- 1Beijing Dongfang Hospital, Beijing University of Traditional Chinese Medicine, Beijing, China
- 2Beijing Hospital of Traditional Chinese Medicine, Affiliated with Capital Medical University, Beijing, China
- 3Beijing Institute of Traditional Chinese Medicine, Beijing, China
Purpose: This meta-analysis aims to identify whether patients with sepsis who have persistent tachycardia despite initial resuscitation can benefit from ultrashort-acting β-blockers.
Materials and methods: Relevant studies from MEDLINE, the Cochrane Library, and Embase were searched by two independent investigators. RevMan version 5.3 (Cochrane Collaboration) was used for statistical analysis.
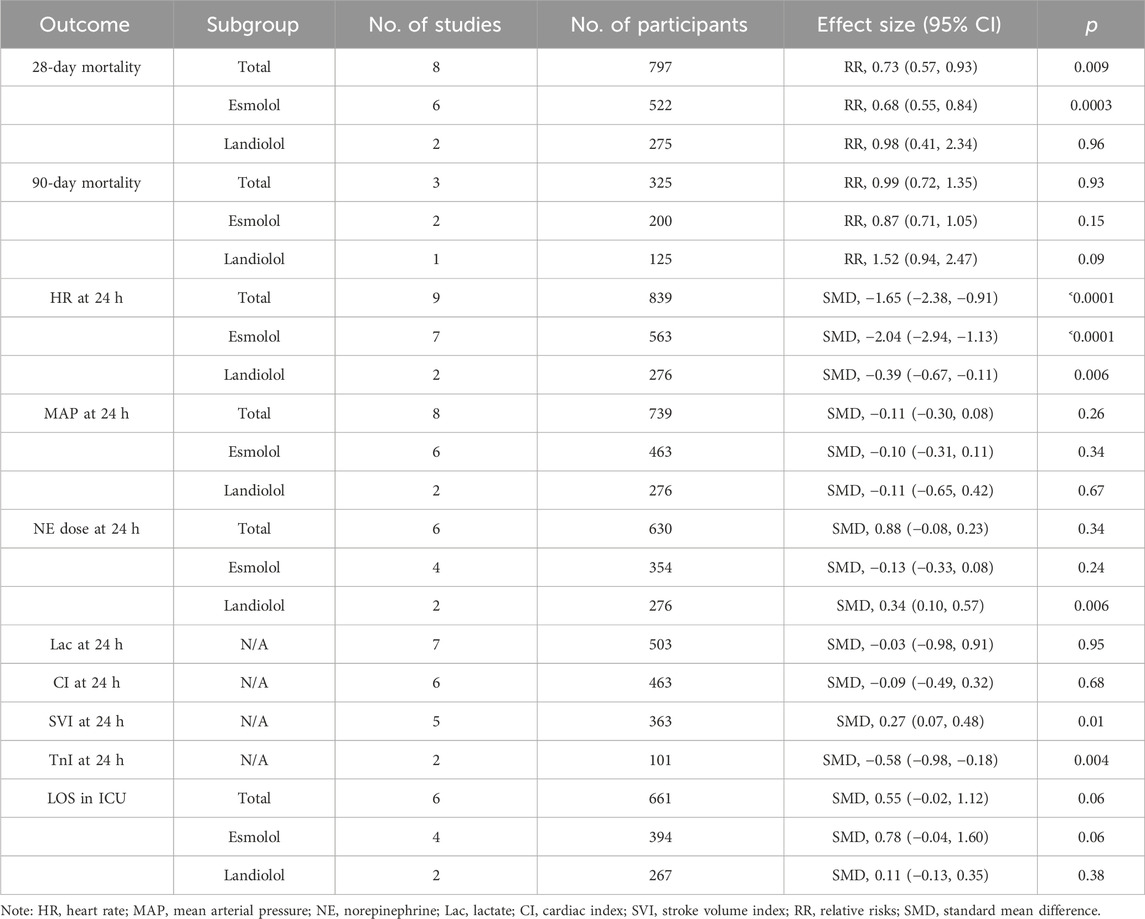
Results: A total of 10 studies were identified and incorporated into the meta-analysis. The results showed that the administration of ultrashort-acting β-blockers (esmolol/landiolol) in patients with sepsis with persistent tachycardia despite initial resuscitation was significantly associated with a lower 28-day mortality rate (risk ratio [RR], 0.73; 95% confidence interval [CI], 0.57–0.93; and p˂0.01). Subgroup analysis showed that the administration of esmolol in patients with sepsis was significantly associated with a lower 28-day mortality rate (RR, 0.68; 95% CI, 0.55–0.84; and p˂0.001), while there was no significant difference between the landiolol and control groups (RR, 0.98; 95% CI, 0.41–2.34; and p = 0.96). No significant differences between the two groups were found in 90-day mortality, mean arterial pressure (MAP), lactate (Lac) level, cardiac index (CI), and troponin I (TnI) at 24 h after enrollment.
Conclusion: The meta-analysis indicated that the use of esmolol in patients with persistent tachycardia, despite initial resuscitation, was linked to a notable reduction in 28-day mortality rates. Therefore, this study advocates for the consideration of esmolol in the treatment of sepsis in cases where tachycardia persists despite initial resuscitation.
Introduction
Currently, sepsis remains a life-threatening condition in emergency and critical care medicine due to its dysregulated inflammatory response to infection (Singer M et al., 2016; Hollenberg and Steven, 2023). Despite early active bundle treatment, mortality rates remain high (Li et al., 2022; Weng et al., 2023). Traditionally, β-blockers have been viewed as having negative effects on myocardial inotropy and the heart rate, potentially impacting hemodynamics and reducing cardiac output in sepsis patients. This has led to debates regarding the use of β-blockers in this patient population. However, recent research studies (Parker et al., 1987; Kuipers et al., 2014; Walkey et al., 2014; Hayase et al., 2016) have indicated that tachycardia significantly increases sepsis mortality, and a reduction in the heart rate (within 24 h) can lead to improved outcomes (Parker et al., 1987). As a result, there is a growing interest in studying the effectiveness of β-blockers in managing septic tachycardia (Walkey et al., 2014; Yang et al., 2023).
Previous research has demonstrated the effectiveness of esmolol in achieving target heart rates and reducing the 28-day mortality of septic patients with tachycardia (Morelli et al., 2013). Subsequent meta-analyses have further supported the benefits of esmolol in sepsis/septic shock patients (Liu et al., 2018; Huang et al., 2021). The potential therapeutic advantages of landiolol, a highly selective β1 receptor blocker, in septic patients with tachycardia warrant additional investigation (Lall et al., 2021; Gangl et al., 2022). A recent multicenter clinical trial (J-Land 3S) revealed that although landiolol did not decrease the 28-day mortality of septic patients, it did significantly lower their heart rates, allowing more patients to reach the desired 60–94 beats/min range within 24 h without an increase in adverse events (Kakihana et al., 2020). However, another multicenter clinical trial (Whitehouse et al., 2023) found that administering landiolol to sepsis patients did not decrease the SOFA score but increased the 28-day mortality and adverse event rates. Consequently, the use of landiolol in sepsis patients is not recommended. Therefore, it is crucial to systematically assess the efficacy of esmolol and landiolol in sepsis. Hasegawa et al. (2021) addressed this, but they evaluated esmolol and landiolol together without a separate analysis of landiolol.
A significant concern about the use of β1-blockers for sepsis is the potential decrease in cardiac output, which may result in hypotension. Therefore, the treatment of tachycardia in septic shock remains a topic of controversy. There is ongoing debate regarding the suitability of both esmolol and landiolol for patients with sepsis and tachycardia, given that they are both β1-blockers. This study aims to evaluate esmolol and landiolol as distinct interventions to determine their impact on the prognosis of sepsis patients with tachycardia.
Materials and methods
This study adhered to the recommendations and checklist outlined in the Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) statement (Moher et al., 2009). The protocol was registered in PROSPERO (www.crd.york.ac.uk) on 15 January 2024 (Registration ID: CRD42024497520).
Search strategy
The databases PubMed, Embase, and the Cochrane Library were searched from their inception to 3 January 2024 using keywords such as esmolol, landiolol, sepsis, and randomized controlled trial (RCT). The theme of word search for tachycardia was initially considered in our search, resulting in 44 items. However, we were concerned that this search strategy might be too narrow and could potentially overlook important clinical trials. To broaden the search scope, we implemented the current search method, ultimately yielding 122 items, as shown in Supplementary Figure S1.
Eligibility criteria of original studies and study selection
In this study, the inclusion criteria consisted of patients aged 18 years or older with sepsis exhibiting persistent tachycardia despite initial resuscitation (after 24 h of hemodynamic optimization aimed at establishing an adequate circulating blood volume, a mixed venous oxygen saturation higher than 65%, and a mean arterial pressure [MAP] of 65 mmHg or higher, while their heart rate persisted at 95/min or higher), meeting sepsis-1, sepsis-2, or sepsis-3 definitions. The interventions included IV esmolol/landiolol, with the control being a placebo or no intervention. Primary outcomes focused on 28-day mortality, while secondary outcomes included 90-day mortality, heart rate (HR), MAP at 24 h post-enrollment treatment, norepinephrine (NE) dose, lactate level, cardiac index (CI), stroke volume index (SVI), troponin I (TnI), length of stay (LOS) in the ICU, and adverse events. The study design was an RCT, with abstracts and titles screened by two independent reviewers (HX and HP), and any disagreements were resolved through discussion to reach a consensus. The authors of the included trials were contacted for clarification when needed.
Data extraction
A pre-defined data extraction form was used in this study, with two reviewers (HX and HP) independently extracting information such as the first author, published year, sample size, intervention, control, and outcomes from the selected trials. Any discrepancies between the two reviewers were resolved through discussion with a third reviewer until a consensus was reached.
Bias risk in individual trials
The methodological quality of the studies was evaluated by independent reviewers (HX and HP) to assess the risk of bias. Any disagreements were resolved through further discussions. In cases where reaching a conclusion was challenging, a third reviewer (XXL) reviewed the entire article and participated in the discussion. The risk of bias in each trial was assessed using the Cochrane Collaboration tool for evaluating bias in randomized trials.
Statistical analysis
Statistical analysis was conducted using RevMan version 5.3 from the Cochrane Collaboration. The Cochrane Handbook of Systematic Reviews guided the selection of risk ratios (RRs) and 95% confidence intervals (CIs) for dichotomous outcome evaluation, while the mean difference and its 95% CI were used for continuous outcomes. Between-study heterogeneity was assessed using an I2 test, where 25% or lower indicated low heterogeneity, 50% indicated moderate heterogeneity, and 75% indicated high heterogeneity. The fixed-effects model was used in cases of no or low heterogeneity, and pooled RRs were calculated using the Mantel–Haenszel method. Publication bias was investigated if there were more than 10 studies for a specific outcome. A significance level of p < 0.05 was applied. Trial sequential analysis (TSA) was conducted for subgroup analysis of esmolol using TSA viewer version 0.9 Beta (http://www.ctu.dk/tsa/). The parameters used were as follows: the control group had a mortality rate of 50%, the experimental group had a mortality rate of 35%, a two-sided α = 0.05 for the difference test, and 1-β = 0.8.
Results
Description and risk of bias of included studies
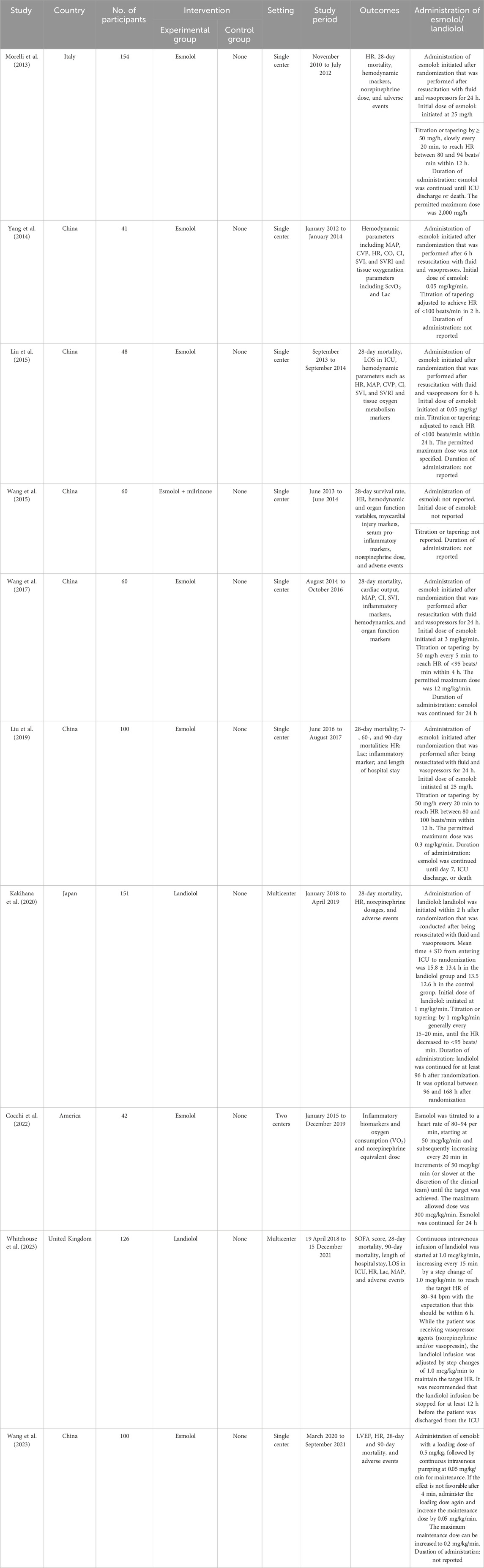
A total of 213 records were identified using the search strategy, with 89 potentially eligible records obtained after removing duplicates. Following the screening of titles and abstracts, 57 studies were excluded, resulting in the inclusion of 10 studies (Morelli et al., 2013; Yang et al., 2014; Liu et al., 2015; Wang et al., 2015; Wang et al., 2017; Liu et al., 2019; Kakihana et al., 2020; Cocchi et al., 2022; Wang et al., 2023; Whitehouse et al., 2023) involving 881 participants (Figure 1). The characteristics of these included studies are given in Table 1. The risk of bias in these studies was assessed using the Cochrane Collaboration tool, with the results given in Supplementary Figure S2. All 10 studies (Morelli et al., 2013; Yang et al., 2014; Liu et al., 2015; Wang et al., 2015; Wang et al., 2017; Liu et al., 2019; Kakihana et al., 2020; Cocchi et al., 2022; Wang et al., 2023; Whitehouse et al., 2023) were deemed to have low risk in terms of random sequence generation, incomplete outcome data, and selective reporting. Five studies (Liu et al., 2019; Kakihana et al., 2020; Cocchi et al., 2022; Wang et al., 2023; Whitehouse et al., 2023) were considered to have a low risk for allocation concealment, while this could not be assessed in the other five studies (Morelli et al., 2013; Yang et al., 2014; Liu et al., 2015; Wang et al., 2015; Wang et al., 2017) due to insufficient information. One study (Wang et al., 2017) was rated as low risk, while five (Morelli et al., 2013; Wang et al., 2015; Liu et al., 2019; Kakihana et al., 2020; Cocchi et al., 2022) were rated as high risk for blinding of participants and personnel. Similarly, two studies (Kakihana et al., 2020; Cocchi et al., 2022) were deemed high risk and four (Morelli et al., 2013; Wang et al., 2015; Liu et al., 2019; Whitehouse et al., 2023) were deemed low risk for blinding of outcome assessment, with the assessment being inconclusive for the remaining four studies (Yang et al., 2014; Liu et al., 2015; Wang et al., 2017; Wang et al., 2023) due to inadequate information. In terms of other biases, four studies (Yang et al., 2014; Kakihana et al., 2020; Cocchi et al., 2022; Whitehouse et al., 2023) were conducted at multiple centers and considered low risk, while the remaining six studies (Morelli et al., 2013; Liu et al., 2015; Wang et al., 2015; Wang et al., 2017; Liu et al., 2019; Wang et al., 2023) conducted at a single center were deemed high risk.
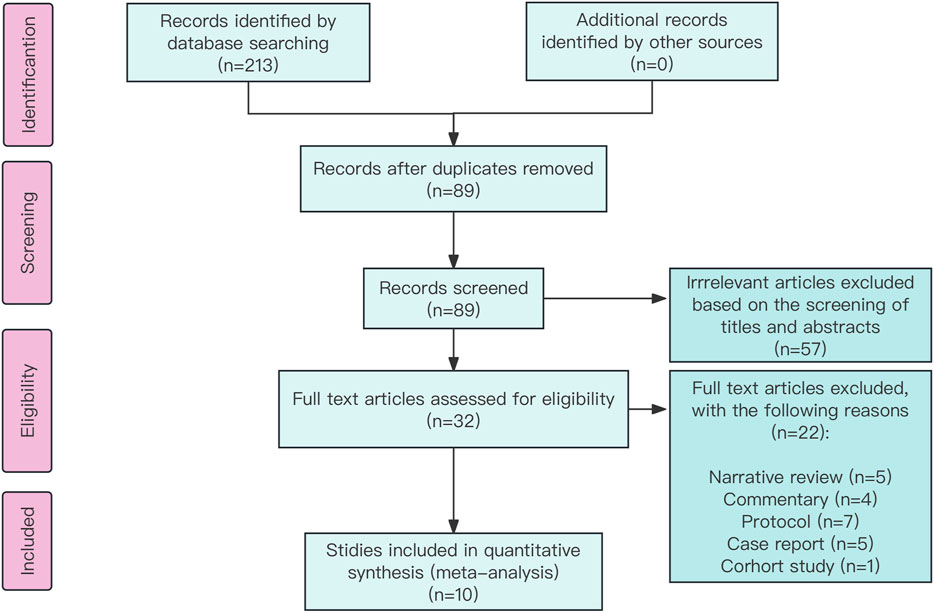
Figure 1. Preferred Reporting Items for Systematic Reviews and Meta-Analyses chart: identification and selection of studies for inclusion.
Primary outcome
A total of 8 (Morelli et al., 2013; Liu et al., 2015; Wang et al., 2015; Wang et al., 2017; Liu et al., 2019; Kakihana et al., 2020; Wang et al., 2023; Whitehouse et al., 2023) out of 10 RCTs with 797 participants reported 28-day mortality outcomes. The results indicated that administering ultrashort-acting β-blockers (esmolol/landiolol) to patients with sepsis who had persistent tachycardia despite initial resuscitation was significantly associated with a lower 28-day mortality rate (RR: 0.73; 95% CI: 0.57–0.93; and p < 0.01). Notably, subgroup analysis revealed differing outcomes. The use of esmolol in sepsis patients was significantly linked to reduced 28-day mortality (RR: 0.68; 95% CI: 0.55–0.84; and p < 0.001), whereas there was no significant difference between the landiolol and control groups (RR: 0.98; 95% CI: 0.41–2.34; and p = 0.96) (see Figure 2). The funnel plot is shown in Supplementary Figure S3.
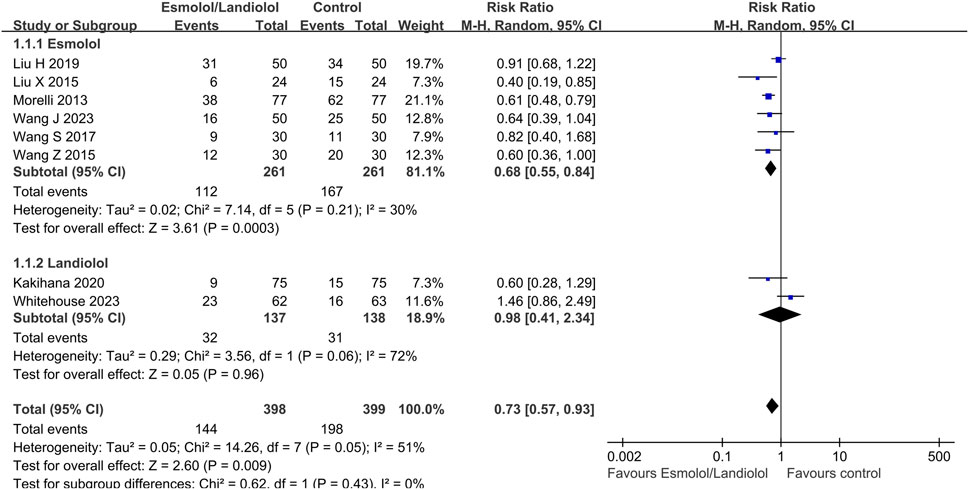
Figure 2. Forest plot of esmolol or landiolol group vs. control group: 28-day mortality. M-H: Mantel–Haenszel.
Secondary outcomes
The study evaluated secondary outcomes including 90-day mortality (Figure 3A), heart rate (Figure 3B), mean arterial pressure (Figure 3C), norepinephrine dose (Figure 3D), lactate levels (Figure 4A), cardiac index (Figure 4B), stroke volume index (Figure 4C), and troponin I levels (Figure 4D) at 24 h after enrollment, and length of stay in the ICU (Figure 5).

Figure 3. Forest plot of esmolol or landiolol group vs. control group: 90-day mortality, heart rate, MAP, and NE dose at 24 h after enrollment. (A) 90-day mortality; (B) heart rate at 24 h after enrollment; (C) MAP at 24 h after enrollment; and (D) NE dose at 24 h after enrollment. MAP, mean arterial pressure; NE, norepinephrine. IV, inverse variance; Std., standardized.
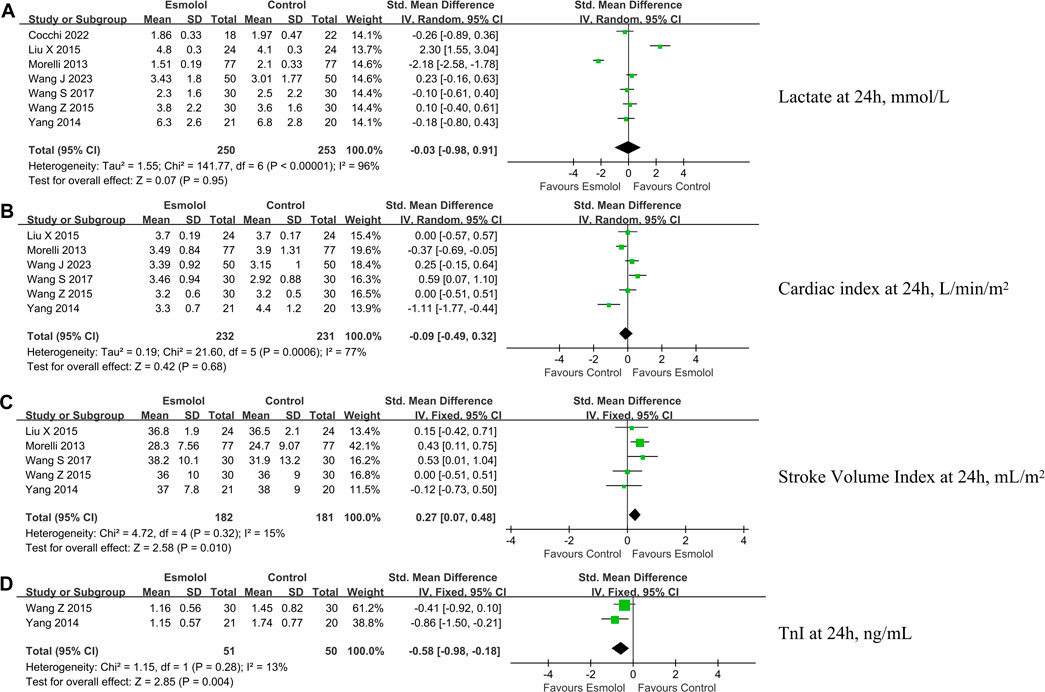
Figure 4. Forest plot of esmolol group vs. control group: Lac, CI, SVI, and TnI at 24 h after enrollment. (A) Lac at 24 h after enrollment; (B) CI at 24 h after enrollment; (C) SVI at 24 h after enrollment; and (D) TnI at 24 h after enrollment. Lac, lactate; CI, cardiac index; SVI, stroke volume index. IV, inverse variance; Std., standardized.
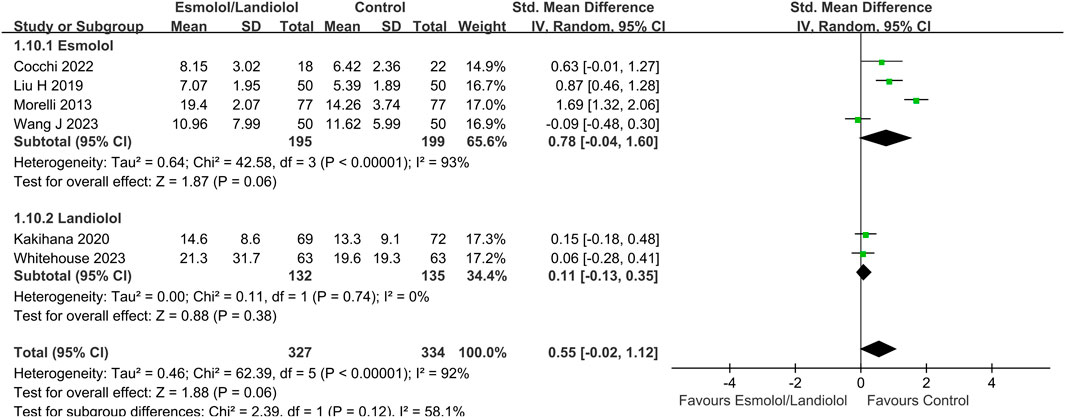
Figure 5. Forest plot of esmolol or landiolol group vs. control group: LOS in ICU. LOS, length of stay; ICU, intensive care unit; IV, inverse variance; Std., standardized.
Four studies (Morelli et al., 2013; Kakihana et al., 2020; Wang et al., 2023; Whitehouse et al., 2023) examined adverse events, with one (Morelli et al., 2013) showing no adverse events in either group and another reporting asymptomatic bradycardia in the experimental group but no significant arrhythmias in the control group. The remaining two studies (Kakihana et al., 2020; Whitehouse et al., 2023) reported adverse events in both the landiolol and control groups. One study (Kakihana et al., 2020) reported that adverse events were observed in 9 (12%) of the 77 patients in the landiolol group and 8 (11%) of the 74 patients in the control group. Another study (Whitehouse et al., 2023) reported that adverse events were observed in 17.5% (10/63) of those receiving landiolol and 12.7% (8/63) of those receiving standard care.
Trial sequential analysis
The TSA results are shown in Figure 6. A total of 6 clinical trials (Morelli et al., 2013; Liu et al., 2015; Wang et al., 2015; Wang et al., 2017; Liu et al., 2019; Wang et al., 2023) involving 522 patients were included in the esmolol subgroup meta-analysis, with an actual sample size of 586 cases. The TSA results indicated that the cumulative Z-curve crossed both the traditional and TSA boundary values simultaneously, leading to a positive conclusion being reached before the expected sample size. This suggests that for patients with sepsis who continue to experience tachycardia after initial fluid resuscitation, esmolol provides a 28-day survival advantage with accurate evidence.
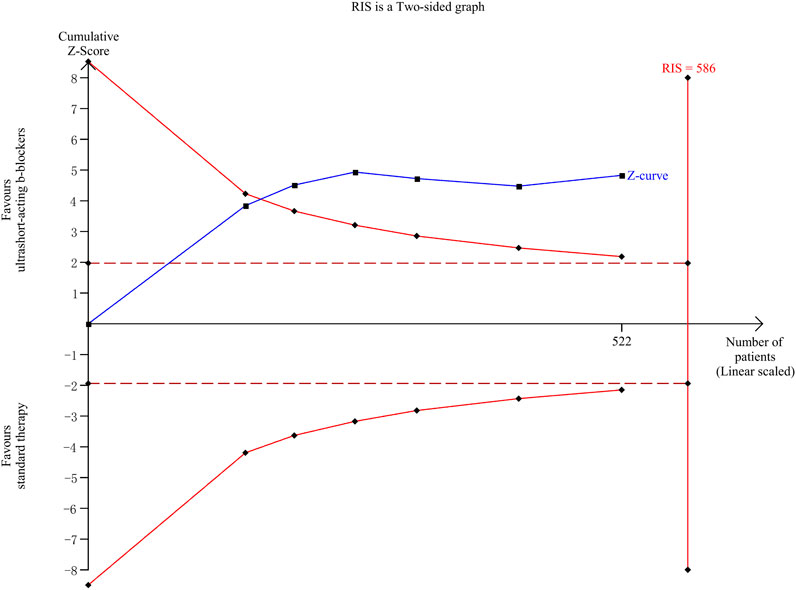
Figure 6. Trial sequential analysis. RIS, required information size. The solid blue line represents the cumulative Z-curve; two symmetrical red solid line curves represent the TSA boundary value; the deep red horizontal dashed line represents the traditional threshold value Z = 1.96; and the red vertical line on the right side represents RIS.
Discussion
The meta-analysis revealed that the use of ultrashort-acting β-blockers (esmolol/landiolol) in septic patients with persistent tachycardia post-resuscitation was linked to reduced 28-day mortality. Subgroup analysis indicated esmolol as the preferred choice for these patients, while the limited sample size prevented the identification of survival benefits with landiolol. This study addresses existing controversies and offers valuable insights for clinical decision-making. The summary of meta-analysis was shown in Table 2.
According to the Surviving Sepsis Guideline (Evans et al., 2021), norepinephrine is recommended as a first-line drug for treating septic shock to maintain stable hemodynamics. However, it is well-recognized that norepinephrine use can elevate catecholamine levels, potentially leading to cardiac dysfunction due to sympathetic hyperstimulation (Monnet et al., 2011; Jozwiak, 2022). Studies (Havaldar, 2018; Prescott Hallie and Angus Derek, 2018) have highlighted that cardiac dysfunction plays a crucial role in the poor prognosis of septic patients, with tachycardia increasing cardiac workload and myocardial oxygen consumption, contributing to cardiac dysfunction (Lanspa et al., 2017; Suzuki et al., 2017). Recent research studies have indicated that reducing the heart rate of septic patients to a specific level, alongside adequate fluid resuscitation and circulatory stability, can effectively enhance patient outcomes. A randomized open-label clinical trial conducted by Morelli et al. (2013) and published in JAMA in 2013 yielded positive results. The study demonstrated that all patients in the esmolol group achieved the target heart rate, with a higher cardiac stroke volume index than that in the control group, aligning with our study's findings.
The traditional belief that β-blockers are unsuitable for patients with sepsis/septic shock due to their cardiac suppressive effects is being challenged. This study compared the effects of esmolol and landiolol on the mean arterial pressure, showing no significant difference compared to the control group. However, the landiolol group may require a higher dose of norepinephrine to maintain a relatively stable mean arterial pressure. Both esmolol and landiolol, as selective β1-blockers with a rapid onset of action, require dose titration for optimal bradycardic effects. Landiolol is also approved for treating ventricular fibrillation or tachycardia (Sakamoto et al., 2012; Nagai et al., 2013; Taenaka and Kikawa, 2013). Evidence from case reports, retrospective studies, and animal research supports the use of landiolol for sepsis-related tachyarrhythmias (Okajima et al., 2015; Mathieu et al., 2018; Gangl et al., 2022). Two clinical trials (Kakihana et al., 2020; Whitehouse et al., 2023) specifically evaluated the efficacy of landiolol in sepsis treatment. The J-land 3S study (Kakihana et al., 2020) showed a higher proportion of patients achieving target heart rates in the landiolol group than in the control group, with a significant reduction in new arrhythmias. The results showed that a total of 41 patients (55%) in the landiolol group had a heart rate of 60–94 beats/min 24 h after enrollment, while only 25 patients (33%) in the control group showed the same. There was a statistical difference between the two groups (p = 0.0031), and the incidence of new arrhythmias within 7 days was significantly reduced (9% vs. 25%, p = 0.015). However, the 28-day mortality rates did not significantly differ between the landiolol and control groups. Another clinical study (Whitehouse et al., 2023) published in JAMA in 2023 (STRESS-L) did not support the use of landiolol in sepsis. The result showed that there was no statistically significant difference between the average SOFA score of the landiolol group (8.8 ± 3.9) and that of the control group (8.1 ± 3.2) (p = 0.24). In addition, the 28-day mortality (37.1%) and 90-day mortality (43.5%) rates in the landiolol group were higher than those in the control group (25.4% and 14.9%), but there was no statistical difference between the groups (p ˃ 0.05). More importantly, the incidence of serious adverse events in the landiolol group (25.4%) was significantly higher than that of the control group (6.4%), with a statistical difference between the groups (p = 0.006).
A previous meta-analysis (Hasegawa et al., 2021) examined the impact of β-blockers on the mortality of patients with sepsis and tachycardia. Our study differs from this previous analysis by conducting a subgroup analysis specifically focusing on the effects of esmolol and landiolol on the mortality of patients with sepsis and tachycardia. Our findings indicate that esmolol significantly reduces the 28-day mortality rate in these patients, whereas landiolol does not show the same effect. Furthermore, the study reveals that landiolol necessitated an increased dose of norepinephrine to maintain mean arterial pressure, suggesting an indirect impact on the hemodynamics of patients with sepsis and tachycardia. Therefore, our study advocates for the use of esmolol in treating patients with septic tachycardia, aligning with findings from previous research. However, the limited sample size prevented the identification of survival benefits with landiolol.
Conclusion
The meta-analysis indicated that the use of esmolol in patients with persistent tachycardia, despite initial resuscitation, was linked to a notable reduction in 28-day mortality rates. Therefore, this study advocates for the consideration of esmolol in the treatment of sepsis in cases where tachycardia persists despite initial resuscitation.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material; further inquiries can be directed to the corresponding authors.
Author contributions
PH: data curation, formal analysis, software, writing–original draft, and writing–review and editing. FL: data curation and writing–review and editing. XH: data curation and writing–review and editing. BL: methodology, software, and writing–review and editing. XX: supervision and writing–review and editing. QL: funding acquisition, supervision, validation, visualization, and writing–review and editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. The present study was supported by the National Natural Science Foundation of China (No. 82205070).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2024.1380175/full#supplementary-material
References
Cocchi, M. N., Dargin, J., Chase, M., Patel, P. V., Grossestreuer, A., Balaji, L., et al. (2022). Esmolol to treat the hemodynamic effects of septic shock: a randomized controlled trial. Shock 57, 508–517. doi:10.1097/SHK.0000000000001905
Evans, L., Rhodes, A., Waleed, A., Antonelli, M., Coopersmith, C. M., French, C., et al. (2021). Surviving sepsis campaign: international guidelines for management of sepsis and septic shock 2021. Intensive Care Med. 47, 1181–1247. doi:10.1007/s00134-021-06506-y
Gangl, C., Krychtiuk Konstantin, A., Schoenbauer, R., and Speidl, W. S. (2022). Landiolol for refractory tachyarrhythmias in the intensive care unit: case reports. Eur. Heart J. Suppl. 24, D43–D49. doi:10.1093/eurheartjsupp/suac026
Hasegawa, D., Ryota, S., Narut, P., Nishida, K., Takahashi, K., Yatabe, T., et al. (2021). Effect of ultrashort-acting β-blockers on mortality in patients with sepsis with persistent tachycardia despite initial resuscitation: a systematic review and meta-analysis of randomized controlled trials. Chest 159, 2289–2300. doi:10.1016/j.chest.2021.01.009
Havaldar, A. A. (2018). Evaluation of sepsis induced cardiac dysfunction as a predictor of mortality. Cardiovasc Ultrasound 16, 31. doi:10.1186/s12947-018-0149-4
Hayase, N., Yamamoto, M., Asada, T., Isshiki, R., Yahagi, N., and Doi, K. (2016). Association of heart rate with N-terminal pro-B-type natriuretic peptide in septic patients: a prospective observational cohort study. Shock 46, 642–648. doi:10.1097/SHK.0000000000000673
Hollenberg Steven, M. (2023). β-Blockers in patients with sepsis: putting the puzzle together, piece by piece. JAMA 330, 1627–1628. doi:10.1001/jama.2023.20455
Huang, P., Zheng, X., Liu, Z., and Fang, X. (2021). The efficacy and safety of esmolol for septic shock: a systematic review and meta-analysis of randomized controlled trials. Front. Pharmacol. 12, 682232. doi:10.3389/fphar.2021.682232
Jozwiak, M. (2022). Alternatives to norepinephrine in septic shock: which agents and when? J. Intensive Med. 2, 223–232. doi:10.1016/j.jointm.2022.05.001
Kakihana, Y., Nishida, O., Takumi, T., Okajima, M., Morimatsu, H., Ogura, H., et al. (2020). Efficacy and safety of landiolol, an ultra-short-acting β1-selective antagonist, for treatment of sepsis-related tachyarrhythmia (J-Land 3S): a multicentre, open-label, randomised controlled trial. Lancet Respir. Med. 8, 863–872. doi:10.1016/S2213-2600(20)30037-0
Kuipers, S., Klein Klouwenberg, P. M., and Cremer, O. L. (2014). Incidence, risk factors and outcomes of new-onset atrial fibrillation in patients with sepsis: a systematic review. Crit. Care 18, 688. doi:10.1186/s13054-014-0688-5
Lall, R., Dipesh, M., Emma, S., Boota, N., Regan, S., Bion, J., et al. (2021). Study into the reversal of septic shock with landiolol (beta blockade): STRESS-L Study protocol for a randomised trial. BMJ Open 11, e043194. doi:10.1136/bmjopen-2020-043194
Lanspa, M. J., Sajid, S., Andrew, H., Wilson, E. L., Olsen, T. D., Hirshberg, E. L., et al. (2017). Associations among left ventricular systolic function, tachycardia, and cardiac preload in septic patients. Ann. Intensive Care 7, 17. doi:10.1186/s13613-017-0240-2
Li, A., Lowell, L., Hanyu, Q., Arabi, Y. M., Myatra, S. N., Egi, M., et al. (2022). Epidemiology, management, and outcomes of sepsis in ICUs among countries of differing national wealth across asia. Am. J. Respir. Crit. Care Med. 206, 1107–1116. doi:10.1164/rccm.202112-2743OC
Liu, H., Ding, X. F., Zhang, S. G., Wang, H. X., Luo, Y. G., Duan, X. G., et al. (2019). Effect of esmolol in septic shock patients with tachycardia: a randomized clinical trial. Zhonghua Yi Xue Za Zhi 99 (17), 1317–1322. doi:10.3760/cma.j.issn.0376-2491.2019.17.009
Liu, P., Qi, W., Tang, Y., Zhou, Z., and Feng, M. (2018). The influence of esmolol on septic shock and sepsis: a meta-analysis of randomized controlled studies. Am. J. Emerg. Med. 36, 470–474. doi:10.1016/j.ajem.2017.11.013
Liu, X., Huang, W., Wen, M., Wenxin, Z., Wenqiang, J., Shenglong, C., et al. (2015). Esmolol improves clinical outcome and tissue oxygen metabolism in patients with septic shock through controlling heart rate. Chin. Crit. Care Med. 27 (9), 759–763.
Mathieu, C., Martine, D., Frank, K., Lalevée, N., Lan, C., Fourny, N., et al. (2018). Sex-mediated response to the beta-blocker landiolol in sepsis: an experimental, randomized study. Crit. Care Med. 46, e684–e691. doi:10.1097/CCM.0000000000003146
Moher, D., Liberati, A., Tetzlaff, J., and Altman, D. G.PRISMA Group (2009). Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann. Intern Med. 151 (4), 264–W64. doi:10.7326/0003-4819-151-4-200908180-00135
Monnet, X., Julien, J., Julien, M., Richard, C., and Teboul, J. L. (2011). Norepinephrine increases cardiac preload and reduces preload dependency assessed by passive leg raising in septic shock patients. Crit. Care Med. 39, 689–694. doi:10.1097/CCM.0b013e318206d2a3
Morelli, A., Ertmer, C., Westphal, M., Rehberg, S., Kampmeier, T., Ligges, S., et al. (2013). Effect of heart rate control with esmolol on hemodynamic and clinical outcomes in patients with septic shock: a randomized clinical trial. JAMA 310 (16), 1683–1691. doi:10.1001/jama.2013.278477
Nagai, R., Kinugawa, K., Inoue, H., Atarashi, H., Seino, Y., Yamashita, T., et al. (2013). Urgent management of rapid heart rate in patients with atrial fibrillation/flutter and left ventricular dysfunction: comparison of the ultra-short-acting β1-selective blocker landiolol with digoxin (J-Land Study). Circ. J. 77, 908–916. doi:10.1253/circj.cj-12-1618
Okajima, M., Masayuki, T., and Takumi, T. (2015). Landiolol, an ultra-short-acting β1-blocker, is useful for managing supraventricular tachyarrhythmias in sepsis. World J. Crit. Care Med. 4, 251–257. doi:10.5492/wjccm.v4.i3.251
Parker, M. M., Shelhamer, J. H., Natanson, C., Alling, D. W., and Parrillo, J. E. (1987). Serial cardiovascular variables in survivors and nonsurvivors of human septic shock: heart rate as an early predictor of prognosis. Crit. Care Med. 15, 923–929. doi:10.1097/00003246-198710000-00006
Prescott Hallie, C., and Angus Derek, C. (2018). Enhancing recovery from sepsis: a review. JAMA 319, 62–75. doi:10.1001/jama.2017.17687
Sakamoto, A., Kitakaze, M., Takamoto, S., Namiki, A., Kasanuki, H., Hosoda, S., et al. (2012). Landiolol, an ultra-short-acting β₁-blocker, more effectively terminates atrial fibrillation than diltiazem after open heart surgery: prospective, multicenter, randomized, open-label study (JL-KNIGHT study). Circ. J. 76, 1097–1101. doi:10.1253/circj.cj-11-1332
Singer, M., Deutschman, C. S., Seymour, C. W., Shankar-Hari, M., Annane, D., Bauer, M., et al. (2016). The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA 315, 801–810. doi:10.1001/jama.2016.0287
Suzuki, T., Suzuki, Y., Jun, O., Kurazumi, T., Suhara, T., Ueda, T., et al. (2017). Sepsis-induced cardiac dysfunction and β-adrenergic blockade therapy for sepsis. J. Intensive Care 5, 22. doi:10.1186/s40560-017-0215-2
Taenaka, N., and Kikawa, S. (2013). The effectiveness and safety of landiolol hydrochloride, an ultra-short-acting β1-blocker, in postoperative patients with supraventricular tachyarrhythmias: a multicenter, randomized, double-blind, placebo-controlled study. Am. J. Cardiovasc Drugs 13, 353–364. doi:10.1007/s40256-013-0035-2
Walkey, A. J., Hammill, B. G., Curtis, L. H., and Benjamin, E. J. (2014). Long-term outcomes following development of new-onset atrial fibrillation during sepsis. Chest 146, 1187–1195. doi:10.1378/chest.14-0003
Wang, J., Gao, X., He, Z., Xu, G., and Li, T. (2023). Evaluating the effects of Esmolol on cardiac function in patients with Septic cardiomyopathy by Speck-tracking echocardiography-a randomized controlled trial. BMC Anesthesiol. 23, 51. doi:10.1186/s12871-023-01983-8
Wang, S., Li, M., Duan, J., Yi, L., Huang, X., Chen, D., et al. (2017). Effect of esmolol on hemodynamics and clinical outcomes in patients with septic shock. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue 29 (5), 390–395. doi:10.3760/cma.j.issn.2095-4352.2017.05.002
Wang, Z., Wu, Q., Nie, X., Guo, J., and Yang, C. (2015). Combination therapy with milrinone and esmolol for heart protection in patients with severe sepsis: a prospective, randomized trial. Clin. Drug Investig. 35 (11), 707–716. doi:10.1007/s40261-015-0325-3
Weng, L., Yang, X., Peng, Y., Wang, Y., Chen, Y., Liu, W., et al. (2023). National incidence and mortality of hospitalized sepsis in China. Crit. Care 27, 84. doi:10.1186/s13054-023-04385-x
Whitehouse, T., Hossain, A., Perkins Gavin, D., Gordon, A. C., Bion, J., Young, D., et al. (2023). Landiolol and organ failure in patients with septic shock: the STRESS-L randomized clinical trial. JAMA 330, 1641–1652. doi:10.1001/jama.2023.20134
Yang, Q., Kong, T., Ziping, B., Yang, S., Chen, X., Zheng, J., et al. (2023). Association between the β-blocker use and patients with sepsis: a cohort study. Front. Med. (Lausanne) 10, 1272871. doi:10.3389/fmed.2023.1272871
Keywords: ultrashort-acting β-blockers, mortality, sepsis, tachycardia, meta-analysis
Citation: Huang P, Liu F, Hu X, Li B, Xu X and Liu Q (2024) Effect of ultrashort-acting β-blockers on 28-day mortality in patients with sepsis with persistent tachycardia despite initial resuscitation: a meta-analysis of randomized controlled trials and trial sequential analysis. Front. Pharmacol. 15:1380175. doi: 10.3389/fphar.2024.1380175
Received: 19 February 2024; Accepted: 20 May 2024;
Published: 20 June 2024.
Edited by:
Marek Nalos, Nepean Hospital, AustraliaReviewed by:
Arnoldo Santos, University Hospital Fundación Jiménez Díaz, SpainKaiquan Tan, MOH Holdings, Singapore
Copyright © 2024 Huang, Liu, Hu, Li, Xu and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaolong Xu, eGlhb2xvbmdfeHUzMDEzQDEyNi5jb20=; Qingquan Liu, bGl1cWluZ3F1YW5fMjAwM0AxMjYuY29t
 Po Huang
Po Huang Fusheng Liu1
Fusheng Liu1 Xiaolong Xu
Xiaolong Xu Qingquan Liu
Qingquan Liu