
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Pharmacol. , 27 June 2024
Sec. Ethnopharmacology
Volume 15 - 2024 | https://doi.org/10.3389/fphar.2024.1402763
 Wei-jian Zhang1
Wei-jian Zhang1 Rui-qi Chen2
Rui-qi Chen2 Xuan Tang2
Xuan Tang2 Pei-bo Li2
Pei-bo Li2 Jian Wang3
Jian Wang3 Hai-ke Wu4
Hai-ke Wu4 Ning Xu5
Ning Xu5 Ming-fei Zou5
Ming-fei Zou5 Sen-rong Luo6
Sen-rong Luo6 Zi-qi Ouyang6
Zi-qi Ouyang6 Zhi-kai Chen6
Zhi-kai Chen6 Xu-xing Liao1*
Xu-xing Liao1* Hao Wu2*
Hao Wu2*Naoxintong Capsule (NXT), a renowned traditional Chinese medicine (TCM) formulation, has been broadly applied in China for more than 30 years. Over decades, accumulating evidences have proven satisfactory efficacy and safety of NXT in treating cardiovascular and cerebrovascular diseases (CCVD). Studies have been conducted unceasingly, while this growing latest knowledge of NXT has not yet been interpreted properly and summarized comprehensively. Hence, we systematically review the advancements in NXT research, from its chemical constituents, quality control, pharmacokinetics, to its profound pharmacological activities as well as its clinical applications in CCVD. Moreover, we further propose specific challenges for its future perspectives: 1) to precisely clarify bioactivities of single compound in complicated mixtures; 2) to evaluate the pharmacokinetic behaviors of NXT feature components in clinical studies, especially drug-drug interactions in CCVD patients; 3) to explore and validate its multi-target mechanisms by integrating multi-omics technologies; 4) to re-evaluate the safety and efficacy of NXT by carrying out large-scale, multicenter randomized controlled trials. In brief, this review aims to straighten out a paradigm for TCM modernization, which help to contribute NXT as a piece of Chinese Wisdom into the advanced intervention strategy for CCVD therapy.
Cardiovascular and cerebrovascular diseases (CCVD) are a large category of diseases that pose a great threat to public health, causing more than 20 million deaths worldwide each year (Hasani et al., 2023). However, the quality of survival management of CCVD is constantly low because CCVD patients often undergo an intricate, multifactorial pathogenesis, leading to high mortality and high degree of disability (Joseph et al., 2017). Nowadays, the conventional strategies for CCVD management mainly include platelet aggregation inhibitors to prevent thrombosis (e.g., aspirin and clopidogrel), hypolipidemic agents to sustain lipids homeostasis (e.g., statins or fibrates), and antihypertensive therapies to maintain blood pressure (e.g., β-blockers and ACE-I/angiotensin receptor blockers) as well. Many patients also need to take drugs such as metformin to control their blood glucose at the same time. Differences in drug use may be one of the main reasons for the large differences in morbidity and mortality of acute myocardial infarction or stroke in different income countries (Leong et al., 2017). However, many patients with CCVD require long-term or even lifelong medication after diagnosis. These kinds of patients often accompanied by a variety of underlying diseases, having various potential health risks under the current drug treatment strategies. Moreover, the complex disease complications also bring a huge healthcare burden to many patients’ families. Therefore, it is necessary to explore new safe and effective treatment options.
Traditional Chinese Medicine (TCM) draws an increasing interest worldwide for its complementary and alternative therapeutic effects compared with Western medicine (WM) and its remarkable ability to address various difficult diseases (Liu et al., 2015; Pan et al., 2022). TCM has been used as an effective intervention for thousands of years in the treatment of CCVD in Asia, accumulating a quantity of valuable medication experience and prescriptions, with at least more than 20 herbs commonly used in TCM reported to have strong cardiovascular and cerebrovascular protective effects (Hao et al., 2017; Zhang et al., 2022). Naoxintong capsule (NXT) is a modern TCM prescription derived from Buyang Huanwu Decoction, approved by the China Food and Drug Administration (CFDA) and included in the Chinese Pharmacopeia (2020 edition). NXT has been used for the treatment of CCVD for about 30 years. Accumulating evidence from animal experiments and clinical applications has confirmed the significant efficacy and safety of NXT (Han et al., 2019). As the representative prescription of co-treatment of CCVD, in addition to the remarkable efficacy and safety, NXT possesses distinct therapeutic advantages compared to traditional strategies for treating CCVD. For instance, compared with the conventional drugs, patients have a good tolerance and little resistance to NXT treatment (Han et al., 2019). Besides, NXT in combination with the conventional interventions helps to improve efficacy while reduce side effects (Yu et al., 2024). Consequently, NXT has achieved excellent efficacy feedback in the Chinese market over the past 30 years, with an annual sales volume of 1 billion yuan, making it a fairly well-known modern application of TCM in the treatment of CCVD. Over the years, TCM, represented by NXT, has accumulated a lot of valuable experience in the clinical treatment of CCVD, but the intrinsic reasons for the efficacy of TCM still need to be studied more deeply and thoroughly.
Post-marketing re-evaluation is an essential part of TCM modernization research, which re-articulates the accumulated experience of TCM with the theories of modern medicine. Re-evaluation of the quality and efficacy of TCM prescriptions after marketing is helpful to continuously improve the pharmaceutical process, interpret the pharmacological mechanism, and ensure the safety of drug use. NXT, as one of the most widely used TCMs with the abundant research base and the vast majority of evidence of efficacy in China, has accumulated a large number of results in modernization research, but it is undeniable that it still lacks some critical studies. In this review, we systematically sort out the current reports of NXT in terms of composition, quality control, pharmacokinetics, pharmacological activity, clinical application, etc. We aim to explore the research paradigm of TCM post-market re-evaluation by taking NXT as an example, as well as to provide ideas and directions for TCM modernization research and provide valuable references for new interventions or therapeutic ideas for CCVD.
NXT is a fine powder mixture containing 11 botanical drugs [Astragalus membranaceus (Fisch.) Bge. [Fabaceae, astragali radix], Paeonia lactiflora Pall. [Paeoniaceae, paeoniae radix rubra], Salvia miltiorrhiza Bunge [Lamiaceae, salviae miltiorrhizae radix et rhizoma], Angelica sinensis (Oliv.) Diels [Apiaceae, angelicae sinensis radix], Conioselinum anthriscoides ‘Chuanxiong’ [Apiaceae, chuanxiong rhizoma], Prunus persica (L.) Batsch [Rosaceae, persicae semen], Carthamus tinctorius L. [Asteraceae, carthami flos], Spatholobus suberectus Dunn [Fabaceae, spatholobi caulis], Achyranthes bidentata Blume [Amaranthaceae, achyranthis bidentatae radix], Neolitsea cassia (L.) Kosterm. [Lauraceae, Cinnamomi ranulus], and Morus alba L. [Moraceae, ramulus mori]], 2 kinds of resin drugs [Boswellia sacra Flück. [Burseraceae, olibanum], Commiphora myrrha (T. Nees) Engl. [Burseraceae, Myrrha]] and 3 kinds of zoological drugs [Pheretima aspergillum (E.Perrier). [Megascolecidae, pheretima], Hirudo nipponica Whitman [Hirudinidae, hirudo], and Buthus martensii Karsch [Buthidae, scorpio]]. The preparation method of NXT includes crushing the above 16 medicinal materials into fine powder, screening through mesh size of 80, then mixing in a certain proportion (details in Table 1), and finally putting into the capsule (Chinese Pharmacopoeia Commission, 2020). NXT is a prescription that fully complies with the principles of TCM formulas.
Astragali Radix (Huangqi in Chinese) plays a key role in the formula of NXT. Astragali Radix mainly contains polysaccharides, flavonoids and saponins. The primary active components in Astragali Radix are astragalosides and iso-astragalosides, such as astragaloside I, II, III, IV, and iso-astragaloside I, II, which account for more than 80% of the total saponins (Zhang et al., 2021). For Astragali Radix, Calycosin-7-O-β-D-glucoside is the component commonly used for the chemical marker in the quality analysis, while astragaloside IV is assigned as a principal quality control index (Ping et al., 2017). The main chemical compositions of Pheretima (Dilong) include protein, amino acid, enzymes, lipids, trace elements and nucleotides. Lumbrukinase is one of the characteristic components of Pheretima, which possess the anticoagulant and thrombolytic effects (Xu et al., 2022). Hirudo (Shuizhi) mainly contains protein polypeptides, pteridines, glycolipids, carboxylic esters and free amino acids. Hirudin is the most active components in Hirudo, which is the strongest natural thrombin specific inhibitor so far (Shi et al., 2023). The main components of Scorpio (Quanxie) include buthotoxin, sterol derivatives, alkaloids, amino acids, lipids and terpenoid (Kyungjin et al., 2018). The primary active constituents of Salviae Miltiorrhizae Radix et Rhizoma (Danshen) are water-soluble phenolic acids and lipid-soluble diterpenoids. Contained phenolic acids mainly include danshensu, protocatechualdehyde, salvianolic acid A, B, C, D, E, F, G, as well as caffeic acid, rosmarinic acid, lithospermic acid, while diterpenoids are mainly tanshinone I, tanshinone IIA, tanshinone IIB, and cryptotanshinone (Su et al., 2015). The primary components in Angelicae Sinensis Radix (Danggui) are phthalides, phenolic acids and their derivatives, polysaccharides, phenylpropanoids and flavonoids. Among them, phenolic acids represented by ferulic acid and their derivatives are the main bioactive components, which are closely related to the pharmacological effect of Angelicae Sinensis Radix (Yi et al., 2009). The main chemical components of Chuanxiong Rhizoma (Chuanxiong) include volatile oils, phenolic acids, alkaloids, and polysaccharides. Ferulic acid, coniferyl ferulate, Z-ligustilide, senkyunolide A, I, H, as well as levistolide A are supposed to be the quality marker of Chuanxiong Rhizoma (Li et al., 2012). Paeoniae Radix Rubra (Chishao) mainly contains terpenoids, tannins, flavonoids and polysaccharides. Paeoniflorin serves as a primary quality control indicator of Paeoniae Radix Rubra (Yue et al., 2022). Persicae Semen (Taoren) mainly contains volatile oils, cyanogenic glycosides, flavonoids, sterols and saponins. Amygdalin is assigned as a principal quality control index of Persicae Semen (Yang et al., 2014). Carthami Flos (Honghua) mainly contains flavonoids, alkaloids, lignans, sterols and polysaccharides and hydroxysafflor yellow A is a chemical marker for its quality control (Qiao et al., 2021). The primary components in Olibanum (Ruxiang) and Myrrha (Moyao) are terpenoids and volatile oils (Xu et al., 2011; Feng et al., 2021). The main active components in Spatholobi Caulis (Jixueteng) are flavonoids, phenolic acids, phenylpropanoids, terpenoids and sterols (Guan et al., 2013). The main active components in Ramulus Mori (Sangzhi) are alkaloids and polysaccharides (Yang et al., 2015). Cinnamomi Ranulus (Guizhi) mainly contains phenylpropanoids, terpenoids, flavonoids and volatile oils (Liu et al., 2020). The primary active components in Achyranthis Bidentatae Radix (Niuxi) are polysaccharides and saponins. β-ecdysterone is assigned as a principal index of quality control of Achyranthis bidentatae (He et al., 2017). A brief illustration of the primary constituents in NXT were shown in Figure 1.
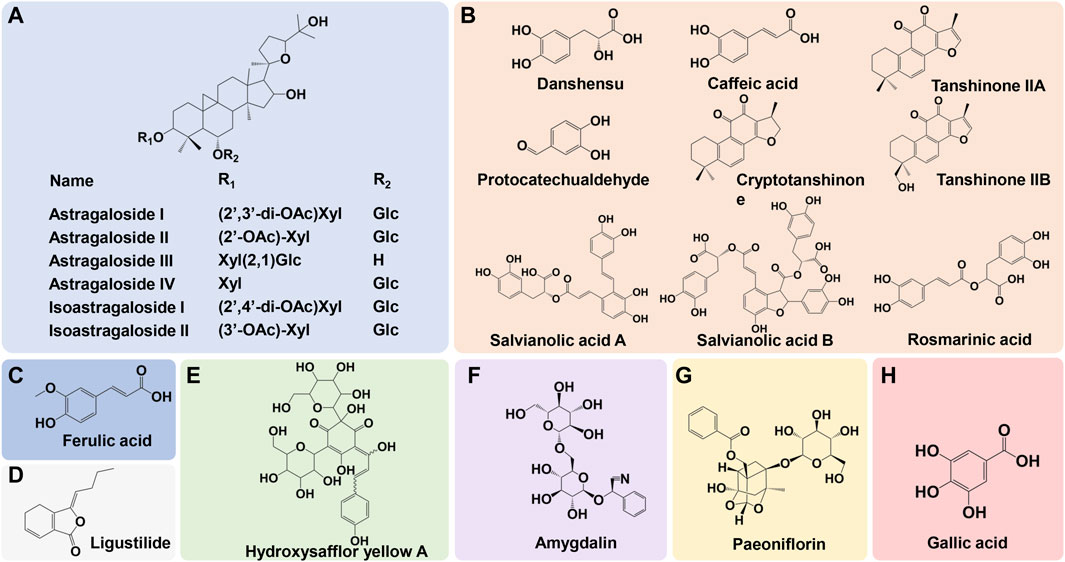
Figure 1. The primary constituents in NXT and corresponding source. (A) Astragali Radix, (B) Salviae Miltiorrhizar Radix et Rhizoma, (C) Angelicae Sinensis Radix/Chuanxiong Rhizoma, (D) Chuanxiong Rhizoma, (E) Carthami Flos, (F) Persicae Semen, (G) Paeoniae Radix Rubra, (H) Spatholobi Caulis/Paeoniae Radix Rubra.
As a mixed mixture, NXT’s chemical composition is extremely complex. With the development of analytical techniques, researchers tried to clarify the chemical profiles of NXT via instruments with high throughput and high resolution in recent years. Using the ultra-high-pressure liquid chromatography coupled with linear ion trap-Orbitrap tandem mass spectrometry (UHPLC-LTQ-Orbitrap), Wang et al. identified the composition of NXT. As a result, a total of 178 compounds were detected, including 21 flavones, 6 flavone glycosides, 18 phenanthraquinones, and 22 terpenoids (Wang et al., 2015), while Ma et al. identified 81 compounds with the method of ultra-performance liquid chromatography/quadrupole time-of-flight mass spectrometry (UPLC/Q-TOF-MS) (Ma et al., 2016). The identified components of both two methods mainly included organic acids, amino acids, flavonoids, lactones and terpenoids. In terms of composition quantification, a method of Matrix Solid-Phase Dispersion (MSPD) combined with high performance liquid chromatography (HPLC) was established to simultaneously determine sixteen compounds. These compounds included phenolic acids (gallic acid, lithospermic acid, chlorogenic acid, ferulic acid, rosmarinic acid, 1,5-dicaffeoylqunic acid, salvianolic acid B, and 3,5-dicaffeoylqunic acid), flavonoids (formononetin, calycosin, and kaempferol-3-O-rutinoside), lactones (butyllidephthalide and ligustilide), monoterpenoids (paeoniflorin), phenanthraquinones (cryptotanshinone), and furans (5-hydroxymethylfurfural), which could be considered as potential chemical markers for quality control of NXT (Wang et al., 2018a). Recently, six active compounds including paeoniflorin, lithospermic acid, salvianolic acid B, Z-ligustilid, 3,5-dicaffeoylquinic acid, rosmarinic acid, were found to be accounted for a large proportion of thrombin/FXa inhibitory activity of NXT, which supposed to be anticoagulant quality markers in NXT (Du et al., 2022).
Quality control is an indispensable part to ensure the safety and effectiveness of TCM. Currently, a series of strategies have been used to establish an appropriate quality control system, including qualitative identification, content determination, finger-prints, etc. Multiple technologies such as thin layer chromatography (TLC), high performance liquid chromatography (HPLC), gas chromatography (GC), and mass spectrometry (MS) have been widely applied to the study on quality standard of TCM.
For NXT, in the Chinese Pharmacopoeia of 2020 edition, microscope is used for the identification of Carthami Flos, Angelicae Sinensis Radix, Astragali Radix, Cinnamomi Ranulus, Paeoniae Radix Rubra, Scorpio, and Spatholobi Caulis, while TLC is used to qualitatively identify Salviae Miltiorrhizar Radix et Rhizoma, Astragali Radix, Angelicae Sinensis Radix, Chuanxiong Rhizoma, Achyranthis Bidentatae Radix, Boswellia Carterii Resina, Spatholobi Caulis, and Cinnamomi Ramulus.
Chromatographic fingerprinting is an effective strategy to evaluate the intact quality of TCM medicinal materials and formula, which can reflect the complex chemical information contained in TCM more comprehensively. Using HPLC coupled with DAD detector, Su et al. established fingerprint-based quality control method for NXT with 33 common characteristic peaks, 13 of which were identified as mulberroside A, ligustrazine, catechin, paeoniflorin, calycosin-7-glucoside, hydroxysafflor yellow A, ferulic acid, rosmarinic acid, calycosin, salvianolic acid B, formononetin, tanshinone Ⅰ, tanshinone Ⅳ(Su et al., 2019), which were derived from twelve plant herbs in NXT, and could provide an effective method for quality control. However, due to the huge difference in components between plant herbs and zoological drugs, the sample preparation methods and the detection conditions are totally different, no components of zoological drugs were detected in this HPLC fingerprint. Indeed, it is extremely difficult for researchers to simultaneously determine the compounds belonging to sixteen medicinal materials in the formula.
Quality control of animal derived ingredients is also an essential approach to ensure the safety and effectiveness of NXT. DNA barcoding technology is a newly developed biological species identification technology in recent years. This technology uses a relatively short standard DNA sequence in the genome to identify species. It has been widely applied in the identification of Chinese medicinal materials from animals and plants. Most of the identified ingredients of NXT in the above studies are derived from the plant herbs. There has been a lack of reports on the identification of three zoological drugs (Hirudo, Scorpio, and Pheretima) in NXT and their protein components. In order to fill this gap, our group used DNA barcoding molecular identification technology to design the specific primers of Hirudo, Scorpio, and Pheretima, which can be used to identify whether the sample of NXT contains the corresponding authentic animal drugs, greatly improving the quality control of NXT. Using DNA barcoding technology, we developed a method to authenticate zoological drugs including Hirudo, Scorpio, and Pheretima in NXT (Zhu et al., 2019a; b; 2020). Specific primers were designed through mitochondrial cytochrome c oxidase subunit I (COI) gene sequence for genuine species, namely, Metaphire and Amynthas generas of Pheretima, Whitmania pigra and Hirudo nipponica of Hirudo, Mesobuthus martensii of Scorpio, respectively. Using specific primers, PCR amplification was performed for the genuine species and the common asulterants of Pheretima, Hirudo and Scorpio, as well as NXT. The obtained amplification sequences were sequenced and assessed by basic local alignment search tool (BLAST) in GenBank database. The species of samples were identified by the sequences with the most similarity. Results showed that the Pheretima’s specific primers MF2R2, Hirudo’s specific primers WF1R2 and WF2R2, Scorpio’s specific primers SF1R1 and SF2R4, could specifically identify the corresponding animal drug that the company used in NXT, namely, Metaphire vulgaris (or Metaphire guillelmi), W. pigra and M. martensii, with amplicons of around 230 bp, 200 bp, 250–260 bp, respectively. This DNA molecular identification method is accurate, simple, with high specificity and sensitivity, thus it could be used as a supplement to the conventional method for origin identification and further improve the quality control of three zoological drugs in NXT.
In the Chinese Pharmacopoeia of 2020 edition, HPLC based quantitative determination is applied to monitor the paeoniflorin and salvianolic acid B, which are derived from Paeoniae Radix Rubra and Salviae Miltiorrhizae Radix et Rhizoma. According to the regulations, the content of paeoniflorin and salvianolic acid B should not be less than 0.40 mg per capsule, respectively. To date, plenty of studies focus on the content determinations in NXT (details in Table 2) and a mass of active ingredients have been detected, mainly including flavonoids, organic acids, and lactones. Moreover, the trace elements in NXT also have been quantified.
Pharmacokinetics plays a vital role in drug development and efficacy evaluation. Pharmacokinetics mainly investigate the characteristic interactions of a drug and the body in terms of absorption, distribution, biochemical conversion (or metabolism) and excretion, as well as the dynamic changes of blood concentration. For most TCM, the active ingredient needs to be absorbed into blood and reach the target organs thus exert pharmacological effects. Hence, clarifying which compounds have distinct exposure in blood or tissue after administration is the primary issue when conducting pharmacokinetic researches of TCM.
To date, a series of pharmacokinetic studies of NXT have been carried out. Li et al. identified eleven components in the plasma of the rats orally administered NXT, including tanshinone IIA, ecdysterone, mulberroside A, amygdalin, salvianolic acid B, caffeic acid, astragaloside IV, ferulic acid, formononetin, cryptotanshinone, and paeoniflorin. In addition, the pharmacokinetics of ferulic acid, caffeic acid, formononetin, cryptotanshinone and tanshinone ⅡA in rat plasma were studied. It was found that caffeic acid, ferulic acid and formononetin were rapidly absorbed into the blood of rats after an oral administration of NXT, whose time to reach maximum drug concentration (Tmax) were all less than1hour (Li J. et al., 2017). In another study, with the method of everted gut sacs, Yang et al. investigated the absorption of chemical components of NXT in different intestinal segments and found that paeoniflorin, hydroxysafflor yellow A, salvianolic acid B and ferulic acid were absorbed in the ileum and jejunum segments, and the absorption of these four components did not reach Tmax within 3 hours, while the mode of transport of these four components remains to be further studied (Huang et al., 2013). Our group systematically studied the metabolism of NXT in beagle dog by UFLC-Q-TOF-MS/MS. As a result, a total of 25 prototype and 15 catabolites of NXT were detected in beagle dog plasma (He et al., 2021), while 36 prototype and 52 metabolites were identified in feces and urine (He et al., 2019). Meanwhile, the pharmacokinetic profiles of six crucial components including cryptotanshinone, ferulic acid, paeoniflorin, prunasin, senkyunolide G, and tanshinone IIA were investigated and the Tmax of these six compounds were determined as 1.29, 1.12, 2.33, 1.91, 1.25, and 1.75h, while corresponding Cmax were 2.32, 18.99, 41.03, 433.88, 2.76, 2.37 ng/mL, respectively (He et al., 2021). Recently, Li et al. compared the difference of pharmacokinetic parameters of the major bio-active components of NXT in normal and acute blood stasis rats. The result showed that Cmax of ferulic acid and formononetin, AUCall of ferulic acid and ferulic acid, and AUCINF_obs of ferulic acid were significantly decreased in blood stasis rats, suggesting the absorption of the four NXT components was weakened in the pathological state of blood stasis, which might be related to the alteration of hemorheology (Li et al., 2022). The metabolic products are summed in Table 3.
Given NXT has been widely used in a co-administered treatment with other prescription drugs, such as dual antiplatelet therapy (clopidogrel and aspirin), statins, or other TCMs in treating CCVD, it is necessary to attach importance to the drug-drug interactions in pharmacokinetic studies. The key enzyme system, CYP450 family, plays a vital role in metabolizing various drugs and the regulation of CYP450 enzymes is a pivotal cause of adverse drug-drug interactions. Chen et al. found that NXT significantly promoted the catalytic activities of CYP2C19 enzyme on drug metabolism in human liver microsomes (Hui, 2011a). Their further study demonstrated that NXT could enhance the promoter activity, expression and metabolic activity of CYP2C19 via pregnane X receptor (PXR) (Sun et al., 2016). And the addition of NXT to clopidogrel exhibited increased antiplatelet effect in patients with CYP2C19∗2 gene mutation after percutaneous coronary intervention (PCI) (Hui, 2011b). Ouyang et al. investigated the potential effects of NXT on the CYP450 activities in rats and evaluated the pharmacokinetics effect of NXT on metoprolol tartrate and found that NXT exerted significant effect on inducing CYP3A4 activity while inhibiting CYP2D6 activity in vivo, thus affecting the metabolism of metoprolol tartrate and its metabolites, suggesting the importance to have a widespread concern regarding the combined use of NXT and western medicine clinically especially those metabolized by CYP3A4 and CYP2D6, so as to prevent drug-drug-related toxicity and side effects (Ouyang et al., 2019). The above pharmacokinetic studies laid a foundation for the research of NXT’s pharmacological mechanism and provide important information and scientific basis for further rational clinical application of NXT.
Atherosclerosis is a profound pattern disease of arteriosclerosis, characterized by a buildup of fibrofatty plaque within the innermost layer of an arterial wall (Tabares-Guevara et al., 2021). These plaques develop to thick and narrow the arterial wall within lesions, reducing the blood flow and oxygen supply. Moreover, a fully developed plaque could rupture, leading to dramatic hemorrhage and blood clot formation. In either case, the artery can be entirely blockage with severe blood flow cutting off. In fact, atherosclerosis results in not only myocardial infarction and stroke but also disabling peripheral artery diseases (Badimon and Vilahur, 2014; Tatsumi and Mackman, 2015).
As for atherosclerotic plaque initiation and progression, low density lipoproteins (LDLs), which take charge of blood cholesterol transport, has been well-characterized as the most profound risk factor. Other risk factors, both for atherosclerosis and its thrombotic complication, includes high levels of circulating cholesterol and triglyceride, aberrant lipid intake and metabolism, obesity, hypertension, cigarette smoking and diabetes mellitus (Yang et al., 2016; Lu et al., 2022). Increasing evidences have demonstrated the potent role of NXT in alleviating hyperlipidemia and excessive LDL particles in plasma, which is a prerequisite for plaque initiation. In high fat diet or genetic modified ApoE knockout mice, 10 weeks of NXT gavage (624 mg/kg bodyweight per day (mpk)) remarkable attenuated the accumulation of total cholesterol (TC), triglyceride (TG), and low-density lipoprotein cholesterol (LDL-C) in circulating blood, while restored the content of serum high density lipoprotein cholesterol (HDL-C) (Yang et al., 2016). In the liver of high-fat diet fed ApoE KO induced atherosclerotic mice, by activating hormone-sensitive lipase (HSL), adipose triglyceride lipase (ATGL) and energy-sensing AMP-activating protein kinase (AMPKα) signaling, NXT downregulated hepatic lipid contents with reduced abundance of TC and TG enriched droplets (Yang et al., 2017).
Atherosclerotic plaque is initiated when circulating LDL particles penetrate and accumulate into the innermost layer, tunica intima, of the arterial wall. This happens when circulating monocytes respond to the Chemoattractant signaling of the inflamed site, recruited into the intima layer where they mature into macrophages that are capable of recognizing and engulfing intima resident LDL particles and turn into foam cells. In the pro-atherogenic model of ApoE−/− mice fed an atherogenic diet, Wang et al. found that 8 weeks of NXT gavage (624 mpk) could significantly decline the accumulation of foam cells in atherosclerotic plaques, which was associated with the reduction of cellular uptake of oxidized LDL (ox-LDL) as well as the decrease of surface expression of scavenger receptor class A (SR-A) and B (SR-B) (Wang et al., 2018). Another study found that NXT downregulated the gene expression of monocyte chemoattractant protein-1, which compromised the penetrate of monocytes into the tunica intima layer and thus alleviated the foam cell formation (Yang et al., 2016; Long-Tao and Chang-Geng, 2018). Using HFD induced atherosclerosis in LDLR deficient mice, Jin et al. reported the inhibitory of NXT on dendritic cell maturation. NXT treatment significantly delayed the maturation of dendritic cells within lesion, as evidenced by reduced abundance of surface molecules CD40, CD86, CD80 as well as compromised production of pro-inflammatory IL-12p70 (Zhao et al., 2013). As dendritic cells actively participate in early foam cell formation, lipid homeostasis as well as efficient T cell activation, this study shed light on the potential effects of NXT in regulating innate-adaptive immune crosstalk during atherosclerotic plaque development.
The most life-threating event in atherosclerosis is the sudden rupture of a vulnerable plaque, which is the leading cause of acute hemorrhage and severe thrombosis. Hence, increasing the stability of vulnerable plaques is of top priority in disease intervention and management (Han et al., 2019; Mushenkova et al., 2020). Vulnerable plaques are often associated with high inflammatory cell content and the presence of a large necrotic core. Within plaques, inflammatory activation of macrophages stimulates the release of pro-inflammatory cytokines, inhibits interstitial collagen synthesis and protease digestion of fiber cap components (Nakajima et al., 2022). Studies demonstrated NXT could help to maintain unstable plaque. In high-fat diet fed apoE (−/−) mice, NXT increased the content of smooth muscle cells/collagen in the cap of aortic wall lesions, while reducing the buried fiber cap, mineralization and macrophage aggregation in the lesions, suggesting that NXT can reduce advanced atherosclerosis while improve plaque stability (Yang et al., 2016).
As atherosclerosis progresses, the unstable plaques may eventually rupture to form arterial thrombi (Han et al., 2019). Such thrombus potentially disrupts or even blocks the blood flow, leading to health-threating complications including myocardial infarction and acute ischemic stroke, depends on different sites of obstruction (Asada et al., 2018; Stark and Massberg, 2021). Currently, the “gold-standard” strategy for thrombotic management is the long-term application of anti-platelet drugs, among which aspirin and clopidogrel are the most widely applied. These two drugs, however, could lead to detrimental consequences. Notably, long-term, even lifelong anti-platelet meditation dramatically increases the risk of accidental bleeding and hemorrhage transformation. Thus, it is of great demand of seeking alternative strategies which can balance the effects between thrombosis and hemorrhage. Using acute stasis rats, we have investigated the antithrombotic effect of NXT in vivo. Compared with model group, NXT treatment indicated a potent improvement of blood coagulation and platelet aggregation, as evidenced by decreased the whole blood viscosity at low shear rates, prolonged prothrombin time and activated partial thromboplastin time, decreased the fibrinogen (FIB) level, and reduced the level of platelet activating factor (Zhang et al., 2020b). Thrombin and factor Xa are two critical modulatory targets of anticoagulants. Recently, Du et al. reported that NXT extract exhibited great activity against thrombin and factor Xa. Six feature components of NXT extract were screened out, namely, paeoniflorin, lithospermic acid, salvianolic acid B, Z-ligustilid, 3,5-dicaffeoylquinic acid, rosmarinic acid, which accounted for the predominant inhibitory activities of thrombin/factor Xa (Du et al., 2022). Based on the carrageenan-induced mouse model, Yang et al. found that NXT treatment could reduce thrombi, which was related to the downregulation of serum TNF-α and P-selectin levels. In vitro, NXT effectively reduced lipopolysaccharide-activated adhesion of THP-1 monocytes to human umbilical vein endothelial cells HUVECs, and inhibited oxidized LDL activated platelet adhesion. Of note, NXT administration could antagonize clopidogrel induced cell death in HUVECs, which shed light on the potential of NXT in combination therapy to recover clopidogrel caused cellular damages (Li et al., 2018). Moreover, using a thrombosis mode in zebrafish, Wang et al. demonstrated the attenuation of NXT treatment in zebrafish tail venous thrombus with enhanced quantity of heart red blood cells through modulating COX2-VEGF/NFκB signaling (Wang et al., 2021).
The role of the gut microbiota in atherosclerosis has begun to be appreciated in recent years. Mounting evidence has indicated the imbalanced gut microbial community in humans is associated with the incidence of atherosclerosis (Jonsson and Bäckhed, 2016). Recent studies have convincingly linked gut microbiota to traits relevant to atherosclerosis, such as dyslipidemia, inflammation and insulin resistance (Org et al., 2015). It is noteworthy that a series of recent studies demonstrated that NXT could alleviate atherosclerotic cardiovascular diseases partially by regulating the structure of gut microbiota. With the high-fat diet (HFD)-fed minipig model, we found that the long-term administration of NXT could regulate blood lipid profiles, inhibit vascular inflammation, enhance antioxidant capacity, and alleviate myocardial injury. NXT could increase the diversity of gut microbiota and reverse the increase of Firmicutes/Bacteroidetes (F/B) ratio caused by high fat diet. Some key bacterium, including Caproiciproducens, Sutterella, Erysipelotrichaceae, and Romboutsia were found to be closely involved in NXT’s effects (Zhang et al., 2019). In another experiment, Li et al. explored the preventive effect of NXT on hyperlipidemia in HFD-fed rat model and also investigated the regulation of gut microbiota, and found that NXT could significantly reduce TC, TG, LDL-C levels and increase HDL-C level in serum. And NXT significantly altered the composition of gut microbiota and changed the fecal levels of gut microbiota related metabolites, including bile acids (BAs) and short chain fatty acids (SCFAs). Some important factors as F/B ratio, the amount of propionic acid and the relative abundance of Collinsella were proposed to be potential therapeutic biomarkers (Lu et al., 2022).
Statins are the first-line lipid-lowering drugs used clinically for the treatment of atherosclerosis. However, long-term statin therapy often causes side effects such as liver injury and rhabdomyolysis. NXT combined with statins not only have beneficial effects on atherosclerosis, but also can reduce the adverse effects of statins. Yang et al. suggested that the combination of NXT and atorvastatin could, on one hand, enhance plaque stability and, on the other hand, attenuate atorvastatin-induced acute liver inflammation (Yang et al., 2017). In addition, we have demonstrated that NXT administration could attenuate atorvastatin induced excessive oxidative stress, hepatic and renal dysfunction, inflammatory activation, and endothelial integrity (Zhang et al., 2020a). These drug combination studies suggest that the co-treatment of NXT to atorvastatin can enhance safety and efficacy.
Cardiovascular disease (CVD) is a category of diseases associated with heart or cardiovascular vessels, including coronary artery diseases, myocardial infarction, post-infarction myocardial remodeling, and cardiomyopathy (Wang et al., 2021). The World Health Organization defines cardiovascular disease as a leading life-threaten cause globally. In 2016, there was an estimated of 17.9 million cardiovascular disease induced death, accounted for 31 percentiles of all-cause mortality globally (Mendis et al., 2015). Among CVD, myocardial infarction has become one of the leading causes of death in the world. It is caused by myocardial ischemia and hypoxia due to coronary artery disease. Myocardial infarction happens when blood flow decreases or even stops, leading to dramatically myocardial ischemia and tissue death. Besides the beneficial outcomes of NXT in treating thrombosis, its cardioprotective effects against myocardial infarction had been systematically explored and highlighted. In one study, using cardiogenic cell line H9c2, Xu et al. suggested the water exact of NXT could significantly protect against superoxide induced cell death via modulating PPARα expression, and promote autophagy and autophagy associated genes, leading to inhibition of mTOR signaling (Xu et al., 2016). Moreover, NXT water extract could potently alleviate cardiomyocyte hypertrophy in H9c2 cells, such effect is achieved, at least in part, through regulating PPAR𝛾-mediated autophagy (Yuan et al., 2017). In another study, the in vivo intestinal absorption liquid was employed instead of drug containing serum or extraction fractions and found that precondition of NXT remarkably enhanced the anti-oxidative capacity, inhibited activation of PPAR/caspase-3, rescued mitochondrial membrane potential, and reduced intracellular Ca2+, thereby protecting myoblasts from oxidative injuries and programmed cell death (Zhang et al., 2013). Recently, Wu et al. assessed the therapeutic outcomes of NXT on acute myocardial infarction induced by ligation of the left anterior descending coronary artery in rats and investigated the pharmacodynamic mechanisms from the perspective of metabolic reprogramming. After NXT treatment, heart function was significantly recovered, with both left ventricular ejection fraction (LVEF) and left ventricular fraction shortening (LVFS) improved. Histology revealed decrease inflammatory infiltration, alleviated myocardial fibrosis, and reduced infarct size in NXT treated rats. Targeted metabolomics further demonstrated NXT treatment could restore myocardial ischemia induced metabolic rewiring, including glycolysis, tricarboxylic acid (TCA) cycle, oxidative phosphorylation, purine metabolism and glutathione metabolism. In line with shifted metabolite profiles, mechanism studies indicated the effects of NXT in regulating metabolism associated crucial proteins, including silent information regulator 1 (SIRT1), peroxisome proliferator-activated receptor gamma coactivator 1α (PGC-1α), and a subunit of mitochondrial ATP synthase (ATP5D). These results together emphasized the cardioprotective effects of NXT against myocardial infarction, and highlighted the regulatory roles of NXT in energy metabolism related metabolic reprogramming (Zhao et al., 2022). Activated platelets is another crucial risk factor for myocardial infarction occurrence. A platelet-fibrin plug often forms at the site of ruptured atherosclerotic plaque, which could lead to myocardial infarction (Oikonomou et al., 2020). Study of Wang et al. revealed that NXT significantly decreased infarct size, decreased lactic dehydrogenase (LDH), creatine kinase (CK), and creatine kinase-MB (CK-MB) values, and improved cardiac function in rats with myocardial infarction. Such beneficial effects were achieved, at least in part, by inhibiting platelets activation via down-regulating ROS levels, decreasing ERK5 phosphorylation, and increasing RAC1 phosphorylation (Zhang et al., 2022).
The brain is one of the most important and subtle organs in the human body, and the cerebral circulation continuously supplies blood to the brain. All diseases affecting brain circulation can be defined into the category of cerebrovascular diseases, with stroke being the deadliest, accounting for more than 10% of all cause deaths worldwide. Cerebral ischemia is caused by embolic occlusion or thrombotic of a cerebral artery, accounting for more than 60% of stroke cases (Collaborators, 2021). On one hand, under ischemic stroke, the blood supply to part of the brain is compromised and oxygen is deprived, often leads to neuronal cell death and functional losses. When the blood supply is restored, on the other hand, reperfusion injury occurs because of increased production of reactive oxygen/nitrogen species, microvascular vasoconstriction as well as cytokine release. NXT has been shown to have a beneficial effect on ischemic stroke. Using a middle cerebral artery occlusion (MCAO) rat model, Yang et al. conducted a UPLC/TOF-MS-based metabolomic study to explore potential biomarkers in plasma associate with cerebral ischemia as well as evaluated the therapeutic effects of NXT and found that NXT treatment could significantly improve neurological deficits and reduce cerebral infarct size. Meanwhile, NXT treatment obviously altered the levels of the potential therapeutic targets, such as glutamine, PE (17:0), LysoPE (20:1), LysoPE (24:0), etc., suggesting that the therapeutic effects of NXT on cerebral ischemia are partially due to interference with glutamine metabolism and lipid metabolism (Liu et al., 2016). Amino acids in cerebrospinal fluid perform critical roles in cerebral ischemia. Recently, focusing on the amino acids in cerebrospinal fluid, the same group conducted an amino acid-protein interaction imbalanced network of cerebral ischemia to further investigated the pharmacological mechanisms of NXT. They observed the dynamic levels of amino acids in cerebrospinal fluid 3,6,12, and 24 h after MCAO, and found that NXT treatment could restore the abnormal level of nine amino acid biomarkers at 24 h after MCAO, including alanine, valine, taurine, leucine, isoleucine, glutamine, glutamic acid, phenylalanine, (S)-tyrosine, and tryptophan. And, aromatic-L-amino-acid decarboxylase (AADC) was found to be one of the putative targets for NXT in the protection against cerebral ischemia (Xu et al., 2019). In a permanent MCAO model, Li et al. conducted combining LC–MS/MS and transcriptome microarray analyses to investigate the protein and mRNA profiles and the changes in the rat cerebral cortex during cerebral ischemic injury and repair processes due to NXT treatment. They have identified 39 potent candidates, signaling pathways and core markers for cerebral ischemic injury modulated by NXT, among which marked MAPK signaling as a major target for NXT (Liu et al., 2020). Using a cerebral ischemia-reperfusion (I/R) injury mice model, Xue et al. evaluated the neuroprotective effect of NXT against I/R as well as relevant mechanism. Compared with model group, NXT treatment had significantly improved neurological defect, reduced infarct volume and brain edema. Meanwhile, NXT downregulated the elevated levels of MDA and SOD. The neuroprotective mechanisms of NXT against cerebral I/R injury may associated with the anti-oxidant and anti-inflammation activities via decreasing the expression of LOX-1, phosphorylation of ERK1/2 and translocation of NF-κB p65 (Xue et al., 2016).
Ischemic stroke, hemorrhagic stroke, hypoxic-ischemic brain injury and other cerebrovascular diseases can cause insufficient blood flow, leading to functional impairment and cognitive defects of the brain, such as vascular dementia (VD). Ma et al. explored the role of NXT on VD in mice model with chronic hypoperfusion damage, which was established by double carotid artery coarctation operation. NXT could recover the loss of space learning and memory, improve the morphological alterations of neural tissues, inhibit the activation of pro-inflammatory astrocytes, and eventually rescue the ischemic damages of the brain (Ma et al., 2015). Other studies revealed that NXT could protect against VD by enhancing hippocampus VEGF expression, inhibiting endothelial apoptosis, sustaining endothelial barrier integrity, promoting angiogenesis (Wei et al., 2006) and regulating choline acetyl transferase in cholinergic neural circuits (Sun and Zeng, 2022). Moreover, in APPswe/PSEN1dE9 double transgenic (APP/PS1) mice, the beneficial outcomes of NXT against cognitive deficit had been further clarified. NXT inhibited neuron atrophy and apoptosis by reducing the contents of pro-inflammatory mediators including IL-1β, IL-6, TNF-α in the brain. Meanwhile, in a mouse hippocampus neuronal cell line HT-22, NXT had been found to mitigate L-glutamic acid enhanced inflammation, reactive oxygen species (ROS) production and apoptosis, partially via down-regulating TLR4/NF-κB/IL-1β pathway (Wang et al., 2021). These studies together emphasize NXT as a promising candidate for treating cerebral vascular diseases as well as vascular associated cognitive disorders.
In addition to cardiovascular and cerebrovascular diseases, other diseases can also be improved by NXT treatment, all of which are reported to be related to diabetes. Diabetes is characterized by long-term hyperglycemia, which can cause multiple complications. In past years, our group first combined the gut microbiome and metabolomics to explore the effects and potential mechanisms of NXT in anti-diabetes. Based on the type 2 diabetes mellitus (T2DM) rat model established by HFD combined with streptozotocin (STZ) injection, 8 weeks of NXT gavage (1,000 mpk) was found to significantly ameliorate hyperlipidemia and hyperglycemia, improve insulin resistance, reduce inflammation and alleviate myocardial injury. Meanwhile, NXT recovered the richness and diversity of microbial community in T2DM rats. At genus, NXT significantly altered the relative abundances of certain gut bacteria, including Erysipelatoclostridium, Oscillibacter, Ruminiclostridium 9 and Ruminococcus 1. Besides, NXT significantly changed the levels of metabolites in serum, mainly including fatty acids, lysophospholipids, bile acids and other metabolites. Several feature pathways, such as arachidonic acid metabolism, fatty acid β-oxidation and glycerophospholipid metabolism, were identified as the key pathways of NXT in anti-diabetes (Yan et al., 2020).
Diabetic nephropathy is one of the complications of diabetes with high morbidity and mortality. Using 6-week-old adolescent db/db mice, Yang et al. first evaluated the protective effects of NXT on diabetic nephropathy, and found NXT significantly reduced diabetes-increased glucose levels, ameliorated serum lipid profiles, and improved renal functions (Yang et al., 2018). Recently, in 12-week-old adult db/db mice, they further explored the therapeutic outcomes of NXT. At late phase of diabetic nephropathy development, NXT treatment was also capable of reducing diabetes-induced systematic hyperglycemia and dyslipidemia. Moreover, after NXT post-treatment, several feature symptoms including high secreted albumin and ratio of albumin to creatinine in urine, kidney atrophy/hypernephrotrophy, mesangial matrix expansion as well as glomerulosclerosis are all remarkably attenuated. Such protective effects were associated, at least in part, with MMP2/9 activation, inactivation of TGF-β1 pathway, inhibitory upregulation of adhesion molecules in the kidney as well as activation of AMPKα-mediated glucose and energy metabolism in liver, adipose tissue, and skeletal muscle (Yang et al., 2019).
Diabetic wound healing is a troublesome in diabetes mellitus. And, nearly one-third of costs are attributed to the management of wound healing associated diabetic foot disorders (Driver et al., 2010). To this concern, effective strategies are urgently needed to mitigate the incidence and severity of these chronic, non-healing diabetic foot ulcers. In one study, Fang et al. investigated the effects of NXT in a murine model of diabetic wound healing and found that NXT could accelerate diabetic wound closure, accompanying by the upregulation of ECM remolding and collagen deposition. For mechanism, NXT promoted neutrophils efferocytosis, which might help to alleviate neutrophils induced tissue damages (Fang et al., 2021). In addition, NXT had been reported to rewire the polarization of M2 macrophages through regulating JAK/SATA cascades, which would facilitate focal inflammatory resolution and macrophage mediated tissue repair (Fang et al., 2021). These results, when taken together, emphasize the promising potential of NXT as a combination therapeutic strategy in the management of diabetic complications. The pharmacological properties of NXT were illustrated in Figure 2.
So far, many studies, both in vitro and in vivo, have proven the beneficial effects of NXT in the prevention and treatment of CCVD in varied pathological stages. These studies have been carried out in various animal models as well as cell models, providing considerable evidences in both pharmacological and mechanism perspective. Multiple protective effects of NXT on CCVD have been reported, including atherosclerosis, thrombosis, myocardial infarction, ischemic stroke, and diabetic nephropathy. Biologically, these protective effects on cardio-cerebrovascular diseases of NXT can be associated with its actions on lipid/glucose metabolism, inflammation, oxidative stress, apoptosis and gut microbiota. As the representative prescription of co-treatment of CCVD, NXT possesses complex components and plays a therapeutic effect through multiple targets and multiple pathways. The pharmacological properties of main active components of NXT are summarized in Table 4. In brief, NXT performed its therapeutic effect on the co-treatment of CCVD through the pattern “multiple components-multiple targets-multiple pathways”.
Actually, cardio-cerebrovascular diseases are commonly caused by the abnormality of “blood” and “blood vessel”, that is, the abnormal change of hemorheology, including the blood viscosity, blood coagulation, etc. and the change of vascular morphology and biologic functions. Modern medicine believes that atherosclerosis is the common pathological basis of cardio-cerebrovascular diseases. From this point of view, the common underlying mechanism of NXT for the co-treatment of cardio-cerebrovascular diseases has been discussed in detail in section 5.1. However, given that these effects are scattered throughout the course of CCVD, randomized controlled trials with long-term follow-up are urgently needed to clarify the precise roles of NXT in personalized medicine and to declare its rationality in combination therapy.
ACS is the leading cause of death in coronary heart disease (CHD), often caused by the instability of atherosclerotic plaque (Zhang et al., 2023). The mainstay treatment for ACS is percutaneous coronary intervention (PCI), which can quickly reconstitute the blood supply and effectively remove vascular stenosis or occlusion (Dong et al., 2023). However, PCI may cause plaque rupture, platelets activation, formation of blood clots and could even lead to a secondary thrombus (Abbate et al., 2012). And, dual antiplatelet therapy (aspirin plus clopidogrel) is the most commonly treatment strategy for ACS patients undergoing PCI. It is worth mentioning that the antiplatelet effect of clopidogrel depends primarily on its activation by the cytochrome P450 (CYP) system, particular CYP2C19 enzyme. The person who has more CYP2C19*2 copies possesses reduced CYP2C19 enzyme activity will be less sensitive to clopidogrel treatment due to the poor metabolism of clopidogrel (Fontana et al., 2008). In many cases, dual antiplatelet therapy does not achieve the ideal therapeutic effect in patients who carried the CYP2C19∗2 polymorphism as the part of clopidogrel does not work well. Interestingly, about 50% population in China possesses CYP2C19*2 the nonfunctional allele (Fontana et al., 2008), which means that the clopidogrel therapy may does not work well in half of Chinese patients. In China, to further enhance the clinical efficacy, some TCMs will be used as the secondary prevention in addition to aspirin and clopidogrel. In the study of Chen et al., ninety patients with CYP2C19*2 polymorphism were randomly assigned to receive either adjunctive NXT (triple group, 45 cases) or dual antiplatelet therapy (dual group (aspirin plus clopidogrel), 45 cases). The authors compared the degree of platelet inhibition at 7 days after treatment and the rate of subsequent major adverse cardiovascular events (MACEs) during a 12-month follow-up. They found that the adjunctive NXT to dual antiplatelet therapy could significantly enhance the antiplatelet effect and decrease subsequent MACE in patients with the CYP2C19*2 polymorphism undergoing PCI(Chen et al., 2014). Despite the sample size in the study was small, the positive results in long follow-up periods (12-month) makes the claim convincing that the addition of NXT to dual antiplatelet therapy helped a lot in patients with the CYP2C19*2 polymorphism. The specific mechanism of action needs further study.
In recent years, NXT has also been reported effective when combined with Danhong injection in ACS patients undergoing PCI base on the secondary prevention. Both of the two clinical trials revealed that the combination of NXT and Danhong injection had better effects on cardiac functions than each of them alone (Lv et al., 2015; Zhao et al., 2018). Thereinto, one study is a multicentered, randomized controlled, double-blind trial, placebo-controlled, and parallel-designed clinical trial with 65 cases in each group. The primary outcomes were clinical curative effects and cardiovascular events. The secondary outcomes included parameters from laboratory examinations: plasma ET-1, NO, VWF, and cardiac function such as left ventricular ejection fraction (LVEF). In the NXT combination group, the authors observed the better signs of clinical efficacy, such as reduced LVEF, higher nitric oxide levels, and lower levels of endothelin-1 (ET-1) and von Willebrand factor (VWF), without detectable cardiovascular events or other adverse reactions. Another study investigated the effect of NXT combination on short-term prognosis by comparing the incidences of major adverse cardiovascular events and cardiac functions, including ejection fraction and 6-min walk test distance, and serum level of the ACS marker (sCD40 L), during hospital discharge and at 3 months after PCI. The conclusion is that the NXT combination exerted significant effect in improving the cardiac functions at 3 months following discharge, but not at the time of discharge. However, some limitations did still exist in both two studies. For instance, they did not examine end points such as all-cause mortality, the sample size was small, and the follow-up time of 3 months is relatively short.
Systematic review and meta-analysis provide an available approach to assess the existing evidence to support the clinical practice. Recently, a meta-analysis of 13 clinical randomized trials (RCTs) involving 1,286 patients after PCI revealed that compared with the control group treating with conventional WM, NXT combined WM group showed better efficacy in decreasing the maximum platelet aggregation rate, lowering the levels of plasma vWF, GPⅡb/Ⅲa, and fibrinogen, and reducing the occurrence of adverse cardiovascular events (Liu et al., 2022). However, because of the limited number and quality of articles included, the findings of meta-analysis need to be validated by additional more high-quality, and double-blinded multicenter RCTs with a larger sample size in the future. Taken together, to some extent, the positive results in clinical trials suggested that NXT could be one of the complimentary drugs used in the secondary prevention for ACS patients after PCI.
CSA, characterized by chest pain or discomfort, is a major symptomatic presentation in about 50% of patients with CHD (Gao et al., 2021). In China, the prevalence rate of CSA was about 3.6% in 2017 and the numbers are still rapidly increasing in recent years due to ageing as well as multiple risk factors such as diabetes mellitus, hypertension, etc., (Wang et al., 2022). Currently, guideline-directed therapy for CSA mainly includes antiplatelet agents, statins, calcium channel blockers and nitrates (Healthcare Engineering, 2023). However, some adverse reactions of the long term anti-atherogenic interventions have been reported (Banach and Mikhailidis, 2018; Qian et al., 2021). Besides, a number of patients are less sensitive to the antiplatelet treatment. Recently, in a clinical study by Qiu et al., NXT (4 capsules/time, 3 times per day) plus ticagrelor showed better total effective rate (92.16%) compared with ticagrelor alone (76.47%). And, NXT combination can better lower the frequency and duration of angina pectoris and reduce the serum cardiac troponinⅠ (cTnⅠ), CK-MB, myoglobin (MYO), myeloperoxidase (MPO) and lipid peroxide (LPO) levels, while elevate the left ventricular ejection fraction (LVEF) and stroke-volume (SV), suggesting that adding NXT to ticagrelor helped a lot in patients with CSA, especially in improving cardiac function and reducing angina pectoris symptoms (Qiu et al., 2023). Though the difference of curative effect between NXT combination and ticagrelor was significant and clear, the study has some flaws in the study design. For instance, this is a small scale RCT study (51 case in each group), with only 8-week intervention period, which is relatively short, and without follow-ups, making it less convincing. It is worth mentioning that CSA affects a large population across the world and has a high mortality rate. Therefore, a large-scale and long-term future study with a longer-term follow-up that assesses mortality as the primary endpoint is expected.
In the past nearly 30 years from 1990 to 2019, at least 10% global population died from ischemic stroke, accounting for half of all deaths in cerebrovascular disease (Group et al., 2020). The main treatment methods of ischemic stroke include craniotomy, minimally invasive intervention and drug therapy. However, both of craniotomy and minimally invasive intervention can be extremely traumatic, which should only be used as a last resort. Pharmacological intervention is the most conventional option, such as aspirin and clopidogrel are often used to treat IS but which can lead to an increased risk of bleeding in the brain. NXT is reported to be promising as a new alternative treatment. There have been several reviews demonstrated the beneficial effect of NXT on the treatment and prognosis of ischemic stroke (Li et al., 2021; Li et al., 2021). A considerable number of clinical studies have also been conducted on the treatment of ischemic stroke by NXT. In a clinical trial study, 153 patients (>65 years old) with non-valvular atrial fibrillation (NVAF) and genetic variants of vitamin K epoxide reductase (VKORC1) were selected to receive oral aspirin combined with NXT or warfarin and were followed for at least 1 year (Tohgi et al., 1991). The result showed that NXT has similar therapeutic effect but less risk of bleeding and all-cause death during the 1-year follow-up. Although the number of participants in the study was relatively small, the long-term follow-up still revealed part of the efficacy of NXT. Another study which focused on stroke risk in 600 patients with NVAF compared with the general population have shown that the incidence of ischemic stroke in the general population increases with age and has no difference between treated and untreated subjects among hypertensives (Wang et al., 2018b). Therefore, for patients who cannot tolerate warfarin, NXT may be considered as an alternative therapy. Only sufficient amount of data can provide more convincing validation, we need more experiments to verify the efficacy of NXT on patients of different ages.
VD is a group of mental and cognitive dysfunction syndromes caused by cerebrovascular accident and is one of the common causes of senile dementia. Drug therapy is the only treatment for VD. The commonly used drugs include acetylcholinesterase inhibitors, non-competitive N-methyl-D-aspartate (NMDA) receptor antagonists, aspirin, and heparin. Compared with them, NXT has fewer side effects (Kombo et al., 2022). Recently, a meta-analysis study revealed that the use of NXT in the treatment of VD can increase the clinical effect, improve cognitive function, enhance the ability of daily living and reduce the degree of clinical dementia, without increasing the risk of adverse reactions (Li et al., 2022). A total of 33 studies comprising 2,947 patients with VD were included in this study. However, the low methodological quality of the included RCTs that reduced the quality of evidence: first, none of the studies mentioned the allocation hiding and blind method, which had the risk of serious bias; secondly, mini-mental state examination (MMSE)score and activities of daily living (ADL) score index may have heterogeneity due to different treatment course and intervention measures in control group. Finally, the sample size of ADL score index is relatively small which would lead to large error in results. Thus, the findings remain to be questioned. The high-quality multicenter RCTs with more scientific experimental designs to derive more scientific and accurate conclusion are urgent needed.
With the prevalence of unhealthy lifestyles, the incidence of type 2 diabetes has been increasing and its related medical burden has been rising accordingly (Guo et al., 2023). In recent years, NXT showed its therapeutic potential in the treatment of diabetes complications in clinic including diabetic nephropathy, diabetic peripheral neuropathy, etc. In a clinical study involving 180 T2DM patients with atherosclerosis, NXT administration (3 capsules/time, 3 times per day) significantly decreased carotid artery intimal medial thickness (IMT) and plaque area, reduced levels of TC, TG, LDL-C, fasting plasma glucose (FPG), glycated hemoglobin A1c (HbA1c), while increased peak systolic velocity (PSV) and end diastolic velocity (EDV) (Cheng et al., 2016). In a clinical observation involving 80 patients, Zhou et al. investigated the effect of NXT (3 capsules/time, 3 times per day) combined with alprostadil in the treatment of diabetic nephropathy. NXT combination significantly decreased the 24 h urinary protein quantification and urinary albumin excretion rate as well as lowered the urea nitrogen and creatinine levels (Zhou et al., 2020). Another clinical study revealed that NXT is capable of improving the therapeutic effects in the treatment of diabetic peripheral neuropathy, such as increasing the motor nerve conduction velocity and sensory nerve conduction velocity (Zhang, 2015). Recently, a real-world retrospective cohort study concerning diabetic kidney disease (DKD) involving 1,798 DKD patients revealed that NXT can slow the decline of estimated glomerular filtration rate and reduce the risk of renal outcomes (Zhang et al., 2022). It is worth mentioning that almost all current RCTs concerning its effect in treating diabetes complications were conducted in China, and the published articles were written in Chinese, which may lead to potential selection bias. Thus, on account of some limitations, more high-quality, and double-blinded multicenter RCTs with a larger sample size are needed to test and verify the positive results of NXT in the treatment of diabetes complications in the future and high quality of clinical evidence is expected.
Benefiting from the effect of promoting blood circulation and dredging collaterals, NXT is also engaged in the treatment of other diseases. Compare with rivaroxaban alone, NXT combination was more effective in improving pulmonary function index, reducing IL-6, CRP and TNF-α levels, and improving the coagulation function in patients with acute pulmonary embolism (Sun et al., 2019). The significant superiority of NXT combination was also documented in treating lower extremity deep venous thrombosis, vertebral artery cervical spondylopathy and lumbar disc herniation (Li et al., 2018). The clinical applications of NXT were outlined in Figure 3.
A long-term preclinical toxicity test observed no adverse reactions in body weight, blood routine, heart, liver and kidney functions, and electrocardiogram (Long-Tao and Chang-Geng, 2018). In clinical application, some adverse events were reported, mainly including mild gastrointestinal reaction, such as anorexia, nausea, and diarrhea (Hou et al., 2014). Very few patients appeared skin pruritus, peeling, papules, lethargy, upset, and dizziness after starting the drug, which will disappear soon after it is withdrawn. On the whole, NXT possesses a very high safety profile in not only animal studies but also clinic trials. Further large-scale in-depth clinical safety evaluation based on big data would be meaningful to confirm the safety of NXT.
Since its inception in the 1993s, NXT has been employed in a mass of researches concerning its post-marketing quality and efficacy, including chemical profiles, quality control, pharmacokinetics, pharmacological properties and clinical applications. This review outlines the existing research findings, points out some typical problems and offers suggestions to contribute to the progress of NXT.
Firstly, the quality control of NXT needs further improvement. Currently, the chemical profiles of NXT have been systematically investigated. A variety of constituents, such as amygdalin, paeoniflorin, salvianolic acid B, ligustilide, gallic acid, hydroxysafflor yellow A, and butylidenephthalide were identified as the major cardioprotective components contained in NXT (Han et al., 2019). And, several quantitative methods for simultaneous determination of multiple components in NXT have been established. However, the existing quality control methods as well as chromatographic fingerprints are still not capable of detecting the components derived from animal medicinal materials in NXT, which are insufficient to reflect the integrity of the formula. For monitoring the overall quality, it is better to further establish the chromatographic fingerprints containing the animal medicinal ingredients, which remains a big challenge. Besides, the effective substances of NXT are not yet clear. It is urgently need to clarify the contribution of the component to the pharmacodynamic effects of NXT, especially those belong to the Zoological drugs. And, the biopotency-associated multi-index content determination method should be developed. In addition, in consideration of safety, it is necessary to monitor the content of amygdalin derived from Persicae Semen and buthatoxin derived from Scorpio in NXT.
Secondly, pharmacokinetic properties of NXT still need further comprehensive study. Indeed, there are a large number of absorbable and poorly absorbed components in NXT, thereby the pharmacokinetic behavior of NXT is extremely complex. The absorption and metabolism of NXT in vivo simply represented by the pharmacokinetic curves of a few or dozens of components is insufficient to fully understand its pharmacokinetic behavior, which still needs further study. Besides, pharmacokinetic process of NXT in humans have not yet been fully elucidated. And, the correlation between pharmacokinetics and pharmacodynamics of NXT remains largely obscured.
Thirdly, in-depth mechanism studies are scarce. Gut-organ axis is a topic gotten more attention in research field of human health in recent years, including the gut-brain axis, gut-heart axis, gut-liver axis and so on. Since NXT is taken orally, it inevitably interacts with gut microbiota. The recent studies addressing the influences of gut microbiota offered new perspectives on the mechanism of NXT in the prevention and treatment of diseases. However, these existing studies just investigated the correlation between gut microbiota and clinical/metabolomic parameters after the NXT intervention and what the result offers is a possibility. The correlations do not prove cause and effect. Further studies should use the germfree animal techniques and fecal microbiota transplantation to confirm the causal effect of NXT on gut microbiota. Besides, further studies could also focus on the specific metabolites via targeted metabolomics technology. Specially, BAs and SCFAs have been reported to be closely associated with glucose and lipid metabolism. It would be valuable to conduct targeted studies on bile acid metabolic pathways or SCFA metabolic pathways since NXT was reported to have effect on these metabolites. And, it is encouraged to clarify functional mechanisms of NXT in human, integrating multi-omics technologies (gut microbiome, metabolomics, etc.), accompanying by verification through the animal or cell experiments.
Fourthly, high-quality RCTs are still lacking. Large-scale, multicenter randomized clinical studies with placebos, long follow-up and well-defined endpoints are urgent need for reconfirming the effectiveness and safety of NXT. Besides, since NXT is usually used in the combination with other drugs in the clinic, it is necessary to pay attention to the drug-drug interactions for ensuring medication safety. Further clinical studies can also focus on the pharmacokinetic and pharmacodynamic interactions between NXT and co-administered drugs, including antiplatelet drugs, statins and other TCMs.
In summary, NXT is a representative TCM formula in the treatment of CCVD, which could exert potent effect in relieving atherosclerosis, myocardial infarction injury, cerebral ischemia and I/R injury, thrombosis, and diabetic nephropathy, but there are also some problems need to be settled. Further studies should focus on the continued improvement of quality control, pharmacokinetic properties, pharmacological mechanism, and the reconfirmation of clinical effectiveness and safety.
W-jZ: Data curation, Formal Analysis, Investigation, Methodology, Writing–original draft, Conceptualization. R-qC: Data curation, Formal Analysis, Investigation, Writing–original draft. XT: Formal Analysis, Investigation, Writing–original draft. P-bL: Investigation, Methodology, Writing–original draft. JW: Formal Analysis, Investigation, Writing–original draft. H-kW: Investigation, Methodology, Writing–original draft. NX: Data curation, Formal Analysis, Writing–original draft. M-fZ: Investigation, Methodology, Writing–original draft. S-rL: Formal Analysis, Investigation, Writing–original draft. Z-qO: Investigation, Methodology, Writing–original draft. Z-kC: Investigation, Methodology, Writing–original draft. X-xL: Data curation, Formal Analysis, Investigation, Methodology, Project administration, Supervision, Writing–review and editing, Writing–original draft. HW: Data curation, Formal Analysis, Funding acquisition, Investigation, Visualization, Writing–review and editing, Writing–original draft.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by Guangdong Basic and Applied Basic Research Foundation under grant number (2019A1515010604).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbate, R., Cioni, G., Ricci, I., Miranda, M., and Gori, A. M. (2012). Thrombosis and acute coronary syndrome. Thromb. Res. 129 (3), 235–240. doi:10.1016/j.thromres.2011.12.026
Ao, H., Feng, W., and Peng, C. (2018). Hydroxysafflor yellow A: a promising therapeutic agent for a broad spectrum of diseases. Evid. Complement. Altern. Med. 2018, 8259280. doi:10.1155/2018/8259280
Asada, Y., Yamashita, A., Sato, Y., and Hatakeyama, K. (2018). Thrombus formation and propagation in the onset of cardiovascular events. J. Atheroscler. Thromb. 25 (8), 653–664. doi:10.5551/jat.RV17022
Badimon, L., and Vilahur, G. (2014). Thrombosis formation on atherosclerotic lesions and plaque rupture. J. Intern. Med. 276 (6), 618–632. doi:10.1111/joim.12296
Banach, M., and Mikhailidis, D. P. (2018). Statin intolerance: some practical hints. Cardiol. Clin. 36 (2018), 225–231. doi:10.1016/j.ccl.2017.12.004
Chang, M., Lei, Y., Zhang, J., Xu, J., Wu, H., Tang, S., et al. (2024). Effect of naoxintong capsule on microglia and proteomics of cortex after myocardial infarction in rats. Mol. Neurobiol. 61 (5), 2904–2920. doi:10.1007/s12035-023-03724-x
Chen, H., Wu, X. Y., Wu, H. X., and Wang, H. (2014). A randomized controlled trial of adjunctive Bunchang Naoxintong Capsule (步长脑心通胶囊) versus maintenance dose clopidogrel in patients with CYP2C19*2 polymorphism. Chin. J. Integr. Med. 20 (12), 894–902. doi:10.1007/s11655-014-2023-z
Chen, J. S., Wang, Z. Y., and Chen, Q. H. (2015). Determination of protocatechu aldehyde in NaoXinTong capsule by HPLC. Guid. J. Tradit. Chin. Med. Pharm. 21 (22), 34–36. doi:10.13862/j.cnki.cn43-1446/r.2015.22.011
Chen, L., Wei, L., Yu, Q., Shi, H., and Liu, G. (2020). Tanshinone IIA alleviates hypoxia/reoxygenation induced cardiomyocyte injury via lncRNA AK003290/miR-124-5p signaling. Bmc. Mol. Cell. Biol. 2020 (1), 20–22. doi:10.1186/s12860-020-00264-3
Cheng, S. H., Wang, J., Xu, G. H., Sun, G. X., Tao, X. J., Yang, X. C., et al. (2016). Effect of Naoxintong capsule on vascular remodeling in type 2 diabetic patients with subclinical vascular disease. Chin. J. Integr. Tradit. West. Med. 36 (12), 1439–1444. doi:10.7661/CJIM.2016.12.1439
Chinese Pharmacopoeia Commission (2020). Pharmacopoeia of the people’s Republic of China. China: China Medical Science Press.
Choi, E. S., Yoon, J. J., Han, B. H., Jeong, D. H., Lee, H. S., Kang, D. G., et al. (2018). Ligustilide attenuates vascular inflammation and activates Nrf2/HO-1 induction and, NO synthesis in HUVECs. Phytomedicine 38, 12–23. doi:10.1016/j.phymed.2017.09.022
Choubey, S., Goyal, S., Varughese, L. R., Kumar, V., Sharma, A. K., and Beniwal, V. (2018). Probing gallic acid for its broad spectrum applications. Mini-rev. Med. Chem. 18 (15), 1283–1293. doi:10.2174/1389557518666180330114010
Collaborators, G. S. (2021). Global, regional, and national burden of stroke and its risk factors, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. Neurol. 20 (10), 795–820. doi:10.1016/s1474-4422(21)00252-0
Dong, S., Zhao, Z., Huang, X., Ma, M., Yang, Z., Fan, C., et al. (2023). Triglyceride-glucose index is associated with poor prognosis in acute coronary syndrome patients with prior coronary artery bypass grafting undergoing percutaneous coronary intervention. Cardiovasc. Diabetol. 22 (1), 286. doi:10.1186/s12933-023-02029-6
Dong, S., Zhu, Z., Zhang, Y., Cheng, J., and Xu, Z. (1997). Determination of the contednt of trace elements in Chinese traditional medicine naoxintong by AAS. Guangdong Trace Elem. Sci. 4 (9), 48–50. doi:10.16755/j.cnki.issn.1006-446x.1997.09.015
Driver, V. R., Fabbi, M., Lavery, L. A., and Gibbons, G. (2010). The costs of diabetic foot: the economic case for the limb salvage team. J. Vasc. Surg. 52 (3 Suppl. l), 17S–22s. doi:10.1016/j.jvs.2010.06.003
Du, K. Z., Cui, Y., Chen, S., Yang, R., Shang, Y., Wang, C., et al. (2022). An integration strategy combined progressive multivariate statistics with anticoagulant activity evaluation for screening anticoagulant quality markers in Chinese patent medicine. J. Ethnopharmacol. 287, 114964. doi:10.1016/j.jep.2021.114964
Duan, J., Wen, A., Guo, C., Miaomaio, D., Yi, Z., Ding, Y., et al. (2017). Protocatechualdehyde protects against cerebral ischemia-reperfusion-induced oxidative injury via protein kinase cε/nrf2/HO-1 pathway. Mol. Neurobiol. 54 (2), 833–845. doi:10.1007/s12035-016-9690-z
Ekbbal, R., Akhtar, M. S., Al-Shaqha, W. M., Kumar, S., and Jaiswal, A. K. (2023). Regulation of interleukin 2, proto-oncogene c-fos and poly (ADP-Ribose) glycohydrolase genomic expression by rosmarinic acid in attenuation of diabetes and associated complications. J. Biol. Regul. Homeost. Agents 37 (6), 3321–3331. doi:10.23812/j.biol.regul.homeost.agents.20233706.329
Fang, L., Chen, L., Song, M., He, J., Zhang, L., Li, C., et al. (2021). Naoxintong accelerates diabetic wound healing by attenuating inflammatory response. Pharm. Biol. 59 (1), 252–261. doi:10.1080/13880209.2021.1877735
Feng, Y., Zhang, Q., and Sun, L. (2021). Five terpenoids from the gum resin of Boswellia carterii and their cytotoxicity. Fitoterapia 154, 105017. doi:10.1016/j.fitote.2021.105017
Fontana, P., Senouf, D., and Mach, F. (2008). Biological effect of increased maintenance dose of clopidogrel in cardiovascular outpatients and influence of the cytochrome P450 2C19*2 allele on clopidogrel responsiveness. Thromb. Res. 121 (4), 463–468. doi:10.1016/j.thromres.2007.06.012
Ganguly, R., Singh, S. V., Jaiswal, K., Kumar, R., and Pandey, A. K. (2023). Modulatory effect of caffeic acid in alleviating diabetes and associated complications. World. J. Diabetes 2023 (2), 62–75. doi:10.4239/wjd.v14.i2.62
Gao, H. j., Cai, H. R., Zhuang, J. Q., Dai, X. Z., Fu, X., Zhang, W. Z., et al. (2021). Efficacy and safety of Naoxintong capsule for treating chronic stable angina: study protocol for a randomized controlled trial. Trials 22 (1), 336. doi:10.1186/s13063-021-05264-y
Group, G. N. J. G., Mensah, G. A., Johnson, C. O., Addolorato, G., Ammirati, E., Baddour, L. M., et al. (2020). Global burden of cardiovascular diseases and risk factors, 1990-2019: update from the GBD 2019 study. J. Am. Coll. Cardiol. 76 (25), 2982–3021. doi:10.1016/j.jacc.2020.11.010
Guan, Y., An, P., Zhang, Z., Zhang, F., Yu, Y., Wu, Q., et al. (2013). Screening identifies the Chinese medicinal plant Caulis Spatholobi as an effective HAMP expression inhibitor. J. Nutr. 143 (7), 1061–1066. doi:10.3945/jn.113.174201
Guo, Y., Chen, L., Li, Y., Duan, J., Liu, C., and Jiang, H. (2023). Research progress of artesunate in diabetes and its complications. Tradit. Med. Res. 8 (5), 30. doi:10.53388/TMR20220811001
Han, J., Tan, H., Duan, Y., Chen, Y., Zhu, Y., Zhao, B., et al. (2019). The cardioprotective properties and the involved mechanisms of NaoXinTong capsule. Pharmacol. Res. 141, 409–417. doi:10.1016/j.phrs.2019.01.024
Hao, P., Jiang, F., Cheng, J., Ma, L., Zhang, Y., and Zhao, Y. (2017). Traditional Chinese medicine for cardiovascular disease: evidence and potential mechanisms. J. Am. Coll. Cardiol. 69 (24), 2952–2966. doi:10.1016/j.jacc.2017.04.041
Hasani, W. S. R., Muhamad, N. A., Hanis, T. M., Maamor, N. H., Chen, X. W., Omar, M. A., et al. (2023). The global estimate of premature cardiovascular mortality: a systematic review and meta-analysis of age-standardized mortality rate. BMC Public Health 23 (1), 1561. doi:10.1186/s12889-023-16466-1
He, X., Wang, X., Fang, J., Chang, Y., Ning, N., Guo, H., et al. (2017). The genus Achyranthes: a review on traditional uses, phytochemistry, and pharmacological activities. J. Ethnopharmacol. 203, 260–278. doi:10.1016/j.jep.2017.03.035
He, Y., Su, W., Chen, T., Zeng, X., Yan, Z., Guo, J., et al. (2019). Identification of prototype compounds and derived metabolites of naoxintong capsule in beagle dog urine and feces by UFLC-Q-TOF-MS/MS. J. Pharm. Biomed. Anal. 176, 112806. doi:10.1016/j.jpba.2019.112806
He, Y., Su, W., He, X., Chen, T., Zeng, X., Yan, Z., et al. (2021). Pharmacokinetics and biotransformation investigation in beagle dog of active compounds from naoxintong capsule. Biomed. Pharmacother. 133, 110940. doi:10.1016/j.biopha.2020.110940
Healthcare Engineering, J. O. (2023). Retracted: data mining-based analysis of modern Chinese medicine for the treatment of stable angina pectoris in coronary heart disease. J. Healthc. Eng. 2023, 9857271. doi:10.1155/2023/9857271
Hou, J. H., Zhang, L. L., Jun-Nong, L. I., Wang, Y. H., Zheng, Y., Cardiology, D. O., et al. (2014). The clinical curative effect and safety of Naoxintong capsules in the treatment of unstable angina: a meta-analysis. Prog. Mod. Biomed. 8 (14), 1545–1549. doi:10.13241/j.cnkipmb.2014.08.040
Huang, B., Li, G., Guo, Y. F., Wang, S. S., Liu, F., Xu, H. Y., et al. (2013). Study on absorption location of four components from Naoxintong capsule. Chin. J. Chin. Mat. Med. 38 (6), 889–893. doi:10.4268/cjcmm20130624
Hui, C. (2011a). Effect of Naoxintong on the activity of cytochrome P2C19 (CYP2C19) in human liver microsome. Int. J. Cardiol. 152 (Suppl. S1), S109. doi:10.1016/j.ijcard.2011.08.829
Hui, C. (2011b). Randomized comparison of adjunctive naoxintong versus standard maintenance dose clopidogrel in patients with CYP2C19*2 gene mutation: results of the ABNJC-CYP2C19*2 (adjunctive buchang naoxintong jiaonang versus standard maintenance dose clopidogrel in patients with CYP2C19*2 gene mutation) randomized study. Int. J. Cardiol. 152 (Suppl. S1), S26. doi:10.1016/j.ijcard.2011.08.547
Hwang, C. H., Jang, E., and Lee, J. H. (2023). Pharmacological benefits and underlying mechanisms ofSalvia miltiorrhizaagainst molecular pathology of various liver diseases: a review. Am. J. Chin. Med. 51 (7), 1675–1709. doi:10.1142/S0192415X23500763
Jin, L., Piao, Z. H., Sun, S., Liu, B., Kim, G. R., Seok, Y. M., et al. (2017). Gallic acid reduces blood pressure and attenuates oxidative stress and cardiac hypertrophy in spontaneously hypertensive rats. Sci. Rep. 7 (1), 15607. doi:10.1038/s41598-017-15925-1
Jonsson, A. L., and Bäckhed, F. (2016). Role of gut microbiota in atherosclerosis. Nat. Rev. Cardiol. 14 (2), 79–87. doi:10.1038/nrcardio.2016.183
Joseph, P., Leong, D., McKee, M., Anand, S. S., Schwalm, J. D., Teo, K., et al. (2017). Reducing the global burden of cardiovascular disease, Part 1: the epidemiology and risk factors. Circ. Res. 121 (6), 677–694. doi:10.1161/CIRCRESAHA.117.308903
Kombo, H., Kumar, S., and Singh, G. (2022). Traditional medicines and experimental analysis methods for Alzheimer’s disease. Tradit. Med. Res. 7 (5), 43. doi:10.53388/TMR20220402002
Kyungjin, L., Bumjung, K., Youngmin, B., Hyejin, J., Meixiang, S., Minwoo, K., et al. (2018). Review on the characteristics of liver-pacifying medicinal in relation to the treatment of stroke: from scientific evidence to traditional medical theory. J. Tradit. Chin. Med. 38 (1), 139–150. doi:10.1016/j.jtcm.2018.01.003
Leong, D. P., Joseph, P. G., McKee, M., Anand, S. S., Teo, K. K., Schwalm, J. D., et al. (2017). Reducing the global burden of cardiovascular disease, Part 2: prevention and treatment of cardiovascular disease. Circ. Res. 121 (6), 695–710. doi:10.1161/CIRCRESAHA.117.311849
Li, G., Meng, F. Y., Yang, H. J., Fang, J., Liu, F., and Fang, M. H. (2013). UPLC simultaneous determination of five components in Naoxintong capsules. Chin. J. Pharm. Anal. 33 (3), 414–418. doi:10.16155/j.0254-1793.2013.03.012
Li, J., Bai, Y., Bai, Y., Zhu, R. C., Liu, W., Jun, C., et al. (2017a). Pharmacokinetics of caffeic acid, ferulic acid, formononetin, cryptotanshinone, and tanshinone IIA after oral administration of naoxintong capsule in rat by HPLC-MS/MS. Evid. Based. Complement. Altern. Med. 2017, 1–12. doi:10.1155/2017/9057238
Li, J., Zhao, X., Zhang, Y., Wan, H., He, Y., Li, X., et al. (2021a). Comparison of traditional Chinese medicine in the long-term secondary prevention for patients with ischemic stroke: a systematical analysis. Front. Pharmacol. 12, 722975. doi:10.3389/fphar.2021.722975
Li, L., Hou, X., Xu, R., Liu, C., and Tu, M. (2017b). Research review on the pharmacological effects of astragaloside IV. Fundam. Clin. Pharmacol. 31 (1), 17–36. doi:10.1111/fcp.12232
Li, L., Zheng, Y., Bao, J., Zhao, Y., Zhang, Q., Li, W., et al. (2022a). Meta-analysis of Naoxintong capsule for patients with vascular dementia. Evid. Based. Complement. Altern. Med. 2022, 5000948. doi:10.1155/2022/5000948
Li, N., Yang, F., Zhao, Z., Jiang, J., and Lei, Y. (2021b). Efficacy and safety of Naoxintong capsule for acute ischemic stroke: a protocol for systematic review and meta-analysis. Med. Baltim. 100 (34), e27120. doi:10.1097/md.0000000000027120
Li, Q., Chen, Y., Zhao, D., Wei, Z., Zhang, S., Chen, Y., et al. (2018a). NaoXinTong capsule inhibits carrageenan-induced thrombosis in mice. J. Cardiovasc. Pharmacol. 72 (1), 49–59. doi:10.1097/fjc.0000000000000592
Li, W., Tang, Y., Chen, Y., and Duan, J. A. (2012). Advances in the chemical analysis and biological activities of chuanxiong. Molecules 17 (9), 10614–10651. doi:10.3390/molecules170910614
Li, W. X., Zhang, S. Q., Li, M. M., Zhang, H., Wang, X. Y., Niu, L., et al. (2022b). Pharmacokinetic comparison of four major bio-active components of Naoxintong capsule in normal and acute blood stasis rats using ultra-performance liquid chromatography coupled with triple-quadrupole mass spectrometry. WJTCM, 008. doi:10.4103/wjtcm.wjtcm_53_21
Li, W. X., Zhang, S. Q., Zhao, Y. D., Tang, J. F., Li, C. X., Wang, X. Y., et al. (2018b). Study progress on chemical compounds, pharmacological action and clinical application of Naoxintong capsule. Chin. J. Chin. Mat. Med. 43 (10), 1998–2005. doi:10.19540/j.cnki.cjcmm.20180208.004
Lin, C. H., Lan, C. C., Chiu, V., Hsieh, P. C., Kuo, C. Y., and Lee, C. T. (2020). Danshensu attenuates hypoxia-induced reactive oxygen species production and inducible nitric oxide synthase expression in RAW 264.7 cells. Curr. Top. Nutraceut. R. 18 (1), 89–95. doi:10.37290/ctnr2641-452X.18:89-95
Liu, H., Zheng, Y. F., Li, C. Y., Zheng, Y. Y., Wang, D. Q., Wu, Z., et al. (2015). Discovery of anti-inflammatory ingredients in Chinese herbal formula kouyanqing granule based on relevance analysis between chemical characters and biological effects. Sci. Rep. 5, 18080. doi:10.1038/srep18080
Liu, J., Zhang, Q., Li, R. L., Wei, S. J., Huang, C. Y., Gao, Y. X., et al. (2020a). The traditional uses, phytochemistry, pharmacology and toxicology of Cinnamomi ramulus: a review. J. Pharm. Pharmacol. 72 (3), 319–342. doi:10.1111/jphp.13189
Liu, M., Liu, X., Wang, H., Xiao, H., Jing, F., Tang, L., et al. (2016). Metabolomics study on the effects of Buchang Naoxintong capsules for treating cerebral ischemia in rats using UPLC-Q/TOF-MS. J. Ethnopharmacol. 180, 1–11. doi:10.1016/j.jep.2016.01.016
Liu, S. N., Shang, J. J., Qiu, S. L., Liu, X. Y., Yang, Y. W., and Wang, Z. (2022). Effect of Naoxintong capsule combined with conventional western medicine on antithrombotic efficacy and safety after PCI: a meta analysis. Chin. J. Integr. Med. Cardio-Cerebrovasc. Dis. 20 (13), 2322–2328. doi:10.12102/j.issn.1672-1349.2022.13.004
Liu, X., Wang, Q., Cui, Y., Hussain, M., Yang, H., and Li, X. (2020b). Multiple protein and mRNA expression correlations in the rat cerebral cortex after ischemic injury and repair due to buchang naoxintong jiaonang (BNJ) intervention. Biomed. Pharmacother. 125, 109917. doi:10.1016/j.biopha.2020.109917
Long-Tao, L., and Chang-Geng, F. U. (2018). Chinese experts consensus on clinical application of Naoxintong capsule(脑心通胶囊). Chin. J. Integr. Med. 24 (3), 232–236. doi:10.1007/s11655-018-2981-6
Lu, Y., Wan, H., Wu, Y., Yang, J., Yu, L., He, Y., et al. (2022). Naoxintong capsule alternates gut microbiota and prevents hyperlipidemia in high-fat-diet fed rats. Front. Pharmacol. 13, 843409. doi:10.3389/fphar.2022.843409
Lv, J., Xiong, W., Lei, T., Wang, H., Sun, M., Hao, E., et al. (2017). Amygdalin ameliorates the progression of atherosclerosis in LDL receptordeficient mice. Mol. Med. Rep. 16 (2017), 8171–8179. doi:10.3892/mmr.2017.7609
Lv, Y., Pan, Y., Gao, Y., Lu, J., Li, Y., Bai, J., et al. (2015). Effect of Danhong injection combined with Naoxintong tablets on prognosis and inflammatory factor expression in acute coronary syndrome patients undergoing percutaneous coronary intervention. Acta. Cardiol. Sin. 31 (4), 301–307. doi:10.6515/acs20150502a
Ma, X., Lv, B., Li, P., Jiang, X., Zhou, Q., Wang, X., et al. (2016). Identification of "multiple components-multiple targets-multiple pathways" associated with Naoxintong capsule in the treatment of heart diseases using UPLC/Q-TOF-MS and network pharmacology. Evid. Based. Complement. Alternat. Med. 2016, 9468087. doi:10.1155/2016/9468087
Ma, Y., Yang, Y., Zhao, B., Jia, L., Zhao, J., Wang, H., et al. (2015). Study on neuroprotective effect of Naoxintong Capsules on vascular dementia mice with chronic hypoperfusion. China Med. Her. 12 (28), 4–8.
Mendis, S., Davis, S., and Norrving, B. (2015). Organizational update: the world health organization global status report on noncommunicable diseases 2014; one more landmark step in the combat against stroke and vascular disease. Stroke 46 (5), e121–e122. doi:10.1161/strokeaha.115.008097
Mushenkova, N. V., Summerhill, V. I., Zhang, D., Romanenko, E. B., Grechko, A. V., and Orekhov, A.A.-O. (2020). Current advances in the diagnostic imaging of atherosclerosis: insights into the pathophysiology of vulnerable plaque. Int. J. Mol. Sci. 21 (8), 2992. doi:10.3390/ijms21082992
Nakajima, A., Libby, P., Mitomo, S., Yuki, H., Araki, M., Seegers, L. M., et al. (2022). Biomarkers associated with coronary high-risk plaques. J. Thromb. Thrombolysis. 54 (4), 647–659. doi:10.1007/s11239-022-02709-2
Oikonomou, E., Leopoulou, M., Theofilis, P., Antonopoulos, A. S., Siasos, G., Latsios, G., et al. (2020). A link between inflammation and thrombosis in atherosclerotic cardiovascular diseases: clinical and therapeutic implications. Atherosclerosis 309, 16–26. doi:10.1016/j.atherosclerosis.2020.07.027
Org, E., Mehrabian, M., and Lusis, A. J. (2015). Unraveling the environmental and genetic interactions in atherosclerosis: central role of the gut microbiota. Atherosclerosis 241 (2), 387–399. doi:10.1016/j.atherosclerosis.2015.05.035
Ouyang, H., Shen, J., Huang, X., Ma, W., Jia, Q., Yao, G., et al. (2019). Effect of Naoxintong capsules on the activities of CYP450 and metabolism of metoprolol tartrate in rats evaluated by probe cocktail and pharmacokinetic methods. Evid. Based. Complement. Altern. Med. 2019, 5242605. doi:10.1155/2019/5242605
Pan, B., Zhang, H., Li, H., Yuan, E., Luo, J., Zhang, T., et al. (2022). Advances in Chinese medicine treatment and research on endocrine diseases in 2021. Tradit. Med. Res. 7 (5), 49. doi:10.53388/TMR20220208001
Ping, L., Haiping, Z., and Yumin, L. (2017). Anti-aging implications of Astragalus Membranaceus (Huangqi): a well-known Chinese tonic. Aging and Dis. 8 (6), 868–886. doi:10.14336/AD.2017.0816
Qian, Y., Xu, B., Qian, X., Cao, L., Cheng, Y., Liu, X., et al. (2021). Incidence and risk factors for antiplatelet therapy-related bleeding complications among elderly patients after coronary stenting: a multicenter retrospective observation. Front. Pharmacol. 12 (2021), 661619. doi:10.3389/fphar.2021.661619
Qiao, Y., Shi, Y., Wu, C., Hou, X., Pan, X., Deng, Z., et al. (2021). Rapid screening and identification of anticoagulation component from carthami flos by two-dimensional thrombin affinity chromatography combined with HPLC-MS/MS. J. Sep. Sci. 44 (16), 3061–3069. doi:10.1002/jssc.202100092
Qiu, X. C., Zhang, Z., Jin, Z. T., Wang, L. L., Wen, T., and Zhou, J. (2023). Clinical study on Naoxintong Capsules combined with ticagrelor in treatment of angina pectoris of coronary heart disease. Drugs and Clin. 38 (5), 1–5. doi:10.7501/j.issn.1674-5515.2023.05.014
Shi, P., Wei, J., You, H., Chen, S., Tan, F., and Lu, Z. (2023). Cloning, characterization, and heterologous expression of a candidate Hirudin gene from the salivary gland transcriptome of Hirudo nipponia. Sci. Rep. 13 (1), 4943. doi:10.1038/s41598-023-32303-2
Srinivasan, M., Sudheer, A. R., and Menon, V. P. (2007). Ferulic Acid: therapeutic potential through its antioxidant property. J. Clin. Biochem. Nutr. 40 (2), 92–100. doi:10.3164/jcbn.40.92
Stark, K., and Massberg, S. (2021). Interplay between inflammation and thrombosis in cardiovascular pathology. Nat. Rev. Cardiol. 18 (9), 666–682. doi:10.1038/s41569-021-00552-1
Su, C. Y., Ming, Q. L., Rahman, K., Han, T., and Qin, L. P. (2015a). Salvia miltiorrhiza: traditional medicinal uses, chemistry, and pharmacology. Chin. J. Nat. Med. 13 (3), 163–182. doi:10.1016/s1875-5364(15)30002-9
Su, H., He, J. B., Hou, P. H., and Kang, X. G. (2015b). Study on HPLC fingerprint and determination of components of Naoxintong Capsule. Hebei Med. J. 37 (5), 764–767. doi:10.3969/j.issn.1002-7386.2015.05.047
Su, R., Tang, Z. S., Liu, F., Chen, Y. B., and Song, Z. X. (2019). HPLC-DAD fingerprint of Naoxintong capsules. Cent. South Pharm. 17 (1), 1–4. doi:10.7539/j.issn.1672-2981.2019.01.001
Sun, H., Lou, X. Y., Wu, X. Y., Wang, H., Qu, Q., Tan, S. L., et al. (2016). Up-regulation of CYP2C19 expression by BuChang NaoXinTong via PXR activation in HepG2 cells. PLoS One 11 (7), e0160285. doi:10.1371/journal.pone.0160285
Sun, J. K., He, L. D., Xue, X. C., and Liu, B. M. (2019). Effect of Naoxintong capsule combined with rivaroxaban in treatment of acute pulmonary embolism and influence on IL-6, CRP and TNF-α. Chin. Arch. Tradit. Chin. Med. 37 (12), 3061–3064.
Sun, K. Z., and Zeng, J. G. (2022). Effect of Naoxintong Capsule on acetylcholinergic nerve in vascular dementia rats. J. Tradit. Chin. Med. 21 (6), 50–53. doi:10.14046/j.cnki.zyytb2002.2022.06.017
Tabares-Guevara, J.A.-O., Villa-Pulgarin, J.A.-O., and Hernandez, J.A.-O. (2021). Atherosclerosis: immunopathogenesis and strategies for immunotherapy. Immunotherapy 13 (14), 1231–1244. doi:10.2217/imt-2021-0009
Tatsumi, K., and Mackman, N. (2015). Tissue factor and atherothrombosis. J. Atheroscler. Thromb. 22 (6), 543–549. doi:10.5551/jat.30940
Tohgi, H., Tajima, T., Konno, T., Towada, S., Kamata, A., and Yamazaki, M. (1991). The risk of cerebral infarction in non-valvular atrial fibrillation: effects of age, hypertension and antihypertensive treatment. Eur. Neurol. 31 (3), 126–130. doi:10.1159/000116661
Wang, D., Sheng, L. S., Song, Y., and Li, P. (2006). Determination of eight astragalosides in BuChang Naoxintong capsule by HPLC-MS. Chin. J. Nat. Med. 4 (4), 287–290.
Wang, H., Jiang, Y., Ding, M., Li, J., Hao, J., He, J., et al. (2018a). Simultaneous determination and qualitative analysis of six types of components in Naoxintong capsule by miniaturized matrix solid-phase dispersion extraction coupled with ultra high-performance liquid chromatography with photodiode array detection and quadrupole time-of-flight mass spectrometry. J. Sep. Sci. 41 (9), 2064–2084. doi:10.1002/jssc.201701411
Wang, H., Zhou, X. K., Zheng, L. F., Wu, X. Y., and Chen, H. (2018b). Comparison of aspirin and Naoxintong capsule with adjusted-dose Warfarin in elderly patients with high-risk of non-valvular atrial fibrillation and genetic variants of vitamin K epoxide reductase. Chin. J. Integr. Med. 24 (4), 247–253. doi:10.1007/s11655-015-2443-4
Wang, M., Sun, G. B., Sun, X., Wang, H. W., Meng, X. B., Qin, M., et al. (2013). Cardioprotective effect of salvianolic acid B against arsenic trioxide-induced injury in cardiac H9c2 cells via the PI3K/Akt signal pathway. Toxicol. Lett. 216 (2-3), 100–107. doi:10.1016/j.toxlet.2012.11.023
Wang, S. S., Xu, H. Y., Ma, Y., Wang, X. G., Yang, S., Huang, B., et al. (2015). Characterization and rapid identification of chemical constituents of NaoXinTong capsules by UHPLC-linear ion trap/Orbitrap mass spectrometry. J. Pharm. Biomed. Anal. 111, 104–118. doi:10.1016/j.jpba.2015.01.020
Wang, T., Zhao, Z., Yu, X., Zeng, T., Xu, M., Xu, Y., et al. (2021a). Age-specific modifiable risk factor profiles for cardiovascular disease and all-cause mortality: a nationwide, population-based, prospective cohort study. Lancet. Reg. Health. West. pac. 17, 100277. doi:10.1016/j.lanwpc.2021.100277
Wang, X., Yin, Z., Cao, P., Zheng, S., Chen, Y., Yu, M., et al. (2021b). NaoXinTong Capsule ameliorates memory deficit in APP/PS1 mice by regulating inflammatory cytokines. Biomed. Pharmacother. 133, 110964. doi:10.1016/j.biopha.2020.110964
Wang, Y., Xu, Y., Zhang, L., Huang, S., Dou, L., Yang, J., et al. (2022). Comparison of Buyang Huanwu granules and Naoxintong capsules in the treatment of stable angina pectoris: rationale and design of a randomized, blinded, multicentre clinical trial. Trials 23 (1), 65. doi:10.1186/s13063-021-05914-1
Wang, Y. B. (2008). Determination of danshensu in Naoxintong capsules by HPLC. Pract. J. Card. Cereb. Pneum. Vasc. Dis. 16 (5), 29–30.
Wang, Z., Liu, P., Hu, M., Lu, S., Lyu, Z., Kou, Y., et al. (2021c). Naoxintong restores ischemia injury and inhibits thrombosis via COX2-VEGF/NFκB signaling. J. Ethnopharmacol. 270, 113809. doi:10.1016/j.jep.2021.113809
Wang, Z., Shi, H., Zhao, H., Dong, Z., Zhao, B., Weng, X., et al. (2018c). Naoxintong retards atherosclerosis by inhibiting foam cell formation through activating Pparα pathway. Curr. Mol. Med. 18 (10), 698–710. doi:10.2174/1566524019666190207143207
Wei, R., He, M. D., Liu, S. M., Li, H., and Wang, Z. (2006). The effect of Buchang Naoxintong on learning memory and vascular endothelial growth factor of vascular dementia. J. A. poplexy Nerv. Dis. 23 (3), 306–308.
Xu, H., Jin, J., Chen, L., Li, C., Xu, Q., Shi, J., et al. (2016a). Naoxintong/PPARα signaling inhibits H9c2 cell apoptosis and autophagy in response to oxidative stress. Evid. Based. Complement. Altern. Med. 2016, 4370381. doi:10.1155/2016/4370381
Xu, H. Y., Shi, Y., Zhang, Y. Q., Jia, Q., Li, D. F., Zhang, Y., et al. (2016b). Identification of key active constituents of Buchang Naoxintong capsules with therapeutic effects against ischemic stroke by using an integrative pharmacology-based approach. Mol. Biosyst. 12 (1), 233–245. doi:10.1039/c5mb00460h
Xu, J., Guo, Y., Li, Y., Zhao, P., Liu, C., Ma, Y., et al. (2011). Sesquiterpenoids from the resinous exudates of Commiphora myrrha and their neuroprotective effects. Planta. Med. 77 (18), 2023–2028. doi:10.1055/s-0031-1280087
Xu, J., Liu, X., Luo, L., Tang, L., Guo, N., Liu, M., et al. (2019). A metabonomics investigation into the therapeutic effects of BuChang NaoXinTong capsules on reversing the amino acid-protein interaction network of cerebral ischemia. Oxid. Med. Cell. Longev. 2019, 7258624. doi:10.1155/2019/7258624
Xu, T., Liu, X., Wang, S., Kong, H., Yu, X., Liu, C., et al. (2022). Effect of Pheretima aspergillum on reducing fibrosis: a systematic review and meta-analysis. Front. Pharmacol. 13, 1039553. doi:10.3389/fphar.2022.1039553
Xue, J., Zhang, X., Zhang, C., Kang, N., Liu, X., Yu, J., et al. (2016). Protective effect of Naoxintong against cerebral ischemia reperfusion injury in mice. J. Ethnopharmacol. 182, 181–189. doi:10.1016/j.jep.2016.02.022
Yan, Z., Wu, H., Zhou, H., Chen, S., He, Y., Zhang, W., et al. (2020). Integrated metabolomics and gut microbiome to the effects and mechanisms of naoxintong capsule on type 2 diabetes in rats. Sci. Rep. 10 (1), 10829. doi:10.1038/s41598-020-67362-2
Yang, C., Zhao, J., Cheng, Y., Li, X., and Rong, J. (2014). Bioactivity-guided fractionation identifies amygdalin as a potent neurotrophic agent from herbal medicine Semen Persicae extract. Biomed. Res. Int. 2014, 306857. doi:10.1155/2014/306857
Yang, S., Chen, Y., Duan, Y., Ma, C., Liu, L., Li, Q., et al. (2019). Therapeutic potential of NaoXinTong Capsule on the developed diabetic nephropathy in db/db mice. Biomed. Pharmacother. 118, 109389. doi:10.1016/j.biopha.2019.109389
Yang, S., Liu, M., Chen, Y., Ma, C., Liu, L., Zhao, B., et al. (2018). NaoXinTong Capsules inhibit the development of diabetic nephropathy in db/db mice. Sci. Rep. 8 (1), 9158. doi:10.1038/s41598-018-26746-1
Yang, S., Wang, B., Xia, X., Li, X., Wang, R., Sheng, L., et al. (2015). Simultaneous quantification of three active alkaloids from a traditional Chinese medicine Ramulus Mori (Sangzhi) in rat plasma using liquid chromatography-tandem mass spectrometry. J. Pharm. Biomed. Anal. 109, 177–183. doi:10.1016/j.jpba.2015.02.019
Yang, X., Li, Y., Sun, L., Liu, Y., Ma, C., Chen, Y., et al. (2017). NaoXinTong enhances atorvastatin-induced plaque stability while ameliorating atorvastatin-induced hepatic inflammation. J. Cardiovasc. Pharmacol. 69 (1), 55–64. doi:10.1097/fjc.0000000000000441
Yang, X., Sun, L., Li, Y., Ma, C., Yang, J., Zhang, W., et al. (2016). NaoXinTong inhibits the advanced atherosclerosis and enhances the plaque stability in apolipoprotein E deficient mice. J. Cardiovasc. Pharmacol. 67 (3), 203–211. doi:10.1097/fjc.0000000000000334
Yi, L., Liang, Y., Wu, H., and Yuan, D. (2009). The analysis of radix angelicae sinensis (danggui). J. Chromatogr. A 1216 (11), 1991–2001. doi:10.1016/j.chroma.2008.07.033
Yu, J., Zhu, X., Qi, X., Che, J., and Cao, B. (2013). Paeoniflorin protects human EA.hy926 endothelial cells against gamma-radiation induced oxidative injury by activating the NF-E2-related factor 2/heme oxygenase-1 pathway. Toxicol. Lett. 218 (2013), 224–234. doi:10.1016/j.toxlet.2013.01.028
Yu, L., Huang, P., Wang, M., Li, Z., Cai, H., Feng, Y., et al. (2024). Comprehensive effect of Naoxintong capsule combined with Western medicine on coronary heart disease after percutaneous coronary intervention: a meta-analysis. Front. Pharmacol. 2024, 1274000. doi:10.3389/fphar.2024.1274000
Yuan, S., Jin, J., Chen, L., Hou, Y., and Wang, H. (2017). Naoxintong/PPARγ signaling inhibits cardiac hypertrophy via activation of autophagy. Evid. Based. Complement. Altern. Med. 2017, 3801976. doi:10.1155/2017/3801976
Yue, L. F., Huang, X. W., Gao, J. F., Zhang, L. H., Wang, D. M., Sun, J. K., et al. (2022). Determination of components in Radix paeoniae rubra based on QAMS. Pak. J. Pharm. Sci. 35 (4), 1037–1041.
Zhang, C. H., Yang, X., Wei, J. R., Chen, N., Xu, J. P., Bi, Y. Q., et al. (2021). Ethnopharmacology, phytochemistry, pharmacology, toxicology and clinical applications of Radix Astragali. Chin. J. Integr. Med. 27 (3), 229–240. doi:10.1007/s11655-019-3032-8
Zhang, F., Huang, B., Zhao, Y., Tang, S., Xu, H., Wang, L., et al. (2013). BNC protects H9c2 cardiomyoblasts from H 2 O 2 -induced oxidative injury through ERK1/2 signaling pathway. Evid. Based. Complement. Altern. Med. 2013, 802784. doi:10.1155/2013/802784
Zhang, H. L., Cui, Y. X., Wang, S. M., Song, C. W., and Chen, G. Q. (2010). Determination of paeoniflorin in Naoxintong capsules by HPLC. JCM 25 (1), 118–119. doi:10.16368/j.issn.1674-8999.2010.01.063
Zhang, L., Chen, L., You, X., Li, M., Shi, H., Sun, W., et al. (2022a). Naoxintong capsule limits myocardial infarct expansion by inhibiting platelet activation through the ERK5 pathway. Phytomedicine 98, 153953. doi:10.1016/j.phymed.2022.153953
Zhang, S. J., Wang, L., and Tian, Z. M. (2019a). Simultaneous determination of seven index components in Naoxintong capsule by HPLC wavelength switching method. J. China. Pharmacol. Univ. 50 (5), 572–578. doi:10.11665/j.issn.1000-5048.20190510
Zhang, W. J., Su, W. W., Li, P. B., Rao, H. Y., Lin, Q. W., Zeng, X., et al. (2019b). Naoxintong capsule inhibits the development of cardiovascular pathological changes in Bama minipig through improving gut microbiota. Front. Pharmacol. 10, 1128. doi:10.3389/fphar.2019.01128
Zhang, W. J., Su, W. W., Lin, Q. W., He, Y., Yan, Z. H., Wang, Y. G., et al. (2020a). Protective effects of Naoxintong capsule alone and in combination with ticagrelor and atorvastatin in rats with Qi deficiency and blood stasis syndrome. Pharm. Biol. 58 (1), 1006–1022. doi:10.1080/13880209.2020.1821066
Zhang, W. J., Su, W. W., Lin, Q. W., Yan, Z. H., Yao, H. L., Zeng, X., et al. (2020b). Protective effects of Naoxintong Capsule on rats with blood stasis syndrome. Biotechnol. Biotec. Eq. 34 (1), 1077–1086. doi:10.1080/13102818.2020.1820377
Zhang, W. W. (2015). Buchang Naoxintong combined with western treatment of diabetic peripheral neuropathy randomized controlled study. J. Pract. Tradit. Chin. Intern. Med. 129 (11), 102–104. doi:10.13729/j.issn.1671-7813.2015.11.47
Zhang, X., Wang, X., Xu, L., Liu, J., Ren, P., and Wu, H. (2023). The predictive value of machine learning for mortality risk in patients with acute coronary syndromes: a systematic review and meta-analysis. Eur. J. Med. Res. 28 (1), 451. doi:10.1186/s40001-023-01027-4
Zhang, Y., Zhang, Y., Yang, C., Duan, Y., Jiang, L., Jin, D., et al. (2022b). Naoxintong capsule delay the progression of diabetic kidney disease: a real-world cohort study. Front. Endocrinol. (Lausanne) 13, 1037564. doi:10.3389/fendo.2022.1037564
Zhang, Y. C. (2008). Determination of tanshinone IIA in buchang naoxintong capsule. Liaoning J. Tradit. Chin. Med. 35 (4), 590. doi:10.13192/j.ljtcm.2008.04.114.zhangych.071
Zhang, Z., Hu, C., Dai, F., Tang, F., and Tang, C. (2022c). Mechanisms and status of research on the protective effects of traditional Chinese medicine against ischemic brain injury. Tradit. Med. Res. 7 (1), 6. doi:10.53388/TMR20211021250
Zhao, J., Ouyang, Y., Wang, H., Lai, H., Hu, S., Tang, L., et al. (2022). An energy metabolism study on the efficacy of Naoxintong capsules against myocardial infarction in a rat model. Oxid. Med. Cell. Longev. 2022, 3712500. doi:10.1155/2022/3712500
Zhao, J., Zhu, H., Wang, S., Ma, X., Liu, X., Wang, C., et al. (2013). Naoxintong protects against atherosclerosis through lipid-lowering and inhibiting maturation of dendritic cells in LDL receptor knockout mice fed a high-fat diet. Curr. Pharm. Des. 19 (33), 5891–5896. doi:10.2174/1381612811319330008
Zhao, S., Tang, Y., Cai, H., Liu, W., Zhang, L., Chen, D., et al. (2018). Treatment of Danhong injection combined with Naoxintong capsule in acute coronary syndrome patients undergoing PCI operation: study for a randomized controlled and double-blind trial. Evid. Based. Complement. Altern. Med. 2018, 8485472. doi:10.1155/2018/8485472
Zhou, X. J., Wu, Y., and Huang, W. (2020). Evaluation of Naoxintong capsule combined with alprostadil in treatment of 80 cases of diabetic nephropathy. Liaoning J. Tradit. Chin. Med. 47 (8), 92–93. doi:10.13192/j.issn.1000-1719.2020.08.026
Zhu, X. X., Wu, H. Y., Shaw, P. C., Peng, W., and Su, W. W. (2019a). Specific DNA identification of pheretima in the naoxintong capsule. Chin. Med. 14, 41. doi:10.1186/s13020-019-0264-7
Zhu, X. X., Wu, H. Y., Shaw, P. C., Peng, W., and Su, W. W. (2019b). Specific DNA identification of Scorpio in naoxintong capsules. Cent. South Pharm. 17 (12), 2015–2020.
Keywords: traditional Chinese medicine (TCM), naoxintong capsule (NXT), cardiovascular and cerebrovascular diseases (CCVD), chemical compositions, pharmacological properties, clinical applications
Citation: Zhang W-j, Chen R-q, Tang X, Li P-b, Wang J, Wu H-k, Xu N, Zou M-f, Luo S-r, Ouyang Z-q, Chen Z-k, Liao X-x and Wu H (2024) Naoxintong capsule for treating cardiovascular and cerebrovascular diseases: from bench to bedside. Front. Pharmacol. 15:1402763. doi: 10.3389/fphar.2024.1402763
Received: 18 March 2024; Accepted: 06 June 2024;
Published: 27 June 2024.
Edited by:
Rong-Rong He, Jinan University, ChinaReviewed by:
Ming Lyu, Tianjin University of Traditional Chinese Medicine, ChinaCopyright © 2024 Zhang, Chen, Tang, Li, Wang, Wu, Xu, Zou, Luo, Ouyang, Chen, Liao and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xu-xing Liao, ZHJsaWFvMjEwNDA5QDE2My5jb20=; Hao Wu, d3VoYW84QG1haWwuc3lzdS5lZHUuY24=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.