- 1Department of Orthopedic, The Second Hospital of Shanxi Medical University, Taiyuan, China
- 2Department of Basic Medicine Sciences, Department of Orthopaedics of Sir Run Run Shaw Hospital, Zhejiang University School of Medicine, Hangzhou, China
- 3College of Biomedical Engineering, Taiyuan University of Technology, Taiyuan, China
Background: Hypertension is a common complication in patients with osteoarthritis (OA). There is increasing interest in the relationship between hypertension and OA. However, hypertension has been reported to negatively affect symptoms and quality of life in patients with OA. Therefore, treating hypertension is crucial for patients with OA. However, there is a lack of real-world studies on the effects of medications for treating hypertension on OA.
Methods: Data from the FAERS database from January 2004 to December 2023 were extracted for disproportionality analyses, and proportional reporting ratios (PRRs) were used to assess the association between medications for hypertension and all types of arthritis. Adverse event signals were identified and determined using reporting odds ratios (RORs) Adverse event signals were considered to have occurred if a drug-induced adverse event was recorded more than or equal to 3 and the lower limit of the ROR confidence interval was more than 1. We selected five classes of drugs including, calcium channel blockers (CCBs), angiotensin converting enzyme inhibitors (ACEIs), angiotensin receptor blockers (ARBs), thiazide diuretics and β-blockers and representative drugs were analysed for osteoarthritis-related adverse reactions, and age and gender subgroups were analysed for drugs of significance. We also analysed the occurrence of AEs in relation to time using the Weibull distribution.
Results: In terms of overall data, we found significant OA adverse reaction signals only for ARBs among the five drug classes.ARB AEs for spinal osteoarthritis (ROR 4.64, 95% CI 3.62–5.94), osteoarthritis (ROR 3.24 95% CI 2.82–3.72) and gouty arthritis (ROR 3.27 95% CI 1.22–8.75) were the three adverse reactions with the loudest signals. Next, we found that valsartan had strong osteoarthritis adverse reaction signals among the three ARBs, namely, irbesartan, cloxartan, and valsartan. We also analysed age and gender subgroups and found that osteoarthritis signals were strongest in the 18–65 and 65+ population, while females seem to be more prone to valsartan-related OA AEs.
Conclusion: ARBs, especially valsartan, have significant positive signals for OA AEs. Therefore, ARB drugs, especially valsartan, should be used with caution when treating patients with OA combined with hypertension.
1 Introduction
Osteoarthritis (OA) is a major cause of reduced quality of life for patients and is the sixth leading cause of disability globally. Various risk factors, including age, smoking, body mass index (BMI), low-density lipoprotein (LDL), and alcohol consumption, contribute to OA (Abramoff and Caldera, 2020). OA is most prevalent in the elderly, where it is the leading cause of chronic pain, functional impairment, and poor quality of life (Peat et al., 2001). It has been demonstrated that patients with OA are at a higher risk of developing hypertensive disorders (Dillon et al., 2006; Veronese et al., 2018). Additionally, patients with OA have a greater risk of experiencing cardiovascular events and all-cause mortality compared to non-OA patients (Nüesch et al., 2011; Haugen et al., 2015; Kendzerska et al., 2017). Previous studies have also revealed the association between hypertension and the development of knee OA (Zhang et al., 2017; Lo et al., 2022; Shi and Schlenk, 2022). Therefore, treating hypertension is crucial for improving the quality of life and prognosis of patients with OA.
Currently, there are five classes of first-line drugs used to treat hypertension: Calcium channel blockers (CCBs), angiotensin converting enzyme inhibitors (ACEIs), angiotensin receptor blockers (ARBs), thiazide diuretics and beta-blockers (Whelton et al., 2017; Al-Makki et al., 2022). It is important to use clear and concise language when discussing medical treatments, and to avoid complex terminology that may be difficult for the reader to understand. Several studies have investigated the potential association between certain drugs and the development of OA. Zhou et al. reported that ACEIs may have potential for the treatment of knee OA (Zhou et al., 2020). Li et al. (2021a) found that the use of calcium channel blockers was associated with narrowing of the knee joint space. Driban et al. (2016) observed symptomatic changes in patients with knee OA who were taking thiazides. It is important to note that these findings are not conclusive and further research is needed to fully understand the relationship between these drugs and OA. However, there are no studies analysing the effect of hypertensive medications on OA using large databases.
FDA Adverse Event Reporting System (FAERS) is a public database of adverse reaction reports from healthcare professionals, manufacturers, and consumers. It contains information on adverse reactions to drug use, including when they occur and their outcomes. The database is used by the FDA for record-keeping and post-market safety oversight of drugs (Sharma and Kumar, 2022; Javed and Kumar, 2024). This study collected testing records of ACEIs, ARBs, CCBs, beta-blockers, and hydrochlorothiazide medications to count the signal values of adverse reactions associated with OA. The aim was to provide a large-sample study of medications used in the treatment of hypertension and joint damage, and effective recommendations for the treatment of patients with OA combined with hypertension.
2 Materials and methods
2.1 Data sources
The study’s data was sourced from the open-source FAERS database, which adheres to the International Safety Reporting Guidelines (ICH E2B) published by the International Conference on Harmonisation (ICH). The FAERS database is updated quarterly and covers seven areas: patient demographics and management information (DEMO), drug information (DRUG), adverse event codes (REAC), patient outcomes (OUTC), reporting source (RPSR), treatment start and end dates (THER) associated with the reported drug, and indication for use (INDI) (Li H. et al., 2021). Additionally, we provided information on duplicate entries. Each adverse event (AE) was coded using the Medical Dictionary of Regulatory Activities (MedDRA, version 26.1) Preferred Terminology (PT). The PT is a unique descriptor for individual medical concepts, such as diagnoses and symptoms. The hierarchy also includes High-Level Terminology (HLT) and High-Level Group Terminology (HLGT). HLGTs are categorised into systemic organ categories according to etiology, site of disease or purpose. HLGTs are classified into systemic organ classes (SOCs) based on etiology, site of disease, or purpose (Brown et al., 1999). For this study, musculoskeletal and connective tissue diseases were selected for SOCs, and arthritis-related disease names were selected for PT. The search criteria used by MedDRA were applied.
2.2 Data process
This study collected report files from Q1 2004 to Q4 2023, excluding reports with documentation errors and missing data. Reports where the role cod attribute of the drug in the DRUG file was designated as ‘PS’ (major suspicion) were screened to improve the confidence of the adverse event (AE) analyses. Representative drugs from the five classes of hypertensive drugs were selected for analysis. The ACEIs we selected included captopril, enalapril and fenazopyridine. The CCB class is represented by nifedipine, diltiazem, and verapamil. Losartan, valsartan, and irbesartan are representative drugs of the ARB class (Burnier and Brunner, 2000). Beta-blockers are represented by propranolol, metoprolol, and labetalol. Thiazide diuretics are represented by hydrochlorothiazide (Mancia et al., 2023). The desired drug names were searched using MeSH terms to ensure completeness. The PT associated with OA were retained and analysed in subgroups based on population-specific, gender, age, and drug use patterns. The time to onset of drug-related OA finding signals was also calculated. Time to onset was defined as the time interval between the date of AE onset and the date of initiation of medication use. The Weibull shape parameter (WSP) test was used to analyse the onset time (Abe et al., 2016). The time to onset data was evaluated based on the median, quartiles, and WSP test. The incidence of AEs after treatment initiation depends on the drug’s mechanism of action and usually varies over time. In contrast, the incidence of AEs unrelated to drug therapy remains constant. Early failure type curves are characterised by β values less than 1 and a 95% CI also less than 1, indicating a decreasing hazard over time. Random failure type curves, on the other hand, have β values equal to or close to 1 and a 95% CI containing 1, indicating a steady and consistent hazard over time. Finally, wear-off type curves have β values greater than 1 and a 95% CI not containing 1, indicating an increasing hazard (Sauzet et al., 2013; Nakamura et al., 2015). A Logistic model was employed to substantiate the hypothesis that age and gender are risk factors for the occurrence of AEs.
2.3 Statistical analysis
Pharmacovigilance signals were measured using proportional disproportionality analysis. Adverse event signals were identified and defined using the reported odds ratio (ROR), which was considered to have occurred if a drug-induced adverse event was recorded as greater than or equal to 3 and the lower limit of the ROR confidence interval was greater than 1 (Sakaeda et al., 2013; Tian et al., 2022). The ROR and its 95% confidence interval were calculated using the formula below
The higher the ROR value, the stronger the adverse effect signal, indicating a stronger correlation between the drug and the AE. Baseline data were described by frequency counts and frequencies (Jain et al., 2023; Sharma et al., 2023). Data were collated and statistically analysed using R (version 4.3.2) and the accompanying Rtools and Rstudio versions (Figure 1).
3 Results
3.1 Five classes of antihypertensive drugs correlate with the occurrence of OA AEs
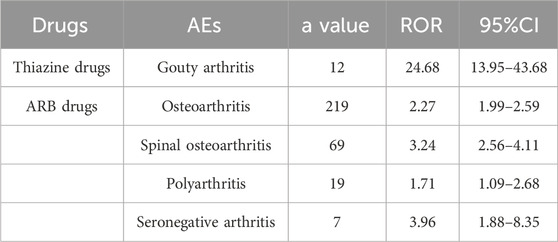
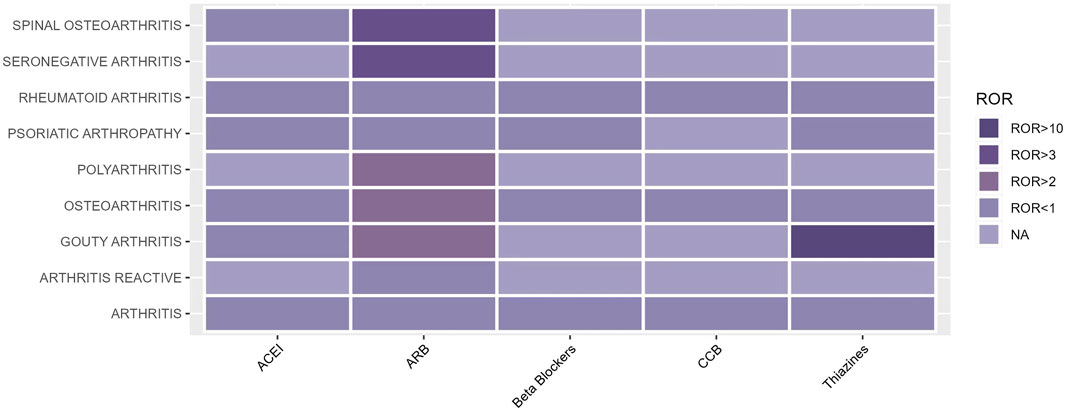
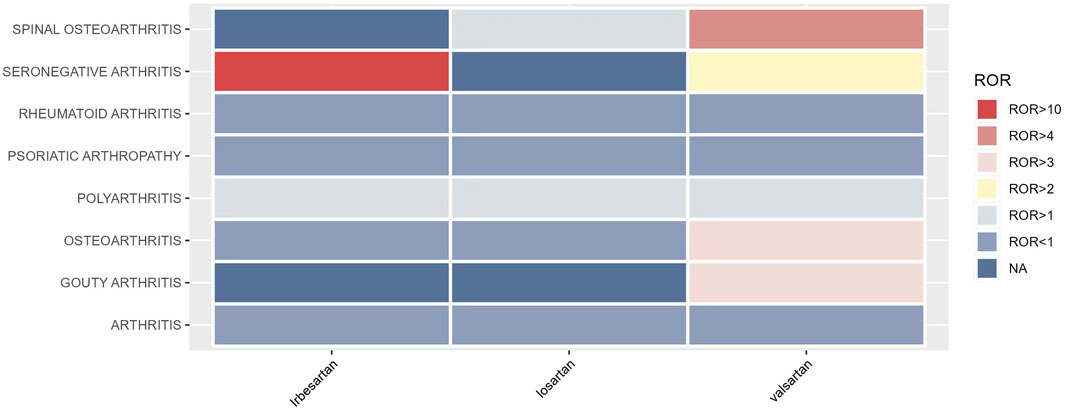
The occurrence of OA AEs in patients treated for hypertension was investigated in the FAERS database from 2004 to 2023 using the descriptive methodology described above. OA AEs in PT included spinal osteoarthritis, seronegative arthritis, rheumatoid arthritis, psoriatic arthropathy, polyarthritis, osteoarthritis, gouty arthritis, arthritis, and reactive arthritis. Figure 1 displays the results. The study found that both ARBs and thiazides have adverse effects related to arthritis. Positive AEs were observed for several ARB drugs, including spinal osteoarthritis (ROR 3.24, 95%CI 2.56–4.11), seronegative arthritis (ROR 3.96, 95%CI 1.88–8.35), polyarthritis (ROR 1.71, 95%CI 1.09–2.68), and osteoarthritis (ROR 2.27, 95%CI 1.99–2.59). Thiazide-related AEs were positively associated with gouty arthritis (ROR 24.68, 95%CI 13.95–43.68). As hyperuricaemia and gout are well-documented adverse effects of hydrochlorothiazide drugs (Hueskes et al., 2012; Dhayat et al., 2023), this study will focus on the OA AEs from ARBs (Table 1; Figure 2).
3.2 Statistical baseline data on valsartan and OA AEs
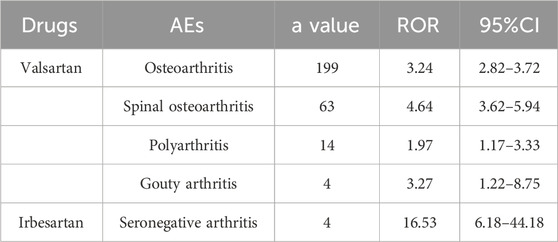
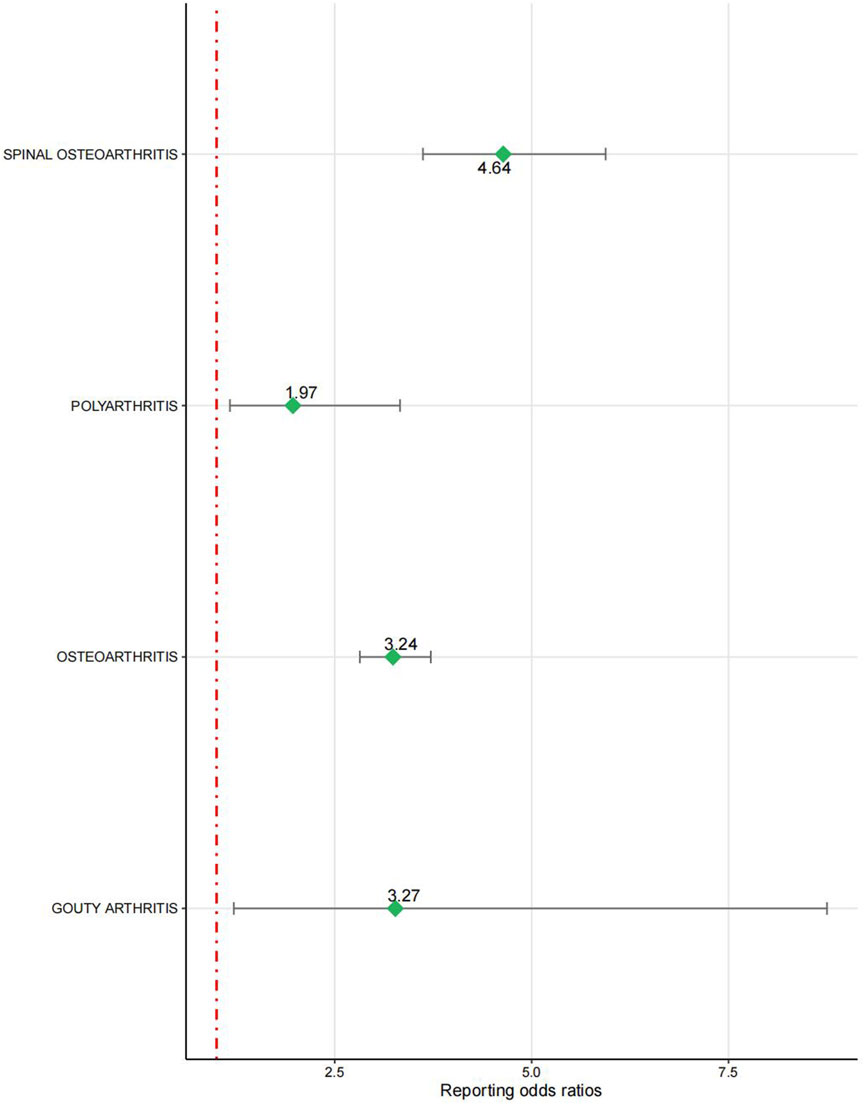
The signal intensity of OA AEs was counted for each of the three drug classes (Table 2; Figure 3). Irbesartan showed a positive signal for seronegative arthritis (ROR 16.53, 95%CI 6.18–44.18), while valsartan showed a positive signal for osteoarthritis (ROR 3.24, 95%CI 2.82–3.72), spinal osteoarthritis (ROR 4.64, 95%CI 3.62–5.94), polyarthritis (ROR 1.97, 95%CI 1.17–3.33), and gouty arthritis (ROR 3.27, 95%CI 1.22–8.75) (Figure 4). In contrast, no arthritis-associated signals were found for cloxartan.
3.3 General characteristics of adverse event reports associated with OA occurring at valsartan
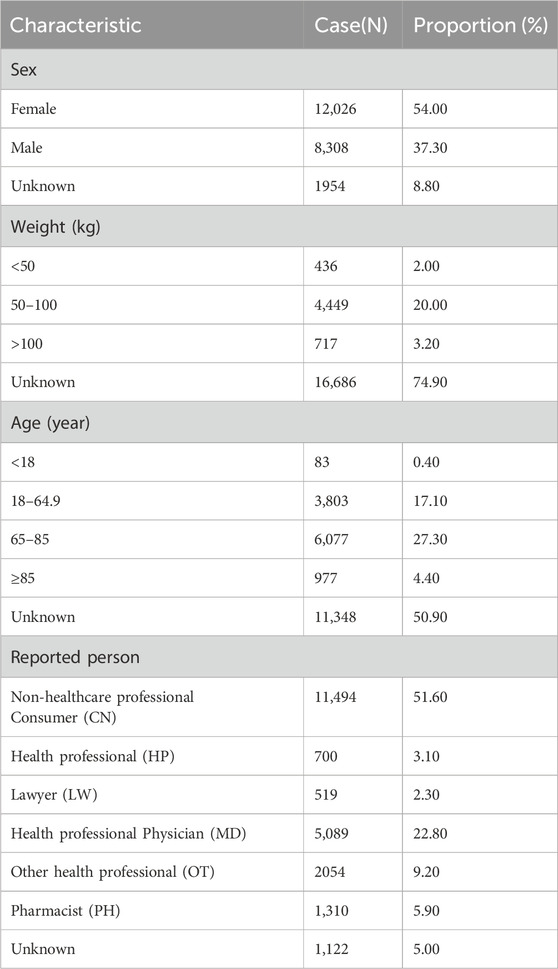
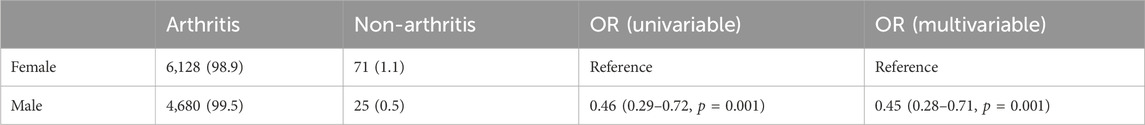
After observing that valsartan produced the highest number of OA-related positive PT, we analysed the valsartan drug data for age and subgroups. We began by counting the valsartan drug data in general (Table 3). A total of 22,288 reports were recorded from the first quarter of 2004 to the third quarter of 2023. It was found that valsartan-related OA AEs were more prevalent in women than in men. Of the gender-specific reports, 12,026 were from women and 8,308 were from men, resulting in a ratio of 1.45 women to 1 man. The highest number of reports came from individuals weighing between 50–100 kg, accounting for 20% of the total number of reports. The age group with the highest number of reports was 65–85 years old, representing 55.6% of the known age reports, which is consistent with the prevalence of hypertension combined with OA. The reporting population consisted mainly of consumers and physicians, who submitted 8,153 reports (43.2%) and 4244 reports (22.5%), respectively. The reporting population consisted mainly of consumers and physicians, who submitted 8,153 reports (43.2%) and 4244 reports (22.5%), respectively. The reporting population consisted mainly of consumers and physicians, who submitted 8,153 reports (43.2%) and 4244 reports (22.5%), respectively. These two groups accounted for more than half of the reports (Table 3).
3.4 Age, gender and temporal characteristics of valsartan-related OA AEs
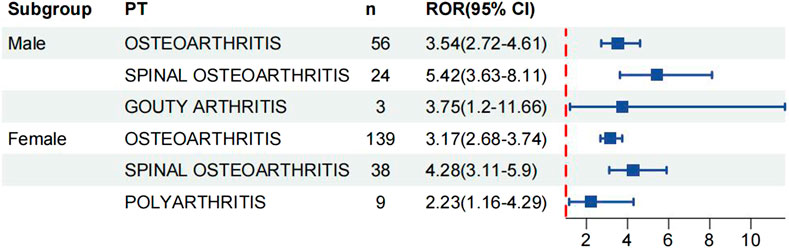
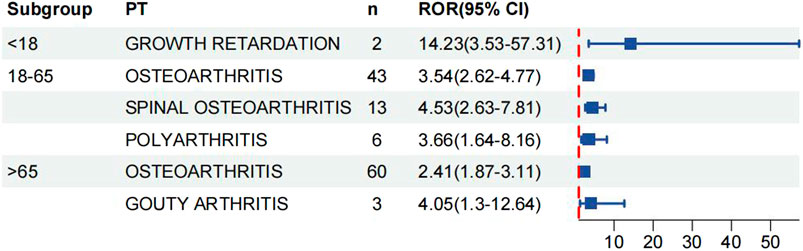
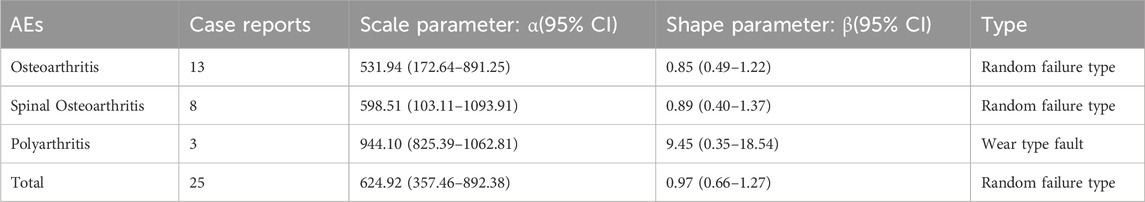
We analysed age and gender subgroups for adverse reactions associated with valsartan and found that the highest number of OA cases was reported in male patients (n = 56, ROR 3.54, 95% CI 2.72–4.61). The most commonly reported condition in females was OA, with a higher incidence than in males (n = 139, ROR 3.17, 95%CI 2.68–3.74) (Figure 5). In the age subgroup analysis for individuals under 18 years old, the only skeletal muscle-related adverse event was growth retardation, with a total of two reports (n = 2, ROR 14.23, 95%CI 3.53–57.31). The report shows that OA was the most commonly reported condition in both age groups, with 43 reports and a ROR of 3.54 (95%CI 2.62–4.77) in the 18–65 year olds and 60 reports and a ROR of 2.41 (95%CI 1.87–3.11) in the over-65 year olds (Figure 6) (23, 95%CI 3.53–57.31). Logistic regression modelling was used to investigate the impact of age and gender on the occurrence of OA AEs. The results showed that gender had a significant effect on the occurrence of valsartan-related OA AEs, with males being a protective factor. However, no significant effect of age on the occurrence of AEs was observed (Table 4). Finally, we analysed the trend of each post-traumatic stressor over time using the Weibull distribution. We found that polyarthritis was part of the wear-and-tear inefficacy model, indicating that the risk of polyarthritis associated with valsartan increased with time. In contrast, all other forms of arthritis and the overall trend belonged to the follow-through failure type (Table 5).
4 Discussion
The prevalence of hypertension in patients with OA is on the rise. Studies have demonstrated that hypertension exacerbates the progression of OA and heightens the likelihood of adverse outcomes (Hall et al., 2016; Zhang et al., 2017; Veronese et al., 2018). Therefore, it is crucial to identify the risks and adverse effects of hypertensive drugs on OA. Administering appropriate hypertensive drugs to control blood pressure in patients with OA can enhance their quality of life and survival rate. Antihypertensive drugs have been associated with the progression or symptoms of OA. However, no study has examined the relationship between antihypertensive drugs and OA in terms of adverse drug reactions. This study searched and analysed five classes of representative first-line drugs for the treatment of hypertension through the FAERS database. The study found an association between ARBs and thiazides with OA, while CCBs, thiazide diuretics, and β-blockers were not associated with OA. Further investigation revealed that the positive signals were mainly from valsartan drugs. Stratification by age and gender showed that the positive signals were stronger in women and people over 65 years of age, while men were less likely to experience ARB-associated OA AEs. We found that the occurrence of polyarthritis associated with valsartan increased with the duration of drug administration, according to Weibull distribution analysis.
Articular cartilage consists of chondrocytes and an extracellular matrix comprising type II collagen and proteoglycans (Cheng et al., 2013). It is a non-vascular tissue, which limits its self-repairing ability after injury, thereby increasing the risk of OA (Alford and Cole, 2005; Liu et al., 2017; Powers et al., 2021). Therefore, blood vessels play a crucial role in cartilage repair. As OA is a chronic disease, it is prevalent among the elderly population and is often associated with hypertension and other diseases (Eymard et al., 2015; Schlenk et al., 2021). Hypertension can cause vascular damage, which affects the inflammatory response and oxidative stress in the body. This, in turn, affects the blood supply and nutrition of skeletal tissues (Fernandes and Valdes, 2015; Mosquera et al., 2019; Merkely et al., 2021). It is important to control hypertension for the quality of life and prognosis of patients with OA (Smith and Cooper-DeHoff, 2019; Zhang et al., 2023). A number of studies have demonstrated that antihypertensive drugs can influence bone metabolism (Song et al., 2024a; Song et al., 2024b; Ma et al., 2024), with a complex relationship also emerging with OA (Bae et al., 2015; Uzieliene et al., 2019). Therefore, it is crucial to determine whether the drugs have adverse effects related to OA. This study covers six classes of first-line drugs used to treat hypertension. The study found that CCBs, ACEIs, and β-blockers do not have OA-associated adverse effects, while ARBs and thiazide diuretics have high signals.
Valsartan is a member of the ARB drugs. It acts by blocking the angiotensin II receptors, thereby dilating the blood vessels and lowering blood pressure. Angiotensin II, the key molecule of RAS, acts in vivo by binding to angiotensin II type 1 (AT1) and type 2 (AT2) receptors (Peach, 1977). It is therefore possible that ARB drugs play a role in the regulation of OA by modulating the level of synovial inflammation and the release of synovial inflammatory factors (Terenzi et al., 2017). Recent studies have demonstrated that angiotensin II is expressed in synovial tissues of both humans and animals and is involved in the pathogenesis of OA (Liu et al., 2016; Forrester et al., 2018; Wang et al., 2018). It has been demonstrated that ARB can facilitate the expression of transforming growth factor-β (TGF-β) (Wu et al., 2016; Zou et al., 2022). Although TGF-β is essential for articular cartilage homeostasis (Blaney Davidson et al., 2007), it has also been found that an excessive amount of TGF-β can be produced during OA (Bakker et al., 2001; van de Laar et al., 2011). In addition, intra-articular knee injections of TGF-β1 induced OA in rats (Serra et al., 1997; Itayem et al., 1999; Shen et al., 2013). Furthermore, it has been demonstrated that ARB drugs may facilitate chondrocyte hypertrophic senescence during skeletal development in mice (Chen et al., 2015). Some studies have further explored the mechanism and found that ARB can participate in OA regulation through the activation of NF-κB and phosphorylation of JNK (Sampson et al., 2016; Yu et al., 2016; Yamagishi et al., 2018). The NF-κB signalling pathway plays a pivotal role in the release of inflammatory factors, which is a crucial step in the progression of OA (Saito and Tanaka, 2017; Cao et al., 2021). Additionally, the phosphorylation of JNK has been observed to result in the enhanced secretion of matrix metalloproteinase 13 (MMP13), a protein that accelerates the breakdown of cartilage tissue (Lin et al., 2021; Sun et al., 2023). Furthermore, evidence indicates that ARB drugs can influence the progression of OA by activating vascular endothelial growth factor (VEGF) molecules (Chen et al., 2017; MacDonald et al., 2018). Our study found that valsartan had a positive signal for OA AEs. It is therefore recommended that valsartan be used with caution when patients with OA and hypertension require treatment with ARBs. However, higher quality RCTs and mechanism studies are needed to provide stronger evidence.
Age and gender are widely acknowledged as risk factors for OA (Felson et al., 2000; Wluka et al., 2000). Age is one of the strongest risk factors for OA in the elderly due to biological changes such as loss of muscle strength, cartilage wear and tear, and deterioration of proprioception that occur with age (Felson and Zhang, 1998; Helmick et al., 2008). In this study, it was also found that the number and proportion of positive reports were highest among people above 65 years of age, compared to other age groups. Age may exacerbate the manifestation and probability of valsartan-related OA AEs. Additionally, women are at a higher risk of developing OA and experiencing more severe symptoms than men (Hannan et al., 1990; Srikanth et al., 2005). This may be due to the effect of endogenous oestrogens and changes during menopause in women. In addition, our findings suggest that females may be more susceptible to valsartan-related OA AEs. However, the data on hypertension revealed a higher number of records for female patients than for male patients. Further high-grade studies are required in the future to investigate the impact of gender on valsartan-related OA AEs. A study demonstrated that the prevalence of hypertension was lower in premenopausal women than in men of the same age, and increased significantly in postmenopausal women (Ji et al., 2020). Furthermore, the prevalence of hypertension is higher in women than in men over the age of 65 years (O'Keeffe et al., 2018). We concluded from Weibull’s analysis that the incidence of AEs associated with valsartan in OA patients increased with the duration of administration. This reinforces our recommendation that valsartan should be used with caution in patients with OA.
Apart from the above, hydrochlorothiazide and irbesartan also demonstrated positive effects. Hydrochlorothiazide, in particular, significantly increases the risk of gout by affecting uric acid excretion (Schrijver and Weinberger, 1979; Dhayat et al., 2023). As a result, this study also found a positive association between hydrochlorothiazide and gouty arthritis. The present study did not investigate or discuss in detail the relationship between irbesartan and OA due to the lack of relevant studies and the small number of reports associating irbesartan only with seronegative arthritis. Therefore, further clinical and experimental studies are needed to establish the relationship between irbesartan and OA. Furthermore, different conclusions have been reached in studies on the relationship between CCBs and β-blockers and OA. For instance, some studies have proposed that CCBs can decelerate the progression of OA by antagonising intra-articular calcium channels (Takamatsu et al., 2014; Bertram et al., 2016). However, other studies have suggested that CCBs may worsen pain and joint stenosis in OA patients (Daniilidis et al., 2015; Li M. et al., 2021). Additionally, there are conflicting findings regarding the effects of β-blockers on OA (Martin et al., 2015; Valdes et al., 2017). The results of this study indicate that there are no discernible positive signals for OA AEs for any of the aforementioned pharmaceutical agents. Further high-quality clinical and mechanistic studies are needed to establish the relationship between them.
Furthermore, initial guidelines recommended monotherapy for the control of hypertension. However, an increasing number of studies have shown that blood pressure is a multi-regulatory variable involving multiple pathophysiological processesand that patient compliance with monotherapy is poor (Berra et al., 2016; Rea et al., 2018). Therefore, monotherapy may not be sufficient to control blood pressure in most patients (Bakris et al., 2014). Among them, ACEI or ARB in combination with CCB or thiazide is the most recommended combination regimen in the guidelines (Mancia et al., 2023). It should be noted that ARBs, β-blockers and thiazide diuretics are all associated with hyperuricemia (Choi et al., 2012; Bardin and Richette, 2017). Therefore, these drugs should be avoided when treating patients with gouty arthritis and hypertension. In addition, the combination of ARBs and ACEIs or two ARB drugs increases the likelihood of nephrotoxicity and acute renal failure, which is also noteworthy (Ontarget et al., 2008; Parving et al., 2009). However, there is a lack of clinical trials on the use of combinations in the treatment of patients with arthritis. The amount of data on co-medication in the FAERS database is also limited, making it difficult to investigate whether co-medication causes OA AEs. In future studies, we will focus on the effect of ARB combination therapy on the occurrence of OA AEs.
There are limitations to this study. Firstly, we only included five first-line and representative anti-hypertensive drugs, which may introduce bias in the results. Secondly, the accuracy of statistical results may be biased during the initial data entry stage due to the self-reporting nature of the FAERS database. Inadequate case reporting can also significantly impact study results. Furthermore, the majority of cases recorded in the FAERS system are from Europe and the United States, indicating a need for more global data entry.
5 Conclusion
Through comprehensive and systematic analysis of the FAERS data, we have identified a strong association between five classes of first-line antihypertensive drugs and OA-related side effects. We have also assessed the severity of AEs across different populations, age groups, and genders. Specifically, we have found a strong adverse effect signal for the valsartan drug in the ARB class. Subgroup analyses showed that the population over 65 years and females had the highest number of positive reports. Females appear to be more susceptible to valsartan-related OA AEs, but higher quality studies are needed to confirm this. Additionally, the incidence of valsartan-associated OA AEs increased with increasing duration of dosing. Therefore, We recommend that patients with OA combined with hypertension should use ARBs with caution, especially valsartan.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
ZG: Writing–original draft, Writing–review and editing. JD: Investigation, Writing–review and editing. ZZ: Data curation, Writing–review and editing. SC: Methodology, Writing–review and editing. XM: Supervision, Writing–review and editing. ZW: Software, Writing–review and editing. ZY: Formal Analysis, Writing–review and editing. XL: Project administration, Writing–review and editing. ZT: Project administration, Writing–review and editing. CM: Validation, Writing–review and editing. CaX: Validation, Writing–review and editing. CuX: Funding acquisition, Resources, Writing–original draft, Writing–review and editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study was supported by a grant from Central-led Local Science and Technology Development Funds (YDZJSX20231A062), Shanxi Provincial Scientific and Technological Achievement Transformation Guidance Special Programme (202204021301067).
Acknowledgments
The presentation of the manuscript should be as preprint. The authors would like to thank the Key Laboratory of Bone and Soft Tissue Injury Repair of Shanxi Province for their help.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
OA, Osteoarthritis; ARB, Angiotensin receptor blocker; ACEI, Angiotensin converting enzyme inhibitors; AT, Angiotensin; CCB, Calcium channel blockers; ARNI, Angiotensin receptor-neprilysin inhibitor; TGF-β, Transforming growthfactor β; RCT, Randomized controlled trial.
References
Abe, J., Umetsu, R., Mataki, K., Kato, Y., Ueda, N., Nakayama, Y., et al. (2016). Analysis of Stevens-Johnson syndrome and toxic epidermal necrolysis using the Japanese Adverse Drug Event Report database. J. Pharm. Health Care Sci. 2, 14. doi:10.1186/s40780-016-0048-5
Abramoff, B., and Caldera, F. E. (2020). Osteoarthritis: pathology, diagnosis, and treatment options. Med. Clin. North Am. 104 (2), 293–311. doi:10.1016/j.mcna.2019.10.007
Alford, J. W., and Cole, B. J. (2005). Cartilage restoration, part 1: basic science, historical perspective, patient evaluation, and treatment options. Am. J. Sports Med. 33 (2), 295–306. doi:10.1177/0363546504273510
Al-Makki, A., DiPette, D., Whelton, P. K., Murad, M. H., Mustafa, R. A., Acharya, S., et al. (2022). Hypertension pharmacological treatment in adults: a world health organization guideline executive summary. Hypertension 79 (1), 293–301. doi:10.1161/HYPERTENSIONAHA.121.18192
Bae, Y. H., Shin, J. S., Lee, J., Kim, M. r., Park, K. B., Cho, J. H., et al. (2015). Association between hypertension and the prevalence of low back pain and osteoarthritis in Koreans: a cross-sectional study. PLoS One 10 (9), e0138790. doi:10.1371/journal.pone.0138790
Bakker, A. C., van de Loo, F. A., van Beuningen, H. M., Sime, P., van Lent, P. L., van der Kraan, P. M., et al. (2001). Overexpression of active TGF-beta-1 in the murine knee joint: evidence for synovial-layer-dependent chondro-osteophyte formation. Osteoarthr. Cartil. 9 (2), 128–136. doi:10.1053/joca.2000.0368
Bakris, G., Sarafidis, P., Agarwal, R., and Ruilope, L. (2014). Review of blood pressure control rates and outcomes. J. Am. Soc. Hypertens. 8 (2), 127–141. doi:10.1016/j.jash.2013.07.009
Bardin, T., and Richette, P. (2017). Impact of comorbidities on gout and hyperuricaemia: an update on prevalence and treatment options. BMC Med. 15 (1), 123. doi:10.1186/s12916-017-0890-9
Berra, E., Azizi, M., Capron, A., Høieggen, A., Rabbia, F., Kjeldsen, S. E., et al. (2016). Evaluation of adherence should become an integral part of assessment of patients with apparently treatment-resistant hypertension. Hypertension 68 (2), 297–306. doi:10.1161/HYPERTENSIONAHA.116.07464
Bertram, K. L., Banderali, U., Tailor, P., and Krawetz, R. J. (2016). Ion channel expression and function in normal and osteoarthritic human synovial fluid progenitor cells. Channels (Austin) 10 (2), 148–157. doi:10.1080/19336950.2015.1116652
Blaney Davidson, E. N., van der Kraan, P. M., and van den Berg, W. B. (2007). TGF-beta and osteoarthritis. Osteoarthr. Cartil. 15 (6), 597–604. doi:10.1016/j.joca.2007.02.005
Brown, E. G., Wood, L., and Wood, S. (1999). The medical dictionary for regulatory activities (MedDRA). Drug Saf. 20 (2), 109–117. doi:10.2165/00002018-199920020-00002
Burnier, M., and Brunner, H. R. (2000). Angiotensin II receptor antagonists. Lancet. 355 (9204), 637–645. doi:10.1016/s0140-6736(99)10365-9
Cao, Y., Tang, S., Nie, X., Zhou, Z., Ruan, G., Han, W., et al. (2021). Decreased miR-214-3p activates NF-κB pathway and aggravates osteoarthritis progression. EBioMedicine 65, 103283. doi:10.1016/j.ebiom.2021.103283
Chen, J. L., Zou, C., Chen, Y., Zhu, W., Liu, W., Huang, J., et al. (2017). TGFβ1 induces hypertrophic change and expression of angiogenic factors in human chondrocytes. Oncotarget 8 (53), 91316–91327. doi:10.18632/oncotarget.20509
Chen, S., Grover, M., Sibai, T., Black, J., Rianon, N., Rajagopal, A., et al. (2015). Losartan increases bone mass and accelerates chondrocyte hypertrophy in developing skeleton. Mol. Genet. Metab. 115 (1), 53–60. doi:10.1016/j.ymgme.2015.02.006
Cheng, T., Zhang, L., Fu, X., Wang, W., Xu, H., Song, H., et al. (2013). The potential protective effects of calcitonin involved in coordinating chondrocyte response, extracellular matrix, and subchondral trabecular bone in experimental osteoarthritis. Connect. Tissue Res. 54 (2), 139–146. doi:10.3109/03008207.2012.760549
Choi, H. K., Soriano, L. C., Zhang, Y., and Rodríguez, L. A. (2012). Antihypertensive drugs and risk of incident gout among patients with hypertension: population based case-control study. BMJ 344, d8190. doi:10.1136/bmj.d8190
Daniilidis, K., Georges, P., Tibesku, C. O., and Prehm, P. (2015). Positive side effects of Ca antagonists for osteoarthritic joints-results of an in vivo pilot study. J. Orthop. Surg. Res. 10, 1. doi:10.1186/s13018-014-0138-8
Dhayat, N. A., Bonny, O., Roth, B., Christe, A., Ritter, A., Mohebbi, N., et al. (2023). Hydrochlorothiazide and prevention of kidney-stone recurrence. N. Engl. J. Med. 388 (9), 781–791. doi:10.1056/NEJMoa2209275
Dillon, C. F., Rasch, E. K., Gu, Q., and Hirsch, R. (2006). Prevalence of knee osteoarthritis in the United States: arthritis data from the third national health and nutrition examination survey 1991-94. J. Rheumatol. 33 (11), 2271–2279.
Driban, J. B., Lo, G. H., Eaton, C. B., Lapane, K. L., Nevitt, M., Harvey, W. F., et al. (2016). Exploratory analysis of osteoarthritis progression among medication users: data from the Osteoarthritis Initiative. Ther. Adv. Musculoskelet. Dis. 8 (6), 207–219. doi:10.1177/1759720X16664323
Eymard, F., Parsons, C., Edwards, M. H., Petit-Dop, F., Reginster, J. Y., Bruyère, O., et al. (2015). Diabetes is a risk factor for knee osteoarthritis progression. Osteoarthr. Cartil. 23 (6), 851–859. doi:10.1016/j.joca.2015.01.013
Felson, D. T., Lawrence, R. C., Dieppe, P. A., Hirsch, R., Helmick, C. G., Jordan, J. M., et al. (2000). Osteoarthritis: new insights. Part 1: the disease and its risk factors. Ann. Intern Med. 133 (8), 635–646. doi:10.7326/0003-4819-133-8-200010170-00016
Felson, D. T., and Zhang, Y. (1998). An update on the epidemiology of knee and hip osteoarthritis with a view to prevention. Arthritis Rheum. 41 (8), 1343–1355. doi:10.1002/1529-0131(199808)41:8<1343::AID-ART3>3.0.CO;2-9
Fernandes, G. S., and Valdes, A. M. (2015). Cardiovascular disease and osteoarthritis: common pathways and patient outcomes. Eur. J. Clin. Invest. 45 (4), 405–414. doi:10.1111/eci.12413
Forrester, S. J., Booz, G. W., Sigmund, C. D., Coffman, T. M., Kawai, T., Rizzo, V., et al. (2018). Angiotensin II signal transduction: an update on mechanisms of physiology and pathophysiology. Physiol. Rev. 98 (3), 1627–1738. doi:10.1152/physrev.00038.2017
Hall, A. J., Stubbs, B., Mamas, M. A., Myint, P. K., and Smith, T. O. (2016). Association between osteoarthritis and cardiovascular disease: systematic review and meta-analysis. Eur. J. Prev. Cardiol. 23 (9), 938–946. doi:10.1177/2047487315610663
Hannan, M. T., Felson, D. T., Anderson, J. J., Naimark, A., and Kannel, W. B. (1990). Estrogen use and radiographic osteoarthritis of the knee in women. The Framingham Osteoarthritis Study. Arthritis Rheum. 33 (4), 525–532. doi:10.1002/art.1780330410
Haugen, I. K., Ramachandran, V. S., Misra, D., Neogi, T., Niu, J., Yang, T., et al. (2015). Hand osteoarthritis in relation to mortality and incidence of cardiovascular disease: data from the Framingham heart study. Ann. Rheum. Dis. 74 (1), 74–81. doi:10.1136/annrheumdis-2013-203789
Helmick, C. G., Felson, D. T., Lawrence, R. C., Gabriel, S., Hirsch, R., Kwoh, C. K., et al. (2008). Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part I. Arthritis Rheum. 58 (1), 15–25. doi:10.1002/art.23177
Hueskes, B. A., Roovers, E. A., Mantel-Teeuwisse, A. K., Janssens, H. J., van de Lisdonk, E. H., and Janssen, M. (2012). Use of diuretics and the risk of gouty arthritis: a systematic review. Semin. Arthritis Rheum. 41 (6), 879–889. doi:10.1016/j.semarthrit.2011.11.008
Itayem, R., Mengarelli-Widholm, S., and Reinholt, F. P. (1999). The long-term effect of a short course of transforming growth factor-beta1 on rat articular cartilage. APMIS 107 (2), 183–192. doi:10.1111/j.1699-0463.1999.tb01543.x
Jain, D., Sharma, G., and Kumar, A. (2023). Adverse effects of proton pump inhibitors (PPIs) on the renal system using data mining algorithms (DMAs). Expert Opin. Drug Saf. 22 (8), 741–752. doi:10.1080/14740338.2023.2189698
Javed, F., and Kumar, A. (2024). Identification of signal of clindamycin associated renal failure acute: a disproportionality analysis. Curr. Drug Saf. 19 (1), 123–128. doi:10.2174/1574886318666230228142856
Ji, H., Kim, A., Ebinger, J. E., Niiranen, T. J., Claggett, B. L., Bairey Merz, C. N., et al. (2020). Sex differences in blood pressure trajectories over the life course. JAMA Cardiol. 5 (3), 19–26. doi:10.1001/jamacardio.2019.5306
Kendzerska, T., Jüni, P., King, L. K., Croxford, R., Stanaitis, I., and Hawker, G. A. (2017). The longitudinal relationship between hand, hip and knee osteoarthritis and cardiovascular events: a population-based cohort study. Osteoarthr. Cartil. 25 (11), 1771–1780. doi:10.1016/j.joca.2017.07.024
Li, H., Sun, X., Sun, D., Zhao, J., Xu, Z., Zhao, P., et al. (2021b). Thromboembolic events associated with immune checkpoint inhibitors: a real-world study of data from the food and drug administration adverse event reporting system (FAERS) database. Int. Immunopharmacol. 98, 107818. doi:10.1016/j.intimp.2021.107818
Li, M., Zeng, Y., Nie, Y., Wu, Y., Liu, Y., Wu, L., et al. (2021a). The effects of different antihypertensive drugs on pain and joint space width of knee osteoarthritis - a comparative study with data from Osteoarthritis Initiative. J. Clin. Hypertens. (Greenwich) 23 (11), 2009–2015. doi:10.1111/jch.14362
Lin, Z., Miao, J., Zhang, T., He, M., Wang, Z., Feng, X., et al. (2021). JUNB-FBXO21-ERK axis promotes cartilage degeneration in osteoarthritis by inhibiting autophagy. Aging Cell 20 (2), e13306. doi:10.1111/acel.13306
Liu, C. F., Samsa, W. E., Zhou, G., and Lefebvre, V. (2017). Transcriptional control of chondrocyte specification and differentiation. Semin. Cell Dev. Biol. 62, 34–49. doi:10.1016/j.semcdb.2016.10.004
Liu, Q., Tian, J., Xu, Y., Li, C., Meng, X., and Fu, F. (2016). Protective effect of RA on myocardial infarction-induced cardiac fibrosis via at1r/p38 MAPK pathway signaling and modulation of the ACE2/ACE ratio. J. Agric. Food Chem. 64 (35), 6716–6722. doi:10.1021/acs.jafc.6b03001
Lo, K., Au, M., Ni, J., and Wen, C. (2022). Association between hypertension and osteoarthritis: a systematic review and meta-analysis of observational studies. J. Orthop. Transl. 32, 12–20. doi:10.1016/j.jot.2021.05.003
Ma, H., Cai, X., Hu, J., Song, S., Zhu, Q., Zhang, Y., et al. (2024). Association of systemic inflammatory response index with bone mineral density, osteoporosis, and future fracture risk in elderly hypertensive patients. Postgrad. Med. 136 (4), 406–416. doi:10.1080/00325481.2024.2354158
MacDonald, I. J., Liu, S. C., Su, C. M., Wang, Y. H., Tsai, C. H., and Tang, C. H. (2018). Implications of angiogenesis involvement in arthritis. Int. J. Mol. Sci. 19 (7), 2012. doi:10.3390/ijms19072012
Mancia, G., Kreutz, R., Brunström, M., Burnier, M., Grassi, G., Januszewicz, A., et al. (2023). 2023 ESH guidelines for the management of arterial hypertension the task force for the management of arterial hypertension of the European society of hypertension: endorsed by the international society of hypertension (ISH) and the European renal association (ERA). J. Hypertens. 41 (12), 1874–2071. doi:10.1097/HJH.0000000000003480
Martin, L. J., Piltonen, M. H., Gauthier, J., Convertino, M., Acland, E. L., Dokholyan, N. V., et al. (2015). Differences in the antinociceptive effects and binding properties of propranolol and bupranolol enantiomers. J. Pain 16 (12), 1321–1333. doi:10.1016/j.jpain.2015.09.004
Merkely, G., Ackermann, J., and Gomoll, A. H. (2021). The role of hypertension in cartilage restoration: increased failure rate after autologous chondrocyte implantation but not after osteochondral allograft transplantation. Cartilage 13 (1_Suppl. l), 1306S–1314S. doi:10.1177/1947603519900792
Mosquera, A., Rego-Pérez, I., Blanco, F. J., and Fernández, J. L. (2019). Leukocyte telomere length in patients with radiographic knee osteoarthritis. Environ. Mol. Mutagen 60 (3), 298–301. doi:10.1002/em.22247
Nakamura, M., Umetsu, R., Abe, J., Matsui, T., Ueda, N., Kato, Y., et al. (2015). Analysis of the time-to-onset of osteonecrosis of jaw with bisphosphonate treatment using the data from a spontaneous reporting system of adverse drug events. J. Pharm. Health Care Sci. 1, 34. doi:10.1186/s40780-015-0035-2
Nüesch, E., Dieppe, P., Reichenbach, S., Williams, S., Iff, S., and Jüni, P. (2011). All cause and disease specific mortality in patients with knee or hip osteoarthritis: population based cohort study. BMJ 342, d1165. doi:10.1136/bmj.d1165
O'Keeffe, L. M., Simpkin, A. J., Tilling, K., Anderson, E. L., Hughes, A. D., Lawlor, D. A., et al. (2018). Sex-specific trajectories of measures of cardiovascular health during childhood and adolescence: a prospective cohort study. Atherosclerosis 278, 190–196. doi:10.1016/j.atherosclerosis.2018.09.030
Ontarget, I., Yusuf, S., Teo, K. K., Janice, P., Leanne, D., Copland, I., et al. (2008). Telmisartan, ramipril, or both in patients at high risk for vascular events. N. Engl. J. Med. 358 (15), 1547–1559.
Parving, H. H., Brenner, B. M., McMurray, J. J., de Zeeuw, D., Haffner, S. M., Solomon, S. D., et al. (2009). Aliskiren trial in type 2 diabetes using cardio-renal endpoints (ALTITUDE): rationale and study design. Nephrol. Dial. Transpl. 24 (5), 1663–1671. doi:10.1093/ndt/gfn721
Peach, M. J. (1977). Renin-angiotensin system: biochemistry and mechanisms of action. Physiol. Rev. 57 (2), 313–370. doi:10.1152/physrev.1977.57.2.313
Peat, G., McCarney, R., and Croft, P. (2001). Knee pain and osteoarthritis in older adults: a review of community burden and current use of primary health care. Ann. Rheum. Dis. 60 (2), 91–97. doi:10.1136/ard.60.2.91
Powers, R. T., Dowd, T. C., and Giza, E. (2021). Surgical treatment for osteochondral lesions of the talus. Arthroscopy 37 (12), 3393–3396. doi:10.1016/j.arthro.2021.10.002
Rea, F., Corrao, G., Merlino, L., and Mancia, G. (2018). Initial antihypertensive treatment strategies and therapeutic inertia. Hypertension 72 (4), 846–853. doi:10.1161/HYPERTENSIONAHA.118.11308
Saito, T., and Tanaka, S. (2017). Molecular mechanisms underlying osteoarthritis development: notch and NF-κB. Arthritis Res. Ther. 19 (1), 94. doi:10.1186/s13075-017-1296-y
Sakaeda, T., Tamon, A., Kadoyama, K., and Okuno, Y. (2013). Data mining of the public version of the FDA adverse event reporting system. Int. J. Med. Sci. 10 (7), 796–803. doi:10.7150/ijms.6048
Sampson, A. K., Irvine, J. C., Shihata, W. A., Dragoljevic, D., Lumsden, N., Huet, O., et al. (2016). Compound 21, a selective agonist of angiotensin AT2 receptors, prevents endothelial inflammation and leukocyte adhesion in vitro and in vivo. Br. J. Pharmacol. 173 (4), 729–740. doi:10.1111/bph.13063
Sauzet, O., Carvajal, A., Escudero, A., Molokhia, M., and Cornelius, V. R. (2013). Illustration of the weibull shape parameter signal detection tool using electronic healthcare record data. Drug Saf. 36 (10), 995–1006. doi:10.1007/s40264-013-0061-7
Schlenk, E. A., Fitzgerald, G. K., Rogers, J. C., Kwoh, C. K., and Sereika, S. M. (2021). Promoting physical activity in older adults with knee osteoarthritis and hypertension: a randomized controlled trial. J. Aging Phys. Act. 29 (2), 207–218. doi:10.1123/japa.2019-0498
Schrijver, G., and Weinberger, M. H. (1979). Hydrochlorothiazide and spironolactone in hypertension. Clin. Pharmacol. Ther. 25 (1), 33–42. doi:10.1002/cpt197925133
Serra, R., Johnson, M., Filvaroff, E. H., LaBorde, J., Sheehan, D. M., Derynck, R., et al. (1997). Expression of a truncated, kinase-defective TGF-beta type II receptor in mouse skeletal tissue promotes terminal chondrocyte differentiation and osteoarthritis. J. Cell Biol. 139 (2), 541–552. doi:10.1083/jcb.139.2.541
Sharma, A., and Kumar, A. (2022). Identification of novel signal of clobazam-associated drug reaction with eosinophilia and systemic symptoms syndrome: a disproportionality analysis. Acta Neurol. Scand. 146 (5), 623–627. doi:10.1111/ane.13690
Sharma, A., Roy, S., Sharma, R., and Kumar, A. (2023). Association of antiviral drugs and their possible mechanisms with DRESS syndrome using data mining algorithms. J. Med. Virol. 95 (3), e28671. doi:10.1002/jmv.28671
Shen, J., Li, J., Wang, B., Jin, H., Wang, M., Zhang, Y., et al. (2013). Deletion of the transforming growth factor β receptor type II gene in articular chondrocytes leads to a progressive osteoarthritis-like phenotype in mice. Arthritis Rheum. 65 (12), 3107–3119. doi:10.1002/art.38122
Shi, X., and Schlenk, E. A. (2022). Association of hypertension with knee pain severity among people with knee osteoarthritis. Pain Manag. Nurs. 23 (2), 135–141. doi:10.1016/j.pmn.2021.08.002
Smith, S. M., and Cooper-DeHoff, R. M. (2019). Fixed-dose combination amlodipine/celecoxib (consensi) for hypertension and osteoarthritis. Am. J. Med. 132 (2), 172–174. doi:10.1016/j.amjmed.2018.08.027
Song, S., Cai, X., Hu, J., Zhu, Q., Shen, D., Heizhati, M., et al. (2024a). Correlation between plasma aldosterone concentration and bone mineral density in middle-aged and elderly hypertensive patients: potential impact on osteoporosis and future fracture risk. Front. Endocrinol. (Lausanne) 15, 1373862. doi:10.3389/fendo.2024.1373862
Song, S., Cai, X., Hu, J., Zhu, Q., Shen, D., Ma, H., et al. (2024b). Effectiveness of spironolactone in reducing osteoporosis and future fracture risk in middle-aged and elderly hypertensive patients. Drug Des. Devel Ther. 18, 2215–2225. doi:10.2147/DDDT.S466904
Srikanth, V. K., Fryer, J. L., Zhai, G., Winzenberg, T. M., Hosmer, D., and Jones, G. (2005). A meta-analysis of sex differences prevalence, incidence and severity of osteoarthritis. Osteoarthr. Cartil. 13 (9), 769–781. doi:10.1016/j.joca.2005.04.014
Sun, K., Hou, L., Guo, Z., Wang, G., Guo, J., Xu, J., et al. (2023). JNK-JUN-NCOA4 axis contributes to chondrocyte ferroptosis and aggravates osteoarthritis via ferritinophagy. Free Radic. Biol. Med. 200, 87–101. doi:10.1016/j.freeradbiomed.2023.03.008
Takamatsu, A., Ohkawara, B., Ito, M., Masuda, A., Sakai, T., Ishiguro, N., et al. (2014). Verapamil protects against cartilage degradation in osteoarthritis by inhibiting Wnt/β-catenin signaling. PLoS One 9 (3), e92699. doi:10.1371/journal.pone.0092699
Terenzi, R., Manetti, M., Rosa, I., Romano, E., Galluccio, F., Guiducci, S., et al. (2017). Angiotensin II type 2 receptor (AT2R) as a novel modulator of inflammation in rheumatoid arthritis synovium. Sci. Rep. 7 (1), 13293. doi:10.1038/s41598-017-13746-w
Tian, X., Chen, L., Gai, D., He, S., Jiang, X., and Zhang, N. (2022). Adverse event profiles of PARP inhibitors: analysis of spontaneous reports submitted to FAERS. Front. Pharmacol. 13, 851246. doi:10.3389/fphar.2022.851246
Uzieliene, I., Bernotiene, E., Rakauskiene, G., Denkovskij, J., Bagdonas, E., Mackiewicz, Z., et al. (2019). The antihypertensive drug nifedipine modulates the metabolism of chondrocytes and human bone marrow-derived mesenchymal stem cells. Front. Endocrinol. (Lausanne) 10, 756. doi:10.3389/fendo.2019.00756
Valdes, A. M., Abhishek, A., Muir, K., Zhang, W., Maciewicz, R. A., and Doherty, M. (2017). Association of beta-blocker use with less prevalent joint pain and lower opioid requirement in people with osteoarthritis. Arthritis Care Res. Hob. 69 (7), 1076–1081. doi:10.1002/acr.23091
van de Laar, I. M., Oldenburg, R. A., Pals, G., Roos-Hesselink, J. W., de Graaf, B. M., Verhagen, J. M. A., et al. (2011). Mutations in SMAD3 cause a syndromic form of aortic aneurysms and dissections with early-onset osteoarthritis. Nat. Genet. 43 (2), 121–126. doi:10.1038/ng.744
Veronese, N., Stubbs, B., Solmi, M., Smith, T. O., Noale, M., Schofield, P., et al. (2018). Knee osteoarthritis and risk of hypertension: a longitudinal cohort study. Rejuvenation Res. 21 (1), 15–21. doi:10.1089/rej.2017.1917
Wang, Y., Kou, J., Zhang, H., Wang, C., Li, H., Ren, Y., et al. (2018). The renin-angiotensin system in the synovium promotes periarticular osteopenia in a rat model of collagen-induced arthritis. Int. Immunopharmacol. 65, 550–558. doi:10.1016/j.intimp.2018.11.001
Whelton, P. K., Carey, R. M., Aronow, W. S., Casey, D. E., Collins, K. J., Dennison Himmelfarb, C., et al. (2017). 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American college of cardiology/American heart association task force on clinical practice guidelines. J. Am. Coll. Cardiol. 71 (19), e127–e248. doi:10.1016/j.jacc.2017.11.006
Wluka, A. E., Cicuttini, F. M., and Spector, T. D. (2000). Menopause, oestrogens and arthritis. Maturitas 35 (3), 183–199. doi:10.1016/s0378-5122(00)00118-3
Wu, M., Peng, Z., Zu, C., Ma, J., Lu, S., Zhong, J., et al. (2016). Losartan attenuates myocardial endothelial-to-mesenchymal transition in spontaneous hypertensive rats via inhibiting TGF-β/smad signaling. PLoS One 11 (5), e0155730. doi:10.1371/journal.pone.0155730
Yamagishi, K., Tsukamoto, I., Nakamura, F., Hashimoto, K., Ohtani, K., and Akagi, M. (2018). Activation of the renin-angiotensin system in mice aggravates mechanical loading-induced knee osteoarthritis. Eur. J. Histochem 62 (3), 2930. doi:10.4081/ejh.2018.2930
Yu, C., Tang, W., Wang, Y., Shen, Q., Wang, B., Cai, C., et al. (2016). Downregulation of ACE2/Ang-(1-7)/Mas axis promotes breast cancer metastasis by enhancing store-operated calcium entry. Cancer Lett. 376 (2), 268–277. doi:10.1016/j.canlet.2016.04.006
Zhang, Y., Wang, Y., Zhao, C., Cai, W., Wang, Z., and Zhao, W. (2023). Effects of blood pressure and antihypertensive drugs on osteoarthritis: a mendelian randomized study. Aging Clin. Exp. Res. 35 (11), 2437–2444. doi:10.1007/s40520-023-02530-8
Zhang, Y. M., Wang, J., and Liu, X. G. (2017). Association between hypertension and risk of knee osteoarthritis: a meta-analysis of observational studies. Med. Baltim. 96 (32), e7584. doi:10.1097/MD.0000000000007584
Zhou, L., Kwoh, C. K., Ran, D., Ashbeck, E. L., and Lo-Ciganic, W. H. (2020). Lack of evidence that beta blocker use reduces knee pain, areas of joint pain, or analgesic use among individuals with symptomatic knee osteoarthritis. Osteoarthr. Cartil. 28 (1), 53–61. doi:10.1016/j.joca.2019.08.008
Keywords: hypertension, osteoarthritis, pharmacovigilance, valsartan, FAERS
Citation: Guo Z, Di J, Zhang Z, Chen S, Mao X, Wang Z, Yan Z, Li X, Tian Z, Mu C, Xiang C and Xiang C (2024) Antihypertensive drug-associated adverse events in osteoarthritis: a study of a large real-world sample based on the FAERS database. Front. Pharmacol. 15:1404427. doi: 10.3389/fphar.2024.1404427
Received: 22 March 2024; Accepted: 22 July 2024;
Published: 02 September 2024.
Edited by:
Xintian Cai, People’s Hospital of Xinjiang Uygur Autonomous Region, ChinaReviewed by:
Di Shen, People’s Hospital of Xinjiang Uygur Autonomous Region, ChinaAnoop Kumar, Delhi Pharmaceutical Sciences and Research University, India
Joao Massud, Independent Researcher, São Paulo, Brazil
Guan Xin, Shanxi Bethune Hospital, Shanxi Medical University, China
Copyright © 2024 Guo, Di, Zhang, Chen, Mao, Wang, Yan, Li, Tian, Mu, Xiang and Xiang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chuan Xiang, Y2h1YW54aWFuZ0BzeG11LmVkdS5jbg==
†ORCID: Chuan Xiang, orcid.org/0000-0002-1121-6443
‡These authors have contributed equally to this work
 Zijian Guo1‡
Zijian Guo1‡ Jingkai Di
Jingkai Di Chuan Xiang
Chuan Xiang