
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Pharmacol., 10 April 2024
Sec. Experimental Pharmacology and Drug Discovery
Volume 15 - 2024 | https://doi.org/10.3389/fphar.2024.1383000
This article is part of the Research TopicActivation of the cGAS-STING Pathway by Extra-Nuclear DNA and its Pharmacognostic Modulation in Human DiseaseView all 6 articles
In this review, we cover the current understanding of how radiation therapy, which uses ionizing radiation to kill cancer cells, mediates an anti-tumor immune response through the cGAS-STING pathway, and how STING agonists might potentiate this. We examine how cGAS-STING signaling mediates the release of inflammatory cytokines in response to nuclear and mitochondrial DNA entering the cytoplasm. The significance of this in the context of cancer is explored, such as in response to cell-damaging therapies and genomic instability. The contribution of the immune and non-immune cells in the tumor microenvironment is considered. This review also discusses the burgeoning understanding of STING signaling that is independent of inflammatory cytokine release and the various mechanisms by which cancer cells can evade STING signaling. We review the available data on how ionizing radiation stimulates cGAS-STING signaling as well as how STING agonists may potentiate the anti-tumor immune response induced by ionizing radiation. There is also discussion of how novel radiation modalities may affect cGAS-STING signaling. We conclude with a discussion of ongoing and planned clinical trials combining radiation therapy with STING agonists, and provide insights to consider when planning future clinical trials combining these treatments.
Radiation therapy is an integral component of cancer treatment and involves the use of ionizing radiation to destroy cancer cells; however, it can also induce significant tumor-directed immune responses (Demaria et al., 2004; Abuodeh et al., 2016). Immune activation could be used to potentiate radiotherapy, but unfortunately this process is not well understood (Ngwa et al., 2018). In this context, the cGAS-STING pathway has gained increasing attention, as it has been shown to promote an immune response to the DNA damaging agents used to treat cancer (Yum et al., 2020). The cGas-STING pathway is not only an important cellular defense mechanism against intracellular pathogens, generating pro-inflammatory cytokines in response to pathogen-derived cytosolic double-stranded DNA (dsDNA) (Kato et al., 2017), it is now appreciated for its role in several processes, including senescence, genomic instability, autophagy, and relevant to this review, the immunosurveillance of cancer (Beernaert and Parkes, 2023).
To discuss the role of cGAS-STING signaling in the tumor microenvironment, it is important to understand the overall pathway (Figure 1). Cyclic GMP-AMP synthase (cGAS) is a nucleotidyltransferase activated by binding dsDNA (Gao et al., 2013). cGAS has been shown to be predominantly localized to the plasma membrane in the resting state (Barnett et al., 2019), although there is also evidence for localization free in the cytoplasm, around micronuclei, or within nuclei in response to stress signals, like DNA damage (Liu H. et al., 2022). It combines guanine monophosphate (GMP) and adenosine monophosphate (AMP) to form cyclic GMP-AMP (cGAMP) (Sun et al., 2013), bound at the 3′ OH of AMP and 5′ phosphate of GMP at one end, and the 2′ OH of GMP and 5’ phosphate of AMP at the other end (Ablasser et al., 2013). Specifically, cGAS activation involves two strands of dsDNA binding with two cGAS proteins (Li et al., 2013; Zhang et al., 2014). cGAS can bind to RNA and single stranded DNA, but it is considered to be only significantly activated by dsDNA greater than around 45 base pairs, and is actually inhibited by dsDNA in the approximately 20–40 base pair range. (Chen et al., 2016; Liu et al., 2023b). The binding of cGAS is independent of dsDNA sequence, as it is due to the interactions between the protein and the sugar-phosphate backbone of dsDNA, but not the nitrogen base (Chen et al., 2016; Kato et al., 2017). However, the degree of cGAS activation upon binding DNA appears to be governed by the specific DNA sequence, its length, and any damage, due to the influence these factors can have on the mechanical flexibility of dsDNA, important for the optimal sensing of dsDNA by cGAS (Wang et al., 2022).
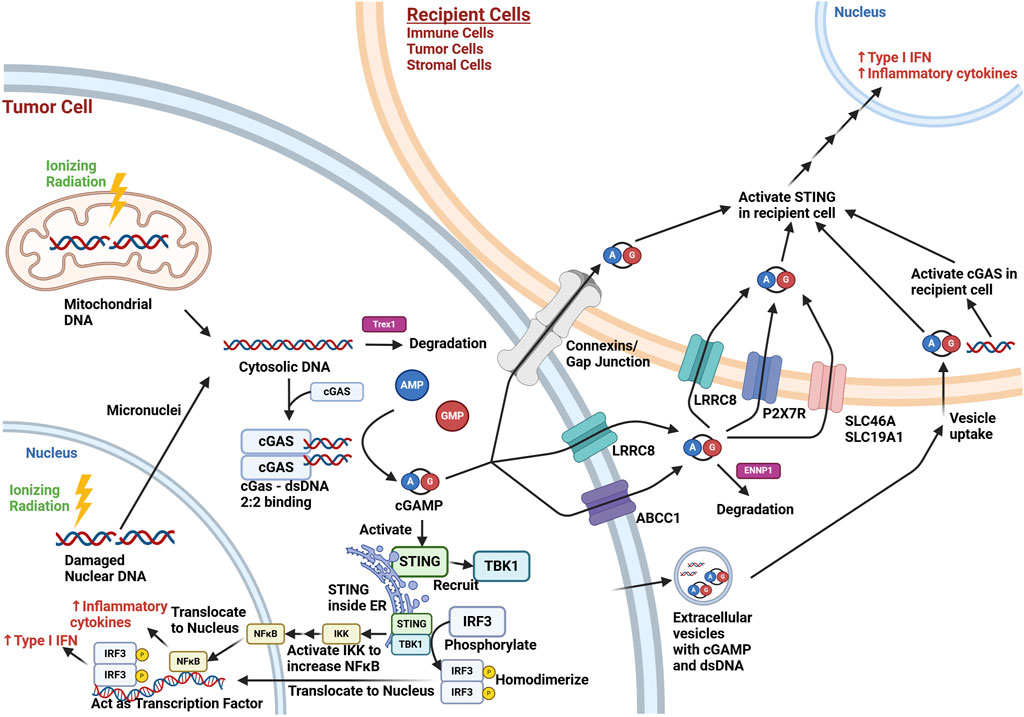
Figure 1. Overview of cGAS-STING Signaling in the Tumor Microenvironment after Ionizing Radiation Exposure. Ionizing radiation (lightning bolt) results in cytoplasmic release of mitochondrial dsDNA immediately and nuclear dsDNA eventually through micronuclei formation during cell division. This cytosolic dsDNA may be degraded by TREX1, a cytoplasmic endonuclease. cGAS activation involves two molecules of dsDNA bind to a dimer of cGAS (2:2 binding), which allows cGAS to form cGAMP from AMP and GMP. cGAMP intracellularly can activate STING in the endoplasmic reticulum (ER), which can then recruit TBK1. This complex brings in and phosphorylates IRF3, which then homodimerizes and translocates to the nucleus, where it acts as a transcription factor for the production of type I interferons. STING can also act through the NF-κB pathway to lead to increased inflammatory cytokine production (e.g., TNF-α, IL-1, IL-6, IL-8). cGAMP may also exit the cell via connexins/gap junctions, membrane transporters (e.g., LRCC8, ABCC1), or extracellular vesicles. dsDNA may also be contained in extracellular vesicles. Extracellular cGAMP not contained in vesicles is exposed to possible degradation by ENNP1, an endonuclease. cGAMP may be taken up by cells in the microenvironment via connexins/gap junctions, membrane transporters (LRRC8, P2x7R, SCL46A, SCL19A1), or via uptake of extracellular vesicles. The cGAMP can activate STING in recipient cells, and dsDNA uptake can lead to STING activation through recipient cell cGAS activation. This results in the paracrine/autocrine production of type I interferons and inflammatory cytokines in the tumor microenvironment. While the irradiated cell in this schematic was set to be a tumor cell, other irradiated cells in the tumor microenvironment (e.g., immune and stromal cells) may undergo similar cGAS-STING pathway signaling. Created with BioRender.com.
The cGAMP produced by cGAS activates an endoplasmic reticulum protein called stimulator of interferon genes (STING) (Wu et al., 2013; Shang et al., 2019). The conformational change induced by cGAMP binding results in STING oligomerization, with migration towards the Golgi apparatus (Dobbs et al., 2015; Ergun et al., 2019). STING recruits TANK Binding Kinase 1 (TBK1), which leads to the phosphorylation of interferon regulatory factor 3 (IRF3) (Ishikawa et al., 2009; Zhao et al., 2016; Zhao et al., 2019). This allows IRF3 to homodimerize, translocate to the nucleus, and act as a transcription factor (Tanaka and Chen, 2012), promoting type I interferon (interferon α/β) production (Yum et al., 2021). TBK1 also leads to increased nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) signaling via activating the inhibitor of NF-κB (IκB) kinase (IKK) complex, which can phosphorylate IκB family proteins, releasing NF-κB for downstream signaling (Yum et al., 2021). As a nuclear transcription factor, NF-κB also increases inflammatory cytokines (e.g., TNF-α, IL-1, IL-6, IL-8) (Hoesel and Schmid, 2013). The activation of both IRF3 and NF-κB transcription factors results in a synergistic increase in downstream activation, making cGAS-STING a potent pro-inflammatory response pathway (Panne et al., 2007).
In this review, we will cover the effects of cGAS-STING signaling in the tumor microenvironment, with a focus on its role in the response of tumors to radiation therapy. We will also examine the current and future potential for drugs targeting the cGAS-STING pathway to potentiate radiation therapy.
Multiple studies have helped clarify the role of the cGAS-STING pathway in the tumor microenvironment. Most of the focus has been on cancer cells and immune cells; however, this pathway should be relatively agnostic to cell type (Motwani et al., 2019). For cells in the tumor microenvironment, DNA damaging treatments (Yang et al., 2017), such as chemotherapy or radiation, can generate dsDNA fragments, which can become cytosolic dsDNA. Cancer cells can also generate cytosolic dsDNA through genomic instability (Bakhoum et al., 2018). The sensing of this cytosolic dsDNA by cGAS leads to cGAMP production, which can have several different fates ultimately leading to the production of type I interferon in the tumor. For instance, cGAMP can activate STING signaling directly within tumor cells, leading to an inflammatory response through production of type 1 interferon (Kumar et al., 2023).
However, interferon production can also occur through STING activation in other cells in the tumor microenvironment (Mekers et al., 2022). One mechanism for this is the secretion of cGAMP, where it can act as a paracrine signal, activating STING in recipient cells. For example, cGAMP can be secreted and taken up by volume-regulated anion channels, such as the leucine-rich repeat containing protein (LRRC8) (Lahey et al., 2020; Zhou et al., 2020; Concepcion et al., 2022). ATP binding cassette subfamily C member 1 (ABCC1), known for contributing to chemotherapy resistance by transporting chemotherapeutics out of cancer cells, also has been shown to export cGAMP (Maltbaek et al., 2022). This cGAMP can be then be taken up by cells with the plasma membrane transporters solute carrier family 19 member 1 (SLC19A1) (Luteijn et al., 2019; Ritchie et al., 2019) or solute carrier family 46 (SLC46A) (Cordova et al., 2021), with species and cell type specificity. The purinergic P2X7 receptor (P2X7R), a ligand-gated ion channel, has also been shown to have a role in cGAMP uptake, though it is not clear if this is direct or through its association with the formation of transmembrane channels via pannexins (Iglesias et al., 2008). Others have shown that connexin proteins can play a role in the intercellular transfer of cGAMP(Schadt et al., 2019; Pépin et al., 2020). Connexins can form hemi-channels on the plasma membrane capable of secreting contents into the extracellular space as well as connect cells for direct transfer of molecules. Both functions may contribute to cGAMP transport. Extracellular vesicles are membrane bound particles secreted by cells either directly from the plasma membrane or by fusion of internal compartments with the plasma membrane, and have been shown to carry cGAMP(Tkach et al., 2022). It has also been reported that dsDNA is contained in extracellular vesicles, which may contribute to the cGAS-STING activation in recipient cells (Diamond et al., 2018). Similarly, activated STING has been shown to be released in the extracellular vesicles of tumor cells stimulated with a STING agonist, and these extracellular vesicles were shown to promote anti-tumor immunity in mouse tumor models (Gao et al., 2022). Extracellular cGAMP is degraded by the phosphodiesterase activity of ectonucleotide pyrophosphatase/phosphodiesterase 1 (ENPP1), limiting its effectiveness as a paracrine signal for an immune response (Li et al., 2014; Kato et al., 2018; Li J. et al., 2021). It has also been demonstrated that dying cancer cells release dsDNA that can be taken up by phagocytic cells in the tumor, leading to cGAS/STING activation (Klarquist et al., 2014).
Whether immune cells are recipients of tumor-derived cGAMP or have their cGAS directly activated, both pathways result in activation of STING leading to downstream IRF3 signaling. This activation of IRF3 by STING leads to type I interferon production, which mediates an important immune signaling response in the microenvironment. Type 1 interferon has been shown to mediate an anti-cancer immune response through signaling on the interferon α/β receptor 1 (IFNAR1) on dendritic cells (Diamond et al., 2011). Specifically, the subset of dendritic cells that are CD8α+ appears to be important for mediating this effect (Fuertes et al., 2011), as these cells perform antigen cross presentation and priming of CD8+ T-cells, which are responsible for the cytotoxic anti-tumor immune response (Woo et al., 2014). Interestingly, there is evidence that acute type I interferon responses lead to these immunostimulatory responses, while persistent signaling can lead to a suppressive phenotype through immune exhaustion (Boukhaled et al., 2021). It is also important to remember that the immune response from cGAS-STING signaling may not be only due to cytokines and interferon signaling for a cytotoxic T-cell response. For instance, lymphoma cells have been shown to increase expression of retinoic acid early transcript 1 (RAE1) ligands in response to cGAS-STING signaling from cytosolic dsDNA, which targets them for elimination by NK cells (Lam et al., 2014). Considering the relative contribution of the different cellular subsets in the tumor microenvironment, it should be noted that the CD45+ immune cells, specifically the dendritic cell subset, have been shown to contribute to most of the type I interferon production in tumors (Deng et al., 2014; Schadt et al., 2019).
However, non-immune elements in the tumor microenvironment may also influence the immune response. In one model, endothelial cells were shown to be important in responding to cGAMP in the tumor microenvironment by producing interferon-β(Demaria et al., 2015). Similarly, cancer-associated fibroblasts (CAFs) can have STING activation leading to interferon release, contributing to the immune response (Arwert et al., 2020). The significance of CAFs is difficult to assess, as it has been shown that tumor-promoting CAFs(Ma et al., 2022) and tumor-suppressive CAFs(Kabashima et al., 2022) can be induced from cGAS-STING signaling in the cancer cells, while others have shown that a STING agonist can contribute to downregulation of CAFs as part of the immune response (Hajiabadi et al., 2023). Non-cellular elements in the tumor can also participate in the immune response. For example, the extracellular matrix has been shown to signal for an immunosuppressive phenotype in tumor cells via mechanotransduction from stress fibers leading to autophagic degradation of cGAS (Liu et al., 2023c).
It is important to recognize that cancer cells can circumvent cGAS-STING signaling and the subsequent immune response through a number of mechanisms. A study evaluating The Cancer Genome Atlas (TCGA) and other human tumor genome databases revealed that tumors frequently contain loss-of-function mutations as well as epigenetic silencing of cGAS and STING (Konno et al., 2018). For example, loss of liver kinase B1 (LKB1) was shown in the context of KRAS-driven lung cancers to silence STING expression (Kitajima et al., 2019). At the protein level, post-translational modifications, such as ubiquitination, acetylation, phosphorylation, and SUMOylation, can also modify the activity of STING and cGAS (Liu J. et al., 2022). Even mutations in tumor protein 53 (p53) can block STING signaling (Ghosh et al., 2021). Another mechanism of suppressing STING signaling is upregulation of three prime repair exonuclease 1 (TREX1), an enzyme that can prevent cGAS activation by degrading cytosolic dsDNA (Vanpouille-Box et al., 2017). Persistent cGAS-STING signaling, as happens in cancers with chromosomal instability, can lead to decreased interferon release via reduced STING levels as a form of tachyphylaxis (Li et al., 2023). STING activation has also been shown to increase PD-L1 expression in cervical cancer cells through the NF-κB pathway, which could be clinically meaningful if this translated into improved immune evasion, and would suggest a benefit for pharmaceuticals targeting the STING pathway to be given with PD-L1 inhibitors (Cai et al., 2020). There is also evidence that cancer cells can respond to the type I interferon in their environment, leading to resistance to cytotoxic T-cells via inhibition of granzyme B (Chen et al., 2019) and increased PD-L1 levels (Jacquelot et al., 2019).
Taken together, these interactions demonstrate the complex interplay between cGAS-STING signaling and the tumor microenvironment, as well as the significance of these factors when considering pharmacologic interventions.
In addition to its central role in the immune response to cancer, STING signaling has been implicated in processes independent of its immunostimulatory effect. For example, the type I interferons released as a result of STING signaling may elicit a pro-apoptotic and anti-proliferative autocrine/paracrine response in some cancer cells. Studies of exogenous administration of type I interferons in vitro have demonstrated that these effects of interferons are variable based on cancer cell origin, mutations, and interferon type (Rodríguez-Villanueva and McDonnell, 1995; Chawla-Sarkar et al., 2001). There is also evidence for more direct STING induction of apoptosis through IRF3 activation leading to loss of mitochondrial outer membrane permeability (Zierhut et al., 2019). Cancer cells may in part develop resistance to interferon-induced apoptosis through mutations in the apoptotic pathways downstream of the interferon-α,β receptor (Chawla-Sarkar, 2003). Interferons have also been shown to act as a pro-survival signal for some cancer cells, providing resistance to chemotherapy (Gaston et al., 2016) and radiation (Khodarev et al., 2004) in vitro. These findings suggest that while interferon signaling can induce apoptosis in sensitive cancer cells, apoptosis-resistant cancer cells may develop pro-survival signaling through signal transducer and activator of transcription 1 (STAT1) or other pathways. Other cell death and signaling pathways may be involved as well, although the bulk of the literature focuses on how interferon signaling affects apoptosis. For instance, colon cancer cells have been shown to undergo necroptosis in response to autocrine/paracrine interferon resulting from cGAS-STING signaling (Chen et al., 2018). Ultimately, the biological relevance of these direct effects of interferons on cancer cells is not clear, as there appears to be variability in the extent to which cancer cells express interferon receptors (Yano et al., 1999).
The activation of STING in cancer cells may also affect the survival response independent of downstream interferon production. For instance, the cGAS-STING pathway can activate autophagy, which can reduce cytosolic dsDNA and promote cell survival (Gui et al., 2019). However, STING signaling was also found to result in ferroptotic cell death in pancreatic cancer cells in an interferon-independent manner (Li C. et al., 2021). Cheradame et al. found increased DNA damage by the comet assay and decreased clonogenic survival of breast cancer cells to mafosfamide or radiation in the context of STING knockdown (Cheradame et al., 2021). This was independent of silencing IFNAR1, suggesting it is independent of interferon signaling. Knockdown of STING in multiple patient-derived xenografts resulted in reduced cell viability even in the absence of genotoxic stress. In contrast to these findings, Haymen et al. found that STING knockout decreased damage by comet assay and increased clonogenic survival of prostate cancer cells to cisplatin or radiation (Hayman et al., 2021). They demonstrated in nude mice that prostate cancer cells with STING knockout injected subcutaneously were more resistant to ionizing radiation, as assessed by tumor growth delay. The use of nude mice suggests this was independent of T cells. When attempting to reconcile these apparently contradictory studies, several factors should be considered. First, the use of different cell types can lead to different conclusions. Similarly, the difference between performing a gene knockdown or knock-out is significant, as signaling pathways can be sensitive to the difference between reduced versus no signaling. Lastly, Cheradame et al. found their effect was independent of paracrine/autocrine interferon signaling, while Hayman et al. noted that interferon-stimulated gene 15 levels were increased in response to radiation in the wild-type prostate cancer cells, but abrogated in the STING knockout cells, suggesting that the pro-survival effect of STING knockout they observed may be mediated by the tumor cells responding to STING-mediated interferon production.
Overall, this section emphasizes the challenges inherent in attempts to manipulate these signaling pathways for therapeutic benefit: The net effect of cancer cell intrinsic responses as well as tumor microenvironment and immune responses must be considered when evaluating whether a therapeutic intervention is expected to be beneficial.
Radiation therapy to treat cancer uses ionizing radiation to slow or prevent the growth and spread of tumors as well as microscopic neoplastic disease. According to classical radiobiology, the principal mechanism is through direct and indirect damage to genomic DNA in the nucleus causing preferential damage and killing of cancer cells; however, ionizing radiation also activates numerous signaling pathways and genetic programs in both neoplastic and non-neoplastic cells (Joiner and van der Kogel, 2018). Although the exact mechanism is a current area of investigation, one such byproduct of cellular irradiation is the generation of cytosolic dsDNA, which can be from nuclear (Mackenzie et al., 2017) or mitochondrial (Yamazaki et al., 2020) origin. The relative importance of these two sources of cytoplasmic dsDNA in initiating radiation-induced inflammation is an area of active research and controversy. Although nuclear dsDNA and micronuclei appear to correlate with radiation-induced inflammation, it may take days for this to stimulate a response, since mitotic progression is an important component of micronuclei formation (Harding et al., 2017). In contrast, mitochondrial dsDNA enters the cytosol within hours after irradiation (Patrushev et al., 2006), and therefore may potentially be more important for initial cGAS activation. Cytoplasmic dsDNA generated by radiation may be an especially potent inducer of cGAS signaling due to the oxidative damage and modifications caused by ionizing radiation (Goodhead, 1989), potentially making it more resistant to cytosolic nucleases like TREX1 (Gehrke et al., 2013). The resultant cGAMP leads to an inflammatory response that is thought to have a role in radiation-induced inflammation and the abscopal effect, wherein radiation treatment at one site of disease causes shrinking of distant sites via the immune system (Galluzzi et al., 2023).
In preclinical models, the anti-tumor immune effect of radiation appears dependent on STING activation leading to type I interferon production in dendritic cells. This can occur from cGAMP produced by cGAS directly in dendritic cells (Deng et al., 2014) or via cGAMP secreted from tumor cells (Schadt et al., 2019). The cGAMP released from cancer cells can be degraded by ENPP1 present in the microenvironment, limiting the relative contribution of cGAMP from the tumor cells (Carozza et al., 2020a). This may in part explain the difference in Deng et al. and Schadt et al. regarding the relative importance of cGAMP derived from cancer cells versus dendritic cells in mediating an anti-tumor immune response. dsDNA released by the tumor cells can also be taken up by dendritic cells, resulting in cGAS activation (Diamond et al., 2018; Fang et al., 2021). It has been shown that canonical NF-κB signaling in dendritic cells is important for the STING-dependent anti-tumor immunity seen with ionizing radiation, while non-canonical NF-κB signaling was found to be inhibitory (Hou et al., 2018). It was demonstrated in vivo that a non-canonical NF-κB pathway inhibitor could produce tumor growth delay with 20 Gy ionizing radiation in an interferon-dependent manner using an immunocompetent mouse with subcutaneous tumors (Hou et al., 2018). These results support the role of dendritic cells in promoting anti-tumor immunity in response to ionizing radiation. Interestingly, one study found that radiation exposure to normal tissue, not the tumor microenvironment, can still elicit a significant antitumor effect in a p53-dependent manner, although the exact mechanism was not clarified (Camphausen et al., 2003). Given the role of STING signaling in normal tissue inflammation and immune surveillance in response to ionizing radiation (Dou et al., 2017), it is reasonable to consider if cGAS-STING signaling is involved. Moreover, the p53 dependence in the host mice that was necessary for the anti-tumor effect in Camphausen et al. is consistent with recent findings that p53 signals for TREX1 degradation, resulting in cytoplasmic dsDNA accumulation, cGAS-STING pathway activation, and ultimately immune-dependent tumor regression (Ghosh et al., 2021; Ghosh et al., 2023).
There is debate about the optimal radiation dose and fractionation for stimulating an anti-tumor immune response stemming from apparently conflicting observations in different studies. Pivotal work by Vanpouille-Box et al. provided a mechanistic underpinning for the observation that moderate hypofractionation (e.g., 8 Gy x three fractions) is more effective at inducing a systemic anti-tumor response than treatment with a larger dose of 20 Gy delivered in a single fraction (Vanpouille-Box et al., 2017). Using a syngeneic TSA breast cancer model, they demonstrated that a higher 20 Gy radiation dose had a reduced immune response relative to multiple fractions of lower doses (e.g., 8 Gy x three fractions) due to reduced secretion of type I interferon. This was attributed to the induction of TREX1, a cytosolic endonuclease that degraded dsDNA in the cytosol, preventing cGAS activation. The improved response to multiple fractions of lower doses relative to a single high dose fraction was corroborated by Yamazaki et al., who demonstrated that autophagy-competent cancer cells could avoid this response through enhanced clearing of cytosolic dsDNA (Yamazaki et al., 2020). More specifically, it appears autophagy can clear micronuclei (Bartsch et al., 2017) and damaged mitochondria (Lindqvist et al., 2018) before they can release dsDNA into the cytosol. However, it should be noted that studies exist showing that higher doses (e.g., over 20 Gy) can generate an interferon response in a tumor model, though without CTLA-4 blockade (Burnette et al., 2011; Filatenkov et al., 2015). These discordant observations suggest there is additional nuance to be explored in this area; perhaps that there may be significant variation in the threshold dose for TREX1 activation in different cancers. What has been consistent, however, is that dendritic cells and the interferon receptor IFNAR1 are important for mediating the cytotoxic T-cell immune response in mouse models (Burnette et al., 2011; Vanpouille-Box et al., 2017). Collectively, these results emphasize that details of the radiation treatment (e.g., dose and fractionation) as well as the cancer cells (e.g., autophagy, TREX1 activation) are important considerations for evaluating radiation-induced immunogenic responses.
There are potential limitations to using radiation to stimulate cGAS-STING. Radiation generates significant oxidative stress, which can activate the nuclear factor erythroid 2-related factor 2 (NRF2) pathway. NRF2 is a transcription factor that is released from inhibition in response to oxidative stress. While this pathway can increase the levels of reactive oxygen species scavengers, helping to reduce damage mediated from oxidative stress, it has been shown to suppress STING expression, thereby reducing interferon signaling (Olagnier et al., 2018; Jessen et al., 2020). The nuances of how the transcription factor achieves this effect have not yet been clarified. However, this suggests that in cancer cells that do not already have defective STING signaling, oxidative stress from chemotherapy or radiation treatment could lead to reduced STING signaling. It would be valuable for potential clinical applications to clarify whether this is a transient or persistent effect in response to NRF2 signaling, as it may inform the timing of STING agonists relative to these treatments. Cancer cells exposed to ionizing radiation have also been shown to use caspases to reduce their type 1 interferon production as well as the tumor-directed immune response in vivo (Rodriguez-Ruiz et al., 2019; Han et al., 2020). To the extent that interferon production from cancer cells is significant, these pathways represent potential barriers to the radiation-induced interferon response. Other effects of radiation include the observation that irradiated mesenchymal stem cells can promote tumor metastasis in a mouse lung metastasis model, which could be blocked by knocking down the cGAS-STING pathway in the irradiated mesenchymal stem cells (Zheng et al., 2020). Radiation may also activate inflammatory cGAS-STING in normal non-immune cells, which may lead to normal tissue toxicity via pyropotosis; however, it was demonstrated in an intestinal model that ultrahigh dose rates may spare normal tissue from this effect (Shi et al., 2022).
A variety of STING agonists have been developed to promote an immunogenic response through STING activation (Hines et al., 2023). This has led several groups to test whether STING agonists could be used to promote the immunogenic response seen with radiation treatment (Table 1). In vitro, intratumoral administration of cGAMP results in enhanced tumor regression with increased interferon-related T-cell response (Deng et al., 2014). Synthetic cGAMP analogues were created to circumvent limitations associated with direct cGAMP administration, such as improving stability and efficacy (Motedayen Aval et al., 2020). Even before the flavone acetic acid derivative called 5,6-dimethylxanthenone-4-acetic acid (DMXAA) was known to be a STING agonist, it was understood to elicit anti-cancer activity through anti-vascular and anti-immune effects (Daei Farshchi Adli et al., 2018), and demonstrated synergy with ionizing radiation (Pedley et al., 1996; Wilson et al., 1998). Eventually, DMXAA was shown to be a STING agonist capable of significant immune-related tumor regression in vivo (Corrales et al., 2015), although it is notable for several disappointing clinical trials (Hines et al., 2023), attributed to its binding of STING in mice, but not humans (Conlon et al., 2013). ADU-S100, a synthetic cyclic dinucleotide (CDN), was shown to have an effect on tumor size but did not appear to have significant synergy with radiation treatment in a rat model of esophageal adenocarcinoma, comparing tumor size before radiation treatment and 8 weeks afterwards (Zaidi et al., 2021). Interestingly, only the groups receiving ADU-S100 prevented relative tumor growth at 8 weeks, and the radiation treatment alone produced no tumor growth delay. Without more information about the tumor growth kinetics or a description of the radiation technique, it is difficult to draw definitive conclusions about this finding. Another type of STING agonist, a small molecule dimeric amidobenzimidazole (diABZI), was shown to work with radiation in cultured non-small cell lung cancer cells, with the combination reducing clonogenic survival through increased apoptosis (Xue et al., 2022). A synthetic phosphodiesterase-resistant cGAMP derivative was injected concurrently and 24h after mice received 10 Gy in one fraction, causing an early TNFα dependent response from the tumor microenvironment followed by T-cell mediated tumor regression, with control of distant, non-irradiated tumors (Baird et al., 2016). This early TNFα effect mediated by the tumor microenvironment has also been shown for STING agonists in the absence of radiation treatment (Francica et al., 2018), indicating the importance of understanding how STING agonists affect normal tissue in order to optimize clinical implementation.
Other pre-clinical studies have explored using nanoparticles containing cGAMP, with the idea that nanoparticles can accumulate in tumors and stabilized cGAMP(Motedayen Aval et al., 2020). Inhalable nanoparticles with cGAMP were able to synergize with radiation in a mouse model of lung metastases through enhanced antigen presenting cell and T-cell activation, leading to synergistic anti-tumor responses (Liu et al., 2019). diABZI was also tagged to CD47/PD-L1 targeting nanoparticles for intratumoral injection into a mouse glioblastoma model, leading to improved T cell infiltration in the brain and synergy with radiation delivered as 9 Gy in three fractions, using survival as the endpoint (Zhang et al., 2023). A STING-activating nanoparticle-based vaccine had STING-dependent synergy with radiation treatments both on the primary irradiated tumor as well as in implanted distant tumors, using a subcutaneous mouse flank model (Luo et al., 2019). Another group used a nanoscale metal-organic layer (MOL) impregnated with cGAMP to take advantage of the radiosensitizing properties of the MOL in addition to its ability to retain cGAMP in the tumor microenvironment (Luo et al., 2022). Intratumoral injection of cGAMP-MOL in a mouse model led to improved STING activation and regression in tumors irradiated with 12 Gy in six fractions.
Manganese has also been shown to activate STING, however, it is difficult to localize to the tumor. Several attempts have been made to overcome this using intratumorally injected scaffolds. Wang et al. demonstrated that intratumorally injected alginate with manganese leads to increased radiation-induced immunity using 10 Gy in two fractions (Wang et al., 2021), while Yan et al. used a metal-phenolic network to coordinate intratumoral administration of a radiationsensitizer (high-Z material) with manganese, and demonstrated tumor growth inhibition with ionizing radiation delivered as 18 Gy in three fractions (Yan J. et al., 2022).
Another way to promote the inflammatory response downstream of STING would be to use small molecule inhibitors of ENPP1, which can degrade extracellular cGAMP (Carozza et al., 2020a; Carozza et al., 2020b). This approach is significant as we gain more appreciation of the role of ectonucleotidases in reducing the effectiveness of cancer treatments (Stagg et al., 2023). A poster presentation provided preliminary results showing that the orally administered ENPP1 inhibitor, MV-626, acted synergistically with radiation treatment, resulting in tumor eradication in the six mice tested (Baird et al., 2018). Drugs targeting both ataxia telangiectasia mutated (ATM) and ataxia telangiectasia mutated and Rad3 related (ATR) proteins, involved in DNA damage repair, were administered with ionizing radiation in mouse tumor models and demonstrated increased CD8+ T-cell infiltration through canonical and non-canonical STING activation (Liu C. et al., 2023; Jin et al., 2023). This effect is presumably mediated through increased cytosolic dsDNA produced by blocking these DNA repair process proteins.
These studies collectively demonstrate the potential for STING agonists with radiation therapy. However, there needs to be caution when extrapolating preclinical results, especially in mice, to what could be expected in clinical trials. Similarly, the radiation doses in these studies were mostly large, single fractions of radiation. Notably, the studies using the largest single doses, such as 20 Gy in one fraction, seemed to find synergy between radiation and STING agonists, whereas the studies using lower doses often found just additive effects. This would align with the results of Vanpouille-Box et al., discussed previously, who found that larger single doses of radiation resulted in TREX1 activation, leading to degradation of cytoplasmic DNA and a reduced tumor-directed immune response (Vanpouille-Box et al., 2017). It may be that STING agonists could be a way to overcome the effects of this potential TREX1 activation at high doses. This observation is also important to emphasize because these large doses are not comparable to current and historical standards of radiation therapy. Radiation treatments are typically delivered in multiple smaller doses (e.g., 2 Gy per fraction), although larger fraction sizes can be used in stereotactic body radiotherapy (SBRT). These findings suggest that the role of STING agonists may be more meaningful in the context of SBRT, but need additional preclinical studies to more intensely evaluate the role of radiation dose and fractionation. Regarding the timing of STING agonists relative to the radiation treatment, it is difficult to draw any definitive conclusions. However, it may be that delivering STING agonists only before radiation treatment, and not after, is associated with additive responses. This aspect of the timing should be tested directly in preclinical studies, as it is relevant to future clinical trials. Another consideration is that several of these studies used tumor cells modified to express tumor-specific antigens, such as OVA and SIY, or were HPV-induced, all of which will lead to the stronger potential for an immune response. In these studies, there remains the question of whether the responses would still be as potent for a more immunologically cold tumor. However, many studies discussed did not use these, and still demonstrated that synergistic responses were possible. Despite these limitations, these studies can help guide future preclinical works, as well as provide some early insights for clinical trial design.
At this time, the formula for significant anti-tumor immune responses from radiation treatment remains elusive (Galluzzi et al., 2023). Findings from early phase clinical trials suggest that immune modifying agents might support radiation-induced abscopal effects (Golden et al., 2015; Welsh et al., 2019; Curti et al., 2020). However, local radiation treatments leading to distant tumor response in the clinic remain largely anecdotal (Abuodeh et al., 2016; Dagoglu et al., 2019). STING agonists are one approach to amplifying this radiation-related immune response. Currently, there are no available clinical results regarding the efficacy of STING agonists with radiation therapy, but herein we will evaluate the currently available clinical and preclinical information in the literature.
A synthetic cyclic dinucleotide (CDN) analog called TAK-676 (eazostinag disodium) is a well-characterized and potent STING agonist (Carideo Cunniff et al., 2022). In contrast to many other CDN-based drugs that utilize intratumor delivery, limiting treatment to accessible tumors, TAK-676 can be delivered intravenously. This is being used in a Phase I trial of patients with advanced non-small cell lung cancer, triple negative breast cancer, or squamous cell head and neck cancers who progressed on checkpoint inhibitor treatment and have two or more lesions. This trial will have one lesion treated with radiation therapy as 24 Gy in three fractions, followed by PD-1 inhibitor on day 1 and dose-escalated TAK-676 at day 1, 8, 15 (Cooper et al., 2022). Although the primary endpoint of the trial is safety, the secondary endpoints of tumor response at the irradiated site as well as at the non-irradiated lesions will provide early insights into the resultant anti-tumor immune response from this regimen.
Given what we reviewed so far, optimal STING agonist trial design should consider the role of radiation dose and fractionation, as high doses may activate TREX1, hampering any natural STING signaling through cGAMP generation (Vanpouille-Box et al., 2017). Further, the timing and dose of STING agonists may be important, as persistent STING agonism may lead to immune cell apoptosis, inadvertently decreasing immune responses. For instance, T-cells can respond to STING agonism with apoptosis, leading to a dampened immune response to tumors (Gulen et al., 2017). It appears that calibrated, lower levels of STING activation are needed to promote immunogenicity and avoid this effect (Sivick et al., 2018). Preclinical work shows that cancer cells can develop compensatory mechanisms to resist the effects of STING agonists (Lemos et al., 2020). As mentioned in the previous section, radiation activates the NRF2 response, which can lead to decreased STING expression and poorer responses to STING agonists (Olagnier et al., 2018). STING also appears to have a role in DNA damage repair, and it remains to be clarified whether adding STING agonists to radiation treatment would be protective or sensitizing to cancer cells in this respect. The timing of STING agonist administration relative to radiation treatment may also be important. It is clear that additional preclinical data are needed to help clarify the optimal timing, frequency, and dosing of STING agonists, as well as the radiation treatments.
The effect of dose rate on STING signaling is another area of practical importance, particularly in the context of low dose rate brachytherapy and targeted radionuclide therapy. Unfortunately, there is limited evidence in the literature regarding the effects of low dose rates on STING signaling. Patel et al. were able to show that yttrium-90 (90Y), a β-particle emitter with a 2.7 days half life, could be preferentially targeted to tumors by tagging it to an alkylphosphocholine called NM600 (Patel et al., 2021). When combined with immune checkpoint inhibitors (ICIs), this radionuclide resulted in complete responses in 45%–66% of mice using an immunologically cold syngeneic tumor cell line. Notably, this effect was STING dependent. This group also published a comparison of this 90Y-tagged NM600 with standard external beam radiation therapy (EBRT) in a mouse model (Jagodinsky et al., 2021). Using their syngeneic mouse model, they demonstrated that type 1 interferon release was similar between the two approaches, but the increase was delayed with the use of the radionuclide relative to EBRT. This delay may be due to the low dose rate, requiring more time to cause enough damage to stimulate cGAS-STING signaling. Interestingly, they also found in their mouse model that the CD8+ T cells had increased type 2 interferon release, but that it was unaffected by knockout of STING in the tumor cells. This suggests that cGAS-STING signaling in the microenvironment, or even a STING-independent mechanism in the tumor or its microenvironment, may be contributing to the observed intratumoral immune response. Yang et al. explored the role of STING signaling at low dose rates using radium-223 (223Ra), an α-particle emitter with an 11.43 days half-life that targets to bone, in a mouse model with syngeneic tumor cells in the bone marrow (Yang et al., 2024). They found that STING knockout in the tumor cells increased tumor cell viability and allowed greater tumor growth in response to 223Ra treatment. They attributed this difference to the tumor cells with STING knockout or antagonism having reduced pyroptosis. The immunomodulatory effects of these low dose rates treatments will be important to help optimize radionuclide treatments, particularly as lutetium-177, a β-particle emitter with a 6.6 days half-life, is gaining more clinical applications (Jia et al., 2022; Constanzo et al., 2023; Fallah et al., 2023). In the context of this review, if radionuclides could be tagged to STING agonists, then their combined effects could contribute to increased efficacy through enhanced tumor-directed immune responses. However, additional preclinical studies are needed in this area to help clarify the role of STING signaling using low dose rate radiotherapy techniques.
There are preclinical findings that could be considered when attempting to improve clinical efficacy. For instance, loading CDNs in extracellular vesicles has been demonstrated in mouse tumor models to improve the effectiveness of CDNs(Jang et al., 2021). A Phase I/II clinical trial (NCT04592484) involving intratumor injection of CDNs loaded into extracellular vesicles called exoSTING for advanced/metastatic solid tumors, by Codiak Bioscience, had a press release mentioning signs of clinical efficacy, but the study has been halted with no published results to date (Ducrot et al., 2023). These results, even if negative, will be helpful in guiding the field towards optimal drug delivery.
Novel radiation delivery techniques may also lead to synergy with STING agonists through differential effects on the immune system relative to conventionally delivered radiation therapy. FLASH radiotherapy and lattice/grid therapy both allow higher doses of radiation to be delivered while avoiding normal tissue toxicity (Bertho et al., 2023). FLASH delivers ultrahigh dose rates (>40 Gy/s compared to 2 Gy/min with standard radiotherapy) of ionizing radiation, which through mechanisms not fully understood, leads to preferential sparing of the normal tissue (Limoli and Vozenin, 2023). For instance, in a preclinical model, delivering radiation at ultrahigh dose rates was able to avoid the toxicity from inflammation caused by STING activation in the normal tissue that was seen with conventional dose rates, while preserving efficacy against the tumor (Shi et al., 2022). Lattice therapy and its counterpart GRID deliver spatially-separated high doses of radiation within a tumor such that the normal tissue, including the intratumoral lymphatic and immune structures, have reduced toxicity. This potential preservation of immune cells in the microenvironment, while still damaging tumors, has been shown in at least one preclinical model to lead to immune effects even in distant, unirradiated tumors (Kanagavelu et al., 2014), and there is at least one case report demonstrating efficacy against a large (63cc) tumor combined with PD-1 immunotherapy (Jiang et al., 2021). Personalized ultrafractionated stereotactic adaptive radiation therapy (PULSAR) delivers larger doses of radiation spaced farther apart in time, to capitalize on tumor shrinking and changes in the tumor microenvironment. PULSAR was used in mouse tumor models with PD-L1 antibodies to demonstrate a synergistic response of radioimmunotherapy (Moore et al., 2021). They also demonstrated that when delivering two fractions of 15 Gy spaced over 10 days, delivery of PD-L1 with the second fraction, but not the first, was important for the anti-tumor immune response. These findings lead to questions about whether the addition of STING agonists to this regimen could further promote an immunogenic response. There are currently two active clinical trials looking to combine PULSAR treatment with the STING agonist IMSA101 (NCT05846659, NCT05846646). Lastly, pre-clinical evidence suggests that carbon ion irradiation of cancer cells produce similar immune responses through the STING pathway relative to x-rays (Du et al., 2021). However, the signaling may be stronger with carbon ions (Guo et al., 2023), possibly related to their enhanced radiobiological effectiveness (Schlaff et al., 2014). Ultimately, the radiobiology of these novel radiation modalities and delivery techniques is still under investigation, and the effect of these on tumor control and immune activation are active research questions, complicating studies using these techniques in combination with pharmacological immune activators.
There should also be some consideration for the potential normal tissue toxicity from STING agonists. While current trials seem to demonstrate mild toxicity (Hines et al., 2023), preclinical data suggests the potential for STING-related injury. For instance, cGAS-STING can signal for pyroptosis in normal tissues (Yan M. et al., 2022; Zhang et al., 2022), and this has been demonstrated to play a role in intestinal toxicity to ionizing radiation exposure through pyroptosis (Shi et al., 2022). Similarly, cGAS-STING signaling in macrophages can also lead to necroptosis through proinflammatory cytokine production, and the STING agonist DMXAA was shown to result in sterile shock in mice, which was not present when administered to STING deficient mice (Brault et al., 2018). These finding suggest that cytokines induced from STING agonists could cause a cytokine release syndrome (Khadka et al., 2019). Methods of targeting STING agonists to tumors (Su et al., 2019; Wu et al., 2022), or simply having tocilizumab available (Le et al., 2018), may be meaningful clinically to prevent potential systemic inflammatory responses.
In this review, we have discussed the significance of cGAS-STING signaling in the context of radiation therapy. We covered its activation by genotoxic stress resulting in cytosolic dsDNA. The various modes of intercellular signaling mediated by cGAMP and the role of STING activation in cancer cells and in immune cells were considered. The immune stimulating versus immunosuppressive potential was explored, including the role of shorter exposures to STING agonists to avoid immunosuppression. We covered the complexity of STING signaling in the DNA damage response, both in tumor cells and their microenvironment. We applied these ideas specifically to the cellular response to radiation, exploring the nuances of the pathways involved. We discussed the promising results of combining radiation treatments with STING agonists in preclinical studies, reviewed ongoing studies combining STING agonists and radiation, and provided considerations for how these may influence study design for future clinical trials. We also evaluated the potential role for new radiation treatment technologies, and how they may capitalize on cGAS-STING signaling. We look forward to future works clarifying the complex interplay of cGAS-STING signaling in cancer cells and their microenvironment, and the immense potential for combining STING agonists with radiation therapy.
NC: Conceptualization, Writing–original draft, Writing–review and editing. NG: Writing–review and editing. RV: Writing–review and editing. BC: Conceptualization, Supervision, Writing–original draft, Writing–review and editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Ablasser, A., Goldeck, M., Cavlar, T., Deimling, T., Witte, G., Röhl, I., et al. (2013). cGAS produces a 2′-5′-linked cyclic dinucleotide second messenger that activates STING. Nature 498 (7454), 380–384. doi:10.1038/nature12306
Abuodeh, Y., Venkat, P., and Kim, S. (2016). Systematic review of case reports on the abscopal effect. Curr. Problems Cancer 40 (1), 25–37. doi:10.1016/j.currproblcancer.2015.10.001
Arwert, E. N., Milford, E. L., Rullan, A., Derzsi, S., Hooper, S., Kato, T., et al. (2020). STING and IRF3 in stromal fibroblasts enable sensing of genomic stress in cancer cells to undermine oncolytic viral therapy. Nat. Cell Biol. 22 (7), 758–766. doi:10.1038/s41556-020-0527-7
Baird, J., Dietsch, G., Florio, V., Gallatin, M., Knox, C., Odingo, J., et al. (2018). MV-626, a potent and selective inhibitor of ENPP1 enhances STING activation and augments T-cell mediated anti-tumor activity in vivo. Soc. Immunother. Cancer 2018 Annu. Meet. Posters 7.
Baird, J. R., Friedman, D., Cottam, B., Dubensky, T. W., Kanne, D. B., Bambina, S., et al. (2016). Radiotherapy combined with novel STING-targeting oligonucleotides results in regression of established tumors. Cancer Res. 76 (1), 50–61. doi:10.1158/0008-5472.CAN-14-3619
Bakhoum, S. F., Ngo, B., Laughney, A. M., Cavallo, J.-A., Murphy, C. J., Ly, P., et al. (2018). Chromosomal instability drives metastasis through a cytosolic DNA response. Nature 553 (7689), 467–472. doi:10.1038/nature25432
Barnett, K. C., Coronas-Serna, J. M., Zhou, W., Ernandes, M. J., Cao, A., Kranzusch, P. J., et al. (2019). Phosphoinositide interactions position cGAS at the plasma membrane to ensure efficient distinction between self- and viral DNA. Cell 176 (6), 1432–1446. doi:10.1016/j.cell.2019.01.049
Bartsch, K., Knittler, K., Borowski, C., Rudnik, S., Damme, M., Aden, K., et al. (2017). Absence of RNase H2 triggers generation of immunogenic micronuclei removed by autophagy. Hum. Mol. Genet. 26 (20), 3960–3972. doi:10.1093/hmg/ddx283
Beernaert, B., and Parkes, E. E. (2023). cGAS–STING signalling in cancer: striking a balance with chromosomal instability. Biochem. Soc. Trans. 51 (2), 539–555. doi:10.1042/bst20220838
Bertho, A., Iturri, L., and Prezado, Y. (2023). “Chapter Two - radiation-induced immune response in novel radiotherapy approaches FLASH and spatially fractionated radiotherapies,” in International review of cell and molecular biology. Editors C. Mirjolet, and L. Galluzzi (Academic Press), 37–68.
Boukhaled, G. M., Harding, S., and Brooks, D. G. (2021). Opposing roles of type I interferons in cancer immunity. Annu. Rev. Pathology Mech. Dis. 16 (1), 167–198. doi:10.1146/annurev-pathol-031920-093932
Brault, M., Olsen, T. M., Martinez, J., Stetson, D. B., and Oberst, A. (2018). Intracellular nucleic acid sensing triggers necroptosis through synergistic type I IFN and TNF signaling. J. Immunol. 200 (8), 2748–2756. doi:10.4049/jimmunol.1701492
Burnette, B. C., Liang, H., Lee, Y., Chlewicki, L., Khodarev, N. N., Weichselbaum, R. R., et al. (2011). The efficacy of radiotherapy relies upon induction of type I interferon–dependent innate and adaptive immunity. Cancer Res. 71 (7), 2488–2496. doi:10.1158/0008-5472.can-10-2820
Cai, H., Yan, L., Liu, N., Xu, M., and Cai, H. (2020). IFI16 promotes cervical cancer progression by upregulating PD-L1 in immunomicroenvironment through STING-TBK1-NF-kB pathway. Biomed. Pharmacother. 123, 109790. doi:10.1016/j.biopha.2019.109790
Camphausen, K., Moses, M. A., Ménard, C., Sproull, M., Beecken, W.-D., Folkman, J., et al. (2003). Radiation abscopal antitumor effect is mediated through p53. Cancer Res. 63 (8), 1990–1993. doi:10.1016/s0360-3016(02)03449-1
Carideo Cunniff, E., Sato, Y., Mai, D., Appleman, V. A., Iwasaki, S., Kolev, V., et al. (2022). TAK-676: a novel stimulator of interferon genes (STING) agonist promoting durable IFN-dependent antitumor immunity in preclinical studies. Cancer Res. Commun. 2 (6), 489–502. doi:10.1158/2767-9764.crc-21-0161
Carozza, J. A., Böhnert, V., Nguyen, K. C., Skariah, G., Shaw, K. E., Brown, J. A., et al. (2020a). Extracellular cGAMP is a cancer-cell-produced immunotransmitter involved in radiation-induced anticancer immunity. Nat. Cancer 1 (2), 184–196. doi:10.1038/s43018-020-0028-4
Carozza, J. A., Brown, J. A., Böhnert, V., Fernandez, D., Alsaif, Y., Mardjuki, R. E., et al. (2020b). Structure-aided development of small-molecule inhibitors of ENPP1, the extracellular phosphodiesterase of the immunotransmitter cGAMP. Cell Chem. Biol. 27 (11), 1347–1358. doi:10.1016/j.chembiol.2020.07.007
Chawla-Sarkar, M., Leaman, D. W., and Borden, E. C. (2001). Preferential induction of apoptosis by interferon (IFN)-beta compared with IFN-alpha2: correlation with TRAIL/Apo2L induction in melanoma cell lines. Clin. Cancer Res. 7 (6), 1821–1831.
Chawla-Sarkar, M., Lindner, D. J., Liu, Y. F., Williams, B. R., Sen, G. C., Silverman, R. H., et al. (2003). Apoptosis and interferons: role of interferon-stimulated genes as mediators of apoptosis. Apoptosis 8 (3), 237–249. doi:10.1023/a:1023668705040
Chen, D., Tong, J., Yang, L., Wei, L., Stolz, D. B., Yu, J., et al. (2018). PUMA amplifies necroptosis signaling by activating cytosolic DNA sensors. Proc. Natl. Acad. Sci. 115 (15), 3930–3935. doi:10.1073/pnas.1717190115
Chen, J., Cao, Y., Markelc, B., Kaeppler, J., Vermeer, J. A. F., and Muschel, R. J. (2019). Type I IFN protects cancer cells from CD8+ T cell–mediated cytotoxicity after radiation. J. Clin. Investigation 129 (10), 4224–4238. doi:10.1172/jci127458
Chen, Q., Sun, L., and Chen, Z. J. (2016). Regulation and function of the cGAS–STING pathway of cytosolic DNA sensing. Nat. Immunol. 17 (10), 1142–1149. doi:10.1038/ni.3558
Cheradame, L., Guerrera, I. C., Gaston, J., Schmitt, A., Jung, V., Goudin, N., et al. (2021). STING protects breast cancer cells from intrinsic and genotoxic-induced DNA instability via a non-canonical, cell-autonomous pathway. Oncogene 40 (49), 6627–6640. doi:10.1038/s41388-021-02037-4
Concepcion, A. R., Wagner, L. E., Zhu, J., Tao, A. Y., Yang, J., Khodadadi-Jamayran, A., et al. (2022). The volume-regulated anion channel LRRC8C suppresses T cell function by regulating cyclic dinucleotide transport and STING–p53 signaling. Nat. Immunol. 23 (2), 287–302. doi:10.1038/s41590-021-01105-x
Conlon, J., Burdette, D. L., Sharma, S., Bhat, N., Thompson, M., Jiang, Z., et al. (2013). Mouse, but not human STING, binds and signals in response to the vascular disrupting agent 5,6-dimethylxanthenone-4-acetic acid. J. Immunol. 190 (10), 5216–5225. doi:10.4049/jimmunol.1300097
Constanzo, J., Bouden, Y., Godry, L., Kotzki, P. O., Deshayes, E., and Pouget, J. P. (2023). “Chapter Four - immunomodulatory effects of targeted radionuclide therapy,” in International review of cell and molecular biology. Editors C. Mirjolet, and L. Galluzzi (Academic Press), 105–136.
Cooper, B. T., Chmura, S. J., Luke, J. J., Shiao, S. L., Basho, R. K., Iams, W. T., et al. (2022). TAK-676 in combination with pembrolizumab after radiation therapy in patients (pts) with advanced non–small cell lung cancer (NSCLC), triple-negative breast cancer (TNBC), or squamous-cell carcinoma of the head and neck (SCCHN): phase 1 study design. American Society of Clinical Oncology.
Cordova, A. F., Ritchie, C., Böhnert, V., and Li, L. (2021). Human SLC46A2 is the dominant cGAMP importer in extracellular cGAMP-sensing macrophages and monocytes. ACS Central Sci. 7 (6), 1073–1088. doi:10.1021/acscentsci.1c00440
Corrales, L., Laura, S., David, K., Kanne, D. B., Sivick, K. E., Katibah, G. E., et al. (2015). Direct activation of STING in the tumor microenvironment leads to potent and systemic tumor regression and immunity. Cell Rep. 11 (7), 1018–1030. doi:10.1016/j.celrep.2015.04.031
Curti, B., Crittenden, M., Seung, S. K., Fountain, C. B., Payne, R., Chang, S., et al. (2020). Randomized phase II study of stereotactic body radiotherapy and interleukin-2 versus interleukin-2 in patients with metastatic melanoma. J. Immunother. Cancer 8 (1), e000773. doi:10.1136/jitc-2020-000773
Daei Farshchi Adli, A., Jahanban Esfahlan, R., Seidi, K., Samandari Rad, S., and Zarghami, N. (2018). An overview on Vadimezan (DMXAA): the vascular disrupting agent. Chem. Biol. Drug Des. 91 (5), 996–1006. doi:10.1111/cbdd.13166
Dagoglu, N., Karaman, S., Caglar, H. B., and Oral, E. N. (2019). Abscopal effect of radiotherapy in the immunotherapy era: systematic review of reported cases. Cureus 11 (2), e4103. doi:10.7759/cureus.4103
Demaria, O., De Gassart, A., Coso, S., Gestermann, N., Di Domizio, J., Flatz, L., et al. (2015). STING activation of tumor endothelial cells initiates spontaneous and therapeutic antitumor immunity. Proc. Natl. Acad. Sci. 112 (50), 15408–15413. doi:10.1073/pnas.1512832112
Demaria, S., Ng, B., Devitt, M. L., Babb, J. S., Kawashima, N., Liebes, L., et al. (2004). Ionizing radiation inhibition of distant untreated tumors (abscopal effect) is immune mediated. Int. J. Radiat. Oncology*Biology*Physics 58 (3), 862–870. doi:10.1016/j.ijrobp.2003.09.012
Deng, L., Liang, H., Xu, M., Yang, X., Burnette, B., Arina, A., et al. (2014). STING-dependent cytosolic DNA sensing promotes radiation-induced type I interferon-dependent antitumor immunity in immunogenic tumors. Immunity 41 (5), 843–852. doi:10.1016/j.immuni.2014.10.019
Diamond, J. M., Vanpouille-Box, C., Spada, S., Rudqvist, N.-P., Chapman, J. R., Ueberheide, B. M., et al. (2018). Exosomes shuttle TREX1-sensitive IFN-stimulatory dsDNA from irradiated cancer cells to DCs. Cancer Immunol. Res. 6 (8), 910–920. doi:10.1158/2326-6066.Cir-17-0581
Diamond, M. S., Kinder, M., Matsushita, H., Mashayekhi, M., Dunn, G. P., Archambault, J. M., et al. (2011). Type I interferon is selectively required by dendritic cells for immune rejection of tumors. J. Exp. Med. 208 (10), 1989–2003. doi:10.1084/jem.20101158
Dobbs, N., Burnaevskiy, N., Chen, D., Vijay, N., and Yan, N. (2015). STING activation by translocation from the ER is associated with infection and autoinflammatory disease. Cell Host Microbe 18 (2), 157–168. doi:10.1016/j.chom.2015.07.001
Dou, Z., Ghosh, K., Vizioli, M. G., Zhu, J., Sen, P., Wangensteen, K. J., et al. (2017). Cytoplasmic chromatin triggers inflammation in senescence and cancer. Nature 550 (7676), 402–406. doi:10.1038/nature24050
Du, J., Kageyama, S.-I., Hirata, H., Motegi, A., Nakamura, M., Hirano, Y., et al. (2021). Comparative analysis of the immune responses in cancer cells irradiated with X-ray, proton and carbon-ion beams. Biochem. Biophysical Res. Commun. 585, 55–60. doi:10.1016/j.bbrc.2021.11.004
Ducrot, C., Loiseau, S., Wong, C., Madec, E., Volatron, J., and Piffoux, M. (2023). Hybrid extracellular vesicles for drug delivery. Cancer Lett. 558, 216107. doi:10.1016/j.canlet.2023.216107
Ergun, S. L., Fernandez, D., Weiss, T. M., and Li, L. (2019). STING polymer structure reveals mechanisms for activation, hyperactivation, and inhibition. Cell 178 (2), 290–301. doi:10.1016/j.cell.2019.05.036
Fallah, J., Agrawal, S., Gittleman, H., Fiero, M. H., Subramaniam, S., John, C., et al. (2023). FDA approval summary: lutetium Lu 177 vipivotide tetraxetan for patients with metastatic castration-resistant prostate cancer. Clin. Cancer Res. 29 (9), 1651–1657. doi:10.1158/1078-0432.ccr-22-2875
Fang, C., Mo, F., Liu, L., Du, J., Luo, M., Men, K., et al. (2021). Oxidized mitochondrial DNA sensing by STING signaling promotes the antitumor effect of an irradiated immunogenic cancer cell vaccine. Cell. Mol. Immunol. 18 (9), 2211–2223. doi:10.1038/s41423-020-0456-1
Filatenkov, A., Baker, J., Mueller, A. M. S., Kenkel, J., Ahn, G. O., Dutt, S., et al. (2015). Ablative tumor radiation can change the tumor immune cell microenvironment to induce durable complete remissions. Clin. Cancer Res. 21 (16), 3727–3739. doi:10.1158/1078-0432.ccr-14-2824
Francica, B. J., Ghasemzadeh, A., Desbien, A. L., Theodros, D., Sivick, K. E., Reiner, G. L., et al. (2018). TNFα and radioresistant stromal cells are essential for therapeutic efficacy of cyclic dinucleotide STING agonists in nonimmunogenic tumors. Cancer Immunol. Res. 6 (4), 422–433. doi:10.1158/2326-6066.cir-17-0263
Fuertes, M. B., Kacha, A. K., Kline, J., Woo, S.-R., Kranz, D. M., Murphy, K. M., et al. (2011). Host type I IFN signals are required for antitumor CD8+ T cell responses through CD8{alpha}+ dendritic cells. J. Exp. Med. 208 (10), 2005–2016. doi:10.1084/jem.20101159
Galluzzi, L., Aryankalayil, M. J., Coleman, C. N., and Formenti, S. C. (2023). Emerging evidence for adapting radiotherapy to immunotherapy. Nat. Rev. Clin. Oncol. 20 (8), 543–557. doi:10.1038/s41571-023-00782-x
Gao, P., Ascano, M., Wu, Y., Barchet, W., Gaffney, B. L., Zillinger, T., et al. (2013). Cyclic [G(2',5')pA(3',5')p] is the metazoan second messenger produced by DNA-activated cyclic GMP-AMP synthase. Cell 153 (5), 1094–1107. doi:10.1016/j.cell.2013.04.046
Gao, Y., Zheng, X., Chang, B., Lin, Y., Huang, X., Wang, W., et al. (2022). Intercellular transfer of activated STING triggered by RAB22A-mediated non-canonical autophagy promotes antitumor immunity. Cell Res. 32 (12), 1086–1104. doi:10.1038/s41422-022-00731-w
Gaston, J., Cheradame, L., Yvonnet, V., Deas, O., Poupon, M.-F., Judde, J.-G., et al. (2016). Intracellular STING inactivation sensitizes breast cancer cells to genotoxic agents. Oncotarget 7 (47), 77205–77224. doi:10.18632/oncotarget.12858
Gehrke, N., Mertens, C., Zillinger, T., Wenzel, J., Bald, T., Zahn, S., et al. (2013). Oxidative damage of DNA confers resistance to cytosolic nuclease TREX1 degradation and potentiates STING-dependent immune sensing. Immunity 39 (3), 482–495. doi:10.1016/j.immuni.2013.08.004
Ghosh, M., Saha, S., Bettke, J., Nagar, R., Parrales, A., Iwakuma, T., et al. (2021). Mutant p53 suppresses innate immune signaling to promote tumorigenesis. Cancer Cell 39 (4), 494–508.e5. doi:10.1016/j.ccell.2021.01.003
Ghosh, M., Saha, S., Li, J., Montrose, D. C., and Martinez, L. A. (2023). p53 engages the cGAS/STING cytosolic DNA sensing pathway for tumor suppression. Mol. Cell 83 (2), 266–280.e6. doi:10.1016/j.molcel.2022.12.023
Golden, E. B., Chhabra, A., Chachoua, A., Adams, S., Donach, M., Fenton-Kerimian, M., et al. (2015). Local radiotherapy and granulocyte-macrophage colony-stimulating factor to generate abscopal responses in patients with metastatic solid tumours: a proof-of-principle trial. Lancet Oncol. 16 (7), 795–803. doi:10.1016/s1470-2045(15)00054-6
Goodhead, D. T. (1989). The initial physical damage produced by ionizing radiations. Int. J. Radiat. Biol. 56 (5), 623–634. doi:10.1080/09553008914551841
Gui, X., Yang, H., Li, T., Tan, X., Shi, P., Li, M., et al. (2019). Autophagy induction via STING trafficking is a primordial function of the cGAS pathway. Nature 567 (7747), 262–266. doi:10.1038/s41586-019-1006-9
Gulen, M. F., Koch, U., Haag, S. M., Schuler, F., Apetoh, L., Villunger, A., et al. (2017). Signalling strength determines proapoptotic functions of STING. Nat. Commun. 8 (1), 427. doi:10.1038/s41467-017-00573-w
Guo, Y., Shen, R., Wang, F., Wang, Y., Xia, P., Wu, R., et al. (2023). Carbon ion irradiation induces DNA damage in melanoma and optimizes the tumor microenvironment based on the cGAS–STING pathway. J. Cancer Res. Clin. Oncol. 149 (9), 6315–6328. doi:10.1007/s00432-023-04577-6
Hajiabadi, S., Alidadi, S., Montakhab Farahi, Z., Ghahramani Seno, M. M., Farzin, H., and Haghparast, A. (2023). Immunotherapy with STING and TLR9 agonists promotes synergistic therapeutic efficacy with suppressed cancer-associated fibroblasts in colon carcinoma. Front. Immunol. 14, 1258691. doi:10.3389/fimmu.2023.1258691
Han, C., Liu, Z., Zhang, Y., Shen, A., Dong, C., Zhang, A., et al. (2020). Tumor cells suppress radiation-induced immunity by hijacking caspase 9 signaling. Nat. Immunol. 21 (5), 546–554. doi:10.1038/s41590-020-0641-5
Harding, S. M., Benci, J. L., Irianto, J., Discher, D. E., Minn, A. J., and Greenberg, R. A. (2017). Mitotic progression following DNA damage enables pattern recognition within micronuclei. Nature 548 (7668), 466–470. doi:10.1038/nature23470
Hayman, T. J., Baro, M., MacNeil, T., Phoomak, C., Aung, T. N., Cui, W., et al. (2021). STING enhances cell death through regulation of reactive oxygen species and DNA damage. Nat. Commun. 12 (1), 2327. doi:10.1038/s41467-021-22572-8
Hines, J. B., Kacew, A. J., and Sweis, R. F. (2023). The development of STING agonists and emerging results as a cancer immunotherapy. Curr. Oncol. Rep. 25 (3), 189–199. doi:10.1007/s11912-023-01361-0
Hoesel, B., and Schmid, J. A. (2013). The complexity of NF-κB signaling in inflammation and cancer. Mol. Cancer 12 (1), 86. doi:10.1186/1476-4598-12-86
Hou, Y., Liang, H., Rao, E., Zheng, W., Huang, X., Deng, L., et al. (2018). Non-canonical NF-κB antagonizes STING sensor-mediated DNA sensing in radiotherapy. Immunity 49 (3), 490–503. doi:10.1016/j.immuni.2018.07.008
Iglesias, R., Locovei, S., Roque, A., Alberto, A. P., Dahl, G., Spray, D. C., et al. (2008). P2X7 receptor-Pannexin1 complex: pharmacology and signaling. Am. J. Physiology-Cell Physiology 295 (3), C752–C760. doi:10.1152/ajpcell.00228.2008
Ishikawa, H., Ma, Z., and Barber, G. N. (2009). STING regulates intracellular DNA-mediated, type I interferon-dependent innate immunity. Nature 461 (7265), 788–792. doi:10.1038/nature08476
Jacquelot, N., Yamazaki, T., Roberti, M. P., Duong, C. P. M., Andrews, M. C., Verlingue, L., et al. (2019). Sustained Type I interferon signaling as a mechanism of resistance to PD-1 blockade. Cell Res. 29 (10), 846–861. doi:10.1038/s41422-019-0224-x
Jagodinsky, J. C., Jin, W. J., Bates, A. M., Hernandez, R., Grudzinski, J. J., Marsh, I. R., et al. (2021). Temporal analysis of type 1 interferon activation in tumor cells following external beam radiotherapy or targeted radionuclide therapy. Theranostics 11 (13), 6120–6137. doi:10.7150/thno.54881
Jang, S. C., Economides, K. D., Moniz, R. J., Sia, C. L., Lewis, N., McCoy, C., et al. (2021). ExoSTING, an extracellular vesicle loaded with STING agonists, promotes tumor immune surveillance. Commun. Biol. 4 (1), 497. doi:10.1038/s42003-021-02004-5
Jessen, C., Kreß, J. K. C., Baluapuri, A., Hufnagel, A., Schmitz, W., Kneitz, S., et al. (2020). The transcription factor NRF2 enhances melanoma malignancy by blocking differentiation and inducing COX2 expression. Oncogene 39 (44), 6841–6855. doi:10.1038/s41388-020-01477-8
Jia, A. Y., Kashani, R., Zaorsky, N. G., Spratt, D. E., Kiess, A. P., Michalski, J. M., et al. (2022). Lutetium-177 dotatate: a practical review. Pract. Radiat. Oncol. 12 (4), 305–311. doi:10.1016/j.prro.2022.02.002
Jiang, L., Li, X., Zhang, J., Li, W., Dong, F., Chen, C., et al. (2021). Combined high-dose LATTICE radiation therapy and immune checkpoint blockade for advanced bulky tumors: the concept and a case report. Front. Oncol. 10, 548132. doi:10.3389/fonc.2020.548132
Jin, W. J., Zangl, L. M., Hyun, M., Massoud, E., Schroeder, K., Alexandridis, R. A., et al. (2023). ATM inhibition augments type I interferon response and antitumor T-cell immunity when combined with radiation therapy in murine tumor models. J. Immunother. Cancer 11 (9), e007474. doi:10.1136/jitc-2023-007474
Joiner, M. C., and van der Kogel, A. J. (2018). Basic clinical radiobiology. Boca Raton, Florida: CRC Press.
Kabashima, A., Matsuo, Y., Ito, S., Akiyama, Y., Ishii, T., Shimada, S., et al. (2022). cGAS-STING signaling encourages immune cell overcoming of fibroblast barricades in pancreatic cancer. Sci. Rep. 12 (1), 10466. doi:10.1038/s41598-022-14297-5
Kanagavelu, S., Gupta, S., Wu, X., Philip, S., Wattenberg, M. M., Hodge, J. W., et al. (2014). In vivo effects of lattice radiation therapy on local and distant lung cancer: potential role of immunomodulation. Radiat. Res. 182 (2), 149–162. doi:10.1667/RR3819.1
Kato, K., Nishimasu, H., Oikawa, D., Hirano, S., Hirano, H., Kasuya, G., et al. (2018). Structural insights into cGAMP degradation by Ecto-nucleotide pyrophosphatase phosphodiesterase 1. Nat. Commun. 9 (1), 4424. doi:10.1038/s41467-018-06922-7
Kato, K., Omura, H., Ishitani, R., and Nureki, O. (2017). Cyclic GMP–AMP as an endogenous second messenger in innate immune signaling by cytosolic DNA. Annu. Rev. Biochem. 86 (1), 541–566. doi:10.1146/annurev-biochem-061516-044813
Khadka, R. H., Sakemura, R., Kenderian, S. S., and Johnson, A. J. (2019). Management of cytokine release syndrome: an update on emerging antigen-specific T cell engaging immunotherapies. Immunotherapy 11 (10), 851–857. doi:10.2217/imt-2019-0074
Khodarev, N. N., Beckett, M., Labay, E., Darga, T., Roizman, B., and Weichselbaum, R. R. (2004). STAT1 is overexpressed in tumors selected for radioresistance and confers protection from radiation in transduced sensitive cells. Proc. Natl. Acad. Sci. 101 (6), 1714–1719. doi:10.1073/pnas.0308102100
Kitajima, S., Ivanova, E., Guo, S., Yoshida, R., Campisi, M., Sundararaman, S. K., et al. (2019). Suppression of STING associated with LKB1 loss in KRAS-driven lung cancer. Cancer Discov. 9 (1), 34–45. doi:10.1158/2159-8290.cd-18-0689
Klarquist, J., Hennies, C. M., Lehn, M. A., Reboulet, R. A., Feau, S., and Janssen, E. M. (2014). STING-mediated DNA sensing promotes antitumor and autoimmune responses to dying cells. J. Immunol. 193 (12), 6124–6134. doi:10.4049/jimmunol.1401869
Konno, H., Yamauchi, S., Berglund, A., Putney, R. M., Mulé, J. J., and Barber, G. N. (2018). Suppression of STING signaling through epigenetic silencing and missense mutation impedes DNA damage mediated cytokine production. Oncogene 37 (15), 2037–2051. doi:10.1038/s41388-017-0120-0
Kumar, V., Bauer, C., and Stewart IV, J. H. (2023). Cancer cell-specific cGAS/STING Signaling pathway in the era of advancing cancer cell biology. Eur. J. Cell Biol. 151338, 151338. doi:10.1016/j.ejcb.2023.151338
Lahey, L. J., Mardjuki, R. E., Wen, X., Hess, G. T., Ritchie, C., Carozza, J. A., et al. (2020). LRRC8A:C/E heteromeric channels are ubiquitous transporters of cGAMP. Mol. Cell 80 (4), 578–591. doi:10.1016/j.molcel.2020.10.021
Lam, A. R., Le Bert, N., Ho, S. S. W., Shen, Y. J., Tang, M. L. F., Xiong, G. M., et al. (2014). RAE1 ligands for the NKG2D receptor are regulated by STING-dependent DNA sensor pathways in lymphoma. Cancer Res. 74 (8), 2193–2203. doi:10.1158/0008-5472.can-13-1703
Le, R. Q., Li, L., Yuan, W., Shord, S. S., Nie, L., Habtemariam, B. A., et al. (2018). FDA approval summary: tocilizumab for treatment of chimeric antigen receptor T cell-induced severe or life-threatening cytokine release syndrome. Oncol. 23 (8), 943–947. doi:10.1634/theoncologist.2018-0028
Lemos, H., Ou, R., McCardle, C., Lin, Y., Calver, J., Minett, J., et al. (2020). Overcoming resistance to STING agonist therapy to incite durable protective antitumor immunity. J. Immunother. Cancer 8 (2), e001182. doi:10.1136/jitc-2020-001182
Li, C., Zhang, Y., Liu, J., Kang, R., Klionsky, D. J., and Tang, D. (2021a). Mitochondrial DNA stress triggers autophagy-dependent ferroptotic death. Autophagy 17 (4), 948–960. doi:10.1080/15548627.2020.1739447
Li, J., Duran, M. A., Dhanota, N., Chatila, W. K., Bettigole, S. E., Kwon, J., et al. (2021b). Metastasis and immune evasion from extracellular cGAMP hydrolysis. Cancer Discov. 11 (5), 1212–1227. doi:10.1158/2159-8290.cd-20-0387
Li, J., Hubisz, M. J., Earlie, E. M., Duran, M. A., Hong, C., Varela, A. A., et al. (2023). Non-cell-autonomous cancer progression from chromosomal instability. Nature 620 (7976), 1080–1088. doi:10.1038/s41586-023-06464-z
Li, L., Yin, Q., Kuss, P., Maliga, Z., Millán, J. L., Wu, H., et al. (2014). Hydrolysis of 2′3′-cGAMP by ENPP1 and design of nonhydrolyzable analogs. Nat. Chem. Biol. 10 (12), 1043–1048. doi:10.1038/nchembio.1661
Li, X., Shu, C., Yi, G., Chaton, C. T., Shelton, C. L., Diao, J., et al. (2013). Cyclic GMP-AMP synthase is activated by double-stranded DNA-induced oligomerization. Immunity 39 (6), 1019–1031. doi:10.1016/j.immuni.2013.10.019
Limoli, C. L., and Vozenin, M.-C. (2023). Reinventing radiobiology in the light of FLASH radiotherapy. Annu. Rev. Cancer Biol. 7 (1), 1–21. doi:10.1146/annurev-cancerbio-061421-022217
Lindqvist, L. M., Frank, D., McArthur, K., Dite, T. A., Lazarou, M., Oakhill, J. S., et al. (2018). Autophagy induced during apoptosis degrades mitochondria and inhibits type I interferon secretion. Cell Death Differ. 25 (4), 784–796. doi:10.1038/s41418-017-0017-z
Liu, C., Wang, X., Qin, W., Tu, J., Li, C., Zhao, W., et al. (2023a). Combining radiation and the ATR inhibitor berzosertib activates STING signaling and enhances immunotherapy via inhibiting SHP1 function in colorectal cancer. Cancer Commun. 43 (4), 435–454. doi:10.1002/cac2.12412
Liu, H., Wang, F., Cao, Y., Dang, Y., and Ge, B. (2022a). The multifaceted functions of cGAS. J. Mol. Cell Biol. 14 (5), mjac031. doi:10.1093/jmcb/mjac031
Liu, J., Rui, K., Peng, N., Luo, H., Zhu, B., Zuo, X., et al. (2022b). The cGAS-STING pathway: post-translational modifications and functional implications in diseases. Cytokine and Growth Factor Rev. 68, 69–80. doi:10.1016/j.cytogfr.2022.09.003
Liu, Y., Chen, X., Zhao, Y., Wang, X.-Y., Luo, Y.-W., Chen, L., et al. (2023b). Small cytosolic double-stranded DNA represses cyclic GMP-AMP synthase activation and induces autophagy. Cell Rep. 42 (8), 112852. doi:10.1016/j.celrep.2023.112852
Liu, Y., Crowe, W. N., Wang, L., Lu, Y., Petty, W. J., Habib, A. A., et al. (2019). An inhalable nanoparticulate STING agonist synergizes with radiotherapy to confer long-term control of lung metastases. Nat. Commun. 10 (1), 5108. doi:10.1038/s41467-019-13094-5
Liu, Y., Yao, X., Zhao, Y., Fang, D., Shi, L., Yang, L., et al. (2023c). Mechanotransduction in response to ECM stiffening impairs cGAS immune signaling in tumor cells. Cell Rep. 42 (10), 113213. doi:10.1016/j.celrep.2023.113213
Luo, M., Liu, Z., Zhang, X., Han, C., Samandi, L. Z., Dong, C., et al. (2019). Synergistic STING activation by PC7A nanovaccine and ionizing radiation improves cancer immunotherapy. J. Control. Release 300, 154–160. doi:10.1016/j.jconrel.2019.02.036
Luo, T., Nash, G. T., Jiang, X., Feng, X., Mao, J., Liu, J., et al. (2022). A 2D nanoradiosensitizer enhances radiotherapy and delivers STING agonists to potentiate cancer immunotherapy. Adv. Mater. 34 (39), 2110588. doi:10.1002/adma.202110588
Luteijn, R. D., Zaver, S. A., Gowen, B. G., Wyman, S. K., Garelis, N. E., Onia, L., et al. (2019). SLC19A1 transports immunoreactive cyclic dinucleotides. Nature 573 (7774), 434–438. doi:10.1038/s41586-019-1553-0
Ma, Z., Li, X., Mao, Y., Wei, C., Huang, Z., Li, G., et al. (2022). Interferon-dependent SLC14A1+ cancer-associated fibroblasts promote cancer stemness via WNT5A in bladder cancer. Cancer Cell 40 (12), 1550–1565.e7. doi:10.1016/j.ccell.2022.11.005
Mackenzie, K. J., Carroll, P., Martin, C.-A., Murina, O., Fluteau, A., Simpson, D. J., et al. (2017). cGAS surveillance of micronuclei links genome instability to innate immunity. Nature 548 (7668), 461–465. doi:10.1038/nature23449
Maltbaek, J. H., Cambier, S., Snyder, J. M., and Stetson, D. B. (2022). ABCC1 transporter exports the immunostimulatory cyclic dinucleotide cGAMP. Immunity 55 (10), 1799–1812.e4. doi:10.1016/j.immuni.2022.08.006
Mekers, V. E., Kho, V. M., Ansems, M., and Adema, G. J. (2022). cGAS/cGAMP/STING signal propagation in the tumor microenvironment: key role for myeloid cells in antitumor immunity. Radiotherapy Oncol. 174, 158–167. doi:10.1016/j.radonc.2022.07.014
Moore, C., Hsu, C.-C., Chen, W.-M., Chen, B. P. C., Han, C., Story, M., et al. (2021). Personalized ultrafractionated stereotactic adaptive radiotherapy (PULSAR) in preclinical models enhances single-agent immune checkpoint blockade. Int. J. Radiat. Oncology*Biology*Physics 110 (5), 1306–1316. doi:10.1016/j.ijrobp.2021.03.047
Motedayen Aval, L., Pease, J. E., Sharma, R., and Pinato, D. J. (2020). Challenges and opportunities in the clinical development of STING agonists for cancer immunotherapy. J. Clin. Med. 9 (10), 3323. doi:10.3390/jcm9103323
Motwani, M., Pesiridis, S., and Fitzgerald, K. A. (2019). DNA sensing by the cGAS–STING pathway in health and disease. Nat. Rev. Genet. 20 (11), 657–674. doi:10.1038/s41576-019-0151-1
Ngwa, W., Irabor, O. C., Schoenfeld, J. D., Hesser, J., Demaria, S., and Formenti, S. C. (2018). Using immunotherapy to boost the abscopal effect. Nat. Rev. Cancer 18 (5), 313–322. doi:10.1038/nrc.2018.6
Olagnier, D., Brandtoft, A. M., Gunderstofte, C., Villadsen, N. L., Krapp, C., Thielke, A. L., et al. (2018). Nrf2 negatively regulates STING indicating a link between antiviral sensing and metabolic reprogramming. Nat. Commun. 9 (1), 3506. doi:10.1038/s41467-018-05861-7
Panne, D., Maniatis, T., and Harrison, S. C. (2007). An atomic model of the interferon-beta enhanceosome. Cell 129 (6), 1111–1123. doi:10.1016/j.cell.2007.05.019
Patel, R. B., Hernandez, R., Carlson, P., Grudzinski, J., Bates, A. M., Jagodinsky, J. C., et al. (2021). Low-dose targeted radionuclide therapy renders immunologically cold tumors responsive to immune checkpoint blockade. Sci. Transl. Med. 13 (602), eabb3631. doi:10.1126/scitranslmed.abb3631
Patrushev, M., Kasymov, V., Patrusheva, V., Ushakova, T., Gogvadze, V., and Gaziev, A. I. (2006). Release of mitochondrial DNA fragments from brain mitochondria of irradiated mice. Mitochondrion 6 (1), 43–47. doi:10.1016/j.mito.2005.12.001
Pedley, R. B., Boden, J. A., Boden, R., Boxer, G. M., Flynn, A. A., Keep, P. A., et al. (1996). Ablation of colorectal xenografts with combined radioimmunotherapy and tumor blood flow-modifying agents. Cancer Res. 56 (14), 3293–3300.
Pépin, G., De Nardo, D., Rootes, C. L., Ullah, T. R., Al-Asmari, S. S., Balka, K. R., et al. (2020). Connexin-dependent transfer of cGAMP to phagocytes modulates antiviral responses. mBio 11 (1), e03187-19. doi:10.1128/mbio.03187-19
Ritchie, C., Cordova, A. F., Hess, G. T., Bassik, M. C., and Li, L. (2019). SLC19A1 is an importer of the immunotransmitter cGAMP. Mol. Cell 75 (2), 372–381. doi:10.1016/j.molcel.2019.05.006
Rodriguez-Ruiz, M. E., Buqué, A., Hensler, M., Chen, J., Bloy, N., Petroni, G., et al. (2019). Apoptotic caspases inhibit abscopal responses to radiation and identify a new prognostic biomarker for breast cancer patients. OncoImmunology 8 (11), e1655964. doi:10.1080/2162402x.2019.1655964
Rodríguez-Villanueva, J., and McDonnell, T. J. (1995). Induction of apoptotic cell death in non-melanoma skin cancer by interferon-alpha. Int. J. Cancer 61 (1), 110–114. doi:10.1002/ijc.2910610119
Schadt, L., Sparano, C., Schweiger, N. A., Silina, K., Cecconi, V., Lucchiari, G., et al. (2019). Cancer-cell-intrinsic cGAS expression mediates tumor immunogenicity. Cell Rep. 29 (5), 1236–1248. doi:10.1016/j.celrep.2019.09.065
Schlaff, C. D., Krauze, A., Belard, A., O’Connell, J. J., and Camphausen, K. A. (2014). Bringing the heavy: carbon ion therapy in the radiobiological and clinical context. Radiat. Oncol. 9 (1), 88. doi:10.1186/1748-717x-9-88
Shang, G., Zhang, C., Chen, Z. J., Bai, X.-C., and Zhang, X. (2019). Cryo-EM structures of STING reveal its mechanism of activation by cyclic GMP–AMP. Nature 567 (7748), 389–393. doi:10.1038/s41586-019-0998-5
Shi, X., Yang, Y., Zhang, W., Wang, J., Xiao, D., Ren, H., et al. (2022). FLASH X-ray spares intestinal crypts from pyroptosis initiated by cGAS-STING activation upon radioimmunotherapy. Proc. Natl. Acad. Sci. U. S. A. 119 (43), e2208506119. doi:10.1073/pnas.2208506119
Sivick, K. E., Desbien, A. L., Glickman, L. H., Reiner, G. L., Corrales, L., Surh, N. H., et al. (2018). Magnitude of therapeutic STING activation determines CD8+ T cell-mediated anti-tumor immunity. Cell Rep. 25 (11), 3074–3085. doi:10.1016/j.celrep.2018.11.047
Stagg, J., Golden, E., Wennerberg, E., and Demaria, S. (2023). The interplay between the DNA damage response and ectonucleotidases modulates tumor response to therapy. Sci. Immunol. 8 (85), eabq3015. doi:10.1126/sciimmunol.abq3015
Su, T., Zhang, Y., Valerie, K., Wang, X.-Y., Lin, S., and Zhu, G. (2019). STING activation in cancer immunotherapy. Theranostics 9 (25), 7759–7771. doi:10.7150/thno.37574
Sun, L., Wu, J., Du, F., Chen, X., and Chen, Z. J. (2013). Cyclic GMP-AMP synthase is a cytosolic DNA sensor that activates the type I interferon pathway. Science 339 (6121), 786–791. doi:10.1126/science.1232458
Tanaka, Y., and Chen, Z. J. (2012). STING specifies IRF3 phosphorylation by TBK1 in the cytosolic DNA signaling pathway. Sci. Signal. 5 (214), ra20. doi:10.1126/scisignal.2002521
Tkach, M., Thalmensi, J., Timperi, E., Gueguen, P., Névo, N., Grisard, E., et al. (2022). Extracellular vesicles from triple negative breast cancer promote pro-inflammatory macrophages associated with better clinical outcome. Proc. Natl. Acad. Sci. 119 (17), e2107394119. doi:10.1073/pnas.2107394119
Vanpouille-Box, C., Alard, A., Aryankalayil, M. J., Sarfraz, Y., Diamond, J. M., Schneider, R. J., et al. (2017). DNA exonuclease Trex1 regulates radiotherapy-induced tumour immunogenicity. Nat. Commun. 8, 15618. doi:10.1038/ncomms15618
Wang, C., Sun, Z., Zhao, C., Zhang, Z., Wang, H., Liu, Y., et al. (2021). Maintaining manganese in tumor to activate cGAS-STING pathway evokes a robust abscopal anti-tumor effect. J. Control. Release 331, 480–490. doi:10.1016/j.jconrel.2021.01.036
Wang, L., Li, S., Wang, K., Wang, N., Liu, Q., Sun, Z., et al. (2022). DNA mechanical flexibility controls DNA potential to activate cGAS-mediated immune surveillance. Nat. Commun. 13 (1), 7107. doi:10.1038/s41467-022-34858-6
Welsh, J. W., Tang, C., De Groot, P., Naing, A., Hess, K. R., Heymach, J. V., et al. (2019). Phase II trial of ipilimumab with stereotactic radiation therapy for metastatic disease: outcomes, toxicities, and low-dose radiation–related abscopal responses. Cancer Immunol. Res. 7 (12), 1903–1909. doi:10.1158/2326-6066.cir-18-0793
Wilson, W., Li, A., Cowan, D. M., and Siim, B. (1998). Enhancement of tumor radiation response by the antivascular agent 5,6-dimethylxanthenone-4-acetic acid. Int. J. Radiat. Oncology*Biology*Physics 42 (4), 905–908. doi:10.1016/S0360-3016(98)00358-7
Woo, S.-R., Corrales, L., Spranger, S., Michael, M., Furdyna, M. J., Leung, M. Y. K., et al. (2014). STING-dependent cytosolic DNA sensing mediates innate immune recognition of immunogenic tumors. Immunity 41 (5), 830–842. doi:10.1016/j.immuni.2014.10.017
Wu, J., Sun, L., Chen, X., Du, F., Shi, H., Chen, C., et al. (2013). Cyclic GMP-AMP is an endogenous second messenger in innate immune signaling by cytosolic DNA. Science 339 (6121), 826–830. doi:10.1126/science.1229963
Wu, Y.-T., Fang, Y., Wei, Q., Shi, H., Tan, H., Deng, Y., et al. (2022). Tumor-targeted delivery of a STING agonist improves cancer immunotherapy. Proc. Natl. Acad. Sci. 119 (49), e2214278119. doi:10.1073/pnas.2214278119
Xue, A., Shang, Y., Jiao, P., Zhang, S., Zhu, C., He, X., et al. (2022). Increased activation of cGAS-STING pathway enhances radiosensitivity of non-small cell lung cancer cells. Thorac. Cancer 13 (9), 1361–1368. doi:10.1111/1759-7714.14400
Yamazaki, T., Kirchmair, A., Sato, A., Buqué, A., Rybstein, M., Petroni, G., et al. (2020). Mitochondrial DNA drives abscopal responses to radiation that are inhibited by autophagy. Nat. Immunol. 21 (10), 1160–1171. doi:10.1038/s41590-020-0751-0
Yan, J., Wang, G., Xie, L., Tian, H., Li, J., Li, B., et al. (2022a). Engineering radiosensitizer-based metal-phenolic networks potentiate STING pathway activation for advanced radiotherapy. Adv. Mater. 34 (10), 2105783. doi:10.1002/adma.202105783
Yan, M., Li, Y., Luo, Q., Zeng, W., Shao, X., Li, L., et al. (2022b). Mitochondrial damage and activation of the cytosolic DNA sensor cGAS–STING pathway lead to cardiac pyroptosis and hypertrophy in diabetic cardiomyopathy mice. Cell Death Discov. 8 (1), 258. doi:10.1038/s41420-022-01046-w
Yang, H., Wang, H., Ren, J., Chen, Q., and Chen, Z. J. (2017). cGAS is essential for cellular senescence. Proc. Natl. Acad. Sci. 114 (23), E4612–E4620. doi:10.1073/pnas.1705499114
Yang, M., Liu, H., Lou, J., Zhang, J., Zuo, C., Zhu, M., et al. (2024). Alpha-emitter radium-223 induces STING-dependent pyroptosis to trigger robust antitumor immunity. Small 20 (9), 2307448. doi:10.1002/smll.202307448
Yano, H., Iemura, A., Haramaki, M., Ogasawara, S., Takayama, A., Akiba, J., et al. (1999). Interferon alfa receptor expression and growth inhibition by interferon alfa in human liver cancer cell lines. Hepatology 29 (6), 1708–1717. doi:10.1002/hep.510290624
Yum, S., Li, M., and Chen, Z. J. (2020). Old dogs, new trick: classic cancer therapies activate cGAS. Cell Res. 30 (8), 639–648. doi:10.1038/s41422-020-0346-1
Yum, S., Li, M., Fang, Y., and Chen, Z. J. (2021). TBK1 recruitment to STING activates both IRF3 and NF-κB that mediate immune defense against tumors and viral infections. Proc. Natl. Acad. Sci. 118 (14), e2100225118. doi:10.1073/pnas.2100225118
Zaidi, A. H., Kelly, R. J., Gorbunova, A., Omstead, A. N., Salvitti, M. S., Zheng, P., et al. (2021). Intratumoral immunotherapy with STING agonist, ADU-S100, induces CD8+ T-cell mediated anti-tumor immunity in an esophageal adenocarcinoma model. Oncotarget 12 (4), 292–303. doi:10.18632/oncotarget.27886
Zhang, P., Rashidi, A., Zhao, J., Silvers, C., Wang, H., Castro, B., et al. (2023). STING agonist-loaded, CD47/PD-L1-targeting nanoparticles potentiate antitumor immunity and radiotherapy for glioblastoma. Nat. Commun. 14 (1), 1610. doi:10.1038/s41467-023-37328-9
Zhang, W., Li, G., Luo, R., Lei, J., Song, Y., Wang, B., et al. (2022). Cytosolic escape of mitochondrial DNA triggers cGAS-STING-NLRP3 axis-dependent nucleus pulposus cell pyroptosis. Exp. Mol. Med. 54 (2), 129–142. doi:10.1038/s12276-022-00729-9
Zhang, X., Wu, J., Du, F., Xu, H., Sun, L., Chen, Z., et al. (2014). The cytosolic DNA sensor cGAS forms an oligomeric complex with DNA and undergoes switch-like conformational changes in the activation loop. Cell Rep. 6 (3), 421–430. doi:10.1016/j.celrep.2014.01.003
Zhao, B., Du, F., Xu, P., Shu, C., Sankaran, B., Bell, S. L., et al. (2019). A conserved PLPLRT/SD motif of STING mediates the recruitment and activation of TBK1. Nature 569 (7758), 718–722. doi:10.1038/s41586-019-1228-x
Zhao, B., Shu, C., Gao, X., Sankaran, B., Du, F., Shelton, C. L., et al. (2016). Structural basis for concerted recruitment and activation of IRF-3 by innate immune adaptor proteins. Proc. Natl. Acad. Sci. 113 (24), E3403–E3412. doi:10.1073/pnas.1603269113
Zheng, Z., Jia, S., Shao, C., and Shi, Y. (2020). Irradiation induces cancer lung metastasis through activation of the cGAS–STING–CCL5 pathway in mesenchymal stromal cells. Cell Death Dis. 11 (5), 326. doi:10.1038/s41419-020-2546-5
Zhou, C., Chen, X., Planells-Cases, R., Chu, J., Wang, L., Cao, L., et al. (2020). Transfer of cGAMP into bystander cells via LRRC8 volume-regulated anion channels augments STING-mediated interferon responses and anti-viral immunity. Immunity 52 (5), 767–781. doi:10.1016/j.immuni.2020.03.016
Keywords: CGAS, STING, radiation, review, immunotherapy, oncology, STING agonists, microenvironment
Citation: Colangelo NW, Gerber NK, Vatner RE and Cooper BT (2024) Harnessing the cGAS-STING pathway to potentiate radiation therapy: current approaches and future directions. Front. Pharmacol. 15:1383000. doi: 10.3389/fphar.2024.1383000
Received: 06 February 2024; Accepted: 15 March 2024;
Published: 10 April 2024.
Edited by:
Souren Paul, University of Minnesota Twin Cities, United StatesReviewed by:
Lindsey Sloan, University of Minnesota Twin Cities, United StatesCopyright © 2024 Colangelo, Gerber, Vatner and Cooper. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Benjamin T. Cooper, YmVuamFtaW4uY29vcGVyQG55dWxhbmdvbmUub3Jn
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.