- West China School of Pharmacy, Sichuan University, Chengdu, China
Objective: Anaprazole, an innovative drug, has shown promise in initial clinical trials for patients with duodenal ulcers (DU) in China. This study aimed to evaluate the potential effects, safety, and cost-effectiveness of Anaprazole compared to Ilaprazole in the treatment of DU and the budgetary impact on the healthcare system.
Methods: Two multicentre, randomized controlled trials were used as data sources. The efficacy and safety of Anaprazole and Ilaprazole were compared using an anchored matching-adjusted indirect comparison (MAIC). A cost-utility analysis (CUA) was performed using a Markov model. A budget impact analysis (BIA) was conducted to evaluate the impact on the expenditure of the Chinese healthcare system. Deterministic and probabilistic sensitivity analyses were undertaken to test the uncertainty.
Results: The study findings indicated that Anaprazole and Ilaprazole have similar efficacy and safety in treating DU (OR = 1.05; 95% CI, 0.94–1.01; p = 0.35; OR = 0.63; 95% CI, 0.39–1.08; p = 0.12). The ICUR was 2,995.41¥/QALY, which is below the WTP threshold. The CUA results showed that Anaprazole is a cost-effective intervention with a probability of 85% at a given threshold. The results demonstrated strong robustness in the sensitivity analysis. Anaprazole imposed a low burden on the Chinese healthcare budget in the BIA.
Conclusion: Compared with Ilaprazole, Anaprazole has similar efficacy, safety, and high cost-effectiveness, while also impacting the total expenditure of the healthcare system.
Clinical Trial Registration:: ClinicalTrials.gov, identifier NCT04215653 and NCT02847455
Introduction
Approximately 5%–10% of the global population suffers from peptic ulcer disease (PUD), with an annual incidence rate of about 0.1%–0.3% (Tarasconi et al., 2020). According to the Global Burden of Disease (GBD) 2019 statistics, PUD in China has a higher incidence rate, mortality, and Disability-Adjusted Life Years (DALYs) compared to other countries (GBD, 2020). Peptic ulcer mainly includes two types of duodenal ulcer and gastric ulcer, among which duodenal ulcer (DU) and gastric ulcer (GU) occur in a ratio of about 3:1. DU is a common chronic disease characterized by defects in the mucosal and muscular layers of the duodenum (Lanas and Chan, 2017). It can occur at any age but is most common between the ages of 20 and 50, with a higher prevalence in males (Levenstein et al., 1997). DU often presents with recurrent attacks, especially during seasonal changes. Symptoms include severe abdominal pain, and in severe cases, complications such as bleeding, perforation, and obstruction may occur. The severity of symptoms is directly related to the extent of the ulcer. The main causes of DU include abnormal gastric acid secretion, Helicobacter pylori infection, and the use of non-steroidal anti-inflammatory drugs (NSAIDs) and aspirin (Chan and Leung, 2002).
Gastric acid suppression therapy is an important component of the current treatment plan for DU. Proton pump inhibitors (PPIs) are a mainstay of peptic ulcer treatment that act by irreversibly inhibiting H+/K +- ATPase. They are strongly recommended as the first-line treatment for acid suppression and the treatment of peptic ulcers in guidelines and consensus statements such as the “Evidence-based Clinical Practice Guidelines for Peptic Ulcers (2020)” in Japan (Kamada et al., 2021).
Anaprazole sodium enteric-coated tablet (Anaprazole) is a domestically developed PPI in China, with international patent. In June 2023, Anaprazole was approved as a class 1 of innovative drugs in China for the treatment of duodenal ulcer based on a multicenter randomized controlled trial (RCT) (ClinicalTrials.gov NCT04215653) and has been included in the updated National Reimbursement Drug List (NRDL, 2023). It inhibits gastric acid secretion and is used in the treatment of DU. Compared to other PPIs that rely mainly on CYP2C19 enzyme metabolism, Anaprazole achieves co-metabolism through targeted structural design, involving multiple enzymes (7 CYP enzymes) and non-enzyme pathways. The main metabolic enzyme is CYP3A4, similar to llaprazole, but its contribution rate is only 24.6% (Liu et al., 2023). This makes Anaprazole a potentially safer choice for elderly patients and other populations who are using multiple medications.
Currently, the economics of Anaprazole as a new generation of PPI drugs have not been effectively evaluated. Under the background of rising medical costs and limited medical resources, pharmacoeconomics is helpful to reasonably control the rise of drug costs and optimize the allocation of medical resources (Dai et al., 2024). According to recommendations of guideline (Liu et al., 2020), Ilaprazole enteric coated tablet (Ilaprazole) was selected as the reference drug because of the same indication and extensive clinical use. As the first novel PPI in development for peptic ulcer treatment, Anaprazole has the advantages of multi-enzyme plus non-enzyme metabolism, intestinal and renal double-channel excretion, etc., which can provide a safer choice for patients with multiple drugs and renal dysfunction. Furthermore, Anaprazole is based on data from the entire Chinese population and has shown a good safety profile in both Chinese duodenal patients and healthy individuals (Wang et al., 2024).
Although both Anaprazole and Ilaprazole are new generation PPI drugs, Ilaprazole was launched earlier in China (2007) and occupied a large market share in the Chinese market. Compared with the western market, China has a higher incidence of duodenal ulcer. Anaprazole RCT trial is conducted based on the data of the whole Chinese population. By comparing the safety, effectiveness of Anaprazole and Ilaprazole, it may provide a new clinical treatment option suitable for the Chinese population. Additionally, conducting an economic analysis of Anaprazole will assess its impact on the Chinese healthcare system. Understanding the cost-effectiveness and affordability of Anaprazole is vital for healthcare decision-makers to evaluate its financial implications and ascertain its value relative to other treatments. Such evaluations are crucial for informed resource allocation and policy-making, enhancing the sustainability and efficiency of healthcare in China.
Materials and methods
Interventions
This study employed a multicenter RCT comparing Anaprazole versus Rabeprazole (Zhu et al., 2022) (ClinicalTrials.gov, NCT04215653) and another RCT comparing Rabeprazole versus Ilaprazole (Li et al., 2019) (ClinicalTrials ID: NCT02847455) as sources of clinical data. The experimental group comprised 220 patients using Anaprazole, while the control group included 129 patients using Ilaprazole.
Anaprazole is available in tablet form, with a dosage of 20 mg per tablet. In the experimental group, patients were instructed to take one 20 mg tablet of Anaprazole orally once daily before breakfast for a duration of 4 weeks.
Ilaprazole, also in tablet form, comes with a dosage of 5 mg per tablet. In the control group, patients were instructed to take two 5 mg tablets of Ilaprazole orally once daily before breakfast for a duration of 4 weeks.
Comparison of efficacy and safety
Adjusted indirect comparisons are recommended by various decision-making bodies and guidelines for studies lacking head-to-head clinical trials (Phillippo et al., 2018). Given the absence of direct comparative RCTs between Anaprazole and Ilaprazole, an anchored MAIC was conducted to analyze the effect and safety between the two treatments. This approach, as depicted in Figure 1, accounts for confounding factors and provides a more reliable comparison than unadjusted indirect comparisons.
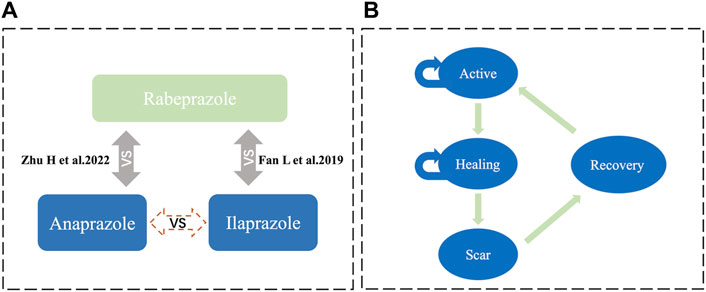
Figure 1. Model structure (A) illustrates the MAIC analysis framework, facilitating an indirect comparison between Anaprazole and Ilaprazole using Rabeprazole as a bridge. (B) depicts the Markov model.
Data from the Ilaprazole control group were matched by weighting individual case data from multicenter clinical trials of Anaprazole. Using a logistic regression model to calculate propensity scores and performing inverse probability weighted matching, the baseline distribution between groups reached equilibrium, allowing for efficacy and safety comparisons within a study population with balanced baseline distributions post-matching (Liu, 2022). Using Bucher’s indirect comparison method (Bucher et al., 1997), the relative efficacy differences between the treatment regimens were quantified: the effect of Anaprazole vs. Rabeprazole was represented as logORAC, the effect of Ilaprazole vs. Rabeprazole as logORBC, and the effect of Anaprazole vs. Ilaprazole (logORAB), which is calculated by Eq. 1:
SPSS 18.0® was utilized to process the data, with count data expressed in percentages and analyzed using the χ2 test. Measurement data are presented as x ± s and analyzed using the t-test, where p ≤ 0.05 indicates a statistically significant difference. The outcome indicators for binary variables in MAIC analysis are represented by odds ratio (OR), and analyzed using R® 4.3.0 software to evaluate the effectiveness and safety of the Anaprazole group.
Model description and structure
This study employed short-term simulations of patient health status transitions using a Markov model from the perspective of the Chinese healthcare system, implemented in Excel Microsoft (Figure 1). The Markov model encompassed four disease states: active (stage A), healing (stage H), scar (stage S), and recovery, aligned with relevant diagnostic criteria (Chun et al., 2014). The probabilities of transitioning between states were calculated using clinical trial data for Anaprazole and clinical literature for Ilaprazole. The cycle length was set at 2 weeks, with the study extending over 24 cycles.
The assumptions of this model included: (Tarasconi et al., 2020): Drawing from clinical trials and an anchored MAIC, 179 patients were included in the Anaprazole group and 139 in the Ilaprazole group, with all patients initially in Stage A at the time of model entry, transitioning through treatment phases until recovery. (GBD, 2020). Given the short cycle length, mortality was not considered; however, the recurrence rate within 6 months was included. (Lanas and Chan, 2017). It was assumed that patients in the active phase must progress to the healing phase before advancing to the scar phase, rather than moving directly to the scar phase.
Data sources
Clinical efficacy
This study employed clinical research outcome indicators as measures of effectiveness. The primary clinical outcome indicator was the ulcer healing rate (Eq. 2), assessed using endoscopic images after 4 weeks of treatment. Efficacy evaluation criteria: patients were assessed via endoscopic images after 4 weeks of medication to determine the transition of ulcer status from stage A to stage S, which was defined as healing.
Safety indicators
The incidence rate of ADRs was used as a safety evaluation parameter (Eq. 3).
Resource use and costs
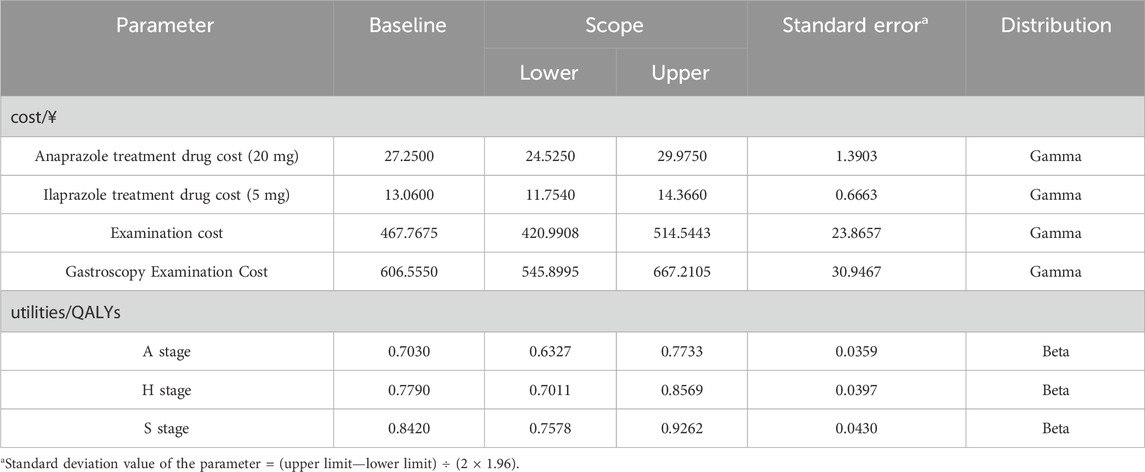
The pharmacoeconomic evaluation primarily considered direct medical costs, direct non-medical costs, indirect costs, and intangible costs (Liu et al., 2020). This study adopted a health system perspective, focusing exclusively on direct medical costs (Table 1). These included costs for drug treatment, registration fees, laboratory tests, and expenses related to adverse reactions (Garg et al., 2022). The price of Anaprazole was provided by the sponsoring enterprise, while the price of Ilaprazole was sourced from the local market (Yaozhinet, 2016). The pricing of other PPIs in the Chinese market was considered in the BIA (18). The total cost was calculated based on the frequency and dosage described in the medication guidelines and the actual circumstances of the clinical trials. Charges for registration and testing were derived from the price list of public medical services available on the hospital’s official website during clinical trials. Where hospitals lacked a public pricing list, prices from medical service lists at comparable hospitals within the same province were used.
Health state utilities
A quality-adjusted life year (QALY) is an index used to measure the effects in this model and was calculated by multiplying life expectancy with health preference utility (Hu et al., 2022). The QALYs for each cycle represent the sum of the QALYs for each health state, calculated as the product of the number of life years for each state and the corresponding quality of life weight (Eq. 4) (Freath et al., 2022). Owing to the absence of utility data in the clinical trials, health utility values in this study were derived from a Markov model pharmacoeconomic analysis of PUD, (Xie and Tang, 2022), based on the European Five Dimensional Health Scale. In this study, the health utility values for the active, healing, and scar stages were 0.7030, 0.7790, and 0.8420, respectively (Table 1).
Discount rate
Guidelines (Liu et al., 2020) explicitly require the discounting of future costs and health outcomes when the research period exceeds 1 year. Due to the short duration of this study (less than 1 year), discounting was not applied.
Transition probabilities
The transition probabilities, representing the likelihood of patients moving from one treatment state to another, were estimated from various sources. For the Anaprazole group, probabilities were derived from individual patient data (Study Details, 2022), with a transfer probability of 0.9253 from the active phase to the healing phase and 0.9661 from the healing phase to the scar phase. For the Ilaprazole group, these probabilities were obtained from clinical studies (Ho et al., 2009), with a transfer probability of 0.8957 from the active phase to the healing phase and 0.9344 from the healing phase to the scar phase. The recurrence rate (the transition probability from the recovery stage back to stage A) was sourced from a large-scale, multicenter prospective cohort study on long-term follow-up of H. pylori reinfection (Xie et al., 2020), reported at 0.13%.
CUA
This study conducted a CUA for economic evaluation. Published RCTs have documented short-term treatment effects for patients over 4 weeks, yet duodenal ulcers entail a certain degree of recurrence probability. To assess the long-term effects of treatment, this study conducted a CUA for economic evaluation. The incremental cost-utility ratio (ICUR) served as the principal outcome measure (Eq. 5), compared against the willingness-to-pay (WTP) threshold to assess the cost-effectiveness of the interventions. The WTP threshold was set at 1–3 times the per capita gross domestic product (GDP), representing the amount willing to be paid for each QALY (9). If the ICUR is less than the WTP, the intervention is considered cost-effective. This study used one times the per capita GDP of China in 2022 as the WTP threshold, amounting to 85,698¥/QALY.
Sensitivity analysis
Pharmacoeconomic evaluations using model methods often encounter uncertainties, including parameter and model uncertainty. To manage these uncertainties, this study utilized deterministic sensitivity analysis (DSA) and probability sensitivity analysis (PSA). In DSA, each cost parameter was varied within a range of ± 10% to assess its impact on the outcomes, depicted through a cyclone chart which visually represents the influence of multiple uncertain factors. PSA involved 1,000 second-order Monte Carlo simulations to sample the distribution of parameters. The results of PSA were displayed in incremental cost-utility plane scatter plots and cost-utility acceptance curves (CUAC), offering insights into the uncertainties of the results and aiding decision-makers in understanding the range of potential outcomes. These sensitivity analyses facilitated a more comprehensive evaluation of the pharmacoeconomic implications of the interventions.
BIA
This study assessed the potential impact of Anaprazole on the health insurance fund from the perspective of health insurance administrators and decision-making departments over the next 3 years following its inclusion in the China National Reimbursement Drug List (NRDL) (Sullivan et al., 2014), using 2023 as the baseline year. The analysis included all PPIs drugs in the Chinese market as potential substitutes for Anaprazole. Target population parameters were derived from peptic ulcer epidemiology literature (Zhang, 2022) and the GBD database (GBD, 2019). Market share and cost data were sourced from the China Medical Economic Information Network (MENET) (MENET, 2022). China’s health insurance fund budget was reported on the official website of National Healthcare Security Administration (National Healthcare, 2022). The projected expenditures of the Chinese health insurance system on PPIs drugs from 2024 to 2026 were calculated to assess the impact of Anaprazole on health insurance budgets.
Results
Basic results
Baseline characteristics of patients included in MAIC analysis
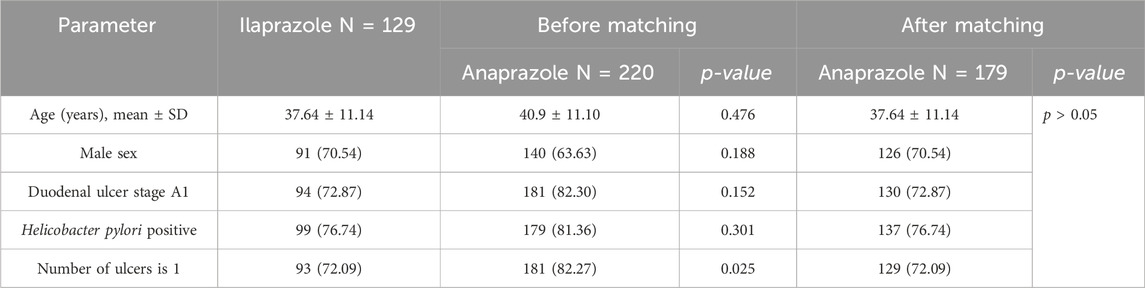
Table 2 presents the baseline comparisons between the Anaprazole and Ilaprazole groups. Prior to matching, both groups exhibited similar distributions in age, gender, ulcer stage, and H. pylori infection rate. However, the proportion of patients with a single ulcer was lower in the Anaprazole group than in the Ilaprazole group. An indirect comparison was performed using anchor matching adjustment based on clinical ulcer characteristics, including age distribution, sex ratio, number of ulcers, and their location and rhythmicity. Following matching, the baseline characteristics of the two groups reached equilibrium.
Effectiveness comparison
After 4 weeks of treatment, the ulcer healing rates in the matched Anaprazole and Ilaprazole groups were 87.9% and 83.7%, respectively. The effectiveness of Anaprazole for duodenal ulcer (DU) treatment was comparable to that of Ilaprazole, with an OR of 1.05, 95% CI [0.94, 1.01], and p = 0.35, as illustrated in Figure 2.
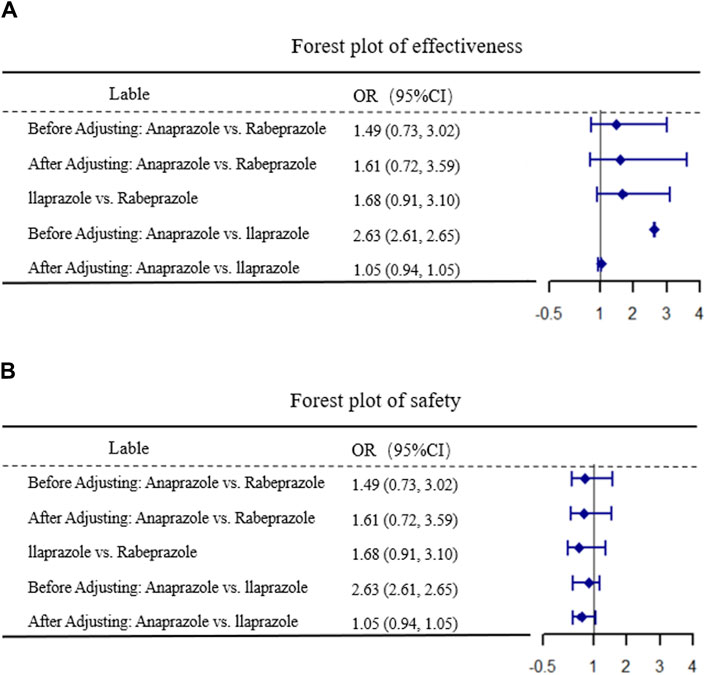
Figure 2. Results of MAIC. (A,B) display the comparative results of efficacy and safety, respectively.
Safety comparison
The incidence of ADR after 4 weeks was 8.21% in the Anaprazole group and 6.98% in the Ilaprazole group. The safety of Anaprazole in treating DU was comparable to Ilaprazole, with an OR of 0.63, 95% CI [0.39, 1.08], and p = 0.12, as depicted in Figure 2.
CUA
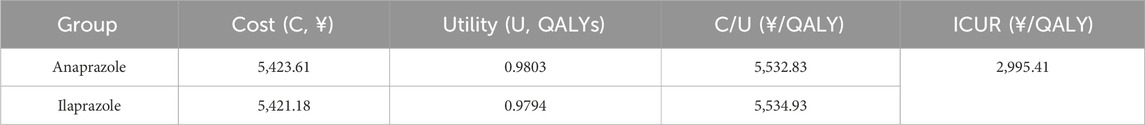
The results of the CUA, shown in Table 3, indicate that the composition and utility value of the Anaprazole group were higher than those of the Ilaprazole group. The ICUR was 2,995.41¥/QALY, which is below the WTP threshold. Thus, Anaprazole is deemed a cost-effective intervention compared to Ilaprazole at the established payment threshold.
Sensitivity analysis
Deterministic sensitivity analysis (DSA)
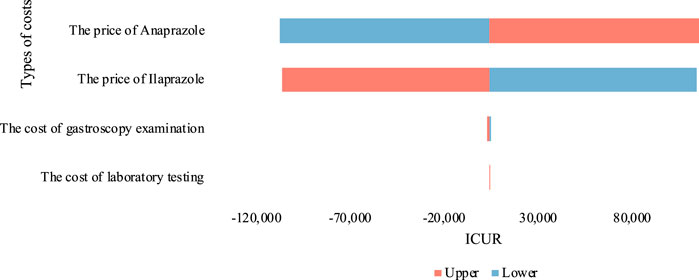
By assigning the cost parameters of both treatments to a range of ± 10% from the baseline value, we conducted a single-factor sensitivity analysis and depicted the outcomes using a tornado chart (Figure 3). The results were somewhat sensitive to the price parameters of Anaprazole and Ilaprazole. Despite fluctuations in drug prices, the ICUR value remained approximately equal to one time the GDP per capita, indicating robustness in our baseline analysis.
Probability sensitivity analysis (PSA)
The cost-utility scatter plot, presented in Figure 4, shows that most PSA values fall below one time the GDP per capita. This suggests that at a WTP threshold equal to one time the GDP per capita, Anaprazole has a higher probability of being economically viable compared to Ilaprazole. The economic justification for treating DU with Anaprazole increases with the WTP threshold. When the WTP is twice the GDP per capita, the likelihood that Anaprazole is cost-effective rises to 85%. The PSA findings align with the baseline analysis.
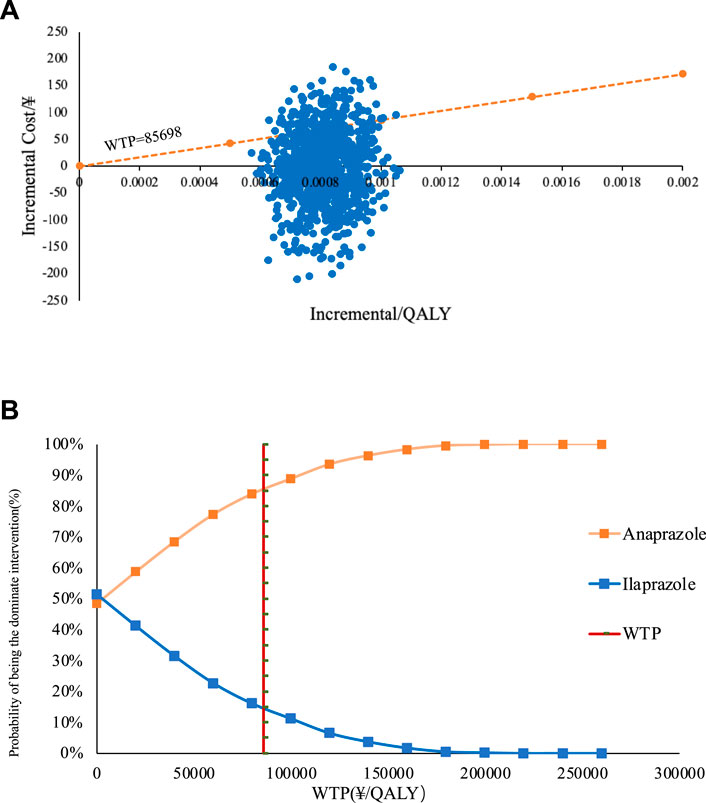
Figure 4. (A) The straight line in the graph represents the WTPs when the scatter of Anaprazole falls in the lower right of the line it means that the Anaprazole is economical compared to Ilaprazole at that WTP. (B) depicts the cost-effectiveness acceptability curve (CEAC).
BIA
In Scenario 1, where Anaprazole was not included in the NRDL, the projected annual Medicare fund expenditures for treating DU from 2024 to 2026 were ¥228.771 million, ¥226.274 million, and ¥238.034 million, respectively. In Scenario 2, assuming Anaprazole sodium enteric-coated tablets are included in the NRDL, the expenditures remain unchanged. The incremental costs represent 0.001957%, 0.003205%, and 0.005247% of the total budget of the China Health Insurance Fund, respectively. Factors such as consultation rate, prevalence of duodenal ulcer patients, and market share of PPI drugs may influence these results.
Discussion
Research has pinpointed the diminished protective capacity of the gastric mucosa, excessive gastric acid secretion, and H. pylori infection as the main causative factors of peptic ulcers (el-Omar et al., 1995). Clinically, PUD is treated with a regimen combining PPIs, gastrointestinal mucosal protectors, and antibiotics. PPI is the first choice for the treatment of peptic ulcer. The metabolism of PPI in the early market is affected by CYP2C19 gene polymorphism, and individual differences are large, and it cannot be used with CYP2C19 enzyme substrate drugs such as Clopidogrel. On the one hand, multidrug patients need PPIs with a lower risk of drug interaction; On the other hand, special patients need PPIs with less burden on the kidneys. Unlike other PPIs, Anaprazole possesses a dual-channel excretion mechanism through both the intestines and kidneys, reducing the drug’s retention time in the body and potentially decreasing toxic side effects (Liu et al., 2023). This characteristic makes it a safer option for patients with renal insufficiency and the elderly. In addition, Anaprazole sodium is less affected by CYP enzyme gene polymorphism, which is conducive to stable efficacy and improve safety.
With the ongoing reform of China’s healthcare system, the rational use of drugs, encompassing safety, effectiveness, and economic considerations, is becoming increasingly critical. Anaprazole, an independently developed PPI in China, has demonstrated favorable safety and symptom relief effects in clinical trials. This study aims to assess the economic viability of Anaprazole and Ilaprazole for treating DU, providing economic insights and support for clinical decision-making. A pharmacoeconomic evaluation was conducted from the healthcare system perspective, focusing on DU patients to identify a more cost-effective treatment strategy without compromising efficacy. The findings revealed that the ulcer healing rate in the Anaprazole group was similar to that in the Ilaprazole group. Additionally, the incremental cost-utility ratio (ICUR) for Anaprazole was below the willingness-to-pay threshold (WTP), suggesting that Anaprazole may offer greater clinical value in DU treatment than Ilaprazole. Given its favorable safety profile, efficacy, and the economic considerations of medical insurance, Anaprazole demonstrates superior clinical value. In conclusion, Anaprazole provides significant clinical efficacy, cost-efficiency, and enhanced economic benefits in DU treatment. Its inclusion in the NRDL minimally impacts the total health insurance expenditure, and the effect on the health insurance fund could be further mitigated through negotiated reductions in the prices of health insurance-covered drugs in exchange for volume increases.
There are several limitations in the methodology of this study. First, due to the absence of direct head-to-head clinical trials comparing Anaprazole and Ilaprazole, this study relied on a multicenter RCT comparing Anaprazole with Rabeprazole, and another RCT comparing Rabeprazole with Ilaprazole. This method of indirect comparison may introduce bias and limit the generalizability of the findings. Second, the MAIC analysis included a limited number of participants, which not only weakened the statistical power but also restricted the extrapolation of the results (Hatswell et al., 2020). To address these limitations, further analysis and long-term head-to-head comparative trials between Anaprazole and Ilaprazole are necessary to provide more reliable evidence. Additionally, Anaprazole and Ilaprazole use CYP3A4 as the main metabolic enzyme, both of which belong to the new generation of PPI drugs. However, due to the short launch time of the new generation of PPI drugs, the safety and effect data under long-term use may be relatively insufficient. However, how to translate the value of innovation into clinical value needs more long-term data support. Future studies may need to systematically evaluate the safety, effectiveness and economy of all PPI drugs in the treatment of duodenal ulcer, so as to provide a richer and more reliable evidence-based basis for clinical decision-making.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
Author contributions
HN: Writing–review and editing, Writing–original draft, Software, Methodology, Formal Analysis, Data curation. JS: Writing–review and editing, Writing–original draft, Software, Methodology, Investigation, Formal Analysis, Data curation. MH: Writing–review and editing, Supervision, Resources, Project administration. NZ: Writing–review and editing, Supervision, Resources, Project administration, Funding acquisition, Conceptualization. SY: Writing–review and editing, Supervision, Resources, Funding acquisition, Conceptualization.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study was supported by the State Scholarship Fund of China (No. 202006240013).
Acknowledgments
Thanks to the five researchers of the research team for their contributions and support to this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer XL declared a shared parent affiliation with the authors to the handling editor at the time of review.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Bucher, H. C., Guyatt, G. H., Griffith, L. E., and Walter, S. D. (1997). The results of direct and indirect treatment comparisons in meta-analysis of randomized controlled trials. J. Clin. Epidemiol. 50 (6), 683–691. doi:10.1016/s0895-4356(97)00049-8
Chan, F. K., and Leung, W. K. (2002). Peptic-ulcer disease. Lancet 360 (9337), 933–941. doi:10.1016/s0140-6736(02)11030-0
H. J. Chun, S. K. Yang, and M. G. Choi (2014). Clinical gastrointestinal endoscopy: a comprehensive atlas (Berlin, Heidelberg: Springer Berlin Heidelberg). Available at: https://link.springer.com/10.1007/978-3-642-35626-1.
Dai, Z., Xu, J., Chang, F., Zhou, W., Ren, T., Qiu, J., et al. (2024). The cost-effectiveness of iruplinalkib versus alectinib in anaplastic lymphoma kinase-positive crizotinib-resistant advanced non-small-cell lung cancer patients in China. Front. Public Health 12, 1333487. doi:10.3389/fpubh.2024.1333487
el-Omar, E. M., Penman, I. D., Ardill, J. E., Chittajallu, R. S., Howie, C., and McColl, K. E. (1995). Helicobacter pylori infection and abnormalities of acid secretion in patients with duodenal ulcer disease. Gastroenterology 109 (3), 681–691. doi:10.1016/0016-5085(95)90374-7
Freath, L. L., Curry, A. S., Cork, D. M. W., Audhya, I. F., and Gooch, K. L. (2022). QALYs and ambulatory status: societal preferences for healthcare decision making. J. Med. Econ. 25 (1), 888–893. doi:10.1080/13696998.2022.2090152
Garg, R., Cheng, V., Ellis, U., Verma, V., McTaggart-Cowan, H., Peacock, S., et al. (2022). Direct medical costs of young-onset colorectal cancer: a worldwide systematic review. BMC Health Serv. Res. 22 (1), 1100. doi:10.1186/s12913-022-08481-6
GBD (2019). Global burden of disease (GBD). Available at: https://www.healthdata.org/research-analysis/gbd.
GBD (2020). Global burden of 369 diseases and injuries in 204 countries and territories, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Lond Engl. 396 (10258), 1204–1222. doi:10.1016/S0140-6736(20)30925-9
Hatswell, A. J., Freemantle, N., and Baio, G. (2020). The effects of model misspecification in unanchored matching-adjusted indirect comparison: results of a simulation study. Value Health J. Int. Soc. Pharmacoeconomics Outcomes Res. 23 (6), 751–759. doi:10.1016/j.jval.2020.02.008
Ho, K. Y., Kuan, A., Zaño, F., Goh, K. L., Mahachai, V., Kim, D. Y., et al. (2009). Randomized, parallel, double-blind comparison of the ulcer-healing effects of ilaprazole and omeprazole in the treatment of gastric and duodenal ulcers. J. Gastroenterol. 44 (7), 697–707. doi:10.1007/s00535-009-0072-4
Hu, M., Ding, P., Ma, J., Yang, N., Zheng, J., and Zhou, N. (2022). Cost-effectiveness analysis of the TCM “yupingfeng granules” in the treatment of acute exacerbations of COPD based on a randomized clinical trial. Int. J. Chron. Obstruct Pulmon Dis. 17, 2369–2379. doi:10.2147/COPD.S374782
Kamada, T., Satoh, K., Itoh, T., Ito, M., Iwamoto, J., Okimoto, T., et al. (2021). Evidence-based clinical practice guidelines for peptic ulcer disease 2020. J. Gastroenterol. 56 (4), 303–322. doi:10.1007/s00535-021-01769-0
Lanas, A., and Chan, F. K. L. (2017). Peptic ulcer disease. Lancet Lond Engl. 390 (10094), 613–624. doi:10.1016/S0140-6736(16)32404-7
Levenstein, S., Kaplan, G. A., and Smith, M. W. (1997). Psychological predictors of peptic ulcer incidence in the Alameda County Study. J. Clin. Gastroenterol. 24 (3), 140–146. doi:10.1097/00004836-199704000-00004
Li, F., Qi, X., and Wang, L. (2019). Ilaprazole compared with rabeprazole in the treatment of duodenal ulcer: a randomized, double-blind, active-controlled, multicenter study. J. Clin. Dastroenterology 53 (9), 641–647. doi:10.1097/MCG.0000000000001186
Liu, F., Xu, Y., Wang, L., Ma, X., Zhang, Z., and Zhuang, X. (2023). Combined contributions of cytochrome P450s (CYPs) and non-enzymatic metabolism in the in vitro biotransformation of anaprazole, a novel proton pump inhibitor. Naunyn Schmiedeb. Arch. Pharmacol. 396 (8), 1759–1771. doi:10.1007/s00210-023-02415-7
Liu, G., Hu, S., and Wu, J. (2020). China guidelines for pharmacoeconomic evaluations,China. Available at: https://www.ispor.org/heor-resources/more-heor-resources/pharmacoeconomic-guidelines/pe-guideline-detail/china-mainland.
Liu, Y. (2022). Introduction of matching-adjusted indirect comparison in medical research. Chin. J. Evid-Based Med. doi:10.7507/1672-2531.202204127
MENET (2022). MENET. Available at: https://www.menet.com.cn/.
National Healthcare (2022). National healthcare security administration, China. Available at: http://www.nhsa.gov.cn/.
Phillippo, D. M., Ades, A. E., Dias, S., Palmer, S., Abrams, K. R., and Welton, N. J. (2018). Methods for population-adjusted indirect comparisons in health technology appraisal. Med. Decis. Mak. Int. J. Soc. Med. Decis. Mak. 38 (2), 200–211. doi:10.1177/0272989X17725740
Study Details (2022) Study Details | A phase 3 randomised controlled trial to evaluate efficacy and safety of Anaprazole in patients with duodenal ulcers. USA: ClinicalTrials.gov. Available at: https://clinicaltrials.gov/study/NCT04215653?cond=NCT04215653&rank=1.
Sullivan, S. D., Mauskopf, J. A., Augustovski, F., Jaime Caro, J., Lee, K. M., Minchin, M., et al. (2014). Budget impact analysis-principles of good practice: report of the ISPOR 2012 budget impact analysis good practice II task force. Value Health J. Int. Soc. Pharmacoeconomics Outcomes Res. 17 (1), 5–14. doi:10.1016/j.jval.2013.08.2291
Tarasconi, A., Coccolini, F., Biffl, W. L., Tomasoni, M., Ansaloni, L., Picetti, E., et al. (2020). Perforated and bleeding peptic ulcer: WSES guidelines. World J. Emerg. Surg. WJES 15, 3. doi:10.1186/s13017-019-0283-9
Wang, F., Niu, X., Liu, F., Ma, X., Cheng, F., Xu, H., et al. (2024). Safety, tolerability, and pharmacokinetics of anaprazole, a novel proton pump inhibitor, in healthy Chinese subjects. Clin. Pharmacol. Drug Dev. doi:10.1002/cpdd.1405
Xie, W., and Tang, M. 2022. Pharmacoeconomic analysis of Markov model in the treatment of peptic ulcer diseases. China J. Pharm. Econ. doi:10.12010/j.issn.1673-5846.2022.10.008
Xie, Y., Song, C., Cheng, H., Xu, C., Zhang, Z., Wang, J., et al. (2020). Long-term follow-up of Helicobacter pylori reinfection and its risk factors after initial eradication: a large-scale multicentre, prospective open cohort, observational study. Emerg. Microbes Infect. 9 (1), 548–557. doi:10.1080/22221751.2020.1737579
Yaozhinet (2016). Yaozhinet. Available at: https://www.yaozh.com/.
Zhang, X. (2022). Disease burden analysis of peptic ulcer disease from 1990 to 2019(in Chinese). Chin. J. Evid-Based Med.
Keywords: duodenal ulcers, Anaprazole, Ilaprazole, anchored matching-adjusted indirect comparison, cost-effectiveness analysis
Citation: Ni H, Shi J, Hu M, Zhou N and Yang S (2024) Cost-effectiveness analysis of Anaprazole versus Ilaprazole for the treatment of duodenal ulcers in China. Front. Pharmacol. 15:1407435. doi: 10.3389/fphar.2024.1407435
Received: 26 March 2024; Accepted: 22 May 2024;
Published: 07 June 2024.
Edited by:
Marcus Tolentino Silva, University of Brasilia, BrazilReviewed by:
Xiaosi Li, Hospital of Chengdu Office of People’s Government of Tibetan Autonomous Region, ChinaJianwei Xuan, Sun Yat-Sen University, China
Copyright © 2024 Ni, Shi, Hu, Zhou and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Naitong Zhou, em50QHNjdS5lZHUuY24mI3gwMjAwYTs=; Shu Yang, eWFuZ3NodTExMDZAc2N1LmVkdS5jbg==
†These authors have contributed equally to this work and share first authorship
 Huitong Ni
Huitong Ni Jiaqi Shi
Jiaqi Shi Ming Hu
Ming Hu Naitong Zhou
Naitong Zhou Shu Yang*
Shu Yang*