- 1Department of Physiology, Rahbar Medical and Dental College, Lahore, Pakistan
- 2Department of Biomedical Sciences, King Edward Medical University, Lahore, Pakistan
- 3Department of Biochemistry, King Edward Medical University, Lahore, Pakistan
- 4Institute of TB and Chest Medicine, Mayo Hospital, Lahore, Pakistan
Background: Chronic obstructive pulmonary disease (COPD) imposes a substantial burden on patients and healthcare systems. Spirometry is the most widely used test to diagnose the disease; however, a surrogate marker is required to predict the disease pattern and progression.
Objectives: The aim of the current study was to explore the association of elevated levels of plasma surfactant protein D (SP-D) with gene expression of osteoclast-associated receptor (OSCAR) and lung functions as potential diagnostic biomarkers of COPD.
Methods: This cross-sectional study employed convenience sampling. As men compose the majority of patients in the outpatient department and with smoking being common among Pakistani men, choosing men offered a representative sample. Using a post-bronchodilator forced expiratory volume in the first second (FEV1) to a forced vital capacity (FVC) of less than 0.70 (FEV1/FVC <0.7), COPD patients were diagnosed on spirometry (n = 41). Controls were healthy individuals with FEV1/FVC >0.7 (n = 41). Plasma SP-D levels were measured using an enzyme-linked immunosorbent assay (ELISA). The gene expression of OSCAR was determined by real-time polymerase chain reaction (qPCR) and subsequently analyzed by the threshold cycle (Ct) method. Statistical Package for Social Sciences (SPSS) version 20 was used for statistical analysis.
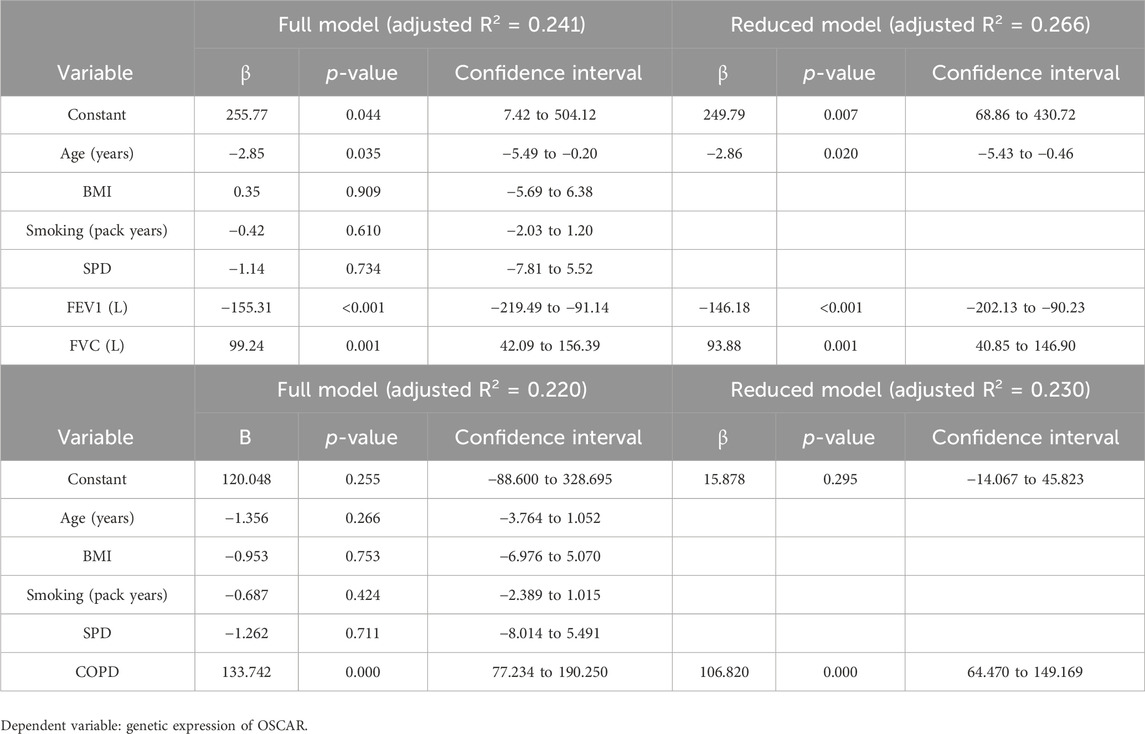
Results: The mean BMI of controls (25.66 ± 4.17 kg/m2) was higher than that of cases (23.49 ± 2.94 kg/m2 (p = .008)). The median age of controls was 49 years (interquartile range (IQR) 42.0–65.0 years) and that of cases was 65 years (IQR = 57.50–68.50). SP-D concentration was not significantly higher in COPD patients [4.96 ng/mL (IQR 3.26–7.96)] as compared to controls [3.64 ng/mL (IQR 2.60–8.59)] (p = .209). The forced expiratory ratio (FEV1/FVC) and FEV1 were related to gene expression of OSCAR (p = <.001). The gene expression of OSCAR was significantly related to SP-D (p = .034). A multiple regression model found FEV1 and FVC to have a significant effect on the gene expression of OSCAR (p-values <0.001 and 0.001, respectively).
Conclusion: Gene expression of OSCAR was increased in COPD patients and related directly to SP-D levels and inversely to lung functions in cohort of this study, suggesting that OSCAR along with SP-D may serve as a diagnostic biomarker of COPD.
1 Introduction
Chronic obstructive pulmonary disease (COPD) is a chronic respiratory syndrome characterized by symptoms such as cough, sputum production, and breathlessness. This syndrome results in respiratory symptoms like inflammation, mucus hypersecretion in the airways, and limited airflow (Bartolomé and Celli, 2018). COPD is mostly observed in smokers and people exposed to noxious gases (NICE, 2022). It is an under-diagnosed disease globally due to a lack of knowledge about its cause(s), prevalence, and progression. COPD has become an ongoing epidemic in both developed and underdeveloped countries, causing 3.23 million deaths in 2019, making it the third leading cause of mortality globally (World Health Organization, 2023). Pakistan has a high COPD prevalence of 18.5% among its population aged 40 years and above (Jarhyan et al., 2022).
COPD causes an enhanced inflammatory response that damages lung tissues and manifests systemic comorbidities such as cardiovascular damage, musculoskeletal dysfunction, osteoporosis, anemia, gastrointestinal symptoms, neuronal damage, and behavioral effects (Cordova-Rivera et al., 2019). The disease progression is marked by episodes of exacerbation characterized by acute aggravation of symptoms of dyspnea, sputum volume, and purulence. The progression results in compromised quality of life and requires frequent hospital visits or admissions (Kjaergaard et al., 2020) Specific biomarkers are required for its early detection, diagnosis, treatment, and drug development. Surfactant protein D (SP-D), a glycoprotein of the collectin family, is secreted by type II alveolar cells. SP-D causes opsonization, neutralization, and agglutination of pathogens by phagocytes (Liao et al., 2021). It has been extensively studied as a diagnostic biomarker for COPD. SP-D concentration increases in plasma due to its transmigration from the alveolar compartment into systemic circulation following smoke exposure. Studies indicate that the average level of SP-D in COPD patients is significantly higher than that in normal controls (Dalgard et al., 2021).
Increasing inflammation, lung parenchymal injury, and leaky capillaries facilitate the shift of SP-D from the pulmonary milieu to systemic circulation in COPD (Tiwari et al., 2024). This SP-D dysregulation and deficiency lead to hampered clearance of apoptotic and necrotic tissue and inflammation flare-up in the lung tissue, worsening the disease (Arroyo et al., 2018).
Recent evidence hints at the role of SP-D in activating circulating monocytes to become tissue macrophages by interacting with the osteoclast-associated receptor (OSCAR). OSCAR is an immunoglobulin-G (IgG)-like activating receptor for collagen and modulates the innate and adaptive responses by enhancing proliferation and maturation of immune cells (Elango et al., 2022). It has been observed that OSCAR is enhanced in the peripheral blood monocytes of patients with chronic inflammatory conditions like rheumatoid arthritis (Jung et al., 2019). Interestingly, SP-D is a ligand for OSCAR, and their interaction (OSCAR-SP-D interaction) triggers TNF-α production by inflammatory monocytes. The circulating monocytes exposed to leaked SP-D then release TNF-α and become mature alveolar macrophages. The increased alveolar macrophage infiltration initiates a chronic inflammatory response, leading to tissue destruction and alveolar damage (Schultz et al., 2015; Sorensen, 2018).
The current study aims to evaluate the expression of OSCAR in blood leukocytes in relation to SP-D levels and lung function in COPD patients. Blood was collected from COPD patients after performing spirometry under sterilized conditions. Plasma was isolated to determine SP-D levels using ELISA. RNA was isolated from plasma to synthesize cDNA, and the expression of OSCAR was quantified with the fold-change method. Results indicated that OSCAR expression was higher in the white blood cells of COPD patients than in smoker controls, was positively associated with circulating SP-D levels, and was negatively associated with lung functions, indicating a relation between OSCAR and chronic inflammation and deteriorating lung function.
Anything that can prevent the exacerbations or even predict future progression of the disease would be pivotal in the management of COPD. The current study will help open new avenues for rapid detection, diagnosis, and possible development of novel biomarkers for disease severity. Once proven, this would help foster advancement toward benefiting the community by reducing the burden of disease.
2 Methods
2.1 Subjects
From November 2021 to May 2022, 82 patients were enrolled from the Outpatient Department of Chest Medicine, Mayo Hospital, Lahore (Shein-Chung Chow et al., 2018). This was a cross-sectional study in which non-probability convenience sampling was adopted to enroll patients. The research was approved by the Institutional Review Board (IRB), Ethical Committee, and Advanced Studies and Research Board (AS&RB) of King Edward Medical University/Mayo Hospital, Lahore.
In this study, 41 patients were enrolled based on history (including chronic cough, sputum production, and breathlessness), examination, and spirometry. Those with a post-bronchodilator ratio (after the administration of 200 mcg salbutamol) of less than 0.70 (FEV1/FVC <0.70) were labeled as having COPD. Post-bronchodilator response was considered significant if there was a 12% increase from baseline and/or a 200-mL improvement in FEV₁, and the patient was excluded (Bhatt et al., 2022). Due to the overlap of symptoms and disease processes between COPD and asthma, the study also excluded individuals with asthma or those having symptoms suggestive of bronchial hyper-reactivity and diagnosed cases of asthma COPD overlap syndrome (ACOS). Patients with active tuberculosis were not included owing to its association with changes in structural, inflammatory, and genetic expression independently of COPD. Rheumatoid arthritis (RA) and osteoarthritis (OA) patients were also excluded as the associated chronic systemic inflammation and medication could interfere with inflammatory biomarkers and genetic expression of OSCAR. Lastly, patients on oral steroid therapy were not included in the study as systemic steroids are known to influence gene expression, hence impacting the study outcomes. Healthy controls (n = 41) were recruited from the general population of Lahore and attendants of patients visiting the hospital. All patients were enrolled after obtaining informed consent. Matching was done in terms of gender, socioeconomic and smoking status, and ethnicity. Both cases and controls included Pakistani men aged between 35 and 76 years. As smoking is more frequent in men in Pakistan, where it is seen as a sign of masculinity and superiority, and is socially unacceptable for women, most COPD patients are men (Masud and Oyebode, 2018). The only female visiting the Chest Department was not selected due to the convenience sampling technique, resulting in an all-male cohort.
2.2 Spirometry
Height (centimeters), weight (kilograms), ethnic background, and smoking status of all participants (cases and controls) were recorded, and spirometry was performed on an electronic spirometer (MIR Spirodoc) (Italy, software version 2.6). In brief, to perform spirometry, patients were advised to fully inhale, seal their mouth around the sterile mouthpiece, and breathe out as hard and as much as possible for at least 6 sec. Cases inhaled 200 µg of salbutamol (Salbo, Getz Pharma, KIA, Karachi, Pakistan) via a spacer device after getting a first record. After a 15-min waiting period, the process was repeated until technically acceptable curves were obtained, and the best of three readings were considered to calculate lung functions (Sim et al., 2017).
2.3 Blood sampling and processing
To obtain a blood sample, venipuncture was conducted, and 5 mL of blood was drawn. Blood (2 mL and 3 mL) was transferred into two EDTA-containing tubes separately. Tubes were placed on ice and transported to the research laboratory within 4 h of collection for RNA isolation. The remaining 3 mL of blood was used to separate the plasma and perform ELISA for the detection of SP-D concentration.
2.4 Genetic expression
2.4.1 RNA isolation
RNA was isolated using the commercially available GeneJET RNA Purification Kit according to the manufacturer’s instructions (Thermo Fisher Scientific). In brief, the collected blood sample stored at 4°C was used for RNA isolation. Whole blood (0.5 mL) was centrifuged at 400 g for 5 min at 4°C, and the clear supernatant (plasma) was discarded using a pipette to obtain cells as pellets. The cell pellet was re-suspended in 600 µL of lysis buffer (supplemented with β-mercaptoethanol) and mixed thoroughly using a vortex. Next, 450 µL of 96% ethanol was added and mixed using a vortex. A measure of 700 μL of the lysate was transferred to the GeneJET RNA Purification Column inserted in a collection tube and centrifuged for 1 min at ≥12,000 × g. The flow-through was discarded, and the purification column was placed back into the collection tube. The GeneJET RNA Purification Column was then placed into a new 2-mL collection tube, and 700 µL of Wash Buffer 1 (supplemented with ethanol) was added to it. The column was centrifuged for 1 min at ≥12,000 × g, and the flow-through was discarded. The purification column was placed back into the collection tube. Wash Buffer 2 (supplemented with ethanol) was added to the GeneJET RNA Purification Column twice, and each time it was centrifuged for 1 min at ≥12,000 × g, with the flow-through being discarded after each wash. Finally, 250 µL of Wash Buffer 2 was added to the column and centrifuged for 2 min at ≥12,000 × g. To elute RNA, 50 µL of nuclease-free water was added to the center of the GeneJET RNA Purification Column membrane, and the column was centrifuged for 1 min at ≥12,000 × g. Purified RNA was stored at −20°C until used for quantification and cDNA synthesis.
2.4.2 cDNA synthesis
RNA (1 µg) was used for cDNA synthesis using the commercially available RevertAid First Strand cDNA Synthesis Kit (Thermo Fisher Scientific) as per the manufacturer’s instructions. In brief, 1 µg of purified RNA was taken in a PCR tube and mixed with buffer, random primers, dNTPs, and reverse transcriptase enzyme. Nuclease-free water was added to adjust the total reaction volume to 10 μL. A negative template control (NTC) was also included, which contained all components but no DNA to rule out any contamination or primer dimers.
The thermal cycling protocol consisted of a hot start at 95°C for 3 min. Then, 30 cycles were repeated as follows: 95°C for 40 sec, 52°C for 30 sec, and 72°C for 40 sec and heating at 72°C for 5 min and 4°C ∞ hold until further analysis.
2.4.3 Real-time PCR
cDNA was used for qRT-PCR using the SYBR green-based kit Maxima SYBR Green/ROX qPCR Master Mix (2X) (Thermo Fisher Scientific). Forward and reverse primers for qRT-PCR are shown in Figure 1. GAPDH, a housekeeping (HK) gene, was used as an internal control in the analysis. Real-time PCR was then performed in triplicate using the AriaMx Real-time PCR System. The master mix was prepared after thawing and gently vortexing all the solutions. The master mix contained the Maxima SYBR Green/ROX qPCR Master Mix (2X) = µL, forward primer = 0.5 µL, reverse primer = 0.5 µL, template DNA = 1 µg, and nuclease-free water to make a total volume of up to 12.5 µL. The master mix was dispensed into PCR tubes, mixed gently, and placed in a thermal cycler. cDNA (5 μL) was added to each tube. The thermal cycler was programmed for an initial denaturation at 95°C for 3 min (one cycle), followed by 40X cycles of denaturation at 95°C for 15 sec, annealing at 60°C for 30 sec, and extension at 72°C for 30 sec.

Figure 1. Forward and reverse primers for OSCAR and housekeeping gene glyceraldehyde 3-phosphate dehydrogenase (GAPDH).
Melting curves were obtained, and threshold cycle (CT) values of both housekeeping genes were used to determine and calculate relative gene expression. The 2−ΔΔCT method was used for interpretation as follows:
where internal control represents the HK gene, sample A represents the cases, and sample B represents the controls (Kuang et al., 2018).
2.5 ELISA
The Invitrogen human SP-D ELISA Kit (EH436RB) was used to detect SP-D levels in the plasma of the cohort. Standards, quality control, and samples were run in duplicates. Plasma samples were diluted two-fold using 1X assay diluent. Wash buffers, diluent, biotin conjugate, and streptavidin–HRP solution were prepared according to the manufacturer’s instructions. Standards were serially diluted for the standard curve. Samples and standards were added to the wells after thawing, and ELISA plates were incubated for 2.5 h at room temperature. After the addition of the biotin conjugate, the samples were incubated for another hour with gentle shaking. Streptavidin HRP was added to wells after discarding the previous solution and washing thrice with wash buffer. Incubation with gentle shaking was done for 45 min. The solution was discarded, and wells were washed three times with wash buffer. The TMB substrate was added and incubated for 30 min in the dark, after which stop solution was added and gently tapped. Color change was observed, and absorbance was measured at 450 nm using the ELISA Reader (Multiskan FC Microplate Photometer) within 30 min (Hayrapetyan et al., 2023).
3 Statistical analysis
The study data are expressed as the mean ± standard deviation and median (interquartile range, IQR) for continuous variables and as frequency for categorical variables. The Shapiro–Wilk test of normality suggested a non-normal distribution of continuous data. A non-parametric Mann–Whitney U test was used for independent group comparisons. The chi-square test was used for categorical variables like smoking status and time since the last cigarette. The Spearman rank correlation was applied to compute the relationship between OSCAR and SP-D and lung functions. Multiple linear regression was performed to quantify the relationship between SP-D and lung functions and OSCAR gene expression.
4 Results
The study included all men aged between 35 and 73 years. The median age of controls was 49 years (interquartile range (IQR) 42.0–65.0), and that of cases was 65 years with an IQR of 57.50–68.50.
Among the controls (n = 41), 27 were never smokers (65%), 3 were ex-smokers (7.3%), and 11 were current smokers (26.8%). The COPD group (n-41) had 2 never-smokers (4.9%), 19 ex-smokers (46.3%), and 20 current smokers (48.8%). The mean BMI of controls was 25.66 ± 4.17 kg/m2, and that of cases was 23.49 ± 2.94 kg/m2. There were 9 (21.9%), 14 (34%), and 18 (43.9%) controls belonging to the upper-middle, lower-middle, and upper-lower classes, respectively. A total of 5 (12.2%), 11 (26.8%), and 25 (60.9%) patients with COPD were from the upper-middle, lower-middle, and upper-lower classes, respectively Table 1.
The average smoking history of controls was 0.0 (median) with an IQR of 0.0–4.1 pack years, while smokers had an average smoking history of 22.5 and an IQR of 10–40.0 median pack years. One pack year is defined as 20 cigarettes per day for 1 year.
Furthermore, 10 (24.39%) of controls and 20 (48.78%) of COPD patients were current smokers. No (0%) control and 5 (12.2%) of COPD patients were smoking for less than 1 year; 01 (2.4%) normal and 8 (19.5%) patients had smoked for less than 10 years; 02 (4.9%) of the control group and 6 (14.63%) of the patient cohort had smoked cigarettes for more than 10 years; 28 (68.29%) controls and 2 (4.88%) were non-smokers. A total of 5 (12.2%) controls and 14 (34.1%) cases had family members affected with COPD, while 36 (87.8%) controls and 27 (65.9%) cases did not have any close family member suffering from COPD (Table 1).
As shown in Table 1, no significant correlation (p = 0.209) was observed between higher surfactant protein D (SP-D) levels of 4.96 ng/mL (IQR 3.26–7.96) in COPD patients as compared to 3.64 ng/mL with an IQR of 2.60–8.59 in controls. The limited number of observations can account for at least some of this conclusion.
The forced expiratory ratio (FEV1/FVC) was 100.63% ± 6.65% of the expected value for the controls, while their forced expiratory volume in the first second (FEV1) was 97.88 ± 18.93 percent of the expected value. For the patients, FEV1/FVC was 63.67 ± 6.62 of the expected value, while their FEV1 was 50.78% ± 16.32% of the expected value. Expected values were estimated according to the age, body mass index, and ethnic background of the subjects. FEF2575 was 2.72 ± 0.95 in controls and 0.66 ± 0.33 in cases. FEV1, FVC, FEV1/FVC, and FEF2575 of controls were significantly different from those of cases (p = <.001 and 0.000). The lung functions were depressed in COPD patients as compared to healthy controls. Detailed lung functions are given in Table 2.
Genetic expression of the OSCAR gene was 1.1 (0.15–4.50) in controls and significantly increased to 77.30 (24.85–180) in COPD patients with a p-value of <0.001.
A direct but weak relationship between OSCAR gene and surfactant protein D level (r = 0.234, p = .034) was found (Table 3). FEF2575% of predicted and surfactant protein D had a weak indirect relation (r = −0.233 and p = 0.035) among the study subjects as shown in Figure 2. Figure 3 shows an inverse and moderate relation between FEV1/FVC and OSCAR gene expression (r = −0.630 and p = .001).
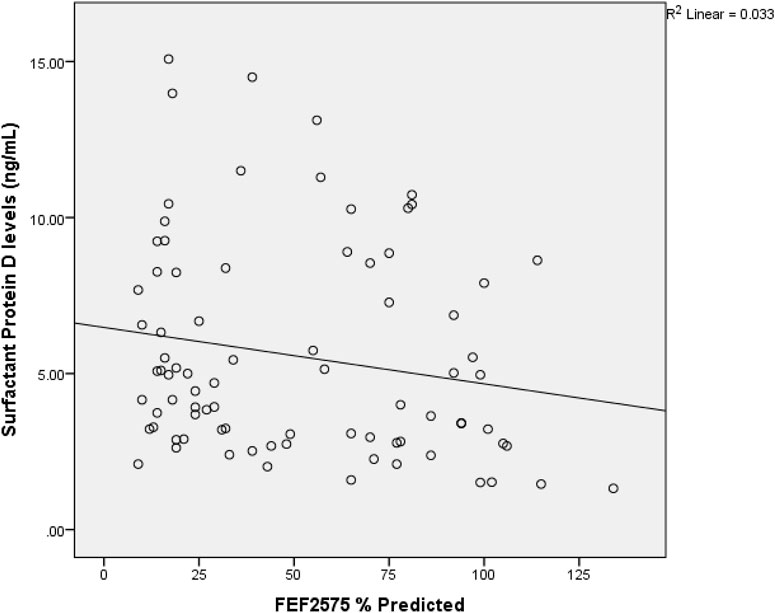
Figure 2. Spearman rank correlation between FEF2575% of predicted and surfactant protein D. r = −0.233 and p = 0.035 among the study subjects.
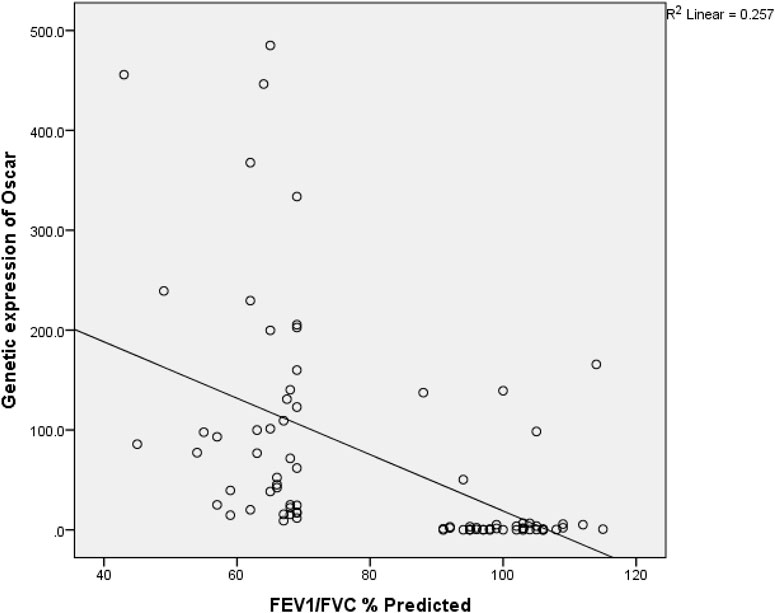
Figure 3. Spearman rank correlation between FEV1/FVC and OSCAR gene expression. r (79) = −0.645 and p = <0.001 among the study subjects.
There was a negative correlation of FEV1, FVC, and FEF2575 with OSCAR, as shown in Table 3. The relation between disease severity and OSCAR gene expression was significant r (79) = .51, p = < .001).
The regression analysis was used to see whether the gene expression has a certain level when linked with lung functions and SPD in the presence of age, BMI, and smoking exposure. It was observed that in the full model, age, FEV1, and FVC were found to have a significant effect on gene expressions as shown in Table 4, with p-values of 0.035, <0.001, and 0.001, respectively. Then, a reduced model was applied by eliminating insignificant variables, and the adjusted R2 increased to 0.266 as compared to 0.244 for the full model. Age had a negative impact, with a p-value of 0.020 and a coefficient of −2.86, explaining that gene expression decreases by 2.86-fold with each year increase after adjusting for low age in the control group. FEV1 had a coefficient of −146.18, indicating that each liter increase in FEV1 may reduce the gene expression by approximately 146-fold, while a per liter increase in FVC may increase the gene expression by 93.88-fold.
The second regression analysis was performed by taking COPD status as a variable and removing lung functions (as COPD status is determined based on lung functions). Here, in the full model, only COPD appeared to have a significant impact on gene expressions, showing all other factors like age, BMI, smoking status, and SPD as insignificant with an adjusted R2 of 0.220, and the reduced model included only COPD status as a variable with an adjusted R2 0.230. So, here, it can be predicted that those with COPD are supposed to have 106.8-fold higher gene expression than those without COPD, even if not considered for age, BMI, smoking, or SPD (Table 4).
5 Discussion
COPD, being a heterogeneous disease with a multifactorial origin, diverse progression, and variation in structural manifestation, clinical presentation, and treatment response, poses a challenge to clinicians and health authorities alike.
Recent endeavors have been directed toward identifying and measuring certain biomarkers that, alone or in concert with spirometry or other inflammatory chemokines, would be helpful in diagnosing and predicting the course of disease progression in patients with COPD. The relationship between biomarkers and lung functions (FEV1, FVC, and FEV1/FVC) has been extensively studied with promising results. Interestingly, SP-D is one such lung-specific biomarker that has not been fully investigated. SP-D, along with many other candidates like interleukin-6 (IL-6), club cell 16 (CC16), serum soluble receptor for advanced glycation end products (sRAGE), C reactive protein (CRP), and fibrinogen, has been studied to a great extent in COPD patients for disease presentation, progression, and treatment results (Stockley et al., 2019).
SP-D, owing to its lung specificity, easy assay in blood and sputum, and inherent functional and biological properties signifying a crucial role in the pathogenesis of COPD, appears to be a candidate in the quest for substantiating it as a disease marker of COPD. Obeidat et al. in their genome-wide association study (GWAS), established the protective role of SP-D on risk of COPD and enhanced decline in FEV1 (Obeidat et al., 2017). Keeping in view the important role of SP-D in COPD, plasma SP-D levels in patients with stable COPD and healthy controls were explored.
An increased (although non-significant) trend in plasma levels of SP-D in patients with COPD compared to controls was observed, as reported by Lv et al. (2022). Smoking status was also not significantly related to levels of SP-D. Serum SP-D concentrations of the COPD group were higher than in the control group. The patients in the current study had stable COPD status compared to those with COPD exacerbations in other studies. It might explain the non-significant increase in plasma SP-D levels, in line with the results of a non-significant relationship between SP-D and smokers with and without COPD, as shown by Andreeva et al. (2021). Liao published similar findings showing higher SP-D levels with no significant relationship in patients with incident COPD compared to those without the disease. In the current study, lung functions were significantly deteriorated in COPD patients as compared to controls, but the association of lung functions with SP-D appeared non-significant, similar to that proposed by Liao et al. (2021). Comparable trends, i.e., higher SP-D levels and deteriorating lung functions in COPD patients as compared to controls, were also observed in a similar study (Çiftci EŞ et al., 2018). Such findings suggest that SP-D plays an important role in maintaining surfactant hemostasis and possesses antioxidant, anti-inflammatory, and immunologic functions.
In vitro results of the current studies show that SP-D regulates the activities of immune cells, epithelial cells, fibrocytes, and smooth muscle cells. It has been shown that blood monocytes have chemokine and adhesion receptors, which allow them to migrate from the circulation to areas of infection or injury. Once on site, activated monocytes secrete pro-inflammatory cytokines such as interleukin 1 (IL-1), IL-6, tumor necrosis factor (TNF), and other inflammatory mediators, which orchestrate the recruitment of additional immune cell types. Monocytes differentiate into macrophages or dendritic cells depending on the local milieu (Kapellos et al., 2019).
Distinctive cell clusters of peripheral blood mononuclear cells (PBMCs) derived from COPD patients and healthy controls were identified, suggesting that impaired immune function may be linked to the progression of COPD (Pei et al., 2022). Increased OSCAR expression on monocytes is associated with disease activity in rheumatoid arthritis (RA) patients. As a costimulatory receptor, OSCAR promotes the production of osteoclasts from precursor monocytes. RA patients with active illness have monocytes with an increased expression of OSCAR (Schultz et al., 2016). In humans, OSCAR is reported to be expressed in monocytes, macrophages, neutrophils, and dendritic cells. The presence of OSCAR on human inflammatory cells leads to an investigation to elucidate its role in innate immunity, and thus, Barrow et al. (2015)identified SP-D as a candidate ligand of OSCAR and suggested that their interaction stimulated TNF-α production from monocytes (Barrow et al., 2015). Human OSCAR enhances the pro-inflammatory response of monocytes to develop into monocyte-derived macrophages (Belchamber et al., 2020). OSCAR can functionally interact with SP-D in inflammatory C-C chemokine receptor type 2 positive (CCR2+) monocytes and macrophages, resulting in a pro-inflammatory response. TNF-α is released from CCR2+ monocytes as a result of this interaction, and the SP-D/OSCAR complex appears to be internalized in alveolar macrophages, which orchestrate innate and adaptive immune responses in the lungs following lung injury by smoke or pollutants (Barrow et al., 2015; Watson et al., 2020). Interestingly, the findings of this study suggest a strong relationship between plasma SP-D levels and OSCAR gene expression, which partially explains the activation of blood monocytes and pulmonary interstitial macrophages. OSCAR gene expression was significantly increased in COPD patients and positively correlated with serum SP-D levels. Lung functions (FEV1, FVC, FEV1/FVC, and FEF2575) also showed a negative correlation with OSCAR gene expression. It is possible that SP-D released in airway lung surfactants would not be encountered by CCR2+ monocytes that patrol lung interstitial tissues in a healthy state. On the other hand, in response to pro-inflammatory or chemotactic stimuli like SP-D, monocytes gradually penetrate the airways. These monocytes replenish the alveolar macrophages and cause an increase in pro-inflammatory macrophages (M1) in comparison to anti-inflammatory macrophages (M2) in COPD, leading to worsening of pulmonary inflammation and subsequent deterioration of lung functions (Wu et al., 2022). This study is one of the first to show a positive correlation between gene expression of OSCAR and serum SP-D levels and a negative correlation between OSCAR gene expression and lung functions in stable COPD patients, hinting toward a more detailed role of SP-D in COPD disease pathogenesis and progression.
There were certain limitations to this work. First, it was a cross-sectional, single-center study with a limited number of samples and participants being exclusively men. The collection of blood for SP-D analysis and genetic expression of OSCAR was performed at a single point in time due to limitations in patient cooperation and follow-up. In the next phases, a prospective cohort study with random or systematic sampling of COPD patients and controls could be done to authenticate the findings of this study. Furthermore, the relationship between SP-D and inflammatory markers, especially TNF-α and ILs, can be investigated. Patients could be followed up to compare plasma SP-D levels and OSCAR gene expression in exacerbation and stable phases of COPD in a multicenter and large-sample study. Second, age could not be matched due to convenience sampling; although it was adjusted with multiple regression analysis, more accurate sampling techniques could be employed to minimize the selection bias. Third, all the patients were of the same ethnicity, due to which the data cannot be generalized to other populations, as the ethnicity may affect serum SP-D levels, lung functions, and gene expression. Fourth, SP-D and genetic expression were measured in stable COPD patients and controls without including patients having exacerbation of COPD. If a significant number of exacerbation patients were included and checked for the serum levels and relation between SP-D and gene expression of OSCAR, it might have provided an insight into the link between the two with greater evidence and confidence.
6 Conclusion
COPD patients exhibit a variety of clinical manifestations, treatment outcomes, exercise stamina, exacerbation risk, and airway damage. In the quest for a prognostic, diagnostic, or predictive biomarker, SP-D and OSCAR show the potential to impact disease diagnosis and patient care and reduce the burden on the healthcare system. The role of OSCAR in bone metabolism is well-established. This study found elevated OSCAR gene expression in the blood of COPD patients in the absence of any bone disease, like rheumatoid arthritis or osteoarthritis. It can aid in the early diagnosis of disease, identification of persons at risk, stratification of individuals on the basis of severity, monitoring of disease progression, and treatment. By validating the integrated relationship between SP-D and OSCAR, knowledge on the pathogenesis of COPD can be improved. New precision therapies and medications can be developed to modulate the activity of SP-D and OSCAR, thereby helping reduce pulmonary and systemic inflammation.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the Ethical Committee and Advanced Studies and Research Board (AS&RB) of King Edward Medical University/Mayo Hospital, Lahore referencing letter no. 133/RC/KEMU dated: 09/02/2021 by Institutional Review Board. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
SM: data curation, formal analysis, investigation, methodology, writing–original draft, and writing–review and editing. NC: conceptualization, data curation, formal analysis, and writing–review and editing. SS: methodology and writing–review and editing. AH: formal analysis, methodology, and writing–original draft. AG: investigation and writing–review and editing. NY: formal analysis and writing–original draft.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. Funding for the purchase of laboratory items was provided by King Edward Medical University, Lahore under Research Grant No. 01/2021-22/RGC/RC/KEMU (Dr. Saima Mukhtar), King Edward Medical University Lahore under PPRA Rule No. 38(2)a, for the Financial Year 2021-22.
In memoriam
I would like to express my heartfelt gratitude to my late mentor and teacher, Professor Dr. Tania A. Shakoori, from the Physiology Department at the University of Health Sciences, Lahore, Pakistan. Her guidance, support, and unwavering dedication to science have been an immense source of inspiration for me. Her passion for teaching and research has profoundly influenced my work and career. This acknowledgment is a humble tribute to her memory and the lasting impact she has had on my professional journey.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Andreeva, E., Pokhasnikova, M., Lebedev, A., Moiseeva, I., Kozlov, A., Kuznetsova, O., et al. (2021). Inflammatory parameters and pulmonary biomarkers in smokers with and without chronic obstructive pulmonary disease (COPD). J. Thorac. Dis. 13 (8), 4812–4829. doi:10.21037/jtd-20-1580
Arroyo, R., Martín-González, A., Echaide, M., Jain, A., Brondyk, W. H., Rosenbaum, J., et al. (2018). Supramolecular assembly of human pulmonary surfactant protein SP-D. J. Mol. Biol. 430 (10), 1495–1509. doi:10.1016/j.jmb.2018.03.027
Barrow, A. D. P. Y., Palarasah, Y., Bugatti, M., Holehouse, A. S., Byers, D. E., Holtzman, M. J., et al. (2015). OSCAR is a receptor for surfactant protein D that activates TNF-α release from human CCR2+ inflammatory monocytes. J. Immunol. 194 (7), 3317–3326. doi:10.4049/jimmunol.1402289
Bartolomé, R., and Celli, A. A. (2018). COPD: time to improve its taxonomy? ERJ Open Res. 4, 00132–02017. doi:10.1183/23120541.00132-2017
Belchamber, K. B. R., Walker, E. M., Stockley, R. A., and Sapey, E. (2020). Monocytes and macrophages in alpha-1 antitrypsin deficiency. Int. J. Chron. Obstruct Pulmon Dis. 15, 3183–3192. doi:10.2147/COPD.S276792
Bhatt, S. P., Fortis, S., and Bodduluri, S. (2022). New guidelines for bronchodilator responsiveness in COPD: a test in search of a use. Am. J. Respir. Crit. Care Med. 206 (8), 1042–1044. doi:10.1164/rccm.202203-0458LE
Çiftci Eş, F., Arkan, G., Acar, D., Saryal, S., and Saryal, S. (2018). The relation between serum surfactant protein-d level and chronic obstructive pulmonary disease severity. Biomed. Res. 29 (7), 1290–1297. doi:10.4066/biomedicalresearch.29-17-1659
Cordova-Rivera, L., Gibson, P. G., Gardiner, P. A., Hiles, S. A., and McDonald, V. M. (2019). Extrapulmonary associations of health status in severe asthma and bronchiectasis: comorbidities and functional outcomes. Respir. Med. 154, 93–101. doi:10.1016/j.rmed.2019.06.010
Dalgard, C., Wang, F., Titlestad, I. L., Kyvik, K. O., Vestbo, J., and Sorensen, G. L. (2021). Increased serum SP-D in identification of high-risk smokers at high risk of COPD. Am. J. Physiol. Lung Cell Mol. Physiol. 320 (6), L1005–L1010. doi:10.1152/ajplung.00604.2020
Dharmapatni, A. A., Algate, K., Coleman, R., Lorimer, M., Cantley, M. D., Smith, M. D., et al. (2017). Osteoclast-associated receptor (OSCAR) distribution in the synovial tissues of patients with active RA and TNF-α and RANKL regulation of expression by osteoclasts in vitro. Inflammation 40, 1566–1575. doi:10.1007/s10753-017-0597-2
Elango, J., Hou, C., Bao, B., Wang, S., Mate Sanchez de Val, J. E., and Wenhui, W. (2022). The molecular interaction of collagen with cell receptors for biological function. Polym. (Basel) 14 (5), 876. doi:10.3390/polym14050876
Hayrapetyan, H., Tran, T., Tellez-Corrales, E., and Madiraju, C. (2023). “Enzyme-linked immunosorbent assay: types and applications,” in ELISA: methods and protocols. Editor R. S. Matson (New York, NY: Springer US), 1–17.
Jarhyan, P., Hutchinson, A., Khaw, D., Prabhakaran, D., and Mohan, S. (2022). Prevalence of chronic obstructive pulmonary disease and chronic bronchitis in eight countries: a systematic review and meta-analysis. Bull. World Health Organ 100 (3), 216–230. doi:10.2471/BLT.21.286870
Jung, Y. K., Kang, Y. M., and Han, S. (2019). Osteoclasts in the inflammatory arthritis: implications for pathologic osteolysis. Immune Netw. 19 (1), e2. doi:10.4110/in.2019.19.e2
Kapellos, T. S., Bonaguro, L., Gemünd, I., Reusch, N., Saglam, A., Hinkley, E. R., et al. (2019). Human monocyte subsets and phenotypes in major chronic inflammatory diseases. Front. Immunol. 10, 2035. doi:10.3389/fimmu.2019.02035
Kjaergaard, J. L., Juhl, C. B., Lange, P., and Wilcke, J. T. (2020). Early pulmonary rehabilitation after acute exacerbation of COPD: a randomised controlled trial. ERJ Open Res. 6 (1), 00173–02019. doi:10.1183/23120541.00173-2019
Kuang, J., Yan, X., Genders, A. J., Granata, C., and Bishop, D. J. (2018). An overview of technical considerations when using quantitative real-time PCR analysis of gene expression in human exercise research. PloS one 13 (5), e0196438. doi:10.1371/journal.pone.0196438
Liao, J. P., Wang, X., Liu, F., Cheng, Y., Hu, Z. W., Zhang, L. N., et al. (2021). Serum surfactant protein D, lung function decline, and incident chronic obstructive pulmonary disease: a longitudinal study in Beijing. J. Thorac. Dis. 13 (1), 92–100. doi:10.21037/jtd-20-1675
Lv, M. Y., Qiang, L. X., Wang, B. C., Zhang, Y. P., Li, Z. H., Li, X. S., et al. (2022). Complex evaluation of surfactant protein A and D as biomarkers for the severity of COPD. Int. J. Chron. Obstruct Pulmon Dis. 17, 1537–1552. doi:10.2147/COPD.S366988
Masud, H., and Oyebode, O. (2018). Inequalities in smoking prevalence: a missed opportunity for tobacco control in Pakistan. J. Public Health (Oxf) 40 (2), 271–278. doi:10.1093/pubmed/fdx044
Obeidat, M., Li, X., Burgess, S., Zhou, G., Fishbane, N., Hansel, N. N., et al. (2017). Surfactant protein D is a causal risk factor for COPD: results of Mendelian randomisation. Eur. Respir. J. 50 (5), 1700657. doi:10.1183/13993003.00657-2017
Pei, Y., Wei, Y., Peng, B., Wang, M., Xu, W., Chen, Z., et al. (2022). Combining single-cell RNA sequencing of peripheral blood mononuclear cells and exosomal transcriptome to reveal the cellular and genetic profiles in COPD. Respir. Res. 23 (1), 260. doi:10.1186/s12931-022-02182-8
Schultz, H. S., Guo, L., Keller, P., Fleetwood, A. J., Sun, M., Guo, W., et al. (2016). OSCAR-collagen signaling in monocytes plays a proinflammatory role and may contribute to the pathogenesis of rheumatoid arthritis. Eur. J. Immunol. 46 (4), 952–963. doi:10.1002/eji.201545986
Schultz, H. S., Nitze, L. M., Zeuthen, L. H., Keller, P., Gruhler, A., Pass, J., et al. (2015). Collagen induces maturation of human monocyte-derived dendritic cells by signaling through osteoclast-associated receptor. J. Immunol. 194 (7), 3169–3179. doi:10.4049/jimmunol.1402800
Shein-Chung Chow, J. S., Wang, H., and Lokhnygina, Y. (2018). Sample size calculations in clinical research. 3rd ed. New York, United States: Taylor and Francis.
Sim, Y. S., Lee, J.-H., Lee, W.-Y., Suh, D. I., Oh, Y.-M., Yoon, J.-s., et al. (2017). Spirometry and bronchodilator test. Tuberc. Respir. Dis. 80 (2), 105–112. doi:10.4046/trd.2017.80.2.105
Sorensen, G. L. (2018). Surfactant protein D in respiratory and non-respiratory diseases. Front. Med. 5 (18), 18–37. doi:10.3389/fmed.2018.00018
Stockley, R. A., Halpin, D. M. G., Celli, B. R., and Singh, D. (2019). Chronic obstructive pulmonary disease biomarkers and their interpretation. Am. J. Respir. Crit. Care Med. 199 (10), 1195–1204. doi:10.1164/rccm.201810-1860SO
Tiwari, S., Fatima, D., and Gowardipe, P. S. (2024). Study of serum surfactant protein D levels in chronic obstructive pulmonary disease patients. Med. J. Dr DY Patil Vidyapeeth 17 (2), 359–362. doi:10.4103/mjdrdypu.mjdrdypu_704_22
Wahid, G., Ahmad, A., Tariq, R., Tamkeen, N., Samad, M., and Rehman, M. (2022). Evaluation of the socioeconomic status of patients received at radiology department, hayatabad medical complex peshawar. J. Postgrad. Med. Inst. 36 (2), 79–84. doi:10.54079/jpmi.36.2.2949
Wang, X., Liu, A., Hou, D., Dong, X., Chu, C., Ju, W., et al. (2023). Epigenetic impact of long non-coding RNA lnc-ADAM9 on extracellular matrix pathway in preterm syndrome through down-regulation of mRNA-ADAM9. J. Transl. Genet. Genomics 7, 126–140. doi:10.20517/jtgg.2023.07
Watson, A., Madsen, J., and Clark, H. W. (2020). SP-A and SP-D: dual functioning immune molecules with antiviral and immunomodulatory properties. Front. Immunol. 11, 622598. doi:10.3389/fimmu.2020.622598
Wu, J., Zhao, X., Xiao, C., Xiong, G., Ye, X., Li, L., et al. (2022). The role of lung macrophages in chronic obstructive pulmonary disease. Respir. Med. 205, 107035. doi:10.1016/j.rmed.2022.107035
Keywords: chronic obstructive pulmonary disease, osteoclast-associated receptor, lung function test, surfactant protein D, gene expression
Citation: Mukhtar S, Choudhry N, Saeed S, Hanif A, Gondal AJ and Yasmin N (2024) Exploring the associations between elevated plasma SP-D levels and OSCAR gene expression as potential biomarkers in patients with COPD: a cross-sectional study. Front. Pharmacol. 15:1376394. doi: 10.3389/fphar.2024.1376394
Received: 20 February 2024; Accepted: 24 June 2024;
Published: 18 July 2024.
Edited by:
Rafał Pawliczak, Medical University of Lodz, PolandReviewed by:
Riffat Mehboob, Lahore Medical Research Center, PakistanVitaliano Nicola Quaranta, University of Bari Aldo Moro, Italy
Copyright © 2024 Mukhtar, Choudhry, Saeed, Hanif, Gondal and Yasmin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Saima Mukhtar, c3ltYXJiQGdtYWlsLmNvbQ==, c2FpbWFtdWtodGFyMTk3MkBvdXRsb29rLmNvbQ==
 Saima Mukhtar
Saima Mukhtar Nakhshab Choudhry3
Nakhshab Choudhry3 Saqib Saeed
Saqib Saeed Aamir J. Gondal
Aamir J. Gondal Nighat Yasmin
Nighat Yasmin