
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Pharmacol. , 21 May 2024
Sec. Ethnopharmacology
Volume 15 - 2024 | https://doi.org/10.3389/fphar.2024.1404738
Background: The efficacy of Chinese herbal medicine (CHM) in managing irritable bowel syndrome with diarrhea (IBS-D) accompanied by anxiety and depression remains uncertain. Thus, a systematic review was carried out employing meta-analysis and network pharmacology to ascertain the efficacy and underlying mechanisms of CHM therapy.
Methods: By conducting a systematic review, including literature search, screening, and data extraction, we identified 25 randomized controlled trials to assess CHM’s effectiveness in treating irritable bowel syndrome alongside anxiety and depression. Network pharmacology was utilized to scrutinize the metabolite utility of CHM in addressing this condition. Potential primary mechanisms were synthesized using information sourced from the PubMed database.
Results: Twenty-five studies, including 2055 patients, were analyzed, revealing significant treatment efficacy for IBS-D in the trial group compared to controls [OR = 4.01, 95% CI (2.99, 5.36), I2 = 0%] Additionally, treatment for depression [SMD = −1.08, 95% CI (-1.30, −0.86), p < 0.00001, I2 = 68%; SDS: SMD = -1.69, 95% CI (-2.48, −0.90), p < 0.0001, I2 = 96%] and anxiety [HAMA: SMD = -1.29, 95% CI (-1.68, −0.91), p < 0.00001, I2 = 89%; SAS: SMD = -1.75, 95% CI (-2.55, −0.95), p < 0.00001, I2 = 96%] significantly improved in the trial group. Furthermore, the trial group exhibited a significantly lower disease relapse rate [OR = 0.30, 95% CI (0.20, 0.44), p < 0.00001, I2 = 0%]. CHM treatment consistently improved IBS severity (IBS-SSS) and symptom scores. Network pharmacology analysis identified key chemical metabolites in traditional Chinese medicine formulations, including Beta-sitosterol, Stigmasterol, Quercetin, Naringenin, Luteolin, Kaempferol, Nobiletin, Wogonin, Formononetin, and Isorhamnetin. Utilizing the STRING database and Cytoscape v3.9.0 software, a protein-protein interaction (PPI) network revealed the top eight key targets: IL-6, TNF, PPARG, PTGS2, ESR1, NOS3, MAPK8, and AKT1, implicated in anti-inflammatory responses, antioxidant stress modulation, and neurotransmitter homeostasis maintenance.
Conclusion: Chinese Herbal Medicine (CHM) offers a promising and safe treatment approach for patients dealing with Diarrheal Irritable Bowel Syndrome (IBS-D) accompanied by anxiety and depression; thus, indicating its potential for practical implementation. The most active metabolites of CHM could simultaneously act on the pathological targets of IBS-D, anxiety, and depression.The diverse scope of CHM’s therapeutic role includes various aspects and objectives, underscoring its potential for broad utilization.
Abdominal pain or discomfort and alterations in defecation patterns are prevalent symptoms of Irritable Bowel Syndrome (IBS), a common functional gastrointestinal disorder. IBS can be categorized into diarrhea-type, constipation-type, mixed-type, and indeterminate-type based on abnormal defecation patterns (Drossman, 2016). Its etiology is complex, involving visceral hypersensitivity, abnormal gastrointestinal motility, and psychological stress (Ford et al., 2017). Many individuals with IBS-D often encounter psychological symptoms alongside gastrointestinal ones. Growing evidence highlights the strong link between IBS-D and anxiety as well as depression (Chen et al., 2022). The prevalence of anxiety symptoms among IBS patients is estimated at 1.28%, while that of depression symptoms is 8.11% (Zamani et al., 2019). Notably, individuals with IBS-D are three times more likely to experience anxiety or depression compared to healthy individuals (Kurokawa et al., 2018). Anxiety and depressive symptoms may exacerbate gastrointestinal and extra-gastrointestinal symptoms by altering visceral hypersensitivity and the intestinal microenvironment, influencing the microbiota-intestinal-brain axis (Moloney et al., 2016; Ford et al., 2017). Moreover, psychological factors have the potential to disrupt intestinal mucosal integrity, modify gut microbiota composition, impair mucosal barrier function, and modulate immune responses. Taken together, these factors play a collective role in the manifestation of symptoms such as diarrhea in individuals with IBS-D, thereby complicating the management of IBS-D patients. Currently, Western medicine lacks specific medication for treating IBS-D. Clinical management typically involves symptomatic supportive treatment, including antispasmodics, antidiarrheals, antibiotics, anxiolytics, and probiotics, alongside dietary adjustments and psychological interventions.
In modern medicine, IBS-D is classified based on its clinical manifestations. However, in traditional Chinese medicine, it is categorized into broader categories such as “diarrhea,” and “abdominal pain.” Traditional Chinese Medicine (TCM) provides a comprehensive understanding of IBS-D, emphasizing personalized treatment tailored to the individual’s constitution, environment, and specific symptoms. Treatment approaches in TCM are varied, including internal administration of herbal medicine, acupuncture, tuina massage, acupoint patches, and herbal enemas. TCM views IBS-D as primarily affecting the small and large intestines, intricately linked with the liver, spleen, and kidney. Core pathogenesis involves spleen-stomach weakness and liver dysfunction in dispersing and regulating. In clinical practice, liver qi stagnation and spleen deficiency with dampness accumulation are commonly observed syndromes in IBS-D. Herbal medicine treatment adjusts medications based on different syndrome patterns to alleviate symptoms such as diarrhea, abdominal distention, and pain. The mechanism of TCM treatment of IBS-D is multifaceted, including the regulation of intestinal function, neuroendocrine, immune system and other pathways. Through multi-target and multi-mechanism regulation, patients’ diarrhea, abdominal pain, and emotional symptoms can be improved to achieve the purpose of treatment. Most studies suggest that CHM treatments for IBS-D yield better outcomes compared to Western medicine. However, many research reports on CHM treatment of IBS-D have limitations, such as small sample sizes and inconsistencies in clinical efficacy evaluation standards. The clinical efficacy of CHM in managing IBS-D with depression and anxiety requires clarification through influential research. Therefore, this paper comprehensively analyzes published research, conducts meta-analysis and systematic review, and provides a rational, evidence-based medical foundation for exploring the efficacy and mechanisms of CHM in treating IBS-D with depression and anxiety.
Search terms for the databases specified included “irritable bowel syndrome or IBS-D″ and “anxiety or anxiety disorder” and “depression or depressive disorder” and “Chinese medicine or herbal medicine” and “randomized controlled trial,” along with their synonyms. The databases to be searched included China National Knowledge Infrastructure (CNKI), Wanfang Database, VIP Database, Chinese Biomedical Literature Database, PubMed, Embase, and Cochrane Library, up to 10 April 2024. Both Chinese and English will be used for retrieval.
1) All participants met the diagnostic criteria for IBS-D (Longstreth et al., 2006; Mearin et al., 2016) with comorbid anxiety and depression. 2) Each group included no fewer than 30 subjects. 3) The trial group received oral CHM alone or CHM in combination with Western Medicine, while the control group received Western Medicine excluding Chinese medicine (multiple interventions allowed). 4) Assessment of anxiety and depressive symptoms in patients utilized the Hamilton Depression Scale (HAMD) or Self-rating Depression Scale (SDS), as well as the Hamilton Anxiety Scale (HAMA) or Self-rating Anxiety Scale (SAS). 5) Efficacy indicators included ① Severity of irritable bowel syndrome (IBS-SSS) score, ② TCM symptom score, ③ Clinical efficacy, ④ Recurrence rate, ⑤ Adverse effects.
1) Non-clinical investigations, case reports, non-randomized controlled trials, and reviews; 2) patients with unclear diagnostic criteria and methods for assessing effectiveness; 3) investigations where the comparison group received CHM treatment; 4) investigations lacking data on reliable endpoint indicators or having inadequately designed experimental protocols; and 5) interventions incorporating additional traditional Chinese medicine physical therapies (e.g., acupuncture, massage, music, etc.).
Two researchers independently evaluated the gathered literature according to predefined inclusion and exclusion criteria. Information was collected from the selected studies, including the primary author’s name, publication year, sample size, age distribution, gender composition, treatment protocol, treatment duration, form and metabolites of herbal dosage, as well as outcome measures.
The evaluation of bias risk utilized the bias risk assessment tool suggested in the Cochrane 5.1.0 manual for randomized controlled trials, as outlined by (Cumpston et al., 2019). This assessment included six key aspects: random allocation sequence, concealed allocation scheme, blinding, incomplete outcome data, selective outcome reporting, and “other issues” for methodological quality appraisal. Two researchers performed quality assessment independently, cross-checking each other’s evaluations. Any discrepancies were resolved through consultation with a third researcher.
The meta-analysis, conducted using Cochrane Collaboration’s RevMan 5.3 software, involved separate entry of outcome indicators for data processing and analysis. Odds ratio (OR) and standard mean difference (SMD) were utilized to evaluate combined effects for dichotomous outcomes and continuous variables, respectively. Heterogeneity was assessed using the chi-squared test, with I2 indicating the degree of heterogeneity. For studies with low heterogeneity (I2 < 50%), a fixed-effects model was employed; whereas for those with significant heterogeneity (I2 ≥ 50%), subgroup analyses were conducted to explore potential sources. If heterogeneity persisted, a random-effects model was applied for effect size combination, with subgroup analysis based on TCM evidence type, interventions, and intervention time. Publication bias was examined using a funnel plot subsequent to presenting results via a forest plot in the meta-analysis. A significance level of p < 0.05 was used to determine statistical significance.
The compositions of the formulations and patented drugs are detailed in Supplementary Table S2. The frequency analysis of each CHM is shown in Supplementary Table S3. Network pharmacology analysis was performed on CHMs with a frequency of at least five to identify the main active metabolites and disease targets.
Active metabolites from Chinese herbal medicine were gathered using the Traditional Chinese Medicine Systems Pharmacology Database (TCMSP) analysis platform, adhering to criteria of oral bioavailability (OB) > 30% and drug-likeness (DL) ≥ 0.18. Subsequently, the corresponding targets of these active metabolites were assembled and refined utilizing UniProt data, excluding non-human genes and redundant or ineffective targets.
Keyword searches for “irritable bowel syndrome,” “depression,” and “anxiety” were performed using the GeneCards database and DisGeNET to retrieve pertinent targets linked with these conditions. Next, all identified targets from these databases were consolidated in Excel, eliminating duplicate genes. The gathered information was then cross-referenced and refined utilizing the UniProt database to ensure accurate gene information for disease targets.
The acquired targets of drug metabolites were mapped against disease targets, and then a Venn diagram was generated to obtain the intersecting genes. These intersection targets were inputted into Cytoscape software (version 3.9.0) to construct the herb-metabolite-target network. Additionally, the primary potential mechanisms of action of the top 10 main agents were summarized from the PubMed database.
The TCM-disease targets were inputted into the STRING online software, with Homo sapiens selected as the species for filtering conditions. This process facilitated the construction of a protein-protein interaction network (PPI network) for drug-disease interactions. A minimum interaction score of 0.4 was set. The degree of each node in the network indicates the protein’s significance in interactions, with a higher number of connections reflecting greater importance within the PPI network.
A total of 1,475 relevant original studies were retrieved from eight databases, including the China National Knowledge Internet (150), VIP (225), Wan Fang (269), SinoMed (757), PubMed (1 study), Web of Science (16), Cochrane Library (43), and Embase (14). A total of 549 duplicates were eliminated. After excluding 843 articles by screening the title and abstract, 83 articles remained for full-text analysis. In total, 58 papers were rejected, and 25 items were included in this study (Figure 1).
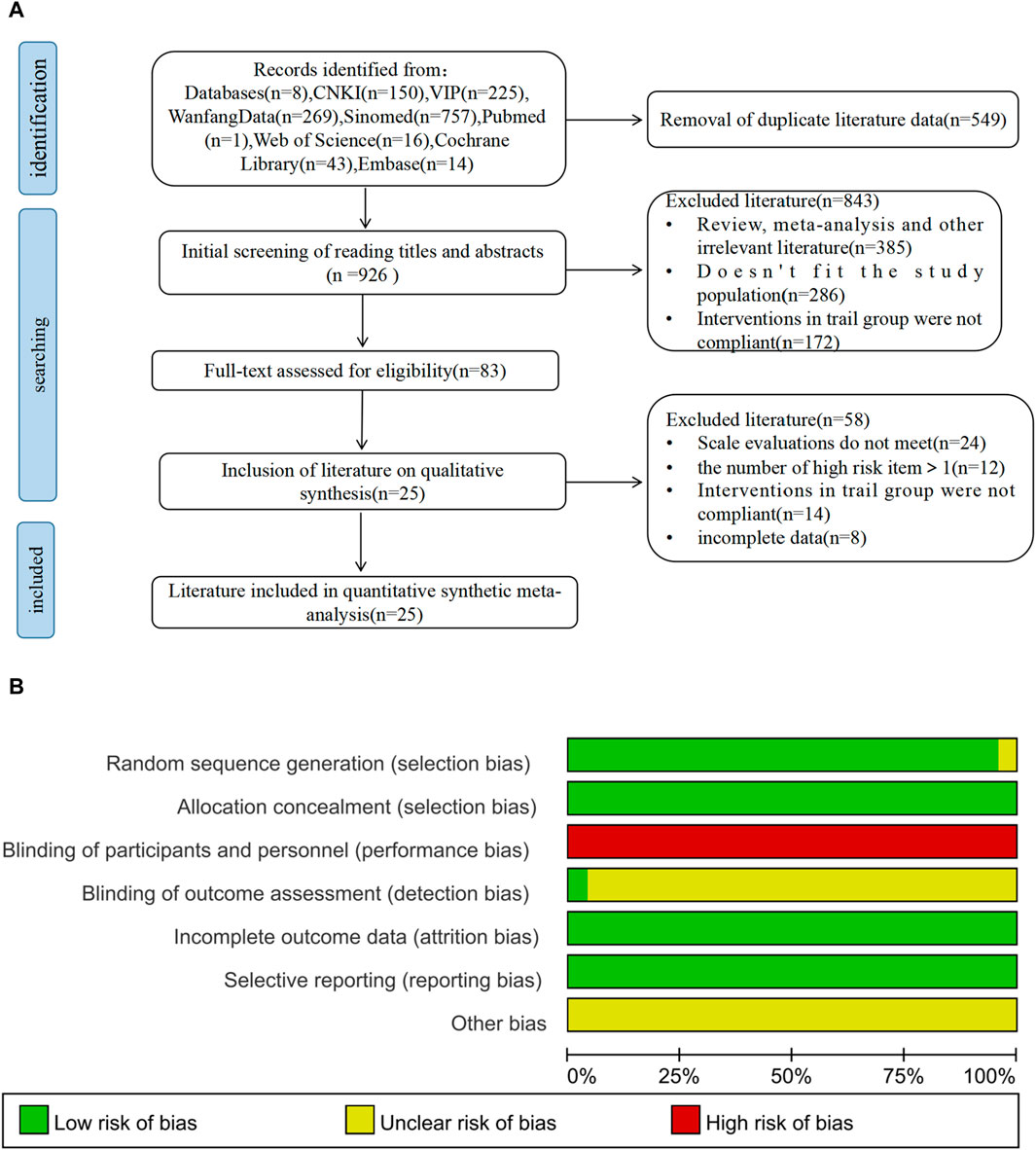
Figure 1. PRISMA flow diagram and risk-of-bias assessment; (A) literature screening process; (B) risk-of-bias summary.
The selected RCTs included 2055 people, 1,028 of whom were in the trial groups and 1,027 of whom were in the control groups. The baseline information for the trial and control groups was similar. The treatment course in all studies varied from 2 weeks to 3 months. The main characteristics of the included studies are summarized in Table 1.
Among the 25 studies, 15 studies (Fu and Xu, 2013; Zhu, 2013; Guo, 2019; Majing, 2019; Wu, 2019; Cai et al., 2020; Liu, 2020; Feng, 2021; He et al., 2021; Li, 2021; Yang, 2021; Liu, 2022; Su and Zhang, 2022; Zhang, 2023; Chen et al., 2024) used the HAMD and HAMA scales to assess anxiety and depressive conditions, while 10 studies (Nie et al., 2014; Zhu et al., 2015; Zhang, 2016; Xu, 2017; Sun et al., 2020; Zhou and Chu, 2020; Ding et al., 2021; Lu and Wang, 2021; Mou, 2021; Gu and Xu, 2022) employed the SDS and SAS scales for the same purpose. Among the eight studies (Zhu et al., 2015; Guo, 2019; Cai et al., 2020; Ding et al., 2021; Lu and Wang, 2021; Mou, 2021; Liu, 2022; Zhang, 2023), the trial group received a combination of Western medicine and CHM treatment. In contrast, in 17 studies (Fu and Xu, 2013; Zhu, 2013; Nie et al., 2014; Zhang, 2016; Xu, 2017; Majing, 2019; Wu, 2019; Liu, 2020; Sun et al., 2020; Zhou and Chu, 2020; Feng, 2021; He et al., 2021; Li, 2021; Yang, 2021; Gu and Xu, 2022; Su and Zhang, 2022; Chen et al., 2024), the trial group was treated solely with CHM. All control groups received conventional Western medicine treatment.
All studies incorporated in this analysis employed random assignment. Among them, 24 studies utilized either the random number table method or computerized randomization grouping, while one study (Zhou and Chu, 2020) did not provide specific details regarding the process of randomization grouping. Two studies (Li, 2021; Chen et al., 2024) implemented allocation concealment, whereas 23 studies did not clearly state its use. Complete data and reliable results were accessible for all included articles. No disparities were observed in the baseline data of these studies. The outcomes of the risk of bias assessment are depicted in Figure 1.
In the majority of studies, significant improvements were observed in HAMA, HAMD, SDS, SAS, IBS-SSS, and TCM symptom scores within the trial group. Consequently, the primary outcome indicators were consolidated to elucidate the efficacy of CHM in treating IBS-D with anxiety and depression.
There is a pressing need for more standardized efficacy assessment criteria in treating IBS-D. The included studies conducted this investigation to establish their judgment criteria for determining treatment effectiveness. They categorized effective indicators such as cure, apparent effect, and effectiveness, while ineffective indicators were classified accordingly. A total of 21 studies (Fu and Xu, 2013; Zhu, 2013; Nie et al., 2014; Zhu et al., 2015; Zhang, 2016; Guo, 2019; Majing, 2019; Wu, 2019; Cai et al., 2020; Liu, 2020; Sun et al., 2020; Zhou and Chu, 2020; Feng, 2021; He et al., 2021; Li, 2021; Lu and Wang, 2021; Liu, 2022; Su and Zhang, 2022; Gu and Xu, 2022; Zhang et al., 2023; Chen et al., 2024) reported clinical effectiveness rates. The heterogeneity test results (p = 1.00, I2 = 0%) indicated no statistical heterogeneity among the studies; thus, a fixed-effects model was utilized for the combined analysis. The difference was statistically significant in the test of combined statistics (Z = 9.32, p < 0.00001, OR = 4.01, 95% CI [2.99,5.36], I2 = 0%), suggesting that the trial group exhibited a higher total effective rate of clinical improvement in IBS-D compared to the control group. These results are illustrated in Figure 2.
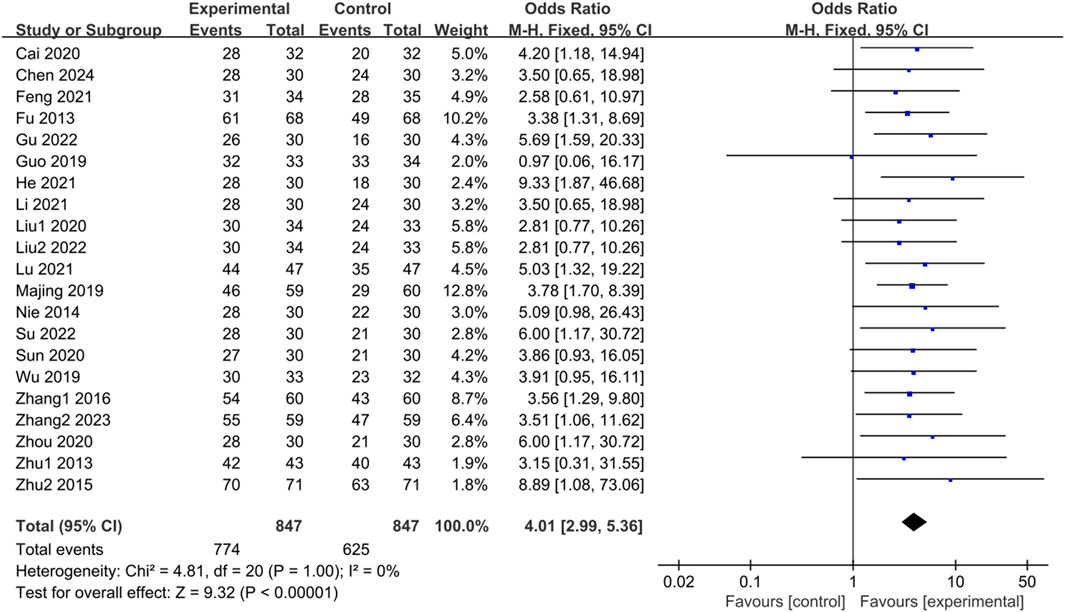
Figure 2. Forest plot comparing the clinical effectiveness of the trial group and the control group after treatment.
Fifteen studies (Fu and Xu, 2013; Zhu, 2013; Guo, 2019; Majing, 2019; Wu, 2019; Cai et al., 2020; Liu, 2020; Feng, 2021; He et al., 2021; Li, 2021; Yang, 2021; Liu, 2022; Su and Zhang, 2022; Zhang, 2023; Chen et al., 2024) examined HAMD scores of IBS-D patients treated with CHM. Initially, a test for heterogeneity was conducted, revealing significant diversity among the studies (p < 0.0001, I2 = 68%). Consequently, a random-effects model was applied for the combined analysis. The difference was statistically significant in the combined statistic test (Z = 9.60, p < 0.00001). Meta-analysis results indicated that HAMD scores of the trial group were lower than those of the control group (SMD = -1.08, 95% CI [-1.30, −0.86], p < 0.00001). Subgroup analyses were carried out to investigate the source of heterogeneity based on intervention time, TCM syndrome of included patients, and interventions (Supplementary Table S4). Subgroup analysis by intervention time revealed: for interventions ≤ 4 weeks, SMD = -0.98, 95% CI [-1.16, −0.80], p < 0.00001, I2 = 7%; for interventions > 4 weeks, SMD = -1.21, 95% CI [-1.67, −0.74], p < 0.00001, I2 = 85%. These findings demonstrated significant improvement in reducing HAMD scores with herbal treatment in the trial group compared to the control group, with statistically significant differences. The results are presented in Supplementary Table S4; Figure 3.
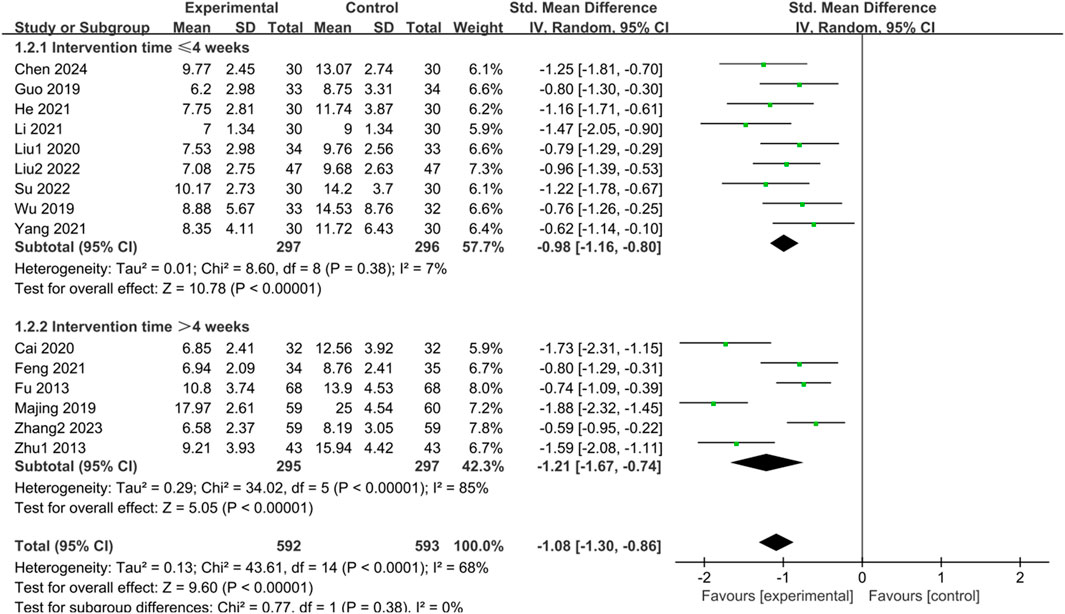
Figure 3. Forest plot comparing the HAMD scores of the trial group and the control group after treatment.
Fifteen studies (Fu and Xu, 2013; Zhu, 2013; Guo, 2019; Majing, 2019; Wu, 2019; Cai et al., 2020; Liu, 2020; Feng, 2021; He et al., 2021; Li, 2021; Yang, 2021; Liu, 2022; Su and Zhang, 2022; Zhang, 2023; Chen et al., 2024) reported HAMA scores of IBS-D patients treated with CHM. Initially, a heterogeneity test was conducted, revealing significant heterogeneity among the studies (p < 0.00001, I2 = 89%). Therefore, a random-effects model was employed for the combined analysis. The difference was statistically significant in the combined statistic test (Z = 6.60, p < 0.00001). Subgroup analyses of intervention time, TCM syndrome of included patients, and interventions were conducted to explore the source of heterogeneity, but none of them were found to be significant sources (Supplementary Table S5). A sensitivity analysis was performed to investigate the source of heterogeneity further, and excluding individual studies did not reduce heterogeneity. Meta-analysis results indicated that the HAMA scores of the trial group were lower than those of the control group (SMD = -1.29, 95% CI [-1.68, −0.91], p < 0.00001). This suggests that CHM treatment significantly reduced HAMA scores in the trial group compared with the control group, with a statistically significant difference. The results are presented in Supplementary Table S5; Figure 4.
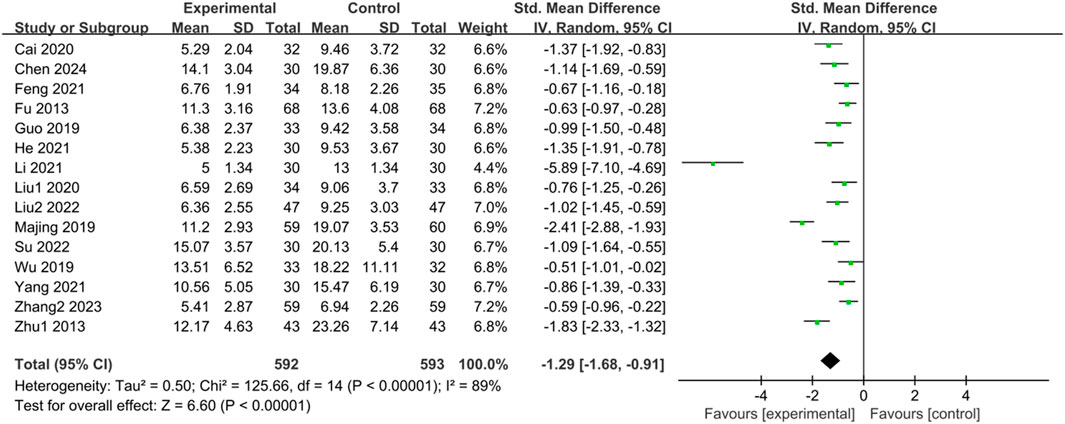
Figure 4. Forest plot comparing HAMA scores of the trial group and the control group after treatment.
Ten studies (Nie et al., 2014; Zhu et al., 2015; Zhang, 2016; Xu, 2017; Sun et al., 2020; Zhou and Chu, 2020; Ding et al., 2021; Lu and Wang, 2021; Mou, 2021; Gu and Xu, 2022) reported SDS scores of IBS-D patients treated with CHM. Initially, a heterogeneity test was conducted, revealing significant heterogeneity among the studies (p < 0.0001, I2 = 96%). Consequently, a random-effects model was applied for the combined analysis, with a statistically significant difference observed in the combined statistic test (Z = 4.20, p < 0.00001). Meta-analysis results showed that SDS scores of the trial group were lower than those of the control group (SMD = -1.69, 95% CI [-2.48, −0.90], p < 0.0001). Subgroup analyses were conducted to explore the source of heterogeneity based on intervention time, TCM syndrome of included patients, and interventions (Supplementary Table S6). Subgroup analysis by TCM syndrome revealed: for liver depression and spleen deficiency, SMD = -0.58, 95% CI [-0.76, −0.40], p < 0.00001, I2 = 12%; for other syndrome types, SMD = -4.01, 95% CI [-6.46, −1.56], p < 0.00001, I2 = 98%. Subgroup analyses based on interventions indicated: for CHM vs. Western medicine, SMD = -0.51, 95% CI [-0.71, −0.32], p < 0.00001, I2 = 30%; for CHM + Western medicine vs. Western medicine, SMD = -4.03, 95% CI [-6.17, −1.89], p = 0.0002, I2 = 98%. The results of the meta-analysis demonstrated that SDS scores of the trial group were lower than those of the control group, indicating that CHM treatment reduced SDS scores in the trial group compared with the control group, with statistically significant differences. The results are presented in Supplementary Table S6; Figure 5.
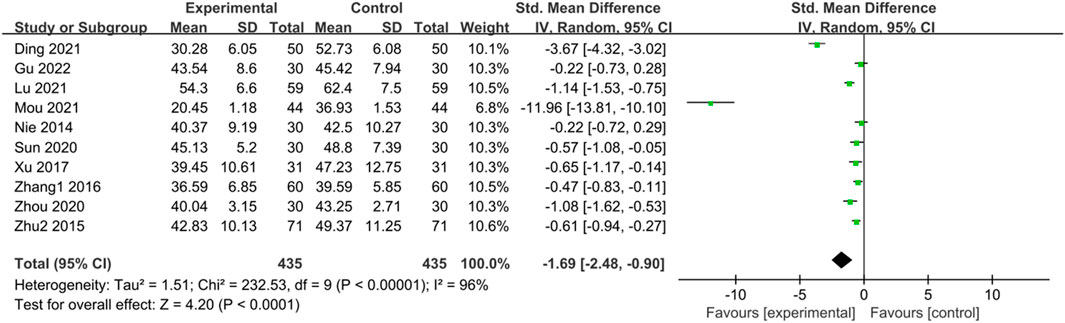
Figure 5. Forest plot comparing the SDS scores of the trial group and the control group after treatment.
Ten studies (Nie et al., 2014; Zhu et al., 2015; Zhang, 2016; Xu, 2017; Sun et al., 2020; Zhou and Chu, 2020; Ding et al., 2021; Lu and Wang, 2021; Mou, 2021; Gu and Xu, 2022) reported SAS scores of IBS-D patients treated with CHM. Initially, a heterogeneity test was conducted, revealing significant statistical heterogeneity among the studies (p < 0.00001, I2 = 96%). Therefore, a random-effects model was applied for the combined analysis, with the combined statistic test yielding a statistically significant difference (Z = 4.28, p < 0.0001). Subgroup analyses of intervention time, evidence type of the included patients, and intervention were conducted to explore the source of heterogeneity, but none of them were found to be significant sources (Supplementary Table S7). A sensitivity analysis was performed to investigate the source of heterogeneity further, but no reduction in heterogeneity was observed after individual studies were excluded. Meta-analysis results indicated that the SAS scores of the trial group were lower than those of the control group (SMD = -1.75, 95% CI [-2.55, −0.95], p < 0.00001). This suggests that CHM treatment exhibited significant advantages in reducing SAS scores in the trial group compared with the control group, with a statistically significant difference. The results are presented in Supplementary Table S7; Figure 6.
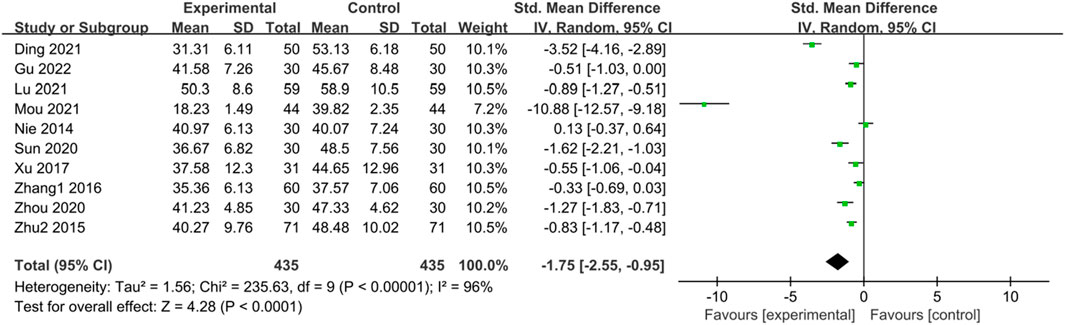
Figure 6. Forest plot comparing SAS scores of the trial group and the control group after treatment.
Eleven studies (Fu and Xu, 2013; Xu, 2017; Guo, 2019; Wu, 2019; Liu, 2020; Ding et al., 2021; He et al., 2021; Yang, 2021; Liu, 2022; Su and Zhang, 2022; Chen et al., 2024) reported the IBS-SSS score of IBS-D patients treated with CHM. Initially, a heterogeneity test was conducted, revealing significant statistical heterogeneity among the studies (p < 0.00001, I2 = 82%). Therefore, a random-effects model was employed for the combined analysis, with the combined statistic test showing a statistically significant difference (Z = 6.70, p < 0.00001). Subgroup analyses of intervention time, evidence type of the included patients, and intervention were conducted to explore the source of heterogeneity, but none of them were identified as significant sources (Supplementary Table S8). Sensitivity analysis was performed to investigate the source of heterogeneity further, but heterogeneity could not be reduced after individual studies were excluded one by one. Meta-analysis results indicated that IBS-SSS scores of the trial group were lower than those of the control group (SMD = -1.24, 95% CI [-1.60, −0.88], p < 0.00001), suggesting that CHM treatment had a significant advantage in reducing IBS-SSS scores in the trial group compared with the control group, with a statistically significant difference. The results are presented in Supplementary Table S8; Figure 7.
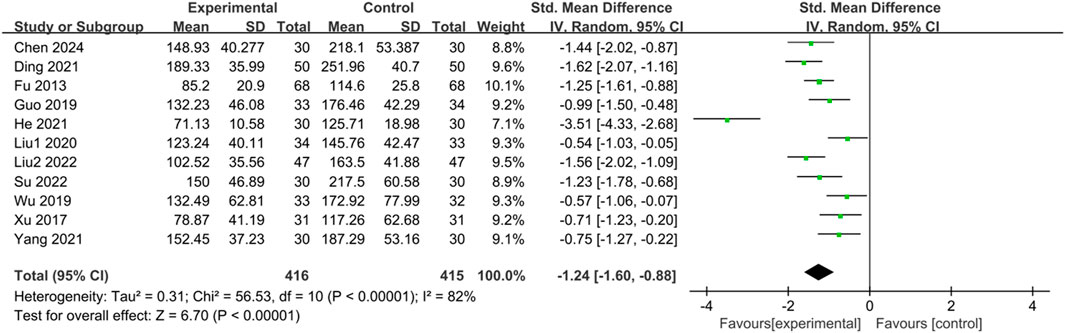
Figure 7. Forest plot comparing the IBS-SSS scores of the trial group and the control group after treatment.
Seventeen studies (Nie et al., 2014; Xu, 2017; Guo, 2019; Majing, 2019; Wu, 2019; Liu, 2020; Zhou and Chu, 2020; Ding et al., 2021; Feng, 2021; He et al., 2021; Li, 2021; Mou, 2021; Gu and Xu, 2022; Liu, 2022; Su and Zhang, 2022; Zhang, 2023; Chen et al., 2024) reported the TCM symptom scores of IBS-D patients treated with CHM. Due to scoring bias across different studies, significant heterogeneity was observed in the TCM symptom scores. The results of the heterogeneity test (p < 0.00001, I2 = 95%) indicated statistically significant heterogeneity among the studies. Therefore, a random-effects model was utilized for the merged analysis, with the combined statistic test showing a statistically significant difference (Z = 6.38, p < 0.00001). Subgroup analyses of intervention time, evidence type of included patients, and intervention were conducted to explore the source of heterogeneity, but none of them were identified as significant sources (Supplementary Table S9). Sensitivity analysis was employed to further investigate the source of heterogeneity, but heterogeneity could not be reduced after excluding individual studies one by one. Meta-analysis results revealed that TCM symptom scores in the trial group were lower than those in the control group (SMD = -1.90, 95% CI [-2.48, −1.31], p < 0.00001), indicating that treatment in the trial group had a significant advantage in reducing TCM symptom scores compared with the control group, with a statistically significant difference. The results are presented in Supplementary Table S9; Figure 8.
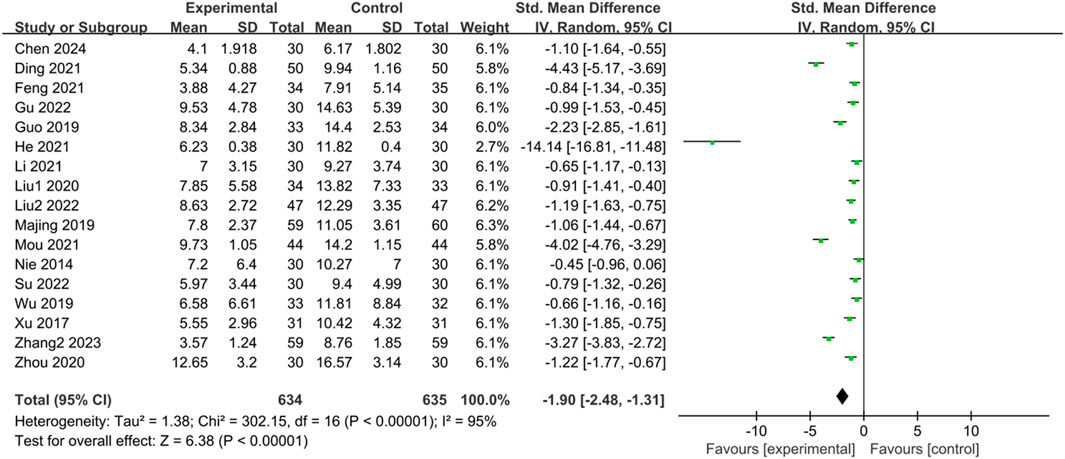
Figure 8. Forest plot comparing TCM symptom scores of the trial group and the control group after treatment.
Ten studies (Fu and Xu, 2013; Guo, 2019; Majing, 2019; Wu, 2019; Sun et al., 2020; Zhou and Chu, 2020; Ding et al., 2021; Feng, 2021; Li, 2021; Gu and Xu, 2022) reported recurrence rates, and the heterogeneity test results (p = 0.64, I2 = 0%), which indicated no statistical heterogeneity among the studies, were combined and analyzed using a fixed-effects model. The results showed that the difference between the trial group and the control group was statistically significant and that CHM treatment could significantly reduce the recurrence rate of IBS-D (Z = 6.11, p < 0.00001, OR = 0.30, 95% CI [0.20, 0.44], I² = 0%). The results are shown in Figure 9.
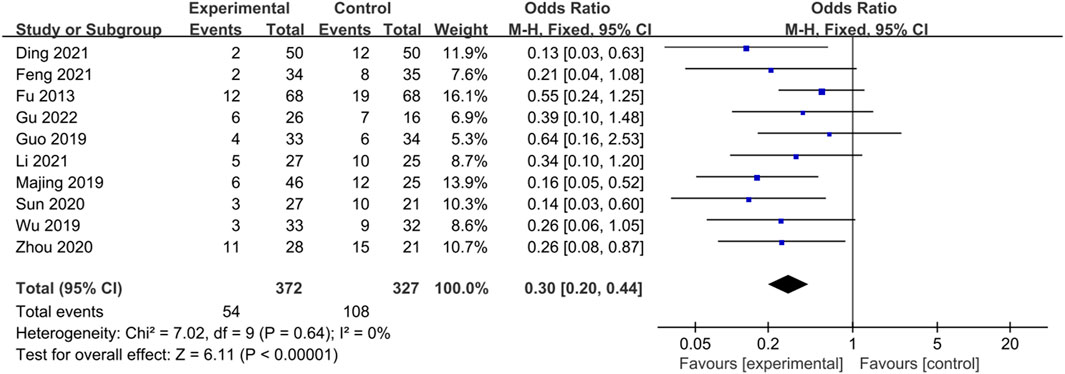
Figure 9. Forest plot comparing the recurrence rates of the trial group and the control group after treatment.
Fifteen trials (Zhang, 2016; Xu, 2017; Guo, 2019; Majing, 2019; Wu, 2019; Liu, 2020; Sun et al., 2020; Zhou and Chu, 2020; Ding et al., 2021; Feng, 2021; He et al., 2021; Lu and Wang, 2021; Yang, 2021; Su and Zhang, 2022; Chen et al., 2024) assessed adverse occurrences in the 25 included studies. Among them, eleven trials reported no significant adverse reactions. However, four trials (Guo, 2019; Zhou and Chu, 2020; Ding et al., 2021; Lu and Wang, 2021) documented adverse reactions, which included neurological symptoms such as headache, gastrointestinal symptoms such as dry mouth, nausea, vomiting, and constipation, and dermatologic symptoms such as skin rash. The most common adverse reactions are summarized in Table 2.
Publication bias was assessed by generating a funnel plot with OR values on the horizontal axis and the standard error (SE) of LogOR on the vertical axis for the primary outcome indicator of this study, which is clinical effectiveness. The funnel plot revealed some asymmetry between the left and right sides, suggesting the presence of publication bias that could influence the combined effect size to some degree. This bias may be attributed to factors such as inconsistent study evaluation, low quality, and small sample size. The results are depicted in Figure 10.
Given the predominance of literature indicating the effectiveness of CHM in treating IBS-D, statistical analysis was conducted to ascertain the frequency of CHM usage and identify commonly employed medications across various groups. CHM metabolites with a frequency of occurrence equal to or greater than five were selected as the primary active metabolites (Supplementary Table S3). The results revealed that Atractylodes macrocephala Koidz., [Asteraceae; Atractylodis macrocephalae rhizoma], Paeonia lactiflora Pall., [Paeoniaceae; Paeoniae radix alba], Citrus × aurantium L., [Rutaceae; Citri reticulatae pericarpium], Glycyrrhiza uralensis Fisch. ex DC., [Fabaceae; Glycyrrhizae radix et rhizoma praeparata cum melle], Bupleurum chinensis DC., [Apiaceae; Bupleuri Radix], Poria cocos (Schw.)Wolf., [Polyporaceae; Poria], Saposhnikovia divaricata (Turcz. ex Ledeb.) Schischk., [Apiaceae; Radix saposhnikoviae], Dolomiaea costus (Falc.)., [Asteraceae; aucklandiae radix], Codonopsis pilosula (Franch.) Nannf., [Campanulaceae; Codonopsis radix], Dioscorea oppositifolia L., [Dioscoreaceae; Dioscoreae rhizoma], Zingiber officinale Roscoe., [Zingiberaceae; Zingiberis rhizoma recens], Atractylodes lancea (Thunb.) DC., [Asteraceae; Atractylodis rhizoma], Prunus mume (Siebold) Siebold & Zucc., [Rosaceae; Fructus mume], Cyperus rotundus L., [Cyperaceae; Cyperi rhizoma], Coptis chinensis Franch., [Ranunculaceae; Coptidis rhizoma] are commonly used to treat IBS-D patients with anxiety and depression. After screening, 194 active metabolites of CHM were obtained, along with 294 unique targets. Subsequently, the gene names of the screened targets were converted into gene symbols using the UniProt database.
Utilizing the GeneCards and DisGeNet disease databases, we conducted a screening for target information associated with IBS-D, anxiety, and depression. The target information from these different diseases was intersected. Subsequently, intersection processing was carried out between the target genes corresponding to the ultimately obtained effective active metabolites of CHM and the disease targets. Consequently, 115 common genes were identified as crucial targets for Traditional Chinese Medicine in treating IBS-D along with anxiety and depression.
We generated a network diagram of CHM, active metabolites, and targets using Cytoscape 3.9.0. According to topological analysis, active metabolites like beta-sitosterol, stigmasterol, quercetin, kaempferol, luteolin, naringenin, isorhamnetin, nobiletin, wogonin, and formononetin could target factors linked to IBS-D, anxiety, and depression concurrently. Among spleen-tonifying medications, key chemical metabolites include quercetin, kaempferol, luteolin, wogonin, and formononetin. Meanwhile, liver-soothing medications mainly contain beta-sitosterol, stigmasterol, naringenin, isorhamnetin, and nobiletin.
We utilized a combined_score threshold of 0.4 in the String database to generate a network diagram of target interactions. Following this, the genes underwent network topology analysis using Cytoscape 3.9.0. According to the topological analysis, genes such as IL-6, TNF, PPARG, PTGS2, ESR1, NOS3, MAPK8, and AKT1 are identified as potential targets for CHM in the treatment of IBS-D along with anxiety and depression.
The actions and mechanisms of the top 10 major active metabolites, as presented in Table 3, were investigated by searching the PubMed database.
The PubMed database was queried to elucidate the mechanism of action of the primary active metabolites (top 10). As indicated in Table 3, beta-sitosterol, quercetin, and luteolin were investigated in studies related to IBS-D, anxiety, and depression. Additionally, studies focusing on anxiety and depression have explored stigmasterol, naringenin, nobiletin, wogonin, and kaempferol. Commonly utilized animal models for IBS-D include the maternally separated (MS) IBS-D rat model, the 2,4,6-trinitrobenzene sulfonic acid (TNBS)-induced post-inflammatory IBS-D rat model, and models induced by water avoidance stress (WAS). Furthermore, animal models commonly used for depression and anxiety include multiple-stress mice, LPS/CORT mice, and Danio rerio models.
Table 3 illustrates the mechanisms attributed to these ten active metabolites concerning IBS-D, anxiety, and depression. These metabolites effectively address visceral sensitivity, normalize gastrointestinal dynamics, diminish inflammatory responses, and regulate mood. They exhibit anti-inflammatory and antioxidative stress effects while maintaining neurotransmitter balance. IBS-D is currently associated with various mechanisms like gut microbiota, visceral hypersensitivity, low-grade inflammation, and brain-gut axis interactions. Beta-sitosterol, quercetin, and luteolin alleviate intestinal symptoms by targeting visceral hypersensitivity, gastrointestinal infections, inflammation, and psychosocial aspects. They regulate sensory nerve pathways and neurotransmitter signaling, reducing gastrointestinal discomfort such as abdominal pain. Additionally, their anti-inflammatory properties alleviate chronic gastrointestinal inflammation, easing symptoms like diarrhea and rectal bleeding. Moreover, these metabolites modulate neuroendocrine and neuroimmune pathways linked to stress response, anxiety, and depression, thereby improving psychosocial wellbeing and gastrointestinal health overall.
With the exception of stigmasterol, wogonin, and formononetin, the other active metabolites exhibit potent anti-inflammatory and antioxidant properties. These metabolites inhibit the release of inflammatory mediators, diminish inflammatory cell activity, and improve immune system function. They play a crucial role in mitigating oxidative stress, a contributing factor to various diseases. Kaempferol, abundant in plant-based foods, demonstrates robust anti-inflammatory effects by inhibiting pro-inflammatory cytokines and scavenging free radicals, thereby safeguarding against tissue damage induced by oxidative stress. Naringenin scavenges free radicals and reactive oxygen species, shielding cells from oxidative harm and modulating inflammatory signaling pathways. Isorhamnetin suppresses NF-κB activation and dampens inflammatory gene expression while possessing antioxidant properties that protect against cellular damage induced by oxidative stress, particularly in gastrointestinal and neuronal tissues affected by psychological stressors. Nobiletin, present in citrus CHM, alleviates gastrointestinal inflammation, restores intestinal barrier integrity, and mitigates stress-induced neuroinflammation and mood disorders by modulating inflammatory pathways and oxidative stress responses.
The imbalance of adrenocorticotropic hormone, serotonin, dopamine, and tryptophan contributes to neurochemical disturbances seen in anxiety and depression. Beta-sitosterol, stigmasterol, and quercetin play a role in modulating these neurotransmitter systems, showing promise in managing anxiety and depression. Isorhamnetin influences synaptic protein expression, impacting synaptic communication. Stigmasterol, quercetin, luteolin, kaempferol, naringenin, nobiletin, and formononetin regulate neurogenesis and brain-derived neurotrophic factor (BDNF) expression, supporting neuronal growth, synaptic plasticity, and mood stability. Quercetin and naringenin alleviate depression and anxiety by addressing mitochondrial dysfunction, restoring mitochondrial function, and reducing oxidative stress. The therapeutic mechanisms of CHM metabolites in IBS-D with concurrent anxiety and depression involve complex signaling pathways, including NLRP3 inflammatory vesicle, cAMP/PKA, BDNF-TrkB, PI3K/AKT/NF-κB, MEK/ERK, and FoxG1/CREB/BDNF pathways. Understanding these intricate signaling networks could inform the development of new therapeutic strategies for managing gastrointestinal disorders and associated neuropsychiatric symptoms.
There is increasing evidence indicating a high prevalence of depression and anxiety among patients with IBS-D. Current medical approaches for treating IBS-D include pharmacological symptomatic treatments, dietary adjustments, and psychotherapies. Chinese medicine has emerged as a significant modality in managing IBS-D with comorbid anxiety and depression, offering enhanced efficacy and fewer side effects compared to conventional treatments. Several clinical studies have highlighted its precise efficacy and safety. However, further validation of CHM’s effectiveness in treating IBS-D with anxiety and depression is needed, particularly due to concerns regarding methodological quality.
This study conducted a comprehensive review of domestic and international research on treating IBS-D with depression and anxiety using CHM. The analysis included 25 studies, comprising 22 CHM metabolite prescriptions and involving 2055 patients. The findings revealed that the CHM-based trial group exhibited superior efficacy compared to the control group (p < 0.05). Additionally, the trial group showed significant improvements in depression scale scores (HAMD and SDS) and anxiety scale scores (HAMA and SAS) compared to the control group (p < 0.05), indicating the potential of CHM treatment to ameliorate mood disorders in IBS-D patients. Moreover, CHM treatment demonstrated advantages in alleviating clinical gastrointestinal discomfort, accompanying symptoms (evaluated by IBS-SSS and total TCM symptom scores), and improving patients’ quality of life (p < 0.05). The recurrence rates in the trial group were lower (14.52%) compared to the control group (33.03%), suggesting a reduced recurrence risk of irritable bowel syndrome with depression and anxiety following CHM intervention. In conclusion, CHM exhibits clinical effectiveness in managing IBS-D with depression and anxiety without increasing the risk of adverse effects.
The gastrointestinal tract operates under a complex network involving central, autonomic, and enteric nervous systems, making it susceptible to influences from adverse emotions and psychological factors (Aburto and Cryan, 2024). This disruption in the brain-gut axis can lead to gastrointestinal dysfunction due to imbalances between the hypothalamus and limbic system, as well as reduced vagal nerve excitability. The mechanism of TCM in the management of IBS-D involves various aspects. Firstly, Chinese herbs alleviate patients’ symptoms by regulating intestinal function, modulating intestinal motility and peristalsis, possibly affecting the smooth muscle of the intestines to promote the normalization of peristalsis. Secondly, TCM can rebalance the yin and yang imbalance in the body by regulating the neuroendocrine system. Through the microbiota-intestinal-brain axis, CHM can ameliorate patients’ abdominal discomfort and psychological symptoms. Additionally, certain CHM enhance the function of the digestive system, promoting the absorption of nutrients, thereby alleviating symptoms in patients with IBS-D. Finally, several herbal medicines possess anti-inflammatory and antioxidant properties, aiding in reducing inflammation reactions in the intestinal mucosa, improving the intestinal environment, and thereby alleviating symptoms. CHM offers a promising approach to managing IBS-D alongside depression and anxiety by employing a multifaceted, multitarget strategy. To investigate the utilization of CHM further, this study analyzed commonly used herbal medications using network pharmacology. The findings revealed several frequently used herbs, including A. macrocephala Koidz., [Asteraceae; Atractylodis macrocephalae rhizoma], P. lactiflora Pall., [Paeoniaceae; Paeoniae radix alba], G. uralensis Fisch. ex DC., [Fabaceae; Glycyrrhizae radix et rhizoma praeparata cum melle], Citrus × aurantium L., [Rutaceae; Citri reticulatae pericarpium], P. cocos (Schw.)Wolf., [Polyporaceae; Poria], Bupleurum chinense DC., [Apiaceae; Bupleuri radix], D. oppositifolia L., [Dioscoreaceae; Dioscoreae rhizoma], C. pilosula (Franch.) Nannf., [Campanulaceae; Codonopsis radix], D. costus (Falc.) Kasana & A.K.Pandey., [Asteraceae; aucklandiae radix],A. lancea (Thunb.) DC., [Asteraceae; Atractylodis rhizoma], C. rotundus L., [Cyperaceae; Cyperi rhizoma]. These herbs contain active metabolites that, according to network pharmacology, can concurrently target disease-related pathways associated with IBS-D, anxiety, and depression. Notable among these active metabolites are beta-sitosterol, stigmasterol, quercetin, naringenin, luteolin, kaempferol, nobiletin, wogonin, formononetin, and isorhamnetin. These metabolites exhibit potential therapeutic effects on the interconnected targets of IBS-D, anxiety, and depression, underscoring the holistic approach of CHM in addressing these complex conditions.
Herbal metabolites like beta-sitosterol, quercetin, and luteolin are pivotal in addressing the intricate relationship between IBS-D and concurrent depression and anxiety. On the other hand, stigmasterol, naringenin, kaempferol, nobiletin, and wogonin target depression and anxiety symptoms. Additionally, formononetin and isorhamnetin play essential roles in treating depression. Beta-sitosterol, a phytosterol, exhibits anti-inflammatory and immunomodulatory effects. Quercetin, known for its anti-inflammatory, antioxidant, and anticancer properties, positively influences immune function by activating AMP-activated protein kinase (AMPK) (Chiang et al., 2023). Luteolin regulates the Nrf2 signaling pathway, protecting against excessive intestinal motility and diarrhea (Xia et al., 2024). Naringenin enhances cell survival by reducing apoptosis rates induced by CORT (Zhang et al., 2023). Stigmasterol demonstrates anti-inflammatory, antioxidant, and neuroprotective characteristics, potentially alleviating depression and anxiety symptoms by maintaining neurotransmitter balance. Kaempferol exhibits anti-ulcerative colitis effects, suggesting promising therapeutic mechanisms (Qu et al., 2021). Nobiletin inhibits pro-inflammatory cytokines and enzymes like COX-2 and iNOS, scavenges free radicals, and reduces oxidative stress. Wogonin suppresses NF-κB activation and the production of inflammatory mediators such as TNF-α and IL-6. Formononetin displays antioxidant effects by mitigating neuronal damage and promoting neurogenesis (Zhang et al., 2022). Isorhamnetin possesses antioxidant, anti-inflammatory, and neuroprotective attributes, hindering the production of inflammatory cytokines and mediators, reducing oxidative stress, and enhancing neuronal survival and synaptic plasticity. Inflammation significantly contributes to the pathophysiology of IBS-D and its associated psychiatric comorbidities, exacerbating symptoms and fostering mood disorders like depression and anxiety. The bidirectional communication of the gut-brain axis is pivotal, wherein gut-derived inflammatory signals influence central nervous system function and mood regulation. CHM metabolites exhibit anti-inflammatory and neurological protection effects by modulating signaling molecules and oxidative stress, alongside antioxidant properties by neutralizing free radicals and curtailing cellular damage in order to improve diarrhea, abdominal discomfort, and mood in patients.
Based on the intersection of metabolite-disease targets, these CHM can target multiple receptors, including PPARG, PTGS2, ESR1, NOS3, MAPK8,1L-6, TNF, and AKT1, to elicit synergistic treatment of diseases effects. PPARG, expressed in various tissues, regulates lipid catabolism and exhibits anti-inflammatory effects when activated, potentially ameliorating colitis symptoms. PTGS2, encoding a crucial cellular protein, modulates anti-inflammatory responses and immune regulation; thus, impacting mood regulation. AKT1, a ubiquitous intracellular kinase, regulates cell metabolism, survival, and proliferation. Phosphorylated AKT1 can activate NLRP3 inflammatory vesicles, contributing to inflammation in colitis-related diseases (Guo et al., 2014). TNF enhances chemokine and cytokine production, amplifying the inflammatory cascade and organ damage, while IL-6, a key cytokine in inflammation induction and maintenance, may contribute to systemic inflammatory responses and mood dysregulation (Ridker, 2016). ESR1, functioning as a transcription factor, regulates gene expression, affecting processes like cell proliferation, differentiation, and apoptosis. NO, a signaling molecule involved in neurotransmission and immune response, plays a crucial role in inflammation, with NOS3-derived NO potentially impacting mood regulation through its involvement in inflammatory processes. MAPK8 responds to various extracellular stimuli, including stress and cytokines, regulating gene expression implicated in inflammation and neuronal plasticity, thereby modulating depression and anxiety.
In summary, the effectiveness of CHM in treating IBS-D patients with anxiety and depression is evident. Importantly, CHM appears to exert multi-metabolite and multi-targeted effects on signaling pathways involved in various aspects of the biology of IBS-D patients with anxiety and depression, including anti-injury/apoptosis, anti-inflammation, antioxidative stress, and neurotransmitter homeostasis maintenance. CHM may ameliorate symptoms of IBS-D that cooccur with anxiety and depression by addressing different facets of the condition.
Firstly, the quality of the included studies was subpar, characterized by low methodological quality. Most studies lacked details on allocation concealment and blinding, and some exhibited selective reporting bias. In future clinical trials, we aim to adhere to the international CONSORT standards to ensure robust study design and reporting. Secondly, many of the included studies had small sample sizes, diminishing the statistical power of our analysis. Moreover, the absence of rigorous sample size estimation in these studies undermines the validity of the findings. Additionally, variations in conventional interventions introduced clinical heterogeneity, such as differing choices of conventional Western medicine, varying intervention durations, and diverse criteria for evaluating efficacy. Subgroup analyses to identify factors influencing heterogeneity were inadequate. Future research endeavors should prioritize rigorous, multicenter, large-sample size randomized controlled trials to furnish high-quality evidence for clinical practice.
CHM demonstrates efficacy in ameliorating symptoms associated with irritable bowel syndrome (IBS-D) in individuals suffering from anxiety and depression. The principal mechanisms underlying the actions of these herbal active metabolites likely involve anti-inflammatory and antioxidative stress effects, along with the regulation of neurotransmitter homeostasis and modulation of autophagy.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
CB: Conceptualization, Investigation, Writing–original draft. JW: Data curation, Writing–original draft. YW: Data curation, Writing–original draft. HL: Methodology, Writing–review and editing. JL: Software, Writing–original draft. SW: Formal Analysis, Writing–original draft. ZB: Validation, Writing–review and editing. RG: Formal Analysis, Supervision, Writing–review and editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by the National Natural Science Foundation of China (Grant No. U21A200276). The funding agency was not involved in any aspect of the study design, data collection, data analysis, or manuscript writing.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2024.1404738/full#supplementary-material
Aburto, M. R., and Cryan, J. F. (2024). Gastrointestinal and brain barriers: unlocking gates of communication across the microbiota-gut-brain axis. Nat Rev Gastroenterol Hepatol 21 (4), 222–247. doi:10.1038/s41575-023-00890-0
Adeoluwa, O. A., Eduviere, A. T., Adeoluwa, G. O., Otomewo, L. O., and Adeniyi, F. R. (2023). The monoaminergic pathways are involved in the antidepressant-like effect of quercetin. Naunyn Schmiedeb. Arch Pharmacol 397, 2497–2506. doi:10.1007/s00210-023-02789-8
Ahmad, H., Rauf, K., Zada, W., Mccarthy, M., Abbas, G., Anwar, F., et al. (2020). Kaempferol facilitated extinction learning in contextual fear conditioned rats via inhibition of fatty-acid amide hydrolase. Molecules 25 (20), 4683. doi:10.3390/molecules25204683
Bansal, Y., Singh, R., Saroj, P., Sodhi, R. K., and Kuhad, A. (2018). Naringenin protects against oxido-inflammatory aberrations and altered tryptophan metabolism in olfactory bulbectomized-mice model of depression. Toxicol Appl Pharmacol 355, 257–268. doi:10.1016/j.taap.2018.07.010
Cai, L. K., Huang, S., Peng, Z. Y., Huang, Y. L., Tao, L. F., and Lan, S. Y. (2020). Clinical response and changes in intestinal barrier Function,Inflammatory markers and neuropeptide Y among diarrhea-predominant irritable bowel syndrome with liver-qi stagnation and spleen deficiency syndrome patients treated with anchang decoction. Chin. General Pract. 23 (9), 1169–1174.
Chen, F., Sun, J., Chen, C., Zhang, Y., Zou, L., Zhang, Z., et al. (2022). Quercetin mitigates methamphetamine-induced anxiety-like behavior through ameliorating mitochondrial dysfunction and neuroinflammation. Front Mol Neurosci 15, 829886. doi:10.3389/fnmol.2022.829886
Chen, Y., Lian, B., Li, P., Yao, S., and Hou, Z. (2022). Studies on irritable bowel syndrome associated with anxiety or depression in the last 20 years: a bibliometric analysis. Front Public Health 10, 947097. doi:10.3389/fpubh.2022.947097
Chen, Y. F., Fan, M., and Ma, Y. T. (2024). Clinical observation on lipi qushi recipe in the treatment of diarrhea-predominant irritable bowel syndrome of spleen deficiency and dampness superabundance type. J. Guangzhou Univ. Chin. Med. 41 (1), 61–67. doi:10.13359/j.cnki.gzxbtcm.2024.01.010
Chiang, M. C., Tsai, T. Y., and Wang, C. J. (2023). The potential benefits of quercetin for brain health: a review of anti-inflammatory and neuroprotective mechanisms. Int. J. Mol. Sci. 24 (7), 6328. doi:10.3390/ijms24076328
Chtourou, Y., Slima, A. B., Gdoura, R., and Fetoui, H. (2015). Naringenin mitigates iron-induced anxiety-like behavioral impairment, mitochondrial dysfunctions, ectonucleotidases and acetylcholinesterase alteration activities in rat Hippocampus. Neurochem. Res. 40 (8), 1563–1575. doi:10.1007/s11064-015-1627-9
Cumpston, M., Li, T., Page, M. J., Chandler, J., Welch, V. A., Higgins, J. P., et al. (2019). Updated guidance for trusted systematic reviews: a new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst Rev 10 (10), ED000142. doi:10.1002/14651858.ED000142
Ding, Y. F., Yuan, W. M., and Xiao, Y. H. (2021). Clinical study of Hewei Huashi tablets in the treatment of diarrhea-predominant irritable bowel syndrome. China Pract. Med. 16 (2), 25–28. doi:10.14163/j.cnki.11-5547/r.2021.02.009
Drossman, D. A. (2016). Functional gastrointestinal disorders: history, pathophysiology, clinical features and Rome IV. Gastroenterology 150, 1262–1279.e2. doi:10.1053/j.gastro.2016.02.032
Feng, S. Y. (2021). “Clinical efficacy observation of jianpi shenshi decoction in the treatment of diarrhea-predominant irritable bowel syndrome with spleen deficiency and dampness-heat syndrome,” (Hubei,China: Hubei University of Chinese Medicine). master’s thesis. doi:10.27134/d.cnki.ghbzc.2021.000277
Fong, S., Li, C., Ho, Y. C., Li, R., Wang, Q., Wong, Y. C., et al. (2017). Brain uptake of bioactive flavones in scutellariae radix and its relationship to anxiolytic effect in mice. Mol Pharm 14 (9), 2908–2916. doi:10.1021/acs.molpharmaceut.7b00029
Ford, A. C., Lacy, B. E., and Talley, N. J. (2017). Irritable bowel syndrome. N Engl J Med 376 (26), 2566–2578. doi:10.1056/NEJMra1607547
Fu, Q., and Xu, D. S. (2013). Shugan liqi zhixie tang in treatment of 68 patients with diarrhea-predominant irritable bowel syndrome. Chin. J. Exp. Traditional Med. Formulae 19 (16), 301–304.
Gammoh, O., Qnais, E. Y., Athamneh, R. Y., Al-Jaidi, B., Al-Tawalbeh, D., Altaber, S., et al. (2023). Unraveling the potential of isorhamnetin as an adjuvant in depression treatment with escitalopram. Curr. Issues Mol. Biol. 45 (9), 7668–7679. doi:10.3390/cimb45090484
Gao, W., Wang, W., Peng, Y., and Deng, Z. (2019). Antidepressive effects of kaempferol mediated by reduction of oxidative stress, proinflammatory cytokines and up-regulation of AKT/β-catenin cascade. Metab. Brain Dis. 34 (2), 485–494. doi:10.1007/s11011-019-0389-5
Ge, C., Wang, S., Wu, X., and Lei, L. (2023). Quercetin mitigates depression-like behavior via the suppression of neuroinflammation and oxidative damage in corticosterone-induced mice. J. Chem. Neuroanat. 132, 102313. doi:10.1016/j.jchemneu.2023.102313
Ghosh, S., Kumar, A., Sachan, N., and Chandra, P. (2022). Evaluation of the antidepressant-like effect of total sterols fraction and stigmasterol isolated from leaves of aegle marmelos and possible mechanism(s) of action involved. Curr Drug Discov Technol 19 (2), e290721195144. doi:10.2174/1570163818666210729165310
Gu, H. R., and Xu, L. Z. (2022). Clinical effect of Baishi Wenpi decoction in treatment of diarrhea-predominant irritable bowel syndrome: an analysis of 30 cases. Hunan J. Traditional Chin. Med. 38 (3), 13–17. doi:10.16808/j.cnki.issn1003-7705.2022.03.004
Guo, K. (2019). Clinical study of guchang zhixie pill combined with jinshuanggiin treating diarrhea-type lrritable bowel syndrome. Hubei,China,Hubei Univ. Chin. Med. master's thesis.
Guo, W., Sun, Y., Liu, W., Wu, X., Guo, L., Cai, P., et al. (2014). Small molecule-driven mitophagy-mediated NLRP3 inflammasome inhibition is responsible for the prevention of colitis-associated cancer. Autophagy 10 (6), 972–985. doi:10.4161/auto.28374
He, J. Y., Huang, S., Zhou, Y. H., Wang, K., Xie, X. L., Qin, C. F., et al. (2021). Clinical study on chaihu guizhi ganjiang decoction in treating IBS-D with liver stagnation and spleen deficiency. J. Emerg. Traditional Chin. 30 (4), 645–648.
Hui, K. M., Huen, M. S., Wang, H. Y., Zheng, H., Sigel, E., Baur, R., et al. (2002). Anxiolytic effect of wogonin, a benzodiazepine receptor ligand isolated from Scutellaria baicalensis Georgi. Biochem. Pharmacol. 64 (9), 1415–1424. doi:10.1016/s0006-2952(02)01347-3
Ishisaka, M., Kakefuda, K., Yamauchi, M., Tsuruma, K., Shimazawa, M., Tsuruta, A., et al. (2011). Luteolin shows an antidepressant-like effect via suppressing endoplasmic reticulum stress. Biol. Pharm. Bull. 34 (9), 1481–1486. doi:10.1248/bpb.34.1481
Islam, M. S., Hossain, R., Ahmed, T., Rahaman, M. M., Al-Khafaji, K., Khan, R. A., et al. (2022). Anxiolytic-like effect of quercetin possibly through GABA receptor interaction pathway: in vivo and in silico studies. Molecules 27 (21), 7149. doi:10.3390/molecules27217149
Karim, N., Khan, I., Abdelhalim, A., Halim, S. A., Khan, A., and Al-Harrasi, A. (2021). Stigmasterol can be new steroidal drug for neurological disorders: evidence of the GABAergic mechanism via receptor modulation. Phytomedicine 90, 153646. doi:10.1016/j.phymed.2021.153646
Kurokawa, S., Kishimoto, T., Mizuno, S., Masaoka, T., Naganuma, M., Liang, K. C., et al. (2018). The effect of fecal microbiota transplantation on psychiatric symptoms among patients with irritable bowel syndrome, functional diarrhea and functional constipation: an open-label observational study. J Affect Disord 235, 506–512. doi:10.1016/j.jad.2018.04.038
Lee, B., Yeom, M., Shim, I., Lee, H., and Hahm, D. H. (2020). Protective effects of quercetin on anxiety-like symptoms and neuroinflammation induced by lipopolysaccharide in rats. Evid Based Complement Altern. Med 2020, 4892415. doi:10.1155/2020/4892415
Lee, H. W., Ryu, H. W., Kang, M. G., Park, D., Lee, H., Shin, H. M., et al. (2017). Potent inhibition of monoamine oxidase A by decursin from Angelica gigas Nakai and by wogonin from Scutellaria baicalensis Georgi. Int. J. Biol. Macromol. 97, 598–605. doi:10.1016/j.ijbiomac.2017.01.080
Lee, I. A., Kim, E. J., and Kim, D. H. (2012). Inhibitory effect of β-sitosterol on TNBS-induced colitis in mice. Planta Med. 78 (9), 896–8. doi:10.1055/s-0031-1298486
Li, H. Y., Wang, J., Liang, L. F., Shen, S. Y., Li, W., Chen, X. R., et al. (2022). Sirtuin 3 plays a critical role in the antidepressant- and anxiolytic-like effects of kaempferol. Antioxidants (Basel) 11 (10), 1886. doi:10.3390/antiox11101886
Li, J., Zhang, S. X., Wang, W., Cheng, K., Guo, H., Rao, C. L., et al. (2017). Potential antidepressant and resilience mechanism revealed by metabolomic study on peripheral blood mononuclear cells of stress resilient rats. Behav. Brain Res. 320, 12–20. doi:10.1016/j.bbr.2016.11.035
Li, J., Zhou, Y., Liu, B. B., Liu, Q., Geng, D., Weng, L. J., et al. (2013). Nobiletin ameliorates the deficits in hippocampal BDNF, TrkB, and synapsin I induced by chronic unpredictable mild stress. Evid Based Complement Altern. Med 2013, 359682. doi:10.1155/2013/359682
Li, M. Q. (2021). “Clinical Observation of Shugan Hezhong Decoction in treating liver qi stagnation and spleen deficiency type irritable bowel syndrome in aspects of curative effect, the scores of HAMA and HAMD17,” (Nanjing,China: Nanjing University of Chinese Medicine). master’s thesis. doi:10.27253/d.cnki.gnjzu.2021.000253
Liu, X. Z. (2022). Forty-seven cases of diarrhea irritable bowel syndrome of liver depression and spleen deficiency with intestine-calming decoction. Henan Tradit. Chin. Med. 42 (3), 423–427. doi:10.16367/j.issn.1003-5028.2022.03.0091
Liu, Z. (2020). “Observation on treatment of irritable bowel syndrome with diarrhea (liver stagnation and spleen deficiency syndrome) by tongxie lizhong decoction,” (Hebei,China: North China University of Science and Technology). master's thesis. doi:10.27108/d.cnki.ghelu.2020.000248
Longstreth, G. F., Thompson, W. G., Chey, W. D., Houghton, L. A., Mearin, F., and Spiller, R. C. (2006). Functional bowel disorders. Gastroenterology 130 (5), 1480–1491. doi:10.1053/j.gastro.2005.11.061
Lu, H., and Wang, D. X. (2021). Efficacy of Chinese medicine evidence and effects on serum 5-hydroxytryptamine, brain-derived neurotrophic factor, and neuropeptide Y in the treatment of diarrhea-type irritable bowel syndrome with spleen deficiency and dampness in Xiangsha Liu Jun Zi Tang. Hebei Tradit. Chin. Med. 43 (2), 256–260. doi:10.3969/j.issn.1002-2619.2021.02.018
Ma, Z. X., Zhang, R. Y., Rui, W. J., Wang, Z. Q., and Feng, X. (2021). Quercetin alleviates chronic unpredictable mild stress-induced depressive-like behaviors by promoting adult hippocampal neurogenesis via FoxG1/CREB/BDNF signaling pathway. Behav. Brain Res. 406, 113245. doi:10.1016/j.bbr.2021.113245
Majing, M. Y. (2019). “Clinical Study on Jieyu Tang Tiaochang decoction in treating irritable bowel syndrome type of Diarrhea(Liver stagnation and spleen syndrome),” (Shanxi,China: Shanxi University of Chinese Medicine). master's thesis.
Mearin, F., Lacy, B. E., Chang, L., Chey, W. D., Lembo, A. J., Simren, M., et al. (2016). Bowel disorders. Gastroenterology 150, 1393–1407.e5. doi:10.1053/j.gastro.2016.02.031
Mokhtari, T., Lu, M., and El-Kenawy, A. E. (2023). Potential anxiolytic and antidepressant-like effects of luteolin in a chronic constriction injury rat model of neuropathic pain: role of oxidative stress, neurotrophins, and inflammatory factors. Int. Immunopharmacol. 122, 110520. doi:10.1016/j.intimp.2023.110520
Moloney, R. D., Johnson, A. C., O'Mahony, S. M., Dinan, T. G., Greenwood-Van, M. B., and Cryan, J. F. (2016). Stress and the microbiota-gut-brain Axis in visceral pain: relevance to irritable bowel syndrome. CNS Neurosci. Ther. 22 (2), 102–117. doi:10.1111/cns.12490
Mou, X. X. (2021). Observation on the clinical effect of baishi wenpi decoction in the treatment of diarrhea-predominant irritable bowel syndrome. Reflexology Rehabilitation Med. 2 (11), 40–42.
Nachammai, V., Jeyabalan, S., and Muthusamy, S. (2021). Anxiolytic effects of silibinin and naringenin on zebrafish model: a preclinical study. Indian J. Pharmacol. 53 (6), 457–464. doi:10.4103/ijp.IJP_18_20
Nie, W., Zhang, L. P., Meng, J., Hu, L. M., Zhang, X. Y., Wang, H. M., et al. (2014). Study on the effect of fortifying spleen and soothing liver on diarrhea-predominant irritable bowel syndrome. Mod. J. Integr. Traditional Chin. West. Med. 23 (31), 3421–3423+3427.
Olugbemide, A. S., Ben-Azu, B., Bakre, A. G., Ajayi, A. M., Femi-Akinlosotu, O., and Umukoro, S. (2021). Naringenin improves depressive- and anxiety-like behaviors in mice exposed to repeated hypoxic stress through modulation of oxido-inflammatory mediators and NF-kB/BDNF expressions. Brain Res. Bull. 169, 214–227. doi:10.1016/j.brainresbull.2020.12.003
Panayotis, N., Freund, P. A., Marvaldi, L., Shalit, T., Brandis, A., Mehlman, T., et al. (2021). β-sitosterol reduces anxiety and synergizes with established anxiolytic drugs in mice. Cell Rep Med 2 (5), 100281. doi:10.1016/j.xcrm.2021.100281
Qin, H. Y., Zang, K. H., Zuo, X., Wu, X. A., and Bian, Z. X. (2019). Quercetin attenuates visceral hypersensitivity and 5-hydroxytryptamine availability in postinflammatory irritable bowel syndrome rats: role of enterochromaffin cells in the colon. J. Med. Food. 22 (7), 663–671. doi:10.1089/jmf.2018.4264
Qu, Y., Li, X., Xu, F., Zhao, S., Wu, X., Wang, Y., et al. (2021). Kaempferol alleviates murine experimental colitis by restoring gut microbiota and inhibiting the LPS-TLR4-NF-κb Axis. Front Immunol 12, 679897. doi:10.3389/fimmu.2021.679897
Ridker, P. M. (2016). From C-reactive protein to interleukin-6 to interleukin-1: moving upstream to identify novel targets for atheroprotection. Circ. Res. 118 (1), 145–156. doi:10.1161/CIRCRESAHA.115.306656
Ryu, D., Jee, H. J., Kim, S. Y., Hwang, S. H., Pil, G. B., and Jung, Y. S. (2022). Luteolin-7-O-Glucuronide improves depression-like and stress coping behaviors in sleep deprivation stress model by activation of the BDNF signaling. Nutrients 14 (16), 3314. doi:10.3390/nu14163314
Shi, H., He, J., Li, X., Han, J., Wu, R., Wang, D., et al. (2018). Isorhamnetin, the active constituent of a Chinese herb Hippophae rhamnoides L, is a potent suppressor of dendritic-cell maturation and trafficking. Int. Immunopharmacol. 55, 216–222. doi:10.1016/j.intimp.2017.12.014
Socała, K., and Wlaź, P. (2016). Evaluation of the antidepressant- and anxiolytic-like activity of α-spinasterol, a plant derivative with TRPV1 antagonistic effects, in mice. Behav. Brain Res. 303, 19–25. doi:10.1016/j.bbr.2016.01.048
Su, G. Y., Yang, J. Y., Wang, F., Ma, J., Zhang, K., Dong, Y. X., et al. (2014). Antidepressant-like effects of Xiaochaihutang in a rat model of chronic unpredictable mild stress. J. Ethnopharmacol. 152 (1), 217–226. doi:10.1016/j.jep.2014.01.006
Su, J. B., and Zhang, L. (2022). Clinical observation of self-prescribed peitu shunmu decoction for the treatment of diarrhea-predominated irritable bowel syndrome of liver stagnation and spleen deficiency type. J. Guangzhou Univ. Traditional Chin. Med. 39 (12), 2776–2781. doi:10.13359/j.cnki.gzxbtcm.2022.12.008
Sun, D. J., You, X. P., and Chi, L. L. (2020). Discussion on curative effect and mechanism of treating diarrhea predominant irritable bowel syndrome with liver-stagnation and spleen-deficiency based on "purging wood from earth. J. Nanjing Univ. Traditional Chin. Med. 36 (2), 193–196. doi:10.14148/j.issn.1672-0482.2020.0193
Sur, B., and Lee, B. (2022). Luteolin reduces fear, anxiety, and depression in rats with post-traumatic stress disorder. Anim Cells Syst Seoul. 26 (4), 174–182. doi:10.1080/19768354.2022.2104925
Tayyab, M., Farheen, S., Khanam, N., Mobarak, H. M., and Shahi, M. H. (2019). Antidepressant and neuroprotective effects of naringenin via sonic hedgehog-GLI1 cell signaling pathway in a rat model of chronic unpredictable mild stress. Neuromolecular Med 21 (3), 250–261. doi:10.1007/s12017-019-08538-6
Tsai, P. H., Wu, P. C., Li, H. R., Senthil, K. K., and Wang, S. Y. (2023). Hirami lemon (Citrus reticulata var. depressa) modulates the gut-brain axis in a chronic mild stress-induced depression mouse model. Food Funct 14 (16), 7535–7549. doi:10.1039/d3fo01301d
Umukoro, S., Kalejaye, H. A., Ben-Azu, B., and Ajayi, A. M. (2018). Naringenin attenuates behavioral derangements induced by social defeat stress in mice via inhibition of acetylcholinesterase activity, oxidative stress and release of pro-inflammatory cytokines. Biomed. Pharmacother. 105, 714–723. doi:10.1016/j.biopha.2018.06.016
Wang, H., Guo, Y., Qiao, Y., Zhang, J., and Jiang, P. (2020). Nobiletin ameliorates NLRP3 inflammasome-mediated inflammation through promoting autophagy via the AMPK pathway. Mol. Neurobiol. 57 (12), 5056–5068. doi:10.1007/s12035-020-02071-5
Wang, R., Hu, X., Liu, S., Wang, J., Xiong, F., Zhang, X., et al. (2023). Kaempferol-3-O-sophoroside (PCS-1) contributes to modulation of depressive-like behaviour in C57BL/6J mice by activating AMPK. Br J Pharmacol 181, 1182–1202. doi:10.1111/bph.16283
Wu, X., Xu, H., Zeng, N., Li, H., Yao, G., Liu, K., et al. (2023). Luteolin alleviates depression-like behavior by modulating glycerophospholipid metabolism in the hippocampus and prefrontal cortex of LOD rats. Ther 30, e14455. doi:10.1111/cns.14455
Wu, Y. N. (2019). Clinical observation of the pingwei capsule in the treatment of diarrhea-predominant Irritable bowel syndrome and its effect on intestional floras. Gansu,China Gansu Univ. Chin. Med. master’s thesis. doi:10.27026/d.cnki.ggszc.2019.000082
Xia, C. X., Gao, A. X., Dong, T. T., and Tsim, K. W. (2023). Flavonoids from Seabuckthorn (Hippophae rhamnoides L.) mimic neurotrophic functions in inducing neurite outgrowth in cultured neurons: signaling via PI3K/Akt and ERK pathways. Phytomedicine 115, 154832. doi:10.1016/j.phymed.2023.154832
Xia, C. X., Gao, A. X., Zhu, Y., Dong, T. T., and Tsim, K. W. (2023). Flavonoids from Seabuckthorn (Hippophae rhamnoides L.) restore CUMS-induced depressive disorder and regulate the gut microbiota in mice. Food Funct 14 (16), 7426–7438. doi:10.1039/d3fo01332d
Xia, Y., Tan, W., Yuan, F., Lin, M., and Luo, H. (2024). Luteolin attenuates oxidative stress and colonic hypermobility in water avoidance stress rats by activating the Nrf2 signaling pathway. Mol. Nutr. Food Res. 68 (1), e2300126. doi:10.1002/mnfr.202300126
Xu, D., Gao, L. N., Song, X. J., Dong, Q. W., Chen, Y. B., Cui, Y. L., et al. (2023). Enhanced antidepressant effects of BDNF-quercetin alginate nanogels for depression therapy. J Nanobiotechnology 21 (1), 379. doi:10.1186/s12951-023-02150-4
Xu, L. (2017). Clinical observation of Shuganfupihuashi Decoction in treating diarrhea-predominant irritable bowel syndrome (liver stagnation and spleen deficiency type). Hubei,China:Hubei Univ. Chin. Med. master's thesis.
Yang, F. (2021). “Clinical efficacy and mechanism of JIAWEIJIAOTAIWAN(JWJTW) in the treatment of irritable bowel syndrome with heart-kidney disinterchange combined with liver depression and spleen deficiency,” (Nanjing,China: Nanjing University of Chinese Medicine). master's thesis. doi:10.27253/d.cnki.gnjzu.2021.000058
Yin, Y., Liu, X., Liu, J., Cai, E., Zhao, Y., Li, H., et al. (2018). The effect of beta-sitosterol and its derivatives on depression by the modification of 5-HT, DA and GABA-ergic systems in mice. RSC Adv 8 (2), 671–680. doi:10.1039/c7ra11364a
Zamani, M., Alizadeh-Tabari, S., and Zamani, V. (2019). Systematic review with meta-analysis: the prevalence of anxiety and depression in patients with irritable bowel syndrome. Aliment Pharmacol Ther 50 (2), 132–143. doi:10.1111/apt.15325
Zhai, L, Yang, W, Li, D, Zhou, W, Cui, M, and Yao, P (2023). Network pharmacology and molecular docking reveal the immunomodulatory mechanism of rhubarb peony decoction for the treatment of ulcerative colitis and irritable bowel syndrome. J. Pharm. Pharm. Sci. 26, 11225. doi:10.3389/jpps.2023.11225
Zhang, C., Zhu, L., Lu, S., Li, M., Bai, M., Li, Y., et al. (2022). The antidepressant-like effect of formononetin on chronic corticosterone-treated mice. Brain Res. 1783, 147844. doi:10.1016/j.brainres.2022.147844
Zhang, J. L., Liu, M., Cui, W., Yang, L., and Zhang, C. N. (2020). Quercetin affects shoaling and anxiety behaviors in zebrafish: involvement of neuroinflammation and neuron apoptosis. Fish Shellfish Immunol. 105, 359–368. doi:10.1016/j.fsi.2020.06.058
Zhang, K., He, M., Su, D., Pan, X., Li, Y., Zhang, H., et al. (2019). Quantitative proteomics reveal antidepressant potential protein targets of xiaochaihutang in corticosterone induced model of depression. J. Ethnopharmacol. 231, 438–445. doi:10.1016/j.jep.2018.11.020
Zhang, L., Lu, R. R., Xu, R. H., Wang, H. H., Feng, W. S., and Zheng, X. K. (2023). Naringenin and apigenin ameliorates corticosterone-induced depressive behaviors. Heliyon 9 (5), e15618. doi:10.1016/j.heliyon.2023.e15618
Zhang, X. W. (2023). Tongxie yaofang and wandai decoction in treating diarrhea-predominant irritable bowel syndrome based on zhonghe thought. Guangming Chin. Med. 38 (13), 2477–2480. doi:10.3969/j.issn.1003-8914.2023.13.009
Zhang, Y. X. (2016). Treatment of diarrhea-type irritable bowel syndrome by adding Angelica sinensis and Paeonia lactiflora powder in 60 cases. Glob. Tradit. Chin. Med. 9 (1), 100–102.
Zhou, X. Y., and Chu, L. (2020). Efficacy of tongxie yaofang on irritable bowel syndrome of the ganyu pixu fuxie type. Clin. J. Chin. Med. 12 (11), 56–58.
Zhu, M. J., Zhang, K., Cao, R. Y., Li, F. P., and Mou, X. D. (2015). Clinical study on 71 cases of diarrhea-type irritable bowel syndrome treated with combination of Chinese and Western medicine. Jiangsu Chin. Med. (12), 43–44.
Zhu, Q., Han, Y., He, Y., Fu, Y., Yang, H., Chen, Y., et al. (2023). Kaempferol improves breast cancer-related depression through the COX-2/PGE2 pathway. Front Biosci (Landmark Ed.) 28 (11), 311. doi:10.31083/j.fbl2811311
Keywords: diarrheal irritable bowel syndrome, anxiety, depression, Chinese herbal medicine, meta-analysis
Citation: Bai C, Wang J, Wang Y, Liu H, Li J, Wang S, Bai Z and Guo R (2024) Exploration of the mechanism of Traditional Chinese Medicine for anxiety and depression in patients with diarrheal irritable bowel syndrome based on network pharmacology and meta-analysis. Front. Pharmacol. 15:1404738. doi: 10.3389/fphar.2024.1404738
Received: 21 March 2024; Accepted: 26 April 2024;
Published: 21 May 2024.
Edited by:
Javier Echeverria, University of Santiago, ChileCopyright © 2024 Bai, Wang, Wang, Liu, Li, Wang, Bai and Guo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rongjuan Guo, ZGZndW9yb25nanVhbkAxNjMuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.