
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pharmacol., 03 May 2024
Sec. Cardiovascular and Smooth Muscle Pharmacology
Volume 15 - 2024 | https://doi.org/10.3389/fphar.2024.1378010
Objective: As a novel drug formulation, antibody drug conjugates (ADCs) are widely used in various types of cancer. However, clinically, there is a lack of attention to the CVD produced by them, as well as a lack of research on the real-world situation. Using the Food and Drug Administration Adverse Event Reporting System (FAERS) database, to ensure its clinical safety application, we analyzed post-marketing data on antitumor ADCs to identify risk factors and drugs associated with the risk of cardiovascular events.
Research design and methods: We used OpenVigil 2.1 to conduct a database query for adverse events (AEs) reported to the FAERS database between the time the drug was launched and the second quarter of 2023. Cardiovascular adverse events (AEs) were grouped into fourteen narrow categories using the Standardized Medical Dictionary for Regulatory Activities (MedDRA) Queries (SMQs), and the reporting odds ratio (ROR) and the proportional reporting ratio (PRR) for reporting the association between different drugs and cardiovascular disease (CVD) risk were calculated.
Results: In the FAERS database, 1863 AEs associated with CVD we studied were identified in patients receiving ADC therapy. Most reports came from people aged ≥65, but a significant number of cases were found to be unknown. The number of patients with antibody-drug conjugates (ADCs)-related CVD cases aged <18 years, 18–64 years, and≥ 65 years was 52 (2.79%), 586 (31.45%), and 613 (32.90%), respectively. The proportion of female patients (834, 44.77%) was higher than that of male patients (752, 40.37%). Death (770 reports), disability (9 reports), Hospitalization initial or prolonged (407 reports), and life-threatening reactions (187 reports). Of the 770 deaths reported, 103 (31.7%) were associated with brentuximab vedotin, 10 (24.4%) with sacituzumab govitecan, 22 (19.3%) with enfortumab vedotin, and 35 (34.7%) with trastuzumab emtansine.49 (41.2%) cases were associated with polatuzumab vedotin, 62 (29%) with trastuzumab deruxtecan, 423 (54.3%) with gemtuzumab ozogamicin, and 66 (38.8%) with inotuzumab ozogamicin. In a disproportionate number of SMQS, cardiac failure (n = 277) and embolic and thrombotic events, venous (n = 446) were the most frequently reported CVD-related AEs in ADCs.
Conclusion: By mining the FAERS database, we provided relevant information on the association between ADC use and cardiovascular-associated AEs. ADCs were associated with increased cardiovascular toxicity, deserving distinct monitoring and appropriate management. Further research is needed to confirm these findings and assess causality.
Antibody drug conjugates (ADCs), as a new type of anti-cancer drugs, are widely used in various cancers, including hematologic neoplasms and solid tumors, and have been described as a “magic bullets” for cancer treatment (Chen et al., 2022; Johns and Campbell, 2022; Dumontet et al., 2023). ADC consists of a linker, payload, and monoclonal antibody (mAb) (Fu et al., 2022). It combines the advantages of high-specific targeting ability and strong killing effect, enabling precise and efficient elimination of cancer cells, and has become one of the hotspots in anticancer drug research and development (Fu et al., 2022). Due to the fact that, compared to traditional cytotoxic chemotherapy, ADCs enhance the cytotoxic effect and targeting ability, while reducing the toxicity of anti-tumor drugs, ADCs have been developed (Beck et al., 2017). However, although ADCs are a type of targeted chemotherapy, higher toxicity and adverse side effects have been reported (Tarantino et al., 2022). Adverse cardiovascular events will have a serious impact on the quality of life and long-term survival time of patients and may also cause serious and life-threatening outcomes (Markina et al., 2023), which will seriously affect the prognosis of tumor patients. Evidence from the FAERS database suggests that ADCs cause cardiovascular toxicity. A recent study showed that the cardiotoxicity of ADCs with a payload of Trastuzumab deruxtecan (T-DXd) was consistently reported (Soares et al., 2023). Although ADCs are commonly prescribed and widely used in clinical settings, the risk of their development into CVD remains controversial. In some studies, the correlation between Trastuzumab deruxtecan and Trastuzumab emtansine and cardiotoxicity has been reported; however, it has not yet been confirmed (Ma et al., 2023; Soares et al., 2023). In addition, the cardiotoxicity risk of other ADCs remains unclear.
Components of cardiovascular toxicity include cardiomyopathy and heart failure, myocarditis, arrhythmias, coronary artery disease, early-onset valvular disease, hypertension, and thromboembolism (Alexandre et al., 2020). In the past, there have been studies on the mining of ADCs adverse events based on the FAERS database, both at home and abroad. One study only mined the signal of adverse events for two ADCs; another study focused on liver injury from ADCs (Ma et al., 2023; Sun et al., 2023), but no study has yet analyzed cardiovascular adverse events related to ADCs using the FAERS database. Therefore, it is necessary for this study to attempt to analyze cardiovascular adverse events related to ADCs.
A comprehensive summary of ADCs based on real-world data is therefore necessary to identify safety risk signals, particularly adverse drug reactions (ADRs), that are not included in safety instructions or specifications. Limited information has been reported regarding the detection and evaluation of cardiovascular associations related to ADRs signals associated with ADCs. Currently, the FAERS database is the largest repository worldwide for spontaneously reported adverse events and is widely recognized for its extensive and standardized data. FAERS can be utilized for ADRs analysis, while data mining algorithms have been employed to conduct post-marketing safety monitoring and reevaluation of drugs from AEs databases. Hence, this pharmacovigilance analysis was designed to systematically investigate potential red flags indicating cardiovascular adverse events associated with ADCs. The objective of this study is to enhance the safe prescribing practices of ADCs by mining and evaluating relevant signals from the FAERS database, which contains anonymous patient information confirmed by hospital ethics committees as not requiring ethical approval.
Following approval from the U.S. Food and Drug Administration (FDA) in 2000, the first ADC, mylotarg (gemtuzumab ozogamicin), was introduced. Since then, a total of 15 ADCs have been approved by the FDA for treating various types of cancer (Table 1). This retrospective study analyzed publicly available AEs data from the FAERS database from the time after each drug was launched to the second quarter of 2023 using OpenVigil 2.1. This tool, intended for healthcare professionals, enables visual access to FAERS pharmacovigilance data. OpenVigil 2.1 is a web-based query tool for physicians and pharmacists that visually accesses FAERS pharmacovigilance data. The pharmacovigilance tool OpenVigil2.1 enables researchers to extract, clean, mine, and analyze structured AEs information directly from the FAERS database through an API, transforming large raw FAERS datasets into a form that can be extracted and analyzed (Böhm et al., 2012). The FAERS database pharmacovigilance tool OpenVigil2.1, embedded with MedDRA version 24.0, also provides a variety of AEs retrieval methods, including standard MedDRA analytical queries (broad), standard MedDRA analytical queries (narrow), high, preferred, and low. All searches in this study were conducted using standard MedDRA analytical queries (narrow) in the OpenVigil2.1 tool. The operation is relatively simple, and the data is summarized every quarter. In this study, we searched for the gender of the patient, the age at the onset of AEs, the date the report was received, the country in which it was reported, the event, and the outcome. However, the diversity of patient ethnicities and regions may affect the study’s generalizability.
Reports involving the fifteen antibody-drug conjugates (ADCs) were identified by a text-string search of each drug by generic name through the FDA public database during a data mining process. We then searched for 15 ADCs one by one, and finally selected eight FDA-approved ADCs. These include gemtuzumab ozogamicin, brentuximab vedotin, trastuzumab emtansine, inotuzumab ozogamicin, polatuzumab vedotin, enfortumab vedotin, trastuzumab deruxtecan, and sacituzumab govitecan. Used as investigational agents to identify AEs associated with cardiovascular disease. Extract AEs markers about these drugs as primary suspects. In openvigil 2.1, information is collected by selecting “ADCs” from the “drug” menu box and setting “role of drug” to “primary suspect.” For cardiovascular disease determination, we conducted a search based on the preferred terms of the Medical Dictionary for drug regulatory activities (MedDRA, version 24.0), through which we selected the categories of cardiovascular events that occurred at a higher rate. It was finally determined that in this study, cardiovascular AEs were divided into 14 narrow SMQs categories as follows:cardiac failure, hypertension, cardiomyopathy, (embolic and thrombotic events, venous), (embolic and thrombotic events, arterial), (embolic and thrombotic events, vessel type unspecified and mixed arterial and venous), haemorrhagic central nervous system vascular conditions, shock-associated circulatory or cardiac conditions (excl torsade de pointes), supraventricular tachyarrhythmias, ischaemic central nervous system vascular conditions, torsade de pointes/QT prolongation, ventricular tachyarrhythmias, other ischaemic heart disease, (cardiac arrhythmia terms, nonspecific) (Table 2). The operation in openvigil 2.1 is as follows: In the adverse event search module, we select “SMQ-narrow” and enter the customized event term “cardiovascular disease” described above. Finally, records were counted according to the Personal Safety Report (ISR). Clinical characteristics of patients were collected independently, including sex, age, report, country and date of receipt of the report, and AEs outcome.

Table 2. Cardiovascular adverse events grouped into 14 narrow categories of Standardized MedDRA Queries (SMQs) according to MedDRA 24.0.
In addition, disproportionation analysis (also known as case-non-case analysis) is a signal detection method based on two-by-two contingency tables (Table 3) that is widely used in pharmacovigilance studies (Ang et al., 2016). It detects potential adverse drug reaction signals by comparing the proportion of target events occurring with the proportion of target events occurring with all other drugs, a table that can be easily created on OpenVigil. Reporting odds ratio (ROR) and proportional reporting ratio of IC are calculated (PRR) to assess the association between adverse events and the drug, their calculation formula (Li et al., 2023), ROR = (a/c)/(b/d), ROR 95% CI = eln ROR ± 1.96 (1/a + 1/b + 1/c + 1/d) 0.5, signal detection threshold: If A ≥ 3 and the lower threshold of 95% CI is greater than 1, an ADR signal is generated. PRR = a/(a + b)]/[c/(c + d)], = (ad-bc) 2 (a + b + c + d)/(a + b) (c + d) (a + c) (b + d)], signal detection threshold: a ≥ 3, PRR≥2and
Among them, gemtuzumab ozogamicin reported 779 (41.81%) cases, brentuximab vedotin reported 325 (17.44%) cases, and trastuzumab emtansine reported 101 (5.42%) cases. There were 170 (9.13%) reports related to inotuzumab ozogamicin, 119 (6.39%) reports related to polatuzumab vedotin, 114 (6.12%) reports related to enfortumab vedotin, and 41 (2.2%) reports related to sacituzumab govitecan. Trastuzumab deruxtecan was associated with 214 (11.49%) cases (Supplementary Table S1). The characteristics of 1,863 reports of AEs associated with cardiovascular events submitted for ADCs are shown in (Table 4). Most reports came from people aged ≥65, but a significant number of cases were found to be unknown. The number of patients with antibody-drug conjugates (ADCs)-related CVD cases aged <18 years, 18–64 years, and ≥65 years was 52 (2.79%), 586 (31.45%), and 613 (32.90%), respectively. The proportion of female patients (834, 44.77%) was higher than that of male patients (752, 40.37%). The majority of these reports came from the United States (563, representing 30.22% of the total), followed by 449 (accounting for 24.10%) from Japan. Additionally, 156 reports (constituting 8.38%) were received from France and Canada, while 89 (comprising 4.78%) originated from Germany. Finally, 488 reports (representing 26.19% of the total) came from all other countries. AEs reports peaks in 2022 and 2023, with peaks of 369 and 223, respectively (Figure 1). In addition, adverse reactions leading to hospitalization, whether initial or prolonged, and death were most commonly reported. The characteristics of the AEs report are based on demographics and the severity of the results. In cardiovascular reports with ADCs, men treated with brentuximab vedotin, enfortumab vedotin, polatuzumab vedotin, inotuzumab ozogamicin, and gemtuzumab ozogamicin were more likely to develop CVD than women. However, men treated with trastuzumab emtansine, trastuzumab deruxtecan, and sacituzumab govitecan were less likely to develop CVD than women (Supplementary Table S2).
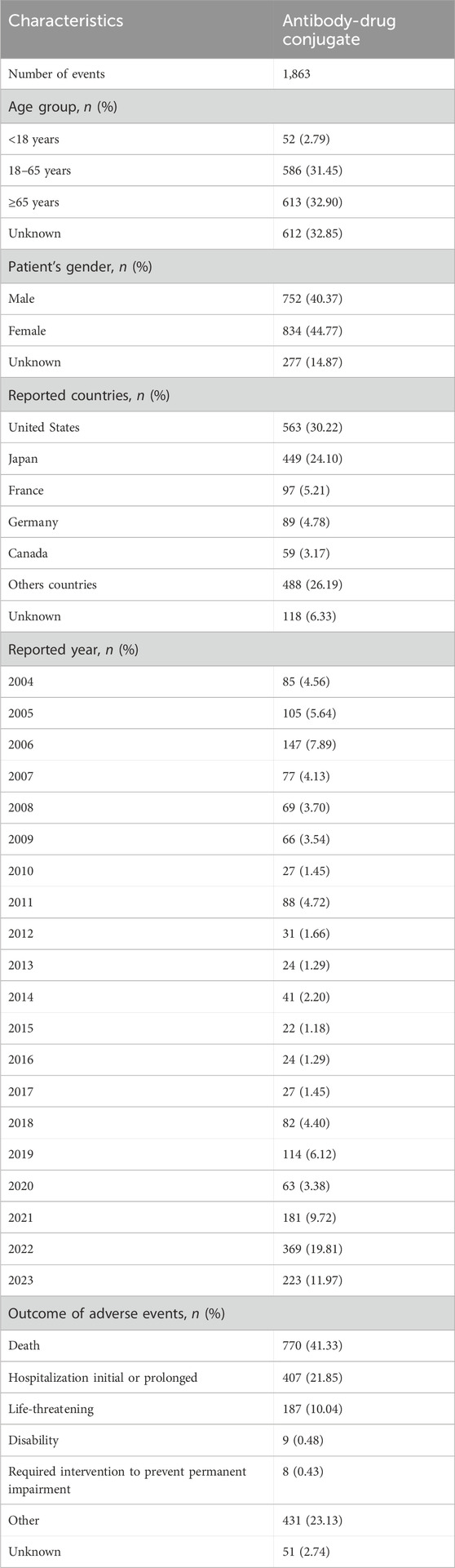
Table 4. Reported characteristics associated with eight antibody-drug conjugates (ADCs) from the first quarter of 2004 to the third quarter of 2023.
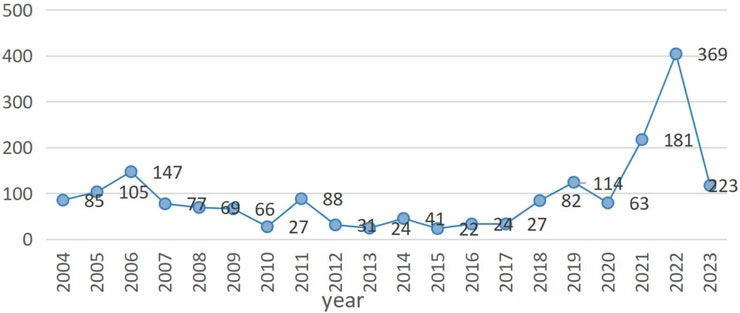
Figure 1. The number of 14 reported cardiovascular adverse events per year after marketing of antibody-drug conjugates (ADCs) in 14 narrow SMQ categories. Note: The blue line represents the eight antibody drug conjugates related to our research. In this figure shows the reported number of cardiovascular events associated with ADCs over time, from 2004 to the second quarter of 2023.
Gemtuzumab ozogamicin, brentuximab vedotin, trastuzumab emtansine, inotuzumab ozogamicin, polatuzumab vedotin, enfortumab vedotin, sacituzumab govitecan, and trastuzumab deruxtecan The number of CVD-related SMQS with suspicious signals was 10, 2, 5, 5, 9, 2, 3, and 3 (Supplementary Table S1), respectively. Embolic and thrombotic events, venous (n = 446), and cardiac failure (n = 277) were the most frequently reported CVD-related AEs in the ADCs class. These PTs differ in their signaling levels in the various ADCs. In terms of longitudinal self-comparison, we examined different signaling levels of cardiovascular disease SMQ across all reported ADC classes of drugs. Gemtuzumab ozogamicin found the most adverse signals among 14 narrow categories of SMQ. Gemtuzumab ozogamicin had the strongest signal for embolic and thrombotic events, venous. ROR and 95% CI, 11.806 (10.259; 13.587). Disproportionate analysis also revealed (Supplementary Table S1) that brentuximab vedotin had the strongest potential association with cardiac failure. ROR and 95% CI, 1.748 (1.351; 2.262) In addition, trastuzumab emtansine had the strongest potential association with cardiomyopathy, ROR and 95% CI, 4.766 (2.759; 8.235). Inotuzumab ozogamicin had the strongest signal for embolic and thrombotic events, venous. ROR and 95% CI, 29.67 (24.242; 36.314). Of the cardiovascular disease-related AEs associated with polatuzumab vedotin, ventricular tachyarrhythmias showed the strongest signal strength (ROR) and 95% CI, 2.302 (0.862; 6.144). Among cardiovascular disease-related AEs associated with enfortumab vedotin, embolic and thrombotic events, venous showed the strongest signal strength of ROR and 95% CI, 1.628 (1.023; 2.591). Among the cardiovascular disease-related adverse events associated with sacituzumab govitecan, ventricular tachyarrhythmias, venous showed the strongest signal strength ROR and 95% CI, 3.013 (0.969; 9.367). Among cardiovascular disease-related AEs associated with trastuzumab deruxtecan, cardiomyopathy showed the strongest signal strength, ROR and 95% CI, 4.433 (3.144; 6.251). Thus, ADCs show a strong signal in embolic and thrombotic events, venous. The other reporting odds ratio (ROR) and proportional reporting ratio (PRR) of the correlation between different drugs and cardiovascular disease (CVD) are shown in (Supplementary Table S1).
Of note, of all 14 narrow SMQ-class cardiovascular AEs associated with antibody-drug conjugates (ADCs), in 1863 ADC-associated CVD cases (Table 4), the final outcomes were as follows: Death 770 (41.3%)reports, disability 9 (0.5%)reports, Hospitalization initial or prolonged 407 (21.8%)reports, and life-threatening reactions187 (10.0%) reports. Of the 770 (41.3%) deaths reported, 103 (31.7%) were associated with brentuximab vedotin, 10 (24.4%) with sacituzumab govitecan, 22 (19.3%) with enfortumab vedotin, and 35 (34.7%) with trastuzumab emtansine.49 (41.2%) cases were associated with polatuzumab vedotin, 62 (29%) with trastuzumab deruxtecan, 423 (54.3%) with gemtuzumab ozogamicin, and 66 (38.8%) with inotuzumab ozogamicin (Supplementary Table S2). No actual cause-and-effect assessment was performed between ADCs and death, and the data only provided a safety signal, not a real risk. At most 770 (41.3%) deaths. Specifically, the outcomes of higher risk levels, death, life-threatening events, the proportion of hospitalizations corresponding to different signal-significant SMQS, and those resulting in longer hospitalizations. Notably, among the adverse events, hypertension (SMQ) accounts for the lowest proportion in both disability (0.05%) and life-threatening (0.27%) events. However, the adverse events that cause higher mortality are embolic and thrombotic events, venous (SMQ), cardiac failure (SMQ), haemorrhagic central nervous system vascular conditions (SMQ), embolic and thrombotic events, vessel type unspecified and mixed arterial and venous (SMQ), and shock-associated circulatory or cardiac conditions (excl torsade de pointes) (SMQ). The number of deaths caused by these adverse events is 166, 112, 110, 92, and 90, respectively.
With the escalating use of ADCs, an increasing number of adverse reactions related to cardiac toxicity have been reported. The toxic effects of ADCs can be divided into on-target toxicity and off-target toxicity. A typical example of ADC’s on-target toxicity is the cardiac toxicity of anti-HER2 drugs. Cardiotoxicity is a common toxicity of anti-HER2 drugs, which is usually manifested as a decrease in left ventricular ejection fraction (LVEF) and occasionally as congestive heart failure (CHF) (Pondé et al., 2016). The cardiac toxicity of anti-HER2 drugs may be related to the important role of HER2 signaling pathway in myocardial cell function (Pondé et al., 2016). In the EMILIA study, the incidence of LVEF decline >15% or LVEF <50% in the trastuzumab emtansine group was 1.7% (Bregni et al., 2016). Long-term follow-up results showed that the incidence of ≥ grade 3 cardiac insufficiency was <1% (Moilanen et al., 2018; Li et al., 2023). In the KATHERINE study, the incidence of cardiac events in the trastuzumab emtansine group was 0.1% (von Minckwitz et al., 2019). In addition, adverse reactions related to cardiac toxicity have also been reported in previous clinical trials of other ADCs, for instance. The safety and efficacy evaluation of gemtuzumab ozogamicin as monotherapy was performed in the AML-19 and MyloFrance-1 studies. The AEs that occurred were mainly cardiovascular, with a 6% incidence (Amadori et al., 2016). In addition, in a phase 3 trial of inotuzumab ozogamicin (InO), among patients undergoing follow-up HSCT, 53 of 79 (67.1%) in the InO group died of cardiac disease [4 of 29 (13.8%)] (Kantarjian et al., 2019). From the current clinical data, it can be seen that ADCs can lead to cardiovascular adverse reactions, its cardiac toxicity can seriously affect the survival and prognosis of patients. Therefore, it is important for us to raise awareness of its cardiac toxicity. It is also very important for clinicians to rationalize the use of ADCs in clinical practice, strengthen the effective management of adverse reactions to cardiac toxicity, and maximize the clinical effectiveness of the drugs, which also affects the survival and outcome of patients.
Currently, there is a lack of real-world data, and ADCs are gradually being launched in China and widely used in clinical practice. Therefore, we need to conduct signal mining and analysis based on databases to explore the cardiac safety issues after their launch, providing a reference for safe and rational clinical use of drugs. This study explores the risk status of ADCs from the perspective of signal risk by accessing cardiovascular AEs data from the FAERS database and conducting signal mining on the FAERS database. The results of this study showed that cardiovascular AEs were collected in 8 out of 15 ADCs. The reason for zero reports registering belantamab madotin (approved in 2020), tisatumab vedotin (approved in 2021), cetuximab saratolacan (approved in 2020), disitamab vedotin (approved in 2021), mirvetuximab soravtansine (approved in 2022), and loncastuximab tesirine (approved in 2021) is mainly due to the short time they have been on the market. The reason for zero reports reporting on moxetumab pasudotox may be related to the small number of patients who received treatment after the drug was marketed. Among the 1,863 disease lines collected from 8 ADCs, gemtuzumab ozogamycin had the highest number of cases, and gemtuzumab ozogamycin was strongly associated with CVD according to the PRR and ROR methods. PRR and ROR methods, The sacituzumab-govitecan signal is weak. This may be related to the short launch time, with gemtuzumab ozogamicin being the earliest to be launched. Sacituzumab govitecan was only approved for listing in 2020. However, the signals we observed indicate that the strength of signals corresponding to different cardiovascular types varies among different drugs. For example, gemtuzumab ozogamicin exhibits the strongest signal for embolic and thrombotic events, venous, while brentuximab vedotin demonstrates the strongest signal for cardiac failure. This suggests that the manifestations of cardiovascular toxicity vary among different drugs. According to the year-end analysis, the report reached its peak in 2022. The reason may be that over time, more and more ADCs have been launched, and in 2023 (Figure 1), there has been a decline. In fact, this is because only two-quarters were collected in 2023. Further research is needed to verify the actual needs. Most ADCs have been listed since 2011. Gemtuzumab ozogamicin (Wyeth/Pfizer) received accelerated approval for relapsed CD33+ acute myeloid leukemia (AML) in 2000 but was voluntarily withdrawn from the market in 2010 after post-marketing studies. In 2017, the FDA re-approved alternative doses of the drug.
Through gender and age analysis, the proportion of cardiovascular disease in women is higher than that in men; patients over 65 years old are more prone to cardiovascular AEs. The higher proportion of female patients may be related to the indications of ADC drugs. Trastuzumab deruxtecan and trastuzumab emtansine are used to treat breast cancer patients, which may be the reason for the higher number of female patients than male patients. Although the proportion of female patients is higher than male patients, it is currently unclear whether the actual proportion of men and women using these ADC drugs is balanced. The reason for the high proportion of patients over 65 years old may be that patients under 65 years old have better tolerance. Although the number of cardiovascular events caused by ADCs together is higher in patients over 65 years old and more in women than men, there are differences between different drugs. As far as the country is concerned, the United States has the highest number of reported cases. This may be related to the fact that most ADCs have been approved for marketing in the United States. In terms of outcomes, of the 1863 patients who developed ADC-related cardiovascular events, 770 (41.33%) died during treatment. The correlation between this death outcome and ADCs needs further research.
Therefore, our research indicates that there is a certain signal link between these eight ADCs and cardiovascular issues, which proves that these eight ADC drugs pose a cardiovascular risk. Maintaining a healthy cardiovascular system is essential for the survival and wellbeing of mammalian life. Because the development of cardiovascular disease (CVD) is a major determinant of human morbidity and mortality, cardiovascular disease (CVD) has become the leading cause of non-relapse-related death among cancer survivors (Wong-Siegel et al., 2023). Cardiovascular toxicity (CT) includes but is not limited to hypertension, arrhythmia, and cardiomyopathy, with 1,000 definitions of cardiovascular relevance initially screened in the International ICD10 Diagnostic Code. In our study, we also provided additional information on the association between ADC use and cardiovascular-associated AEs. Mortality from CVD and other causes is clinically significant in the long-term follow-up of cancer patients (Clèries et al., 2022). Because cardiovascular disease severity is common in patients, further research into ADC cardiovascular disease is of clear importance for human health. Our research is based on the FAERS database, but there are also some limitations in our study. These limitations include the following points. 1) Antibody-drug conjugates (ADCs) have been on the market for a short time worldwide, and ADR monitoring data are relatively incomplete. 2) The hypothesis generated by the pharmacovigilance database only observed signals, but it cannot verify the signal detection related to the hypothesis, and the relevant detection needs to be verified through prospective studies. 3) As the FAERS database is a self-reporting system, there may be omissions, biases, and inconsistencies in the vast amount of information, and the resulting biases may affect the outcome of data mining. Similar to all drug safety studies, this database does not provide diagnostic information, therefore, it is not possible to analyze and prove the relationship between adverse reactions and drugs. 4) Currently, ADCs are gradually being launched in China. Due to the short period of time since their launch and the limited types available, there is a lack of real-world data. However, as the use of these drugs increases domestically in the future, we can utilize domestic real-world data for further exploration. 5) The analysis based on the existing information in the FAERS database is not clear about whether patients have cardiovascular disease at baseline, which may affect the analysis of the results.
Although this study identified many adverse effects not mentioned in the description of the ADCs, causality needs to be further evaluated. Future studies can be considered.
The FAERS data mining has shown that cardiovascular AEs are associated with Ado-gemtuzumab ozogamicin, brentuximab vedotin, trastuzumab emtansine, inotuzumab ozogamicin, polatuzumab vedotin, enfortumab vedotin, trastuzumab deruxtecan, and sacituzumab govitecan. ADCs have not been analyzed in a large post-marketing population. Based on the FAERS database, the ADRs of ADCs can be deeply detected from various aspects, which can be used as a supplement to the drug label or specification. We recommend that clinicians regularly monitor ADCs and be alert for adverse reactions associated with cardiovascular disease in patients receiving ADCs. The safety of ADCs must be continually explored in the real world to better protect patients.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
PL: Writing–original draft, Visualization, Formal Analysis, Data curation. SL: Writing–review and editing. LP: Writing–review and editing. YW: Writing–review and editing, Supervision. WC: Supervision, Writing–review and editing. XW: Methodology, Conceptualization, Writing–review and editing, Supervision.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This research was financially supported by Chongqing medical scientific research project (Joint project of Chongqing Health Commission and Science and Technology Bureau) (grant numbers 2024QNXM016 and 2024ZDXM025), and the construction project of the key specialty of Chongqing clinical pharmacy.
All the data were available from the FAERS database managed by the U.S. FDA. The conclusion in our study do not represent the opinion of the FDA.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2024.1378010/full#supplementary-material
Alexandre, J., Cautela, J., Ederhy, S., Damaj, G. L., Salem, J. E., Barlesi, F., et al. (2020). Cardiovascular toxicity related to cancer treatment: a pragmatic approach to the American and European cardio-oncology guidelines. J. Am. Heart Assoc. 9 (18), e018403. doi:10.1161/JAHA.120.018403
Amadori, S., Suciu, S., Selleslag, D., Aversa, F., Gaidano, G., Musso, M., et al. (2016). Gemtuzumab ozogamicin versus best supportive care in older patients with newly diagnosed acute myeloid leukemia unsuitable for intensive chemotherapy: results of the randomized phase III EORTC-GIMEMA AML-19 trial. J. Clin. Oncol. 34 (9), 972–979. doi:10.1200/JCO.2015.64.0060
Ang, P. S., Chen, Z., Chan, C. L., and Tai, B. C. (2016). Data mining spontaneous adverse drug event reports for safety signals in Singapore - a comparison of three different disproportionality measures. Expert Opin. Drug Saf. 15 (5), 583–590. doi:10.1517/14740338.2016.1167184
Beck, A., Goetsch, L., Dumontet, C., and Corvaïa, N. (2017). Strategies and challenges for the next generation of antibody-drug conjugates. Nat. Rev. Drug Discov. 16 (5), 315–337. doi:10.1038/nrd.2016.268
Böhm, R., Höcker, J., Cascorbi, I., and Herdegen, T. (2012). OpenVigil--free eyeballs on AERS pharmacovigilance data. Nat. Biotechnol. 30 (2), 137–138. doi:10.1038/nbt.2113
Bregni, G., Galli, G., Gevorgyan, A., de Braud, F., and Di Cosimo, S. (2016). Trastuzumab cardiac toxicity: a problem we put our heart into. Tumori 102 (1), 1–5. doi:10.5301/tj.5000393
Chen, Y. F., Xu, Y. Y., Shao, Z. M., and Yu, K. D. (2022). Resistance to antibody-drug conjugates in breast cancer: mechanisms and solutions. Cancer Commun. 43 (3), 297–337. doi:10.1002/cac2.12387
Clèries, R., Ameijide, A., Buxó, M., Vilardell, M., Martínez, J. M., Font, R., et al. (2022). Ten-year probabilities of death due to cancer and cardiovascular disease among breast cancer patients diagnosed in north-eastern Spain. Int. J. Environ. Res. Public Health 20 (1), 405. doi:10.3390/ijerph20010405
Dumontet, C., Reichert, J. M., Senter, P. D., Lambert, J. M., and Beck, A. (2023). Antibody-drug conjugates come of age in oncology. Nat. Rev. Drug Discov. 22 (8), 641–661. doi:10.1038/s41573-023-00709-2
Fu, Z., Li, S., Han, S., Shi, C., and Zhang, Y. (2022). Antibody drug conjugate: the "biological missile" for targeted cancer therapy. Signal Transduct. Target Ther. 7 (1), 93. doi:10.1038/s41392-022-00947-7
Johns, A. C., and Campbell, M. T. (2022). Toxicities from antibody-drug conjugates. Cancer J. 28 (6), 469–478. doi:10.1097/PPO.0000000000000626
Kantarjian, H. M., DeAngelo, D. J., Stelljes, M., Liedtke, M., Stock, W., Gökbuget, N., et al. (2019). Inotuzumab ozogamicin versus standard of care in relapsed or refractory acute lymphoblastic leukemia: final report and long-term survival follow-up from the randomized, phase 3 INO-VATE study. Cancer 125 (14), 2474–2487. doi:10.1002/cncr.32116
Li, H., Zhang, M., Jiao, X., Zhu, Y., Liu, Y., Zeng, L., et al. (2023). Using disproportionality analysis to explore the association between periostitis and triazole antifungals in the FDA Adverse Event Reporting System Database. Sci. Rep. 13 (1), 4475. doi:10.1038/s41598-023-27687-0
Ma, P., Tian, H., Shi, Q., Liu, R., Zhang, Y., Qi, X., et al. (2023). High risks adverse events associated with trastuzumab emtansine and trastuzumab deruxtecan for the treatment of HER2-positive/mutated malignancies: a pharmacovigilance study based on the FAERS database. Expert Opin. Drug Saf. 22 (8), 685–696. doi:10.1080/14740338.2023.2204228
Markina, Y. V., Kirichenko, T. V., Tolstik, T. V., Bogatyreva, A. I., Zotova, U. S., Cherednichenko, V. R., et al. (2023). Target and cell therapy for atherosclerosis and CVD. Int. J. Mol. Sci. 24 (12), 10308. doi:10.3390/ijms241210308
Moilanen, T., Jokimäki, A., Tenhunen, O., and Koivunen, J. P. (2018). Trastuzumab-induced cardiotoxicity and its risk factors in real-world setting of breast cancer patients. J. Cancer Res. Clin. Oncol. 144 (8), 1613–1621. doi:10.1007/s00432-018-2682-9
Pondé, N. F., Lambertini, M., and de Azambuja, E. (2016). Twenty years of anti-HER2 therapy-associated cardiotoxicity. ESMO Open 1 (4), e000073. doi:10.1136/esmoopen-2016-000073
Soares, L. R., Vilbert, M., Rosa, V. D. L., Oliveira, J. L., Deus, M. M., and Freitas-Junior, R. (2023). Incidence of interstitial lung disease and cardiotoxicity with trastuzumab deruxtecan in breast cancer patients: a systematic review and single-arm meta-analysis. ESMO Open 8 (4), 101613. doi:10.1016/j.esmoop.2023.101613
Sun, C., Yang, X., Tang, L., and Chen, J. (2023). A pharmacovigilance study on drug-induced liver injury associated with antibody-drug conjugates (ADCs) based on the food and drug administration adverse event reporting system. Expert Opin. Drug Saf., 1–12. doi:10.1080/14740338.2023.2277801
Tang, S., Wu, Z., Xu, L., Wen, Q., and Zhang, X. (2022). Adverse reaction signals mining and hemorrhagic signals comparison of ticagrelor and clopidogrel: a pharmacovigilance study based on FAERS. Front. Pharmacol. 13, 970066. doi:10.3389/fphar.2022.970066
Tarantino, P., Carmagnani Pestana, R., Corti, C., Modi, S., Bardia, A., Tolaney, S. M., et al. (2022). Antibody-drug conjugates: smart chemotherapy delivery across tumor histologies. CA Cancer J. Clin. 72 (2), 165–182. doi:10.3322/caac.21705
von Minckwitz, G., Huang, C. S., Mano, M. S., Loibl, S., Mamounas, E. P., Untch, M., et al. (2019). Trastuzumab emtansine for residual invasive HER2-positive breast cancer. N. Engl. J. Med. 380 (7), 617–628. doi:10.1056/NEJMoa1814017
Keywords: FAERS, disproportionality analysis, antibody-drug conjugate, cardiovascular toxicity, adverse events
Citation: Long P, Li S, Pan L, Wang Y, Chen W and Wang X (2024) Cardiovascular adverse events associated with antibody-drug conjugates (ADCs): a pharmacovigilance study based on the FAERS database. Front. Pharmacol. 15:1378010. doi: 10.3389/fphar.2024.1378010
Received: 29 January 2024; Accepted: 15 April 2024;
Published: 03 May 2024.
Edited by:
Simone Brogi, University of Pisa, ItalyReviewed by:
Nazareno Paolocci, Johns Hopkins University, United StatesCopyright © 2024 Long, Li, Pan, Wang, Chen and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yuanqiang Wang, d2FuZ3lxbm5AY3F1dC5lZHUuY24=; Wanyi Chen, Y2hlbndhbnlpQGNxdS5lZHUuY24=; Xiaoxiao Wang, d2FuZ3hpYW94aWFvODkxMDIxQDE2My5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.