- 1Department of Hepatobiliary Surgery, Guangdong Provincial Hospital of Chinese Medicine, Guangzhou, China
- 2Department of Anesthesia, The Second Affiliated Hospital of Guangzhou Medical University, Guangzhou, China
Background: Hepatitis B, often leading to Hepatocellular carcinoma (HCC), poses a major global health challenge. While Tenofovir (TDF) and Entecavir (ETV) are potent treatments, their comparative effectiveness in improving recurrence-free survival (RFS) and overall survival (OS) rates in HBV-related HCC is not well-established.
Methods: We conducted an individual patient data meta-analysis using survival data from randomized trials and high-quality propensity score-matched studies to compare the impact of Tenofovir (TDF) and Entecavir (ETV) on RFS and OS in HBV-related HCC patients. Data from six databases and gray literature up to 30 August 2023, were analyzed, utilizing Kaplan-Meier curves, stratified Cox models, and shared frailty models for survival rate assessment and to address between-study heterogeneity. The study employed restricted mean survival time analysis to evaluate differences in RFS and OS between TDF-treated and ETV-treated patients. Additionally, landmark analyses compared early (<2 years) and late (≥2 years) tumor recurrence in these cohorts.
Results: This study incorporated seven research articles, covering 4,602 patients with HBV-related HCC (2,082 on TDF and 2,520 on ETV). Within the overall cohort, TDF recipients demonstrated significantly higher RFS (p = 0.042) and OS (p < 0.001) than those on ETV. The stratified Cox model revealed significantly improved OS for the TDF group compared to the ETV group (hazard ratio, 0.756; 95% confidence interval, 0.639–0.896; p = 0.001), a result corroborated by the shared frailty model. Over a follow-up period of 1–8 years, no significant difference was noted in the mean time to death between the TDF and ETV groups. The rates of early recurrence did not significantly differ between the groups (p = 0.735). However, TDF treatment was significantly associated with a reduced risk of late recurrence compared to ETV (p < 0.001). In the HCC resection subgroup, the disparities in OS, early, and late recurrence rates between the two treatments paralleled those seen in the overall cohort.
Conclusion: Compared to ETV, TDF may enhance OS and reduce late tumor recurrence risk in HBV-related HCC patients receiving curative treatment. However, there was no statistically significant distinction in the timing of tumor recurrence and mortality between patients administered TDF and those prescribed ETV.
Systematic Review Registration: http://www.crd.york.ac.uk/prospero/.
Introduction
Hepatitis B is a global health concern, affecting approximately 296 million people worldwide with chronic hepatitis B infection (Hsu et al., 2023). Hepatocellular carcinoma (HCC) is one of the primary and most lethal outcomes of chronic hepatitis B (CHB). It is the third leading cause of cancer-related mortality globally and it is estimated that around 830,000 deaths occur annually worldwide due to HCC (Vogel et al., 2022). Despite the introduction of numerous treatment modalities over the past few decades, including hepatic resection, liver transplantation, ablative therapies, transarterial chemoembolization, radiotherapy, and systemic antineoplastic treatments (Zhou et al., 2023), it is unfortunate that a high level of hepatitis B virus (HBV) DNA remains an independent risk factor for the recurrence of HCC, even in cases undergoing curative liver resection, consequently leading to diminished postoperative survival rates (Wang et al., 2020). Previous research has demonstrated that nucleos(t)ide analogues (NAs) treatment not only significantly reduces the incidence of HBV-related HCC (Wu et al., 2014), but also markedly prolongs the overall survival of patients with HBV-related HCC and reduces tumor recurrence by lowering viral load (Huang et al., 2015; Lee et al., 2016). While NAs therapy contributes to improved prognoses in HBV-related HCC, it fails to cure HBV or completely prevent HCC recurrence. This is attributed to the inability of NAs to eliminate covalently closed circular DNA (cccDNA) within HBV-infected hepatocytes, which can persist and potentially reactivate within the liver cells (Yang and Kao, 2014). Until the advent of novel antiviral agents targeting cccDNA or hepatocytes harboring cccDNA, optimizing NAs therapy remains a primary focus.
Tenofovir (TDF) and Entecavir (ETV), as potent NAs with high resistance barriers, are recommended in major clinical guidelines as primary treatments for chronic HBV infection (Sarin et al., 2016; Lampertico et al., 2017; Terrault et al., 2018; You et al., 2023a). Although both TDF and ETV have similar antiviral efficacy, their relative impacts on the prognosis of HBV-related HCC patients are debated. Recent meta-analysis suggests that compared with ETV, TDF has the advantage of improving recurrence-free survival (RFS) and overall survival (OS) of patients with HBV-related HCC who underwent resection (Kong et al., 2023; Liu et al., 2023). However, this analysis is limited by its reliance on aggregated study-level data, which overlooks individual patient differences, and by the lack of individual patient time-to-event data, making it impossible to accurately calculate survival rates and risks at each time point, thus affecting the precision of RFS and OS estimates. To address these limitations, this study conducts an individual patient data meta-analysis (IPDMA) using randomized controlled trial or high-quality propensity score-matched cohort study data. This approach provides more accurate RFS and OS estimates and resolves ongoing debates. IPDMA is considered the gold standard for pooled analysis of time-to-event data, as it comprehensively accounts for censoring and effectively addresses both between-study and within-study heterogeneity (De Jong et al., 2020). Importantly, it also allows for testing violations of the proportional hazards assumption, an aspect not feasible in traditional meta-analyses (Rahman et al., 2019).
Methods
Search strategy and selection criteria
We conducted a literature synthesis in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines for individual participant data systematic reviews (Stewart et al., 2015; Page et al., 2021). Six databases (Web of Science, PubMed, Embase, Scopus, Cochrane CENTRAL, LILACS) and gray literature (OpenGrey, ProQuest Dissertations and Theses Global) were searched without language restriction from inception to 30 August 2023. Keywords and Medical Subject Headings (MeSH) terms pertaining to HBV-related HCC, TDF, and ETV were integrated into the search strategy. Details of the full search strategy are available in Supplementary Methods. Two reviewers independently screened titles and abstracts and subsequently performed full-text reviews. Any disagreements were resolved through discussion, involving a senior reviewer when necessary. The inclusion criteria for this study were patients diagnosed with HBV-related HCC, studies comparing the effects of TDF versus ETV, and reporting outcomes as RFS and/or OS. The study types included randomized controlled trials or high-quality PSM studies. Sufficient data were required, including Kaplan-Meier survival curves, time-to-event data, and detailed patient characteristics (Tan et al., 2022). Studies employing other confounder control methods, including covariance adjustment, stratification, and inverse probability of treatment weighting, were excluded. Although these techniques are typically effective in reducing bias, their balancing effect is lost in meta-analyses using reconstructed individual patient data, since meta-analysts are unaware of the patient-level covariates or propensity scores used for bias control (Syn et al., 2021). For studies with overlapping patient populations in multiple papers, we selected the article that provided the most data with either the largest patient sample, the most subgroup data, and/or the most updated data.
Data extraction and study quality assessment
Two reviewers independently extracted study characteristics, encompassing patient demographics, tumor profiles, and biochemical parameters, in addition to covariates employed for propensity score matching. Disagreements were resolved through discussion, in cases where consensus was not reached, a senior reviewer served as an arbitrator. Individual patient data were reconstructed from published survival curves utilizing the methodology proposed by Guyot and colleagues (Guyot et al., 2012). Additionally, two reviewers independently evaluated the quality of included studies employing the Newcastle-Ottawa Scale for cohort studies (Wells et al., 2023), with any disagreements resolved through consensus or consultation with a senior reviewer.
Statistical analysis
All analyses were conducted using R statistical software (version 4.2.3, R Foundation for Statistical Computing, 2023). For the baseline characteristics included in the study (if data is available), dichotomous variables were pooled using means and 95% confidence intervals. When median data were reported, they were converted to means and standard deviations using established methodologies prior to being pooled (Wan et al., 2014; Luo et al., 2018). For continuous variables, the data were pooled using constituent ratios and 95% confidence intervals. The heterogeneity of the included studies was assessed using Cochran’s Q statistic and the I2 metric. When I2 was less than 50%, a fixed-effect model was employed; otherwise, a random-effect model was utilized for pooling the results. Kaplan-Meier curves were used to plot the RFS rates and OS rates of HBV-related HCC, with intergroup differences assessed using the log-rank test. Sensitivity analysis was also conducted to evaluate the stability of the results using a “leave-one-out” analysis. We employed both stratified Cox models and shared frailty models to address between-study heterogeneity. Both models need to satisfy the proportional hazards assumption and cannot directly handle time-dependent covariates. We used the Grambsch-Therneau test to assess the proportionality assumption and visually assessed the non-zero slope using Schoenfeld residuals. The restricted mean survival time (RMST) analysis evaluated differences in RFS and OS between patients who received TDF and those who received ETV over time. Landmark analyses were conducted based on a prespecified landmark point at 2 years, comparing early (<2 years) and late (≥2 years) tumor recurrence between patients treated with TDF and ETV, respectively (Yoo et al., 2022). Risk of publication bias was evaluated with a Funnel plot, Begg’s rank correlation test, and Egger’s regression test.
Results
Summary of included articles
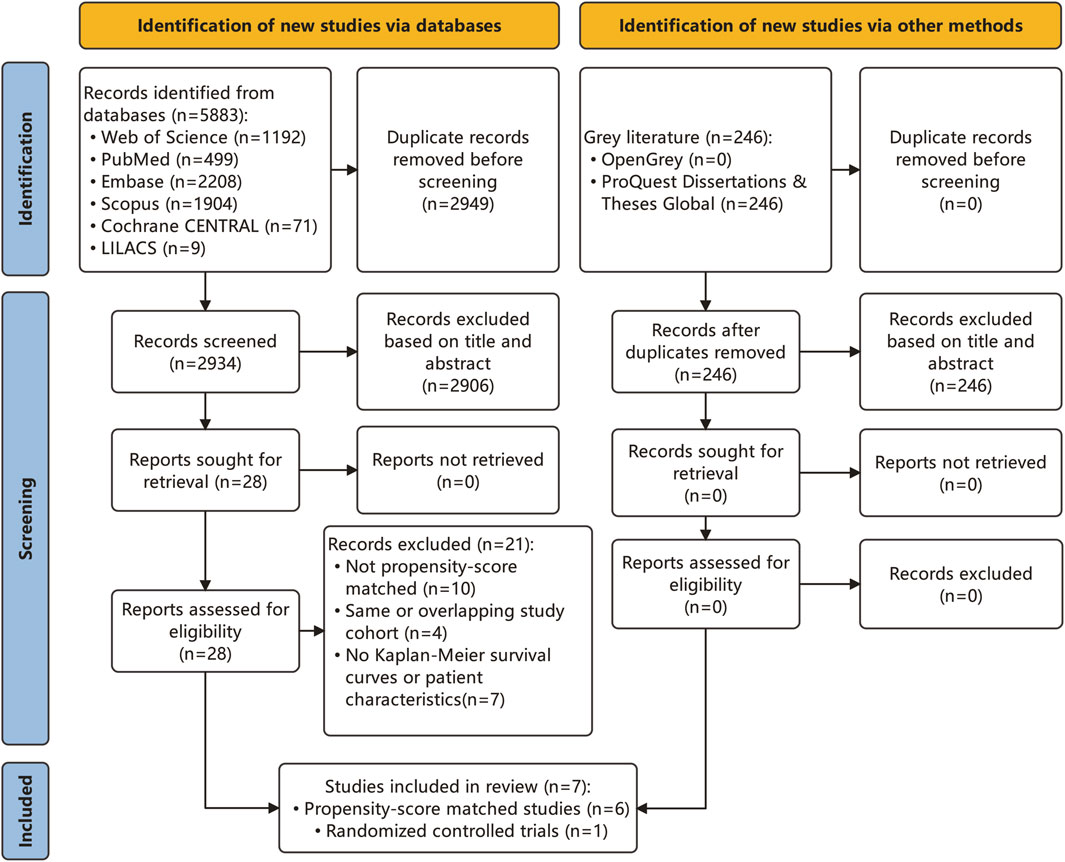
A comprehensive search across six databases identified 5,883 articles (Figure 1). After duplicate removal, 2,934 articles remained for consideration. Subsequent title and abstract review led to the exclusion of 2,906 articles, leaving 28 for full-text evaluation. Additionally, a gray literature search revealed 246 articles, with none meeting the inclusion criteria. Eventually, seven articles (Qi et al., 2021; Hu et al., 2022; Tsai et al., 2022; Wang et al., 2022; Yun et al., 2022; Linye et al., 2023; Yang et al., 2023) fulfilled the study’s inclusion criteria and were incorporated into the analysis, as detailed in Supplementary Table S1. These studies, published between 2021 and 2023, included five conducted in mainland China (Qi et al., 2021; Hu et al., 2022; Wang et al., 2022; Linye et al., 2023; Yang et al., 2023), one in Taiwan (Tsai et al., 2022), and one in South Korea (Yun et al., 2022). The study designs comprised six retrospective studies and one randomized controlled trial. Four studies were single-center, and the other three were multi-center, with two deriving from administrative databases and one from a clinical cohort. The patient interventions in these studies varied: five studies conducted HCC resection surgery (Qi et al., 2021; Tsai et al., 2022; Wang et al., 2022; Yun et al., 2022; Linye et al., 2023), and two implemented treatments other than HCC resection (Hu et al., 2022; Yang et al., 2023), including liver transplantation and radiofrequency ablation (RFA). The original and reconstructed survival curves from these studies are depicted in Supplementary Figure S1. The quality of the included studies was high, with each scoring 8 or higher on the Newcastle Ottawa Scale, as shown in Supplementary Table S2. Visual inspection of funnel plots, along with Begg’s rank correlation test and Egger’s regression test, indicated no evidence of publication bias (Supplementary Figure S2).
Baseline characteristics
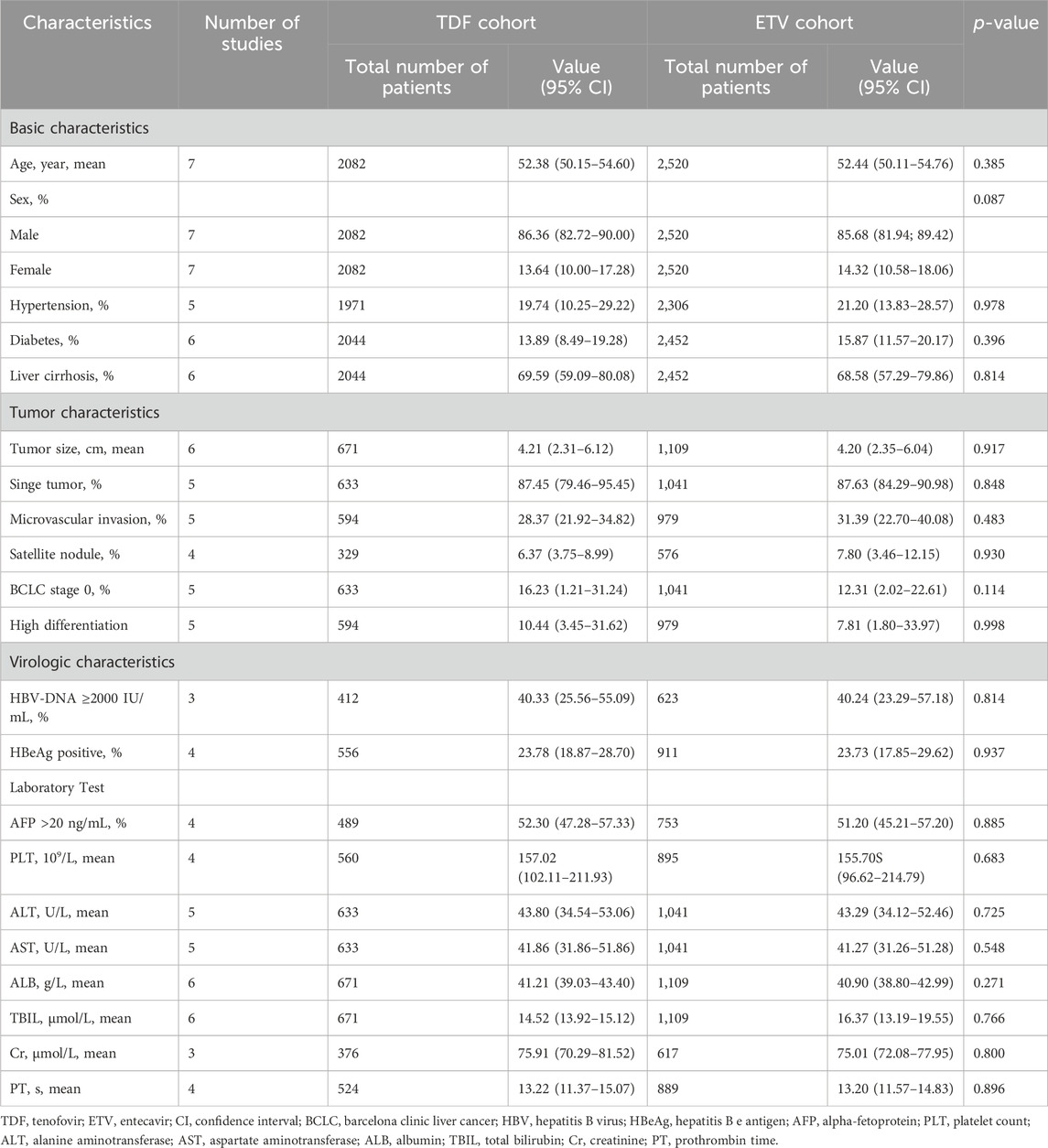
Table 1 outlines the baseline characteristics of HBV-related HCC patients who received TDF and those who underwent ETV treatment following propensity score matching. The characteristics included a mean age of 52.42 (95% confidence interval [CI], 50.87–53.97) years and a sex distribution of 85.96% (95% CI, 83.43%–88.50%) male and 14.04% (95% CI, 11.50%–16.57%) female. No significant differences in these characteristics were noted between the TDF and ETV groups. The median follow-up period was 39.92 (IQR, 27.2–55.84) months for the TDF group and 45.77 (IQR, 28.32–63.94) months for the ETV group.
Analysis of the overall cohort
RFS and OS in overall cohort
In the overall cohort, our analysis encompassed 4,602 patients with HBV-related HCC from seven studies, comprising 2,082 patients treated with TDF and 2,520 with ETV. The 1-, 3-, and 5-year RFS rates for the TDF group were 80.5% (95% CI, 78.8%–82.3%), 65.8% (95% CI, 63.7%–68.0%), and 60.2% (95% CI, 57.8%–62.8%), respectively. Conversely, the ETV group reported RFS rates of 80.2% (95% CI, 78.6%–81.8%), 63.5% (95% CI, 61.6%–65.5%), and 55.6% (95% CI, 53.5%–57.9%). Patients receiving TDF antiviral therapy demonstrated a significantly higher RFS compared to those on ETV therapy (p = 0.042, Figure 2A). The OS rates at these intervals for the TDF group were 97.4% (95% CI, 96.7%–98.1%), 90.8% (95% CI, 89.5%–92.2%), and 86.5% (95% CI, 84.6%–88.4%), while the ETV group had OS rates of 95.2% (95% CI, 94.4%–96.1%), 87.4% (95% CI, 86.0%–88.7%), and 81.4% (95% CI, 79.7%–83.2%). The TDF group exhibited a markedly improved OS compared to the ETV group (p < 0.001, Figure 2B). A sensitivity analysis verified the robustness of the RFS and OS outcomes (Supplementary Figure S3A,B). In the stratified Cox model, which accounts for inter-study heterogeneity, the OS was significantly greater in the TDF group compared to the ETV group (hazard ratio [HR], 0.756; 95% CI, 0.639–0.896; p = 0.001). Subsequent analysis using the shared frailty model produced similar findings (Table 2). Regarding RFS, the proportionality assumption was not satisfied (p = 0.001); consequently, analyses using the stratified Cox model and the shared frailty model were not conducted. The RMST analysis assessed differences in RFS and OS among patients receiving TDF versus those receiving ETV in the overall cohort over time. This analysis found no significant differences in the mean time to recurrence between the TDF and ETV groups during a follow-up period of 1–8 years (RMST difference at 1 year, −0.001 years [95% CI, −0.002–0.001], p = 0.404; RMST difference at 8 years, −0.025 years [95% CI, −0.103–0.053], p = 0.528) (Supplementary Table S3). Similarly, no significant differences were observed in the mean time to death between the TDF and ETV groups over the same period (RMST difference at 1 year, 0.000 years [95% CI, −0.001–0.000], p = 0.317; RMST difference at 8 years, 0.022 years [95% CI, −0.004–0.048], p = 0.092) (Supplementary Table S4).
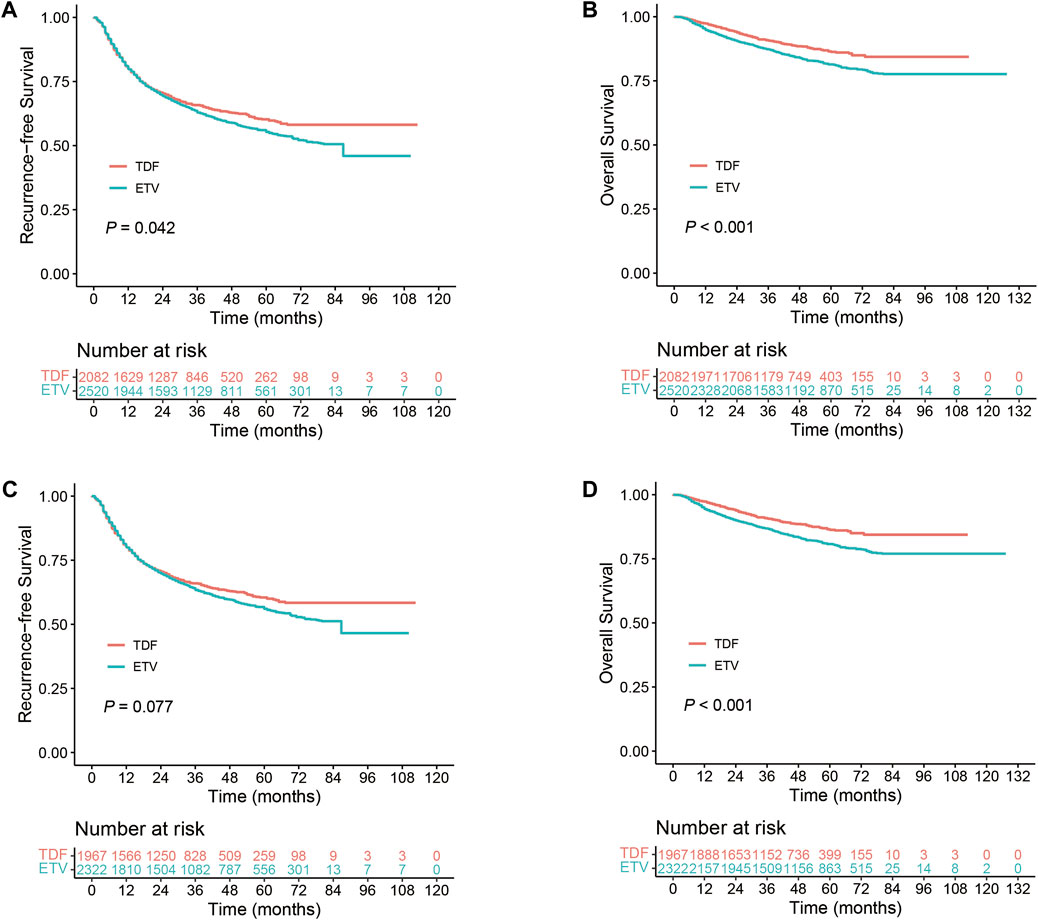
Figure 2. Comparison of TDF and ETV in HBV-related HCC patients. (A) RFS in the overall cohort; (B) OS in the overall cohort; (C) RFS in the Resection Subgroup; (D) OS in the Resection Subgroup.
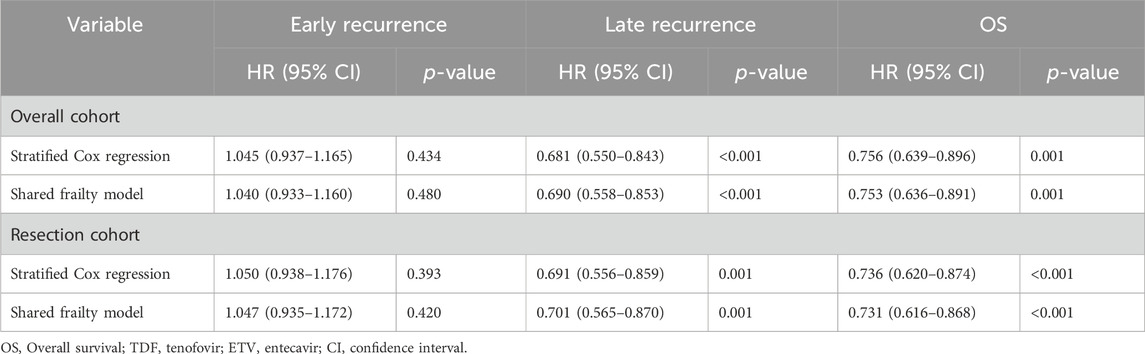
Table 2. Summary of the analysis on early and late recurrence and OS in patients treated with TDF and ETV.
Early and late recurrence in overall cohort
HCC recurrence is commonly classified as either early or late, typically defined by a 2-year cutoff following surgery (Imamura et al., 2003; Wu et al., 2009). We conducted a landmark analysis to evaluate recurrence in HBV-related HCC patients from the overall cohort who received TDF or ETV. Supplementary Figure S4A illustrates that early recurrence rates did not significantly differ between the groups (p = 0.735). However, TDF treatment, in comparison to ETV, was significantly associated with a reduced risk of late recurrence (p < 0.001). The stratified Cox model revealed no significant difference in early tumor recurrence between the TDF and ETV groups (HR, 1.045; 95% CI, 0.937–1.165; p = 0.434). However, TDF was associated with a significantly lower risk of late tumor recurrence compared to ETV (HR, 0.681; 95% CI, 0.550–0.843; p < 0.001). Analysis employing the shared frailty model yielded consistent results (Table 2).
Analysis of the resection subgroup
RFS and OS in resection subgroup
In the resection subgroup, our study included 4,289 patients with HBV-related HCC, with 1,967 receiving TDF and 2,322 undergoing ETV therapy. RFS rates in the TDF group at 1-, 3-, and 5-year were 80.7% (95% CI, 79.0%–82.5%), 66.0% (95% CI, 63.9%–68.2%), and 60.5% (95% CI, 58.0%–63.1%), respectively. In the ETV group, these rates were 80.4% (95% CI, 78.8%–82.1%), 64.0% (95% CI, 62.0%–66.1%), and 56.3% (95% CI, 54.1%–58.7%). The differences in recurrence-free survival between the therapies were not statistically significant. However, TDF therapy demonstrated a potential improvement in RFS (p = 0.077, Figure 2C). In terms of OS, the 1-, 3-, and 5-year rates for the TDF group were 97.4% (95% CI, 96.7%–98.1%), 90.8% (95% CI, 89.5%–92.2%), and 86.5% (95% CI, 84.6%–88.5%). For the ETV group, the rates were 94.9% (95% CI, 93.9%–95.8%), 86.9% (95% CI, 85.4%–88.3%), and 80.8% (95% CI, 79.0%–82.6%). OS was significantly higher in patients treated with TDF compared to ETV (p < 0.001, Figure 2D). A sensitivity analysis confirmed the robustness of the RFS and OS findings in the resection subgroup (Supplementary Figure S3C,D). In the stratified Cox model, a significant increase in OS was observed in the TDF group compared to the ETV group (HR, 0.736; 95% CI, 0.620–0.874; p < 0.001). Analysis using the shared frailty model revealed similar results (Table 2). Concerning RFS, the proportionality assumption was not met; therefore, analyses employing the stratified Cox model and the shared frailty model were not performed. The RMST analysis showed no significant differences in the mean time to recurrence between the TDF and ETV groups, even after 8 years of follow-up (RMST difference at 8 years, −0.030 [95% CI, −0.11–0.049] years; p = 0.454). The mean time to death also exhibited a similar pattern (Supplementary Table S4).
Early and late recurrence in resection subgroup
As shown in Supplementary Figure S4B, the landmark analysis of recurrence in HBV-related HCC patients within the resection subgroup, treated with either TDF or ETV, revealed no significant difference in early recurrence rates between the groups (p = 0.866). However, TDF treatment, as opposed to ETV, significantly correlated with a lower risk of late recurrence (p < 0.001). The stratified Cox regression analysis showed no significant disparity in early tumor recurrence rates between the TDF and ETV groups (HR, 1.050; 95% CI, 0.938–1.176; p = 0.393). In contrast, TDF treatment was significantly associated with a reduced risk of late tumor recurrence compared to ETV (HR, 0.691; 95% CI, 0.556–0.859; p = 0.001). The application of the shared frailty model corroborated these findings, as detailed in Table 2.
Discussion
In this study, we conducted a meta-analysis using reconstructed IPD from 4,602 patients with HBV-related HCC. Among these patients, 2,082 received TDF treatment, while 2,520 received ETV treatment. The aim was to investigate the impact of TDF compared to ETV on the RFS and OS of individuals with HBV-related HCC. Given the absence of multicenter, large-sample randomized controlled clinical trials, this analysis represents the most robust evidence available to date for assessing the effects of TDF vs ETV on the prognosis of patients with HBV-related HCC. In the overall cohort, we observed that patients treated with TDF had a lower risk of tumor recurrence and higher overall survival rates compared to those treated with ETV. These findings align with previous meta-analysis results (Liu et al., 2023). A network meta-analysis, involving 13,517 participants from 16 studies (including 11 randomized controlled trials and five propensity-matched cohort studies), compared the efficacy of TDF and ETV in the treatment of chronic hepatitis B patients over a 48-week period. The results demonstrated that at the 48-week mark, patients receiving TDF treatment exhibited a higher virological response rate than those receiving ETV treatment, with a more pronounced difference noted in patients positive for hepatitis B e antigen (Con et al., 2021). Moreover, TDF-treated patients exhibited elevated levels of serum interferon (IFN)-λ3 (Murata et al., 2018; Umemura et al., 2022), a factor known to have the potential to directly or indirectly inhibit tumor growth (Stiff and Carson, 2015). Additionally, an in vitro study suggested that TDF could restore the function of T cells and natural killer cells by downregulating interleukin (IL)-10 and upregulating IL-12, thereby playing crucial roles in antiviral and antitumor immunity (MURATA et al., 2020). Research also shows that TDF can inhibit the translocation of Akt to the plasma membrane, thereby blocking the mammalian target of rapamycin pathway, which is commonly activated in most cancer cells (Murata and Mizokami, 2023). These findings offer plausible explanations for the superior prognosis associated with TDF compared to ETV in the treatment of patients with HBV-related HCC. Nevertheless, further research is warranted to elucidate these differences fully. Due to the violation of the proportional hazards assumption (p = 0.001), we refrained from employing stratified Cox models and shared frailty models to analyze the occurrence of HCC recurrence in the overall cohort. However, the RMST analysis revealed that by the 8 years of treatment, no statistically significant differences were observed in the times to tumor recurrence and death between patients treated with TDF and those treated with ETV, with reductions of 9 days and extensions of 8 days, respectively. The divergence between the RMST results and those of the log-rank test can be attributed to the distinct statistical properties and sensitivities of these methodologies. The log-rank test, which is sensitive to differences at any point during the follow-up period, indicated a benefit with TDF treatment. In contrast, the RMST, which calculates a summary measure of survival within a predefined period and is robust against violations of the proportional hazards assumption, suggested no significant long-term differences between the treatments over an 8-year span. This suggests that although patients receiving TDF demonstrate a decreased incidence of tumor recurrence and enhanced OS compared to those receiving ETV, the reduction in tumor recurrence time and the extension in survival time are not significant. This is consistent with the current Guidelines for the Prevention and Treatment of Chronic Hepatitis B (2022 edition), which do not explicitly prefer TDF over ETV (You et al., 2023b). Consequently, when deciding between TDF and ETV, factors such as drug suitability, comorbidities, and cost-effectiveness should take precedence. For instance, research has indicated that TDF may be a more appropriate choice for HBeAg-positive patients, especially those with high viral loads, as it demonstrates more robust suppression of HBV DNA levels (Gao et al., 2014). Additionally, it is widely acknowledged that TDF may induce nephrotoxic and osteotoxic side effects (Lim et al., 2019; Jung et al., 2022). Therefore, ETV may represent a preferable option for patients with renal impairment or osteoporosis, given that TDF could exacerbate renal or skeletal issues.
In our analysis of HCC recurrence, we categorized cases into early and late stages, utilizing a 2-year landmark point. The Grambsch-Therneau test yielded a p-value of 0.041 for the stratified Cox model applied to the early recurrence cohort. Although this p-value falls below the commonly accepted threshold of 0.05, it signifies a noteworthy enhancement when compared to the Grambsch-Therneau test’s p-value for the Overall Cohort. Consequently, the decision was made to utilize the stratified Cox model for our analysis. We performed log-rank tests, stratified Cox models, and shared frailty models for both early and late recurrence cohorts, consistently revealing that TDF mitigated the risk of late recurrence rather than early recurrence. This suggests that the beneficial effects of TDF on HCC may require some time to become evident. Conventionally, early recurrence is often associated with the invasiveness of the primary tumor and the presence of microvascular invasion (Poon et al., 2000; Imamura et al., 2003). The potential of TDF and ETV to reduce the risk of early recurrence in HBV-related HCC by modifying the tumor microenvironment remains uncertain and necessitates further investigation. In contrast, late recurrence is typically attributed to multicentric tumor growth or the development of de novo cancer (Xu et al., 2019), influenced by ongoing hepatitis B virus infection and/or cirrhosis. While NAs effectively suppress HBV-DNA replication, they fall short of completely clearing HBsAg. Research indicates that preoperative HBsAg levels exceeding 1000 IU/mL independently elevate the risk of HCC recurrence in patients with low HBV DNA levels (Huang et al., 2014). Moreover, cirrhosis represents a significant complication of HBV infection and a pivotal factor in HCC development (Rizzo et al., 2022). TDF diminishes the risk of late recurrence, potentially attributed to its superior viral suppression and liver function preservation compared to ETV (Park et al., 2017; Zheng et al., 2023). TDF positively influences liver health by maintaining a sustained and stable antiviral effect, enhancing liver function, mitigating inflammatory responses, and averting fibrosis progression (Marcellin et al., 2013; Park et al., 2017; Zheng et al., 2023). Inflammation seems to play a substantial role in both the onset and advancement of HCC (Yu et al., 2018). Recent investigations have suggested that aspirin use for ≥90 days significantly lowers HCC incidence in CHB patients (Jang et al., 2022). The concurrent utilization of aspirin and TDF may represent a promising approach, yet further research is warranted to explore this possibility in future studies.
Within the HCC resection subgroup, patients who received TDF treatment exhibited a trend toward improved RFS compared to those treated with ETV, despite the absence of a statistically significant difference in tumor recurrence risk. This hints at a potential disparity in HCC recurrence risk associated with these two treatment modalities. The discrepancies in OS, early, and late recurrence rates between the two groups closely mirrored those observed in the overall cohort. Given that all seven studies included in this analysis exclusively involved curative interventions, comprising five instances of surgical resection, one liver transplantation case, and one curative RFA procedure, the study’s conclusions are specifically relevant to patients undergoing curative treatments. Curative interventions are typically most suitable for HCC patients with smaller and limited tumors, as well as those in good liver and overall health (Cucchetti et al., 2023). Among these cases, those undergoing HCC resection surgery constituted the majority, accounting for 93.2% (4,289/4,602) of the entire cohort. Consequently, the differences between the two drugs within the overall cohort primarily reflect variations following HCC resection surgery. As only two articles discussed treatments other than liver resection surgery, data from these studies were not incorporated into the pooled analysis.
Our study addresses previous limitations in research concerning the prognosis of patients with HBV-related HCC treated with TDF vs ETV. We accomplished this by aggregating event-time data from individual patient-level datasets, exclusively incorporating randomized controlled trials and high-quality propensity score-matched studies. In contrast to prior meta-analyses on this subject, our approach takes into account issues such as patient attrition and low study quality. Notably, this study represents the inaugural meta-analysis on this subject that utilizes reconstructed individual participant data. Furthermore, we performed additional analyses using stratified Cox models and shared frailty models to elucidate heterogeneity among the studies. Additionally, we employed RMST analysis to elucidate treatment effects over time, especially considering the shorter follow-up duration in most TDF cohorts compared to ETV cohorts. In cases where the proportional hazards assumption was violated, RMST differences emerged as a popular alternative to hazard ratios, consistently yielding robust estimates (Uno et al., 2014). Nonetheless, we acknowledge several limitations in our study. First, although all included studies achieved a score of eight or higher on the Newcastle-Ottawa Scale, only one was a randomized controlled trial; the rest were retrospective cohort studies, potentially introducing selection bias (Sessler and Imrey, 2015). Despite our efforts to mitigate this by exclusively including high-quality propensity score-matched retrospective cohort studies, complete elimination of selection bias may not have been achieved (Heinze and Jüni, 2011). Additionally, all included articles focused on Asian populations, raising the possibility of regional bias. Moreover, while gender and age were balanced across all studies at baseline, equilibrium in tumor characteristics, virologic attributes, and laboratory tests was attained only in the majority, not all, of the studies. Furthermore, residual confounding factors such as the degree of liver fibrosis, surgical data, history of alcohol use, family history of HCC, virus genotype, previous use of NAs, and adherence to antiviral treatment may impact the results. However, these factors were not collected or were collected in only a few studies, making it impossible for us to further balance them. In future research, it is essential to conduct large-scale, prospective, multicenter studies involving diverse clinical populations to validate the differences in therapeutic effects of TDF vs ETV in patients with HBV-related HCC. Additionally, future studies should investigate the cost-effectiveness of TDF vs ETV to fully assess the economic impacts of these treatment methods in patients with HBV-related HCC.
Conclusion
In summary, when compared to ETV, TDF demonstrates the potential to improve the OS of patients with HBV-related HCC undergoing curative treatment, concurrently reducing the risk of late tumor recurrence. Notably, at the 8th year of treatment, there was no statistically significant distinction in the timing of tumor recurrence and mortality between patients administered TDF and those prescribed ETV, with a reduction of 9 days and an extension of 8 days, respectively. The selection between TDF and ETV should be guided by individual patient-specific factors and convenience. However, it is imperative to underscore the necessity for additional large-scale, prospective, multicenter studies to corroborate these findings.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
Author contributions
J-XP: Conceptualization, Investigation, Methodology, Writing–original draft, Writing–review and editing. L-ZW: Data curation, Formal Analysis, Investigation, Writing–review and editing. Q-TW: Formal Analysis, Software, Writing–review and editing. H-LL: Writing–review and editing. L-JL: Supervision, Writing–review and editing. J-MH: Funding acquisition, Project administration, Resources, Supervision, Writing–review and editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study was supported by two grant from by The International Cooperation Project of the Science and Technology Planning Project of Guangdong Province (No 2022A0505050065), Guangdong Natural Science Foundation (No 2022A1515011632).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2024.1393861/full#supplementary-material
References
Con, D., Goodwin, T., Majeed, A., Roberts, S., and Kemp, W. (2021). Comparison of 48-week efficacy of tenofovir vs entecavir for patients with chronic hepatitis B: a network meta-analysis. J. Viral Hepat. 28 (1), 40–50. doi:10.1111/jvh.13400
Cucchetti, A., Elshaarawy, O., Han, G., Chong, C. C. N., Serra, C., O'Rourke, J. M., et al. (2023). Potentially curative therapies’ for hepatocellular carcinoma: how many patients can actually be cured? Br. J. Cancer 128 (9), 1665–1671. doi:10.1038/s41416-023-02188-z
De Jong, V. M. T., Moons, K. G. M., Riley, R. D., Tudur Smith, C., Marson, A. G., Eijkemans, M. J. C., et al. (2020). Individual participant data meta-analysis of intervention studies with time-to-event outcomes: a review of the methodology and an applied example. Res. Synthesis Methods 11 (2), 148–168. doi:10.1002/jrsm.1384
Gao, L., Trinh, H. N., Li, J., and Nguyen, M. H. (2014). Tenofovir is superior to entecavir for achieving complete viral suppression in HBeAg-positive chronic hepatitis B patients with high HBV DNA. Alimentary Pharmacol. Ther. 39 (6), 629–637. doi:10.1111/apt.12629
Guyot, P., Ades, A., Ouwens, M. J., and Welton, N. J. (2012). Enhanced secondary analysis of survival data: reconstructing the data from published Kaplan-Meier survival curves. BMC Med. Res. Methodol. 12 (1), 9. doi:10.1186/1471-2288-12-9
Heinze, G., and Jüni, P. (2011). An overview of the objectives of and the approaches to propensity score analyses. Eur. Heart J. 32 (14), 1704–1708. doi:10.1093/eurheartj/ehr031
Hsu, Y. C., Huang, D. Q., and Nguyen, M. H. (2023). Global burden of hepatitis B virus: current status, missed opportunities and a call for action. Nat. Rev. Gastroenterology Hepatology 20 (8), 524–537. doi:10.1038/s41575-023-00760-9
Huang, G., Lau, W. Y., Wang, Z. G., Pan, Z. Y., Yuan, S. X., Shen, F., et al. (2015). Antiviral therapy improves postoperative survival in patients with hepatocellular carcinoma: a randomized controlled trial. Ann. Surg. 261 (1), 56–66. doi:10.1097/SLA.0000000000000858
Huang, G., Lau, W. Y., Zhou, W. P., Shen, F., Pan, Z. X., Yuan, S. X., et al. (2014). Prediction of hepatocellular carcinoma recurrence in patients with low hepatitis B virus DNA levels and high preoperative hepatitis B surface antigen levels. JAMA Surg. 149 (6), 519–527. doi:10.1001/jamasurg.2013.4648
Hu, Z., Zeng, H., Hou, J., Wang, J., Xu, L., Zhang, Y., et al. (2022). Tenofovir vs. Entecavir on outcomes of hepatitis B virus-related hepatocellular carcinoma after radiofrequency ablation. Viruses 14 (4), 656. doi:10.3390/v14040656
Imamura, H., Matsuyama, Y., Tanaka, E., Ohkubo, T., Hasegawa, K., Miyagawa, S., et al. (2003). Risk factors contributing to early and late phase intrahepatic recurrence of hepatocellular carcinoma after hepatectomy. J. Hepatology 38 (2), 200–207. doi:10.1016/s0168-8278(02)00360-4
Jang, H., Lee, Y. B., Moon, H., Chung, J. W., Nam, J. Y., Cho, E. J., et al. (2022). Aspirin use and risk of hepatocellular carcinoma in patients with chronic hepatitis B with or without cirrhosis. Hepatology 76 (2), 492–501. doi:10.1002/hep.32380
Jung, C. Y., Kim, H. W., Ahn, S. H., Kim, S. U., and Kim, B. S. (2022). Tenofovir is associated with higher risk of kidney function decline than entecavir in patients with chronic hepatitis B. Clin. Gastroenterology Hepatology 20 (4), 956–958.e2. doi:10.1016/j.cgh.2021.05.032
Kong, Q., Yi, M., Teng, F., et al. (2023). Enhanced prognosis of HCC patients undergoing radical treatments with tenofovir versus entecavir: a meta-analysis based on propensity score matching studies. Asian J. Surg. S1015-9584 (23), 01472. doi:10.1016/j.asjsur.2023.09.057
Lampertico, P., Agarwal, K., Berg, T., Buti, M., Janssen, H. L., Papatheodoridis, G., et al. (2017). EASL 2017 clinical practice guidelines on the management of hepatitis B virus infection. J. Hepatology 67 (2), 370–398. doi:10.1016/j.jhep.2017.03.021
Lee, T., Lin, J., Zeng, Y., Chen, Y. J., Wu, M. S., and Wu, C. Y. (2016). Association between nucleos(t)ide analog and tumor recurrence in hepatitis B virus–related hepatocellular carcinoma after radiofrequency ablation. Hepatology 63 (5), 1517–1527. doi:10.1002/hep.28266
Lim, Y. S., Gwak, G. Y., Choi, J., Lee, Y. S., Byun, K. S., Kim, Y. J., et al. (2019). Monotherapy with tenofovir disoproxil fumarate for adefovir-resistant vs. entecavir-resistant chronic hepatitis B: a 5-year clinical trial. J. Hepatology 71 (1), 35–44. doi:10.1016/j.jhep.2019.02.021
Linye, H., Zijing, X., Xiaoyun, Z., et al. (2023). Tenofovir vs entecavir on the prognosis of hepatitis B-Related hepatocellular carcinoma after surgical resection: a randomised controlled trial. London, England: International journal of surgery.
Liu, H., Han, C. L., Tian, B. W., Ding, Z. N., Yang, Y. F., Ma, Y. L., et al. (2023). Tenofovir versus entecavir on the prognosis of hepatitis B virus-related hepatocellular carcinoma: a systematic review and meta-analysis. Expert Rev. Gastroenterology Hepatology 17 (6), 623–633. doi:10.1080/17474124.2023.2212161
Luo, D., Wan, X., Liu, J., and Tong, T. (2018). Optimally estimating the sample mean from the sample size, median, mid-range, and/or mid-quartile range. Stat. Methods Med. Res. 27 (6), 1785–1805. doi:10.1177/0962280216669183
Marcellin, P., Gane, E., Buti, M., Afdhal, N., Sievert, W., Jacobson, I. M., et al. (2013). Regression of cirrhosis during treatment with tenofovir disoproxil fumarate for chronic hepatitis B: a 5-year open-label follow-up study. Lancet 381 (9865), 468–475. doi:10.1016/S0140-6736(12)61425-1
Murata, K., Asano, M., Matsumoto, A., Sugiyama, M., Nishida, N., Tanaka, E., et al. (2018). Induction of IFN-λ3 as an additional effect of nucleotide, not nucleoside, analogues: a new potential target for HBV infection. Gut 67 (2), 362–371. doi:10.1136/gutjnl-2016-312653
Murata, K., and Mizokami, M. (2023). Possible biological mechanisms of entecavir versus tenofovir disoproxil fumarate on reducing the risk of hepatocellular carcinoma. J. Gastroenterology Hepatology 38 (5), 683–691. doi:10.1111/jgh.16178
Murata, K., Tsukuda, S., Suizu, F., Kimura, A., Sugiyama, M., Watashi, K., et al. (2020). Immunomodulatory mechanism of acyclic nucleoside phosphates in treatment of hepatitis B virus infection. Hepatology 71 (5), 1533–1545. doi:10.1002/hep.30956
Page, M. J., Mckenzie, J. E., Bossuyt, P. M., Boutron, I., Hoffmann, T. C., Mulrow, C. D., et al. (2021). The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 372, n71. doi:10.1136/bmj.n71
Park, J. W., Kwak, K. M., Kim, S. E., Jang, M. K., Suk, K. T., Kim, D. J., et al. (2017). Comparison of the long-term efficacy between entecavir and tenofovir in treatment-naïve chronic hepatitis B patients. BMC Gastroenterol. 17 (1), 39. doi:10.1186/s12876-017-0596-7
Poon, R. T., Fan, S. T., Ng, I. O., Lo, C. M., Liu, C. L., and Wong, J. (2000). Different risk factors and prognosis for early and late intrahepatic recurrence after resection of hepatocellular carcinoma. Cancer 89 (3), 500–507. doi:10.1002/1097-0142(20000801)89:3<500::aid-cncr4>3.0.co;2-o
Qi, W., Shen, J., Dai, J., Wu, Y., Zhang, Y., Leng, S., et al. (2021). Comparison of nucleoside and nucleotide analogs in the recurrence of hepatitis B virus-related hepatocellular carcinoma after surgical resection: a multicenter study. Cancer Med. 10 (23), 8421–8431. doi:10.1002/cam4.4348
Rahman, R., Fell, G., Ventz, S., Arfé, A., Vanderbeek, A. M., Trippa, L., et al. (2019). Deviation from the proportional hazards assumption in randomized phase 3 clinical trials in oncology: prevalence, associated factors, and implications. Clin. Cancer Res. 25 (21), 6339–6345. doi:10.1158/1078-0432.CCR-18-3999
Rizzo, G. E. M., Cabibbo, G., and Craxì, A. (2022). Hepatitis B virus-associated hepatocellular carcinoma. Viruses 14 (5), 986. doi:10.3390/v14050986
Sarin, S. K., Kumar, M., Lau, G. K., Abbas, Z., Chan, H. L. Y., Chen, C. J., et al. (2016). Asian-pacific clinical practice guidelines on the management of hepatitis B: a 2015 update. Hepatol. Int. 10 (1), 1–98. doi:10.1007/s12072-015-9675-4
Sessler, D. I., and Imrey, P. B. (2015). Clinical research methodology 1: study designs and methodologic sources of error. Anesth. Analgesia 121 (4), 1034–1042. doi:10.1213/ANE.0000000000000815
Stewart, L. A., Clarke, M., Rovers, M., Riley, R. D., Simmonds, M., Stewart, G., et al. (2015). Preferred reporting Items for systematic review and meta-analyses of individual participant data: the PRISMA-IPD statement. JAMA 313 (16), 1657–1665. doi:10.1001/jama.2015.3656
Stiff, A., and Carson, I. I. I. W. (2015). Investigations of interferon-lambda for the treatment of cancer. J. Innate Immun. 7 (3), 243–250. doi:10.1159/000370113
Syn, N. L., Cummings, D. E., Wang, L. Z., Lin, D. J., Zhao, J. J., Loh, M., et al. (2021). Association of metabolic–bariatric surgery with long-term survival in adults with and without diabetes: a one-stage meta-analysis of matched cohort and prospective controlled studies with 174 772 participants. Lancet 397 (10287), 1830–1841. doi:10.1016/S0140-6736(21)00591-2
Tan, B. K. J., Han, R., Zhao, J. J., Tan, N. K. W., Quah, E. S. H., Tan, C. J. W., et al. (2022). Prognosis and persistence of smell and taste dysfunction in patients with covid-19: meta-analysis with parametric cure modelling of recovery curves. BMJ 378, e069503. doi:10.1136/bmj-2021-069503
Terrault, N. A., Lok, A. S. F., Mcmahon, B. J., Chang, K. M., Hwang, J. P., Jonas, M. M., et al. (2018). Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology 67 (4), 1560–1599. doi:10.1002/hep.29800
Tsai, M. C., Wang, C. C., Lee, W. C., Lin, C. C., Chang, K. C., Chen, C. H., et al. (2022). Tenofovir is superior to entecavir on tertiary prevention for BCLC stage 0/A hepatocellular carcinoma after curative resection. Liver Cancer 11 (1), 22–37. doi:10.1159/000518940
Umemura, M., Ogawa, K., Morikawa, K., Kubo, A., Tokuchi, Y., Yamada, R., et al. (2022). Effects of nucleos(t)ide analogs on hepatitis B surface antigen reduction with interferon-lambda 3 induction in chronic hepatitis B patients. Hepatology Res. 52 (7), 586–596. doi:10.1111/hepr.13768
Uno, H., Claggett, B., Tian, L., Inoue, E., Gallo, P., Miyata, T., et al. (2014). Moving beyond the hazard ratio in quantifying the between-group difference in survival analysis. J. Clin. Oncol. 32 (22), 2380–2385. doi:10.1200/JCO.2014.55.2208
Vogel, A., Meyer, T., Sapisochin, G., Salem, R., and Saborowski, A. (2022). Hepatocellular carcinoma. Lancet 400 (10360), 1345–1362. doi:10.1016/S0140-6736(22)01200-4
Wang, M. D., Li, C., Liang, L., Xing, H., Sun, L. Y., Quan, B., et al. (2020). Early and late recurrence of hepatitis B virus-associated hepatocellular carcinoma. Oncol. 25 (10), e1541–e1551. doi:10.1634/theoncologist.2019-0944
Wang, X. H., Hu, Z. L., Fu, Y. Z., Hou, J. Y., Li, W. X., Zhang, Y. J., et al. (2022). Tenofovir vs. entecavir on prognosis of hepatitis B virus-related hepatocellular carcinoma after curative resection. J. Gastroenterology 57 (3), 185–198. doi:10.1007/s00535-022-01855-x
Wan, X., Wang, W., Liu, J., and Tong, T. (2014). Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med. Res. Methodol. 14 (1), 135. doi:10.1186/1471-2288-14-135
Wells, G. A., Shea, B., O’Connell, D., et al. (2023). The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses[EB/OL]. Available at: https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
Wu, C. Y., Lin, J. T., Ho, H. J., Su, C. W., Lee, T. Y., Wang, S. Y., et al. (2014). Association of nucleos(t)ide analogue therapy with reduced risk of hepatocellular carcinoma in patients with chronic hepatitis B—a nationwide cohort study. Gastroenterology 147 (1), 143–151.e5. doi:10.1053/j.gastro.2014.03.048
Wu, J. C., Huang, Y. H., Chau, G. Y., Su, C. W., Lai, C. R., Lee, P. C., et al. (2009). Risk factors for early and late recurrence in hepatitis B-related hepatocellular carcinoma. J. Hepatology 51 (5), 890–897. doi:10.1016/j.jhep.2009.07.009
Xu, X. F., Xing, H., Han, J., Li, Z. L., Lau, W. Y., Zhou, Y. H., et al. (2019). Risk factors, patterns, and outcomes of late recurrence after liver resection for hepatocellular carcinoma: a multicenter study from China. JAMA Surg. 154 (3), 209–217. doi:10.1001/jamasurg.2018.4334
Yang, H. C., and Kao, J. H. (2014). Persistence of hepatitis B virus covalently closed circular DNA in hepatocytes: molecular mechanisms and clinical significance. Emerg. Microbes Infect. 3 (1), e64–e67. doi:10.1038/emi.2014.64
Yang, J., Chen, Y., Sun, H., Zhang, X., Wang, J., Liang, Z., et al. (2023). Tenofovir versus entecavir on decreasing risk of HBV-related hepatocellular carcinoma recurrence after liver transplantation. Infect. Agents Cancer 18 (1), 2. doi:10.1186/s13027-022-00478-4
Yoo, S., Kim, J. Y., Lim, Y. S., Han, S., and Choi, J. (2022). Impact of HBsAg seroclearance on late recurrence of hepatitis B virus-related hepatocellular carcinoma after surgical resection. J. Hepatology 77 (4), 939–946. doi:10.1016/j.jhep.2022.05.014
You, H., Wang, F., Li, T., Xu, X., Sun, Y., Nan, Y., et al. (2023a). Guidelines for the prevention and treatment of chronic hepatitis B (version 2022). J. Clin. Transl. Hepatology 11 (6), 1425–1442. doi:10.14218/JCTH.2023.00320
You, H., Wang, F., Li, T., Xu, X., Sun, Y., Nan, Y., et al. (2023b). Guidelines for the prevention and treatment of chronic hepatitis B (version 2022). J. Clin. Transl. Hepatology 000 (000), 000. doi:10.14218/jcth.2023.00320
Yu, L. X., Ling, Y., and Wang, H. Y. (2018). Role of nonresolving inflammation in hepatocellular carcinoma development and progression. npj Precis. Oncol. 2 (1), 6–10. doi:10.1038/s41698-018-0048-z
Yun, B., Ahn, S. H., Oh, J., Yoon, J. H., and Kim, B. K. (2022). Association of tenofovir and entecavir use with prognosis after surgical resection for hepatitis B virus-related hepatocellular carcinoma. Eur. J. Intern. Med. 103, 122–125. doi:10.1016/j.ejim.2022.07.003
Zheng, Z., Wang, J., Wu, T., Wang, J., Pan, Y., et al. (2023). Tenofovir versus entecavir on outcomes of hepatitis B virus-related hepatocellular carcinoma after FOLFOX-hepatic arterial infusion chemotherapy. J. Hepatocell. Carcinoma 10, 2117–2132. doi:10.2147/JHC.S436062
Keywords: hepatocellular carcinoma, hepatitis B, tenofovir, entecavir, overall survival rates, recurrence-free survival rate, meta-analysis
Citation: Peng J-X, Wang L-Z, Wang Q-T, Li H-L, Lin L-J and He J-M (2024) Tenofovir versus entecavir on the prognosis of hepatitis B virus-related hepatocellular carcinoma: a reconstructed individual patient data meta-analysis. Front. Pharmacol. 15:1393861. doi: 10.3389/fphar.2024.1393861
Received: 29 February 2024; Accepted: 16 July 2024;
Published: 22 August 2024.
Edited by:
Kalicharan Sharma, Jamia Hamdard University, IndiaReviewed by:
Sara Ashtari, Shahid Beheshti University, IranWenjun Wang, Xi’an Jiaotong University, China
Copyright © 2024 Peng, Wang, Wang, Li, Lin and He. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jun-Ming He, aGVqdW5taW5nMDEwMUBzaW5hLmNvbQ==
 Jian-Xin Peng1
Jian-Xin Peng1 Ling-Zhi Wang
Ling-Zhi Wang Jun-Ming He
Jun-Ming He