- 1Department of Endocrinology, Hospital of Chengdu University of Traditional Chinese Medicine, Chengdu, China
- 2State Key Laboratory of Southwestern Chinese Medicine Resources, College of Pharmacy, Chengdu University of Traditional Chinese Medicine, Chengdu, China
- 3TCM Regulating Metabolic Diseases Key Laboratory of Sichuan Province, Hospital of Chengdu University of Traditional Chinese Medicine, Chengdu, China
- 4College of Basic Medicine, Chengdu University of Traditional Chinese Medicine, Chengdu, China
- 5Department of Dermatology, Hospital of Chengdu University of Traditional Chinese Medicine, Chengdu, China
- 6Sichuan Provincial Engineering Research Center of Innovative Re-development of Famous Classical Formulas, Tianfu TCM Innovation Harbour, Chengdu University of Traditional Chinese Medicine, Chengdu, China
The incidence of inflammatory bowel disease (IBD) and the associated risk of colon cancer are increasing globally. Traditional Chinese medicine (TCM) treatment has unique advantages. The Sishen Pill, a common Chinese patented drug used to treat abdominal pain and diarrhea, consists mainly of Psoraleae Fructus, Myristicae Semen, Euodiae Fructus, and Schisandra Chinensis. Modern research has confirmed that Sishen Pill and its active secondary metabolites, such as psoralen, myristicin, evodiamine, and schisandrin, can improve intestinal inflammation and exert antitumor pharmacological effects. Common mechanisms in treating IBD and colon cancer mainly include regulating inflammation-related signaling pathways such as nuclear factor-kappa B, mitogen-activated protein kinase, phosphatidylinositol 3-kinase, NOD-like receptor heat protein domain-related protein 3, and wingless-type MMTV integration site family; NF-E2-related factor 2 and hypoxia-inducible factor 1α to inhibit oxidative stress; mitochondrial autophagy and endoplasmic reticulum stress; intestinal immune cell differentiation and function through the Janus kinase/signal transducer and activator of transcription pathway; and improving the gut microbiota and intestinal barrier. Overall, existing evidence suggests the potential of the Sishen pill to improve IBD and suppress inflammation-to-cancer transformation. However, large-scale randomized controlled clinical studies and research on the safety of these clinical applications are urgently required.
1 Introduction
Inflammatory bowel disease (IBD) is a chronic disease of the intestine that mainly includes ulcerative colitis (UC) and Crohn’s disease (CD). Its incidence has shown an upward trend worldwide (Ng et al., 2017). UC, the most important type of IBD, progresses gradually from the rectum to the proximal segments of the colon, with its lesions often localized to the mucosal epithelium. UC can occur in any part of the gastrointestinal tract and is commonly found in the terminal ileum and right colon. In addition to common discomfort symptoms, such as abdominal pain, diarrhea, and bloody stools, IBD is associated with an increased risk of various metabolic diseases, such as diabetes (Lu et al., 2020; Li et al., 2021b), acute coronary syndrome (D'Ascenzo et al., 2023; Zaka et al., 2023), nonalcoholic fatty liver disease (Chen et al., 2020c), and autoimmune skin diseases (Fu et al., 2018) such as rheumatoid arthritis and psoriasis. More importantly, IBD can increase the risk of various cancers, such as colon cancer (Gatenby et al., 2021; Piovani et al., 2022). A survey of patients with UC revealed that the estimated cumulative risk of UC-associated colorectal cancer was 0.7% within 10 years, but by 30 years, the risk rose to 33.2% (Kim et al., 2009). Treatment of IBD with 5-aminosalicylates can significantly reduce the incidence of colon cancer (Bonovas et al., 2017; Hsiao et al., 2022). In recent studies, targeted nutritional interventions (Cassotta et al., 2023), probiotics, and other intestinal microecological agents (Lee et al., 2022) were found to be effective in treating colitis-associated colon cancer (CACC). The process of IBD transformation into cancer involves complex molecular mechanisms, such as gene mutations, epigenetic alterations, persistent chronic inflammation, gut microbiota disorders, and others (Xue et al., 2018). Further exploration is warranted to limit intestinal inflammation and inhibit its transformation into colon tumors.
Natural botanical drugs have the therapeutic advantage of multiple pathways and multiple targets; numerous studies have confirmed that botanical drugs or their extracts could improve IBD, inhibit its progression to colon cancer, exerting an integrated pharmacological “anti-inflammatory + anti-cancer” effect (Yang et al., 2023b). Traditionally, the Sishen Pill is a Chinese patent drug commonly used to treat diarrhea and is mainly composed of Psoraleae Fructus, Myristicae Semen, Euodiae Fructus, and Schisandrae Chinensis at a dosage ratio of 4 : 2: 2 : 1. Jujubae Fructus and Zingiberis Rhizoma were also used as excipients in this formula. In traditional Chinese medicine (TCM), Sishen Pill is believed to “warm the kidneys to dispel cold and astringing the intestines to stop diarrhea.” modern clinical studies showed that it could effectively treat IBD and other intestinal inflammatory injury (Li et al., 2018; Long and Cao, 2021b; Xu et al., 2022b). The main active metabolites in this formula, such as myristicin (Ismail Abo El-Fadl and Mohamed, 2022), psoralen (Zhou, 2020), deoxyschizandrin (Zhang et al., 2016), evodiamine (Ding et al., 2020), and others could improve the intestinal mucosal damage caused by IBD through various molecular mechanisms. Recent studies also found that the Sishen Pill can effectively treat colon cancer (Jiang et al., 2023) and prevent the progression of inflammatory cancer transformation (Cao et al., 2012; Cao, 2013; Cao et al., 2013); various metabolites in this formula could also suppress the growth of colonic tumor cells. This review comprehensively summarizes the experimental research on the treatment of IBD and colon cancer with Sishen Pill and its active phytochemicals, screens for core effective phytochemicals, clarifies key targets of action, generalizes the potential common molecular mechanism of Sishen Pill to treat IBD and colon cancer, and proposes a future research outlook based on the current research.
2 Metabolites investigation of Sishen Pill
The earliest records of the Sishen Pill can be traced back to the Hua Tuo Shen Yi Mi Zhuan during the Han Dynasty. The main disease it treats is “predawn diarrhea” (Wang et al., 2015). Modern research has found that this formula not only treats diarrhea but also has curative effects on multiple intestinal diseases such as UC (Long and Cao, 2021a), irritable bowel syndrome (Li et al., 2018a), colorectal cancer (Sun et al., 2021), and extraintestinal diseases such as depression (Luo et al., 2023) and breast cancer (Xu et al., 2022a). The multiple active metabolites contained in the Sishen Pill determine its multi-target therapeutic effects. Several studies have applied advanced technology to analyze qualitative or quantitative the metabolites in the Sishen Pill. Briefly, high performance liquid chromatography (HPLC) (Wei et al., 2021; Guo et al., 2023), HPLC-electrospray ionization-tandem mass spectrometry (HPLC-ESI-MS/MS) (Zhang et al., 2018), and flash evaporation-gas chromatography/mass spectrometry (FE-GC/MS) (Huang et al., 2019) have been used to identify effective metabolites in this formula. Sishen Pill contains various effective metabolites such as coumarins, lignin, alkaloids, terpenoids, and others (Guo et al., 2023). The main metabolites with potential therapeutic effects on IBD and/or colon cancer are shown in Figure 1.
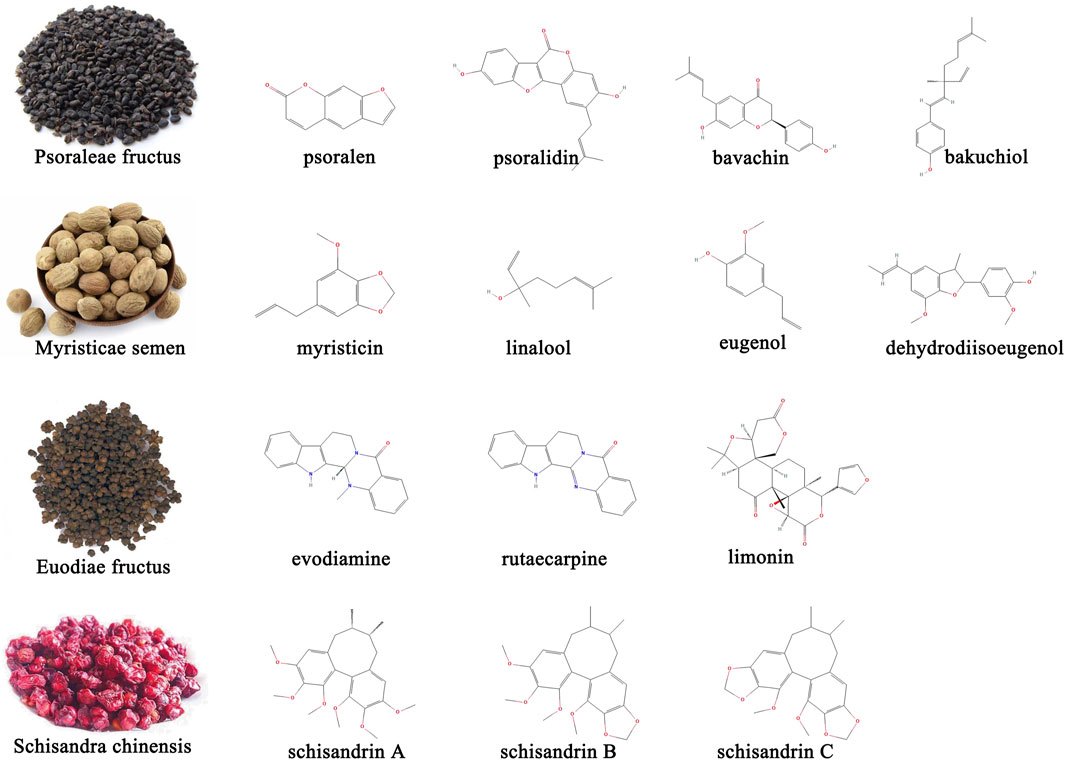
Figure 1. Representative active metabolites in Sishen Pill with potential therapeutic effects on IBD and/or colon cancer.
Psoraleae Fructus is the dried ripe fruit of Psoralea corylifolia Linn. The Leguminosae family and its metabolites include coumarins, flavonoids, benzofurans, monoterpenes, and some trace elements (Mu et al., 2018). Other studies focused on psoralen, isopsoralen, and psoralidin in coumarins; bavachin, bavachinin, and neobavaisoflavone in flavonoids; and bakuchiol in monoterpenoids (Chopra et al., 2013). In addition to antibacterial, anti-inflammatory, antitumor, antiviral, and antioxidant effects, Psoraleae Fructus can regulate bone cell metabolism, enhance skin pigmentation, and act like estrogen, expanding its utility in orthopedics, dermatology, and gynecology (Ren et al., 2020; Sharifi-Rad et al., 2020). The coumarin content in Psoraleae Fructus is an important indicator that the Sishen Pill meets quality standards (Huang et al., 2019). Several studies have confirmed that intestinal bacteria play an important role in metabolic processes. Wang et al. developed a rapid, sensitive, and selective ultra-performance liquid chromatography-tandem mass spectrometry (UPLC-MS/MS) method and found that psoralenoside and isopsoralenoside could be metabolized to psoralen and isopsoralen by gut microbiota through de-glucosylation (Wang et al., 2014). Furthermore, Liu et al. investigated the metabolic profiles of psoralen and isopsoralen under intestinal conditions and confirmed that some metabolites, such as 6,7-furano-hydrocoumaric acid and 5,6-furano-hydrocoumaric acid, have stronger activities in antioxidant stress and as anti-inflammatories (Liu L. et al., 2019).
Myristica Semen, the dried seeds of Myristica fragrans Houtt. plants in the Myristiceae family are a common TCM medicinal and edible homologous that contains lignans such as dehydrodiisoeugenol and macelignan; phenylpropanoids such as myristicin, eugenol, isoeugenol, and elemicin; and terpene alcohols such as linalool, all found to have multiple pharmacological properties (Liu et al., 2023). In addition to the therapeutic effects on the digestive system, such as peptic ulcer and diarrhea, Myristicae Semen has also been shown to be active against Parkinson’s disease and has anti-depressant, anti-epileptic, and anti-dementia effects (Liu et al., 2023). The combination of Myristicae Semen and Psoraleae Fructus, known as the traditional Ershen Pill formula, is also used to treat intestinal diseases such as diarrhea and abdominal cold pain. Gao et al. (2017) used HPLC to “fingerprint” Ershen Pill-medicated serum and found that psoralen, isopsoralen, bakuchiol, corylin, and dehydrodiisoeugenol were the main metabolites absorbed into the blood.
Euodiae Fructus is a nearly ripe, dry fruit of the Rutaceae plant Euodia rutaecarpa (Juss.) Benth. or E. rutaecarpa (Juss.) var. officinalis (Dode) Huang, or E. rutaecarpa (Juss.) Benth. var. bodinieri (Dode) Huang; it contains mainly alkaloids, terpenoids, flavonoids, phenylpropanoids, anthraquinone, and sterols; research has now focused on metabolites such as evodiamine, rutaecarpine, rutaevine, and limonin (Kong et al., 2023). Euodiae Fructus is widely used in clinical practice and has multiple effects, including pain relief, anti-inflammatory effects, gastrointestinal protection, antitumor effects, central nervous system protection, cardiovascular protection, and glycolipid metabolism regulation. Recently, to solve the problems of low solubility and bioavailability of evodiamine, attempts have been made to develop novel phospholipid and nanocomplex drug carriers to deliver evodiamine, achieve better clinical efficacy and reduce side effects (Luo et al., 2021).
Schisandrae Chinensis originates from the dried and ripe fruits of the Magnoliaceae plant Schisandra Chinensis (Turcz.) Baill, or Schisandra Sphenanthera Rehd. et Wils; the former is called Schisandrae Sphenantherae Fructus, whereas the latter is called Schisandrae Chinensis Fructus. The effective metabolites of Schisandrae Chinensis contain lignans, volatile oils, polysaccharides, organic acids, terpenoids, and flavonoids. Among them, lignans are considered the primary active metabolites, including mainly schizandrin A, schizandrin B, schizandrin C, schizandrol A, schizandrol B, schistenherin A, and schistenherin B. Studies found that schisandrins could regulate the central nervous, cardiovascular, digestive, endocrine, and immune systems, and are often used for sleep promotion, regulation of glucose and lipid metabolism, and as anti-inflammatory and anti-diarrhea agents (Xing et al., 2021). Similar to evodiamine, schisandrins have relatively low bioavailability; new technologies such as self-emulsifying drug delivery systems and solubility have been improved to some extent (Shao et al., 2010).
3 Research progress on Sishen Pill in the treatment of IBD and colon cancer
3.1 Sishen Pill in the treatment of IBD
The clinical efficacy of the Sishen Pill in treating UC has been confirmed by multiple clinical studies. Long et al. conducted a meta-analysis of nine randomized controlled trials (RCTs) including 680 patients and found that, compared to sulfasalazine and mesalazine, the combined use of Sishen Pill could effectively improve the effectiveness of treatment, reduce C-reactive protein levels, and have a lower incidence of adverse reactions (Long and Cao, 2021b), however, among the original studies included in this meta-analysis, different studies adopted different forms of administration of Sishen Pills (oral or enema), and it remains to be further explored which administration route can achieve better therapeutic effects. Zhang et al. used network pharmacology and bioinformatics methods to screen 22 key targets of the Sishen Pill in treating UC (Zhang et al., 2019) and suggested that it could improve intestinal inflammatory state, repair intestinal mucosal injury, and inhibit disease progression by regulating multiple targets, however, further experimental research is needed to confirm the relevant conclusions based on bioinformatics analysis. Table 1 lists the relevant basic research progress on the Sishen Pill for the treatment of IBD. Briefly, several studies focused on the inhibitory effects of Sishen Pill on the toll-like receptor (TLR): Huang (Huang et al., 2021) and Zhao (Zhaohua et al., 2022) found that the formula could inhibit expression levels of myeloid differentiation factor 88 (MyD88), interleukin-1 receptor associated kinase 4 (Irak4), and nuclear factor-kappa B(NF-κB) by down-regulating the activation of TLR2. Wang (Wang et al., 2019a) and Ge (Ge et al., 2022) confirmed that TLR4 was the key target, and downregulating TLR4 could inhibit the occurrence of subsequent inflammatory responses through MyD88-dependent and MyD88-independent pathways. In addition, Zhang (Zhang et al., 2021c), Wang (Wang et al., 2022a) and Zhao (Zhao et al., 2013) explored the molecular mechanism of the Sishen Pill in inhibiting the inflammatory response and promoting intestinal mucosal repair via phosphatidylinositol 3-kinase (PI3K)/protein kinase B (PKB/Akt), Janus kinase (JAK)/signal transducer and activator of transcription 5 (STAT5), and mitogen activated protein kinase (MAPK) signal pathways. Moreover, the regulation of intestinal immune cells by Sishen Pill mainly manifests in different subsets of T lymphocytes and regulatory T cells (Treg) (Liu et al., 2016), helper T cells (Th) (Liu et al., 2016), follicular helper T cells (Tfh) (Liu et al., 2020), follicular regulatory T cells (Tfr) (Huang et al., 2022; Kang et al., 2022), memory T cells (TM) (Ge et al., 2020), and dendritic cells (Liu et al., 2022). For regulating the gut microbiota, Sishen Pill has been shown to increase the relative abundance of beneficial bacteria, such as Lactobacillus and Akkermansia, and to promote an increase in intestinal butyrate content (Chen et al., 2020b; Wang et al., 2022b; Ge et al., 2022). In summary, the above studies have elucidated the mechanism of action of Sishen Pill in treating IBD from different perspectives, but there is still a lack of deeper exploration on the key targets of action, and the application of molecular inhibitors/activators or gene knockout animal model and other experimental methods is necessary and anticipated in future research.
3.2 Sishen Pill in the treatment of colon cancer
The RCT carried out by Sun et al. confirmed that the additional application of Sishen Pill in chemotherapy could significantly improve the patient’s discomfort symptoms, enhance the treatment effectiveness, reduce the probability of chemotherapy side effects (e.g., leukopenia, thrombocytopenia, liver and kidney function injury), and regulate immune cell levels (CD8+ cells↓, CD3+ and CD4+ cells↑) (Sun et al., 2021). Another clinical study revealed the therapeutic mechanism of the Sishen Pill in treating colon cancer from the perspective of gut microbiota. Researchers found that the richness and diversity of fecal microbiota in postoperative colon cancer patients were lower compared to healthy individuals; the Sishen Pill could improve this trend (Tan et al., 2023). For patients undergoing radical resection of colorectal cancer, Sishen Pill could not only alleviate clinical symptoms such as abdominal distension, tiredness, knee pain, waist acid, and cold but also reduce the tumor marker CEA and immune function indexes CD8+ and CD8+/CD4+ and increase the level of CD4+ (Zhang Y. et al., 2021). It should be pointed out that all three clinical studies mentioned above exist some methodological flaws, for example, not using a double-blind study design, and lacking relevant descriptions about allocation concealment, which to some extent reduces the reliability of clinical trial results; besides, these studies have not adopted the core indicators of clinical research on colon cancer, such as whether it can improve the survival rate of patients? Longer follow-up periods are necessary in the further research. The cell experiment on the molecular mechanism of Sishen Pill treating colon cancer showed that serum medicated with 10% Sishen Pill could downregulate the viability of HCT116 cells and the expression level of glucose transporter 1 (GLUT-1) and promote the activation of enzymes related to aerobic glycolysis, such as hexokinase and fructose-6-phosphate kinase. It could also decrease the overexpression of methyltransferase-like 3 protein and inhibit m6A RNA methylation, suggesting that Sishen Pill could regulate the glycolysis process by intervening in epigenetic modification, thereby inhibiting the proliferation of colon cancer cells (Jiang et al., 2023). In addition to the direct anticancer effect, Cao et al. also carried out research on the molecular mechanism of the Sishen Pill in inhibiting the transformation of colonic inflammatory lesions to colon cancer and found that the formula could downregulate the expression levels of nuclear factor e2-related factor 2 (Nrf2) and cyclooxygenase-2 (COX-2) in colon tissue, reducing the cancer formation rate of dextran sodium sulfate (DSS)-induced colitis mice; both oral and enema administrations had significant curative effects (Cao et al., 2012; Cao, 2013). In short, some studies also indicated the clinical effectiveness and molecular mechanism of Sishen Pill in the treatment of colon cancer; however, higher-quality clinical research and deeper mechanistic explorations are needed.
4 Research progress on metabolites of Sishen Pill in the treatment of IBD and colon cancer
4.1 Psoralea fructus
Zhou et al. studied the pharmacological effects and molecular mechanisms of psoralen, isopsoralen, and bakuchiol in treating IBD and confirmed that psoralen is the core pharmacodynamic substance and that the mechanism might be associated with the homeostasis of bile acids regulated by farnesoid X receptor (FXR)-fibroblast growth factor 15 (FGF15) pathways (Zhou, 2020). Ami et al. applied network pharmacology to identify 13 metabolites with good bioavailability after the oral administration of Psoraleae Fructus; and 11 metabolites could significantly reduce the overproduction of nitric oxide (NO), tumor necrosis factor- α (TNF-α) and interleukin-6 (IL-6) in macrophages induced by lipopolysaccharide (LPS) (Lee et al., 2023). Besides, network pharmacology analysis has also been used to explore molecular mechanism of isobavachalcone, an active metabolite of Psoralea Fructus, in the treatment of IBD, and Yang et al. confirmed AKT1, matrix metalloprotein 9 (MMP9), epidermal growth factor receptor (EGFR), insulin-like growth factor 1(IGF1), and steroid receptor coactivator (SRC) were its core targets (Yang et al., 2023a). The identification of effective metabolites is a key focus of botanical drug research, and the above studies is mainly based on the drug concentration in the blood, or bioavailability as the main screening criterion, which may inevitably overlook the indispensable effects of some metabolites with poor bioavailability, and they may exer intestinal protective effect by regulating the gut microbiota or microbial metabolites, rather than entering the peripheral loop. In addition, bakuchiol (Lim et al., 2019) and bavachin (Hung et al., 2019) have been shown to have ideal anti-inflammatory activities, and can they effectively treat IBD? Further research is needed for confirmation.
In recent years, the anticancer activity of various metabolites contained in Psoraleae Fructus has received widespread attention. For example, psoralen can inhibit the invasion and metastasis of human colon cancer HCT-116 cells, and its mechanism may be related to the downregulation of β-catenin, TCF4 proteins, their downstream target genes, vascular endothelial growth factor (VEGF), and MMP-9. Similarly, psoralidin was confirmed to reduce cell viability and enhance cell apoptosis by inhibiting the NF-κB and Bcl-2/BCL-2 associated X (Bax) signaling pathways (Jin et al., 2016); concurrently, it could also trigger oxidative damage-mediated apoptosis via rapidly boosting reactive oxygen species (ROS) generation (Sun et al., 2022). Bakuchiol can activate c-Jun N-terminal kinase (JNK) phosphorylation, induce ROS generation, and regulate the expression of death receptors and various anti-apoptotic proteins (Park et al., 2016). In addition, bavacin and 8-methoxypsoralen activate caspases by suppressing the MAPK and PI3K/AKT pathways, thereby promoting cancer cell apoptosis (Bartnik et al., 2017; Wang et al., 2023a) (see Table 2 for further details).
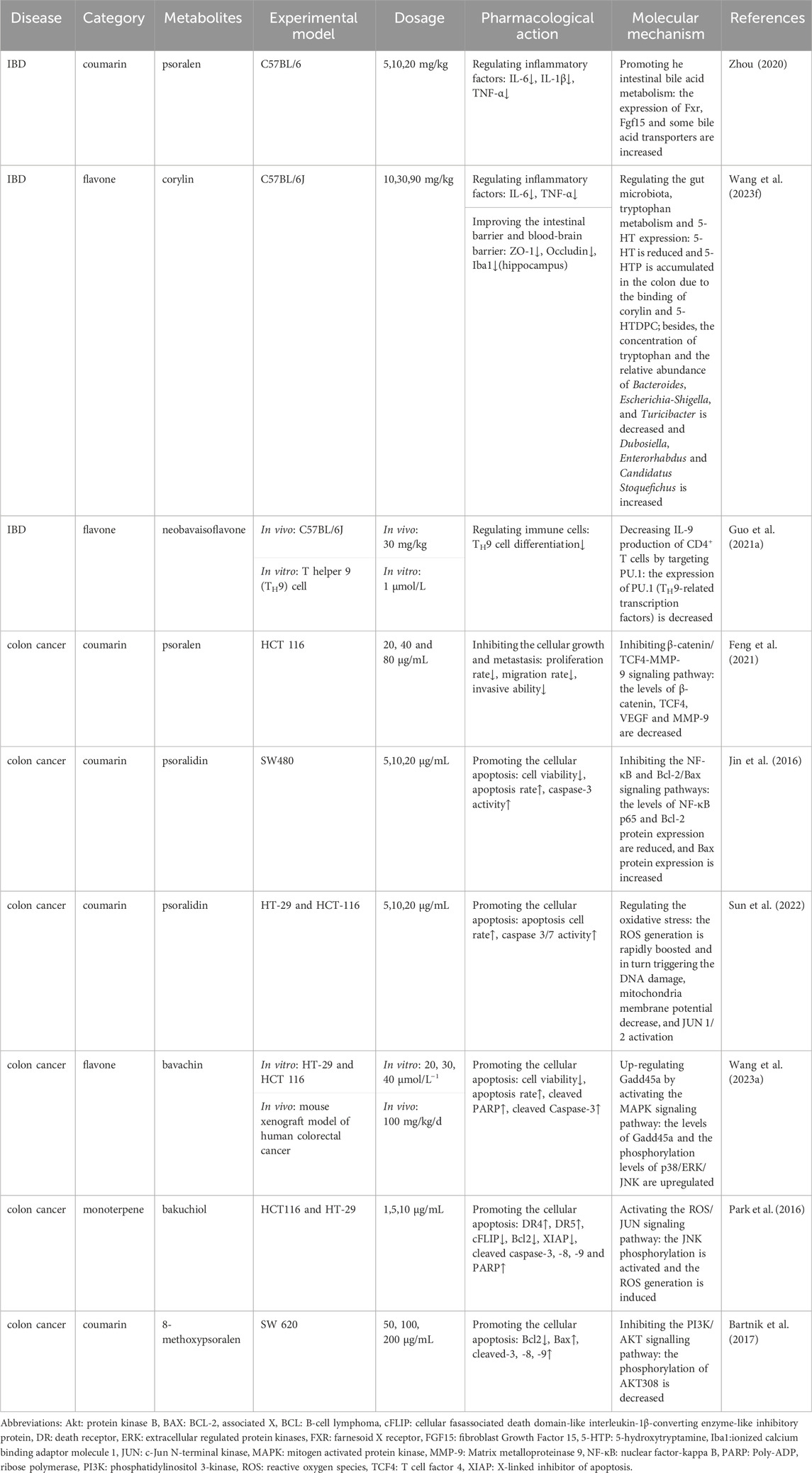
Table 2. Pharmacological effects and molecular mechanisms of the metabolites of Psoralea fructus in the treatment of IBD and colon cancer.
4.2 Myristica semen
Studies found that myristicin and linalool, two main metabolites in Myristica Semen, could exert anti-inflammatory and antioxidant effects by regulating the expression levels of NF-κ B and Nrf-2 (Tekeli et al., 2018; Ismail Abo El-Fadl and Mohamed, 2022). Compared to using diclofenac alone, the composite formulation of diclofenac and eugenol could better inhibit the nuclear translocation of NF-κB by activating the Nrf2/heme oxygenase-1 (HO-1) signaling pathway, thereby demonstrating better therapeutic effects against UC (Wang et al., 2023c). Zhang et al. developed a new phospholipid nanovesicle containing the volatile oil medicine eugenol to treat UC and confirmed that it was more conducive to percutaneous absorption and had better clinical efficacy (Zhang et al., 2020c).
In investigating Myristica Semen in treating colon cancer, Chen et al. used a network pharmacology method to screen nine active metabolites including galbacin and 24 core targets (Chen et al., 2023). Piras et al. (2012) identified the anticancer activity of essential oils and myristicin extracted from Myristicae Semen, confirming that they had a significant inhibitory effect on the growth of a colon cancer cell line (undifferentiated Caco-2 cells). In addition, Duan et al. (2020) showed that myristicin could inhibit the proliferation, migration, and invasion of colon cancer cells and induce cellular apoptosis by regulating the mitogen-activated protein kinase (MAPKK/MEK)/extracellular regulated protein kinase (ERK) signaling pathway. In addition, the regulatory effect of linalool on the oxidative response was beneficial for the treatment of colon cancer; it was confirmed that cellular apoptosis was induced by promoting the production of hydroxyl radicals and 4-HNE (a marker of oxidative stress due to increased lipid peroxidation) (Iwasaki et al., 2016). Moreover, some studies focused on the inhibitory effects of eugenol, isoeugenol, and dihydrodiiisoeugenol on colon cancer cells, showing that their anti-cancer mechanisms involve the regulation of metabolic pathways, apoptosis/metastasis-related gene expression, and the activation of endoplasmic reticulum stress-induced inhibition of autophagy (Li et al., 2021a; Ghodousi-Dehnavi et al., 2021; Bilgin et al., 2023). In above studies, Iwasaki et al. (Iwasaki et al., 2016) and Li et al. (Li et al., 2021b) used the tumor xenograft model in their experiments, while evidence from other studies mainly came from cell experiments. Generally speaking, the anti-tumor effects of botanical drugs are not single target or single pathway, and there exist complex interactions between different molecular pathways. It is crucial to explore the overall effects in tumor xenograft animal models, which greatly increases the credibility of research results. Further details are presented in Table 3.
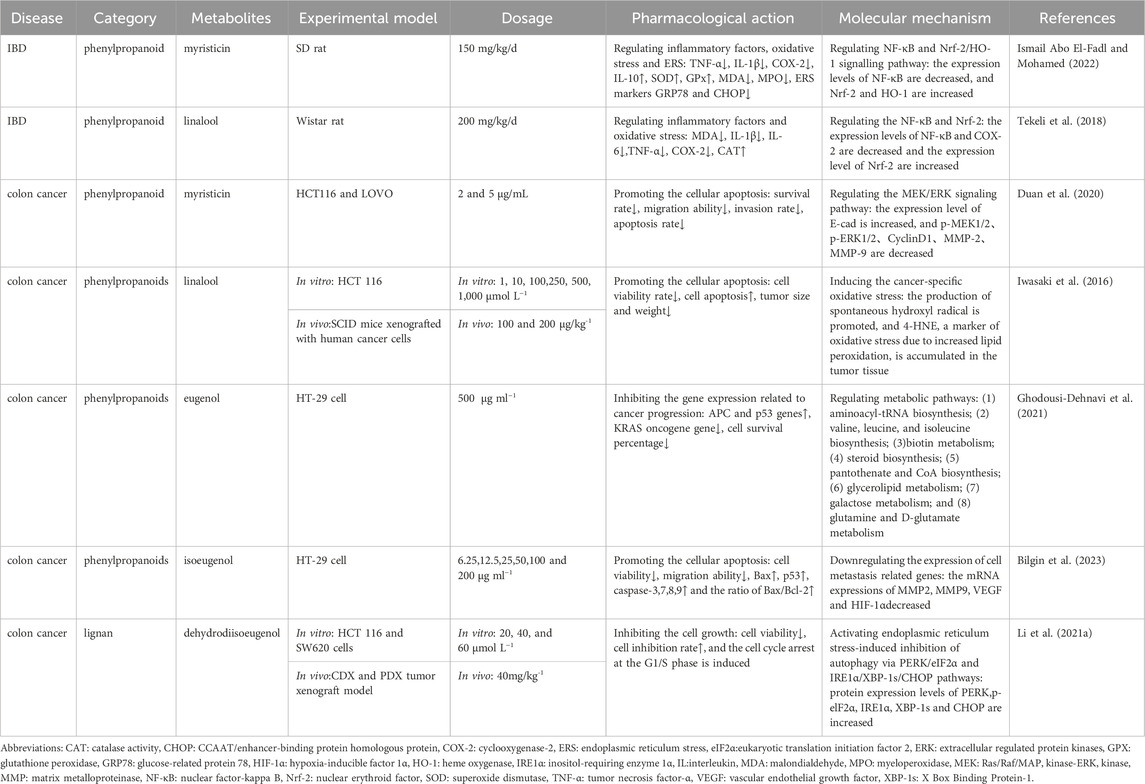
Table 3. Pharmacological effects and molecular mechanisms of the metabolites of Myristicae semen in the treatment of IBD and colon cancer.
4.3 Euodiae fructus
Studies suggested that NF-κB was the key target of evodiamine in the treatment of IBD, and downregulating NF-κB pathway proteins and inhibiting NOD like receptor heat protein domain related protein 3 (NLRP3) expression could alleviate inflammation-induced cell damage and repair the intestinal mucosal barrier (Shen et al., 2019; Ding et al., 2020). Simultaneously, evodiamine could also promote the regulation of the gut microbiota, especially by increasing the relative abundance of the beneficial bacterium Lactobacillus and intestinal acetate content, inhibiting the proliferation of the pathogenic Escherichia coli, and reducing plasma LPS and various inflammatory factor levels (Shen et al., 2019; Wang et al., 2020c; Ding et al., 2020). Another study suggested that kelch-like ECH-associated protein 1 (KEAP1) is a key target of rutecarpine, inhibiting the interactions between KEAP1 and Nrf2 by binding to the KEAP1 kelch domain, thereby activating Nrf2, promoting its nuclear translocation, upregulating the Nrf2-mediated antioxidant response, and achieving the pharmacological effect of improving intestinal mucosal injury (Zhang et al., 2020b).
In parallel, Chien et al. found that evodiamine could activate the MAPK signaling pathway and induce cell apoptosis and G2/M arrest by upregulating the phosphorylation levels of ERK and JNK proteins (Chien et al., 2014). Other researchers (Huang et al., 2015), Zhu (Zhu et al., 2021), and Zhang (Zhang et al., 2022) suggested that the anti-inflammatory effects of evodiamine also involved PI3K, STAT3, and NF-κB signaling pathways. In addition, evodiamine can reverse the epithelial-mesenchymal transition of tumor-associated fibroblasts induced by promoting the phosphorylation of Smad2 and Smad3, thereby reducing the migration and invasion abilities of tumor cells (Yang et al., 2019). In addition, Woong et al.’s research confirmed that rutecarpine could also inhibit wingless-type MMTV integration site family (Wnt)/β-catenin-mediated signaling pathway, thereby downregulating the expression levels of epithelial mesenchymal transition biomarkers such as MMP-7, Snail, and N-cadherin (Byun et al., 2022). Li et al. developed an EGFR targeting evodiamine-encapsulated polyamino acid nanoparticles to resolve the issue of low solubility and bioavailability and compared it with the traditional evodiamine formulation. The new preparation significantly increased the cytotoxicity of colon cancer cells and inhibited cell adhesion, invasion, and migration (Li et al., 2019a). Others have designed new metabolites based on evodiamine that have shown promising antitumor activity (Wang et al., 2020a; Li et al., 2020b). It should be pointed out that the dosage of evodiamine is still controversial in different studies. The minimum dose is 1 mg/kg, while the maximum dose is 40 mg/kg. It can be seen that a more detailed dose-response and dose-toxicity relationships of evodiamine needs to be further determined, which is crucial for guiding the clinical application. Further details are presented in Table 4.
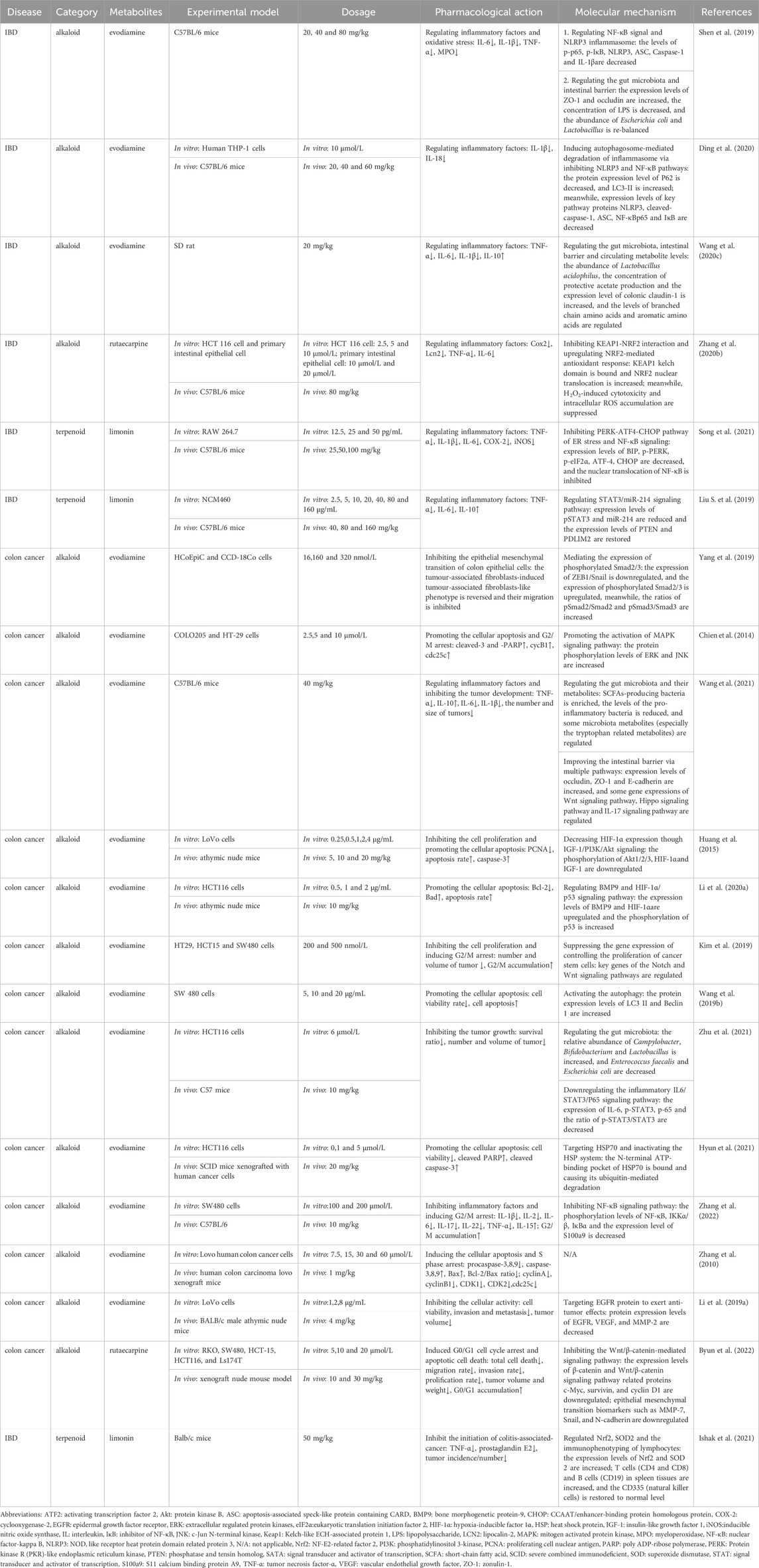
Table 4. Pharmacological effects and molecular mechanisms of the metabolites of Euodiae fructus in the treatment of IBD and colon cancer.
4.4 Schisandra chinensis
Multiple schisandrins have definite therapeutic effects in IBD. For example, schisandrin A (Wang et al., 2023b), schisandrin B (Liu et al., 2015), schisandrin C (Kim et al., 2022) and deoxyschizandrin (Yu and Qian, 2021) inhibited the NF-κB nuclear translocation and downstream pro-inflammatory signaling pathway activation; schisandrin B could also regulate AMPK/Nrf2, affect NLRP3 inflammasome, and then alleviate cell pyroptosis and intestinal epithelial damage caused by immune inflammation. In addition, Wang et al. conducted a pharmacokinetic analysis of seven different types of lignin in Schisandrae Chinensis and found that Cmax and AUC0-∞ of schisandrin were significantly higher than those of other lignans; they also confirmed that it could treat UC by inhibiting the serum/glucocorticoid regulated kinase 1 (SGK1)/NLRP3 pathway and regulating the gut microbiota (Wang et al., 2023d).
Some studies focused on the therapeutic effects of metabolites in Schisandra Chinensis on colon cancer. Casarin et al. found that two types of lignins in Schisandrae Chinensis, (+)-deoxyschisandrin (1) and (−)-gomisin N, could induce the apoptosis of colon adenocarcinoma cells (LoVo); the mechanism was related to the downregulation of cyclin B protein expression, mediating G2/M phase arrest (Casarin et al., 2014). Schisandrin A has also been proven to have a regulatory effect on the cell cycle; it could downregulate the expression of HO-1 protein through the Nrf-2 signaling pathway, thereby reducing the production of reactive oxygen species and nitrogen oxides. However, it could also block NF-κB nuclear translocation and the activation of MAPKs to inhibit inflammatory response (Wan et al., 2019). Some studies focused on the therapeutic potential of Schisandrae Chinensis in inhibiting the “inflammation-cancer transformation.” Li et al.’s experiment confirmed that schisandrin B could inhibit the occurrence of colitis-associated cancer by regulating the gut microbiota and activating the phosphorylation of focal adhesion kinase and its downstream kinase (Li et al., 2019b). Pu et al. (2021) found that the pharmacological effect of schisandrin B in inhibiting the proliferation and metastasis of colitis-related tumors was related to the downregulation of silencing regulatory protein 1(SIRT1) and inducing the expression of smad ubiquitination regulatory factor 2 (SMURF2) (Pu et al., 2021). The anti-tumor activity of some non-specific active substances of Schisandrae Chinensis, such as citral, cannot be ignored either; the experiment by Sheikh et al. confirmed that citral could inhibit the proliferation of HCT116 and HT29 cells in a dose-dependent and time-dependent manner; its mechanism was related to mediating the phosphorylation of p53 protein and promoting the mitochondrial release of apoptogenic factors (Sheikh et al., 2017). See Table 5 for more details.
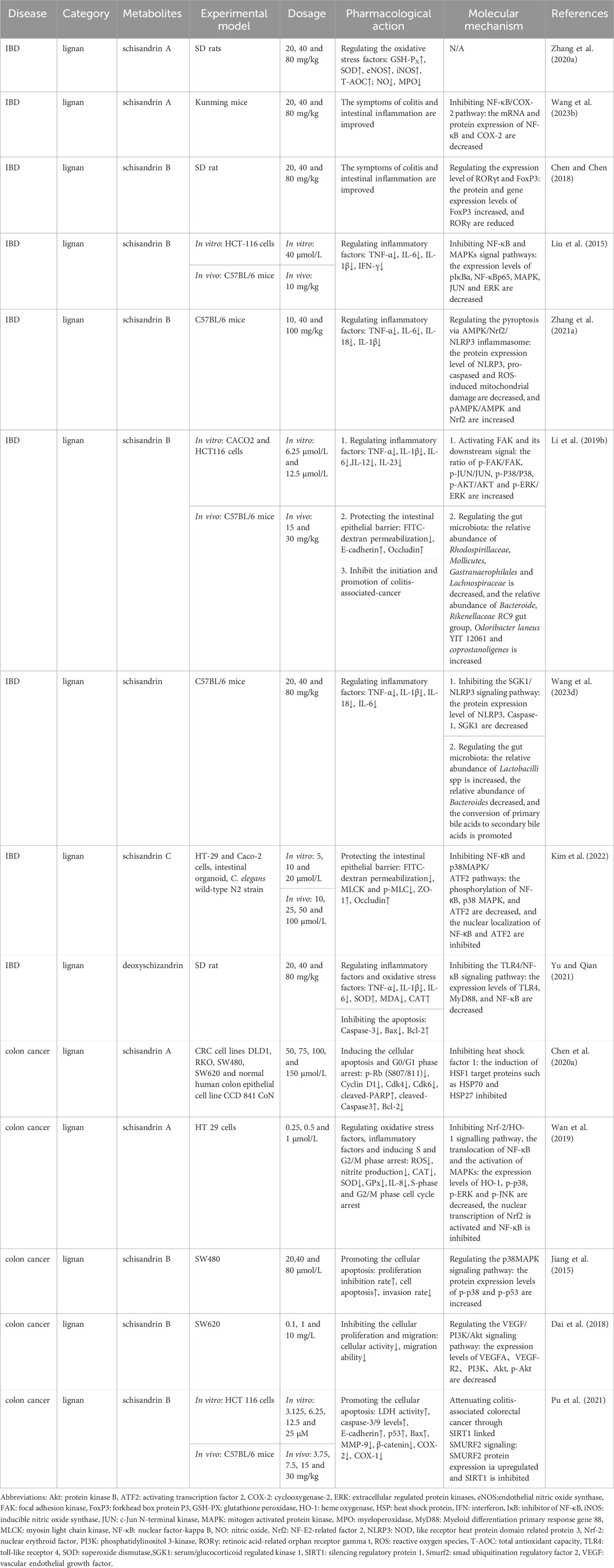
Table 5. Pharmacological effects and molecular mechanisms of the metabolites of Schisandra chinensis in the treatment of IBD and colon cancer.
5 Discussion on the common molecular mechanism of Sishen Pill in treating IBD and colon cancer
5.1 Regulating inflammation related signaling pathways
Chronic inflammation is not only an important feature of IBD but also a driver of the onset and development of colon cancer. Studies have found that chronic intestinal inflammation can cause DNA double chain breaks, oxidative stress damage, and epigenetic changes in intestinal epithelial cells, upregulate oncogenes, downregulate cancer suppressor genes, and promote the occurrence of dysplasia and cancer (Shah and Itzkowitz, 2022). Regulation of the inflammatory response is the core mechanism of Sishen Pill in treating IBD and inhibiting inflammation-cancer transformation, which involves multiple inflammation-related signaling pathways (Figure 2).
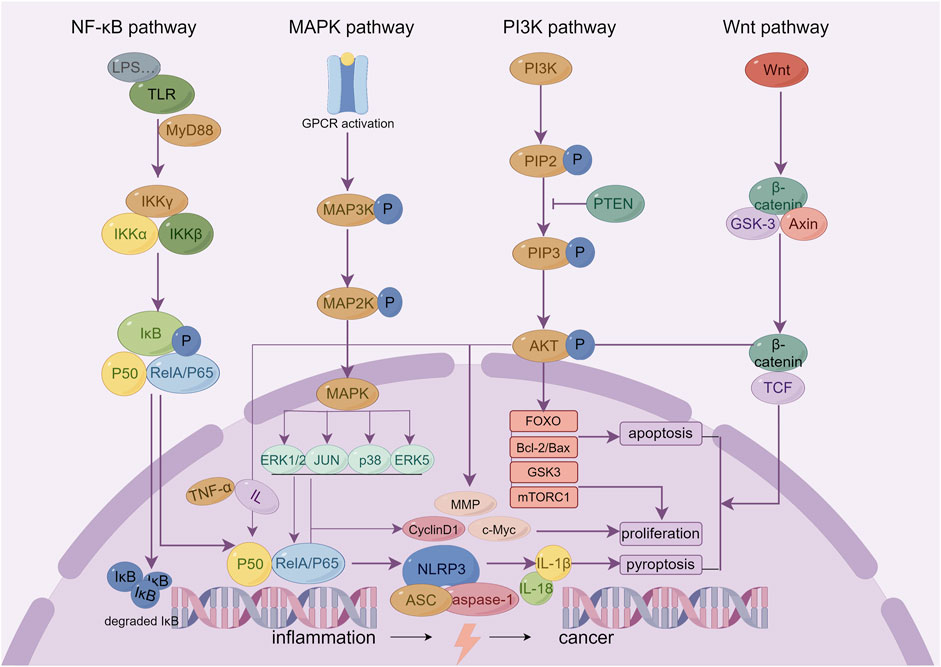
Figure 2. The key inflammatory related signaling pathways of Sishen Pill and its effective metabolites in treating IBD and colon cancer.
Many mechanistic studies on Sishen Pills focus on the regulatory effects of the NF-κB pathway (Wang et al., 2019d; Ge et al., 2022; Zhaohua et al., 2022). NF-κB is a classical key inflammatory modulator. LPS and other pro-inflammatory factors activate TLRs, induce NF-κB nuclear translocation, and regulate the gene expression of a variety of inflammatory mediators. In particular, LPS can promote the increase of TNF-α and multiple interleukins that act on macrophages to produce many inflammatory mediators and continuously induce NF-κB nuclear translocation to form a positive feedback cascade amplification effect. The basic activity of NF-κB is necessary for the normal proliferation and differentiation of cells to maintain the immune balance of epithelial tissue and inhibit the interference of inflammation on pithelial tissue homeostasis (Iacobazzi et al., 2023). Recently, a number of studies have focused on the role of NF-κB in promoting tumor cell apoptosis. As a landmark cell cycle protein, cyclinD1 is also the target gene of NF-κB. The continuous activation of NF-κB can initiate cyclinD1 transcription, promote the G1/G0 phase-to-S phase transition, and lead to abnormal cell proliferation and cancer. Thus, inhibiting NF-κB activation or blocking its downstream key proteins is considered an important target for developing new antitumor drugs (Taniguchi and Karin, 2018; Deka and Li, 2023).
The pharmacological effects of the Sishen Pill in the treatment of IBD and colon cancer are also related to the regulation of the MAPK and PI3K/Akt signaling pathways. Extracellular regulated protein kinases (ERK)1/2, c-JNK, p38, and ERK5 are the main members of the MAPK family, and there is also extensive cross talk between different pathways: ERK mainly regulates cell growth and differentiation; JNK and p38 play more important roles in stress responses such as inflammation and cell apoptosis; and ERK5 can regulate pathological processes such as cell cycle acceleration and endothelial cell proliferation caused by growth factors and stress (Ronkina and Gaestel, 2022). In general, the MAPK signaling pathway can mediate the release of TNF-α, IL-1, IL-6, IL-8, and other inflammatory factors, cell apoptosis, and neutrophil activation, induce the expression of intracellular nitric oxide, improve the activity of intracellular inducible nitric oxide synthase, and induce the occurrence and development of IBD and colon cancer (Yong et al., 2009). In addition, PI3K is a key target that is closely related to inflammation and tumor development. In the inflammatory state, PI3K phosphorylates phosphatidylinositol 4,5-bisphosphate (PIP2) to generate phosphatidylinositol-3,4,5-triphosphate (PIP3), recruiting downstream proteins such as Akt. The activated AKT subsequently phosphorylates multiple downstream substrate proteins. PI3K can also promote the activation of NF-κB and regulate the inflammatory response by phosphorylating and inhibiting IκB kinase (IKK); it can also regulate biological processes such as cell proliferation, survival, apoptosis, and metabolism and then promote tumor progression. Briefly, it can 1) act on mammalian rapamycin target protein complex 1 (mTORC1) to promote protein synthesis and cell growth; 2) phosphorylate forkhead box O (FOXO) transcription factors, inhibit its transcriptional activity, and affect cell cycle, apoptosis, and metabolism; 3) inhibit the activity of glycogen synthase kinase 3 (GSK3) and regulate glycogen synthesis and cell cycle; 4) phosphorylate and activate the pro-apoptotic protein Bad, making it unable to bind to Bcl-2 or Bcl-XL, and thus reducing the occurrence of normal cell apoptosis (Mayer and Arteaga, 2016; Wang et al., 2023e). In the above study, Sishen Pill improved intestinal inflammatory factors, immune cell disorders, and a series of symptoms of IBD by downregulating the expression of key proteins in the MAPK (Zhao et al., 2013) and PI3K/Akt (Ge et al., 2020; Zhang et al., 2021c; Liu et al., 2021; Liu et al., 2022) signaling pathways, and its active metabolites bavachin (Wang et al., 2023a), myristicin (Duan et al., 2020), evodiamine (Chien et al., 2014), schisandrin B (Jiang et al., 2015), 8-methoxypsoralen (Bartnik et al., 2017), and schisandrin B (Dai et al., 2018). Consequently, the Sishen Pill could inhibit the progression of colon cancer by regulating the MAPK and PI3K pathways.
The NLRP3 inflammasome and Wnt signaling pathways affect pyroptosis and cell differentiation/apoptosis, respectively, and are potential targets for regulating colon inflammation-cancer transformation. NLRP3 inflammasome is composed of NLRP3, apoptosis-associated speck-like protein containing CARD (ASC) and effector pro-caspase-1, and can affect the occurrence and development of IBD and even cancer via regulating the maturation, secretion, and pyroptosis of IL-1β and IL-18. Studies found that for IBD patients during the active period, the production of IL-1β and IL-18 and the activity of caspase-1 increase, thereby mediating the occurrence of intestinal cell apoptosis (Qi et al., 2021). Cell apoptosis is an important pathological basis for the transformation from inflammation to cancer and can induce the release of pro-inflammatory cytokines and promote tumor infiltration into local tissues, thus increasing the risk of tumor occurrence and metastasis (He et al., 2024). In addition, the Wnt/β-catenin signaling pathway has been confirmed to influence the differentiation fate of cell development to a certain extent, affecting cancer cell proliferation, stemness, apoptosis, autophagy, and metabolism. The modification and degradation of β -catenin are key events in the occurrence and development of colon cancer (Zhao et al., 2022). The research indicates that Sishen Pills (Zhao et al., 2019) and their active metabolites, schisandrin B (Zhang et al., 2021a), schisandrin (Wang et al., 2023d), evodiamine (Kim et al., 2019; Shen et al., 2019; Ding et al., 2020), and rutaecarpine (Byun et al., 2022) could affect cell fate by regulating the NLRP3 and Wnt signaling pathways, thus offering a therapeutic role in IBD and colon cancer.
5.2 Inhibiting the oxidative stress
Research has shown that during chronic inflammation, innate immune cells such as macrophages produce large amounts of reactive oxygen species (ROS) and reactive nitrogen species (RNS), leading to the aggravation of oxidative stress. During the active phase of IBD, the expression of ROS in the intestinal mucosa increases, and the subsequent reaction of ROS with DNA can lead to chromosomal breakage, carcinogenesis, and tumor cell proliferation (Wu and Liu, 2022). Regulating the Nrf2/HO-1 pathway may be a way to treat IBD with Sishen Pills (Zhang et al., 2021b), involving their active metabolites such as myristicin (Ismail Abo El-Fadl and Mohamed, 2022), linalool (Tekeli et al., 2018), rutaecarpine (Zhang et al., 2020b) and schisandrin B (Zhang et al., 2021a). Under normal physiological conditions, the Nrf2 in cells binds to the kelch-like ECH-associated protein 1 (Keap1) in the cytoplasm and remains in a steady state; when Keap1 receives an oxidative stress signal, it can release Nrf2 and then transfer it to the nucleus and upregulate the expression of downstream antioxidant proteins, such as HO-1 (Ibrahim et al., 2023). At the same time, HO-1 can also block the NF-κB activation and downregulate the transcription of inflammatory factors and chemokines by inhibiting the production of cytokines and ROS (Wang and He, 2022); this may contribute to the treatment of IBD by inhibiting intestinal inflammation-cancer transformation (Lu et al., 2023).
As the α subunit of hypoxia inducible factor-1 (HIF-1), HIF-1α mediates the adaptive response of cells to a hypoxic environment. In general, HIF-1 is activated under cellular hypoxia; it can activate multiple target genes involved in regulating the cellular redox status to reduce ROS generation; it can also regulate the expression levels of mitochondria-specific genes to adapt to hypoxic environments and improve mitochondrial function. As the above studies showed, the anti-colon cancer effects of evodiamine (Huang et al., 2015; Li et al., 2020a) and isoeugenol may be mediated by HIF-1α. The activation of HIF-1α and its signaling pathway has bidirectional regulatory effects. Studies have confirmed that moderate activation can promote cell survival and increase the protective effect against injury stimuli, whereas excessive activation can aggravate damage to intestinal cells (Taylor and Colgan, 2007). Due to the abnormal vascular microenvironment and inadequate local blood and oxygen supply to the tumor, hypoxia is a common feature of colon cancer; HIF-1α in the activated state can supply energy to tumor cells by upregulating glucose transporters and glycolysis related enzymes, helping cells adapt to the hypoxic environment. Previous studies confirmed that regulating aerobic glycolysis is an important factor for Sishen Pill to treat colon cancer (Zhang et al., 2021d; Jiang et al., 2023); whether it is related to HIF-1α remains to be determined. In addition, the activation of HIF-1α can also promote the tumor angiogenesis and metastasis by up regulating the expression of VEGF and MMP. As the above studies showed, several effective metabolites of Sishen Pill, such as psoralen, isoeugenol, and evodiamine, can downregulate the expression of VEGF and MMP proteins (Li et al., 2019a; Feng et al., 2021; Bilgin et al., 2023), thus promoting the apoptosis and having a therapeutic effect in colon cancer (Figure 3A).
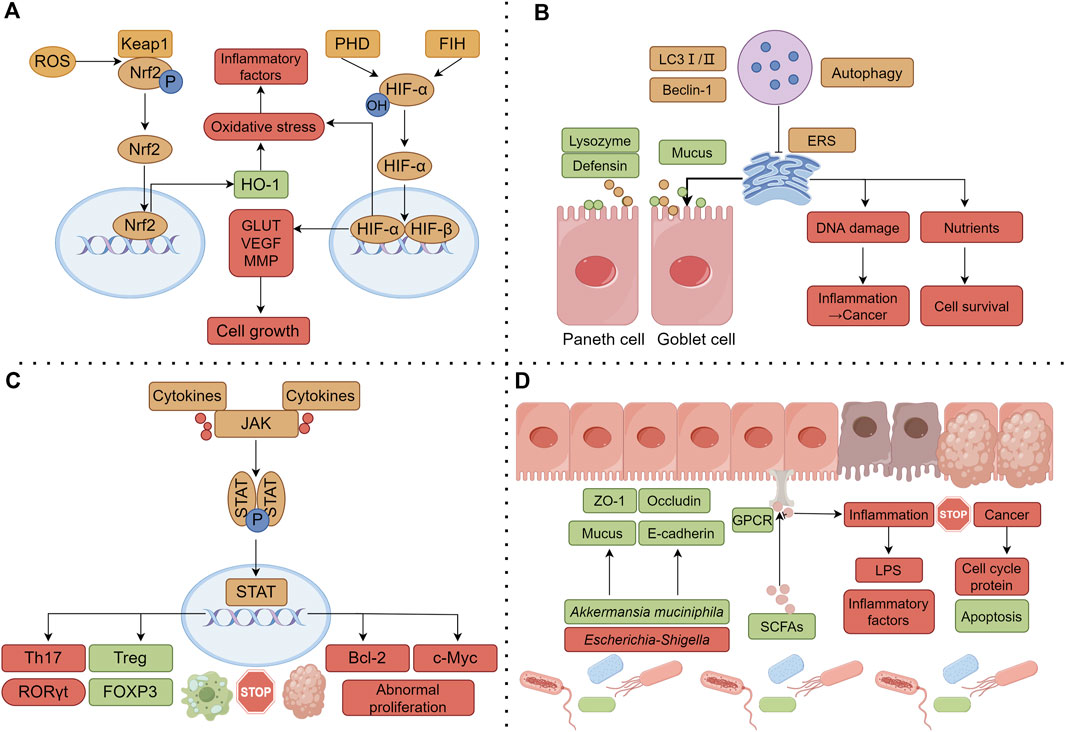
Figure 3. Common molecular mechanisms of Sishen Pill and its effective components in treating IBD and colon cancer. (A) Regulating the oxidative stress; (B) Regulating the mitochondrial autophagy; (C) Regulating intestinal immune cells; (D) Regulating the gut microbiota and internal barrier. Note: the green box represents the upregulated or promoted molecule, and the red box represents the downregulated or inhibited molecule.
5.3 Regulating the mitochondrial autophagy
Research has shown that impaired autophagy can disrupt the function of intestinal epithelial cells and affect innate and adaptive immune responses, ROS production, and endoplasmic reticulum stress (ERS), ultimately promoting the occurrence or progression of IBD (Alula and Theiss, 2023). Antimicrobial peptides secreted by intestinal Paneth cells are an important component of the intestinal mucus layer; however, owing to autophagy dysfunction, patients would experience decreased secretion of defensins and lysozymes by Paneth cells, leading to a weakened ability of the intestinal mucosa to resist the colonization of bacteria in the gut, hindering bacterial clearance, and damaging the intestinal mucosal barrier (Cray et al., 2021). At the same time, autophagy also participates in the mucus secretion and degradation metabolism of goblet cells, maintaining a stable balance of interactions between the intestinal mucosa and the gut microbiota (Naama et al., 2023). In the pathological environment of IBD, sustained inflammatory stimulation can lead to protein imbalance and abnormal folding in the lumen of the endoplasmic reticulum, exacerbating ERS. Autophagy can reduce the negative effects of ERS by clearing abnormal proteins and damaged organelles. Previous studies found that the Sishen Pill (Yu et al.) and its metabolites, myristicin (Ismail Abo El-Fadl and Mohamed, 2022) and evodiamine (Ding et al., 2020) promote autophagy and exert a positive influence on downregulating intestinal inflammatory responses.
Other studies have shown that dehydrodiisoeugenol (Li et al., 2021a) and evodiamine (Wang et al., 2019b) promote tumor cell apoptosis and exert anti-colon cancer effects by activating autophagy. Current research suggests that autophagy has a dual role in cancer occurrence. Autophagy is a surveillance system in normal cells that removes damaged organelles and aggregated proteins through lysosomes, consequently reducing DNA damage and protecting cells from malignant transformation (Yamazaki et al., 2021). Clinical studies have shown that a lack of autophagy-related proteins such as LC-3II, ATG5, and Beclin 1 can indicate poor prognosis in colon cancer patients (Choi et al., 2014). Autophagy can also provide key nutrients for tumor growth and metabolism and support tumor formation by inhibiting apoptosis (Galluzzi et al., 2015). Autophagy plays different roles in the different stages of malignant tumor development; a deeper exploration of the pharmacological mechanism of inflammation-cancer transformation as a whole is needed (Figure 3B).
5.4 Regulating intestinal immune cells
The regulatory effects of Sishen Pill on intestinal immune cells are also important for inhibiting the progression of IBD inflammation and its transformation into colon cancer. Taking Tregs as an example, the number of Tregs in the inflammatory mucosa of patients with IBD often shows a compensatory increasing trend, but the degree of increase is insufficient to control mucosal inflammation, leading to a relatively insufficient state (Hovhannisyan et al., 2011). Peripheral blood cell analysis has shown that the number of Tregs decreases, and the number of pro-inflammatory Th17 cells increases in IBD patients (Eastaff-Leung et al., 2010). Animal experiments have shown that the adoptive transfer of Tregs can alleviate enteritis by inhibiting Th1 and Th17 inflammatory responses, further confirming the regulatory effects of Tregs on intestinal inflammation (Boschetti et al., 2017). Multiple studies have shown that Sishen Pill can upregulate Tregs while downregulating the proportion of Th17 cells, thereby inhibiting the progression of IBD (Liu et al., 2016; Wang et al., 2022b; Huang et al., 2022). Schisandrin B upregulates the expression of forkhead box protein P3 (FoxP3) and promotes Treg proliferation and differentiation, regulating the intestinal immunity of IBD (Liu et al., 2015). The possible role of Tregs in treating intestinal tumors has also received widespread attention, but there is still debate over how Tregs affect the occurrence and progression of tumors. Some studies suggest that Tregs can lead to tumor growth and deterioration by inhibiting anti-tumor immune responses (Saito et al., 2016), associated with poor prognosis of the disease; however, further research is needed to investigate the effect of the Sishen Pill on Tregs in colon cancer models.
Memory T cells (Tms) are a crucial part of inflammatory immune responses. Tms can usually be divided into three main groups: central memory T cells (Tcm), effective memory T cells (Tem), and tissue-resident memory T cells (Trm). Zhao et al. found that the specific activation of Tm can prevent the recurrence of Crohn’s disease (Zhao et al., 2020). Two studies found that Sishen Pills can increase the level of Tcm cells and inhibit intestinal inflammatory factors through the PI3K/Akt and JAK/STAT5 pathways (Ge et al., 2020; Wang et al., 2022a). In addition, Tcm has self-renewal and replication capabilities, can recognize tumor antigens, and exerts long-lasting anti-tumor effects (Wang et al., 2020d). However, there is currently no relevant report on the Sishen Pill and its active metabolites in treating colon cancer by regulating Tcm; a deeper understanding of the pharmacological mechanisms is urgently needed.
JAK/STAT is one of the central communication nodes of cell function and is essential for initiating innate immunity, coordinating adaptive immune mechanisms, and regulating inflammatory responses. In intestinal-associated lymphoid tissues, dendritic cells and other antigen-presenting cells initiate antigen-specific immune responses, determining the activation of B cells and the differentiation of initial T helper cells, driven by the cytokine-receptor interaction of JAK-STAT signaling. Furthermore, different subtypes of helper T cells (Th1, Th2, Th9, and Th17) regulate Tregs, macrophages, and dendritic cells, among other immune cells, thereby regulating the intestinal inflammatory response and inhibiting tumor occurrence (Hu et al., 2021). JAK/STAT pathway inhibitors have been used to treat IBD, showing good therapeutic potential in preclinical studies (Salas et al., 2020). As mentioned above, multiple studies have confirmed that Sishen Pill can regulate intestinal cellular immunity by regulating JAK-STAT and the expression of its downstream protein suppressor of cytokine signaling (SOCS), one of the key signaling pathways by which the pill inhibits IBD immune inflammation and inflammation-cancer transformation (Liu et al., 2016; Liu et al., 2020; Wang et al., 2022a; Kang et al., 2022) (Figure 3C).
5.5 Regulating the gut microbiota and intestinal barrier
Research has shown widespread dysbiosis in the gut microbiota of both IBD and colon cancer patients and that Sishen Pill can affect the integrity of the intestinal barrier by regulating the gut microbiota and its metabolites. The decrease of Akkermansia muciniphila (AKK) and the increase of Escherichia-Shigella are significant characteristics of the gut microbiota in IBD population (Morgan et al., 2012; Alam et al., 2016); animal experiments have shown that Akk can promote the production of intestinal mucus, regulate the expression of tight junction proteins, and reduce the expression levels of inflammatory and chemotactic factors in the colon and serum (Bian et al., 2019). In addition, oral administration of inactivated Akk or the outer membrane protein of Akk (Amuc_1100) can also regulate CD8+ T cells, improving IBD and preventing the occurrence of CACC (Wang et al., 2020b). Another study suggested that AKK bacteria could enrich M1-like tumor associated macrophages in the colon cancer microenvironment in NLRP3 dependent way, thereby inhibiting tumor formation and development (Fan et al., 2021a). On the contrary, certain types of Escherichia-Shigella can escape host immunity, adhere to and invade intestinal epithelium and macrophages in hosts with genetic susceptibility to IBD, and initiate IBD development (Zangara et al., 2023). Also, the genotoxin produced by Escherichia-Shigella can penetrate the colon cell membrane and migrate to the nucleus, causing DNA double strand breaks, cell cycle arrest, chromosomal aberrations, intestinal epithelial damage, and eventually leading to cancer (Fan et al., 2021b). The above studies show that Sishen Pill can increase the relative abundance of AKK in the intestine (Chen et al., 2020b; Ge et al., 2022; Jin et al., 2023) and its effective metabolites, evodiamine, and corylin, leading to significant inhibition of Escherichia-Shigella the proliferation (Zhu et al., 2021; Wang et al., 2023f) thereby exerting anti-inflammatory and anticancer pharmacological activities.
Some metabolites of the gut microbiota, such as short-chain fatty acids (SCFAs), are also important for developing colitis and tumors. Studies have shown a decreasing trend in intestinal SCFAs in both IBD and colon cancer populations (Wang et al., 2019c; Dalile et al., 2019). As the main source of energy for intestinal epithelial cells, SCFAs not only promote the proliferation and differentiation of intestinal epithelial cells, reduce cell apoptosis, and maintain the mechanical barrier of the intestinal mucosa but also improve the secretion of intestinal mucoproteins, lubricate the intestine, block the adhesion of pathogens to the intestinal mucosa, and inhibit the occurrence of intestinal immune inflammation (Sun et al., 2017). In addition, butyrate in SCFAs has been proven to promote apoptosis and inhibit the proliferation of human colon cancer cells by activating G-protein coupled receptor 109A (GPR109A) (Moniri and Farah, 2021). The above research indicates that while regulating the gut microbiota, Sishen Pill can increase the contents of total SCFAs and butyrate in the intestine, thereby improving the inflammatory microenvironment of the intestine (Wang et al., 2022b).
Gut microbiota can affect the morphology and function of the intestinal barrier through various pathways; the integrity of the intestinal barrier is of great significance for the treatment of IBD and colon cancer. Post et al. detected 28 mucin proteins in the colonic mucosa of UC patients and found that seven mucin proteins, such as mucin 2 (MUC2), were significantly reduced; 30% of UC patients had abnormal permeability of the mucus layer, suggesting that abnormal colonic barrier function promotes the occurrence of UC (van der Post et al., 2019). Rath et al. showed that healing of the intestinal barrier has a high predictive value for the course of patients with remission-phase IBD; predictive ability of intestinal barrier healing might far exceed established or emerging parameters, such as endoscopic and histological remission (Rath et al., 2023). In addition, intestinal barrier damage and microbial translocation can activate chronic inflammation, further promoting the secretion of pro-inflammatory factors by immune cells and accelerating the process of colonic inflammation-cancer transformation (Shalapour and Karin, 2020). Another study found that when the intestinal vascular barrier is damaged, intestinal bacteria are more likely to spread to the liver, promoting the formation of a pre-metastatic niche for “colon-liver” metastasis, thereby promoting the recruitment of metastatic cells (Bertocchi et al., 2021). As mentioned earlier, Sishen Pills (Zhang et al., 2021c), schisandrin B (Li et al., 2019b), schisandrin C (Kim et al., 2022), and corylin (Wang et al., 2023f) can regulate the secretion of intestinal epithelial tight junction proteins and mucin, repair damaged intestinal mucosal barriers and inhibit the progression of IBD (Figure 3D).
6 Limitations and outlook
As a classic proprietary Chinese medicine for treating diarrhea, the curative effect of the Sishen Pill on IBD and colon cancer has been widely studied. Briefly, TCM formulas can regulate multiple targets simultaneously and exert integrated pharmacological effects; they can not only regulate intestinal immune inflammation disorders and fight against tumors but can also improve various symptoms, such as abdominal pain and diarrhea, enhancing the patient’s quality of life. In addition, preventive treatment of disease is a characteristic and an advantage of TCM; the application of the Sishen Pill in the early stage of IBD can effectively inhibit the transformation from inflammation to colon cancer. In summary, based on Western medical treatment, accumulating evidence suggests that the use of TCM represented by Sishen Pills can often bring more clinical benefits to patients. However, in terms of the current research on Sishen Pills, many limitations still need to be addressed.
Firstly, the above mentioned clinical studies and experimental studies have preliminarily confirmed the evidence that Sishen Pill can effectively treat IBD and colon cancer, however, the standardization of study design and reporting still needs further improvement. For example, 1) most studies do not provide quality testing reports and specific preparation methods of Sishen Pill; 2) there is a lack of description in the report regarding the experimental design methods and bias control strategies, such as specific measures for randomization of groups and baseline data of different groups before intervention; 3) there is a lack of description of animal or cell model selection criteria and modeling methods. In future studies, we recommend that: 1) design and report rigorously according to the requirements of the Cochrane Handbook (clinical study) (PT and Sally, 2024) and ARRIVE guidelines (animal experiments) (Kilkenny et al., 2012); 2) clinical studies should adopt internationally recognized major outcome measures, and basic experiments are necessary to observe the overall therapeutic effect of Sishen Pill on experimental animals, rather than just cell experimental evidence.
Second, there is still insufficient evidence on the safety of the Sishen Pill, posing a hidden danger in its clinical application. In recent years, liver damage caused by Psoraleae Fructus has become a focus of attention (Xu and Xiao, 2023). Guo et al. confirmed that the mechanism of liver injury could be associated with oxidative stress and mitochondrial damage-mediated apoptosis (Guo et al., 2021b) and that it may also be involved in liver regeneration, bile metabolism, energy metabolism, and other processes (Fan et al., 2024; Feng et al., 2024). Compared to bakuchiol, psoralen and isopsoralen have been confirmed to have stronger liver toxicity in vivo; their toxic effects are positively correlated with dosage (Mu et al., 2018). Similarly, Euodiae Fructus has also been shown to pose a potential risk of liver injury (Kong et al., 2023); its mechanism of action may be related to peroxidation injury, inflammatory factor mediation, mitochondrial damage, and drug‒ protein adduct formation (Wei et al., 2020). Other studies have reported that evodiamine exerts potential nephrotoxicity and cardiotoxicity (Yang et al., 2021). The impact of Myristicae Semen on the liver is two-sided; some studies confirmed that myristicin has a protective effect on drug-induced liver injury (Sohn et al., 2008; Yimam et al., 2016; Yang et al., 2018), while others found that Myristicae Semen extracts can also damage liver cells, increase serum transaminase levels and that the toxic effects are time- and dose-dependent (Cao et al., 2020). We believe that the “dose-effect-toxicity” relationship of the Sishen Pill should be further clarified through basic research to evaluate the clinical efficacy and safety. Further research should be conducted to enhance efficacy and detoxification using methods such as the processing and rational compatibility.
Third, although current research suggests that multiple metabolites in Sishen Pill have therapeutic effects on IBD and colon cancer, the active metabolites of this formula still need to be clarified. The currently elucidated molecular mechanisms may provide important links to its integrated pharmacological effects; however, the most critical target of action and differential pathways among different metabolites still require further exploration. We suggest using a more precise research approach as the next step in basic research. Luo and others (Luo et al., 2022) confirmed that MyD88 is the specific target for the anti-inflammatory effects of schisandrin B using target knockout models, carrying out target-metabolites binding assays, molecular docking, and experimental verification. In future research, high-throughput screening of the proteome and target-metabolites binding assays, vital for determining deeper pharmacological mechanisms of action and revealing the scientific connotations of TCM compatibility, should be widely used.
Fourth, clinical evidence of the Sishen Pill in treating colon cancer and inhibiting inflammation-cancer transformation is still lacking. As mentioned above, there are relatively few clinical and basic studies of Sishen Pill on treating colon cancer; more evidence is needed on its effective metabolites. However, there are complex interactions between different chemical metabolites, so the efficacy evaluation and mechanism exploration of Sishen Pill in treating colon cancer need to be further carried out. Besides, the transformation of colonic “inflammation-cancer” is a dynamic process; at present, only a few studies have focused on the effect of Sishen Pill and its metabolites on CACC. Specific clinical application strategies, including the best application nodes and treatment courses, are also urgently needed. In addition to the traditional dosage forms, the efficacy and safety of decoction enemas and volatile oils have also been preliminary confirmed; however, the differences in the indications of different dosage forms and how better market transformations can be performed need to be addressed stepwise through a series of studies. Large sample sizes, long-term follow-up RCTs, and real-world post-marketing reevaluations of formulas are necessary to better address the above issues.
7 Conclusion
Sishen Pills and its metabolites show great potential in the treatment of IBD, colon cancer and the inhibition of colonic inflammation-cancer transformation. Modern pharmacological research has confirmed that Sishen Pills molecular mechanisms mainly involve regulating inflammatory signaling pathways, inhibiting oxidative stress, improving mitochondrial mitophagy, regulating intestinal immune cells, and modulating the gut microbiota. Meanwhile, we should not overlook the limitations of current research. Due to the lack of rigorously designed large-sample RCTs, it is still difficult to answer questions about the long-term effectiveness and safety of the Sishen Pill in treating IBD and colon cancer. In future research, we recommend combining RCTs with real-world clinical studies to obtain stronger clinical evidence. At the same time, it is important to strengthen research on potential core metabolites of Sishen Pill, such as evodiamine and schisandrins, and clarify their key molecular targets, in order to lay the foundation for the new drug development.
Author contributions
BZ: Supervision, Writing–original draft, Writing–review and editing. YC: Writing–original draft, Writing–review and editing. QJ: Writing–original draft. SX: Writing–original draft. QX: Writing–original draft. CW: Writing–original draft. CY: Conceptualization, Supervision, Writing–original draft, Writing–review and editing. JL: Conceptualization, Supervision, Writing–original draft, Writing–review and editing. CZ: Conceptualization, Funding acquisition, Supervision, Writing–original draft, Writing–review and editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This research was funded by the Sichuan Science and Technology Program (2023NSFSC1994) and China Postdoctoral Science Foundation (2022MD713683).
Acknowledgments
The figures in the article were drawn using FigDraw (www.figdraw.com).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Alam, A., Leoni, G., Quiros, M., Wu, H., Desai, C., Nishio, H., et al. (2016). The microenvironment of injured murine gut elicits a local pro-restitutive microbiota. Nat. Microbiol. 1, 15021. doi:10.1038/nmicrobiol.2015.21
Alula, K. M., and Theiss, A. L. (2023). Autophagy in Crohn's disease: converging on dysfunctional innate immunity. Cells 12 (13), 1779. doi:10.3390/cells12131779
Bartnik, M., Sławińska-Brych, A., Żurek, A., Kandefer-Szerszeń, M., and Zdzisińska, B. (2017). 8-methoxypsoralen reduces AKT phosphorylation, induces intrinsic and extrinsic apoptotic pathways, and suppresses cell growth of SK-N-AS neuroblastoma and SW620 metastatic colon cancer cells. J. Ethnopharmacol. 207, 19–29. doi:10.1016/j.jep.2017.06.010
Bertocchi, A., Carloni, S., Ravenda, P. S., Bertalot, G., Spadoni, I., Lo Cascio, A., et al. (2021). Gut vascular barrier impairment leads to intestinal bacteria dissemination and colorectal cancer metastasis to liver. Cancer Cell 39 (5), 708–724.e11. doi:10.1016/j.ccell.2021.03.004
Bian, X., Wu, W., Yang, L., Lv, L., Wang, Q., Li, Y., et al. (2019). Administration of Akkermansia muciniphila ameliorates dextran sulfate sodium-induced ulcerative colitis in mice. Front. Microbiol. 10, 2259. doi:10.3389/fmicb.2019.02259
Bilgin, S., Erden Tayhan, S., Yıldırım, A., and Koç, E. (2023). Investigation of the effects of isoeugenol-based phenolic compounds on migration and proliferation of HT29 colon cancer cells at cellular and molecular level. Bioorg Chem. 130, 106230. doi:10.1016/j.bioorg.2022.106230
Bonovas, S., Fiorino, G., Lytras, T., Nikolopoulos, G., Peyrin-Biroulet, L., and Danese, S. (2017). Systematic review with meta-analysis: use of 5-aminosalicylates and risk of colorectal neoplasia in patients with inflammatory bowel disease. Aliment. Pharmacol. Ther. 45 (9), 1179–1192. doi:10.1111/apt.14023
Boschetti, G., Kanjarawi, R., Bardel, E., Collardeau-Frachon, S., Duclaux-Loras, R., Moro-Sibilot, L., et al. (2017). Gut inflammation in mice triggers proliferation and function of mucosal Foxp3+ regulatory T cells but impairs their conversion from CD4+ T cells. J. Crohns Colitis 11 (1), 105–117. doi:10.1093/ecco-jcc/jjw125
Byun, W. S., Bae, E. S., Kim, W. K., and Lee, S. K. (2022). Antitumor activity of rutaecarpine in human colorectal cancer cells by suppression of wnt/β-catenin signaling. J. Nat. Prod. 85 (5), 1407–1418. doi:10.1021/acs.jnatprod.2c00224
Cao, Y. (2013). Preventive effect of traditional Chinese medicine "Si-Shen bolus" on colitis associated cancer in mice. Doctor. master’s thesis. Liaoning: Liaoning University of Traditional Chinese Medicine.
Cao, Y., Wang, Y.-J., Xie, X., and Tian, Z.-G. (2013). Effect of Sishen Pill on the expression of CD133 protein in colon cancer induced by colitis in mice. Chin. Med. Mod. Distance Educ. China 11 (08), 145–146.
Cao, Y., Zhao, D.-Y., Chai, J.-Y., and Tian, Z.-G. (2012). Chemopreventive effect of sishen pill on experimental colon cancer in rats. J. Liaoning Univ. Traditional Chin. Med. 14 (11), 127–129. doi:10.13194/j.jlunivtcm.2012.11.129.caoy.063
Cao, Z., Xia, W., Zhang, X., Yuan, H., Guan, D., and Gao, L. (2020). Hepatotoxicity of nutmeg: a pilot study based on metabolomics. Biomed. Pharmacother. 131, 110780. doi:10.1016/j.biopha.2020.110780
Casarin, E., Dall'Acqua, S., Smejkal, K., Slapetová, T., Innocenti, G., and Carrara, M. (2014). Molecular mechanisms of antiproliferative effects induced by Schisandra-derived dibenzocyclooctadiene lignans (+)-deoxyschisandrin and (-)-gomisin N in human tumour cell lines. Fitoterapia 98, 241–247. doi:10.1016/j.fitote.2014.08.001
Cassotta, M., Cianciosi, D., De Giuseppe, R., Navarro-Hortal, M. D., Armas Diaz, Y., Forbes-Hernández, T. Y., et al. (2023). Possible role of nutrition in the prevention of inflammatory bowel disease-related colorectal cancer: a focus on human studies. Nutrition 110, 111980. doi:10.1016/j.nut.2023.111980
Chen, B. C., Tu, S. L., Zheng, B. A., Dong, Q. J., Wan, Z. A., and Dai, Q. Q. (2020a). Schizandrin A exhibits potent anticancer activity in colorectal cancer cells by inhibiting heat shock factor 1. Biosci. Rep. 40 (3). doi:10.1042/bsr20200203
Chen, F., Yin, Y. T., Zhao, H. M., Wang, H. Y., Zhong, Y. B., Long, J., et al. (2020b). Sishen pill treatment of DSS-induced colitis via regulating interaction with inflammatory dendritic cells and gut microbiota. Front. Physiol. 11, 801. doi:10.3389/fphys.2020.00801
Chen, L.-L., and Chen, R.-J. (2018). Therapeutical effect of schizandrin B on ulcerative colitis in rats and underlying mechanism. China Pharm. 21 (11), 1941–1945.
Chen, X., Xu, H., Li, R.-N., Li, X.-L., Zhao, L.-N., and Xu, Z.-L. (2023). Mechanism of Myristica fragrans Houtt.Against colorectal carcinoma based on network pharmacology and molecular docking. J. Med. Inf. 36 (14), 16–21.
Chen, Y., Chen, L., Xing, C., Deng, G., Zeng, F., Xie, T., et al. (2020c). The risk of rheumatoid arthritis among patients with inflammatory bowel disease: a systematic review and meta-analysis. BMC Gastroenterol. 20 (1), 192. doi:10.1186/s12876-020-01339-3
Chien, C. C., Wu, M. S., Shen, S. C., Ko, C. H., Chen, C. H., Yang, L. L., et al. (2014). Activation of JNK contributes to evodiamine-induced apoptosis and G2/M arrest in human colorectal carcinoma cells: a structure-activity study of evodiamine. PLoS One 9 (6), e99729. doi:10.1371/journal.pone.0099729
Choi, J. H., Cho, Y. S., Ko, Y. H., Hong, S. U., Park, J. H., and Lee, M. A. (2014). Absence of autophagy-related proteins expression is associated with poor prognosis in patients with colorectal adenocarcinoma. Gastroenterol. Res. Pract. 2014, 179586. doi:10.1155/2014/179586
Chopra, B., Dhingra, A. K., and Dhar, K. L. (2013). Psoralea corylifolia L. (Buguchi) - folklore to modern evidence: review. Fitoterapia 90, 44–56. doi:10.1016/j.fitote.2013.06.016
Cray, P., Sheahan, B. J., and Dekaney, C. M. (2021). Secretory sorcery: Paneth cell control of intestinal repair and homeostasis. Cell Mol. Gastroenterol. Hepatol. 12 (4), 1239–1250. doi:10.1016/j.jcmgh.2021.06.006
Dai, G.-L., Gong, T., Li, Y., Ding, K., Wu, K.-L., Li, Z.-W., et al. (2018). Effect of schisandrin B on proliferation and migration of human SW620 colon cancer cell via VEGF/PI3K/Akt signaling pathway. Chin. Pharm. J. 53(14), 1186–1191.
Dalile, B., Van Oudenhove, L., Vervliet, B., and Verbeke, K. (2019). The role of short-chain fatty acids in microbiota-gut-brain communication. Nat. Rev. Gastroenterol. Hepatol. 16 (8), 461–478. doi:10.1038/s41575-019-0157-3
D'Ascenzo, F., Bruno, F., Iannaccone, M., Testa, G., De Filippo, O., Giannino, G., et al. (2023). Patients with inflammatory bowel disease are at increased risk of atherothrombotic disease: a systematic review with meta-analysis. Int. J. Cardiol. 378, 96–104. doi:10.1016/j.ijcard.2023.02.042
Deka, K., and Li, Y. (2023). Transcriptional regulation during aberrant activation of NF-κB signalling in cancer. Cells 12 (5), 788. doi:10.3390/cells12050788
Ding, W., Ding, Z., Wang, Y., Zhu, Y., Gao, Q., Cao, W., et al. (2020). Evodiamine attenuates experimental colitis injury via activating autophagy and inhibiting NLRP3 inflammasome assembly. Front. Pharmacol. 11, 573870. doi:10.3389/fphar.2020.573870
Duan, C.-Y., He, N.-N., Zhu, L., Fan, L., Yi, F., Wang, T., et al. (2020). The mechanism of myristicin lnhibiting proliferation, migration and invasion of colon cancer cell lines. Mod. Traditional Chin. Med. Materia Medica-World Sci. Technol. 22 (04), 907–913.
Eastaff-Leung, N., Mabarrack, N., Barbour, A., Cummins, A., and Barry, S. (2010). Foxp3+ regulatory T cells, Th17 effector cells, and cytokine environment in inflammatory bowel disease. J. Clin. Immunol. 30 (1), 80–89. doi:10.1007/s10875-009-9345-1
Fan, B.-B., Zhong, R.-Z., Ma, Z., Han, Y., and Shu, T. (2024). Progress in pharmacological studies of buguzhi (Psoralea). Chin. Archives Traditional Chin. Med., 1–8.
Fan, L., Xu, C., Ge, Q., Lin, Y., Wong, C. C., Qi, Y., et al. (2021a). A. Muciniphila suppresses colorectal tumorigenesis by inducing TLR2/NLRP3-mediated M1-like TAMs. Cancer Immunol. Res. 9 (10), 1111–1124. doi:10.1158/2326-6066.Cir-20-1019
Fan, X., Jin, Y., Chen, G., Ma, X., and Zhang, L. (2021b). Gut microbiota dysbiosis drives the development of colorectal cancer. Digestion 102 (4), 508–515. doi:10.1159/000508328
Feng, K.-R., Wu, Y.-L., Li, W.-X., Wang, X.-Y., Zhang, H., Yang, L.-G., et al. (2024). Research progress on hepatotoxicity of Psoralea and its attenuation methods. China J. Traditional Chin. Med. Pharm. 1-8.
Feng, Y.-Y., Zhou, L.-H., Liu, N.-N., Sun, X.-T., Jia, R., and Li, Q. (2021). Effects of psoralen on invasion and metastasis of human colon cancer cells and β-catenin/TCF4-MMP-9 signaling pathway. China J. Traditional Chin. Med. Pharm. 36 (12), 7033–7037.
Fu, Y., Lee, C. H., and Chi, C. C. (2018). Association of psoriasis with inflammatory bowel disease: a systematic review and meta-analysis. JAMA Dermatol 154 (12), 1417–1423. doi:10.1001/jamadermatol.2018.3631
Galluzzi, L., Pietrocola, F., Bravo-San Pedro, J. M., Amaravadi, R. K., Baehrecke, E. H., Cecconi, F., et al. (2015). Autophagy in malignant transformation and cancer progression. Embo J. 34 (7), 856–880. doi:10.15252/embj.201490784
Gao, J.-R., Xu, S.-Z., Han, Y.-Q., Wei, L.-B., and Song, J.-M. (2017). Serum fingerprint of drug-couple Psoralea corylifolia-Myristica fragrants. Chin. Traditional Herb. Drugs 48 (12), 2401–2406.
Gatenby, G., Glyn, T., Pearson, J., Gearry, R., and Eglinton, T. (2021). The long-term incidence of dysplasia and colorectal cancer in a Crohn's colitis population-based cohort. Colorectal Dis. 23 (9), 2399–2406. doi:10.1111/codi.15756
Ge, W., Wang, H. Y., Zhao, H. M., Liu, X. K., Zhong, Y. B., Long, J., et al. (2020). Effect of sishen pill on memory T cells from experimental colitis induced by dextran sulfate sodium. Front. Pharmacol. 11, 908. doi:10.3389/fphar.2020.00908
Ge, W., Zhou, B. G., Zhong, Y. B., Liu, S. Q., Huang, J. Q., Yuan, W. Y., et al. (2022). Sishen pill ameliorates dextran sulfate sodium (DSS)-Induced colitis with spleen-kidney yang deficiency syndromes: role of gut microbiota, fecal metabolites, inflammatory dendritic cells, and TLR4/NF-κB pathway. Evid. Based Complement. Altern. Med. 2022, 6132289. doi:10.1155/2022/6132289
Ghodousi-Dehnavi, E., Hosseini, R. H., Arjmand, M., Nasri, S., and Zamani, Z. (2021). A metabolomic investigation of eugenol on colorectal cancer cell line HT-29 by modifying the expression of APC, p53, and KRAS genes. Evid. Based Complement. Altern. Med. 2021, 1448206. doi:10.1155/2021/1448206
Guo, J., Qiao, C., Zhou, J., Hu, S., Lin, X., Shen, Y., et al. (2021a). Neobavaisoflavone-mediated T(H)9 cell differentiation ameliorates bowel inflammation. Int. Immunopharmacol. 101 (Pt A), 108191. doi:10.1016/j.intimp.2021.108191
Guo, M., Liang, D.-Y., Huang, R., and Pan, X. (2023). The application of one test and multiple evaluation method in the content determinationand quality evaluation of 9 components in Sishen pills. J. South-Central Univ. Natl. Sci. Ed. 42 (02), 174–179. doi:10.20056/j.cnki.ZNMDZK.20230205
Guo, Z., Li, P., Wang, C., Kang, Q., Tu, C., Jiang, B., et al. (2021b). Five constituents contributed to the Psoraleae fructus-induced hepatotoxicity via mitochondrial dysfunction and apoptosis. Front. Pharmacol. 12, 682823. doi:10.3389/fphar.2021.682823
He, Z., Feng, D., Zhang, C., Chen, Z., Wang, H., Hou, J., et al. (2024). Recent strategies for evoking immunogenic Pyroptosis in antitumor immunotherapy. J. Control Release 366, 375–394. doi:10.1016/j.jconrel.2023.12.023
Hovhannisyan, Z., Treatman, J., Littman, D. R., and Mayer, L. (2011). Characterization of interleukin-17-producing regulatory T cells in inflamed intestinal mucosa from patients with inflammatory bowel diseases. Gastroenterology 140 (3), 957–965. doi:10.1053/j.gastro.2010.12.002
Hsiao, S. W., Yen, H. H., and Chen, Y. Y. (2022). Chemoprevention of colitis-associated dysplasia or cancer in inflammatory bowel disease. Gut Liver 16 (6), 840–848. doi:10.5009/gnl210479
Hu, X., Li, J., Fu, M., Zhao, X., and Wang, W. (2021). The JAK/STAT signaling pathway: from bench to clinic. Signal Transduct. Target Ther. 6 (1), 402. doi:10.1038/s41392-021-00791-1
Huang, J., Chen, Z. H., Ren, C. M., Wang, D. X., Yuan, S. X., Wu, Q. X., et al. (2015). Antiproliferation effect of evodiamine in human colon cancer cells is associated with IGF-1/HIF-1α downregulation. Oncol. Rep. 34 (6), 3203–3211. doi:10.3892/or.2015.4309
Huang, J., Jin, J., Kang, Z.-P., Liu, D.-Y., Cheng, S.-M., Zhong, Y.-B., et al. (2022). Effect of sisheng pill and its disassembly on expression of Treg cells and PD-1/PD-L1 in mice with colitis. Lishizhen Med. Materia Medica Res. 33 (06), 1284–1287.
Huang, J.-Q., Jiang, Q.-Q., Zhong, Y.-B., Wang, M.-X., Long, J., Zhao, H.-M., et al. (2021). Regulatory effect of volatile oil from sishenwan on TLR/MyD88 signaling pathway in mice with chronic ulcerative colitis. Chin. J. Exp. Traditional Med. Formulae 27 (23), 19–25. doi:10.13422/j.cnki.syfjx.20212301
Huang, Y.-L., Huang, Z.-P., and Wang, L.-L. (2019). Simultaneous determination of 8 active components in Sishen pills by flash evaporation-gas chromatography/mass spectrometry. Chin. J. Pharm. Analysis 39 (03), 510–517. doi:10.16155/j.0254-1793.2019.03.19
Hung, Y. L., Wang, S. C., Suzuki, K., Fang, S. H., Chen, C. S., Cheng, W. C., et al. (2019). Bavachin attenuates LPS-induced inflammatory response and inhibits the activation of NLRP3 inflammasome in macrophages. Phytomedicine 59, 152785. doi:10.1016/j.phymed.2018.12.008
Hyun, S. Y., Le, H. T., Min, H. Y., Pei, H., Lim, Y., Song, I., et al. (2021). Evodiamine inhibits both stem cell and non-stem-cell populations in human cancer cells by targeting heat shock protein 70. Theranostics 11 (6), 2932–2952. doi:10.7150/thno.49876
Iacobazzi, D., Convertini, P., Todisco, S., Santarsiero, A., Iacobazzi, V., and Infantino, V. (2023). New insights into NF-κB signaling in innate immunity: focus on immunometabolic crosstalks. Biol. (Basel) 12 (6), 776. doi:10.3390/biology12060776
Ibrahim, L., Stanton, C., Nutsch, K., Nguyen, T., Li-Ma, C., Ko, Y., et al. (2023). Succinylation of a KEAP1 sensor lysine promotes NRF2 activation. Cell Chem. Biol. 30 (10), 1295–1302.e4. doi:10.1016/j.chembiol.2023.07.014
Ishak, N. I. M., Mohamed, S., Madzuki, I. N., Mustapha, N. M., and Esa, N. M. (2021). Limonin modulated immune and inflammatory responses to suppress colorectal adenocarcinoma in mice model. Naunyn Schmiedeb. Arch. Pharmacol. 394 (9), 1907–1915. doi:10.1007/s00210-021-02101-6
Ismail Abo El-Fadl, H. M., and Mohamed, M. F. A. (2022). Targeting endoplasmic reticulum stress, Nrf-2/HO-1, and NF-κB by myristicin and its role in attenuation of ulcerative colitis in rats. Life Sci. 311 (Pt B), 121187. doi:10.1016/j.lfs.2022.121187
Iwasaki, K., Zheng, Y. W., Murata, S., Ito, H., Nakayama, K., Kurokawa, T., et al. (2016). Anticancer effect of linalool via cancer-specific hydroxyl radical generation in human colon cancer. World J. Gastroenterol. 22 (44), 9765–9774. doi:10.3748/wjg.v22.i44.9765
Jiang, E.-P., Li, H., Yu, C.-Y., and Zhu, W. (2015). Influence of schisandrin B in apoptosis and invasion of SW480 cells via p38MAPK signaling pathway. J. Jilin Univ. Ed. 41 (04), 675–679+885. doi:10.13481/j.1671-587x.20150401
Jiang, Y.-F., Huang, Y., Xiao, C., Zhou, S.-W., Zheng, L.-L., and You, F.-M. (2023). Inhibitory effect and mechanism of sishenwan-containing serum on aerobic glycolysis in human colon cancer cells. Chin. J. Exp. Traditional Med. Formulae 29 (19), 26–33. doi:10.13422/j.cnki.syfjx.20230130
Jin, J., Liu, D.-Y., Huang, J., Kang, Z.-P., Zhong, Y.-B., Long, J., et al. (2023). Regulation of sishen pills, ershen pills and wuweizi powder on intestinal microflora lmbalance in mice with colitis. Chin. Archives Traditional Chin. Med. 41 (04), 169–292. doi:10.13193/j.issn.1673-7717.2023.04.033
Jin, Z., Yan, W., Jin, H., Ge, C., and Xu, Y. (2016). Differential effect of psoralidin in enhancing apoptosis of colon cancer cells via nuclear factor-κB and B-cell lymphoma-2/B-cell lymphoma-2-associated X protein signaling pathways. Oncol. Lett. 11 (1), 267–272. doi:10.3892/ol.2015.3861
Kang, Z.-P., Jin, J., Jiang, Q.-Q., Zhao, H.-M., Cheng, S.-M., Zhong, Y.-B., et al. (2022). Effect of Sishen Pills and its split prescriptions on Tfr/Tfh9/Tfh17 cells in colitis mice. China J. Chin. Materia Medica 47 (05), 1300–1306. doi:10.19540/j.cnki.cjcmm.20211102.401
Kilkenny, C., Browne, W. J., Cuthill, I. C., Emerson, M., and Altman, D. G. (2012). Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. Osteoarthr. Cartil. 20 (4), 256–260. doi:10.1016/j.joca.2012.02.010
Kim, B. J., Yang, S. K., Kim, J. S., Jeen, Y. T., Choi, H., Han, D. S., et al. (2009). Trends of ulcerative colitis-associated colorectal cancer in Korea: a KASID study. J. Gastroenterol. Hepatol. 24 (4), 667–671. doi:10.1111/j.1440-1746.2008.05730.x
Kim, H., Yu, Y., Choi, S., Lee, H., Yu, J., Lee, J. H., et al. (2019). Evodiamine eliminates colon cancer stem cells via suppressing notch and wnt signaling. Molecules 24 (24), 4520. doi:10.3390/molecules24244520
Kim, M. R., Cho, S. Y., Lee, H. J., Kim, J. Y., Nguyen, U. T. T., Ha, N. M., et al. (2022). Schisandrin C improves leaky gut conditions in intestinal cell monolayer, organoid, and nematode models by increasing tight junction protein expression. Phytomedicine 103, 154209. doi:10.1016/j.phymed.2022.154209
Kong, Y.-D., Qi, Y., Cui, N., Zhang, Z.-H., Sun, Y.-P., Zeng, Y.-N., et al. (2023). Research progress on modern chemical constituents and pharmacological effects of evodia rutaecarpa. Inf. Traditional Chin. Med. 40 (05), 79–83+89. doi:10.19656/j.cnki.1002-2406.20230513
Lee, A., Chung, Y. C., Song, K. H., Ryuk, J. A., Ha, H., and Hwang, Y. H. (2023). Network pharmacology-based identification of bioavailable anti-inflammatory agents from Psoralea corylifolia L. in an experimental colitis model. J. Ethnopharmacol. 313, 116534. doi:10.1016/j.jep.2023.116534
Lee, S. Y., Lee, D. Y., Kang, J. H., Kim, J. H., Jeong, J. W., Kim, H. W., et al. (2022). Relationship between gut microbiota and colorectal cancer: probiotics as a potential strategy for prevention. Food Res. Int. 156, 111327. doi:10.1016/j.foodres.2022.111327
Li, C., Cai, G., Song, D., Gao, R., Teng, P., Zhou, L., et al. (2019a). Development of EGFR-targeted evodiamine nanoparticles for the treatment of colorectal cancer. Biomater. Sci. 7 (9), 3627–3639. doi:10.1039/c9bm00613c
Li, C., Zhang, K., Pan, G., Ji, H., Li, C., Wang, X., et al. (2021a). Dehydrodiisoeugenol inhibits colorectal cancer growth by endoplasmic reticulum stress-induced autophagic pathways. J. Exp. Clin. Cancer Res. 40 (1), 125. doi:10.1186/s13046-021-01915-9
Li, F. S., Huang, J., Cui, M. Z., Zeng, J. R., Li, P. P., Li, L., et al. (2020a). BMP9 mediates the anticancer activity of evodiamine through HIF-1α/p53 in human colon cancer cells. Oncol. Rep. 43 (2), 415–426. doi:10.3892/or.2019.7427
Li, J., Lu, Y., Wang, D., Quan, F., Chen, X., Sun, R., et al. (2019b). Schisandrin B prevents ulcerative colitis and colitis-associated-cancer by activating focal adhesion kinase and influence on gut microbiota in an in vivo and in vitro model. Eur. J. Pharmacol. 854, 9–21. doi:10.1016/j.ejphar.2019.03.059
Li, X., Wu, S., Dong, G., Chen, S., Ma, Z., Liu, D., et al. (2020b). Natural product evodiamine with borate trigger unit: discovery of potent antitumor agents against colon cancer. ACS Med. Chem. Lett. 11 (4), 439–444. doi:10.1021/acsmedchemlett.9b00513
Li, Y.-G., Deng, N., and Lin, X.-Y. (2018). Meta-analysis of sishen decoction on diarrhea-predominant lrritable bowel syndrome. J. Emerg. Traditional Chin. Med. 27 (02), 215–218.
Li, Z., Qiao, L., Yun, X., Du, F., Xing, S., and Yang, M. (2021b). Increased risk of ischemic heart disease and diabetes in inflammatory bowel disease. Z Gastroenterol. 59 (2), 117–124. doi:10.1055/a-1283-6966
Lim, H. S., Kim, Y. J., Kim, B. Y., and Jeong, S. J. (2019). Bakuchiol suppresses inflammatory responses via the downregulation of the p38 MAPK/ERK signaling pathway. Int. J. Mol. Sci. 20 (14), 3574. doi:10.3390/ijms20143574
Liu, D.-Y., Xu, R., Huang, M.-F., Wang, X., Zou, Y., Yue, H.-Y., et al. (2016). Mechanism of sishen wan in regulating balance of T lymphocyte subsets and Treg/Th17 in colitis rats. Chin. J. Exp. Traditional Med. Formulae 22 (03), 107–111. doi:10.13422/j.cnki.syfjx.2016030107
Liu, L., Zhang, L., Cui, Z. X., Liu, X. Y., Xu, W., and Yang, X. W. (2019). Transformation of psoralen and isopsoralen by human intestinal microbial in vitro, and the biological activities of its metabolites. Molecules 24 (22), 4080. doi:10.3390/molecules24224080
Liu, R., Wang, Y., Zhu, X.-D., Gao, Y.-K., Wang, H., and Zhong, X.-T. (2021). Effect of sishenwan on PI3K/Akt/mTOR signal pathway in colonic tissue of rats with ulcerative colitis model of spleen kidney yang deficiency. Chin. J. Exp. Traditional Med. Formulae 27 (04), 16–23. doi:10.13422/j.cnki.syfjx.20210437
Liu, R.-R., Sun, A.-Q., Yu, X.-J., He, M.-Y., Xie, H.-B., Gao, P., et al. (2023). Research progress on chemical composition and pharmacological effects of Myristicae Semen and predictive analysis on its quality marker. Chin. Traditional Herb. Drugs 54 (14), 4682–4700.
Liu, S., Zhang, S., Lv, X., Lu, J., Ren, C., Zeng, Z., et al. (2019). Limonin ameliorates ulcerative colitis by regulating STAT3/miR-214 signaling pathway. Int. Immunopharmacol. 75, 105768. doi:10.1016/j.intimp.2019.105768
Liu, S.-P., Ge, W., Cheng, S.-M., Yuan, W.-Y., Zhao, H.-M., Liu, D.-Y., et al. (2022). Regulation of Sishen Pill on the surface costimulatory molecules of dendritic cells in mice with spleen kidney yang deficiency colitis. Lishizhen Med. Materia Medica Res. 33 (12), 2878–2881.
Liu, W., Liu, Y., Wang, Z., Yu, T., Lu, Q., and Chen, H. (2015). Suppression of MAPK and NF-κ B pathways by schisandrin B contributes to attenuation of DSS-induced mice model of inflammatory bowel disease. Pharmazie 70 (9), 598–603.
Liu, X. K., Zhao, H. M., Wang, H. Y., Ge, W., Zhong, Y. B., Long, J., et al. (2020). Regulatory effect of sishen pill on Tfh cells in mice with experimental colitis. Front. Physiol. 11, 589. doi:10.3389/fphys.2020.00589
Long, C.-W., and Cao, H. (2021a). A meta-analysis on efficacy of modified sishen pill or combined with retention enema in treatment of ulcerative colitis compared with western medicine. J. Pract. Traditional Chin. Intern. Med. 35 (08), 147–148. doi:10.13729/j.issn.1671-7813.Z20201290
Long, C.-W., and Cao, H. (2021b). A meta-analysis on efficacy of modified sishen pill or combinedwith retention enema in treatment of ulcerative colitis compared with western medicine. J. Pract. Traditional Chin. Intern. Med. 35 (08), 147–148. doi:10.13729/j.issn.1671-7813.Z20201290
Lu, C., Xue, L., Luo, K., Liu, Y., Lai, J., Yao, X., et al. (2023). Colon-accumulated gold nanoclusters alleviate intestinal inflammation and prevent secondary colorectal carcinogenesis via nrf2-dependent macrophage reprogramming. ACS Nano 17 (18), 18421–18432. doi:10.1021/acsnano.3c06025
Lu, S., Gong, J., Tan, Y., and Liu, D. (2020). Epidemiologic association between inflammatory bowel diseases and type 1 diabetes mellitus: a meta-analysis. J. Gastrointestin Liver Dis. 29 (3), 407–413. doi:10.15403/jgld-798
Luo, C., Ai, J., Ren, E., Li, J., Feng, C., Li, X., et al. (2021). Research progress on evodiamine, a bioactive alkaloid of Evodiae fructus: focus on its anti-cancer activity and bioavailability (Review). Exp. Ther. Med. 22 (5), 1327. doi:10.3892/etm.2021.10762
Luo, W., Lin, K., Hua, J., Han, J., Zhang, Q., Chen, L., et al. (2022). Schisandrin B attenuates diabetic cardiomyopathy by targeting MyD88 and inhibiting MyD88-dependent inflammation. Adv. Sci. (Weinh) 9 (31), e2202590. doi:10.1002/advs.202202590
Luo, Y., Deng, Y., Shen, X.-M., Zhang, Y., Li, J.-C., Li, S., et al. (2023). Antidepressant effect of Sishen Wan and its effect on central monoamine nervous system. Chin. J. Pharmacol. Toxicol. 37 (02), 105–111.
Mayer, I. A., and Arteaga, C. L. (2016). The PI3K/AKT pathway as a target for cancer treatment. Annu. Rev. Med. 67, 11–28. doi:10.1146/annurev-med-062913-051343
Moniri, N. H., and Farah, Q. (2021). Short-chain free-fatty acid G protein-coupled receptors in colon cancer. Biochem. Pharmacol. 186, 114483. doi:10.1016/j.bcp.2021.114483
Morgan, X. C., Tickle, T. L., Sokol, H., Gevers, D., Devaney, K. L., Ward, D. V., et al. (2012). Dysfunction of the intestinal microbiome in inflammatory bowel disease and treatment. Genome Biol. 13 (9), R79. doi:10.1186/gb-2012-13-9-r79
Mu, G.-H., Shi, Y., Shen, M.-M., Wu, L.-T., Zhao, Q.-Z., and Liu, Y.-F. (2018). Overview and thoughts on on the main side effects of fructus Psoraleae. World Chin. Med. 13 (04), 1038–1042.
Naama, M., Telpaz, S., Awad, A., Ben-Simon, S., Harshuk-Shabso, S., Modilevsky, S., et al. (2023). Autophagy controls mucus secretion from intestinal goblet cells by alleviating ER stress. Cell Host Microbe 31 (3), 433–446.e4. doi:10.1016/j.chom.2023.01.006
Ng, S. C., Shi, H. Y., Hamidi, N., Underwood, F. E., Tang, W., Benchimol, E. I., et al. (2017). Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet 390 (10114), 2769–2778. doi:10.1016/s0140-6736(17)32448-0
Park, M. H., Kim, J. H., Chung, Y. H., and Lee, S. H. (2016). Bakuchiol sensitizes cancer cells to TRAIL through ROS- and JNK-mediated upregulation of death receptors and downregulation of survival proteins. Biochem. Biophys. Res. Commun. 473 (2), 586–592. doi:10.1016/j.bbrc.2016.03.127
Piovani, D., Hassan, C., Repici, A., Rimassa, L., Carlo-Stella, C., Nikolopoulos, G. K., et al. (2022). Risk of cancer in inflammatory bowel diseases: umbrella review and reanalysis of meta-analyses. Gastroenterology 163 (3), 671–684. doi:10.1053/j.gastro.2022.05.038
Piras, A., Rosa, A., Marongiu, B., Atzeri, A., Dessì, M. A., Falconieri, D., et al. (2012). Extraction and separation of volatile and fixed oils from seeds of Myristica fragrans by supercritical CO₂: chemical composition and cytotoxic activity on Caco-2 cancer cells. J. Food Sci. 77 (4), C448–C453. doi:10.1111/j.1750-3841.2012.02618.x
Pt, H. J., and Sally, G. (2024). Cochrane Handbook for systematic reviews of interventions:cochrane book series. John Wiley & Sons, Ltd.
Pu, Z., Zhang, W., Wang, M., Xu, M., Xie, H., and Zhao, J. (2021). Schisandrin B attenuates colitis-associated colorectal cancer through SIRT1 linked SMURF2 signaling. Am. J. Chin. Med. 49 (7), 1773–1789. doi:10.1142/s0192415x21500841
Qi, Y.-T., Zhang, F.-R., and Miao, Y.-L. (2021). Pyropotosis and inflammatory bowel disease. Chin. J. Inflamm. Bowel Dis. 05 (1), 92–95. doi:10.3760/cma.j.cn101480-20191022-00127
Rath, T., Atreya, R., Bodenschatz, J., Uter, W., Geppert, C. E., Vitali, F., et al. (2023). Intestinal barrier healing is superior to endoscopic and histologic remission for predicting major adverse outcomes in inflammatory bowel disease: the prospective ERIca trial. Gastroenterology 164 (2), 241–255. doi:10.1053/j.gastro.2022.10.014
Ren, Y., Song, X., Tan, L., Guo, C., Wang, M., Liu, H., et al. (2020). A review of the pharmacological properties of psoralen. Front. Pharmacol. 11, 571535. doi:10.3389/fphar.2020.571535
Ronkina, N., and Gaestel, M. (2022). MAPK-activated protein kinases: servant or partner? Annu. Rev. Biochem. 91, 505–540. doi:10.1146/annurev-biochem-081720-114505
Saito, T., Nishikawa, H., Wada, H., Nagano, Y., Sugiyama, D., Atarashi, K., et al. (2016). Two FOXP3(+)CD4(+) T cell subpopulations distinctly control the prognosis of colorectal cancers. Nat. Med. 22 (6), 679–684. doi:10.1038/nm.4086
Salas, A., Hernandez-Rocha, C., Duijvestein, M., Faubion, W., McGovern, D., Vermeire, S., et al. (2020). JAK-STAT pathway targeting for the treatment of inflammatory bowel disease. Nat. Rev. Gastroenterol. Hepatol. 17 (6), 323–337. doi:10.1038/s41575-020-0273-0
Shah, S. C., and Itzkowitz, S. H. (2022). Colorectal cancer in inflammatory bowel disease: mechanisms and management. Gastroenterology 162 (3), 715–730.e3. doi:10.1053/j.gastro.2021.10.035
Shalapour, S., and Karin, M. (2020). Cruel to Be kind: epithelial, microbial, and immune cell interactions in gastrointestinal cancers. Annu. Rev. Immunol. 38, 649–671. doi:10.1146/annurev-immunol-082019-081656
Shao, B., Tang, J., Ji, H., Liu, H., Liu, Y., Zhu, D., et al. (2010). Enhanced oral bioavailability of Wurenchun (Fructus Schisandrae Chinensis extracts) by self-emulsifying drug delivery systems. Drug Dev. Ind. Pharm. 36 (11), 1356–1363. doi:10.3109/03639045.2010.480975
Sharifi-Rad, J., Kamiloglu, S., Yeskaliyeva, B., Beyatli, A., Alfred, M. A., Salehi, B., et al. (2020). Pharmacological activities of psoralidin: a comprehensive review of the molecular mechanisms of action. Front. Pharmacol. 11, 571459. doi:10.3389/fphar.2020.571459
Sheikh, B. Y., Sarker, M. M. R., Kamarudin, M. N. A., and Mohan, G. (2017). Antiproliferative and apoptosis inducing effects of citral via p53 and ROS-induced mitochondrial-mediated apoptosis in human colorectal HCT116 and HT29 cell lines. Biomed. Pharmacother. 96, 834–846. doi:10.1016/j.biopha.2017.10.038
Shen, P., Zhang, Z., Zhu, K., Cao, H., Liu, J., Lu, X., et al. (2019). Evodiamine prevents dextran sulfate sodium-induced murine experimental colitis via the regulation of NF-κB and NLRP3 inflammasome. Biomed. Pharmacother. 110, 786–795. doi:10.1016/j.biopha.2018.12.033
Sohn, J. H., Han, K. L., Kim, J. H., Rukayadi, Y., and Hwang, J. K. (2008). Protective Effects of macelignan on cisplatin-induced hepatotoxicity is associated with JNK activation. Biol. Pharm. Bull. 31 (2), 273–277. doi:10.1248/bpb.31.273
Song, C., Chen, J., Li, X., Yang, R., Cao, X., Zhou, L., et al. (2021). Limonin ameliorates dextran sulfate sodium-induced chronic colitis in mice by inhibiting PERK-ATF4-CHOP pathway of ER stress and NF-κB signaling. Int. Immunopharmacol. 90, 107161. doi:10.1016/j.intimp.2020.107161
Sun, C., Zhao, L., Wang, X., Hou, Y., Guo, X., Lu, J. J., et al. (2022). Psoralidin, a natural compound from Psoralea corylifolia, induces oxidative damage mediated apoptosis in colon cancer cells. J. Biochem. Mol. Toxicol. 36 (7), e23051. doi:10.1002/jbt.23051
Sun, H.-H., Yang, J., and Wang, D.-J. (2021). Clinical study of Lizhong decoction and Sishen pill modified and subtractedcombined with FOLFIRl chemotherapy on elderly patients with advanced colon cancer with spleen and kidney Yang deficiency syndrome. Chin. J. Integr. Traditional West. Med. Dig. 29 (04), 276–279.
Sun, M., Wu, W., Liu, Z., and Cong, Y. (2017). Microbiota metabolite short chain fatty acids, GPCR, and inflammatory bowel diseases. J. Gastroenterol. 52 (1), 1–8. doi:10.1007/s00535-016-1242-9
Tan, Y.-B., Jiang, L.-J., Liao, Z.-J., and Zhou, Q. (2023). Clinical effect of Sishen pill combined with Lizhong decoction in the treatment of patients with spleen-kidney Yang deficiency syndrome after colorectal cancer surgery based on intestinal flora. China Med. 18 (07), 1054–1058.
Taniguchi, K., and Karin, M. (2018). NF-κB, inflammation, immunity and cancer: coming of age. Nat. Rev. Immunol. 18 (5), 309–324. doi:10.1038/nri.2017.142
Taylor, C. T., and Colgan, S. P. (2007). Hypoxia and gastrointestinal disease. J. Mol. Med. Berl. 85 (12), 1295–1300. doi:10.1007/s00109-007-0277-z
Tekeli, I. O., Ateşşahin, A., Sakin, F., Aslan, A., Çeribaşı, S., and Yipel, M. (2018). Protective effects of conventional and colon-targeted lycopene and linalool on ulcerative colitis induced by acetic acid in rats. Inflammopharmacology 27, 313–322. doi:10.1007/s10787-018-0485-x
van der Post, S., Jabbar, K. S., Birchenough, G., Arike, L., Akhtar, N., Sjovall, H., et al. (2019). Structural weakening of the colonic mucus barrier is an early event in ulcerative colitis pathogenesis. Gut 68 (12), 2142–2151. doi:10.1136/gutjnl-2018-317571
Wan, M. L. Y., Turner, P. C., Co, V. A., Wang, M. F., Amiri, K. M. A., and El-Nezami, H. (2019). Schisandrin A protects intestinal epithelial cells from deoxynivalenol-induced cytotoxicity, oxidative damage and inflammation. Sci. Rep. 9 (1), 19173. doi:10.1038/s41598-019-55821-4
Wang, A.-H., He, L.-J., and Zhu, X.-D. (2019a). Effect of sishenwan on toll-like receptor 4 and lRAK-M expression in colonic tissue of rats with ulcerative colitis of spleen-kidney yang deficiency type. Chin. J. Exp. Traditional Med. Formulae 25 (14), 70–76. doi:10.13422/j.cnki.syfjx.20191439
Wang, D., Ge, S., Chen, Z., and Song, Y. (2019b). Evodiamine exerts anticancer effects via induction of apoptosis and autophagy and suppresses the migration and invasion of human colon cancer cells. J. buon 24 (5), 1824–1829.
Wang, G., Yu, Y., Wang, Y. Z., Wang, J. J., Guan, R., Sun, Y., et al. (2019c). Role of SCFAs in gut microbiome and glycolysis for colorectal cancer therapy. J. Cell Physiol. 234 (10), 17023–17049. doi:10.1002/jcp.28436
Wang, H. Y., Zhao, H. M., Wang, Y., Liu, Y., Lu, X. Y., Liu, X. K., et al. (2019d). Sishen Wan® ameliorated trinitrobenzene-sulfonic-acid-induced chronic colitis via NEMO/NLK signaling pathway. Front. Pharmacol. 10, 170. doi:10.3389/fphar.2019.00170
Wang, L., Fang, K., Cheng, J., Li, Y., Huang, Y., Chen, S., et al. (2020a). Scaffold hopping of natural product evodiamine: discovery of a novel antitumor scaffold with excellent potency against colon cancer. J. Med. Chem. 63 (2), 696–713. doi:10.1021/acs.jmedchem.9b01626
Wang, L., and He, C. (2022). Nrf2-mediated anti-inflammatory polarization of macrophages as therapeutic targets for osteoarthritis. Front. Immunol. 13, 967193. doi:10.3389/fimmu.2022.967193
Wang, L., Tang, L., Feng, Y., Zhao, S., Han, M., Zhang, C., et al. (2020b). A purified membrane protein from Akkermansia muciniphila or the pasteurised bacterium blunts colitis associated tumourigenesis by modulation of CD8(+) T cells in mice. Gut 69 (11), 1988–1997. doi:10.1136/gutjnl-2019-320105
Wang, L.-H., Ma, D.-C., and Sun, L.-X. (2015). The origin and new exploration of SiShen pills. West. J. Traditional Chin. Med. 28 (03), 47–49.
Wang, M., Huang, X., Kang, Z., Huang, J., Wei, S., Zhao, H., et al. (2022a). Mechanism of sishen-pill-regulated special memory T and mTfh cell via involving JAK/STAT5 pathway in colitis mice. Evid. Based Complement. Altern. Med. 2022, 6446674. doi:10.1155/2022/6446674
Wang, M., Tian, B., Shen, J., Xu, S., Liu, C., Guan, L., et al. (2023a). Bavachin induces apoptosis in colorectal cancer cells through Gadd45a via the MAPK signaling pathway. Chin. J. Nat. Med. 21 (1), 36–46. doi:10.1016/s1875-5364(23)60383-8
Wang, M., Zhou, B., Cong, W., Zhang, M., Li, Z., Li, Y., et al. (2021). Amelioration of AOM/DSS-Induced murine colitis-associated cancer by evodiamine intervention is primarily associated with gut microbiota-metabolism-inflammatory signaling Axis. Front. Pharmacol. 12, 797605. doi:10.3389/fphar.2021.797605
Wang, M. X., Lin, L., Chen, Y. D., Zhong, Y. P., Lin, Y. X., Li, P., et al. (2020c). Evodiamine has therapeutic efficacy in ulcerative colitis by increasing Lactobacillus acidophilus levels and acetate production. Pharmacol. Res. 159, 104978. doi:10.1016/j.phrs.2020.104978
Wang, S.-J., Zhang, Y., Liang, H.-Y., Zhang, M.-B., and Xu, Z.-L. (2023b). Influence of deoxyschizandrin on NF KB/COX-2 signal pathway in colon of mice with inflammatory bowel disease. West. J. Traditional Chin. Med. 36 (09), 35–39.
Wang, T., Shen, Y., Luyten, S., Yang, Y., and Jiang, X. (2020d). Tissue-resident memory CD8(+) T cells in cancer immunology and immunotherapy. Pharmacol. Res. 159, 104876. doi:10.1016/j.phrs.2020.104876
Wang, W., Wang, S. K., Wang, Q., Zhang, Z., Li, B., Zhou, Z. D., et al. (2023c). Diclofenac and eugenol hybrid with enhanced anti-inflammatory activity through activating HO-1 and inhibiting NF-κB pathway in vitro and in vivo. Eur. J. Med. Chem. 259, 115669. doi:10.1016/j.ejmech.2023.115669
Wang, X., Shen, C., Wang, X., Tang, J., Wu, Z., Huang, Y., et al. (2023d). Schisandrin protects against ulcerative colitis by inhibiting the SGK1/NLRP3 signaling pathway and reshaping gut microbiota in mice. Chin. Med. 18 (1), 112. doi:10.1186/s13020-023-00815-8
Wang, Y., Zhang, T., and He, X. (2023e). Advances in the role of microRNAs associated with the PI3K/AKT signaling pathway in lung cancer. Front. Oncol. 13, 1279822. doi:10.3389/fonc.2023.1279822
Wang, Y., Zhu, X., Liang, Y., Li, X., Wang, Y., and Li, J. (2022b). Sishen wan treats ulcerative colitis in rats by regulating gut microbiota and restoring the Treg/Th17 balance. Evid. Based Complement. Altern. Med. 2022, 1432816. doi:10.1155/2022/1432816
Wang, Y. F., Liu, Y. N., Xiong, W., Yan, D. M., Zhu, Y., Gao, X. M., et al. (2014). A UPLC-MS/MS method for in vivo and in vitro pharmacokinetic studies of psoralenoside, isopsoralenoside, psoralen and isopsoralen from Psoralea corylifolia extract. J. Ethnopharmacol. 151 (1), 609–617. doi:10.1016/j.jep.2013.11.013
Wang, Z. J., Chen, L. H., Xu, J., Xu, Q. X., Xu, W., and Yang, X. W. (2023f). Corylin ameliorates chronic ulcerative colitis via regulating the gut-brain axis and promoting 5-hydroxytryptophan production in the colon. Phytomedicine 110, 154651. doi:10.1016/j.phymed.2023.154651
Wei, S.-T., Liu, Y.-Q., Huang, J., Sheng, Y.-H., and Tang, L.-M. (2020). Research progress of chemical component,Medicinal efficacy and liver toxicity of fructus evodiae. World Chin. Med. 15 (23), 3580–3585+3592.
Wei, W., Hou, J.-Z., Zhu, S.-J., Sheng, X.-Y., and Guo, H.-R. (2021). Study on quality control of multi-components in sishen pills based on fingerprints. Chin. J. Inf. Traditional Chin. Med. 28 (10).
Wu, W., and Liu, Z.-J. (2022). Mechanisms of the carcinogenesis of inflammatory bowel disease and current status of animal models researches. Chin. J. Inflamm. Bowel Dis. 06 (4), 281–286. doi:10.3760/cma.j.cn101480-20220913-00146
Xing, N.-N., Qu, H.-D., Ren, W.-C., and Ma, W. (2021). Main chemical constituents and modern pharmacological action of Schisandrae chinensis fructus:A review. Chin. J. Exp. Traditional Med. Formulae 27 (15), 210–218. doi:10.13422/j.cnki.syfjx.20211407
Xu, B., and Xiao, L.-B. (2023). Research Progress on hepatotoxicity and attenuation of Psoralea corylifolia. Lishizhen Med. Materia Medica Res. 34 (01), 159–161.
Xu, J., Zhang, Q., Wang, H., Zhang, Y., Cheng, P.-Y., and Gao, F. (2022a). Clinical observation on the efficacy and adverse reaction of modified sishen pill in combination with abemaciclib and endocrine therapy in patients with HR+/HER2-Advanced breast cancer. Chin. J. Ration. Drug Use 19 (12), 38–43.
Xu, Z.-L., Xu, H., Chen, X., Li, R.-N., Tao, X.-J., and Li, X.-L. (2022b). Meta-analysis of Sishen-pill alone or in combination in the treatment of inflammatory bowel disease. China Mod. Dr. 60 (29), 72–75+93.
Xue, M., Shi, L., Wang, W., Chen, S., and Wang, L. (2018). An overview of molecular profiles in ulcerative colitis-related cancer. Inflamm. Bowel Dis. 24 (9), 1883–1894. doi:10.1093/ibd/izy221
Yamazaki, T., Bravo-San Pedro, J. M., Galluzzi, L., Kroemer, G., and Pietrocola, F. (2021). Autophagy in the cancer-immunity dialogue. Adv. Drug Deliv. Rev. 169, 40–50. doi:10.1016/j.addr.2020.12.003
Yang, C.-Q., Lian, W.-Y., Wang, Y.-G., and Gao, Y. (2021). Research progress in pharmacology and toxicology of evodiamine. China J. Chin. Materia Medica 46 (20), 5218–5225. doi:10.19540/j.cnki.cjcmm.20210518.602
Yang, T., Li, S., and Deng, S.-Q. (2023a). Mechanism of isobavachalcone in treatment of ulcerative colitis. J. Shanghai Univ. Sci. Ed. 29 (02), 253–263.
Yang, W., Gong, X., Wang, X., and Huang, C. (2019). A mediator of phosphorylated Smad2/3, evodiamine, in the reversion of TAF-induced EMT in normal colonic epithelial cells. Invest New Drugs 37 (5), 865–875. doi:10.1007/s10637-018-0702-x
Yang, X. N., Liu, X. M., Fang, J. H., Zhu, X., Yang, X. W., Xiao, X. R., et al. (2018). PPARα mediates the hepatoprotective effects of nutmeg. J. Proteome Res. 17 (5), 1887–1897. doi:10.1021/acs.jproteome.7b00901
Yang, Y., Liu, Z., Lyu, H., Guo, X., Jiang, H., Liu, L., et al. (2023b). Traditional Chinese medicine-induced treatment in colitis-associated colorectal cancer. Chin. Med. J. Engl. 136 (10), 1249–1250. doi:10.1097/cm9.0000000000002667
Yimam, M., Jiao, P., Hong, M., and Jia, Q. (2016). Hepatoprotective activity of an herbal composition, MAP, a standardized blend comprising Myristica fragrans, Astragalus membranaceus, and poria cocos. J. Med. Food 19 (10), 952–960. doi:10.1089/jmf.2016.0048
Yong, H. Y., Koh, M. S., and Moon, A. (2009). The p38 MAPK inhibitors for the treatment of inflammatory diseases and cancer. Expert Opin. Investig. Drugs 18 (12), 1893–1905. doi:10.1517/13543780903321490
Yu, S., and Qian, H. (2021). Deoxyschizandrin treats mice with ulcerative colitis possibly via the TLR4/NF-κB signaling pathway. Am. J. Transl. Res. 13 (4), 3856–3863.
Yu, W.-L., Wang, Y., Cheng, J., Niu, L.-H., Chen, G.-Y., An, F.-L., et al. (2024). Effect of sishen pill and disassembling prescription on experimental ulcerative colitis in rats. Pharmacol. Clin. Chin. Materia Medica, 1–17. doi:10.13412/j.cnki.zyyl.20231020.001
Zaka, A., Mridha, N., Subhaharan, D., Jones, M., Niranjan, S., Mohsen, W., et al. (2023). Inflammatory bowel disease patients have an increased risk of acute coronary syndrome: a systematic review and meta-analysis. Open Heart 10 (2), e002483. doi:10.1136/openhrt-2023-002483
Zangara, M. T., Darwish, L., and Coombes, B. K. (2023). Characterizing the pathogenic potential of Crohn's disease-associated adherent-invasive Escherichia coli. EcoSal Plus 11, eesp00182022. doi:10.1128/ecosalplus.esp-0018-2022
Zhang, C., Fan, X., Xu, X., Yang, X., Wang, X., and Liang, H. P. (2010). Evodiamine induces caspase-dependent apoptosis and S phase arrest in human colon lovo cells. Anticancer Drugs 21 (8), 766–776. doi:10.1097/CAD.0b013e32833d26a9
Zhang, G., Yu, Y., Huang, H.-L., and Zhang, S.-C. (2019). Mechanism of sishenwan in treatment of ulcerative colitis based on network pharmacology and bioinformatics. Chin. J. Exp. Traditional Med. Formulae 25 (24), 142–149. doi:10.13422/j.cnki.syfjx.20192438
Zhang, W., Wang, W., Shen, C., Wang, X., Pu, Z., and Yin, Q. (2021a). Network pharmacology for systematic understanding of Schisandrin B reduces the epithelial cells injury of colitis through regulating pyroptosis by AMPK/Nrf2/NLRP3 inflammasome. Aging (Albany NY) 13 (19), 23193–23209. doi:10.18632/aging.203611
Zhang, W. F., Yang, Y., Su, X., Xu, D. Y., Yan, Y. L., Gao, Q., et al. (2016). Deoxyschizandrin suppresses dss-induced ulcerative colitis in mice. Saudi J. Gastroenterol. 22 (6), 448–455. doi:10.4103/1319-3767.195552
Zhang, X.-X., Jin, J.-W., Liu, C.-H., Zhou, M., He, Y.-X., Wang, F., et al. (2021b). Effect of Nrf2/HO-1 signaling pathway in intestinal protection by Sishen Pills against ulcerative colitis in mice. China J. Chin. Materia Medica 46 (16), 4187–4192. doi:10.19540/j.cnki.cjcmm.20210524.402
Zhang, X.-X., Li, X.-N., Hu, S., and Chang, R.-M. (2018). Simultaneous determination of nine bioactive components in Sishen Pills by HPLC-ESI-MS/MS. Chin. Traditional Herb. Drugs 49 (09), 2070–2075.
Zhang, X.-Y., Zhao, H.-M., Liu, Y., Liu, X.-K., Liu, F.-C., Chen, F., et al. (2020a). Regulation of schisandrin A on oxidative stress and ulcerative colitis in rats. Chin. Archives Traditional Chin. Med. 38 (02), 166–169+283. doi:10.13193/j.issn.1673-7717.2020.02.041
Zhang, X. Y., Zhao, H. M., Liu, Y., Lu, X. Y., Li, Y. Z., Pan, Q. H., et al. (2021c). Sishen pill maintained colonic mucosal barrier integrity to treat ulcerative colitis via Rho/ROCK signaling pathway. Evid. Based Complement. Altern. Med. 2021, 5536679. doi:10.1155/2021/5536679
Zhang, Y., Dai, H.-B., and Zhu, P.-F. (2021d). Observation on the curative effect of shenling baizhu powder and sishen Pill Combined with chemotherapy for radical resection of colorectal cancer. Chin. J. Surg. Integr. Traditional West. Med. 27 (04), 592–596.
Zhang, Y., Yan, T., Sun, D., Xie, C., Wang, T., Liu, X., et al. (2020b). Rutaecarpine inhibits KEAP1-NRF2 interaction to activate NRF2 and ameliorate dextran sulfate sodium-induced colitis. Free Radic. Biol. Med. 148, 33–41. doi:10.1016/j.freeradbiomed.2019.12.012
Zhang, Y., Zhang, H., Zhang, K., Li, Z., Guo, T., Wu, T., et al. (2020c). Co-hybridized composite nanovesicles for enhanced transdermal eugenol and cinnamaldehyde delivery and their potential efficacy in ulcerative colitis. Nanomedicine 28, 102212. doi:10.1016/j.nano.2020.102212
Zhang, Y., Zhang, Y., Zhao, Y., Wu, W., Meng, W., Zhou, Y., et al. (2022). Protection against ulcerative colitis and colorectal cancer by evodiamine via anti-inflammatory effects. Mol. Med. Rep. 25 (5), 188. doi:10.3892/mmr.2022.12704
Zhao, H., Ming, T., Tang, S., Ren, S., Yang, H., Liu, M., et al. (2022). Wnt signaling in colorectal cancer: pathogenic role and therapeutic target. Mol. Cancer 21 (1), 144. doi:10.1186/s12943-022-01616-7
Zhao, H. M., Huang, X. Y., Zhou, F., Tong, W. T., Wan, P. T., Huang, M. F., et al. (2013). Si shen wan inhibits mRNA expression of apoptosis-related molecules in p38 MAPK signal pathway in mice with colitis. Evid. Based Complement. Altern. Med. 2013, 432097. doi:10.1155/2013/432097
Zhao, H. M., Liu, Y., Huang, X. Y., Liu, X. K., Chen, F., Zhang, X. Y., et al. (2019). Pharmacological mechanism of Sishen Wan® attenuated experimental chronic colitis by inhibiting wnt/β-catenin pathway. J. Ethnopharmacol. 240, 111936. doi:10.1016/j.jep.2019.111936
Zhao, Q., Duck, L. W., Huang, F., Alexander, K. L., Maynard, C. L., Mannon, P. J., et al. (2020). CD4(+) T cell activation and concomitant mTOR metabolic inhibition can ablate microbiota-specific memory cells and prevent colitis. Sci. Immunol. 5 (54), eabc6373. doi:10.1126/sciimmunol.abc6373
Zhaohua, Z., Rong, L., Nana, D. U., and Xiangdong, Z. (2022). Efficacy of Sishen Wan on dinitrobenzene sulfonic acid-induced ulcerative colitis and its effect on toll-like receptor 2/interleukin-1 receptor-associated kinase-4/nuclear factor-κB signal pathway. J. Tradit. Chin. Med. 42 (4), 565–575. doi:10.19852/j.cnki.jtcm.20220608.001
Zhou, Z.-X. (2020). The screening of active ingredients and underlying mechanism in Psoraleae Fructus treatment of ulcerative colitis. doctor. doctor’s thesis. Liaoning: Shenyang Pharmaceutical University.
Keywords: inflammatory bowel disease, colon cancer, Sishen Pill, molecular mechanism, natural product
Citation: Zhang B, Cheng Y, Jian Q, Xiang S, Xu Q, Wang C, Yang C, Lin J and Zheng C (2024) Sishen Pill and its active phytochemicals in treating inflammatory bowel disease and colon cancer: an overview. Front. Pharmacol. 15:1375585. doi: 10.3389/fphar.2024.1375585
Received: 24 January 2024; Accepted: 21 March 2024;
Published: 08 April 2024.
Edited by:
Xianyu Li, China Academy of Chinese Medical Sciences, ChinaReviewed by:
Shengpeng Wang, University of Macau, ChinaNianrong Zhang, China-Japan Friendship Hospital, China
Copyright © 2024 Zhang, Cheng, Jian, Xiang, Xu, Wang, Yang, Lin and Zheng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chuan Yang, ODIzOTI0MjlAcXEuY29t; Junzhi Lin, bGluanVuemhpQGNkdXRjbS5lZHUuY24=; Chuan Zheng, emhlbmdjaHVhbkBjZHV0Y20uZWR1LmNu
†These authors have contributed equally to this work
 Boxun Zhang
Boxun Zhang Yingying Cheng2†
Yingying Cheng2† Sirui Xiang
Sirui Xiang Junzhi Lin
Junzhi Lin