
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Pharmacol. , 18 July 2024
Sec. Pharmacology of Anti-Cancer Drugs
Volume 15 - 2024 | https://doi.org/10.3389/fphar.2024.1401017
Background: The incidence of uveitis has risen with the use of targeted therapies, particularly prevalent in the administration of immune checkpoint inhibitors and MAP-kinase pathway inhibitors. We report the first case of VKH-like uveitis linked to Donafenib employed for the primary hepatocellular carcinoma, highlighting the necessity of ophthalmological follow-up in patients undergoing treatment with Donafenib.
Case presentation: A 55-year-old man developed VKH-like symptoms, including sporadic white patches, tinnitus, headache, and mild bilateral vision reduction, after 18 months of treatment with Donafenib and Sintilimab for hepatocellular carcinoma. Based on ophthalmological examinations that fundus fluorescein angiography images demonstrating multiple focal areas of pinpoint hyperfluorescence, along with pooling indicative of neurosensory detachment and disc leakage in both eyes, choroid thickening in swept-source optical coherence tomography, and “sunset-glow” fundus appearance, a tentative diagnosis of VKH-like uveitis was made. Initially, his best-corrected visual acuity (BCVA) was 20/200 in the right eye and 20/80 in the left eye. Upon discontinuing Donafenib and starting a 3-month course of oral glucocorticoids, his BCVA improved to 20/30 in the right eye and 20/40 in the left eye.
Conclusion: Targeted drugs have been commonly used for cancer treatment in recent years, but challenges of ocular side effects emerged gradually. To optimize patient outcomes, regular ophthalmological follow-ups are essential for those undergoing treatment with targeted therapies like Donafenib.
Immune checkpoint inhibitors (ICIs) and MAP-kinase pathway inhibitors (MAPKis) are primarily responsible for the high occurrence of drug-induced uveitis (DIU) (Thibault et al., 2024). ICIs have been identified as causative agents for several ocular conditions, including dry eye, conjunctivitis, uveitis, scleritis and choroidal retinitis (Liu et al., 2020; Fortes et al., 2021). Ocular side effects associated with MAPKis primarily affect the retina and include chorioretinopathy and serous retinal detachment, leading the symptoms like blurred vision, halos around lights (Jeng-Miller et al., 2024; Thibault et al., 2024). Additionally, reports indicate that MAPKis cause ocular adverse effects more frequently than ICIs (Sada et al., 2023).
Donafenib, a novel small molecule multi-kinase inhibitor, targets multiple receptors, including the vascular endothelial growth factor receptor, platelet-derived growth factor receptor, various Raf kinases, to exert its anti-tumor effects (Li et al., 2020; ECoLCoCSoC, 2022). Previous studies have identified adverse events associated with Donafenib, including hand-foot skin reactions, diarrhea, elevated aspartate aminotransferase levels, and decreased white blood cells counts (Qin et al., 2021). However, reports on adverse ocular manifestations of Donafenib are not yet comprehensive.
Vogt-Koyanagi-Harada (VKH), characterized as bilateral granulomatous panuveitis, is an inflammatory condition affecting both eyes and can lead to symptoms such as meningeal irritation, hearing impairment, vitiligo, and hair loss or whitening (Greco et al., 2013; Lavezzo et al., 2016). The precise causes and mechanisms of VKH remain unclear. However, studies have indicated that the overexpression of HLA-DR4/DR53, along with certain environmental factors such as cytomegalovirus infections, could activate the adaptive immune response, leading to the activation of Th1 and Th17 cells (Greco et al., 2013; Du et al., 2016; O'Keefe and Rao, 2017). Ultimately, these immune responses culminate in an acute autoimmune reaction affecting multiple organs, primarily due to the direct targeting of self-antigens, such as tyrosinase, which are expressed by melanocytes (Du et al., 2016; O'Keefe and Rao, 2017; Sakata et al., 2014).
We report a case of VKH-like uveitis caused by Donafenib in the treatment of primary hepatocellular carcinoma, which has not been reported before, emphasizing the need for ophthalmological follow-ups for tumor patients receiving Donafenib and other novel targeted inhibitors.
A 55-year-old man presented with a complaint of persistently reduced vision for over a month. The patient was diagnosed with hepatocellular carcinoma (stage Ia, China Liver Cancer Staging System (Xie et al., 2020)) in right lobe, and underwent laparoscopic hepatectomy and cystectomy 3 years ago. Following surgery, he was treated by Entecavir Dispersible Tablets (RuiFuEn, 0.5 mg/tablet, Suzhou Dawnrays Pharmaceutical Co., Ltd.) orally 0.5 mg/d. However, the disease relapsed one and a half years later, manifesting as indistinct shadows on liver in the CT examination. Consequently, he underwent radiofrequency ablation, leading to his current treatment with a combination of Donafenib (Zepsun, 100 mg, Suzhou Zelgen Biopharmaceuticals Co., Ltd) and Sintilimab (PD-1 inhibitor, 100 mg, Innovent) instead of Entecavir Dispersible Tablets. He was administered Donafenib orally 400 g/d, and the Sintilimab was administered by intravenous injection at a dose of 200 mg every 21 days. Unfortunately, after 18 months of using Donafenib and the Sintilimab, he experienced sporadic white patches on his body, tinnitus, headaches, and mild bilateral vision reduction. He sought medical attention because his vision had dramatically worsened in the past 4 weeks. The patient was diagnosed as hepatitis B for over 10 years, and had no history of systematic disease like hypertension, diabetes, drug allergy.
Fundus fluorescein angiography (FFA) and swept-source optical coherence tomography (SS-OCT, SVison) revealed VKH-like manifestations, including exudative retinal detachment and choroidal thickening. The FFA images demonstrated multiple focal areas of pinpoint hyperfluorescence, along with pooling indicative of neurosensory detachment (NSD) and disc leakage in both eyes (Figure 1). The SS-OCT images of the patient revealed NSD in the right eye (Figure 2A), as well as choroidal folds and thickened choroid in both eyes (Figures 2A, B). We measured the patient’s subfoveal choroid thickness (SFCT), which was 800 μm in the right eye and 836 μm in the left eye.
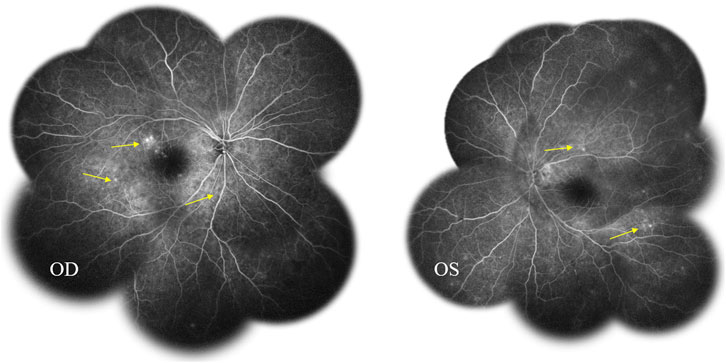
Figure 1. Fundus fluorescein angiography (FFA) displayed multifocal hyperfluorescent lesions at the level of retinal pigment epithelium pooled into the sub-neurosensory retinal space (arrows).
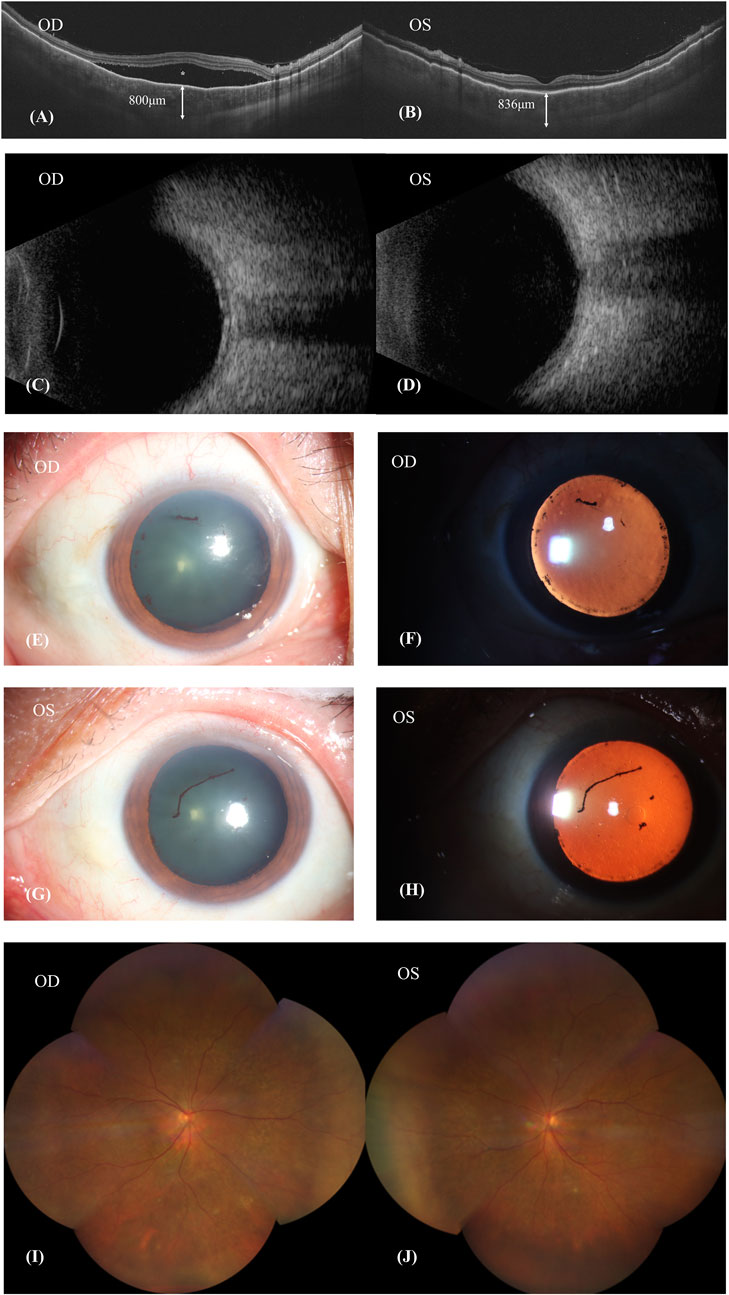
Figure 2. Patient’s first visit. (A,B): SS-OCT showed the neurosensory retinal detachment (NSD) in the right eye (asterisk), choroid folds and thickened choroid in both eyes. The subfoveal choroid thickness (SFCT) were 800 μm in the right eye and 836 μm in the left eye. (C,D): Ultrasound B scan showed thickening of choroid and clear eyeballs. (E–H): Both eyes showed signs of iris synechiae and anterior uveitis with fibrin formation. (I,J): Patient’s fundus image was basically normal.
The patient’s BCVA is 20/200 in his right eye and 20/80 in his left eye. Ultrasound B-scan imaging revealed a clear vitreous body but demonstrated choroidal thickening in both eyes (Figures 2C, D). Ophthalmic anterior segment imaging displayed signs of iris synechiae and anterior uveitis, along with fibrin formation (Figures 2E–H). However, the patient’s fundus imaging appeared basically normal (Figures 2I, J). Following comprehensive examinations, it was considered that the patient had uveitis and was positive for the HLA-B27 antigen. The patient’s erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) levels were elevated at 42.0 mm/h and 7.01 mg/L, respectively, indicative of inflammation. Evidence of an active immune response was indicated by elevated levels of immunoglobulins IgG (19.00 g/L), IgA (5710 mg/L), and IgE (612 IU/mL). Additionally, he tested positive for hepatitis B surface antigen (HBsAg) and hepatitis B e antigen (HBeAg). Analysis of his intraocular fluid revealed high levels of interleukins IL-6 (6090.44 pg/mL), IL-8 (1357.48 pg/mL), and IL-10 (36.83 pg/mL), indicating ocular inflammation. Considering the necessity of Sintilimab for hepatocellular carcinoma and previous reports on the ocular side effectives from sorafenib whose mechanism was similar to Donafenib, we recommended discontinuation of Donafenib and regular follow-ups at the eye clinic.
One week after discontinuing Donafenib, his BCVA remained unchanged, but he experienced relief from his headache and tinnitus. Examination revealed synechiae involving the pupil and iris in both eyes, and anterior uveitis was observed in the left eye (Figures 3A–D). His fundus image showed choroidal depigmentation as sun-set fundus (Figures 3E, F). Considering his VKH-like symptoms, we initiated treatment with oral glucocorticoids. This involved weekly tapering by 5 mg of prednisolone acetate orally, starting at a dose of 80 mg (1 mg/kg body weight). Additionally, neocyspin (25 mg) was prescribed to be taken orally twice a day (morning and evening) for 3 months.
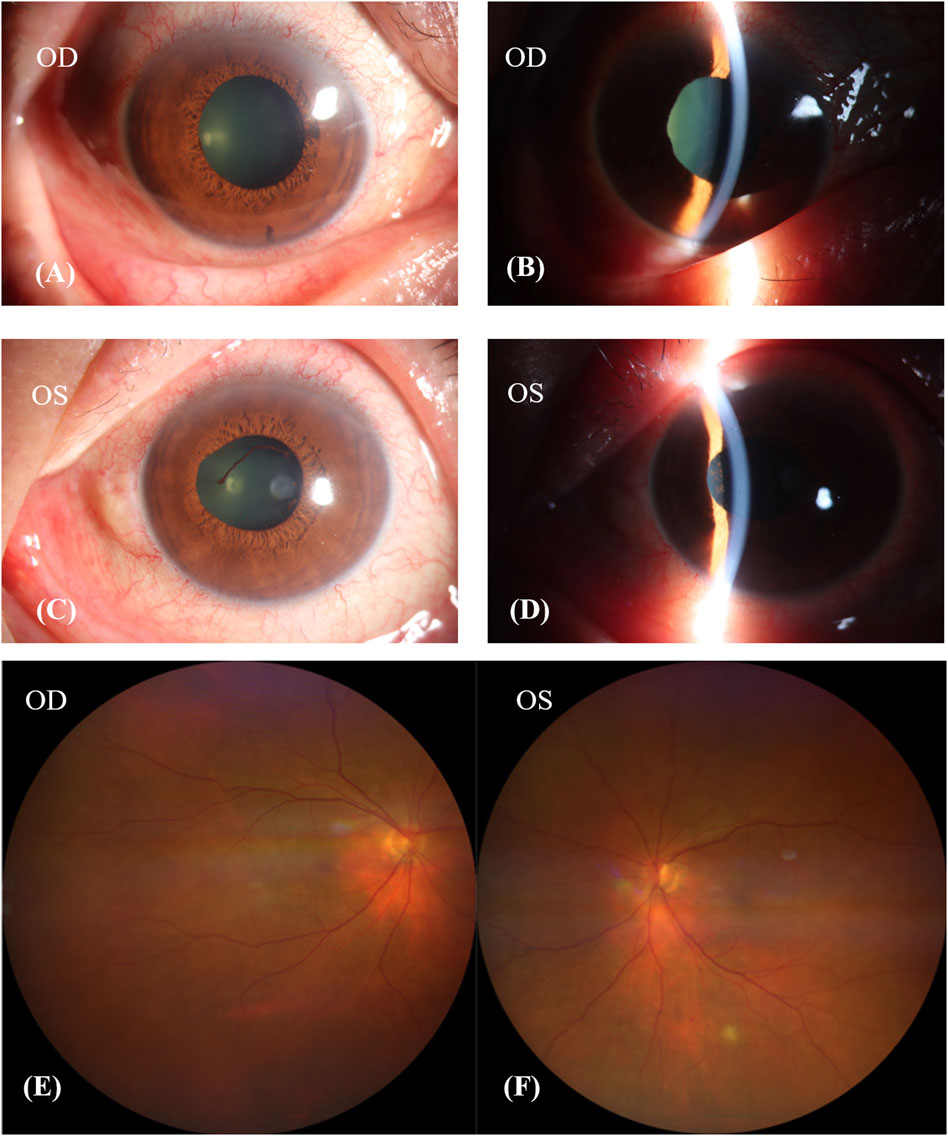
Figure 3. Patient’s condition after discontinuing donafenib 1 week. (A–D): The pupil and iris had synechiae in both eyes, and the anterior uveitis of left eye. (E,F): Patient’s fundus image showed choroidal depigmentation, sun-set fundus.
After discontinuing Donafenib and receiving oral glucocorticoid and neocyspin for 3 months, the patient’s BCVA improved to 20/30 in the right eye and 20/40 in the left eye. Ophthalmic anterior segment imaging showed the obvious red-light reflex, suggesting fundus of the patient had a sunset-glow change, and the lens’ surface of the left eye had anterior synechiae (Figures 4A–D). The fundus imaging depicted a pale optic nerve surrounded by red-orange choroidal depigmentation, characteristic of a “sunset-glow” fundus appearance (Figures 4E, F). He responded well to the oral corticosteroid therapy, as evidenced by the resolution of exudative detachment and diminishing fluid observed in the SS-OCT (Figures 4G, H). The SS-OCT images showed a flattened retina and thinning of the choroid, where the choroidoscleral border became visible. The SFCT was 261 μm in the right eye and 263 μm in the left eye.
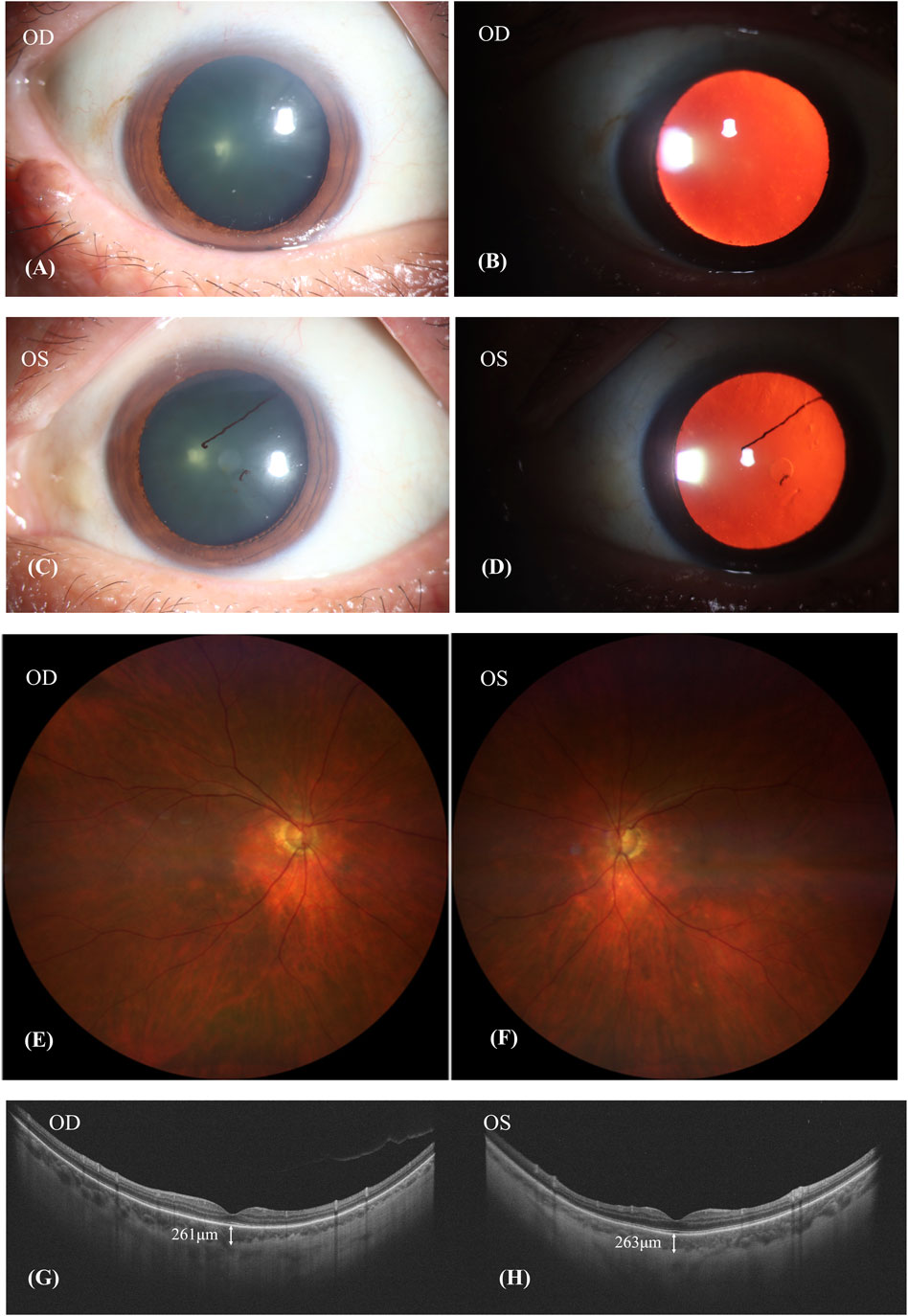
Figure 4. Patient’s condition after discontinuing donafenib and receiving oral glucocorticoid and neocyspin for 3 months. (A–D): The obvious red-light reflex suggested that the fundus of the patient had a sunset-glow change, and the lens surface of the left eye had anterior synechiae. (E,F): A pale nerve surrounded by red-orange choroidal depigmentation was referred to as a “sunset-glow” fundus. (G,H): SS-OCT showed retina flatted. The choroid was thinned out and the choroidoscleral border was visible. The subfoveal choroid thickness (SFCT) was 261 μm in the right eye and 263 μm in the left eye.
With the improvement of the patient’s ocular symptoms, we collaborated with the multidisciplinary team to continue the treatment of hepatocellular carcinoma with the lowest effective oral dose of Donafenib (200 mg/day) and recommended regular ophthalmological follow-ups. One month after resuming Donafenib, the patient returned for ophthalmologic examinations, which indicated that his condition remained stable. His BCVA was 20/25 in the right eye and 20/30 in the left eye.
In the literature, we firstly reported case of VKH-like uveitis caused by Donafenib. Various types of drugs result in DIU, including cidofovir (Davis et al., 1997; Lopez et al., 2006), rifabutin (Bhagat et al., 2001; Trone et al., 2018), bisphosphonates (Etminan et al., 2012; Chartrand et al., 2023), sulfonamides (Arola et al., 1998; Pathak and Power, 2007), nonsteroidal anti-inflammatory drugs (Mandeville et al., 2001; Okafor et al., 2017), tumor necrotic factor-α inhibitors (Ramos-Casals et al., 2008; Seve et al., 2012), fluoroquinolones (Forooghian et al., 2013; Sandhu et al., 2016), immune checkpoint inhibitors (Arai et al., 2017; Conrady et al., 2018), and BRAF/MERK inhibitors (Moorthy et al., 2018; Agarwal et al., 2020; Dimitriou et al., 2021). Additionally, the advent of targeted therapy has given rise to cases of targeted drug-induced uveitis. Uveitis has been reported as a consequence of using sorafenib and sunitinib (Fraunfelder and Fraunfelder, 2018). In the treatment of metastatic melanoma, the BRAF inhibitor vemurafenib has been associated with VKH-like symptoms (Lim et al., 2018; Xiaotong et al., 2022), resulting in drug-induced uveitis and secondary glaucoma (Xiaoli et al., 2022).
Given the patient’s medical history and clinical presentations, we considered the possibility of DIU. The patient received PD-1 immune therapy, known to cause VKH-like responses (Cunningham et al., 2020), concurrently with Donafenib. It was crucial to determine which drug was responsible for the VKH-like manifestations in this case or the potential interaction of the two drugs. Considering the potential risks associated with novel targeted therapies, and Sorafenib with similar mechanism to Donafenib, had potential risk of inducing uveitis reported before (Fraunfelder and Fraunfelder, 2018), we opted to discontinue Donafenib for 1 week to evaluate whether this would result in an improvement in the patient’s fundus condition. After discontinuing Donafenib, the patient reported improved headache and tinnitus. We initiated corticosteroid therapy to confirm the diagnosis. Three months after discontinuing Donafenib, we observed an improvement in the VKH-like manifestations. As a result, we speculated that the VKH-like symptoms were triggered by the use of Donafenib.
Donafenib is employed as a first-line targeted therapy for hepatocellular carcinoma in China (LCCoCMD, 2022). It is an oral small molecule inhibitor, effectively suppressing tumor growth and angiogenesis, achieved by targeting several receptor tyrosine kinases, including those linked to vascular endothelial growth factor and platelet-derived growth factor. Additionally, Donafenib targets various Raf kinases and the Raf/MEK/ERK signaling pathway, leading to dual inhibition and multi-target blockade, thereby effectively combating cancer (Li et al., 2020; Qin et al., 2021; ECoLCoCSoC, 2022). During the follow-up, except for ophthalmological performance, we discovered that the patient experienced tinnitus, headache, hearing loss, and sporadic white patches, which are typical symptoms of VKH. After stopping the use of Donafenib and starting corticosteroid therapy, his symptoms showed a positive response to it. Consequently, we speculated that the use of Donafenib led to the development of uveitis, which mimicked the VKH effects on ophthalmological performance.
Although the exact mechanism remains unclear, we have developed several hypotheses to explain this process. One hypothesis posits that Donafenib’s inhibition of the BRAF pathway may activate the immune system, potentially triggering an immune reaction that results in VKH-like uveitis (Xiaotong et al., 2022). Furthermore, the anti-tumor effect of BRAF inhibitors leads to widespread apoptosis of tumor cells, resulting in the release of a significant number of antigens (Apivatthakakul et al., 2020). This may cause systemic hypersensitivity and ocular involvement, manifesting as uveitis.
Our study had several limitations. Firstly, we discontinued Donafenib for only 1 week, which was not sufficient to fully observe the progression of the patient’s condition. Secondly, our study included only one case and future research should involve larger sample sizes. Thirdly, it was challenging to discern the potential interaction between Donafenib and Sintilimab by stopping Donafenib only. However, in the real clinical practice, Sintilimab was essential for the patient’s therapy and we could not discontinue or alter it. In the future research, we aim to explore the potential interaction Donafenib and Sintilimab using cell lines and animal models.
In conclusion, we reported the first case of VKH-like uveitis induced by Donafenib, underscoring the necessity of ophthalmological follow-ups during targeted therapy. The patient’s condition improved following the discontinuation of Donafenib, and the addition of prednisolone acetate further accelerated this improvement. We successfully observed the entire progression of fundus changes associated with the VKH-like uveitis. To definitively determine the mechanism by which Donafenib causes VKH-like uveitis, further rigorous experiments and more substantial evidence are required.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Written informed consent was obtained from the patient for publication of this case report and any accompanying images.
RL: Conceptualization, Data curation, Formal Analysis, Writing–original draft, Writing–review and editing. GL: Validation, Writing–review and editing. FL: Resources, Writing–review and editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Agarwal, M., Dutta Majumder, P., Babu, K., Konana, V. K., Goyal, M., Touhami, S., et al. (2020). Drug-induced uveitis: a review. Indian J. Ophthalmol. 68 (9), 1799–1807. doi:10.4103/ijo.IJO_816_20
Apivatthakakul, A., Kunavisarut, P., Rothova, A., and Pathanapitoon, K. (2020). Development of acute vogt-koyanagi-harada-like syndrome during the treatment course with vemurafenib for metastatic melanoma. Ocul. Immunol. Inflamm. 28 (3), 505–508. doi:10.1080/09273948.2019.1597896
Arai, T., Harada, K., Usui, Y., Irisawa, R., and Tsuboi, R. (2017). Case of acute anterior uveitis and Vogt-Koyanagi-Harada syndrome-like eruptions induced by nivolumab in a melanoma patient. J. Dermatol 44 (8), 975–976. doi:10.1111/1346-8138.13612
Arola, O., Peltonen, R., and Rossi, T. (1998). Arthritis, uveitis, and Stevens-Johnson syndrome induced by trimethoprim. Lancet. 351 (9109), 1102. doi:10.1016/S0140-6736(05)79382-X
Bhagat, N., Read, R. W., Rao, N. A., Smith, R. E., and Chong, L. P. (2001). Rifabutin-associated hypopyon uveitis in human immunodeficiency virus-negative immunocompetent individuals. Ophthalmology. 108 (4), 750–752. doi:10.1016/s0161-6420(00)00586-8
Chartrand, N. A., Lau, C. K., Parsons, M. T., Handlon, J. J., Ronquillo, Y. C., Hoopes, P. C., et al. (2023). Ocular side effects of bisphosphonates: a review of literature. J. Ocul. Pharmacol. Ther. 39 (1), 3–16. doi:10.1089/jop.2022.0094
Chinese Medical Doctor Association liver cancer professional committee (2022). Chinese expert consensus on clinical application of molecular targeted drugs in hepatocellular carcinoma (2022 edition). Chin. Med. J. 102 (34), 2655–2668. doi:10.3760/cma.cn112137-20220623-01387
Conrady, C. D., Larochelle, M., Pecen, P., Palestine, A., Shakoor, A., and Singh, A. (2018). Checkpoint inhibitor-induced uveitis: a case series. Graefes Arch. Clin. Exp. Ophthalmol. 256 (1), 187–191. doi:10.1007/s00417-017-3835-2
Cunningham, E. T., Moorthy, R. S., and Zierhut, M. (2020). Immune checkpoint inhibitor-induced uveitis. Ocul. Immunol. Inflamm. 28 (6), 847–849. doi:10.1080/09273948.2020.1801286
Davis, J. L., Taskintuna, I., Freeman, W. R., Weinberg, D. V., Feuer, W. J., and Leonard, R. E. (1997). Iritis and hypotony after treatment with intravenous cidofovir for cytomegalovirus retinitis. Arch. Ophthalmol. 115 (6), 733–737. doi:10.1001/archopht.1997.01100150735008
Dimitriou, F., Urner-Bloch, U., Eggenschwiler, C., Mitsakakis, N., Mangana, J., Dummer, R., et al. (2021). The association between immune checkpoint or BRAF/MEK inhibitor therapy and uveitis in patients with advanced cutaneous melanoma. Eur. J. Cancer 144, 215–223. doi:10.1016/j.ejca.2020.11.027
Du, L., Kijlstra, A., and Yang, P. (2016). Vogt-Koyanagi-Harada disease: novel insights into pathophysiology, diagnosis and treatment. Prog. Retin Eye Res. 52, 84–111. doi:10.1016/j.preteyeres.2016.02.002
Etminan, M., Forooghian, F., and Maberley, D. (2012). Inflammatory ocular adverse events with the use of oral bisphosphonates: a retrospective cohort study. Cmaj 184 (8), E431–E434. doi:10.1503/cmaj.111752
Forooghian, F., Maberley, D., Albiani, D. A., Kirker, A. W., Merkur, A. B., and Etminan, M. (2013). Uveitis risk following oral fluoroquinolone therapy: a nested case-control Study. Ocul. Immunol. Inflamm. 21 (5), 390–393. doi:10.3109/09273948.2013.808351
Fortes, B. H., Liou, H., and Dalvin, L. A. (2021). Ophthalmic adverse effects of immune checkpoint inhibitors: the Mayo Clinic experience. Br. J. Ophthalmol. 105 (9), 1263–1271. doi:10.1136/bjophthalmol-2020-316970
Fraunfelder, F. T., and Fraunfelder, F. W. (2018). Oral anti-vascular endothelial growth factor drugs and ocular adverse events. J. Ocul. Pharmacol. Ther. 34 (6), 432–435. doi:10.1089/jop.2018.0019
Greco, A., Fusconi, M., Gallo, A., Turchetta, R., Marinelli, C., Macri, G. F., et al. (2013). Vogt-Koyanagi-Harada syndrome. Autoimmun. Rev. 12 (11), 1033–1038. doi:10.1016/j.autrev.2013.01.004
Jeng-Miller, K. W., Miller, M. A., and Heier, J. S. (2024). Ocular effects of MEK inhibitor therapy: literature review, clinical presentation, and best practices for mitigation. Oncologist 29 (5), e616–e621. doi:10.1093/oncolo/oyae014
Lavezzo, M. M., Sakata, V. M., Morita, C., Rodriguez, E. E. C., Abdallah, S. F., da Silva, F. T. G., et al. (2016). Vogt-Koyanagi-Harada disease: review of a rare autoimmune disease targeting antigens of melanocytes. Orphanet J. Rare Dis. 11, 29. doi:10.1186/s13023-016-0412-4
Li, X., Qiu, M., Wang, S., Zhu, H., Feng, B., and Zheng, L. (2020). A Phase I dose-escalation, pharmacokinetics and food-effect study of oral donafenib in patients with advanced solid tumours. Cancer Chemother. Pharmacol. 85 (3), 593–604. doi:10.1007/s00280-020-04031-1
Lim, J., Lomax, A. J., McNeil, C., and Harrisberg, B. (2018). Uveitis and papillitis in the setting of dabrafenib and trametinib therapy for metastatic melanoma: a case report. Ocul. Immunol. Inflamm. 26 (4), 628–631. doi:10.1080/09273948.2016.1246666
Liu, X., Wang, Z., Zhao, C., Wang, H., Guo, X., Zhou, J., et al. (2020). Clinical diagnosis and treatment recommendations for ocular toxicities of targeted therapy and immune checkpoint inhibitor therapy. Thorac. Cancer 11 (3), 810–818. doi:10.1111/1759-7714.13327
Liver Cancer Expert Committee of Chinese Society of Clinical Oncology, Expert Committee on Safety Management of Antitumor Drugs of Chinese Society of Clinical Oncology (2022). Expert consensus on the clinical application of donafenib in the treatment of hepatocellular carcinoma. J. Clin. Oncol. 27 (8), 749–757. doi:10.3969/j.issn.1009-0460.2022.08.013
Lopez, V., Sola, E., Gutierrez, C., Burgos, D., Cabello, M., García, I., et al. (2006). Anterior uveitis associated with treatment with intravenous cidofovir in kidney transplant patients with BK virus nephropathy. Transpl. Proc. 38 (8), 2412–2413. doi:10.1016/j.transproceed.2006.08.067
Mandeville, J. T., Levinson, R. D., and Holland, G. N. (2001). The tubulointerstitial nephritis and uveitis syndrome. Surv. Ophthalmol. 46 (3), 195–208. doi:10.1016/s0039-6257(01)00261-2
Moorthy, R. S., Moorthy, M. S., and Cunningham, E. T. (2018). Drug-induced uveitis. Curr. Opin. Ophthalmol. 29 (6), 588–603. doi:10.1097/ICU.0000000000000530
Okafor, L. O., Hewins, P., Murray, P. I., and Denniston, A. K. (2017). Tubulointerstitial nephritis and uveitis (TINU) syndrome: a systematic review of its epidemiology, demographics and risk factors. Orphanet J. Rare Dis. 12 (1), 128. doi:10.1186/s13023-017-0677-2
O'Keefe, G. A., and Rao, N. A. (2017). Vogt-Koyanagi-Harada disease. Surv. Ophthalmol. 62 (1), 1–25. doi:10.1016/j.survophthal.2016.05.002
Pathak, S., and Power, B. (2007). Bilateral acute anterior uveitis as a side effect of trimethoprim. Eye (Lond). 21 (2), 252–253. doi:10.1038/sj.eye.6702483
Qin, S., Bi, F., Gu, S., Bai, Y., Chen, Z., Wang, Z., et al. (2021). Donafenib versus sorafenib in first-line treatment of unresectable or metastatic hepatocellular carcinoma: a randomized, open-label, parallel-controlled phase II-III trial. J. Clin. Oncol. 39 (27), 3002–3011. doi:10.1200/JCO.21.00163
Ramos-Casals, M., Brito-Zerón, P., Soto, M. J., Cuadrado, M. J., and Khamashta, M. A. (2008). Autoimmune diseases induced by TNF-targeted therapies. Best. Pract. Res. Clin. Rheumatol. 22 (5), 847–861. doi:10.1016/j.berh.2008.09.008
Sada, I., Harada, Y., Hiyama, T., Mizukami, M., Kan, T., Kawai, M., et al. (2023). Uveitis associated with immune checkpoint inhibitors or BRAF/MEK inhibitors in patients with malignant melanoma. Melanoma Res. 33 (6), 539–546. doi:10.1097/CMR.0000000000000933
Sakata, V. M., da Silva, F. T., Hirata, C. E., de Carvalho, J. F., and Yamamoto, J. H. (2014). Diagnosis and classification of Vogt-Koyanagi-Harada disease. Autoimmun. Rev. 13 (4-5), 550–555. doi:10.1016/j.autrev.2014.01.023
Sandhu, H. S., Brucker, A. J., Ma, L., and VanderBeek, B. L. (2016). Oral fluoroquinolones and the risk of uveitis. JAMA Ophthalmol. 134 (1), 38–43. doi:10.1001/jamaophthalmol.2015.4092
Seve, P., Varron, L., Broussolle, C., Denis, P., and Kodjikian, L. (2012). Sarcoid-related uveitis occurring during adalimumab therapy. Ocul. Immunol. Inflamm. 20 (1), 59–60. doi:10.3109/09273948.2011.623213
Thibault, T., Ben Ghezala, I., Freppel, R., Rajillah, A., Boulay, C., Brunel, P., et al. (2024). Drug-induced uveitis related to checkpoint inhibitors and MAP-kinase inhibitors. Ophthalmology 131 (2), 249–251. doi:10.1016/j.ophtha.2023.10.004
Trone, M. C., Manoli, P., Mattei, J., Thuret, G., Gain, P., and Stephan, J. L. (2018). Rifabutin-associated uveitis in a 10-year-old child with HIV: case report and review of the literature. J. Fr. Ophtalmol. 41 (8), e391–e393. doi:10.1016/j.jfo.2017.11.035
Xiaoli, S., Mingying, L., and Hongbin, X. (2022). Vemurafenib induced drug-uveitis and secondary glaucoma. Chin. Med. Care Repos. (01), E03071–E. doi:10.3760/cma.j.cmcr.2022.e03071
Xiaotong, R., Tong, G., and Fan, Y. (2022). Vogt-Koyanagi-Harada syndrome in the setting of vemurafenib therapy for metastatic melanoma: a case report. Chin. J. Ophthalmol. (11), 925–928. doi:10.3760/cma.j.cn112142-20220215-00055
Keywords: VKH-like uveitis, donafenib-induced uveitis, hepatocellular carcinoma, targeted therapy, ocular side effects of cancer therapy
Citation: Liu R, Liu G and Lu F (2024) VKH-like uveitis during donafenib therapy for hepatocellular carcinoma: a case report and review of the literature. Front. Pharmacol. 15:1401017. doi: 10.3389/fphar.2024.1401017
Received: 14 March 2024; Accepted: 25 June 2024;
Published: 18 July 2024.
Edited by:
Jianqiang Xu, Dalian University of Technology, ChinaReviewed by:
Ujjalkumar Subhash Das, University of Pennsylvania, United StatesCopyright © 2024 Liu, Liu and Lu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fang Lu, bHVmYW5nQHdjaHNjdS5jbg==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.