
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Pharmacol. , 30 May 2024
Sec. Ethnopharmacology
Volume 15 - 2024 | https://doi.org/10.3389/fphar.2024.1407212
Areca nut (AN), the fruit or seed of Areca catechu Linn, has many uses, including chewing and medicinal purposes. It has sparked worries about health due to the presence of alkaloids. Chewing AN may have a variety of negative consequences; however, the medicinal use of AN has no notable adverse effects. To completely understand and effectively use AN, researchers have investigated its chemical makeup or biological activity, analyzed the variations between different AN species and different periods, and improved extraction and processing procedures. Today, an increasing number of researchers are exploring the underlying reasons for AN variations, as well as the molecular mechanisms of biosynthesis of chemical components, to comprehend and change AN at the genetic level. This review presents an overview of the clinical study, pharmacology, and detection of the main bioactive components in AN, and the main factors influencing their content, delving into the omics applications in AN research. On the basis of the discussions and summaries, this review identifies current research gaps and proposes future directions for investigation.
Areca catechu is a monoecious, perennial and evergreen tree belonging to the palm family (Figure 1A). It grows in the tropical climates of Asia, East Africa, and the Pacific, particularly in South and Southeast Asian countries. It is now believed that A. catechu originated in Indonesia, Malaysia, or the Philippines (Rooney, 1995; Lim, 2012; World Health Organization. Regional Office for the Western Pacific, 2012; Manimekalai et al., 2018; Joo et al., 2020). After thousands of years of evolution, it has become a significant economic tree species in certain regions, such as Indonesia and Vietnam. Hainan Province is the largest producer of A. catechu in China (Yang et al., 2018). In addition to its contribution to the agricultural economies of the countries where it is grown, A. catechu has an important place in the religious, cultural, and social milieus of the rural people. Areca nut (AN) is the fruit of A. catechu (Figures 1B, C), and it is not only used to chew; however, different parts of it have different medicinal values. The immature fruit is boiled and dried, and the husk obtained after removing the seeds is called arecae pericarpium (“Da Fu Pi” in Chinese) (Figure 1D), whereas the husk obtained after processing the mature fruit is called “Da Fu Mao” (Figure 1G). Their primary functions include moving qi, soothing the middle, disinhibiting water, and relieving swelling. The mature fruit is boiled and dried, and the husk is removed, resulting in therapeutic seeds known as arecae semen (Figure 1E). The charred seeds are known as arecae semen tostum (Figure 1F). For seeds, the arecae semen is mainly used to kill parasites, and the arecae semen tostum is mainly used to promote digestion. In this review, the term AN is used to refer to both the fruit and the seed of A. catechu without distinction. The fresh young fruit (usually harvested at around 4 months) are chewed with various ingredients in different regions. It is estimated that more than 600 million people chew AN (Warnakulasuriya and Chen, 2022). Unfortunately, AN is considered the world’s fourth most addictive substance and is classified as a Group 1 carcinogen by the International Agency for Research on Cancer (IARC) of the World Health Organization (IARC Working Group on the Evaluation of Carcinogenic Risks to Humans, 2007). In traditional medicinal applications, the processing of traditional Chinese medicine has been proven by numerous studies to enhance efficacy and reduce toxicity. To cater to individuals with diverse constitutions and address various primary symptoms, China has long employed the technique of charcoal roasting to transform arecae semen into arecae semen tostum. Arecae semen has the strongest medicinal properties and is not recommended for frail individuals. By contrast, Arecae semen tostum offers milder effects with less adverse effects while alleviating food stagnation. Researchers have already compared the chemical compositions of AN, in addition to the influence of different processing methods on these chemical components. Distinct parts of AN and various processing techniques can lead to significant changes in the active ingredients.
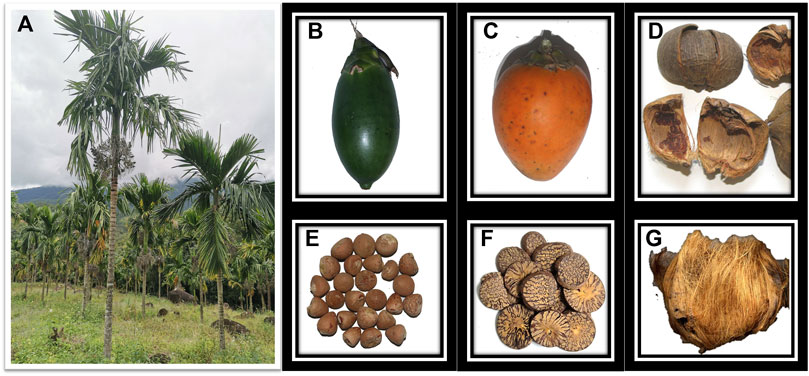
Figure 1. The plant, fruit, husk, and seed of Areca catechu. (A) Plant A. catechu. (B) Fresh and tender fruit. (C) Ripe fruit. (D) Arecae pericarpium. (E) Arecae semen. (F) Arecae semen tostum. (G) Da Fu Mao.
Although it is true that the medicinal and edible uses of seeds and fruit should not be confused, alkaloids, the main active ingredients that cause the positive effects, also play a major role in the negative effects. As a result, the public is afraid of pharmaceuticals and preparations that contain arecae semen or arecae semen tostum. Nevertheless, the adverse clinical events associated with medicinal seeds are relatively rare and controllable. Ancient books contain no records of arecae semen’s toxicity and usually consider it nontoxic. Only a few records mention likely side effects, such as “fever and injury to vital energy”, emphasizing that it should not be used by those with weak constitutions or those who do not have specific indications for its use (Sun et al., 2017).
This review provides an overview of the clinical study and observation of AN, and two main categories of bioactive compounds in the fruit and seeds of A. catechu: alkaloids and polyphenolic substances. It describes their pharmacological effects and potential adverse effects, and summarizes recent literature on the main detected substances, quantification methods, and influencing factors. Subsequently, the application of omics technologies in AN research is discussed, encompassing aspects such as A. catechu germplasm, metabolites, and bioactive properties, with the aim of offering a reference for better utilization and quality determination of AN.
AN is commonly used in therapeutic settings as a proprietary Chinese medicine. These formulas were proposed by traditional Chinese physicians a hundred or a thousand years ago and were enhanced during use. There have been no reports of clinically significant toxic side effects of the use of AN for medicinal purposes. “Muxiang Binglang Pill” has the effect of eliminating food-induced stagnation, and its combination with Western medicine in the comprehensive treatment of mild acute pancreatitis can effectively promote the recovery of intestinal function and improve its overall clinical efficacy (Wang, 2024). The clinical efficacy of employing “Binglang Powder” in conjunction with acupoint massage in the treatment of patients with spleen and stomach diseases is remarkable, effectively relieving symptoms, reducing inflammatory reactions in the spleen and stomach, and improving spleen and stomach function (Liu, 2021). Moreover, AN can promote the recovery of gastrointestinal function after gynecological abdominal surgery, and its mechanism of action may be related to the modulation of the level of motilin, a brain-gut peptide hormone (Liang et al., 2020). “Food Retention Relieve Oral Liquid” is composed of 10 medicines, including Galli Gigerii Endothelium Corneum, Sparganii Rhizoma, Curcumae Rhizoma, and Arecae semen. It has good clinical efficacy in the treatment of anorexia nervosa in children (Wan, 2010; Sun et al., 2014). “Jinyingzi Binglang Decoction” is effective in treating patients with chronic, intractable diarrhea (Zhang, 2014). “Food Retention and Cough Relieve Oral Liquid for Children” is composed of Crataegi Fructus, Arecae semen, and eight other medicines that are effective in the treatment of pediatric cough and have the advantages of few toxic side effects and ease of use (Di, 2015). “Binglang Shisanwei Pill,” in combination with psychological care, can effectively improve the symptoms of depression and increase satisfaction in clinical care (Gu, 2021). Furthermore, in animal parasite therapy, arecoline hydrobromide is useful in the eradication of cattle tick Rhipicephalus microplus infections in calves, and clinical safety has been determined by examining hematological characteristics and performing skin irritation assays in calves infested with ticks (Jain et al., 2021).
AN chewing often leads to oral problems. Clinical analysis of 189 cases of oral submucosal fibrous degeneration in a hospital in Zhengzhou (a city in China) showed that the onset of oral submucous fibrosis (OSF) predominantly occurs in males, with a tendency for rejuvenation. Age, chewing time, and chewing amount are related to OSF disease severity (Yu et al., 2022). Tang et al. (2018) conducted a clinical analysis of 86 cases of OSF caused by AN chewing; the higher the frequency and duration of AN chewing, the greater the probability of the disease. The majority of patients with OSF have initial symptoms of mucosal irritation pain and limited mouth opening. Most patients also have a smoking habit, which has a synergistic effect on the occurrence of OSF. In oral squamous cell carcinoma (OSCC) patients with an chewing habit, tumors are relatively well differentiated, and the buccal and tongue areas are the most common sites of OSCC (Shao et al., 2021). Long-term AN chewing may lead to different degrees of tooth abrasion. By reviewing the medical records of patients with oropharyngeal squamous cell carcinoma (OPSCC) between 1999 and 2013, Chen et al. (2020) found that HPV-OPSCC accounted for the majority of OPSCC in areas where AN chewing is endemic. The combination of alcohol/betel nut chewing/smoking exposure has a significant negative impact on disease-free survival and overall survival.
Alkaloids display a wide range of biological functions and may pose potential health threats. They are typical constituents and bioactive compounds found in AN, with presence primarily limited to A. catechu in the palm family. Presently, at least 18 alkaloids have been identified in AN (Table 1) (Peng et al., 2015; Tang et al., 2017; Cao et al., 2019); however, there is a notable dearth of dedicated research on the isolation and identification of alkaloids in the seed. The total alkaloid content in AN varies between 0.3% and 0.6% (Han, 2010). Arecoline is commonly considered the most abundant alkaloid, followed by guvacine, arecaidine, and guvacoline. These four alkaloids not only constitute a significant portion of the alkaloids in AN but are also major active substances (Oliveira et al., 2021). Hence, researchers often rely on measuring the content of arecoline or these four alkaloids as evidence of AN efficacy or toxicity. Furthermore, experiments have shown that different regions, growth stages, and processing methods can significantly influence the alkaloid content of AN.
The alkaloids in AN exhibit a wide range of biological effects, including anthelmintic, antimicrobial, anti-inflammatory, and analgesic effects and effects on the cardiovascular, nervous, digestive, and endocrine systems.
The Chinese Pharmacopoeia documents that arecae semen can be used for anthelmintic purposes (National Pharmacopoeia Committee, 2020). Alkaloids present in AN have exhibited significant anthelminthic effects against various endoparasites in the body, such as hydatid worms, tapeworms, and acute Toxoplasma gondii parasites (Batham, 1946; Zheng et al., 1998; Xu, 2018). In a study conducted by Puyathorn et al. (2022), the methanol and water crude extracts from AN, as well as the arecoline hydrobromide solution, were capable of damaging the tegument (surface membrane) of flukes and effectively eliminating them in the early stages.
The alkaloids in AN also display inhibitory effects on Proteusbacillus vulgaris, Candida albicans, and Bacillus anthracis spores, with a minimal inhibitory concentration (MIC) of 0.8 mg/mL (Luo et al., 2010). The alkaloids and polyphenolic compounds in AN extracts can also effectively control the occurrence of anthracnose diseases (Ahmad Rusdan et al., 2015).
AN primarily contains arecoline and condensed tannins, and these components have a relaxing effect on the aortas of rats with an intact endothelium. Thus, arecoline can reduce vascular tone in a concentration-dependent manner, triggering a blood pressure-lowering effect (Goto et al., 1997; Chen et al., 2020).
Arecoline is an agonist of the α7 nicotinic acetylcholine receptor and an effective inflammation inhibitor. Papke et al. (2015) found that when used in combination with the positive allosteric modulator PNU120696, arecoline and PNU120696 together significantly upregulate the expression of this receptor, thereby achieving a synergistic anti-inflammatory effect.
Arecoline is the primary substance responsible for the effects on the central and autonomic nervous systems. Arecoline has a stimulating effect on the sympathetic nervous system and can simultaneously target M and N receptors. Alkaloids found in AN, including arecoline, arecaidine, guvacine, and guvacoline, can induce behavioral changes in zebrafish (Siregar et al., 2022). These changes include an increase in the sense of wellbeing, stamina, and euphoria (Garg et al., 2014). Research suggests that the injection of arecoline significantly shortens sleep duration in mice exposed to alcohol but prolongs their resistance to sleep following pentobarbital injection (Sun et al., 2005; Xiao et al., 2013). Nevertheless, experiments targeting the righting reflex induced by pentobarbital and ethanol have shown that arecoline can reduce the sleep duration induced by ethanol but does not affect the sleep duration induced by pentobarbital at the doses used. Additionally, at the same dosage levels, arecoline does not significantly affect the latency of sleep induced by ethanol or pentobarbital in mice (Sun et al., 2010). When administered in the early stages of aging, arecoline exhibits potential pharmacological properties for extending lifespan. Studies suggest that arecoline reduces muscle tissue through the GAR2/PLCβ pathway, leading to lifespan extension (Ching et al., 2020). Furthermore, doses of 125 and 175 mg/kg of the dichloromethane extract of AN significantly inhibit withdrawal symptoms in morphine-dependent mice. Arecoline also improves some of the negative symptoms of mental illness (Kumarnsit et al., 2005). In zebrafish, arecoline increases serotonin levels, social preference, and brain norepinephrine while reducing serotonin turnover, leading to anxiolytic effects. Thus, it can be seen that arecoline possesses anxiolytic-like activity (Serikuly et al., 2021). Arecoline enhances cognition, memory, and some behavioral disorders in patients with schizophrenia or Alzheimer’s disease by activating postsynaptic muscarinic M1 receptors (Avery et al., 1997; Brunetti et al., 2020).
Previous research has reported that a water extract containing 0.06% arecoline significantly enhances the contraction of gastric smooth muscle and muscle strips in the duodenum, ileum, and colon (Ni et al., 2004). Wang et al. (2020) found that hydrobromide arecoline noticeably increased the contractile ability of gastric and intestinal muscle strips in various regions of the gastrointestinal tract in wild-type C57BL/6 mice. Correspondingly, Arecae pericarpium extract and arecoline can induce contractions in the lower esophageal sphincter sling and clasp muscles in pigs, and this effect is dose-dependent. The contractions induced by the extract and arecoline are mediated by muscarinic receptors, suggesting the possibility of developing alternative treatments for gastroesophageal reflux disease (Tey et al., 2021).
AN has an impact on the human endocrine system. Arecoline can increase the release of corticotropin-releasing hormones by activating the hypothalamic–pituitary–adrenal axis (Calogero et al., 1989; Wang and Hu, 2010). Dasgupta’s team found that arecoline can exacerbate thyroid dysfunction in mice under metabolic stress, while also improving thyroid hyperactivity induced by cold stress (Dasgupta et al., 2017; Dasgupta et al., 2018). Wang et al. (2008) discovered that by activating L-type calcium channels, arecoline increases the activity of 17β-hydroxysteroid dehydrogenase and enhances the expression of Steroidogenic Acute Regulatory Protein in interstitial cells of the testes. This directly stimulates the production of testicular hormones. Qi (2010) found that arecoline may activate insulin receptor substrates in the insulin signaling pathway through the phosphatidylinositol 3-kinase/protein kinase B (PI3K/AKT) pathway, which promotes insulin secretion to lower blood glucose levels.
Arecoline can reduce the levels of the epithelial tumor cell survival factor IL-6, increase the levels of tumor suppressor P53, induce cell cycle arrest, promote apoptosis, and thereby prevent basal cell carcinoma (Huang et al., 2012).
Nevertheless, the higher alkaloid content in the seed than in the husk makes the seed the primary carcinogenic component, especially in oral cancer development. In vitro and in vivo experiments have shown that arecoline can induce the generation of reactive oxygen species, cell cycle arrest, apoptosis, DNA damage, upregulation of transcriptional proteins, and cytotoxicity in human endothelial cells (Tseng et al., 2012; Peng et al., 2015; Wang et al., 2016; Tu et al., 2019).
It has been reported that four alkaloids, namely, arecoline (the most potent), arecaidine, guvacine, and guvacoline, stimulate fibroblasts to produce collagen, which is believed to represent the main pathogenic mechanism of oral submucous fibrosis (Angadi and Rao, 2011; Arakeri and Brennan, 2013). Among these, arecoline, the key compound initiating the process of submucous fibrosis in the oral mucosa, operates in conjunction with Transforming Growth Factor-β, which plays a pathological role in organ fibrosis (Li et al., 2019; Ku et al., 2021). Arecoline promotes the activation of buccal mucosal fibroblasts, which is induced by transforming growth factor-β1 through the enhancement of phosphodiesterase 4A activity (Zhang et al., 2021). Additionally, arecoline causes cardiac fibrosis, regulates renal fibrosis in renal tubular epithelial cells, and ultimately contributes to the progression of chronic kidney disease (Ding et al., 2018; Hsieh et al., 2020; Ku et al., 2021).
Arecoline has been shown to enhance cervical lymph node metastasis in tongue xenografted models using nude mice, indicating that it induces the epithelial–mesenchymal transition and promotes metastasis in oral cancer (Ren et al., 2021). Additionally, arecoline has demonstrated the ability to stimulate proliferation and enhance the migratory potential of HepG2 cells, upregulating the expression of PI3K-AKT pathway factors. This suggests that arecoline can promote the migration and proliferation of human hepatocellular carcinoma cells by activating the phosphatidylinositol 3-kinase/protein kinase B/mammalian target of the rapamycin (PI3K/AKT/mTOR) pathway (Xie et al., 2022). However, there is no direct evidence of arecoline-induced carcinogenesis in animal models (Shen et al., 2020).
Arecoline stimulates collagen production in cultured cells at concentrations as low as 0.1 μg/mL and is cytotoxic at concentrations above 10 μg/mL (Cox et al., 2010). It suppresses epithelial cell viability through the AKT/mTOR signaling pathway in oral cancer, with minimum effective concentrations ranging from 0.025 to 12.5 μg/mL (Chen et al., 2014; Gu et al., 2019). AN’s ethanolic extract exhibited cytotoxicity in a lung cancer cell line at a concentration of 1.7 µg arecoline per mg of extract (Abbas et al., 2018). In a breast cancer cell line study, arecoline inhibited cell proliferation and induced apoptosis at a concentration of 100 μM/L (Feng et al., 2016). Arecoline reduces the aminobutyric acid functional pathway in the nervous system and induces neuronal damage or neuronal apoptotic death by attenuating antioxidant defense and enhancing oxidative stress (Huang et al., 2003; Shih et al., 2010). When intraperitoneally injected into rats, arecoline induces cardiac apoptosis through the Fas/Fas ligand pathway (Lin et al., 2021). Additionally, different doses of arecoline exhibit varying degrees of impact on murine splenic lymphocytes, human liver cells, C2C12 myoblasts, and other cell types (Chou et al., 2008; Dasgupta et al., 2010; Chang et al., 2012; Chang et al., 2013; Gu et al., 2013). These findings collectively indicate that arecoline has cytotoxic and apoptotic effects on various cell types and can impact different signaling pathways and biological processes. These effects can vary depending on the cell type and concentration of arecoline used. Arecoline is a major alkaloid found in AN, and its health effects are of concern due to its association with oral cancers and other health problems in individuals who consume AN.
In recent years, comprehensive research has been conducted on the toxic effects of AN and arecoline on reproduction. Garcia-Algar et al. (2005) reported the birth outcomes of newborns whose mothers consumed AN during pregnancy, with two exhibiting adverse outcomes; however, no clear conclusions can be drawn regarding the correlation between fetal exposure to arecoline and clinical outcomes. Moreover, an experiment conducted by Liu et al. (2011) demonstrated that arecoline is toxic to embryos during the peri-implantation stages in mice. Additionally, alkaloids significantly decrease the motility of human sperm in a dose-dependent manner (Yuan et al., 2012). Another study showed that a dose of 50 mg/kg body weight of AN has contraceptive effects in male rats (Rahman et al., 2020).
Arecoline, in particular, is one of the genotoxic alkaloids in AN. Arecoline could inhibit p53 by its expression and transactivation function. Consequently, this inhibits DNA repair and induces the DNA damage response (Tsai et al., 2008). In addition, it may trigger DNA chain disruption, chromosome distortion, and sister chromatid exchange, affect DNA repair, and cause oxidative stress (IARC Monographs Vol 128 group., 2021).
In addition, arecoline can induce cardiac toxicity and heart damage through various pathways, including the activation of JAK2/STAT3 induced by IL-6, MEK5/ERK5, and Mitogen-Activated Protein Kinase (Ho et al., 2022). Arecoline disrupts mouse oocytes’ low adenosine 5′-triphosphate levels, increases oxidative stress, and exacerbates intestinal inflammation by affecting the abundance of gut microbiota, which regulates serum metabolite concentrations in mice (Li et al., 2020; Zhao et al., 2023). In addition, arecoline may induce bronchial smooth muscle contraction and spasm, causing chest tightness; affect lipid metabolism thus leading to obesity; and may also cause insulin resistance and diabetes mellitus (Taylor et al., 1992; Hsu et al., 2010; Hsieh et al., 2011). The beneficial and adverse effects of AN are shown in Table 2 and Figure 2.
Researchers typically evaluate the effectiveness and safety of AN by measuring the alkaloid content. There is a thorough summary of the methodologies for liquid chromatography (LC) separation of alkaloids in herbal medicines provided by Zhao et al. (2020), including reversed-phase LC, ion exchange chromatography, hydrophilic interaction chromatography, two-dimensional LC, and mixed-mode LC. High-performance liquid chromatography (HPLC) is the primary method used for quantitative analysis in AN.
Xue et al. (2011) established a near-infrared spectroscopy model to predict the dynamic changes in the content of three alkaloids (arecoline, arecaidine, and guvacine) during the drying process of AN. This model reveals the quality changes in AN during the drying process, providing a basis for rapid online analysis and quality control. Yuan et al. (2012) improved HPLC by employing a cation exchange column to determine the content of three major alkaloids, arecoline, arecaidine, and guvacine, in fresh, dried, shell, and nut parts to investigate the impact of AN on male sperm. Tian et al. (2018) used HPLC to determine the content of four alkaloid components (arecoline, arecaidine, guvacine, and guvacoline) in AN materials. Their findings indicated that arecoline, arecaidine, guvacine, and guvacoline could serve as quality control markers for AN materials, with specific recommendations for the contents in various parts of the nut. Yuan et al. (2018) reported the use of both HPLC-UV and ultra-high-performance liquid chromatography-tandem mass spectrometry (UHPLC-MS/MS) methods for the quantitative analysis of four characteristic AN alkaloids. They introduced an UHPLC-MS/MS method to assess typically overlooked components in AN products, including eight phenolic compounds and a trace number of alkaloids. Gheddar et al. (2020) developed two methods for analyzing four alkaloids in hair to assess long-term AN exposure. The presence of these substances in hair serves as a reliable marker of AN consumption. Hu et al. (2010) conducted a high-throughput analysis of five urinary metabolites of AN and tobacco alkaloids using online solid-phase extraction liquid chromatography–tandem mass spectrometry to aid in the study of the carcinogenic effects of AN and tobacco exposure. The detected urinary metabolites of AN included arecoline, arecaidine, and N-methylnipecotic acid. Osborne et al. (2022), to determine the levels of five alkaloids as markers to evaluate the safety of chewing AN, used UHPLC-MS/MS. Huang (2022) used HPLC to measure four cytotoxic alkaloids in AN. Pasupuleti et al. (2022) presented a green analytical methodology combining in-syringe-assisted vortex-induced salt-enhanced liquid-liquid microextraction with UHPLC-MS/MS to simultaneously monitor five AN alkaloids (arecaidine, guvacine, guvacoline, arecoline, and N-Nitrosoguvacoline) in saliva and urine, aiming to predict the potential health risks of short- and long-term betel quid chewing. Liu et al. (2022) established a liquid chromatography-ion trap-time of flight mass spectrometry method for the simultaneous determination of four areca-specific alkaloids and the analysis of the two main toxic alkaloids (arecoline and arecaidine) during AN blanching. Additionally, a combination technique of HPLC coupled with ion mobility spectrometry (IMS) was established by Lu et al. (2022) to detect the contents of arecoline, guvacine, and arecaidine in husks, seeds, green fruit, smoked fruit, and simotang oral liquid. Wu et al. (2022) used matrix-assisted laser desorption/ionization mass imaging spectrometry (MALDI-MSI) to detect 10 physiologically active alkaloids in different AN fruit developmental stages, with LC-MS/MS validation and quantification to assess the distribution of these alkaloids in various tissue types. Furthermore, given that the detection of arecoline relies primarily on time-consuming and expensive chromatography-based methods, Wang et al. (2022) developed an indirect competitive enzyme-linked immunosorbent assay (icELISA) method using mAb-A5H12 for the detection of arecoline in traditional Chinese medicine and fresh AN. Detailed information is summarized in Table 3.
The alkaloid content in AN is influenced by various factors, such as the variety, cultivation practices, climate, geographic location, harvesting time (growth period), processing methods, and storage. In this connection, the impact of geographic location, growth period, processing methods, and extraction techniques based on existing research are discussed.
Although the AN used worldwide comes from the same species, various factors such as growing regions and weather conditions, have led to the development of different varieties of AN. As a result, there is significant variation in the alkaloid content among arecae semen varieties sold worldwide, ranging from 2 to 10 mg/g dry weight (Gupta et al., 2020). Jantarat et al. (2013) found that the content of arecoline in AN obtained from northeast Thailand ranged from 0.02% to 0.12%, whereas the content of arecoline in AN grown in China ranged from 0.22% to 0.56%. Even within the same country, regional differences in AN cultivation can have a significant impact on the content of AN metabolites. For example, Lin et al. (1992) showed that the arecoline content of AN obtained from different producing areas in China exhibited high variability. A study of AN from four regions of India (Banda Aceh, North Sumatra, West Kalimantan and West Papua) found that samples from the West Papua region had higher values than those from other regions (Sari et al., 2020). Zhang et al. (2022) investigated the metabolomics of betel quid components from four different regions in Indonesia and observed significant geographical variations in the content and concentration of metabolites. These findings underscore the importance of considering geographical and regional factors when evaluating the alkaloid content and its potential effects.
The content of arecoline, the most abundant alkaloid in AN, varies throughout its growth period. Research has shown that the arecoline content increases as the nut matures, reaches its maximum level, and then drastically decreases in the mature nut. When evaluating the safety and efficacy of AN consumption, this change in alkaloid content during growth is a crucial consideration. Studies have revealed that after pollination, the total alkaloid content in AN seeds initially increases and then decreases, with the highest content observed around 110 days. However, the alkaloid content in the nut’s pericarp (the outer covering) reaches its peak at around 80 days and gradually decreases as the nut continues to develop (Yan et al., 2023). This variation in alkaloid content during growth has been visualized using methods such as electrospray ionization mass spectrometry (ESI MS) and desorption electrospray ionization mass spectrometry (DESI MS) imaging. These techniques have shown that as the nut matures, white and brown regions form within the nut, and the distribution of alkaloids changes (Srimany et al., 2016). At the final stage of maturity, arecoline, arecaidine, and guvacoline tend to accumulate in the brown region of the nut, whereas guvacine is more concentrated in the white region of the nut. This segregation of alkaloids in different regions of the nut indicates that the white portion of the ripened nut may be a safer choice for consumption, as a higher proportion of alkaloids, including arecoline, is found in the brown region. Separating the white and brown regions of ripened AN can potentially allow for the safer utilization of its medicinal properties while reducing exposure to the higher alkaloid content in the brown region.
The processing of fresh AN can considerably affect the alkaloid content. Studies have shown variations in alkaloid levels based on different processing methods. Here are some examples: Dutta et al. (2017) examined various AN preparations consumed in India, including raw, boiled, and roasted varieties. The study found that raw AN contained the highest arecoline concentration, while boiled AN had the least, with roasted varieties exhibiting an intermediate level. In traditional Chinese medicine, AN is also processed in different ways, such as raw (fresh nuts boiled, peeled, and dried) or charred. These different processing methods can affect the content of various alkaloids. Hou et al. (2023) found that AN had the highest content of arecoline, followed by dehydroarecoline, arecaidine, and dehydroarecaidine. Roasted AN had a slightly lower alkaloid content, with differences noted in arecaidine and dehydroarecaidine. In summary, the arecoline content followed the order: raw AN > shade-dried slices > sun-exposed slices > roasted AN > charcoal.
The choice of solvent can have a significant impact on the efficiency of extracting bioactive compounds from natural products, including AN. Solvent polarity is one of the key factors influencing the extraction efficiency of phytochemicals (Suchinina et al., 2011; Zhu et al., 2020). In the context of AN seed extracts, researchers have found that extracts obtained using selected deep eutectic solvents have a higher total alkaloid content than other solvents. Ethyl acetate extracts have the lowest total phenolic content (Wang et al., 2021). Multivariate analysis results have confirmed that the choice of extraction solvent significantly influences the phytochemical composition and biological activities of AN extracts. This emphasizes the importance of carefully selecting the appropriate solvent to obtain the desired bioactive compounds from AN or other natural products, as different solvents can yield varying chemical profiles and extraction efficiencies. Researchers should consider the specific compounds they aim to extract and the properties of the solvent that will be most effective for their purposes.
Polyphenols, being one of the two most biologically active substances, are the most abundant secondary metabolites in arecae semen, with an approximate 31% total polyphenol content. At least 30 polyphenol compounds have been isolated from AN (Celimuge and Xu, 2023).
Reportedly, polyphenols exhibit various beneficial effects, including antidepressant, antihypertensive, antioxidant, anti-inflammatory, and antibacterial activities.
He et al. (2013) examined the antidepressant activity of total polyphenols from AN in a mouse model of depression. The results suggest that the total polyphenol extract from AN may exert a significant antidepressant effect, possibly by influencing the concentrations of monoamine neurotransmitters in brain tissue. Zhou et al. (2020) indicated that the gut microbiota could enhance the bioavailability of polyphenols by breaking them down into metabolites with higher activity and better absorption. This, in turn, can improve depression through the microbiota–gut–brain axis.
AN contains a substantial amount of proanthocyanidins. Huang et al. (2010) discovered its anti-inflammatory properties and showed that can inhibit edematous inflammation induced by carrageenan and the formation of prostaglandin E2. Proanthocyanidins from AN also have effective anti-allergic properties, reducing food allergy reactions. The administration of polyphenols isolated from AN (0.05% and 0.1%, w/w) can decrease ovalbumin-induced allergic responses, including diarrhea and infiltration and degranulation of mast cells in the duodenum, as well as reduce serum ovalbumin-specific IgE and interleukin-4 levels in the duodenum (Wang et al., 2013).
Catechins found in AN also exhibit anti-hypertensive effects. Gilani et al. (2006) found that catechins not only dilate blood vessels but also possess antiplatelet activity. In a study by Ghayur et al. (2011), catechins isolated from AN demonstrated a clear antiplatelet effect, with IC50 values of 3.630, 1.876, 2.551, 1.541, and 2.640 mg/mL for platelet aggregation induced by arachidonic acid, adenosine diphosphate, platelet-activating factor, epinephrine, and calcium ionophore, respectively.
Polyphenols possess antioxidant, anti-inflammatory, and antibacterial properties. Recent research has indicated that they provide protection against oxidative stress induced by hypoxia by scavenging excess free radicals and improving the blood gas parameters of hypoxic organisms (Ma et al., 2022a). The proanthocyanidins in AN, as well as flavonoids, are effective components in exerting antioxidant effects. The antioxidant capacity is related to the total polyphenol and total flavonoid content (Zhang et al., 2015). In 2022, Chaikhong et al. (2022) found that arecoline ethanol extracts, which are rich in phenolic content, demonstrated potent free radical scavenging activity. Additionally, Ma et al. (2022a) showed that arecoline acetone extract, containing a high concentration of proanthocyanidins, exhibited a significant downregulation of TPA-induced cyclooxygenase-2 expression, inhibited UVB-induced photo-damage, and mitigated premature skin aging induced by solar ultraviolet radiation.
HPLC analysis has shown that the major components in the extract are catechins and quercetin. Therefore, the phenolic compounds in the extract exhibit immunomodulatory activity against Staphylococcus aureus infection (Sari et al., 2020). The polyphenols in the ethanol extract, including catechins, epicatechin, and epicatechin gallate, possess infection-resistance activity against Mycobacterium tuberculosis, Gram-positive S. aureus, and Gram-negative Escherichia coli (Raju et al., 2021). Catechins also display significant antifungal properties against anthracnose fungi (Yenjit et al., 2010).
Furthermore, the acetone extracts of AN, which are rich in procyanidins, significantly inhibit cyclic adenosine monophosphate/dexamethasone-induced gluconeogenesis in mouse primary hepatocytes (Huang et al., 2013). The pro-apoptotic effect of the polyphenol-enriched AN extract on splenic lymphocytes was examined. The results indicate that the polyphenol-enriched AN extract markedly induces lymphocyte apoptosis, and the primary active constituents might be oligomeric procyanidins (Wang et al., 2009).
As Table 4 shows, the determination of polyphenolic compound content, primarily focusing on total polyphenols, is commonly performed using UV spectrophotometry. Wang et al. (2011) employed HPLC to determine the presence of nine polyphenolic compounds in AN fruit and seed. These compounds included gallic acid, ellagic acid, epicatechin, epicatechin gallate, catechin, chlorogenic acid, epicatechin gallate ester of ellagic acid, epicatechin, and epicatechin ester of ellagic acid. All nine components were detected in the fruit, while epicatechin, chlorogenic acid, and epicatechin ester of ellagic acid were not detected in the seeds. Among the polyphenols in the seeds, epicatechin was the most abundant (1,610 mg/kg), followed by gallic acid (622 mg/kg). Xing et al. (2019) employed ellagic acid as a reference standard and Folin reagent as the colorimetric reagent to measure the total polyphenolic content in AN using spectrophotometry. Similarly, Wang et al. (2022) employed ellagic acid as a reference standard and used the Folin colorimetric method to determine the total polyphenolic content in AN, comparing the polyphenol yield under different extraction conditions to optimize the polyphenol extraction process. Ma et al. (2022b) employed UV spectrophotometry using ferrous tartrate as a colorimetric reagent and gallic acid as the control standard to measure the total polyphenolic content in AN extracts. Additionally, to determine the content of catechin, epicatechin and protocatechuic acid in AN polyphenolic extracts, they established an HPLC analytical method. These methods aim to provide experimental evidence for the quality control and standardization of AN polyphenolic extracts. Song et al. (2022) used UHPLC-MS/MS technology to analyze and partially quantify flavonoid components in AN extracts. Their findings revealed variations in the types and quantities of flavonoid compounds among different AN parts. Hesperidin and quercetin were the predominant flavonoid components in the husk extract, whereas the seed extract contained higher levels of flavonoid compounds, including epicatechin, procyanidin B1, procyanidin B2, and L-epicatechin. Furthermore, the seed extract exhibited the highest observed antioxidant activity, the most potent inhibition of α-amylase and α-glucosidase enzymes, and the ability to inhibit angiotensin-converting enzymes relative to other extracts.
These research methodologies are indispensable for understanding the polyphenolic content in AN, in addition to their biological activities and potential medicinal effects. Variations in components and concentrations can significantly impact the therapeutic properties of AN. By applying these methods, researchers can obtain a better understanding of the polyphenolic compounds in AN and provide data support for the quality control of related products in academic research.
Based on existing research, this section mainly introduces the effects of geographic location, growth period, and extraction methods on the polyphenol content of AN.
In a study by Sari et al. (2020), AN samples from four different regions, namely, Banda Aceh, North Sumatra, West Kalimantan, and West Papua, were selected to measure the polyphenolic content in AN husks. The findings indicated that there were no significant differences in polyphenolic content among the husks from Banda Aceh, North Sumatra, and West Kalimantan. The West Papua region exhibited a significantly higher polyphenolic content in husks than the other three regions. Although all four regions share a tropical rainforest climate, they differ significantly in terms of altitude and latitude. Remarkably, West Papua, with the highest altitude and being situated in the southern hemisphere, may be the primary factor contributing to the variations in AN polyphenolic content among these regions. Correspondingly, a study of the polyphenol content of different parts of the elderberry from different altitudes and locations confirmed this conclusion (Senica et al., 2017).
Similar to the alkaloids, the polyphenol content in AN also changed with the growth period. Wang et al. (1997) showed that polyphenol content gradually increased with an increase in maturity. However, in another experiment, immature seeds showed a higher polyphenol content than mature seeds (Hamsar et al., 2011). Additionally, in a study by Yan et al. (2022), metabolomics analysis using gas chromatography-mass spectrometry (GC-MS) was conducted to determine the metabolites in AN husks and seeds at different developmental stages. The total flavonoid content in AN seeds was significantly higher than that in the fruit husk, with seeds reaching a maximum of 47.92% and husk reaching a maximum of 0.83%. In AN, the total flavonoid content in the seeds exhibited an increasing trend followed by a decrease, reaching its peak at 110 days of development. However, the husk showed a decreasing trend followed by an increase, with the highest content at 140 days. These studies collectively suggest that as the maturation period progresses, the polyphenolic content in AN initially increases and then gradually decreases. This result may be because different researchers have different judgments of AN maturity.
There are various methods for extracting polyphenols from AN, with the most commonly used methods being organic solvent extraction and ultrasound-assisted extraction. Fan et al. (2022) used supercritical fluid extraction to extract phenolic compounds from dried AN husks and seeds. They optimized the conditions for ultrasound-microwave synergistic extraction (UMSE). Compared with traditional liquid-solid extraction (CLSE), UMSE is a non-traditional method for the solid-phase extraction of phenolic compounds, and it offers advantages such as short reaction times, increased efficiency, and reduced energy consumption compared to CLSE. Experimental results showed that UMSE significantly improved the total phenolic content, purity, antibacterial activity, and antioxidant activity of phenolic compounds compared to CLSE (Han, 2010).
Substances identified from AN extraction also include triterpenes, steroids, fatty acids, physcion, chrysophanol, and epoxyconiferyl alcohol in Table 1. (Peng et al., 2015; Liu and Chang, 2023). Terpenoids have pharmacological effects, such as antipyretic, analgesic, anti-inflammatory, antibacterial, antiviral, and anticancer effects. Steroids regulate lipid metabolism, and fatty acids are key anti-inflammatory mediators. These ingredients, together with alkaloids and polyphenols, affect the life activities of the human body, treat diseases, and threaten health. Since the content of these is not high and they are not the main pharmacologically active substance, there is almost no research on these ingredients in AN.
Currently, in quality and safety research on AN, the technologies applied extend beyond the evaluation of biological activity and the determination of major pharmacological components. They also encompass genomic, metabolomic, and transcriptomic techniques.
The genome of AN has recently been published. Yang et al. (2021) presented a chromosome-scale reference genome assembly with 92.92% of the genes functionally annotated. This annotation included genes responsible for the biosynthesis of flavonoids, anthocyanins, monoterpenoids, and their derivatives, indicating an enrichment and expansion of gene families. However, despite the recognition of 40–50 Areca species, the geographic origins of AN, and its subsequent diffusion and diversification remain inadequately documented. A gene-typing method targeting 34 chloroplast DNA microsatellites was developed to unravel the historical aspects of AN evolution (Raimondeau et al., 2021). This method can be used to trace maternal lineage propagation, proving effective in the analysis of plant specimens. It is essential to elucidate the regulatory mechanisms governing fruit shape in the pursuit of cultivating superior AN varieties. Ding et al. (2023) categorized fruit from 137 AN germplasm resources into three types (spherical, elliptical, and cylindrical) based on the fruit shape index. They identified 86 candidate genes associated with fruit morphology. These candidate genes encode proteins, such as UDP-glucose transferase 85A2, ABA-responsive element-binding factor GBF4, E3 ubiquitin-protein ligase SIAH1, and leucine-rich repeat receptor-like serine/threonine-protein kinase ERECTA. Furthermore, in A. catechu, the frequent occurrence of small fruit detachments leading to significant yield losses has been observed. Researchers found that the genes encoding plant-specific DNA binding with one finger (DOF) transcription factors exhibited a uniform upregulation in the abscission zone, suggesting the potential role of DOF transcription factors in the regulation of fruitlet abscission in A. catechu (Li et al., 2022).
Non-targeted metabolomics methods have been employed to identify new compounds with potential medicinal and physiological activities in AN (Wu et al., 2021). The metabolic profile of Indonesian betel quid was analyzed using non-targeted GC-MS, and 92 plant chemical substances with the exception of alkaloids, primarily including benzene ring compounds, terpenes, acids, aldehydes, alcohols, and esters, were identified (Zhang et al., 2022). Lai et al. (2023) utilized non-targeted metabolomics to identify 331 metabolites from the roots, stems, and leaves of A. catechu, including 107 flavonoids, 71 lipids, 44 amino acids and derivatives, and 33 alkaloids. Thirty-six genes were identified using combined transcriptome and metabolomic analysis examining the biosynthetic processes underlying metabolic variations in A. catechu tissues. Flavonoid biosynthesis was controlled by transcription factors AcMYB5 and AcMYB194. Zhou et al. (2023) conducted a comprehensive analysis of the mechanisms underlying the accumulation of B vitamins during the development of AN through a combination of transcriptomics and targeted metabolomics. A total of 88 structural genes related to B vitamin biosynthesis were identified. The study obtained the metabolic profiles of 6 B vitamins at different AN developmental stages. The key transcription factors responsible for regulating the accumulation of thiamine and riboflavin in AN were identified, including AcbZIP21, AcMYB84, and AcARF32.
A comprehensive approach integrating metabolomics and network pharmacology was employed by Li et al. (2023) to elucidate the potential mechanisms underlying AN addiction. Network pharmacology analysis revealed that all crucial targets associated with AN addiction were modulated by arecoline, highlighting the significance of the G protein-coupled receptor signaling pathway. Furthermore, analysis of plasma and fecal metabolomes following arecoline intervention in mice suggested that this component may affect the dopamine and 5-hydroxytryptamine systems by modulating the biosynthesis of phenylalanine, tyrosine, and tryptophan, as well as the metabolism of phenylalanine, primary bile acids, glycerophospholipids, and the structure of the intestinal microbiota.
In a serum metabolomics study examining the toxicity of arecae semen and its underlying mechanisms, arecae semen demonstrated distinct cardiotoxic effects and hindered normal growth in Wistar male rats (Lin et al., 2020). The differentiation in metabolic profiles revealed 19 metabolites identified as potential biomarkers in rats treated with arecae semen.
Using ultra-high-performance liquid chromatography-time-of-flight mass spectrometry (UHPLC-TOF-MS) analysis of urine in mice, a metabolomics approach was employed to study the metabolism of arecoline and arecaidine (Giri et al., 2006). Eleven arecoline metabolites were identified, including arecaidine, arecoline N-oxide, arecaidine N-oxide, N-methylnipecotic acid, N-methylnipecotylglycine, arecaidinylglycine, arecaidinylglycerol, arecaidine mercapturic acid, arecoline mercapturic acid, and arecoline N-oxide mercapturic acid, as well as nine unidentified metabolites. The predominant metabolite for both arecoline and arecaidine was N-methylnipecotic acid. An additional noteworthy metabolite detected was the monoacylglyceride of arecaidine.
A previous study on AN chewing and OSCC suggested that exposure to carcinogenic substances present in AN may lead to genomic instability detected through oral tumor subsets by SSR PCR (Zienolddiny et al., 2004). Later research on the mutational characteristics of AN chewing-related tongue cancer confirmed that in the general population, AN chewing-related tongue cancer exhibits distinct mutational features compared to non-AN chewing-related tongue cancer. These features are associated with frequent mutations in the RASA1 gene and CpG islands throughout the entire genome (Zhang et al., 2019). Another study investigated the impact of AN chewing on salivary proteomics through MS (Sultan et al., 2020). MALDI-MSI was employed to generate a profile of peptides in AN consumers and a control group. Thirteen peptide peaks were significantly altered (p < 0.05) in AN addicts compared to the control group. These significant peptides corresponded to proteins such as cystatin SN, cystatin S, α2 macroglobulin, complement C3, apolipoprotein E, serum albumin, matrix metalloproteinase-9 deleted in malignant brain tumor protein 1, zinc-alpha-2-glycoprotein, and protein S100A8. Most of these proteins interact with each other, and some serve as biomarkers for malignant oral tumors.
The intestinal microbiota exhibits a significant response to arecoline intake. Based on shotgun metagenomic sequencing using a metagenomic shotgun approach, Xu et al. (2023a) investigated the impact of arecoline on intestinal microbiota. The results indicated that arecoline promoted lipid metabolism in mice, manifested by a significant reduction in serum total cholesterol, triglycerides, and hepatic total cholesterol levels, leading to a decrease in abdominal fat accumulation. Arecoline intake significantly modulates the levels of the neurotransmitters serotonin and norepinephrine in the brain. Notably, arecoline intervention markedly increases serum interleukin-6 and lipopolysaccharide levels, inducing inflammation in the body. Arecoline significantly reduces hepatic glutathione levels and increases malondialdehyde levels at a high dose, causing hepatic oxidative stress. Arecoline intake leads to intestinal damage by stimulating the release of IL-6 and IL-1β. In another study, arecoline demonstrated an ameliorative effect on intestinal damage induced by constipation (Xu et al., 2023b). By measuring symptoms related to constipation, intestinal microbiota, short-chain fatty acid content in the cecum, and gene expression in the colon, the impact of arecoline on constipation was explored. Arecoline intervention significantly downregulated gene expression associated with intestinal diseases, alleviating constipation induced by loperamide. Furthermore, an experiment on non-alcoholic fatty liver disease rats revealed through analysis of intestinal metabolomics and 16S rRNA sequencing that arecoline has lipid-lowering effects (Zhu et al., 2023). This effect may be mediated through intestinal metabolites, gut microbiota, and the Butyricicoccus/Christensenella/Coriobacteriaceae-COX2/PGE2 pathway.
The mainstream view is that chewing AN causes oral cancer, and the alkaloid components in AN play a key role. Although alkaloids found in AN are associated with various toxic effects, in clinical practice, traditional Chinese physicians prescribe AN-based treatments based on traditional Chinese medicine theory and individual patient conditions. AN is often processed through decoction (boiling in water) before being administered to patients, and the dosages used are significantly lower than those consumed when chewing AN. Investigations reveal that there have been no reported cases of severe adverse reactions associated with the use of AN-containing Chinese herbal medicines, such as “Simo Decoction” (Sun et al., 2017). However, it is essential for patients to follow the guidance of trained healthcare professionals and exercise caution when using any herbal treatment, including those containing AN, as individual reactions may vary.
In AN, the four primary alkaloids are arecoline, arecaidine, guvacine, and guvacoline, with arecoline having the highest concentration. Arecoline is the predominant and leading inducer of addiction and carcinogenic effects. Consequently, researchers and experts have directed significant attention to understanding the content and biological effects of arecoline in AN. Generally, arecaidine, guvacine, and guvacoline have lower concentrations than arecoline; however, these proportions are not absolute. The relative content of these four alkaloids varies based on factors such as AN variety, geographical location, degree of ripeness, and processing methods. Therefore, it is not entirely reasonable to focus exclusively on studying the content and biological effects of arecoline. The lower concentrations of arecaidine, guvacine, and guvacoline should not be disregarded. In research, comprehensive assessments should include all four alkaloids to accurately reflect the safety and efficacy of AN. Given these findings, for diverse purposes, users may select AN of different varieties, growth periods, and origins and use various processing methods to either reduce or increase specific components.
AN has been used in China since the Western Han dynasty, as documented in ancient Chinese medical texts. These texts record various medicinal combinations involving AN, demonstrating its efficacy in treating conditions such as oppressive sensations in the chest, heartache, lower back pain, urinary and bowel irregularities, intestinal parasitism, athlete’s foot, and oral ulcers. Subsequent scholarly investigations into the pharmacological properties of AN extracts have revealed their antidepressant, antimicrobial, anti-aging, insecticidal, anti-osteoporotic, hepatoprotective, and anti-inflammatory effects (Dar et al., 1997; Lee and Choi, 1999; Pithayanukul et al., 2009; Li et al., 2017; Jam et al., 2021; Sharaf et al., 2021; Kweon et al., 2022; Puyathorn et al., 2022). In a recent study, extracts from arecae semen demonstrated inhibitory effects on snake venom (More et al., 2022). Summarizing modern pharmacological experiments on AN alkaloids and polyphenols has revealed their diverse biological functions, including insecticidal, anti-inflammatory, antimicrobial, hypotensive, anxiolytic, antidepressant, gastrointestinal improvement, and antioxidant properties. Alkaloids and polyphenols are the primary substances responsible for the therapeutic effects of AN. Although other components in AN also possess a variety of biological functions, their lower concentrations relegate them to secondary roles. Thus, they have not garnered as much attention from researchers. Furthermore, the therapeutic effects or toxicity of AN vary depending on the solvent and extraction methods employed. This variation is ascribed to alterations in the content, proportion, and types of different components in the extracts resulting from these processing methods. The question of how to optimize processing to maximize efficacy while minimizing side effects remains a subject that requires further exploration.
Presently, omics studies have become the primary means for discovering active compounds in AN and for understanding the pathways and mechanisms of AN metabolite synthesis and accumulation. They are also applied to tracing the historical spread of AN and cultivating superior varieties. Concurrently, integrated multi-omics research plays a crucial role in enhancing our understanding of AN metabolism in the body and its impact on the internal microenvironment.
The discovery of potential medicinal and physiologically active compounds in AN using metabolomics is just the beginning. It is crucial to employ network pharmacology to identify potential targets, conduct structure–activity relationship studies, isolate and identify components, and subsequently proceed with efficacy research, which will help to explore and develop the value of AN. Additionally, future research can leverage omics technologies for comprehensive comparative studies on AN from different regions of the world and growth stages. Based on this foundation, researchers can optimize the use of different AN varieties or engage in breeding efforts to meet diverse needs. Simultaneously, it is crucial to define the optimal harvesting period for AN. Studying the changes in metabolites or pharmacological effects during the maturation process across different production areas can guide the selection of suitable harvesting times for specific purposes. Further, establishing industry-wide consensus or standards for harvest times is essential. For AN used in traditional Chinese medicine, systematic research should be conducted to support regulations regarding harvesting times and processing methods.
Researchers need to be mindful that the medicinal and chewing forms of AN consumption are not the same when using omics technologies to study the potential effects of AN on the human body. Research conducted on the correct form of use can more accurately reflect its impact on the human body. Additionally, whether consumed as food or for medicinal purposes, AN is used in its entirety. Therefore, studying individual components cannot be entirely rational for representing the holistic nature of AN.
Quality is the primary factor influencing the value of products and people’s choices regardless of whether it is used for consumption or medicinal purposes. Quality can be affected by factors such as the growing environment, variety, processing methods, and extraction techniques. Currently, the presence and content of medicinal constituents are key indicators for evaluating their quality. However, unlike chemical pharmaceuticals with simpler compositions, traditional Chinese medicine contains a multitude of components and medicinal constituents, making it challenging for researchers to determine the criteria for assessing the quality of herbal medicines.
Alkaloids and tannin concentrates are considered the characteristic components of AN, and alkaloids and phenolic substances are the main bioactive components. Alkaloids have received the most attention in research on A. catechu, with arecoline accounting for the vast majority and other alkaloids being investigated rather infrequently. Polyphenolic flavonoids, primarily total polyphenol and other components of polyphenols, have also received attention. There is a significant change in the content and types of polyphenols in different parts of A. catechu. Schaftoside and diosmetin are the most abundant in husks, and catechins, followed by tannic acid, are the most abundant in seeds (Song et al., 2022). Therefore, arecoline, arecaidine, guvacine, guvacoline, and catechin are potential quality markers and can be used as index components to evaluate the quality of arecae semen. In addition, the detection of a single substance is always limited in representativeness when considering the multi-component and multi-target characteristics of traditional Chinese medicine. However, too many components will make the detection work time consuming and laborious. A simpler and more comprehensive method of AN quality determination needs to be explored.
YS: Conceptualization, Data curation, Formal Analysis, Investigation, Visualization, Writing–original draft, Writing–review and editing. JF: Project administration, Writing–review and editing, Supervision. WH: Project administration, Writing–review and editing, Supervision. HQ: Project administration, Writing–review and editing, Supervision. YL: Conceptualization, Project administration, Supervision, Writing–review and editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. Project of Medical and Health Science and Technology Innovation Engineering of the Chinese Academy of Medical Sciences, Grant/Award Number: 2021-1-I2M-032; Major Science and Technology Project of Hainan Province, China, Grant/Award Numbers: ZDKJ2021034, ZDYF2021SHFZ047, ZDYF2022XDNY162; Major Science and Technology Project of Wanning County, Grant/Award Number: 2022wnkj09; Natural Science Foundation of Hainan Province, Grant/Award Number: 324QN337.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
OSF, oral submucous fibrosis; OSCC, oral squamous cell carcinoma; OPSCC, oropharyngeal squamous cell carcinoma; MIC, minimal inhibitory concentration; PI3K/AKT, phosphatidylinositol 3-kinase/protein kinase B; PI3K/AKT/mTOR, phosphatidylinositol 3-kinase/protein kinase B/mammalian target of rapamycin; HPLC, high-performance liquid chromatography; UV, ultraviolet; UHPLC-MS/MS, ultra-high-performance liquid chromatography-tandem mass spectrometry; IMS, ion mobility spectrometry; MALDI-MSI, matrix-assisted laser desorption/ionization mass imaging spectrometry; IC50, half maximal inhibitory concentration; ESI, electrospray ionization; DESI, desorption electrospray ionization; GC-MS, gas chromatography-mass spectrometry DOF, DNA-binding with one finger; SSR PCR. simple sequence repeats polymerase chain reaction.
Abbas, G., Kashif, M., Khan, T. A., Bhatti, H. A., Haque, S., Naqvi, S., et al. (2018). Cytotoxic, embryotoxic, insecticidal and anti-microbial activities of standardized Areca catechu nut. Pak. J. Pharm. Sci. 31 (2), 385–392.
Ahmad Rusdan, A. I., Kadir, J., Muda Mohammed, M. T., and Cheng Lian, G. E. (2015). Potential of the extract from the nut of areca catechu to control mango anthracnose. Pertanika J. Trop. Agric. Sci. 38 (3), 375–388.
Angadi, P. V., and Rao, S. S. (2011). Areca nut in pathogenesis of oral submucous fibrosis: revisited. Oral Maxillofac. Surg. 15 (1), 1–9. doi:10.1007/s10006-010-0219-8
Arakeri, G., and Brennan, P. A. (2013). Oral submucous fibrosis: an overview of the aetiology, pathogenesis, classification, and principles of management. Br. J. Oral Maxillofac. Surg. 51 (7), 587–593. doi:10.1016/j.bjoms.2012.08.014
Avery, E. E., Baker, L. D., and Asthana, S. (1997). Potential role of muscarinic agonists in Alzheimer's disease. Drugs Aging 11 (6), 450–459. doi:10.2165/00002512-199711060-00004
Batham, E. (1946). Testing arecoline hydrobromide as an anthelminthic for hydatid worms in dogs. Parasitology 37 (3-4), 185–191. doi:10.1017/s0031182000013342
Brunetti, P., Lo Faro, A. F., Tini, A., Busardò, F. P., and Carlier, J. (2020). Pharmacology of herbal sexual enhancers: a review of psychiatric and neurological adverse effects. Pharmaceuticals 13 (10), 309. doi:10.3390/ph13100309
Calogero, A. E., Kamilaris, T. C., Gomez, M. T., Johnson, E. O., Tartaglia, M. E., Gold, P. W., et al. (1989). The muscarinic cholinergic agonist arecoline stimulates the rat hypothalamic-pituitary-adrenal Axis through a centrally-mediated corticotropin-releasing hormone-dependent mechanism. Endocrinology 125 (5), 2445–2453. doi:10.1210/endo-125-5-2445
Cao, M. R., Yuan, H. W., Daniyal, M., Yu, H. H., Xie, Q. L., Liu, Y. K., et al. (2019). Two new alkaloids isolated from traditional Chinese medicine Binglang the fruit of Areca catechu. Fitoterapia 138, 104276. doi:10.1016/j.fitote.2019.104276
Celimuge, X. U., and Xu, L. (2023). Research progress in chemical composition,pharmacology and toxicity of Areca Semen and prediction and analysis of its quality markers. Nat. Prod. Res. Dev. 35 (8), 1431–1441. doi:10.16333/j.1001-6880.2023.8.016
Chaikhong, K., Chumpolphant, S., Rangsinth, P., Sillapachaiyaporn, C., Chuchawankul, S., Tencomnao, T., et al. (2022). Antioxidant and anti-skin aging potential of selected Thai plants: in vitro evaluation and in silico target prediction. Plants 12 (1), 65. doi:10.3390/plants12010065
Chang, Y. F., Liu, T. Y., and Liu, S. T. (2013). Arecoline inhibits and destabilizes agrin-induced acetylcholine receptor cluster formation in C2C12 myotubes. Food Chem. Toxicol. 60, 391–396. doi:10.1016/j.fct.2013.07.079
Chang, Y. F., Liu, T. Y., Liu, S. T., and Tseng, C. N. (2012). Arecoline inhibits myogenic differentiation of C2C12 myoblasts by reducing STAT3 phosphorylation. Food Chem. Toxicol. 50 (10), 3433–3439. doi:10.1016/j.fct.2012.07.032
Chen, P. H., Lee, K. W., Hsu, C. C., Chen, J. Y. F., Wang, Y. H., Chen, K. K., et al. (2014). Expression of a splice variant of CYP26B1 in betel quid-related oral cancer. Sci. World J. 2014, 810561–810568. doi:10.1155/2014/810561
Chen, T. C., Wu, C. T., Ko, J. Y., Yang, T. L., Lou, P. J., Wang, C. P., et al. (2020a). Clinical characteristics and treatment outcome of oropharyngeal squamous cell carcinoma in an endemic betel quid region. Sci. Rep. 10 (1), 526. doi:10.1038/s41598-019-57177-1
Chen, Y. F., Zeng, T., Xiang, Z. W., Hao, L., Hu, L., and Bao, M. H. (2020b). Study on the effect of arecoline on the tension of isolated thoracic aortic rings in rats. Bus. Inf. 19, 171–172.
Ching, T. T., Chen, Y. C., Li, G., Liu, J. F., Xu, X. Z. S., and Hsu, A. L. (2020). Short-term enhancement of motor neuron synaptic exocytosis during early aging extends lifespan in Caenorhabditis elegans. Exp. Biol. Med. 245 (17), 1552–1559. doi:10.1177/1535370220950639
Chou, W. W., Guh, J. Y., Tsai, J. F., Hwang, C. C., Chen, H. C., Huang, J. S., et al. (2008). Arecoline-induced growth arrest and p21WAF1 expression are dependent on p53 in rat hepatocytes. Toxicology 243 (1-2), 1–10. doi:10.1016/j.tox.2007.09.003
Cox, S., Vickers, E. R., Ghu, S., and Zoellner, H. (2010). Salivary arecoline levels during areca nut chewing in human volunteers. J. Oral Pathol. Med. 39 (6), 465–469. doi:10.1111/j.1600-0714.2009.00881.x
Dar, A., Khatoon, S., Rahman, G., and Atta-Ur-Rahman, (1997). Anti-depressant activities of Areca catechu fruit extract. Phytomedicine 4 (1), 41–45. doi:10.1016/S0944-7113(97)80026-8
Dasgupta, R., Chatterjee, A., Sarkar, S., and Maiti, B. R. (2017). Arecoline aggravates hypothyroidism in metabolic stress in mice. Arch. Physiol. Biochem. 123 (2), 105–111. doi:10.1080/13813455.2016.1267228
Dasgupta, R., Chatterji, U., Nag, T. C., Chaudhuri-Sengupta, S., Nag, D., and Maiti, B. R. (2010). Ultrastructural and hormonal modulations of the thyroid gland following arecoline treatment in albino mice. Mol. Cell. Endocrinol. 319 (1-2), 1–7. doi:10.1016/j.mce.2010.01.005
Dasgupta, R., Saha, I., Maity, A., Ray, P. P., and Maiti, B. R. (2018). Arecoline ameliorates hyperthyroid condition in mice under cold stress. Arch. Physiol. Biochem. 124 (5), 436–441. doi:10.1080/13813455.2017.1420665
Di, Z. M. (2015). Observation on the clinical effect of food retention and cough Relieve oral liquid for children in treating cough in children. New J. Tradit. Chin. Med. 47 (08), 177–179. doi:10.13457/j.cnki.jncm.2015.08.082
Ding, H., Bai, F., Cao, H. D., Xu, J., Fang, L., Wu, J. N., et al. (2018). PDE/cAMP/Epac/C/EBP-β signaling cascade regulates mitochondria biogenesis of tubular epithelial cells in renal fibrosis. Antioxid. Redox Signal. 29 (7), 637–652. doi:10.1089/ars.2017.7041
Ding, H., Zhou, G. Z., Zhao, L., Li, X. Y., Wang, Y. C., Xia, C. C., et al. (2023). Genome-wide association analysis of fruit shape-related traits in areca catechu. Int. J. Mol. Sci. 24 (5), 4686. doi:10.3390/ijms24054686
Dutta, D., Ramanna, C., and Kamath, V. (2017). Estimation of arecoline content of various forms of areca nut preparations by high-pressure thin-layer chromatography. J. Adv. Clin. Res. Insights 4 (2), 31–37. doi:10.15713/ins.jcri.153
Fan, X. Y., Jiang, C. Y., Dai, W. N., Jing, H. J., Du, X. J., Peng, M. C., et al. (2022). Effects of different extraction on the antibacterial and antioxidant activities of phenolic compounds of areca nut (husks and seeds). J. Food Meas. Charact. 16 (2), 1502–1515. doi:10.1007/s11694-021-01244-7
Feng, S. D., Wu, D., Yang, S. S., He, J. Q., Zhang, K. F., and Ling, H. Y. (2016). Effect of arecoline on proliferation and apoptosis of MCF-7 human breast cancer cells. Chin. J. Appl. Physiol. 32 (4), 370–372. doi:10.13459/j.cnki.cjap.2016.04.021
Garcia-Algar, O., Vall, O., Alameda, F., Puig, C., Pellegrini, M., Pacifici, R., et al. (2005). Prenatal exposure to arecoline (areca nut alkaloid) and birth outcomes. Arch. Dis. Child. Fetal Neonatal Ed. 90 (3), F276–FF277. doi:10.1136/adc.2004.061325
Garg, A., Chaturvedi, P., and Gupta, P. C. (2014). A review of the systemic adverse effects of areca nut or betel nut. Indian J. Med. Paediatr. Oncol. 35 (01), 3–9. doi:10.4103/0971-5851.133702
Ghayur, M. N., Kazim, S. F., Rasheed, H., Khalid, A., Jumani, M. I., Choudhary, M. I., et al. (2011). Identification of antiplatelet and acetylcholinesterase inhibitory constituents in betel nut. J. Chin. Integr. Med. 9 (6), 619–625. doi:10.3736/jcim20110607
Gheddar, L., Ricaut, F. X., Ameline, A., Brucato, N., Tsang, R., Leavesley, M., et al. (2020). Testing for betel nut alkaloids in hair of Papuans abusers using UPLC–MS/MS and UPLC–Q-Tof-MS. J. Anal. Toxicol. 44 (1), 41–48. doi:10.1093/jat/bkz045
Gilani, A. H., Ghayur, M. N., Houghton, P. J., Jabeen, Q., Kazim, S. F., Jumani, M. I., et al. (2006). Studies on the hypotensive, cardio-suppressant, vasodilator and antiplatelet activities of betel nut crude extract and its constituents. Int. J. Pharmacol. 2 (1), 33–41. doi:10.3923/ijp.2006.33.41
Giri, S., Idle, J. R., Chen, C., Zabriskie, T. M., Krausz, K. W., and Gonzalez, F. J. (2006). A metabolomic approach to the metabolism of the areca nut alkaloids arecoline and arecaidine in the mouse. Chem. Res. Toxicol. 19 (6), 818–827. doi:10.1021/tx0600402
Goto, H., Tanaka, N., Tanigawa, K., Shimada, Y., Itoh, T., and Terasawa, K. (1997). Endothelium-dependent vasodilator effect of extract prepared from the seeds of Areca catechu on isolated rat aorta. Phytother. Res. 11 (6), 457–459. doi:10.1002/(SICI)1099-1573(199709)11:6<457::AID-PTR123>3.0.CO;2-J
Gu, G. H., Hu, H., Zeng, W., Xu, F. Z., and Yuan, J. S. (2013). Effect of the aqueous extract of betel nut and arecoline on hepatocyte apoptosis in mice. Pharmacol. Clin. Chin. Mat. Med. 29 (2), 56–59. doi:10.13412/j.cnki.zyyl.2013.02.022
Gu, L. L. (2021). Clinical observation on Mongolian medicine Binglang Shisanwei Pill combined with psychological nursing in the treatment of depression. J. Med. Pharm. Chin. Minor. 27 (07), 79–80. doi:10.16041/j.cnki.cn15-1175.2021.07.038
Gu, L. Q., Xie, C. Q., Peng, Q., Zhang, J. M., Li, J. D., and Tang, Z. G. (2019). Arecoline suppresses epithelial cell viability through the Akt/mTOR signaling pathway via upregulation of PHLPP2. Toxicol 419, 32–39. doi:10.1016/j.tox.2019.03.006
Guo, Z. K., Mei, W. L., Zeng, Y. B., Wang, H., and Dao, H. F. (2012). GC-MSAnalysis of liposoluble constituents in fruits of Areca catechu L. Mod. Chin. Med. 14 (06), 1–3. doi:10.13313/j.issn.1673-4890.2012.06.012
Gupta, A. K., Tulsyan, S., Thakur, N., Sharma, V., Sinha, D. N., and Mehrotra, R. (2020). Chemistry, metabolism and pharmacology of carcinogenic alkaloids present in areca nut and factors affecting their concentration. Regul. Toxicol. Pharmacol. 110, 104548. doi:10.1016/j.yrtph.2019.104548
Hamsar, M. N., Ismail, S., Mordi, M. N., Ramanathan, S., and Mansor, S. M. (2011). Antioxidant activity and the effect of different parts of areca catechu extracts on Glutathione-S-Transferase activity in vitro. Free Radicals Antioxid. 1 (1), 28–33. doi:10.5530/ax.2011.1.6
Han, L. (2010). “Studies on extraction and separation of antioxidant constituents from areca nut,” (Hainan: Hainan University). master's thesis.
He, J. Y., Huang, B., Xin, Z. T., Chen, J. T., Chen, X. Y., Chen, X. J., et al. (2013). Study on the antidepressant effects of total phenolics from betel nut seeds. J. Chin. Med. Mat. 36 (8), 1331–1334. doi:10.13863/j.issn1001-4454.2013.08.035
Ho, T. J., Tsai, B. C. K., Kuo, C. H., Luk, H. N., Day, C. H., Hsieh, D. J. Y., et al. (2022). Arecoline induces cardiotoxicity by upregulating and activating cardiac hypertrophy-related pathways in Sprague–Dawley rats. Chem. Biol. Interact. 354, 109810. doi:10.1016/j.cbi.2022.109810
Hou, Q. Y., Fu, L. S., Liang, H. Y., Zhou, Y. K., Yang, X. Q., and Zhao, X. S. (2023). Determination of four alkaloids components in arecae semen, arecae semen tostum and arecae pericarpium by LC-PDA. Chin. J. Mod. Appl. Pharm. 40 (4), 443–447. doi:10.13748/j.cnki.issn1007-7693.2023.04.002
Hsieh, T. J., Hsieh, P. C., Wu, M. T., Chang, W. C., Hsiao, P. J., Lin, K. D., et al. (2011). Betel nut extract and arecoline block insulin signaling and lipid storage in 3T3-L1 adipocytes. Cell Biol. Toxicol. 27 (6), 397–411. doi:10.1007/s10565-011-9195-5
Hsieh, Y. H., Syu, R. J., Lee, C. C., Lin, S. H., Lee, C. H., Cheng, C. W., et al. (2020). Arecoline induces epithelial mesenchymal transition in HK2 cells by upregulating the ERK-mediated signaling pathway. Environ. Toxicol. 35 (9), 1007–1014. doi:10.1002/tox.22937
Hsu, H. F., Tsou, T. C., Chao, H. R., Shy, C. G., Kuo, Y. T., Tsai, F. Y., et al. (2010). Effects of arecoline on adipogenesis, lipolysis, and glucose uptake of adipocytes-A possible role of betel-quid chewing in metabolic syndrome. Toxicol. Appl. Pharmacol. 245 (3), 370–377. doi:10.1016/j.taap.2010.04.008
Hu, C. W., Chang, Y. Z., Wang, H. W., and Chao, M. R. (2010). High-throughput simultaneous analysis of five urinary metabolites of areca nut and tobacco alkaloids by isotope-dilution liquid chromatography-tandem mass spectrometry with on-line solid-phase extraction. Cancer Epidemiol. Biomarkers Prev. 19 (10), 2570–2581. doi:10.1158/1055-9965.Epi-10-0483
Huang, L. W., Hsieh, B. S., Cheng, H. L., Hu, Y. C., Chang, W. T., and Chang, K. L. (2012). Arecoline decreases interleukin-6 production and induces apoptosis and cell cycle arrest in human basal cell carcinoma cells. Toxicol. Appl. Pharmacol. 258 (2), 199–207. doi:10.1016/j.taap.2011.11.001
Huang, P. L., Chi, C. W., and Liu, T. Y. (2010). Effects of Areca catechu L. containing procyanidins on cyclooxygenase-2 expression in vitro and in vivo. Food Chem. Toxicol. 48 (1), 306–313. doi:10.1016/j.fct.2009.10.014
Huang, P. L., Chi, C. W., and Liu, T. Y. (2013). Areca nut procyanidins ameliorate streptozocin-induced hyperglycemia by regulating gluconeogenesis. Food Chem. Toxicol. 55, 137–143. doi:10.1016/j.fct.2012.12.057
Huang, X. (2022). Determination of four kinds of cytotoxic alkaloids in arecae semen by HPLC. Food Drug 24 (003), 245–249. doi:10.3969/j.issn.1672-979X.2022.03.012
Huang, Z. L., Xiao, B. X., Wang, X. Q., Li, Y. Y., and Deng, H. B. (2003). Betel nut indulgence as a cause of epilepsy. Seizure 12 (6), 406–408. doi:10.1016/s1059-1311(02)00377-1
IARC Monographs Vol 128 group (2021). Carcinogenicity of acrolein, crotonaldehyde, and arecoline. Lancet Oncol. 22 (1), 19–20. doi:10.1016/s1470-2045(20)30727-0
IARC Working Group on the Evaluation of Carcinogenic Risks to Humans (2007). Smokeless tobacco and some tobacco-specific N-nitrosamines. Int. Agency Res. Cancer.
Jain, P., Satapathy, T., and Pandey, R. K. (2021). Acaricidal activity and clinical safety of arecoline hydrobromide on calves infested with cattle tick Rhipicephalus microplus (Acari: ixodidae). Vet. Parasitol. 298, 109490. doi:10.1016/j.vetpar.2021.109490
Jam, N., Hajimohammadi, R., Gharbani, P., and Mehrizad, A. (2021). Evaluation of antibacterial activity of aqueous, ethanolic and methanolic extracts of areca nut fruit on selected bacteria. Biomed. Res. Int. 2021, 6663399. doi:10.1155/2021/6663399
Jantarat, C., Sirathanarun, P., Songsrm, W., Srinornate, W., and Daengprom, S. (2013). A simple and rapid HPLC technique for determination of arecoline in areca nut (Areca catechu L.) extract. Walailak J. Sci. Technol. 10 (1), 57–66. doi:10.2004/wjst.v10i1.439
Joo, Y. J., Newcombe, D., Nosa, V., and Bullen, C. (2020). Investigating betel nut use, antecedents and consequences: a review of literature. Subst. Use Misuse 55 (9), 1422–1442. doi:10.1080/10826084.2019.1666144
Ku, C. W., Day, C. H., Ou, H. C., Ho, T. J., Chen, R. J., Kumar, V. B., et al. (2021). The molecular mechanisms underlying arecoline-induced cardiac fibrosis in rats. Open Life Sci. 16 (1), 1182–1192. doi:10.1515/biol-2021-0116
Kumarnsit, E., Keawpradub, N., Vongvatcharanon, U., Sawangjaroen, K., and Govitrapong, P. (2005). Suppressive effects of dichloromethane fraction from the Areca catechu nut on naloxone-precipitated morphine withdrawal in mice. Fitoterapia 76 (6), 534–539. doi:10.1016/j.fitote.2005.04.015
Kweon, B., Kim, D. U., Oh, J. Y., Oh, H., Kim, Y. C., Mun, Y. J., et al. (2022). Arecae pericarpium water extract alleviates chronic pancreatitis by deactivating pancreatic stellate cells. Front. Pharmacol. 13, 941955. doi:10.3389/fphar.2022.941955
Lai, J., Li, C., Zhang, Y. R., Wu, Z. Y., Li, W. G., Zhang, Z. H., et al. (2023). Integrated transcriptomic and metabolomic analyses reveal the molecular and metabolic basis of flavonoids in areca catechu L. J. Agric. Food Chem. 71 (12), 4851–4862. doi:10.1021/acs.jafc.2c08864
Lee, K. K., and Choi, J. D. (1999). The effects of Areca catechu L extract on anti-aging. Int. J. Cosmet. Sci. 21 (4), 285–295. doi:10.1046/j.1467-2494.1999.196563.x
Li, J., Jia, X. C., Yang, Y. D., Chen, Y. C., Wang, L. K., Liu, L. Y., et al. (2022). Genome-wide identification of the DOF gene family involved in fruitlet abscission in areca catechu L. Int. J. Mol. Sci. 23 (19), 11768. doi:10.3390/ijms231911768
Li, J., Yao, M., Zhu, X., Li, Q., He, J., Chen, L., et al. (2019). YAP-induced endothelial-mesenchymal transition in oral submucous fibrosis. J. Dent. Res. 98 (8), 920–929. doi:10.1177/0022034519851804
Li, M. Y., Pang, X., Guo, Z. T., Yang, Y. L., Gu, Z. H., and Zhang, L. (2023). Integrated metabolomics and network pharmacology to reveal the mechanism of areca nut addiction. Addict. Biol. 28 (12), e13352. doi:10.1111/adb.13352
Li, S. Y., Chen, R., Luo, K. L., Guo, Y., Xiao, M., and Du, G. K. (2017). Areca nut extract protects against ovariectomy-induced osteoporosis in mice. Exp. Ther. Med. 13 (6), 2893–2899. doi:10.3892/etm.2017.4362
Li, W. D., Zang, C. j., Yin, S., Shen, W., Sun, Q. Y., and Zhao, M. H. (2020). Metformin protects against mouse oocyte apoptosis defects induced by arecoline. Cell Prolif. 53 (7), e12809. doi:10.1111/cpr.12809
Liang, X. F., Sun, J., Zheng, W. L., Chen, Z. Q., and Cao, L. X. (2020). The clinical study of efficacy of arecae semen on recovery of gastrointestinal function after gynecological surgery. Tradit. Chin. Drug Res. Clin. Pharmacol. 31 (07), 862–866. doi:10.19378/j.issn.1003-9783.2020.07.017
Lin, L., Xu, H. H., Deng, P. F., and Lu, W. Q. (1992). A thin layer chromatography densitometric method for the determination of arecoline content in semen Arecae from different producing areas. China J. Chin. Mat. Med. 17 (8), 491–492.
Lin, Q. H., Wang, C. G., Jia, Z., Xiong, H., Xue, X., Liu, M. N., et al. (2020). UPLC-HDMS-based on serum metabolomics reveals the toxicity of arecae semen. J. Ethnopharmacol. 247, 112223. doi:10.1016/j.jep.2019.112223
Lin, W. Y., Tsai, B. C. K., Day, C. H., Chiu, P. L., Chen, R. J., Chen, M. Y. C., et al. (2021). Arecoline induces heart injure via Fas/Fas ligand apoptotic pathway in heart of Sprague–Dawley rat. Environ. Toxicol. 36 (8), 1567–1575. doi:10.1002/tox.23153
Liu, G. K. (2021). Analysis of the clinical efficacy of Binglang Powder combined with acupoint massage in the treatment of spleen and stomach diseases. Chin. J. Conval. Med. 30 (03), 300–302. doi:10.13517/j.cnki.ccm.2021.03.028
Liu, P. F., and Chang, Y. F. (2023). The controversial roles of areca nut: medicine or toxin? Int. J. Mol. Sci. 24 (10), 8996. doi:10.3390/ijms24108996
Liu, S. T., Young, G. C., Lee, Y. C., and Chang, Y. F. (2011). A preliminary report on the toxicity of arecoline on early pregnancy in mice. Food Chem. Toxicol. 49 (1), 144–148. doi:10.1016/j.fct.2010.10.009
Liu, X. L., Jiang, L., Zhang, Q., Zhao, Z. D., and Zhang, H. D. (2022). Arecoline and arecaidine lixiviation in areca nut blanching: liquid chromatography-ion trap-time of flight hybrid mass spectrometry determination and kinetic modeling. J. Food Process. Preserv. 46 (11), e16942. doi:10.1111/jfpp.16942
Lu, Y., Yu, J. N., Liu, W., Jing, G. X., Li, W. S., and Liu, W. J. (2022). Quantitative determination of major alkaloids in areca nut products by high-performance liquid chromatography coupled with ion mobility spectrometry. J. Sep. Sci. 45 (24), 4469–4477. doi:10.1002/jssc.202200361
Luo, S. S., Zhang, H. D., Liu, X. L., and Zhu, L. (2010). Study on antimicrobial activity of arecoline from betel nut in vitro. Innov. Ed. Farm Prod. Process, 47–50. doi:10.3969/j.issn.1671-9646(C).2010.09.002
Ma, J. H., Du, X., Zhao, A. P., Wang, Z. H., Guo, Q. W., Qin, N. N., et al. (2022a). Anti-hypoxic pharmacological effects of betelnut polyphenols. J. Cent. South Univ. Med. Sci. 47 (4), 512–520. doi:10.11817/j.issn.1672-7347.2022.210582
Ma, J. H., Wang, R., Du, X., Zhao, A. P., Wang, Z. H., and Zhang, H. B. (2022b). Determination of total polyphenols and catechins in betel nut polyphenol extract. J. Pharm. Pract. Serv., 040. doi:10.12206/j.issn.1006-0111.202106013
Manimekalai, R., Nair, S., Naganeeswaran, A., Karun, A., Malhotra, S., and Hubbali, V. (2018). Transcriptome sequencing and de novo assembly in arecanut, Areca catechu L elucidates the secondary metabolite pathway genes. Biotechnol. Rep. (Amst). 17, 63–69. doi:10.1016/j.btre.2017.12.005
More, V., Muhsinah, A. B., Latha, G. S., Alhazmi, A. Y. M., Ibrahim, O. A., S. Binshaya, A., et al. (2022). Evaluation of anti-venom potential of areca catechu seed extract on Bungarus caeruleus venom. Separations 9 (11), 360. doi:10.3390/separations9110360
Mu, X. N., Yang, W. Q., Wang, W. J., Liu, G., Zhang, X. Q., and Ye, W. C. (2014). Chemical constituents from the fruits of Areca catechu. J. Jinan Univ. (1), 56–60. doi:10.3969/j.issn.1000-9965.2014.01.010
National Pharmacopoeia Committee (2020) Pharmacopoeia of the people's Republic of China 2020. Beijing: China Medical Science Press.
Ni, Y. D., Wang, J. H., and Wang, R. J. (2004). Comparative study on the gastrointestinal effects of areca nut and arecoline. Pharmacol. Clin. Chin. Mat. Med. 20 (2), 2. doi:10.3969/j.issn.1001-859X.2004.02.008
Oliveira, N. G., Ramos, D. L., and Dinis-Oliveira, R. J. (2021). Genetic toxicology and toxicokinetics of arecoline and related areca nut compounds: an updated review. Arch. Toxicol. 95 (2), 375–393. doi:10.1007/s00204-020-02926-9
Osborne, P. G., Pasupuleti, R. R., Wu, M. T., Lee, C. H., and Ponnusamy, V. K. (2022). LC-MS/MS measurement of alkaloids in alkaline extracts of Areca nut preparations and their physiological effects. Process Biochem. 118, 65–73. doi:10.1016/j.procbio.2022.04.018
Papke, R. L., Horenstein, N. A., and Stokes, C. (2015). Nicotinic activity of arecoline, the psychoactive element of" Betel Nuts", suggests a basis for habitual use and anti-inflammatory activity. PLoS One 10 (10), e0140907. doi:10.1371/journal.pone.0140907
Pasupuleti, R. R., Lee, C. H., Osborne, P. G., Wu, M. T., and Ponnusamy, V. K. (2022). Rapid green analytical methodology for simultaneous biomonitoring of five toxic areca nut alkaloids using UHPLC-MS/MS for predicting health hazardous risks. J. Hazard. Mat. 422, 126923. doi:10.1016/j.jhazmat.2021.126923
Peng, W. (2018). “Processing mechanisms of the charred areca nut's characteristics of "good at relieving dyspepsia" and the suitable arecoline content in charred areca nut,” (Chengdu: Chengdu University of Chinese Medicine). dissertation.
Peng, W., Liu, Y. J., Wu, N., Sun, T., He, X. Y., Gao, Y. X., et al. (2015). Areca catechu L. (Arecaceae): a review of its traditional uses, botany, phytochemistry, pharmacology and toxicology. J. Ethnopharmacol. 164, 340–356. doi:10.1016/j.jep.2015.02.010
Pithayanukul, P., Nithitanakool, S., and Bavovada, R. (2009). Hepatoprotective potential of extracts from seeds of Areca catechu and nutgalls of Quercus infectoria. Molecules 14 (12), 4987–5000. doi:10.3390/molecules14124987
Puyathorn, N., Sukonpan, C., Toungsuwan, P., Narakornwit, W., and Phaechamud, T. (2022). Effect of arecoline, aqueous and methanolic areca nut extracts on rumen fluke. IOP Conf. Ser. Mat. Sci. Eng. 1234 (1), 012019. doi:10.1088/1757-899X/1234/1/012019
Qi, Z. Q. (2010). “Protection effect of Arecoline on pancreas cells in type 2 diabetic rats and its mechanism,” (Hunan: University of South China). master's thesis.
Rahman, A. O., Purwakanthi, A., and Dewi, H. (2020). Antifertility effect of betel nut (Areca catechu L) in male rat. Medisains 18 (2), 52–57. doi:10.30595/medisains.v18i2.7588
Raimondeau, P., Manzi, S., Brucato, N., Kinipi, C., Leavesley, M., Ricaut, F.-X., et al. (2021). Genome skims analysis of betel palms (Areca spp., Arecaceae) and development of a profiling method to assess their plastome diversity. Gene 800, 145845. doi:10.1016/j.gene.2021.145845
Raju, A., De Subrata, S., Ray, M. K., and Degani, M. S. (2021). Antituberculosis activity of polyphenols of Areca catechu. Int. J. Mycobacteriol. 10 (1), 13–18. doi:10.4103/ijmy.ijmy_199_20
Ren, H., He, G. Q., Lu, Z. Y., He, Q. T., Li, S. A., Huang, Z. X., et al. (2021). Retracted: arecoline induces epithelial-mesenchymal transformation and promotes metastasis of oral cancer by SAA1 expression. Cancer Sci. 112 (6), 2173–2184. doi:10.1111/cas.14866
Rooney, D. F. (1995). Betel chewing in South-East Asia. Available at: http://rooneyarchive.net/lectures/betel_chewing_in_south-east_asia.htm (Accessed April 28, 2024).
Sari, E. F., Prayogo, G. P., Loo, Y. T., Zhang, P., McCullough, M. J., and Cirillo, N. (2020a). Distinct phenolic, alkaloid and antioxidant profile in betel quids from four regions of Indonesia. Sci. Rep. 10 (1), 16254. doi:10.1038/s41598-020-73337-0
Sari, L. M., Hakim, R. F., Mubarak, Z., and Andriyanto, A. (2020b). Analysis of phenolic compounds and immunomodulatory activity of areca nut extract from Aceh, Indonesia, against Staphylococcus aureus infection in Sprague-Dawley rats. Vet. World 13 (1), 134–140. doi:10.14202/vetworld.2020.134-140
Senica, M., Stampar, F., Veberic, R., and Mikulic-Petkovsek, M. (2017). The higher the better? Differences in phenolics and cyanogenic glycosides in Sambucus nigra leaves, flowers and berries from different altitudes. J. Sci. Food Agric. 97 (8), 2623–2632. doi:10.1002/jsfa.8085
Serikuly, N., Alpyshov, E. T., Wang, D. M., Wang, J. T., Yang, L. E., Hu, G. J., et al. (2021). Effects of acute and chronic arecoline in adult zebrafish: anxiolytic-like activity, elevated brain monoamines and the potential role of microglia. Prog. Neuropsychopharmacol. Biol. Psychiatry 104, 109977. doi:10.1016/j.pnpbp.2020.109977
Shao, X. J., Zhu, Q., Liu, Y., Han, X. D., Lin, X. Z., and Xi, Q. (2021). Correlation of betel nut chewing and clinicopathologic factors of oral squamous cell carcinoma. Shanghai J. Stomatol. 30 (03), 268–272. doi:10.19439/j.sjos.2021.03.009
Sharaf, S., Chinchu, J. U., Rahitha Devi, S. J., and Prakash Kumar, B. (2021). Identification of bioactive compounds with anti-inflammatory potential in the methanolic fruit extract of Areca catechu L.(Palmaceae). Indian J. biochem. Biophys. 58 (5), 464–471. doi:10.56042/ijbb.v58i5.31325
Shen, X. L., and Duan, L. L. (2009). Research on chemical composition and pharmacological of arecae. J. Yichun Coll. 31 (2), 95–97.
Shen, Y. W., Shih, Y. H., Fuh, L. J., and Shieh, T. M. (2020). Oral submucous fibrosis: a review on biomarkers, pathogenic mechanisms, and treatments. Int. J. Mol. Sci. 21 (19), 7231. doi:10.3390/ijms21197231
Shih, Y. T., Chen, P. S., Wu, C. H., Tseng, Y. T., Wu, Y. C., and Lo, Y. C. (2010). Arecoline, a major alkaloid of the areca nut, causes neurotoxicity through enhancement of oxidative stress and suppression of the antioxidant protective system. Free Radic. Bio.l Med. 49 (10), 1471–1479. doi:10.1016/j.freeradbiomed.2010.07.017
Siregar, P., Audira, G., Castillo, A. L., Roldan, M. J. M., Suryanto, M. E., Liu, R. X., et al. (2022). Comparison of the psychoactive activity of four primary Areca nut alkaloids in zebrafish by behavioral approach and molecular docking. Biomed. Pharmacother. 155, 113809. doi:10.1016/j.biopha.2022.113809
Song, F., Tang, M. M., Wang, H., Zhang, Y. F., Zhu, K. X., Chen, X. A., et al. (2022). UHPLC-MS/MS identification, quantification of flavonoid compounds from Areca catechu L. extracts and in vitro evaluation of antioxidant and key enzyme inhibition properties involved in hyperglycemia and hypertension. Ind. Crops Prod. 189, 115787. doi:10.1016/j.indcrop.2022.115787
Srimany, A., George, C., Naik, H. R., Pinto, D. G., Chandrakumar, N., and Pradeep, T. (2016). Developmental patterning and segregation of alkaloids in areca nut (seed of Areca catechu) revealed by magnetic resonance and mass spectrometry imaging. Phytochemistry 125, 35–42. doi:10.1016/j.phytochem.2016.02.002
Suchinina, T. V., Shestakova, T. S., Petrichenko, V. M., and Novikova, V. (2011). Solvent polarity effect on the composition of biologically active substances, UV spectral characteristics, and antibacterial activity of Euphrasia brevipila herb extracts. Pharm. Chem. J. 44, 683–686. doi:10.1007/s11094-011-0542-x
Sultan, R., Mirza, M. R., Choudhary, M. I., Alam, M., and Haque, I.-u. (2020). Salivary proteomic analysis of betel nut (areca catechu) consumers by mass spectrometry revealed primary indication of oral malignancies. Int. J. Pept. Res. Ther. 26, 1073–1084. doi:10.1007/s10989-019-09909-0
Sun, H. Y., Shao, J. H., and Gao, H. N. (2014). Observation on the clinical efficacy of fuchu combined with food retention Relieve oral liquid in the treatment of anorexia in children. Med. Forum 18 (07), 947–948.
Sun, L., Song, H. B., Zhang, L., Huang, J. K., Wang, J. X., Yang, X. H., et al. (2017). Systematic evaluation for safety of traditional Chinese medicine Areca catechu and its preparations. China J. Chin. Mat. Med. 42 (21), 4067–4073. doi:10.19540/j.cnki.cjcmm.20170919.014
Sun, Y. P., Han, R., Luo, J., Chen, F., and Liang, J. H. (2005). Effects of arecoline on central suppression in mice treatedacutely with ethanol. Chin. J. Drug Depend. 14 (5), 333–337. doi:10.3969/j.issn.1007-9718.2005.05.004
Sun, Y. P., Liu, Q., Luo, J., Guo, P., Chen, F., Lawrence, A. J., et al. (2010). Systemic administration of arecoline reduces ethanol-induced sleeping through activation of central muscarinic receptor in mice. Alcohol. Clin. Exp. Res. 34 (1), 150–157. doi:10.1111/j.1530-0277.2009.01076.x
Tang, S. N., Zhang, J., Liu, D., Liu, Z. W., Zhang, X. Q., and Ye, W. C. (2017). Three new areca alkaloids from the nuts of Areca catechu. J. Asian. Nat. Prod. Res. 19 (12), 1155–1159. doi:10.1080/10286020.2017.1307187
Tang, Y. F., Dong, Q. S., Zhao, J., and Li, X. H. (2018). Clinical analysis of 86 cases with oral submucous fibrosis induced by chewing areca in troops stationed in wuhan. Mil. Med. Jt. Logist. 32 (11), 796–799. doi:10.13730/j.issn.1009-2595.2018.11.014
Taylor, R. F., al-Jarad, N., John, L. M., Conroy, D. M., and Barnes, N. C. (1992). Betel-nut chewing and asthma. Lancet 339 (8802), 1134–1136. doi:10.1016/0140-6736(92)90732-i
Tey, S. L., Li, C. Y., Lin, L. W., Chang, L. C., Chen, Y. L., Chang, F. R., et al. (2021). Arecae pericarpium extract induces porcine lower-esophageal-sphincter contraction via muscarinic receptors. BMC Complement. Med. Ther. 21 (1), 275. doi:10.1186/s12906-021-03442-8
Tian, L. C., Qin, S. R., Yi, H., Li, C., Ma, H. W., Ren, C. T., et al. (2018). Comparative study on contents of four alkaloids in homologous herbal medicines--Arecae Pericarpium and Arecae Semen. China J. Chin. Mat. Med. 43 (14), 2850–2856. doi:10.19540/j.cnki.cjcmm.20180514.009
Tsai, Y. S., Lee, K. W., Huang, J. L., Liu, Y. S., Juo, S. H., Kuo, W. R., et al. (2008). Arecoline, a major alkaloid of areca nut, inhibits p53, represses DNA repair, and triggers DNA damage response in human epithelial cells. Toxicology 249 (2-3), 230–237. doi:10.1016/j.tox.2008.05.007
Tseng, S. K., Chang, M. C., Su, C. Y., Chi, L. Y., Chang, J. Z. C., Tseng, W. Y., et al. (2012). Arecoline induced cell cycle arrest, apoptosis, and cytotoxicity to human endothelial cells. Clin. Oral. Investig. 16 (4), 1267–1273. doi:10.1007/s00784-011-0604-1
Tu, H. F., Chen, M. Y., Lai, J. C. Y., Chen, Y. L., Wong, Y. W., Yang, C. C., et al. (2019). Arecoline-regulated ataxia telangiectasia mutated expression level in oral cancer progression. Head. Neck 41 (8), 2525–2537. doi:10.1002/hed.25718
Wan, H. X. (2010). 100 cases of anorexia in children treated with Yunpi Zengshi Decoction. China Mod. Med. 17 (05), 82. doi:10.3969/j.issn.1674-4721.2010.05.057
Wang, C. C., Huang, P. L., Liu, T. Y., and Jan, T. R. (2009). Highly oligomeric procyanidins from areca nut induce lymphocyte apoptosis via the depletion of intracellular thiols. Toxicol. Vitro. 23 (7), 1234–1241. doi:10.1016/j.tiv.2009.07.033
Wang, C. C., Lin, Y. R., Liao, M. H., and Jan, T. R. (2013). Oral supplementation with areca-derived polyphenols attenuates food allergic responses in ovalbumin-sensitized mice. BMC Complement. Altern. Med. 13, 154. doi:10.1186/1472-6882-13-154
Wang, C. K., Lee, W. H., and Peng, C. H. (1997). Contents of phenolics and alkaloids in Areca catechu Linn. during maturation. J. Agric. Food Chem. 45 (4), 1185–1188. doi:10.1021/jf960547q
Wang, G., and Hu, B. (2010). Research progress of arecoline. J. Clin. Pathol. Res. (2), 171–175. doi:10.3969/j.issn.1673-2588.2010.02.018
Wang, J. (2024). “Evaluate the clinical efficacy of Muxiang Binglang Pill in the treatment of mild acute pancreatitis with visceral excess heat accumulation syndrome,” (Hunan: Hunan University of Chinese Medicine). master's thesis.
Wang, M. Y., Luo, J. H., and Li, J. G. (2011). Determination of polyphenols in areca catechu by HPLC. Nat. Prod. Res. Dev. 23 (1), 101–104. doi:10.16333/j.1001-6880.2011.01.032
Wang, R. M., Pan, F. B., He, R. P., Kuang, F. J., Wang, L., and Lin, X. (2021). Arecanut (Areca catechu L.) seed extracts extracted by conventional and eco-friendly solvents: relation between phytochemical compositions and biological activities by multivariate analysis. J. Appl. Res. Med. Aromat. Plants 25, 100336. doi:10.1016/j.jarmap.2021.100336
Wang, S. W., Hwang, G. S., Chen, T. J., and Wang, P. S. (2008). Effects of arecoline on testosterone release in rats. Am. J. Physiol. Endocrinol. Metab. 295 (2), E497–E504. doi:10.1152/ajpendo.00045.2008
Wang, T., Chen, Q. C., Cao, L. X., Zhou, L., and Chen, Z. Q. (2020). Effect of arecoline hydrobromide on motility of gastrointestinal smooth muscle strips lsolated from C57BL/6 and W/Wv mutant mice. Tradit. Chin. Drug Res. Clin. Pharmacol. 31 (4), 10. doi:10.19378/j.issn.1003-9783.2020.04.010
Wang, T. Y., Peng, C. Y., Lee, S. S., Chou, M. Y., Yu, C. C., and Chang, Y. C. (2016). Acquisition cancer stemness, mesenchymal transdifferentiation, and chemoresistance properties by chronic exposure of oral epithelial cells to arecoline. Oncotarget 7 (51), 84072–84081. doi:10.18632/oncotarget.11432
Wang, Y., Liu, S. W., Zhang, T. T., Hou, Y. N., and Shen, M. X. (2022a). Optimization of extraction technology of polyphenols from betel nut. J. Hainan Trop. Ocean. Univ. (002), 029. doi:10.13307/j.issn.2096-3122.2022.02.05
Wang, Y. H., Ding, M. Y., Ma, H. Q., Wu, J., Zhao, H. W., and Wan, Y. L. (2022b). Development of a specific monoclonal antibody-based icELISA for detection of arecoline in traditional Chinese medicines and fresh areca nuts. Food Agric. Immunol. 33 (1), 113–126. doi:10.1080/09540105.2021.2025347
Warnakulasuriya, S., and Chen, T. H. H. (2022). Areca nut and oral cancer: evidence from studies conducted in humans. J. Dent. Res. 101 (10), 1139–1146. doi:10.1177/00220345221092751
World Health Organization. Regional Office for the Western Pacific (2012). Review of areca (betel) nut and tobacco use in the pacific: a technical report. Available at: https://iris.who.int/handle/10665/206910 (Accessed April 28, 2024).
Wu, J., Cui, C., Zhang, H., Liu, D., Schreiber, L., Qin, W., et al. (2021). Identifying new compounds with potential pharmaceutical and physiological activity in Areca catechu and Areca triandra via a non-targeted metabolomic approach. Phytochem. Anal. 32 (6), 970–981. doi:10.1002/pca.3039
Wu, J., Cui, C., Zhao, H. W., Zhou, G. Z., Qin, L., Li, X. Y., et al. (2022). In-situ detection and imaging of Areca catechu fruit alkaloids by MALDI-MSI. Ind. Crops Prod. 188, 115533. doi:10.1016/j.indcrop.2022.115533
Xiao, B. M., Xiao, N. u., Peng, M. J., Gong, L. M., Li, C., and Liin, L. M. (2013). Research on effects of arecoline on refreshing and acute toxicity test. China J. Mod. Med. 20 (20), 3. doi:10.3969/j.issn.1674-4721.2013.20.005
Xie, H., Jing, R., Liao, X. T., Chen, H. S., Xie, X. L., Dai, H. J., et al. (2022). Arecoline promotes proliferation and migration of human HepG2 cells through activation of the PI3K/AKT/mTOR pathway. Hereditas 159 (1), 29. doi:10.1186/s41065-022-00241-0
Xing, J. H., Guan, D., and Zhu, X. Y. (2019). Determination of polyphenol in areca extract. Chem. Res. Toxicol. 30 (1), 38–41. doi:10.14002/j.hxya.2019.01.004
Xu, J. J. (2018). “Effect of arecanut extract on anti-toxoplasma gondii,” (Jilin: Yanbian University). master's thesis.
Xu, M., Su, S. Y., Jiang, S. M., Li, W. G., Zhang, Z., Zhang, J. C., et al. (2023a). Short-term arecoline exposure affected the systemic health state of mice, in which gut microbes played an important role. Ecotoxicol. Environ. Saf. 259, 115055. doi:10.1016/j.ecoenv.2023.115055
Xu, M., Wang, W. J., Su, S. Y., Li, W. G., Hu, X. S., and Zhang, J. C. (2023b). Arecoline alleviated loperamide induced constipation by regulating gut microbes and the expression of colonic genome. Ecotoxicol. Environ. Saf. 264, 115423. doi:10.1016/j.ecoenv.2023.115423
Xue, J. T., Wu, C. J., Wang, L. L., Jiang, S., Huang, G., Zhang, J. L., et al. (2011). Dynamic prediction models for alkaloid content using NIR technology for the study and online analysis of parching in Areca Seed. Food Chem. 126 (2), 725–730. doi:10.1016/j.foodchem.2010.11.036
Yan, H. Q., Yu, J., Zheng, W., Li, Y., Liu, L. Y., and Li, Z. G. (2022). Analysis of metabolites and study on antioxidant activity of Areca catechu L. in different developmental stages. China Food Addit. 33 (8), 72–79. doi:10.19804/j.issn1006-2513.2022.08.010
Yan, H. Q., Yu, J., Zheng, W., Li, Y., Liu, L. Y., and Li, Z. G. (2023). Analysis of transcriptome and screening of differential genes of areca catechu L. At different developmental stages. Mol. Plant Breed. 21 (14), 4595–4605. doi:10.13271/j.mpb.021.004595
Yang, L. Z., Liu, X. X., and Li, Z. P. (2018). Production and planting techniques of areca nut. World Agric. (7), 121–128. doi:10.13856/j.cn11-1097/s.2018.07.018
Yang, W. Q., Wang, H. C., Wang, W. J., Wang, Y., Zhang, X. Q., and Ye, W. C. (2012). Chemical constituents from the fruits of Areca catechu. J. Chin. Med. Mat. 35 (3), 400–403.
Yang, Y. D., Huang, L. Y., Xu, C. Y., Qi, L., Wu, Z. Y., Li, J., et al. (2021). Chromosome-scale genome assembly of areca palm (Areca catechu). Mol. Ecol. Resour. 21 (7), 2504–2519. doi:10.1111/1755-0998.13446
Yenjit, P., Issarakraisila, M., Intana, W., and Chantrapromma, K. (2010). Fungicidal activity of compounds extracted from the pericarp of Areca catechu against Colletotrichum gloeosporioides in vitro and in mango fruit. Postharvest Biol. Tec. 55 (2), 129–132. doi:10.1016/j.postharvbio.2009.09.003
Yin, M. S., Pan, F. B., Guo, J. X., Ji, X. L., and Liu, Y. Q. (2021). Research into chemical constituents and pharmacological activities in Areca catechu L. Food Res. Dev. 42 (15), 219–224.
Yu, C. L., Yue, L. J., and Huo, Y. H. (2022). Clinical analysis of 189 cases of oral submucous fibrosis diagnosed in a hospital in Zhengzhou. Mod. Dis. Control Prev. 33 (09), 721–724. doi:10.13515/j.cnki.hnjpm.1006-8414.2022.09.021
Yuan, H. W., Cao, M. R., Yi, P., Xie, Q. L., Jian, Y. Q., Li, B., et al. (2018). Determination of alkaloids and phenols in the chewable husk products of Areca catechu L. Using HPLC-UV and UHPLC-MS/MS. J. Liq. Chromatogr. Relat. Technol. 41 (10), 612–620. doi:10.1080/10826076.2018.1486326
Yuan, J. S., Yang, D. J., Liang, Y. H., Gao, W. P., Ren, Z. P., Zeng, W., et al. (2012). Alkaloids from areca (betel) nuts and their effects on human sperm motility in vitro. J. Food Sci. 77 (4), T70–T78. doi:10.1111/j.1750-3841.2012.02653.x
Zeng, Q. (2012). “Study on the chemical constituents of areca nut,” (Changsha: Central South University of Forestry and Technology). master's thesis.
Zhang, B., Gao, L. H., Shao, C. S., Deng, M. S., and Chen, L. J. (2021). Arecoline enhances phosphodiesterase 4A activity to promote transforming growth factor-β-induced buccal mucosal fibroblast activation via cAMP-Epac1 signaling pathway. Front. Pharmacol. 12, 722040. doi:10.3389/fphar.2021.722040
Zhang, D., Li, D., Xu, Q. T., and Kang, W. Y. (2015). Comparative study of antioxidant activity and ingredient in different parts ofAreca catechu L. extract. Sci. Tec. Food Ind. 36 (2), 102–104. doi:10.13386/j.issn1002-0306.2015.02.013
Zhang, P. Z., Sari, E. F., McCullough, M. J., and Cirillo, N. (2022). Metabolomic profile of Indonesian betel quids. Biomolecules 12 (10), 1469. doi:10.3390/biom12101469
Zhang, W. (2014). Observation on the clinical efficacy of Jinyingzi Binglang Decoction in the treatment of chronic refractory diarrhea. Asia-Pac. Tradit. Med. 10 (12), 99–100.
Zhang, W. L., Wang, M., Wu, Q. F., Zhu, Q., Jiao, Y. C., Zhu, Y. M., et al. (2019). Mutational signatures and the genomic landscape of betel quid chewing-associated tongue carcinoma. Cancer Med. 8 (2), 701–711. doi:10.1002/cam4.1888
Zhao, H., Ding, T. H., Chen, Y. L., Yang, W. B., Rao, J., Liu, D., et al. (2023). Arecoline aggravates acute ulcerative colitis in mice by affecting intestinal microbiota and serum metabolites. Front. Immunol. 14, 1197922. doi:10.3389/fimmu.2023.1197922
Zhao, W. J., Chen, X. Y., Liu, Y. Q., Li, P., and Li, H. J. (2020). Liquid chromatographic separation of alkaloids in herbal medicines: current status and perspectives. J. Sep. Sci. 43 (9-10), 1755–1772. doi:10.1002/jssc.202000081
Zheng, H. Z., Dong, Z. H., and She, J. (1998) Modern research and application of traditional Chinese medicine. Beijing: Xueyuan press.
Zhou, G. Z., Jiang, W. X., Luo, H. F., Li, X. Y., and Wan, Y. L. (2023). Transcriptome and targeted metabolomic integrated analysis reveals mechanisms of B vitamin accumulation in Areca catechu nut development. Int. J. Biol. Macromol. 241, 124570. doi:10.1016/j.ijbiomac.2023.124570
Zhou, N., Gu, X. Y., Zhuang, T. X., Xu, Y., Yang, L., and Zhou, M. M. (2020). Gut microbiota: a pivotal hub for polyphenols as antidepressants. J. Agric. Food Chem. 68 (22), 6007–6020. doi:10.1021/acs.jafc.0c01461
Zhu, H. L., Zhang, J. C., Li, C. F., Liu, S. X., and Wang, L. (2020). Morinda citrifolia L. leaves extracts obtained by traditional and eco-friendly extraction solvents: relation between phenolic compositions and biological properties by multivariate analysis. Ind. Crop. Prod. 153, 112586. doi:10.1016/j.indcrop.2020.112586
Zhu, L. P., Li, D., and Yang, X. F. (2023). Gut metabolomics and 16S rRNA sequencing analysis of the effects of arecoline on non-alcoholic fatty liver disease in rats. Front. Pharmacol. 14, 1132026. doi:10.3389/fphar.2023.1132026
Keywords: areca nut, active components, pharmacology, quality detection, influencing factors, omics, clinical study
Citation: Sun Y, Feng J, Hou W, Qi H and Liu Y (2024) Comprehensive insights into areca nut: active components and omics technologies for bioactivity evaluation and quality control. Front. Pharmacol. 15:1407212. doi: 10.3389/fphar.2024.1407212
Received: 26 March 2024; Accepted: 06 May 2024;
Published: 30 May 2024.
Edited by:
Wei Peng, Chengdu University of Traditional Chinese Medicine, ChinaReviewed by:
Qin Wan Huang, Chengdu University of Traditional Chinese Medicine, ChinaCopyright © 2024 Sun, Feng, Hou, Qi and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yangyang Liu, eXlsaXVAaW1wbGFkLmFjLmNu
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.