
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pharmacol., 12 June 2024
Sec. Pharmacology of Anti-Cancer Drugs
Volume 15 - 2024 | https://doi.org/10.3389/fphar.2024.1395951
 Ioana Creanga-Murariu1,2,3
Ioana Creanga-Murariu1,2,3 Leontina-Elena Filipiuc1,2
Leontina-Elena Filipiuc1,2 Maria-Raluca Gogu1
Maria-Raluca Gogu1 Mitica Ciorpac1
Mitica Ciorpac1 Carmen Marinela Cumpat2,4
Carmen Marinela Cumpat2,4 Bogdan-Ionel Tamba1,2*
Bogdan-Ionel Tamba1,2* Teodora Alexa-Stratulat2,3
Teodora Alexa-Stratulat2,3Introduction: Chemotherapy-induced peripheral neuropathy (CIPN) is a shared burden for 68.1% of oncological patients undergoing chemotherapy with Paclitaxel (PTX). The symptoms are intense and troublesome, patients reporting paresthesia, loss of sensation, and dysesthetic pain. While current medications focus on decreasing the symptom intensity, often ineffective, no medication is yet recommended by the guidelines for the prevention of CIPN. Cannabinoids are an attractive option, as their neuroprotective features have already been demonstrated in neuropathies with other etiologies, by offering the peripheral neurons protection against toxic effects, which promotes analgesia.
Methods: We aim to screen several new cannabinoids for their potential use as neuroprotective agents for CIPN by investigating the cellular toxicity profile and by assessing the potential neuroprotective features against PTX using a primary dorsal root ganglion neuronal culture.
Results: Our study showed that synthetic cannabinoids JWH-007, AM-694 and MAB-CHMINACA and phytocannabinoids Cannabixir® Medium dried flowers (NC1) and Cannabixir® THC full extract (NC2) preserve the viability of fibroblasts and primary cultured neurons, in most of the tested dosages and time-points. The combination between the cannabinoids and PTX conducted to a cell viability of 70%–89% compared to 40% when PTX was administered alone for 48 h. When assessing the efficacy for neuroprotection, the combination between cannabinoids and PTX led to better preservation of neurite length at all tested time-points compared to controls, highly drug and exposure-time dependent. By comparison, the combination of the cannabinoids and PTX administered for 24 h conducted to axonal shortening between 23% and 44%, as opposed to PTX only, which shortened the axons by 63% compared to their baseline values.
Discussion and Conclusion: Cannabinoids could be potential new candidates for the treatment of paclitaxel-induced peripheral neuropathy; however, our findings need to be followed by additional tests to understand the exact mechanism of action, which would support the translation of the cannabinoids in the oncological clinical practice.
Chemotherapy-induced peripheral neuropathy (CIPN) is a common side effect of anticancer drugs, with the incidence varying from as low as 12.1% to as high as 96.2% (Masocha, 2018). Some chemotherapy drugs are known to be associated with a high incidence of CIPN, such as platinum derivates, vinca alkaloids, Bortezonib, or Thalidomide. Taxanes, in particular, are associated with a high incidence of CIPN, with paclitaxel (PTX) responsible for more than 70% of the cases (Seretny et al., 2014). Moreover, a significant proportion of patients developing CIPN after PTX have persisting symptoms for up to 1 year after the end of the treatment (Seretny et al., 2014; Burgess et al., 2021), most often reported as paresthesia, loss of sensation, and dysesthetic pain in the feet and hands with a “stocking and glove” distribution (Staff et al., 2020). Particularly noteworthy for the clinical practice is that due to the disturbing and persistent symptoms patients perceive, the physician is often forced to reduce the dosage of Paclitaxel, even though reductions below 85% are known to decrease survival rates significantly (Manfredi and Horwitz, 1984).
The pathology of CIPN is complex and still poorly understood. However, the direct effects of the chemotherapy on the peripheral nerves are responsible for the patient’s painful sensation. PTX’s primary mode of action is hyper-stabilizing the microtubules, preventing the normal cycles of microtubule depolymerisation and repolymerization within the cytoskeleton (Manfredi and Horwitz, 1984). PXT’s effects vary between different types of cells, such as tumoral cells, where the tubulin polymerisation hinders the formation of the mitotic spindle, causing the arrest of the cells in the G2/M-phase of the cell cycle, whereas in non-dividing cells, such as neurons, paclitaxel-induced tubulin polymerisation is thought to interfere with axonal transport, causing peripheral neuropathy (Manfredi and Horwitz, 1984). Besides the effects on the microtubules, the neurotoxicity of PTX could also be exerted by other mechanisms, such as mitochondrial dysfunction, immune response induction, and calcium ion disruption (Zajączkowska et al., 2019; Desforges et al., 2022).
The current treatment options for CIPN evolve around painful symptom control. The oncology practice guidelines developed by the European Society of Medical Oncology (ESMO) and the American Society of Clinical Oncology (ASCO) advise the prescription of anticonvulsants, tricyclic antidepressants, serotonin-norepinephrine reuptake inhibitors and opioids (Jordan et al., 2020; Loprinzi et al., 2020). The clinical management of CIPN is difficult, given the fact that the efficacy of the named medications is limited, varies greatly between the patients and can cause serious side effects (Jordan et al., 2019). Nevertheless, the medication aims only to decrease the symptom intensity and doesn’t provide a curative approach to the pain. Current practices for the prevention of high-grade CIPN is to provide special attention to the onset of painful symptoms when administering the neurotoxic treatment in order to reduce the chemotherapy dose if necessary or to change the class of medication (Hausheer et al., 2006). However, this approach is not ideal, given the fact that reductions below 85% of the effective antitumoral dosages are known to decrease survival rates significantly (Manfredi and Horwitz, 1984). On the other hand, targeting neuroprotection as an alternative option for preventing and treating CIPN is attractive, however it is a strategy that is not yet included in the guidelines (Loprinzi et al.).
The need for new drugs in this setting shifted the attention of clinicians and researchers to cannabinoids, as they have been studied for their neuroprotective activity in a range of diseases and pathological conditions, including Alzheimer’s disease, Parkinson’s, multiple sclerosis, dementia and nevertheless neuropathic pain of various etiologies (Pacher and Kunos, 2013). Endogenous or exogenous cannabinoids interact with the endocannabinoid system, heterogeneously present in different structures of the central and peripheric nervous system, including essential regions of pain processing, such as the spinal cord, thalamus, amygdala and dorsal root ganglions (DRGs) (Finn et al., 2021). A necessary part of the endocannabinoid system is represented by the cannabinoid receptors 1 and 2 (CB1R and CB2R), present in pain pathways from the peripheral sensory nerve endings up to the brain (Barinaga, 2001). CB1R are mainly expressed throughout the central nervous system (CNS), in key areas responsible for pain transmission. They are involved in the attenuation of synaptic transmission and modulation of another neuronal mechanism due to their expression on the primary afferent neurons (Ibrahim et al., 2003). The activation of CB1R from primary afferent neurons, DRGs and brain regions involved in pain processing is associated with a decrease in neuronal excitability and a dampening of neurotransmission (Brown and Winterstein, 2019). Also, the activation causes a decrease in the release of neurotransmitters such as dopamine and GABA, conducting to a neuroinhibitory state (Piomelli et al., 2000). Contrary, CB2R expression is non-neuronal and is found in glia, immune cells and peripheral tissues. For this reason, CB2 selective ligands could modulate pain by inhibiting the release of proinflammatory factors by non-neuronal cells located near nociceptive neuron terminals (Guindon and Hohmann, 2009; Vera et al., 2013).
Exogenous ligands of the cannabinoid receptors are either naturally sourced, derived from plants belonging to the Cannabis genus or synthetically engineered to mimic the structure and function of some phytocannabinoids (PCs) (Creanga-Murariu et al., 2023). The first discovered were the PCs, of which the most known active molecules are Δ9-tetrahydrocannabinol (Δ9-THC) and cannabidiol (CBD) (Mechoulam and ShvoHashish, 1963). Each cannabis plant has a vast assortment of active compounds that vary in composition, concentration and ratio depending on environmental factors, genetic background and even morpho-spatial position of the plant (Danziger and Bernstein, 2021). There are more than 100 lipid-soluble molecules within each cannabis plant, which could explain the versatility of medicinal use (analgesic, anticonvulsant, antispasmodic, diuretic, expectorant etc. (Zuardi, 2006)) and the unique feature of synergistic effects (the “entourage effect”) with the cannabinoids (Filipiuc et al., 2021). Synthetic cannabinoids (SCs) are compounds functionally similar to PCs but entirely different in composition, lacking the vast combinations of active molecules. They were initially designed as ligands for the identification of endogenous cannabinoid system, particularly the CRs. These chemically engineered drugs mimic the PC’s mode of action by acting on the same receptors. However, they are more potent due to their activity as full agonists and their higher affinity for cannabinoid receptors (Tamba et al., 2020). Due to the intense effects, SCs entered the black market as recreational drugs, administered incorrectly, with important side effects. As such, acute, severe or unpredictable side effects have been reported following SC abuse, and hospital admission rates are consistently higher for SC use than for natural cannabinoid consumption (Tamba et al.). SCs come with the advantage of predictable pharmacokinetics and pharmacodynamics as their synthetization is usually conducted in established laboratories. On the other hand, cannabis plants can come from farmers that don’t always subject to the regulation of Good Manufacturing Practices to ensure the standardisation of the product, which can conduct the growing of plants genotypically and phenotypically different than the originals (de Souza et al., 2022).
The prevalence of cancer patients using any form of cannabinoids was between 25% and 40%, as reported by a recent study (Pergam et al., 2017). The drugs were either physician-prescribed or illicitly procured, and the majority of the patients reported using them to manage pain symptoms (Tringale et al., 2019). Although widespread, one needs to be aware of the multitude of side effects, especially the increased risk of psychosis, cognition impairment, sedation, and cardiovascular and pulmonary function alteration (Creanga-Murariu et al., 2023). These side effects are highly dependent on the ratio between active molecules, potency, dosages, age of the patients, naïve vs. experienced users and acute vs. chronic exposure (Creanga-Murariu et al., 2023). Consequently, the scientific field must keep up with every new candidate by thoroughly testing each one’s toxicity and efficacy to distinguish between beneficial and life-threatening effects.
During the last years, cannabinoids have been studied as a valuable option to successfully modulate the endocannabinoid system in preclinical models with the outcome of neuroprotection, bringing an alternative for cancer patients suffering from neuropathic pain (Rahn et al., 2007; Yamamoto et al., 2008; Scott et al., 2014; Romero-Sandoval et al., 2017). Fewer studies have been conducted in the human population. However, the patients showed a decrease in the symptom intensity when cannabis was added to their standard therapy (Lynch et al., 2014; Creanga-Murariu et al., 2023).
As pain is the most common and troublesome symptom of CIPN and nerve dysfunction is an important pillar observed in the pathogenesis, it leads to the hypothesis that neuropathic pain is, in fact, a symptom of neurodegeneration (Bordet and Pruss, 2009). Nonetheless, suppose neurodegeneration is a mechanism responsible for the onset of neuropathic pain. In that case, it seems logical to consider neuroprotective drugs as tools to prevent the onset, control progression, or even reverse the nerve damage leading to neuropathic pain. However, the clinical practice still lacks efficient strategies for the prevention of neuropathy, hence pain, in the case of patients exposed to known neurotoxic chemotherapy. Only a few drugs have been explored with the aim of preventing or delaying the onset of CIPN (e.g., Acetyl-L-carnitine, alpha-lipoic acid, or cryotherapy). However, no firm recommendation for clinical use is endorsed by the guidelines (Loprinzi et al., 2020).
In the present study, we are motivated by the high burden of cancer patients experiencing CIPN, the poorly understood pathogenesis of neuropathic pain, the absence of efficient treatment and the attractive potential of cannabinoids as new analgesic agents for cancer patients. Therefore, we aim to screen three SCs JWH-007, AM-694, MAB-CHMINACA and two phytocannabinoids, Cannabixir® Medium dried flowers (NC1) and Cannabixir® THC full extract (NC2), which have not been tested before with this indication, for their general toxicity and efficacy for neuroprotection, thus analgesia. Using a primary dorsal root ganglion neural culture, our experiment design will assess neuroprotection regarding overall neuronal morphology and neurite length. We chose the model given that unbalanced synaptic communication in the dorsal horn of the spinal cord is one of the leading causes of neuropathic pain consolidation (Campos et al., 2021). We hypothesise that cannabinoids offer neuroprotection for neurons directly exposed to PTX and could be potentially translated to the clinic as new agents for the prevention or delay of the CIPN.
Synthetic cannabinoids JWH-007, AM-694, and MAB-CHMINACA were purchased from Cayman Chemical (Cat # 155471-10-6; 335161-03-0; 22047; Ann Arbor, MI, United States). Natural cannabinoids, Cannabixir® Medium dried flowers (NC1) was sourced from Cansativa GmbH (Mörfelden-Walldorf, Germany); Cannabixir® THC full extract (NC2) was sourced from FYTA Company B.V., (Netherlands). The compounds were dissolved in DMSO (0.1%). An antineoplastic agent from the taxane group, semisynthetic Paclitaxel 98% purity, was purchased from Sigma Aldrich (Cat # 33069-62-4, Darmstadt, Germany).
For the toxicity studies, V79 cell line was purchased from ATCC (Cat # CCL-93; Virginia, United States); substrate 3-(4,5-dimethylthiazol-2-yl) 2,5 diphenyl tetrazolium bromide (MTT powder, Cat # M5655), Dimethylsulfoxide (DMSO, Cat # 67-68-5), Dulbecco’s Modified Eagle Medium (DMEM, Cat # D6429) and Fetal Bovine Serum (FBS, Cat # F7524) were purchased from Sigma-Aldrich (Saint Louis, United States). For efficacy studies (primary DRG neurons culture), dissection and culture media (RPMI 1640, Cat # R8758; Neurobasal A* Cat #10888022), supplements (Penicillin-Streptomycin Cat # P4333, B27* Cat # 17504044, Glutamax* Cat # 35050061), enzymes (Collagenase Cat #C9697, Trypsin EDTA 0.25%* Cat # 25200056, DNAse* Cat # 18047019, Trypsin Inhibitor Cat T6522), Percoll (Cat # P1644), Poly-D-Lysine (Cat # P3513) and Laminin (Cat # L2020) were also purchased from Sigma-Aldrich (Saint Louis, United States) and ThermoFisher Scientific* (Waltham, MA, United States) respectivley.
Animal anaesthesia: Isoflurane 2% (ISOFLUTEK 1000 mg/g inhalation vapour, liquid) used for animals’ anaesthesia was obtained from Laboratorios KARIZOO, S.A. (Caldes de Montbui, Spain) and used according to literature (Marquardt et al., 2018).
Swiss Albino female mice, 8–12 weeks old, 20–40 g weight were kept in a controlled environment (20°C ± 4°C room temperature, 50% ± 5% relative humidity, and a 12 h artificial light-dark cycle, 07.00 a.m./07.00 p.m.), in individually ventilated cages (IVCs), with ad libitum access to food and water, in the CEMEX animal research facility of the Grigore T. Popa University of Medicine and Pharmacy, Iasi, Romania. The experimental study was carried out in compliance with European Directive 2010/63/EU. It was authorised by the university’s Research Ethics Committee (no. 47/17.02.2021) and approved by the Romanian National Sanitary Veterinary and Food Safety Authority (no. 34/07.04.2021).
Preliminary toxicity tests were performed using fibroblast culture (V79 cell line). In each well of a 96-well plate, 3000 cells were incubated for 24 h at 37°C, 5% carbon dioxide. Serial dilutions (5 µM, 10 μM, 15 μM, 20 μM, and 25 µM) of each cannabinoid was administered for another 24 h. The growth media of the controls contained a similar concentration of DMSO (0.1%), which was used for treatment formulation to exclude any solvent effects on cell viability. We chose our dosages based on similar work published (Cooper and Goldstein, 1976; Miyato et al., 2009; Kosgodage et al., 2018; Saliba et al., 2019; Griffiths et al., 2021; Kim et al., 2021). Treated cell lines were examined microscopically to detect morphological changes or detached cells.
Cell viability was evaluated using the MTT assay, which involves the conversion of the water-soluble yellow dye MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] to insoluble purple formazan by the action of mitochondrial lactate dehydrogenase enzymes (LDH). Further, DMSO was used to dissolve the insoluble purple formazan product into a coloured solution. Treated cells were washed once with phosphate-buffered saline (pH 7.2 0.2), and residual live cells were stained with 0.5% MTT solution. After 2 hours of incubation with the dye, MTT was discarded, and the newly formed intra-cytoplasmic MTT formazan crystals were dissolved using DMSO. The experiment was conducted in triplicate.
The viability of the cells was evaluated using a plate reader (EZ Read 400, Microplate Reader, Biochrom, UK), where optical densities were determined at 570 nm. The viability percentage was calculated as follows: cell viability percentage = (OD of treated cells/OD of untreated cells) × 100. Results are expressed as mean ± SD. The experimental design is presented in Figure 1.
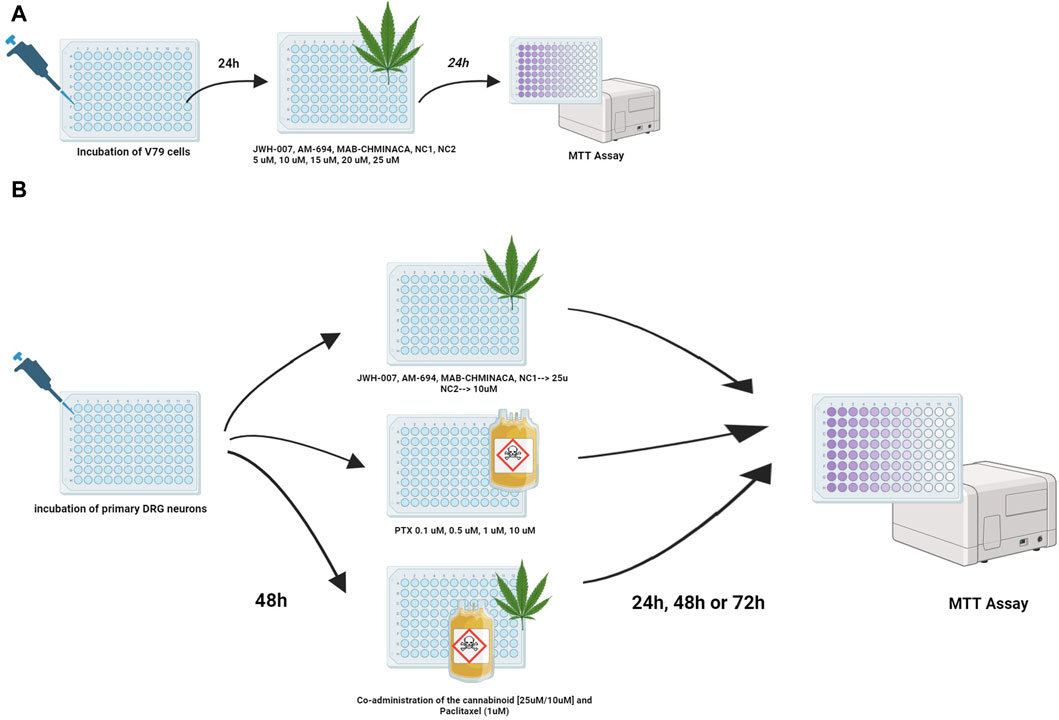
Figure 1. Treatment protocols for studying the viability of the cannabinoids and/or PTX on (A) fibroblasts or (B) DRG neurons were investigated using the MTT test. Viability was assessed at 24 h for fibroblasts and 24, 48, and 72 h post-treatment for neurons.
DRG primary cultures are a suitable, simple, well-accepted method to assess the toxicity of pharmaceutical agents. Their ability to outgrow neurites in vitro and to react by decreasing the axonal length when in contact with neurotoxic agents makes DRG culture an excellent model for studying peripheral neuropathy induced by antineoplastic agents (Windebank et al., 1994). The presented method is adapted by Öztürk et al. (2013).
Swiss Albino female mice were deeply anaesthetised with isoflurane by inhalation followed by scarification. They were placed in a ventral position, and under aseptic conditions, the skin and soft tissues were removed along the vertebral column. Using a corneoscleral punch, the spinous apoptosis was crushed, and the spinal cord was exposed and scooped out. Parallel to the vertebral bodies, each costo-vertebral joint was cut using surgical scissors, clearing out the muscles, fat or other surrounding tissues. The dissection pieces were placed on ice, and using the stereomicroscope, DRGs were identified and collected from the intervertebral foramina on both sides. All collected DRGs were placed in a sterile dish containing the dissection media (RPMI-1640 with 1% Antibiotic/Antimycotic solution) until 15–20 were collected. After dissection, DRGs were moved to the culture media (Neurobasal A, 2% B27, 1% GlutaMax and 1% Antibiotic/Antimycotic solution), where Collagenase 1 mg/mL was added for the first enzymatic reaction (45 min at 37°C, 5% CO2).
At the end of the incubation, collagenase was removed by gently washing three times with 1 mL of HBSS. The second enzymatic reaction started with adding Trypsin EDTA at 0.25% for 15 min at 37°C, with 5% CO2. Soon after, 10 µL DNAse was added to the same tube for the third enzymatic reaction, and DRGs were gradually triturated for approximately 10 min with 1,000 µL pipette tip, 200 µL pipette tip and finally with insulin needle until the tissue was homogenised, followed by another incubation of 30 min. The enzyme activity was stopped by the action of the trypsin inhibitor solution and then removed by centrifugation at 180 g for 3 min. The DRG neurons were collected between the 35% and 10% layers of the Percoll gradient, washed with warm culture media and separated from debrides by centrifugation at 180 g for 20 min 24 h before harvesting, 12-well plates were pre-coated with 1 mg/mL Poly-D-lysine and 0.1 mg/mL laminin overnight at 4°C for the adhesion and neurite elongation of the cultured neurons. They were washed once with distilled H2O before seeding the cells in a culture medium. Separated neurons were seeded onto the centre of the coated coverslips for 2 h in an incubator with 37°C and 5% CO2, followed by adding the warm culture medium. The growth and morphology of neurons were monitored after 24 and 48 h.
DRG neurons (9,000–10,000/well) were incubated for 48 h in 96-well plates and treated with different concentrations of Paclitaxel (0.1 µM, 0.5 µM, 1 μM, and 10 µM) and JWH-007 (25 µM), AM-694 (25 µM), MAB-CHMINACA (25 µM), NC1 (25 µM) and NC2 (10 µM) either individually or simultaneously combined to study the effects on cell viability. For the co-administration of the cannabinoids and PTX, the 1 µM dosage was chosen. Cell viability (%) was measured at 24 h, 48 h or 72 h using the abovementioned MTT assay. DRG neurons cultured in normal media free of PTX or/and the cannabinoids were used as control groups. Controls contained the similar highest concentration of DMSO (0.1%) to exclude any solvent effects on cell viability. All experiments were performed in duplicate. The experimental design is presented in Figure 1.
The cannabinoid’s neuroprotective efficacy was assessed in three different settings (Figure 2): i) Assessment of the toxic/protective effects of each cannabinoid administered in monotherapy; ii) Determination of the toxic dose of PTX that induced shortening in the axonal length; iii) Assessment of the effects of the combination between each cannabinoid and PTX.
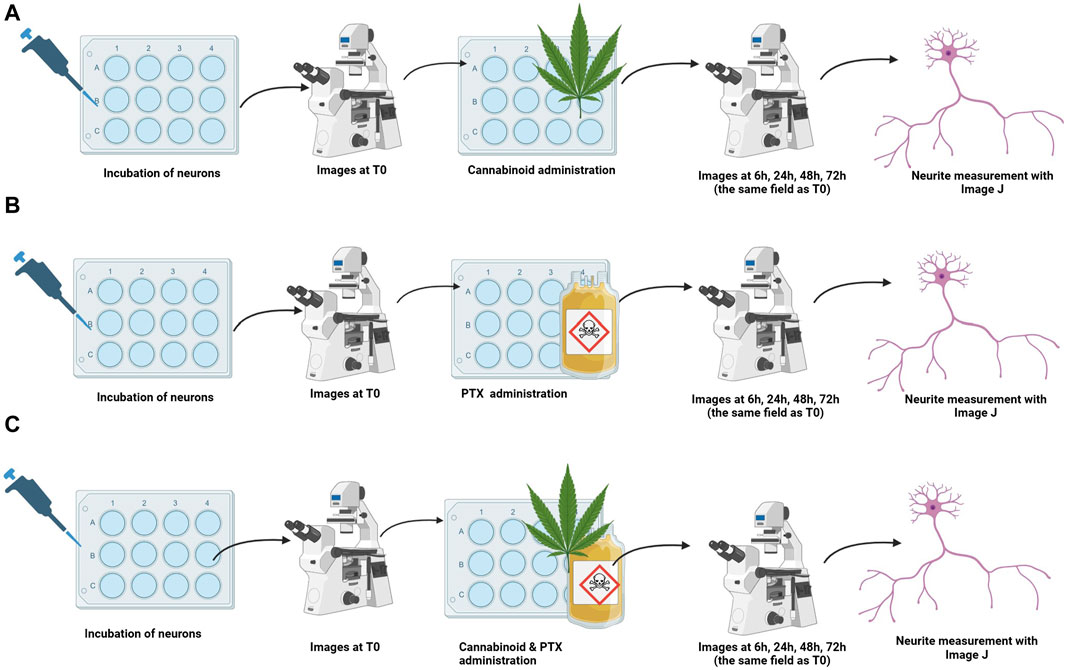
Figure 2. Treatment protocols for studying the efficacy of cannabinoids for neuroprotection. Neurons were incubated, and baseline images were taken. Treatments were administered (A) cannabinoid monotherapy, (B) PTX monotherapy, and (C) co-administration of the cannabinoids and PTX; neuron’s overall morphology and neurite length of the same microscopical field identified at T0 were assessed at 6 h, 24 h, 48 h, and 72 h after treatment administration.
After complete adhesion, maturing and neurite elongation of the cultured neurons was observed, pictures of different microscope fields were taken using a microscope camera (Mshot, CMOS camera MD50-T). The corresponding place on the plate was marked using a fine-tip marker. Immediately after, treatments were administered. To identify the neurotoxic dose of the PTX, neurons were treated with different concentrations (0.1 µM, 0.5 µM, 1 µM, and 10 µM). These dosages were chosen based on previously reported studies (Guo et al., 2017; Akin et al., 2021; Park et al., 2021). The cannabinoid’s neuroprotective features when administered in monotherapy were tested by the addition of 25 µM of the SCs and NC1 or 10 µM of the NC2 on top of the cultured neurons and incubated at the same physiological conditions as mentioned before. We chose these dosages based on our preliminary findings regarding general fibroblast and neural toxicity. 6 h, 24 h, 48 h, and 72 h after the treatment administration, the same microscopic fields identified at T0 were monitored, and pictures were again taken.
For the co-administration of cannabinoids and PTX, fully matured neurons were simultaneously treated with either JWH-007 (25 µM), AM-694 (25 µM), MAB-CHMINACA (25 µM), NC1 (25 µM) or NC2 (10 µM) combined with1µM Paclitaxel and incubated at 37°C, 5% CO2. We chose this dosage of PTX, as we observed that it induced the highest neurite shortening while still keeping the targeted neurons viable at 48 h. As for the previous tests, images of the same neurites were obtained before any treatment and 6 h, 24 h, 48 h, 72 h after administration. For each set, neuronal death was qualitatively assessed and appreciated by the total disappearance of the neuron’s cellular body and neurites in the targeted microscopic field. The media of the controls contained either 1 µM of PTX or normal culture media with a similar concentration of DMSO (0.1%) used for treatment formulation to exclude any solvent effects on cell viability. Data comes from three independent experiments performed with three replicates, where five to eight microscopic regions were randomly recorded per plate, each field containing 1 to 5 measured neurons.
The neurotoxicity of the treatments was assessed by measuring the neurite length of the targeted neurons. To calibrate distances, we followed the instructions provided by Pemberton et al., where an image with a known scale was used. The hemocytometer was chosen as a tool for calibration, as it has a known distance of 200 μm between two adjacent lines (Pemberton et al., 2018). Briefly, a sample image from treated DRG neurons was opened in ImageJ, (v1.46r (National Institutes of Health, Laboratory for Optical and Computational Instrumentation, University of Wisconsin, Madison, WI, United States) subtracted the background, converted to 8-bit grayscale and then individually opened in Neurite Tracer plugin. Each axon was semi-manually traced, and the lengths were automatically calculated. In addition, to ensure consistency and track temporal changes across the experimental timeline, measurements were taken at 6 h, 24 h, 48 h, and 72 h post-administration from the same fields and, consequently, the same neurons as those initially recorded.
For the effects of the cannabinoids on the viability of the cells, results are expressed as %cell viability relative to controls (untreated cells) and represent the mean ±SD of two or three independent experiments. The difference from the controls was assessed using the one-way ANOVA test; a p-value<0.05 was considered significant.
The neuroprotective effects of the treatments were assessed relative to the baseline neurite length (T0). The analysis was focused on the axon shortening percentage for each measurement time (6 h, 24 h, 48 h, and 72 h), computed as the percentage change from the baseline for the neurite length of DRG neurons. Each neuron was compared to its baseline values. Data comes from two independent experiments performed with four replicates, where five to eight regions were recorded randomly per coverslip, each microscopic field containing 1 to 5 measured neurons. The normality of the data was evaluated using the Shapiro-Wilk Normality Test. Furthermore, Levene’s test for homogeneity of variances was conducted, and significant differences between groups were observed. A two-way ANOVA analysis was performed further to investigate the treatments’ significance on neurite outgrowth, followed by pairwise comparisons using T-tests. Bonferroni adjustment was applied to the p-values to address the issue of multiple comparisons. Additionally, pairwise comparisons were conducted assuming unequal variances, necessitating the Welch (or Satterthwaite) approximation to determine the degrees of freedom. p-value<0.05 was considered significant.
Fibroblast cells (V79 cell line) were treated for 24 h with different concentrations (5 µM, 10 μM, 15 μM, 20 μM, and 25 µM) of each of the cannabinoids. We found that the percentage of the viable cells varied with the dose and the type of cannabinoid administered (Figure 3). Each synthetic cannabinoid and NC1 had a good toxicity profile; even at the highest dosages, the viability of the cells remained higher than 70%, as seen in Figure 1. On the other hand, NC2 preserved the fibroblasts viability above 70% only for dosages lower than 10 µM. An additional 5 µM was conducted to a viability of 68%; in the case of the maximum tested dosage, 25 μM, the viability of the cells was 36%.
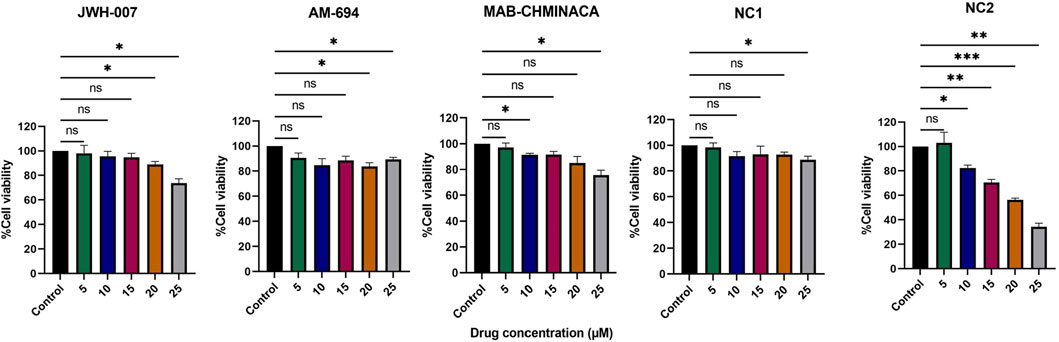
Figure 3. Effects of different concentrations of the cannabinoids (5, 10, 15, 20, and 25 µM) on viability (%) of the V79 fibroblast cell line, relative to controls (fibroblasts in normal culture media). Data is expressed as mean ± SD, performed in triplicate using the MTT assay (*p < 0.05, **p < 0.01, ***p < 0.001, ns = no statistical significance).
When looking at the toxicity evaluated on primary neuron culture, 10 µM Paclitaxel had a clear cytotoxic effect on the cells at all tested time points, with viability dropping to less than 20% at 72 h post-administration, as seen in Figure 4A. On the other hand, the 0.1 µM, 0.5 µM, and 1 µM had cytotoxic features on neurons in a time-dependent, but not concentration-dependent manner, more robust starting with 48 h post-administration. The cannabinoids, as expected, showed no important effects on the viability of the cells when administered in monotherapy, except for MAB-CHMINACA, which conducted a cell viability of almost 70% 72 h post-administration. Combining the cannabinoids and PTX (1 µM) resulted in a clear difference in terms of % of viable cells, more importantly at 48 h and 72 h, where all tested cannabinoids preserved the survival of the neurons compared to chemotherapy alone (Figure 5).
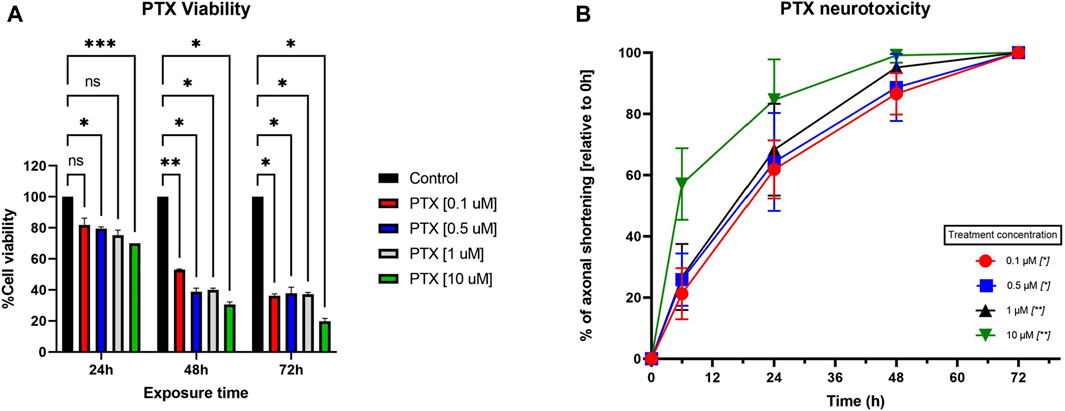
Figure 4. (A) Effects of different concentrations of PTX (0.1 µM, 0.5 µM, 1 μM, and 10 µM) on the viability of neurons, assessed with MTT. The results are expressed as % of viable cells and were measured at 3 different time points (24 h, 48 h, and 72 h post-administration). Data is expressed as mean ± SD; the results come from three different experiments performed in duplicate (*p < 0.05, **p < 0.01, ***p < 0.001, ns = no statistical significance). (B) Effects of different Paclitaxel concentrations (0.1 µM, 0.5 µM, 1 μM, and 10 µM) on neurite shortening of DRG neurons, measured at 6 h, 24 h, 48 h, and 72 h after treatment, compared to their initial lengths. Different concentrations of Paclitaxel had toxic effects leading to a reduction in neurite length, starting as soon as 6 h, compared to baseline values. Data is expressed as % of axonal shortening relative to each neuron’s baseline value and comes from three independent experiments performed with three replicates, where five to eight regions were recorded randomly per coverslip, each microscopic field containing 1–5 measured neurons (*p < 0.05, **p < 0.01, ns = no statistical significance).
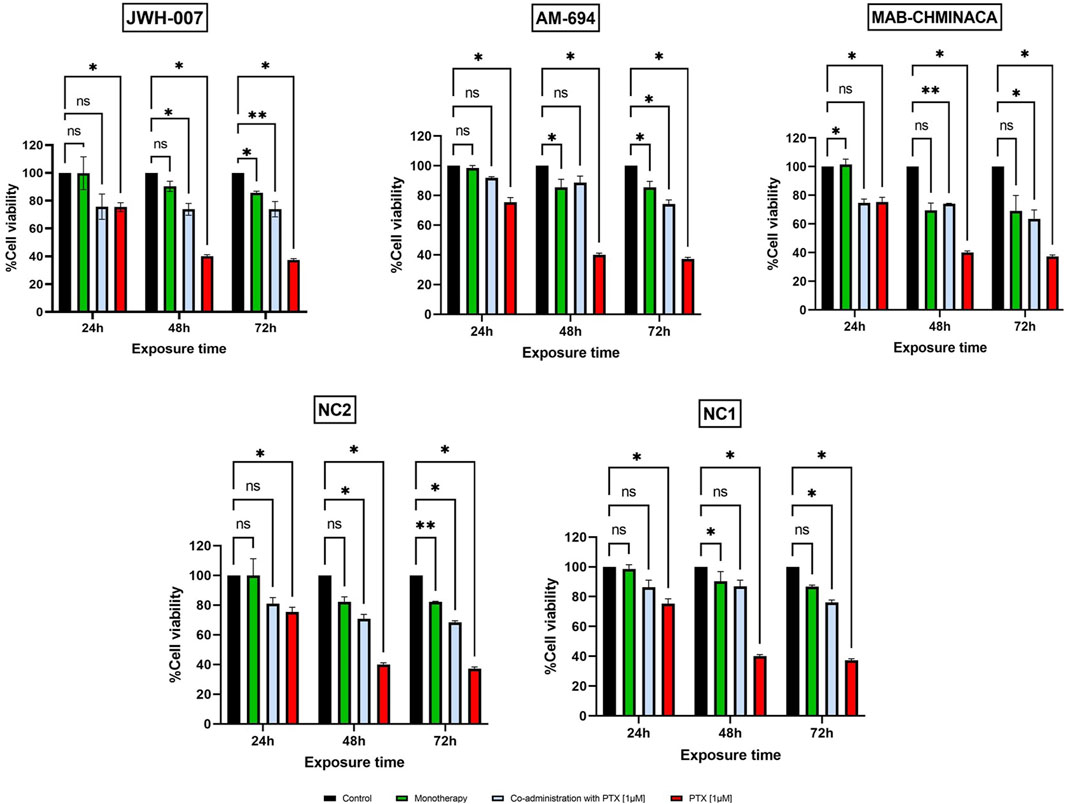
Figure 5. Effects of JWH-007 (25 µM), AM-694 (25 µM), MAB-CHMINACA (25 µM), NC1 (25 µM) and NC2 (10 µM) on neuron viability in monotherapy or co-administered with PTX (1 µM The results are expressed as % of viable cells and were measured at 3 different time points (24 h, 48 h, and 72 h post administration). Data is expressed as mean ± SD; the results come from three different experiments performed in duplicate (*p < 0.05, **p < 0.01, ns = no statistical significance).
Established primary DRG neuron cultures were examined under a phase-contrast microscope at various time points (before treatment (T0) and at 6, 24, 48, and 72 h after treatment) to analyse the overall cellular morphology and track the axonal length. At each time point, the same microscope field was analysed. At T0, all neuron somas appeared round, bright, and refractile, with a large nucleus. The neurons had long, extended, thin neurites that connected and formed networks. Afterwards, due to the action of the treatments, the neurites’ lengths began to modify in a time-dependent manner, as seen in Figure 6.
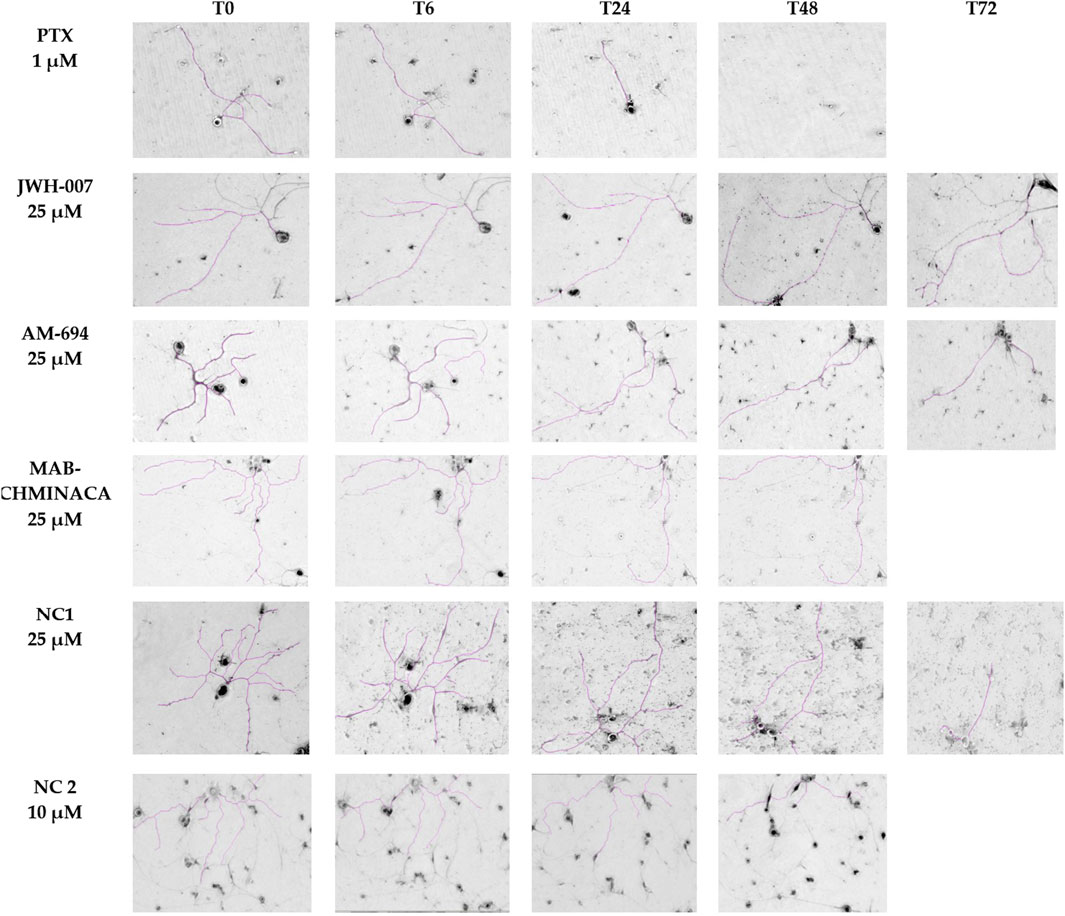
Figure 6. Neurons’ morphological features and neurite outgrowth measurements with ImageJ. On each row, the time-dependent effects of each compound administered in monotherapy are presented, scale bars 200 µm.
PTX’s toxic effects on the neurites were visible starting from the first assessment (6 h), with total neuronal death at 72 h post-administration, as observed in the targeted microscopic fields, regardless of the dose (Figure 4B). In the case of 0.1 µM and 0.5 µM PTX, 24 h after the treatment administration, more than half of the initial axonal length was lost, as seen in Figure 3. 48 h after treatment initiation, cellular death appeared for 13% and respectively 25% of the targeted neurons. The 10 µM concentration had the most toxic effect on the cells; at 24 h, only 63% of the neurons were still viable, and the remaining ones had a preserved neuronal length of almost 20% of their initial values. In the case of the neurons treated with 1 μM, we saw a time-dependent axonal shortening, with the most balanced proportion between maximal axonal shortening and cellular death. Compared to the initial values, the percentages of the remaining axons varied between the ones of the 0.5 µM and 10 µM treated groups at each time point tested. Following the same trend as the rest of the treated groups regarding neuronal death, only 25% of the cells were still identifiable on the targeted microscopic fields 48 h after treatment administration.
JWH-007 had a neuroprotective effect as soon as 6 h after administration, with an almost 6% increase in neurite length (Figure 7). However, this neurotrophic effect was soon over, at the 24 h timepoint the neurites began to shorten compared to untreated neurons that continued to elongate their neurites with an additional 10% from the initial values. In contrast to the known neurotoxic agent PTX, this shortening effect was significantly lower (p < 0.0001) at 24 h, with neurites preserving 91% of their initial length, compared to the PTX group, which preserved 32%. This effect (p < 0.0001) was preserved for the T48 time-point. Interestingly, 72 h after the treatment initiation, neurons were still viable, and the axons were visible and measurable.
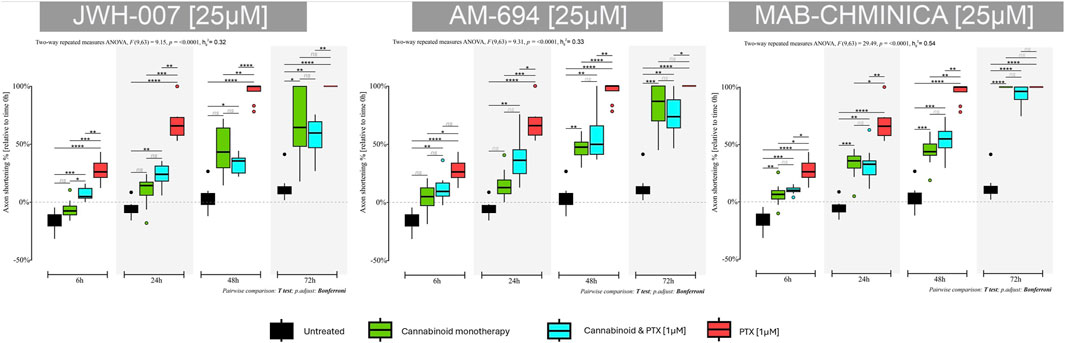
Figure 7. Effects of either 25 µM JWH-007, 25 µM AM-694, 25 µM MAB-CHMINACA on axonal lengths of DRG neurons, measured at 6 h, 24 h, 48 h, and 72 h after treatment, either alone or combined with 1 µM PTX. Data is expressed as % of axonal shortening relative to each neuron’s baseline value. Controls are neurons in normal media and neurons treated with 1 µM PTX. Data comes from two independent experiments performed with four replicates, where five to eight regions were recorded randomly per coverslip, each microscopic field containing 1 to 5 measured neurons. (*p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001, ns = no statistical significance).
Adding PTX to the JWH-007 solution was associated with a more pronounced shortening of the axons than monotherapy but still significantly better than PTX alone (p < 0.001), as seen in the 24-h timepoint. Paradoxically, 48 h and 72 h into the treatment, the combination led to better neurite preservation than cannabinoid monotherapy (65% vs. 55% at 48 h); however, it was not statistically significant.
AM-694 induced neurotrophic activity 6 h after the treatment administration, with a downward trajectory soon after (p < 0.01) (Figure 7). As with the JWH-007, the % of axonal shortening was significantly lower compared to the PTX-only group (p < 0.001 at 24 h, p < 0.0001 at 48 h). At 72 h, neurons remained viable and had a preserved neurite length of 18% from the initial value. However, the untreated neurons kept 87% of their initial length.
The addition of PTX to AM-694 accelerated the neurite shortening, preserving the axonal length of 63% and 43% for the combination group compared to the 84% and 53% of the monotherapy at 24 h and 48 h. Still, the combination was much better than PTX in terms of axonal length preservation (p < 0.01 at 24 h, p < 0.001 at 48 h and p < 0.01 at 72 h). The same paradoxical effect of better combination effects compared to monotherapy was seen in this group of AM-694 treated neurons at 72 h post-therapy initiation.
MAB-CHMINACA induced axonal shortening at all tested time points; however, at 24 h, it reduced the lengths by 22% compared to the PTX group and their 68% shortening (p < 0.01) (Figure 7). The same effect was seen at 48 h (p < 0.01), in contrast to the untreated neurons that preserved 95% of their initial values—also, the cannabinoid-induced cellular death at 72 h post-treatment initiation, similar to the PTX.
The coadministration of MAB-CHMINACA and PTX was similar in axonal shortening compared to the monotherapy; however, it was better than PTX alone. At 24 h, 67% of the initial neurite’s length was preserved, compared to 78% in the monotherapy and 32% in the PTX group (p < 0.01). Similarly, at 48 h, more of the neurite length was preserved compared to the PTX group (p < 0.01).
When administered alone, the NC1 phytocannabinoid had the highest neuroprotective effect at 6 h and 24 h (Figure 8). The neurite’s length increased in total size by 7% compared to their initial values (p < 0.01). More prolonged exposure led to axonal shortening, but the lengths were significantly higher than in the case of PTX. 79% of the axonal length was preserved at 48 h compared to 95% in the untreated group and 3% in the PTX.
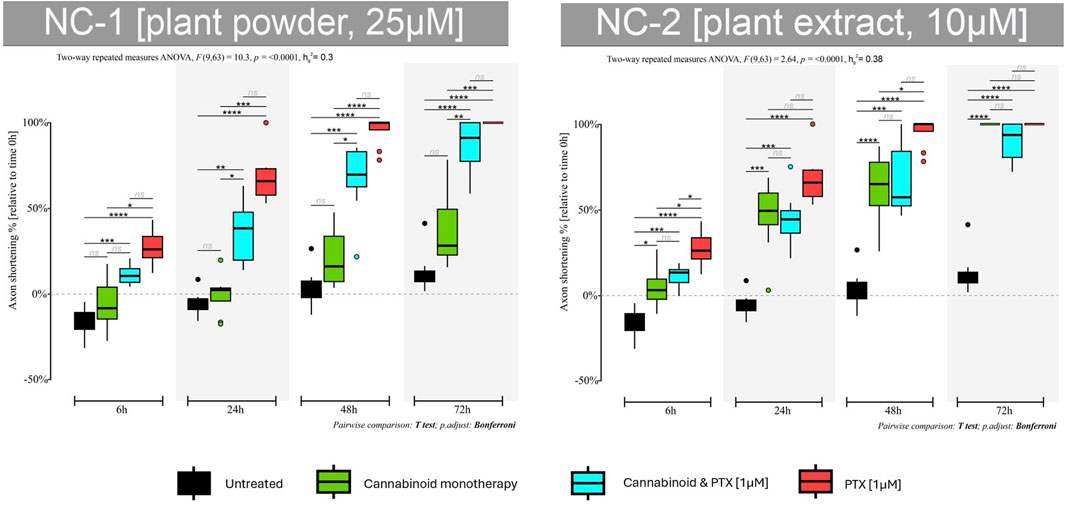
Figure 8. Effects of either 25 µM NC1 or 10 µM NC2 on axonal lengths of DRG neurons, measured at 6 h, 24 h, 48 h, and 72 h after treatment, either alone or combined with 1 µM PTX. Data is expressed as % of axonal shortening relative to each neuron’s baseline value. Controls are neurons in normal media and neurons treated with 1 µM PTX. Data comes from two independent experiments performed with four replicates, where five to eight regions were recorded randomly per coverslip, each microscopic field containing 1 to 5 measured neurons (*p < 0.05,**p < 0.01, ***p < 0.001, ****p < 0.0001, ns = no statistical significance).
When adding the neurotoxic agent, some of the neuroprotective features of the NC1 were obviously lost. However, the cannabinoid protected the neurites from accelerated shortening, and the axons preserved 63% of their initial lengths at 24 h, compared to 32% in the PTX-treated group (p < 0.001) and 33% at 48 h vs. 3% (p < 0.001). Moreover, neurons were still viable at 72 h and also preserved 14% of their initial axonal length.
NC2 induced axonal shortening at all tested time points, but the results were much better than those of the PTX group (Figure 8). The axons shortened by 46% at 24 h compared to the PTX group that lost 32% of their length (p < 0.01). The same trend was seen at 48 h (p < 0.01)—moreover, the phytocannabinoid induced cellular death at 72 h after treatment initiation, similar to PTX.
NC2 protected the neurons from the toxic effects of the PTX. Combining the two agents caused neurites to shorten less than when using PTX only. This group had a 55% and 32% preserved axonal length at 24 h and 48 h compared to 32% and 3%, respectively.
Phytocannabinoids include more than 110 natural compounds, with more than 100 lipophilic molecules identified in their structure, Δ9-THC and CBD being the best-known representatives. Historically, synthetic cannabinoids have been synthesised to localise CBRs, being 2-100 times more potent than Δ9-THC, even though they are similar in chemical structure. Because of the increased risk of abuse and serious side effects, SCs became overlooked for their medicinal benefits (Tamba et al., 2020).
Given the immense variability in composition, thorough safety and efficacy tests must be conducted if translation of the cannabinoids in the clinic is to be achieved. In our study, the tested cannabinoids had an acceptable toxicity profile on fibroblasts and neurons. Even at the highest tested dosages, the SCs and NC1 showed no important cytotoxicity in fibroblast toxicity. NC2 had a good toxicity profile up to the 10 µM dose, and then the viability of the fibroblasts dropped below the 70% threshold. When measuring the cytotoxicity of the compounds on neurons, our data showed that PTX had a toxic effect on the viability of the cells in a time-dependent manner but concentration-dependent for only the 10 µM dosage, in line with similar studies (Guo et al., 2017; Elfarnawany and Dehghani, 2022; Elfarnawany and Dehghani, 2023). However, when PTX was combined with the cannabinoids, the percentage of cytotoxicity decreased, suggesting a protective role that cannabinoids have for preserving neurons. In addition, the viability of the neurons was qualitatively assessed, along with the neurite outgrowth measurements. We could observe that the intense toxicity of PTX was conducted not only to axonal shortening but also to complete neuronal death (the disappearance of the targeted cell), as opposed to the cannabinoids. We could see the same effect as observed with the MTT assay; the addition of cannabinoids on the PTX-treated neurons was conducted to prolong the survival of the cells. Notably, the best results were observed with JWH-007, AM-694 and NC1, with the lowest neuron viability being more than 65% at 72 h after the treatment.
Advances in neuropathic pain therapies are challenging due to the ethical and practical difficulties of pain studies in the human population. Thus, preclinical models are commonly used to mimic similar pathological modifications encountered in neuropathic pain. Moreover, establishing relevant preclinical neuropathic pain models allows the development of clinically efficient pain prophylactic drugs (Sousa et al., 2016). High concentrations of PTX were found to accumulate in the dorsal root ganglia (DRG) and not in the peripheral nerves, suggesting that changes in the peripheral nerve fibres may be secondary to DRG neuron involvement (Cavaletti et al., 2000). Animal studies are often used to investigate the systemic modifications produced by neuropathic pain and the effects of different therapies on peripheral nerves. Still, they are only sometimes suitable to study local interactions. Therefore, various in vitro culture systems have been developed to understand the mechanisms of neurotoxicity/neuroprotection. Primary neuronal cell culture is commonly employed in research to retain the cell’s original structure and physiology (Amini and White, 2013).
Up to 68.1% of cancer patients treated with Paclitaxel develop peripheral neuropathy (Klein and Lehmann, 2021). Although neurons are not dividing cells, they are also a target of PTX’s toxicity, with DRG neurons highly susceptible to chemotherapeutic drug accumulation (Cavaletti et al., 2000). In the current study, PTX caused axon shortening, thus neurotoxicity of the cultured neurons in a dose- and exposure-time-dependent manner, which aligns with similar studies (Carlson and Ocean, 2011; Jaggi and Singh, 2012). The toxic effects of PTX were rapid, with a significant reduction in neurite length of neurons as early as 6 h post-treatment administration. The toxicity is caused by the accumulation of the drug at the neuronal level, which leads to higher susceptibility or vulnerability of neurites to toxins than neuronal somata (Scuteri et al., 2006; Guo et al., 2017).
Reports of the neuroprotective activity of cannabinoids were published decades ago when various cannabinoids were shown to exert neuroprotection upon several disease models such as Parkinson’s Disease, Alzheimer’s Disease, Multiple Sclerosis, and, nevertheless, CIPN (Sarne and Mechoulam, 2005; Galve-Roperh et al., 2008). Using cannabinoids as drugs that target neuroprotection makes them potential candidates to reverse or prevent the neurodegenerative processes that underlie neuropathic pain. To mention a few examples exploring this indication, synthetic cannabinoid WIN-55,212-2, a mixed CB1R/CB2R-receptor agonist, was tested on DRG neurons, and authors found significant results for neuropathic pain modulation through the inhibition of the TNF-α-induced expression/activity of NOS in the treated neurons (Tan and Cao, 2018) The same WIN-55,212-2 confers neuroprotection in a model of neonatal hypoxic-ischemic encephalopathy (Fernández-López et al., 2006) and protects the rat hippocampal neurons from excitotoxicity (Blanton et al., 2019). Incubation of DRG neurons with cisplatin URB597 and JZL184, inhibitors of the endocannabinoid 2-arachidonoylglycerol (2-AG) hydrolysis, attenuated the neurite shortening changes through activation of CB1R (Khasabova et al., 2011; Khasabova et al., 2012). Also, THC administration reduces neuronal loss and brain damage in excitotoxicity and ischemia models (van der Stelt et al., 2001), while 2-arachidonoylglycerol (2AG) protects neurons in traumatic brain injury (Panikashvili et al., 2001).
DRG neurons have diversified morphology and functions responsible for neuropathic pain signal transduction and modulation by the expression of various ion channels and receptors (Berta et al., 2017). The alterations caused by PTX were commonly observed in the transient receptor potential channels (TRP), voltage-gated ion channels, glutamate, and ATP-sensitive receptors in the DRG neurons (Krames, 2014). The molecular mechanisms underlying the neuroprotective action of cannabinoids are related to the direct action of ligands on the endocannabinoid system by activating CB1R and CB2R (Ramírez et al., 2005). Other structures involved are the nuclear receptors of the PPAR family, transcription factors, serotonin 1A receptors and the adenosine signalling pathway (Antonazzo et al., 2019).
Agonists of the CB1R can provide neuroprotective effects by normalising glutamate homeostasis, as the receptors are located at neuronal glutamatergic terminals, and their activation reduces glutamate release (Mechoulam et al., 2002). Moreover, cannabinoids can modulate the activity of voltage-sensitive calcium channels, which consequently could diminish the calcium influx (Demuth and Molleman, 2006). Another neuroprotection mechanism is restoring the equilibrium between oxidative and antioxidant mechanisms within the neurons. It is related to the cannabinoid’s ability to reduce excessive production of the reactive oxygen species by acting as scavengers and antagonising lipid peroxidation (Chen et al., 2000).
Our results clearly show that cannabinoids offer neuroprotection in delaying or preventing neurite shortening, the marker for neuronal suffering. This effect is shown in all the tested cannabinoids when administered together with the PTX. The cannabinoids seemed to be reversing part of the damage that PTX does, with the neurite lengths being significantly longer when a combination of the drugs was administered. When assessing the neurites length of the cannabinoids administered in monotherapy, compared to the untreated controls, we could, in fact, see that they have a toxic impact on their own when administered for more than 24 h. Only two of our tested cannabinoids, JWH-007 and the phytocannabinoid NC1, had a neurotrophic effect in terms of stimulating the axonal elongation, similar to the untreated neurons, but this effect was emphasised only briefly. However, the toxicity of the cannabinoids administered in monotherapy is incomparable with the damage that PTX does.
This “yin-yang” feature of neuroprotection/neurotoxicity of cannabinoids present in our study has also been identified by other research groups. It has been shown to be highly dependent on experimental factors. The dual (neurostimulator and neuroinhibitory) effects of cannabinoids depend, in most cases, on the concentration of the drug tested. Regular (high) concentrations induced the conventional inhibitory (neuroprotective) effects, and low concentrations of cannabinoids induced stimulatory (neurotoxic) effects (Sarne and Keren, 2004). Moreover, short-time exposure to the cannabinoids results in a high concentration of the drug on neurons and, therefore, will protect the cells from damage, while longer exposure times promote nerve damage (Lawston et al., 2000; Panikashvili et al., 2001; van der Stelt et al., 2001). Although in vitro studies are suitable for assessing the local effects of cannabinoids, neuroprotection does not rely solely on neurons but also on glial cells or vascular endothelium, which is why cannabinoid’ neuroprotection is sometimes more evident in vivo studies (Guzman, 2003). Some results worth mentioning are WIN 55,212-2, which has been found to reduce cold allodynia and thermal hyperalgesia symptoms with only one administration on a nerve ligation neuropathy murine model (Rahn et al., 2007). CBD has also demonstrated an antinociceptive effect in mechanical and cold allodynia induced by CIPN using paclitaxel (Ward et al., 2011; Ward et al., 2014). Mixed agonists (∆9 -THC, CP55,940, and WIN55,212-2) reversed mechanical and cold allodynia and plantar heat hyperalgesia in CIPN models (Rahn et al., 2014; Deng et al., 2015a; Deng et al., 2015b).
One interesting finding in our study was that after 48 h of treatment, monotherapy with the SCs caused a higher shortening of the axons compared to the combination of the cannabinoids and PTX. One explanation could be found in the mechanisms mentioned earlier; however, another possibility could be seen when looking at the similarities between the cannabinoids and PTX mechanisms of action. In the case of PTX, besides the activity on the microtubules, the mitochondrion has been put forth as a potential mediator of toxicity due to its ability to alter its structure and function. Studies have shown that PTX evokes immediate mitochondrial depolarisation, and due to the opening of the mitochondrial permeability transition pore, it induces dysregulation of the axonal transport through the voltage-gated calcium channels (Gornstein and Schwarz, 2014). Also, mitochondrion is important in regulating intracellular calcium homeostasis, where increased calcium oscillations are observed after PTX treatment (Kidd et al., 2002). Consequently, the changes that PTX induces in the mitochondrial structure are related to increased calcium-mediated neuronal excitability (Jaggi and Singh, 2012). On the other hand, the interaction of the cannabinoids with G-coupled receptors on the neuronal surface attenuates the cAMP production, which causes a reduction in neuronal activity by modulating the potassium channels and inhibiting voltage-gated calcium channels (Howlett et al., 2010). The modulation of the voltage-dependent calcium channels reduces the elevation of intracellular calcium and, consequently, the release of glutamate, as described in previous paragraphs (Mechoulam et al., 2002; Demuth and Molleman, 2006). On the contrary, some cannabinoids potentiate the internalisation of calcium; thus, high levels of intracellular calcium initiate a complex cascade of intracellular events, such as the stimulation of various proapoptotic enzymes, which affect cell homeostasis and lead to paradoxically, neuronal suffering (Sarne and Mechoulam, 2005). These common mechanisms of action between cannabinoids and PTX could raise the hypothesis of whether there is a non-competitive/competitive antagonistic relationship between the drugs, causing the cannabinoids to temperate part of the harmful action of the chemotherapeutic drug. However, this hypothesis needs further exploration.
When comparing the effects of the best candidate from the SCs, JWH-007 and the best phytocannabinoid, NC1, we observed better effects for preserving the neurite length of the natural cannabinoid when administered in monotherapy. Each strain of cannabis plant can have variations in the concentration of the substances within. It usually contains over 500 other chemical compounds such as cannabinoid phenols, non-cannabinoid phenols, alcohols, aldehydes, n-alkanes, alkaloids, flavonoids, terpenoids, wax esters and steroids, which in turn may modulate the effect of cannabinoids, the so-called “entourage effect” (Ben-Shabat et al., 1998). All these molecules are believed to have synergistic interactions and not only enhance the cannabinoid’s activity but also independently modulate some beneficial effects. Even though SCs are more potent, they do not benefit from the entourage effect, as the accompanying molecules are not incorporated during manufacturing. In contrast, this “simple” structure of JWH-007 was the key to better neuroprotective features when co-administered with PTX, as opposed to NC1, which showed more modest results, probably due to the unknown interactions of some of the plant’s components with the chemotherapeutic drug.
Based on our knowledge and literature search, these cannabinoids were never tested for their potential neuroprotective features and, consequently, analgesic effects for in vitro CIPN models. JWH-007 is a chemical from the naphthoylindole family, which acts as an agonist at both the CB1R, with a binding affinity of 9.5 nM and CB2R, with a binding affinity of 2.94 nM. AM-694 and MAB-CHMINACA are CB1R agonists, but their binding affinity differs. AM-694 has a Ki of 0.08 nM at CB1R and 18 times selectivity over CB2R with a Ki of 1.44 Nm. MAB-CHMINACA is a potent agonist of the CB1R with a binding affinity of Ki = 0.289 nM and was initially developed by Pfizer® in 2009 as an analgesic medication. However, no results regarding its efficacy have been published. The class of synthetic substances known as JWH contains over 100 compounds, all having a much higher affinity for the CBR than natural cannabinoids. Some of the representatives, JWH133 and JWH015, have been found to decrease mechanical allodynia in a nerve ligation neuropathic murine model (Romero-Sandoval et al., 2017). JWH-007s and AM-694s increased affinity for the CB1R could explain the better results obtained in our study regarding neuroprotection, in contrast to the others with lower affinity for the receptor. As for the phytocannabinoids, Cannabixir® Medium dried flowers (NC1) are in the form of a dried inflorescence of Cannabis sativa L. and have a 15.6% THC and <1% CBD content. Cannabixir® THC full extract (NC2), a full THCA-enriched extract of female Cannabis buds, contains ∼20% THC. Nevertheless, Δ9-THC is considered a mixed CB1R/CB2R agonist and has also been shown to alleviate pain, as observed in our study (Rahn et al., 2014; Deng et al., 2015a).
Our results contribute to the knowledge of the benefits that cannabinoids can have for oncological patients, with a particular focus on neuropathic pain. Using cannabinoids as agents that prevent or delay the installation of CIPN is a major contributor to the analgesia and, consequently, the quality of life of cancer patients. Moreover, preventing CIPN allows clinicians to use more aggressive therapeutic schemes, which would give better tumour control while minimizing the side effects, with significantly high input on patient survival. Altogether, neuroprotection of our tested cannabinoids is not a feature desirable only for neuropathic pain and analgesia of cancer patients. These neuroprotective effects could be translated to other models where damage to the peripheral neurons is implied, primarily neurological disorders. Our results are promising for a larger cohort of pathologies and could be further explored using various disease models.
Even though the presented results are promising, we acknowledge that our study isn’t without limitations. The neuroprotection of candidate drugs can be assessed in multiple ways, neurite length dynamics being the most used; however, it is not the only one available (Lehmann et al., 2020). More studies need to be conducted to evaluate the effects of cannabinoids on glial cells, vascular endothelium, and other structures involved in pathogenesis. Additionally, further studies need to investigate the mechanism of action of cannabinoids for neuroprotection. As neurotoxicity could be considered a syndrome due to the multiple mechanisms, cell types, receptors, and neurotransmitters involved, the in vitro studies must be followed by investigations of the effects in living organisms, contributing to the overall knowledge on the topic. Our study’s purpose is exploratory, as it is the first study to screen the chosen cannabinoids in the setting of paclitaxel-induced toxicity; of course, all experimental studies raise questions and hypotheses and must be followed by other mechanism-oriented studies.
Our study paves the way for the benefits of either synthetic cannabinoids or phytocannabinoids for the palliation of chemotherapy-induced peripheral neuropathy. We found that three synthetic cannabinoids (JWH-007, AM-694 and MAB-CHMINACA) and two phytocannabinoids (Cannabixir® Medium dried flowers (NC1) and Cannabixir® THC full extract (NC2)) had an acceptable toxicity profile on fibroblasts and primary neuronal culture and can be an effective option for paclitaxel-induced peripheral neuropathy. The synthetic cannabinoid JWH-007 and the phytocannabinoid Cannabixir® Medium dried flowers (NC1) had the best results in their class. They presented good neurotrophic and neuroprotective activity on the primary dorsal root ganglion culture model. The present findings must be followed by additional tests to understand the exact mechanism of action better and further investigate these results in the in vivo setting. Cannabinoids are at a critical tipping point in science; however, there is a need for more high-quality basic science, which would ensure the successful translation of the cannabinoids into clinical trials.
The raw data supporting the conclusion of this article will be made available by the authors, without undue reservation.
The animal study was approved by the experimental study was performed in accordance with the European Directive 2010/63/EU and has been approved by the university’s Research Ethics Committee (no. 47/17.02.2021) and authorized by Romanian National Sanitary Veterinary and Food Safety Authority (no. 34/07.04.2021). The study was conducted in accordance with the local legislation and institutional requirements.
IC-M: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Validation, Visualization, Writing–original draft, Writing–review and editing. L-EF: Investigation, Writing–review and editing. M-RG: Data curation, Investigation, Writing–original draft. MC: Conceptualization, Writing–review and editing, Formal Analysis. CC: Conceptualization, Writing–review and editing. B-IT: Writing–review and editing, Conceptualization, Funding acquisition, Project administration, Resources, Supervision, Validation, Visualization. TA-S: Conceptualization, Supervision, Visualization, Writing–review and editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by a grant from the Ministry of Research, Innovation and Digitization, CNCS/CCCDI—UEFISCDI, project number PN-III-P4-ID-PCE-2020-1247, within PNCDI III.
We are grateful for the transfer of knowledge regarding DRG culture initiation provided by Professor G. Öztürk and Dr E. Yldiz from the SABITA Laboratory at the Medipol University in Istanbul, Turkey.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
CIPN, chemotherapy-induced peripheral neuropathy; PC, phytocannabinoid; SC, synthetic cannabinoid; CBD, cannabidiol; THC, tetrahydrocannabinol; Δ9 -THC, delta-9-tetrahydrocannabinol; 2AG, 2-arachidonoylglycerol; CB, cannabinoid receptor; CB1R, cannabinoid receptor 1; CB2R, cannabinoid receptor 2; TRPV1, transient receptor potential vanilloid 1; PPARs, peroxisome proliferator-activated receptors; NC1, cannabixir® medium dried flowers; NC2, cannabixir® THC full extract; PTX, paclitaxel; DMSO, dimethylsulfoxide; DRG, dorsal root ganglion; GABA, γ-aminobutyric acid; TNF-α, tumor necrosis factor-α; NOS, nitric oxide synthase; cAMP, cyclic adenosine monophosphate; ESMO, European society of medical oncology; ASCO, American society of clinical oncology.
Akin, E. J., Alsaloum, M., Higerd, G. P., Liu, S., Zhao, P., Dib-Hajj, F. B., et al. (2021). Paclitaxel increases axonal localization and vesicular trafficking of Nav1.7. Brain 144 (6), 1727–1737. doi:10.1093/brain/awab113
Antonazzo, M., Botta, M., Bengoetxea, H., Ruiz-Ortega, J. Á., and Morera-Herreras, T. (2019). Therapeutic potential of cannabinoids as neuroprotective agents for damaged cells conducing to movement disorders. Int. Rev. Neurobiol. 146, 229–257. doi:10.1016/bs.irn.2019.06.012
Barinaga, M. (2001). Neurobiology. How cannabinoids work in the brain. Science 291 (5513), 2530–2531. doi:10.1126/science.291.5513.2530
Ben-Shabat, S., Fride, E., Sheskin, T., Tamiri, T., Rhee, M. H., Vogel, Z., et al. (1998). An entourage effect: inactive endogenous fatty acid glycerol esters enhance 2-arachidonoyl-glycerol cannabinoid activity. Eur. J. Pharmacol. 353 (1), 23–31. doi:10.1016/s0014-2999(98)00392-6
Berta, T., Qadri, Y., Tan, P.-H., and Ji, R.-R. (2017). Targeting dorsal root ganglia and primary sensory neurons for the treatment of chronic pain. Expert Opin. Ther. Targets 21 (7), 695–703. doi:10.1080/14728222.2017.1328057
Blanton, H. L., Brelsfoard, J., DeTurk, N., Pruitt, K., Narasimhan, M., Morgan, D. J., et al. (2019). Cannabinoids: current and future options to treat chronic and chemotherapy-induced neuropathic pain. Drugs 79 (9), 969–995. doi:10.1007/s40265-019-01132-x
Bordet, T., and Pruss, R. M. (2009). Targeting neuroprotection as an alternative approach to preventing and treating neuropathic pain. Neurotherapeutics 6 (4), 648–662. doi:10.1016/j.nurt.2009.07.001
Brown, J. D., and Winterstein, A. G. (2019). Potential adverse drug events and drug-drug interactions with medical and consumer cannabidiol (CBD) use. J. Clin. Med. 8 (7), 989. doi:10.3390/jcm8070989
Burgess, J., Ferdousi, M., Gosal, D., Boon, C., Matsumoto, K., Marshall, A., et al. (2021). Chemotherapy-induced peripheral neuropathy: epidemiology, pathomechanisms and treatment. Oncol. Ther. 9 (2), 385–450. doi:10.1007/s40487-021-00168-y
Campos, R. M. P., Aguiar, A. F. L., Paes-Colli, Y., Trindade, P. M. P., Ferreira, B. K., de Melo Reis, R. A., et al. (2021). Cannabinoid therapeutics in chronic neuropathic pain: from animal research to human treatment. Front. Physiol. 12, 785176. doi:10.3389/fphys.2021.785176
Carlson, K., and Ocean, A. J. (2011). Peripheral neuropathy with microtubule-targeting agents: occurrence and management approach. Clin. Breast Cancer 11 (2), 73–81. doi:10.1016/j.clbc.2011.03.006
Cavaletti, G., Cavalletti, E., Oggioni, N., Sottani, C., Minoia, C., D’Incalci, M., et al. (2000). Distribution of paclitaxel within the nervous system of the rat after repeated intravenous administration. Neurotoxicology 21 (3), 389–393.
Chen, Y., McCarron, R. M., Ohara, Y., Bembry, J., Azzam, N., Lenz, F. A., et al. (2000). Human brain capillary endothelium: 2-arachidonoglycerol (endocannabinoid) interacts with endothelin-1. Circ. Res. 87 (4), 323–327. doi:10.1161/01.res.87.4.323
Cooper, J. T., and Goldstein, S. (1976). Toxicity testing in vitro. I. The effects of delta 9-tetrahydrocannabinol and aflatoxin B1 on the growth of cultured human fibroblasts. Can. J. Physiol. Pharmacol. 54 (4), 541–545. doi:10.1139/y76-075
Creanga-Murariu, I., Filipiuc, L. E., Cuciureanu, M., Tamba, B.-I., and Alexa-Stratulat, T. (2023). Should oncologists trust cannabinoids? Front. Pharmacol. 14, 1211506. doi:10.3389/fphar.2023.1211506
Danziger, N., and Bernstein, N. (2021). Shape matters: plant architecture affects chemical uniformity in large-size medical cannabis plants. Plants 10 (9), 1834. doi:10.3390/plants10091834
Demuth, D. G., and Molleman, A. (2006). Cannabinoid signalling. Life Sci. 78 (6), 549–563. doi:10.1016/j.lfs.2005.05.055
Deng, L., Cornett, B. L., Mackie, K., and Hohmann, A. G. (2015b). CB1 knockout mice unveil sustained CB2-mediated antiallodynic effects of the mixed CB1/CB2 agonist CP55,940 in a mouse model of paclitaxel-induced neuropathic pain. Mol. Pharmacol. 88 (1), 64–74. doi:10.1124/mol.115.098483
Deng, L., Guindon, J., Cornett, B. L., Makriyannis, A., Mackie, K., and Hohmann, A. G. (2015a). Chronic cannabinoid receptor 2 activation reverses paclitaxel neuropathy without tolerance or cannabinoid receptor 1-dependent withdrawal. Biol. Psychiatry 77 (5), 475–487. doi:10.1016/j.biopsych.2014.04.009
Desforges, A. D., Hebert, C. M., Spence, A. L., Reid, B., Dhaibar, H. A., Cruz-Topete, D., et al. (2022). Treatment and diagnosis of chemotherapy-induced peripheral neuropathy: an update. Biomed. Pharmacother. 147, 112671. doi:10.1016/j.biopha.2022.112671
de Souza, M. R., Henriques, A. T., and Limberger, R. P. (2022). Medical cannabis regulation: an overview of models around the world with emphasis on the Brazilian scenario. J. Cannabis Res. 4 (1), 33. doi:10.1186/s42238-022-00142-z
Elfarnawany, A., and Dehghani, F. (2022). Palmitoylethanolamide mitigates paclitaxel toxicity in primary dorsal root ganglion neurons. Biomolecules 12 (12), 1873. doi:10.3390/biom12121873
Elfarnawany, A., and Dehghani, F. (2023). Time- and concentration-dependent adverse effects of paclitaxel on non-neuronal cells in rat primary dorsal root ganglia. Toxics 11 (7), 581. doi:10.3390/toxics11070581
Fernández-López, D., Martínez-Orgado, J., Nuñez, E., Romero, J., Lorenzo, P., Moro, M. A., et al. (2006). Characterization of the neuroprotective effect of the cannabinoid agonist WIN-55212 in an in vitro model of hypoxic-ischemic brain damage in newborn rats. Pediatr. Res. 60 (2), 169–173. doi:10.1203/01.pdr.0000228839.00122.6c
Filipiuc, L. E., Ababei, D. C., Alexa-Stratulat, T., Pricope, C. V., Bild, V., Stefanescu, R., et al. (2021). Major phytocannabinoids and their related compounds: should we only search for drugs that act on cannabinoid receptors? Pharmaceutics 13 (11), 1823. doi:10.3390/pharmaceutics13111823
Finn, D. P., Haroutounian, S., Hohmann, A. G., Krane, E., Soliman, N., and Rice, A. S. C. (2021). Cannabinoids, the endocannabinoid system, and pain: a review of preclinical studies. Pain 162 (Suppl. 1), S5–S25. doi:10.1097/j.pain.0000000000002268
Galve-Roperh, I., Aguado, T., Palazuelos, J., and Guzmán, M. (2008). Mechanisms of control of neuron survival by the endocannabinoid system. Curr. Pharm. Des. 14 (23), 2279–2288. doi:10.2174/138161208785740117
Gornstein, E., and Schwarz, T. L. (2014). The paradox of paclitaxel neurotoxicity: mechanisms and unanswered questions. Neuropharmacology 76 Pt A, 175–183. doi:10.1016/j.neuropharm.2013.08.016
Griffiths, C., Aikins, J., Warshal, D., and Ostrovsky, O. (2021). Can cannabidiol affect the efficacy of chemotherapy and epigenetic treatments in cancer? Biomolecules 11 (5), 766. doi:10.3390/biom11050766
Guindon, J., and Hohmann, A. G. (2009). The endocannabinoid system and pain. CNS Neurol. Disord. Drug Targets 8 (6), 403–421. doi:10.2174/187152709789824660
Guo, L., Hamre, J., Eldridge, S., Behrsing, H. P., Cutuli, F. M., Mussio, J., et al. (2017). Editor’s highlight: multiparametric image analysis of rat dorsal root ganglion cultures to evaluate peripheral neuropathy-inducing chemotherapeutics. Toxicol. Sci. 156 (1), 275–288. doi:10.1093/toxsci/kfw254
Guzman, M. (2003). Neurons on cannabinoids: dead or alive? Br. J. Pharmacol. 140 (3), 439–440. doi:10.1038/sj.bjp.0705465
Hausheer, F. H., Schilsky, R. L., Bain, S., Berghorn, E. J., and Lieberman, F. (2006). Diagnosis, management, and evaluation of chemotherapy-induced peripheral neuropathy. Semin. Oncol. 33 (1), 15–49. doi:10.1053/j.seminoncol.2005.12.010
Howlett, A. C., Blume, L. C., and Dalton, G. D. (2010). CB(1) cannabinoid receptors and their associated proteins. Curr. Med. Chem. 17 (14), 1382–1393. doi:10.2174/092986710790980023
Ibrahim, M. M., Deng, H., Zvonok, A., Cockayne, D. A., Kwan, J., Mata, H. P., et al. (2003). Activation of CB2 cannabinoid receptors by AM1241 inhibits experimental neuropathic pain: pain inhibition by receptors not present in the CNS. Proc. Natl. Acad. Sci. U. S. A. 100 (18), 10529–10533. doi:10.1073/pnas.1834309100
Jaggi, A. S., and Singh, N. (2012). Mechanisms in cancer-chemotherapeutic drugs-induced peripheral neuropathy. Toxicology 291 (1–3), 1–9. doi:10.1016/j.tox.2011.10.019
Jordan, B., Jahn, F., Sauer, S., and Jordan, K. (2019). Prevention and management of chemotherapy-induced polyneuropathy. Breast Care (Basel) 14 (2), 79–84. doi:10.1159/000499599
Jordan, B., Margulies, A., Cardoso, F., Cavaletti, G., Haugnes, H. S., Jahn, P., et al. (2020). Systemic anticancer therapy-induced peripheral and central neurotoxicity: ESMO-EONS-EANO Clinical Practice Guidelines for diagnosis, prevention, treatment and follow-up. Ann. Oncol. 31 (10), 1306–1319. doi:10.1016/j.annonc.2020.07.003
Khasabova, I. A., Gielissen, J., Chandiramani, A., Harding-Rose, C., Odeh, D. A., Simone, D. A., et al. (2011). CB1 and CB2 receptor agonists promote analgesia through synergy in a murine model of tumor pain. Behav. Pharmacol. 22 (5–6), 607–616. doi:10.1097/FBP.0b013e3283474a6d
Khasabova, I. A., Khasabov, S., Paz, J., Harding-Rose, C., Simone, D. A., and Seybold, V. S. (2012). Cannabinoid type-1 receptor reduces pain and neurotoxicity produced by chemotherapy. J. Neurosci. 32 (20), 7091–7101. doi:10.1523/JNEUROSCI.0403-12.2012
Kidd, J. F., Pilkington, M. F., Schell, M. J., Fogarty, K. E., Skepper, J. N., Taylor, C. W., et al. (2002). Paclitaxel affects cytosolic calcium signals by opening the mitochondrial permeability transition pore. J. Biol. Chem. 277 (8), 6504–6510. doi:10.1074/jbc.M106802200
Kim, J., Choi, H., Kang, E. K., Ji, G. Y., Kim, Y., and Choi, I. S. (2021). In vitro studies on therapeutic effects of cannabidiol in neural cells: neurons, glia, and neural stem cells. Molecules 26 (19), 6077. doi:10.3390/molecules26196077
Klein, I., and Lehmann, H. C. (2021). Pathomechanisms of paclitaxel-induced peripheral neuropathy. Toxics 9 (10), 229. doi:10.3390/toxics9100229
Kosgodage, U. S., Mould, R., Henley, A. B., Nunn, A. V., Guy, G. W., Thomas, E. L., et al. (2018). Cannabidiol (CBD) is a novel inhibitor for exosome and microvesicle (EMV) release in cancer. Front. Pharmacol. 9, 889. doi:10.3389/fphar.2018.00889
Krames, E. S. (2014). The role of the dorsal root ganglion in the development of neuropathic pain. Pain Med. 15 (10), 1669–1685. doi:10.1111/pme.12413
Lawston, J., Borella, A., Robinson, J. K., and Whitaker-Azmitia, P. M. (2000). Changes in hippocampal morphology following chronic treatment with the synthetic cannabinoid WIN 55,212-2. Brain Res. 877 (2), 407–410. doi:10.1016/s0006-8993(00)02739-6
Lehmann, H. C., Staff, N. P., and Hoke, A. (2020). Modeling chemotherapy induced peripheral neuropathy (CIPN) in vitro: prospects and limitations. Exp. Neurol. 326, 113140. doi:10.1016/j.expneurol.2019.113140
Loprinzi, C. L., Lacchetti, C., Bleeker, J., Cavaletti, G., Chauhan, C., Hertz, D. L., et al. (2020). Prevention and management of chemotherapy-induced peripheral neuropathy in survivors of adult cancers: ASCO guideline update. J. Clin. Oncol. 38 (28), 3325–3348. doi:10.1200/JCO.20.01399
Lynch, M. E., Cesar-Rittenberg, P., and Hohmann, A. G. (2014). A double-blind, placebo-controlled, crossover pilot trial with extension using an oral mucosal cannabinoid extract for treatment of chemotherapy-induced neuropathic pain. J. Pain Symptom Manage 47 (1), 166–173. doi:10.1016/j.jpainsymman.2013.02.018
Manfredi, J. J., and Horwitz, S. B. (1984). Taxol: an antimitotic agent with a new mechanism of action. Pharmacol. Ther. 25 (1), 83–125. doi:10.1016/0163-7258(84)90025-1
Marquardt, N., Feja, M., Hünigen, H., Plendl, J., Menken, L., Fink, H., et al. (2018). Euthanasia of laboratory mice: are isoflurane and sevoflurane real alternatives to carbon dioxide? PLoS ONE 13 (9), e0203793. doi:10.1371/journal.pone.0203793
Masocha, W. (2018). Targeting the endocannabinoid system for prevention or treatment of chemotherapy-induced neuropathic pain: studies in animal models. Pain Res. Manag. 2018, 5234943. doi:10.1155/2018/5234943
Mechoulam, R., Panikashvili, D., and Shohami, E. (2002). Cannabinoids and brain injury: therapeutic implications. Trends Mol. Med. 8 (2), 58–61. doi:10.1016/s1471-4914(02)02276-1
Mechoulam, R., and ShvoHashish, Y. I. (1963). Hashish. I. The structure of cannabidiol. Tetrahedron. 19 (12), 2073–2078. doi:10.1016/0040-4020(63)85022-x
Miyato, H., Kitayama, J., Yamashita, H., Souma, D., Asakage, M., Yamada, J., et al. (2009). Pharmacological synergism between cannabinoids and paclitaxel in gastric cancer cell lines. J. Surg. Res. 155 (1), 40–47. doi:10.1016/j.jss.2008.06.045
Öztürk, G., Cengiz, N., Erdoğan, E., Him, A., Oğuz, E. K., Yenidünya, E., et al. (2013). Two distinct types of dying back axonal degeneration in vitro. Neuropathol. Appl. Neurobiol. 39 (4), 362–376. doi:10.1111/j.1365-2990.2012.01295.x
Pacher, P., and Kunos, G. (2013). Modulating the endocannabinoid system in human health and disease--successes and failures. FEBS J. 280 (9), 1918–1943. doi:10.1111/febs.12260
Panikashvili, D., Simeonidou, C., Ben-Shabat, S., Hanus, L., Breuer, A., Mechoulam, R., et al. (2001). An endogenous cannabinoid (2-AG) is neuroprotective after brain injury. Nature 413 (6855), 527–531. doi:10.1038/35097089
Park, S. H., Eber, M. R., Fonseca, M. M., Patel, C. M., Cunnane, K. A., Ding, H., et al. (2021). Usefulness of the measurement of neurite outgrowth of primary sensory neurons to study cancer-related painful complications. Biochem. Pharmacol. 188, 114520. doi:10.1016/j.bcp.2021.114520
Pemberton, K., Mersman, B., and Xu, F. (2018). Using imagej to assess neurite outgrowth in mammalian cell cultures: research data quantification exercises in undergraduate neuroscience lab. J. Undergrad. Neurosci. Educ. 16 (2), A186–A194.
Pergam, S. A., Woodfield, M. C., Lee, C. M., Cheng, G.-S., Baker, K. K., Marquis, S. R., et al. (2017). Cannabis use among patients at a comprehensive cancer center in a state with legalized medicinal and recreational use. Cancer 123 (22), 4488–4497. doi:10.1002/cncr.30879
Piomelli, D., Giuffrida, A., Calignano, A., and Rodríguez de Fonseca, F. (2000). The endocannabinoid system as a target for therapeutic drugs. Trends Pharmacol. Sci. 21 (6), 218–224. doi:10.1016/s0165-6147(00)01482-6
Rahn, E. J., Deng, L., Thakur, G. A., Vemuri, K., Zvonok, A. M., Lai, Y. Y., et al. (2014). Prophylactic cannabinoid administration blocks the development of paclitaxel-induced neuropathic nociception during analgesic treatment and following cessation of drug delivery. Mol. Pain 10, 27. doi:10.1186/1744-8069-10-27
Rahn, E. J., Makriyannis, A., and Hohmann, A. G. (2007). Activation of cannabinoid CB1 and CB2 receptors suppresses neuropathic nociception evoked by the chemotherapeutic agent vincristine in rats. Br. J. Pharmacol. 152 (5), 765–777. doi:10.1038/sj.bjp.0707333
Ramírez, B. G., Blázquez, C., Gómez del Pulgar, T., Guzmán, M., and de Ceballos, M. L. (2005). Prevention of Alzheimer’s disease pathology by cannabinoids: neuroprotection mediated by blockade of microglial activation. J. Neurosci. 25 (8), 1904–1913. doi:10.1523/JNEUROSCI.4540-04.2005
Romero-Sandoval, E. A., Kolano, A. L., and Alvarado-Vázquez, P. A. (2017). Cannabis and cannabinoids for chronic pain. Curr. Rheumatol. Rep. 19 (11), 67. doi:10.1007/s11926-017-0693-1
Saliba, S. W., Bonifacino, T., Serchov, T., Bonanno, G., de Oliveira, A. C. P., and Fiebich, B. L. (2019). Neuroprotective effect of AM404 against NMDA-induced hippocampal excitotoxicity. Front. Cell Neurosci. 13, 566. doi:10.3389/fncel.2019.00566
Sarne, Y., and Keren, O. (2004). Are cannabinoid drugs neurotoxic or neuroprotective? Med. Hypotheses 63 (2), 187–192. doi:10.1016/j.mehy.2004.02.043
Sarne, Y., and Mechoulam, R. (2005). Cannabinoids: between neuroprotection and neurotoxicity. Curr. Drug Targets CNS Neurol. Disord. 4 (6), 677–684. doi:10.2174/156800705774933005
Scott, K. A., Dalgleish, A. G., and Liu, W. M. (2014). The combination of cannabidiol and Δ9-tetrahydrocannabinol enhances the anticancer effects of radiation in an orthotopic murine glioma model. Mol. Cancer Ther. 13 (12), 2955–2967. doi:10.1158/1535-7163.MCT-14-0402
Scuteri, A., Nicolini, G., Miloso, M., Bossi, M., Cavaletti, G., Windebank, A. J., et al. (2006). Paclitaxel toxicity in post-mitotic dorsal root ganglion (DRG) cells. Anticancer Res. 26 (2A), 1065–1070.
Seretny, M., Currie, G. L., Sena, E. S., Ramnarine, S., Grant, R., MacLeod, M. R., et al. (2014). Incidence, prevalence, and predictors of chemotherapy-induced peripheral neuropathy: a systematic review and meta-analysis. Pain 155 (12), 2461–2470. doi:10.1016/j.pain.2014.09.020
Sousa, A. M., Lages, G. V., Pereira, C. L., and Slullitel, A. (2016). Experimental models for the study of neuropathic pain. Rev. dor 17. doi:10.5935/1806-0013.20160043
Staff, N. P., Fehrenbacher, J. C., Caillaud, M., Damaj, M. I., Segal, R. A., and Rieger, S. (2020). Pathogenesis of paclitaxel-induced peripheral neuropathy: a current review of in vitro and in vivo findings using rodent and human model systems. Exp. Neurol. 324, 113121. doi:10.1016/j.expneurol.2019.113121
Tamba, B. I., Stanciu, G. D., Urîtu, C. M., Rezus, E., Stefanescu, R., Mihai, C. T., et al. (2020). Challenges and opportunities in preclinical research of synthetic cannabinoids for pain therapy. Med. Kaunas. 56 (1), 24. doi:10.3390/medicina56010024
Tan, R., and Cao, L. (2018). Cannabinoid WIN-55,212-2 mesylate inhibits tumor necrosis factor-α-induced expression of nitric oxide synthase in dorsal root ganglion neurons. Int. J. Mol. Med. 42 (2), 919–925. doi:10.3892/ijmm.2018.3687
Tringale, K. R., Huynh-Le, M.-P., Salans, M., Marshall, D. C., Shi, Y., and Hattangadi-Gluth, J. A. (2019). The role of cancer in marijuana and prescription opioid use in the United States: a population-based analysis from 2005 to 2014. Cancer 125 (13), 2242–2251. doi:10.1002/cncr.32059
van der Stelt, M., Veldhuis, W. B., Bär, P. R., Veldink, G. A., Vliegenthart, J. F., and Nicolay, K. (2001). Neuroprotection by Delta9-tetrahydrocannabinol, the main active compound in marijuana, against ouabain-induced in vivo excitotoxicity. J. Neurosci. 21 (17), 6475–6479. doi:10.1523/JNEUROSCI.21-17-06475.2001
Vera, G., Cabezos, P. A., Martín, M. I., and Abalo, R. (2013). Characterization of cannabinoid-induced relief of neuropathic pain in a rat model of cisplatin-induced neuropathy. Pharmacol. Biochem. Behav. 105, 205–212. doi:10.1016/j.pbb.2013.02.008
Ward, S. J., McAllister, S. D., Kawamura, R., Murase, R., Neelakantan, H., and Walker, E. A. (2014). Cannabidiol inhibits paclitaxel-induced neuropathic pain through 5-HT(1A) receptors without diminishing nervous system function or chemotherapy efficacy. Br. J. Pharmacol. 171 (3), 636–645. doi:10.1111/bph.12439
Ward, S. J., Ramirez, M. D., Neelakantan, H., and Walker, E. A. (2011). Cannabidiol prevents the development of cold and mechanical allodynia in paclitaxel-treated female C57Bl6 mice. Anesth. Analg. 113 (4), 947–950. doi:10.1213/ANE.0b013e3182283486
Windebank, A. J., Smith, A. G., and Russell, J. W. (1994). The effect of nerve growth factor, ciliary neurotrophic factor, and ACTH analogs on cisplatin neurotoxicity in vitro. Neurology 44 (3 Pt 1), 488–494. doi:10.1212/wnl.44.3_part_1.488
Yamamoto, W., Mikami, T., and Iwamura, H. (2008). Involvement of central cannabinoid CB2 receptor in reducing mechanical allodynia in a mouse model of neuropathic pain. Eur. J. Pharmacol. 583 (1), 56–61. doi:10.1016/j.ejphar.2008.01.010
Zajączkowska, R., Kocot-Kępska, M., Leppert, W., Wrzosek, A., Mika, J., and Wordliczek, J. (2019). Mechanisms of chemotherapy-induced peripheral neuropathy. Int. J. Mol. Sci. 20 (6), 1451. doi:10.3390/ijms20061451
Keywords: neuropathic pain, cannabis, THC, CBD, antalgic, palliative care, chemotherapy, cellular model
Citation: Creanga-Murariu I, Filipiuc L-E, Gogu M-R, Ciorpac M, Cumpat CM, Tamba B-I and Alexa-Stratulat T (2024) The potential neuroprotective effects of cannabinoids against paclitaxel-induced peripheral neuropathy: in vitro study on neurite outgrowth. Front. Pharmacol. 15:1395951. doi: 10.3389/fphar.2024.1395951
Received: 04 March 2024; Accepted: 27 May 2024;
Published: 12 June 2024.
Edited by:
Mohammed Abu El-Magd, Kafrelsheikh University, EgyptReviewed by:
Mustafa Shukry, Kafrelsheikh University, EgyptCopyright © 2024 Creanga-Murariu, Filipiuc, Gogu, Ciorpac, Cumpat, Tamba and Alexa-Stratulat. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bogdan-Ionel Tamba, Ym9nZGFuLnRhbWJhQHVtZmlhc2kucm8=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.