior to CTs alone or CTs plus other injections in improving patients’ CER, NIHSS, CSS, and BI scores, suggesting that SXNI combined with CTs can significantly improve clinical efficacy, reduce neurological deficits, and promote the recovery of ADL in patients with AIS. Subgroup analysis and mixed-effect meta-regression analysis indicated that duration of intervention, daily dose of SXNI, total dose of SXNI, sample size, mean age, different injections used in the control group, quality of studies, and publication year were not sources of heterogeneity. Except for the BI score between SXNI plus CTs and CTs plus other injections, the sensitivity analysis of the remaining indicators demonstrated that the meta-analysis results were robust. Sensitivity analysis revealed that the difference in BI scores between SXNI plus CTs and CTs plus other injections was not significant when five studies were removed one at a time (Chen, 2021; Li, 2014; Ma, 2016; Zhang et al., 2009; Zhao, 2015). In terms of safety, based on the results of this study, we cautiously believe that SXNI is relatively safe and reliable. Publication bias was found in the CER and NIHSS scores. Only 15 (12.9%) studies were judged to be of moderate to high quality. The levels of evidence for the CER, NIHSS, CSS, and BI scores were very low to low quality. Therefore, the results of this study should be considered with caution.
According to traditional Chinese medicine theory, the main pathological changes in AIS are qi deficiency and blood stasis. Qi is regarded as the main material basement that constitutes the human body and maintains life activities and an energy which manifests simultaneously on the physical and emotional-mental-spiritual level, and it has functions of promoting, warming, defense, transformative action and containment) (Jiang et al., 2023; Tian et al., 2024). Therefore, clinical treatment of this disease is mainly carried out by supplementing qi, promoting blood circulation, and dredging meridians and collaterals. SXNI is an extract of G. biloba leaves that promotes blood circulation, eliminates blood stasis, unblocks meridians, tonifies qi, and strengthens the brain (Ding, 2018; Zhang, 2019). From the perspective of Western medicine, SXNI can improve blood rheology, inhibit platelet aggregation, and reduce blood viscosity (Zhang et al., 2005). Ginkgolides can inhibit glutamate receptor-gated calcium channels, significantly reduce Ca2+ content in brain cells, prevent intracellular Ca2+ overload, and reduce cascade reaction-induced brain cell necrosis (Chandrasekaran et al., 2001). SXNI can improve the energy metabolism and nutrition of hypoxic brain cells via its antioxidant properties (Smith et al., 2002; Nash and Shah, 2015) and reduce the apoptosis of neuronal cells in brain tissue through the inhibition of multiple inflammatory responses and the modulation of oxidative stress levels (Dong et al., 2023). Moreover, SXNI can increase superoxide dismutase activity against oxidative damage caused by ischemia to protect muscle cells (Gao et al., 2007; Koyama et al., 2013; Powers and Lennon, 1999), and Ginkgolide B also can promote muscle regeneration by reviving osteocalcin–GPRC6A signalling (Wang et al., 2023). This may be the potential mechanism by which SXNI can reduce neurological deficits and improve limb function in AIS patients.
A centralized hospital monitoring study of the safety of SXNI injection in 9,735 patients showed that SXNI had a low incidence of AEs and good safety (Qu et al., 2023), which is consistent with our results. However, with the expanding application scope and increasing use frequency of SXNI in clinical practice, it is often abused. Reports of AEs related to SXNI were not uncommon. More than 80% of patients experienced AEs within 2 hours after administration (Chen, 2018; Gong and Shang, 2023), which prompted us to focus on monitoring the patient’s condition during this time period. The occurrence of AEs to SXNI may be related to many factors, such as patient age, type of solvent, dosage, over-the-counter use, and coadministration (Wu, 2019). Therefore, we should pay attention to selecting the appropriate drug solvent, strictly follow the instructions for use, and try to avoid using other injections in combination.
In this review, 12.9% of the included studies were defined as moderate to high quality. Correct randomization and allocation concealment are important conditions for ensuring the minimization of selection bias; however, only 15 (12.9%) studies reported correct randomization methods, and none of the studies reported detailed allocation concealment, blinding, or implementation. Of all studies, 2.6% did not conduct intention-to-treat analysis and did not specify the reason for case dropout, 7.8% may not have completely reported outcomes, and none was registered in advance. Although all outcomes except CER showed significant heterogeneity, our subgroup analysis and mixed-effect regression analysis did not reveal the source of heterogeneity. Sensitivity analysis revealed differences in BI scores between SXNI plus CTs and CTs plus other injections when five studies (Chen, 2021; Li, 2014; Ma, 2016; Zhang et al., 2009; Zhao, 2015) were excluded one by one. The different manufacturers of SXNI, large sample size spans, different age-inclusion criteria, and populations in the different regions of these five studies may be potential sources of heterogeneity. The overall level of evidence for each outcome ranged from very low to low quality. The reasons for this were mainly the low methodological quality of the included studies, high heterogeneity among the included studies, and potential publication bias. Consequently, future RCTs with large sample sizes from multiple centers should be rigorously conducted in accordance with the consolidated standards of reporting trials (CONSORT) and should be registered in advance on the registration platform (Schulz et al., 2010).
4.2 Compared with previous studies
Our results suggested that SXNI in the treatment of AIS could not only increase CER and improve neurological function and ADL but also had good safety, which was consistent with the results of five systematic reviews previously published in 2011, 2012, and 2016 (Yan, et al., 2011; Xi et al., 2012; Zheng et al., 2012; Cai and Jiang, 2012; Tan, et al., 2016). We included patients who had AIS within 14 days; however, Xi et al. and Zheng et al. included patients whose stroke occurred within 72 h and 7 days, respectively. According to the use requirements of SXNI, we included only adult stroke patients, but previous studies did not limit the age of AIS patients, which may have affected the scope of its application. Compared with previous meta-analyses (Yan, et al., 2011; Xi et al., 2012; Zheng et al., 2012; Cai and Jiang, 2012; Tan, et al., 2016), our study used more comprehensive outcomes (including CER, neurological function, ADL, and AEs) to evaluate the efficacy and safety of SXNI as an add-on therapy for AIS. However, Yan et al. included only 14 RCTs and did not report neurological deficit scores for outcome measures. Cai et al. only observed the CER of SXNI in AIS patients based on 22 RCTs. Tan et al. did not evaluate the effect of SXNI on the ability of patients with AIS to perform ADL. Moreover, the studies included in the previous meta-analyses (Yan, et al., 2011; Xi et al., 2012; Zheng et al., 2012; Cai and Jiang, 2012; Tan, et al., 2016) were all published before 2016, and many RCTs focusing on SXNI for AIS have been reported since 2016; therefore, our literature search deadline was 2023 to update the evidence.
4.3 Implications for practice and research
Our findings supported the clinical use of SXNI as an add-on treatment for AIS patients with a low level of evidence. Moreover, because no long-term follow-up studies have been performed to assess the effect of SXNI on the long-term prognosis of AIS patients, our study can only confirm that an intervention duration of 14–30 days with SXNI can effectively improve the neurological function and ADL of AIS patients. The usual daily dose of SXNI was 20 mL, and the most frequent treatment duration was 14 days. The five most common symptoms related to AEs were dizziness, flushing, palpitations, nausea, and headache, which is consistent with previous studies (Qu et al., 2023; Gong and Shang, 2023). Cardiovascular system events were the most common AEs of SXNI for AIS patients. Therefore, in clinical practice, SXNI should be used in strict accordance with the instructions, and patients should be monitored within 2 h after SXNI administration.
In this review, most (87.1%) included studies were judged as low quality. The lack of a detailed description of allocation concealment and blinding methods is a common problem in most studies. In all, 7.8% of the studies may have had a selective reporting bias. The included studies were all single-center RCTs conducted in China, which may mask the efficacy of SXNI in stroke patients of different ethnicities. Most of the studies were conducted in cities, and there is a lack of studies on SXNI for AIS in rural or primary medical institutions, while AIS patients in rural and primary medical institutions are in urgent need of effective and convenient drugs or technologies. SXNI has the advantages of being easy to use and safe. If SXNI is indeed effective in improving the prognosis of AIS patients, it will compensate for the deficiency caused by limited access to IVT and/or EVT. Large-sample, multicenter, placebo-controlled RCTs, especially in rural or primary medical institutions, should be conducted in the future. Moreover, all RCTs should be registered in an internationally recognized clinical trial registration platform and reported in accordance with the CONSORT guidelines.
4.4 Limitations and advantages
Some limitations of this study should be noted. First, all included trials were conducted in China and published in Chinese, which may have led to selection bias and publication bias and reduced the generalizability of the results to the applicable population. Second, most of the included studies were of low quality and had significant heterogeneity, which led to a low level of evidence.
There are also some advantages in our study. First, we carried out an extensive literature search, including seven databases and two clinical trial registration platforms, to obtain as many relevant studies as possible. Second, two reviewers independently performed literature screening, data extraction, and quality evaluation and cross-checked to ensure the accuracy of the data. Third, we conducted a sensitivity analysis, subgroup analysis, and mixed-effects meta-regression analysis to test the robustness of the meta-analysis results. We also assessed the level of evidence for each outcome based on the GRADE tool.
5 Conclusion
SXNI, as an add-on therapy, maybe safe and significantly improved the clinical efficacy, neurological function, and ADL of patients with AIS. However, due to the low quality of the included studies and the very low to low level of evidence in the meta-analysis results, more standardized, large sample, multicenter, and long follow-up RCTs are needed to confirm the efficacy and safety of SXNI for AIS.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Author contributions
JZ: Writing–review and editing, Writing–original draft, Methodology, Funding acquisition, Data curation, Conceptualization. XX: Writing–original draft, Project administration. YZ: Writing–original draft, Project administration. LL: Writing–original draft, Data curation, Project administration. HC: Writing–review and editing, Funding acquisition. LZ: Writing–review and editing, Conceptualization.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study was funded by Medical Scientific Research Foundation of Guangdong Province (No. A2022239), special project of Science and Technology of Traditional Chinese Medicine of Guangdong Provincial Hospital of Chinese Medicine (No. YN2020QN23), Guangdong Basic and Applied Basic Research Foundation (No. 2022A1515220025), and special project of Lingnan modernization of traditional Chinese medicine in 2019 Guangdong Provincial R & D Program (No. 2020B1111100008). This study was also funded by the project of Traditional Chinese medicine rehabilitation talent training program of the Traditional Chinese Medicine Bureau of Guangdong Province (No. 01020184). The funders had no influence on the study design, data collection and analysis, decision to publish, or manuscript preparation.
Acknowledgments
We would like to thank all graduate students in the Department of Rehabilitation from Guangdong Provincial Hospital of Chinese Medicine for their assistance in finishing this paper. We also thank Home for Researchers editorial team (www.home-for-researchers.com) for English language editing.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2024.1407669/full#supplementary-material
Abbreviations
ADL, activities of daily living; AEs, adverse events; AIS, acute ischemic stroke; BI, Barthel index; CBM, Chinese biological medicine database; CI, confidence interval; CER, clinical effective rate; CNKI, China national knowledge infrastructure; CONSORT, consolidated standards of reporting trials; CSS, Chinese Stroke Scale; CT, computed tomography; CTs, conventional treatments; EVT, endovascular thrombectomy; GBE, Ginkgo biloba extract; GRADE, Grading of Recommendation, Assessment, Development, and Evaluation; IVT, intravenous thrombolysis; MRI, magnetic resonance imaging; CND, clinical neurological deficit; NIHSS, National Institutes of Health Stroke Scale; RCTs, randomized controlled trials; ROB, risk of bias; RR, risk ratio; SMD, standardized mean difference; SXNI, shuxuening injection; PAF, platelet-activating factor; PRISMA, preferred reporting items for systematic reviews and meta-analysis; VIP, Chinese scientific journal database; WMD, weighted mean difference.
References
Ahlemeyer, B., and Krieglstein, J. (2003). Neuroprotective effects of Ginkgo biloba extract. Cell Mol. Life Sci. 60 (9), 1779–1792. doi:10.1007/s00018-003-3080-1
Bhatia, R., Hill, M. D., Shobha, N., Menon, B., Bal, S., Kochar, P., et al. (2010). Low rates of acute recanalization with intravenous recombinant tissue plasminogen activator in ischemic stroke: real-world experience and a call for action. Stroke 41 (10), 2254–2258. doi:10.1161/STROKEAHA.110.592535
Brott, T., Adams, H. P., Olinger, C. P., Marler, J. R., Barsan, W. G., Biller, J., et al. (1989). Measurements of acute cerebral infarction: a clinical examination scale. Stroke 20 (7), 864–870. doi:10.1161/01.str.20.7.864
Cai, L. M., and Jiang, J. Z. (2012). Summary analysis of the efficacy of Shuxuening injection for acute cerebral infarction. Hebei Med. J. 34 (8), 1217–1219. doi:10.3969/j.issn.1002-7386.2012.08.058
Chandrasekaran, K., Mehrabian, Z., Spinnewyn, B., Drieu, K., and Fiskum, G. (2001). Neuroprotective effects of bilobalide, a component of the Ginkgo biloba extract (EGb 761), in gerbil global brain ischemia. Brain Res. 922 (2), 282–292. doi:10.1016/s0006-8993(01)03188-2
Chen, J. (2018). Clinical analysis of adverse reactions of Shuxuening injection. J. Med. Theory Pract. 31 (3), 425–426. doi:10.19381/j.issn.1001-7585.2018.03.065
Chen, L. Y. (2021). Clinical effect of Shuxuening injection in the adjuvant treatment of acute cerebral infarction. Chin. J. Clin. Ration. Drug Use 14 (22), 47–49. doi:10.15887/j.cnki.13-1389/r.2021.22.017
Chinese Society of Neurology, Chinese Stroke Society (2018). Chinese guidelines for diagnosis and treatment of acute ischemic stroke. Chin. J. Neurol. 51, 666–682. doi:10.3760/cma.j.issn.1006-7876.2018.09.004
Cui, Q., Zhang, Y. L., Ma, Y. H., Yu, H. Y., Zhao, X. Z., Zhang, L. H., et al. (2020). A network pharmacology approach to investigate the mechanism of Shuxuening injection in the treatment of ischemic stroke. J. Ethnopharmacol. 257, 112891. doi:10.1016/j.jep.2020.112891
Diamond, B. J., Shiflett, S. C., Feiwel, N., Matheis, R. J., Noskin, O., Richards, J. A., et al. (2000). Ginkgo biloba extract: mechanisms and clinical indications. Archives Phys. Med. rehabilitation 81 (5), 668–678. doi:10.1016/s0003-9993(00)90052-2
Ding, Y. B. (2018). Effect of Shuxuening injection combined with Xingnao Kaiqiao acupuncture on blood lipid and neurological function in patients with cerebral infarction. Clin. Res. Pract. 3 (18), 119–120. doi:10.19347/j.cnki.2096-1413.201818055
Dong, W., Gong, T., Zhao, S., Wen, S., Chen, Q., Jiang, M., et al. (2023). A novel extract from Ginkgo biloba inhibits neuroinflammation and maintains white matter integrity in experimental stroke. Neuroscience 523, 7–19. doi:10.1016/j.neuroscience.2023.05.015
Fourth Session of Chinese National Cerebrovascular Conference (1996). Scoring criteria for clinical neurological deficit in stroke patients (1995). Chin. J. Neurol. 29 (6), 381–383.
Gao, J., Zhou, N., Hu, J. J., and Zhang, H. X. (2007). Protection of shuxuening injection on focal cerebral ischemic in rat. Chin. J. Rehabilitation Theory Pract. (08), 712–713. doi:10.3969/j.issn.1006-9771.2007.08.005
GBD 2019 Stroke Collaborators (2021). Global, regional, and national burden of stroke and its risk factors, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Neurol. 20 (10), 795–820. doi:10.1016/S1474-4422(21)00252-0
Gong, T., and Shang, B. (2023). Rationality and safety evaluation of the use of Shuxuening injection. J. Navy Med. 44 (5), 524–526. doi:10.3969/j.issn.1009-0754.2023.05.023
Guidetti, C., Paracchini, S., Lucchini, S., Cambieri, M., and Marzatico, F. (2001). Prevention of neuronal cell damage induced by oxidative stress in-vitro: effect of different Ginkgo biloba extracts. J. Pharm. Pharmacol. 53 (3), 387–392. doi:10.1211/0022357011775442
Gumbinger, C., Gruschka, P., Böttinger, M., Heerlein, K., Barrows, R., Hacke, W., et al. (2012). Improved prediction of poor outcome after thrombolysis using conservative definitions of symptomatic hemorrhage. Stroke 43 (1), 240–242. doi:10.1161/STROKEAHA.111.623033
Hasan, T. F., Hasan, H., and Kelley, R. E. (2021). Overview of acute ischemic stroke evaluation and management. Biomedicines 9 (10), 1486. doi:10.3390/biomedicines9101486
Higgins, J. P. T., and Green, S. (2011). Cochrane handbook for systematic reviews of interventions. London: The Cochrane Collaboration.
Jiang, J. C., Jiang, Y. L., and Wen, J. (2023). Cognitive load theory perspectives on Ma wanli's fundamentals of traditional Chinese medicine the inspiration of English translation and interpretation of the core concept of qi. J. Guangxi Univ. Chin. Med. 26 (02), 86–91. doi:10.3969/j.issn.2095-4441.2023.02.022
Jørgensen, H. S., Nakayama, H., Raaschou, H. O., and Olsen, T. S. (1999). Stroke. Neurologic and functional recovery the Copenhagen stroke study. Phys. Med. rehabilitation Clin. N. Am. 10 (4), 887–906. doi:10.1016/s1047-9651(18)30169-4
Koyama, H., Nojiri, H., Kawakami, S., Sunagawa, T., Shirasawa, T., and Shimizu, T. (2013). Antioxidants improve the phenotypes of dilated cardiomyopathy and muscle fatigue in mitochondrial superoxide dismutase-deficient mice. Molecules 18 (2), 1383–1393. doi:10.3390/molecules18021383
Kwah, L. K., and Diong, J. (2014). National Institutes of Health stroke scale (NIHSS). J. Physiother. 60 (1), 61. doi:10.1016/j.jphys.2013.12.012
Li, L. D., Zhou, Y., and Shi, S. F. (2023). Edaravone combined with Shuxuening versus edaravone alone in the treatment of acute cerebral infarction: a systematic review and meta-analysis. Medicine 102 (9), e32929. doi:10.1097/MD.0000000000032929
Li, S., Zhang, X., Fang, Q., Zhou, J., Zhang, M., Wang, H., et al. (2017). Ginkgo biloba extract improved cognitive and neurological functions of acute ischaemic stroke: a randomised controlled trial. Stroke Vasc. neurology 2 (4), 189–197. doi:10.1136/svn-2017-000104
Li, S. J. (2014). Clinical effect of Shuxuening injection in the treatment of acute cerebral infarction. Jilin Med. J. 35 (12), 2560. doi:10.3969/j.issn.1004-0412.2014.12.050
Ma, Y. X. (2016). Clinical effect and safety analysis of Shuxuening in the treatment of cerebral infarction. China Health Care & Nutr. 26 (29), 49. doi:10.3969/j.issn.1004-7484.2016.29.057
Mahoney, F. I., and Barthel, D. W. (1965). Functional evaluation: the barthel index. Md. state Med. J. 14, 61–65.
Nash, K. M., and Shah, Z. A. (2015). Current perspectives on the beneficial role of Ginkgo biloba in neurological and cerebrovascular disorders. Integr. Med. insights 10, 1–9. doi:10.4137/IMI.S25054
National Administration of Traditional Chinese Medicine (1994). Standard for diagnosis and efficacy of Chinese medicine syndrome. Nanjing: Nanjing University Press.
Oskouei, D. S., Rikhtegar, R., Hashemilar, M., Sadeghi-Bazargani, H., Sharifi-Bonab, M., Sadeghi-Hokmabadi, E., et al. (2013). The effect of Ginkgo biloba on functional outcome of patients with acute ischemic stroke: a double-blind, placebo-controlled, randomized clinical trial. J. Stroke Cerebrovasc. Dis. 22 (8), e557–e563. doi:10.1016/j.jstrokecerebrovasdis.2013.06.010
Ovbiagele, B., Goldstein, L. B., Higashida, R. T., Howard, V. J., Johnston, S. C., Khavjou, O. A., et al. American Heart Association Advocacy Coordinating Committee and Stroke Council (2013). Forecasting the future of stroke in the United States: a policy statement from the American heart association and American stroke association. Stroke 44 (8), 2361–2375. doi:10.1161/STR.0b013e31829734f2
Oyama, Y., Chikahisa, L., Ueha, T., Kanemaru, K., and Noda, K. (1996). Ginkgo biloba extract protects brain neurons against oxidative stress induced by hydrogen peroxide. Brain Res. 712 (2), 349–352. doi:10.1016/0006-8993(95)01440-3
Page, M. J., McKenzie, J. E., Bossuyt, P. M., Boutron, I., Hoffmann, T. C., Mulrow, C. D., et al. (2021). The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 372, n71. doi:10.1136/bmj.n71
Powers, S. K., and Lennon, S. L. (1999). Analysis of cellular responses to free radicals: focus on exercise and skeletal muscle. Proc. Nutr. Soc. 58 (4), 1025–1033. doi:10.1017/s0029665199001342
Qu, Y., Ma, X. J., Li, Q. H., Li, H. L., Zhao, X. C., Sun, X. H., et al. (2023). Intensive hospital monitoring study on safety of 9735 cases of Shuxuening Injection. Chin. Traditional Herb. Drugs 54 (10), 3253–3260. doi:10.7501/j.issn.0253-2670.2023.10.022
Rochmah, T. N., Rahmawati, I. T., Dahlui, M., Budiarto, W., and Bilqis, N. (2021). Economic burden of stroke disease: a systematic review. Int. J. Environ. Res. Public Health 18 (14), 7552. doi:10.3390/ijerph18147552
Rosa, J. L., Alves, M., Ferreira, P., Papoila, A. L., and Nunes, A. P. (2022). Previous disability and benefit of acute phase therapy in functional prognosis of selected patients with ischemic stroke. J. Stroke Cerebrovasc. Dis. 31 (1), 106183. doi:10.1016/j.jstrokecerebrovasdis.2021.106183
Schulz, K. F., Altman, D. G., and Moher, D.CONSORT Group (2010). CONSORT 2010 statement: updated guidelines for reporting parallel group randomized trials. Ann. Intern Med. 152 (11), 726–732. doi:10.7326/0003-4819-152-11-201006010-00232
Shi, C., Zhao, L., Zhu, B., Li, Q., Yew, D. T., Yao, Z., et al. (2009). Protective effects of Ginkgo biloba extract (EGb761) and its constituents quercetin and ginkgolide B against beta-amyloid peptide-induced toxicity in SH-SY5Y cells. Chem. Biol. Interact. 181 (1), 115–123. doi:10.1016/j.cbi.2009.05.010
Singh, S. K., Srivastav, S., Castellani, R. J., Plascencia-Villa, G., and Perry, G. (2019). Neuroprotective and antioxidant effect of Ginkgo biloba extract against AD and other neurological disorders. Neurotherapeutics 16 (3), 666–674. doi:10.1007/s13311-019-00767-8
Smith, J. V., Burdick, A. J., Golik, P., Khan, I., Wallace, D., and Luo, Y. (2002). Anti-apoptotic properties of Ginkgo biloba extract EGb 761 in differentiated PC12 cells. Cell Mol. Biol(Noisy-le-grand) 48 (6), 699–707.
Smith, P. F., Maclennan, K., and Darlington, C. L. (1996). The neuroprotective properties of the Ginkgo biloba leaf: a review of the possible relationship to platelet-activating factor (PAF). J. Ethnopharmacol. 50 (3), 131–139. doi:10.1016/0378-8741(96)01379-7
Tan, D., Wu, J. R., Liu, S., and Zhang, B. (2016). Meta-analysis of efficacy of shuxuening injection in the treatment of cerebral infarction. Chin. J. Pharmacoepidemiol. 25 (8), 492–498.
Tian, J., Chen, L., Huang, X. L., Ding, Y. R., Wang, W., Huang, X. Y., et al. (2024). Understanding and relationship of qi and xue in traditional Chinese and western medicine. Chin. J. Med. Guide 26 (02), 171–175. doi:10.3969/j.issn.1009-0959.2024.02.020
Wang, B. Y., Chen, Y. F., Hsiao, A. W., Chen, W. J., Lee, C. W., and Lee, O. K. (2023). Ginkgolide B facilitates muscle regeneration via rejuvenating osteocalcin-mediated bone-to-muscle modulation in aged mice. J. Cachexia Sarcopenia Muscle 14 (3), 1349–1364. doi:10.1002/jcsm.13228
Wassélius, J., Arnberg, F., von Euler, M., Wester, P., and Ullberg, T. (2022). Endovascular thrombectomy for acute ischemic stroke. J. Intern. Med. 291 (3), 303–316. doi:10.1111/joim.13425
Wu, T. T. (2019). Retrospective analysis of adverse drug reaction of shuxuening injection in xi'an from 2013 to 2017. Her. Med. 38 (1), 49–53. doi:10.3870/j.issn.1004-0781.2019.01.011
Xi, B. C., Zhang, C., and Sun, L. L. (2012). Meta analysis of Shuxuening injection in treatment of patients with acute cerebral infarction. Pharm. Care Res. 12 (5), 354–357. doi:10.5428/pcar20120512
Yan, J., Wang, X. W., Zhou, J., Jing, J., Wang, C. X., and Li, J. J. (2011). Meta-analysis of shuxuening injection in the treatment for cerebral infarction. Chin. J. Rehabil. Theory Pract. 17 (9), 884–886. doi:10.3969/j.issn.1006-9771.2011.09.025
Zhang, A. L., Zhang, J. W., Sun, J. N., and Xu, Q. P. (2005). Progress on the mechanism of anti-ischemic action of Ginkgo biloba extracts. China Pharm. 16 (08), 626–629. doi:10.3969/j.issn.1001-0408.2005.08.031
Zhang, H., Zhou, M., and Zhang, J. J. (2009). The therapeutic effect of Shuxuening injection on acute cerebral infarction and its mechanism. China J. Traditional Chin. Med. Pharm. 24 (01), 81–84. doi:10.3969/j.issn.1004-7484(s).2013.09.542
Zhang, Z. W. (2019). Clinical observation of Pingganditantongluotang combined with Shuxuening injection in the treatment of cerebral infarction in the convalescent phase. Guangming J. Chin. Med. 34 (21), 3336–3338. doi:10.3969/j.issn.1003-8914.2019.21.047
Zhao, Y. X. (2015). Clinical observation of Shuxuening in the treatment of cerebral infarction. J. Front. Med. 5 (36), 87–88. doi:10.3969/j.issn.2095-1752.2015.36.086
Zheng, W. K., Zhang, L., and Shang, H. C. (2012). Systematic review on shuxuening injection in treating acute cerebral infarction. China Licens. Pharm. 9 (12), 33–41. doi:10.3969/j.issn.1672-5433.2012.12.007
Keywords: shuxuening injection, acute ischemic stroke, traditional Chinese medicine, systematic review, meta-analysis
Citation: Zhan J, Xu X, Zhu Y, Liu L, Chen H and Zhan L (2024) Shuxuening injection for treating acute ischemic stroke: a PRISMA-compliant systematic review and meta-analysis of randomized controlled trials. Front. Pharmacol. 15:1407669. doi: 10.3389/fphar.2024.1407669
Received: 15 April 2024; Accepted: 07 October 2024;
Published: 22 October 2024.
Edited by:
Viola B. Morris, Emory University, United StatesReviewed by:
Mina Bagheri Varzaneh, University of Illinois Chicago, United StatesYongsheng Chen, Jinan University, China
Copyright © 2024 Zhan, Xu, Zhu, Liu, Chen and Zhan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lechang Zhan, emxjNjYxMkAxNjMuY29t
 Jie Zhan
Jie Zhan Xiaowen Xu
Xiaowen Xu Yanzhen Zhu
Yanzhen Zhu Lin Liu3
Lin Liu3