
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pharmacol., 07 January 2025
Sec. Drugs Outcomes Research and Policies
Volume 15 - 2024 | https://doi.org/10.3389/fphar.2024.1402423
Objective: The combination of pembrolizumab and chemotherapy has demonstrated notable clinical advantages in improving overall survival than chemotherapy alone for patients with untreated advanced pleural mesothelioma. The purpose of this study was to assess its cost-effectiveness.
Materials and methods: A Markov state-transition model was constructed using data from the IND227 phase 3 randomized clinical trial. Utility values for health states were taken from the IND227 trial, and direct medical costs were from the pertinent literature and local pricing data. Outcomes measured included quality-adjusted life years (QALYs), incremental cost-effectiveness ratio (ICER), incremental net health benefit (INHB), and incremental net monetary benefit (INMB). To manage the uncertainty in the model, both probabilistic sensitivity analysis (PSA) and one-way sensitivity analysis (OWSA) were used.
Results: In the base-case analysis, pembrolizumab plus chemotherapy resulted in an incremental gain of 0.23 QALYs at an additional cost of $18,199.63, resulting in an ICER of $80,557.23/QALY. This was not favorable compared to China’s willingness-to-pay (WTP) threshold of $38,042.49/QALY, with an INHB of −0.25 QALYs and an INMB of $-9,605.00. Subgroup analyses showed ICERs for pembrolizumab plus chemotherapy versus chemotherapy of $33,917.61 and $99,536.73 in non-epithelioid and epithelioid patients, respectively. PSA indicated probabilities of cost-effectiveness for pembrolizumab plus chemotherapy at 0.55%, 69.41%, and 0.14% for the entire population and the non-epithelioid and epithelioid subgroups, respectively.
Conclusion: In the Chinese healthcare system, the combination of pembrolizumab and chemotherapy did not prove to be more cost-effective than chemotherapy alone as an initial treatment for untreated advanced pleural mesothelioma, with the exception of patients who have non-epithelioid histology.
In 2020, mesothelioma, a rare but highly aggressive form of cancer, resulted in 30,870 new cases and 26,278 deaths globally (Siegel et al., 2021; Sung et al., 2021). The predominant type of this cancer, malignant pleural mesothelioma (MPM), is often identified in advanced stages. MPM is classified into three histologic categories: epithelioid, sarcomatoid, and biphasic (Yap et al., 2017). For those with inoperable MPM, the preferred treatment approach is a chemotherapy combination of pemetrex- and platinum-based drugs (Popat et al., 2022). Although the pemetrexed-cisplatin combo offers clinical advantages compared to cisplatin alone, the median overall survival (OS) remains limited to approximately 12.1 months (Vogelzang et al., 2003). Some mesotheliomas express programmed death-ligand 1 (PD-L1), with the PD-L1 and PD-L2 ligands being present in tumor cells and their microenvironment. Blocking the interaction between PD-L1 and the PD-1 receptor in T cells can promote cancer suppression. Monoclonal antibodies that target the PD-1/PD-L1 pathway are gaining attention as a potential treatment option (Mansfield et al., 2014; Chapel et al., 2019; Sahu et al., 2023). Recent multicenter, randomized phase III clinical trials have indicated that immune checkpoint inhibitors, especially in combination therapies, can improve OS in patients with malignant mesotheliomas, exceeding the results of standard treatments (Baas et al., 2021; Fennell et al., 2021).
Pembrolizumab, a humanized monoclonal IgG4 kappa antibody targeting human PD-1, attaches to PD-1 without activating Fc-receptors or complement, thus exhibiting no cytotoxic effects (Wang and Khan, 2023). In the IND227 clinical trial (Chu et al., 2023), integrating pembrolizumab, an inhibitor of PD-1, into the standard chemotherapy regimen of platinum-pemetrexed significantly improved treatment outcomes for patients with advanced pleural mesothelioma, as compared to chemotherapy alone, leading to a 21% decrease in mortality risk. This improvement was evident in most subgroups, regardless of PD-L1 expression, even with a higher incidence of salvage immunotherapy in the group receiving only chemotherapy. The overall survival results achieved with this treatment approach were on par with those seen in studies using nivolumab-ipilimumab (Baas et al., 2021). Additionally, progression-free survival (PFS) and objective response rates (ORR) were more favorable. The safety profile of this combination in pleural mesothelioma patients mirrored that of advanced cases of non-small cell lung cancer. Although the inclusion of pembrolizumab resulted in a higher rate of adverse events, it did not negatively impact quality of life as reported by patients.
During the CheckMate-743 trial, analysis of subgroups revealed that individuals with non-epithelioid MPM experienced more significant improvements in OS. Patients with sarcomatoid MPM, who typically face a grimmer prognosis and less effective responses to chemotherapy, benefited more substantially from the combined treatment with nivolumab and ipilimumab. Similarly, the IND227 trial demonstrated a more notable advantage in patients with non-epithelioid histology. This underscores the importance of immunotherapy in the treatment regimen for these patients, especially since chemotherapy alone tends to be less effective (National Comprehensive Cancer Network, 2024).
Although the combination of pembrolizumab and chemotherapy has shown greater clinical effectiveness than chemotherapy alone, the financial rationale for this new treatment approach has not been determined. This study was conducted to evaluate the cost-effectiveness of pembrolizumab plus chemotherapy versus chemotherapy alone as a first-line treatment for advanced pleural mesothelioma in China. Considering the different clinical outcomes for patients with epithelioid or non-epithelioid histology, this analysis also focused on assessing cost-effectiveness within these specific subgroups.
This study adhered to the Consolidated Health Economic Evaluation Reporting Standards (CHEERS) checklist (Husereau et al., 2022). It targeted patients with advanced pleural mesothelioma deemed unsuitable for surgery and who had not undergone previous systemic therapy for their advanced condition. However, [neo] adjuvant chemotherapy was allowed more than a year before treatment. Eligibility criteria included an Eastern Cooperative Oncology Group performance status score of 0 or 1, measurable disease as defined by the Response Evaluation Criteria in Solid Tumours (RECIST) version 1.1, modified for use in pleural mesothelioma (mRECIST) and the provision of a tumor sample for correlative analysis. Exclusion criteria included patients with untreated CNS metastases, pneumonitis, current glucocorticoid treatment equivalent to more than 10 mg daily of prednisone (within 7 days before the first dose of study treatment), or those suffering from concurrent serious illnesses or other cancers.
Eligible patients were treated with intravenous cisplatin (75 mg/m2) and pemetrexed (500 mg/m2) every 3 weeks for up to six cycles. Those randomly assigned to the pembrolizumab group also received intravenous pembrolizumab (200 mg) every 3 weeks for up to 2 years. Patient evaluations were conducted before each cycle, 4 weeks after discontinuation, every 12 weeks until progression, and biannually until death. Imaging for both groups was scheduled every 6 weeks for the first three assessments, followed by 12-week intervals.
Subsequent treatment regimens were based on the Chinese expert consensus on diagnosing and treating malignant pleural mesothelioma (2023 edition) (Wang et al., 2023), the IND227 trial, and clinical practice. In the pembrolizumab plus chemotherapy group, patients received bevacizumab plus gemcitabine plus carboplatin, while the chemotherapy group received nivolumab. The doses of chemotherapy agents were calculated using a reference patient weighing 65.00 kg with a height of 1.64 m (body surface area of 1.72 m2). The current pembrolizumab and nivolumab patient assistance programs were considered for their effect on price reductions for these drugs.
The cost implications of adverse events (AEs) were determined based on data from the IND227 trial. In our analysis, only grade 3/4 AEs, classified as serious adverse events (SAEs), were considered, and this was restricted to those with incidence rates exceeding 5%. These SAEs included fatigue, anemia, thrombocytopenia, neutropenia, increased lipase levels, and hyperglycemia.
The development and outcome analysis of the model was conducted using TreeAge Pro 2022 software (Williamstown, MA, USA) and R software (version 4.2.3, Vienna, Austria). A three-state Markov model was implemented (Freitag et al., 2024; Wei et al., 2024), which comprised the following health states: progression-free survival (PFS), progressive disease (PD), and death (Figure 1).
All subjects entered the model in the PFS state and were either treated with chemotherapy alone or a combination of pembrolizumab and chemotherapy until disease progression or the emergence of intolerable toxicity. Patients whose disease advanced were transitioned to the PD state, where they received additional therapies following the discontinuation of pembrolizumab or placebo. The fraction of patients in the PD state was determined based on the area under the OS curve, the proportion of surviving patients, the proportion of patients with PFS, and the difference between the OS and PFS curves.
The simulation was performed over a period of 10 years, thereby encompassing over 99% of the mortality events in both treatment cohorts. The analysis was conducted from the vantage point of the Chinese healthcare systems, integrating all costs associated with healthcare services.
The primary outcomes measured were quality-adjusted life years (QALYs) and costs. To account for the value of time and adjust future costs and benefits, a 5% annual discount rate was used, as recommended by the World Health Organization (WHO) guidelines for pharmacoeconomic evaluations (Chinese Pharmaceutical Association, 2024). All costs were adjusted for 2023 prices using the local consumer price index and converted to US dollars at an exchange rate of $1 = ¥7.0467. The analysis included a cost-effectiveness evaluation, expressed in terms of incremental cost-effectiveness ratios (ICERs). The ICER was calculated as follows: ICER = [Cost (pembrolizumab plus chemotherapy) - Cost (chemotherapy)]/[QALY (pembrolizumab plus chemotherapy) - QALY (chemotherapy)]. The willingness-to-pay (WTP) threshold was three times China’s per capita gross domestic product (GDP) in 2023, $38,042.49, according to WHO recommendations. Furthermore, this study incorporated assessments of the incremental net health benefit (INHB) and the incremental net monetary benefit (INMB). These values were computed using the following equations: INHB (λ) = (μE1 - μE0) - (μC1 - μC0)/λ = ΔE - ΔC/λ and INMB (λ) = (μE1 - μE0) × λ - (μC1 - μC0) = ΔE × λ - ΔC. In these equations, μCi and μEi represent the costs and utility associated with pembrolizumab plus chemotherapy (i = 1) or chemotherapy alone (i = 0), respectively, with λ denoting the WTP threshold.
Survival curves for OS and FS in the IND227 trial were constructed using the method proposed by Guyot et al. (2012). Time-to-event data points for the Kaplan-Meier survival curves for OS and PFS were extracted using GetData Graph Digitizer version 2.26 (www.getdata.graph.digitizer.com). These data points were then applied to fit a range of parametric survival models, including Exponential, Weibull, WeibullPH, Gamma, Log-normal, Gompertz, Generalized Gamma, and Log-logistic distributions.
To determine the most suitable survival curves for PFS and OS, the fits were evaluated using both the Akaike Information Criterion (AIC) and the Bayesian Information Criterion (BIC), along with a visual review of the results (as presented in Tables 3-5). The estimated shape parameters (g) and scale parameters (l) for these curves are outlined in Table 1. Additionally, extensive details on long-term survival data can be found in Table 1 and Figures 2–7.
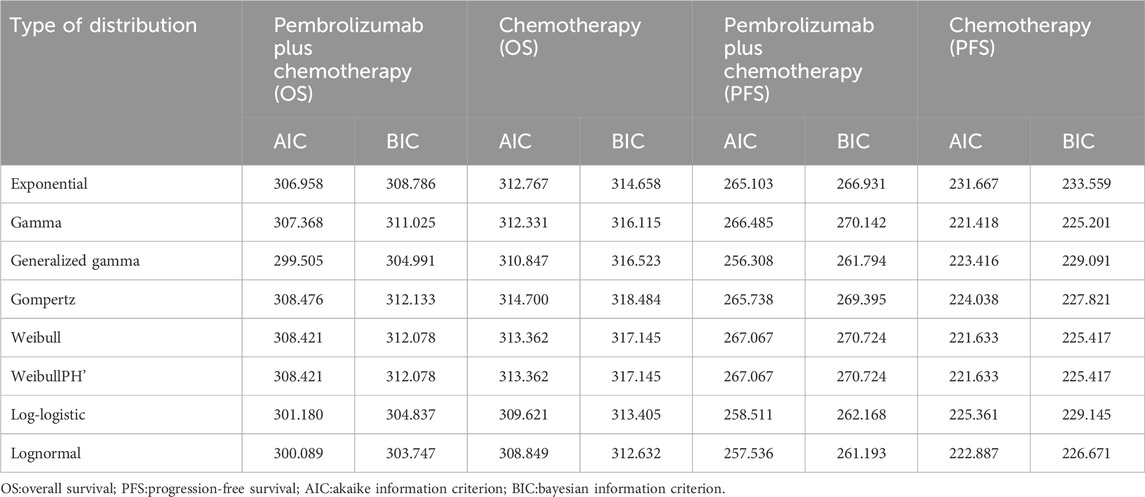
Table 4. The Akaike information criteria (AIC) and Bayesian information criteria (BIC) (Non-epithelioid).
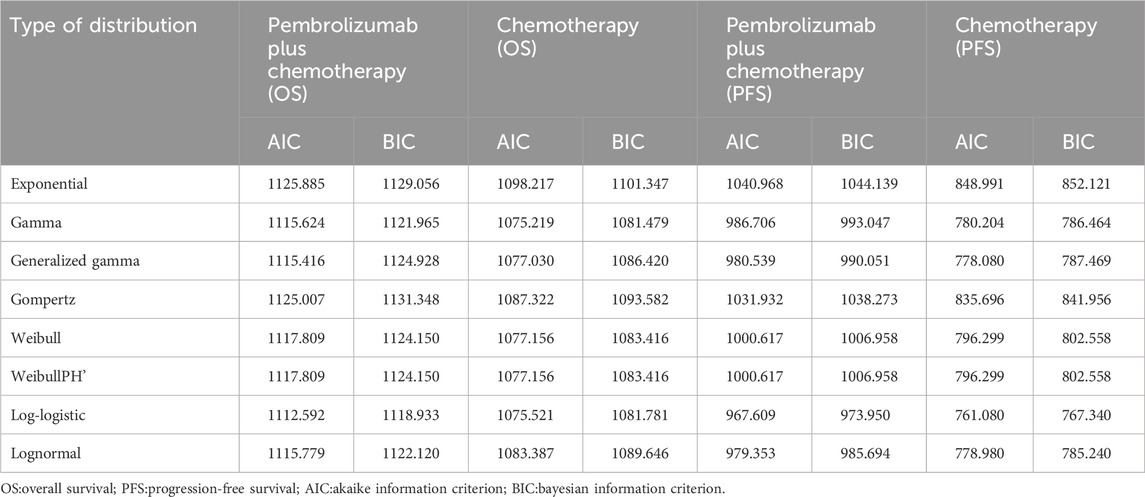
Table 5. The Akaike information criteria (AIC) and Bayesian information criteria (BIC) (Epithelioid).
This analysis was conducted from the perspective of the Chinese healthcare system, emphasizing direct costs. These include drug expenses, laboratory test fees, costs associated with enhanced chest and abdomen CT scans, preventive medications for each intravenous administration, end-of-life expenses, infusion fees per intravenous administration, subsequent treatment costs, and expenses related to managing grades 3 and 4 AEs. Drug costs were derived from national medical insurance negotiation prices and local charges, while other cost data were from previously published research studies and relevant literature.
The drug doses for the patients were determined using the IND227 study protocol. Treatment costs per cycle were determined based on dose schedules and the local price (Table 1) (Wu et al., 2014; Li et al., 2021; Lin et al., 2021; Liu L. et al., 2023; Zhu et al., 2023). The cost associated with each AE was calculated by multiplying the incidence rate of the AE by the per-occurrence cost of managing these events (Wu et al., 2020; Shu et al., 2022; Liu S. et al., 2023). We assumed that all AEs occurred during the first cycle of treatment. Table 2 presents the incidence rates for each AE in detail.
The health utility scores in our model were assigned on a scale ranging from death (0) to perfect health (1). Due to the unavailability of the European Quality of Life-5 Dimensions-5 Level (EQ-5D-5L) data from the IND227 trial, direct quality-of-life data could not be obtained. Consequently, the utility values were from the relevant published literature (Table 1). The utility values for PFS and PD were set at 0.706 and 0.565, respectively (Zhan et al., 2017; Scherpereel et al., 2022; Yang L. et al., 2022). AEs were considered to decrease health utility, a concept known as disutility (Morris et al., 2014; Yang J. et al., 2022; Zhu et al., 2022; Liu S. et al., 2023). This disutility associated with AEs was incorporated only in the first cycle of the model and was assumed to occur once every 3 weeks.
Two sensitivity analyses were conducted to address the inherent uncertainty: one-way sensitivity analysis (OWSA) and probabilistic sensitivity analysis (PSA). In the OWSA, the parameter ranges were from the existing published literature, and the parameter variations were set to ±20% of the baseline values (Morris et al., 2014; Wu et al., 2014; Zhan et al., 2017; Wu et al., 2020; Li et al., 2021; Lin et al., 2021; Scherpereel et al., 2022; Shu et al., 2022; Yang J. et al., 2022; Yang L. et al., 2022; Zhu et al., 2022; Liu L. et al., 2023; Liu S. et al., 2023; Zhu et al., 2023). For PSA, the model parameters were altered simultaneously across 10,000 Monte Carlo simulations, allowing an evaluation of the probability that each intervention would be cost-effective in varying the WTP thresholds for an additional QALY. In these simulations, beta distributions were assigned to utility parameters, and gamma distributions were used for cost variables. The outcomes of this analysis are shown in a scatter plot and a cost-effectiveness acceptability curve.
In subgroup analyses, the cost-effectiveness of pembrolizumab plus chemotherapy versus chemotherapy alone as first-line treatment for untreated advanced pleural mesothelioma was assessed separately for patients with epithelioid and non-epithelioid histologies. This assessment used base-case analysis, OWSA, and PSA methods. Due to the lack of distinct data in the IND227 trial in the follow-up treatment plan, drug selection, and the occurrence of adverse reactions specific to the epithelioid and non-epithelioid subgroups, it was assumed that these factors were consistent with those observed in the general study population.
During a 10-year analysis horizon, base-case findings revealed that the pembrolizumab plus chemotherapy group achieved an additional 0.99 QALYs at an incremental cost of $35,560.57. In contrast, the chemotherapy-only group gained 0.77 QALYs, with associated costs totaling $17,360.94. Comparative analysis between pembrolizumab plus chemotherapy and chemotherapy alone indicated a mean incremental effect of 0.23 QALYs and an additional cost of $18,199.63. This resulted in an ICER for pembrolizumab plus chemotherapy versus chemotherapy of $80,557.23 per QALY, as shown in Table 5. In particular, when evaluated against the China WTP cost-effectiveness threshold of $38,042.49 per QALY, pembrolizumab plus chemotherapy was not more cost-effective than chemotherapy. Additionally, at this WTP threshold, pembrolizumab plus chemotherapy, relative to chemotherapy, demonstrated an INHB of −0.25 QALYs and an INMB of $-9,605.00 (Table 5).
In the subgroup analyses, the ICER for pembrolizumab plus chemotherapy versus chemotherapy alone was $33,917.61 per QALY gained for patients with non-epithelioid histology and $99,536.73 per QALY for those with epithelioid histology. In particular, in the non-epithelioid subgroup, ICER was below the China WTP threshold of $38,042.49 per QALY, as shown in Table 6. Furthermore, the INHB associated with pembrolizumab plus chemotherapy was 0.05 QALYs for non-epithelioid patients and −0.29 QALYs for epithelioid patients. Regarding INMB, the values were $2,085.28 and $-11,127.42, respectively, at the WTP threshold of $38,042.49 per QALY compared to chemotherapy, as detailed in Table 6.
Figure 8 presents a tornado diagram from OWSA in the analysis that covers the entire population. This diagram shows the factors that exert the most significant influence on the base-case outcomes. The cost of pembrolizumab, the utility value of PD, and the cost of bevacizumab had the most pronounced impact on the base-case results. Figure 9 indicates that, for patients with non-epithelioid histology, ICER was mainly influenced by the utility of PFS, and the costs of pembrolizumab and nivolumab. In contrast, in patients with epithelioid histology, ICER was predominantly affected by three factors: the utility of PD, the cost of pembrolizumab, and the cost of bevacizumab, as shown in Figure 9. However, due to the marked differences in health outcomes between the two treatment strategies in all three groups, no variations in parameter values altered the study outcomes for these groups.
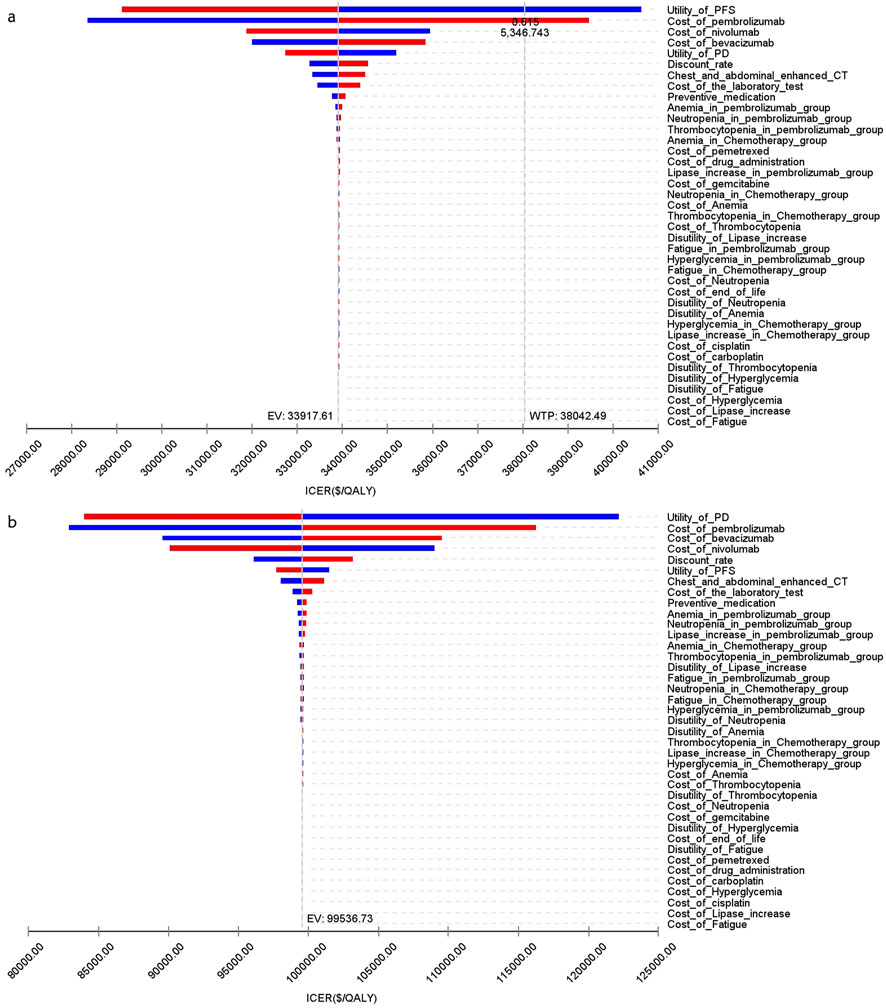
Figure 9. The tornado diagram of one-way sensitivity analysis (A) Patients with non-epithelioid group, (B) Patients with epithelioid group.
Figures 10–13 show acceptability curves and probabilistic scatter plots, which indicate the cost-effectiveness landscape. PSA outcomes revealed significant probabilities that pembrolizumab plus chemotherapy is deemed cost-effective. These probabilities were 0.55% for the entire population, 69.41% for those with non-epithelioid histology, and 0.14% for those with epithelioid histology, evaluated against a WTP threshold of three times China’s GDP per capita ($38,042.49).
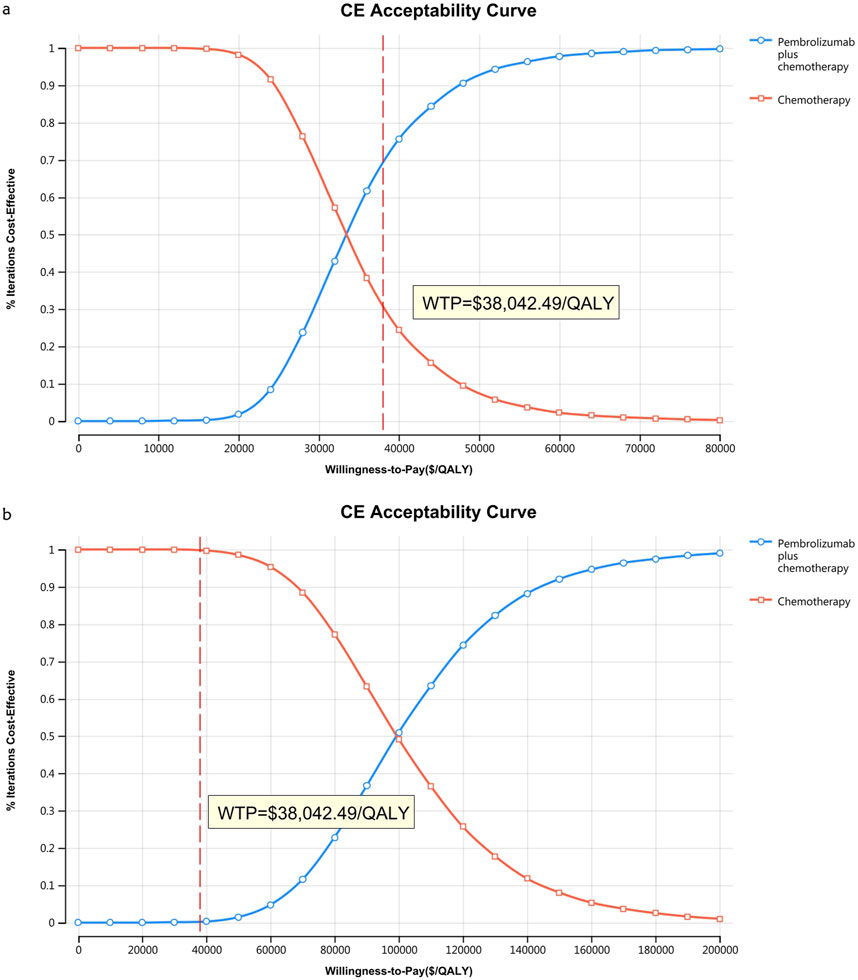
Figure 11. The cost-effectiveness acceptability curve (B) Patients with non-epithelioid group, (B) Patients with epithelioid group.
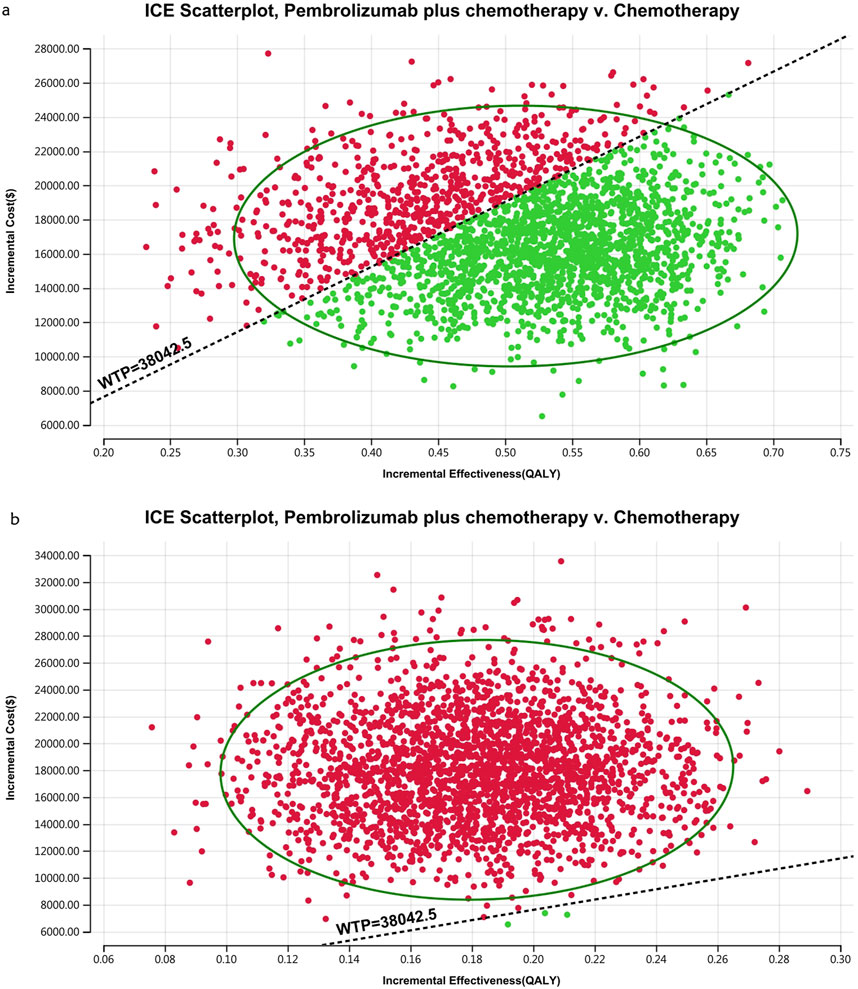
Figure 13. The cost-effectiveness probabilistic scatter plot (A) Patients with non-epithelioid group, (B) Patients with epithelioid group.
Previous studies have suggested that immune checkpoint inhibitors are not cost-effective as first-line therapy in patients with advanced pleural mesothelioma (Yang L. et al., 2022; Ye et al., 2022; Barbier et al., 2023; Yang et al., 2023; Lang et al., 2024). However, the IND227 trial demonstrated that adding pembrolizumab to platinum and pemetrexed chemotherapy significantly improved the primary endpoint of overall survival and other key efficacy outcomes in these patients. In our study, compared to conventional treatment, pembrolizumab plus chemotherapy resulted in ICERs of $80,557.23 and $99,536.73 for the entire population and the epithelioid subgroup, respectively. However, in the non-epithelioid group, the ICER was $33,917.61. At current prices and the WTP threshold, pembrolizumab plus chemotherapy is more cost-effective than chemotherapy alone in advanced pleural mesothelioma. This assertion is supported by findings from INHB and INMB analyses, showing INHBs of −0.25, 0.05, and −0.29, and INMBs of $-9,605.00, $2,085.28, and $-11,127.42 in the respective patient subgroups compared to chemotherapy. Sensitivity analyses corroborated these base-case results.
However, no parameter value change altered the study results due to a significant health outcome gap between the two strategies in patients with the entire population and epithelioid histology. In contrast, for the non-epithelioid subgroup, with an ICER of $33,917.61/QALY, nearly every parameter influenced the study outcome, as this value is close to the WTP threshold of $38,042.49/QALY. The cost-effectiveness acceptability curve supports this, indicating a probability of 69.41% that this combination is cost-effective at a threshold of three times the GDP per capita per QALY. Therefore, reducing the prices of immune checkpoint inhibitors would benefit more patients.
In China, the National Healthcare Security Administration (NHSA) has engaged in multiple rounds of negotiations with pharmaceutical enterprises, predominantly focusing on the re-evaluation of the pricing structures of anticancer drugs. These negotiations have led to a significant reduction in the prices of various anticancer medications, thereby augmenting the cost-effectiveness of pembrolizumab from the perspective of the Chinese healthcare system. In the Chinese healthcare framework, the combination of pembrolizumab and chemotherapy was not more cost-effective than chemotherapy alone as an initial treatment for untreated advanced pleural mesothelioma, with the exception of patients with non-epithelioid histology. Hence, further validation of the cost-effectiveness of pembrolizumab plus chemotherapy would be requisite in the healthcare settings of developed countries, especially in the United States.
In the IND227 study, consistent with other research, patients with non-epithelioid histology had longer overall survival (Baas et al., 2021). The strengths of this study include being the first to evaluate the cost-effectiveness of pembrolizumab plus chemotherapy versus chemotherapy alone as first-line therapy for advanced pleural mesothelioma from the perspective of the Chinese health service system. It also uniquely assessed cost-effectiveness in the non-epithelioid and epithelioid subgroups, finding that immune checkpoint inhibitor plus chemotherapy is more cost-effective than chemotherapy alone in patients with non-epithelioid advanced pleural mesothelioma. The choices for second-line treatments are complex and varied, including surgery, chemotherapy, targeted therapy, immunotherapy, and radiation therapy. The IND227 trial was deficient in specific plans for second-line treatment, merely providing the utilization rate of second-line immunotherapy, which was three times higher in the chemotherapy group than in the pembrolizumab plus chemotherapy group. However, considering the Chinese context, the most pragmatic treatment plan was selected: bevacizumab plus gemcitabine plus carboplatin in the pembrolizumab plus chemotherapy group and nivolumab in the chemotherapy group. The one-way sensitivity analysis (OWSA) indicated that as the cost of second-line treatments in the pembrolizumab plus chemotherapy group escalated, the ICER also increased, while the converse was true in the chemotherapy group.
This study has several limitations. First, Quality of Life (EQ-5D) and cost per QALY data were not reported in the IND227 study, necessitating the use of utility values derived from the literature for PFS and PD, which could introduce uncertainties in the modeled results. Second, clinical data were obtained from a phase 3 trial conducted in Canada, Italy, and France, potentially introducing bias. Third, the lack of distinct data in the IND227 trial on the subsequent treatment plan, drug selection, and occurrence of AEs for non-epithelioid and epithelioid patients required assumptions to be made, assuming that these aspects were consistent with the general patient population. Despite these limitations, a cost-effectiveness analysis based on data from the IND227 study remains feasible and provides valuable information for treatment decision-making.
In summary, this investigation demonstrates that pembrolizumab plus chemotherapy is a cost-effective therapy compared to chemotherapy for the first-line therapy of untreated advanced pleural mesothelioma patients with non-epithelioid histology from the Chinese healthcare system, but in entire population and those with non-epithelioid was not. The insights derived from this analysis provide a promising guide for decision-makers and medical professionals and evidence to support the broader application of pembrolizumab in clinical settings.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
WL: Conceptualization, Data curation, Formal Analysis, Methodology, Resources, Software, Visualization, Writing–original draft, Writing–review and editing. YH: Conceptualization, Data curation, Writing–review and editing. CH: Funding acquisition, Project administration, Writing–review and editing. HL: Conceptualization, Data curation, Writing–original draft. QJ: Conceptualization, Data curation, Writing–original draft. LM: Formal Analysis, Funding acquisition, Methodology, Writing–original draft.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2024.1402423/full#supplementary-material
Baas, P., Scherpereel, A., Nowak, A. K., Fujimoto, N., Peters, S., Tsao, A. S., et al. (2021). First-line nivolumab plus ipilimumab in unresectable malignant pleural mesothelioma (CheckMate 743): a multicentre, randomised, open-label, phase 3 trial. Lancet 397 (10272), 375–386. doi:10.1016/s0140-6736(20)32714-8
Barbier, M. C., Fengler, A., Pardo, E., Bhadhuri, A., Meier, N., and Gautschi, O. (2023). Cost effectiveness and budget impact of nivolumab plus ipilimumab versus platinum plus pemetrexed (with and without bevacizumab) in patients with unresectable malignant pleural mesothelioma in Switzerland. Pharmacoeconomics 41 (12), 1641–1655. doi:10.1007/s40273-023-01305-3
Chapel, D. B., Stewart, R., Furtado, L. V., Husain, A. N., Krausz, T., and Deftereos, G. (2019). Tumor PD-L1 expression in malignant pleural and peritoneal mesothelioma by Dako PD-L1 22C3 pharmDx and Dako PD-L1 28-8 pharmDx assays. Hum. Pathol. 87, 11–17. doi:10.1016/j.humpath.2019.02.001
Chinese Pharmaceutical Association (2024). China pharmacoeconomic evaluation guideline 2020 (Draft for comments). Available at: https://www.cpa.org.cn/cpadmn/attached/file/20200929/1601363750111497.pdf (Accessed November 23, 2024).
Chu, Q., Perrone, F., Greillier, L., Tu, W., Piccirillo, M. C., Grosso, F., et al. (2023). Pembrolizumab plus chemotherapy versus chemotherapy in untreated advanced pleural mesothelioma in Canada, Italy, and France: a phase 3, open-label, randomised controlled trial. Lancet 402 (10419), 2295–2306. doi:10.1016/s0140-6736(23)01613-6
Fennell, D. A., Ewings, S., Ottensmeier, C., Califano, R., Hanna, G. G., Hill, K., et al. (2021). Nivolumab versus placebo in patients with relapsed malignant mesothelioma (CONFIRM): a multicentre, double-blind, randomised, phase 3 trial. Lancet Oncol. 22 (11), 1530–1540. doi:10.1016/s1470-2045(21)00471-x
Freitag, A., Sarri, G., Ta, A., Gurskyte, L., Cherepanov, D., and Hernandez, L. G. (2024). A systematic review of modeling approaches to evaluate treatments for relapsed refractory multiple myeloma: critical review and considerations for future health economic models. Pharmacoeconomics 42 (9), 955–1002. doi:10.1007/s40273-024-01399-3
Guyot, P., Ades, A. E., Ouwens, M. J., and Welton, N. J. (2012). Enhanced secondary analysis of survival data: reconstructing the data from published Kaplan-Meier survival curves. BMC Med. Res. Methodol. 12, 9. doi:10.1186/1471-2288-12-9
Husereau, D., Drummond, M., Augustovski, F., de Bekker-Grob, E., Briggs, A. H., Carswell, C., et al. (2022). Consolidated health economic evaluation reporting standards 2022 (CHEERS 2022) statement: updated reporting guidance for health economic evaluations. Value Health 25 (1), 3–9. doi:10.1016/j.jval.2021.11.1351
Lang, W., Wei, J., Jiang, Q., Ai, Q., Zhao, X., Xiao, L., et al. (2024). Cost-effectiveness analysis of nivolumab versus placebo for relapsed malignant mesothelioma. Int. J. Clin. Pharm. 46 (1), 158–165. doi:10.1007/s11096-023-01662-1
Li, W., Guo, H., Li, L., and Cui, J. (2021). Comprehensive comparison between adjuvant targeted therapy and chemotherapy for EGFR-mutant nsclc patients: a cost-effectiveness analysis. Front. Oncol. 11, 619376. doi:10.3389/fonc.2021.619376
Lin, Y. T., Chen, Y., Liu, T. X., Kuang, F., and Huang, P. (2021). Cost-effectiveness analysis of camrelizumab immunotherapy versus docetaxel or irinotecan chemotherapy as second-line therapy for advanced or metastatic esophageal squamous cell carcinoma. Cancer Manag. Res. 13, 8219–8230. doi:10.2147/cmar.S335515
Liu, L., Wang, L., Chen, L., Ding, Y., Zhang, Q., and Shu, Y. (2023a). Cost-effectiveness of sintilimab plus chemotherapy versus chemotherapy alone as first-line treatment of locally advanced or metastatic oesophageal squamous cell carcinoma. Front. Immunol. 14, 1092385. doi:10.3389/fimmu.2023.1092385
Liu, S., Jiang, N., Dou, L., and Li, S. (2023b). Cost-effectiveness analysis of serplulimab plus chemotherapy in the first-line treatment for PD-L1-positive esophageal squamous cell carcinoma in China. Front. Immunol. 14, 1172242. doi:10.3389/fimmu.2023.1172242
Mansfield, A. S., Roden, A. C., Peikert, T., Sheinin, Y. M., Harrington, S. M., Krco, C. J., et al. (2014). B7-H1 expression in malignant pleural mesothelioma is associated with sarcomatoid histology and poor prognosis. J. Thorac. Oncol. 9 (7), 1036–1040. doi:10.1097/jto.0000000000000177
Morris, S., Gurusamy, K. S., Patel, N., and Davidson, B. R. (2014). Cost-effectiveness of early laparoscopic cholecystectomy for mild acute gallstone pancreatitis. Br. J. Surg. 101 (7), 828–835. doi:10.1002/bjs.9501
National Comprehensive Cancer Network (2024). Clinical practice guidelines in Oncology: mesothelioma pleural. Available at: www.nccn.org/patients.
Popat, S., Baas, P., Faivre-Finn, C., Girard, N., Nicholson, A. G., Nowak, A. K., et al. (2022). Malignant pleural mesothelioma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up(☆). Ann. Oncol. 33 (2), 129–142. doi:10.1016/j.annonc.2021.11.005
Sahu, R. K., Ruhi, S., Jeppu, A. K., Al-Goshae, H. A., Syed, A., Nagdev, S., et al. (2023). Malignant mesothelioma tumours: molecular pathogenesis, diagnosis, and therapies accompanying clinical studies. Front. Oncol. 13, 1204722. doi:10.3389/fonc.2023.1204722
Scherpereel, A., Antonia, S., Bautista, Y., Grossi, F., Kowalski, D., Zalcman, G., et al. (2022). First-line nivolumab plus ipilimumab versus chemotherapy for the treatment of unresectable malignant pleural mesothelioma: patient-reported outcomes in CheckMate 743. Lung Cancer 167, 8–16. doi:10.1016/j.lungcan.2022.03.012
Shu, Y., Ding, Y., and Zhang, Q. (2022). Cost-effectiveness of nivolumab plus chemotherapy vs. Chemotherapy as first-line treatment for advanced gastric cancer/gastroesophageal junction cancer/esophagel adenocarcinoma in China. Front. Oncol. 12, 851522. doi:10.3389/fonc.2022.851522
Siegel, R. L., Miller, K. D., Fuchs, H. E., and Jemal, A. (2021). Cancer statistics, 2021. CA Cancer J. Clin. 71 (1), 7–33. doi:10.3322/caac.21654
Sung, H., Ferlay, J., Siegel, R. L., Laversanne, M., Soerjomataram, I., Jemal, A., et al. (2021). Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 71 (3), 209–249. doi:10.3322/caac.21660
Vogelzang, N. J., Rusthoven, J. J., Symanowski, J., Denham, C., Kaukel, E., Ruffie, P., et al. (2003). Phase III study of pemetrexed in combination with cisplatin versus cisplatin alone in patients with malignant pleural mesothelioma. J. Clin. Oncol. 21 (14), 2636–2644. doi:10.1200/jco.2003.11.136
Wang, Q., Xu, C., Wang, W., Zhang, Y., Li, Z., Song, Z., et al. (2023). Chinese expert consensus on the diagnosis and treatment of malignant pleural mesothelioma. Thorac. Cancer 14 (26), 2715–2731. doi:10.1111/1759-7714.15022
Wang, S., and Khan, F. I. (2023). Investigation of molecular interactions mechanism of pembrolizumab and PD-1. Int. J. Mol. Sci. 24 (13), 10684. doi:10.3390/ijms241310684
Wei, Q., Liang, Y., Mao, J., and Guan, X. (2024). Cost-effectiveness analysis of adjuvant alectinib versus platinum-based chemotherapy in resected ALK-positive non-small-cell lung cancer in the Chinese health care system. Cancer Med. 13 (22), e70405. doi:10.1002/cam4.70405
Wu, B., Li, T., Cai, J., Xu, Y., and Zhao, G. (2014). Cost-effectiveness analysis of adjuvant chemotherapies in patients presenting with gastric cancer after D2 gastrectomy. BMC Cancer 14, 984. doi:10.1186/1471-2407-14-984
Wu, Q., Liao, W., Zhang, M., Huang, J., Zhang, P., and Li, Q. (2020). Cost-effectiveness of tucatinib in human epidermal growth factor receptor 2-positive metastatic breast cancer from the US and Chinese perspectives. Front. Oncol. 10, 1336. doi:10.3389/fonc.2020.01336
Yang, J., Han, J., Zhang, Y., Muhetaer, M., Chen, N., and Yan, X. (2022a). Cost-effectiveness analysis of trastuzumab deruxtecan versus trastuzumab emtansine for HER2-positive breast cancer. Front. Pharmacol. 13, 924126. doi:10.3389/fphar.2022.924126
Yang, L., Cao, X., Li, N., Zheng, B., Liu, M., and Cai, H. (2022b). Cost-effectiveness analysis of nivolumab plus ipilimumab versus chemotherapy as the first-line treatment for unresectable malignant pleural mesothelioma. Ther. Adv. Med. Oncol. 14, 17588359221116604. doi:10.1177/17588359221116604
Yang, L., Song, X., Zeng, W., Zheng, Z., and Lin, W. (2023). First-line nivolumab plus ipilimumab for unresectable MPM in China: a cost-effectiveness analysis. Orphanet J. Rare Dis. 18 (1), 326. doi:10.1186/s13023-023-02925-w
Yap, T. A., Aerts, J. G., Popat, S., and Fennell, D. A. (2017). Novel insights into mesothelioma biology and implications for therapy. Nat. Rev. Cancer 17 (8), 475–488. doi:10.1038/nrc.2017.42
Ye, Z. M., Tang, Z. Q., Xu, Z., Zhou, Q., and Li, H. (2022). Cost-effectiveness of nivolumab plus ipilimumab as first-line treatment for American patients with unresectable malignant pleural mesothelioma. Front. Public Health 10, 947375. doi:10.3389/fpubh.2022.947375
Zhan, M., Zheng, H., Xu, T., Yang, Y., and Li, Q. (2017). Cost-effectiveness analysis of additional bevacizumab to pemetrexed plus cisplatin for malignant pleural mesothelioma based on the MAPS trial. Lung Cancer 110, 1–6. doi:10.1016/j.lungcan.2017.05.012
Zhu, Y., Liu, K., Wang, M., Wang, K., and Zhu, H. (2022). Trastuzumab deruxtecan versus trastuzumab emtansine for patients with human epidermal growth factor receptor 2-positive metastatic breast cancer: a cost-effectiveness analysis. Breast 66, 191–198. doi:10.1016/j.breast.2022.10.010
Keywords: cost-effectiveness, pleural mesothelioma, non-epithelioid, pembrolizumab, markov mode
Citation: Lang W, He Y, Hou C, Li H, Jiang Q and Mei L (2025) Cost-effectiveness analysis of pembrolizumab plus chemotherapy versus chemotherapy in untreated advanced pleural mesothelioma in the Chinese healthcare system. Front. Pharmacol. 15:1402423. doi: 10.3389/fphar.2024.1402423
Received: 19 March 2024; Accepted: 19 December 2024;
Published: 07 January 2025.
Edited by:
Bernd Rosenkranz, Fundisa African Academy of Medicines Development, South AfricaReviewed by:
Xingchen Peng, Sichuan University, ChinaCopyright © 2025 Lang, He, Hou, Li, Jiang and Mei. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wenwang Lang, MjkwNzAyMDYyQHFxLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.