- 1Graduate School, Hunan University of Chinese Medicine, Changsha, China
- 2Department of Acupuncture Rehabilitation, Changsha Hospital of Traditional Chinese Medicine, Changsha, China
- 3Department of Neurology, Hunan Provincial Hospital of Integrated Traditional Chinese and Western Medicine (Hunan Academy of Chinese Medicine Affiliated Hospital), Changsha, China
Objective: To conduct a meta-analysis of the effectiveness and safety of ginkgo biloba extract combined with donepezil hydrochloride vs. donepezil for the treatment of vascular dementia (VaD).
Methods: Four English databases (PubMed, EMBASE, Web of Science, Cochrane Library) and four Chinese databases [the China National Knowledge Infrastructure Wanfang DATA, the Chongqing VIP Database (VIP), China Biomedical Database (CBM)] were manually searched for literature published from dates of the inception of the databases to September 2023. The randomized controlled trials (RCTs) of ginkgo biloba extract with donepezil hydrochloride vs. donepezil for the treatment of VaD were included. Relevant literature was screened, and the data in the included studies were extracted for quality assessment according to the Risk of bias tool.
Results: A total of 1,309 participants were enrolled in the 15 RCTs. Of these, 656 participants were in the experimental group (ginkgo biloba extract combined with donepezil) and 653 participants were in the control group (donepezil).The results showed that combination therapy was superior to donepezil alone, and there were statistically significant differences in several outcomes including RR in change for total effective rate (1.28, 95% confidence intervals 1.20, 1.38, p < 0.001), MD in change for Mini-Mental State Examination score (2.98, 95%CI 2.31, 3.65, p < 0.001), Barthel Index score (8.55,95%CI 1.11, 15.99, p = 0.024), Activity of Daily Living Scale (ADL)score (10.11,95% CI 7.16,13.07,p < 0.001).
Conclusion: Ginkgo biloba extract combined with donepezil dramatically improved the total effective rate, MMSE, BI and ADL scores, and decreased homocysteine (HCY), plasma viscosity (PV), whole blood viscosity at high cut (BVH) and whole blood viscosity at low cut (BVL) in VaD patients, while the effect on mean flow velocity and pulse index (PI) of middle cerebral artery (MCA) is not obvious. However, more relevant high-quality RCTs are needed to validate these results.
Systematic Review Registration: Identifier CRD42023474678.
1 Introduction
Vascular dementia (VaD), which caused by cerebrovascular disease, is a common type of dementia in clinical practice. It becomes the second most common type of dementia after Alzheimer’s disease (AD) in recent years, accounting for about 15% of dementia cases (Kalaria, 2018; OBrien and Thomas, 2015). The main clinical manifestations include cognitive, psychological, and behavioral symptoms, such as repetitive questioning, restlessness, depression, apathy, confusion, aggression, sleep disorders, and various misbehaviors (Kales et al., 2015). Consequently, the occurrence of VaD has a negative impact on the living quality and safety of patients, and brings a huge burden to the family and society, such as self-care problems or communication disorders.
Currently, there is no uniformed standard on the treatment of dementia. Generally recognized therapeutic drugs include acetylcholinesterase inhibitors and NMDA receptor antagonists (Farooq et al., 2017). Donepezil, galantamine, memantine, etc., are commonly used in clinical treatment of the disease, which are proved to improve the cognitive function on patients with different stages (Orgogozo et al., 2002; Wilkinson et al., 2003; Auchus et al., 2007).
In addition, since ginkgo biloba extract has been reported as a natural medicine in the 1980s (Subhan and Hindmarch, 1984), it is widely used in the management of cardiovascular disease and coronary heart disease (Shaito et al., 2020). Morever, experts have reached a consensus on the use of ginkgo biloba extract to treat cognitive disorders (Kandiah et al., 2019). Flavonoids and terpenoids are considered as pharmacologically active components of Ginkgo biloba (Smith et al., 1996; Shi et al., 2009). At present, Ginkgo biloba preparation mainly includes oral preparation and injection preparation. The oral formulation contains 19.2 mg of total flavonoid glycoside and 4.8 mg of terpenoid lactone or 9.6 mg of total flavonoid glycoside and 2.4 mg of terpenoid lactone. Shuxuening is a commonly used ginkgo biloba injection. Every 5 mL Shuxuening contains 17.5 mg of ginkgo biloba extract (4.2 mg of total flavonoid glycosides and 0.70 mg of ginkgolide) (Yang et al., 2016). The neuroprotective mechanism of ginkgo biloba extract is clear in two aspects: on the one hand, it can reduce the damage caused by platelet activating factor (PAF) during cerebral ischemia by antagonizing PAF; On the other hand, it can remove free radicals in the body, produce antioxidant effect, and reduce the damage of brain tissue caused by excessive free radicals produced in the body during cerebral ischemia (Noorbala and Akhondzadeh, 2006). Besides, some studies have mentioned that ginkgo biloba extract can treat dementia patients by regulating serum HCY concentration and hemorheology (Diamond et al., 2000).
A lot of clinical studies have proven its effectiveness and safety in the treatment of dementia, both AD and VaD (Ihl et al., 2012). Besides, a meta-analysis indicated that ginkgo biloba extract could improve cognitive function and the ability to live daily in people with VaD, and this meta-analysis included RCTs of ginkgo biloba extract combined with VaD drugs and control group with VaD drugs alone. (The drugs for VaD treatment mainly include donepezil, nimodipine, huperzine A, oxiracetam, piracetam, butylphthalide and other drugs that promote microcirculation and improve cognition. Ginkgo biloba extract includes common ginkgo biloba products, namely, tablets, capsules, and soft capsules (Zhan et al., 2021). However, there is still a lack of meta-analysis on conventional western medicine, represented by donepezil, compared with ginkgo biloba extract combined with donepezil for VaD treatment. Therefore, to synthesize more evidence for the role of ginkgo biloba combined with donepezil in the treatment of this disease, we included articles published in recent years in a more comprehensive meta-analysis evaluating the efficacy and safety of this regimen.
2 Methods
The protocol of this review has been registered in the International Prospective Register Of Systematic Reviews, and the approval number for registration is CRD42023474678. It was reported following the statement guidelines of preferred reporting items for systematic reviews and meta-analyses protocols (Moher et al., 2015).
2.1 Search strategy
The literature search was conducted in database, including four English databases (PubMed, EMBASE, Web of Science, Cochrane Library) and four Chinese databases [the China National Knowledge Infrastructure (CNKI), Wanfang DATA, the Chongqing VIP Database (VIP), China Biomedical Database (CBM)], were manually searched for literature published from dates of the inception of the databases to 7 September 2023(Beijing time).In order to comprehensively search and obtain relevant literature, a manual search was carried out using the following search terms and their variants: ginkgo biloba, ginkgo leaf extract, donepezil and VaD. Detailed retrieval strategies are provided in Supplementary Material S1.
2.2 Eligibility criteria
2.2.1 Inclusion criteria
(1) Participants: Patients with a clinical diagnosis of VaD were considered regardless of nationality, race, gender, occupation, or educational background.
Although the causes of VaD are not limited, all patients should be diagnosed with VaD according to at least one of the current or past VaD definitions or guidelines, such as:
① Diagnostic and Statistical Manual of Mental Disorder fourth, DSM-IV (Gmitrowicz and Kucharska, 1994).
② Draft diagnostic criteria for vascular dementia, Chinese Medical Association Branch of Neurology (Branch CMAN, 2002).
③ Rockwood clinical diagnostic criteria for VCI (Erkinjuntti and Rockwood, 2003)
④ Discussion on clinical diagnostic Criteria of Vascular Dementia (Jia and Wang, 2010).
(2) Intervention: The experimental group was given ginkgo biloba preparation combined with donepezil.
(3) Comparision: The control group was only given donepezil.
(4) Outcomes: One of the following outcomes was reported: Primary outcome including total effective rate and Mini-Mental State Examination (MMSE) score, Barthel Index (BI) score and Activity of Daily Living Scale (ADL) score; secondary outcome including homocysteine (HCY), hemorheological indicators [whole blood viscosity at high cut (BVH), whole blood viscosity at low cut (BVL), plasma viscosity (PV), fibrinogen (FIB)], mean flow velocity and pulse index (PI) of middle cerebral artery (MCA) and adverse events as safety outcomes.
(5) Study design:Randomized controlled trials (RCTs) involving patients diagnosed with VaD.
2.2.2 Exclusion criteria
(1) Reviews, case reports, research protocols or conference papers.
(2) Animal and in vitro studies.
(3) Duplication and lack of access to full texts.
(4) Literature with outcome indicators or data errors could not be extracted.
Two reviewers Liangyi Xiao and Jie Tang independently screened the literature according to the above criteria, and the different opinions encountered during the research screening process were resolved through discussion or by the third reviewer Yao Xie.
2.3 Data extraction and quality assessment
Two reviewers (Liangyi Xiao and Jie Tang) independently extracted data from the final included literature, including first author, publication year, country, intervention and control measures, duration of treatment, basic information about study subjects, outcome measures, etc. Included studies will be evaluated by two independent reviewers (Liangyi Xiao and Jie Tang) using the Cochrane Bias Risk Assessment Tool (RoB2.0) (Sterne et al., 2019) on five aspects of included randomised controlled studies: Bias arising from randomization, bias from deviations from established interventions, bias from missing outcome data, bias from outcome measurement, bias from selective reporting of results. For each study, an independent quality assessment was conducted by two researchers, who rated the five aspects as “low risk,” “high risk,” and “possibly risky.” The diverging literature was evaluated through discussion or the suggestion of a third investigator, and the evaluation results were shown in the bias risk map.
2.4 Data integration and statistical analysis
The main outcome indicator are total effective rate, MMSE score, BI score and ADL score. The secondary outcome indicator are HCY, hemorheological indicators (BVH, BVL, PV, FIB), mean flow velocity and PI of MCA and adverse events as safety outcomes. We performed a meta-analysis using stata15.1. For continuous data, when using the same scale, weighted mean differences (WMD) were calculated and 95% confidence intervals (CI) were reported. For binary categorical variables, RR was used as the effect index for meta-analysis. Heterogeneity tests were based on p-value obtained from Q tests combined with the I2 statistic. Among them, the I2 statistic is an important indicator of heterogeneity, the value of 25%, 50% and 75%, representing low, medium and high heterogeneity (Higgins et al., 2003). If there is no significant heterogeneity between each study, i.e., I2 < 50% and p > 0.1, a meta-analysis is performed using a fixed-effect model (Mantel-Haenszel method). Instead, a random effects model (DerSimonian-Laird method) will be used.Subgroup analysis and regression analysis were based on efficacy evaluation criteria and treatment duration to determine the magnitude and source of heterogeneity among studies. Sensitivity analysis was used to evaluate the robustness of the meta-analysis results. Funnel plots were created to assess whether publication bias existed in the included literature, and Egger or Begg methods were used for statistical testing (the number of studies should be ≥ 5). For the results with significant publication bias, the shear compensation method was used to measure the impact of publication bias on the results.
3 Results
3.1 Literature screening results and flow charts
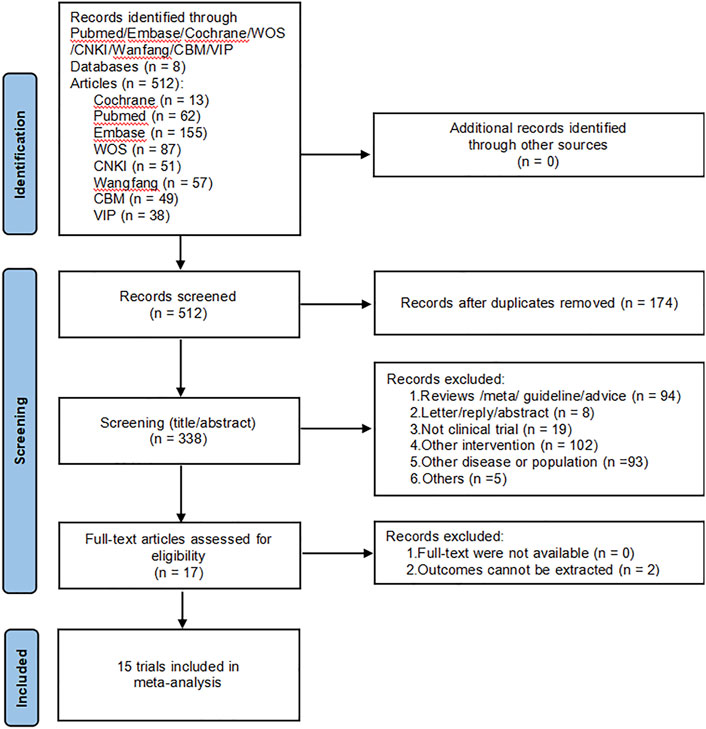
A total of 512 papers were retrieved from the initial database search, and no additional studies were identified from the reference scan. After removing duplicates, 338 articles were examined by title and abstract. Of these, 323 articles were excluded because they did not meet the inclusion criteria, 17 articles have been carefully reviewed for full text. Finally, 15 studies were included in this meta-analysis (Guo, 2010; Chen, 2011; Bao and He, 2012; Yu, 2014; He et al., 2016; Guo, 2018; Guan, 2019; Xu, 2019; Chen and Li, 2020; Gao et al., 2020; Miao, 2020; Zheng, 2020; Duan et al., 2021; Tong, 2021; Shen et al., 2023). Figure 1 shows the literature screening process.
3.2 Studies’ characteristics
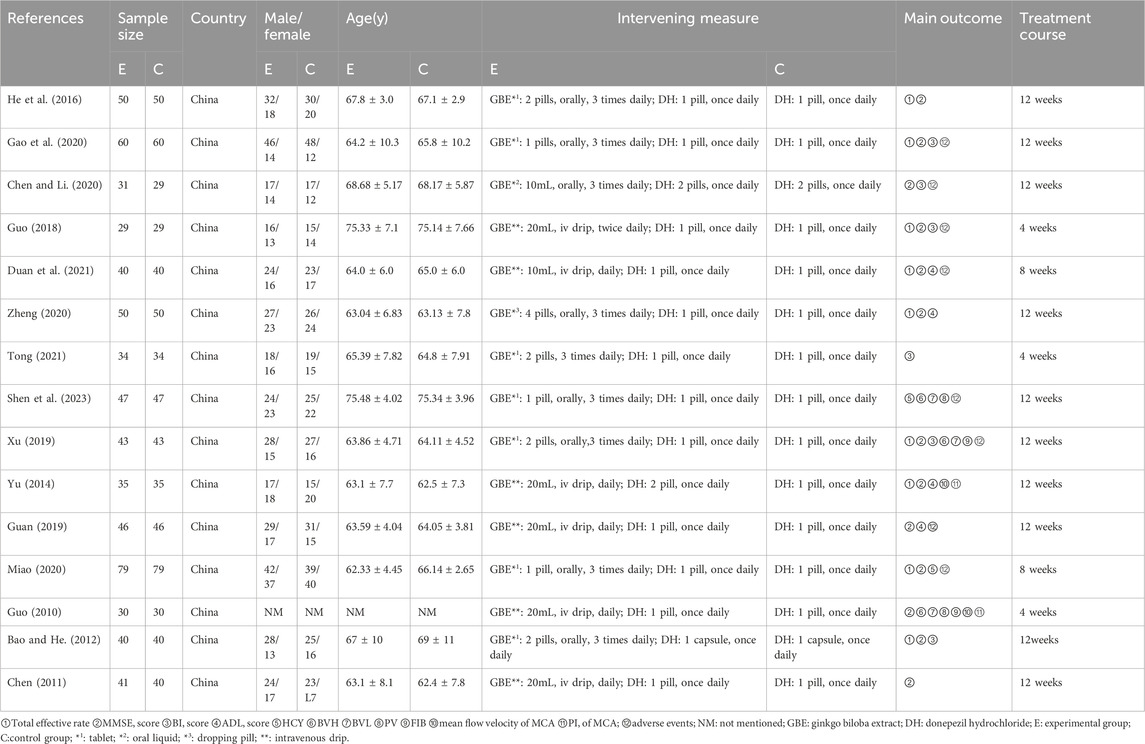
The basic characteristics of the included studies are in Table 1.
The fifteen included studies involved 1,309 patients from China, ranging in age from 62.33 ± 4.45 to 75.48 ± 4.02 years old. Of these, 656 participants were in the experimental group (ginkgo biloba extract combined with donepezil) and 653 participants were in the control group (donepezil), published from 2010 to 2023. The size of samples included in the study varies from 58 cases to 158 cases. The outcome indicators reported are as follows: total effective rate (n = 9), MMSE score (n = 13), BI score (n = 6), ADL score (n = 4), HCY (n = 2), BVH(n = 3), BVL (n = 3), PV(n = 2), FIB(n = 2) and mean flow velocity and PI (n = 2) of MCA (n = 2). In addition, the treatment course ranges from 4 weeks to 12 weeks.
3.3 Quality evaluation
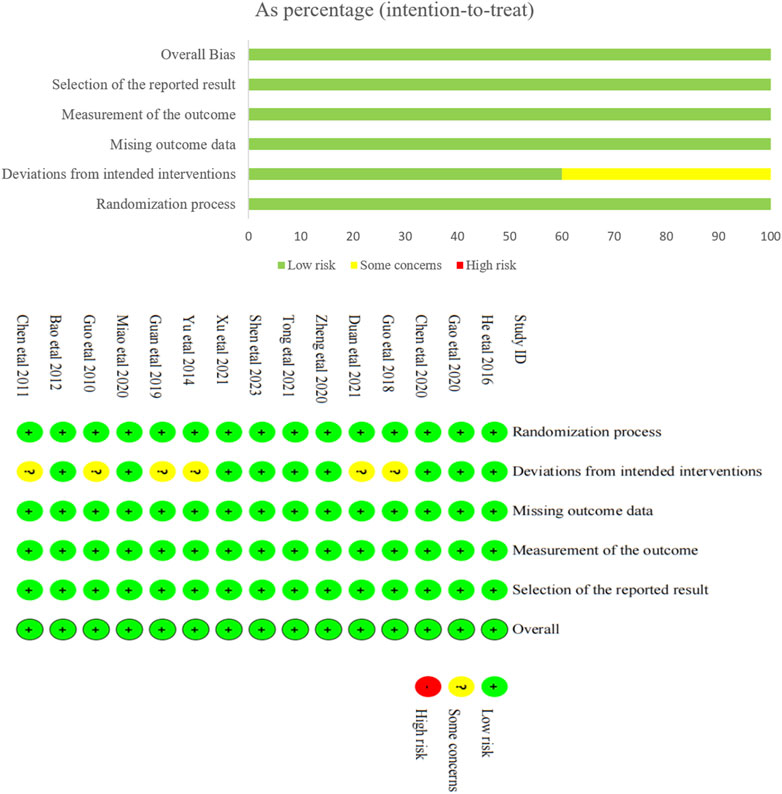
The results of bias risk assessment for the 15 included studies are shown in Figure 2. Among the bias generated during randomization, all the included studies were randomly assigned, which was a low risk bias. Of the bias from the established interventions, six studies were assessed as potentially risky because they did not implement double-blind method, but were assessed as low-risk bias after reasonable analytical methods provided, while the remaining nine studies were assessed as low-risk. All studies had low risk bias in missing outcome data and measurement outcomes. It was not clear from all studies whether there was selective reporting, and this risk of bias is a possible risk. Taken together, the risk of bias in the included literature was small.
3.4 Results of meta-analysis
3.4.1 Total effective rate
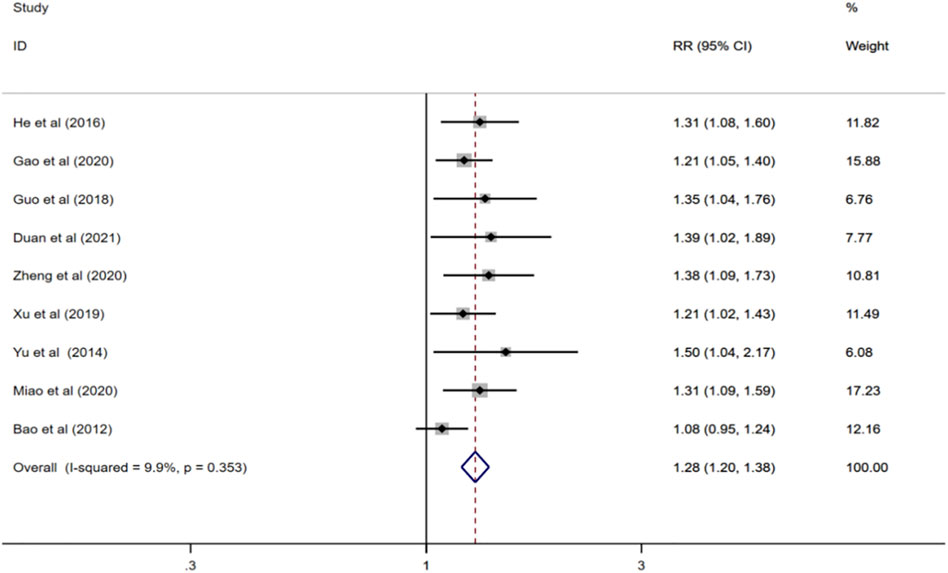
Nine reported total effective rate (Bao and He, 2012; Yu, 2014; He et al., 2016; Guo, 2018; Xu, 2019; Gao et al., 2020; Miao, 2020; Zheng, 2020; Duan et al., 2021), and meta-analysis was performed using a fixed-effect model (I2 = 9.9%, p = 0.353). The results showed that compared with donepezil, ginkgo biloba extract combined with donepezil could improve the total effective rate (RR = 1.28; CI:1.20 to1.38; p < 0.001) Figure 3.
3.4.2 MMSE score
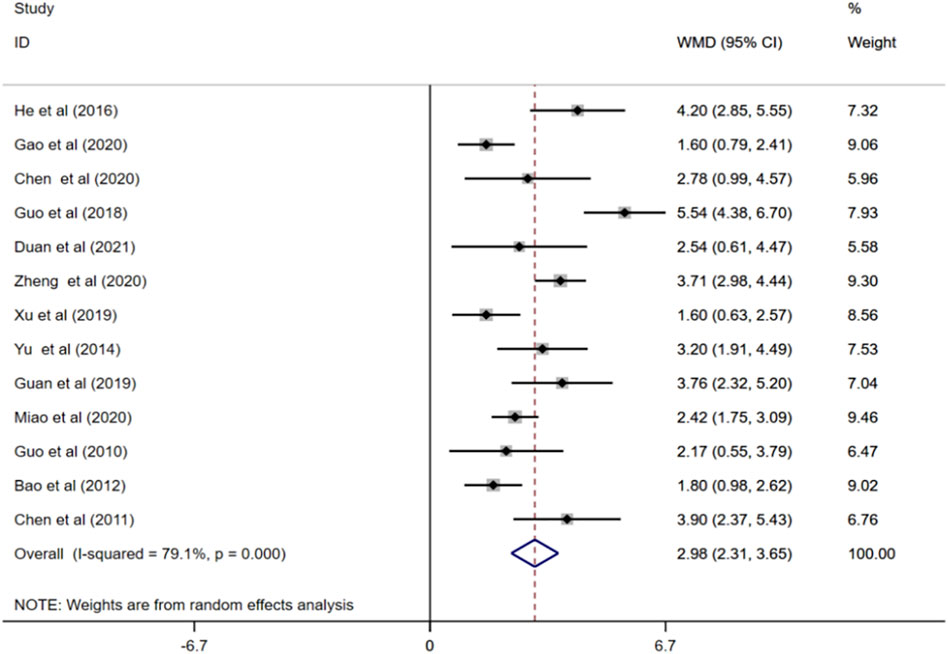
MMSE scores were reported in 13 studies (Guo, 2010; Chen, 2011; Bao and He, 2012; Yu, 2014; He et al., 2016; Guo, 2018; Guan, 2019; Xu, 2019; Chen and Li, 2020; Gao et al., 2020; Miao, 2020; Zheng, 2020; Duan et al., 2021). Random effects model showed that treatment with ginkgo biloba extract combined with donepezil remarkably increased patients’ MMSE scores compared to treatment of donepezil alone. (WMD = 2.98; 95%CI:2.31 to 3.65; p < 0.001; I2 = 79.1%, p < 0.001), Figure 4.
3.4.3 BI score
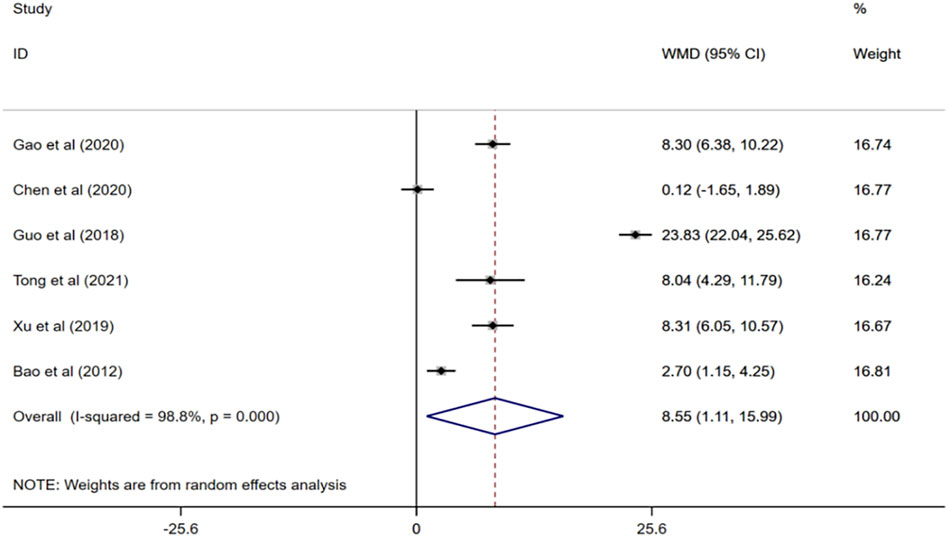
Six randomized controlled trials (Bao and He, 2012; Guo, 2018; Xu, 2019; Chen and Li, 2020; Gao et al., 2020; Tong, 2021) evaluated the effects of ginkgo biloba extract combined with donepezil on BI scores in patients with VaD. Meta-analysis of the random effects model showed that BI score of the experimental group was higher than that of the control group, and the difference was statistically significant (WMD = 8.55; 95% CI: 1.11 to 15.99; I2 = 98.8%, p < 0.001) Figure 5.
3.4.4 ADL score
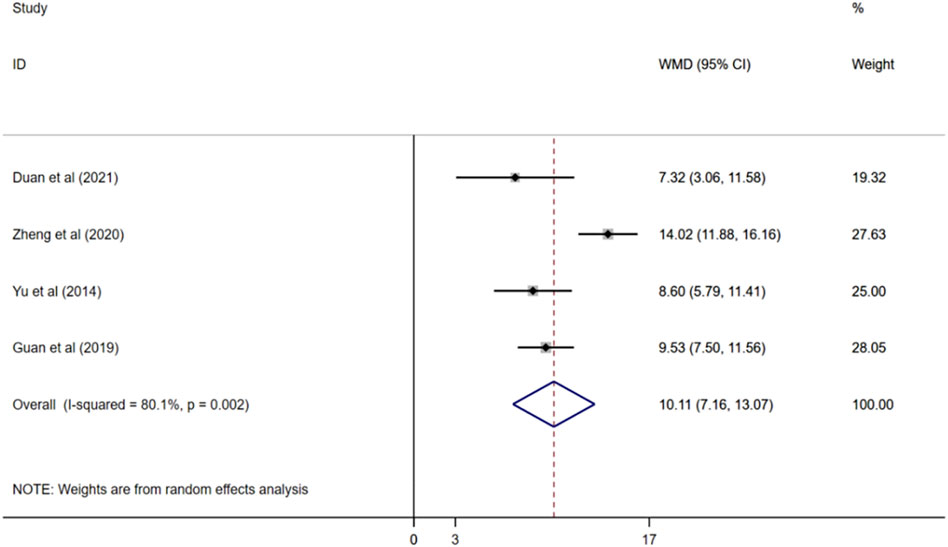
Four studies reported ADL scores (Yu, 2014; Guan, 2019; Zheng, 2020; Duan et al., 2021), and a random effects model was used for meta-analysis (I2 = 80.1%, p < 0.01). The results demonstated that compared with donepezil, ginkgo biloba extract combined with donepezil could improve ADL score observably. (WMD = 10.11; 95%CI:7.16 to13.07; p < 0.001) Figure 6.
3.4.5 Homocysteine
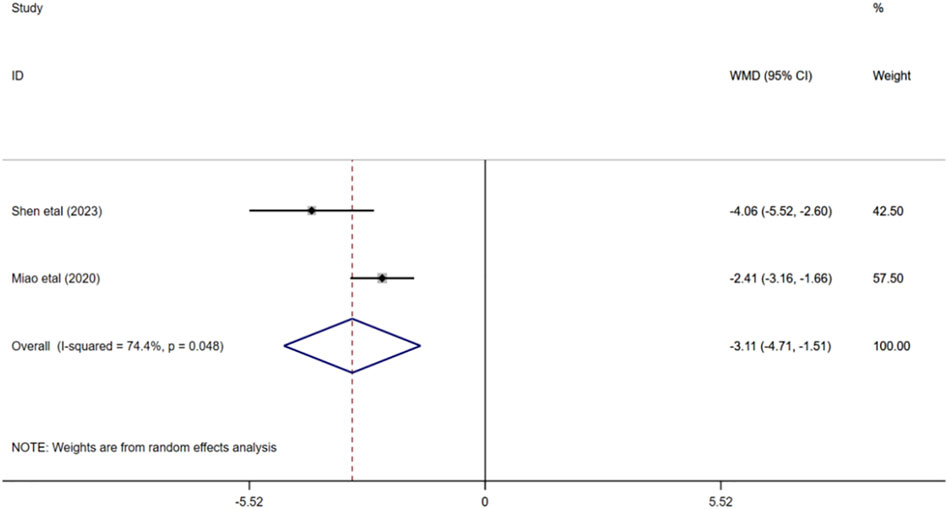
A total of 252 participants were enrolled from two studies (Miao, 2020; Shen et al., 2023), with 126 participants in the experimental group and 126 in the control group. Q test for heterogeneity showed heterogeneity in effect size (I2 = 74.4%, p < 0.05), indicating high heterogeneity between studies. In consequence, the random effects model is used for analysis. The results of meta-analysis manifested that HCY in experimental group was significantly lower than that in control group (WMD = −3.11; 95% CI: -4.71 to −1.51; p < 0.001) Figure 7.
3.4.6 Hemorheological indicators
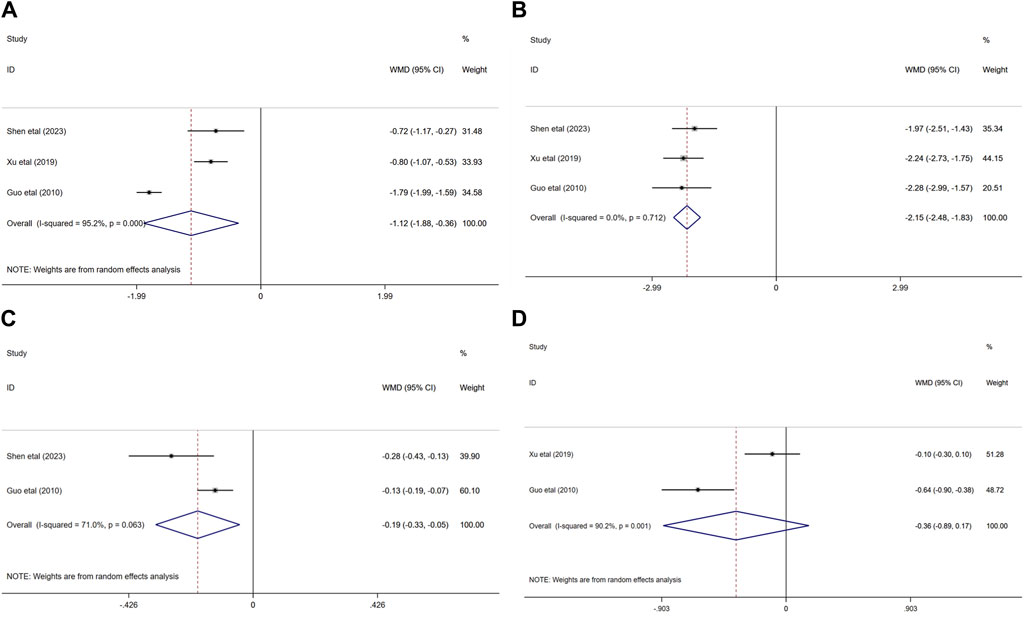
Three randomized controlled trials (Guo, 2018; Xu, 2019; Shen et al., 2023) evaluated the effects of ginkgo biloba extract combined with donepezil on hemorheology in patients with VaD. In terms of the influence on BVH and BVL, the result demonstated that compared with donepezil, ginkgo biloba extract combined with donepezil could reduce the BVH and BVL (WMD = −1.12; 95% CI: −1.88 to −0.36; p < 0.01, WMD = −2.15; 95% CI: −2.48 to −1.83; p < 0.001, respectively).
Two of the three studies researched the effects of combination medication on FIB(33, 37) and PV(32, 37) in patients with VaD. The results indicated that the PV of the experimental group was significantly lower than that of the control group (WMD = −0.19; 95% CI: −0.33 to −0.05; p < 0.05). Nevertheless, FIB decreased in both experimental group and control group (WMD = −0.36; 95% CI: −0.89 to −0.17; p > 0.05) Figure 8.
3.4.7 Mean blood flow velocity and pulse index of MCA
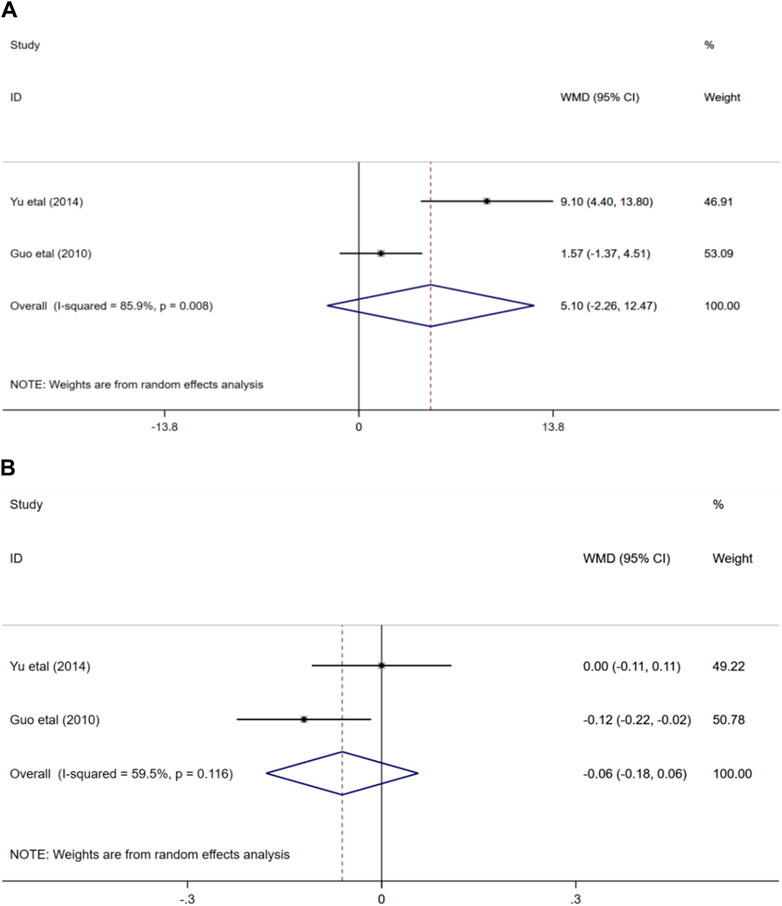
Two randomized controlled trials (Guo, 2010; Yu, 2014) evaluated the effects of ginkgo biloba extract combined with donepezil on mean blood flow velocity and pulse index in patients with VaD. In terms of the impact on the mean blood flow velocity of MCA, the result showed that the combination of medication or medication alone could improve the mean blood flow velocity of MCA in participants, and the difference was not statistically significant (WMD = 5.1; 95% CI: −2.26 to 12.47; p > 0.05). Similarly, the outcome also demonstrated that there was no statistical difference in the effects of the two treatment options on middle cerebral artery pulse index (WMD = −0.06; 95% CI: −0.18 to 0.06; p > 0.05) Figure 9.
3.4.8 Adverse event
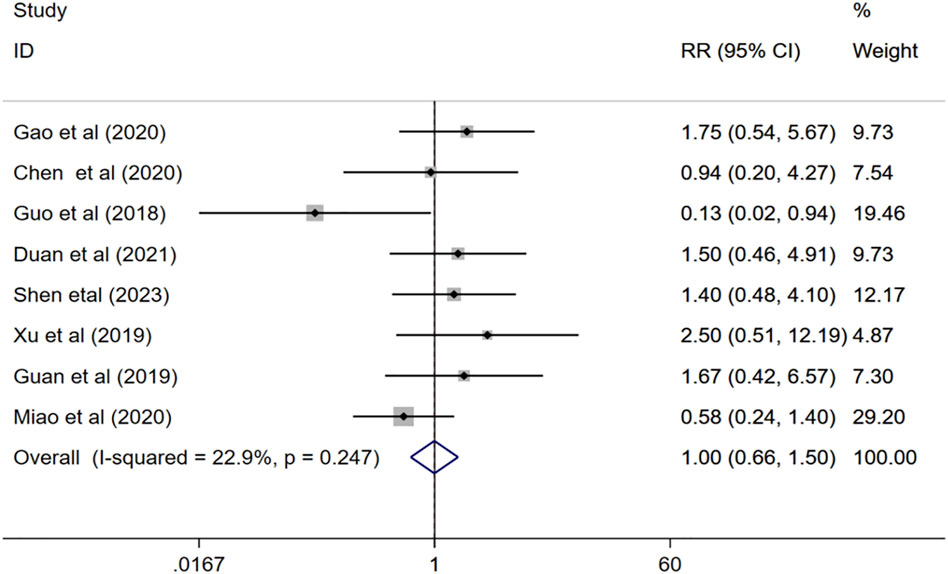
Eight studies (Guo, 2018; Guan, 2019; Xu, 2019; Chen and Li, 2020; Gao et al., 2020; Miao, 2020; Duan et al., 2021; Shen et al., 2023) evaluated the adverse effects of combination therapy for VaD. The meta-analysis of the fix effects model showed that (I2 = 22.9%, p = 0.247), there is no significant difference between the incidence of adverse event of the experimental group and that of the control group (RR = 1.00, 95%CI 0.66 to 1.50, p = 0.981) Figure 10.
3.5 Subgroup analysis
To explore the source of heterogeneity, we performed subgroup analysis of total effective rate based on efficacy evaluation criteria, treatment duration and administration route, and subgroup analysis of MMSE score, BI score, and ADL score based on treatment duration and administration route.
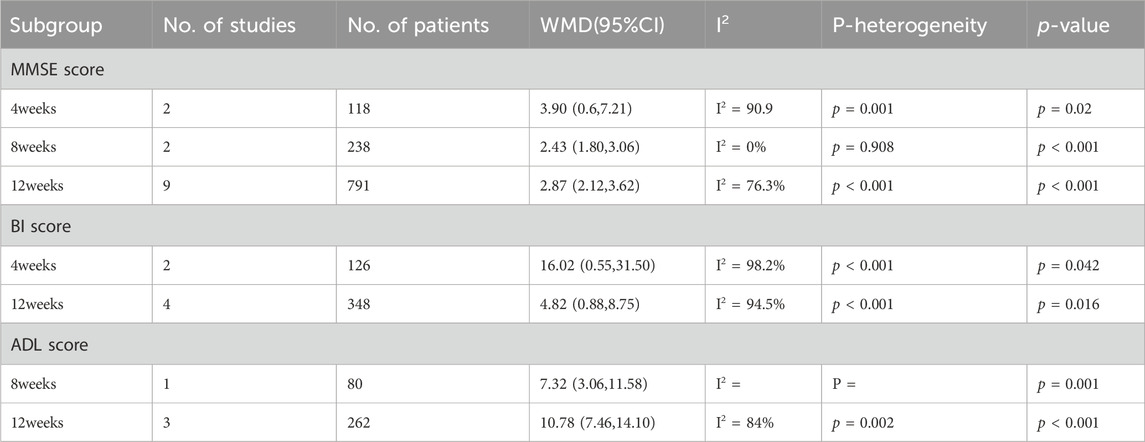
The results of subgroup analysis showed that under efficacy evaluation criteria (a 1-point increase in the MMSE score, or a 12% or 15% increase in the MMSE score), treatment duration (4 weeks, 8 weeks or 12 weeks) and administration route (po or ivgtt), the total effective rate of the combination group was significantly higher than that of the donepezil group (Table 2). Regression results further indicated that efficacy evaluation criteria and treatment duration were not the sources of heterogeneity (p = 0.606, p = 0.237, p = 0.158,respectively).

Table 2. Subgroup analysis of total effective rate based on efficacy evaluation criteria, treatment duration and administration route.
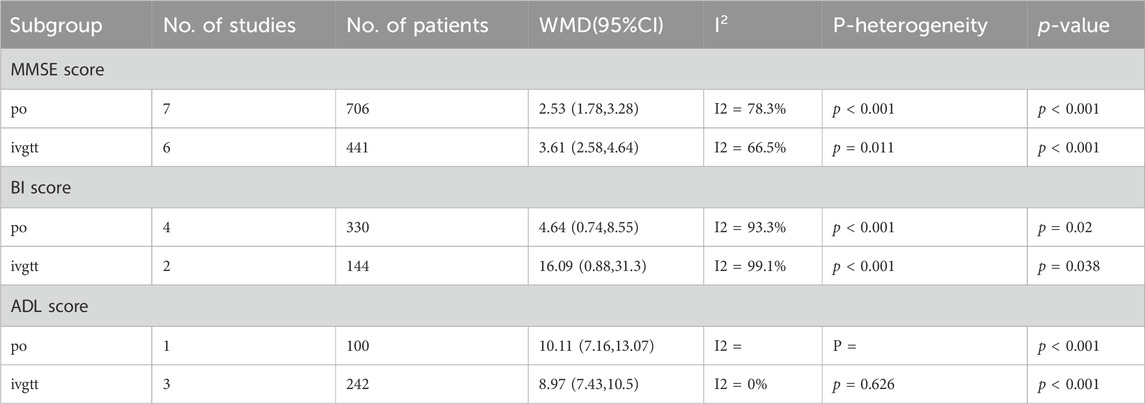
After subgroup analysis of MMSE score based on treatment time, the results showed that MMSE scores were higher in the combination group than in the donepezil group regardless of the duration of treatment at 4, 8, or 12 weeks (p = 0.02, p < 0.001, p < 0.001, respectively) (Table 3). Regression analysis further demonstrated that treatment duration was not the source of heterogeneity (p = 0.37). Similarly, subgroup analyses of MMSE scores according to administration route led to higher MMSE scores with combination therapy than with donepezil, whether administered orally or intravenously (p < 0.001, p < 0.001, respectively) (Table 4).Morever, regression analysis further indicated administration route is not the source of the heterogeneity (p = 0.086).
We performed a subgroup analysis of BI scores based on treatment duration, and the results illustrated that after 4 or 12 weeks of treatment, patients in the combination group had higher BI scores than those in the donepezil group (p = 0.042, p = 0.016, respectively). Moreover, we found in the regression analysis that the BI score increased more after 12 weeks than 4 weeks (p = 0.046). In addition, subgroup analysis of BI scores according to the administration route of ginkgo biloba extract showed that BI scores were higher in the combination group than in the donepezil group (p = 0.02, p = 0.038, respectively) (Table 4). We found in regression analysis that BI scores were higher with intravenous than with oral administration (p = 0.044).
However, an analysis of ADL scores based on the same subgroup factors showed that the combination therapy significantly improved ADL scores in patients with VaD at either 8 or 12 weeks of treatment, but the difference was not significant (p = 0.378). Besides, based on the administration route of ginkgo biloba extract on ADL scores subgroup analysis, results show that the combination group ADL scores higher than that in donepezil group (p < 0.001, p < 0.001, respectively). But the interesting thing is, in regression analysis, we found that the ADL score was higher with oral than with intravenous ginkgo biloba extract (p < 0.001).
3.6 Sensitivity analysis
We performed a sensitivity analysis for the total effective rate, MMSE, BI and ADL scores and assessed the impact of each study on the pooled results through a one-by-one exclusion method. The analysis showed that none of the combined results were significantly affected by any single study. This suggests that the results of this meta-analysis are generally relatively reliable. Sensitivity analysis was shown in following Figure 11.

Figure 11. Sensitivity analysis of (A) total effective rate, (B) MMSE scores, (C) BI scores, (D) ADL scores.
3.7 Publication bias
To ensure the validity of the meta-analysis results, we used funnel plots, Egger’s and Begg’s tests to identify publication bias in total effective rate, MMSE, BI and ADL scores. The results manifested that MMSE, BI and ADL scores had no significant publication bias (p > 0.05). On the contrary, there was significant publication bias in the total effective rate (p < 0.01). After supplementing four literature with shear compensation method, the conclusions did not change, which further confirmed the reliability of our results (Supplementary Material S2).
4 Discussion
Our study found that ginkgo biloba extract combined with donepezil significantly improved the total effective rate, MMSE, BI and ADL scores, and decreased serum homocysteine, plasma viscosity, whole blood high and low tangency viscosity in patients with VaD. The results are similar to previous studies. A meta-analysis of 18 randomized controlled studies (Li et al., 2023) of ginkgo biloba extract combined with donepezil in the treatment of Alzheimer’s disease showed that the combination improved cognitive function in patients, consistent with our findings. A retrospective study concluded that ginkgo biloba extract EGb 761 combined with acetylcholinesterase inhibitors is more effective in the treatment of VaD, which provides real evidence for the treatment of VaD with combined medication (García-Alberca et al., 2022).
Donepezil hydrochloride, it is a kind of acetyl cholinesterase inhibitor, is by far the most commonly used prescription for the treatment of mild-to-moderate AD (42). Existing studies have confirmed that cholinergic deficits are also present in patients with VaD. Therefore, acetylcholinesterase inhibitors may also provide benefit to patients with VaD (Farlow, 2006). Two 6-month randomized, double-blind, placebo-controlled trials investigated the effect of donepezil at either 5 mg/d or 10 mg/d for VaD treatment, each involving more than 600 patients. The results of both studies demonstrated statistically significant dose-related improvements in cognitive function, as measured by changes in ADAS-cog scores, in patients treated with donepezil compared with patients treated with placebo (Wilkinson et al., 2003; Kales et al., 2015). Furthermore, in animal experimental studies, cholinergic dysfunction occurs in mouse models of cerebrovascular injury and in VaD patients (Román, 2005; Román and Kalaria, 2006). A number of studies of cholinergic deficits in cerebral ischemia-associated VaD models have shown persistent reductions in several cholinergic markers. In rats, chronic cerebral hypoperfusion resulting from bilateral common carotid artery occlusion (BCCAO) has been shown to result in the loss of cholinergic neurons, as manifested by reduced choline acetyltransferase (ChAT) and acetylcholinesterase activity (Ni et al., 1995; Tanaka et al., 1996). Another study reported that rats with multiple small emboli have been shown to develop multiple infarcts and show reduced cholinergic markers (Naritomi, 1991). In fact, the application of acetylcholinesterase inhibitors has shown advantages in improving learning and memory impairment and other deficits in VaD patients and VaD animal models (Erkinjuntti et al., 2003; Borlongan et al., 2005; Birks, 2006).
Ginkgo biloba preparations are commonly used in the treatment of ischemic cerebrovascular diseases. A number of studies have shown that ginkgo biloba extract combined with acetylcholinesterase inhibitors is more effective in the treatment of cognitive impairment (Zhang et al., 2019; Zhao et al., 2021). Studies have shown (Zhao et al., 2021) that compared with ginkgo biloba extract (EGb761) or donepezil, the combined application of EGb761 and donepezil has a better anti-amnestic effect on scopolamine-induced cognitive impairment rats, and the mechanism may be that EGb761 enhances the therapeutic effect of donepezil by increasing the pro-cholinergic and antioxidant activities. However, the risks associated with the use of ginkgo biloba preparations are the focus of attention in the medical community. Some researchers have suggested that there is a serious risk of bleeding when ginkgo biloba preparation is combined with anticoagulant or antiplatelet drugs, but existing studies have reported that ginkgo biloba extract EGb761 has no significant effect on the efficacy of aspirin or warfarin combination, and has no adverse effect on safety (Bone, 2008). Due to the limited number of relevant studies and the small sample size and low quality of the available studies, the possibility that ginkgo biloba preparation caused idiosyngenic bleeding events cannot be ruled out based on the available information. In addition, in the existing VaD studies, we found that the serum HCY level was often used as the observation index, and most cross-sectional studies reported that the serum HCY concentration of dementia patients was significantly higher than that of the control group (Clarke et al., 1998; McCaddon et al., 1998; Lehmann et al., 1999). The results of a clinical study showed that HCY concentrations were higher in VaD patients than in controls, suggesting that mildly elevated HCY levels may increase the risk of VaD (McIlroy et al., 2002).
Many studies have used ginkgo biloba preparation combined with conventional western medicine to treat cognitive impairment of cerebral small vessel disease, and the results show that the combined treatment can significantly improve the cognitive function of patients with cerebral small vessel disease and reduce serum HCY, suggesting that ginkgo biloba may treat cognitive impairment of cerebral small vessel disease by reducing HCY (McIlroy et al., 2002; Cao et al., 2018; Hou et al., 2019; Qu, 2019). This is consistent with the results of our study. The mechanism may be related to the regulation of glycogen synthase kinase 3β(GSK-3β) and protein phosphatase 2a (PP2A) activities by ginkgo biloba extract (Zeng et al., 2015).
Subgroup analysis was performed according to efficacy rating criteria and duration of treatment.This study suggested that the evaluation criteria of efficacy did not affect the total effective rate of combination drugs in the treatment of VaD. Besides, treatment duration of 4, 8, 12 weeks or administration route of oral or intravenous did not alter the result of combination therapy on total effective rate and MMSE score. Among them, we found that the treatment duration of 12 weeks was more beneficial to the increase of BI score in patients with VaD than that of 4 weeks, whereas there was little difference in improvement in ADL scores between 12 and 8 weeks of treatment. More interestingly, the results showed that the BI score was higher when ginkgo biloba extract was administered intravenously than when it was administered orally, but the ADL score was higher when it was administered orally than when it was administered intravenously. Therefore, in the treatment of vascular dementia, attention should be paid to shorten the treatment time, reduce the treatment cost, and rationally allocate medical resources. However, due to the limited number of included references, the results should be interpreted with caution.
The limitations of this study are as follows: First, the articles included in this study were all single-center studies and carried out in China, which may bring resistance to the international promotion of combination drugs; Second, the heterogeneity is high, which may be related to the differences of drug dosage form (tablets, capsules, injections), active ingredient content (terpenolides: ginkgolides, ginkgolactones; ginkgo flavonoids), medication route (oral, intravenous drip), and age, gender and race of the study population.
5 Conclusion
In summary, the available evidence suggests that compared with Donepezil alone, ginkgo biloba extract combined with Donepezil is more effective in the treatment of vascular dementia and has a better effect on cognitive function and daily living ability in these patients. In addition, combination therapy may decrease hemorheological indicators and serum HCY content in patients with vascular dementia, but no combination therapy was found to directly improve cerebral hemodynamic parameters in participants. Multicenter randomized controlled studies with high quality and large samples are needed for validation.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author contributions
LX: Writing–original draft. JT: Writing–review and editing. HT: Writing–review and editing. YX: Writing–review and editing. SW: Writing–review and editing. LX: Writing–review and editing. DW: Writing–review and editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Study on the protective mechanism of hippocampal neurons in VD and the intervention effect of Zishenhuoxuang decoction by targeting Wnt/Ca2+ involved in tunneling nanotubes-mediated mitochondrial transfer between cells. General Project of National Natural Science Foundation of China, 82374441.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2024.1374482/full#supplementary-material
References
Auchus, A. P., Brashear, H. R., Salloway, S., Korczyn, A. D., De Deyn, P. P., Gassmann-Mayer, C., et al. (2007). Galantamine treatment of vascular dementia: a randomized trial. Neurology 69 (5), 448–458. doi:10.1212/01.wnl.0000266625.31615.f6
Bao, N., and He, Y. (2012). Clinical efficacy of donepezil hydrochloride combined with ginkgo biloba leaves in the treatment of vascular dementia. China Med. 07 (8), 965–966. doi:10.3760/cma.j.issn.1673-4777.2012.08.021
Birks, J. (2006). Cholinesterase inhibitors for Alzheimer’s disease. Cochrane Database Syst. Rev. 2006 (1), Cd005593. doi:10.1002/14651858.Cd005593
Bone, K. M. (2008). Potential interaction of Ginkgo biloba leaf with antiplatelet or anticoagulant drugs: what is the evidence? Mol. Nutr. Food Res. 52 (7), 764–771. doi:10.1002/mnfr.200700098
Borlongan, C. V., Sumaya, I. C., and Moss, D. E. (2005). Methanesulfonyl fluoride, an acetylcholinesterase inhibitor, attenuates simple learning and memory deficits in ischemic rats. Brain Res. 1038 (1), 50–58. doi:10.1016/j.brainres.2005.01.028
Branch CMAN (2002). Draft diagnostic criteria for vascular dementia. Chin. J. NEUROLOGY 35 (4), 246. doi:10.3760/j.issn:1006-7876.2002.04.036
Cao, B., Wang, T., Kang, T., and Xie, J. (2018). The clinical effect of ginkgo biloba combined with nimodipine on the Hcy,S100B and cognitive function of patients with mild to moderate vascular dementia. Shaanxi Med. J. 47 (3), 379–381. doi:10.3969/j.issn.1000-7377.2018.03.034
Chen, S. (2011). Clinical observation on the combination of ginkgo biloba injection and donepezil in the treatment of 41 cases of elderly vascular dementia. Chin. J. Clin. Ration. DRUG USE 4 (13), 50–51. doi:10.3969/j.issn.1674-3296.2011.13.037
Chen, T., and Li, A. (2020). Clinical effect of donepezil hydrochloride combined with ginkgo leaf extract and armillariella mellea powders oral solution in the treatment of vascular dementia. Chin. Community Dr. 36 (1), 83–84. doi:10.3969/j.issn.1007-614x.2020.01.051
Clarke, R., Smith, A. D., Jobst, K. A., Refsum, H., Sutton, L., and Ueland, P. M. (1998). Folate, vitamin B12, and serum total homocysteine levels in confirmed Alzheimer disease. Arch. Neurol. 55 (11), 1449–1455. doi:10.1001/archneur.55.11.1449
Diamond, B. J., Shiflett, S. C., Feiwel, N., Matheis, R. J., Noskin, O., Richards, J. A., et al. (2000). Ginkgo biloba extract: mechanisms and clinical indications. Arch. Phys. Med. Rehabil. 81 (5), 668–678. doi:10.1016/s0003-9993(00)90052-2
Duan, X., Yang, Z., ZhongboZhang, G. L., Qu, J., and Liu, W. (2021). Efficacy of ginkgolides injection combined with donepezil on vascular dementia and its influence on oxidative stress. Mod. J. Integr. Traditional Chin. West. Med. 30 (11), 1172–1175. doi:10.3969/j.issn.1008-8849.2021.11.007
Erkinjuntti, T., Kurz, A., Small, G. W., Bullock, R., Lilienfeld, S., Damaraju, C. V., et al. (2003). An open-label extension trial of galantamine in patients with probable vascular dementia and mixed dementia. Clin. Ther. 25 (6), 1765–1782. doi:10.1016/s0149-2918(03)80168-6
Erkinjuntti, T., and Rockwood, K. (2003). Vascular dementia. Semin. Clin. Neuropsychiatry 8 (1), 37–45. doi:10.1053/scnp.2003.50004
Farlow, M. R. (2006). Use of antidementia agents in vascular dementia: beyond Alzheimer disease. Mayo Clin. Proc. 81 (10), 1350–1358. doi:10.4065/81.10.1350
Farooq, M. U., Min, J., Goshgarian, C., and Gorelick, P. B. (2017). Pharmacotherapy for vascular cognitive impairment. CNS Drugs 31 (9), 759–776. doi:10.1007/s40263-017-0459-3
Gao, W., Li, Y., and Tang, X. (2020). Analysis of cognitive function and quality of life of patients with vascular dementia treated with donepezil hydrochloride and ginkgo leaf. Chin. J. Pract. Nerv. Dis. 23 (19), 1723–1727. doi:10.12083/sysj.2020.19.012
García-Alberca, J. M., Mendoza, S., and Gris, E. (2022). Benefits of treatment with ginkgo biloba extract EGb 761 alone or combined with acetylcholinesterase inhibitors in vascular dementia. Clin. Drug Investig. 42 (5), 391–402. doi:10.1007/s40261-022-01136-8
Gmitrowicz, A., and Kucharska, A. (1994). Developmental disorders in the fourth edition of the American classification: diagnostic and statistical manual of mental disorders (DSM IV -- optional book). Psychiatr. Pol. 28 (5), 509–521.
Guan, Y. (2019). Clinical study on ginkgo biloba extract injection combined with donepezil hydrochloride tablets in the treatment of vascular dementia. Med. Forum 23 (20), 2948–2949. doi:10.19435/j.1672-1721.2019.20.094
Guo, N. (2018). Clinical observation on the combination of ginkgo biloba injection and donepezil hydrochloride in the treatment of vascular dementia patients. Mod. Diagnosis Treat. 29 (8), 1258–1259. doi:10.3969/j.issn.1001-8174.2018.08.043
Guo, X. (2010). Clinical observation of aricept combined with cinepazide maleate on vascular dementia. Med. INNOVATION CHINA 7 (19), 12–14. doi:10.3969/j.issn.1674-4985.2010.19.006
He, D., Qin, X., Zhao, D., Cai, L., Bai, J., and Wen, H. (2016). Aricept combined with Ginkgo Biloba Leaves extract tablets in treatment of vascular cognitive impairment. J. Changchun Univ. Traditional Chin. Med. 32 (4), 765–767. doi:10.13463/j.cnki.cczyy.2016.04.037
Higgins, J. P., Thompson, S. G., Deeks, J. J., and Altman, D. G. (2003). Measuring inconsistency in meta-analyses. Bmj 327 (7414), 557–560. doi:10.1136/bmj.327.7414.557
Hou, J., Sun, J., Shi, J., Qi, G., Heng, X., and Guo, C. (2019). Clinical study on the adjuvant treatment of yinxing damo injection in cognitive impairment of cerebral small vessel disease. Chin. J. Integr. Traditional West. Med. 39 (11), 1336–1339. doi:10.7661/j.cjim.20190929.327
Ihl, R., Tribanek, M., and Bachinskaya, N.GOTADAY Study Group (2012). Efficacy and tolerability of a once daily formulation of Ginkgo biloba extract EGb 761® in Alzheimer’s disease and vascular dementia: results from a randomised controlled trial. Pharmacopsychiatry 45 (2), 41–46. doi:10.1055/s-0031-1291217
Jia, J., and Wang, S. (2010). Exploration of clinical diagnostic criteria for vascular dementia. Chin. J. Neuroimmunol. NEUROLOGY 17 (6), 387–389. doi:10.3969/j.issn.1006-2963.2010.06.001
Kalaria, R. N. (2018). The pathology and pathophysiology of vascular dementia. Neuropharmacology 134 (Pt B), 226–239. doi:10.1016/j.neuropharm.2017.12.030
Kales, H. C., Gitlin, L. N., and Lyketsos, C. G. (2015). Assessment and management of behavioral and psychological symptoms of dementia. Bmj 350, h369. doi:10.1136/bmj.h369
Kandiah, N., Ong, P. A., Yuda, T., Ng, L. L., Mamun, K., Merchant, R. A., et al. (2019). Treatment of dementia and mild cognitive impairment with or without cerebrovascular disease: expert consensus on the use of Ginkgo biloba extract, EGb 761®. CNS Neurosci. Ther. 25 (2), 288–298. doi:10.1111/cns.13095
Lehmann, M., Gottfries, C. G., Regland, B., and Ouml, R. N. (1999). Identification of cognitive impairment in the elderly: homocysteine is an early marker. Dement. Geriatr. Cogn. Disord. 10 (1), 12–20. doi:10.1159/000017092
Li, D., Ma, J., Wei, B., Gao, S., Lang, Y., and Wan, X. (2023). Effectiveness and safety of ginkgo biloba preparations in the treatment of Alzheimer’s disease: a systematic review and meta-analysis. Front. Aging Neurosci. 15, 1124710. doi:10.3389/fnagi.2023.1124710
McCaddon, A., Davies, G., Hudson, P., Tandy, S., and Cattell, H. (1998). Total serum homocysteine in senile dementia of Alzheimer type. Int. J. Geriatr. Psychiatry 13 (4), 235–239. doi:10.1002/(sici)1099-1166(199804)13:4<235::aid-gps761>3.0.co;2-8
McIlroy, S. P., Dynan, K. B., Lawson, J. T., Patterson, C. C., and Passmore, A. P. (2002). Moderately elevated plasma homocysteine, methylenetetrahydrofolate reductase genotype, and risk for stroke, vascular dementia, and Alzheimer disease in Northern Ireland. Stroke 33 (10), 2351–2356. doi:10.1161/01.str.0000032550.90046.38
Miao, G. (2020). Observation on the therapeutic effect of Ginkgo biloba leaf preparation combined with Doperidone hydrochloride in the treatment of vascular dementia. Sichuan J. Anat. 28 (1), 92–93. doi:10.3969/j.issn.1005-1457.2020.01.045
Moher, D., Shamseer, L., Clarke, M., Ghersi, D., Liberati, A., Petticrew, M., et al. (2015). Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst. Rev. 4 (1), 1. doi:10.1186/2046-4053-4-1
Naritomi, H. (1991). Experimental basis of multi-infarct dementia: memory impairments in rodent models of ischemia. Alzheimer Dis. Assoc. Disord. 5 (2), 103–111. doi:10.1097/00002093-199100520-00007
Ni, J. W., Matsumoto, K., Li, H. B., Murakami, Y., and Watanabe, H. (1995). Neuronal damage and decrease of central acetylcholine level following permanent occlusion of bilateral common carotid arteries in rat. Brain Res. 673 (2), 290–296. doi:10.1016/0006-8993(94)01436-l
Noorbala, A. A., and Akhondzadeh, S. (2006). Attention-deficit/hyperactivity disorder: etiology and pharmacotherapy. Arch. Iran. Med. 9 (4), 374–380.
OBrien, J. T., and Thomas, A. (2015). Vascular dementia. Lancet 386 (10004), 1698–1706. doi:10.1016/s0140-6736(15)00463-8
Orgogozo, J. M., Rigaud, A. S., Stöffler, A., Möbius, H. J., and Forette, F. (2002). Efficacy and safety of memantine in patients with mild to moderate vascular dementia: a randomized, placebo-controlled trial (MMM 300). Stroke 33 (7), 1834–1839. doi:10.1161/01.str.0000020094.08790.49
Qu, J. (2019). Clinical study on Yinxingye Capsules combined with mecobalamin in treatment of cerebrovascular cognitive impairment. J. Article 34, 2946–2950.
Román, G. C. (2005). Cholinergic dysfunction in vascular dementia. Curr. Psychiatry Rep. 7 (1), 18–26. doi:10.1007/s11920-005-0019-2
Román, G. C., and Kalaria, R. N. (2006). Vascular determinants of cholinergic deficits in Alzheimer disease and vascular dementia. Neurobiol. Aging 27 (12), 1769–1785. doi:10.1016/j.neurobiolaging.2005.10.004
Shaito, A., Thuan, D. T. B., Phu, H. T., Nguyen, T. H. D., Hasan, H., Halabi, S., et al. (2020). Herbal medicine for cardiovascular diseases: efficacy, mechanisms, and safety. Front. Pharmacol. 11, 422. doi:10.3389/fphar.2020.00422
Shen, D., Li, J., and Zou, C. (2023). Effects of ginkgo biloba leaf combined with donepezil on cognitive function,hemorheology and serum related in-dexes in patients with vascular dementia. Fujian Med. J. 45 (3), 22–26. doi:10.3969/j.issn.1002-2600.2023.03.008
Shi, C., Zhao, L., Zhu, B., Li, Q., Yew, D. T., Yao, Z., et al. (2009). Protective effects of Ginkgo biloba extract (EGb761) and its constituents quercetin and ginkgolide B against beta-amyloid peptide-induced toxicity in SH-SY5Y cells. Chem. Biol. Interact. 181 (1), 115–123. doi:10.1016/j.cbi.2009.05.010
Smith, P. F., Maclennan, K., and Darlington, C. L. (1996). The neuroprotective properties of the Ginkgo biloba leaf: a review of the possible relationship to platelet-activating factor (PAF). J. Ethnopharmacol. 50 (3), 131–139. doi:10.1016/0378-8741(96)01379-7
Sterne, J. A. C., Savović, J., Page, M. J., Elbers, R. G., Blencowe, N. S., Boutron, I., et al. (2019). RoB 2: a revised tool for assessing risk of bias in randomised trials. Bmj 366, l4898. doi:10.1136/bmj.l4898
Subhan, Z., and Hindmarch, I. (1984). The psychopharmacological effects of Ginkgo biloba extract in normal healthy volunteers. Int. J. Clin. Pharmacol. Res. 4 (2), 89–93.
Tanaka, K., Ogawa, N., Asanuma, M., Kondo, Y., and Nomura, M. (1996). Relationship between cholinergic dysfunction and discrimination learning disabilities in Wistar rats following chronic cerebral hypoperfusion. Brain Res. 729 (1), 55–65. doi:10.1016/0006-8993(96)00400-3
Tong, Z. (2021). Improvement of cognitive function and effects of serum NSE and BDNF in patients with vascular dementia by Ginkgo biloba leaves. Pract. Clin. J. Integr. Traditional Chin. West. Med. 21 (6), 51–52. doi:10.13638/j.issn.1671-4040.2021.06.025
Wilkinson, D., Doody, R., Helme, R., Taubman, K., Mintzer, J., Kertesz, A., et al. (2003). Donepezil in vascular dementia: a randomized, placebo-controlled study. Neurology 61 (4), 479–486. doi:10.1212/01.wnl.0000078943.50032.fc
Xu, Y. (2019). Therapeutic effect of ginkgo tablet combined with donepezil in patients with vascular dementia. Med. Innovation China 16 (18), 121–124. doi:10.3969/j.issn.1674-4985.2019.18.032
Yang, Y., Zhou, B., and Zhao, W. (2016). Ginkgo biloba leaves: a model for the research and development of traditional Chinese medicine/plant medicine. Chin. Traditional Herb. Drugs 47 (15), 2579–2591. doi:10.7501/j.issn.0253-2670.2016.15.001
Yu, G. (2014). Curative effect on vascular dementia disease and mechanism of action of extract of ginkgo biloba injection combined with donepezil. Chin. J. Exp. Traditional Med. Formulae 20 (20), 210–213. doi:10.13422/j.cnki.syfjx.2014200210
Zeng, K., Huang, F., Bao, J., Li, M., and Wang, X. (2015). The preventive and therapeutic effects of Ginkgo biloba leaf extract on Alzheimer’s disease and its mechanism. Chin. J. Pathophysiol. (10), 1898. doi:10.3969/j.issn.1000-4718.2015.10.331
Zhan, M., Sun, L., Liu, J., Zeng, Z., Shen, W., Li, H., et al. (2021). EGb in the treatment for patients with VCI: a systematic review and meta-analysis. Oxid. Med. Cell Longev. 2021, 8787684. doi:10.1155/2021/8787684
Zhang, J., Wang, J., Zhou, G. S., Tan, Y. J., Tao, H. J., Chen, J. Q., et al. (2019). Studies of the anti-amnesic effects and mechanisms of single and combined use of donepezil and ginkgo ketoester tablet on scopolamine-induced memory impairment in mice. Oxid. Med. Cell Longev. 2019, 8636835. doi:10.1155/2019/8636835
Zhao, J., Li, K., Wang, Y., Li, D., Wang, Q., Xie, S., et al. (2021). Enhanced anti-amnestic effect of donepezil by Ginkgo biloba extract (EGb 761) via further improvement in pro-cholinergic and antioxidative activities. J. Ethnopharmacol. 269, 113711. doi:10.1016/j.jep.2020.113711
Keywords: ginkgo biloba extract, donepezil, vascular dementia, meta-analysis, systematic review
Citation: Xiao L, Tang J, Tan H, Xie Y, Wang S, Xie L and Wu D (2024) Efficacy and safety of ginkgo biloba extract combined with donepezil hydrochloride in the treatment of Chinese patients with vascular dementia: A systematic review meta-analysis. Front. Pharmacol. 15:1374482. doi: 10.3389/fphar.2024.1374482
Received: 22 January 2024; Accepted: 29 May 2024;
Published: 03 July 2024.
Edited by:
Jacob Raber, Oregon Health and Science University, United StatesReviewed by:
Pietro Gareri, ASP Catanzaro, ItalyHui Pei, China Academy of Chinese Medical Sciences, China
Copyright © 2024 Xiao, Tang, Tan, Xie, Wang, Xie and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dahua Wu, ODkzMDQ5MzUyQHFxLmNvbQ==
†Present address: Liangyi Xiao, Department of Neurology, Hunan Provincial Hospital of Integrated Traditional Chinese and Western Medicine (Hunan Academy of Chinese Medicine Affiliated Hospital), Changsha, China
 Liangyi Xiao
Liangyi Xiao Jie Tang1
Jie Tang1 Yao Xie
Yao Xie Le Xie
Le Xie Dahua Wu
Dahua Wu