- 1Tianjin University of Traditional Chinese Medicine, Tianjin, China
- 2Chinese EQUATOR Centre, Hong Kong Baptist University, Kowloon, China
- 3Guangdong Provincial Hospital of Chinese Medicine in Guizhou, Guizhou, China
- 4School of Chinese Medicine, Hong Kong Baptist University, Kowloon, China
- 5Vincent V.C. Woo Chinese Medicine Clinical Research Institute, Hong Kong Baptist University, Kowloon, China
- 6Centre for Chinese Herbal Medicine Drug Development, School of Chinese Medicine, Hong Kong Baptist University, Kowloon, China
Objectives: The impact of the Standard Protocol Items: Recommendations for Interventional Trials of Traditional Chinese Medicine (SPIRIT-TCM) Extension 2018 statement on the reporting quality of randomized controlled trial (RCT) protocols in traditional Chinese medicine (TCM) is not clear. This review aimed to assess the reporting characteristics and quality of RCT protocols involving interventions such as Chinese herbal medicine formulas (CHMF), acupuncture, and moxibustion published in the last 3 years.
Methods: We conducted an extensive search among multiple databases, including All EBM Reviews, Allied and Complementary Medicine (AMED), Embase, Ovid MEDLINE(R), PubMed, Web of Science, Google Scholar, and ClinicalTrials.gov for publications in English from 1 January 2020 to 10 August 2023. Two reviewers independently assessed the eligibility of the publications, extracted predetermined information, and evaluated the reporting based on the SPIRIT-TCM Extension 2018 checklist.
Results: Of the 420 eligible protocols (comprising 163 studies on CHMF, 239 on acupuncture, and 18 on moxibustion), the average reporting compliance rate was only 35.4%. Approximately half of the assessed items fell into the category of poorly reported, demonstrating a compliance rate below 65%. Notably, reporting compliance in acupuncture and moxibustion interventional studies exhibited higher scores than compliance in CHMF studies.
Conclusion: Continued, concerted, and coordinated efforts are required by journals, editors, reviewers, and investigators to improve the application and promotion of the SPIRIT-TCM Extension 2018 reporting guideline.
1 Introduction
Numerous countries have increasingly recognized the potential value of traditional Chinese medicine (TCM) in preventing and treating both infectious and non-infectious diseases. In the realm of infectious diseases, artemisinin, derived from TCM, is widely employed to treat malaria in Africa (Daily et al., 2022). Addressing the global infectious disease, Coronavirus Disease 2019-related pneumonia, the lung cleansing and detoxifying decoction has demonstrated efficacy in alleviating symptoms like fever, cough, headache, and severity of illness rates (Liu et al., 2020). In the context of non-communicable diseases, arsenic, extracted from TCM, transformed the outlook for acute monocytic leukemia, previously deemed incurable, and is now recommended as a first-line drug (Burnett et al., 2015; Wang et al., 2018). Notably, TCM was incorporated into the International Statistical Classification of Diseases and Related Health Problems by the World Health Organization in 2019 (Lam et al., 2019). Despite these advancements, TCM faces scrutiny regarding its efficacy and safety in Western countries because it is an empirical medicine with complex herbal compositions.
Randomized controlled trials (RCTs) are widely regarded as the gold standard for assessing drug efficacy, particularly in TCM-related trials. To ensure the quality of RCTs at the design stage, the SPIRIT (Standard Protocol Items: Recommendations for Interventional Trials) statements were introduced in 2013. Comprising 33 items, including research background, objectives, and participant information, SPIRIT 2013 serves as a guideline for the minimum content of a clinical trial protocol, aiming to establish routine standards for completeness and transparency (Chan et al., 2013). As a result, the quality of RCT protocols has improved since the publication of the SPIRIT statement. Scholars have observed a significant enhancement in reporting quality by comparing the checklist items of 300 protocols before and after introducing the SPIRIT statement (Tan et al., 2020). This underscores the positive impact of standardized guidelines on promoting transparency and completeness in clinical trial protocols.
Despite a sharp increase in the number of TCM clinical trials registered on ClinicalTrials.gov after the publication of the SPIRIT statement, concerns persisted regarding transparency and repeatability. This is attributed to the fact that the suggestions outlined in the statement are not fully applicable to the distinctive characteristics of TCM (Cheng et al., 2017; Dai et al., 2019; Zhang et al., 2020). Several reporting guidelines for TCM, including acupuncture, moxibustion, and CHMF interventional studies, were developed in 2010 (MacPherson et al., 2010), 2013 (Cheng et al., 2013), and 2018 (Dai et al., 2019), respectively to address this issue. These efforts aimed to provide more comprehensive standards and enhance the quality of RCTs in TCM research. After that, the SPIRIT-TCM Extension 2018 was issued (Dai et al., 2019). Several critical factors were included in this extension, such as TCM pattern, criteria for practitioners, and various TCM interventions.
The introduction of SPIRIT-TCM Extension 2018 marked a significant advancement in the sophistication of research programs for RCTs in TCM. It not only guides the implementation of TCM protocols but also facilitates other scholars in retracing and building upon these protocols. For instance, following SPIRIT-TCM Extension 2018, some researchers reported more comprehensive details, including TCM pattern, quality control and safety assessment of CHMF interventions, and TCM-relevant rationale, in their clinical trial of TCM treatments for irritable bowel syndrome (Zheng et al., 2021). This underscores the positive impact of tailored guidelines on enhancing the rigor and transparency of TCM clinical trials.
Despite the publication of the SPIRIT-TCM Extension 2018 5 years ago, there remains a noticeable absence of detailed analyses regarding its impact on TCM clinical trial protocols. Additionally, there is a scarcity of systematic studies addressing this issue. Previous research has highlighted the inadequate utilization and endorsement of the CONSORT extensions for TCM reporting guidelines (Zhang et al., 2022). To bridge this gap, our objective is to scrutinize a selection of TCM RCT protocols from the past 3 years, with a particular focus on CHMF, acupuncture, and moxibustion—areas covered by the SPIRIT-TCM Extension 2018 guideline. The primary aim of this review is to pinpoint potential shortcomings for enhancement, thereby offering valuable insights to foster greater awareness, application, and dissemination of the SPIRIT-TCM 2018 guideline.
2 Materials and methods
This review is conducted and reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 checklist (Page et al., 2021). The study has been registered on the Open Science Framework (https://osf.io/573c2/).
2.1 Eligibility criteria
The inclusion criteria comprised RCT protocols published in English from 1 January 2020 to 10 August 2023. The inclusion criteria were the following: i) The interventions were limited to CHMF, acupuncture, and moxibustion. According to the CONSORT-2017-CHMF (Gagnier et al., 2006a; Gagnier et al., 2006b; Cheng et al., 2017), the CHMF dosage form included decoctions, granules, powders, and botanical hybrid preparations (Panossian et al., 2024). The administration routes of CHMF were oral and external. ii) The protocol of RCTs. iii) There must be a control group, but no limitations were imposed on the types of control groups or the assessed outcomes. iv) Published in English including all EBM Reviews (Cochrane Database of Systematic Reviews, American College of Physicians Journal Club, Database of Abstracts of Reviews of Effects, Cochrane Clinical Answers, Cochrane Methodology Register, Health Technology Assessments, Cochrane Central Register of Controlled Trials and NHS Economic Evaluation Database), AMED (Allied and Complementary Medicine), Embase, Ovid MEDLINE(R), PubMed, Web of Science, Google scholar, and ClinicalTrials.gov.
The exclusion criteria were the following: i) the final results of an RCT or review; ii) duplicated records; iii) without studied interventions; iv) lack of a control group; v) complex intervention (with more than two studied interventions).
The systematic searches were conducted across the electronic databases, as noted in the detailed search strategy for each database provided in Supplementary Material S1.
2.2 Screening
Two reviewers (HL and LHH) independently assessed the publications during two phases. The initial phase involved the evaluation of titles and abstracts, while the subsequent phase entailed a comprehensive assessment of the complete texts of selected studies. The inclusion and exclusion criteria were consistently applied throughout both phases. Throughout the entire process, two reviewers conducted a meticulous verification to ensure the utmost precision. Any disagreements between the reviewers were resolved through discussion or consultation with the third reviewer (XZ).
2.3 Data extraction
A pre-designed data extraction form with four parts and 16 specific items was developed (Supplementary Material S2). Extracted data mainly addressed two categories: i) general characteristics (e.g., publication year, information of authors, journal, and funding), and ii) TCM-related characteristics (e.g., studied disease with or without TCM patterns and TCM-related outcomes). Before formally commencing the extraction process, a third reviewer (XZ) conducted training sessions on the extraction form rules. Moreover, a pilot test was administered on nine randomly selected articles, covering all three interventions of CHMF, acupuncture, and moxibustion. Two independent reviewers (LHH and HZT) were responsible for extracting data from each article. Any disagreements between the reviewers were resolved through discussion.
2.4 Assessment of reporting quality
In the SPIRIT-TCM Extension 2018 checklist, 15 items were customized for interventions involving CHMF, acupuncture, and moxibustion, respectively (Dai et al., 2019). These items included study rationale, eligibility criteria, intervention details, and outcome indicator measures. To enhance the efficiency of the assessment process, each item in SPIRIT-TCM Extension 2018 has been redesigned into one or more questions to facilitate more complex assessments. Thus, a specially designed quality assessment form was developed by the researcher (XZ) based on the SPIRIT-TCM Extension 2018 checklist. The formulation of the questions was primarily guided by the distinctive features inherent in TCM intervention modalities, such as i) essential elements of TCM in clinical practice; ii) revealing the TCM utilization of individualization in a transparent manner, elucidating the underlying reasons for its implementation, and providing comprehensive insights into its operational intricacies; and iii) the rationale for the selection of TCM. The assessment form utilized in this study incorporates the 15 primary items outlined in SPIRIT-TCM Extension 2018. Specifically, it encompasses 29 sub-questions for CHMF intervention, 21 sub-questions for acupuncture intervention, and 20 sub-questions for moxibustion intervention (Table 1-3). It was notable that item 6a.3 is not applicable for assessment as this review excluded complex interventions.
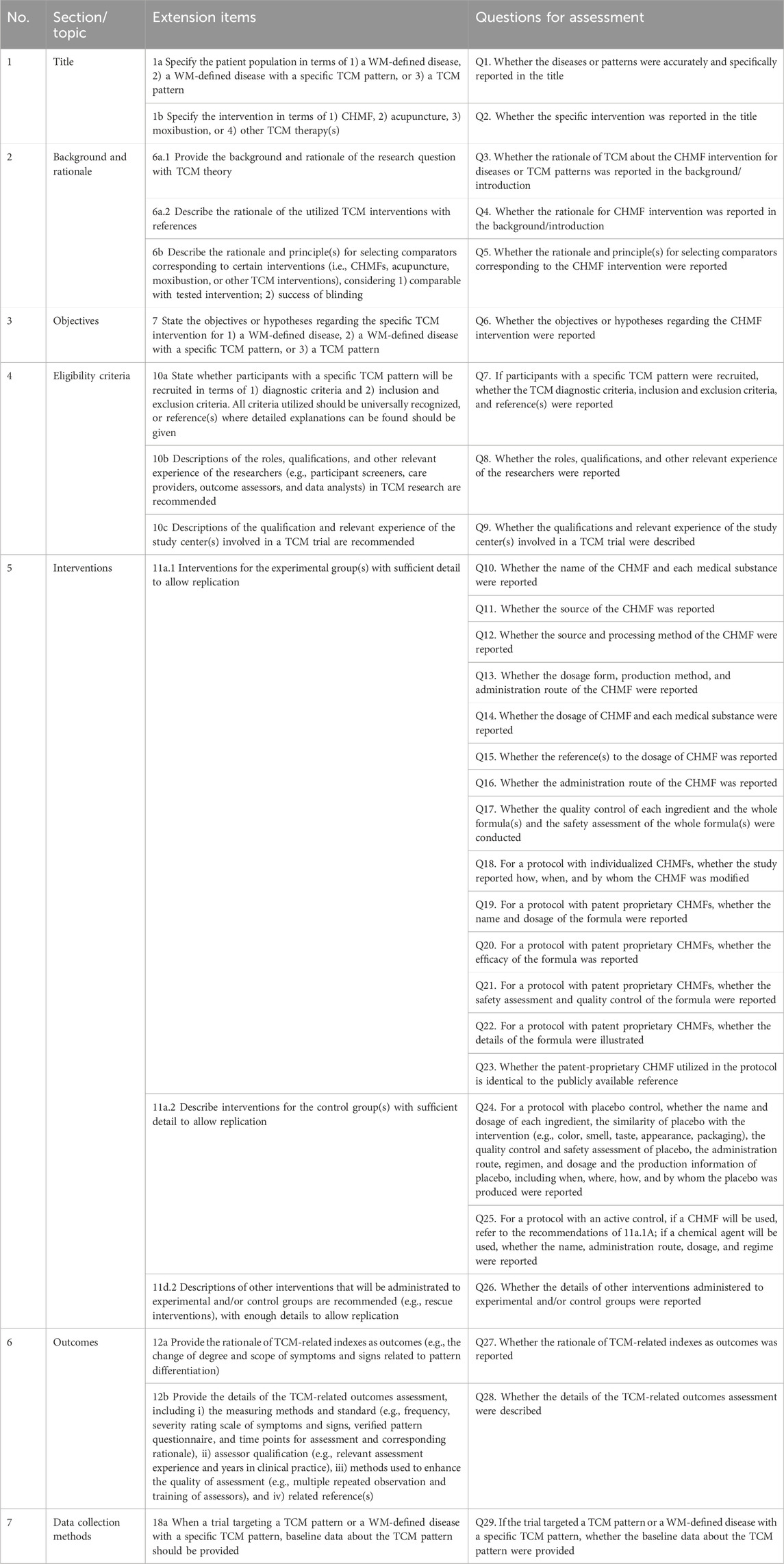
Table 1. Twenty-nine sub-questions for CHMF interventional studies based on SPIRIT-TCM Extension 2018.
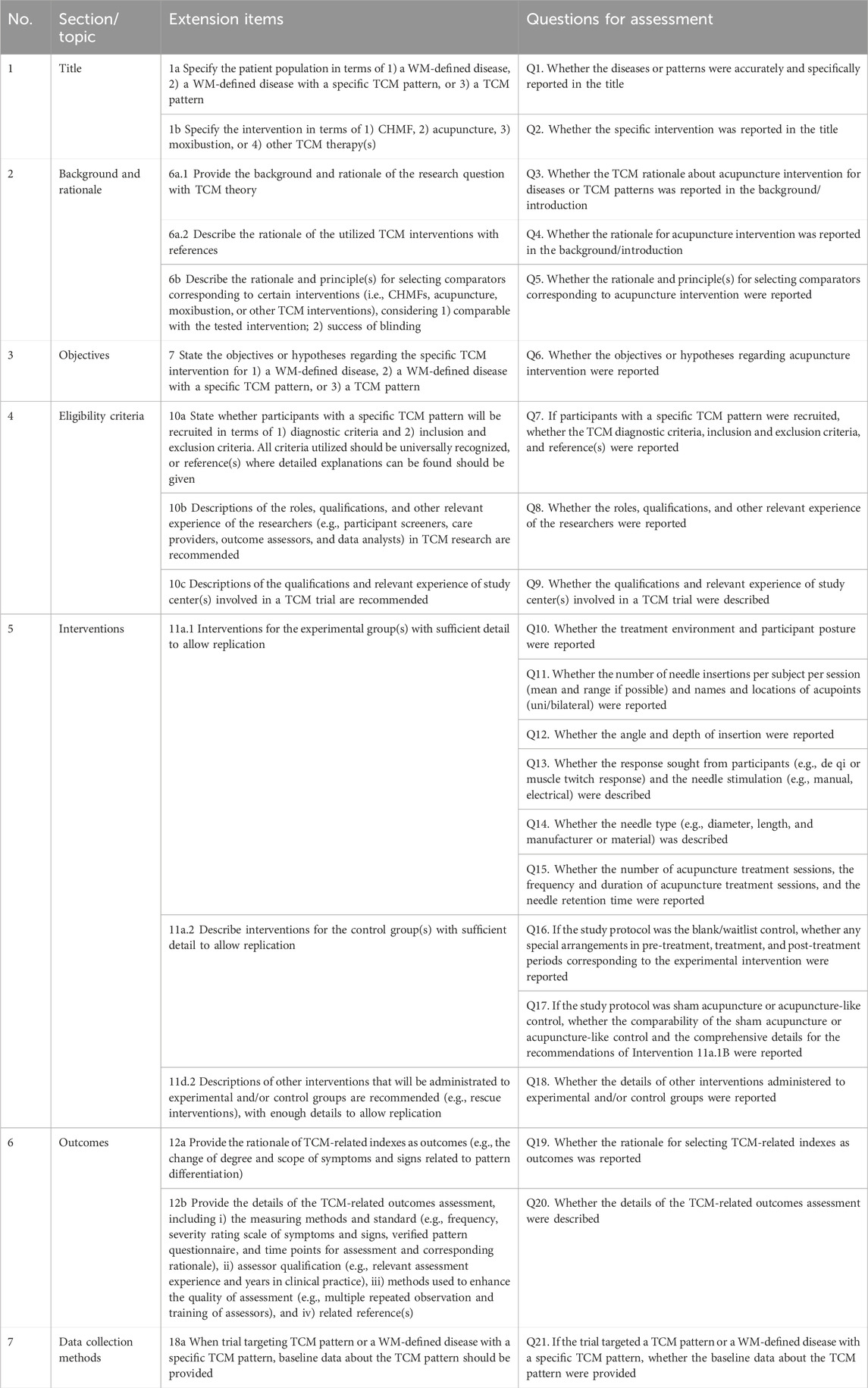
Table 2. Twenty-one sub-questions for acupuncture interventional studies based on SPIRIT-TCM Extension 2018.
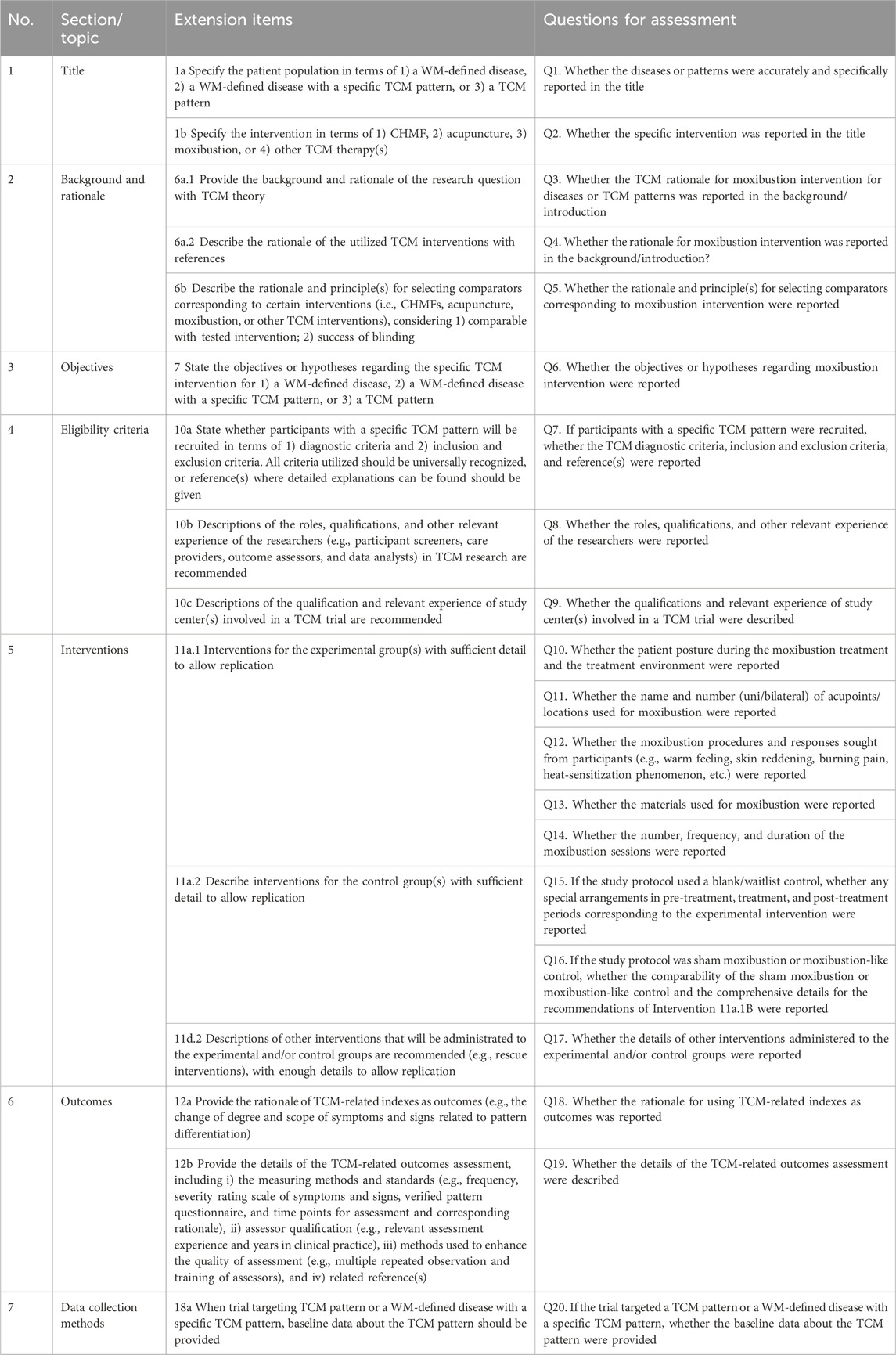
Table 3. Twenty sub-questions for moxibustion interventional studies based on SPIRIT-TCM Extension 2018.
To facilitate quality assessment, each question offers four scoring options: 2, 1, 0, and Not Applicable (NA). In the assessment process, each question will be awarded a score of “2” only if all relevant details of the information specified in SPIRIT-TCM Extension 2018 are fully reported. Conversely, a score of “1” was awarded if only some of the required information was disclosed, and a score of “0” was awarded if no information was disclosed at all. Cases marked “NA” indicate that the particular item or sub-question is not relevant to the particular RCT. Details of the standard operating procedures (SOP), including the original items and specific questions, scoring rules, and examples, are presented in Supplementary Material S3. All reviewers were trained by a senior reviewer (XZ) and participated in pilot evaluations of ten randomly selected articles from each type of intervention to ensure consistency in scoring. Each question was scored by two independent reviewers (e.g., CHMF articles: HZT and LHH, acupuncture articles: HL and LHH, and moxibustion articles: HZT and HL). Cohen’s kappa was used to identify the level of agreement, including poor agreement (0%–40%), moderate agreement (41%–60%), substantial agreement (61%–80%), and almost complete agreement (81%–100%) (Viera and Garrett, 2005). Any inconsistencies were resolved by the senior reviewer (XZ).
2.5 Data analysis
Descriptive statistics were performed to analyze the characteristics and reporting compliance of the included articles. Categorical variables are summarized in frequencies and percentages. For the reported compliance rate (CR, calculated as CR = n/N*100%), the count of “Not Applicable” instances was excluded from the calculation, while continuous variables are expressed as mean and standard deviation (SD). To assess the reporting quality, each item or question was evaluated based on the number of reports that received a score of “2” out of the total number of included reports, and the compliance rate was calculated according to the proportion. The compliance level was then categorized into three groups: excellent compliance (>90%), good compliance (between 65% and 90%), and poor compliance (<65%). The report compliance rate for each intervention was calculated separately by time of publication and by specific items. Data analysis was performed using SPSS software, version 26.0, and the results were clearly demonstrated using figures to illustrate trends and comparisons. All data were collected and recorded in Microsoft Office Excel 365.
3 Results
3.1 Search
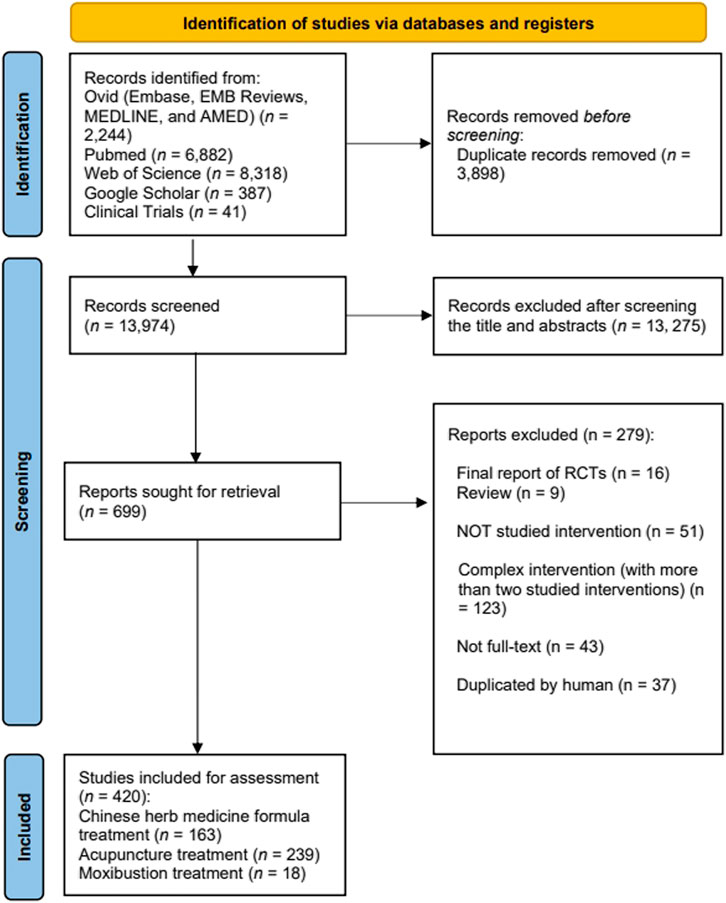
The initial search yielded 17,872 records, from which 17,452 relevant studies were retained after excluding duplicates and screening the titles and abstracts. Following a thorough review of full-text articles, we identified 420 eligible protocols, including 163 Chinese herbal medicine formula studies, 239 acupuncture studies, and 18 moxibustion studies (Figure 1). The RCTs included in this analysis are listed in Supplementary Material S4.
3.2 Characteristics of included studies
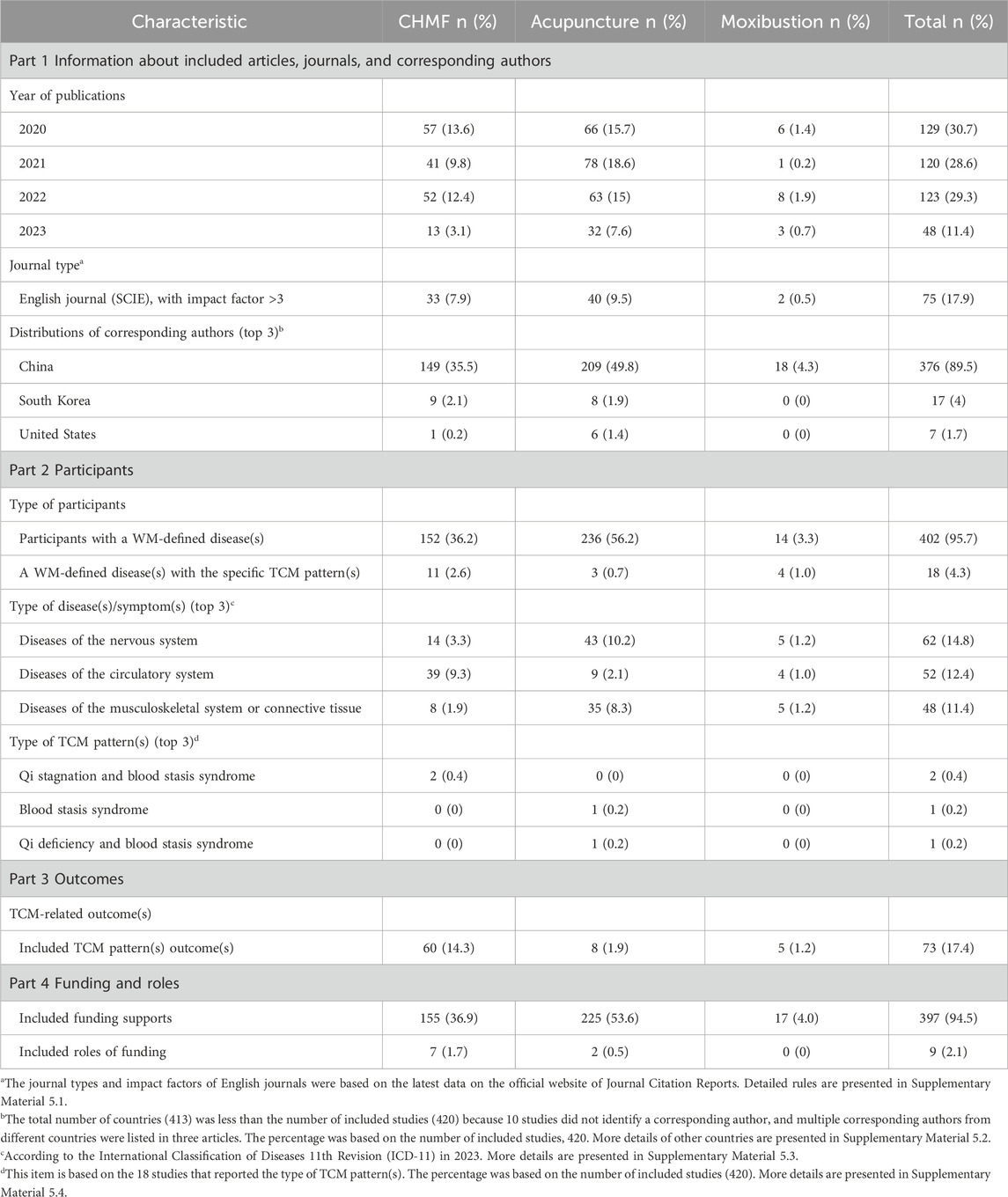
This study included 420 protocols published from 2020 to 2023. All were published in English journals, of which 17.9% had an impact factor greater than 3. The corresponding authors of all studies were distributed in 12 countries, and 89.5% of the corresponding authors were from China. Other countries included South Korea, the United States, France, Germany, Portugal, Brazil, the United Kingdom, Australia, Canada, and Poland. Only 4.3% of the participants were identified as having a Western medicine (WM)-defined disease(s) with a specific TCM pattern(s), while the remaining 95.7% were only identified as having a WM-defined disease(s). The most commonly studied disease was the disease of the nervous system (14.8%). In terms of TCM pattern(s) types, 0.4% were patterns of qi stagnation and blood stasis. In addition, 17.4% of the studies used TCM-related indicators in the outcomes. Funding support was reported for 94.5% of the protocols. However, only 2.1% of the roles of funding were described. More details are provided in Table 4 and Supplementary Material S5.
3.3 Reporting quality assessment of all included protocols
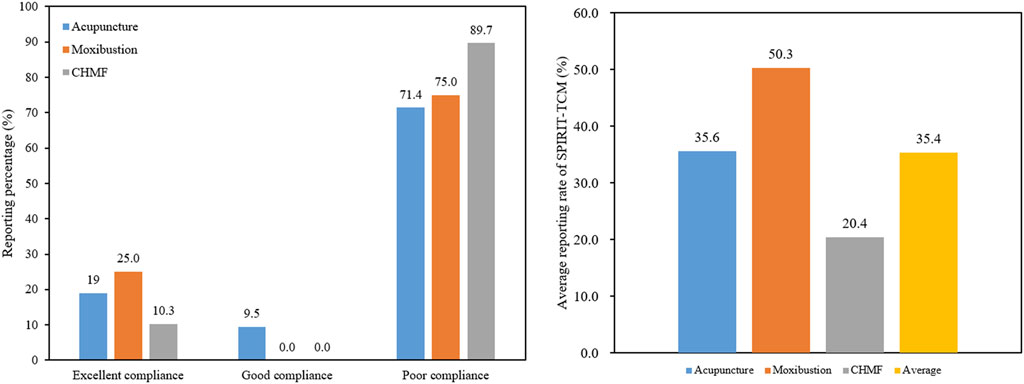
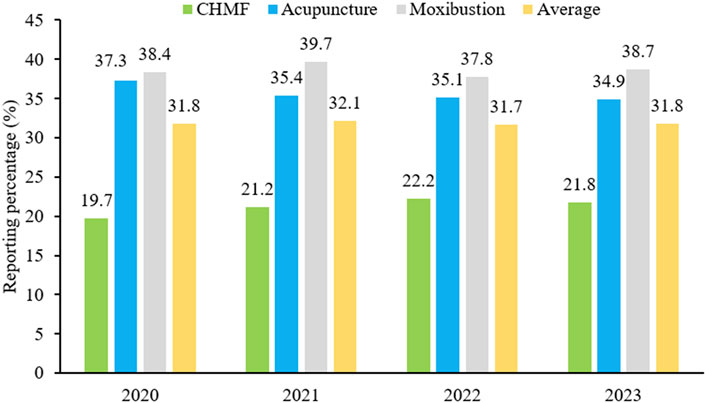
SPIRIT-TCM Extensions 2018 was used to evaluate the quality of 420 articles that met the inclusion criteria, with an average reporting rate of 35.4%. Among the specific interventions, moxibustion demonstrated the highest reporting rate at 50.3%, followed by acupuncture at 35.6% and CHMF at 20.4%. The inter-rater agreement for each item exceeded 85% among the two reviewers (Supplementary Material S6). Concerning the compliance level, protocols of moxibustion were categorized as demonstrating excellent compliance, while CHMF protocols displayed poor compliance (Figure 2). In terms of year of publication, the average reporting rates from 2020 to 2023 were 31.8%, 32.1%, 31.7%, and 31.8%. Among the three interventions, moxibustion had the highest average annual reporting rate, and CHMF had the lowest (Figure 3). Focusing on specific items, the average reporting rate of each intervention in title and objectives exhibited a relatively higher rate above 90%. Conversely, the average compliance rate for eligibility criteria, outcomes, and data collection methods was below 30%, particularly for data collection methods, standing at a mere 2.3%. For acupuncture intervention, no literature report on this item considered TCM patterns in the collection of baseline data or data statistics (Figure 4).
3.4 Reporting quality assessment of CHMF protocols
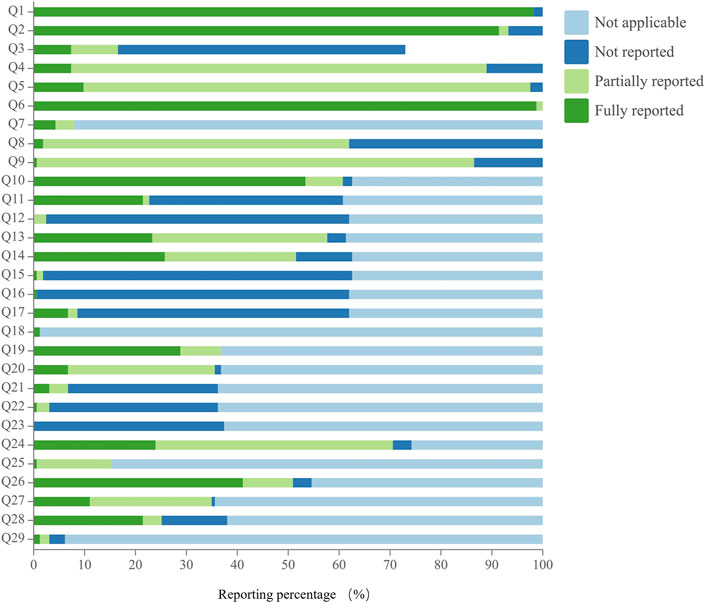
The reporting quality of CHMF studies assessed using the SPIRIT-TCM extension 2018 checklist varied across 29 specific questions, with excellent compliance observed in 10.3% of cases, good compliance in 0%, and poor compliance in 89.7%. Regarding poor reporting, there were 26 specific issues assessed, and their reporting rates were ranked in descending order as follows: Q10 (53.4%), Q26 (41.1%), Q20 (28.8%), Q19 (28.8%), Q14 (25.8%), Q24 (23.9%), Q13 (23.3%), Q28 (21.5%), Q11 (21.5%), Q27 (11.0%), Q5 (9.8%), Q4 (7.4%), Q3 (7.4%), Q17 (6.7%), Q7 (4.3%), Q21 (3.1%), Q8 (1.8%), Q29 (1.2%), Q18 (1.2%), Q25 (0.6%), Q22 (0.6%), Q16 (0.6%), Q15 (0.6%), Q9 (0.6%), Q23 (0%), and Q12 (0%). Notably, the reporting rates for 24 of these specific issues were below 30%, especially for principles of CHMF, individualized treatment, details of the control, relevant experience of study center(s), details of patent proprietary CHMF, reference(s) to dosage of CHMF, administration route, origin and processing method of the CHMF, and whether the patent proprietary CHMF utilized in the protocol was identical to the public applicable reference. Details are shown in Figure 5 and Supplementary Material S7.
3.5 Reporting quality assessment of acupuncture protocols
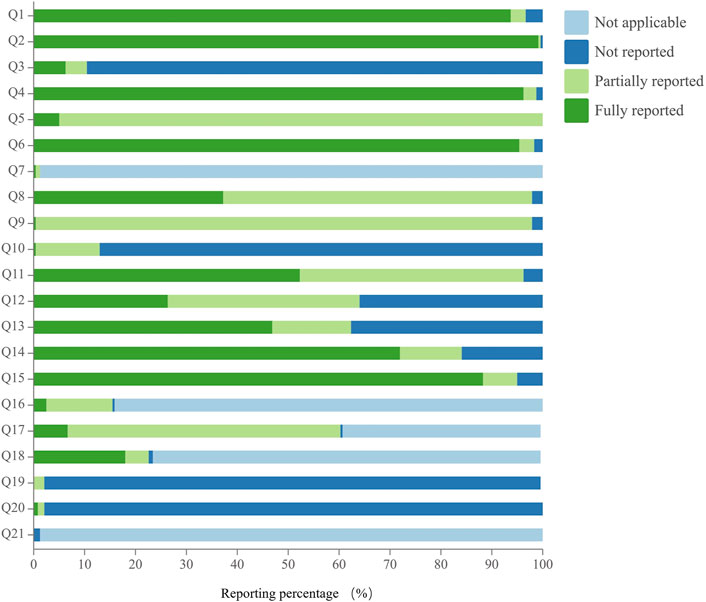
The reporting quality of acupuncture studies assessed using the SPIRIT-TCM checklist varied across 21 specific questions, with excellent compliance observed in 19.0% of cases, good compliance in 9.5%, and poor compliance in 71.4%. Regarding poor reporting, there were 15 specific issues assessed, and their reporting rates were ranked in descending order as follows: Q11 (52.3%), Q13 (46.9%), Q8 (37.2%), Q12 (26.4%), Q18 (18.0%), Q17 (6.7%), Q3 (6.3%), Q5 (5.0%), Q16 (2.5%), Q20 (0.8%), Q9 (0.4%), Q7 (0.4%), Q10 (0.4%), Q21 (0%), and Q19 (0%). Notably, the reporting rates for 12 of these specific issues were below 30%, especially for angle and depth of insertion, qualification and relevant experience of study center(s), TCM diagnostic criteria, inclusion and exclusion criteria, and reference(s), treatment environment and participant posture, rationale of TCM-related indexes as outcomes, and data collection methods. Details are shown in Figure 6 and Supplementary Material S8.
3.6 Reporting quality assessment of moxibustion protocols
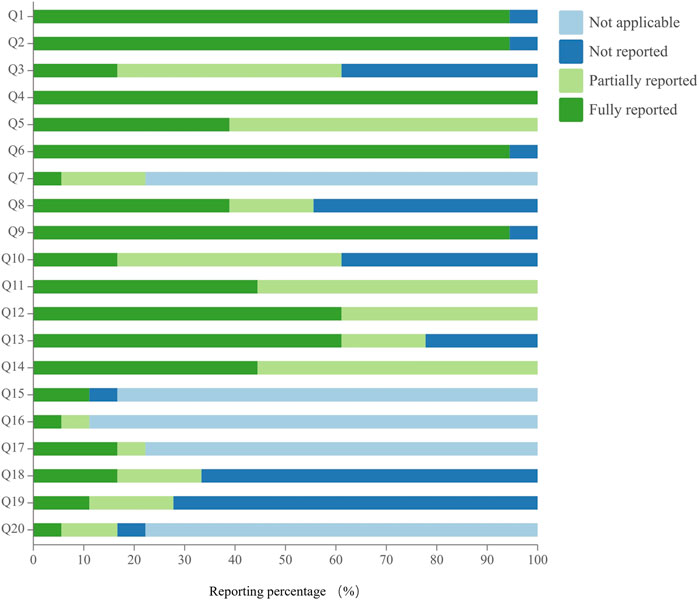
The reporting quality of moxibustion studies assessed using the SPIRIT-TCM checklist varied across 20 specific questions, with excellent compliance observed in 25.0% of cases, good compliance in 0%, and poor compliance in 75.0%. Regarding poor reporting, there were 15 specific issues assessed, and their reporting rates were ranked in descending order as follows: Q12 (61.1%), Q13 (61.1%), Q11 (44.4%), Q14 (44.4%), Q5 (38.9%), Q8 (38.9%), Q18 (16.7%), Q17 (16.7%), Q10 (16.7%), Q3 (16.7%), Q19 (11.1%), Q15 (11.1%), Q20 (5.6%), Q16 (5.6%), and Q7 (5.6%). Notably, the reporting rates for nine of these specific issues were below 30%, especially for TCM diagnostic criteria, inclusion and exclusion criteria and reference(s), comparability of the sham moxibustion or moxibustion-like control, details of the intervention, and data collection methods. Details are shown in Figure 7 and Supplementary Material S9.
4 Discussion
SPIRIT-TCM Extension 2018 underscored the significance of TCM syndromes in clinical trials by providing examples and explanations for different TCM interventions. Reporting of the protocols included in this item fell short, particularly in either ignoring during the study design or incomplete reporting of the TCM syndrome (if used) information about diagnosis criteria and measurement methods. In clinical practice, TCM places a crucial emphasis on treatment guided by syndrome differentiation, considering it as vital as disease diagnosis. To illustrate, in the context of the evolution of coronavirus disease 2019, different TCM syndromes may manifest, each requiring varied medications. The accurate differentiation of TCM patterns is essential for ensuring clinical efficacy, and an incorrect differentiation may result in ineffective intervention and even adverse effects (Zhao et al., 2020; Zhao et al., 2021). Therefore, it is imperative to highlight the importance of TCM syndrome types, as they not only serve as a guarantee for therapeutic effectiveness but also play a pivotal role in the reproducibility of experiments.
Only 17.4% of the studies adopted TCM-related outcomes. TCM pattern score is the most common type of TCM-related outcome report and can evaluate the composite efficacy of treatments according to a series of symptom evaluations. Generally, the higher the symptom score is, the more severe the disease will be (Jiang et al., 2016). In previous studies, some scholars have also indicated a lack of TCM symptom outcomes in most RCTs of TCM. For instance, a breast cancer-related study (Ji et al., 2020) accesses the TCM clinical value with a series of Western clinical indicators but without TCM symptom indicators. TCM symptom scores focus on subjective feelings and symptoms, while the Western clinical indicators highlight the laboratory report. Sometimes, there is no clear correlation between subjective symptoms and laboratory tests. To illustrate, acute myocardial infarction patients might have severe clinical symptoms but a normal electrocardiograph and laboratory indicators (Zhang et al., 2023). Hence, neglecting TCM-related indicators may introduce bias, potentially yielding inaccurate results. Special consideration should be given to TCM symptom/pattern scores, which not only serve as a crucial metric for emphasizing the overall efficacy of TCM therapies but also serve as a focal point for evaluating the effectiveness or ineffectiveness of a TCM treatment program in alleviating specific symptoms.
SPIRIT-TCM Extension 2018 also placed significant emphasis on providing specific and detailed descriptions of TCM interventions, particularly in CHMF, acupuncture, and moxibustion. Notably, TCM interventions pose a unique challenge for quantification, in contrast to Western medicine. This study, however, reveals a concerning gap in the reporting of details. Specifically, less than 30% of CHMF protocols detail the principles underlying CHMFs, individualized treatment approaches, specific control groups, particulars of patent proprietary CHMFs, references to dosage, and the origin and processing methods of the CHMF. The quality control of Chinese herbs (e.g., the origin and method of preparation) is a key factor in the efficacy. For example, “Dihuang,” Rehmannia, produced in Henan Province, is divided into “raw Dihuang” and “processed Dihuang” (Su et al., 2016) with different functions. The raw Dihuang is mainly used to clear heat and cool the blood, nourish Yin, and promote the production of body fluid, while the processed Dihuang is mainly used to tonify the blood and nourish Yin. The TCM-related RCTs must detail the origin, processing, formula, and so on. The lack of processing information on TCM may lead to unrepeatable results. The reason may be that most first authors are clinicians or statisticians who do not have a background related to TCM (Zhang et al., 2019). Therefore, including professionals with a background in TCM is necessary to ensure quality control of CHMF.
Similarly, less than 30% of the acupuncture protocols provided information on the angle and depth of insertion, qualifications and relevant experience of study center(s), TCM diagnostic criteria, inclusion and exclusion criteria with references, treatment environment specifics, and participant posture. The angle and depth of insertion are the main guarantees of efficacy and safety. For example, in terms of treatment effectiveness, deeper insertion had a better analgesic effect than shallow insertion in the treatment of chronic lumbar myofascial pain (Ceccherelli et al., 2002). In terms of safety, pneumothorax will be triggered by straight thrusts rather than oblique thrusts in the acupuncture of the chest (Nishie et al., 2021). Therefore, the description of the angle and depth of insertion is essential for acupuncture RCTs, but this aspect is not commonly reported in RCTs. The reason that the demerits of the acupuncture RCTs about the angle and depth of insertion may have two sides: On the one hand, for practitioners, different acupuncturists operate the same acupuncture point with different depths and angles; on the other hand, for patients, different patients have different body mass indexes and physical characteristics. Therefore, for the details of the intervention to be better and replicable, the characteristics of the included population (e.g., height, weight, etc.) need to be strictly defined, and the practitioner needs to be tarined in the operation of acupuncture.
In moxibustion, less than 30% of protocols reported the comparability of sham moxibustion or moxibustion-like control and provided detailed intervention information. However, the selection of the comparator will influence the interpretations of trial outcomes according to the SPIRIT-TCM Extension 2018, and the safety and efficacy cannot be proved and will be doubted by Western medicine specialists (Wang et al., 2022). The reason for the absence of sham moxibustion or moxibustion-like controls is that moxibustion, unlike other drugs, cannot be set up in the same way as the test drug in terms of the drug’s color, taste, or smell. For example, it is not only made in various forms (e.g., moxa cone moxibustion, warm needle moxibustion, etc.) but also has a different mechanism of treatment (e.g., the external smoke directly eliminates germs, and the burning temperature reaches 43° to achieve the desired therapeutic effect, and the therapeutic effect of infrared light radiation). Therefore, the moxibustion control group needs to be set up to meet the conditions of smoke, heat, and radiation insulation at the same time. For example, aluminum foil can be utilized for smoke and radiation insulation, and an asbestos heat-resistant layer can be used for heat insulation (Pan et al., 2019).
Among the three typical TCM intervention methods, moxibustion presented the highest reporting rate at 50.3%, followed by acupuncture at 35.6%, and finally, CHMF at 20.4%. Delving into specific items, the average reporting rate in the title and objectives stands relatively high. In contrast, the average reporting rate for eligibility criteria, outcomes, and data collection methods is below 30%, particularly for data collection methods, which is remarkably low at 2.3%. Notably, acupuncture studies exhibited the poorest performance in this regard, particularly in the collection of baseline data or data statistics related to TCM patterns. Similarly, a previous analysis of RCT reports of acupuncture for low back pain between 2010 and 2020 showed that the quality was moderate (Cohen’s κ-statistic 0.4 <κ ≤ 0.6) (Liu et al., 2021). The documentation about the quality of herbal medicine formulas was poor according to the CONSORT checklist in 2006 (Bian et al., 2006)4. In addition, a systematic review (Li et al., 2020) of Chinese patent medicine for eczema showed the mean overall quality score was not good with reference to the CONSORT-CHMF 2017 statement.
This study is subject to certain limitations. First, the review focused on identifying TCM RCT protocols published between 1 January 2020 and 10 August 2023 across eight designated databases. Any records beyond the specified cutoff period or not included in these databases were excluded. Additionally, the study only considered publications in English, potentially overlooking trials published in other languages. Second, the quality assessment was strictly based on the SPIRIT-TCM 2018 checklists, limiting the scope to three TCM interventions: CHMF, acupuncture, and moxibustion. When acknowledging these constraints, we believe that the findings presented in this study reflect significant and reliable trends in TCM protocol within the specified parameters.
5 Conclusion
This review first set SPIRIT-TCM Extension 2018 as a reference to evaluate the reported characteristics and quality of protocols of RCT that involve interventions such as CHMF, acupuncture, and moxibustion published in the last 3 years and to provide a basis for the refinement of subsequent RCTs in TCM. The results showed that the average compliance rate of TCM RCTs was only 35.4%, with only 4.3% identifying TCM pattern-based relevant diseases, 17.4% of the studies used TCM outcome-related indicators, and all TCM intervention details were described in less than 30% of the studies. Therefore, in the future, the following methods should be adopted to promote the impact and application of the SPIRIT-TCM Extension 2018: 1) In the selection of diseases and interventions, follow the guiding principles of TCM diagnosis and treatment to determine the disease, TCM patterns, treatment principles, and treatment protocols, and strictly determine the inclusion criteria according to the requirements of the diseases; (2) in terms of the details of the intervention, the quality and safety of the CHMF, the operation methods of acupuncture and moxibustion, and the setting of an appropriate control group or placebo group need to be strictly controlled; (3) in terms of outcome indicators, TCM-related indicators are indispensable to highlight the clinical efficacy of TCM.
Data availability statement
All data analysed during this study are included in the article/Supplementary Materials. More information can be found in the registration dataset at Open Science Framework (https://osf.io/573c2/).
Author contributions
LZ: writing–original draft, visualization, software, formal analysis, and data curation. HL: writing–original draft, visualization, software, formal analysis, and data curation. LH: writing–original draft, visualization, software, formal analysis, and data curation. XQ: writing–original draft, validation, and methodology. HT: writing–original draft, validation, methodology. XZ: writing–original draft, validation, and methodology. CL: writing–original draft, validation, and methodology. AL: writing–review and editing, supervision, resources, and funding acquisition. ZB: writing–review and editing, supervision, resources, and funding acquisition. XZ: writing–review and editing, writing–original draft, visualization, validation, supervision, project administration, methodology, formal analysis, data curation, and conceptualization.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work is supported by the Chinese Medicine Development Fund, Hong Kong, China (20B2/027A and 23B2/027A_R1); the Chinese Medicine Development Fund, Hong Kong, China (23B2/027A_R1); the Center for Evidence-based Traditional Chinese Medicine, CCEBTM (2020YJSZX-5); Donation funding of the Vincent V.C. Woo Chinese Medicine Clinical Research Institute; and the Health@InnoHK Initiative Fund of the Hong Kong Special Administrative Region Government (ITC RC/IHK/4/7).
Acknowledgments
The authors thank all the financial support from the Chinese Medicine Development Fund, Hong Kong, China (20B2/027A and 23B2/027A_R1); the Chinese Medicine Development Fund, Hong Kong, China (23B2/027A_R1); the Center for Evidence-based Traditional Chinese Medicine, CCEBTM (2020YJSZX-5); Donation funding of the Vincent V.C. Woo Chinese Medicine Clinical Research Institute; and the Health@InnoHK Initiative Fund of the Hong Kong Special Administrative Region Government (ITC RC/IHK/4/7).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2024.1389808/full#supplementary-material
References
Bian, Z. X., Moher, D., Dagenais, S., Li, Y. P., Wu, T. X., Liu, L., et al. (2006). Improving the quality of randomized controlled trials in Chinese herbal medicine, part IV: applying a revised CONSORT checklist to measure reporting quality. Zhong Xi Yi Jie He Xue Bao 4 (3), 233–242. doi:10.3736/jcim20060303
Burnett, A. K., Russell, N. H., Hills, R. K., Bowen, D., Kell, J., Knapper, S., et al. (2015). Arsenic trioxide and all-trans retinoic acid treatment for acute promyelocytic leukaemia in all risk groups (AML17): results of a randomised, controlled, phase 3 trial. Lancet Oncol. 16 (13), 1295–1305. doi:10.1016/S1470-2045(15)00193-X
Ceccherelli, F., Rigoni, M. T., Gagliardi, G., and Ruzzante, L. (2002). Comparison of superficial and deep acupuncture in the treatment of lumbar myofascial pain: a double-blind randomized controlled study. Clin. J. Pain 18 (3), 149–153. doi:10.1097/00002508-200205000-00003
Chan, A. W., Tetzlaff, J. M., Altman, D. G., Laupacis, A., Gøtzsche, P. C., Krleža-Jerić, K., et al. (2013). SPIRIT 2013 statement: defining standard protocol items for clinical trials. Ann. Intern Med. 158 (3), 200–207. doi:10.7326/0003-4819-158-3-201302050-00583
Cheng, C. W., Fu, S. F., Zhou, Q. H., Wu, T. X., Shang, H. C., Tang, X. D., et al. (2013). Extending the CONSORT statement to moxibustion. J. Integr. Med. 11 (1), 54–63. doi:10.3736/jintegrmed2013009
Cheng, C. W., Wu, T. X., Shang, H. C., Li, Y. P., Altman, D. G., Moher, D., et al. (2017). CONSORT extension for Chinese herbal medicine formulas 2017: recommendations, explanation, and elaboration. Ann. Intern Med. 167 (2), 112–121. doi:10.7326/M16-2977
Dai, L., Cheng, C. W., Tian, R., Zhong, L. L., Li, Y. P., Lyu, A. P., et al. (2019). Standard protocol items for clinical trials with traditional Chinese medicine 2018: recommendations, explanation and elaboration (SPIRIT-TCM extension 2018). Chin. J. Integr. Med. 25 (1), 71–79. doi:10.1007/s11655-018-2999-x
Daily, J. P., Minuti, A., and Khan, N. (2022). Diagnosis, treatment, and prevention of malaria in the us: a review. Jama 328 (5), 460–471. doi:10.1001/jama.2022.12366
Gagnier, J. J., Boon, H., Rochon, P., Moher, D., Barnes, J., Bombardier, C., et al. (2006a). Recommendations for reporting randomized controlled trials of herbal interventions: explanation and elaboration. J. Clin. Epidemiol. 59 (11), 1134–1149. doi:10.1016/j.jclinepi.2005.12.020
Gagnier, J. J., Boon, H., Rochon, P., Moher, D., Barnes, J., Bombardier, C., et al. (2006b). Reporting randomized, controlled trials of herbal interventions: an elaborated CONSORT statement. Ann. Intern Med. 144 (5), 364–367. doi:10.7326/0003-4819-144-5-200603070-00013
Ji, Y., Li, S., Zhang, X., Liu, Y., Lu, Q., Li, Q., et al. (2020). The prophylactic and therapeutic effects of moxibustion combined with traditional Chinese medicine decoction for treating chemotherapy-induced myelosuppression in early-stage breast cancer: study protocol for a randomized controlled trial. Trials 21 (1), 844. doi:10.1186/s13063-020-04749-6
Jiang, H., Liu, W., Li, G., Fan, T., and Mao, B. (2016). Chinese medicinal herbs in the treatment of upper airway cough syndrome: a systematic review of randomized, controlled trials. Altern. Ther. Health Med. 22 (3), 38–51.
Lam, W. C., Lyu, A., and Bian, Z. (2019). ICD-11: impact on traditional Chinese medicine and World healthcare systems. Pharm. Med. 33 (5), 373–377. doi:10.1007/s40290-019-00295-y
Li, M., Zhou, B., Zhou, L., and Li, L. (2020). Reporting quality of randomized controlled trials for the treatment of eczema with Chinese patent medicine based on the CONSORT-CHM formulas 2017. Evid. Based Complement. Altern. Med. 2020, 2949125. doi:10.1155/2020/2949125
Liu, M., Gao, Y., Yuan, Y., Yang, K., Shi, S., Zhang, J., et al. (2020). Efficacy and safety of integrated traditional Chinese and western medicine for corona virus disease 2019 (COVID-19): a systematic review and meta-analysis. Pharmacol. Res. 158, 104896. doi:10.1016/j.phrs.2020.104896
Liu, X., Xu, Z., Wang, Y., Luo, H., Zou, D., Zhou, Z., et al. (2021). Evaluating the quality of reports about randomized controlled trials of acupuncture for low back pain. J. Pain Res. 14, 1141–1151. doi:10.2147/jpr.s308006
MacPherson, H., Altman, D. G., Hammerschlag, R., Youping, L., Taixiang, W., White, A., et al. (2010). Revised STandards for reporting interventions in clinical trials of acupuncture (STRICTA): extending the CONSORT statement. PLoS Med. 7 (6), e1000261–ST14. doi:10.1089/acm.2010.1610
Nishie, M., Masaki, K., Kayama, Y., and Yoshino, T. (2021). Bilateral pneumothorax after acupuncture treatment. BMJ Case Rep. 14 (3), e241510. doi:10.1136/bcr-2020-241510
Page, M. J., McKenzie, J. E., Bossuyt, P. M., Boutron, I., Hoffmann, T. C., Mulrow, C. D., et al. (2021). The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Bmj 372, n71. doi:10.1136/bmj.n71
Pan, J. H., Lu, L., Wang, C., and Fu, W. B. (2019). A preliminary discussion of the necessity of controlled setting of placebo moxibustion. Zhen Ci Yan Jiu 44 (12), 922–925. doi:10.13702/j.1000-0607.180444
Panossian, A., Lemerond, T., and Efferth, T. (2024). State-of-the-Art review on botanical hybrid preparations in phytomedicine and phytotherapy research: background and perspectives. Pharm. (Basel) 17 (4), 483. doi:10.3390/ph17040483
Su, X., Yao, Z., Li, S., and Sun, H. (2016). Synergism of Chinese herbal medicine: illustrated by danshen compound. Evid. Based Complement. Altern. Med. 2016, 7279361. doi:10.1155/2016/7279361
Tan, Z. W., Tan, A. C., Li, T., Harris, I., Naylor, J. M., Siebelt, M., et al. (2020). Has the reporting quality of published randomised controlled trial protocols improved since the SPIRIT statement? A methodological study. BMJ Open 10 (8), e038283. doi:10.1136/bmjopen-2020-038283
Viera, A. J., and Garrett, J. M. (2005). Understanding interobserver agreement: the kappa statistic. Fam. Med. 37 (5), 360–363.
Wang, J., Wong, Y. K., and Liao, F. (2018). What has traditional Chinese medicine delivered for modern medicine? Expert Rev. Mol. Med. 20, e4. doi:10.1017/erm.2018.3
Wang, Z., Xu, M., Shi, Z., Bao, C., Liu, H., Zhou, C., et al. (2022). Mild moxibustion for irritable bowel syndrome with diarrhea (IBS-D): a randomized controlled trial. J. Ethnopharmacol. 289, 115064. doi:10.1016/j.jep.2022.115064
Zhang, L., Liu, Y., Wang, K., Ou, X., Zhou, J., Zhang, H., et al. (2023). Integration of machine learning to identify diagnostic genes in leukocytes for acute myocardial infarction patients. J. Transl. Med. 21 (1), 761. doi:10.1186/s12967-023-04573-x
Zhang, X., Aixinjueluo, Q. Y., Li, S. Y., Song, L. L., Lau, C. T., Tan, R., et al. (2019). Reporting quality of Cochrane systematic reviews with Chinese herbal medicines. Syst. Rev. 8 (1), 302. doi:10.1186/s13643-019-1218-y
Zhang, X., Chung, W. C. A., Lau, C. T., and Wang, N. (2022). Reporting guidelines of Chinese medicine: current situation and future development. J. Traditional Chin. Med. Sci. 9 (03), 209–216. doi:10.1016/j.jtcms.2022.06.008
Zhang, X., Lan, L., Chan, J. C. P., Zhong, L. L. D., Cheng, C. W., Lam, W. C., et al. (2020). WHO Trial Registration Data Set (TRDS) extension for traditional Chinese medicine 2020: recommendations, explanation, and elaboration. BMC Med. Res. Methodol. 20 (1), 192. doi:10.1186/s12874-020-01077-w
Zhao, H., Zhao, L., Wu, F., and Shen, L. (2021). Clinical research on traditional Chinese medicine treatment for bacterial vaginosis. Phytother. Res. 35 (9), 4943–4956. doi:10.1002/ptr.7123
Zhao, Z. H., Zhou, Y., Li, W. H., Huang, Q. S., Tang, Z. H., and Li, H. (2020). Analysis of traditional Chinese medicine diagnosis and treatment strategies for COVID-19 based on "the diagnosis and treatment program for coronavirus disease-2019" from Chinese authority. Am. J. Chin. Med. 48 (5), 1035–1049. doi:10.1142/S0192415X20500500
Zheng, Y., Ching, J., Cheng, C. W., Lam, W. C., Chan, K. L., Zhang, X., et al. (2021). Efficacy and safety of Chinese medicine JCM-16021 for diarrhea-predominant irritable bowel syndrome: study protocol for a multi-center, randomized, double-blind, placebo controlled clinical trial. Chin. Med. 16 (1), 117. doi:10.1186/s13020-021-00530-2
Keywords: evidence synthesis, randomized controlled trial, reporting quality, SPIRIT guideline, traditional Chinese medicine registration: open science framework
Citation: Zhang L, Li H, Hu L, Ou X, Tan H, Zhang X, Lau CT, Lyu A, Bian Z and Zhang X (2024) Reporting characteristics and quality of randomized controlled trial protocols in traditional Chinese medicine: a cross-sectional study. Front. Pharmacol. 15:1389808. doi: 10.3389/fphar.2024.1389808
Received: 23 February 2024; Accepted: 14 May 2024;
Published: 07 June 2024.
Edited by:
Alexander George Panossian, Phytomed AB, SwedenCopyright © 2024 Zhang, Li, Hu, Ou, Tan, Zhang, Lau, Lyu, Bian and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xuan Zhang, zhangxuan87418@126.com
†These authors share first authorship
 Lin Zhang1†
Lin Zhang1† Xuan Zhang
Xuan Zhang