- 1College of Food Science and Pharmaceutical Engineering, Zaozhuang University, Zaozhuang, China
- 2College of Pharmacy, Shandong University of Traditional Chinese Medicine, Jinan, China
Pomegranate seeds (PS) are the dried seeds derived from pomegranate fruit, accounting for approximately 20% of the fruit’s total weight, and are a by-product of pomegranate juice extraction. These seeds hold significance in traditional medicine among Uyghurs and Tibetan cultures, featuring diverse clinical applications within traditional Chinese medicine. These applications include management of gastric coldness and acidity, abdominal distension, liver and gallbladder fever, and pediatric enteritis. PS demonstrates properties such as stomach tonicity, qi regulation, analgesia, and anti-inflammatory effects. Extensive research underscores the richness of PS in various phytochemical compounds and metabolites, notably unsaturated fatty acids (particularly linolenic acid and linoleic acid), phenolic compounds tocopherols, proteins, and volatile oils. Notably, among these bioactive compounds, punicic acid (PA), found within PS, demonstrates potential in the prevention and treatment of cancers, diabetes, obesity, and other ailments. Despite extensive literature on pomegranate as a botanical entity, a comprehensive review focusing specifically on the chemical composition and pharmacological effects of PS remains elusive. Therefore, this review aimed to consolidate knowledge regarding the medicinal properties of PS, summarizing its chemical composition, traditional uses, and pharmacological effects in treating various diseases, thereby laying a foundation for the advancement and application of PS in the field of pharmacology.
1 Introduction
Pomegranate (Punica granatum L.), a member of the family Punicaceae, is a deciduous shrub or small tree with a longstanding history of cultivation in China. The first documented medicinal use of various pomegranate parts, including the peel, seeds, flowers, leaves, and roots, dates back to the Han Dynasty, as recorded in Min-Yi-Bie-Lu (名医别录Han Dynasty). Therefore, pomegranate has a very long history of use not only in traditional Chinese medicine but also in diverse clinical practices among Tibetans, Uyghurs, Miaos, and other ethnic groups. Apart from its nutritional significance, pomegranate has important medicinal attributes. Research indicates that different components of the pomegranate, such as the juice, pericarp, seeds, flowers, leaves, and peels, are rich in biologically active compounds with anti-diabetic, anti-tumor, anti-inflammatory, anti-malarial, and anti-fibrotic properties, which are widely recognized and utilized globally (Maphetu et al., 2022; Yisimayili and Chao, 2022). Moreover, studies have revealed that the bark of the pomegranate tree is a natural green corrosion inhibitor (Marsoul et al., 2020). Pomegranate also finds clinical application in Hmong medicine for treating conditions such as chronic diarrhea, dysentery, and roundworms. Furthermore, pomegranate flowers are used to treat nosebleeds, whereas whole pomegranate dried fruit is used in Mongolian medicine for treating conditions such as gastritis, indigestion, and abdominal distension. The Pharmacopoeia of the People’s Republic of China (2020 edition) acknowledges the efficacy of pomegranate peel in astringent and antidiarrheal, hemostatic, and anthelmintic capacities. Various pharmaceutical properties attributed to pomegranate peels include anti-proliferative, anti-inflammatory, and anti-cancer effects (Wong et al., 2021). Pomegranate juice, which is rich in antioxidants, demonstrates cholesterol oxidation resistance, atherosclerosis prevention, anti-inflammatory and anti-aging effects, along with potential to prevent Alzheimer’s disease and diabetes (Sreeja et al., 2012). Consequently, the utilization of pomegranate by-products continues to expand, enhancing the economic efficiency of the pomegranate industry.
Pomegranate seeds (PS), derived from the pomegranate fruit in dried form, constitute approximately 20% of its total fruit weight (Gao et al., 2023), serving as the primary by-product of pomegranate juice processing. Despite this, PS are relatively under-researched and underutilized, leading to substantial amounts of annual waste estimated at 70,000–100,000 tons. Although, PS represent a significant amount among waste products in the fruit processing industry, they hold considerable value as a resource for pharmaceuticals and nutraceuticals (Khoddami et al., 2014). In Tibetan medicine, PS is commonly known as“Saizhu”and is recognized for its sweet and sour taste, warm properties, and therapeutic effects in ailments of stomach, digestion, and lungs. With heat-clearing and damp-drying effects, PS are used in traditional medicine for treating conditions such as hemorrhage, dampness-heat syndrome, and other diseases (Huo et al., 2023). Research indicates diverse pharmacological effects associated with PS, including anti-tumor and anti-osteoporosis properties, possibly attributable to its constituents such as unsaturated fatty acids, phenols, sterols, proteins, and volatile oils (Min et al., 2010; Kaseke et al., 2021; Guzmán-Lorite et al., 2022a). Pomegranate seed oil (PSO), extracted from PS, constitutes 12%–20% of PS’s total weight and is notably rich in punicic acid (PA), a rare isomer of conjugated linoleic acid known for its efficacy against breast cancer, prostate cancer, diabetes, and obesity (Aruna et al., 2016; Zielińska et al., 2022). PA exhibits promising potential as an alternative treatment (Franczyk-Żarów et al., 2023), and the extraction of PSO not only minimizes waste but also enhances the economic and health benefits associated with pomegranate (Fourati et al., 2020).
The full potential of PS remains largely untapped due to an inadequate understanding of its value and a dearth of development in its applications. Despite being a valuable resource, PS have not been sufficiently exploited for their benefits. Although some recent reviews of pomegranate research have briefly addressed studies related to PS, a comprehensive examination of their chemical composition is yet to be achieved (Kori et al., 2020; Ge et al., 2021; Maphetu et al., 2022). In recent years, several studies have investigated the chemical composition of PS (Hernández-Corroto et al., 2022; Iriti et al., 2023); however, these studies have focused on only one or a few components of PS. This paper presents a comprehensive and systematic review of the chemical composition of PS. Moreover, there is a notable absence of systematic summaries concerning the pharmacological effects of PS, as well as a lack of comprehensive reviews detailing their traditional uses, clinical applications, and chemical metabolites. The absence of such reviews hampers a broader and updated perspective. Hence, there is a need for systematic and comprehensive reviews that could provide a foundation for leveraging PS effectively and to stimulate further research and development of PS applications in the pharmaceutical, health product, food, and other industries.
2 Methods
Literature search was conducted using the keywords “pomegranate seed,” “pharmacological properties, or activities, or effects, or roles,” “ethnopharmacology,” “traditional uses,” “botanical characteristics,” “chemical composition” in major scientific literature databases such as PubMed, Web of Science, Wiley, Francis & Taylor, Hindawi, SciFinder, Science Direct, Springer, ACS, CNKI, Google Scholar, and Baidu Scholar. Additionally, books, MSc theses, and Ph.D. From approximately 200 identified studies, a total of 115 studies, which met the inclusion criteria, were preserved in this survey.
3 Botanical characteristics
Pomegranate (P. granatum L.), a member of the Punicaceae family, thrives in tropical and subtropical regions worldwide. Originating from ancient Mediterranean areas characterized by cool winters and warm dry summers, this climate fosters optimal growth conditions for the plant (Sarkhosh et al., 2020). Cultivated across diverse regions, the pomegranate assumes various names. In Asian countries such as China, Georgia, and Afghanistan, a wide array of pomegranate varieties has been reported (Ge et al., 2021).
The pomegranate plant reach heights of 4–5 m, adorned with thorny branches (Sarkhosh et al., 2020). Additionally, the plant features flaky bark and glossy, crinkled petal leaves (Guerrero-Solano et al., 2020). Upon ripening, the fruit forms a berry measuring approximately 5–12 cm in diameter, boasting a round shape and thick, reddish skin. Each fruit contains anywhere from 200 to 1400 seeds enveloped in water-laden pulp (Stover and Mercure, 2007), which varies in color from white to deep red or purple.
4 Traditional uses
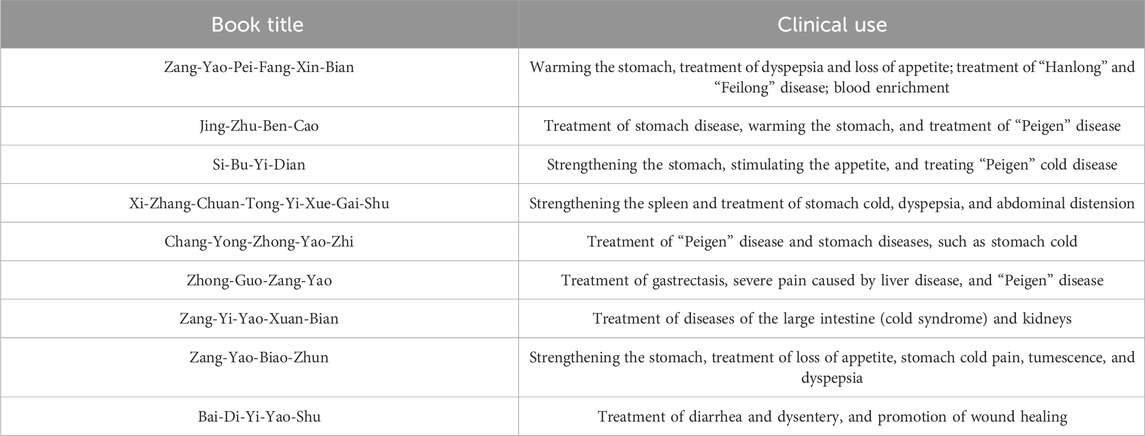
Bai-Di-Yi-Yao-Shu (拜地依药书) documented pomegranate peel, seeds, and flowers as possessing a sour and astringent taste, prescribed for managing diarrhea, dysentery, and facilitating wound healing (Editorial Board of Chinese Materia Medica of National Administration of Traditional Chinese Medicine, 2005). Jing Zhu Ben Cao (晶珠本草) indicates that PS can effectively address stomach ailments, warm the stomach, and alleviate symptoms of Peigen disease. In Si Bu Yi Dian (四部医典), pomegranate seeds are hailed as “the king of warm medicine,” utilized for fortifying the stomach, enhancing appetite, and managing “Peigen” cold disease.
In traditional Uyghur and Tibetan medicine, PS serves primarily in the treatment of gastric disorders and is esteemed for its bioactive metabolites and functional lipid abundance. Contemporary research demonstrates that pomegranate seeds exhibit antioxidant, anti-inflammatory, anti-tumor, hypoglycemic, hypolipidemic, and cardiovascular protective properties (Chen et al., 2023a). Traditional medicinal applications of PS are summarized in Table 1.
As per Zhong Hua Ben Cao’s Tibetan Medicine Volume (中华本草·藏药卷), Shunqi thirteen-flavored San, incorporating pomegranate, dried ginger, pepper, Piper longum, nutmeg, round cardamom, Amomum tsao-ko, safflower, Cinnamomum cassia, myrobalan, Halite, Halite Violaceous, and Nigella glandulifera, is prescribed for intestinal distension and bloating (State Administration of Traditional Chinese Medicine, 2002). Pomegranate Stomach Pill composed of PS, Cinnamomum cassia, Piper longum, safflower, and round cardamom; proven effective in treating gastroenteritis (Kong et al., 2021). In contemporary medicine, PS is often combined with other Chinese medicines to enhance their efficacy in stomach and kidney warming and in managing cholecystitis and enteritis (Supplementary Table S1). Top of Form.
5 Chemical composition
PS contains a wide array of phytochemicals and bioactive compounds. Li et al. (2020) effectively identified flavonoids and phenolic acids within PS. Furthermore, in 2023, the same research team identified fatty acids, sterols, and other metabolites in PS (Li et al., 2023). The principal metabolites present in PS include fatty acids, flavonoids, phenolic acids, proteins (Hernández-Corroto et al., 2022), volatile oils (Min et al., 2010), phytosterols, and others such as squalene, carotenoids, and vitamin E (Iriti et al., 2023).
5.1 Fatty acids
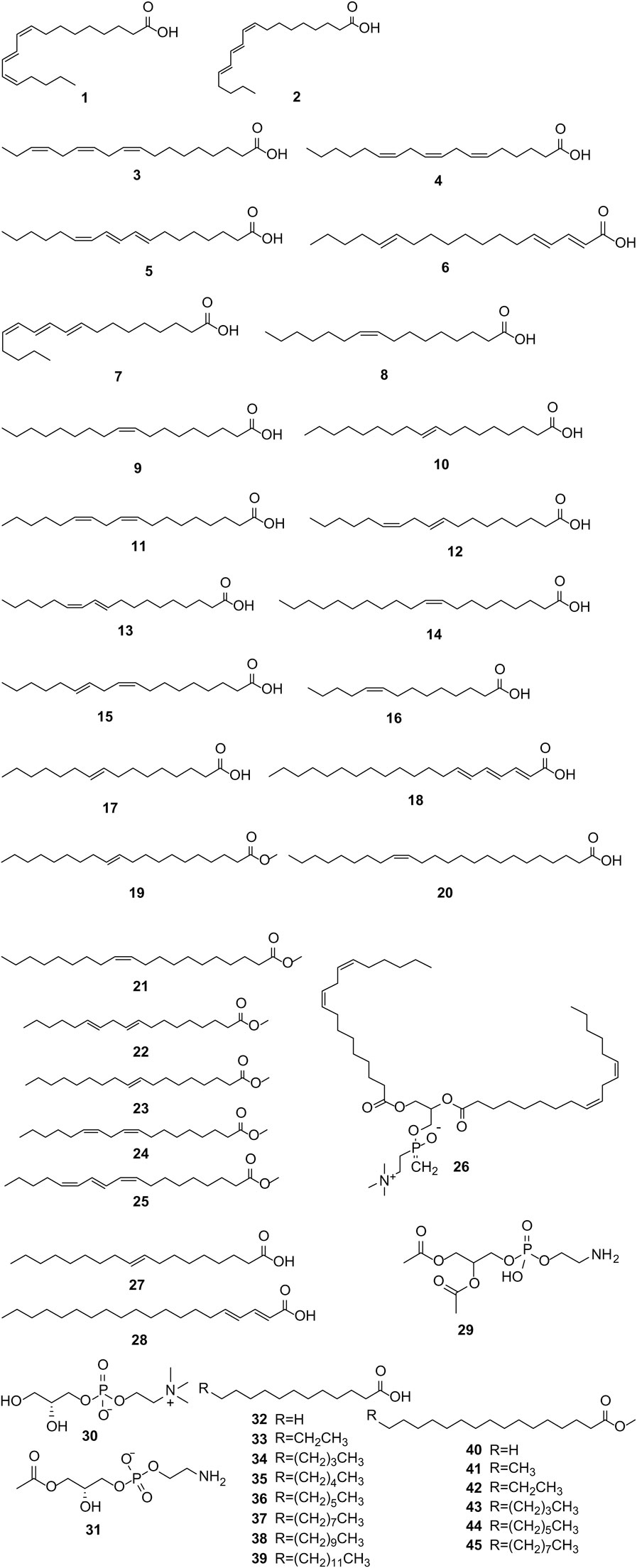
PS is abundant in various fatty acids (Eikani et al., 2012), characterized by significant levels of monounsaturated fatty acids (MUFAs) and polyunsaturated fatty acids (PUFAs), and lesser quantities of saturated fatty acids (SFAs), as elucidated by Siano et al. (2016). The total lipid content in PS ranges from 7.9% to 16%, with PSO particularly rich in conjugated linolenic acids (CLnAs), notably PA, constituting 74%–85% of the total fatty acid content. Other prominent fatty acids include oleic, linoleic, and palmitic acids, alongside certain phospholipids (Verardo et al., 2014). The fatty acid composition of PS is detailed in Supplementary Table S2, with the structural formulas of these fatty acids depicted in Figure 1.
5.2 Phenolic metabolites
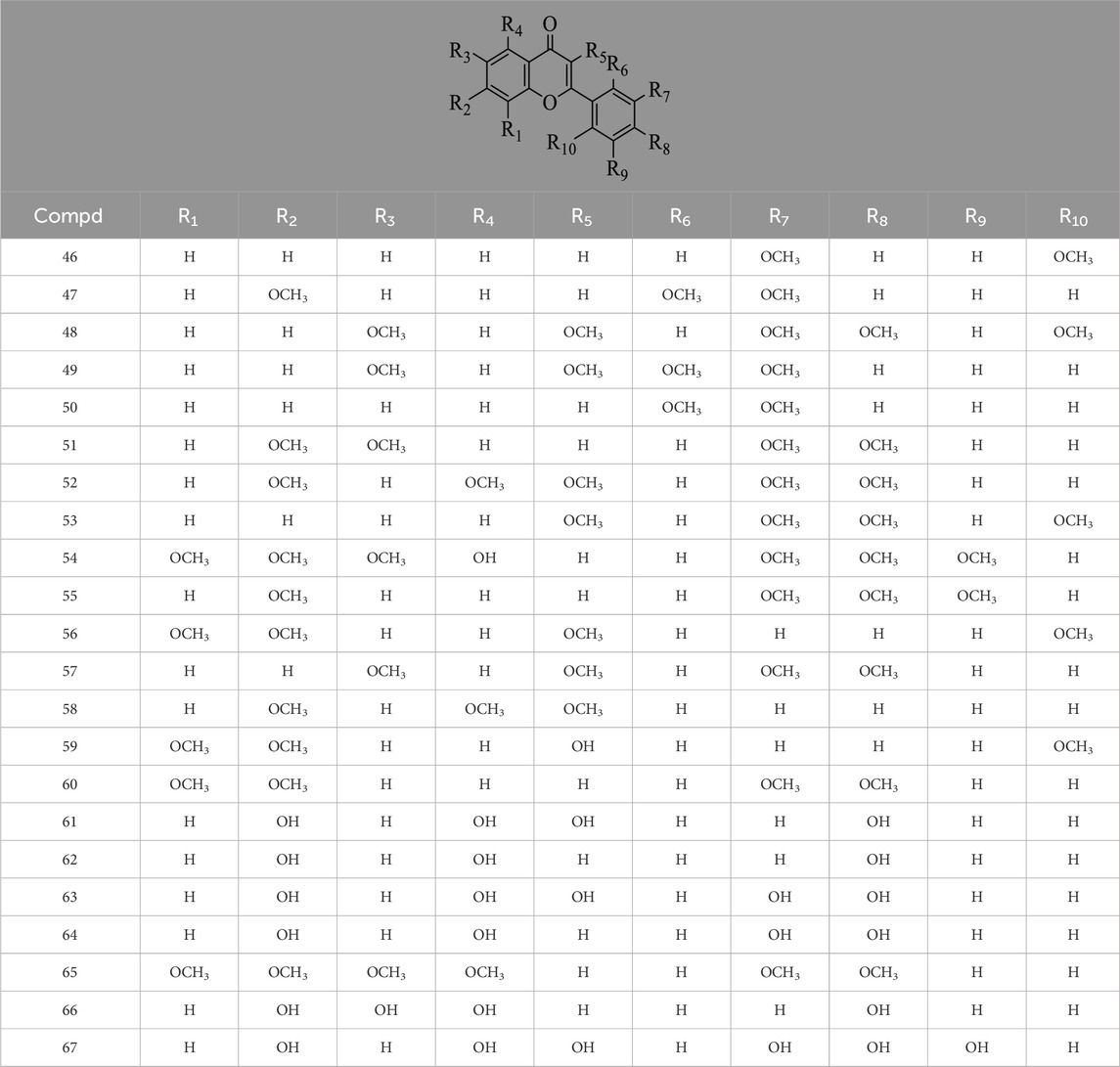
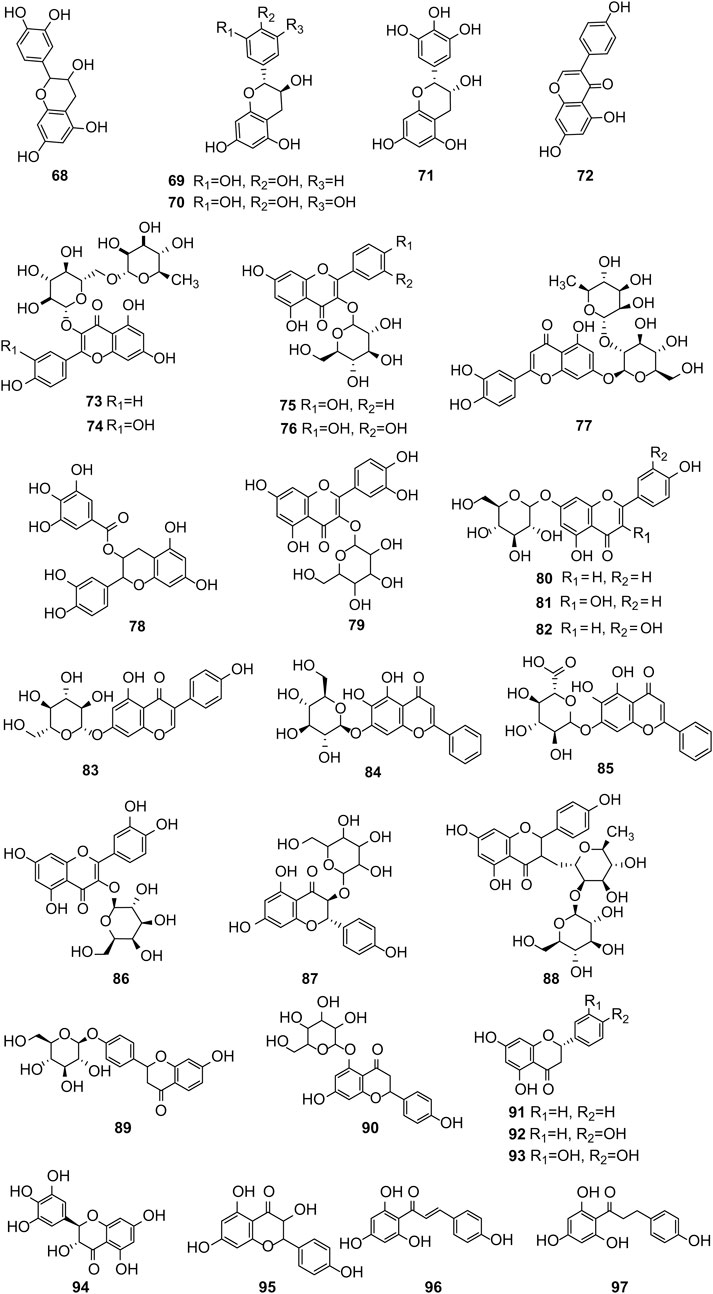
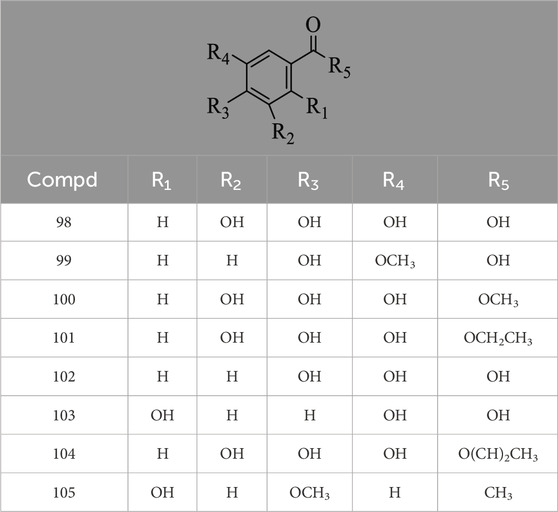
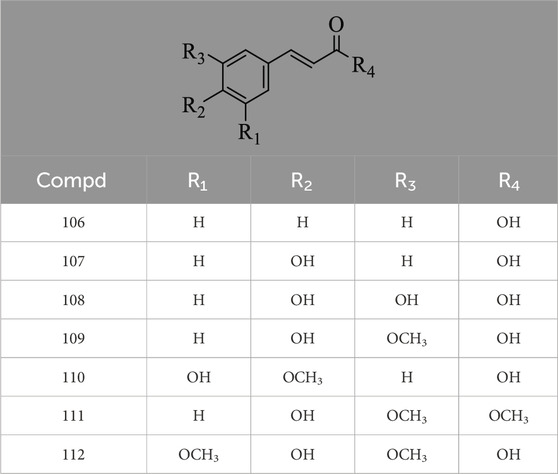
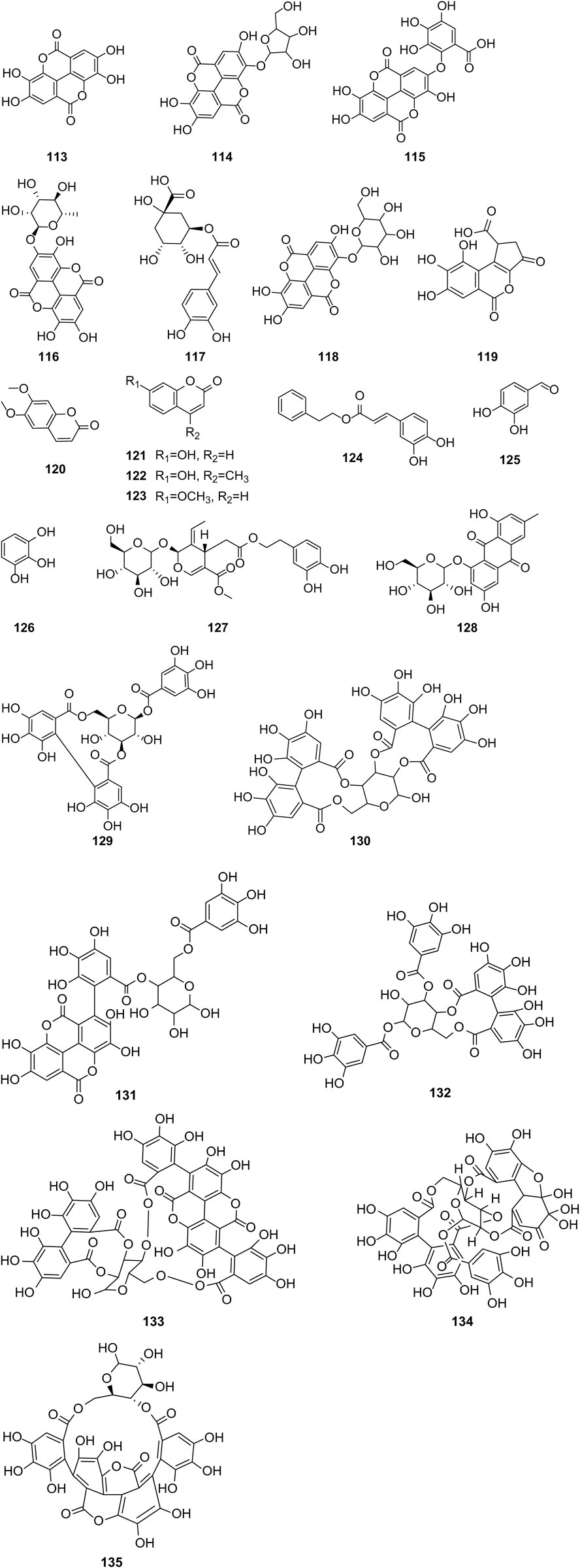
Hernández-Corroto et al. (2022) identified 30 different phenolic metabolites. Among these, ellagic acid emerges as a key phenolic metabolite in PS, renowned for its anti-cancer, anti-inflammatory, antioxidant, and hypolipidemic pharmacological effects (Ranjha et al., 2021). Ambigaipalan et al. (2017) identified 47 phenolic metabolites in PS, encompassing various flavonoids renowned for their antioxidant activity. Notably, naringenin hexoside and catechins were the most abundant flavonoids. The flavonoid composition of PS is detailed in Supplementary Table S3, with structural formulas presented in Table 2 and Figure 2. Phenolics in PS, excluding flavonoids, are shown in Supplementary Table S4, while the structural formulas of phenolic metabolites other than flavonoids are presented in Tables 3, 4 and Figure 3.
5.3 Volatile oil metabolites
Min et al. (2010) employed GC/MS to examine the volatile oil metabolites in sour and sweet PS, identifying 14 and 15 fractions, respectively. Both varieties of PS contained volatile oils such as geranial, carvacrol, and eucalyptol, albeit with notable differences in relative contents between them.
5.4 Other classes
PS contains 10%–20% protein (Talekar et al., 2018). Elfalleh et al. (2012) investigated the free amino acid and protein contents of Tunisian and Chinese PS, revealing 18 common free amino acids in both varieties, including all essential, sulfur-containing, and aromatic amino acids, with glutamic acid, arginine, and aspartic acid prevailing as the major amino acids. These amino acids are often lacking in most foods (Bar-Ya’akov et al., 2019). Guzmán-Lorite et al. (2022b) devised an environmentally friendly method for the comprehensive recovery of proteins from PS using natural deep eutectic solvent choline chloride and glacial acetic acid (ChCl:HAC), alongside continuous pressurized liquid extraction under alkaline conditions, facilitating the eco-friendly and complete retrieval of proteins from PS.
Additionally, PS comprises steroids, carotenoids, tocopherols, trace elements, and other metabolites. Górnaś and Rudzińska (2016) identified numerous phytosterols in PSO, including glycosterol, stigmasterol, glutosterol, sterol, 7-stigmasterol, and cyclic oxysterol. Furthermore, PS is rich in trace elements. Using flame atomic absorption spectrometry, Yin et al. (2011) detected nine trace elements in sour and sweet PS, including Cu, Mn, Zn, Fe, Cr, K, Ca, Na, and Mg.
6 Pharmacological activities
Numerous studies have showcased the anti-tumor effects (Costantini et al., 2014) and anti-osteoporosis properties (Yogesh et al., 2020) of PS. Esther Lydia et al. (2020) developed a functional yogurt by employing PSO as a reducing agent to cap gold nanoparticles, demonstrating its in vitro antioxidant and anti-cancer activities.
6.1 Anti-tumor and anti-cancer effects
The primary metabolite in the hydrophilic extract of PSO is PA. In vitro experiments treating two breast cancer cell lines, MDA-MB-231 and MCF-7, with 0.5 μL and 0.6 μL of the extract led to an increase in the number of cells in the G0/G1 phase of the cell cycle compared to untreated cells. Moreover, treatment with higher doses of the extract showed more pronounced effects. Additionally, levels of vascular endothelial growth factor and pro-inflammatory cytokines decreased, indicating anti-inflammatory properties and preventive effects on tumor formation and metastasis (Costantini et al., 2014). Another in vitro study on human breast cancer cell lines MCF-7 and MDA-MB-468 using PSO yielded similar results (Mahmoudi et al., 2017). PSO inhibits the proliferative activity of breast cancer cells through various molecular proteins or signaling pathways, potentially by regulating the expression of Cox-2, Bcl-2, Bax, cystatinase-3 (zymosan), and P53 in cells (Fu et al., 2015).
Mete et al. (2019) conducted in vitro experiments to evaluate the toxic effects of PA on T98 cells. After 24 h of exposure to doses of 1, 5, 10, 50, and 100 μL/mL, they determined that the IC50 dose was 9.85 μL/mL. Applying the IC50 dose of PA to an in vitro wound model inhibited the migration and proliferation of T98 glioblastoma cells. Additionally, PA treatment reduced angiogenesis, tumor progression, and induced apoptosis. Notably, PA treatment significantly decreased the levels of PI3K and AKT1 in T98 cells, thereby inhibiting the proliferation of glioblastoma cells, possibly through the PI3K/Akt/mTOR signaling pathway.
Nasr et al. (2023) conducted in vitro experiments using fresh medium containing two-fold serial dilutions of PS and methanol extract of pomegranate peel, added to pre-cultured plates of human hepatocellular carcinoma cells (HepG2 cells) at a concentration of 0.1 mL/mL, respectively. Their findings revealed that pomegranate seeds exerted a significant toxic effect on HepG2 cells compared to peel extracts. Furthermore, there was notable cell cycle blockade and cell death in the G0/G1 and S phases, with cell cycle arrest and cell death not observed in the G2/M phase. Cell arrest was followed by an increase in reactive oxygen species (ROS) and MDA levels, alongside a decrease in superoxide dismutase (SOD), GSH, and catalase levels. Among apoptosis-related genes, the expression of pro-apoptotic genes (P53, Cy-C, Bax, casp-3) was significantly upregulated, while the expression of the anti-apoptotic gene (Bcl-2) was significantly downregulated.
Another study demonstrated that the total flavonoids in PS possess cancer-preventive effects by effectively eliminating nitrite, a precursor for nitrosamine synthesis, and inhibiting nitrosamine synthesis (Wang et al., 2018).
Astrocytic glioblastoma (GBM) is considered resistant to chemotherapy. The utilization of nanocarrier systems to traverse the blood-brain barrier has the potential to enhance therapeutic efficacy and mitigate the side effects of conventional chemotherapy. PSO nanoemulsions (n = 3) containing Laplacin were formulated via spontaneous emulsification solvent diffusion. These nanoemulsions were then utilized for the preparation of ketoprofen, a non-steroidal anti-inflammatory drug, followed by in vitro testing on C6 cells. The results of the study revealed that the nanoemulsions enhanced the photostability of the drug against UVC radiation and improved its solubility. Furthermore, these formulations have exhibited suitability for intravenous administration and have demonstrated significant activity against glioma cells in vitro (Ferreira et al., 2015).
6.2 Hypoglycemic effects
PA has been shown to exert anti-diabetic effects through various mechanisms, including the reduction of inflammatory cytokines, regulation of glucose homeostasis, and contains antioxidant properties (Khajebishak et al., 2019a). PSO exhibits good digestibility, bio-accessibility and anti-inflammatory properties (Bañares et al., 2023). Harzallah et al. (2016) conducted in vitro experiments on male C57Bl/6 mice, administering PSO (2 mL/kg/d) and assessing parameters such as body weight, body fat, energy expenditure, food and fluid intake, blood glucose, plasma insulin, and lipids. Their findings revealed that PSO improves insulin sensitivity. PSO’s therapeutic efficacy in patients with type 2 diabetes mellitus was investigated by Khajebishak et al. (2019b) in a randomized clinical trial involving 52 obese type 2 diabetes mellitus patients over an 8-week period. Participants were divided into an intervention group (n = 26; receiving three capsules containing 1 g of PSO per day) and a placebo group (n = 26; receiving equal amounts of paraffin). GLUT-4 gene expression and glycemic index were evaluated using standard methods, demonstrating an increase in GLUT-4 gene expression in diabetic patients without any observed side effects. However, further clinical studies are warranted to validate the findings. In a study by Wu et al. (2015b), the effect of PSO on plasma phospholipids in mice with type 2 diabetes mellitus was examined. They found that the pharmacological mechanism may involve the activation of AMP-activated protein kinase. Nevertheless, additional research is required to elucidate the underlying biological mechanisms.
6.3 Antioxidant, anti-inflammatory, and analgesic effects
PS serves as a rich source of various antioxidants, including flavonoids, PA, and α-tocopherol (Jing et al., 2012). Flavonoids found in PS neutralize free radicals and have strong DPPH scavenging activity. Notably, the concentration of DPPH significantly decreased with increasing levels of ethanol extract from PS (Al-Huqail et al., 2018). PSO also demonstrates antioxidant activity by scavenging free radicals and DPPH radicals in a dose-dependent manner (Harzallah et al., 2016). This antioxidant capacity is likely attributed to PA, a natural antioxidant known for its potent free radical scavenging activity (Rojo-Gutiérrez et al., 2021). PA has been shown to upregulate the expression of peroxisome proliferator-activated receptor (PPAR), thereby reducing oxidative damage and inflammation (Guerra-Vázquez et al., 2022). However, research by Đurđević et al. (2018) indicates that PSO exhibits strong antioxidant effects but is highly susceptible to oxidative deterioration when exposed to light, moisture, and oxygen. Nevertheless, the application of Oliveria decumbens essential oil (ODEO) has been shown to enhance the oxidative stability of PSO (Golmakani et al., 2021).
Li et al. (2018) conducted an experiment assessing the effects of PSO on aging model mice. They observed enhanced G6PD activity and increased NADPH content in the liver and kidney across all dose groups (75, 250, and 750 mg/kg). Administration of PSO reversed body weight loss, reduced MDA content in the liver, kidney, brain, and serum, increased GSH content, and improved the activities of T-AOC and the antioxidant enzymes SOD and GSH-Px. These findings suggest that PSO exerts significant antagonistic effects against D-galactose-induced oxidative stress in aging model mice, indicating its potential application in the development of anti-aging health foods. Hamouda and Felemban (2023) conducted an in vivo study administering PSO orally to rats at a dose of 250 mL/kg body weight daily for 21 days. Their results demonstrated that PSO reduced levels of collagenase, elastase, hyaluronidase, tyrosinase, cyclooxygenase-2, lipid peroxidation, and nitric oxide in the rats. Subsequently, they conducted a human study involving 60 women with skin problems, such as calluses on the hands, nail inflammation, and extra skin tags around the nails. Each participant received PSO for 21 days (250 mL/kg), confirming the results observed in the rat study. Moreover, incorporation of PSO nanocapsules into Laplacian films facilitated topical delivery of PSO and improved drug bioavailability in the treatment of atopic dermatitis (Ferrari Cervi et al., 2021). Ferreira et al. (2016) performed in vivo experiments with PSO-ketoprofen nanoemulsions on male adult Swiss mice. Mechanical anomalous pain testing using Von Frey Hair revealed that the duration of action of free ketoprofen was up to 6 h, whereas that of the nanoemulsions was up to 10 h, indicating prolonged anti-injury perception effects. Additionally, acute toxicity assessment based on alanine aminotransferase (ALT) and aspartate aminotransferase (AST) activity and urea levels after repeated use of the nanoemulsion over 7 days showed no toxic effects in the animals. The results suggest that the formulation holds promise as an alternative treatment for inflammatory and pain-related disorders such as arthritis.
Mizrahi et al. (2014) developed a nanodroplet formulation of PSO aimed at treating mice with genetic prion disease. Administering Nano-PSO via gavage five times (150 μL/day) to mice, they observed that Nano-PSO delayed the onset and progression of the disease within a shorter timeframe and at a lower dose compared to natural PSO. The treatment reduced lipid oxidation and neuronal loss, demonstrating neuroprotective effects. In another study, PS hydro-ethanolic extract (administered at doses of 200, 400, 800 mg/kg via gavage) was given to scopolamine-induced amnesic rats over a period of 3 weeks. During the third week, scopolamine was administered 30 min before conducting Morris water maze (MWM) and passive avoidance tests. The PS hydro-ethanolic extract notably reduced the time (up to 173%) and distance (up to 332%) required to reach the plateau during MWM learning (p < 0.001). Moreover, it led to a decrease in the expression of TNF-α, IL-1β, and AChE in hippocampal tissues (with maximum reductions of 114%, 137%, and 106%, respectively; p < 0.01). These findings indicated that PS could mitigate scopolamine-induced memory and learning deficits in rats by enhancing the function of the cholinergic system, suppressing oxidative stress, and modulating inflammatory factors (Akbarian et al., 2022).
6.4 Anti-osteoporotic effects
PSO extract is abundant in phytoestrogens and antioxidant metabolites. In a study by Sheng et al. (2016), PSO was administered daily via gavage to de-ovulated rats at doses of 0.95 g/kg and 1.27 g/kg. The results indicated a reduction in serum calcium and phosphorus levels, as well as alkaline phosphatase activity. Conversely, serum SOD levels increased, while malondialdehyde (MDA) levels decreased. Moreover, there was an increase in bone mineral density at various skeletal sites, including the mandible, mid-point of the femur, and distal end of the femur (Sheng et al., 2016). In another study utilizing bilateral de-ovulated female western albino rats as postmenopausal models, PSO n-hexane extract (administered at 500 μL/kg body weight/day) showed promising results in improving bone structure, density, and markers. This extract exhibited therapeutic effects in osteoporosis without adverse effects on lipid levels, uric acid levels, or liver function (Shaban et al., 2017). These findings suggest that PS could serve as a safe, effective, and cost-efficient alternative for preventing and treating osteoporosis in postmenopausal women. Furthermore, administration of 30 mg of pomegranate seed extract (PSE) (approximately 100–120 mg/kg) per day to de-ovulated rats led to significantly elevated levels of estradiol and notable improvements in vaginal atrophy and tibial thickness compared to those fed standard food. The results suggest that PS or its extracts may serve as effective complementary or alternative therapies for preserving bone mineral density and treating vaginal epithelial atrophy during menopause (Kaban et al., 2018).
Zhang et al. (2016) investigated the impact of aqueous extract of PS (AE-PS) on bone loss in glucocorticoid (GC)-induced osteoporosis (GIOP) mice. They administered AE-PS at a dose of 0.1 mg/kg/d for 12 weeks in combination with dexamethasone (DXM) and examined its effects on calcium homeostasis. The results revealed that AE-PS decreased bone loss, effectively curbed urinary calcium loss, and mitigated bone tissue loss in mice. In a subsequent study by Zhang et al. (2019), an in vivo experimental investigation was conducted using pure PSO at doses of 0.95, 1.27, and 1.59 g/kg via gavage in Sprague Dawley (SD) rats categorized into experimental groups 1, 2, and 3. Compared to group 1, groups 2 and 3 exhibited a reduction in calcium content and serum malondialdehyde (MDA) activity, along with an increase in SOD activity. These findings suggested that PSO protects cartilage by enhancing oxygen radical scavenging activity and suppressing oxidative stress. Moreover, it hindered cartilage degeneration in the knee joints of osteoarthritic rats and inhibited the deposition of calcium and phosphorus in the cartilage and subchondral bone, thereby promoting the restoration of chondrocyte elasticity. Liu et al. (2021) administered pure PSO in doses of 0.95 g/kg, 1.27 g/kg, and 1.59 g/kg to rats with osteoarthritis via gavage. They compared these doses with a model group that received 1.59 g/kg of normal saline via gavage. The mRNA expression of matrix metalloproteinase-1 in chondrocytes decreased significantly (p < 0.01), while mRNA expression of type II collagen (CoⅡ) increased significantly (p < 0.01). These results suggested that the mechanism of action of PSO in osteoarthritis may involve inhibiting cartilage matrix-degrading enzymes by down-regulating the mRNA expression of matrix metalloproteinase-1 in articular chondrocytes. This action slows down the breakdown of the articular cartilage matrix, promotes the recovery of cartilage elasticity, and improves cartilage degeneration by up-regulating the mRNA expression of type II collagen (Co II) in chondrocytes.
6.5 Anti-cardiovascular disease effects
Ambigaipalan et al. (2017) demonstrated that PS exhibits significant antioxidant activity, thereby safeguarding the cardiovascular system against free radical-induced damage. It inhibits copper-induced low-density lipoprotein-cholesterol peroxidation, reduces the activity levels of α-glucosidase and lipase, as well as mitigates blood pressure, cholesterol levels, and vascular inflammatory responses. These findings suggest that PS can effectively prevent cardiovascular and cerebrovascular diseases. Additionally, PS confers protective effects against methotrexate-induced alterations in serum oxidative stress (SOD and GPx) and lipids (total cholesterol, high-density lipoproteins, and low-density lipoproteins) in rats (Doostan et al., 2017). The consumption of PSO may enhance cardiovascular health by decreasing plasma total cholesterol and low-density lipoprotein (LDL) levels. Teh et al. (2019) conducted an in vivo experimental study by administering PSO to hamsters, demonstrating its efficacy in lowering plasma triglyceride levels and elevating HDL/LDL ratio. Moreover, Bihamta et al. (2017) revealed that PSO pretreatment at concentrations of 50, 100, and 200 μg/mL increased H9c2 cell viability significantly to 60% ± 2.1% (p < 0.01), 67% ± 2.7% (p < 0.001), 80.25% ± 2% (p < 0.001), and 88% ± 1.9% (p < 0.001), respectively. The findings highlight the protective effect of PSO against oxidative stress-induced cardiomyocyte damage. The protective mechanism involves the reduction of ROS production and lipid peroxidation, indicating the potential of PSO as a natural cardioprotective agent for preventing cardiovascular diseases.
6.6 Anti-obesity effects
PSO supplementation has also shown promising results in mitigating diet-induced obesity and insulin resistance in mice. Vroegrijk et al. (2011) conducted an in vivo experiment where mice were subjected to the same high-fat diet as controls, but with 1 g of fat per 100 g of food replaced by pomegranate seed oil. Over a 12-week high-fat dietary intervention, mice receiving PSO exhibited lower body weights, measuring 30.5 ± 2.9 g compared to 33.8 ± 3.2 g for controls (p = 0.02). This reduction in body weight was primarily attributed to a decrease in body fat mass, measuring 3.3 ± 2.3 g and 6.7 ± 2.7 g, respectively (p = 0.02). Notably, the insulin clamp assay revealed that PSO supplementation did not impact hepatic insulin sensitivity but significantly improved peripheral insulin sensitivity by 164% ± 52% and 92% ± 24%, respectively (p = 0.01). PSO has been found to decrease visceral adipose tissue weight in rodents, associated with enhanced hepatic fatty acid β-oxidation. Furthermore, it combats obesity-associated inflammation and insulin resistance by activating PPARγ receptors (Hontecillas et al., 2009). PSO also regulates body weight gain in high-fat diet-fed mice by up-regulating the expression of the beige adipose tissue-associated gene uncoupling protein 1 (UCP1). This action promotes the formation of white adipose beige-like tissue, thus controlling the size of lipid droplets in adipose tissue cells and influencing abdominal fat mass and the abdominal fat ratio (Guo et al., 2019).
Additionally, a combination of PSO and perilla seed oil has been shown to enhance blood lipids and aid in body weight management in mice (Li et al., 2015). Amri et al. (2017) conducted research on the beneficial effects of PSO with regard to brain cholinesterase activity, brain oxidative stress, and lipid profile in rats subjected to a high-fat, high-fructose diet (HFD), in both in vivo and in vitro experiments. Their findings revealed a dose-dependent inhibitory effect of PSO on cholinesterase activity. In the in vivo study, the HFD regimen induced a significant 17.4% increase in brain cholinesterase activity compared to normal rats. However, treatment with PSO decreased brain cholinesterase activity by 15.48% in HFD rats compared to their untreated counterparts. Furthermore, PSO regulated lipid distribution in the bloodstream and prevented lipid accumulation in both cerebral and somatic tissues relative to in untreated HFD rats. Moreover, administration of the extracts shielded the brain from oxidative stress by reducing malondialdehyde (MDA) and protein carbonylation levels while increasing SOD and glutathione peroxidase (GPx) levels. The results suggest that PSE possesses neuroprotective effects, possibly attributed to cholinesterase inhibition and antioxidant capacity enhancement.
6.7 Anti-microbial effects
Wound infection stands as a pivotal postoperative complication in surgical patients, with the dwindling efficacy of anti-microbial chemicals and antibiotics due to drug resistance. Consequently, the exploration of alternative natural compounds or metabolites for controlling wound infections has garnered significant attention. Recent studies have demonstrated that PSE combined with electrostatically spun poly (vinyl alcohol) (PVA) inhibits the growth of Staphylococcus aureus and expedites wound healing. This PVA nanofiber dressing, infused with an herbal extract possessing antibacterial and antioxidant properties, holds promise as a substitute for antibiotics in managing wound infections (Reisi et al., 2023). Treatment parameters were evaluated through in vivo experiments involving the application of PSO to post-excision wounds in rats once daily for 14 days. The findings indicate that PSO partially aids in treating excision wounds in rats and may be suitable for clinical treatment in humans, albeit requiring large-scale controlled studies (Atsü Md et al., 2023). Furthermore, an effective dental scaffold, crafted with PSE, pomegranate peel extract (PPE), polyvinyl alcohol, and starch using 3D printing technology, has been developed for treating periodontal disease. This scaffold covers the damaged area and contributes to healing. The incorporation of PSE and PPE enhances bacteriostatic activity against S. aureus and Enterococcus faecalis, thereby improving anti-microbial efficacy and demonstrating promising potential for clinical applications (Karabulut et al., 2023).
7 Discussion
This study presents a systematic review of the traditional uses and chemical composition of pomegranate seeds (PS) and the current literature on its pharmacological effects (Figure 4). PS is a by-product of pomegranate processing, and China serves as the primary producer of pomegranates. Currently, China’s utilization of PS remains relatively low, leading to significant waste generation from pomegranate cultivation and processing. PS and PSO, are abundant in fatty acids, flavonoids, sterols, tocopherols, and other active phytochemicals and metabolites. Bioactive compounds such as PA found in PSs exhibit potential to inhibit breast cancer and glioblastoma, reducing the risk of type II diabetes mellitus, and addressing insulin resistance. Understanding the chemical composition of these seeds can facilitate the exploration of their potential applications, leading to the development of valuable products and offering insights for the utilization of other plant seeds. Therefore, it is imperative to explore strategies for the development and effective utilization of the medicinal potential of PS as a valuable resource to enhance its utilization rate. Moreover, several studies have demonstrated the antioxidant, anti-diabetic, anti-obesity, anti-microbial, and anti-inflammatory properties of PS, along with its beneficial effects against cardiovascular diseases, breast cancer, prostate cancer, and osteoporosis. However, despite these findings, the potential of PS in disease prevention and treatment, as well as the molecular targets underlying its pharmacodynamic effects, remain unclear. The unknown relationship between the chemical composition and pharmacological activity of PS hampers its further development and application. To address the gap, there is a need for more in-depth exploration of the relationship between the bioactive compounds and metabolites in PS and their mechanisms of action. This requires extensive in vivo and in vitro research work, as well as clinical analyses, to assess the correlation between the chemical composition of PS and its mechanisms of action in treating various diseases. Although some experimental data support the pharmacological effects of PS, its safety and efficacy are yet to be verified adequately. Consequently, it is essential to gather sufficient safety and efficacy data to support its route of administration, dosage, and duration of administration. Ultimately, verifying the therapeutic efficacy of approved actives in animal models through human trials is crucial for validating their clinical use.
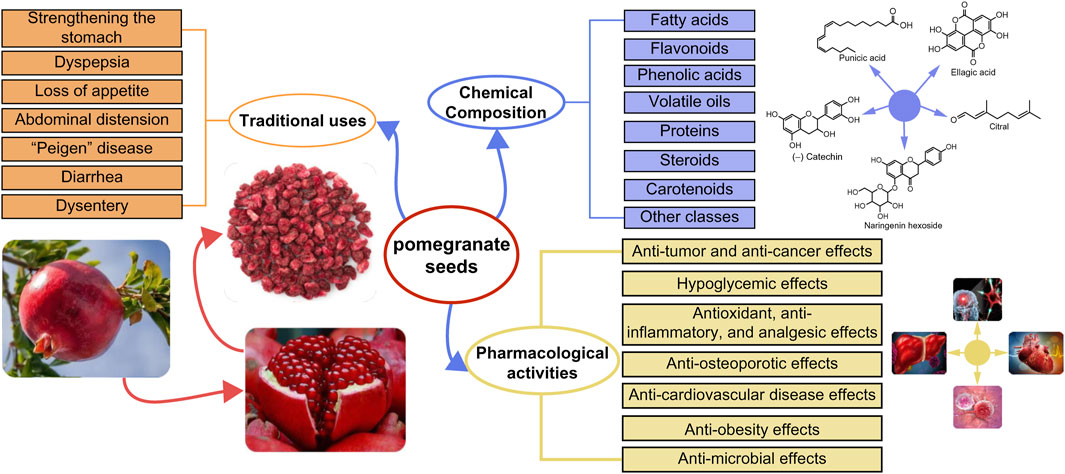
Figure 4. Summary of the traditional uses, chemical composition and pharmacological activities of pomegranate seeds.
Currently, obtaining pure substances for chemical composition analysis of herbal medicines and natural products requires time-consuming and laborious sample extraction and purification processes. Identifying bioactive metabolites from traditional medicinal botanical drugs poses a challenge in developing new medicines. Notably, extracting active metabolites from PS also encounters these obstacles. Additionally, the bioavailability of some active metabolites in PS is limited. Therefore, further research is necessary to enhance both extraction techniques and the bioavailability of active metabolites. Advanced drug delivery systems such as micelles, liposomes, nanoparticles, and nanoemulsions offer promising avenues for improvement. PSO nanoemulsions have demonstrated potent anti-tumor activity by inducing apoptosis in tumor cells through DNA breaks, suggesting their potential as an alternative to chemotherapy (Mohamed et al., 2023). Sahafi et al. (2021) utilized ultrasonic emulsification to create PSO nanoemulsions loaded with alpha-tocopherol, revealing their favorable properties even in harsh environmental conditions. In a study by Petrou et al. (2021), the effects of the brain-targeted PSO nanoformulation GranaGard were investigated in patients with multiple sclerosis (MS). They concluded that GranaGard administration may enhance and stabilize cognitive dysfunction in MS patients, although further confirmation through a larger, randomized clinical trial with longer follow-up is necessary. This finding is particularly significant given the neglected research area of cognitive impairment in MS, where no approved drugs effectively address this issue. Hence, additional research is warranted to enhance both the extraction and bioavailability of active metabolites.
As modern pharmacological research advances, the scientific basis of traditional medicinal efficacy becomes increasingly clear. Traditional Chinese medicine (TCM) is gradually validated by modern science, enriching the development of contemporary medicine with TCM principles and experiences. However, some traditional applications of PS lack support from modern pharmacological research. For example, according to the Jing Zhu Ben Cao, PS is purported to treat stomach diseases, warm the stomach, and address Peigen disease. While traditional PS formulations such as “Jiebai Pills,” “Shi Wei Hei Bing Pian Wan,” and “Shiyiwei Golden Pills” are practically used for gastrointestinal disorders, contemporary pharmacological studies have predominantly explored PS’s efficacy in treating cardiovascular diseases, cancer, and osteoporosis. Notably, there is a lack of pharmacological research on the role of PS in the treatment of gastric diseases. It is suggested that modern pharmacology can be employed to explore the relevant mechanism pathways and pharmacological metabolites of PS in traditional clinically applied formulas. By doing so, we can interpret the principles of TCM with modern science while adhering to TCM’s fundamental principles. This approach facilitates the integration and compatibility of TCM with modern science, which is essential for advancing scientific research and innovation in Chinese medicine. In conclusion, PS represents a sustainable residue that merits development for its utilization. With a long history as a traditional medicine and being a natural remedy, PS holds vast potential for the prevention and treatment of various diseases. Moreover, it serves as a foundational basis for the comprehensive development of PS in the food, nutraceuticals, pharmaceuticals, and cosmetics industries.
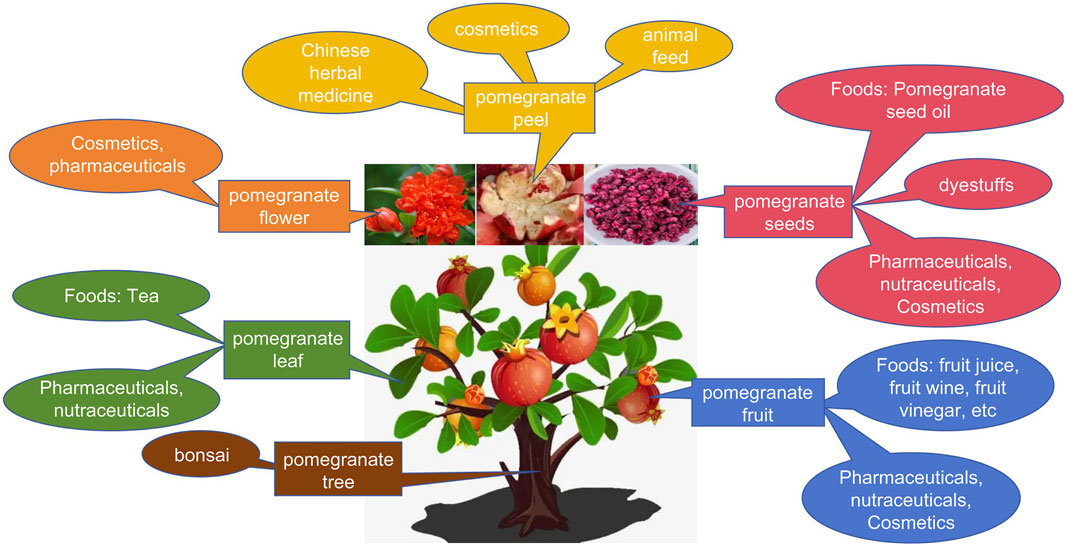
Pomegranate is currently listed in China’s Catalog of Medicinal and Food Supplements, recognized for its dual role as both a medicinal herbal remedy and a health food supplement. Beyond its culinary use, researchers worldwide are increasingly investigating its medicinal properties and applications in the food and nutraceutical industries. Pomegranate fruit is not only edible but also utilized in beverage production (Mohagheghi et al., 2011). Pomegranate fruit extract, known for its tannins and astringent qualities, is utilized in hair color cosmetics as a metabolite. Pomegranate fruit, flowers, and rinds find common application in cosmetics (Liu et al., 2009). Pomegranate peel waste serves as feed for ruminants, while extracts from its flowers are employed for reducing injuries and swelling, and as dyes in cosmetics and textile (Jurenka, 2008). PSEs are also under consideration for wound healing in chitosan dressings (do Nascimento et al., 2020). Furthermore, PSO stands as a functional metabolite with potential applications in the fruit juice and beverage industry. While PSO is commonly utilized as a food metabolite or additive in lubricants, fuels, and paint formulations, seed oils have recently garnered increased attention due to their rich content of hydrophilic and lipophilic bioactive metabolites. These compounds hold substantial potential for nutritional, pharmaceutical, and cosmetic applications (Kalamara et al., 2015). Numerous studies have highlighted the ability of polyunsaturated fatty acids (PUFAs), particularly PA, to inhibit breast cancer and glioblastoma, as well as to reduce the risk of type II diabetes mellitus and insulin resistance. In addition to PA, PS contains a wealth of phenolic metabolites, with ellagic acid being a prominent example. This compound exhibits significant antioxidant, anti-inflammatory, and anti-microbial properties. Supplementing animal feed with ellagic acid has been shown to enhance animal performance, improve meat quality, enhance disease resistance, holding potential for application in the animal husbandry industry (Huang et al., 2024). Additionally, PSO contains phytosterols, notably β-sitosterol. Owing to its chemical composition resembling that of cholesterol, β-sitosterol finds applications across diverse fields including medicine, agriculture, and chemical industries, owing to its unique biological and physicochemical properties (Bao et al., 2022). Furthermore, PSO contains high levels of tocopherols, which are potent antioxidants that protect fats and oils from degradation and mitigate oxidative stress in the body after consumption (Barouh et al., 2022). Owing to their robust antioxidant properties, tocopherols have been suggested to reduce the risk of cancer (Das Gupta and Suh, 2016). In addition, PSO contains high levels of triterpenes, particularly squalene, a well-known dietary supplement for reducing cholesterol and triglyceride levels. Although squalene is primarily extracted from fish, it can also be found in oil-rich fruits and grains, making it crucial in vegan diets as a substitute for animal-derived sources (Caligiani et al., 2010).
Understanding the chemical composition of pomegranate seeds holds promise for their potential applications in developing valuable products and inspiring the utilization of other seeds. Our study outlines the comprehensive development of pomegranate resources, as illustrated in Figure 5. Ancillary industries related to pomegranates continue to emerge, substantially enhancing the economic benefits of the pomegranate industry and extending its value chain. This research serves as a model for developing the pomegranate industry chain, offering guidance and insights to enhance its competitiveness and ensure sustainable growth.
Author contributions
JW: Conceptualization, Writing–original draft, Writing–review and editing. MS: Writing–original draft. JY: Writing–original draft. JW: Writing–review and editing. QC: Writing–review and editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study was supported by the Key Research and Development Program of Shandong Province, China [grant no. 2022TZXD0019]; the Doctoral Research Initiation Fund of Zaozhuang University [grant no. 1020712]; and the Science and Technology Project of Zaozhuang [grant no. 2019GX18].
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2024.1401826/full#supplementary-material
References
Akbarian, M., Mirzavi, F., Amirahmadi, S., Hosseini, M., Alipour, M., Feizi, H., et al. (2022). Amelioration of oxidative stress, cholinergic dysfunction, and neuroinflammation in scopolamine-induced amnesic rats fed with pomegranate seed. Inflammopharmacology 30, 1021–1035. doi:10.1007/s10787-022-00971-7
Al-Huqail, A. A., Elgaaly, G. A., and Ibrahim, M. M. (2018). Identification of bioactive phytochemical from two Punica species using GC-MS and estimation of antioxidant activity of seed extracts. Saudi J. Biol. Sci. 25, 1420–1428. doi:10.1016/j.sjbs.2015.11.009
Ambigaipalan, P., De Camargo, A. C., and Shahidi, F. (2017). Identification of phenolic antioxidants and bioactives of pomegranate seeds following juice extraction using HPLC-DAD-ESI-MSn. Food Chem. 221, 1883–1894. doi:10.1016/j.foodchem.2016.10.058
Amri, Z., Ghorbel, A., Turki, M., Akrout, F. M., Ayadi, F., Elfeki, A., et al. (2017). Effect of pomegranate extracts on brain antioxidant markers and cholinesterase activity in high fat-high fructose diet induced obesity in rat model. BMC Complement. Altern. Med. 17, 339. doi:10.1186/s12906-017-1842-9
Aruna, P., Venkataramanamma, D., Singh, A. K., and Singh, R. P. (2016). Health benefits of punicic acid: a review. Compr. Rev. Food Sci. Food Saf. 15, 16–27. doi:10.1111/1541-4337.12171
Atsü Md, A. N., Tosuner Md, Z., and Bilgiç Md, T. (2023). Evaluation of the effect of pomegranate seed oil on healing in a rat wound model with antioxidant, vascular, and histopathological parameters. Int. J. Low. Extrem. Wounds. 22, 661–671. doi:10.1177/15347346211040593
Bañares, C., Carballeda-Sangiao, N., Chabni, A., García-Cordero, J., Reglero, G., de Pascual-Teresa, S., et al. (2023). Anti-inflammatory effect of two pomegranate seed oils obtained by green technologies in Caco-2 cells using the bioaccessible fraction from in vitro gastrointestinal digestion. Food Res. Int. 165, 112475. doi:10.1016/j.foodres.2023.112475
Bao, X., Zhang, Y., Zhang, H., and Xia, L. (2022). Molecular mechanism of β-sitosterol and its derivatives in tumor progression. Front. Oncol. 12, 926975. doi:10.3389/fonc.2022.926975
Barouh, N., Bourlieu-Lacanal, C., Figueroa-Espinoza, M. C., Durand, E., and Villeneuve, P. (2022). Tocopherols as antioxidants in lipid-based systems: the combination of chemical and physicochemical interactions determines their efficiency. Compr. Rev. Food Sci. Food Saf. 21 (1), 642–688. doi:10.1111/1541-4337.12867
Bar-Ya’akov, I., Tian, L., Amir, R., and Holland, D. (2019). Primary metabolites, anthocyanins, and hydrolyzable tannins in the pomegranate fruit. Front. Plant Sci. 10, 620. doi:10.3389/fpls.2019.00620
Bihamta, M., Hosseini, A., Ghorbani, A., and Boroushaki, M. T. (2017). Protective effect of pomegranate seed oil against H2O2-induced oxidative stress in cardiomyocytes. Avicenna J. Phytomed. 7, 46–53. doi:10.22038/AJP.2016.6919
Caligiani, A., Bonzanini, F., Palla, G., Cirlini, M., and Bruni, R. (2010). Characterization of a potential nutraceutical ingredient: pomegranate (Punica granatum L.) seed oil unsaponifiable fraction. Plant Foods Hum. Nutr. 65, 277–283. doi:10.1007/s11130-010-0173-5
Chen, H. X., Zang, Y. L., Lin, S. Q., Lvu, X. D., Cui, H. J., Hou, L., et al. (2023a). Preparation and quality evaluation of pomegranate seed oil soy isoflavones soft capsules. Hubei Agric. Sci. 62, 108–112. doi:10.14088/j.cnki.issn0439-8114.2023.09.020
Costantini, S., Rusolo, F., De Vito, V., Moccia, S., Picariello, G., Capone, F., et al. (2014). Potential anti-inflammatory effects of the hydrophilic fraction of pomegranate (Punica granatum L.) seed oil on breast cancer cell lines. Molecules 19, 8644–8660. doi:10.3390/molecules19068644
Das Gupta, S., and Suh, N. (2016). Tocopherols in cancer: an update. Mol. Nutr. Food Res. 60 (6), 1354–1363. doi:10.1002/mnfr.201500847
do Nascimento, M. F., Cardoso, J. C., Santos, T. S., Tavares, L. A., Pashirova, T. N., Severino, P., et al. (2020). Development and characterization of biointeractive gelatin wound dressing based on extract of Punica granatum Linn. Pharmaceutics 12, 1204. doi:10.3390/pharmaceutics12121204
Doostan, F., Vafafar, R., Zakeri-Milani, P., Pouri, A., Amini Afshar, R., and Mesgari Abbasi, M. (2017). Effects of pomegranate (Punica granatum L.) seed and peel methanolic extracts on oxidative stress and lipid profile changes induced by methotrexate in rats. Adv. Pharm. Bull. 7, 269–274. doi:10.15171/apb.2017.032
Đurđević, S., Šavikin, K., Živković, J., Böhm, V., Stanojković, T., Damjanović, A., et al. (2018). Antioxidant and cytotoxic activity of fatty oil isolated by supercritical fluid extraction from microwave pretreated seeds of wild growing Punica granatum L. J. Supercrit. Fluids. 133, 225–232. doi:10.1016/j.supflu.2017.10.021
Editorial Board of Chinese Materia Medica of National Administration of Traditional Chinese Medicine (2005). Chinese Materia Medica: Uyghur medicinal roll. Shanghai: Shanghai Scientific & Technical Publishers, 118–119.
Eikani, M. H., Golmohammad, F., and Homami, S. S. (2012). Extraction of pomegranate (Punica granatum L.) seed oil using superheated hexane. Food Bioprod. process. 90, 32–36. doi:10.1016/j.fbp.2011.01.002
Elfalleh, W., Hannachi, H., Guetat, A., Tlili, N., Guasmi, F., Ferchichi, A., et al. (2012). Storage protein and amino acid contents of Tunisian and Chinese pomegranate (Punica granatum L.) cultivars. Genet. Resour. Crop Evol. 59, 999–1014. doi:10.1007/s10722-011-9739-9
Esther Lydia, D. E., Khusro, A., Immanuel, P., Esmail, G. A., Al-Dhabi, N. A., and Arasu, M. V. (2020). Photo-activated synthesis and characterization of gold nanoparticles from Punica granatum L. seed oil: an assessment on antioxidant and anticancer properties for functional yoghurt nutraceuticals. J. Photochem. Photobiol. B 206, 111868. doi:10.1016/j.jphotobiol.2020.111868
Ferrari Cervi, V., Parcianello Saccol, C., Henrique Marcondes Sari, M., Cristóvão Martins, C., Saldanha da Rosa, L., Dias Ilha, B., et al. (2021). Pullulan film incorporated with nanocapsules improves pomegranate seed oil anti-inflammatory and antioxidant effects in the treatment of atopic dermatitis in mice. Int. J. Pharm. 609, 121144. doi:10.1016/j.ijpharm.2021.121144
Ferreira, L. M., Cervi, V. F., Gehrcke, M., da Silveira, E. F., Azambuja, J. H., Braganhol, E., et al. (2015). Ketoprofen-loaded pomegranate seed oil nanoemulsion stabilized by pullulan: selective antiglioma formulation for intravenous administration. Colloids Surf. B Biointerfaces. 130, 272–277. doi:10.1016/j.colsurfb.2015.04.023
Ferreira, L. M., Sari, M. H. M., Cervi, V. F., Gehrcke, M., Barbieri, A. V., Zborowski, V. A., et al. (2016). Pomegranate seed oil nanoemulsions improve the photostability and in vivo antinociceptive effect of a non-steroidal anti-inflammatory drug. Colloids Surf. B Biointerfaces. 144, 214–221. doi:10.1016/j.colsurfb.2016.04.008
Fourati, M., Smaoui, S., Hlima, H. B., Elhadef, K., Braïek, O. B., Ennouri, K., et al. (2020). Bioactive compounds and pharmacological potential of pomegranate (Punica granatum) seeds – a review. Plant Foods Hum. Nutr. 75, 477–486. doi:10.1007/s11130-020-00863-7
Franczyk-Żarów, M., Tarko, T., Drahun-Misztal, A., Czyzynska-Cichon, I., Kus, E., and Kostogrys, R. B. (2023). Pomegranate seed oil as a source of conjugated linolenic acid (CLnA) has no effect on atherosclerosis development but improves lipid profile and affects the expression of lipid metabolism genes in apoE/LDLR−/− mice. Int. J. Mol. Sci. 24, 1737. doi:10.3390/ijms24021737
Fu, G. Q., Liu, L., Zhang, L., Gao, Y., Xv, X. N., Xie, F., et al. (2015). Punica granatum seed oil inhibits malignant behavior of breast cancer cells. Mil. Med. Sci. 39, 438–442.
Gao, L., Zhang, L., Liu, J., Zhang, X., and Lu, Y. (2023). Analysis of the volatile flavor compounds of pomegranate seeds at different processing temperatures by GC-IMS. Molecules 28, 2717. doi:10.3390/molecules28062717
Ge, S., Duo, L., Wang, J., GegenZhula, Y., Yang, J., Li, Z., et al. (2021). A unique understanding of traditional medicine of pomegranate, Punica granatum L. and its current research status. J. Ethnopharmacol. 271, 113877. doi:10.1016/j.jep.2021.113877
Golmakani, M. T., Mansouri, Z., Ansari, S., and Alavi, N. (2021). Improving oxidative stability of pomegranate seed oil using Oliveria decumbens essential oil. J. Food Process. Preserv. 45, e15483. doi:10.1111/jfpp.15483
Górnaś, P., and Rudzińska, M. (2016). Seeds recovered from industry by-products of nine fruit species with a high potential utility as a source of unconventional oil for biodiesel and cosmetic and pharmaceutical sectors. Ind. Crops Prod. 83, 329–338. doi:10.1016/j.indcrop.2016.01.021
Guerra-Vázquez, C. M., Martínez-Ávila, M., Guajardo-Flores, D., and Antunes-Ricardo, M. (2022). Punicic acid and its role in the prevention of neurological disorders: a review. Foods 11, 252. doi:10.3390/foods11030252
Guerrero-Solano, J. A., Jaramillo-Morales, O. A., Jiménez-Cabrera, T., Urrutia-Hernández, T. A., Chehue-Romero, A., Olvera-Hernández, E. G., et al. (2020). Punica protopunica balf., the forgotten sister of the common pomegranate (Punica granatum L.): features and medicinal properties-A review. Plants (Basel). 9, 1214. doi:10.3390/plants9091214
Guo, Q., Yv, Z. Y., Chen, J., Wang, H., and Wang, J. H. (2019). Effects of pomegranate seed oil on body weight and UCP1 expression of mice fed with high fat diet. J. Dalian Polytech. Univ. 38, 244–247.
Guzmán-Lorite, M., Marina, M. L., and García, M. C. (2022a). Pressurized liquids vs. high intensity focused ultrasounds for the extraction of proteins from a pomegranate seed waste. Innov. Food Sci. Emerg. Technol. 77, 102958. doi:10.1016/j.ifset.2022.102958
Guzmán-Lorite, M., Marina, M. L., and García, M. C. (2022b). Successive extraction using natural deep eutectic solvents and pressurized liquids for a greener and holistic recovery of proteins from pomegranate seeds. Food Res. Int. 161, 111862. doi:10.1016/j.foodres.2022.111862
Hamouda, A. F., and Felemban, S. (2023). Biochemical pilot study on effects of pomegranate seed oil extract and cosmetic Cream on neurologically mediated skin inflammation in animals and humans: a comparative observational study. Molecules 28, 903. doi:10.3390/molecules28020903
Harzallah, A., Hammami, M., Kępczyńska, M. A., Hislop, D. C., Arch, J. R. S., Cawthorne, M. A., et al. (2016). Comparison of potential preventive effects of pomegranate flower, peel and seed oil on insulin resistance and inflammation in high-fat and high-sucrose diet-induced obesity mice model. Arch. Physiol. Biochem. 122, 75–87. doi:10.3109/13813455.2016.1148053
Hernández-Corroto, E., Boussetta, N., Marina, M. L., García, M. C., and Vorobiev, E. (2022). High voltage electrical discharges followed by deep eutectic solvents extraction for the valorization of pomegranate seeds (Punica granatum L). Innov. Food Sci. Emerg. Technol. 79, 103055. doi:10.1016/j.ifset.2022.103055
Hontecillas, R., O'Shea, M., Einerhand, A., Diguardo, M., and Bassaganya-Riera, J. (2009). Activation of PPAR gamma and alpha by punicic acid ameliorates glucose tolerance and suppresses obesity-related inflammation. J. Am. Coll. Nutr. 28 (2), 184–195. doi:10.1080/07315724.2009.10719770
Huang, Y. C., Cao, J., and Dai, C. S. (2024). Biological functions of ellagic acid and its application in animal production. China Food (01), 12–16. doi:10.15906/j.cnki.cn11-2975/s.2023010015-12
Huo, S. X., Kang, Y. T., Zhang, S. S., Wusiman, Z., Aibai, S., Li, Z., et al. (2023). Pharmacological effects and metabonomics of Shiliuzi compound capsules on ulcerative colitis. Pharmacol. Clin. Chin. Mat. Med. 39, 36–43.
Iriti, G., Bonacci, S., Lopreiato, V., Frisina, M., Oliverio, M., and Procopio, A. (2023). Functional compounds of cold-pressed pomegranate seed oil: fatty acids and phytosterols profile as quality biomarkers for origin discrimination. Foods 12, 2599. doi:10.3390/foods12132599
Jing, P., Ye, T., Shi, H., Sheng, Y., Slavin, M., Gao, B., et al. (2012). Antioxidant properties and phytochemical composition of China-grown pomegranate seeds. Food Chem. 132, 1457–1464. doi:10.1016/j.foodchem.2011.12.002
Jurenka, J. S. (2008). Therapeutic applications of pomegranate (Punica granatum L.): a review. Altern. Med. Rev. 13, 128–144. doi:10.1136/aim.26.2.121
Kaban, I., Kaban, A., Tunca, A. F., Aka, N., Kavak, H., and Akar, F. (2018). Effect of pomegranate extract on vagina, skeleton, metabolic and endocrine profiles in an ovariectomized rat model. J. Obstet. Gynaecol. Res. 44, 1087–1091. doi:10.1111/jog.13642
Kalamara, E., Goula, A. M., and Adamopoulos, K. G. (2015). An integrated process for utilization of pomegranate wastes—seeds. Innov. Food Sci. Emerg. Technol. 27, 144–153. doi:10.1016/j.ifset.2014.12.001
Karabulut, H., Ulag, S., Dalbayrak, B., Arisan, E. D., Taskin, T., Guncu, M. M., et al. (2023). A novel approach for the fabrication of 3D-printed dental membrane scaffolds including antimicrobial pomegranate extract. Pharmaceutics 15, 737. doi:10.3390/pharmaceutics15030737
Kaseke, T., Opara, U. L., and Fawole, O. A. (2021). Quality and antioxidant properties of cold-pressed oil from blanched and microwave-pretreated pomegranate seed. Foods 10, 712. doi:10.3390/foods10040712
Khajebishak, Y., Payahoo, L., Alivand, M., and Alipour, B. (2019a). Punicic acid: a potential compound of pomegranate seed oil in type 2 diabetes mellitus management. J. Cell. Physiol. 234, 2112–2120. doi:10.1002/jcp.27556
Khajebishak, Y., Payahoo, L., Alivand, M., Hamishehkar, H., Mobasseri, M., Ebrahimzadeh, V., et al. (2019b). Effect of pomegranate seed oil supplementation on the GLUT-4 gene expression and glycemic control in obese people with type 2 diabetes: a randomized controlled clinical trial. J. Cell. Physiol. 234, 19621–19628. doi:10.1002/jcp.28561
Khoddami, A., Man, Y. B. C., and Roberts, T. H. (2014). Physico-chemical properties and fatty acid profile of seed oils from pomegranate (Punica granatum L.) extracted by cold pressing. Eur. J. Lipid Sci. Technol. 116, 553–562. doi:10.1002/ejlt.201300416
Kong, X. W., Luo, M., Jv, Y. K., Xv, J. H., Zheng, Z., Xiong, H., et al. (2021). Exploring the mechanism of action of pomegranate stomach-enhancing pills in the treatment of gastroenteritis based on network pharmacology. Mod. Traditional Chin. Med. Materia Medica-World Sci. Technol. 23, 1415–1427.
Kori, A. H., Mahesar, S. A., Sherazi, S. T. H., Laghari, Z. H., and Panhwar, T. (2020). A review on techniques employed for encapsulation of the bioactive components of Punicagranatum L. J. Food Process. Preserv. 44 (11), e14848. doi:10.1111/jfpp.14848
Li, G., Chen, J., Yang, Q., Yang, X., Wang, P., Lei, H., et al. (2023). Identification of chemical constituents in pomegranate seeds based on ultra-high-performance supercritical fluid chromatography coupled with quadrupole time-of-flight mass spectrometry. Rapid Commun. Mass Spectrom. 37 (Suppl. 1), e9482. doi:10.1002/rcm.9482
Li, G., Chen, M., Chen, J., Shang, Y., Lian, X., Wang, P., et al. (2020). Chemical composition analysis of pomegranate seeds based on ultra-high-performance liquid chromatography coupled with quadrupole-Orbitrap high-resolution mass spectrometry. J. Pharm. Biomed. Anal. 187, 113357. doi:10.1016/j.jpba.2020.113357
Li, W., Hao, J., Zhang, L., Huang, X., Dang, X. K., and Shu, G. W. (2018). Antioxidant activity of Punica granatum seed oil on aging model mice induced by D-galactose. China Oils Fats 43, 55–59.
Li, Y., Gao, Y., and Li, W. M. (2015). Blood lipid regulating effect of compound pomegranate seed oil and perilla seed oil extract in diabetic C57BL/6J mice. Chin. J. New Drugs. 24, 2231–2234.
Liu, G., Xu, X., Hao, Q., and Gao, Y. (2009). Supercritical CO2 extraction optimization of pomegranate (Punica granatum l.) seed oil using response surface methodology. LWT Food Sci. Technol. 42, 1491–1495. doi:10.1016/j.lwt.2009.04.011
Liu, X. W., Zhang, T., Shao, G., Ma, Z. P., and Wang, R. J. (2021). Effects of pomegranate seed oil on pathological morphology and mRNA levels of metabolic factors of knee cartilage in rats with osteoarthritis. Xv, W.S. J. Baotou Med. Coll. 37, 53–57.
Mahmoudi, R., Servatkhah, M., Fallahzadeh, A. R., Abidi, H., Shirazi, H. R. G., Delaviz, H., et al. (2017). Pomegranate seed oil shows inhibitory effect on invasion of human breast cancer cell lines. J. Clin. Diagn. Res. 11, 5–10. doi:10.7860/JCDR/2017/25963.10847
Maphetu, N., Unuofin, J. O., Masuku, N. P., Olisah, C., and Lebelo, S. L. (2022). Medicinal uses, pharmacological activities, phytochemistry, and the molecular mechanisms of Punica granatum L. (pomegranate) plant extracts: a review. Biomed. Pharmacother. 153, 113256. doi:10.1016/j.biopha.2022.113256
Marsoul, A., Ijjaali, M., Elhajjaji, F., Taleb, M., Salim, R., and Boukir, A. (2020). Phytochemical screening, total phenolic and flavonoid methanolic extract of pomegranate bark (Punica granatum L): evaluation of the inhibitory effect in acidic medium 1 M HCl. Mat. Today Proc. 27, 3193–3198. doi:10.1016/j.matpr.2020.04.202
Mete, M., Ünsal, Ü. Ü., Aydemir, I., Sönmez, P. K., and Tuglu, M. I. (2019). Punicic acid inhibits glioblastoma migration and proliferation via the PI3K/AKT1/mTOR signaling pathway. Anti Cancer Agents Med. Chem. 19, 1120–1131. doi:10.2174/1871520619666190405112507
Min, Y., Wang, H., Zhang, W., and Liu, W. (2010). Analysis on microelement contents and volatile oil compositions in pomegranate seeds. Anhui Agric. Sci. 38, 15096–15097.
Mizrahi, M., Friedman-Levi, Y., Larush, L., Frid, K., Binyamin, O., Dori, D., et al. (2014). Pomegranate seed oil nanoemulsions for the prevention and treatment of neurodegenerative diseases: the case of genetic CJD. Nanomedicine 10, 1353–1363. doi:10.1016/j.nano.2014.03.015
Mohagheghi, M., Rezaei, K., Labbafi, M., and Ebrahimzadeh Mousavi, S. M. (2011). Pomegranate seed oil as a functional ingredient in beverages. Eur. J. Lipid Sci. Technol. 113, 730–736. doi:10.1002/ejlt.201000334
Mohamed, H. R. H., Tulbah, F. S. A., El-Ghor, A. A., and Eissa, S. M. (2023). Suppression of tumor growth and apoptosis induction by pomegranate seed nano-emulsion in mice bearing solid Ehrlich carcinoma cells. Sci. Rep. 13, 5525. doi:10.1038/s41598-023-32488-6
Nasr, M., Naeem, S. A., El-Shenbaby, I., Mohamed, F. M. A., Mahmoud, S. M., Abuamara, T. M. M., et al. (2023). Pomegranate seeds and peel ethanolic extracts anticancer potentials and related genetic, histological, immunohistochemical, apoptotic and oxidative stress profiles: in vitro study. J. Exp. Pharmacol. 15, 191–205. doi:10.2147/JEP.S404321
Petrou, P., Ginzberg, A., Binyamin, O., and Karussis, D. (2021). Beneficial effects of a nanoformulation of pomegranate seed oil, GranaGard, on the cognitive function of multiple sclerosis patients. Mult. Scler. Relat. Disord. 54, 103103. doi:10.1016/j.msard.2021.103103
Ranjha, M. M. A. N., Kanwal, R., Shafique, B., Arshad, R. N., Irfan, S., Kieliszek, M., et al. (2021). A critical review on pulsed electric field: a novel technology for the extraction of phytoconstituents. Molecules 26, 4893. doi:10.3390/molecules26164893
Reisi, F., Kafshdouzan, K., Moslemi, H. R., Nourbakhsh, M. S., and Khaligh, S. G. (2023). The electrospun fibrous membrane containing pomegranate seed extract/polyvinyl alcohol improves infectious wound healing in Wistar rats. Fibers Polym. 24, 1225–1235. doi:10.1007/s12221-023-00076-0
Rojo-Gutiérrez, E., Carrasco-Molinar, O., Tirado-Gallegos, J. M., Levario-Gómez, A., Chávez-González, M. L., Baeza-Jiménez, R., et al. (2021). Evaluation of green extraction processes, lipid composition and antioxidant activity of pomegranate seed oil. Food Meas. 15, 2098–2107. doi:10.1007/s11694-020-00804-7
Sahafi, S. M., Goli, S. A. H., Kadivar, M., Varshosaz, J., and Shirvani, A. (2021). Pomegranate seed oil nanoemulsion enriched by α-tocopherol; the effect of environmental stresses and long-term storage on its physicochemical properties and oxidation stability. Food Chem. 345, 128759. doi:10.1016/j.foodchem.2020.128759
Sarkhosh, A., Yavari, A. M., and Zamani, Z. (Editors) (2020). The pomegranate: botany, production and uses. Boston, MA: CABI Publishing.
Shaban, N. Z., Talaat, I. M., Elrashidy, F. H., Hegazy, A. Y., and Sultan, A. S. (2017). Therapeutic role of Punica granatum (pomegranate) seed oil extract on bone turnover and resorption induced in ovariectomized rats. J. Nutr. Health Aging 21, 1299–1306. doi:10.1007/s12603-017-0884-5
Sheng, X. W., Tao, Z., Lei, K. W., Jun, C. Y., and Hua, Y. Z. (2016). Effect of pomegranate seed oil on bone metabolism in ovariectomized rats. Chin. J. Gerontol. 36, 4956–4957.
Siano, F., Straccia, M. C., Paolucci, M., Fasulo, G., Boscaino, F., and Volpe, M. G. (2016). Physico-chemical properties and fatty acid composition of pomegranate, cherry and pumpkin seed oils. J. Sci. Food Agric. 96, 1730–1735. doi:10.1002/jsfa.7279
Sreeja, S., Santhosh Kumar, T. R. S., Lakshmi, B. S., and Sreeja, S. (2012). Pomegranate extract demonstrate a selective estrogen receptor modulator profile in human tumor cell lines and in vivo models of estrogen deprivation. J. Nutr. Biochem. 23, 725–732. doi:10.1016/j.jnutbio.2011.03.015
State Administration of Traditional Chinese Medicine (2002). Tibetan medicine. Shanghai: Shanghai Scientific & Technical Publishers, 128–306.
Stover, E. D., and Mercure, E. W. (2007). The pomegranate: a new look at the fruit of paradise. Hortscience 42, 1088–1092. doi:10.21273/HORTSCI.42.5.1088
Talekar, S., Patti, A. F., Singh, R., Vijayraghavan, R., and Arora, A. (2018). From waste to wealth: high recovery of nutraceuticals from pomegranate seed waste using a green extraction process. Ind. Crops Prod. 112, 790–802. doi:10.1016/j.indcrop.2017.12.023
Teh, H. E., Yokoyama, W. H., German, J. B., McHugh, T. H., and Pan, Z. (2019). Hypocholesterolemic effects of expeller-pressed and solvent-extracted fruit seed oils and defatted pomegranate seed meals. J. Agric. Food Chem. 67, 6150–6159. doi:10.1021/acs.jafc.8b07186
Verardo, V., Garcia-Salas, P., Baldi, E., Segura-Carretero, A., Fernandez-Gutierrez, A., and Caboni, M. F. (2014). Pomegranate seeds as a source of nutraceutical oil naturally rich in bioactive lipids. Food Res. Int. 65, 445–452. doi:10.1016/j.foodres.2014.04.044
Vroegrijk, I. O. C. M., Van Diepen, J. A., Van Den Berg, S., Westbroek, I., Keizer, H., Gambelli, L., et al. (2011). Pomegranate seed oil, a rich source of punicic acid, prevents diet-induced obesity and insulin resistance in mice. Food Chem. Toxicol. 49, 1426–1430. doi:10.1016/j.fct.2011.03.037
Wang, Z. Y., Zhang, L. H., Wang, Y. H., Dai, B., Wang, F., and Wang, J. L. (2018). Microwave-assisted ionic liquid extraction of total flavonoids from fermented pomegranate seeds and their inhibition of nitrosation reaction. Chin. Trad. Pat. Med. 40, 213–218.
Wong, T. L., Strandberg, K. R., Croley, C. R., Fraser, S. E., Nagulapalli Venkata, K. C. N., Fimognari, C., et al. (2021). Pomegranate bioactive constituents target multiple oncogenic and oncosuppressive signaling for cancer prevention and intervention. Semin. Cancer Biol. 73, 265–293. doi:10.1016/j.semcancer.2021.01.006
Wu, X., Li, Y., Wang, Q., Li, W., and Feng, Y. (2015b). Effects of berberine and pomegranate seed oil on plasma phospholipid metabolites associated with risks of type 2 diabetes mellitus by U-HPLC/Q-TOF-MS. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 1007, 110–120. doi:10.1016/j.jchromb.2015.11.008
Yin, P. L., Guan, M., Hou, L. Q., Du, W. J., and Liu, C. (2011). Determination of trace elements in pomegranate seeds by flame atomic absorption spectrometry. Phys. Test. Chem. Analysis 47, 360–361.
Yisimayili, Z., and Chao, Z. (2022). A review on phytochemicals, metabolic profiles and pharmacokinetics studies of the different parts (juice, seeds, peel, flowers, leaves and bark) of pomegranate (Punica granatum L.). Food Chem. 395, 133600. doi:10.1016/j.foodchem.2022.133600
Yogesh, H. S., Kuppasamy, S., Buripad, G., and Hallu, R. L. S. (2020). Evaluation of antiosteoporosis activity of ethanolic extract of Punica granatum Linn. seeds in ovariectomized-induced osteoporosis rats. Int. J. Green Pharm. 14, 66–72. doi:10.22377/IJGP.V14I1.2776
Zhang, T., Xv, W. S., Liu, X. W., Wang, Z., Zheng, L. N., Zhang, W. S., et al. (2019). Effects and significance of pomegranate seed oil on chondral and subchondral bone elements and serum oxidative stress indicators in osteoarthritis rats. Mod. Med. J. 47, 1476–1481.
Zhang, Y., Shao, J., Wang, Z., Yang, T., Liu, S., Liu, Y., et al. (2016). Aqueous extract of pomegranate seed attenuates glucocorticoid-induced bone loss and hypercalciuria in mice: a comparative study with alendronate. Int. J. Mol. Med. 38, 491–498. doi:10.3892/ijmm.2016.2622
Keywords: pomegranate seeds, chemical composition, unsaturated fatty acids, pharmacological effects, anti-tumor activity
Citation: Wang J, Sun M, Yu J, Wang J and Cui Q (2024) Pomegranate seeds: a comprehensive review of traditional uses, chemical composition, and pharmacological properties. Front. Pharmacol. 15:1401826. doi: 10.3389/fphar.2024.1401826
Received: 16 March 2024; Accepted: 24 June 2024;
Published: 11 July 2024.
Edited by:
Karim Hosni, Institut National de Recherche et d’Analyse Physico-Chimique (INRAP), TunisiaReviewed by:
Mariana Martinez-Avila, Monterrey Institute of Technology and Higher Education (ITESM), MexicoBojana B. Vidovic, University of Belgrade, Serbia
Copyright © 2024 Wang, Sun, Yu, Wang and Cui. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jian Wang, aHdhbmdqaWFuMDhAMTYzLmNvbQ==
 Jian Wang
Jian Wang Mengjie Sun1
Mengjie Sun1 Jinglong Wang
Jinglong Wang