
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Pharmacol., 11 July 2024
Sec. Inflammation Pharmacology
Volume 15 - 2024 | https://doi.org/10.3389/fphar.2024.1376262
Objective: To compare the risk of infection in inflammatory arthritis patients treated with tumor necrosis factor (TNF) inhibitors.
Methods: PubMed, Embase, and the Cochrane Library were systematically searched from inception to 28 December 2023 for randomized controlled trials (RCTs) assessing TNF inhibitors and reporting infections. Subsequently, pairwise and network meta-analyses were conducted to determine odds ratios (ORs) and the corresponding 95% confidence intervals (CIs).
Results: A total of 61 RCTs involving 20,458 patients were included. Pairwise meta-analysis revealed that certolizumab pegol was significantly associated with an increased risk of serious infection compared to placebo (OR:2.28, 95% CI: 1.13–4.62). Both adalimumab and certolizumab pegol were also significantly associated with an increased risk of any infection compared to placebo (OR:1.18, 95% CI: 1.06 to 1.30 and OR:1.40, 95% CI: 1.11 to 1.76, respectively). Moreover, a network meta-analysis indicated that certolizumab pegol and infliximab were associated with a higher risk of serious infection compared to other TNF inhibitors. In the cumulative ranking of any infection risk, certolizumab pegol had the highest risk compared with others. TNF inhibitors increased the risk of tuberculosis but not that of herpes zoster.
Conclusion: Available evidence indicates etanercept and golimumab are likely associated with a lower risk of infection compared to other TNF inhibitors in inflammatory arthritis. For patients at a heightened risk of infection, prioritizing the use of etanercept and golimumab may be advisable to minimize patient risk.
Systematic Review Registration: identifier CRD42022316577
Inflammatory arthritis (IA) is a heterogeneous pathology inducing motor system impairment, joint function loss, and joint ankylosis. Additionally, persistent inflammation may affect other organs, considerably impacting the quality of life. The global prevalence of inflammatory arthritis approximates 3% (Pfeffer et al., 1993; Fraenkel et al., 2021). Rheumatoid arthritis (RA), psoriatic arthritis (PsA), and ankylosing spondylitis (AS) are the most common subtypes of inflammatory arthritis.
Currently, multiple guidelines recommend prompt initiation of treatment with TNF inhibitors in individuals with rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis who exhibit an inadequate response to standard or other conventional treatments (Singh et al., 2019; Ward et al., 2019; Smolen et al., 2020; Fraenkel et al., 2021). TNF inhibitors are effective therapeutics in inflammatory arthritis (Maini et al., 1999; Gorman et al., 2002; Weinblatt et al., 2003; Mease et al., 2005). Multiple studies have shown infection is the most common adverse event of TNF inhibitors (Burmester et al., 2013; Husni et al., 2022). This may result from the suppression of TNF-α-mediated immune responses, likely increasing the risk of infection. The developed infection may result in prolonged hospitalization or even be life-threatening.
Five TNF inhibitors have received FDA approval and are extensively used in the treatment of inflammatory arthritis, all of which target TNF-α, with etanercept additionally suppressing TNF-β. It remains controversial whether the five TNF inhibitors are different in terms of infection incidence. Previous reports have indicated TNF inhibitors do not differ in infection risk (Rubbert-Roth et al., 2018). However, the growing number of patients receiving TNF inhibitors has led to gradual observations of distinctions in infection risk among the five TNF inhibitors (Bongartz et al., 2006; Saliba et al., 2016).
Because of the absence of head-to-head studies, direct evidence is lacking to compare the current TNF inhibitors for both efficacy and safety. Notably, the first large-scale study was published directly comparing adalimumab and certolizumab pegol for efficacy and safety, with comparable incidence rates of infection, tuberculosis, and opportunistic infections among them in 2016 (Smolen et al., 2016). However, it is crucial to acknowledge that this is the sole study providing a direct comparison, and large-scale data comparisons are currently insufficient. Consequently, we aimed to assess the risk of infection associated with TNF inhibitors by network meta-analysis to select drugs with a lower risk of infection and provide medication suggestions for susceptible patients.
This network meta-analysis was conducted based on the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) statement complemented with the PRISMA extension for NMAs (Hutton et al., 2015; Page et al., 2021) and has been registered with PROSPERO (No. CRD42022316577).
PubMed, Embase and the Cochrane Library were systematically searched to collect relevant studies assessing TNF inhibitors for the treatment of inflammatory arthritis from inception to 27 December 2023. The literature search was restricted to human studies published in English. Our English search terms encompassed “Adalimumab,” “Etanercept,” “Infliximab,” “Golimumab,” “Certolizumab pegol,” “Rheumatoid arthritis,” “Ankylosing spondylitis,” “Psoriatic arthritis” and “Randomized Controlled Trial.” The specific search strategy is detailed in Supplementary Table S1. Additionally, we identified further potential trials by manual searching the references of the included trials and relevant meta-analyses. Two reviewers (ZJ and YZ) selected the included studies according to the following criteria: 1) patients received the recommended standard dosages of TNF inhibitors (adalimumab, etanercept, golimumab, infliximab, and certolizumab) (see Table 1 for specific details). In patients administered background therapy, the latter was required to be the same; 2) primary outcomes included serious infection (defined as a diagnosis of infection requiring antimicrobial therapy and/or hospitalization) and any infection; secondary outcome measures included opportunistic infection (including Candida spp., Pseudomonas spp., Pneumocystis spp., etc., excluding tuberculosis infection), tuberculosis (including pulmonary tuberculosis, peritoneal tuberculosis, disseminated tuberculosis, etc.) and herpes zoster; 3) study design was RCT.
Two reviewers (ZJ and YZ) independently performed the literature search and data extraction. In case of disagreement, a third reviewer (GL) was involved in arbitration. Missing data were diligently sought as much as possible by contacting the corresponding author.
After screening the literature, the titles and abstracts were first read to exclude irrelevant studies. Then, full texts were further read for final inclusion. The data extracted mainly included: 1) basic information about the included studies (study title, publication year, country, and NCT number); 2) basic patient characteristics (age and gender); 3) sample size; 4) key information for risk of bias assessment; 5) information related to the outcome measures.
Two investigators (ZJ and YZ) independently assessed the risk of bias of all included studies with the Cochrane risk of bias tool (Cumpston et al., 2019). In case of disagreement, a third investigator (GL) was involved in arbitration. The quality evaluation included random sequence generation (selection bias), allocation concealment (selection bias), blinding (performance and detection biases), incomplete data (attrition bias), selective reporting (reporting bias), and other biases. We used the Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) approach to assess the certainty of the evidence (Puhan et al., 2014).
In pairwise meta-analysis, the random-effects model was used to determine odds ratios (ORs) and 95% confidence intervals (CIs) for the risk of infection. The degree of heterogeneity was assessed by the I2 value, with I2 < 25% considered as no heterogeneity, 25% ≤ I2 < 50% as low heterogeneity, 50% ≤ I2 < 75% as moderate heterogeneity, and I2 ≥ 75% as high heterogeneity (Higgins et al., 2003). The risk of infection for the five TNF inhibitors was preliminarily predicted by pairwise comparison. To assess the robustness of the primary results, sensitivity analysis was performed by excluding trials with a follow-up ⩾52 weeks and a sample size <50. Funnel plots and Egger’s test were used to assess publication bias (Sterne et al., 2011).
To further compare the risk of infection for the five TNF inhibitors, a network meta-analysis was performed with the random-effects model using the “mvmeta” command. ORs and 95% CIs were obtained for different interventions. The ranking of TNF inhibitor infection risk was assessed by surface under the cumulative ranking curve (SUCRA) analysis (Shim et al., 2017). Inconsistency tests were performed to check for discrepancies between direct and indirect evidence in closed loops (Song et al., 2011). Small sample effects were assessed by comparing adjusted funnel plots (Chaimani and Salanti, 2012). Additionally, meta-regression and subgroup analyses were utilized to examine the effects of covariates, including trial features (disease type, follow-up time, age, and background therapy) on the pooled effect size. Statistical analysis was carried out with RevMan 5.4 (The Cochrane Collaboration, 2020) and STATA 17 (Stata Corp., College Station, Texas).
Upon systematic search, 3,298 records were retrieved; of these, 1,264 repeated records were eliminated and 61 were finally included based on title, abstract, and full-text reading. The study flowchart is depicted in Figure 1. Available direct and indirect comparisons are represented by network plots (Figure 2). This study included 20,458 patients, 16,183 with rheumatoid arthritis, 2,113 with ankylosing spondylitis and 2,162 with psoriatic arthritis. Basic characteristics are shown in Table 1. Totally 61 studies were included by random grouping. Among them, three included trials did not perform blinding, resulting in a judgment of high risk of bias. A trial utilized single blinding. None of the included studies reported concealment, incomplete data, or selective reporting of outcome measures with other risks of bias. The literature quality is shown in Supplementary Figure S1.
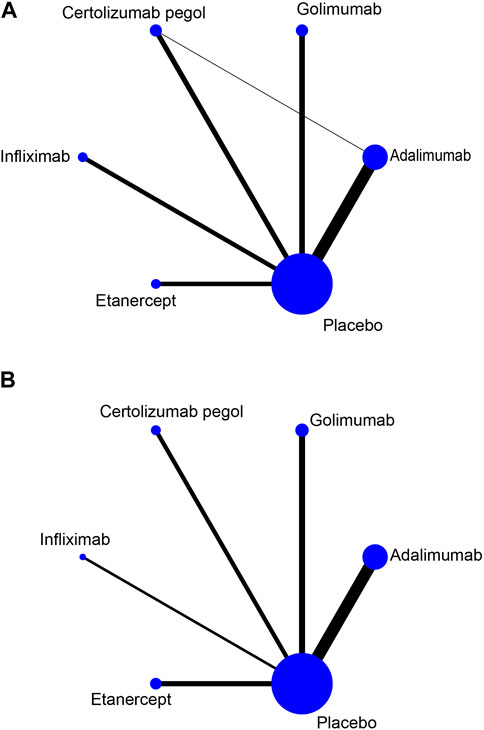
Figure 2. Network plots for TNF inhibitors and the risk of infection. The node size reflects the number of patients in the treatment group and the line thickness reflects the number of trials for the given comparison. No connecting line between the two treatments indicates no direct comparison (A) Risk of serious infection. (B) Risk of any infection.
Pairwise meta-analysis indicated that certolizumab pegol was significantly associated with an increased risk of serious infection compared to placebo (OR:2.28, 95%CI:1.13–4.62). Conversely, no significant differences were detected between adalimumab (OR:1.27, 95%CI:0.85–1.88), etanercept (OR:0.79, 95%CI:0.46–1.34), golimumab (OR:0.69, 95%CI:0.36–1.35), infliximab (OR:1.39, 95%CI:0.72–2.68) and placebo. Notably, both adalimumab and certolizumab pegol were associated with a significantly higher risk of any infection compared to placebo (OR:1.18, 95%CI:1.06 to 1.30 and OR:1.40, 95%CI:1.11 to 1.76, respectively).
Our analysis revealed no significant association between TNF inhibitors and opportunistic infections. However, in terms of tuberculosis, TNF inhibitors significantly increased the risk of infection compared to placebo (OR:2.21, 95%CI:1.05–4.66). Conversely, in terms of herpes zoster, TNF inhibitors were not significantly associated with the risk of infection compared to placebo (OR:1.50, 95%CI:0.72–3.11).
Overall, no significant heterogeneity was observed, as indicated in Supplementary Figure S2. Furthermore, no significant publication bias was detected by Egger’s test (p > 0.05), and visual inspection of funnel plots was carried out for the four outcome measures (see Supplementary Figure S3). Sensitivity analysis, detailed in Supplementary Figure S4, did not significantly alter the main results, suggesting robustness in our findings.
In network meta-analysis, certolizumab pegol had a significantly increased risk of serious infection in comparison with placebo, golimumab, and etanercept (OR:1.85, 95%CI:1.09 to 3.14; OR:2.67, 95%CI:1.14 to 6.26 and OR:2.41, 95%CI:1.14 to 5.08, respectively; Figure 3). We performed a ranking of infection risk based on the surface under the cumulative ranking curves (Supplementary Figure S5). The cumulative probability ranking for the risk of serious infection was highest for certolizumab pegol (90.7%), followed by infliximab (73.1%), adalimumab (69.5%), etanercept (17.0%) and golimumab (12.8%). Direct and indirect comparisons revealed no significant differences (p = 0.377).
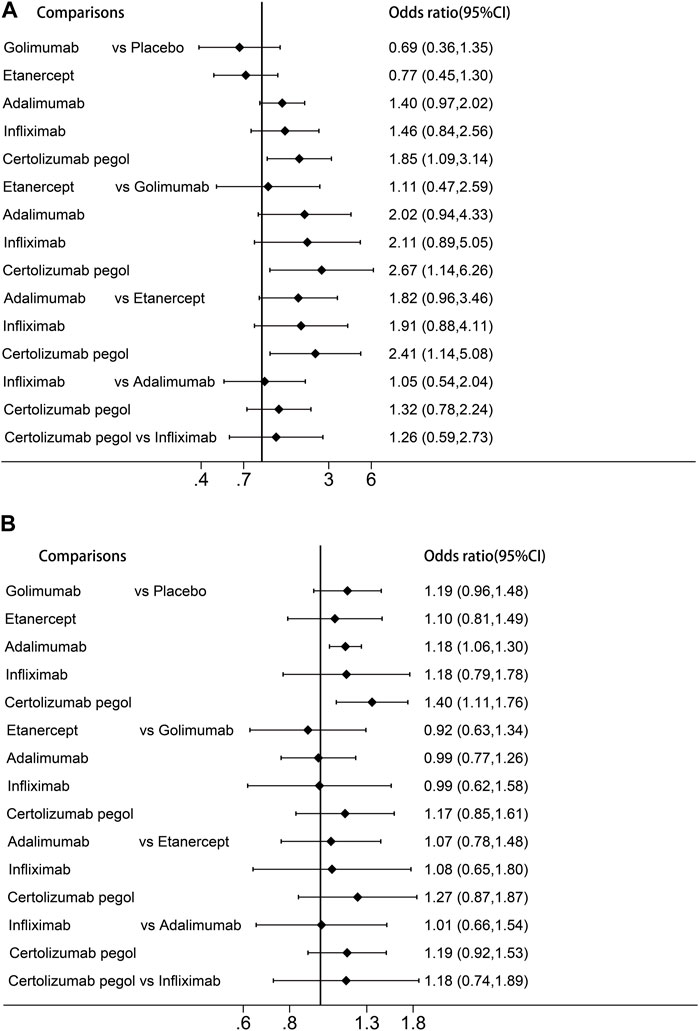
Figure 3. Forest plots of the network meta-analysis (A) Risk of serious infection. (B) Risk of any infection.
For any infection, adalimumab was associated with a significantly higher risk compared to placebo (OR:1.18, 95%CI:1.06–1.30). The results of the cumulative ranking of any infection risk demonstrated certolizumab pegol had the highest risk of infection (87.7%), followed by golimumab (56.5%), infliximab (53.3%), adalimumab (53.6%) and etanercept (38.1%). Adjusted funnel plots for comparison indicated the absence of publication bias and a low sample effect, as illustrated in Supplementary Figure S3. However, due to limited data availability, network meta-analyses could not be performed for opportunistic infections, herpes zoster infection, and tuberculosis.
A meta-regression analysis was performed based on disease type, follow-up time, age, and background therapy. The results showed that the latter factors may had no significant effects on the results (p > 0.05) (Supplementary Table S2). Meanwhile, detailed subgroup analyses were carried out and no possible influencing factors were found (Supplementary Table S3).
The GRADE assessments are presented in the Supplementary Table S4. As all included studies were RCTs, the quality of starting evidence was high. We performed sensitivity analyses that did not identify any studies with a high risk of bias, indicating that there is no need to downgrade because of risk of bias. In addition, most comparisons were downgraded due to imprecisions. Consequently, the quality of our comparisons was moderate.
In this study, we evaluated of the infection risk among patients with inflammatory arthritis receiving treatment with five different TNF inhibitors. The results demonstrated all TNF inhibitors increased the risk of any infection, with certolizumab pegol displaying the highest risk. Etanercept and golimumab had a lower risk of serious infection. TNF inhibitors increased the risk of tuberculosis but not that of opportunistic infections and herpes zoster. In addition, we performed a detailed meta-regression analysis to assess the effects of the five TNF inhibitors on the risk of infection for various disease types, ages, and background therapies. None of these factors affected the results.
Several studies have shown that TNF inhibitors significantly increase the risk of serious infections and any infection (Minozzi et al., 2016; Chiu and Chen, 2020). Our conclusions are generally aligned with previous studies. In terms of serious infections, patients using adalimumab, certolizumab, and infliximab had an increased risk of serious infections in a study comparing safety in rheumatoid arthritis. A similar trend was observed for golimumab in combination with methotrexate. In contrast, serious infection rates tended to be lower with etanercept (Michaud et al., 2014). Data from the Dutch Rheumatoid Arthritis Monitoring (DREAM) registry revealed that patients with rheumatoid arthritis treated with etanercept had a lower risk of serious infection than adalimumab and infliximab (van Dartel et al., 2013). In a head-to-head clinical trial directly comparing the efficacy and safety of certolizumab and adalimumab in patients with rheumatoid arthritis, it was found that there was no significant difference in the occurrence of serious infections between adalimumab and certolizumab (Smolen et al., 2016). Galloway et al. (2011) concluded that the highest risk of infection occurs within the first 6 months of treatment, stabilizing between 24 and 36 months. Factors such as a history of serious infections, glucocorticoid dose, smoking, diabetes, and older age were identified as significant predictors of serious infections in patients treated with biologics (Singh, 2016). Therefore, extra caution should be exercised regarding the occurrence of serious infections in these patients. In terms of any infection, a meta-analysis included 71 RCTs involving 22,760 adult rheumatology patients and seven open-label extension (OLE) studies involving 2,236 patients found that TNF inhibitors significantly increased the risk of any infection (Minozzi et al., 2016). Overall, these findings and our study suggest that etanercept is a potentially safer option for infections, followed by golimumab.
Patients administered TNF inhibitors for immune-mediated inflammatory disorders are known to be susceptible to infection by diverse opportunistic pathogens, including Coccidioides, Histoplasma, Nontuberculous mycobacteria, and Mycobacterium tuberculosis (Winthrop and Chiller, 2009). Many trials included in published meta-analyses have demonstrated that TNF-α inhibitors significantly increase the risk of tuberculosis in patients with rheumatic diseases (RA, AS, PsA) (Ai et al., 2015; Minozzi et al., 2016). Related reports have further identified structural and functional differences between antibodies and soluble receptors, resulting in distinct affinities for binding to TNF-α and effects on T cell proliferation and apoptosis. Additionally, etanercept slightly affects membrane-bound TNF, with a lower risk of tuberculosis compared with monoclonal antibodies (Dixon et al., 2010; Marotte and Cimaz, 2014). As a result, physicians must screen for primary tuberculosis or latent tuberculosis infection (LTBI) before initiating TNF-α therapy to decrease the risk of LTBI reactivation (Gardam et al., 2003; Fonseca et al., 2008). In patients with LTBI or previously treated tuberculosis, etanercept rather than other TNF inhibitors is recommended to reduce the risk of tuberculosis infection (Godfrey and Friedman, 2019).
Herpes zoster results from varicella-zoster virus (VZV) reactivation (Schmader and Dworkin, 2008). Currently, whether TNF inhibitors increase the risk of herpes zoster remains controversial. Contrary to some retrospective studies that suggested an association, our study did not find that the use of TNF inhibitors significantly increased the risk of herpes zoster infection. A retrospective study on the treatment of psoriatic arthritis and the risk of herpes zoster included 3,128 patients to explore the association of traditional antirheumatic drugs and TNF inhibitors with herpes zoster (Schmader and Dworkin, 2008). Conversely, several meta-analyses have also confirmed that TNF inhibitors increase the risk of herpes zoster infection, especially monoclonal antibodies (Strangfeld et al., 2009; Che et al., 2014; Redeker et al., 2022). We consider that the discrepancy may be related to the duration of follow-up. Studies have shown that the average time between initiation of TNF inhibitor therapy and the occurrence of a herpes zoster event of 8–19 months, with incidence peaking in the first 2 years after initiating biologic therapy (Zisman et al., 2016). In contrast, the trials we included had study durations of mostly 6 months, which prevented some events outside the study duration from being recorded. In 2021, the ACIP in the United States updated vaccine recommendations to administer two doses of recombinant herpes zoster vaccine (RZV) to prevent herpes zoster and its complications in adults ≥19 years who are or may be immunodeficient or immunosuppressed due to disease or therapy (Anderson et al., 2022).
We consider the difference in infection risk for TNF inhibitors to be mostly explained by their different molecular structures, leading to differences in pharmacokinetics and mechanisms of action (Wallis, 2009). Firstly, the difference in affinity among various TNF inhibitors is obvious. Certolizumab pegol improves pharmacokinetics, increases affinity, and prolongs half-life by forming pegylated structures (Pasut, 2014). It binds TNF with a higher affinity than other TNF inhibitors. The stronger affinity may predict more effective TNF inhibition. Secondly, the direct cytotoxicity of certolizumab differs from that of other TNF inhibitors in that it directly induces the death of transmembrane cells expressing TNF-α (Ueda et al., 2013). Finally, monoclonal antibodies represented by infliximab can also directly eliminate activated T cells and monocytes/macrophages by cell lysis or induction of apoptosis (Furst et al., 2006). These aspects could explain the trend that certolizumab and infliximab seem to be more susceptible to infection.
The strength of this study is the use of a network meta-analysis to assess risk differences between TNF inhibitors in the absence of direct head-to-head studies. Moreover, this network meta-analysis provided sufficient evidence for a relationship between TNF inhibitors and infection. However, this study had some limitations. First, we included RA, AS, PsA patients, which allowed the inclusion of more trials but may also increase the risk of bias. However, we conducted a meta-regression analysis to demonstrate the current conclusions may be applied to all patients with rheumatic disorders. Second, most evidence came from randomized controlled trials versus placebo, with only one head-to-head study. To generate more accurate results, more head-to-head trials are required. Third, all the studies had a certain time frame, so some infections that occurred after the study may not have been captured. Fourth, although the definitions in different versions of MedDRA may vary, this does not affect the accuracy of our results. Finally, rheumatoid arthritis had the most studies, while ankylosing spondylitis and psoriatic arthritis had relatively fewer studies. Therefore, rheumatoid arthritis may have a more significant impact on the final safety results.
In summary, this network meta-analysis showed that both golimumab and etanercept might have a lower risk of infection compared with other TNF inhibitors. These findings offer a foundation for drug selection in susceptible patients, aiming to reduce the risk of infection and promote individualized medication.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
ZJ: Conceptualization, Formal Analysis, Writing–original draft. YZ: Investigation, Software, Writing–review and editing. GL: Data curation, Methodology, Writing–review and editing. SZ: Writing–review and editing. WZ: Conceptualization, Supervision, Writing–review and editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
We extend our sincere gratitude to Professor Chao Zhang for her expert guidance and unwavering support in the study design and data analysis.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2024.1376262/full#supplementary-material
Ai, J. W., Zhang, S., Ruan, Q. L., Yu, Y. Q., Zhang, B. Y., Liu, Q. H., et al. (2015). The risk of tuberculosis in patients with rheumatoid arthritis treated with tumor necrosis factor-α antagonist: a metaanalysis of both randomized controlled trials and registry/cohort studies. J. Rheumatol. 42 (12), 2229–2237. doi:10.3899/jrheum.150057
Anderson, T. C., Masters, N. B., Guo, A., Shepersky, L., Leidner, A. J., Lee, G. M., et al. (2022). Use of recombinant zoster vaccine in immunocompromised adults aged ≥19 Years: recommendations of the advisory committee on immunization practices - United States, 2022. MMWR-Morb. Mortal. Wkly. Rep. 71 (3), 80–84. doi:10.15585/mmwr.mm7103a2
Bongartz, T., Sutton, A. J., Sweeting, M. J., Buchan, I., Matteson, E. L., and Montori, V. (2006). Anti-TNF antibody therapy in rheumatoid arthritis and the risk of serious infections and malignancies: systematic review and meta-analysis of rare harmful effects in randomized controlled trials. JAMA-J. Am. Med. Assoc. 295 (19), 2275–2285. doi:10.1001/jama.295.19.2275
Burmester, G. R., Panaccione, R., Gordon, K. B., Mcilraith, M. J., and Lacerda, A. P. (2013). Adalimumab: long-term safety in 23 458 patients from global clinical trials in rheumatoid arthritis, juvenile idiopathic arthritis, ankylosing spondylitis, psoriatic arthritis, psoriasis and Crohn's disease. Ann. Rheum. Dis. 72 (4), 517–524. doi:10.1136/annrheumdis-2011-201244
Chaimani, A., and Salanti, G. (2012). Using network meta-analysis to evaluate the existence of small-study effects in a network of interventions. Res. Synth. Methods. 3 (2), 161–176. doi:10.1002/jrsm.57
Che, H., Lukas, C., Morel, J., and Combe, B. (2014). Risk of herpes/herpes zoster during anti-tumor necrosis factor therapy in patients with rheumatoid arthritis. Systematic review and meta-analysis. Jt. Bone Spine 81 (3), 215–221. doi:10.1016/j.jbspin.2013.07.009
Chiu, Y. M., and Chen, D. Y. (2020). Infection risk in patients undergoing treatment for inflammatory arthritis: non-biologics versus biologics. Expert Rev. Clin. Immunol. 16 (2), 207–228. doi:10.1080/1744666X.2019.1705785
Cumpston, M., Li, T., Page, M. J., Chandler, J., Welch, V. A., Higgins, J. P., et al. (2019). Updated guidance for trusted systematic reviews: a new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst. Rev. 10 (10), ED000142. doi:10.1002/14651858.ED000142
Dixon, W. G., Hyrich, K. L., Watson, K. D., Lunt, M., Galloway, J., Ustianowski, A., et al. (2010). Drug-specific risk of tuberculosis in patients with rheumatoid arthritis treated with anti-TNF therapy: results from the British Society for Rheumatology Biologics Register (BSRBR). Ann. Rheum. Dis. 69 (3), 522–528. doi:10.1136/ard.2009.118935
Fonseca, J. E., Lucas, H., Canhao, H., Duarte, R., Santos, M. J., Villar, M., et al. (2008). Recommendations for the diagnosis and treatment of latent and active tuberculosis in inflammatory joint diseases candidates for therapy with tumor necrosis factor alpha inhibitors: march 2008 update. Acta Reumatol. Port. 33 (1), 77–85.
Fraenkel, L., Bathon, J. M., England, B. R., St, C. E., Arayssi, T., Carandang, K., et al. (2021). 2021 American College of rheumatology guideline for the treatment of rheumatoid arthritis. Arthritis Care Res. 73 (7), 924–939. doi:10.1002/acr.24596
Furst, D. E., Wallis, R., Broder, M., and Beenhouwer, D. O. (2006). Tumor necrosis factor antagonists: different kinetics and/or mechanisms of action may explain differences in the risk for developing granulomatous infection. Semin. Arthritis Rheum. 36 (3), 159–167. doi:10.1016/j.semarthrit.2006.02.001
Galloway, J. B., Hyrich, K. L., Mercer, L. K., Dixon, W. G., Fu, B., Ustianowski, A. P., et al. (2011). Anti-TNF therapy is associated with an increased risk of serious infections in patients with rheumatoid arthritis especially in the first 6 months of treatment: updated results from the British Society for Rheumatology Biologics Register with special emphasis on risks in the elderly. Rheumatology 50 (1), 124–131. doi:10.1093/rheumatology/keq242
Gardam, M. A., Keystone, E. C., Menzies, R., Manners, S., Skamene, E., Long, R., et al. (2003). Anti-tumour necrosis factor agents and tuberculosis risk: mechanisms of action and clinical management. Lancet Infect. Dis. 3 (3), 148–155. doi:10.1016/s1473-3099(03)00545-0
Godfrey, M. S., and Friedman, L. N. (2019). Tuberculosis and biologic therapies: anti-tumor necrosis factor-α and beyond. Clin. Chest Med. 40 (4), 721–739. doi:10.1016/j.ccm.2019.07.003
Gorman, J. D., Sack, K. E., and Davis, J. J. (2002). Treatment of ankylosing spondylitis by inhibition of tumor necrosis factor alpha. N. Engl. J. Med. 346 (18), 1349–1356. doi:10.1056/NEJMoa012664
Higgins, J. P., Thompson, S. G., Deeks, J. J., and Altman, D. G. (2003). Measuring inconsistency in meta-analyses. BMJ-British Med. J. 327 (7414), 557–560. doi:10.1136/bmj.327.7414.557
Husni, M. E., Deodhar, A., Schwartzman, S., Chakravarty, S. D., Hsia, E. C., Leu, J. H., et al. (2022). Pooled safety results across phase 3 randomized trials of intravenous golimumab in rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis. Arthritis Res. Ther. 24 (1), 73. doi:10.1186/s13075-022-02753-6
Hutton, B., Salanti, G., Caldwell, D. M., Chaimani, A., Schmid, C. H., Cameron, C., et al. (2015). The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann. Intern. Med. 162 (11), 777–784. doi:10.7326/M14-2385
Maini, R., St, C. E., Breedveld, F., Furst, D., Kalden, J., Weisman, M., et al. (1999). Infliximab (chimeric anti-tumour necrosis factor alpha monoclonal antibody) versus placebo in rheumatoid arthritis patients receiving concomitant methotrexate: a randomised phase III trial. ATTRACT Study Group. Lancet. 354 (9194), 1932–1939. doi:10.1016/s0140-6736(99)05246-0
Marotte, H., and Cimaz, R. (2014). Etanercept - TNF receptor and IgG1 Fc fusion protein: is it different from other TNF blockers? Expert Opin. Biol. Ther. 14 (5), 569–572. doi:10.1517/14712598.2014.896334
Mease, P. J., Gladman, D. D., Ritchlin, C. T., Ruderman, E. M., Steinfeld, S. D., Choy, E. H., et al. (2005). Adalimumab for the treatment of patients with moderately to severely active psoriatic arthritis: results of a double-blind, randomized, placebo-controlled trial. Arthritis Rheum. 52 (10), 3279–3289. doi:10.1002/art.21306
Michaud, T. L., Rho, Y. H., Shamliyan, T., Kuntz, K. M., and Choi, H. K. (2014). The comparative safety of tumor necrosis factor inhibitors in rheumatoid arthritis: a meta-analysis update of 44 trials. Am. J. Med. 127 (12), 1208–1232. doi:10.1016/j.amjmed.2014.06.012
Minozzi, S., Bonovas, S., Lytras, T., Pecoraro, V., Gonzalez-Lorenzo, M., Bastiampillai, A. J., et al. (2016). Risk of infections using anti-TNF agents in rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis: a systematic review and meta-analysis. Expert Opin. Drug Saf. 15 (Suppl. 1), 11–34. doi:10.1080/14740338.2016.1240783
Page, M. J., Mckenzie, J. E., Bossuyt, P. M., Boutron, I., Hoffmann, T. C., Mulrow, C. D., et al. (2021). The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ-British Med. J. 372, n71. doi:10.1136/bmj.n71
Pasut, G. (2014). Pegylation of biological molecules and potential benefits: pharmacological properties of certolizumab pegol. Biodrugs 28 (Suppl. 1), S15–S23. doi:10.1007/s40259-013-0064-z
Pfeffer, K., Matsuyama, T., Kundig, T. M., Wakeham, A., Kishihara, K., Shahinian, A., et al. (1993). Mice deficient for the 55 kd tumor necrosis factor receptor are resistant to endotoxic shock, yet succumb to L. monocytogenes infection. Cell. 73 (3), 457–467. doi:10.1016/0092-8674(93)90134-c
Puhan, M. A., Schunemann, H. J., Murad, M. H., Li, T., Brignardello-Petersen, R., Singh, J. A., et al. (2014). A GRADE Working Group approach for rating the quality of treatment effect estimates from network meta-analysis. BMJ-British Med. J. 349, g5630. doi:10.1136/bmj.g5630
Redeker, I., Albrecht, K., Kekow, J., Burmester, G. R., Braun, J., Schafer, M., et al. (2022). Risk of herpes zoster (shingles) in patients with rheumatoid arthritis under biologic, targeted synthetic and conventional synthetic DMARD treatment: data from the German RABBIT register. Ann. Rheum. Dis. 81 (1), 41–47. doi:10.1136/annrheumdis-2021-220651
Rubbert-Roth, A., Atzeni, F., Masala, I. F., Caporali, R., Montecucco, C., and Sarzi-Puttini, P. (2018). TNF inhibitors in rheumatoid arthritis and spondyloarthritis: are they the same? Autoimmun. Rev. 17 (1), 24–28. doi:10.1016/j.autrev.2017.11.005
Saliba, L., Moulis, G., Abou, T. M., Rousseau, V., Chebane, L., Petitpain, N., et al. (2016). Tumor necrosis factor inhibitors added to nonbiological immunosuppressants vs. nonbiological immunosuppressants alone: a different signal of cancer risk according to the condition. A disproportionality analysis in a nationwide pharmacovigilance database. Fundam. Clin. Pharmacol. 30 (2), 162–171. doi:10.1111/fcp.12171
Schmader, K. E., and Dworkin, R. H. (2008). Natural history and treatment of herpes zoster. J. Pain. 9 (1 Suppl. 1), S3–S9. doi:10.1016/j.jpain.2007.10.002
Shim, S., Yoon, B. H., Shin, I. S., and Bae, J. M. (2017). Network meta-analysis: application and practice using Stata. Epidemiol. Health 39, e2017047. doi:10.4178/epih.e2017047
Singh, J. A. (2016). Infections with biologics in rheumatoid arthritis and related conditions: a scoping review of serious or hospitalized infections in observational studies. Curr. Rheumatol. Rep. 18 (10), 61. doi:10.1007/s11926-016-0609-5
Singh, J. A., Guyatt, G., Ogdie, A., Gladman, D. D., Deal, C., Deodhar, A., et al. (2019). Special article: 2018 American College of rheumatology/national psoriasis foundation guideline for the treatment of psoriatic arthritis. Arthritis Rheumatol. 71 (1), 5–32. doi:10.1002/art.40726
Smolen, J. S., Burmester, G. R., Combe, B., Curtis, J. R., Hall, S., Haraoui, B., et al. (2016). Head-to-head comparison of certolizumab pegol versus adalimumab in rheumatoid arthritis: 2-year efficacy and safety results from the randomised EXXELERATE study. Lancet 388 (10061), 2763–2774. doi:10.1016/S0140-6736(16)31651-8
Smolen, J. S., Landewe, R., Bijlsma, J., Burmester, G. R., Dougados, M., Kerschbaumer, A., et al. (2020). EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2019 update. Ann. Rheum. Dis. 79 (6), 685–699. doi:10.1136/annrheumdis-2019-216655
Song, F., Xiong, T., Parekh-Bhurke, S., Loke, Y. K., Sutton, A. J., Eastwood, A. J., et al. (2011). Inconsistency between direct and indirect comparisons of competing interventions: meta-epidemiological study. BMJ-British Med. J. 343, d4909. doi:10.1136/bmj.d4909
Sterne, J. A., Sutton, A. J., Ioannidis, J. P., Terrin, N., Jones, D. R., Lau, J., et al. (2011). Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ-British Med. J. 343, d4002. doi:10.1136/bmj.d4002
Strangfeld, A., Listing, J., Herzer, P., Liebhaber, A., Rockwitz, K., Richter, C., et al. (2009). Risk of herpes zoster in patients with rheumatoid arthritis treated with anti-TNF-alpha agents. JAMA-J. Am. Med. Assoc. 301 (7), 737–744. doi:10.1001/jama.2009.146
Ueda, N., Tsukamoto, H., Mitoma, H., Ayano, M., Tanaka, A., Ohta, S., et al. (2013). The cytotoxic effects of certolizumab pegol and golimumab mediated by transmembrane tumor necrosis factor α. Inflamm. Bowel Dis. 19 (6), 1224–1231. doi:10.1097/MIB.0b013e318280b169
van Dartel, S. A., Fransen, J., Kievit, W., Flendrie, M., den Broeder, A. A., Visser, H., et al. (2013). Difference in the risk of serious infections in patients with rheumatoid arthritis treated with adalimumab, infliximab and etanercept: results from the Dutch Rheumatoid Arthritis Monitoring (DREAM) registry. Ann. Rheum. Dis. 72 (6), 895–900. doi:10.1136/annrheumdis-2012-201338
Wallis, R. S. (2009). Infectious complications of tumor necrosis factor blockade. Curr. Opin. Infect. Dis. 22 (4), 403–409. doi:10.1097/QCO.0b013e32832dda55
Ward, M. M., Deodhar, A., Gensler, L. S., Dubreuil, M., Yu, D., Khan, M. A., et al. (2019). 2019 update of the American College of rheumatology/spondylitis association of America/spondyloarthritis research and treatment network recommendations for the treatment of ankylosing spondylitis and nonradiographic axial spondyloarthritis. Arthritis Rheumatol. 71 (10), 1599–1613. doi:10.1002/art.41042
Weinblatt, M. E., Keystone, E. C., Furst, D. E., Moreland, L. W., Weisman, M. H., Birbara, C. A., et al. (2003). Adalimumab, a fully human anti-tumor necrosis factor alpha monoclonal antibody, for the treatment of rheumatoid arthritis in patients taking concomitant methotrexate: the ARMADA trial. Arthritis Rheum. 48 (1), 35–45. doi:10.1002/art.10697
Winthrop, K. L., and Chiller, T. (2009). Preventing and treating biologic-associated opportunistic infections. Nat. Rev. Rheumatol. 5 (7), 405–410. doi:10.1038/nrrheum.2009.105
Keywords: tumor necrosis factor inhibitors, inflammatory arthritis, infection, network meta-analysis, systematic review
Citation: Jiang Z, Zou Y, Li G, Zhao S and Zhang W (2024) Comparisons of infection events associated with tumor necrosis factor inhibitors in patients with inflammatory arthritis: A systematic review and network meta-analysis. Front. Pharmacol. 15:1376262. doi: 10.3389/fphar.2024.1376262
Received: 25 January 2024; Accepted: 10 June 2024;
Published: 11 July 2024.
Edited by:
Annalisa Bruno, University of Studies G. d’Annunzio Chieti and Pescara, ItalyReviewed by:
Eduardo Monguilhott Dalmarco, Federal University of Santa Catarina, BrazilCopyright © 2024 Jiang, Zou, Li, Zhao and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wei Zhang, anN0emhhbmd3ZWlAMTI2LmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.