
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Pharmacol. , 23 July 2024
Sec. Neuropharmacology
Volume 15 - 2024 | https://doi.org/10.3389/fphar.2024.1395867
 Marie-Lou Dessus-Gilbert1‡
Marie-Lou Dessus-Gilbert1‡ Mikail Nourredine2,3†‡
Mikail Nourredine2,3†‡ Luc Zimmer2,4†
Luc Zimmer2,4† Benjamin Rolland4,5†
Benjamin Rolland4,5† Marie-Maude Geoffray5,6†
Marie-Maude Geoffray5,6† Marine Auffret2,3†
Marine Auffret2,3† Lucie Jurek5,6*†
Lucie Jurek5,6*†Aims: This systematic review and meta-analysis aimed to assess the efficacy of NMDA antagonists in ASD (Autism Spectrum Disorder) on the core (communication and social interaction, repetitive behavior) and associated symptoms (irritability) of ASD, as well as their safety.
Methods: PubMed, CENTRAL, CINHAL, EMBASE, and PsycINFO databases were searched until November 2023. Two authors independently selected the studies and extracted data. Randomized controlled trials assessing the efficacy of NMDA receptor antagonists in participants with ASD aged <18 years were included. The quality of the studies was assessed using the Risk of Bias-2 tool. A random-effect meta-analysis model was used to calculate standardized mean differences (SMD) or odds ratios (OR) using meta package in R.
Results: This systematic review included ten studies (588 participants). Most studies did not report scales assessing core symptoms of ASD. Meta-analysis of efficacy on ASD core symptoms included three studies (248 participants). NMDA antagonists were not superior to placebo [SMD = 0.29; CI 95% (−1,94; 1.35); I2 = 0%]. NMDA antagonists was not superior to placebo concerning response (four studies, 189 participants) [OR = 2.4; CI 95% (0.69; 8.38); I2 = 35%]. Meta-analysis of efficacy on irritability included three studies (186 participants); NMDA antagonists were not superior to placebo [MD irritability = −1.94; CI 95% (−4.66; 0.77); I2 = 0%]. Compared with placebo, significantly more participants in the NMDA antagonist group reported at least one adverse event (five studies, 310 participants) [OR = 2.04; CI 95% (1.17; 3.57); I2 = 0%].
Conclusion: Current evidence does not support the effectiveness of NMDA antagonists in the treatment of ASD symptoms or irritability. Further research is needed due to the limited and low quality data available.
Systematic Review Registration: PROSPERO CRD42018110399.
Autism Spectrum Disorder (ASD) is a neurodevelopmental disorder characterized by communication and social interaction deficits, as well as repetitive behaviors and/or restricted interests, referred to as core symptoms of ASD (American Psychiatric Association and éditeur, 2015). The proportion of autistic children in the general pediatric population is estimated to be 0.6% in Europe and 0.4% worldwide (Salari et al., 2022).
While only two medications are approved by the FDA (risperidone and aripiprazole) for the treatment of irritability associated with ASD, no pharmacological treatment is indicated for the decrease in ASD core symptoms (Center for Drug Evaluation and Research and U.S. Food and Drug Administration, 2018).
Studies of neurotransmitters and autism have suggested that aberrant glutamatergic transmission may play a role in ASD. Glutamate is an important excitatory neurotransmitter essential to cognitive function and neuronal development. Its action on neuroglial cells has various effects, such as neuronal migration, differentiation, and development. The glutamatergic system contributes to neural plasticity and cognitive functions. However, excess glutamate can be neurotoxic, leading to cellular death (Rojas, 2014; Uzunova et al., 2014).
Several experimental studies have found abnormalities in the glutamate system in ASD, mainly focusing on the NMDA (N-methyl-D-aspartate) receptor, an ionotropic receptor that enhances glutamatergic excitation. Postmortem studies of the brain tissues of autistic patients, for example, have shown lower levels of glutamate decarboxylase, the catalyst that converts glutamate to GABA, and increased NMDA receptor density (Purcell et al., 2001; Rojas, 2014). Dysfunction in NMDA receptors at excitatory synapses has been associated with ASD (Ghanizadeh, 2011; Burnashev and Szepetowski, 2015). Genetic studies have shown alterations in NMDA receptor subunit in ASD (Paoletti et al., 2013). Animal models of ASD suggest bidirectional dysfunction of NMDA receptors by showing, among other things, that modulators of NMDA receptors can normalize ASD-like behaviors in animal models (Kang and Kim, 2015; Lee et al., 2015; Chung et al., 2019).
Different NMDA antagonist drugs that act on the glutamatergic system by blocking glutamate entry into cells have been assessed in ASD. This review aims to appraise the efficacy and safety of NMDA antagonists on ASD symptom severity in autistic children. Efficacy on behavioral problem outcomes (irritability/hyperactivity) will also be evaluated.
The recommendations of the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA 2020) (Page et al., 2021) reporting guideline were followed herein (see Supplementary Material). The protocol of this review was registered in PROSPERO in October 2018 (CRD42018110399). Deviations from the preregistered protocol are described below.
We have searched the following electronic bibliographic databases: PubMed, the Cochrane Central Register of Controlled Trials (CENTRAL), CINHAL, EMBASE, and PsycINFO, from their creation until October 2, 2020.
A combination of terms related to ASD and NMDA antagonists were used. The complete algorithm is presented in the Supplementary Material. The reference lists of the included articles were manually checked to identify any additional relevant studies.
Alerts were used to retrieve new eligible articles up to November 2023. No new studies were included.
Two independent reviewers (L.J. and M.D.) conducted the literature search with the help of the Covidence website (www.covidence.com) to process the double-blind selection and to manage the duplicates.
Each reviewer checked the relevance of the different studies through their titles and abstracts. The full texts were read to determine their eligibility. Disagreements were resolved by referral to a third author (M.N).
The inclusion criteria were as follows: 1) original articles written in English or French, published in a peer-reviewed journal reporting randomized controlled studies (RCT) or unpublished trials retrieved from CENTRAL if results were available; 2) the population was composed of children (under 18 years of age) with a clinical diagnosis of ASD (or Pervasive Developmental Disorders, PDD) corresponding to the criteria of the Diagnostic and Statistical Manual for Mental Disorders (DSM-IV), Fourth or Fifth (DSM-5) Editions, or the International Classification of Disease, 10 th Revision (ICD-10), or using a standardized diagnostic instrument. Genetic syndromes and ADHD were accepted if associated with a documented diagnosis of ASD or PDD; 3) The intervention was a pharmacological intervention with an NMDA antagonist (e.g., memantine, dextromethorphan, atomoxetine, ketamine, amantadine, acamprosate, felbamate, minocycline, d-cycloserine, lanicomine, nitrous oxide, taxoprodil, or rapastinel). Any dosage, duration, or administration frequency of the drug was considered. The control procedure was a placebo.
Animal studies, studies including adults and the elderly, or studies on autistic symptoms without ASD (or PDD) were excluded from this review.
For each included study, two reviewers (L.J. and ML.D.) extracted the following variables using a standardized extraction form: study design, sample size, population characteristics, ASD diagnosis method, adverse events, and study results.
The extracted data were verified by a third author (M. N.). In case of missing data or additional details, the primary authors were contacted by mail or directly by telephone. Eight authors were contacted, but no responses were received.
Two independent reviewers (L. J. and M. N.) assessed the risk of bias of the included studies using the Revised Cochrane risk-of-bias tool for randomized trials (RoB 2). Any discrepancies were resolved through discussion with a third author (M.G.). The ROB2 contains five domains, as follows: 1) Bias arising from the randomization process, 2) Bias due to deviations from intended interventions, 3) Bias due to missing outcome data, 4) Bias in measurement of the outcome, 5) Bias in selection of the reported result. Each domain is divided into signage questions. The response options for the signaling questions are: 1) Yes, 2) Probably yes, 3) Probably no, 4) No, and 5) No information. These responses allow us to determine a Low, Unclear, or High risk of bias.
Data were analyzed using R studio (R software version 4.1.2) with the “meta” package (version 6.5–0). We used random-effects models because they allow the true population effect size to differ among studies. The effect size was the odds ratio for dichotomous outcomes and mean differences for continuous outcomes. The standardized mean difference was used when all studies assessed the same outcome but used different scales. Restricted maximum likelihood estimator for tau2 was used. The Hartung Knapp method (IntHout et al., 2014) was used to compute confidence intervals of the summary effect. Heterogeneity was analyzed using tau2 and I2.
The initially planned meta-analysis could not be carried out with all the included studies due to the clinical heterogeneity of the available data. Therefore, we focused on four criteria: efficacy of NMDA antagonists on autism core symptoms, number of responders, efficacy on irritability, and the number of adverse events.
Although initially planned, none of the subgroup or sensitivity analyses were performed because of the small number of available studies (Higgins and Thompson, 2004). Similarly, as previously recommended, publication bias was not assessed because less than ten studies were included in the meta-analysis (Lau et al., 2006).
The literature search generated 560 articles. Thirteen additional records were identified through manual searches of bibliographies. After removing duplicates, the titles and abstracts of 405 records were screened, and 35 records were assessed for eligibility by full-text review. Nineteen articles were excluded as they did not meet the inclusion criteria. The reasons for exclusion are outlined in Figure 1 and the Supplementary Material. An additional search performed before the final analysis did not retrieve any additional articles. Two publications reported the same study (Minshawi et al., 2016; Wink et al., 2017). The main publication of the RCT (Minshawi et al., 2016) was included; however, information from both publications was used. Four reports, each corresponding to one peer-reviewed publication, were retrieved from the trial registers. Ten unique studies were included in the systematic review, with a total of 588 participants (314 with intervention and 256 with placebo).
The characteristics and main results of the included studies are summarized in Table 1.
The search retrieved 5 studies from the United States (Woodard et al., 2007; Minshawi et al., 2016; Aman et al., 2017; Gagan, 2019; Hardan et al., 2019), 3 from Iran (Ghaleiha et al., 2013; Mohammadi et al., 2013; Karahmadi et al., 2018), 1 from the UK (King et al., 2001), and 1 from Ukraine (Martsenkovsky and Martsenkovska, 2016). Six studies were unicentric (Gagan, 2019; Woodard et al., 2007; Ghaleiha et al., 2013; Karahmadi et al., 2018; Mohammadi et al., 2013; Martsenkovsky and Martsenkovska, 2016) and four were multicenter trials (King et al., 2001; Minshawi et al., 2016; Aman et al., 2017; Hardan et al., 2019), ranging from two to six different centers. The diagnosis of ASD was validated by DSM IV or 5 criteria and/or by ADI-R and/or ADOS, while some studies considered CARS (Woodard et al., 2007), GARS (Karahmadi et al., 2018), or ICD 10 (King et al., 2001) criteria.
Six studies were double-blind randomized controlled trials with intervention versus placebo. Two studies compared NMA antagonist + risperidone versus placebo plus risperidone (Ghaleiha et al., 2013; Mohammadi et al., 2013), one study was conducted using the ABAB scheme (Woodard et al., 2007), and one was a withdrawal study (Hardan et al., 2019). Eight studies had follow-up durations of ≥ 10 weeks.
The treatments evaluated were memantine in six studies (Ghaleiha et al., 2013; Martsenkovsky and Martsenkovska, 2016; Aman et al., 2017; Gagan, 2019; Karahmadi et al., 2018; Hardan et al., 2019), amantadine in two studies (King et al., 2001; Mohammadi et al., 2013), dextromethorphan in one study (Woodard et al., 2007), and d-cycloserine in one study (Minshawi et al., 2016). Risperidone was associated with amantadine in one study (Mohammadi et al., 2013) and memantine in one study (Ghaleiha et al., 2013). Two interventions were combined with behavioral therapy (Martsenkovsky and Martsenkovska, 2016; Karahmadi et al., 2018) and one with social skills training (Wink et al., 2017).
The main scales used were the Aberrant Behavior Checklist (ABC) (n = 7), Clinical Global Impression (CGI) (n = 6), Social Responsiveness Scale (SRS) (n = 5), and Children’s Communication Checklist (CCC) (n = 3). ABC, SRS, and CCC were completed by the parents and/or caregivers. CGI was rated by the investigator. No self-administered questionnaire was administered.
Participant samples ranged from 16 to 121 and were mostly male. One study did not report sex distribution (Martsenkovsky and Martsenkovska, 2016). The patients were aged between 7 and 10 years, except for one study with participants aged 18–36 months (Martsenkovsky and Martsenkovska, 2016).
Two studies excluded patients with intellectual disability (IQ < 70) (Minshawi et al., 2016; Gagan, 2019), and two did not provide any details on IQ (Martsenkovsky and Martsenkovska, 2016; Karahmadi et al., 2018). Other studies selected participants with IQs ≥ 35. Only two studies used ADHD (Attention Deficit Hyperactivity Disorder) as an exclusion criterion (Mohammadi et al., 2013; Karahmadi et al., 2018). In other studies, this diagnosis was not sought in the inclusion criteria, the exclusion criteria, or the description of the population. Methylphenidate and other psychotropic drugs were additional exclusion criteria in some studies (Gagan, 2019; Ghaleiha et al., 2013; King et al., 2001). Only one study searched for and reported four methylphenidate users (Aman et al., 2017).
The severity of ASD according to the DSM 5 (Rosen et al., 2021) was not specified. Severity was usually assessed by CGI-Severity, but results on this scale were never reported.
The overall quality of each study is reported in Figure 2. The risk of bias was rated as low in two studies (Mohammadi et al., 2013; Hardan et al., 2019), some concerns were reported in two studies (Minshawi et al., 2016; Aman et al., 2017), and a high risk of bias was reported in six studies (Gagan, 2019; Woodard et al., 2007; Ghaleiha et al., 2013; Karahmadi et al., 2018; King et al., 2001; Martsenkovsky and Martsenkovska, 2016). For these studies, there was a risk that the reported results had been selected post hoc, as they did not provide a registered protocol before the end of the study. One study (Gagan, 2019) had a registered protocol, but precision on the primary outcome was added after the end of the study. One study (Karahmadi et al., 2018) reported a subscale analysis as the primary result (ABC subscale-irritability), in contrast to the outcome described in their registered protocol (ABC total score).
On the ten included studies, three authors did not precisely determine the main outcome of the study (Woodard et al., 2007; Martsenkovsky and Martsenkovska, 2016; Karahmadi et al., 2018).
Two studies found a significant effect of a NMDA-antagonist on irritability (rated with the ABC-irritability subscale) at week 10. In the first study, SRS-irritability at the end line was lower in the mematine + risperidone group (8.90; SD = 3.05) than in the placebo + risperidone group (12.75; SD = 3.05) (p ≤ 0.01) (Ghaleiha et al., 2013). In the second study, difference from baseline was higher in amantadine + risperidone group (8.60; SD = 4.65) than in placebo + risperidone group (5.35; SD = 3.95) with a mean difference of 3.2 (95%CI 0.48–6.01) (Mohammadi et al., 2013).
No significant difference in response was observed in the King et al. study, with 47% responders in the amantadine group and 37% in the placebo group {OR = 1.5 [95% CI(0.4; 5.9)]} (King et al., 2001), while Gagan (2019) reported 47% of responders in the memantine group and 19% in the placebo group.
Two studies chose the SRS-total score as the main outcome and found no significant differences between the active and placebo group. Aman et al. (2017) estimated a mean difference of −0.1 (95% CI = −7.2 to 6.6) between memantine and placebo group. Minshawi et al. (2016) estimated a mean difference of 3.61 (CI 95% = −5.95–13.17) between d-cycloserine + social skills training and placebo + social-skills training.
In the withdrawal study, no significant difference in loss of therapeutic response was observed between full-dose of memantine and placebo [OR = 1.1 (95 CI% = 0.7; 1.8)] or between reduced-dose of memantine and placebo [OR = 1.1 (95 CI% = 0.7; 1.7)] (Hardan et al., 2019).
The secondary outcomes are presented in Table 1.
Three studies reported serious adverse events (SAE). Aman et al. reported three SAE in the memantine group: irritability, choking, and affective disorders (Aman et al., 2017). In a study by Hardan et al. (2019), four SAE were reported in the placebo group and two in the memantine group. Suicidal thoughts were reported in one study in the placebo group (Minshawi et al., 2016).
Since many studies did not report the results of scales assessing the core symptoms of autism, three studies were included in the meta-analysis, with a total of 248 participants (Figure 3). NMDA antagonists were not superior to placebo [SMD = 0.29; CI95% (−1.94; 1.35); I2 = 0%].
Four studies were included in the meta-analysis of responses to NMDA antagonists, with a total of 189 participants (Figure 4). The response rate was not higher in the NMDA antagonist group than in the placebo group [OR = 2.4; CI 95% (0.69; 8.38); I2 = 35%].
Three studies were included in the meta-analysis, assessing the efficacy of NMDA antagonists on irritability, with a total of 186 participants (Figure 5). NMDA antagonists were not superior to placebo [MD irritability = −1.94; CI 95%(−4.66; 0.77); I2 = 0%].
Five studies were included in the meta-analysis of adverse events (at least one adverse event) with a total of 310 patients (Figure 6). NMDA participants had a significantly higher risk of at least one adverse event than placebo participants [OR = 2.04; CI 95% (1.17; 3.57); I2 = 0%].
The meta-analysis found no efficacy of NMDA antagonists either on the core symptoms of autism, on the overall clinical response, or on the irritability of autistic children.
These results were consistent with those reported in the literature. Two recent meta-analyses assessed the efficacy of memantine, a specific NMDA antagonist, in autism (Brignell et al., 2022; Elnaiem et al., 2022). Both studies concluded that memantine did not improve the core symptoms of autism, even if the certainty of evidence was rated as very low. No significant effects on irritability were observed.
Our review of the literature shows encouraging publications on studies with a long follow-up period (>10 weeks) using NMDA receptor antagonists as an adjuvant to other therapies (behavioral therapy, antipsychotics). These results were not synthesized in the meta-analysis because insufficient data were available.
The variability in the results of these studies may be related to the heterogeneity of methodologies in terms of treatment, duration, population characteristics, and assessment tools (Figure 7). The methodological quality of the different studies was heterogeneous, with a high risk of bias for six studies over ten. We must point out that on several occasions, the outcomes were not ideally chosen or misinterpreted. Some studies reported subscale scores as primary outcomes, whereas the power of the study was calculated based on the total score. Other researchers have reported this observation in the ASD field (Provenzani et al., 2020). Details on the characteristics of the included population were regularly lacking; for example, the age ranges were wide, sweeping across broad levels of development and IQ. The groups did not spread out on the level of autism severity. Very little information was available on comorbidities, particularly ADHD and sleep disorders, two pathologies that could explain irritability and hyperactivity symptoms (Johnson et al., 2018; Thomas et al., 2018).
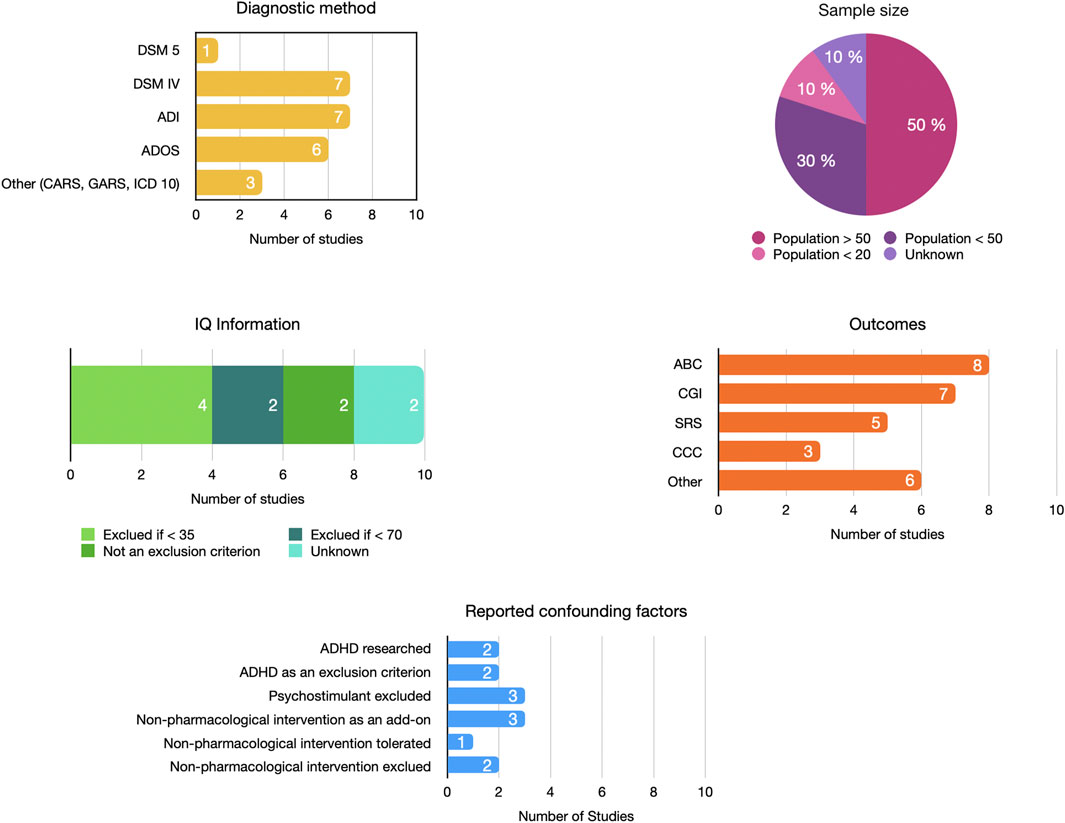
Figure 7. Heterogeneity in the design of included studies. ABC, Aberrant Behavior Checklist; ADHD, Attention Deficit/Hyperactivity Disorder; ADI, Autism Diagnostic Interview; ADOS, Autism Diagnostic Observation Scale; CARS, Childhood Autism Rating Scale; CCC, Children’s Communication Checklist; CGI, Clinical Global Impressions Scale; DSM, Diagnostic and Statistical Manual of Mental Disorders; GARS, Gilliam Autism Rating Scale; ICD, International Classification of Diseases; IQ, Intellectual Quotient; SRS, Social Responsiveness scale.
None of the included studies used self-report questionnaires completed by the children. The studies primarily relied on questionnaires filled out by parents, along with professional evaluations. This choice could be partially justified by a lack of feasibility given the high proportion of young participants and/or those with intellectual disabilities.
Concerning the safety aspects, our meta-analysis showed a higher number of patients with at least one adverse event in the NMDA antagonist group than in the control group. Some severe adverse events were reported in different studies, but none were described as “related to the drug.” These results are consistent with those of two recent meta-analyses on memantine (Brignell et al., 2022; Elnaiem et al., 2022).
Our study has several limitations. As the number of included studies was small, we could not proceed to the subgroup and sensibility analyses we planned. Our primary outcome (core autistic symptoms) was not described in every study, and most of the included studies focused on comorbid autism symptoms. Therefore, the meta-analyses are based on a few studies. The interventions were heterogeneous because we decided to study all NMDA receptor antagonists to understand the potential applicability of the hypothesis of a role of the NMDA system in autism.
Publication bias common to literature reviews was reduced with multiple study sources, including Clinical Trial registries, to broaden the search. Nevertheless, we could not quantitatively analyze publication bias because of the small number of included studies. Moreover, our attempts to contact different authors to retrieve missing data were unsuccessful.
Our study has several strengths. It is the first systematic review to assess the efficacy of NMDA receptor antagonists in autistic symptoms. We followed a previously published protocol on PROSPERO, and the two authors’ screening and data extractions were performed independently. The screening was updated immediately before the final analyses to retrieve potential studies for inclusion. We followed PRISMA recommendations for reporting.
To facilitate the evaluation of therapies for autism and future study synthesis, assessment tools should be standardized, and non-validated questionnaires should not be used as the only outcome in such important studies (Provenzani et al., 2020).
It is necessary to conduct subgroup studies to evaluate treatment efficacy and tolerance according to IQ level, age, or comorbidities. In RCT concerning neurodevelopmental disorders, it is unacceptable to have an imprecise record of comorbidities (IQ, ADHD, etc.) and co-prescribed medications. There is a global need to improve study reporting to enable interpretation, comparison, and application in clinical practice.
As autism is a neurodevelopmental disorder, the literature emphasizes the importance of timing when initiating treatment to modulate brain development. Treatment effectiveness varies according to the degree of maturation and brain plasticity (Uzunova et al., 2014). Indeed, some animal models favor early treatment with NMDA antagonists to attenuate autistic symptoms (Chung et al., 2019). Due to the small number of included studies, we could not analyze the impact of different timing of treatment initiation in this review.
In conclusion, the results of this meta-analysis and literature review are insufficient to confirm nor infirm the efficacy of NDMA antagonists on ASD core symptoms. The NMDA pathway remains interesting for treating behavioral symptoms associated with ASD; however, it is currently insufficiently evaluated and requires more and better-constructed studies.
The current data do not allow us to recommend the prescription of drugs for their NMDA receptor antagonist properties in the indication of ASD.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
M-LD-G: Data curation, Investigation, Visualization, Writing–original draft, Writing–review and editing. MN: Conceptualization, Formal Analysis, Methodology, Supervision, Validation, Writing–original draft, Writing–review and editing. LZ: Conceptualization, Validation, Writing–original draft, Writing–review and editing. BR: Conceptualization, Validation, Writing–original draft, Writing–review and editing. M-MG: Validation, Writing–original draft, Writing–review and editing. MA: Conceptualization, Validation, Writing–original draft, Writing–review and editing. LJ: Conceptualization, Investigation, Methodology, Supervision, Validation, Writing–original draft, Writing–review and editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2024.1395867/full#supplementary-material
Aman, M. G., Findling, R. L., Hardan, A. Y., Hendren, R. L., Melmed, R. D., Kehinde-Nelson, O., et al. (2017). Safety and efficacy of memantine in children with autism: randomized, placebo-controlled study and open-label extension. J. Child Adolesc. Psychopharmacol. 27, 403–412. doi:10.1089/cap.2015.0146
American Psychiatric Association, éditeur (2015). Diagnostic and statistical manual of mental disorders: DSM-5. 5th ed. Washington, D.C: American Psychiatric Association.
Brignell, A., Marraffa, C., Williams, K., and May, T. (2022). Memantine for autism spectrum disorder. Cochrane Database Syst. Rev. 8(8):CD013845. doi:10.1002/14651858.CD013845.pub2
Burnashev, N., and Szepetowski, P. (2015). NMDA receptor subunit mutations in neurodevelopmental disorders. Curr. Opin. Pharmacol. 20, 73–82. doi:10.1016/j.coph.2014.11.008
Center for Drug Evaluation and Research, U.S. Food and Drug Administration (2018). The Voice of the Patient. A series of reports from the. U.S. Food and Drug Administration’s Patient-Focused Drug Development Initiative.
Chung, C., Ha, S., Kang, H., Lee, J., Um, S. M., Yan, H., et al. (2019). Early correction of N-Methyl-D-Aspartate receptor function improves autistic-like social behaviors in adult Shank2−/− mice. Biol. Psychiatry 85, 534–543. doi:10.1016/j.biopsych.2018.09.025
Elnaiem, W., Benmelouka, A. Y., Elgendy, A. M. N., Abdelgalil, M. S., Brimo Alsaman, M. Z., Mogheeth, A., et al. (2022). Evaluation of memantine’s efficacy and safety in the treatment of children with autism spectrum disorder: a systematic review and meta-analysis. Hum. Psychopharmacol. 37, e2841. doi:10.1002/hup.2841
Gagan, J. (2019). Behavioral and neural response to memantine in adolescents with autism spectrum disorder. Available at: https://clinicaltrials.gov/ct2/show/NCT01972074.
Ghaleiha, A., Asadabadi, M., Mohammadi, M.-R., Shahei, M., Tabrizi, M., Hajiaghaee, R., et al. (2013). Memantine as adjunctive treatment to risperidone in children with autistic disorder: a randomized, double-blind, placebo-controlled trial. Int. J. Neuropsychopharmacol. 16, 783–789. doi:10.1017/S1461145712000880
Ghanizadeh, A. (2011). Targeting of Glycine site on NMDA receptor as a possible new strategy for autism treatment. Neurochem. Res. 36, 922–923. doi:10.1007/s11064-010-0381-2
Hardan, A. Y., Hendren, R. L., Aman, M. G., Robb, A., Melmed, R. D., Andersen, K. A., et al. (2019). Efficacy and safety of memantine in children with autism spectrum disorder: results from three phase 2 multicenter studies. Autism 23, 2096–2111. doi:10.1177/1362361318824103
Higgins, J. P. T., and Thompson, S. G. (2004). Controlling the risk of spurious findings from meta-regression. Stat. Med. 23, 1663–1682. doi:10.1002/sim.1752
IntHout, J., Ioannidis, J. P., and Borm, G. F. (2014). The Hartung-Knapp-Sidik-Jonkman method for random effects meta-analysis is straightforward and considerably outperforms the standard DerSimonian-Laird method. BMC Med. Res. Methodol. 14, 25. doi:10.1186/1471-2288-14-25
Johnson, C. R., Smith, T., DeMand, A., Lecavalier, L., Evans, V., Gurka, M., et al. (2018). Exploring sleep quality of young children with autism spectrum disorder and disruptive behaviors. Sleep. Med. 44, 61–66. doi:10.1016/j.sleep.2018.01.008
Kang, J., and Kim, E. (2015). Suppression of NMDA receptor function in mice prenatally exposed to valproic acid improves social deficits and repetitive behaviors. Front. Mol. Neurosci. 8, 17. doi:10.3389/fnmol.2015.00017
Karahmadi, M., Tarrahi, M., Vatankhah Ardestani, S., Omranifard, V., and Farzaneh, B. (2018). Efficacy of memantine as adjunct therapy for autism spectrum disorder in children aged <14 years. Adv. Biomed. Res. 7, 131. doi:10.4103/abr.abr_100_18
King, B. H., Wright, D. M., Handen, B. L., Sikich, L., Zimmerman, A. W., Mcmahon, W., et al. (2001). Double-blind, placebo-controlled study of amantadine hydrochloride in the treatment of children with autistic disorder. J Am Acad Child Adolesc Psychiatry. 40(6):658-65. doi:10.1097/00004583-200106000-00010
Lau, J., Ioannidis, J. P. A., Terrin, N., Schmid, C. H., and Olkin, I. (2006). The case of the misleading funnel plot. BMJ 333, 597–600. doi:10.1136/bmj.333.7568.597
Lee, E.-J., Choi, S. Y., and Kim, E. (2015). NMDA receptor dysfunction in autism spectrum disorders. Curr. Opin. Pharmacol. 20, 8–13. doi:10.1016/j.coph.2014.10.007
Martsenkovsky, I., and Martsenkovska, I. (2016). The safety and efficacy of memantine hydrochloride versus placebo for children under 3 years old with autism spectrum disorders. Eur. Neuropsychopharmacol. 26, S729. doi:10.1016/s0924-977x(16)31879-x
Minshawi, N. F., Wink, L. K., Shaffer, R., Plawecki, M. H., Posey, D. J., Liu, H., et al. (2016). A randomized, placebo-controlled trial of d-cycloserine for the enhancement of social skills training in autism spectrum disorders. Mol. Autism 7, 2. doi:10.1186/s13229-015-0062-8
Mohammadi, M.-R., Yadegari, N., Hassanzadeh, E., Farokhnia, M., Yekehtaz, H., Mirshafiee, O., et al. (2013). Double-blind, placebo-controlled trial of risperidone plus amantadine in children with autism: a 10-week randomized study. Clin. Neuropharmacol. 36, 179–184. doi:10.1097/WNF.0b013e3182a9339d
Page, M. J., McKenzie, J. E., Bossuyt, P. M., Boutron, I., Hoffmann, T. C., Mulrow, C. D., et al. (2021). The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 372, n71. doi:10.1136/bmj.n71
Paoletti, P., Bellone, C., and Zhou, Q. (2013). NMDA receptor subunit diversity: impact on receptor properties, synaptic plasticity and disease. Nat. Rev. Neurosci. 14, 383–400. doi:10.1038/nrn3504
Provenzani, U., Fusar-Poli, L., Brondino, N., Damiani, S., Vercesi, M., Meyer, N., et al. (2020). What are we targeting when we treat autism spectrum disorder? A systematic review of 406 clinical trials. Autism 24, 274–284. doi:10.1177/1362361319854641
Purcell, A. E., Jeon, O. H., Zimmerman, A. W., Blue, M. E., and Pevsner, J. (2001). Postmortem brain abnormalities of the glutamate neurotransmitter system in autism. Neurology 57, 1618–1628. doi:10.1212/wnl.57.9.1618
Rojas, D. C. (2014). The role of glutamate and its receptors in autism and the use of glutamate receptor antagonists in treatment. J. Neural Transm. 121, 891–905. doi:10.1007/s00702-014-1216-0
Rosen, N. E., Lord, C., and Volkmar, F. R. (2021). The diagnosis of autism: from kanner to DSM-III to DSM-5 and beyond. J. Autism Dev. Disord. 51, 4253–4270. doi:10.1007/s10803-021-04904-1
Salari, N., Rasoulpoor, S., Rasoulpoor, S., Shohaimi, S., Jafarpour, S., Abdoli, N., et al. (2022). The global prevalence of autism spectrum disorder: a comprehensive systematic review and meta-analysis. Ital. J. Pediatr. 48, 112. doi:10.1186/s13052-022-01310-w
Thomas, S., Lycett, K., Papadopoulos, N., Sciberras, E., and Rinehart, N. (2018). Exploring behavioral sleep problems in children with ADHD and comorbid autism spectrum disorder. J. Atten. Disord. 22, 947–958. doi:10.1177/1087054715613439
Uzunova, G., Hollander, E., and Shepherd, J. (2014). The role of ionotropic glutamate receptors in childhood neurodevelopmental disorders: autism spectrum disorders and fragile X syndrome. CN. 12, 71–98. doi:10.2174/1570159X113116660046
Wink, L. K., Minshawi, N. F., Shaffer, R. C., Plawecki, M. H., Posey, D. J., Horn, P. S., et al. (2017). d-Cycloserine enhances durability of social skills training in autism spectrum disorder. Mol. Autism 8, 2. doi:10.1186/s13229-017-0116-1
Keywords: autism, d-cycloserine, amantadine, memantine, meta-analysis, NMDA
Citation: Dessus-Gilbert M-L, Nourredine M, Zimmer L, Rolland B, Geoffray M-M, Auffret M and Jurek L (2024) NMDA antagonist agents for the treatment of symptoms in autism spectrum disorder: a systematic review and meta-analysis. Front. Pharmacol. 15:1395867. doi: 10.3389/fphar.2024.1395867
Received: 04 March 2024; Accepted: 04 July 2024;
Published: 23 July 2024.
Edited by:
Harry Pantazopoulos, University of Mississippi Medical Center, United StatesReviewed by:
Deborah Sokol, Indiana University, Purdue University Indianapolis, United StatesCopyright © 2024 Dessus-Gilbert, Nourredine, Zimmer, Rolland, Geoffray, Auffret and Jurek. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lucie Jurek, bHVjaWUuanVyZWtAY2gtbGUtdmluYXRpZXIuZnI=
†ORCID: Mikail Nourredine, orcid.org/0000-0002-0952-0091; Luc Zimmer, orcid.org/0000-0002-2805-7098; Benjamin Rolland, orcid.org/0000-0002-8666-3635; Marie-Maude Geoffray, orcid.org/0000-0002-5528-3613; Marine Auffret, orcid.org/0000-0002-5236-9083; Lucie Jurek, orcid.org/0000-0001-7568-568X
‡These authors share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.