- 1College of Korean Medicine, Wonkwang University, Iksan, Republic of Korea
- 2Mapo Hongik Korean Medicine Clinic, Seoul, Republic of Korea
- 3Research Center of Traditional Korean Medicine, College of Korean Medicine, Wonkwang University, Iksan, Republic of Korea
Introduction: Despite the widespread use of ephedra in various forms, including food supplements and herbal prescriptions, comprehensive studies reviewing its efficacy and safety across different countries are lacking.
Methods: We systematically searched 5 electronic databases and conducted a meta-analysis of 16 randomized controlled trials (RCTs) on ephedra-containing oral medications (EOMs), performing a dose–response analysis for weight loss.
Results: The meta-analysis results revealed a statistically significant reduction in the body mass index (BMI) (MD: 1.5 kg/m2; 95% CI: −2.46 to −0.54) and secondary outcomes like body weight (BW) and waist circumference (WC). The dose–response analysis indicated a correlation between ephedra and weight reduction. The safety analysis showed no significant difference in adverse effects between the treatment and control groups (RR = 0.99, 95% CI = 0.80 ∼ 1.21, and p = 0.90).
Discussion: In conclusion, EOMs demonstrated effectiveness in promoting weight loss, and the dose–response analysis indicated a correlation between ephedra and weight reduction. However, additional research is necessary due to the limited number of studies and inconsistent results among the assessment criteria. Moreover, if prescribed by traditional medicine physicians within the permissible daily ephedrine dosage range of 150 mg set by the Food and Drug Administration (FDA) and monitored by healthcare professionals, the risk of severe adverse events is likely to be minimal.
Systematic Review Registration:: https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=387895, identifier CRD42023387895.
1 Introduction
In recent years, the number of obese people has increased significantly owing to sedentary lifestyles and high-calorie diets (Manson et al., 2004). The World Health Organization (WHO) defines being overweight as having a body mass index (BMI) of 25 or more and obesity as a BMI of 30 or more (WHO Regional Office for Europe, 2022). Globally, the population of overweight and obese adults has increased by 27.5% between 1980 and 2013 (Ng et al., 2014), representing 30% of the global population in 2015 (Bomberg et al., 2017). Obesity is a metabolic disorder characterized by the abnormal accumulation of excess adipose tissue in the body, extending beyond excess body weight (Engin et al., 2017). It can cause hemodynamic problems and abnormalities in the heart’s structure and function. Individuals with metabolic syndrome, including obesity, have a 40%–60% higher mortality rate from cardiovascular disease (Sundström et al., 2006; Powell-Wiley et al., 2021). Obesity is also a risk factor for increasing the prevalence of other diseases, such as metabolic syndrome, type 2 diabetes, hypertension, coronary artery disease, cancer, and stroke (Hasani-Ranjbar et al., 2009; Spiotta and Luma, 2008). According to a 2006 survey, individuals with obesity spend more than 42% more on healthcare annually than those with a healthy weight (Caroline and Apovian, 2016). Based on current trends, healthcare expenses related to obesity in the United States are expected to reach $48–66 billion annually by 2030 (Wang YC. et al., 2011). In conclusion, obesity has become a serious problem in modern society, reducing life expectancy and adding to social and economic burdens such as increased healthcare costs and decreased productivity (Wang YC. et al., 2011; Blackburn and Walker, 2005; Flegal et al., 2005; Kim et al., 2018).
Pharmacological, surgical, and lifestyle interventions are commonly used to treat obesity (Kissane and Pratt, 2011). Sibutramine, which is frequently prescribed and approved for long-term use, may lead to elevated blood pressure and cause side effects such as insomnia and nausea (Tziomalos et al., 2009). Orlistat causes gastrointestinal upset, and rimonabant is known to increase the incidence of mental conditions, such as depression and anxiety (Rucker et al., 2007). Furthermore, the use of phentermine–topiramate is contraindicated in individuals with cardiovascular disease, thereby limiting its application to specific patient populations (Hasani-Ranjbar et al., 2009; Ioannides-Demos et al., 2006). Surgical therapies are more invasive, require a long recovery period, and may lead to long-term metabolic complications such as osteoporosis, hypoglycemia, and nutrient imbalances (Jammah, 2015). Behavioral modifications, such as regular exercise, dietary adjustments, and low-energy diets, augment energy expenditure compared to physical inactivity or limited calorie consumption (Wadden et al., 2020). Although effective in achieving sustained weight loss while minimizing adverse effects and weight regain, lifestyle interventions are challenging to uphold during and after treatment because of issues with personal adherence (Hasani-Ranjbar et al., 2009; Leibel et al., 1995). The usage of dietary supplements, alongside lifestyle interventions, is steadily increasing. However, there is a significant lack of clinical evidence regarding their effectiveness and safety (Poddar et al., 2011).
Therefore, the demand for East Asian traditional medicines (EATMs) is growing among the general public (Davis et al., 2011; Ojukwu et al., 2015). Herbal medicine is gaining popularity as a treatment option for obesity management in individuals seeking EATMs (Park et al., 2012). Herbal medicine treats obesity through various mechanisms, including augmentation of metabolic rates, carbohydrate metabolism modulation, fat absorption inhibition, appetite suppression, and serotonin modulation (He et al., 2020). Comparing clinical trials of herbal medicine and lifestyle interventions for obesity treatment showed that the combined utilization of herbal medicine and lifestyle interventions demonstrated a more substantial weight loss effect than other interventions (Park et al., 2012).
Among other herbal medicines, ephedra has gained popularity as a treatment for obesity in the United States and other countries, particularly since the 1972 report of its weight-loss effects with caffeine. However, concerns surrounding the adverse effects of ephedra prompted the Food and Drug Administration (FDA) to ban its use in foods in 2004 (Miao et al., 2020; Mehendale et al., 2004). Although adverse events associated with ephedra have decreased following its use after the FDA ban (Zell-Kanter et al., 2015), it is still used in traditional medicine for weight loss and other therapeutic purposes in Asian countries, such as China, Republic of Korea, and Japan, often in combination with other herbs (EBM-based Obesity KMCPG Development Committee, 2016; Uneda et al., 2022; Lee et al., 2020). Ephedra contains alkaloids, flavonoids, and tannins, with the primary bioactive compounds being alkaloids, i.e., 1-ephedrine, 1-methyl-ephedrine, and 1-norephedrine (Miao et al., 2020). Ephedra exerts its effects by stimulating the sympathetic nervous system, inducing energy expenditure, and modifying the gut microbiota in obese individuals (Miao et al., 2020; Alraei, 2010; Kim et al., 2014).
The weight-loss effects and mechanisms of ephedra are well known, leading to their widespread use in clinical practice. Despite the widespread use of ephedra in various forms, including food supplements and herbal prescriptions, comprehensive studies reviewing its efficacy and safety in different countries still need to be performed. Further investigation is necessary to evaluate the effectiveness and safety of ephedra in clinical practice. This study performed a systematic review and meta-analysis to comprehensively evaluate randomized controlled trials (RCTs) of ephedra-containing oral medications (EOMs) for weight loss and obesity treatment, regardless of the formulation or country. This meta-analysis assessed the effectiveness and safety of EOMs in terms of BMI, body weight (BW), and waist circumference (WC). Moreover, considering the insufficient amount of research on the dosage and effects of ephedra, we aimed to explore the dose–response relationship of ephedra using both quantitative and qualitative methodologies.
2 Methods
This systematic review and meta-analysis were conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 statement (Mj et al., 2021) and the recommendations of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins et al., 2019). The study protocol was registered in PROSPERO (crd42023387895).
2.1 Search strategy
The initial search was conducted on 10 August 2022 using the electronic databases PubMed, Excerpta Medica Database (Embase), Cochrane Central Register of Controlled Trials (CENTRAL), Allied and Complementary Medicine Database (AMED), and Cumulative Index of Nursing and Allied Health Literature (CINAHL). The second search was performed on 15 December 2023. The search terms used were “obesity,” “EATM,” “natural products,” “ephedra,” and “RCT” (Supplementary Table S1). These three categories were combined using the AND Boolean operator with keywords appropriate for each database. In addition, clinical practice guidelines and literature reviews on herbal treatments for obesity were examined. Only articles that met the inclusion criteria were selected for review.
2.2 Inclusion and exclusion criteria
2.2.1 Study design
Our study included only RCTs involving human participants. Pilot studies that did not report these results were also excluded. There were no restrictions on the blinding used in the RCTs; however, crossover studies were excluded if the necessary information could not be extracted during data analysis.
2.2.2 Participant characteristics
We included studies of overweight and obese patients with a BMI of 25 or more according to WHO criteria, regardless of sex and age (WHO Regional Office for Europe, 2022). Underlying condition were not restricted; however, individuals who used EOMs for growth or weight gain were excluded from the study.
2.2.3 Types of interventions in the treatment and control groups
The treatment group included all interventions involving ephedra, regardless of whether they were combined with herbal prescriptions, single agents, or food supplements. However, we excluded interventions that contained only a partial component of ephedra, such as ephedrine, and cases where the prescription composition was not presented, making it unclear whether ephedra was included.
We only included study designs that used the same lifestyle interventions, such as exercise and diet, in the treatment and control groups. There were no restrictions on the use of other medications.
2.2.4 Outcome measures
The primary outcome was the BMI, calculated as weight divided by the square of height. Secondary outcome variables were weight (kg) and waist circumference (cm) (Delpino and Figueiredo, 2021). Studies that focused solely on blood test values, including serum leptin concentration, were excluded. The frequency and types of adverse events were also assessed.
2.3 Study selection and data extraction
2.3.1 Study selection
Two independent reviewers, HC and JO, initially screened the abstracts and titles to identify potentially eligible articles, which were further evaluated by reviewing the full text. No language restrictions were imposed. Disagreements between the two reviewers (HC and JO) were discussed with a third independent reviewer (JL) to reach a consensus.
2.3.2 Data extraction
HC and JO, summarized the basic information (author, year, and country), blinding, number of participants included (randomized/completed), number of patients by sex, interventions implemented in the treatment and control groups, treatment period, and primary outcome measures. The composition of the intervention, the daily dose, and the pharmaceutical company are described in detail. If necessary data were missing or errors were identified, we contacted the original authors. We also documented the type and number of adverse events, the number of dropouts, and the reasons for dropping out. If any of these details were not explicitly reported in the article, we indicated them as “NR” (not reported).
2.4 Assessment of risk of bias
Two reviewers, HC and JO, independently assessed the risk of bias (ROB) according to the RoB 2 tool published by the Cochrane Collaboration (Jac et al., 2019). The tool assesses the risk of bias in five areas: randomization process, deviations from the intended interventions, missing outcome data, measurement of the outcome, and selection of the reported results. Each of the five areas and the risk of bias were rated as low, of some concern, or high. In accordance with the methodology for systematic reviews of interventions (Higgins et al., 2019), if the two reviewers could not reach an agreement on the assessment of ROB, the disagreement was discussed with a third independent reviewer (JL) to reach a consensus.
2.5 Data analysis and quantitative synthesis
Statistical analyses were performed using RevMan 5.4 software (Cochrane Training, London, United Kingdom) and R software (version 4.2.1; R Foundation for Statistical Computing, Vienna, Austria).
2.5.1 Assessment of the overall effect size
Each study was evaluated for the total effect size of BMI, BW, and WC changes. A random-effects model accounted for intervention composition, dose, and duration variations between studies. Continuous outcomes of change are presented as mean differences (MDs) with 95% confidence intervals (CIs), and dichotomous outcomes are presented as risk ratios (RRs) with 95% confidence intervals. When standard deviations (SDs) were not reported, they were estimated from confidence intervals, and statistical results were obtained directly from the original authors by acquiring raw data. A random-effects model was also used to assess the number of participants with adverse events and dropouts for the total effect size. Statistical significance was set at p < 0.05.
2.5.2 Assessment of heterogeneity and meta-regression analysis
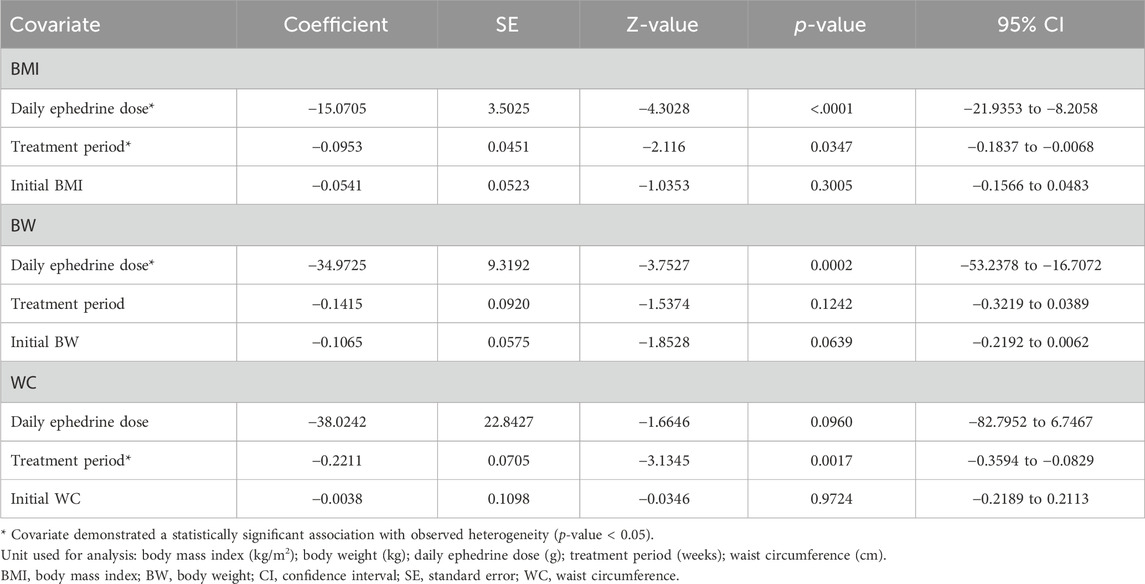
To evaluate the presence of heterogeneity among the included studies, we used the chi-square test and I2 statistic. Statistically significant heterogeneity was defined as p < 0.10 for the chi-square test. Additionally, an I2 value >50% indicated substantial heterogeneity in the study sample (Higgins et al., 2003). Furthermore, we performed meta-regression analyses to investigate potential associations between study-level covariates and the observed statistical heterogeneity (Sg and Jp, 2002). We conducted univariate meta-regression analyses on selected covariates, including daily ephedrine dose, treatment period, baseline BMI, BW, and WC. The analysis used the DerSimonian–Laird methodology (DerSimonian and Laird, 1986; DerSimonian and Laird, 2015), and Wald-type tests were applied for statistical evaluation. The ephedrine dose of ephedra was not reported in the study and was estimated to be 5.25 mg/g, based on the minimum dose specified in the Korean Pharmacopoeia (Jang et al., 2007).
2.5.3 Bubble plot
Two bubble plots were generated to visually depict the relationship between the covariates and changes in the outcome measures. A four-dimensional graph was plotted, in which the x-axis represents the outcome at baseline, the y-axis represents the daily ephedrine dose, the bubble color denotes the treatment period, and the bubble size corresponds to the magnitude of the outcome reduction. In addition, a three-dimensional graph was plotted to represent the relationship between ephedra intake and efficacy. The x-axis in this graph represents the outcome measure at baseline, the y-axis represents the total ephedrine dose, and the bubble size represents the reduction in the outcome measure.
2.5.4 Assessment of publication bias
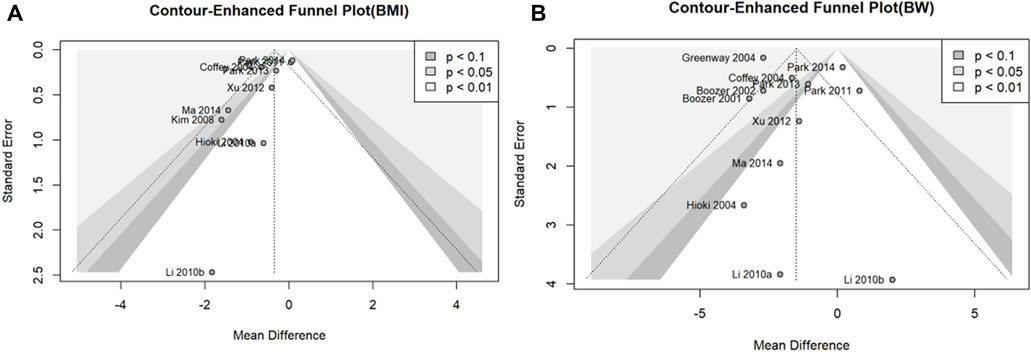
Publication bias was assessed for outcome variables, including the 10 studies in the meta-analysis (Sterne et al., 2011). To determine the potential for publication bias, we presented contour-enhanced funnel plots of the included studies for each outcome (BMI and BW) (Peters et al., 2008). Publication bias was assessed by performing Egger’s test on the observed asymmetry in the funnel plot (Egger et al., 1997).
3 Results
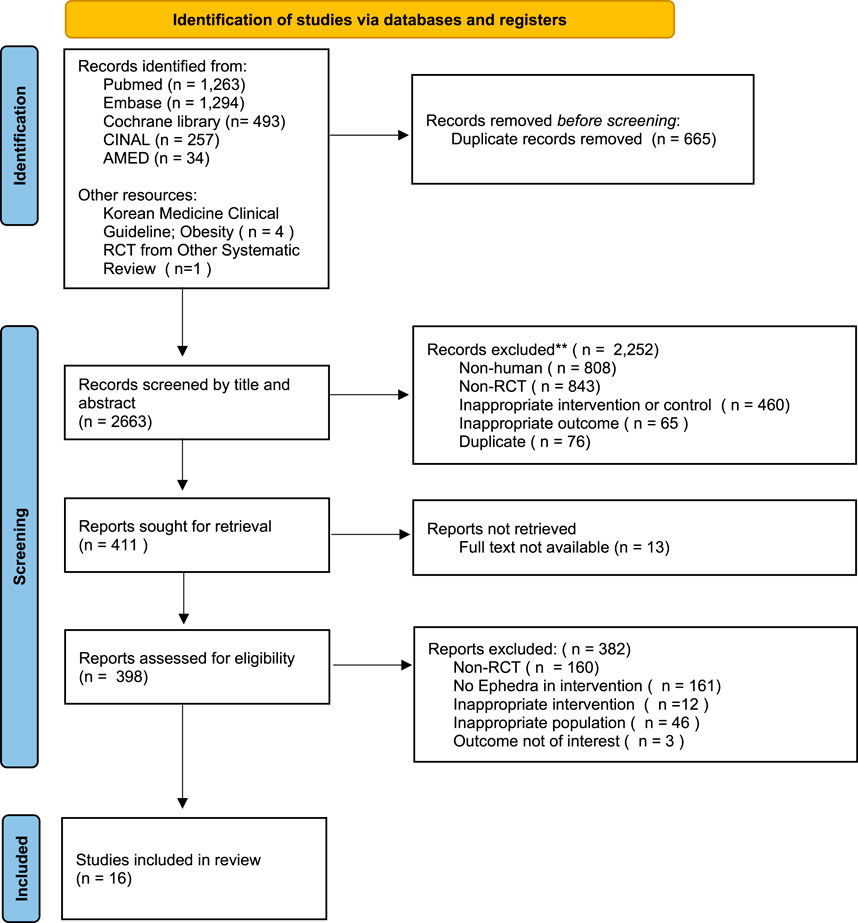
Following the previous search strategy, we searched five electronic databases and identified 3,149 articles, excluding duplicates and retractions. These were reviewed with articles from other sources, resulting in the selection of 16 articles that met the predetermined inclusion criteria (Figure 1).
3.1 General characteristics of the included studies
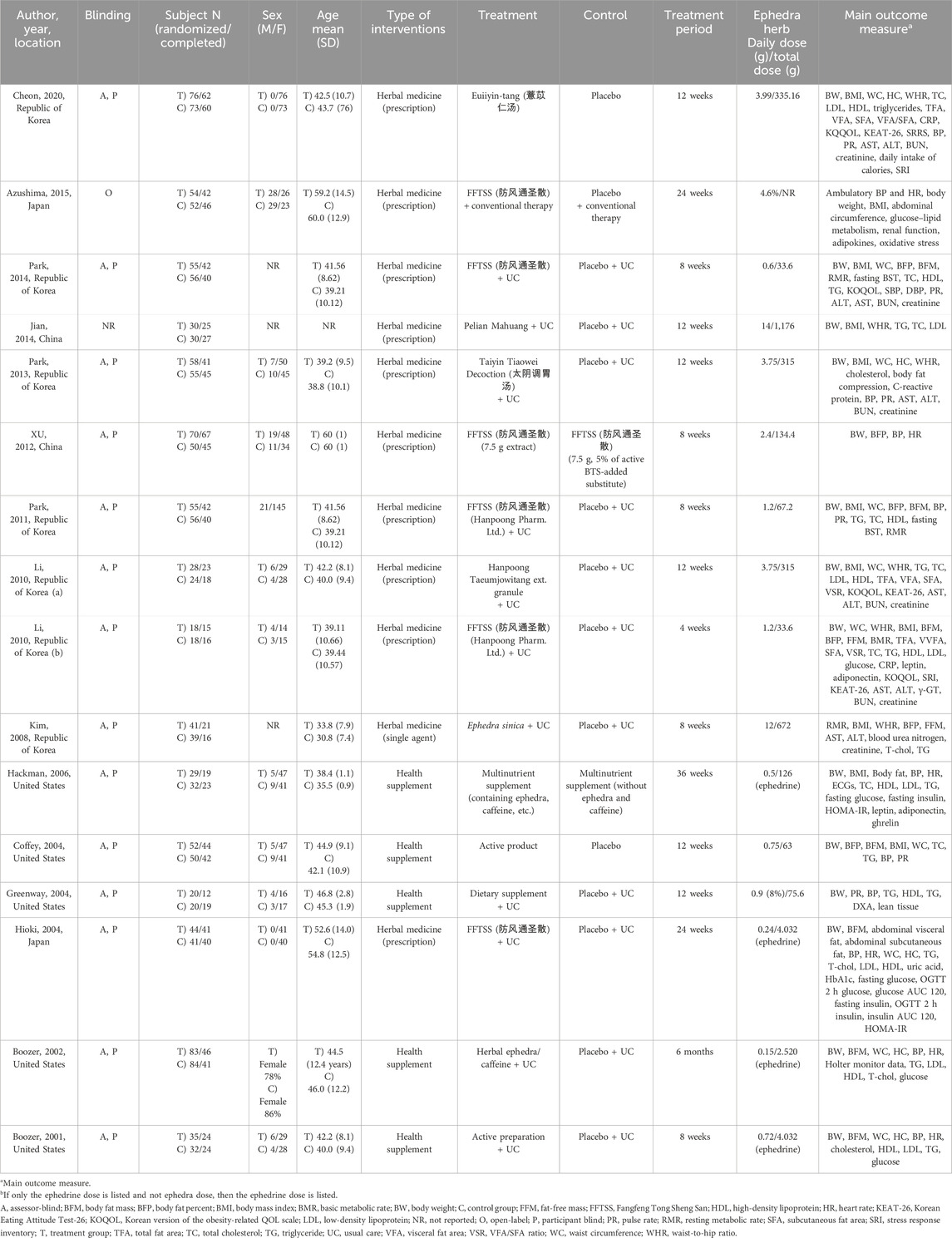
Seven of the 16 studies analyzed were conducted in the Republic of Korea, 5 in the United States, 2 in Japan, and 2 in China, with 1,460 participants (748 in the treatment group and 712 in the control group). Cheon et al. (2020) and Hioki et al. (2004) studied only women, whereas the others neither restricted sex nor reported sex-based criteria. The mean age of the patients in each study ranged from 30.8 to 60 years.
Regarding the intervention of the treatment group, only Kim et al. (2008) used a ephedra as a single herb as the intervention. At the same time, the remaining studies used a combination of ephedra and other ingredients and herbs in forms such as EATM prescriptions or dietary supplements. In addition, all studies used a placebo as a control, except for Hackman et al. [50], who used a multi-nutrient supplement without ephedra or caffeine as a control. Azushima et al. (2015) used conventional therapy for hypertension in both groups, whereas Cheon et al. (2020), Hackman et al. (2006), Coffey et al. (2004), and Feng-Hao et al. (2012) did not use or mention combination therapy. The remaining studies used the usual care for obesity in both the treatment and control groups. The group that received 5% of the treatment group’s dosage was considered the placebo group.
The treatment period varied from 4 to 24 weeks, and the main outcome measures included BMI, BW, and WC, which were also the selection criteria for this study. Additionally, various metabolic and drug toxicity markers were assessed, including serum lipids, blood glucose, aspartate transaminase (AST), alanine transaminase (ALT), and blood urea nitrogen (BUN). The daily dose of ephedra varied from 0.6 to 14 g. The details of each study are summarized in Table 1, and the composition and dosage of the interventions are summarized in Supplementary Table S2.
3.2 Results of the risk of bias assessment
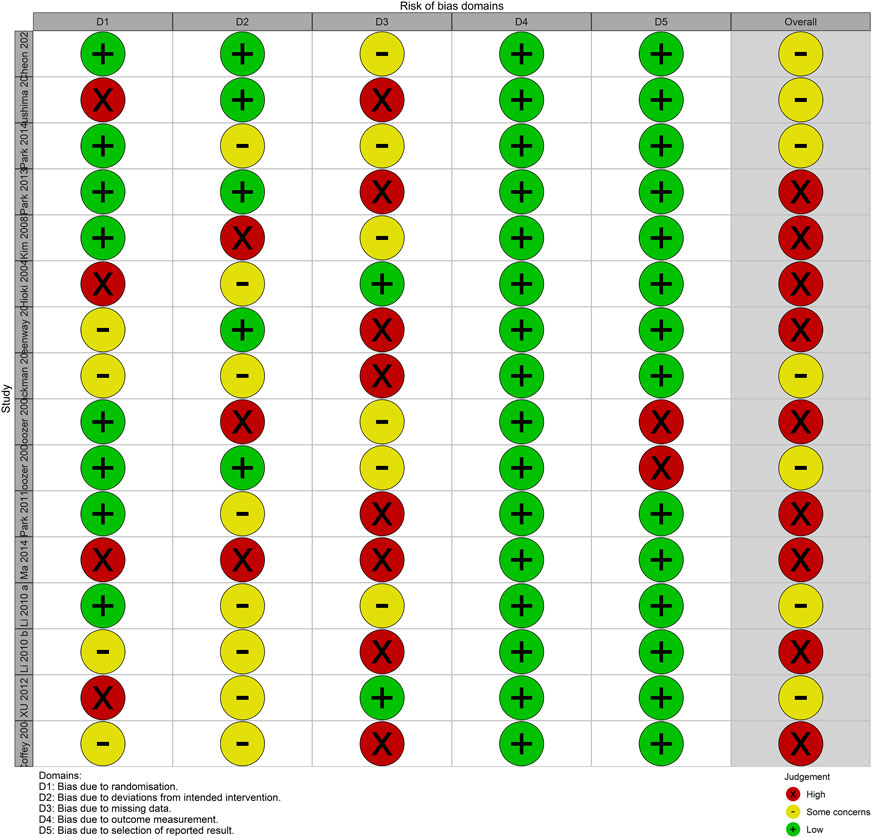
None of the analyzed studies demonstrated a low overall risk of bias, whereas all studies had some concerns or a high overall risk of bias. The risk of bias for the outcome measurement was low because the outcome measure in all studies was an objective metric. Except for two studies (Boozer et al., 2001; Boozer et al., 2002) that reported measurements of height and weight without reporting BMI as an outcome, all other studies reported all outcomes described in the Methods. Therefore, the risk of bias in the selection of reported results was mostly low. However, in some studies (C et al., 2004; Azushima et al., 2015; Feng-Hao et al., 2012; MA et al., 2014), a third party did not perform the randomization process or was not properly blinded, raising concerns about the potential for bias in the randomization process. In addition, the risk of bias was high for deviations from the intended interventions and missing outcome data owing to the occurrence of adverse effects that could be inferred from the intervention, and a high number of dropouts for unclear reasons (Figure 2).
3.3 Meta-analysis of intervention effects
A meta-analysis was conducted to examine the effectiveness of EOMs in reducing BMI, BW, and WC. First, 11 studies reported the change in the BMI as an outcome measure, of which 5 reported the amount of change and SD value, while 6 reported the final value and SD value. Forest plots were generated to present each subgroup’s changes and final values, and the overall effects were pooled. The study by Hackman et al. (2006) was excluded because the SD values and raw data were not available. The meta-analysis revealed that EOMs resulted in a statistically significant additional reduction in the BMI compared to the control group (MD = −0.38 kg/m2; 95% CI= −0.68 to −0.09) (Figure 3A).
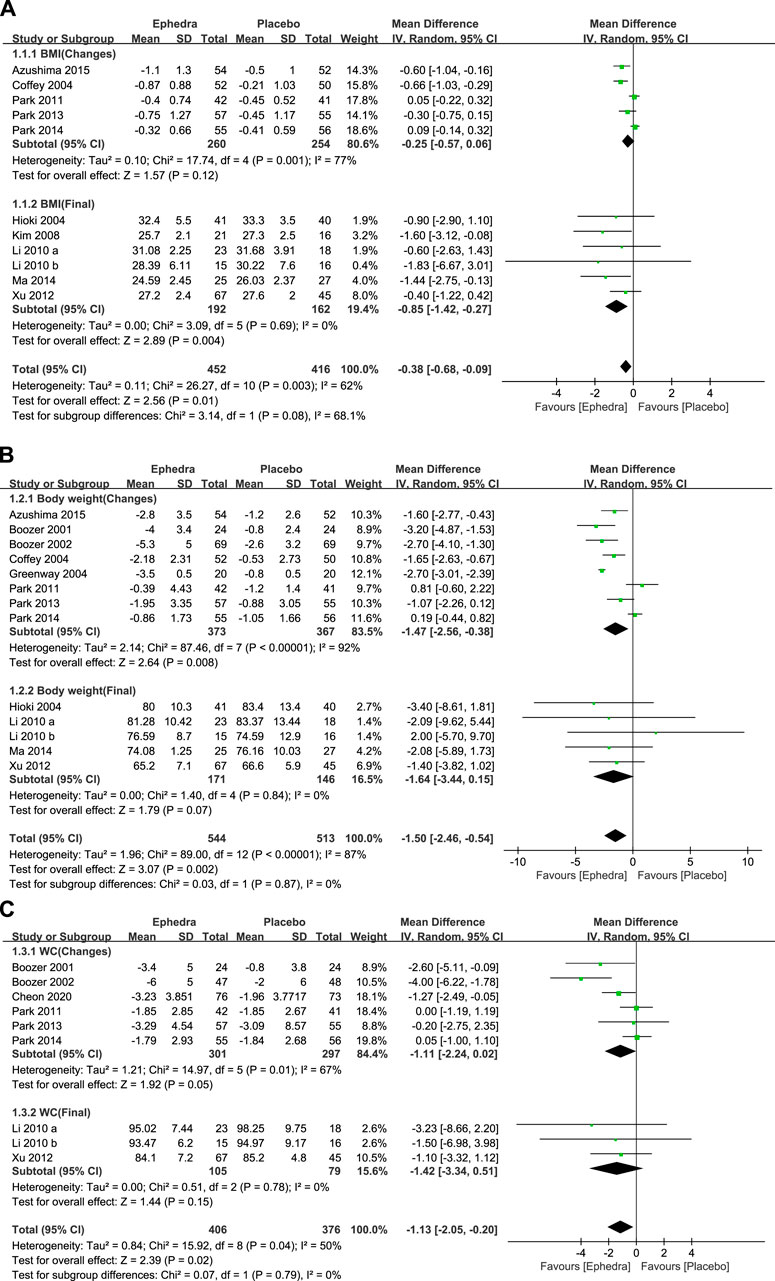
Figure 3. (A) Forest plot showing the effect of ephedra on BMI; (B) forest plot showing the effect of ephedra on BW; and (C) Forest plot showing the effect of ephedra on WC.
In addition, 13 studies reported changes in BW as an outcome measure, of which 8 reported changes and SD values, while 5 reported final values and SD values. The study by Hackman et al. (2006) was excluded for the same reason. The meta-analysis showed that EOMs resulted in a statistically significant additional reduction in BW compared to the control group (MD = −1.5 kg; 95% CI= −2.46 to −0.54) (Figure 3B).
Nine studies reported changes in WC as an outcome measure, of which six reported change and SD values and three reported final and SD values. The meta-analysis presented that EOMs resulted in a statistically significant additional reduction in WC compared to the control group (MD = −1.13 cm; 95% CI= −2.05 to −0.20) (Figure 3C).
3.4 Adverse events reported
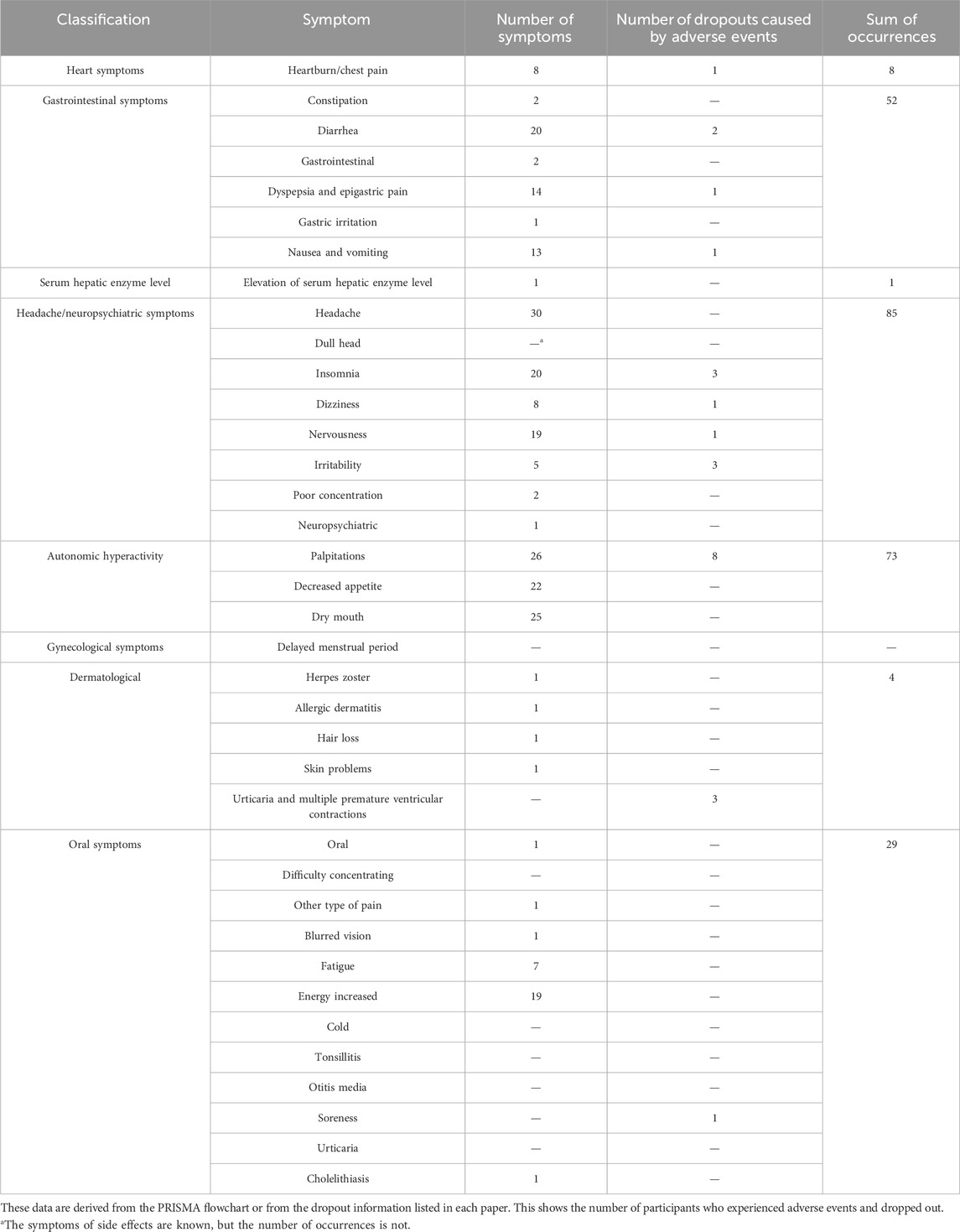
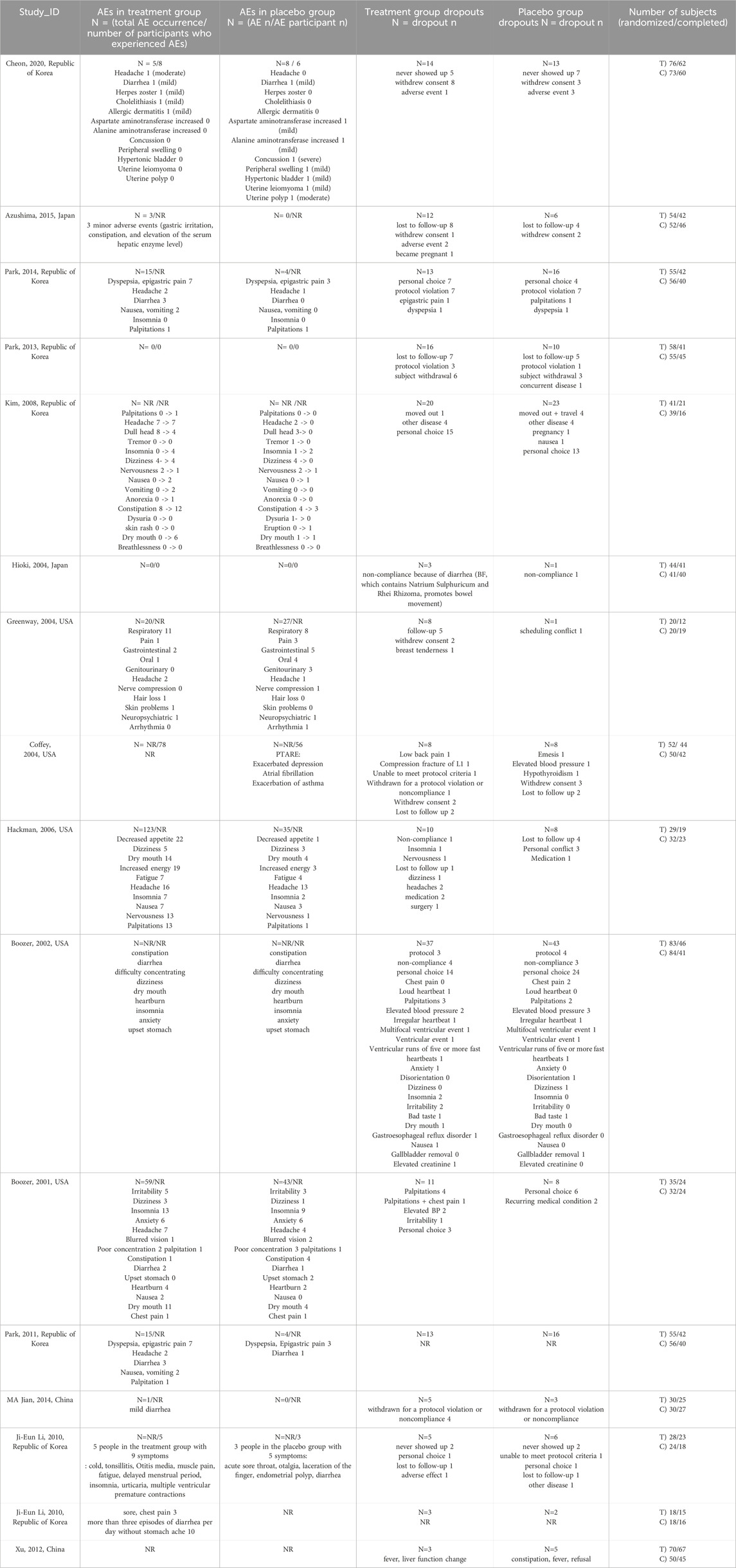
The studies by Kim et al. (2008), Coffey et al. (2004), Boozer et al. (2002), and Feng-Hao et al. (2012), which did not report the number of adverse events in the treatment groups, were excluded, the remaining 12 studies were examined. Adverse events were classified into several categories: cardiac, gastrointestinal, serum hepatic enzyme levels, headache/neuropsychiatric, autonomic hyperactivity, gynecological, and dermatological symptoms. The types and numbers of adverse events are summarized in Table 2. The number of dropouts due to these adverse effects was also included. The most frequently reported adverse events were headache and neuropsychiatric symptoms, with headache being the most common. Additionally, the number of adverse events, participants who experienced adverse events, dropouts, and reasons for dropouts in both the treatment and control groups for all 16 studies are summarized in Table 3.
A meta-analysis was performed using data from five studies that reported the number of participants who experienced adverse events. There was no statistically significant difference between the treatment and control groups (RR = 0.99; 95% CI = 0.80–1.21) (Figure 4A). A meta-analysis of the number of dropouts across all selected studies showed no statistically significant differences between the treatment and control groups (RR = 0.99; 95% CI = 0.83–1.17) (Figure 4B).
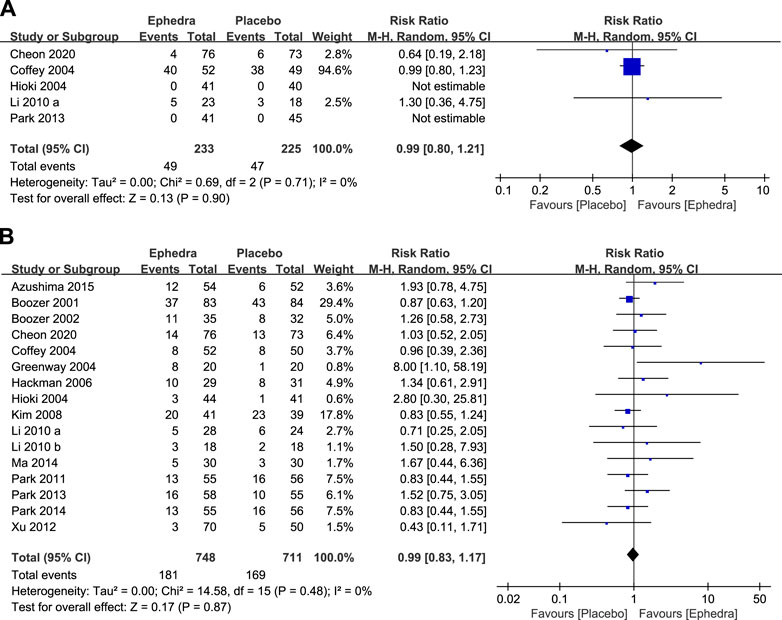
Figure 4. (A) Forest plot for participants who experienced adverse effects and (B) Forest plot for dropouts.
3.5 Meta-regression and bubble plot results
In the meta-analysis of post-treatment changes in the BMI, BW, and WC, the chi2 test yielded a value of p < 0.10, and the I2 values were 62, 87, and 50%, respectively, indicating high heterogeneity among the studies. Therefore, a univariate meta-regression analysis explored the relationship between heterogeneity and covariates. For change in BMI, both the daily dose of ephedrine (QM = 18.5143, p < 0.001, tau2 = 0, I2 = 0%, and R2 = 100%) and duration (QM = 4.4590, p = 0.0347, tau2 = 0.0313, I2 = 29.34%, and R2 = 69.50%) explained the heterogeneity but not the initial BMI (QM = 1.0719, p = 0.3005, tau2 = 0.0625, I2 = 44.22%, and R2 = 39.20%). For changes in BW, the daily dose of ephedrine (QM = 14.0831, p = 0.0002, tau2 = 0.3087, I2 = 37.74%, and R2 = 85.93%) explained the heterogeneity, but the period (QM = 2.3635, p = 0.1242, tau2 = 1.5880, I2 = 83.14%, and R2 = 27.62%) and initial BW (QM = 3.4327, p = 0.0639, tau2 = 1.4732, I2 = 81.47%, and R2 = 32.85%) did not explain the heterogeneity. Finally, for changes in WC, the period (QM = 9.8250, p = 0.0017, tau2 = 0, I2 = 0%, and R2 = 100%) explained heterogeneity as a variable, but the daily dose of ephedrine (QM = 2.7709, p = 0.0960, tau2 = 0.4313, I2 = 33.55%, and R2 = 48.71%) and initial WC (QM = 0.0012, p = 0.9724, tau2 = 1.1167, I2 = 55.66%, and R2 = 0%) did not explain the heterogeneity (Table 4). The results of the meta-regression analysis are visualized using bubble plots (Supplementary Figure S1).
We also generated bubble plots for 10 studies reporting changes in BMI, 12 studies reporting changes in BW, and 9 studies reporting changes in WC, all of which reported the daily doses of ephedra or ephedrine. We developed a four-dimensional graph with the x-axis indicating the baseline measurement of the outcome variable, the y-axis indicating the daily dose of ephedrine, the color of the bubble indicating the study period, and the size of the bubble representing the reduction in the outcome measure (Figures 5A–C). In the same study, we developed another type of bubble plot for each outcome measure. The x-axis represents the outcome measure at baseline, the y-axis represents the total ephedrine dose, and the bubble size represents a reduction in the outcome measure (Supplementary Figure S2).
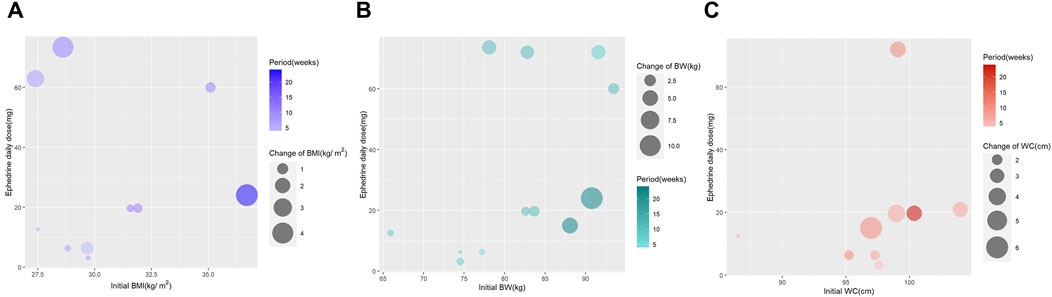
Figure 5. (A) Bubble plot for body mass index change; (B) bubble plot for body weight change; and (C) bubble plot for waist circumference change.
3.6 Assessment of publication bias results
We assessed the potential for publication bias in the outcome variables, BMI and BW, using 10 or more studies included in the meta-analysis. Contour-enhanced funnel plots showed asymmetry in all outcome measures, confirming the possibility of publication bias (Figures 6A, B). Egger’s test was used to assess publication bias. There was no statistically significant publication bias for BW (p = 0.3361); however, there was a possibility of publication bias for BMI (p = 0.0300).
4 Discussion
4.1 Summary of findings
This study aimed to determine the efficacy and safety of EOMs in overweight and obese individuals. The 16 RCTs that included ephedra in interventions for individuals with a BMI of 25 or greater were identified, and their characteristics, interventions, and adverse effects were summarized. A meta-analysis was performed on the outcome measures of BMI, BW, and WC to determine the statistical significance of these effects. The results showed a statistically significant reduction in all outcome measures in the treatment group compared with the control group. A meta-analysis was also performed on adverse events and dropouts and showed no statistically significant difference between the treatment and control groups. Furthermore, a meta-regression analysis was conducted to explore the relationship between heterogeneity in the meta-analysis of effects and covariates. The results indicated that the daily dose of ephedrine and treatment period explained the heterogeneity in BMI change, a daily dose of ephedrine explained the heterogeneity in BW change, and the treatment period explained the heterogeneity in WC change. Additionally, bubble plots were used to visually demonstrate that the covariates were proportionally associated with reductions in the outcome measures (BMI, BW, and WC).
4.1.1 Debate: the efficacy of ephedra on obesity
With the growing popularity of EATM, herbal medicine has received considerable attention as a treatment for obesity. Ephedra is one of the preferred herbal medications for the treatment of obesity, as identified in the Korean Medicine Clinical Practice Guideline for Obesity, which lists several herbal formulas, including Fangfeng Tong Sheng San, Taeumjowitang, Euiiyin-tang, and Chegamuiiyin-tang (Wooltorton and Sibbald, 2002). These formulas have been extensively studied in the Republic of Korea and reported to have clinical effects (Hwang et al., 2007). Previous clinical trials using ephedra have shown that they effectively reduce obesity-related markers such as BW and WC (Boozer et al., 2002). In addition, several systematic reviews have reported statistically significant weight-loss effects in the majority of interventions containing ephedra, further supporting its efficacy as a medication for obesity treatment (Hasani-Ranjbar et al., 2009; Maunder et al., 2020). The results of this systematic review, which synthesized existing studies, support ephedra’s previously reported weight-loss effects. Furthermore, the daily dose of ephedra and the treatment period may have a dose–response relationship, which requires further investigation in additional studies. Moreover, there was a weak association with baseline body weight, suggesting that ephedra may be used for individuals with a BMI of 25 or higher, irrespective of their initial weight status.
The best-known mechanism for the weight-loss effects of ephedra is through the modulation of the sympathetic nervous system to increase metabolism and exercise capacity. In addition, Park et al. (Park SJ. et al., 2022; Park WY. et al., 2022) showed that ephedra plays a dual role in energy metabolism, inhibiting lipogenesis and promoting thermogenesis through browning in the mature state. It has also been reported to exhibit anti-obesity effects by affecting the gut microbiota associated with fat accumulation (Kim et al., 2014). As such, ephedra is a promising drug for treating obesity as it may act not only through sympathetic nervous system stimulation but also other various mechanisms. Research based on the network pharmacology of EOMs is increasingly being conducted. Based on these studies, it is predicted that new mechanisms for the treatment of obesity will be further elucidated that have not been previously predicted and that involve ephedra and other traditional herbal prescriptions, (Jang et al., 2021).
4.1.2 Debate: the safety of ephedra
Ephedra is an herbal medicine that has been the subject of ongoing safety concerns (Bent et al., 2003). Despite these concerns, prior to the 2004 FDA ban, ephedra remained unregulated as a food product in the United States, leading to its widespread misuse. Continued reports of adverse effects eventually prompted the FDA to prohibit the sale of dietary supplements containing ephedra (Palamar, 2011). Similarly, the European Union (EU), Canada, and Australia imposed bans on ephedra-containing food products for the same reasons. Furthermore, the European Food Safety Authority (EFSA) concluded that the variability in ephedrine alkaloid content and distribution across Ephedra species makes it difficult to establish a safe daily intake as a food (EFSA Panel on Food Additives and Nutrient Sources added to Food ANS, 2013).
Ephedra is prohibited by global sports organizations such as the International Olympic Committee (IOC) and the National Collegiate Athletic Association (NCAA) due to its performance-enhancing effects and associated health risks (Powers, 2001; Keisler and Hosey, 2005). Additionally, the World Anti-Doping Agency (WADA) includes ephedrine, the active compound in ephedra, on its list of banned substances (WADA, 2023).
In contrast, ephedra continues to be used in East Asian countries for medicinal purposes, where it is listed in the pharmacopeias of each nation and regulated as a pharmaceutical product under specific standards. Its use is controlled through prescriptions by medical professionals (Tang et al., 2023; WADA, 2023; The Minister of Health and Labour and Welfare, 2016; Kim and Oh, 2020). In the Republic of Korea, the Ministry of Food and Drug Safety (MFDS) has prohibited the distribution of ephedra as a food product and has strictly regulated it as a pharmaceutical product (Kim et al., 2006). While this does not guarantee safety from all potential risks, some reports suggest that using ephedra under medical supervision results in fewer side effects than self-administration of dietary supplements containing ephedra (Lee et al., 2020; Mehendale et al., 2004; Lin et al., 2012).
Before the FDA ban on ephedra, there were reports that dietary supplements containing ephedra caused serious adverse events, including death (Haller and Benowitz, 2000), which led to an FDA ban on the sale of such supplements in 2004 (Miao et al., 2020; Mehendale et al., 2004). Approximately half of the adverse effects of ephedra reported to the FDA are cardiovascular, including coronary artery constriction, vasospasm, arrhythmias, and diseases secondary to hypertension due to the overactivation of the sympathetic nervous system (Wooltorton and Sibbald, 2002). Naik and Freudenberger (2004) clinically demonstrated that coronary artery constriction and vasospasm through sympathomimetic effects have the potential to cause myocarditis. This safety concern has resulted in restrictions on its use based on patient characteristics such as heart disease, hypertension, diabetes, anxiety, and glaucoma (Hsing et al., 2006).
These adverse effects are thought to be due to the toxicity of the alkaloids present in ephedra (Tang et al., 2023). Odaguchi et al. (2019) found that alkaloid-free ephedra extracts may contribute to a lower incidence of adverse effects, suggesting that attention should be paid to alkaloids for their safe use. In addition, endogenous catecholamines released by ephedrine directly or indirectly stimulate the sympathetic nervous system, resulting in central nervous system symptoms, such as mental excitement, insomnia, and wakefulness (Maglione et al., 2005). However, the non-alkaloidal components of ephedra have been used to treat obesity, asthma, and pain through antioxidant, anti-inflammatory, and immunosuppressive mechanisms, suggesting their potential for use in various diseases (Powell-Wiley et al., 2021). Nevertheless, the lack of clarity regarding safety issues may account for the recent reduced use of ephedra.
In this study, we reviewed adverse events in RCTs and found no evidence that the treatment group had a significantly higher dropout rate or adverse events. Although this suggests that its use in a controlled environment, such as a clinical trial, does not result in a higher rate of adverse events, further research is required to assess its safety in the real world. However, when prescribed within the FDA-approved daily ephedrine dose range of 150 mg (Kim et al., 2007), as in this study, the risk of severe adverse events from ephedra is considered to be relatively low, as is the case of herbal medicines with medical monitoring.
Among the studies included in this review, Hackman et al. (2006) reported the highest adverse effects, including decreased appetite and increased energy. However, these are common mechanisms for treating obesity (Christoffersen et al., 2022; Shim et al., 2017), and Miao et al. (2020) and Alraei (2010) reported an increase in energy metabolism as a major mechanism for weight loss. Therefore, depending on how adverse effects are defined, it may be possible to overestimate ephedra’s adverse effects. Further consensus among clinicians and researchers is required to determine which symptoms should be considered adverse effects.
4.1.3 Debate: existing studies on EATM dosage and the dose–response relationship of ephedra in the treatment of obesity
Statistical approaches have been used to determine the optimal dose that maximizes the efficacy of EATM treatments. For instance, various studies have explored dose–response relationships in acupuncture. Qin et al. (2019) explored the dose–response relationship between the number of acupuncture treatments and their effectiveness in chronic prostatitis and pelvic pain syndrome, while Xu et al. (2022) used meta-regression to explore the dose–response relationship in major depressive disorders. Similar attempts have been made for EATM. For example, Tai et al. (2022) utilized real-world data from patients with heart failure to demonstrate the dose–response relationship of the Fuzi (Radix Aconiti Lateralis Preparata) dose with the occurrence of composite cardiovascular events and the association of timing with prognosis.
However, no studies have explored the dose–response relationship between herbal medicines and acupuncture in obesity. This need is particularly pronounced in the case of ephedra, given the significant concerns regarding side effects. Therefore, it is important to determine the optimal dose of ephedra that can safely produce therapeutic effects. Our study found an association between the duration or daily dose of ephedra and treatment outcomes; however, owing to the small number of studies and inconsistent results between endpoints, further research is needed.
4.2 Strengths and limitations
This study has several strengths. Although previous reviews have examined herbal prescriptions for obesity, as well as single agents like ephedra and ephedrine (Park et al., 2012; Shekelle et al., 2003; Sui et al., 2012), no review has focused solely on ephedra. Hence, this systematic review is the first to comprehensively examine the use of ephedra, including single agents, mixed herbal formulas, and food supplements, for weight loss and treating obesity. Moreover, the selection of studies from diverse countries, formulations, and treatment periods allowed us to extensively evaluate their characteristics, therapeutic effects, and adverse effects. Notably, we summarized the types and frequencies of adverse events reported in the included studies to help identify possible trends in adverse events.
Additionally, dosage, duration, and patient baseline are important considerations in clinical practice. This study used meta-regression and bubble plots to explore the relationship between these factors and treatment effects. The results of the meta-regression analysis showed that the daily dose of ephedrine and the treatment period explained a significant amount of heterogeneity in the therapeutic effect. The bubble plot also illustrates the potential relationship between these factors and their therapeutic effects. These findings may provide a basis for further research into the dose–response relationship between treatment period and ephedra dose in treating obesity.
However, this study should be interpreted with caution due to the following limitations. First, the majority of the interventions included in the studies encompassed ephedra and other herbs or chemicals that may have contributed to the treatment effect. Therefore, it cannot be concluded that the results of this meta-analysis are solely attributable to ephedra. In order to evaluate ephedra in combination formulations, further pharmacokinetic and pharmacodynamic studies are required, considering drug interactions between herbal ingredients (Luo et al., 2020). Currently, various studies, including network pharmacology analyses and pharmacokinetic experiments, are being conducted (Miao et al., 2020; Song et al., 2015; Wei et al., 2014). It is deemed necessary to carry out additional research on the synergistic effects of ephedra in order to establish safe guidelines for its clinical use. However, it is noteworthy that EOMs have been reviewed and found to be effective. In clinical studies of EATM, it is impractical to administer ephedra as the sole agent. Therefore, to obtain data on human subjects, an alternative approach would be to analyze studies on EOMs.
Among the various active compounds in ephedra, ephedrine is recognized for its significant role in weight reduction (Miao et al., 2020). To explore the dose effect, this study assumed that ephedra formulations contained the same amount of ephedrine, 5.25 mg/g, the minimum content threshold suggested by the Korean Pharmacopoeia. However, ephedra formulations comprise natural, unprocessed products, and their ephedrine content varies depending on the origin and time of harvest (Matsumoto et al., 2015). There are also variations in the chemical composition of different species of ephedra, and little is known about the clinical differences caused by different alkaloids (Ibragic and Sofić, 2015). Therefore, the results of this study cannot be directly extrapolated to clinical practice. Given the variability due to individual differences between species, along with the effects and interactions when combined with other herbs and prescriptions, further research is necessary, as previously mentioned.
During the study selection process, several limitations were identified in the selection of outcome measures. First, we focused solely on whether the outcome measures included BMI, body weight, and waist circumference without imposing additional criteria. Consequently, the studies we included did not uniformly address the same liver function tests and metabolic markers, such as AST, ALT, BUN, leptin, and adiponectin, with some studies omitting these as outcome measures. As a result, we were unable to comprehensively evaluate the effects of ephedra on metabolic markers and liver function. Second, BMI, which considers only weight and height, is a measure that does not account for other factors, such as muscle mass. Specifically, in older populations, BMI is not an accurate predictor of obesity as it may underestimate obesity due to decreased muscle mass and increased body fat with age (Batsis et al., 2016).
Meta-regression analyses are deemed more meaningful when the number of studies is large, and it is recommended that at least 10 studies should be included (Higgins et al., 2019). However, this study was an exploratory attempt with few studies, especially for WC, and only nine studies met the recommended criteria. Therefore, only univariate meta-regression analyses were performed, and there were differences in significant covariates depending on the outcome measure. Furthermore, Egger’s test for publication bias recommends 10 or more studies in which the WC does not meet the criteria. In addition, bubble plots are solely visual graphs and do not offer conclusive evidence suggesting causality. Owing to the limitations mentioned above (variable ephedrine content of ephedra, insufficient number of studies, and limitations of bubble plots), we limited our meta-regression and bubble plots to exploratory attempts at determining dose–response relationships of ephedra. Nevertheless, as mentioned in the strengths section, these preliminary attempts provide a foundation for further research.
Finally, although we selected the daily dose of ephedrine as a key covariate, this does not mean that we considered the weight-loss effects of ephedra solely dependent on ephedrine. As mentioned earlier, ephedra is a multicomponent herb, and many different mechanisms explain its effects, with non-alkaloidal components contributing to its effects. However, several selected studies reported only the ephedrine dose rather than the ephedra dose. Therefore, we chose ephedrine as the baseline metric to compare the ephedra doses across studies.
4.3 Implications for further research and clinical practice
Adverse effects may be expected to increase with higher doses of ephedra; however, this analysis was limited by the small sample size, which needed to be increased for statistical power. As more high-quality RCTs that include pharmacokinetic studies are conducted, a dose–response analysis of ephedra’s efficacy and adverse effects may suggest a safe and appropriate dose of ephedra using methods such as the restricted cubic spline method (Cj et al., 2022).
However, due to unclear herbal constituents, many RCTs were excluded during the selection process. Even among the selected studies, some did not specify the dose of ephedra. High-quality RCTs that clearly describe the study methods, including constituent herbs and the dose of ephedra, are required to draw useful statistical conclusions in clinical practice. Additionally, there needs to be more uniformity in the presentation of drug ingredients and doses, such as ephedrine or ephedra doses. Thus, an international consensus on labeling standards for intervention composition and dose is needed for RCTs on natural products and herbal medicines.
However, the same drug may elicit different responses. For example, the optimal dosage of warfarin may be influenced by CYP2C9 and VKORC1 genotypes, and leveraging this genetic information in prescribing may mitigate adverse effects (Wang L. et al., 2011). Furthermore, recent studies have attempted to use genetics to predict drug responses, such as detecting drug resistance using gene chips (Yin et al., 2020). In addition, inhibiting and modulating certain genes at the fetal and neonatal stages can lead to a phenotype susceptible to cardiac ischemia (Zhang et al., 2021; Patterson et al., 2010; Lawrence et al., 2011). Integrating these genomic studies with the sensitivity to the effects and adverse reactions of ephedra could eventually lead to the determination of its indications and contraindications through genomic testing.
Exploring the impact of ephedra on different population groups would be valuable as this could offer clinicians a more comprehensive understanding of its use. For example, the effects and pharmacokinetic properties of a drug may vary based on factors such as sex and age (Schwartz, 2003; Davis et al., 2012; Busetto et al., 2009). However, in this study, no restrictions were placed on the population to allow for a more comprehensive analysis. Furthermore, given that our study is a secondary analysis (systematic review) based on a prospective RCT, it does not allow for the detailed assessment of effects across different population groups as we cannot acquire individual patient data. The studies we included aimed to balance the baseline characteristics between treatment and control groups through randomization. Furthermore, our review of existing studies revealed that many had conflicting results or involved small sample sizes, reducing their reliability (Haller and Benowitz, 2000; Samenuk et al., 2002; Gurley et al., 1998). However, as more RCTs incorporate diverse population groups in their designs, or as retrospective studies based on individual patient medical records on ephedra increase, it is anticipated that these avenues will provide broader insights into the effects and use of ephedra.
Metabolic markers such as leptin and adiponectin could serve as alternative indicators of the effect of ephedra on weight reduction or the treatment of metabolic disorders (Klempel and Varady, 2011; Yoon et al., 2011). Conversely, to evaluate safety, several cases have reported that ephedra has the potential to elevate liver enzymes and influence kidney function due to its diuretic effects (Saeed et al., 2019). Therefore, to accurately determine the weight-loss efficacy and safety of ephedra, it is essential to establish guidelines specifying which markers should be included as outcome measures. Incorporating these markers into study designs will contribute to higher-quality research in the future.
In conclusion, this study suggests that EOMs may effectively treat overweight and obesity with higher daily doses or longer treatment periods, possibly resulting in greater efficacy. However, because this may increase the risk of adverse events, clinicians must weigh the tradeoff between effectiveness and adverse effects before prescribing these drugs. We anticipate that further research will contribute to the development of professional and standardized guidelines, ensuring the safe use of ephedra in clinical practice. The necessity to mandate the precise specification of the amounts of ephedra and ephedrine used in clinical research should be thoroughly discussed.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material; further inquiries can be directed to the corresponding authors.
Author contributions
HyC: data curation, formal analysis, investigation, resources, visualization, writing–original draft. JO: data curation, formal analysis, investigation, resources, visualization, writing–original draft. HoC: conceptualization, methodology, writing–review and editing. HJ: methodology, project administration, resources, validation, writing – original draft, writing–review and editing. JL: conceptualization, funding acquisition, methodology, software, supervision, writing–review and editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Korea Basic Science Institute (National research Facilities and Equipment Center) grant funded by the Korea government (MSIT) (No. RS-2024-00403500), and by a grant from the Korea Health Technology Project through the Korea Health Industry Development Institute, funded by the Ministry of Health and Welfare, Republic of Korea (grant no. RS-2023-KH138802).
Acknowledgments
This paper results from research conducted through the Undergraduate Research Program (URP) at Wonkwang University College of Korean Medicine in 2023.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2024.1397247/full#supplementary-material
References
Alraei, R. G. (2010). Herbal and dietary supplements for weight loss. Top. Clin. Nutr. 25 (2), 136–150. doi:10.1097/tin.0b013e3181dbb85e
Azushima, K., Tamura, K., Haku, S., Wakui, H., Kanaoka, T., Ohsawa, M., et al. (2015). Effects of the oriental herbal medicine Bofu-tsusho-san in obesity hypertension: a multicenter, randomized, parallel-group controlled trial (ATH-D-14-01021.R2). Atherosclerosis 240 (1), 297–304. doi:10.1016/j.atherosclerosis.2015.01.025
Batsis, J. A., Mackenzie, T. A., Bartels, S. J., Sahakyan, K. R., Somers, V. K., and Lopez-Jimenez, F. (2016). Diagnostic accuracy of body mass index to identify obesity in older adults: NHANES 1999-2004. Int. J. Obes. (Lond) 40 (5), 761–767. doi:10.1038/ijo.2015.243
Bent, S., Tiedt T, N., Odden M, C., and Shlipak M, G. (2003). The relative safety of ephedra compared with other herbal products. Ann. Intern. Med. 138, 468–471. doi:10.7326/0003-4819-138-6-200303180-00010
Blackburn, G. L., and Walker, W. A. (2005). Science-based solutions to obesity: what are the roles of academia, government, industry, and health care? Am. J. Clin. Nutr. 82 (1 Suppl. l), 207S–210S. doi:10.1093/ajcn/82.1.207S
Bomberg, E., Birch, L., Endenburg, N., German, A. J., Neilson, J., Seligman, H., et al. (2017). The financial costs, behaviour and psychology of obesity: a one health analysis. J. Comp. Pathology 156 (4), 310–325. doi:10.1016/j.jcpa.2017.03.007
Boozer, C., Daly, P., Homel, P., Solomon, J., Blanchard, D., Nasser, J., et al. (2002). Herbal ephedra/caffeine for weight loss: a 6-month randomized safety and efficacy trial. Int. J. Obes. 26 (5), 593–604. doi:10.1038/sj.ijo.0802023
Boozer, C., Nasser, J., Heymsfield, S., Wang, V., Chen, G., and Solomon, J. (2001). An herbal supplement containing Ma Huang-Guarana for weight loss: a randomized, double-blind trial. Int. J. Obes. 25 (3), 316–324. doi:10.1038/sj.ijo.0801539
Busetto, L., Mazza, M., Salvalaio, S., De Stefano, F., Marangon, M., Calò, E., et al. (2009). Obesity treatment in elderly outpatients: predictors of efficacy and drop-out. Eat. Weight Disord. 14 (2–3), e56–e65. doi:10.1007/BF03327801
Caroline, M., and Apovian, M. D. (2016). Obesity: definition, comorbidities, causes, and burden. Suppl. Featur. Publ. 22 (7 Suppl. l).
C, H., K, Y., and T, Y. (2004). Efficacy of bofu-tsusho-san, an oriental herbal medicine, in obese Japanese women with impaired glucose tolerance. Clin. Exp. Pharmacol. and physiology 31 (9), 614–619. doi:10.1111/j.1440-1681.2004.04056.x
Cheon, C., Song, Y. K., and Ko, S. G. (2020). Efficacy and safety of Euiiyin-tang in Korean women with obesity: a randomized, double-blind, placebo-controlled, multicenter trial. Complementary Ther. Med. 51, 102423. doi:10.1016/j.ctim.2020.102423
Christoffersen, B. Ø., Sanchez-Delgado, G., John, L. M., Ryan, D. H., Raun, K., and Ravussin, E. (2022). Beyond appetite regulation: targeting energy expenditure, fat oxidation, and lean mass preservation for sustainable weight loss. Obes. (Silver Spring) 30 (4), 841–857. doi:10.1002/oby.23374
Cj, T., M, E. S., Yh, Y., Yh, T., D, C., J, H., et al. (2022). The effectiveness of Fuzi in combination with routine heart failure treatment on chronic heart failure patients. J. Ethnopharmacol.
Coffey, C. S., Steiner, D., Baker, B. A., and Allison, D. B. (2004). A randomized double-blind placebo-controlled clinical trial of a product containing ephedrine, caffeine, and other ingredients from herbal sources for treatment of overweight and obesity in the absence of lifestyle treatment. Int. J. Obes. 28 (11), 1411–1419. doi:10.1038/sj.ijo.0802784
Davis, C., Fattore, L., Kaplan, A. S., Carter, J. C., Levitan, R. D., and Kennedy, J. L. (2012). The suppression of appetite and food consumption by methylphenidate: the moderating effects of gender and weight status in healthy adults. Int. J. Neuropsychopharmacol. 15 (2), 181–187. doi:10.1017/S1461145711001039
Davis, M. A., West, A. N., Weeks, W. B., and Sirovich, B. E. (2011). Health behaviors and utilization among users of complementary and alternative medicine for treatment versus health promotion. Health Serv. Res. 46 (5), 1402–1416. doi:10.1111/j.1475-6773.2011.01270.x
Delpino, F. M., and Figueiredo, L. M. (2021). Melatonin supplementation and anthropometric indicators of obesity: a systematic review and meta-analysis. Nutrition 91–92, 111399. doi:10.1016/j.nut.2021.111399
DerSimonian, R., and Laird, N. (1986). Meta-analysis in clinical trials. Control Clin. Trials 7 (3), 177–188. doi:10.1016/0197-2456(86)90046-2
DerSimonian, R., and Laird, N. (2015). Meta-analysis in clinical trials revisited. Contemp. Clin. Trials 45 (Pt A), 139–145. doi:10.1016/j.cct.2015.09.002
EBM-based Obesity KMCPG Development Committee (2016). Korean traditional medicinal clinical practice guidelines of obesity.
EFSA Panel on Food Additives and Nutrient Sources added to Food (ANS) (2013). Scientific Opinion on safety evaluation of Ephedra species for use in food. Sci. Opin. Saf. Eval. Ephedra species use food 11 (11). doi:10.2903/j.efsa.2013.3467
Egger, M., Davey Smith, G., Schneider, M., and Minder, C. (1997). Bias in meta-analysis detected by a simple, graphical test. BMJ 315 (7109), 629–634. doi:10.1136/bmj.315.7109.629
Engin, A. (2017). “The definition and prevalence of obesity and metabolic syndrome,” in Obesity and lipotoxicity. Editors A. B. Engin,, and A. Engin (Cham: Springer International Publishing), 1–17.
Feng-Hao, X., Kazuo, U., Hiroko, O., Masayuki, M., and Hideki, O. (2012). Personalized effects of a Kampo herbal formulation on metabolism-A randomized, double-blind, placebo controlled study of Bohu-tsusei-san-. 東方医学 28 (1), 37–59.
Flegal, K. M., Graubard, B. I., Williamson, D. F., and Gail, M. H. (2005). Excess deaths associated with underweight, overweight, and obesity. JAMA 293 (15), 1861–1867. doi:10.1001/jama.293.15.1861
Gurley, B. J., Gardner, S. F., White, L. M., and Wang, P. L. (1998). Ephedrine pharmacokinetics after the ingestion of nutritional supplements containing Ephedra sinica (ma huang). Ther. Drug Monit. 20 (4), 439–445. doi:10.1097/00007691-199808000-00015
G, X., H, L., L, H., Q, X., B, H., Z, Z., et al. (2022). The dose-effect association between acupuncture sessions and its effects on major depressive disorder: a meta-regression of randomized controlled trials. J. Affect. Disord. 310, 318–327. doi:10.1016/j.jad.2022.04.155
Hackman, R. M., Havel, P. J., Schwartz, H. J., Rutledge, J. C., Watnik, M. R., Noceti, E. M., et al. (2006). Multinutrient supplement containing ephedra and caffeine causes weight loss and improves metabolic risk factors in obese women: a randomized controlled trial. Int. J. Obes. 30 (10), 1545–1556. doi:10.1038/sj.ijo.0803283
Haller, C. A., and Benowitz, N. L. (2000). Adverse cardiovascular and central nervous system events associated with dietary supplements containing ephedra alkaloids. N. Engl. J. Med. 343 (25), 1833–1838. doi:10.1056/NEJM200012213432502
Hasani-Ranjbar, S., Nayebi, N., Larijani, B., and Abdollahi, M. (2009). A systematic review of the efficacy and safety of herbal medicines used in the treatment of obesity. World J. Gastroenterology WJG 15 (25), 3073–3085. doi:10.3748/wjg.15.3073
He, Z., Barrett, L. A., Rizvi, R., Tang, X., Payrovnaziri, S. N., and Zhang, R. (2020). Assessing the use and perception of dietary supplements among obese patients with national health and nutrition examination survey. AMIA Jt. Summits Transl. Sci. Proc. 2020, 231–240.
Higgins, J. P. T., Thomas, J., Chandler, J., Cumpston, M., Li, T., Page, M. J., et al. (2019). Cochrane Handbook for systematic reviews of interventions. 2nd Edition (Chichester (UK): John Wiley and Sons).
Higgins, J. P. T., Thompson, S. G., Deeks, J. J., and Altman, D. G. (2003). Measuring inconsistency in meta-analyses. BMJ 327 (7414), 557–560. doi:10.1136/bmj.327.7414.557
Hsing, L., Lee, T., Son, D., Yeo, J., Yang, C., Seo, E., et al. (2006). Adverse effects of ma-huang according to dose: a randomized double-blind placebo-controlled pilot study. J. Korean Orient. Intern. Med. 27 (1), 188–196.
Hwang, M.-J., Shin, H.-D., and Song, M.-Y. (2007). Literature review of herbal medicines on treatment of obesity since 2000;Mainly about ephedra herba. J. Soc. Korean Med. Obes. Res. 7 (1), 39–54.
Ibragic, S., and Sofić, E. (2015). Chemical composition of various Ephedra species. Bosn. J. Basic Med. Sci. 15 (3), 21–27. doi:10.17305/bjbms.2015.539
Ioannides-Demos, L. L., Proietto, J., Tonkin, A. M., and McNeil, J. J. (2006). Safety of drug therapies used for weight loss and treatment of obesity. Drug-Safety 29 (4), 277–302. doi:10.2165/00002018-200629040-00001
Jac, S., J, S., Mj, P., Rg, E., Ns, B., I, B., et al. (2019). RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ Clin. Res. 366, l4898. doi:10.1136/bmj.l4898
Jammah, A. A. (2015). Endocrine and metabolic complications after bariatric surgery. Saudi J. Gastroenterology Official J. Saudi Gastroenterology Assoc. 21 (5), 269–277. doi:10.4103/1319-3767.164183
Jang, D., Jeong, H., Kim, C. E., and Leem, J. (2021). A system-level mechanism of anmyungambi decoction for obesity: a network pharmacological approach. Biomolecules 11 (12), 1881. doi:10.3390/biom11121881
Jang, I. S., Yang, C. S., and Hwang, E. H. (2007). The need for clinical practice guidelines in usage of mahuang in weight loss. J. Korean Med. Obes. Res. 7 (1), 23–29.
Keisler, B. D., and Hosey, R. G. (2005). Ergogenic aids: an update on ephedra. Curr. Sports Med. Rep. 4 (4), 231–235. doi:10.1007/s11932-005-0042-4
Kim, B. S., Song, M. Y., and Kim, H. (2014). The anti-obesity effect of Ephedra sinica through modulation of gut microbiota in obese Korean women. J. Ethnopharmacol. 152 (3), 532–539. doi:10.1016/j.jep.2014.01.038
Kim, H., Han, C.-H., Lee, E.-J., Song, Y.-K., Shin, B.-C., and Kim, Y.-K. (2007). A clinical practice guideline for ma-huang(ephedra sinica) prescription in obesity. J. Soc. Korean Med. Obes. Res. 7 (2), 27–37.
Kim, H. J., Park, J. M., Kim, J. A., and Ko, B. P. (2008). Effect of herbal ephedra sinica and evodia rutaecarpa on body composition and resting metabolic rate: a randomized, double-blind clinical trial in Korean premenopausal women. J. Acupunct. Meridian Stud. 1 (2), 128–138. doi:10.1016/S2005-2901(09)60033-9
Kim, J. Y., Kim, D. B., and Lee, H. J. (2006). Regulations on health/functional foods in Korea. Toxicology 221 (1), 112–118. doi:10.1016/j.tox.2006.01.016
Kim, K. Y., and Oh, J. E. (2020). Evaluation of pharmaceutical abuse and illicit drug use in South Korea by wastewater-based epidemiology. J. Hazard. Mater. 396, 122622. doi:10.1016/j.jhazmat.2020.122622
Kim, T. Y., You, S. E., and Ko, Y. S. (2018). Association between Sasang constitutional types with obesity factors and sleep quality. Integr. Med. Res. 7 (4), 341–350. doi:10.1016/j.imr.2018.06.007
Kissane, N. A., and Pratt, J. S. A. (2011). Medical and surgical treatment of obesity. Best Pract. and Res. Clin. Anaesthesiol. 25 (1), 11–25. doi:10.1016/j.bpa.2011.01.001
Klempel, M. C., and Varady, K. A. (2011). Reliability of leptin, but not adiponectin, as a biomarker for diet-induced weight loss in humans. Nutr. Rev. 69 (3), 145–154. doi:10.1111/j.1753-4887.2011.00373.x
Lawrence, J., Chen, M., Xiong, F., Xiao, D., Zhang, H., Buchholz, J. N., et al. (2011). Foetal nicotine exposure causes PKCε gene repression by promoter methylation in rat hearts. Cardiovasc Res. 89 (1), 89–97. doi:10.1093/cvr/cvq270
Lee, E., Yoon, S. H., Kim, H., Kim, Y. D., Leem, J., and Park, J. (2020). Ephedrae Herba in combination with herbal medicine (Zhizichi decoction and Phellodendri Cortex) for weight reduction: a case series. Integr. Med. Res. 9 (2), 100408. doi:10.1016/j.imr.2020.100408
Leibel, R. L., Rosenbaum, M., and Hirsch, J. (1995). Changes in energy expenditure resulting from altered body weight. N. Engl. J. Med. 332 (10), 621–628. doi:10.1056/NEJM199503093321001
Lin, Y., Hu, N., Chang, L., and Hou, M. (2012). P04.13. Population-based case-control study of Chinese herbal products containing ephedra and cardiovascular disease risk. BMC Complementary Altern. Med. 12 (1), P283. doi:10.1186/1472-6882-12-s1-p283
Luo, T. T., Lu, Y., Yan, S. K., Xiao, X., Rong, X. L., and Guo, J. (2020). Network pharmacology in research of Chinese medicine formula: methodology, application and prospective. Chin. J. Integr. Med. 26 (1), 72–80. doi:10.1007/s11655-019-3064-0
Maglione, M., Miotto, K., Iguchi, M., Jungvig, L., Morton, S. C., and Shekelle, P. G. (2005). Psychiatric effects of ephedra use: an analysis of food and drug administration reports of adverse events. AJP 162 (1), 189–191. doi:10.1176/appi.ajp.162.1.189
Ma, J., yan, Y. U. H., Zhao, N., and fang, WANG Y. (2014). Clinical research of TCM Prescription“Peilian Mahuang”for simple obesity. Inf. Traditional Chin. Med. 31 (1), 46–49.
Manson, J. E., Skerrett, P. J., Greenland, P., and VanItallie, T. B. (2004). The escalating pandemics of obesity and sedentary lifestyle. A call to action for clinicians. Arch. Intern Med. 164 (3), 249–258. doi:10.1001/archinte.164.3.249
Matsumoto, M., Hirayama, M., Ohtomi, N., Ohno, T., Nomura, Y., Iida, O., et al. (2015). Influence of genetic factors on the ephedrine alkaloid composition ratio of Ephedra plants. J. Nat. Med. 69 (1), 63–67. doi:10.1007/s11418-014-0863-7
Maunder, A., Bessell, E., Lauche, R., Adams, J., Sainsbury, A., and Fuller, N. R. (2020). Effectiveness of herbal medicines for weight loss: a systematic review and meta-analysis of randomized controlled trials. Diabetes, Obes. Metabolism 22 (6), 891–903. doi:10.1111/dom.13973
Mehendale, S. R., Bauer, B. A., and Yuan, C. S. (2004). Ephedra-containing dietary supplements in the US versus ephedra as a Chinese medicine. Am. J. Chin. Med. 32 (01), 1–10. doi:10.1142/S0192415X04001680
Miao, S. M., Zhang, Q., Bi, X. B., Cui, J. L., and Wang, M. L. (2020). A review of the phytochemistry and pharmacological activities of Ephedra herb. Chin. J. Nat. Med. 18 (5), 321–344. doi:10.1016/S1875-5364(20)30040-6
Mj, P., Je, M., Pm, B., I, B., Tc, H., Cd, M., et al. (2021). The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ Clin. Res., n71. doi:10.1136/bmj.n71
Naik, S. D., and Freudenberger, R. S. (2004). Ephedra-associated cardiomyopathy. Ann. Pharmacother. 38 (3), 400–403. doi:10.1345/aph.1D408
Ng, M., Fleming, T., Robinson, M., Thomson, B., Graetz, N., Margono, C., et al. (2014). Global, regional, and national prevalence of overweight and obesity in children and adults during 1980-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 384 (9945), 766–781. doi:10.1016/S0140-6736(14)60460-8
Odaguchi, H., Hyuga, S., Sekine, M., Nakamori, S., Takemoto, H., Huang, X., et al. (2019). The adverse effects of ephedra herb and the safety of ephedrine alkaloids-free ephedra herb extract (EFE). Yakugaku Zasshi 139 (11), 1417–1425. doi:10.1248/yakushi.19-00122
Ojukwu, M., Mbizo, J., Leyva, B., Olaku, O., and Zia, F. (2015). Complementary and alternative medicine use among overweight and obese cancer survivors in the United States. Integr. Cancer Ther. 14 (6), 503–514. doi:10.1177/1534735415589347
Palamar, J. (2011). How ephedrine escaped regulation in the United States: a historical review of misuse and associated policy. Health Policy 99 (1), 1–9. doi:10.1016/j.healthpol.2010.07.007
Park, J. H., Lee, M. J., Song, M. Y., Bose, S., Shin, B. C., and Kim, H. J. (2012). Efficacy and safety of mixed oriental herbal medicines for treating human obesity: a systematic review of randomized clinical trials. J. Med. Food 15 (7), 589–597. doi:10.1089/jmf.2011.1982
Park, S. J., Shon, D. H., Ryu, Y. H., and Ko, Y. (2022a). Extract of ephedra sinica stapf induces browning of mouse and human white adipocytes. Foods 11 (7), 1028. doi:10.3390/foods11071028
Park, W. Y., Song, G., Boo, M., Kim, H. I., Park, J. Y., Jung, S. J., et al. (2022b). Anmyungambi decoction ameliorates obesity through activation of non-shivering thermogenesis in Brown and white adipose tissues. Antioxidants (Basel) 12 (1), 49. doi:10.3390/antiox12010049
Patterson, A. J., Chen, M., Xue, Q., Xiao, D., and Zhang, L. (2010). Chronic prenatal hypoxia induces epigenetic programming of PKC{epsilon} gene repression in rat hearts. Circ. Res. 107 (3), 365–373. doi:10.1161/CIRCRESAHA.110.221259
Peters, J. L., Sutton, A. J., Jones, D. R., Abrams, K. R., and Rushton, L. (2008). Contour-enhanced meta-analysis funnel plots help distinguish publication bias from other causes of asymmetry. J. Clin. Epidemiol. 61 (10), 991–996. doi:10.1016/j.jclinepi.2007.11.010
Poddar, K., Kolge, S., Bezman, L., Mullin, G. E., and Cheskin, L. J. (2011). Nutraceutical supplements for weight loss: a systematic review. Nutr. Clin. Pract. 26 (5), 539–552. doi:10.1177/0884533611419859
Powell-Wiley, T. M., Poirier, P., Burke, L. E., Després, J.-P., Gorden-Larsen, P., Lavie, C. J., et al. (2021). Obesity and cardiovascular disease: a scientific statement from the American heart association. Circulation 143 (21), e984–e1010. doi:10.1161/CIR.0000000000000973
Powers, M. (2001). Ephedra and its application to sport performance: another concern for the athletic trainer? J. Athl. Train. 36, 420–424.
Qin, Z., Wu, J., Xu, C., and Liu, Z. (2019). Using meta-regression approach to explore the dose-response association between acupuncture sessions and acupuncture effects on chronic prostatitis/chronic pelvic pain syndrome. Ann. Transl. Med. 7 (6), 116. doi:10.21037/atm.2018.11.45
Rucker, D., Padwal, R., Li, S. K., Curioni, C., and Lau, D. C. W. (2007). Long term pharmacotherapy for obesity and overweight: updated meta-analysis. BMJ 335 (7631), 1194–1199. doi:10.1136/bmj.39385.413113.25
saeed, W., Dhamen, M., Ahmad, R., Ahmad, N., and Naqvi, A. (2019). Clinical uses and toxicity of ephedra sinica: an evidence-based comprehensive retrospective review (2004-2017). Pharmacogn. J. 11 (2), 439–444. doi:10.5530/pj.2019.11.68
Samenuk, D., Link, M. S., Homoud, M. K., Contreras, R., Theohardes, T. C., Wang, P. J., et al. (2002). Adverse cardiovascular events temporally associated with ma huang, an herbal source of ephedrine. Mayo Clin. Proc. 77 (1), 12–16. doi:10.4065/77.1.12
Schwartz, J. B. (2003). The influence of sex on pharmacokinetics. Clin. Pharmacokinet. 42 (2), 107–121. doi:10.2165/00003088-200342020-00001
Sg, T., and Jp, H. (2002). How should meta-regression analyses be undertaken and interpreted? Statistics Med. 21 (11), 1559–1573. doi:10.1002/sim.1187
Shekelle, P. G., Hardy, M. L., Morton, S. C., Maglione, M., Mojica, W. A., Suttorp, M. J., et al. (2003). Efficacy and safety of ephedra and ephedrine for weight loss and athletic performance: a meta-analysis. JAMA J. Am. Med. Assoc. 289 (12), 1537–1545. doi:10.1001/jama.289.12.1537
Shim, E. B., Leem, C. H., Kim, J. J., and Kim, J. Y. (2017). Lower cellular metabolic power can be an explanation for obesity trend in Tae-Eum type: hypothesis and clinical observation. Integr. Med. Res. 6 (3), 254–259. doi:10.1016/j.imr.2017.06.006
Song, S., Tang, Q., Huo, H., Li, H., Xing, X., and Luo, J. (2015). Simultaneous quantification and pharmacokinetics of alkaloids in herba ephedrae-radix aconiti Lateralis extracts. J. Anal. Toxicol. 39 (1), 58–68. doi:10.1093/jat/bku113
Spiotta, R. T., and Luma, G. B. (2008). Evaluating obesity and cardiovascular risk factors in children and adolescents. afp 78 (9), 1052–1058.
Sterne, J. A. C., Sutton, A. J., Ioannidis, J. P. A., Terrin, N., Jones, D. R., Lau, J., et al. (2011). Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ 343, d4002. doi:10.1136/bmj.d4002
Sui, Y., Zhao, H. L., Wong, V. C. W., Brown, N., Li, X. L., Kwan, A. K. L., et al. (2012). A systematic review on use of Chinese medicine and acupuncture for treatment of obesity: therapeutic effect of TCM on obesity. Obes. Rev. 13 (5), 409–430. doi:10.1111/j.1467-789X.2011.00979.x
Sundström, J., Risérus, U., Byberg, L., Zethelius, B., Lithell, H., and Lind, L. (2006). Clinical value of the metabolic syndrome for long term prediction of total and cardiovascular mortality: prospective, population based cohort study. BMJ 332 (7546), 878–882. doi:10.1136/bmj.38766.624097.1F
Tang, S., Ren, J., Kong, L., Yan, G., Liu, C., Han, Y., et al. (2023). Ephedrae herba: a review of its phytochemistry, pharmacology, clinical application, and alkaloid toxicity. Molecules 28 (2), 663. doi:10.3390/molecules28020663
The Minister of Health, Labour and Welfare (2016). Japanese pharmacopoeia. 17th edn. Tokyo, Japan: Ministry of Health, Labour and Welfare.
Tziomalos, K., Krassas, G. E., and Tzotzas, T. (2009). The use of sibutramine in the management of obesity and related disorders: an update. Vasc. Health Risk Manag. 5 (1), 441–452. doi:10.2147/vhrm.s4027
Uneda, K., Kawai, Y., Yamada, T., Kaneko, A., Saito, R., Chen, L., et al. (2022). Japanese traditional Kampo medicine bofutsushosan improves body mass index in participants with obesity: a systematic review and meta-analysis. PLoS One 17 (4), e0266917. doi:10.1371/journal.pone.0266917
WADA (2023). Prohibited list. WADA. Montreal, Canada: World Anti-Doping Agency. Available at: https://www.wada-ama.org/en/resources/world-anti-doping-program/2023-prohibited-list (Accessed September 29, 2022).
Wadden, T. A., Tronieri, J. S., and Butryn, M. L. (2020). Lifestyle modification approaches for the treatment of obesity in adults. Am. Psychol. 75 (2), 235–251. doi:10.1037/amp0000517
Wang, L., McLeod, H. L., and Weinshilboum, R. M. (2011b). Genomics and drug response. N. Engl. J. Med. 364 (12), 1144–1153. doi:10.1056/NEJMra1010600
Wang, Y. C., McPherson, K., Marsh, T., Gortmaker, S. L., and Brown, M. (2011a). Health and economic burden of the projected obesity trends in the USA and the UK. Lancet 378 (9793), 815–825. doi:10.1016/S0140-6736(11)60814-3
Wei, P., Huo, H. L., Hai, M. Q., Cheng, L. H., Feng, X. X., Mei, T. X., et al. (2014). Pharmacokinetic comparisons of five ephedrine alkaloids following oral administration of four different Mahuang-Guizhi herb-pair aqueous extracts ratios in rats. J. Ethnopharmacol. 155 (1), 642–648. doi:10.1016/j.jep.2014.05.065
Wooltorton, E., and Sibbald, B. (2002). Ephedra/ephedrine: cardiovascular and CNS effects. CMAJ 166 (5), 633.
Yin, G., Bie, S., Gu, H., Shu, X., Zheng, W., Peng, K., et al. (2020). Application of gene chip technology in the diagnostic and drug resistance detection of Helicobacter pylori in children. J. Gastroenterol. Hepatol. 35 (8), 1331–1339. doi:10.1111/jgh.14980
Yoon, J. H., Park, J. K., Oh, S. S., Lee, K. H., Kim, S. K., Cho, I. J., et al. (2011). The ratio of serum leptin to adiponectin provides adjunctive information to the risk of metabolic syndrome beyond the homeostasis model assessment insulin resistance: the Korean Genomic Rural Cohort Study. Clin. Chim. Acta. 412 (23–24), 2199–2205. doi:10.1016/j.cca.2011.08.003
Zell-Kanter, M., Quigley, M. A., and Leikin, J. B. (2015). Reduction in ephedra poisonings after FDA ban. N. Engl. J. Med. 372 (22), 2172–2174. doi:10.1056/NEJMc1502505
Keywords: ephedra, overweight, obesity, dose–response analysis, systematic review and meta-analysis
Citation: Cho H, Oh J, Chu H, Jin H and Leem J (2024) Efficacy and safety of ephedra-containing oral medications: a systematic review, meta-analysis, and exploratory dose–response analysis for weight reduction. Front. Pharmacol. 15:1397247. doi: 10.3389/fphar.2024.1397247
Received: 07 March 2024; Accepted: 10 October 2024;
Published: 30 October 2024.
Edited by:
Adolfo Andrade-Cetto, National Autonomous University of Mexico, MexicoReviewed by:
Soobin Jang, Daegu Haany University, Republic of KoreaIshtiaq Jeelani, University of California, San Diego, United States
Copyright © 2024 Cho, Oh, Chu, Jin and Leem. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hongmin Chu, Y2hobjI0NDNAd2t1LmFjLmty; Hanbit Jin, aGFuYml0amluMjJAZ21haWwuY29t; Jungtae Leem, anVsY2hvMDkwOEB3a3UuYWMua3I=
†These authors have contributed equally to this work and share first authorship
‡Present address:
Hanbit Jin, Department of Preventive Medicine, College of Korean Medicine, Dongshin University, Naju, Republic of Korea
 Hyeongyu Cho
Hyeongyu Cho Jeewoo Oh
Jeewoo Oh Hongmin Chu
Hongmin Chu Hanbit Jin
Hanbit Jin Jungtae Leem
Jungtae Leem