
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pharmacol., 05 June 2024
Sec. Pharmacoepidemiology
Volume 15 - 2024 | https://doi.org/10.3389/fphar.2024.1385036
Background: Lorlatinib displays marked systemic and intracranial efficacy against anaplastic lymphoma kinase (ALK) positive non-small cell lung cancer (NSCLC). We aimed to establish the safety profile of lorlatinib based on the Food and Drug Administration Adverse Event Reporting System (FAERS).
Methods: Reports from the FAERS between 2019 and 2023 were collected to conduct the disproportionality analysis. Reporting odds ratio (ROR) was employed to detect the potential adverse events (AEs) related to lorlatinib. The clinical characteristics, age and gender differences, time to onset of AEs were also investigated.
Results: A total of 2,941 AE reports were found to be associated with lorlatinib among the 8,818,870 AE reports obtained from the FAERS database. 167 lorlatinib-related AE signals were identified. The frequently reported AEs including hypercholesterolemia, oedema, and cognitive disorder were in line with those observed in clinical trials and drug instruction. However, AEs such as interstitial lung disease and AV block indicated in the drug label require further evaluation. More attention should be paid to the new potential unexpected AEs including pulmonary arterial hypertension and radiation necrosis. Furthermore, we examined the specific high-risk AEs of different ages and genders. In addition, majority of AEs occurred within the first 2 months after lorlatinib initiation with a median onset time of 51 days.
Conclusion: Our study provides valuable insight into the post-marketing safety profile of lorlatinib, which can potentially benefit the rational and safe administration of lorlatinib in the clinic. Further prospective studies are needed to validate the associations between lorlatinib and the identified AEs.
Lung cancer accounts for 13% of total cancer-related deaths globally and has become the leading cause of cancer-related mortality (Ando et al., 2021). Non-small cell lung cancer (NSCLC) that was often complicated with brain metastases accounted for 85%–90% of all lung cancers (Yang and Gong, 2019). Approximately 3%–5% of patients with NSCLC belonged to anaplastic lymphoma kinase (ALK)-positive NSCLC which provided opportunities for targeted therapy (Chen et al., 2021). The first- and second-generation ALK-tyrosine kinase inhibitors (TKIs) such as crizotinib and alectinib have shown promising efficacy against ALK-positive NSCLC. However, these therapies have been frequently hampered by the acquired drug resistance and progression of central nervous system (CNS) metastases (Reed et al., 2020).
As a third-generation ALK-TKI, lorlatinib was designed and developed with good penetration of the blood-brain barrier and broad-spectrum potency against most known resistance mutations during treatment with first- and second-generation ALK-TKIs (Reed et al., 2020). Lorlatinib has shown marked systemic and intracranial activity in patients with ALK-positive advanced NSCLC who were treatment-naive or who had progressed on a range of prior ALK-TKIs (Shaw et al., 2017; Shaw et al., 2020). Lorlatinib has been approved by Food and Drug Administration (FDA) in November 2018 and later by European Medicines Agency (EMA) in March 2019. Despite being generally well tolerated, lorlatinib has a unique and challenging safety profile compared with other ALK-TKIs that is characterized by hyperlipidemia, cognitive effects, mood effects, oedema, and peripheral neuropathy which are documented by both FDA and EMA. Uncommon yet serious adverse events (AEs) including pneumonitis have been reported as well (Harrison et al., 2022). Lorlatinib-related AEs are predominantly mild to moderate in severity and could be effectively managed with dose modification and standard supportive therapy. However, permanent discontinuation due to AEs including cognitive disorder and mood disorder occurred in 2%–7% of the patients in previous studies (Bauer et al., 2019; Shaw et al., 2020). Moreover, most evidence of lorlatinib-related AEs has been generally from clinical studies with strict inclusion criteria and limited sample sizes and case reports. With the increasing use of lorlatinib in clinical practice, a systemic and comprehensive evaluation of the safety profile of lorlatinib in real-world settings is urgently required.
The FDA Adverse Event Reporting System (FAERS) is a publicly accessible database that collects AE reports worldwide to support the post-marketing safety monitoring of drugs and therapeutic biologic products. In a recent post-marketing safety study of ALK inhibitors, metabolism disorders and neoplasms on the system organ class level and hypercholesterolemia and pulmonary arterial hypertension on the preferred term level were identified for lorlatinib (Omar et al., 2021). However, a detailed safety profile and early detection of new potential AEs for lorlatinib are still lacking. In the present study, we performed a pharmacovigilance analysis of lorlatinib based on the FAERS database and aimed to provide a thorough evaluation of lorlatinib-related AEs in real-world settings and promote the rational and safe use of lorlatinib in clinic.
The data of this pharmacovigilance study were collected from the FAERS from 2019 to 2023. Based on the FAERS, seven data files were provided: DEMO (demographic and administrative information), DRUG (information for drug administration), REAC (adverse event coded by preferred terms), OUTC (outcomes of patients), THER (therapy start dates and end dates for reported drugs), INDI (indications for drugs), and PRSR (report sources). These files were linked through the PRIMARYID field in each file. As the reports in FAERS were submitted spontaneously by healthcare professionals (physicians, pharmacists) and non-healthcare professionals (lawyers, consumers), there inevitably were duplicate reports. Following the FDA’s suggestions, we chose the latest FDA_DT when the PRIMARYIDs were the same, and chose the higher PRIMARYID when the FDA_DT and the CASEID were the same, to conduct de-duplication before data analysis. Both generic name (lorlatinib) and brand name (lorbrena) were used to obtain a comprehensive accounting of lorlatinib-associated reports. Only reports in which lorlatinib was considered the primary suspect (PS) drug with the ROLE_COD being “PS” were collected to improve accuracy. AEs in the FAERS database are coded by the preferred term (PT) and subsequently classified to the corresponding system organ classes (SOCs) based on the standardized Medical Dictionary for Regulatory Activities (MedDRA) terminology (version 26.1). Available information on age, sex, indication, reporter country, and patient outcomes was documented to characterize lorlatinib-related AEs in our analysis. Subgroup analysis was performed to investigate the potential difference in safety profiles of lorlatinib in subgroups based on age [aged younger than 18 (pediatric) and aged 18 and older (adult)] and gender (male and female). The onset time was calculated from the onset date of AE (EVENT_DT field in the DEMO file) minus the start date of lorlatinib treatment (START_DT field in the THER file). Reports with input errors (inaccurate entries, missing data, and EVENT_DT earlier than START_DT) were excluded.
In the current pharmacovigilance study, disproportionality analysis was applied to detect potential safety signals for lorlatinib. Reporting odds ratio (ROR) was calculated by standard formulas (Table 1) to investigate the associations between lorlatinib and AEs. Safety signals were considered significant when the lower border of 95% confidence interval exceeded 1.0 with at least 3 AE reports. p-value <0.05 was considered statistically significant. Data mining and statistical analysis were conducted by MYSQL 8.0 and Navicat Premium 15, SPSS 29.0, and Microsoft Excel 2019.
In the present study, 2941 lorlatinib-related reports were obtained from 8,181,870 AE reports collected from the FAERS database during the study period. The clinical characteristics of these reports were illustrated in Table 2. Females and males accounted for 45.70% and 37.47%, respectively. Lorlatinib-related AEs were more likely to occur in patients aged 18 years and older (65.62%). America submitted the most reports (39.65%), followed by Japan (13.60%), India (12.55%), France (4.32%), and Canada (3.91%). The most reported indication was lung neoplasm malignant (13.74%), followed by non-small cell lung cancer (13.33%), lung adenocarcinoma (3.06%), non-small cell lung cancer metastatic (2.86%), and lung cancer metastatic (2.52%), which was in line with the drug instructions. However, off-label uses of lorlatinib were also commonly reported such as neuroblastoma (1.60%) and adenocarcinoma (0.42%). Regarding the severe outcomes of the identified AEs, other medical events was most frequently reported (35.02%), followed by death (25.98%) and hospitalization (16.12%). AE reports gradually increased from 453 (15.40%) reports in 2019 to 911 (30.98%) reports in 2023.
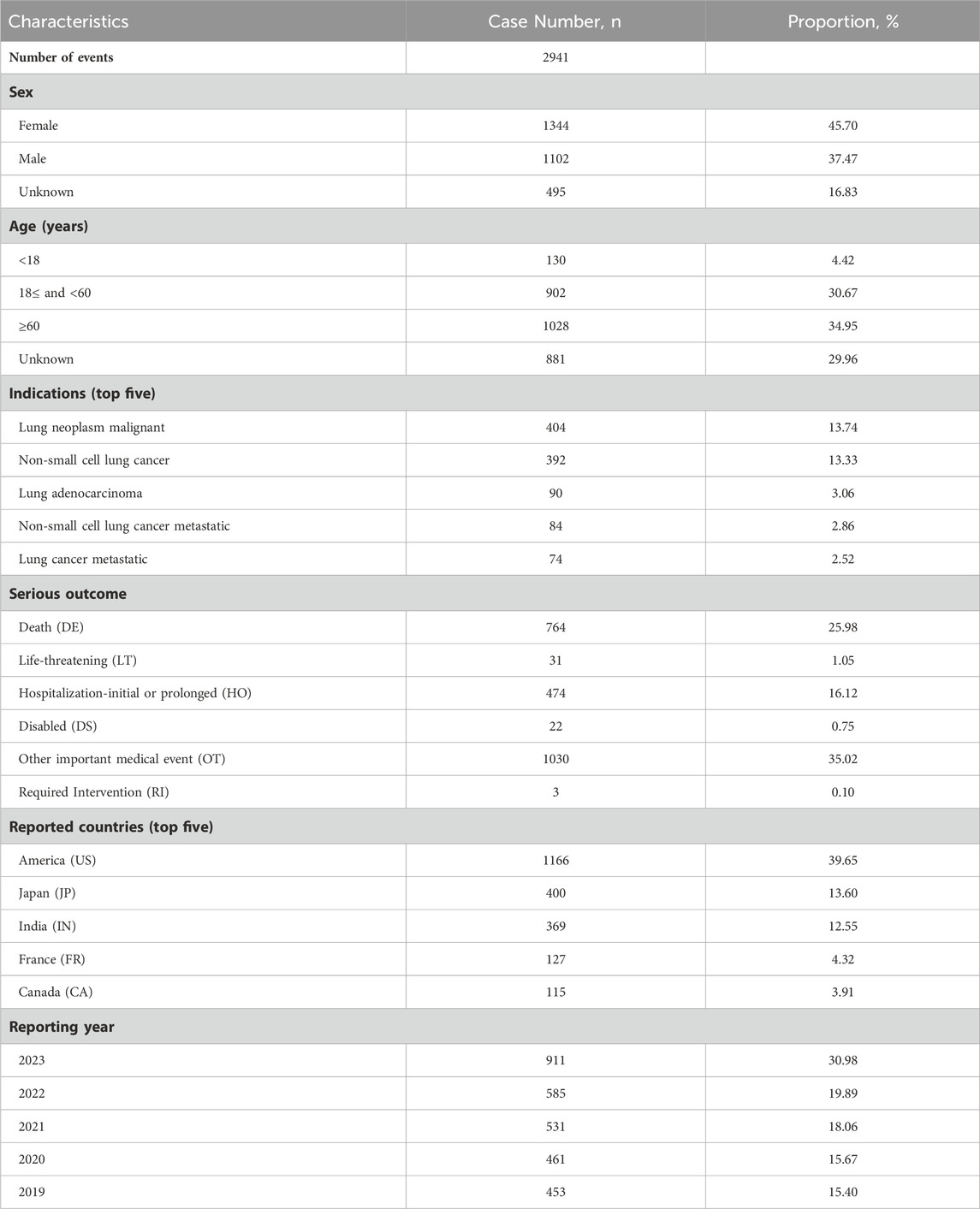
Table 2. Clinical characteristics of lorlatinib-related adverse event reports based on the FAERS database (2019–2023).
Signal detection results are present in Table 3 (Detail in Supplementary Table S1). A total of 167 lorlatinib-related safety signals across 20 SOCs were identified by disproportionality analysis. In our study, commonly reported AEs including hypercholesterolemia (PT: 10020603), hypertriglyceridemia (PT: 10020869), oedema (PT: 10030095), and cognitive disorder (PT: 10057668) were identified which was consistent with the drug instructions and clinical studies. New and unexpected AEs that were not included in the drug label were also observed, such as thrombophlebitis migrans (PT: 10043581), radiation necrosis (PT: 10067362), and pericardial effusion (PT: 10034474). Of note, although thrombophlebitis migrans (n = 6, ROR 64.80) and radiation necrosis (n = 8, ROR 78.00) had small numbers of cases, they showed high signal values, which required further study. Additionally, AEs that are indicated in the label including arthralgia, constipation, and back pain did not meet the criteria for positive signals in our analysis.
Among the top 15 AEs, dyspnoea, hallucination, pleural effusion, and pneumonia that were commonly reported in the subgroup aged 18 and older were not observed in the subgroup of those aged younger than 18. On the other hand, cognitive disorder, febrile neutropenia, mental disorder, and gait disturbance were only reported in the subgroup aged younger than 18 (Table 4). Furthermore, some AEs such as death, dyspnoea, and pleural effusion were more likely to occur in adult patients. High risk AEs in pediatric patients included hydrocephalus, psychotic symptom, and liver function test increased (Table 5, Detail in Supplementary Table S2).
The top 15 AEs were generally similar between females and males. To be specific, blood triglycerides increased and memory impairment were found to be more frequently reported in females, while pneumonia and pyrexia were only reported in males (Table 6). In addition, generalized oedema, delusion, and language disorder were found to be more common in females. On the other hand, a higher incidence of blood urea increased, body mass index increased, and pleural thickening was observed in males compared to females (Table 7, Detail in Supplementary Table S3).
A total of 601 lorlatinib-related AEs with available onset times were included for analysis. The median onset time was 51 days (interquartile range [IQR] 13–152 days). As illustrated in Figure 1, more than half of the AE cases occurred within the first 1 (n = 242, 40.27%) and 2 (n = 80, 13.31%) months after lorlatinib initiation. Of note, a small proportion of AEs (n = 71, 11.81%) still occurred after 1 year of lorlatinib treatment possibly due to the long report period.
As a third-generation ALK-TKI, lorlatinib has a distinct safety profile that is different from those of other ALK-TKIs, including hyperlipidemia, peripheral neuropathy, and central nervous system AEs. In the present study, we conducted a comprehensive pharmacovigilance analysis of the post-marketing safety profile of lorlatinib based on the FAERS database. We identified new potential AEs related to lorlatinib, compared the age and gender differences, and evaluated the onset time of lorlatinib-related AEs. Our results may provide valuable information for the update of drug label and the rational use of lorlatinib in clinical practice.
In the present disproportionality analysis, the most common AEs including hypercholesterolemia, hypertriglyceridemia, oedema, cognitive effects, mood effects, and peripheral neuropathy were in line with the drug instructions and previous studies (Syed, 2019). Further analysis showed that death and neoplasm progression which were not recorded in the drug instruction were reported with the most cases. Similarly, progression of neoplasms in neoplasms was identified as the most frequently reported adverse event for lorlatinib in a recent post-marketing safety study (Omar et al., 2021). Moreover, no lorlatinib-related deaths were reported in previous studies (Shaw et al., 2017; Solomon et al., 2018). Therefore, the frequently reported death and neoplasm progression might be attributed to the characteristics of metastatic NSCLC itself or the patient disease progression rather than lorlatinib.
Lung cancer is associated with a high incidence of brain metastases. Lorlatib was designed to be CNS penetrant and indeed had shown marked intracranial activity in preclinical and clinical studies (Zou et al., 2015; Shaw et al., 2017). However, due to the high brain penetrance and resulting accumulation in the CNS, AEs in nervous system disorders (including memory impairment, cognitive disorder, and amnesia) and psychiatric disorders (including hallucination, confusional state, and affect lability) were frequently reported which were also confirmed in our study. Indeed, CNS AEs occurred in a significantly higher proportion of patients who received lorlatinb (35%) compared to patients who received crizotinib (11%) (Solomon et al., 2022). CNS AEs were mainly mild in severity and improved on dose modifications. Of note, cognitive effects were the most common AEs that led to permanent discontinuation (Yang and Gong, 2019). Therefore, patients should be advised to report any changes in cognitive function, mood, speech, and mental status during treatment with lorlatinib.
Interstitial lung disease (ILD) was a serious adverse event associated with ALK-TKIs with a global incident rate of 2.1% (Suh et al., 2019; Zhao et al., 2023). A recent pharmacovigilance study reported that lorlatinib was statistically associated with ILD and resulted in a high death rate (Zhao et al., 2023). Nevertheless, in the present study, we identified 9 cases of ILD without a positive signal (ROR 1.10, 0.57-2.12). The possible difference might be due to the different data extraction methods. In Min Zhao’s study, ILD was a standardized MedDRA query containing 45 terms at the PT level. However, we focused on the specific term of ILD which might provide a more accurate result. Moreover, our results identified pneumonia (ROR 1.28, 1.01-1.63) and pneumonitis (ROR 5.83, 4.02-8.46) as positive signals with 70 cases and 28 cases, respectively. Interestingly, a recent report presented a case of successful switch to lorlatinib treatment without ILD relapse after the onset of drug-induced ILD caused by alectinib (Myall et al., 2021; Kashizaki et al., 2022). Considering that ILD was indicated in the drug label, the association between lorlatinib and ILD and the safety and administration strategy of lorlatinib in clinical patients require further investigation.
Our analysis identified pulmonary arterial hypertension (PAH) as a positive signal associated with lorlatinib. Consistently, PAH was found to be significantly related to lorlatinib in a recent pharmacovigilance analysis using FAERS (Syed, 2019). Of note, Chabrol et al. (2018) reported that two patients with metastatic NSCLC developed severe PAH within 2 months after lorlatinib treatment, and dramatically improved after lorlatinib withdrawal. Although PAH is not included in the drug insert of lorlatinib so far, our results suggest that more attention should be paid to the potential risk of PAH during lorlatinib treatment and cardiovascular evaluation could be conducted before lorlatinib introduction.
Among the SOC of cardiac disorders, we found 7 significant PTs: 1 PT included in the label and 6 new unexpected PTs not included in the label. Interestingly, 4 new unexpected PTs (cardiac failure, pericardial effusion, cardiac tamponade, and cardiomyopathy) also had positive signals in a previous pharmacovigilance study that focused on the ALK-TKI-associated cardiotoxicity (Liu et al., 2022). It is worth noting that pericardial effusion and cardiac tamponade were life-threatening complications of NSCLC, and severe pericardial effusion was most likely caused by advanced cancer itself (Wang et al., 2000; Mufti et al., 2018). Moreover, the symptoms of pericardial effusion could be effectively improved by other ALK-TKIs (Gadgeel et al., 2014; Matsuo et al., 2016). Currently, the associations between lorlatinib and pericardial effusion and cardiac tamponade are not clear. In addition, we identified 3 cases of AV block, 1 case of AV block first degree, and 1 case of AV block second degree in the present study. However, none of them met the criteria for positive signals. Consistently, no positive signal of AV block was detected in previous pharmacovigilance analysis of FAERS data (Syed, 2019; Liu et al., 2022). In a phase I/II study, no AV block was reported in healthy volunteers who were administrated lorlatinib. However, of the 295 patients treated with lorlatinib, 2 cases of asymptomatic first-degree AV block and 1 case of complete AV block were observed. Moreover, the patient who reported complete AV block had pre-existing second-degree AV block (Bauer et al., 2019). Of note, the FDA drug instruction indicated lorlatinib-induced AV block, but not lorlatinib-induced cardiomyopathy. Our study suggested that further studies were required to confirm the cardiac AEs caused by lorlatinib.
For radiation necrosis, our analysis found a significant signal (ROR 78.00, 38.60-157.63) with lorlatinib though only 8 cases of radiation necrosis were identified. Viola et al. reported two cases of radiation necrosis developed shortly after lorlatinib initiation following progression on the sequential treatment of crizotinib, alectinib, and brigatinib (Zhu et al., 2020). Considering that radiation treatment and NSCLC were identified as risk factors for developing radiation necrosis (Miller et al., 2016; Vellayappan et al., 2018), there remains a possibility that lorlatinib might accelerate the development of radiation necrosis that pre-existed before lorlatinib treatment. Interestingly, Ou et al. (2016) reported a case that radiation necrosis occurred 7 years after the last radiation treatment and 1 year after alectinib introduction. The results of our study and several case reports indicated that radiation necrosis might be a potentially rare but clinically meaningful AE related to lorlatinib. Large-scale studies are needed to recognize whether radiation necrosis is the result of inherent tumor biology, radiation treatment, lorlatinib treatment, or a combination of these factors.
It is well known that age and gender have relevant impacts on the bioavailability, metabolism, and elimination of drugs, leading to different efficacy and AEs (Farkouh et al., 2020; van Kampen et al., 2023). Currently, the report of age-specific and gender-specific AEs related to lorlatinib is unclear. In the present study, we observed a markedly higher number of positive signals in the subgroup aged 18 and older compared to the subgroup aged younger than 18. Moreover, AEs such as pleural effusion and pneumonia were only identified in the subgroup aged 18 and older which might be due to the increasing incidence of pneumonia with age (Henig and Kaye, 2017). Interestingly, pneumonia was more commonly reported in males which might be associated with their longer airways compared to females (Talaminos Barroso et al., 2018). Our results would provide important information for detailed clinical management based on specific subgroup characteristics. Further studies are required to confirm the age and gender differences in the safety profile of lorlatinib for better drug regimens and optimal drug therapy.
Our results indicated that more than half of lorlatinib-related AEs occurred within the first 2 months after the lorlatinib medication with a median time to onset of 51 days. This was compatible with the results from previous studies that lorlatinib-related hyperlipidemia, CNS effects, weight increase, edema, and peripheral neuropathy occurred with a median time to onset of 15 days, 42 days, 64 days, 42 days, and 77 days, respectively (Bauer et al., 2019). In addition, 11.81% of lorlatinib-related AEs still occurred after 365 days. Therefore, the long-term safety of lorlatinib deserves more attention in future clinical studies.
There are several limitations in our current study. First, as the FAERS database is a spontaneous reporting system, potential reporting bias, under-reporting, and over-reporting probably exist. The AE information might be incomplete, inaccurate, and heterogeneous due to the different reporters (professionals and non-professionals). Further, the underlying confounding factors such as drug-drug interactions and patient medication history were unclear. Therefore, a causal relationship between lorlatinib and AE reports could not be established based on our disproportionality analysis. Second, though our present study provided the statistical association between lorlatinib and AEs, the incidence of AEs related to lorlatinib could not be calculated as the total number of cases that occurred in the population was unknown according to the FAERS database. However, our study provided a comprehensive analysis of the safety profile of lorlatinib in real-world settings. In addition, new unexpected AEs such as radiation necrosis were also identified in our study. Based on our results, a general framework of “prepare, monitor, manage, reassess” could be followed to provide practical, actionable, and personalized patient care. Clinicians should keep in mind the likelihood and nature of lorlatinib-related AEs and encourage patients to report any symptoms during lorlatinib treatment. Patients should undergo a series of baseline tests such as biochemical analysis, cardiovascular risk evaluation, neurological evaluation, and contrast-enhanced magnetic resonance imaging of the brain before lorlatinib treatment. Our study added valuable information for the evaluation of the safety profile of lorlatinib in clinical practice.
In conclusion, the present study performed a comprehensive pharmacovigilance analysis of lorlatinib based on the FAERS database. AEs such as ILD and AV block indicated in the drug label require further evaluation. More attention should be paid to the new potential unexpected AEs including PAH and radiation necrosis. Additionally, the potential age and gender differences should be considered for optimal drug therapy in clinical practice. Our study provides valuable information for the rational and safe administration of lorlatinib in the clinic. Further prospective studies are required to confirm the associations between lorlatinib and the identified AEs.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethical approval was not required for the study involving humans in accordance with the local legislation and institutional requirements. Written informed consent to participate in this study was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and the institutional requirements.
HL: Conceptualization, Writing–original draft. CW: Formal Analysis, Writing–original draft. CG: Supervision, Writing–review and editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2024.1385036/full#supplementary-material
AE, adverse event; ALK, anaplastic lymphoma kinase; CI, confidence interval; CNS, central nervous system; EMA, European Medicines Agency; FAERS, Food and Drug Administration Adverse Event Reporting System; FDA, Food and Drug Administration; ILD, interstitial lung disease; IQR, interquartile range; MedDRA, Medical Dictionary for Regulatory Activities; NSCLC, non-small cell lung cancer; PAH, pulmonary arterial hypertension; PS, primary suspect; PT, preferred term; ROR, reporting odds ratio; SOC, system organ classes; TKI, tyrosine kinase inhibitor.
Ando, K., Manabe, R., Kishino, Y., Kusumoto, S., Yamaoka, T., Tanaka, A., et al. (2021). Comparative efficacy and safety of lorlatinib and alectinib for ALK-rearrangement positive advanced non-small cell lung cancer in asian and non-asian patients: a systematic review and network meta-analysis. Cancers (Basel) 13 (15), 3704. doi:10.3390/cancers13153704
Bauer, T. M., Felip, E., Solomon, B. J., Thurm, H., Peltz, G., Chioda, M. D., et al. (2019). Clinical management of adverse events associated with lorlatinib. Oncologist 24 (8), 1103–1110. doi:10.1634/theoncologist.2018-0380
Chabrol, A., Mayenga, M., Hamid, A. M., Friard, S., Salvator, H., Doubre, H., et al. (2018). Lorlatinib - induced pulmonary arterial hypertension. Lung Cancer 120, 60–61. doi:10.1016/j.lungcan.2018.03.023
Chen, J., Ruiz-Garcia, A., James, L. P., Peltz, G., Thurm, H., Clancy, J., et al. (2021). Lorlatinib exposure-response analyses for safety and efficacy in a phase I/II trial to support benefit-risk assessment in non-small cell lung cancer. Clin. Pharmacol. Ther. 110 (5), 1273–1281. doi:10.1002/cpt.2228
Farkouh, A., Riedl, T., Gottardi, R., Czejka, M., and Kautzky-Willer, A. (2020). Sex-related differences in pharmacokinetics and pharmacodynamics of frequently prescribed drugs: a review of the literature. Adv. Ther. 37 (2), 644–655. doi:10.1007/s12325-019-01201-3
Gadgeel, S. M., Gandhi, L., Riely, G. J., Chiappori, A. A., West, H. L., Azada, M. C., et al. (2014). Safety and activity of alectinib against systemic disease and brain metastases in patients with crizotinib-resistant ALK-rearranged non-small-cell lung cancer (AF-002JG): results from the dose-finding portion of a phase 1/2 study. Lancet Oncol. 15 (10), 1119–1128. doi:10.1016/s1470-2045(14)70362-6
Harrison, P. K., Boland, H. E., Aherne, N. J., and Palmieri, D. J. (2022). An unusual case of lorlatinib-induced pneumonitis: a case report. Case Rep. Oncol. 15 (1), 225–230. doi:10.1159/000520158
Henig, O., and Kaye, K. S. (2017). Bacterial pneumonia in older adults. Infect. Dis. Clin. North Am. 31 (4), 689–713. doi:10.1016/j.idc.2017.07.015
Kashizaki, F., Tanaka, A., and Sekido, Y. (2022). Efficacy of lorlatinib after alectinib-induced interstitial lung disease in a patient with anaplastic lymphoma kinase-positive non-small cell lung cancer: a case report. J. Med. Case Rep. 16 (1), 316. doi:10.1186/s13256-022-03556-8
Liu, Y., Chen, C., Rong, C., He, X., and Chen, L. (2022). Anaplastic lymphoma kinase tyrosine kinase inhibitor-associated cardiotoxicity: a recent five-year pharmacovigilance study. Front. Pharmacol. 13, 858279. doi:10.3389/fphar.2022.858279
Matsuo, N., Sekine, A., Kato, T., Hosoda, C., Ito, H., Baba, T., et al. (2016). Promising effect of crizotinib on anaplastic lymphoma kinase (ALK)-Positive non-small cell lung cancer in an elderly patient with a poor performance status: a case report and literature review. Intern Med. 55 (5), 507–509. doi:10.2169/internalmedicine.55.5076
Miller, J. A., Bennett, E. E., Xiao, R., Kotecha, R., Chao, S. T., Vogelbaum, M. A., et al. (2016). Association between radiation necrosis and tumor biology after stereotactic radiosurgery for brain metastasis. Int. J. Radiat. Oncol. Biol. Phys. 96 (5), 1060–1069. doi:10.1016/j.ijrobp.2016.08.039
Mufti, M., Ching, S., Farjami, S., Shahangian, S., and Sobnosky, S. (2018). A case series of two patients presenting with pericardial effusion as first manifestation of non-small cell lung cancer with BRAF mutation and expression of PD-L1. World J. Oncol. 9 (2), 56–61. doi:10.14740/wjon1092w
Myall, N. J., Lei, A. Q., and Wakelee, H. A. (2021). Safety of lorlatinib following alectinib-induced pneumonitis in two patients with ALK-rearranged non-small cell lung cancer: a case series. Transl. Lung Cancer Res. 10 (1), 487–495. doi:10.21037/tlcr-20-564
Omar, N. E., Fahmy Soliman, A. I., Eshra, M., Saeed, T., Hamad, A., and Abou-Ali, A. (2021). Postmarketing safety of anaplastic lymphoma kinase (ALK) inhibitors: an analysis of the FDA Adverse Event Reporting System (FAERS). ESMO Open 6 (6), 100315. doi:10.1016/j.esmoop.2021.100315
Ou, S. H., Weitz, M., Jalas, J. R., Kelly, D. F., Wong, V., Azada, M. C., et al. (2016). Alectinib induced CNS radiation necrosis in an ALK+NSCLC patient with a remote (7 years) history of brain radiation. Lung Cancer 96, 15–18. doi:10.1016/j.lungcan.2016.03.008
Reed, M., Rosales, A. S., Chioda, M. D., Parker, L., Devgan, G., and Kettle, J. (2020). Consensus recommendations for management and counseling of adverse events associated with lorlatinib: a guide for healthcare practitioners. Adv. Ther. 37 (6), 3019–3030. doi:10.1007/s12325-020-01365-3
Shaw, A. T., Bauer, T. M., de Marinis, F., Felip, E., Goto, Y., Liu, G., et al. (2020). First-line lorlatinib or crizotinib in advanced ALK-positive lung cancer. N. Engl. J. Med. 383 (21), 2018–2029. doi:10.1056/NEJMoa2027187
Shaw, A. T., Felip, E., Bauer, T. M., Besse, B., Navarro, A., Postel-Vinay, S., et al. (2017). Lorlatinib in non-small-cell lung cancer with ALK or ROS1 rearrangement: an international, multicentre, open-label, single-arm first-in-man phase 1 trial. Lancet Oncol. 18 (12), 1590–1599. doi:10.1016/s1470-2045(17)30680-0
Solomon, B. J., Bauer, T. M., Ignatius Ou, S. H., Liu, G., Hayashi, H., Bearz, A., et al. (2022). Post hoc analysis of lorlatinib intracranial efficacy and safety in patients with ALK-positive advanced non-small-cell lung cancer from the phase III CROWN study. J. Clin. Oncol. 40 (31), 3593–3602. doi:10.1200/jco.21.02278
Solomon, B. J., Besse, B., Bauer, T. M., Felip, E., Soo, R. A., Camidge, D. R., et al. (2018). Lorlatinib in patients with ALK-positive non-small-cell lung cancer: results from a global phase 2 study. Lancet Oncol. 19 (12), 1654–1667. doi:10.1016/s1470-2045(18)30649-1
Suh, C. H., Kim, K. W., Pyo, J., Hatabu, H., and Nishino, M. (2019). The incidence of ALK inhibitor-related pneumonitis in advanced non-small-cell lung cancer patients: a systematic review and meta-analysis. Lung Cancer 132, 79–86. doi:10.1016/j.lungcan.2019.04.015
Syed, Y. Y. (2019). Lorlatinib: first global approval. Drugs 79 (1), 93–98. doi:10.1007/s40265-018-1041-0
Talaminos Barroso, A., Márquez Martín, E., Roa Romero, L. M., and Ortega Ruiz, F. (2018). Factors affecting lung function: a review of the literature. Arch. Bronconeumol Engl Ed. 54 (6), 327–332. doi:10.1016/j.arbres.2018.01.030
van Kampen, E., van Bussel, M. T. J., Oude Munnink, T. H., Touw, D. J., and Broekman, K. E. (2023). Representation of older patients in the safety analysis of protein kinase inhibitor registration studies. J. Geriatr. Oncol. 14 (8), 101636. doi:10.1016/j.jgo.2023.101636
Vellayappan, B., Tan, C. L., Yong, C., Khor, L. K., Koh, W. Y., Yeo, T. T., et al. (2018). Diagnosis and management of radiation necrosis in patients with brain metastases. Front. Oncol. 8, 395. doi:10.3389/fonc.2018.00395
Wang, P. C., Yang, K. Y., Chao, J. Y., Liu, J. M., Perng, R. P., and Yen, S. H. (2000). Prognostic role of pericardial fluid cytology in cardiac tamponade associated with non-small cell lung cancer. Chest 118 (3), 744–749. doi:10.1378/chest.118.3.744
Yang, J., and Gong, W. (2019). Lorlatinib for the treatment of anaplastic lymphoma kinase-positive non-small cell lung cancer. Expert Rev. Clin. Pharmacol. 12 (3), 173–178. doi:10.1080/17512433.2019.1570846
Zhao, M., Liu, S., Xie, R., Zhang, J., and Li, J. (2023). Interstitial lung disease risk of anaplastic lymphoma kinase tyrosine kinase inhibitor treatment of non-small cell lung cancer: a real-world pharmacovigilance study. Expert Opin. Drug Saf. 22, 1309–1316. doi:10.1080/14740338.2023.2245324
Zhu, V. W., Nagasaka, M., Kubota, T., Raval, K., Robinette, N., Armas, O., et al. (2020). Symptomatic CNS radiation necrosis requiring neurosurgical resection during treatment with lorlatinib in ALK-rearranged NSCLC: a report of two cases. Lung Cancer (Auckl) 11, 13–18. doi:10.2147/lctt.S224991
Keywords: lorlatinib, pharmacovigilance, disproportionality, adverse event, FAERS
Citation: Li H, Wang C and Guo C (2024) Post-marketing safety of lorlatinib: a real-world study based on the FDA adverse event reporting system. Front. Pharmacol. 15:1385036. doi: 10.3389/fphar.2024.1385036
Received: 26 February 2024; Accepted: 21 May 2024;
Published: 05 June 2024.
Edited by:
Anoop Kumar, Delhi Pharmaceutical Sciences and Research University, IndiaReviewed by:
Walid Shalata, Soroka Medical Center, IsraelCopyright © 2024 Li, Wang and Guo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Cuilian Guo, Z3VvY3VpbGlhbjIwMTlAaG90bWFpbC5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.