
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pharmacol. , 23 July 2024
Sec. Pharmacology of Infectious Diseases
Volume 15 - 2024 | https://doi.org/10.3389/fphar.2024.1406454
Objective: To analyze the clinical and laboratory characteristics and to identify predictors of moderate to severe anti-tuberculosis drug-induced liver injury (ATB-DILI) in patients with tuberculosis.
Methods: This prospective study enrolled Tuberculosis (TB) patients treated with first-line anti-tuberculosis drugs at the Affiliated Hospital of Zunyi Medical University between May 2022 and June 2023. The occurrence of ATB-DILI was monitored, and demographic and clinical data were gathered. We analyzed risk factors for the development of moderate to severe ATB-DILI.
Results: ATB-DILI was detected in 120 (10.7%) of the patients, with moderate to severe ATB-DILI occurring in 23 (2.0%) of the 1,124 patients treated with anti-tuberculosis treatment. Multivariate cox regression analysis identified malnutrition (HR = 4.564, 95% CI: 1.029–20.251, p = 0.046) and hemoglobin levels <120 g/L (HR = 2.825, 95% CI: 1.268–11.540, p = 0.017) as independent risk factors for moderate to severe ATB-DILI.
Conclusion: The incidence of moderate to severe ATB-DILI was found to be 2.0%. Malnutrition and hemoglobin levels below 120 g/L emerged as significant independent risk factors for the occurrence of moderate to severe ATB-DILI in this patient population.
Tuberculosis (TB), an ancient infectious disease is caused by infection with the Mycobacterium tuberculosis (MTB) complex. It ranks as the world’s leading cause of infectious diseases and is among the 13 primary causes of death globally (WHO, 2023), posing a serious threat to public health. Annually, there are approximately 10.6 million new tuberculosis cases are reported worldwide (WHO, 2023), according to global estimates, the latest global TB report indicates that in 2022, roughly 1.3 million individuals are expected to die from TB, a figure nearly twice as high as the number of deaths attributed to HIV/AIDS (WHO, 2023), Furthermore, tuberculosis follows COVID-19 as the infectious disease with the second-highest mortality rate. The TB situation in China is particularly concerning, with the country ranking third worldwide in terms of TB burden. In 2022, China is estimated to have 748,000 new TB patients and 30,000 TB-related deaths. The national TB incidence and mortality rates are projected to be 52 and 2.0 cases per 100,000 individuals, respectively (WHO, 2023). Clearly, tuberculosis remians a serious threat to human health globally, and particularly within China.
Anti-tuberculosis chemotherapy is the primary treatment for tuberculosis (Dartois and Rubin, 2022). The first-line drugs, including isoniazid (H), rifampicin (R), pyrazinamide (Z) and ethambutol (E), are commonly used, and the WHO recommends the 2HRZE/4HR regimen as a treatment option for sensitive TB (Who, 2022). Unfortunately, some patients may experience anti-tuberculous drug-induced liver injury (ATB-DILI) during treatment (Devarbhavi et al., 2021), which can lead to hospitalization, discontinuation or restriction of the use of other effective antituberculous drugs in moderate to severe cases. This may result in therapeutic deficiencies (Medina-Caliz et al., 2016; Kullak-Ublick et al., 2017), changes in treatment regimen, prolongation treatment duration, and in hepatic failure (Gafar et al., 2019). Liver transplantation is the most effective treatment for liver failure, yet it is limited due to the scarcity and the cast of liver donors (Ling et al., 2022). Therefore, moderate to severe ATB-DILI not only poses a significant public health concerns but also imposes a substantial financial burden on society. Data indicated that among hospitalized patients with ATB-DILI, 20.1% experience severe liver injury, with a mortality rate of 34.5% due to liver failure during follow-up (Zhao et al., 2020); approximately 35.5% of hospitalized patients present with acute liver failure (ALF), and 9.7% of these patients will die (Wang et al., 2020). Even with prophylactic measures, a considerable number of patients still develop severe ATB-DILI and even die after drug administration (Naidoo et al., 2015; Mehta et al., 2021). This shows that the correct identification of factors for the development of moderate to severe ATB-DILI and identifying appropriate antituberculosis treatment regimens are important for the control of tuberculosis.
Previous research has identified risk factors for the development of moderate to severe ATB-DILI (Shen et al., 2014; Jiang et al., 2021; Zhong et al., 2021), but these studies often suffer from small sample sizes and limited consideration of exposure factors, leading to inconsistent or even contradictory conclusions. This has limited their clinical guidance. Therefore, our study prospectively followed a cohort of TB patients treated with the HRZE regimen to identify clinical risk factors for moderate and severe ATB-DILI. By early and precise identification of patients at high risk for moderate to severe ATB-DILI, we aim to assist clinicians in selecting the most appropriate antituberculosis treatment strategies.
We enrolled tuberculosis patients who were treated with first-line anti-tuberculosis drugs at the Affiliated Hospital of Zunyi Medical University between May 2022 and June 2023. The primary objective was to observe the incidence of ATB-DILI and to analyze the risk factors associated with the development of moderate and severe ATB-DILI.
Inclusion criteria were as follows (had to be met simultaneously): 1) aged ≥18 years; 2) had active tuberculosis (including clinically diagnosed and laboratory-confirmed patients); 3) had an antituberculosis treatment regimen that included HRZ; and 4) had good adherence to medication and follow-up and voluntary participation in this study. Exclusion criteria were as follows: 1) had underlying liver disease, such as viral hepatitis B, cirrhosis, alcoholic hepatitis, or fatty liver, or those with obvious abnormalities in underlying liver function; 2) were using other drugs that may cause abnormalities in liver function, such as acetaminophen, herbal medicines, antitumour drugs or immunosuppressants; 3) had severe cardiac, pulmonary or renal insufficiency; 4) had combined autoimmune diseases, malignant tumors or HIV infection; and 5) were pregnant.
Demographic data (e.g., sex, age), etiological findings for tuberculosis (e.g., acid-fast smear staining, Mycobacterium tuberculosis culture, Mycobacterium tuberculosis nucleic acid test), and initial biochemical parameters (before antituberculous therapy and when ATB-DILI) were meticulously recorded. ATB-DILI was diagnosed based on the criteria established by an international panel of experts (Aithal et al., 2011), which include meeting at least one of the following conditions: (i) alanine aminotransferase (ALT) levels ≥5 times the upper limit of normal (ULN); (ii) Alkaline phosphatase (ALP) ≥2 × ULN, particularly with accompanying elevations in concentrations of 5′- nucleotidase or γ-glutamyl transpeptidase in the absence of known bone pathology driving the rise in ALP level; (iii) More than or equal to threefold elevation in ALT concentration and simultaneous elevation of bilirubin concentration exceeding 2× ULN. The severity of ATB-DILI was categorized as mild, moderate, severe or fatal, in accordance with the International Consensus Criteria (Aithal et al., 2011). According to the simplified RUCAM scoring system, patients with a total score ≥6 points can be diagnosed with ATB-DILI (Ashby et al., 2021). The clinical phenotype of ATB-DILI patients was calculated according to the R value [R value = (serum ALT/ALT ULN)/(serum AKP/AKP ULN)]. Cases were classified as “hepatocellular” if the R value was ≥5, “cholestatic” if the R value was ≤2, and “mixed” if the R value was 2.0–5.0.
Basic demographic information, including sex, age, ethnicity, height, weight, alcohol consumption (both past and current) and smoking status (past and current), was collected through questionnaire prior to the initiation of antituberculosis treatment. All study participants underwent routine blood tests, liver function tests and kidney function tests. In adults, a body mass index (BMI) of less than 18.5 kg/m2 is regarded as an indication of malnutrition (Sinha et al., 2021). A serum albumin level of less than 35 g/L serves as a traditional marker of nutritional status (Keller, 2019). Thus, in this study, patients with a BMI of less than 18.5 kg/m2 or a serum albumin level of less than 35 g/L prior to anti-TB therapy were considered malnourished. Follow-up, including routine blood tests, biochemistry and sedimentation tests, were scheduled every 2 weeks for the first 2 months of anti-tuberculosis drugs use. Following 2 months of treatment, the frequency of follow-up visits and tests were reduced to once a month. Patients were asked to seek medical attention and review the relevant indexes in case of changes in their conditions. This schedule continued until the end of the antituberculosis course. The examination results were extracted from the patients’ electronic medical records. Doctors meticulously documented any liver damage that occurred during treatment and adjusted the antituberculosis treatment plan as necessary, as well as administered hepatoprotective and symptomatic treatments, depending on the severity of the liver damage.
The study design received ethical approval from the Ethics Committee of the Affiliated Hospital of Zunyi Medical University [Approval No.: KLL-2022-735]. Written informed consent was obtained from each subject to participate in this study. However, all the data were anonymous, and the confidentiality of the data was maintained. This study adhered to the Declaration of Helsinki.
Statistical analyses were performed using SPSS 26.0 (SPSS Inc.) and GraphPad Prism 8.0.1 (GraphPad Software). For continuous variables, the mean (x) ± standard deviation (s) is used to represent the normal distribution. Otherwise, median and interquartile range were used. Categorical variables were expressed as percentages. Comparisons between groups were made using the parameterized Student’s t-test or the nonparametric Mann‒Whitney U test (continuous variables) and the χ2 test or Fisher’s exact test (categorical variables). Univariate and multivariate cox regression analyses was used to determine the independent risk factors for ATB-DILI and moderate and severe ATB-DILI. Variables with a p-value less than 0.2 in univariate cox regression analyses and factors considered significant by clinical experts were included in multivariate cox regression analyses by a backward-forward approach to identify risk factors for moderate and severe ATB-DILI. For multivariate cox analyses, the stepwise entry and removal probabilities were set to 0.05 and 0.10 respectively. Differences were considered statistically significant when p < 0.05.
As shown in Figure 1, out of the 1,576 patients initially screened, a total of 1,124 patients who were treated with first-line anti-tuberculosis drugs were followed up until the end of the anti-tuberculosis treatment regimen. During this follow-up period, ATB-DILI was diagnosed in 120 patients. There were no statistically significant differences in mean age and BMI between patients with and without ATB-DILI. However, a lower percentage of patients with ATB-DILI were smokers compared to those without ATB-DILI (20.83% vs. 29.88%, p = 0.043). Before antituberculosis treatment, total lymphocyte count, ALT, aspartate transaminase (AST), gamma glutamyl transferase (GGT), and total bilirubin levels were significantly higher in patients with ATB-DILI than in those without the condition. Additionally, the hemoglobin concentration was significantly lower in patients with ATB-DILI compared to those without it [(123.56 ± 21.73) g/L vs. (127.78 ± 20.26) g/L, p = 0.043] (Table 1).
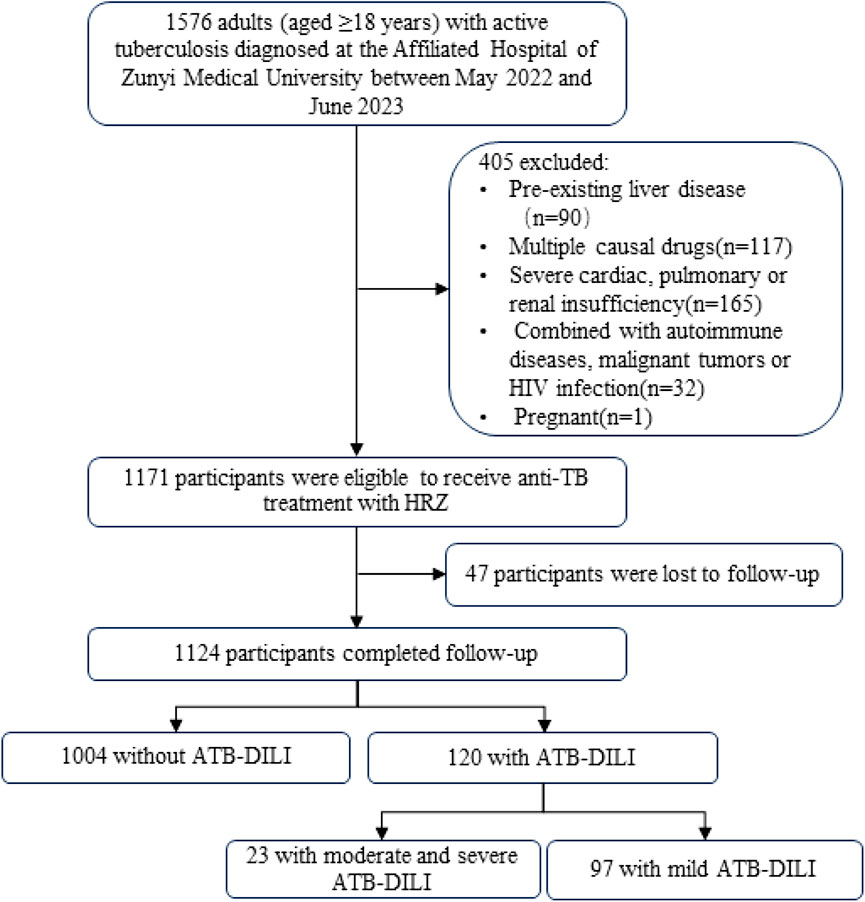
Figure 1. Participant flow chart HIV, human immunodeficiency virus; TB, tuberculosis; H, isoniazid; R, rifampicin; Z, pyrazinamide; ATB-DILI, anti-tuberculous drug-induced liver injury.
Out of the 120 patients with ATB-DILI, 23 cases manifesting as moderate and severe ATB-DILI. The mean age of these patients with moderate and severe ATB-DILI was 51.09 ± 17.20 years, with 6 patients (26.09%) being 65 years of age or older and an 6 patients (26.09%)were female. The average body mass index (BMI) of the patients with moderate and severe ATB-DILI was 19.86 ± 1.98 kg/m2, and 20 patients (86.96%) had a BMI <18.5 kg/m2. The proportion of moderate and severe ATB-DILI patients with BMI lower than 18.5 kg/m2 was significantly higher than that observed in patients with mild ATB-DILI (86.96% vs. 58.76%, p = 0.014). The patients predominantly considered of Han Chinese individuals who were receiving TB treatment for the first time. 6 (26.09%) and 6 (26.09%) patients with TB had a history of smoking and alcohol consumption, respectively. In addition, 3 (13.04%) TB patients had hypertension. The platelet count of patients with moderate and severe ATB-DILI was significantly lower than that of patients with mild ATB-DILI [(210.68 ± 82.11) ×10^9/L vs. 270.91 ± 104.10) ×10^9/L, p = 0.011] (Table 2).
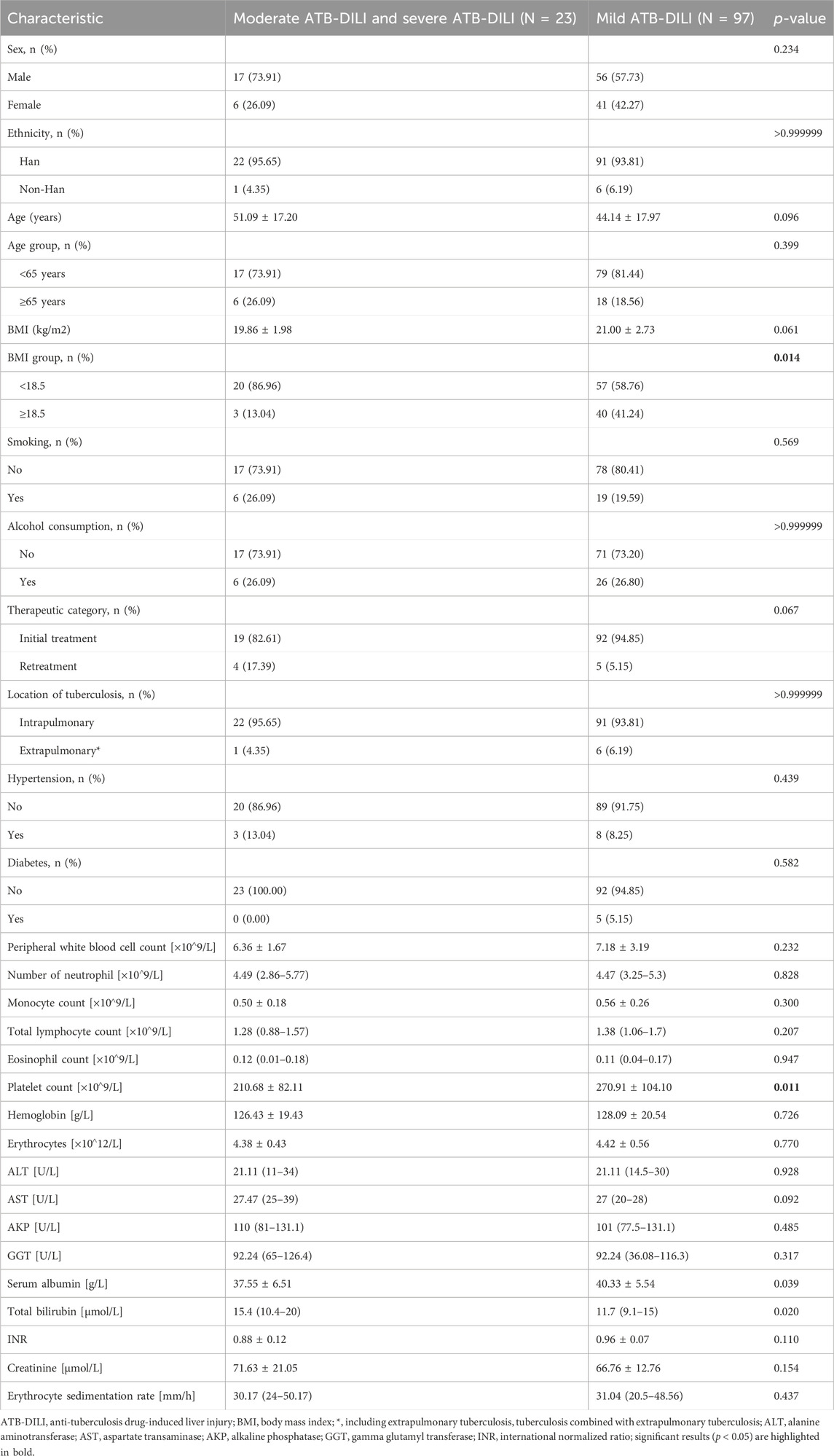
Table 2. Baseline characteristics of patients with mild ATB-DILI versus patients with moderate and severe ATB-DILI.
Among the 1,124 patients who completed anti-tuberculosis treatment, 120 patients developed ATB-DILI, including 23 patients (19.17%) with moderate and severe ATB-DILI and 97 patients (80.83%) with mild ATB-DILI. The overall prevalence of ATB-DILI was 10.7% (120/1,124), with a incidence rate of moderate and severe ATB-DILI was 2.0% (23/1,124). During the follow-up period, there were no deaths due to ATB-DILI.
Patients with moderate and severe ATB-DILI were significantly more likely experience jaundice than those with mild ATB-DILI (43.48% vs. 0%, p < 0.0001). There was no statistical difference in the incidence of antituberculosis drug allergy or the need for hospitalization between the moderate to severe ATB-DILI group and the mild ATB-DILI group. The majority of ATB-DILI cases, regardless of severity, occurred within the first 2 months of anti-TB therapy. However, the incidence of moderate to severe ATB-DILI between days 46–60 was significantly higher than that of mild ATB-DILI (34.78% vs. 9.28%, p = 0.0044). Regardless of the severity of ATB-DILI, the liver injury type was mainly hepatocellular injury type. After the occurrence of ATB-DILI, patients with moderate and severe ATB-DILI has significantly higher mean serum aspartate transaminase (AST) and glutamyl transferase (GGT) concentrations compared to those with mild ATB-DILI, 453 (228–699) U/L vs. 212.5 (117.5–330.25) U/L, p = 0.005 and 141.00 (76.50–256.25) U/L vs. 92.50 (57.00–156.50) U/L, p = 0.033, respectively. In addition, patients with moderate to severe ATB-DILI had significantly higher mean serum total bilirubin concentrations compared to those with mild ATB-DILI [66.40 (45.70–107.70) μmol/L vs15.10 (9.75–19.83) μmol/L. P < 0.0001] (Table 3).
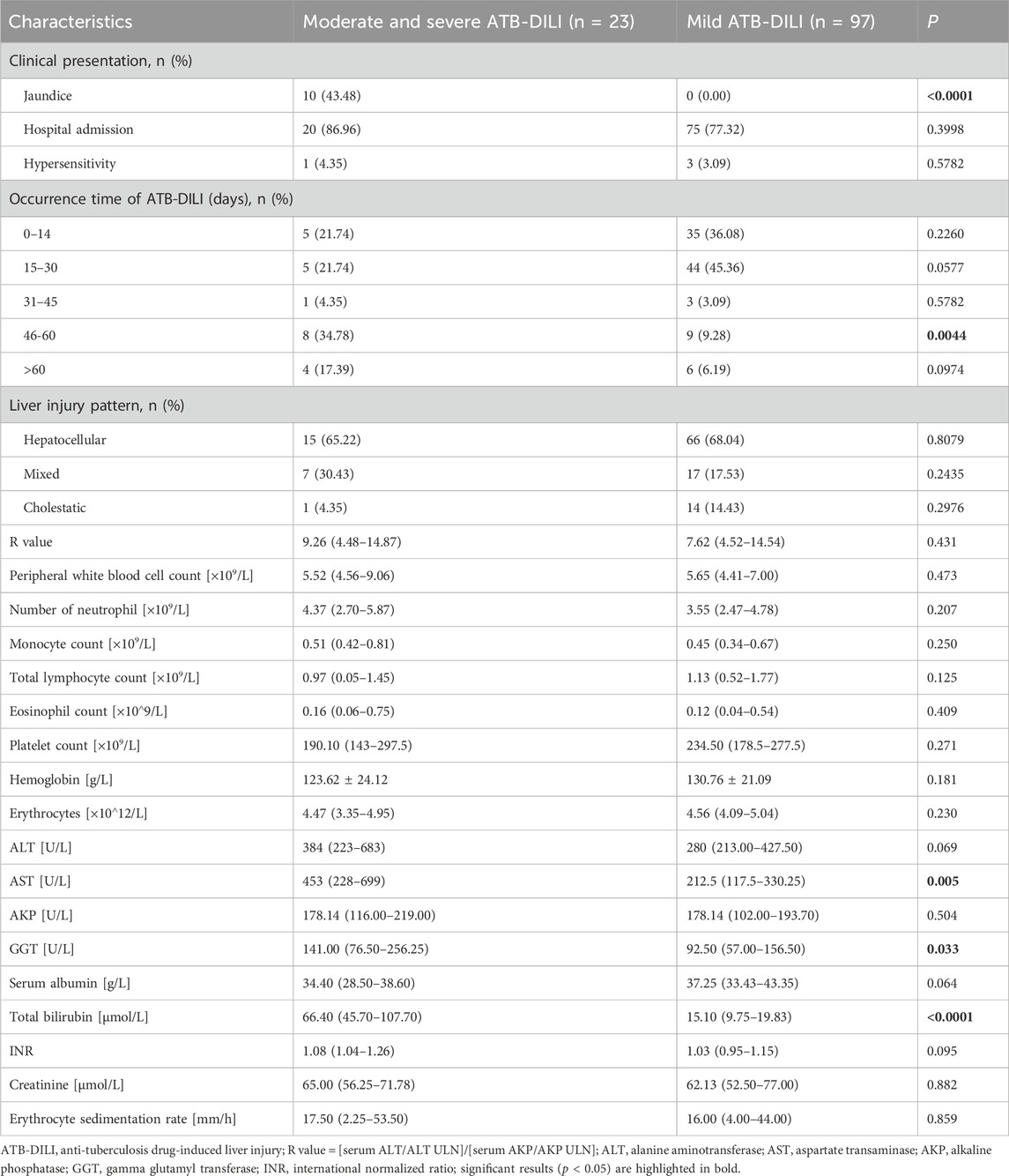
Table 3. Clinical characteristics of patients with moderate and severe ATB-DILI at the time of liver injury compared to patients with mild ATB-DILI.
We performed univariate and multifactorial cox regression analyses to identify potential risk factors for the development of moderate and severe ATB-DILI, using baseline data from prior to the initiation of anti-TB therapy. Univariate cox regression analysis revealed that age ≥65 years, malnutrition (including BMI <18.5 kg/m2 or serum albumin <35 g/L) and hemoglobin <120 g/L were risk factors for ATB-DILI (Table 3). Multivariate cox regression analysis revealed that malnutrition (HR = 2.047, 95% CI: 1.279–3.276, p = 0.003), alcohol consumption (HR = 1.710, 95% CI: 1.033–2.832, p = 0.037) and hemoglobin <120 g/L (HR = 2.289, 95% CI: 1.353–3.870, p = 0.002)were independent risk factors for ATB-DILI in patients with tuberculosis (Table 4).
As shown in Table 5, univariate and multivariate cox regression analyses were performed for risk factors associated with the development of moderate and severe ATB-DILI, also using data from before the start of anti-tuberculosis therapy. Univariate cox regression analysis showed that malnutrition and creatinine >100 μmol/L were risk factors for moderate and severe ATB-DILI. Multivariate cox regression analysis showed that malnutrition (HR = 4.564, 95% CI: 1.029–20.251, p = 0.046) and hemoglobin <120 g/L (HR = 2.825, 95% CI: 1.268–11.540, p = 0.017) were an independent risk factors for moderate and severe ATB-DILI on first-line antituberculosis therapy.
There were no deaths in this study during the entire follow-up period until the end of anti-TB treatment. Of the patients with ATB-DILI, 89.2% (107/120) had their anti-tuberculosis therapy interruption due to ATB-DILI. The duration of anti-tuberculosis therapy interruption was 11 (5–25) days. The TB treatment success rate was 90.2% (906/1,004) for participants without ATB-DILI, 81.7% (98/120) in those with ATB-DILI, and 73.9% (17/23) in those with moderate and severe ATB-DILI. The TB treatment success rate of patients with ATB-DILI was significantly lower than that of patients without ATB-DILI (p = 0.007). However, there was no significant difference in TB treatment success rate between moderate and severe ATB-DILI patients and ATB-DILI patients (p = 0.397).
In China, antituberculosis drugs are a major cause of drug-induced liver injury (Shen et al., 2019). However, the exact mechanisms behind ATB-DILI remain elusive. Among the first-line anti-tuberculosis drugs, isoniazid, rifampicin and pyrazinamide are the main agents associated with ATB-DILI, while reports of liver injury from ethambutol are rare (LiverTox, 2012). The hepatotoxicity of isoniazid and pyrazinamide may be due to their respective metabolites (Lim WS. et al., 2023). Pyrazinamide is metabolized into pyrazinic acid and 5-hydroxypyrazinic acid, which can cause liver injury (Hussain et al., 2021). Isoniazid in vivo metabolites acetylnicotinazid and hydrazide cause liver injury (Metushi et al., 2016). Additionlly, H can be directly oxidized to active metabolites that valently bind to liver macromolecules, leading to liver damage through immune mechanisms (Jenner and Ellard, 1989; Wang et al., 2016). Rifampicin is belived to enhance the hepatotoxicity of other anti-tuberculosis drugs, for example, by providing an acetyl group to H, thereby accelerating its metabolism to acetylhydrazine and causing liver damage (Kim et al., 2017). Rifampicin can also affect bile acid synthesis and transport, leading to cholestasis and promoting liver injury (Zhuang et al., 2022). Beyond these mechanisms, intestinal flora disturbance and intestinal barrier disruption are believed to be involved in the occurrence and development of ATB-DILI. These factors can expose the liver to increased drug or bacterial metabolites, triggering a persistent inflammatory response that promotes liver damage (Chopyk and Grakoui, 2020).
This study revealed that the incidence of ATB-DILI was 10.7%, with a moderate and severe ATB-DILI incidence rate of 2.0%. This incidence rate of ATB-DILI is higher than the 5.4% reported in a previous Chinese study involving 4,562 adult TB patients (Jiang et al., 2021) and the 7.6% in a recent multicenter prospective study in South Korea (Lim J. et al., 2023). However, both the overall ATB-DILI incidence and the incidence of moderate to severe ATB-DILI were lower than the rates reported by Portuguese scholars, which were 44.2% and 28.3% respectively (Cavaco et al., 2022). In Brazil, the incidence of severe ATB-DILI among TB patients with HIV infection reaches as high as 22.1% (Tomich et al., 2015). The discrepancies in incidence rates may be attributed to several factors: 1) variation in diagnostic criteria for ATB-DILI; 2) differences in study populations; 3) variation in prescription habits, and some medical staff may provide preventive liver protection drugs to reduce the incidence of ATB-DILI and severe ATB-DILI; 4) different genetic polymorphisms in different races; 5) different regional factors; and 6) unique metabolomes and the influence of the microbiome. Previous studies have shown that the plasma metabolome and urine microbiome of patients with ATB-DILI and severe ATB-DILI have their own characteristics before liver injury (Wang MG. et al., 2022; Wu et al., 2022). Therefore, it is suggested that the metabolome and microbiome have an impact on the occurrence and severity of ATB-DILI.
The patients in this study developed anti-tuberculosis ATB-DILI overwhelmingly within 2 months therapy, suggesting that the monitoring of liver function indexes should be strengthened during the intensive period of antituberculosis treatment (2 months before antituberculosis treatment). Furthermore, early initiation of liver function monitoring liver function at an early stage (2 weeks after antituberculosis treatment), is beneficial for predicting the occurrence of ATB-DILI (Patterson et al., 2021) and avoiding missed diagnoses of ATB-DILI and adverse outcomes (Hayashi et al., 2015). The proportion of moderate and severe ATB-DILI occurring in 46–60 days is significantly higher than that of mild ATB-DILI, which means that attention should be paid to the monitoring of biochemical indexes of liver function during anti-tuberculosis process to detect severe liver injury early. Predominantly, the type of liver injury observed corresponded to hepatocellular injury, aligning with previous studies (Shen et al., 2019).
It is now widely accepted that old age, alcoholism, hepatitis virus infection or a combination of other liver diseases, malnutrition or hypoproteinaemia and HIV infection are risk factors for ATB-DILI (Ali et al., 2020; Chalasani et al., 2021; Devarbhavi et al., 2021; Jiang et al., 2021). There are few reports in the literature on whether the severity of ATB-DILI severity is related to age. However, it has been found that persistent liver biochemical abnormalities are common after drug-related liver injury in older patients (Fontana et al., 2015). In a retrospective study by Zhao et al. (2020) older age was not found to be a risk factor for severe ATB-DILI (Zhao et al., 2020). Our study also revealed that age ≥65 years do not appear to be at increased risk factor for moderate to severe ATB-DILI, though further studies are needed to confirm this conclusion. Alcohol consumption is recognized as an independent risk factor for severe ATB-DILI, which is consistent with the findings of Ji, who developed a nomogram model to predict the risk of drug-induced liver injury in anti-tuberculosis treatment patients (Ji et al., 2023). The more alcohol consumed, the higher the risk of alcohol-related liver injury and even death (Surial et al., 2021). The effects of hepatitis virus infection, other acute and chronic liver diseases and HIV infection on severe ATB-DILI were not investigated in this study because patients with hepatitis virus infection, other acute and chronic liver diseases and HIV infection were excluded. Tuberculosis combined with malnutrition is very common (Feleke et al., 2019), which have been identified as an important risk factor for the occurrence of ATB-DILI (Lin et al., 2021). There is a significant linear relationship between malnutrition and ATB-DILI (Zhao et al., 2023). Our study extends this understanding by demonstrating that malnutrition is not only a risk factor for ATB-DILI but also for its moderate and severe forms. The relationship between anemia and anti-tuberculosis drug-induced liver injury and its severity is rarely reported in the literature. This study found that hemoglobin <120 g/L is a risk factor for ATB-DILI, and it is also a risk factor for moderate and severe ATB-DILI. The reasons may be as follows: 1) Anemia directly leads to a decrease in systemic oxygen delivery, resulting in liver cell ischemia and hypoxia. 2) Malnutrition leads to decreased liver synthesis of plasma proteins (such as hemoglobin, etc.) and erythropoietin deficiency (Cheung et al., 2012), so anemia patients are often combined with malnutrition, and malnutrition is currently recognized as a risk factor for ATB-DILI.
A large multicenter retrospective study revealed that AST, total bilirubin, platelets, prothrombin time, and gender were not found to be correlated with biochemical nonresolution in patients with drug-induced liver injury (Wang CY. et al., 2022). However, our prospective study did not identify any correlation between AST, total bilirubin, platelets, prothrombin time, and gender with ATB-DILI or moderate and severe ATB-DILI. Future multi-center prospective large sample studies on AST, total bilirubin, platelets, prothrombin time, gender and their correlation with ATB-DILI or moderate and severe ATB-DILI are warranted.
Our research demonstrated that TB patients with malnutrition, and with hemoglobin <120 g/L are more likely to develop moderate and severe ATB-DILI after anti-TB treatment. To some extent, the occurrence of moderate and severe ATB-DILI post-antituberculosis treatment can be prevented by paying attention to these populations. The design of personalized antituberculosis treatment plans for these people can enhance patients compliance and improve efficacy of antituberculosis treatment.
There are several limitations to our study. First, while the prospective follow-up study included 1171 TB patients receiving first-line anti-TB therapy, the sample size for patients with moderate to severe ATB-DILI patients was insufficient. Thus, the conclusion drawn from this study should be interpreted with caution and might not be extrapolated to the wider perspective of patients with tuberculosis worldwide. Second, the study did not investigate the polymorphisms in genes related to drug metabolism, transport, antioxidant response, and immune response, which are known to influence ATB-DILI susceptibility. Future studies should integrate genetic factors with the exploration of risk factors for moderate to severe ATB-DILI. Third, the study did not account for the impact of underlying liver diseases (such as viral hepatitis B, cirrhosis, combined autoimmune diseases, malignant tumors and HIV infection) and pregnancy status all have an impact on moderate and severe ATB-DILI. The exclusion of these populations limits the study’s ability to fully understand the risk factors for moderate to severe ATB-DILI in these groups, necessitating future research. Fourthly, alcohol consumption data were self-reported through questionnaires, which may not capture the full extent of alcohol intake and could introduce bias in the results. Lastly, the absence of routine blood lipid testing among participants precluded the inclusion of blood lipids in the analysis of ATB-DILI and its risk factors for moderate to severe cases.
Our study showed that malnutrition and hemoglobin <120 g/L as risk factors for the development of moderate to severe ATB-DILI. For patients who exhibit these characteristics before antituberculosis treatment, medical staff should be more cautious, and it is recommended that patients with low hepatotoxicity receive antituberculosis treatment to avoid moderate to severe ATB-DILI.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
The studies involving humans were approved by the ethics committee of the Affiliated Hospital of Zunyi Medical University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
QL: Formal Analysis, Writing–original draft. LH: Data curation, Writing–review and editing. HY: Data curation, Writing–review and editing. ZZ: Visualization, Writing–original draft. ZC: Supervision, Writing–review and editing. XW: Data curation, Validation, Writing–review and editing. LC: Conceptualization, Supervision, Writing–review and editing. YL: Conceptualization, Visualization, Writing–review and editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Science and Technology Fund Project of Guizhou Provincial Health Commission (GZWJKJ2019-1-085).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Aithal, G. P., Watkins, P. B., Andrade, R. J., Larrey, D., Molokhia, M., Takikawa, H., et al. (2011). Case definition and phenotype standardization in drug-induced liver injury. Clin. Pharmacol. Ther. 89 (6), 806–815. doi:10.1038/clpt.2011.58
Ali, N., Gupta, N., and Saravu, K. (2020). Malnutrition as an important risk factor for drug-induced liver injury in patients on anti-tubercular therapy: an experience from a tertiary care center in South India. Drug Discov. Ther. 14 (3), 135–138. doi:10.5582/ddt.2020.03029
Ashby, K., Zhuang, W., González-Jimenez, A., Alvarez-Alvarez, I., Lucena, M. I., Andrade, R. J., et al. (2021). Elevated bilirubin, alkaline phosphatase at onset, and drug metabolism are associated with prolonged recovery from DILI. J. Hepatol. 75 (2), 333–341. doi:10.1016/j.jhep.2021.03.021
Cavaco, M. J., Alcobia, C., Oliveiros, B., Mesquita, L. A., Carvalho, A., Matos, F., et al. (2022). Clinical and Genetic Risk Factors for Drug-Induced Liver Injury Associated with Anti-Tuberculosis Treatment-A Study from Patients of Portuguese Health Centers. J. Pers. Med. 12 (5), 790. doi:10.3390/jpm12050790
Chalasani, N. P., Maddur, H., Russo, M. W., Wong, R. J., and Reddy, K. R.Practice Parameters Committee of the American College of Gastroenterology (2021). ACG Clinical Guideline: Diagnosis and Management of Idiosyncratic Drug-Induced Liver Injury. Am. J. Gastroenterol. 116 (5), 878–898. doi:10.14309/ajg.0000000000001259
Cheung, K., Lee, S. S., and Raman, M. (2012). Prevalence and mechanisms of malnutrition in patients with advanced liver disease, and nutrition management strategies. Clin. Gastroenterol. Hepatol. 10 (2), 117–125. doi:10.1016/j.cgh.2011.08.016
Chopyk, D. M., and Grakoui, A. (2020). Contribution of the Intestinal Microbiome and Gut Barrier to Hepatic Disorders. Gastroenterology 159 (3), 849–863. doi:10.1053/j.gastro.2020.04.077
Dartois, V. A., and Rubin, E. J. (2022). Anti-tuberculosis treatment strategies and drug development: challenges and priorities. Nat. Rev. Microbiol. 20 (11), 685–701. doi:10.1038/s41579-022-00731-y
Devarbhavi, H., Aithal, G., Treeprasertsuk, S., Takikawa, H., Mao, Y., Shasthry, S. M., et al. (2021). Drug-induced liver injury: Asia Pacific Association of Study of Liver consensus guidelines. Hepatol. Int. 15 (2), 258–282. doi:10.1007/s12072-021-10144-3
Feleke, B. E., Feleke, T. E., and Biadglegne, F. (2019). Nutritional status of tuberculosis patients, a comparative cross-sectional study. BMC Pulm. Med. 19 (1), 182. doi:10.1186/s12890-019-0953-0
Fontana, R. J., Hayashi, P. H., Barnhart, H., Kleiner, D. E., Reddy, K. R., Chalasani, N., et al. (2015). Persistent liver biochemistry abnormalities are more common in older patients and those with cholestatic drug induced liver injury. Am. J. Gastroenterol. 110 (10), 1450–1459. doi:10.1038/ajg.2015.283
Gafar, F., Arifin, H., Jurnalis, Y. D., Yani, F. F., Fitria, N., Alffenaar, J. W. C., et al. (2019). Antituberculosis Drug-induced Liver Injury in Children: Incidence and Risk Factors During the Two-month Intensive Phase of Therapy. Pediatr. Infect. Dis. J. 38 (1), 50–53. doi:10.1097/INF.0000000000002192
Hayashi, P. H., Fontana, R. J., Chalasani, N. P., Stolz, A. A., Talwalkar, J. A., Navarro, V. J., et al. (2015). Under-reporting and Poor Adherence to Monitoring Guidelines for Severe Cases of Isoniazid Hepatotoxicity. Clin. Gastroenterol. Hepatol. 13 (9), 1676–1682. doi:10.1016/j.cgh.2015.02.024
Hussain, Z., Zhu, J., and Ma, X. (2021). Metabolism and Hepatotoxicity of Pyrazinamide, an Antituberculosis Drug. Drug Metab. Dispos. 49 (8), 679–682. doi:10.1124/dmd.121.000389
Jenner, P. J., and Ellard, G. A. (1989). Isoniazid-related hepatotoxicity: a study of the effect of rifampicin administration on the metabolism of acetylisoniazid in man. Tubercle 70 (2), 93–101. doi:10.1016/0041-3879(89)90033-0
Ji, S., Lu, B., and Pan, X. (2023). A nomogram model to predict the risk of drug-induced liver injury in patients receiving anti-tuberculosis treatment. Front. Pharmacol. 14, 1153815. doi:10.3389/fphar.2023.1153815
Jiang, F., Yan, H., Liang, L., Du, J., Jin, S., Yang, S., et al. (2021). Incidence and risk factors of anti-tuberculosis drug induced liver injury (DILI): Large cohort study involving 4652 Chinese adult tuberculosis patients. Liver Int. 41 (7), 1565–1575. doi:10.1111/liv.14896
Keller, U. (2019). Nutritional Laboratory Markers in Malnutrition. J. Clin. Med. 8 (6), 775. doi:10.3390/jcm8060775
Kim, J. H., Nam, W. S., Kim, S. J., Kwon, O. K., Seung, E. J., Jo, J. J., et al. (2017). Mechanism Investigation of Rifampicin-Induced Liver Injury Using Comparative Toxicoproteomics in Mice. Int. J. Mol. Sci. 18 (7), 1417. doi:10.3390/ijms18071417
Kullak-Ublick, G. A., Andrade, R. J., Merz, M., End, P., Benesic, A., Gerbes, A. L., et al. (2017). Drug-induced liver injury: recent advances in diagnosis and risk assessment. Gut 66 (6), 1154–1164. doi:10.1136/gutjnl-2016-313369
Lim, J., Kim, J. S., Kim, H. W., Kim, Y. H., Jung, S. S., et al. (2023b). Metabolic Disorders Are Associated With Drug-Induced Liver Injury During Antituberculosis Treatment: A Multicenter Prospective Observational Cohort Study in Korea. Open Forum Infect. Dis. 10 (8), ofad422. doi:10.1093/ofid/ofad422
Lim, W. S., Avery, A., Kon, O. M., and Dedicoat, M. (2023a). Anti-tuberculosis drug-induced liver injury. BMJ 383, e074866. doi:10.1136/bmj-2023-074866
Lin, H. S., Lin, M. S., Chi, C. C., Ye, J. J., and Hsieh, C. C. (2021). Nutrition Assessment and Adverse Outcomes in Hospitalized Patients with Tuberculosis. J. Clin. Med. 10 (12), 2702. doi:10.3390/jcm10122702
Ling, S., Jiang, G., Que, Q., Xu, S., Chen, J., and Xu, X. (2022). Liver transplantation in patients with liver failure: Twenty years of experience from China. Liver Int. 42 (9), 2110–2116. doi:10.1111/liv.15288
LiverTox (2012). Clinical and Research Information on Drug-Induced Liver Injury. Bethesda. The National Institute of Diabetes and Digestive and Kidney Diseases.
Medina-Caliz, I., Robles-Diaz, M., Garcia-Munoz, B., Stephens, C., Ortega-Alonso, A., Garcia-Cortes, M., et al. (2016). Definition and risk factors for chronicity following acute idiosyncratic drug-induced liver injury. J. Hepatol. 65 (3), 532–542. doi:10.1016/j.jhep.2016.05.003
Mehta, R., Ive, P., Evans, D., and Menezes, C. N. (2021). Treatment outcomes among patients admitted to hospital with antiretroviral and/or antituberculosis drug-induced liver injury. S. Afr. Med. J. 111 (5), 474–481. doi:10.7196/SAMJ.2021.v111i5.15353
Metushi, I., Uetrecht, J., and Phillips, E. (2016). Mechanism of isoniazid-induced hepatotoxicity: then and now. Br. J. Clin. Pharmacol. 81 (6), 1030–1036. doi:10.1111/bcp.12885
Naidoo, S., Evans, D., Jong, E., Mellet, K., and Berhanu, R. (2015). Outcomes of TB/HIV co-infected patients presenting with antituberculosis drug-induced liver injury. S Afr. Med. J. 105 (5), 393–396. doi:10.7196/samj.8217
Patterson, B., Abbara, A., Collin, S., Henderson, M., Shehata, M., Gorgui-Naguib, H., et al. (2021). Predicting drug-induced liver injury from anti-tuberculous medications by early monitoring of liver tests. J. Infect. 82 (2), 240–244. doi:10.1016/j.jinf.2020.09.038
Shen, T., Liu, Y., Shang, J., Xie, Q., Li, J., Yan, M., et al. (2019). Incidence and Etiology of Drug-Induced Liver Injury in Mainland China. Gastroenterology 156 (8), 2230–2241. doi:10.1053/j.gastro.2019.02.002
Shen, X., Yuan, Z., Mei, J., Zhang, Z., Guo, J., Wu, Z., et al. (2014). Anti-tuberculosis drug-induced liver injury in Shanghai: validation of Hy's Law. Drug Saf. 37 (1), 43–51. doi:10.1007/s40264-013-0119-6
Sinha, P., Lonnroth, K., Bhargava, A., Heysell, S. K., Sarkar, S., Salgame, P., et al. (2021). Food for thought: addressing undernutrition to end tuberculosis. Lancet Infect. Dis. 21 (10), e318–e325. doi:10.1016/S1473-3099(20)30792-1
Surial, B., Bertholet, N., Daeppen, J. B., Darling, K. E. A., Calmy, A., Günthard, H. F., et al. (2021). The Impact of Binge Drinking on Mortality and Liver Disease in the Swiss HIV Cohort Study. J. Clin. Med. 10 (2), 295. doi:10.3390/jcm10020295
Tomich, L. G., Nunez, M., and Mendes-Correa, M. C. (2015). Drug-induced liver injury in hospitalized HIV patients: high incidence and association with drugs for tuberculosis. Ann. Hepatol. 14 (6), 888–894. doi:10.5604/16652681.1171778
Wang, C. Y., Deng, Y., Li, P., Zheng, S., Chen, G., Zhou, G., et al. (2022b). Prediction of biochemical nonresolution in patients with chronic drug-induced liver injury: A large multicenter study. Hepatology 75 (6), 1373–1385. doi:10.1002/hep.32283
Wang, M. G., Wu, S. Q., Zhang, M. M., and He, J. Q. (2022a). Urine metabolomics and microbiome analyses reveal the mechanism of anti-tuberculosis drug-induced liver injury, as assessed for causality using the updated RUCAM: A prospective study. Front. Immunol. 13, 1002126. doi:10.3389/fimmu.2022.1002126
Wang, P., Pradhan, K., Zhong, X. B., and Ma, X. (2016). Isoniazid metabolism and hepatotoxicity. Acta Pharm. Sin. B 6 (5), 384–392. doi:10.1016/j.apsb.2016.07.014
Wang, S., Shangguan, Y., Ding, C., Li, P., Ji, Z., Shao, J., et al. (2020). Risk factors for acute liver failure among inpatients with anti-tuberculosis drug-induced liver injury. J. Int. Med. Res. 48 (1), 300060518811512. doi:10.1177/0300060518811512
WHO (2022). WHO consolidated guidelines on tuberculosis module 4: treatment-drug-susceptible tuberculosis treatment. Geneva: World Health Organization.
Wu, S., Wang, M., Zhang, M., and He, J. Q. (2022). Metabolomics and microbiomes for discovering biomarkers of antituberculosis drugs-induced hepatotoxicity. Arch. Biochem. Biophys. 716, 109118. doi:10.1016/j.abb.2022.109118
Zhao, H., Wang, Y., Zhang, T., Wang, Q., and Xie, W. (2020). Drug-Induced Liver Injury from Anti-Tuberculosis Treatment: A Retrospective Cohort Study. Med. Sci. Monit. 26, e920350. doi:10.12659/MSM.920350
Zhao, L., Li, M., Li, Y., Hao, H., Zhao, S., Ma, A., et al. (2023). Association between macronutrients intake and liver dysfunction among tuberculosis patients in rural China. Asia Pac J. Clin. Nutr. 32 (4), 444–459. doi:10.6133/apjcn.202312_32(4).0009
Zhong, T., Fan, Y., Dong, X. L., Guo, X., Wong, K. H., Wong, W. T., et al. (2021). An Investigation of the Risk Factors Associated With Anti-Tuberculosis Drug-Induced Liver Injury or Abnormal Liver Functioning in 757 Patients With Pulmonary Tuberculosis. Front. Pharmacol. 12, 708522. doi:10.3389/fphar.2021.708522
Keywords: drug-induced liver injury, moderate and severe ATB-DlLl, risk factor, malnutrition, haemoglobin
Citation: Liu Q, Huang L, Yan H, Zong Z, Chen Z, Wu X, Chen L and Lan Y (2024) Clinical risk factors for moderate and severe antituberculosis drug-induced liver injury. Front. Pharmacol. 15:1406454. doi: 10.3389/fphar.2024.1406454
Received: 25 March 2024; Accepted: 08 July 2024;
Published: 23 July 2024.
Edited by:
Minjun Chen, National Center for Toxicological Research (FDA), United StatesCopyright © 2024 Liu, Huang, Yan, Zong, Chen, Wu, Chen and Lan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ling Chen, bGluZ2p1bmNkQDE2My5jb20=; Yuanbo Lan, bHliaXZ5QDE2My5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.