- 1Department of Pharmacy, Affiliated Hospital of North Sichuan Medical College, Nanchong, Sichuan, China
- 2School of Pharmacy, North Sichuan Medical College, Nanchong, Sichuan, China
- 3The First Clinical Medical College, Xuzhou Medical University, Xuzhou, Jiangsu, China
- 4Department of Pharmacy, Hospital of Chengdu Office of People’s Government of Tibetan Autonomous Region (Hospital.C.T), Chengdu, Sichuan, China
Objective: To mine and analyze adverse events (AEs) related to proteasome inhibitors in multiple myeloma based on the FDA Adverse Event Reporting System (FAERS), providing references for rational clinical medication.
Methods: AE data related to multiple myeloma proteasome inhibitors were collected from the FAERS from the first quarter of 2010 to the first quarter of 2024. Signal mining of AEs was conducted using the reporting odds ratio method and Bayesian confidence propagation neural network method.
Results: A total of 8,805 reports for bortezomib, 5,264 for carfilzomib, and 8,771 for ixazomib were collected, with corresponding AE signals of 474, 279, and 287, respectively, involving 23, 21, and 22 System Organ Classes (SOCs). The report information for the three drugs tended to be consistent: more cases were reported in males than in females; the majority of patients were 65 years and over; AEs mostly occurred within 6 months of medication; the outcomes primarily consisted of hospitalization, prolonged hospital stay, and other serious adverse events; the primary reporting country was the United States. The most affected SOCs were infections and infestations, general disorders and administration site conditions, and blood and lymphatic system disorders.
Conclusion: The overall distribution of AEs for the three multiple myeloma proteasome inhibitors was consistent, but there were certain differences in specific AE signal characteristics, which should be noted in clinical applications.
1 Introduction
Multiple myeloma (MM) is the second most common malignant hematologic tumor, characterized by the proliferation of abnormal plasma cells producing monoclonal immunoglobulins, leading to symptoms such as bone pain, anemia, renal impairment, and hypercalcemia (Landgren, 2013; Kazandjian, 2016). It is more common in middle-aged and elderly individuals, and its incidence is rising with the increasing aging population in China (Kyle et al., 2003; Liu et al., 2019). For MM patients, traditional chemotherapy often results in complete remission rate and short median survival (Fu et al., 2013). However, the emergence of new anticancer drugs, proteasome inhibitors (PIs) (Perea et al., 2017), which inhibit the function of tumor proteasomes, disrupt the proliferation and differentiation of tumor cells, and accelerate their collapse and apoptosis, has improved the remission rate and survival period for patients (Rajkumar et al., 2005; Pakjoo et al., 2024). The currently available PIs for multiple myeloma include bortezomib, carfilzomib and ixazomib (Stewart et al., 2015; Ettari et al., 2016). In 2003, bortezomib was approved as the first-generation PI. In 2012, carfilzomib was approved in the United States for patients with MM who had received at least two prior therapies and had relapsed. In 2016, carfilzomib received further approval from the United States for use as a monotherapy or in combination therapy for patients with relapsed or refractory MM. Ixazomib, the world’s first oral PI, was approved for marketing in the United States in 2015. It is commonly used in combination with lenalidomide and dexamethasone for adult MM patients who have received at least one prior therapy. Over the past two decades, PIs have become an essential medication for treating MM patients. Bortezomib has been recommended as a first-line treatment for MM in guidelines in both the US and China, and carfilzomib and ixazomib have also been included in first-line treatment plans (Luo et al., 2018; Witteles and Liedtke, 2019). Therefore, understanding the safety of PIs is of significant importance for their rational clinical use.
The FDA Adverse Event Reporting System (FAERS) is a database established by the FDA to support post-market safety monitoring studies for drugs (DeLoughery and Shatzel, 2019). It includes reports submitted by consumers, healthcare professionals, and drug manufacturers from 1968 to the present. The FDA releases the data to the public every quarter for free download. It offers a broad detection scope and is not limited by time or geography (Huang et al., 2021). The database includes seven data sets, which are linked through the “primaryid” or “ISR” primary link field. Due to its large volume of data, the wealth of information, and free access to the public, this study aims to analyze the adverse event (AE) data of PIs through FAERS to identify AE signals of PIs and to provide references for the rational use of these drugs (Mina et al., 2021).
2 Materials and methods
2.1 Data source
This study collected AE data from FAERS covering 57 quarters from the first quarter of 2010 to the first quarter of 2024, including patient personal information records, drug use records, AE records, drug treatment duration, and AE outcomes.
2.2 Data processing
Bortezomib was searched under the names “Bortezomib,” “Velcae,” “LDP341,” “PS341”; carfilzomib as “Carfilzomib,” “Kyprolis,” “PR171”; and ixazomib as “Ixazomib,” “Ninlaro,” “MLN 9708” to obtain primary suspect (PS) drug reports. FAERS uses the preferred terms (PTs) from the Medical Dictionary for Regulatory Activities (MedDRA) to code AEs (Taher et al., 2021).
2.3 Mining method
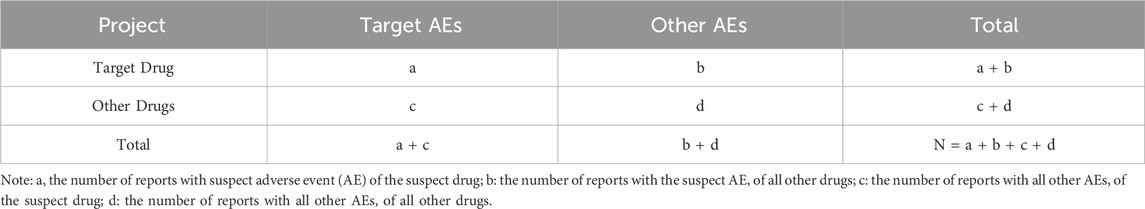
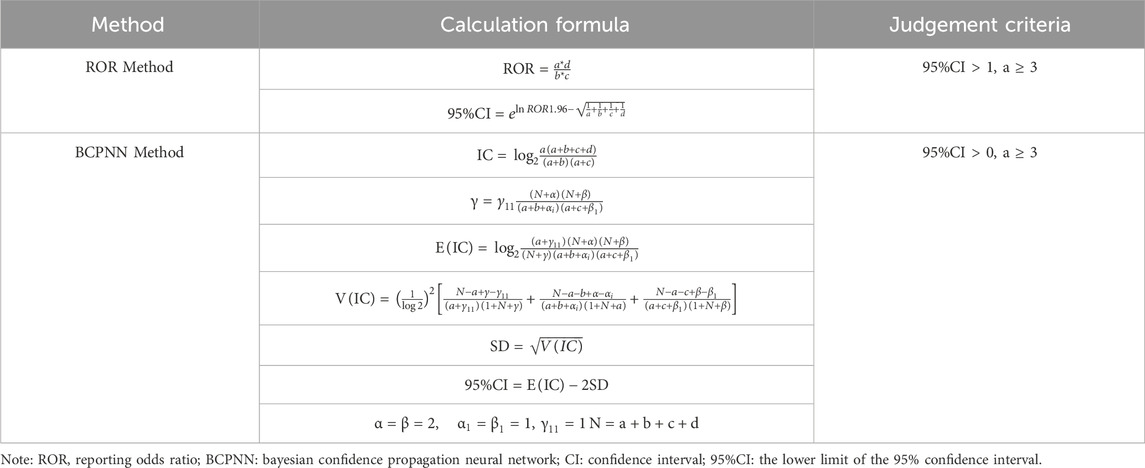
This study used the Reporting Odds Ratio (ROR) method and the Bayesian Confidence Propagation Neural Network (BCPNN) method to mine the data. Calculations for both methods are based on a fourfold table of disproportionality measures, as shown in Table 1. The ROR method has the advantage of eliminating bias and offering high sensitivity, but it is prone to false positives. The BCPNN method, which adds Bayesian law to the fourfold table, makes the results more stable and specific (Ma et al., 2021; Zou et al., 2023). This study used these two methods to investigate the same signals mined, thereby reducing bias caused by a single algorithm, and the algorithms of the specific methods are shown in Table 2.
2.4 Signal categorization
The signals identified were organized using the System Organ Class (SOC) (Liu et al., 2019) of the MedDRA, version 23.1, in English.
3 Results
3.1 Basic information on AE reports
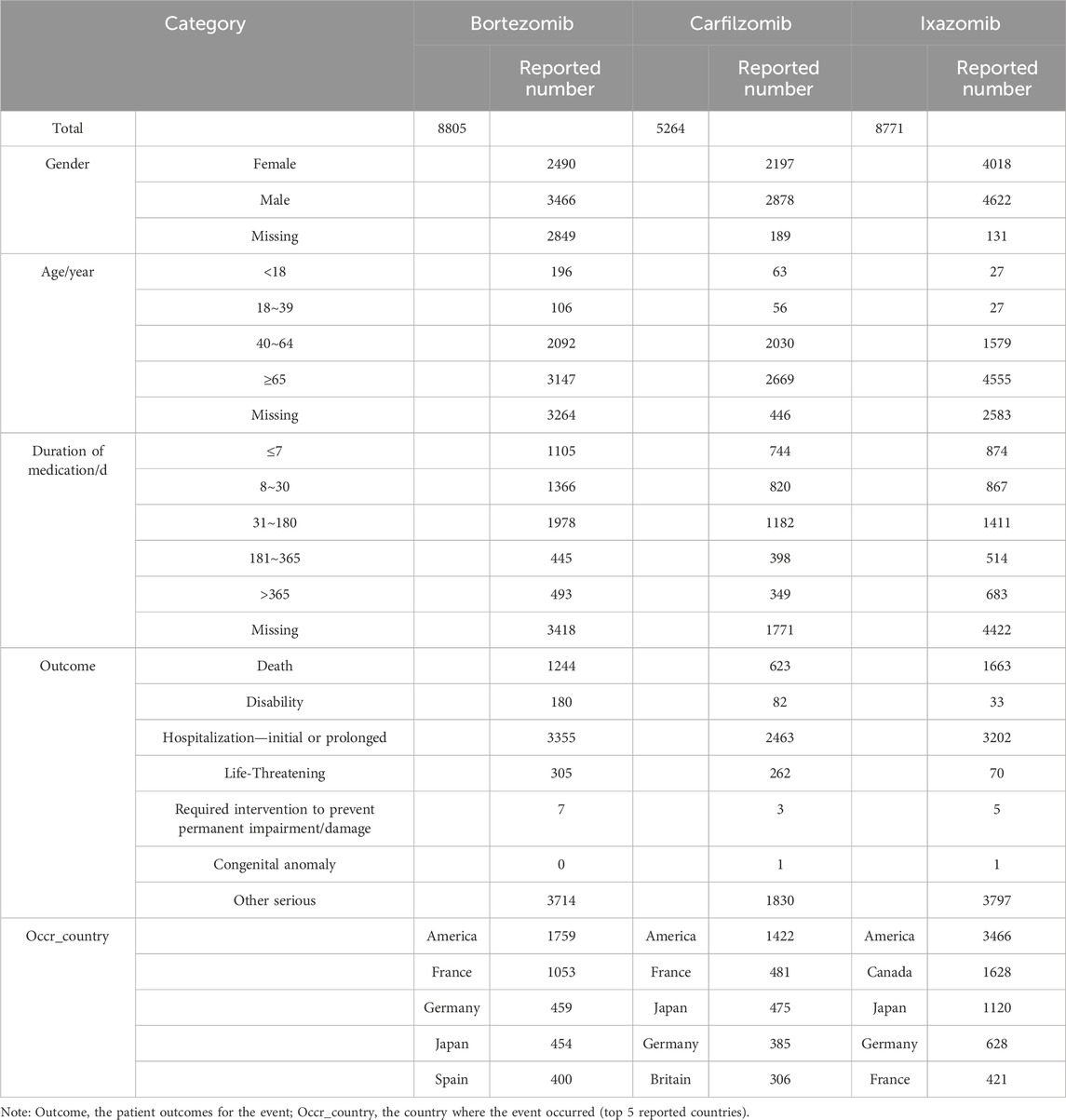
A total of 22,840 AE reports were collected, involving patients with PIs as the PS. Of these, 8,805 were for bortezomib, 5,264 for carfilzomib, and 8,771 for ixazomib. The reports for all three drugs shared the following characteristics: there were more cases in males than females; the main age group was centered around 65 years and older; AEs predominantly occurred within 6 months of treatment; and outcomes primarily involved hospitalization, prolonged hospital stays, and other serious adverse events; the United States was the primary reporting country. Specific information is shown in Table 3.
3.2 AE signals analysis
3.2.1 SOCs distribution
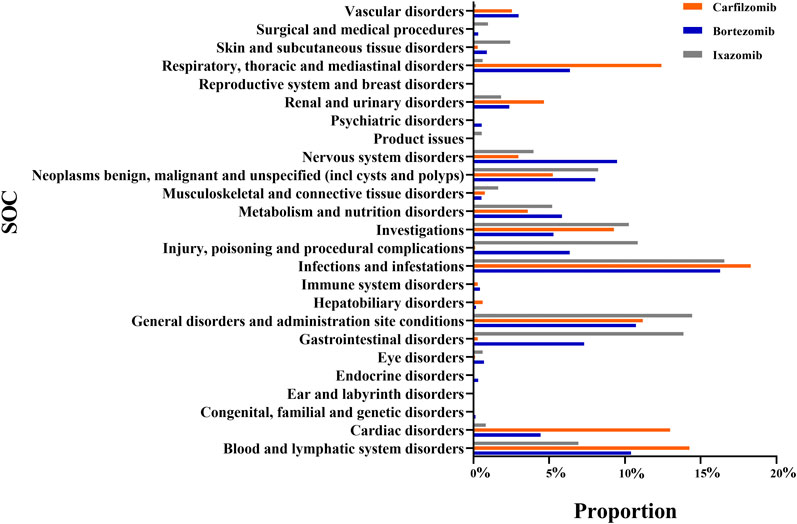
The number of AE signals identified by the ROR method was 714 for bortezomib, 493 for carfilzomib, and 474 for ixazomib. The number of AE signals identified by the BCPNN method was 474 for bortezomib, 279 for carfilzomib, and 287 for ixazomib. A total of 1,040 signals were identified for PIs by both methods in common, with the number and proportion of AE signals for bortezomib, carfilzomib, and ixazomib being 474 (45.58%), 279 (26.83%), and 287 (27.59%), respectively. The number of affected SOC categories was 23 for bortezomib, 21 for carfilzomib, and 22 for ixazomib. The main SOCs were infections and infestations, general disorders and administration site conditions, and blood and lymphatic system disorders. Additionally, bortezomib was associated with nervous system disorders (9.49%), respiratory, thoracic, and mediastinal disorders (6.36%), and gastrointestinal disorders (7.30%). Carfilzomib was associated with cardiac disorders (12.98%), respiratory, thoracic, and mediastinal disorders (12.41%), and others. Ixazomib was associated with gastrointestinal disorders (13.87%), investigations (10.25%), etc. See Figure 1 for details.
3.2.2 Siganls distribution
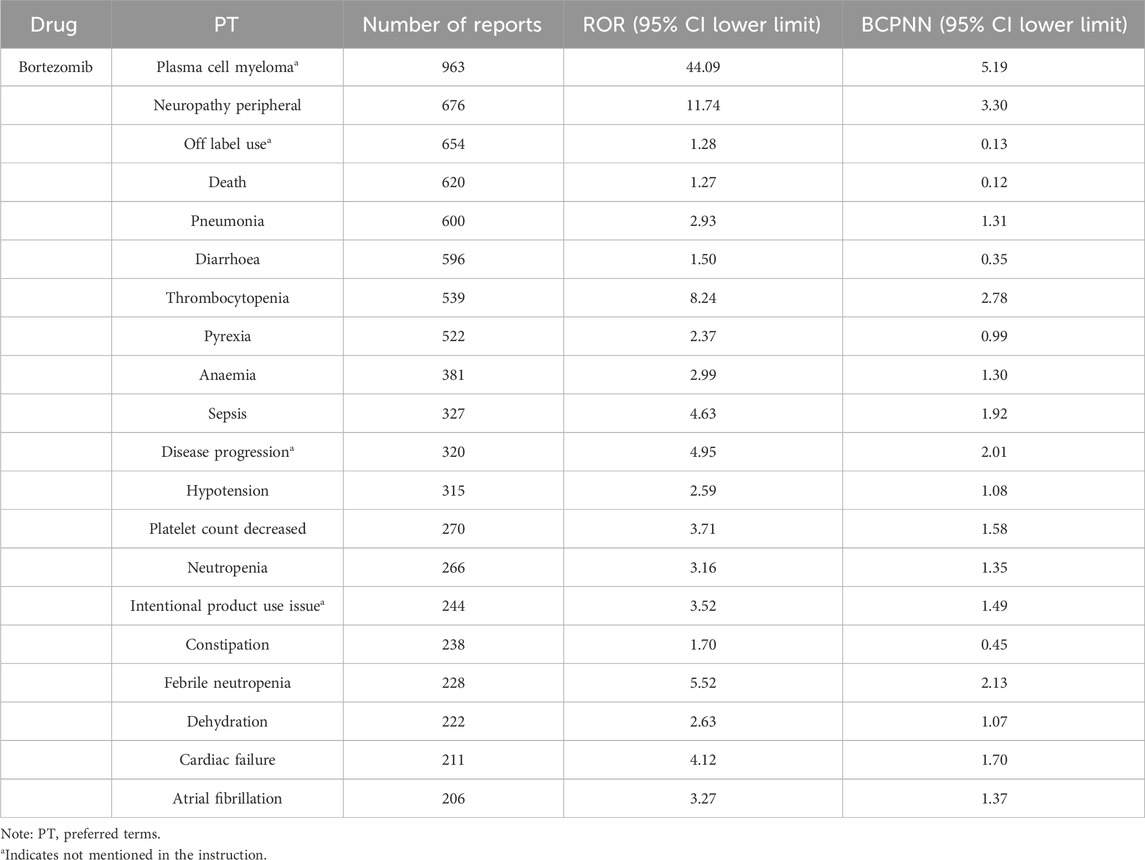
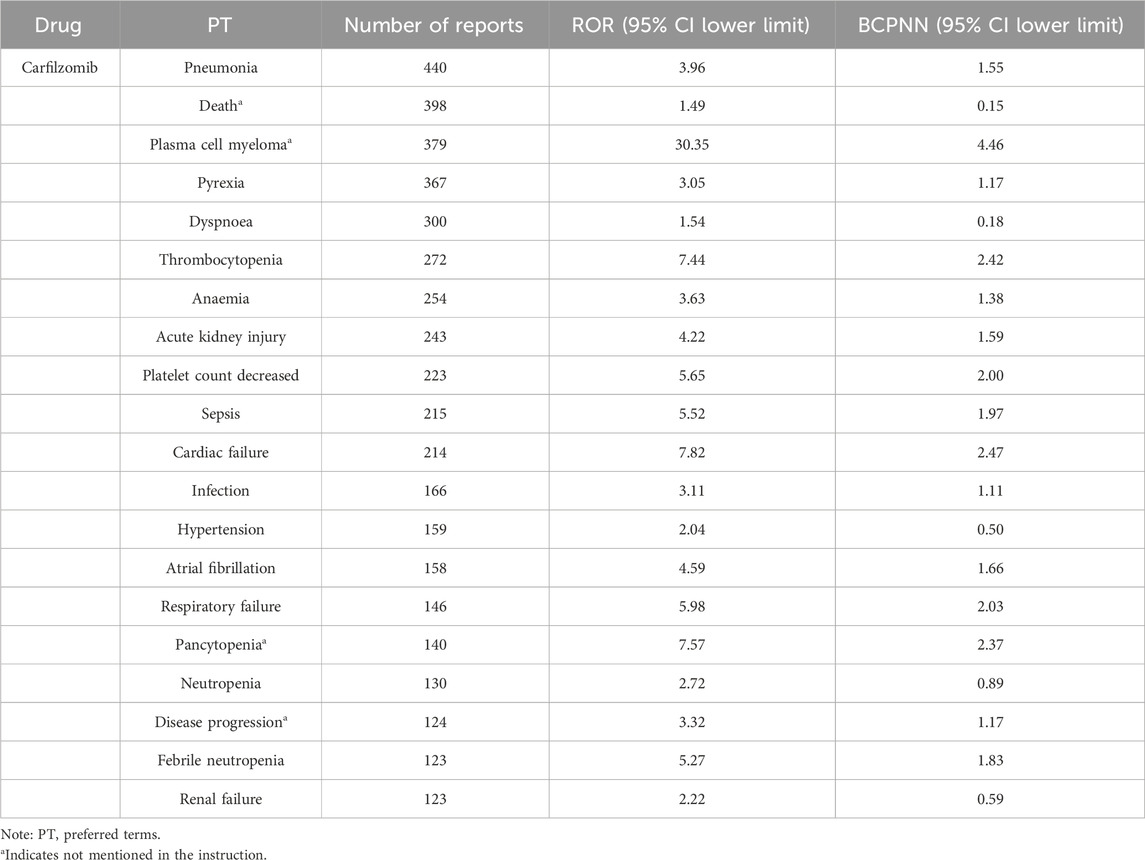
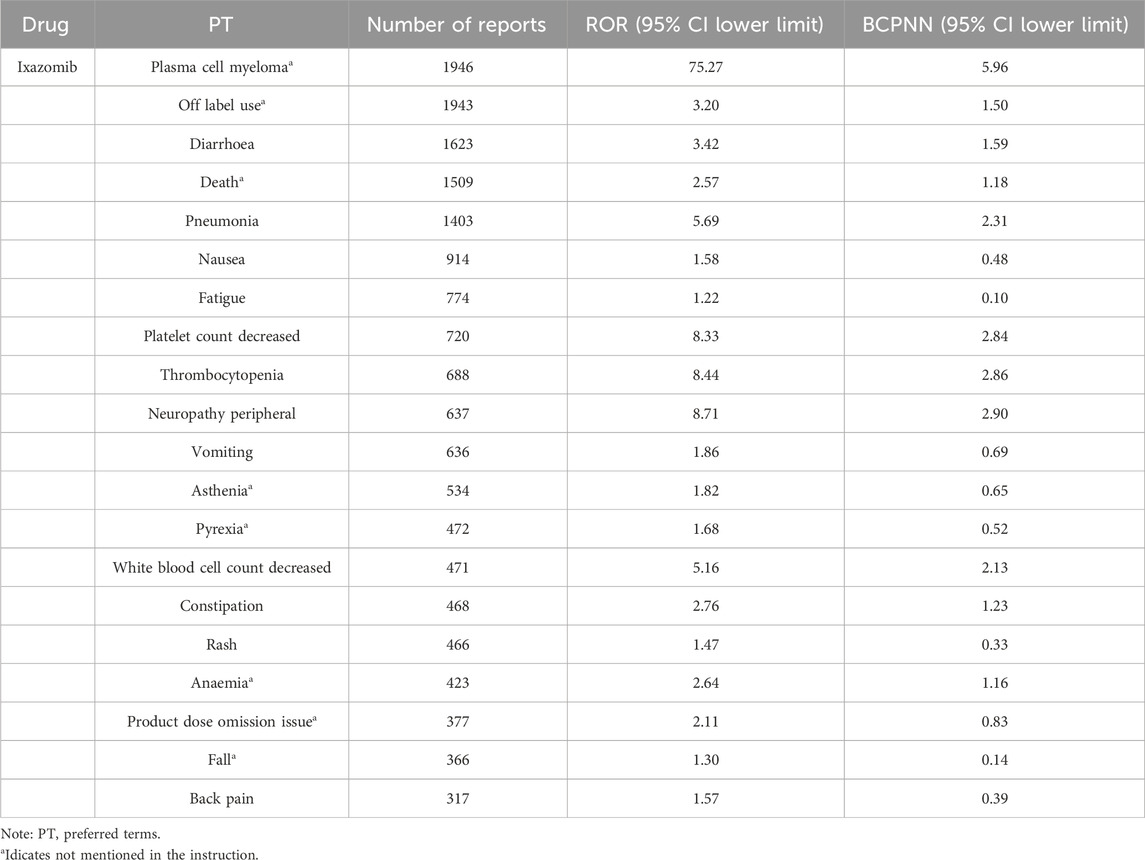
In the top 20 AE reports for bortezomib, carfilzomib, and ixazomib, signals related to blood and lymphatic system disorders were consistently present, including thrombocytopenia and anemia. Additionally, signals such as atrial fibrillation, pneumonia, and sepsis were observed with bortezomib. Carfilzomib was associated with signals like acute kidney injury, cardiac failure, and respiratory failure. Ixazomib showed signals for fatigue, rash, and neuropathy peripheral. For detailed information, see Tables 4–6.
4 Discussion
4.1 Basic situation of AEs
In this study, the number of AE signals identified was 474 for bortezomib, 279 for carfilzomib, and 287 for ixazomib. The most frequently reported signals for bortezomib included neuropathy peripheral, diarrhoea, pneumonia, and thrombocytopenia; for carfilzomib, they were pneumonia, pyrexia, dyspnoea, and acute kidney injury; and for ixazomib, they included diarrhoea, pneumonia, thrombocytopenia, and neuropathy peripheral. These results were consistent with the drug package inserts and related reports, confirming the reliability of this study (Steele, 2013; Bonnet and Moreau, 2017; Cengiz Seval and Beksac, 2018). In the reports for all three drugs, most patients were over 65 years of age, which may be related to the prevalence of the disease in older patients. The reports were primarily from Europe and America, possibly due to the origin of the database and the higher incidence of the disease in these populations (Huang et al., 2022).
4.2 Characteristics of PIs involving SOCs
4.2.1 Infections and infestations
In this category, all three drugs showed signals for pneumonia, respiratory tract infection, and lung infection. The propensity for infections in MM patients may be related to the impact of PIs on the immune system. Studies indicate that PIs can increase the risk of viral infections through mechanisms such as disruption of intracellular antigen presentation, exhaustion of alloreactive T cells, and reduction in cytotoxic T cell responses (Teh et al., 2016). Therefore, infection prevention should be considered during medication.
4.2.2 General disorders and administration site conditions
In this SOC, bortezomib, carfilzomib and ixazomib all had the highest number of death signals. Due to the disease’s prevalence in elderly patients with multiple comorbidities, it is currently uncertain if the deaths are drug-related. Additionally, all three drugs showed a significant number of reports for pyrexia. Pyrexia after bortezomib administration has been associated with pro-inflammatory cytokines secreted by macrophages or fibroblasts (Maruyama et al., 2008). No mechanisms have been reported for the other two drugs, but pyrexia is listed as a common adverse drug reaction (ADR) in the carfilzomib package insert, while ixazomib's package insert does not mention it. Therefore, monitoring body temperature is recommended when using PIs.
4.2.3 Blood and lymphatic system disorders
All three drugs showed signals for thrombocytopenia, anaemia and neutropenia, almost consistent with the ADRs listed in their package inserts. Bortezomib has been reported to regulate the Rho/Rho kinase pathway, a negative regulator of proplatelet formation, thereby leading to thrombocytopenia (Murai et al., 2014). However, since MM itself is a blood system disease with features like thrombocytopenia and anemia, the association with PIs requires further investigation.
4.2.4 Gastrointestinal disorders
Bortezomib and ixazomib showed a higher proportion of signals in this category, mainly for diarrhoea and constipation, consistent with package inserts and clinical trial results. Carfilzomib showed the lowest proportion, with signals only for colitis ischaemic, gastrointestinal amyloidosis, neutropenic colitis, suggesting lower gastrointestinal toxicity compared to bortezomib, aligning with the research by Stansborough RL (Stansborough and Gibson, 2017). Studies have shown that bortezomib can induce time-dependent gastrointestinal damage possibly related to the secretion of pro-inflammatory cytokines. However, the mechanisms of gastrointestinal pathology are complex and diverse, and further exploration is needed to understand the mechanisms of PI-induced gastrointestinal toxicity (Maruyama et al., 2008). With unclear mechanisms of gastrointestinal toxicity from PIs, there are currently no effective preventive measures. In cases of severe gastrointestinal reactions during medication, it is advised to discontinue the current medication or switch to carfilzomib with lower toxicity.
4.2.5 Respiratory, thoracic, and mediastinal disorders
Both bortezomib and carfilzomib showed signals for lung infiltration and acute respiratory distress syndrome, which are listed as ADRs in their package inserts. In such cases, it is advised to discontinue the medication and assess the patient promptly. Ixazomib showed signals for upper respiratory inflammation and respiratory symptoms. Currently, the related toxicity mechanisms are unclear, and preventive and treatment measures are still under research. Patients with respiratory diseases should use these drugs cautiously, weighing the risks and benefits, and if used, their respiratory symptoms should be closely monitored.
4.2.6 Cardiac disorders
All three drugs, bortezomib, carfilzomib, and ixazomib, showed severe signals of cardiac failure, cardiac amyloidosis, and acute coronary syndrome. The signals showed by ixazomib also include cardiac dysfunction, cor pulmonale chronic, degenerative aortic valve disease, left atrial enlargement, and right atrial enlargement, but the package insert for ixazomib only mentions cardiac failure and arrhythmia. Studies suggested that the high sensitivity of cardiomyocytes to damage by bortezomib and carfilzomib may be related to their inhibition of high muscle protein turnover, leading to the induction of apoptosis and myocyte death, and consequently cardiac failure (Hasinoff et al., 2017). The toxicity mechanisms of ixazomib may not have been fully explored due to its shorter time on the market. When using PIs, consider their cardiotoxicity, closely monitor cardiac indicators, and if patients show such symptoms, discontinue the medication and assess them immediately.
4.2.7 Renal and urinary disorders
Of the three drugs, only ixazomib’s package insert does not list signals for acute kidney injury and acute renal failure. Studies have found kidney damage to be associated with an increased risk of early death in MM patients, especially in older patients with more comorbidities (Fan et al., 2021). For bortezomib’s renal toxicity mechanism, it is speculated to increase transcription of pro-apoptotic genes bax and puma, key components of the intrinsic apoptosis pathway, and indirectly affect proteins involved in the extrinsic pathway of cell apoptosis, inducing ischemic renal tubular apoptosis, leading to renal function decline or failure (Huber et al., 2009). No mechanisms have been discussed for ixazomib and carfilzomib. However, carfilzomib’s package insert warns of a greater risk of fatal renal failure in patients with reduced creatinine clearance. When treating patients with PIs, it is advised to monitor renal function regularly, to measure serum creatinine or estimated creatinine clearance, and to take intervention measures promptly if kidney damage or failure occurs.
4.2.8 Nervous system disorders
In this study, all three drugs were associated with multiple nervous system disorders. Studies showed that although bortezomib could not penetrate the blood-brain barrier into the central nervous system, it accumulated in dorsal root ganglia (DRG) causing neurotoxicity and indirectly leading to central nervous system dysfunction, including glial activation, glutamate homeostasis disruption, and inflammation (Yamamoto and Egashira, 2021). No mechanisms have been reported for the other two drugs. However, results from the ENDEAVOR randomized controlled trial showed a significantly lower incidence of grade 2 neuropathy peripheral in the carfilzomib group compared to the bortezomib group (6% vs. 32%) (Goldschmidt et al., 2018). Another study comparing subcutaneous and intravenous bortezomib found better tolerance and lower grade 2 neuropathy peripheral with subcutaneous injection (24% vs. 41%) (Moreau et al., 2011). Consider stopping medication, changing the administration method, or switching treatment plans in cases of severe ADRs in the nervous system during PIs treatment.
5 Conclusion
This study conducted mining and analysis of AE signals for PIs through the FAERS database using the ROR method and BCPNN method, and conducted detailed discussions on the SOCs such as infections and infestations, blood and lymphatic system disorders, nervous system disorders, gastrointestinal disorders in conjunction with existing data. The study found that the AE signals of bortezomib, carfilzomib, ixazomib were essentially the same for infections and infestations, blood and lymphatic system disorders, and nervous system disorders, but differed in respiratory, thoracic, and mediastinal diseases. Additionally, carfilzomib had lower gastrointestinal toxicity, while ixazomib presented numerous signals not mentioned in its package insert, such as pyrexia, kidney and urinary system disorders, and cardiac disorders. This study not only provides a reference for the rational clinical use of PIs but also promotes drug vigilance work for PIs.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/supplementary material.
Author contributions
YP: Writing–review and editing, Writing–original draft, Conceptualization. YYZ: Writing–review and editing, Data curation. KS: Writing–review and editing, Methodology, Formal Analysis. XJ: Writing–review and editing, Methodology, Formal Analysis, Data curation. YZ: Writing–review and editing, Writing–original draft, Conceptualization.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. The study was supported by the Chengdu Municipal Health Commission 2022 Chengdu Medical Research Topic (Nos. 2022349), the Research Project of the Science and Technology Development Program of Affiliated Hospital of North Sichuan Medical College (2022JC027). The funders were not involved in the study design, data acquisition, analysis, or drafting of the manuscript.
Acknowledgments
We would like to thank ChatGPT4.0 (OpenAI, USA) for providing the service of fixing grammar.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
ADR, adverse drug reaction; AE, adverse event; BCPNN, Bayesian Confidence Propagation Neural Network; DRG, dorsal root ganglia; FAERS, The FDA Adverse Event Reporting System; MedDRA, Medical Dictionary for Regulatory Activities; MM, multiple myeloma; PI, proteasome inhibitor; PS, primary suspect; PT, preferred term; ROR, Reporting Odds Ratio; SOC, System Organ Class.
References
Bonnet, A., and Moreau, P. (2017). Safety of ixazomib for the treatment of multiple myeloma. Expert Opin. Drug Saf. 16 (8), 973–980. doi:10.1080/14740338.2017.1344212
Cengiz Seval, G., and Beksac, M. (2018). The safety of bortezomib for the treatment of multiple myeloma. Expert Opin. Drug Saf. 17 (9), 953–962. doi:10.1080/14740338.2018.1513487
DeLoughery, E. P., and Shatzel, J. J. (2019). A comparative analysis of the safety profile of direct oral anticoagulants using the FDA adverse event reporting system. Eur. J. Haematol. 103 (1), 43–46. doi:10.1111/ejh.13240
Ettari, R., Previti, S., Bitto, A., Grasso, S., and Zappalà, M. (2016). Immunoproteasome-selective inhibitors: a promising strategy to treat hematologic malignancies, autoimmune and inflammatory diseases. Curr. Med. Chem. 23 (12), 1217–1238. doi:10.2174/0929867323666160318173706
Fan, L., Ma, Y. P., Chao, Y., and Gao, X. Y. (2021). Clinical characteristic, prognosis and treatment outcome of elderly multiple myeloma patients with impaired renal function. Zhongguo Shi Yan Xue Ye Xue Za Zhi 29 (1), 145–151. doi:10.19746/j.cnki.issn1009-2137.2021.01.023
Fu, C., Wang, J., Xin, X., Liu, H., Xue, S., Ma, X., et al. (2013). Therapeutic effects of autologous hematopoietic stem cell transplantation in multiple myeloma patients. Exp. Ther. Med. 6 (4), 977–982. doi:10.3892/etm.2013.1261
Goldschmidt, H., Moreau, P., Ludwig, H., Niesvizky, R., Chng, W. J., Joshua, D., et al. (2018). Carfilzomib-dexamethasone versus subcutaneous or intravenous bortezomib in relapsed or refractory multiple myeloma: secondary analysis of the phase 3 ENDEAVOR study. Leuk. Lymphoma 59 (6), 1364–1374. doi:10.1080/10428194.2017.1376743
Hasinoff, B. B., Patel, D., and Wu, X. (2017). Molecular mechanisms of the cardiotoxicity of the proteasomal-targeted drugs bortezomib and carfilzomib. Cardiovasc Toxicol. 17 (3), 237–250. doi:10.1007/s12012-016-9378-7
Huang, J., Chan, S. C., Lok, V., Zhang, L., Lucero-Prisno, D. E., Xu, W., et al. (2022). The epidemiological landscape of multiple myeloma: a global cancer registry estimate of disease burden, risk factors, and temporal trends. Lancet Haematol. 9 (9), e670–e677. doi:10.1016/S2352-3026(22)00165-X
Huang, L., Liu, Y., Li, H., Huang, W., Geng, R., Tang, Z., et al. (2021). Bullous pemphigoid and diabetes medications: a disproportionality analysis based on the FDA adverse event reporting system. Int. J. Med. Sci. 18 (9), 1946–1952. doi:10.7150/ijms.55421
Huber, J. M., Tagwerker, A., Heininger, D., Mayer, G., Rosenkranz, A. R., and Eller, K. (2009). The proteasome inhibitor bortezomib aggravates renal ischemia-reperfusion injury. Am. J. Physiol. Ren. Physiol. 297 (2), F451–F460. doi:10.1152/ajprenal.90576.2008
Kazandjian, D. (2016). Multiple myeloma epidemiology and survival: a unique malignancy. Seminars Oncol. 43 (6), 676–681. doi:10.1053/j.seminoncol.2016.11.004
Kyle, R. A., Gertz, M. A., Witzig, T. E., Lust, J. A., Lacy, M. Q., Dispenzieri, A., et al. (2003). Review of 1027 patients with newly diagnosed multiple myeloma. Mayo Clin. Proc. 78 (1), 21–33. doi:10.4065/78.1.21
Landgren, O. (2013). Monoclonal gammopathy of undetermined significance and smoldering multiple myeloma: biological insights and early treatment strategies. Hematol. Am. Soc. Hematol. Educ. Program 2013, 478–487. doi:10.1182/asheducation-2013.1.478
Liu, J., Liu, W., Mi, L., Zeng, X., Cai, C., Ma, J., et al. (2019). Incidence and mortality of multiple myeloma in China, 2006-2016: an analysis of the global burden of disease study 2016. J. Hematol. Oncol. 12 (1), 136. doi:10.1186/s13045-019-0807-5
Luo, X. W., Du, X. Q., Li, J. L., Liu, X. P., and Meng, X. Y. (2018). Treatment options for refractory/relapsed multiple myeloma: an updated evidence synthesis by network meta-analysis. Cancer Manag. Res. 10, 2817–2823. doi:10.2147/CMAR.S166640
Ma, R., Wang, Q., Meng, D., Li, K., and Zhang, Y. (2021). Immune checkpoint inhibitors-related myocarditis in patients with cancer: an analysis of international spontaneous reporting systems. BMC Cancer 21 (1), 38. doi:10.1186/s12885-020-07741-0
Maruyama, D., Watanabe, T., Heike, Y., Nagase, K., Takahashi, N., Yamasaki, S., et al. (2008). Stromal cells in bone marrow play important roles in pro-inflammatory cytokine secretion causing fever following bortezomib administration in patients with multiple myeloma. Int. J. Hematol. 88 (4), 396–402. doi:10.1007/s12185-008-0194-0
Mina, S. A., Muhsen, I. N., Burns, E. A., Sarfraz, H., Pingali, S. R., Xu, J., et al. (2021). Post-marketing analysis of peripheral neuropathy burden with new-generation proteasome inhibitors using the FDA adverse event reporting system. Turk J. Haematol. 38 (3), 218–221. doi:10.4274/tjh.galenos.2021.2021.0052
Moreau, P., Pylypenko, H., Grosicki, S., Karamanesht, I., Leleu, X., Grishunina, M., et al. (2011). Subcutaneous versus intravenous administration of bortezomib in patients with relapsed multiple myeloma: a randomised, phase 3, non-inferiority study. Lancet Oncol. 12 (5), 431–440. doi:10.1016/S1470-2045(11)70081-X
Murai, K., Kowata, S., Shimoyama, T., Yashima-Abo, A., Fujishima, Y., Ito, S., et al. (2014). Bortezomib induces thrombocytopenia by the inhibition of proplatelet formation of megakaryocytes. Eur. J. Haematol. 93 (4), 290–296. doi:10.1111/ejh.12342
Pakjoo, M., Ahmadi, S. E., Zahedi, M., Jaafari, N., Khademi, R., Amini, A., et al. (2024). Interplay between proteasome inhibitors and NF-κB pathway in leukemia and lymphoma: a comprehensive review on challenges ahead of proteasome inhibitors. Cell Commun. Signal 22 (1), 105. doi:10.1186/s12964-023-01433-5
Perea, L., Coll, M., Sanjurjo, L., Blaya, D., Taghdouini, A. E., Rodrigo-Torres, D., et al. (2017). Pentraxin-3 modulates lipopolysaccharide-induced inflammatory response and attenuates liver injury. Hepatology 66 (3), 953–968. doi:10.1002/hep.29215
Rajkumar, S. V., Richardson, P. G., Hideshima, T., and Anderson, K. C. (2005). Proteasome inhibition as a novel therapeutic target in human cancer. J. Clin. Oncol. 23 (3), 630–639. doi:10.1200/JCO.2005.11.030
Stansborough, R. L., and Gibson, R. J. (2017). Proteasome inhibitor-induced gastrointestinal toxicity. Curr. Opin. Support. Palliat. care 11 (2), 133–137. doi:10.1097/SPC.0000000000000266
Steele, J. M. (2013). Carfilzomib: a new proteasome inhibitor for relapsed or refractory multiple myeloma. J. Oncol. Pharm. Pract. 19 (4), 348–354. doi:10.1177/1078155212470388
Stewart, A. K., Rajkumar, S. V., Dimopoulos, M. A., Masszi, T., Spicka, I., Oriol, A., et al. (2015). Carfilzomib, lenalidomide, and dexamethasone for relapsed multiple myeloma. N. Engl. J. Med. 372 (2), 142–152. doi:10.1056/NEJMoa1411321
Taher, M. K., Alami, A., Gravel, C. A., Tsui, D., Bjerre, L. M., Momoli, F., et al. (2021). Systemic quinolones and risk of acute liver failure I: analysis of data from the US FDA adverse event reporting system. JGH Open 5 (7), 778–784. doi:10.1002/jgh3.12585
Teh, B. W., Harrison, S. J., Worth, L. J., Thursky, K. A., and Slavin, M. A. (2016). Infection risk with immunomodulatory and proteasome inhibitor–based therapies across treatment phases for multiple myeloma: a systematic review and meta-analysis. Eur. J. Cancer 67, 21–37. doi:10.1016/j.ejca.2016.07.025
Witteles, R. M., and Liedtke, M. (2019). AL amyloidosis for the cardiologist and oncologist: epidemiology, diagnosis, and management. JACC CardioOncol 1 (1), 117–130. doi:10.1016/j.jaccao.2019.08.002
Yamamoto, S., and Egashira, N. (2021). Pathological mechanisms of bortezomib-induced peripheral neuropathy. Int. J. Mol. Sci. 22 (2), 888. doi:10.3390/ijms22020888
Keywords: proteasome inhibitors, adverse event, signal mining, multiple myeloma, adverse event reporting system
Citation: Peng Y, Zhou Y, Shu K, Jia X and Zhong Y (2024) Signal mining of adverse events of proteasome inhibitors in multiple myeloma based on FAERS. Front. Pharmacol. 15:1396378. doi: 10.3389/fphar.2024.1396378
Received: 05 March 2024; Accepted: 08 August 2024;
Published: 03 September 2024.
Edited by:
Tin Wui Wong, Universiti Teknologi MARA Puncak Alam, MalaysiaReviewed by:
Joanna Gdula-Argasinska, Jagiellonian University Medical College, PolandLin Song, Chongqing Medical University, China
Copyright © 2024 Peng, Zhou, Shu, Jia and Zhong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yan Zhong, MTE3OTM0OTE4MEBxcS5jb20=
†These authors have contributed equally to this work
 Yuan Peng
Yuan Peng Yuying Zhou3†
Yuying Zhou3† Yan Zhong
Yan Zhong