
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Pharmacol. , 22 July 2024
Sec. Ethnopharmacology
Volume 15 - 2024 | https://doi.org/10.3389/fphar.2024.1403823
Immunometabolism has been an emerging hotspot in the fields of tumors, obesity, and atherosclerosis in recent decades, yet few studies have investigated its connection with rheumatoid arthritis (RA). In principle, intracellular metabolic pathways upstream regulated by nutrients and growth factors control the effector functions of immune cells. Dynamic communication and hypermetabolic lesions of immune cells within the inflammatory synovial microenvironment contributes to the development and progression of RA. Hence, targeting metabolic pathways within immune subpopulations and pathological cells may represent novel therapeutic strategies for RA. Natural products constitute a great potential treasury for the research and development of novel drugs targeting RA. Here, we aimed to delineate an atlas of glycolysis, lipid metabolism, amino acid biosynthesis, and nucleotide metabolism in the synovial microenvironment of RA that affect the pathological processes of synovial cells. Meanwhile, therapeutic potentials and pharmacological mechanisms of natural products that are demonstrated to inhibit related key enzymes in the metabolic pathways or reverse the metabolic microenvironment and communication signals were discussed and highlighted.
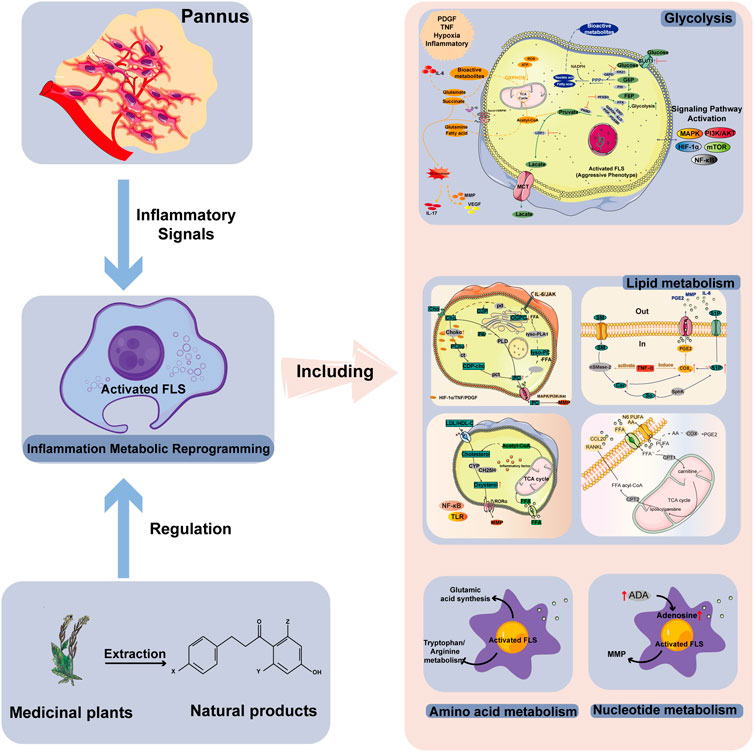
GRAPHICAL ABSTRACT | Activated FLSs in the synovial microenvironment of RA will undergo metabolic reprogramming of glycolysis, lipid, amino acid and nucleotide metabolism under the stimulation of inflammation, hypoxia and other conditions. The bioactive natural products of medicinal plants are able to regulate these four major metabolic modes to reverse the abnormal metabolism in the RA microenvironment.
Rheumatoid arthritis (RA) is a disease caused by the continuous proliferation of fibroblast-like synovial (FLS) cells in the joint synovium, resulting in local tissue hypoxia and inflammatory infiltration. Recent studies show that the cellular metabolism supports cell proliferation and differentiation and thus affects cell function, which is called immunometabolism (Cui et al., 2022). Immunometabolism has been actively studied in the field of tumor, and various types of metabolic modalities have been analyzed and outlined for different types of malignancies. Whereas, studies in RA have not been as extensive.
The RA microenvironment is characterized by low oxygen, acidity, and low nutrient concentrations (Gaber et al., 2005). In pathological states, almost all metabolic pathways, such as gluconeogenesis, lipid, and protein metabolism, exhibit different metabolic activation patterns (Masoumi et al., 2020). Metabolic reprogramming, with some positive correlation between the activation of the aggressive phenotype of FLS cells and subsequent destruction of bone and cartilage (de Oliveira et al., 2019), has a hallmark position in explaining pathogenic cell function and differentiation in RA (Weyand and Goronzy, 2017). As certain metabolic pathways or metabolites can be exploited to trigger abnormal activation of immune cells or stromal cells involved in RA (Okano et al., 2018), regulating immune metabolism or correcting metabolic abnormalities are other ground-breaking treatment strategies for RA.
Conventional, biological, and new non-biological disease-modifying antirheumatic drugs, are frequently used to treat patients with RA. Although the majority of patients now have a positive outlook, several patients still prove treatment resistance. New therapies are so desperately needed (Smolen et al., 2016). Therefore, identifying and creating innovative, efficient, and safer options for long-term use is necessarily needed. More than 75% of the world’s population continues to employ phytotherapy, a traditional medicinal technique, to cure a variety of ailments (Koycheva et al., 2023).
Here, we present the detailed pathogenesis of RA involving immunometabolism processes of glycolysis, lipid, amino acid and nucleotide metabolism. We further summarize natural products as prospective clues for the development of effective therapeutic approaches and how to treat RA by targeting key enzymes or regulating metabolic balance.
Carbohydrate metabolism is the main metabolism that provides biomass and energy after cell activation. During the physiological state, glucose can maintain a dynamic equilibrium of several biomass. Glucose metabolism mainly includes three types of pathways: glucose, which is first transported into the cell through glucose transporter 1 (GLUT1), then broken down by sequential metabolic enzymes, including hexokinase (HK), aldolase, phosphoglycerate kinase 1 (PGK1), and pyruvate kinase, to generate pyruvate, undergoes glycolysis in the cytoplasm to produce lactic acid under oxygen-deficient conditions; pyruvate is transported into the mitochondria under oxygen-enriched conditions and converted into acetyl-CoA in the tricarboxylic acid cycle (TCA cycle). The generated NADH and FADH are further oxidatively phosphorylated (OXPHOS) to generate ATP; and pentose phosphate pathway results in biomass generation. Resting-state cellular metabolism is dominated by OXPHOS.
In RA synovial tissue, platelet-derived growth factors (PDGF), tumor necrosis factor (TNF), and pro-inflammatory mediators can transform resting FLS cells into an aggressive phenotype associated with glycolysis (RAFLS), known as the “Warburg effect” (Biniecka et al., 2016; Garcia-Carbonell et al., 2016; Masoumi et al., 2020). Hypoxia and high expression of HIF-1α in RA can stimulate the metabolic shift of RAFLS cells from oxidative phosphorylation to glycolysis as HIF-1α can upregulate genes involved in glycolysis, such as GLUT-1, HK-2, and LDH (Masoumi et al., 2020). ENO1 is a rate-limiting enzyme in glycolysis. Under hypoxic conditions, ENO1 gene expression was significantly upregulated in RAFLS and promoted its proliferation. The PGK1 catalyzes the transformation of 1,3-diphosphoglycerate into 3-phosphoglycerate, which results in the production of the first adenosine triphosphate in glycolysis (Zhao et al., 2016). Thus, GLUT1 and HK2 are important modulators of the glucose metabolism. Other glycolysis-related rate-limiting enzymes include, but are not limited to, PFKB3 and PKM2, which have garnered remarkable attention as potential anti-arthritis treatments (Bustamante et al., 2018). Silent information regulator 1 (SIRT1) is an energy sensor that controls associated signals like peroxisome proliferator-activated receptor-γ (PPAR-γ) and maintains metabolic homeostasis; along with deacetylates some signals to prevent their transcriptional activity, like NF-κB and HIF-1α, thereby affecting RA (Wu et al., 2021). Patients with RA exhibit SIRT1 deficiency (Wendling et al., 2014), and the SIRT1 is a negative regulator of glycolysis and M1 macrophage polarization (Lei et al., 2021). Enhancement of the glycolytic metabolic pathway leads to an increase in the lactic acid level. Moreover, further transport of lactic acid into joint lumen can stimulate the phenotype transformation, proliferation, and aggressive metastasis of FLS cells, and increases glycolysis as to accelerate the development of RA, ultimately trigging matrix metalloproteinases and further promoting bone and cartilage destruction (Bottini and Firestein, 2013; Garcia-Carbonell et al., 2016; Masuko, 2022; Pucino et al., 2023) (See green arrow route in Figure 1).
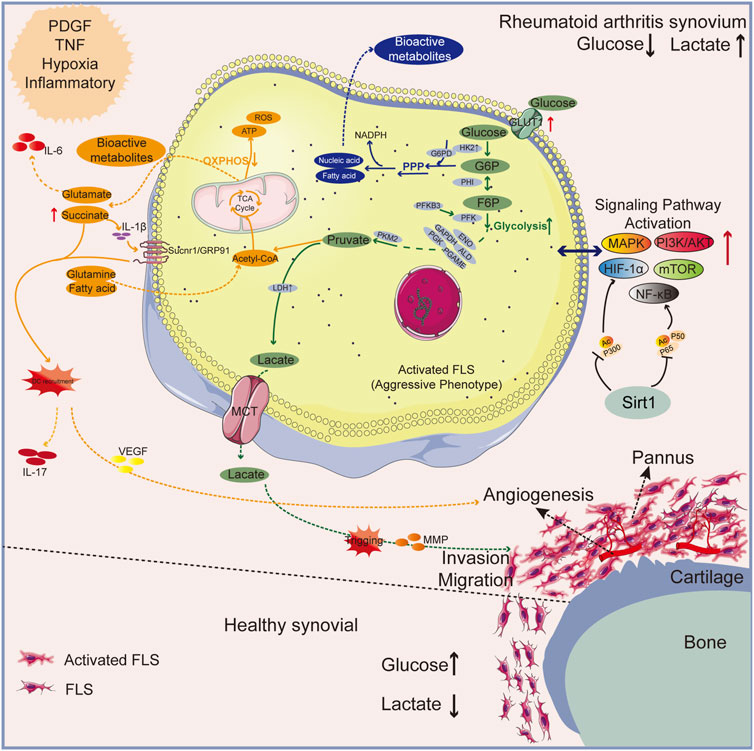
Figure 1. An overview of the mechanisms behind glycolysis of RA pathology. The normal synovial lining has only 2-3 layers of FLS cells; RA’s synovium is a proliferative lining rich in multiple layers of activated FLS cells and phagocytes. Upregulated GLUT1 and HK2 enzymes in synovial fluid owing to the inflammatory microenvironment results in enhanced glycolysis and pentose phosphate pathway (PPP pathway) shunt to produce bioactive metabolites. Lactic acid and succinic acid, by-products of glucose metabolism, can cause angiogenesis and bone destruction. Green arrow: glycolytic pathway; Blue arrow: PPP pathway. Orange arrow: OXPHOS pathway.
As a natural naphthoquinone isolated from Lithospermum erythrorhizon, shikonin alleviated local joint swelling, and lowered lactate levels in peripheral blood of rats in an AA animal model experiment, along with less toxicity to the liver and kidneys. Mechanistically, shikonin could regulate glycolysis and ATP production by inhibiting GLUT1, PKM2 and HK2 alone, which was verified by knocking down PKM2 expression in RAFLSs. Shikonin has the ability to deactivate mTOR, phosphorylated PI3K and AKT to inhibit PI3K/AKT/mTOR pathway and the primary byproduct of glycolysis, lactate (Lu et al., 2018; Li et al., 2021). Shikonin possesses anti-inflammatory and immunomodulatory properties, but this is the first time proposed that its treatment of RA is related by regulating glycolysis. And there seems to be no relevant literature exploring the efficacy of its combination therapy.
Epigallocatechin-3-gallate (EGCG) from green tea has been demonstrated to have therapeutic efficacy in the treatment of IL-1 receptor antagonist knockdown (IL-1RaKO) autoimmune arthritis rats when administered at 40 mg/kg. In fact, mechanistic studies have found that EGCG administration inhibits the production of indicators of glycolysis and mTOR signaling pathway that support Th17 development, including HIF-1a, GLUT-1, MCT4, LDH-a, and GPI, which contributed to the amelioration of arthritis (Yang et al., 2014). Methotrexate is limited by potential hepatotoxicity in the treatment of RA, and EGCG has been identified to improve MTX-induced hepatotoxicity (Pradhan et al., 2023).
An aberrant metabolism environment of increase in PKM enzyme and lactate expression, a considerable decline in triglycerides and pyruvate were reshaped by resveratrol, a SIRT1 agonist, thereby reducing improper glycolytic metabolism to alleviate symptoms of AIA rats at a dose of 50 mg/kg (Wang DD. et al., 2022). Mechanism studies suggest that the therapeutic effect of SIRT1 may be related to the change of monocyte polarization. Overexpression of SIRT1 dramatically boosts p-AMPK expression while suppressing the aberrant increase in PKM1/2 expression. Simultaneously, it significantly inhibits the expression of HIF-1α and its downstream TPI1, nevertheless, new research has demonstrated that resveratrol induced SIRT1 activation inhibits glycolytic facilitated angiogenesis in RA, irrespective of HIF-1α (Jiang TT. et al., 2023). According to certain research, SIRT1 is typically under-expressed in RA patients. Still, some research has cast doubt on this. Therefore, the development of SIRT1-targeted anti-rheumatic therapy depends on the successful confirmation of this issue. In other research reports, resveratrol has long been considered a potential antioxidant for the treatment of RA, which improves RA by activating the SIRT1-Nrf2 signaling pathway (Wang et al., 2020). Although resveratrol has excellent clinical efficacy, patients with multiple myeloma have reported major side effects (Berman et al., 2017).
α-mangostin extracted from mangosteen can effectively inhibit aerobic glycolysis, restore the level of LDH, and abrogate HIF-1α/VEGF expression in AIA rats when the dose is set at 30 mg/kg/day, thereby alleviating inflammation-related hypoxia. This property of α-mangostin is beneficial in blocking synovial pathologic neovascularization (Jiang et al., 2021). There was a more notable reduction in VEGF in the synovium when α-mangostin therapy was employed in place of the typical DMARD leflunomide. This promising discovery suggested that α-mangostin could be more effective than conventional anti-rheumatic medications in regulating angiogenesis (Zuo et al., 2018; Jiang et al., 2021). In addition to these, in AIA models, α-mangostin has been provided to prevent M1 polarization of macrophages and monocytes by concurrently upregulating SIRT1 and PPAR-γ (Wu et al., 2023), and also to reduce inflammation by interfering with the metabolism-immune feedback system mediated by fat cells (Hu et al., 2021). α-mangostin has been demonstrated to be safe in a variety of animal studies. However, increased doses or repeated dosing should be considered for probable adverse effects such as dysbiosis, cardiac insufficiency, and liver toxicity (Do and Cho, 2020).
High levels of ROS and inflammatory mediators boost glycolysis in M1 macrophages, which causes a buildup of intermediates from the TCA cycle, particularly succinate (Peyssonnaux et al., 2007; West et al., 2011; Mills et al., 2016). By producing IL-1β, succinate has a role in recruiting dendritic cells to lymphoid tissue. Th17 cell releasing factors are activated when succinate upregulates the succinate receptor, Sucnr1/GRP91, intensifying the inflammatory milieu (Littlewood-Evans et al., 2016; Saraiva et al., 2018) and inducing VEGF expression, which results in abnormal angiogenesis (Reddy et al., 2018). Furthermore, inflammation promotes the synthesis of the physiologically active metabolite itaconate, which hinders succinate dehydrogenase’s (SDH) ability to operate as an enzyme and causes succinate buildup. When SDH is blocked, the TCA cycle is disrupted, which encourages the generation of ROS inside the mitochondria, the stabilization of HIF-1α, and the prolonged fermentation of glycolysis within the activated cells (Lampropoulou et al., 2016). (See orange arrow route in Figure 1).
Cinnamon is a TCM used to treat RA. Cinnamaldehyde has been found to alleviate the advancement of RA by modulating the succinate/GPR91/HIF-1α axis to limit NLRP3 activation and IL-1β release when administered at 200 mg/kg in vivo and 12.5 μM in vitro (Liu et al., 2020). Although cinnamaldehyde modifies the generation of succinic acid, its major mechanism of attenuating collagen-induced arthritis is via lowering pro-inflammatory cytokines and free radicals (Mateen et al., 2019). For this reason, cinnamaldehyde primarily serves as an adjuvant in the management of RA.
TGF-β1 stimulation of hypoxia increased succinic acid buildup in the synovium of RA rats by reversing SDH activation, and it also triggered the activation of the NLRP3 inflammasome in a way that was dependent on HIF-1α induction. Clematichinenoside AR, a compound isolated from the root of Clematis manshurica Rupr., inhibits TGF-β1-induced hypoxia and the activation of succinic acid-associated NLRP3 inflammasome in the synovium of RA rats by inhibiting SDH activity, thereby preventing myofibroblast activation via the prevention of a cross-talk between inflammation and fibrosis whose dose at 50 mg/kg (Li et al., 2016). Accordingly, succinate as a metabolic signal could be one of the targets for RA intervention.
Additionally, there are other pathologic phenomena in the pathway that triggers RA “Warburg effect” leading to activation of lipid and protein metabolism. Glutamate is elevated in arthritic joints, and the triggering of glutamate receptors induces the release of IL-6 and arthritic pain (Bonnet et al., 2015) (See orange arrow route in Figure 1). Fatty acid and nucleic acid are examples of intermediate metabolites also regulate signaling pathways that trigger immune cells to pick up various signals in the microenvironment and drive pathogenic actions (Weyand and Goronzy, 2017) (See blue arrow route in Figure 1). We go over the related natural products in the section below.
To meet the body’s energy requirements, the synthesis and breakdown of lipids in the body maintain a homeostatic balance. Lipid metabolism is involved in the process of cartilage synthesis (Robinson et al., 2022). However, the RA synovial tissue’s inflammatory milieu induces a dysfunctional state of lipid metabolism. A prior study demonstrated a strong correlation between the presence of inflammatory mediators in the serum of RA patients and CIA mice and lipid metabolism (Arias de la Rosa et al., 2018). Preliminary studies in the 1960s showed a 40%–60% increase in phospholipids and cholesterol in synovial fluid in RA patients compared to corresponding serum samples from healthy controls (Bole, 1962). Phospholipids (categorized as glycerophospholipids and sphingolipids), cholesterol, triglycerides, and plasma lipoproteins account for the majority of biochemical components of post-synthesis fat metabolism.
With the aggravation of inflammation, the choline pathway, where choline kinase (Chokα) catalyzes the conversion of choline into CDP-choline and subsequent formation of phosphatidylcholine (PC), is highly activated in FLS, resulting in the activation of the regulatory MAPK/PI3K/Akt pathway, MMP expression, and promotion of invasive metastasis of RAFLS cells (Glunde et al., 2011; Guma et al., 2015; Bustamante et al., 2017; Miriam Jose and Rasool, 2022). Correspondingly, the metabolite, 1-oleoyl-sn-glycero-3-phosphocholine (OPGC), in the inhibited choline phospholipid metabolism pathway is downregulated in the plasma of RA patients and participates in the pathogenesis of RA by modulating the IL-6/JAK signaling pathway (Figure 2) (Su et al., 2022). The glycerophospholipid metabolic pathway is markedly dysregulated in RA patients, according to multi-omics research. Acetylcholinesterase (ACHE), one of the key genes, showed a substantial enrichment in the glycerophospholipid pathway. Glycerophosphocholine and inflammatory characteristics have a substantial negative association (Jian et al., 2023).
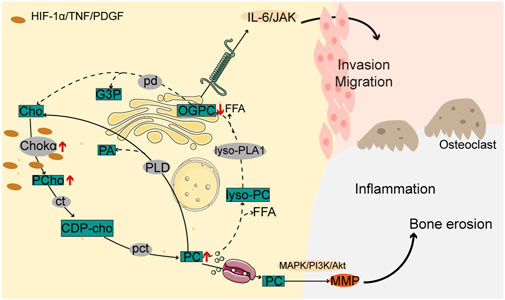
Figure 2. Choline pathway is involved in the pathogenesis of RA. In RAFLS cells, increased choline kinase activity promotes phosphocholine and phosphatidylcholine biosynthesis while reducing glycerophosphocholine metabolites, resulting in MAPK/PI3K/Akt and IL-6/JAK pathway activation along with increased inflammation, and malignant cell invasion and migration, and bone erosion.
Network pharmacological studies have shown that Tripterygium wilfordii glycosides (TWG) can target PLA2 and thus interfere with glycerophospholipid metabolism and ether lipid metabolism, resulting in changes in lysoPC and PC levels. The reduction of lyso-PC levels in CIA rats can be corrected treatment with 6 mg/kg TWG per day (Gao et al., 2023). ChoKα has been reported to regulate the tumor-like phenotype of RA-FLS via activating MAPK and PI3K/Akt proteins. In the IL-21-induced tumor-like phenotype assay of AIA-FLS cells, myricetin, a flavonoid, significantly inhibited the high proliferative and invasive potential of AIA-FLSs at an administered concentration of 10 μM. Mechanistically, myricetin inhibited the IL-21/Ras/ChoKα signaling cascade response (Jose and Rasool, 2023).
Lipids are known to contribute to the progression of chronic inflammation, and of the many lipids formed, sphingolipids are thought to be the most deleterious, with their ability to affect bone homeostasis (Seal et al., 2024). Sphingosine (So), sphingosine 1-phosphate (S1P), and ceramide (Cer) are the central molecules of sphingolipid metabolism (Meshcheryakova et al., 2017). Cer promotes chondrocyte apoptosis (Herr and Debatin, 2001). The balance between them is hypothesized to act as a switch that can control whether cells grow or die (Cuvillier et al., 1996). Sphingosine kinase (SphK) phosphorylates So to S1P (Bustamante et al., 2017), and the synovial fluid of RA patients has been found to contain active SphK and high amounts of S1P (Kitano et al., 2006). Two SphK/S1P pathway-regulated mechanisms may be simultaneously involved in the development of RA: inflammation and bone catabolism. S1P stimulates the migration and invasion of FLS, causing synovial thickening and promoting osteoclast formation and synovitis (El Jamal et al., 2020). Under inflammatory conditions, TNF-α activates neutral sphingomyelinase-2 (nSMase-2), resulting in the hydrolysis of sphingomyelin (SM) to Cer, and activates ceramidase (CDase) and SphK, resulting in increased levels of Cer and S1P. The buildup of Cer and S1P promotes the expression of COX-2 and the release of PGE2, which are involved in the pathogenesis of RA, and enhances the MMP response, leading to accelerated bone destruction (Figure 3) (Qu et al., 2018).
According to a study, treatment with T. wilfordii glycosides (TWG, 6 mg/kg) entirely corrected the high Cer levels and markedly decreased the levels of several SM compounds, which has a positive therapeutic effect on RA and inflammation. CDase is required for the conversion of Cer to SM. Some data suggest that TWG can interact with CDase to modulate Cer levels (Zhu et al., 2022). Nevertheless, a growing number of research investigations have demonstrated that T. wilfordii’s active components frequently have adverse consequences including damage of the skin, gastrointestinal, reproductive, blood, and hepatobiliary systems (Lin et al., 2020). Geniposide extracted from Gardenia jasminoides Ellis, when administered 60 mg/kg daily by gavage, can reverse the levels of Cer, palmitoyl ethanolamine, and glycerophospholipid metabolites in AA rats and further reduce inflammation in AA rats by regulating imbalanced sphingolipid metabolism (Zhan et al., 2020; Ke et al., 2022). Changes in geniposide regulated Cer levels were associated with downregulation of A-SMase expression. In addition, geniposide alleviates VEGF-induced angiogenesis by down-regulating S1P/S1PR1 signaling activation (Wang Y. et al., 2022) and attenuates RAFLS aberrant proliferation by preventing HIF-1α accumulation in synovial anoxic microenvironment (Gan et al., 2022).
Cholesterol biosynthetic activity is increased in chondrocytes, which promotes chondrogenesis by preventing apoptosis (Wu and De Luca, 2004). Patients with RA have upregulated cholesterol hydroxylase (CH25H) that facilitates cholesterol biosynthesis and production of more oxysterol metabolites (Perucha et al., 2019). Cellular homeostasis of cholesterol is controlled by end-product feedback mechanisms performed by the sterol regulatory element binding protein (SREBP) pathway (Yan et al., 2021). Cholesterol metabolism was found to exert catabolic functions in chondrocytes by upregulating matrix-degrading enzymes, leading to the development of arthritis (Figure 4) (Choi et al., 2019).
Berberine is hypothesized to suppress the inflammatory proliferation of RAFLSs by modulating AMPK/lipogenesis, LPA/LPA1/ERK/p38 MAPK (Shen et al., 2020), and ROS-AMPK signaling pathways at 80 μM. TNF-induced RAFLSs overexpress lipid metabolism regulator, SREBP1. Of note, berberine alleviates this expression, thereby reducing the intracellular TNF-stimulated inflammatory proliferation of RAFLSs via mitigating palmitic acid levels (Fan et al., 2018). On the other hand, additional research indicates that the antiarthritic effect of berberine is related to the inhibition of glycolysis. Berberine activates AMPK signaling, downregulates mTORC1/HIF-1α signaling and inhibits the glycolysis of M1 macrophages (Cheng et al., 2023). Berberine reversed the glycolytic reprogramming of CD4+ T cells induced by M1-exo (Cai et al., 2024). Although it has previously been found to cross the placenta to cause harmful effects on the fetus and lead to uterine contractions and miscarriage, berberine has not been found to show any cytotoxic or mutagenic effects at the clinical dosages utilized (Bansod et al., 2021). Recent studies have revealed that certain clinical anti-RA medications are used in conjunction with berberine to reduce side effects. To take one example, the combination of berberine and diclofenac sodium can lessen damage to the intestinal mucosa (Huang et al., 2021).
Free fatty acids are present in the serum and synovial fluid of RA patients, and can impact joint tissues and immune cells involved in the pathophysiology of the disease. According to a study, the synovial environment stimulates carnitine abundance in the mitochondrial FAO pathway of human monocytes under hypoxic conditions, which can trigger a CCL20-mediated inflammatory cascade promoting RA pathogenesis (Rodgers et al., 2020). Fatty acids can be metabolized into bioactive lipid mediators, such as pro-inflammatory prostaglandins (PGs), thromboxanes, and leukotrienes (LTs), that can further damage bone and cartilage, and contribute to the pro-inflammatory environment of FLS in RA (Figure 5A) (Lei et al., 2023). Moreover, RA patients have higher activation of PLA enzymes than healthy individuals, which may lead to higher levels of fatty acids and pro-inflammatory effects of the PC/PLA/LPC axis. Studies have shown that PGE2 and the enzymes involved in its production, PLA2 and COX, are more highly expressed in synovial fluid (Figure 5B) (Brouwers et al., 2015). Arachidonic acid (AA) metabolism is involved in the synthesis of PGs and LTs through cyclooxygenase (COX) and lipoxygenase (LOX) to stimulate inflammatory responses (Figure 5B). Recent clinical results suggest that those individuals at risk for ACPA-positive RA show higher levels of LOX-derived oxylipins (O'Neil et al., 2024).
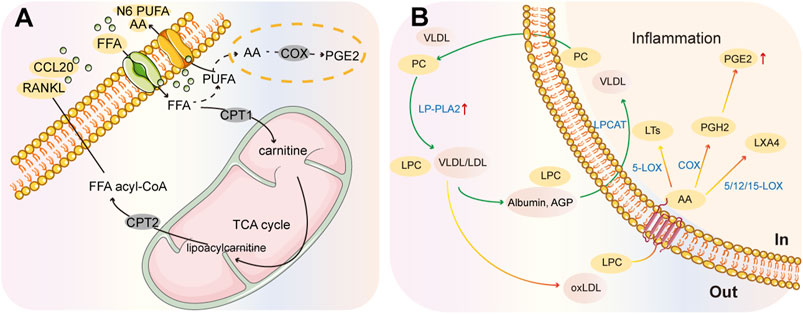
Figure 5. Summary chart of fatty acid metabolism in RA pathology. (A) CCL20-mediated inflammatory cascade response. (B) PC/PLA/LPC metabolic axis. Phospholipases (PLA) can generate LPC from PC and release fatty acids from membrane phospholipids. These fatty acids can be metabolized into inflammatory lipid mediators, such as prostaglandins and PGE2, by different cyclooxygenases (COX) and lipoxygenases (LOX).
PUFA is one of the metabolite fatty acids of triglycerides that is divided into N6 PUFA with a pro-inflammatory effect and N3 PUFA with an anti-inflammatory effect based on the location and role of the double bond. N3 PUFA can regulate gene expression by activating the peroxisome PPAR-γ (Sun et al., 2008). PPAR-γ has been discovered in rat and human cartilage, and activating PPAR-γ prevents human chondrocytes from producing NO and MMP-13 in response to IL-1 (Fahmi et al., 2001; Yang et al., 2021). Thus, PPAR-γ ligands may be of therapeutic value in RA (Ricote et al., 1998).
Thymoquinone (TQ) has been demonstrated to prevent RA by suppressing COX, LOX, inflammatory cytokines, LPO, and NO (Ali et al., 2021). Thymoquinone has regulatory effects on inflammatory molecular signaling pathways like NF-κβ, JAK-STAT, MAPK signaling, immune cells, and epigenetic machinery (Vaillancourt et al., 2011).
Caffeic acid is a potent inhibitor of LT biosynthesis that blocks 5-LOX activity and AA release, thereby improving RA through its anti-inflammatory and anti-lipid peroxidation actions (Boudreau et al., 2012). Furthermore, caffeic acid ameliorate adjuvant-induced arthritis in rats via targeting inflammatory signals, chitinase-3-like protein-1 and angiogenesis at 50 mg/kg/day for 20 days. Notably, caffeic acid has similar efficacy to celecoxib in alleviating assisted induced arthritis with fewer side effects. These findings may advocate the use of caffeic acid as an adjunct strategy for RA management (Fikry et al., 2019).
An earlier experiment revealed that norisoboldine (NOR), isolated from Linderae Radix, was effective at alleviating arthritic rat conditions when administered orally at a dose of 30 mg/kg. Fang et al. proposed that the anti-arthritic effects of NOR were attributed to the restoration of aberrant lipid metabolism, as demonstrated in detail by the upregulation of carnitine palmitoyltransferase 1 expression and downregulation of fatty acid synthase expression by NOR in the spleen and synovial tissues of CIA rats (Fang et al., 2021).
Isosilybin A, a major bioactive constituent of silymarin, is a partial PPARγ agonist that modifies abnormalities of lipid metabolism and stimulates cholesterol efflux from THP-1 macrophages (Wang et al., 2015). Likewise, Morin (Miao et al., 2022) and ginsenoside Rg1 (Zhang et al., 2017) is medications with PPARγ agonist characteristic that can alleviate RA.
The anti-rheumatic effects of TWG may possibly be attributed to the suppression of pro-inflammatory lipid mediators but the stimulation of certain pro-resolving mediators, and the 5-LOX is a direct target of TWG (Zhang et al., 2021). According to an analysis of the chemical constituents of TWG, 5R-Hydroxytriptolide (LLDT-8) is a novel analog of T. wilfordii-derived triptolide. LLDT-8 regulates the expression of stearoyl-CoA desaturase-1 and PPARγ, which contributes to the promotion of significant degradation of lipids and the inhibition of lipid synthesis. T. wilfordii glycosides have been demonstrated to reduce the inflammatory response by down-regulating PGs, metalloproteinases, and inducible COX-2 in previous investigations (Shan et al., 2023). Despite the fact that these results validate TWG’s advantageous characteristics in treating RA, there is still cause for worry given TWG’s widely recognized toxicity as seen in clinical studies.
Fatty acid oxidative (FAO) metabolism is involved in the mechanism of joint destruction in RA (Huang et al., 2022a). Lipid peroxidation might be the defining mechanism of the damage that occurs during RA. Hesperidin (Umar et al., 2013) and quercetin exhibits anti-inflammatory and antioxidant effects in the treatment of arthritis (Jeyadevi et al., 2013). And there are no significant toxicological effects of hesperidin and quercetin were found in cell and animal toxicological evaluation experiments (Ortiz-Andrade et al., 2020). Similarly, compounds, such as protocatechuic acid (Lende et al., 2011) and the extracts of Trigonella foenum graecum (Fenugreek) seeds (Suresh et al., 2012) can minimize lipid peroxidation to treat arthritis.
Glutamine is a potential biomarker in RA patients as it regulates RAFLS proliferation and is involved in the pathogenesis of RA. In RAFLSs, glutaminase 1 expression increased and glutamine metabolism increased (Takahashi et al., 2017). Ornithine is a precursor of glutamate associated with RA; thus, elevated levels of ornithine may affect TNF-α expression via glutamate activity.
Additionally, patients with RA exhibit several other amino acid metabolism, including abnormal tryptophan (Rylance, 1969) and arginine metabolism (Panfili et al., 2020). Metabolomic profiles of synovial fluid from RA patients revealed the downregulation of tryptophan metabolites, which is attributed to the aberrant methylation of the cytotoxic T lymphocyte antigen 4 gene promoter and a hypoxic environment in synovial fluid leading to indoleamine 2,3-dioxygenase 1 (IDO1) low expression (Kaul et al., 2020; Panfili et al., 2020). Tryptophan is converted to kynurenine (KYNA) by the IDO1 enzyme, and IDO1-mediated tryptophan depletion suppresses T helper cells to terminate inappropriate immunological reactions (Tykocinski et al., 2017). The researchers have now conducted tryptophan pathway metabolomics analysis on serum samples from RA patients and normal individuals. Higher serotonin pathway metabolite concentrations and lower kynurenine and indole pathway metabolite concentrations were linked to RA. Quinolinic acid concentrations were higher in RA patients than in healthy individuals within the kynurenine pathway, but kynurenic acid concentrations and the ratio of xanthurenic acid to its precursor 3-hydroxykynurenine were lower. AADAT, also known as kynurenine aminotransferase 2, catalyzes the production of kynurenic acid and xanthurenic acid from precursors of quinolinic acid. Compared to healthy persons, patients with early RA had significantly reduced serum concentrations of AADAT (Phillips, 2024). The latest study has found that RA patients have reduced serum levels of KYNA and xanthurenic, as well as indole derivatives, and increased levels of quinolinic acid. Mechanistically, quinolinic acid favors the proliferation of RAFLSs and affects their cellular metabolism by inducing mitochondrial respiration and glycolysis (Moulin et al., 2024).
Synovial fluid from RA patients has high levels of arginase 1 (Arg1), the rate-limiting enzyme of arginine metabolism, which promotes fibrosis and tissue regeneration (Munder, 2009). iNOS and Arg1 play a major role in controlling the intracellular metabolism of L-arginine (L-Arg) in macrophages. L-Arg catalyzes the conversion of iNOS into NO and L-citrulline. L-citrulline has been discovered as a biomarker for RA (Yang et al., 2020). Early onset of RA is associated with dysregulation of citrullination, a process catalyzed by peptidylarginine deiminase isoform 4 (PADI-4) (Yang et al., 2024). Studies have shown that platelet arginase activity is greater in CIA rats than in control rats, which leads to an inadequate supply of substrate (L-arginine), resulting in nitric oxide synthase isolation, thereby increasing oxidative stress and lipid peroxidation and promoting the development of arthritis (Prati et al., 2011). Based on a latest study, L-Arg helps inflammatory osteoclasts convert glycolysis to oxidative phosphorylation. This process increases ATP synthesis, purine metabolism, and inosine and hypoxanthine levels, which helps to reduce bone erosion and arthritis (Cao et al., 2024).
A clinical trial evaluated the correlation between serum ornithine levels in RA patients and their degree of remission after the administration of sinomenine (SIN, 120 mg, twice daily), and revealed the potential therapeutic mechanism by which SIN could upregulate the gene expression level of Arg1, thereby inducing a reduction in arginine and further downregulation of ornithine (Shi et al., 2022). Another study found that SIN substantially elevated tryptophan metabolites, stimulated aryl hydrocarbon receptors (AhR) in CIA rats, and regulated Th17/Treg balance (Jiang ZM. et al., 2023). SIN can cause gastrointestinal reactions and transient allergic reactions (Huang et al., 2022b). Furthermore, a meta-analysis of ten randomized controlled trials revealed that a combined therapy of MTX and SIN was more successful than MTX alone (Chen et al., 2015). MTX and SIN combination treatment had a therapeutic effectiveness in prior research that was similar to MTX plus leflunomide, but with fewer side effects (Huang et al., 2019).
Eugenol, a phenolic substance, may inhibit pro-inflammatory arthritic responses in arthritis rats by regulating arginase in platelets and preventing lipid peroxidation when administered 2.5 mg/kg orally (Adefegha et al., 2018). At a certain concentration, eugenol impaired the locomotor ability of Drosophila melanogaster (Julio Cesar Silva, 2022).
A paper confirms that inhibition of PADI-4 by vitamin B12 ameliorates RA in CAIA mice (Yang et al., 2024). Atractylodes oil as a novel anti-RA drug (Linghang et al., 2020), and licorice flavonoids (Wei et al., 2018) have regulatory effects on a variety of amino acid metabolites.
Adenosine is produced by intracellular ATP catabolism and rises in hypoxic and inflammatory environments. The purine metabolism enzyme adenosine deaminase (ADA) influences both joint inflammation and adenosine levels. Patients with RA have higher levels of ADA activity in their synovial fluid, and findings indicate a substantial positive association between MMP-9 and ADA isoforms (Atta et al., 2024). According to previous studies, quercetin has an inhibitory effect on lymphocyte ADA activity and modulates nucleoside triphosphate diphosphohydrolase (NTPDase) activity to reduce adenosine levels (Saccol et al., 2019). According to a new concept, from participation in purine biosynthesis to post-translational modification of proteins, 1C metabolism promotes cell development and differentiation through the methionine and folate cycle. The regulation of immune cell function and differentiation, including t-cells, b-cells, and macrophages, is directly affected by 1C metabolism, and thus 1C metabolism could be a determinant of RA, promising to be explored for therapeutic intervention (Dang et al., 2024).
Glycolysis, lipid metabolism, amino acid biosynthesis, and nucleotide metabolism catalyze the disease process to some extent, making metabolic reprogramming of practical importance in the management of RA. This review sought to focus on the products or regulators of metabolic processes that are involved in the disruption of the internal environment to stimulate synovial cytopathology and the natural products that are available to improve or treat arthritis through metabolic reprogramming pathways. Numerous significant metabolic substances and processes, including glycolysis and phenylalanine metabolism, have been the subject of recent research. Studies relating to RA and nucleotide metabolism, still, have not been thoroughly examined. Natural products, as metabolic therapies for RA, can reverse internal environmental metabolic disturbances from multiple angles and targets, with compatibility patterns that rarely cause toxic side effects. The main information was summarized, as shown in Table 1. The rate-limiting enzymes of gluconeogenesis are GLUT1, HK2, G6PD, PFKB3, PKM2, and LDH. Tan et al. (2022) provided a list of organic compounds that might interfere with glycolysis-related transporter proteins and rate-limiting enzymes, which are primarily evaluated for malignancies. In a sense, these natural products may be potential agents that inhibit metabolic reprogramming in the development of RA.
The treatment of RA with Western medicine still entails severe side effects and drug resistance. In the treatment of RA, alternative medicine is becoming increasingly unique. We believe that the role of natural products in immune metabolism modulation in the therapy of RA can give clinicians with novel advice.
Cellular metabolism is an integral biochemical process in the pathogenesis of RA. However, few new mechanistic and clinical studies have been conducted on different metabolic treatment targets, which remain in the early stages of evaluation. Despite the fact that there are several descriptions of metabolic problems in RA, most of them focus on a single metabolism and are insufficiently detailed and clustered. The metabolic regulation processes of herbs in RA have not been completely studied, and the majority of them have only been assessed by metabolomics. Target metabolomics should be employed more in a subsequent study to confirm and investigate these compounds. The majority of the research on metabolomics investigations of bioactive natural products in the treatment mechanisms of RA does not address or clarify whether they have hazardous side effects, which is crucial and necessitates a more comprehensive evaluation including additional clinical trials. Notably, most conventional medications lack specificity, and new therapeutic approaches and research emphasize alternative medicine pathways that can be more precisely targeted to reduce inflammation while preventing the negative metabolic consequences of conventional therapy. Deficiencies of natural drugs, such as short half-life in the human body and susceptibility to cumulative toxicity, can be significantly improved by structural modification using computer-aided drug design and encapsulation of nanomaterials.
SH: Writing–original draft, Writing–review and editing. YL: Writing–original draft. YT: Conceptualization, Writing–original draft. JZ: Visualization, Writing–original draft. YH: Investigation, Writing–review and editing. GL: Investigation, Writing–review and editing. LL: Writing–review and editing, Supervision, Validation. XC: Supervision, Writing–review and editing, Funding acquisition, Project administration.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study was funded by grants from the National Natural Science Foundation of China General Program (82274506 and 82104871), the China Postdoctoral Science Foundation (2022M721128), the Science and Technology Innovation Program of Hunan Province (2020SK1020 and 2021RC4035), the Natural Science Foundation of Guangxi (2020GXNSFAA297053), the Open-competing Disciple Construction Project of Hunan University of Chinese Medicine (HNUCM) (22JBZ003), and was financially supported by the Furong Distinguished Scholar Program of Hunan (XJT [2020]58), the 121 Training Project for Innovative Talents of Hunan (XRSH[2019]192), the Chinese Academy of Engineering Academician Liang Liu’s Workstation Project (KH[2023]3-23YS001), and the World First-class Discipline Incubation Project of HNUCM (XJF[2022]57).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Adefegha, S. A., Okeke, B. M., Oboh, G., Ijomone, O. M., and Oyeleye, S. I. (2018). Modulatory effect of eugenol on arginase, nucleotidase, and adenosine deaminase activities of platelets in a carrageenan-induced arthritis rat model: a possible anti-arthritic mechanism of eugenol. Biomed. Pharmacother. 106, 1616–1623. doi:10.1016/j.biopha.2018.07.143
Ali, M. Y., Akter, Z., Mei, Z., Zheng, M., Tania, M., and Khan, M. A. (2021). Thymoquinone in autoimmune diseases: therapeutic potential and molecular mechanisms. Biomed. Pharmacother. 134, 111157. doi:10.1016/j.biopha.2020.111157
Arias De La Rosa, I., Escudero-Contreras, A., Rodríguez-Cuenca, S., Ruiz-Ponce, M., Jiménez-Gómez, Y., Ruiz-Limón, P., et al. (2018). Defective glucose and lipid metabolism in rheumatoid arthritis is determined by chronic inflammation in metabolic tissues. J. Intern Med. 284, 61–77. doi:10.1111/joim.12743
Atta, A., Salem, M. M., El-Said, K. S., and Mohamed, T. M. (2024). Mechanistic role of quercetin as inhibitor for adenosine deaminase enzyme in rheumatoid arthritis: systematic review. Cell. Mol. Biol. Lett. 29, 14. doi:10.1186/s11658-024-00531-7
Bansod, S., Saifi, M. A., and Godugu, C. (2021). Molecular updates on berberine in liver diseases: bench to bedside. Phytother. Res. 35, 5459–5476. doi:10.1002/ptr.7181
Berman, A. Y., Motechin, R. A., Wiesenfeld, M. Y., and Holz, M. K. (2017). The therapeutic potential of resveratrol: a review of clinical trials. NPJ Precis. Oncol. 1, 35. doi:10.1038/s41698-017-0038-6
Biniecka, M., Canavan, M., Mcgarry, T., Gao, W., Mccormick, J., Cregan, S., et al. (2016). Dysregulated bioenergetics: a key regulator of joint inflammation. Ann. Rheum. Dis. 75, 2192–2200. doi:10.1136/annrheumdis-2015-208476
Bole, G. G. (1962). Synovial fluid lipids in normal individuals and patients with rheumatoid arthritis. Arthritis Rheum. 5, 589–601. doi:10.1002/art.1780050606
Bonnet, C. S., Williams, A. S., Gilbert, S. J., Harvey, A. K., Evans, B. A., and Mason, D. J. (2015). AMPA/kainate glutamate receptors contribute to inflammation, degeneration and pain related behaviour in inflammatory stages of arthritis. Ann. Rheum. Dis. 74, 242–251. doi:10.1136/annrheumdis-2013-203670
Bottini, N., and Firestein, G. S. (2013). Duality of fibroblast-like synoviocytes in RA: passive responders and imprinted aggressors. Nat. Rev. Rheumatol. 9, 24–33. doi:10.1038/nrrheum.2012.190
Boudreau, L. H., Maillet, J., Leblanc, L. M., Jean-François, J., Touaibia, M., Flamand, N., et al. (2012). Caffeic acid phenethyl ester and its amide analogue are potent inhibitors of leukotriene biosynthesis in human polymorphonuclear leukocytes. PLoS One 7, e31833. doi:10.1371/journal.pone.0031833
Brouwers, H., Von Hegedus, J., Toes, R., Kloppenburg, M., and Ioan-Facsinay, A. (2015). Lipid mediators of inflammation in rheumatoid arthritis and osteoarthritis. Best. Pract. Res. Clin. Rheumatol. 29, 741–755. doi:10.1016/j.berh.2016.02.003
Bustamante, M. F., Garcia-Carbonell, R., Whisenant, K. D., and Guma, M. (2017). Fibroblast-like synoviocyte metabolism in the pathogenesis of rheumatoid arthritis. Arthritis Res. Ther. 19, 110. doi:10.1186/s13075-017-1303-3
Bustamante, M. F., Oliveira, P. G., Garcia-Carbonell, R., Croft, A. P., Smith, J. M., Serrano, R. L., et al. (2018). Hexokinase 2 as a novel selective metabolic target for rheumatoid arthritis. Ann. Rheum. Dis. 77, 1636–1643. doi:10.1136/annrheumdis-2018-213103
Cai, W. W., Gao, Y., Cheng, J. W., Yu, Y., Zong, S. Y., Li, Y. H., et al. (2024). Berberine modulates the immunometabolism and differentiation of CD4(+) T cells alleviating experimental arthritis by suppression of M1-exo-miR155. Phytomedicine 124, 155255. doi:10.1016/j.phymed.2023.155255
Cao, S., Li, Y., Song, R., Meng, X., Fuchs, M., Liang, C., et al. (2024). L-arginine metabolism inhibits arthritis and inflammatory bone loss. Ann. Rheum. Dis. 83, 72–87. doi:10.1136/ard-2022-223626
Chen, X. M., Huang, R. Y., Huang, Q. C., Chu, Y. L., and Yan, J. Y. (2015). Systemic review and meta-analysis of the clinical efficacy and adverse effects of zhengqing fengtongning combined with methotrexate in rheumatoid arthritis. Evid. Based Complement. Altern. Med. 2015, 910376. doi:10.1155/2015/910376
Cheng, J. W., Yu, Y., Zong, S. Y., Cai, W. W., Wang, Y., Song, Y. N., et al. (2023). Berberine ameliorates collagen-induced arthritis in mice by restoring macrophage polarization via AMPK/mTORC1 pathway switching glycolytic reprogramming. Int. Immunopharmacol. 124, 111024. doi:10.1016/j.intimp.2023.111024
Choi, W. S., Lee, G., Song, W. H., Koh, J. T., Yang, J., Kwak, J. S., et al. (2019). The CH25H-CYP7B1-RORα axis of cholesterol metabolism regulates osteoarthritis. Nature 566, 254–258. doi:10.1038/s41586-019-0920-1
Cui, H. R., Zhang, J. Y., Cheng, X. H., Zheng, J. X., Zhang, Q., Zheng, R., et al. (2022). Immunometabolism at the service of traditional Chinese medicine. Pharmacol. Res. 176, 106081. doi:10.1016/j.phrs.2022.106081
Cuvillier, O., Pirianov, G., Kleuser, B., Vanek, P. G., Coso, O. A., Gutkind, S., et al. (1996). Suppression of ceramide-mediated programmed cell death by sphingosine-1-phosphate. Nature 381, 800–803. doi:10.1038/381800a0
Dang, S., Jain, A., Dhanda, G., Bhattacharya, N., Bhattacharya, A., and Senapati, S. (2024). One carbon metabolism and its implication in health and immune functions. Cell. Biochem. Funct. 42, e3926. doi:10.1002/cbf.3926
De Oliveira, P. G., Farinon, M., Sanchez-Lopez, E., Miyamoto, S., and Guma, M. (2019). Fibroblast-like synoviocytes glucose metabolism as a therapeutic target in rheumatoid arthritis. Front. Immunol. 10, 1743. doi:10.3389/fimmu.2019.01743
Do, H. T. T., and Cho, J. (2020). Mangosteen pericarp and its bioactive xanthones: potential therapeutic value in alzheimer's disease, Parkinson's disease, and depression with pharmacokinetic and safety profiles. Int. J. Mol. Sci. 2110, 6211. doi:10.3390/ijms21176211
El Jamal, A., Bougault, C., Mebarek, S., Magne, D., Cuvillier, O., and Brizuela, L. (2020). The role of sphingosine 1-phosphate metabolism in bone and joint pathologies and ectopic calcification. Bone 130, 115087. doi:10.1016/j.bone.2019.115087
Fahmi, H., Di Battista, J. A., Pelletier, J. P., Mineau, F., Ranger, P., and Martel-Pelletier, J. (2001). Peroxisome proliferator--activated receptor gamma activators inhibit interleukin-1beta-induced nitric oxide and matrix metalloproteinase 13 production in human chondrocytes. Arthritis Rheum. 44, 595–607. doi:10.1002/1529-0131(200103)44:3<595::aid-anr108>3.0.co;2-8
Fan, X. X., Leung, E. L., Xie, Y., Liu, Z. Q., Zheng, Y. F., Yao, X. J., et al. (2018). Suppression of lipogenesis via reactive oxygen species-AMPK signaling for treating malignant and proliferative diseases. Antioxid. Redox Signal 28, 339–357. doi:10.1089/ars.2017.7090
Fang, Y., Duan, C., Zhang, J., Dai, Y., and Xia, Y. (2021). NMR-based untargeted metabolomics approach to investigate the systemic lipid metabolism regulation of norisoboldine in collagen-induced arthritis rats. Eur. J. Pharmacol. 912, 174608. doi:10.1016/j.ejphar.2021.174608
Fikry, E. M., Gad, A. M., Eid, A. H., and Arab, H. H. (2019). Caffeic acid and ellagic acid ameliorate adjuvant-induced arthritis in rats via targeting inflammatory signals, chitinase-3-like protein-1 and angiogenesis. Biomed. Pharmacother. 110, 878–886. doi:10.1016/j.biopha.2018.12.041
Gaber, T., Dziurla, R., Tripmacher, R., Burmester, G. R., and Buttgereit, F. (2005). Hypoxia inducible factor (HIF) in rheumatology: low O2! See what HIF can do. Ann. Rheum. Dis. 64, 971–980. doi:10.1136/ard.2004.031641
Gan, P., Sun, M., Wu, H., Ke, J., Dong, X., and Chen, F. (2022). A novel mechanism for inhibiting proliferation of rheumatoid arthritis fibroblast-like synoviocytes: geniposide suppresses HIF-1α accumulation in the hypoxic microenvironment of synovium. Inflamm. Res. 71, 1375–1388. doi:10.1007/s00011-022-01636-5
Gao, Y., Qian, Q., Xun, G., Zhang, J., Sun, S., Liu, X., et al. (2023). Integrated metabolomics and network analysis reveal changes in lipid metabolisms of tripterygium glycosides tablets in rats with collagen-induced arthritis. Comput. Struct. Biotechnol. J. 21, 1828–1842. doi:10.1016/j.csbj.2023.02.050
Garcia-Carbonell, R., Divakaruni, A. S., Lodi, A., Vicente-Suarez, I., Saha, A., Cheroutre, H., et al. (2016). Critical role of glucose metabolism in rheumatoid arthritis fibroblast-like synoviocytes. Arthritis Rheumatol. 68, 1614–1626. doi:10.1002/art.39608
Glunde, K., Bhujwalla, Z. M., and Ronen, S. M. (2011). Choline metabolism in malignant transformation. Nat. Rev. Cancer 11, 835–848. doi:10.1038/nrc3162
Guma, M., Sanchez-Lopez, E., Lodi, A., Garcia-Carbonell, R., Tiziani, S., Karin, M., et al. (2015). Choline kinase inhibition in rheumatoid arthritis. Ann. Rheum. Dis. 74, 1399–1407. doi:10.1136/annrheumdis-2014-205696
Herr, I., and Debatin, K. M. (2001). Cellular stress response and apoptosis in cancer therapy. Blood 98, 2603–2614. doi:10.1182/blood.v98.9.2603
Hu, Y. H., Han, J., Wang, L., Shi, C., Li, Y., Olatunji, O. J., et al. (2021). α-Mangostin alleviated inflammation in rats with adjuvant-induced arthritis by disrupting adipocytes-mediated metabolism-immune feedback. Front. Pharmacol. 12, 692806. doi:10.3389/fphar.2021.692806
Huang, D. N., Wu, F. F., Zhang, A. H., Sun, H., and Wang, X. J. (2021). Efficacy of berberine in treatment of rheumatoid arthritis: from multiple targets to therapeutic potential. Pharmacol. Res. 169, 105667. doi:10.1016/j.phrs.2021.105667
Huang, R. Y., Pan, H. D., Wu, J. Q., Zhou, H., Li, Z. G., Qiu, P., et al. (2019). Comparison of combination therapy with methotrexate and sinomenine or leflunomide for active rheumatoid arthritis: a randomized controlled clinical trial. Phytomedicine 57, 403–410. doi:10.1016/j.phymed.2018.12.030
Huang, Z., Luo, R., Yang, L., Chen, H., Zhang, X., Han, J., et al. (2022a). CPT1A-Mediated fatty acid oxidation promotes precursor osteoclast fusion in rheumatoid arthritis. Front. Immunol. 13, 838664. doi:10.3389/fimmu.2022.838664
Huang, Z., Mao, X., Chen, J., He, J., Shi, S., Gui, M., et al. (2022b). The efficacy and safety of zhengqing fengtongning for knee osteoarthritis: a systematic review and meta-analysis of randomized clinical trials. Evid. Based Complement. Altern. Med. 2022, 2768444. doi:10.1155/2022/2768444
Jeyadevi, R., Sivasudha, T., Rameshkumar, A., Ananth, D. A., Aseervatham, G. S., Kumaresan, K., et al. (2013). Enhancement of anti arthritic effect of quercetin using thioglycolic acid-capped cadmium telluride quantum dots as nanocarrier in adjuvant induced arthritic Wistar rats. Colloids Surf. B Biointerfaces 112, 255–263. doi:10.1016/j.colsurfb.2013.07.065
Jian, C., Wei, L., Wu, T., Li, S., Wang, T., Chen, J., et al. (2023). Comprehensive multi-omics analysis reveals the core role of glycerophospholipid metabolism in rheumatoid arthritis development. Arthritis Res. Ther. 25, 246. doi:10.1186/s13075-023-03208-2
Jiang, T. T., Ji, C. F., Cheng, X. P., Gu, S. F., Wang, R., Li, Y., et al. (2021). α-Mangostin alleviated HIF-1α-Mediated angiogenesis in rats with adjuvant-induced arthritis by suppressing aerobic glycolysis. Front. Pharmacol. 12, 785586. doi:10.3389/fphar.2021.785586
Jiang, T. T., Ji, C. L., Yu, L. J., Song, M. K., Li, Y., Liao, Q., et al. (2023a). Resveratrol-induced SIRT1 activation inhibits glycolysis-fueled angiogenesis under rheumatoid arthritis conditions independent of HIF-1α. Inflamm. Res. 72, 1021–1035. doi:10.1007/s00011-023-01728-w
Jiang, Z. M., Zeng, S. L., Huang, T. Q., Lin, Y., Wang, F. F., Gao, X. J., et al. (2023b). Sinomenine ameliorates rheumatoid arthritis by modulating tryptophan metabolism and activating aryl hydrocarbon receptor via gut microbiota regulation. Sci. Bull. (Beijing) 68, 1540–1555. doi:10.1016/j.scib.2023.06.027
Jose, A. M., and Rasool, M. (2023). Myricetin ameliorates the IL-21-induced tumorigenic phenotype of adjuvant-induced arthritis FLS by modulating the choline kinase signaling cascade. Vitro Cell. Dev. Biol. Anim. 59, 811–820. doi:10.1007/s11626-023-00827-6
Julio Cesar Silva, R. L. S. P., Freitas, T. S.De, Rocha, J. E., Macedo, N. S., Nonato, C.De F. A., Linhares, M. L., et al. (2022). Evaluation of antibacterial and toxicological activities of essential oil of Ocimum gratissimum L. and its major constituent eugenol. Food Biosci. 50, 102128. doi:10.1016/j.fbio.2022.102128
Kaul, N. C., Mohapatra, S. R., Adam, I., Tucher, C., Tretter, T., Opitz, C. A., et al. (2020). Hypoxia decreases the T helper cell-suppressive capacity of synovial fibroblasts by downregulating Ido1-mediated tryptophan metabolism. Rheumatol. Oxf. 59, 1148–1158. doi:10.1093/rheumatology/kez587
Ke, J. T., Zhang, H., Bu, Y. H., Gan, P. R., Chen, F. Y., Dong, X. T., et al. (2022). Metabonomic analysis of abnormal sphingolipid metabolism in rheumatoid arthritis synovial fibroblasts in hypoxia microenvironment and intervention of geniposide. Front. Pharmacol. 13, 969408. doi:10.3389/fphar.2022.969408
Kitano, M., Hla, T., Sekiguchi, M., Kawahito, Y., Yoshimura, R., Miyazawa, K., et al. (2006). Sphingosine 1-phosphate/sphingosine 1-phosphate receptor 1 signaling in rheumatoid synovium: regulation of synovial proliferation and inflammatory gene expression. Arthritis Rheum. 54, 742–753. doi:10.1002/art.21668
Koycheva, I. K., Marchev, A. S., Stoykova, I. D., and Georgiev, M. I. (2023). Natural alternatives targeting psoriasis pathology and key signaling pathways: a focus on phytochemicals. Phytochem. Rev. doi:10.1007/s11101-023-09886-9
Lampropoulou, V., Sergushichev, A., Bambouskova, M., Nair, S., Vincent, E. E., Loginicheva, E., et al. (2016). Itaconate links inhibition of succinate dehydrogenase with macrophage metabolic remodeling and regulation of inflammation. Cell. Metab. 24, 158–166. doi:10.1016/j.cmet.2016.06.004
Lei, M., Tao, M. Q., Wu, Y. J., Xu, L., Yang, Z., Li, Y., et al. (2021). Metabolic enzyme triosephosphate isomerase 1 and nicotinamide phosphoribosyltransferase, two independent inflammatory indicators in rheumatoid arthritis: evidences from collagen-induced arthritis and clinical samples. Front. Immunol. 12, 795626. doi:10.3389/fimmu.2021.795626
Lei, Q., Yang, J., Li, L., Zhao, N., Lu, C., Lu, A., et al. (2023). Lipid metabolism and rheumatoid arthritis. Front. Immunol. 14, 1190607. doi:10.3389/fimmu.2023.1190607
Lende, A. B., Kshirsagar, A. D., Deshpande, A. D., Muley, M. M., Patil, R. R., Bafna, P. A., et al. (2011). Anti-inflammatory and analgesic activity of protocatechuic acid in rats and mice. Inflammopharmacology 19, 255–263. doi:10.1007/s10787-011-0086-4
Li, J., Pang, J., Liu, Z., Ge, X., Zhen, Y., Jiang, C. C., et al. (2021). Shikonin induces programmed death of fibroblast synovial cells in rheumatoid arthritis by inhibiting energy pathways. Sci. Rep. 11, 18263. doi:10.1038/s41598-021-97713-6
Li, Y., Zheng, J. Y., Liu, J. Q., Yang, J., Liu, Y., Wang, C., et al. (2016). Succinate/NLRP3 inflammasome induces synovial fibroblast activation: therapeutical effects of clematichinenoside AR on arthritis. Front. Immunol. 7, 532. doi:10.3389/fimmu.2016.00532
Lin, N., Zhang, Y. Q., Jiang, Q., Liu, W., Liu, J., Huang, Q. C., et al. (2020). Clinical practice guideline for tripterygium glycosides/tripterygium wilfordii tablets in the treatment of rheumatoid arthritis. Front. Pharmacol. 11, 608703. doi:10.3389/fphar.2020.608703
Linghang, Q., Yiyi, X., Guosheng, C., Kang, X., Jiyuan, T., Xiong, L., et al. (2020). Effects of atractylodes oil on inflammatory response and serum metabolites in adjuvant arthritis rats. Biomed. Pharmacother. 127, 110130. doi:10.1016/j.biopha.2020.110130
Littlewood-Evans, A., Sarret, S., Apfel, V., Loesle, P., Dawson, J., Zhang, J., et al. (2016). GPR91 senses extracellular succinate released from inflammatory macrophages and exacerbates rheumatoid arthritis. J. Exp. Med. 213, 1655–1662. doi:10.1084/jem.20160061
Liu, P., Wang, J., Wen, W., Pan, T., Chen, H., Fu, Y., et al. (2020). Cinnamaldehyde suppresses NLRP3 derived IL-1β via activating succinate/HIF-1 in rheumatoid arthritis rats. Int. Immunopharmacol. 84, 106570. doi:10.1016/j.intimp.2020.106570
Lu, B., Wang, Z., Ding, Y., Wang, X., Lu, S., Wang, C., et al. (2018). RIP1 and RIP3 contribute to shikonin-induced glycolysis suppression in glioma cells via increase of intracellular hydrogen peroxide. Cancer Lett. 425, 31–42. doi:10.1016/j.canlet.2018.03.046
Masoumi, M., Mehrabzadeh, M., Mahmoudzehi, S., Mousavi, M. J., Jamalzehi, S., Sahebkar, A., et al. (2020). Role of glucose metabolism in aggressive phenotype of fibroblast-like synoviocytes: latest evidence and therapeutic approaches in rheumatoid arthritis. Int. Immunopharmacol. 89, 107064. doi:10.1016/j.intimp.2020.107064
Masuko, K. (2022). Glucose as a potential key to fuel inflammation in rheumatoid arthritis. Nutrients 14, 2349. doi:10.3390/nu14112349
Mateen, S., Shahzad, S., Ahmad, S., Naeem, S. S., Khalid, S., Akhtar, K., et al. (2019). Cinnamaldehyde and eugenol attenuates collagen induced arthritis via reduction of free radicals and pro-inflammatory cytokines. Phytomedicine 53, 70–78. doi:10.1016/j.phymed.2018.09.004
Meshcheryakova, A., Mechtcheriakova, D., and Pietschmann, P. (2017). Sphingosine 1-phosphate signaling in bone remodeling: multifaceted roles and therapeutic potential. Expert Opin. Ther. Targets 21, 725–737. doi:10.1080/14728222.2017.1332180
Miao, Y., Wu, X., Xue, X., Ma, X., Yang, L., Zeng, X., et al. (2022). Morin, the PPARγ agonist, inhibits Th17 differentiation by limiting fatty acid synthesis in collagen-induced arthritis. Cell. Biol. Toxicol. 39, 1433–1452. doi:10.1007/s10565-022-09769-3
Mills, E. L., Kelly, B., Logan, A., Costa, A. S. H., Varma, M., Bryant, C. E., et al. (2016). Succinate dehydrogenase supports metabolic repurposing of mitochondria to drive inflammatory macrophages. Cell. 167, 457–470. doi:10.1016/j.cell.2016.08.064
Miriam Jose, A., and Rasool, M. (2022). Choline kinase: an underappreciated rheumatoid arthritis therapeutic target. Life Sci. 309, 121031. doi:10.1016/j.lfs.2022.121031
Moulin, D., Millard, M., Taïeb, M., Michaudel, C., Aucouturier, A., Lefèvre, A., et al. (2024). Counteracting tryptophan metabolism alterations as a new therapeutic strategy for rheumatoid arthritis. Ann. Rheum. Dis. 83, 312–323. doi:10.1136/ard-2023-224014
Munder, M. (2009). Arginase: an emerging key player in the mammalian immune system. Br. J. Pharmacol. 158, 638–651. doi:10.1111/j.1476-5381.2009.00291.x
Okano, T., Saegusa, J., Takahashi, S., Ueda, Y., and Morinobu, A. (2018). Immunometabolism in rheumatoid arthritis. Immunol. Med. 41, 89–97. doi:10.1080/25785826.2018.1531186
O'neil, L. J., Anaparti, V., Winter, T., Smolik, I., Meng, X., Aukema, H. M., et al. (2024). Lipoxygenase-derived oxylipins are enriched in anti-citrullinated protein antibody (ACPA)-positive individuals at risk for developing rheumatoid arthritis. Arthritis Res. Ther. 26, 51. doi:10.1186/s13075-024-03274-0
Ortiz-Andrade, R., Araujo-León, J. A., Sánchez-Recillas, A., Navarrete-Vazquez, G., González-Sánchez, A. A., Hidalgo-Figueroa, S., et al. (2020). Toxicological screening of four bioactive citroflavonoids: in vitro, in vivo, and in silico approaches. Molecules 25, 5959. doi:10.3390/molecules25245959
Panfili, E., Gerli, R., Grohmann, U., and Pallotta, M. T. (2020). Amino acid metabolism in rheumatoid arthritis: friend or foe?. Biomolecules 10, 1280. doi:10.3390/biom10091280
Perucha, E., Melchiotti, R., Bibby, J. A., Wu, W., Frederiksen, K. S., Roberts, C. A., et al. (2019). The cholesterol biosynthesis pathway regulates IL-10 expression in human Th1 cells. Nat. Commun. 10, 498. doi:10.1038/s41467-019-08332-9
Peyssonnaux, C., Cejudo-Martin, P., Doedens, A., Zinkernagel, A. S., Johnson, R. S., and Nizet, V. (2007). Cutting edge: essential role of hypoxia inducible factor-1alpha in development of lipopolysaccharide-induced sepsis. J. Immunol. 178, 7516–7519. doi:10.4049/jimmunol.178.12.7516
Phillips, R. (2024). Manipulating tryptophan metabolism in arthritis. Nat. Rev. Rheumatol. 20, 67. doi:10.1038/s41584-024-01077-w
Pradhan, A., Sengupta, S., Sengupta, R., and Chatterjee, M. (2023). Attenuation of methotrexate induced hepatotoxicity by epigallocatechin 3-gallate. Drug Chem. Toxicol. 46, 717–725. doi:10.1080/01480545.2022.2085738
Prati, C., Berthelot, A., Wendling, D., and Demougeot, C. (2011). Endothelial dysfunction in rat adjuvant-induced arthritis: up-regulation of the vascular arginase pathway. Arthritis Rheum. 63, 2309–2317. doi:10.1002/art.30391
Pucino, V., Nefla, M., Gauthier, V., Alsaleh, G., Clayton, S. A., Marshall, J., et al. (2023). Differential effect of lactate on synovial fibroblast and macrophage effector functions. Front. Immunol. 14, 1183825. doi:10.3389/fimmu.2023.1183825
Qu, F., Zhang, H., Zhang, M., and Hu, P. (2018). Sphingolipidomic profiling of rat serum by UPLC-Q-TOF-MS: application to rheumatoid arthritis study. Molecules 2310, 1324. doi:10.3390/molecules23061324
Reddy, P. H., Manczak, M., Yin, X., Grady, M. C., Mitchell, A., Tonk, S., et al. (2018). Protective effects of Indian spice curcumin against amyloid-β in alzheimer's disease. J. Alzheimers Dis. 61, 843–866. doi:10.3233/jad-170512
Ricote, M., Li, A. C., Willson, T. M., Kelly, C. J., and Glass, C. K. (1998). The peroxisome proliferator-activated receptor-gamma is a negative regulator of macrophage activation. Nature 391, 79–82. doi:10.1038/34178
Robinson, G., Pineda-Torra, I., Ciurtin, C., and Jury, E. C. (2022). Lipid metabolism in autoimmune rheumatic disease: implications for modern and conventional therapies. J. Clin. Invest 13210, e148552. doi:10.1172/JCI148552
Rodgers, L. C., Cole, J., Rattigan, K. M., Barrett, M. P., Kurian, N., Mcinnes, I. B., et al. (2020). The rheumatoid synovial environment alters fatty acid metabolism in human monocytes and enhances CCL20 secretion. Rheumatol. Oxf. 59, 869–878. doi:10.1093/rheumatology/kez378
Rylance, H. J. (1969). Hypertaurinuria in rheumatoid arthritis. Ann. Rheum. Dis. 28, 41–44. doi:10.1136/ard.28.1.41
Saccol, R., Da Silveira, K. L., Adefegha, S. A., Manzoni, A. G., Da Silveira, L. L., Coelho, A. P. V., et al. (2019). Effect of quercetin on E-NTPDase/E-ADA activities and cytokine secretion of complete Freund adjuvant-induced arthritic rats. Cell. Biochem. Funct. 37, 474–485. doi:10.1002/cbf.3413
Saraiva, A. L., Veras, F. P., Peres, R. S., Talbot, J., De Lima, K. A., Luiz, J. P., et al. (2018). Succinate receptor deficiency attenuates arthritis by reducing dendritic cell traffic and expansion of T(h)17 cells in the lymph nodes. Faseb J. 32, 6550–6558. doi:10.1096/fj.201800285
Seal, A., Hughes, M., Wei, F., Pugazhendhi, A. S., Ngo, C., Ruiz, J., et al. (2024). Sphingolipid-induced bone regulation and its emerging role in dysfunction due to disease and infection. Int. J. Mol. Sci. 2510, 3024. doi:10.3390/ijms25053024
Shan, Y., Zhao, J., Wei, K., Jiang, P., Xu, L., Chang, C., et al. (2023). A comprehensive review of Tripterygium wilfordii hook. f. in the treatment of rheumatic and autoimmune diseases: bioactive compounds, mechanisms of action, and future directions. Front. Pharmacol. 14, 1282610. doi:10.3389/fphar.2023.1282610
Shen, P., Jiao, Y., Miao, L., Chen, J. H., and Momtazi-Borojeni, A. A. (2020). Immunomodulatory effects of berberine on the inflamed joint reveal new therapeutic targets for rheumatoid arthritis management. J. Cell. Mol. Med. 24, 12234–12245. doi:10.1111/jcmm.15803
Shi, Y., Pan, H.-D., Wu, J.-L., Zou, Q.-H., Xie, X.-Y., Li, H.-G., et al. (2022). The correlation between decreased ornithine level and alleviation of rheumatoid arthritis patients assessed by a randomized, placebo-controlled, double-blind clinical trial of sinomenine. Engineering 16, 93–99. doi:10.1016/j.eng.2021.04.014
Smolen, J. S., Aletaha, D., and Mcinnes, I. B. (2016). Rheumatoid arthritis. Lancet 388, 2023–2038. doi:10.1016/s0140-6736(16)30173-8
Su, J., Li, S., Chen, J., Jian, C., Hu, J., Du, H., et al. (2022). Glycerophospholipid metabolism is involved in rheumatoid arthritis pathogenesis by regulating the IL-6/JAK signaling pathway. Biochem. Biophys. Res. Commun. 600, 130–135. doi:10.1016/j.bbrc.2022.02.003
Sun, H., Berquin, I. M., Owens, R. T., O'flaherty, J. T., and Edwards, I. J. (2008). Peroxisome proliferator-activated receptor gamma-mediated up-regulation of syndecan-1 by n-3 fatty acids promotes apoptosis of human breast cancer cells. Cancer Res. 68, 2912–2919. doi:10.1158/0008-5472.can-07-2305
Suresh, P., Kavitha, Ch N., Babu, S. M., Reddy, V. P., and Latha, A. K. (2012). Effect of ethanol extract of Trigonella foenum graecum (Fenugreek) seeds on Freund's adjuvant-induced arthritis in albino rats. Inflammation 35, 1314–1321. doi:10.1007/s10753-012-9444-7
Takahashi, S., Saegusa, J., Sendo, S., Okano, T., Akashi, K., Irino, Y., et al. (2017). Glutaminase 1 plays a key role in the cell growth of fibroblast-like synoviocytes in rheumatoid arthritis. Arthritis Res. Ther. 19, 76. doi:10.1186/s13075-017-1283-3
Tan, C., Li, L., Han, J., Xu, K., and Liu, X. (2022). A new strategy for osteoarthritis therapy: inhibition of glycolysis. Front. Pharmacol. 13, 1057229. doi:10.3389/fphar.2022.1057229
Tykocinski, L. O., Lauffer, A. M., Bohnen, A., Kaul, N. C., Krienke, S., Tretter, T., et al. (2017). Synovial fibroblasts selectively suppress Th1 cell responses through Ido1-mediated tryptophan catabolism. J. Immunol. 198, 3109–3117. doi:10.4049/jimmunol.1600600
Umar, S., Kumar, A., Sajad, M., Zargan, J., Ansari, M. M., Ahmad, S., et al. (2013). Hesperidin inhibits collagen-induced arthritis possibly through suppression of free radical load and reduction in neutrophil activation and infiltration. Rheumatol. Int. 33, 657–663. doi:10.1007/s00296-012-2430-4
Vaillancourt, F., Silva, P., Shi, Q., Fahmi, H., Fernandes, J. C., and Benderdour, M. (2011). Elucidation of molecular mechanisms underlying the protective effects of thymoquinone against rheumatoid arthritis. J. Cell. Biochem. 112, 107–117. doi:10.1002/jcb.22884
Wang, D. D., He, C. Y., Wu, Y. J., Xu, L., Shi, C., Olatunji, O. J., et al. (2022a). AMPK/SIRT1 deficiency drives adjuvant-induced arthritis in rats by promoting glycolysis-mediated monocytes inflammatory polarization. J. Inflamm. Res. 15, 4663–4675. doi:10.2147/jir.s378090
Wang, G., Xie, X., Yuan, L., Qiu, J., Duan, W., Xu, B., et al. (2020). Resveratrol ameliorates rheumatoid arthritis via activation of SIRT1-Nrf2 signaling pathway. Biofactors 46, 441–453. doi:10.1002/biof.1599
Wang, L., Rotter, S., Ladurner, A., Heiss, E. H., Oberlies, N. H., Dirsch, V. M., et al. (2015). Silymarin constituents enhance ABCA1 expression in THP-1 macrophages. Molecules 21, E55. doi:10.3390/molecules21010055
Wang, Y., Wu, H., Gui, B. J., Liu, J., Rong, G. X., Deng, R., et al. (2022b). Geniposide alleviates VEGF-induced angiogenesis by inhibiting VEGFR2/PKC/ERK1/2-mediated SphK1 translocation. Phytomedicine 100, 154068. doi:10.1016/j.phymed.2022.154068
Wei, M., Ma, Y., Liu, Y., Zhou, Y., Men, L., Yue, K., et al. (2018). Urinary metabolomics study on the anti-inflammation effects of flavonoids obtained from Glycyrrhiza. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 1086, 1–10. doi:10.1016/j.jchromb.2018.04.007
Wendling, D., Vidon, C., Khan, K. A., Guillot, X., Godfrin-Valnet, M., Abbas, W., et al. (2014). AB0116 sirt-1 activity in PBMC from patients with rheumatoid arthritis. Ann. Rheumatic Dis. 73, 842. doi:10.1136/annrheumdis-2014-eular.1584
West, A. P., Brodsky, I. E., Rahner, C., Woo, D. K., Erdjument-Bromage, H., Tempst, P., et al. (2011). TLR signalling augments macrophage bactericidal activity through mitochondrial ROS. Nature 472, 476–480. doi:10.1038/nature09973
Weyand, C. M., and Goronzy, J. J. (2017). Immunometabolism in early and late stages of rheumatoid arthritis. Nat. Rev. Rheumatol. 13, 291–301. doi:10.1038/nrrheum.2017.49
Wu, S., and De Luca, F. (2004). Role of cholesterol in the regulation of growth plate chondrogenesis and longitudinal bone growth. J. Biol. Chem. 279, 4642–4647. doi:10.1074/jbc.M305518200
Wu, Y. J., Fang, W. J., Pan, S., Zhang, S. S., Li, D. F., Wang, Z. F., et al. (2021). Regulation of Sirt1 on energy metabolism and immune response in rheumatoid arthritis. Int. Immunopharmacol. 101, 108175. doi:10.1016/j.intimp.2021.108175
Wu, Y. J., Zhang, S. S., Yin, Q., Lei, M., Wang, Q. H., Chen, W. G., et al. (2023). α-Mangostin inhibited M1 polarization of macrophages/monocytes in antigen-induced arthritis mice by up-regulating silent information regulator 1 and peroxisome proliferators-activated receptor γ simultaneously. Drug Des. Devel Ther. 17, 563–577. doi:10.2147/dddt.S397914
Yan, R., Cao, P., Song, W., Li, Y., Wang, T., Qian, H., et al. (2021). Structural basis for sterol sensing by Scap and Insig. Cell. Rep. 35, 109299. doi:10.1016/j.celrep.2021.109299
Yang, C. W., Hsu, H. Y., Lee, Y. Z., and Lee, S. J. (2024). Vitamin B12 inhibits peptidylarginine deiminases and ameliorates rheumatoid arthritis in CAIA mice. Biochem. Biophys. Res. Commun. 704, 149668. doi:10.1016/j.bbrc.2024.149668
Yang, E. J., Lee, J., Lee, S. Y., Kim, E. K., Moon, Y. M., Jung, Y. O., et al. (2014). EGCG attenuates autoimmune arthritis by inhibition of STAT3 and HIF-1α with Th17/Treg control. PLoS One 9, e86062. doi:10.1371/journal.pone.0086062
Yang, X., Chang, Y., and Wei, W. (2020). Emerging role of targeting macrophages in rheumatoid arthritis: focus on polarization, metabolism and apoptosis. Cell. Prolif. 53, e12854. doi:10.1111/cpr.12854
Yang, Y., Wei, J., Li, J., Cui, Y., Zhou, X., and Xie, J. (2021). Lipid metabolism in cartilage and its diseases: a concise review of the research progress. Acta Biochim. Biophys. Sin. (Shanghai) 53, 517–527. doi:10.1093/abbs/gmab021
Zhan, X., Wu, H., and Wu, H. (2020). Joint synovial fluid metabolomics method to decipher the metabolic mechanisms of adjuvant arthritis and geniposide intervention. J. Proteome Res. 19, 3769–3778. doi:10.1021/acs.jproteome.0c00300
Zhang, K., Pace, S., Jordan, P. M., Peltner, L. K., Weber, A., Fischer, D., et al. (2021). Beneficial modulation of lipid mediator biosynthesis in innate immune cells by antirheumatic tripterygium wilfordii glycosides. Biomolecules 11, 746. doi:10.3390/biom11050746
Zhang, L., Zhu, M., Li, M., Du, Y., Duan, S., Huang, Y., et al. (2017). Ginsenoside Rg1 attenuates adjuvant-induced arthritis in rats via modulation of PPAR-γ/NF-κB signal pathway. Oncotarget 8, 55384–55393. doi:10.18632/oncotarget.19526
Zhao, Y., Yan, X., Li, X., Zheng, Y., Li, S., and Chang, X. (2016). PGK1, a glucose metabolism enzyme, may play an important role in rheumatoid arthritis. Inflamm. Res. 65, 815–825. doi:10.1007/s00011-016-0965-7
Zhu, Y., Zhang, L., Zhang, X., Wu, D., Chen, L., Hu, C., et al. (2022). Tripterygium wilfordii glycosides ameliorates collagen-induced arthritis and aberrant lipid metabolism in rats. Front. Pharmacol. 13, 938849. doi:10.3389/fphar.2022.938849
Zuo, J., Yin, Q., Wang, Y. W., Li, Y., Lu, L. M., Xiao, Z. G., et al. (2018). Inhibition of NF-κB pathway in fibroblast-like synoviocytes by α-mangostin implicated in protective effects on joints in rats suffering from adjuvant-induced arthritis. Int. Immunopharmacol. 56, 78–89. doi:10.1016/j.intimp.2018.01.016
Keywords: natural products, rheumatoid arthritis, glycolysis, lipid metabolism, amino acid metabolism, nucleotide metabolism, immunometabolic balance
Citation: Hu S, Lin Y, Tang Y, Zhang J, He Y, Li G, Li L and Cai X (2024) Targeting dysregulated intracellular immunometabolism within synovial microenvironment in rheumatoid arthritis with natural products. Front. Pharmacol. 15:1403823. doi: 10.3389/fphar.2024.1403823
Received: 21 March 2024; Accepted: 01 July 2024;
Published: 22 July 2024.
Edited by:
David M. Pereira, University of Porto, PortugalReviewed by:
Sadiq Umar, University of Illinois Chicago, United StatesCopyright © 2024 Hu, Lin, Tang, Zhang, He, Li, Li and Cai. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiong Cai, Y2FpeGlvbmdAaG51Y20uZWR1LmNu; Liqing Li, bGlsaXFpbmc4N0BxcS5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.