- 1College of Medicine, King Saud Bin Abdulaziz University for Health Sciences, Jeddah, Saudi Arabia
- 2King Abdullah International Medical Research Center, Jeddah, Saudi Arabia
- 3Department of Physiology, Neuroscience Unit, Faculty of Medicine, King Abdulaziz University, Jeddah, Saudi Arabia
- 4Neuroscience and Geroscience Research Unit, King Fahd Medical Research Center, King Abdulaziz University, Jeddah, Saudi Arabia
- 5Department of Pharmacology and Toxicology, College of Pharmacy, Umm Al-Qura University, Makkah, Saudi Arabia
Background: Melatonin is responsible for regulating the sleep-wake cycle and circadian rhythms in mammals. Tramadol, a synthetic opioid analgesic, is used to manage moderate to severe pain but has a high potential for abuse and dependence. Studies have shown that melatonin could be a potential modulator to reduce tramadol addiction.
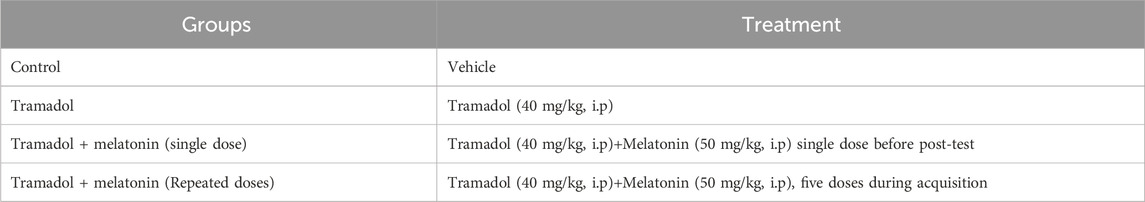
Methods: Male Wistar rats were used to investigate the effect of melatonin on tramadol-induced place preference. The rats were divided into four groups: control, tramadol, tramadol + melatonin (single dose), and tramadol + melatonin (repeated doses). Tramadol was administered intraperitoneally at 40 mg/kg, while melatonin was administered at 50 mg/kg for both the single dose and repeated-dose groups. The study consisted of two phases: habituation and acquisition.
Results: Tramadol administration produced conditioned place preference (CPP) in rats, indicating rewarding effects. However, melatonin administration blocked tramadol-induced CPP. Surprisingly, repeated doses of melatonin were ineffective and did not reduce the expression of CPP compared to that of the single dose administration.
Conclusion: The study suggests that melatonin may be a potential therapeutic option for treating tramadol addiction. The results indicate that melatonin attenuates the expression of tramadol-induced CPP, supporting its uses as an adjunct therapy for managing tramadol addiction. However, further studies are needed to investigate its effectiveness in humans.
Introduction
The hormone melatonin, secreted by the pineal gland in the brain (Claustrat and Leston, 2015), plays a crucial role in regulating the sleep-wake cycle and circadian rhythms of mammals (Claustrat and Leston, 2015). Beyond these functions, melatonin exhibits a broad spectrum of physiological activities, including antioxidant activity, immunomodulation, and neuroprotection (Esposito and Cuzzocrea, 2010; Bantounou et al., 2022). Recent researches have explored its potential therapeutic benefits in sleep disorders, depression, anxiety, and drug addiction (Turek and Gillette, 2004; Papp et al., 2006; Cardinali et al., 2012; Onaolapo and Onaolapo, 2018; Alghamdi and Alshehri, 2021). Additionally, studies have investigated its efficacy in treating cardiovascular diseases, cancer, and Alzheimer’s disease (Sun et al., 2016; Li et al., 2017; Labban et al., 2021). Evidence also suggests that melatonin possesses anti-aging properties and can promote better sleep (Pereira et al., 2020; Bocheva et al., 2022).
Tramadol, a synthetic opioid analgesic, is widely prescribed for moderate to severe pain (Grond and Sablotzki, 2004; Subedi et al., 2019). Despite its effectiveness in pain management, tramadol carries a high potential for abuse and dependence (Roussin et al., 2015; Bassiony et al., 2017; Barbosa et al., 2023). The conditioned place preference (CPP) paradigm measures the drug-rewarding effects in animals (Huston et al., 2013). In CPP, animals learn to associate a specific location with the drug’s euphoric or dysphoric effects, and their preference for that location indicates the drug’s reinforcing properties (Bardo and Bevins, 2000). Studies have demonstrated that tramadol induces CPP in rodents, implying its rewarding effects (Epstein et al., 2006; Huston et al., 2013), which suggests a potential for misuse and abuse. Research has shown that tramadol use can lead to physical dependence and addiction, with individuals who have a history of substance abuse being particularly at risk of developing tramadol addiction (Epstein et al., 2006; Lanier et al., 2010).
The study of melatonin’s modulation of CPP in response to tramadol in rats is critically important, given the escalating concerns about tramadol misuse and abuse (Wood and Dargan, 2021). Tramadol, a commonly prescribed opioid analgesic, is linked to addiction, dependence, and various adverse effects (Reines et al., 2020). Consequently, it is vital to investigate drugs that reduce tramadol’s rewarding effects to prevent addiction and its associated issues. Previous research indicates that melatonin might be an effective treatment for diminishing the effects of several drug addictions (Hemati et al., 2021). Recently, it has been shown that melatonin can block morphine induced CPP through modulating glutamate transporter −1 (GLT-1) and brain-derived neurotrophic factor (BDNF), nuclear factor-kappa B (NF-κB), and cAMP response element-binding protein CREB expression levels (Alghamdi and Alshehri, 2021; Alshehri et al., 2021). Previous research showed evidence that alcohol-dependent humans and rodents experience reduction in melatonin levels and delay in reaching their nocturnal peak concentration of melatonin and activating melatonin receptors using melatonin or agomelatine reduced alcohol seeking in rats (Vengeliene et al., 2015). Other studies have shown melatonin can reduce cocaine (Takahashi et al., 2017), methamphetamine (Clough et al., 2016), and fentanyl seeking behavior (Du et al., 2024). Thus, exploring melatonin’s impact on tramadol-induced CPP in rats is imperative to assess its therapeutic potential for tramadol addiction management.
Melatonin has demonstrated potential effects against the rewarding properties of various drugs of abuse, such as cocaine and morphine, in animal models (Takahashi et al., 2017). However, the impact of melatonin on tramadol-induced CPP remains underexplored. Therefore, this study investigated melatonin as a potential therapeutic compound for treating tramadol addiction.
Materials and methods
Animals
Male Wistar rats weighing 250–300 g were utilized. They were housed in pairs under a 12:12 light/dark cycle, with ad libitum access to food and water. All animals were handled by expert researchers and housed in pairs to minimize stress. The study received approval from the King Fahd Medical Research Center Animal Care and Use Committee. Furthermore, the Biomedical Ethics Research Committee at the King Abdulaziz University (Reference 405-20) the experiments in accordance with the ethical guidelines and research protocols for living organisms established by the King Abdulaziz City for Science and Technology, as authorized by the Royal Decree No. M/59 on 24 August 2010.
Drugs
Tramadol hydrochloride (Sigma Aldrich, USA) and melatonin (Sigma Aldrich, USA), were freshly prepared daily, using 0.5% ethanol and diluted with saline to serve as the vehicle (i.p. 1 mL/kg).
Experimental design and dosing
Phase I
Figure 1 illustrates the habituation phase spanning from day 1 to day 3. Throughout this phase, the animals were allowed to explore the open apparatus for a total of 20 min each day. On day 4, a 20-min pre-test was performed to assess the animals’ preference. The OPTO-MAX Auto-Track software documented various parameters, including the duration, overall activity count, ambulatory count, rest time, and distance covered by the animals in each chamber. A preference for the black chamber was observed among the majority of animals, necessitating the use of a biased approach.

Figure 1. Timeline of the CPP experiment showing the habituation, pre-test, acquisition and post-test.
Phase II
During the acquisition phase, from day 5 to day 14, each animal was placed in the assigned chamber for 45 min. The post-test was conducted on day 15 for 20 min. During this test, the apparatus was open to the animals for a total of 20 min, and the CPP score was calculated. Animals were euthanized using isoflurane on day 16.
Animal groups and dosing
Four groups of animals, each comprising approximately 6-8 rats as detailed in Table 1, were divided as follows: (1) control, (2) tramadol, (3) tramadol + melatonin (single dose), and (4) tramadol + melatonin (repeated doses). The control group was administered vehicle injections throughout the experiment. The tramadol group received tramadol injections (40 mg/kg, i.p.) on alternate days, totaling five injections. The tramadol + melatonin (single dose) group was administered tramadol injections (40 mg/kg, i.p.) on alternate days for five injections, with a single dose of melatonin (50 mg/kg, i. p.) administered 30 min before the post-test. The tramadol + melatonin (repeated doses) group received concurrent injections of tramadol (40 mg/kg, i.p.) and melatonin (50 mg/kg, i.p.) on alternate days, also totaling five injections.
CPP score, total activity, ambulatory count, resting time, and distance traveled
The test utilized a three-compartment apparatus constructed from Plexiglas (Columbus Instruments in Columbus, OH, USA). It comprised two main chambers, each featuring unique visual cues and flooring textures. The white chamber was marked by vertical white stripes and a smooth white floor, whereas the black chamber displayed a pattern of small white and black squares and had a small circle drilled into the floor. A smaller external chamber situated between these two served as a separator. Infrared sensors tracked the animals’ movements and activity throughout the CPP test. The test was recorded for 20 min and all parameters were recorded including the time spent in each chamber, total activity, ambulatory count, resting time, and distance traveled.
Data analysis
All data comprising the CPP score, such as the distance traveled, resting time, ambulatory count, and total activity count, were analyzed using a repeated measure ANOVA followed by the Tukey’s post hoc test. Statistical significance was set at p < 0.05. GraphPad Prism version 10.2.1 was used to analyze the results and create the figures.
Results
The primary objective of this study was to investigate the effect of melatonin on tramadol-induced CPP. We aimed to determine whether melatonin administration could attenuate the CPP induced by tramadol in rats Figure 2. Repeated measures ANOVA revealed significant effect of the days (F (1, 7) = 78.4, p < 0.0001), the effect of treatment (F (3, 21) = 22.79, p < 0.0001), and the interaction between treatment and days (F (3, 21) = 17.82, p < 0.0001). Further analysis using Tukey’s post hoc test indicated a significant increase in CPP scores for animals that received five doses of tramadol during the acquisition phase (tramadol group) compared to the control group (p < 0.0001). However, administering melatonin 30 min before the post-test prevented the tramadol-induced CPP (p < 0.0001). In contrast, repeated doses of melatonin given with tramadol during the acquisition phase did not reduce tramadol-seeking behavior during the post-test (p = 0.5188). Also, post hoc test showed significant increase in CPP score comparing pre-test and post-test in tramadol group (p < 0.0001) and pre-test and post-test in tramadol + MEL (R) group (p < 0.0001).
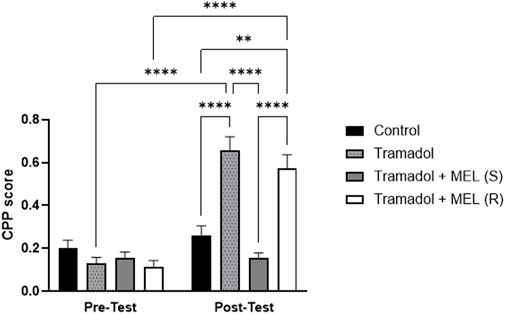
Figure 2. CPP score for the effect of melatonin and tramadol in CPP on all groups (control, tramadol, tramadol + melatonin “single dose”, and tramadol + melatonin “repeated doses”).
In addition to evaluating melatonin’s impact on tramadol-induced CPP, we performed further analyses to examine additional relevant parameters that might influence our results, Figure 3. We quantified the total activity, ambulatory count, resting time, and distance traveled. These measures enabled more comprehensive understanding of the animal’s behavior and more precise assessment of melatonin’s effects.
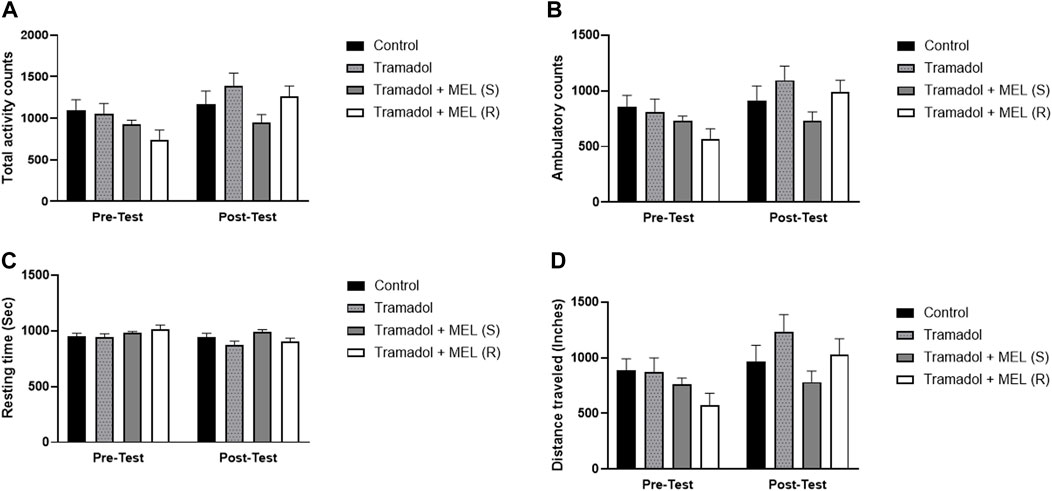
Figure 3. (A) Total activity, (B) ambulatory count, (C) resting time, and (D) distance traveled for the effect of melatonin and tramadol in CPP on all groups (control, tramadol, tramadol + melatonin “single dose”, and tramadol + melatonin “repeated doses”).
First, we assessed melatonin’s impact on tramadol’s total activity, Figure 3A. Repeated measures ANOVA showed significant effect on days (F (1, 7) = 9.321, p = 0.0185), no treatment effect (F (3, 21) = 2.138, p = 0.1258), and no interaction between treatment and days (F (3, 21) = 2.259, p = 0.1113). Second, we evaluated melatonin’s influence on tramadol’s ambulatory count, Figure 3B. Repeated measures ANOVA indicated significant effect on days (F (1, 7) = 8.343, p = 0.0234), no treatment effect (F (3, 21) = 2.033, p = 0.1400), and no effect of treatment and days (F (3, 21) = 2.422, p = 0.0944).
Third, we assessed the impact of melatonin on tramadol-induced resting time, Figure 3C. Repeated measures ANOVA indicated significant effects over days (F (1, 7) = 6.787, p = 0.0352), no significant treatment effect (F (3, 21) = 2.268, p = 0.1103), and no interaction between treatment and days (F (3, 21) = 2.199, p = 0.1182). Lastly, we evaluated the influence of melatonin on the distance traveled, Figure 3D. Repeated measures ANOVA showed significant effects on days (F (1, 7) = 9.295, p = 0.0186), no significant treatment effect (F (3, 21) = 2.261, p = 0.1110), and no interaction between treatment and days (F (3, 21) = 2.250, p = 0.1123).
Discussion
Several preclinical studies have reported the rewarding outcomes of opioids using CPP and other behavioral techniques, such as self-administration (Sim-Selley et al., 2000; Zhang et al., 2012; Mavrikaki et al., 2017; Reeves et al., 2021). Moreover, CPP is considered as one of the most popular non-invasive models for measuring the motivational effects of drugs of abuse in experimental animals (Mavrikaki et al., 2017). Previous reports have consistently demonstrated the rewarding effects of tramadol using the CPP technique (Abdel-Ghany et al., 2015; Sadeghi-Adl et al., 2020; Barbosa et al., 2023). Melatonin, a hormone synthesized by the pineal gland, is essential for maintaining the regular circadian rhythm in mammals (Dubocovich, 2007). Notably, melatonin has played a potential role in attenuating the seeking behavior for several drugs of abuse (Kovanen et al., 2010; Conroy et al., 2012; Alghamdi and Alshehri, 2021; Alshehri et al., 2021). The CPP paradigm can provide further insights into animal behavior beyond the time spent in each chamber, such as the resting time, total activity, ambulatory count, and total distance.
Tramadol, an opioid analgesic, has shown potential for abuse according to epidemiological evidence, coinciding with the increased global demand for opioids over the past 2 decades (Berterame et al., 2016; Dunn et al., 2019). Tramadol also induces physical dependence and withdrawal syndrome upon discontinuation, similar to other opioids (Carroll et al., 2006; Lofwall et al., 2007). Specifically, it has been demonstrated to produce a CPP rewarding effect in rats (Sprague et al., 2002; Tzschentke et al., 2002). Moreover, tramadol affects multiple neurotransmitter systems, including serotonin and norepinephrine, and its effects are partially antagonized by naloxone (Desmeules et al., 1996; Apaydin et al., 2000). Notably, an in vivo microdialysis study provided evidence of a statistically significant increase in dopamine release within the nucleus accumbens shell following a tramadol suggesting preclinical evidence of tramadol’s rewarding effects within the reward circuit (Asari et al., 2018). Consistent with previous findings, this study demonstrated the CPP rewarding effects with tramadol administration in rats.
Studies on melatonin have demonstrated a decrease in dopamine release, primarily through effects on dopamine receptors (Zisapel et al., 1982; Zisapel, 2001). Furthermore, stimulation of melatonin receptors has been shown to reduce alcohol relapse-like behavior in Wistar rats (Vengeliene et al., 2015). A single dose of melatonin significantly attenuated the expression of tramadol-induced CPP. This finding aligns with those of a previous report from our laboratory, which found that administering melatonin 30 min before morphine treatment diminished the morphine CPP effect (Alghamdi and Alshehri, 2021). The same study also revealed that melatonin reversed the expression levels of GLT-1, NF-κB, CREB, and BDNF. Similarly, other studies have indicated that melatonin can restore neuronal impairment induced by methamphetamine in mice (Veschsanit et al., 2021). Therefore, melatonin is recognized for attenuating the rewarding effects and modulating the neuronal impairment caused by drugs of abuse.
Conducting the CPP test during the day, rather than at night, aligns with the pharmacological properties and mechanisms of action of melatonin. Melatonin is a hormone primarily secreted by the pineal gland in response to darkness, with levels typically peaking at night to regulate the sleep-wake cycle and synchronize circadian rhythms in Wistar rats (Sánchez et al., 2004; Sánchez et al., 2008). By administering melatonin and conducting the CPP test during the day is to minimize any potential confounders associated with melatonin release during the night and ensuring the reliability of the results. Furthermore, this study performed several tests including (total activity, ambulatory count, resting time, and distance traveled) to assure the melatonin doses during the day does not affect CPP tests.
The study’s observation that repeated doses of melatonin did not reduce tramadol-seeking behavior during the post-test underscores the complexity of the interaction between melatonin and tramadol in the context of CPP. The initial administration of melatonin 30 min before the acquisition phase test successfully attenuated the seeking behavior induced by tramadol. However, the efficacy of melatonin appeared to diminish with repeated administration. Tramadol is typically considered a mild μ-receptor agonist and also affects other neurotransmitter systems, including serotonergic, noradrenergic, and gamma-aminobutyric acid systems (Bamigbade et al., 1997; Gillen et al., 2000; Jesse et al., 2010). The mechanisms by which tramadol influences each of these systems remain unclear, and limited research is available.
Melatonin produces analgesic properties through a variety of biological pathways (Ambriz-Tututi et al., 2009). Animals and humans studies have demonstrated its efficacy in alleviating nociceptive and neuropathic pain (Srinivasan et al., 2012; Borsani et al., 2017; Kuthati et al., 2019; Shokri et al., 2021). In rodent models, melatonin shows antinociceptive and anti-hyperalgesic effects against a range of stimuli, including inflammation and nerve injury (Yu et al., 2000; Ulugol et al., 2006; Posa et al., 2018). These effects are believed to be a result of the activation of melatonin receptors present in critical pain-regulating regions such as the spinal cord, thalamus, and hypothalamus (Laurido et al., 2002; Lopez-Canul et al., 2015). Activation of these receptors results in the reduction of cyclic AMP levels and inhibition of Ca2+ channels (Vanecek and Vollrath, 1989), consequently lowering intracellular Ca2+ levels (Vanecek, 1995; Vanecek and Watanabe, 1998), which are essential in the central sensitization process associated with inflammatory and neuropathic pain. Furthermore, melatonin modulates various receptor systems, including dopaminergic (Abilio et al., 2003), GABAergic (Golombek et al., 1996), opioidergic (Hemati et al., 2021), and serotonergic pathways (Valdes-Tovar et al., 2018). Melatonin shows anti-inflammatory and antioxidative characteristics (Nabavi et al., 2019; Bantounou et al., 2022), further enhancing its analgesic efficacy (Burchakov and Uspenskaya, 2019). Acting as a potent free radical scavenger, melatonin neutralizes reactive oxygen and nitrogen species and facilitate the activity of antioxidative enzymes such as glutathione peroxidase and superoxide dismutase (Tsia and Hu, 2003; Reiter et al., 2007). On the other hand, tramadol use have been reported to be associated with the activation of proinflammatory cytokines (Kraychete et al., 2009), and glutamatergic involvement (Chetan et al., 2015). Thus, the complex mechanisms of melatonin together with tramadol reduce seeking behavior associated with tramadol use. Using melatonin to attenuate the seeking behavior of tramadol offers advantages in regulating sleep patterns disrupted by tramadol use (Abdullah et al., 2020), and neuroprotective properties, though its direct efficacy in countering tramadol induce CPP. On the other hand, naloxone, as an opioid receptor antagonist, directly blocks opioid effects of tramadol, which may help prevent Tramadol induction of CPP; however, naloxone will participate in the withdrawal symptoms in physically dependent individuals which could limit its suitability for tramadol since tramadol is weak mu opioids against (Lagard et al., 2018). Lastly, the choice between melatonin and naloxone depends on factors such as the severity of addiction, comorbid conditions, and treatment goals.
In this study, various behavioral tests such as total activity, ambulatory count, resting time, and distance traveled serve as important measurement for assessing the rewarding or aversive properties of environmental stimuli. Total activity provides a comprehensive measure of overall locomotor behavior, reflecting the general stimulation level of the rats. Ambulatory count specifically quantifies voluntary movements, to understand the exploratory behavior and activity patterns within the test environment. Resting time, conversely, shows periods of inactivity or grooming potentially indicating the presence of preferred or aversive behavior. Distance traveled serves as a cumulative measure of the spatial exploration undertaken by the subject throughout the conditioning process. These tests collectively contribute to explaining the subtle behavioral responses associated with conditioned preferences, offering valuable insights into the underlying mechanisms of reward and aversion processing.
Limitation, the findings highlight the need to determine the optimal dosing regimen for melatonin and to ascertain whether its impact on drug-seeking behavior diminishes over time, necessitating further research into the dynamics of this interaction. Understanding how melatonin influences tramadol in a repeated dosing context is crucial for explaining its potential therapeutic uses. Additionally, investigating the molecular and neurobiological alterations that occur with chronic melatonin administration in conjunction with tramadol may reveal mechanisms underlying the observed effects. In summary, the immediate influence of melatonin on tramadol-induced CPP underscores the importance of thoroughly understanding the temporal aspects and dose-response relationships to fully understand melatonin’s potential to attenuate tramadol’s drug-seeking behavior
Conclusion
The results of this study suggest that melatonin may offer therapeutic benefits in treating tramadol addiction. Administration of melatonin significantly reduced the expression of tramadol-induced CPP in rats. Furthermore, analysis of relevant parameters, including the total activity, ambulatory count, resting time, and distance traveled, revealed that melatonin did not significantly affect these measures. Thus, the influence of melatonin on tramadol-seeking behavior appears to be specific and not attributable to changes in the overall activity or locomotion. These findings support the potential use of melatonin as an adjunct therapy for managing tramadol addiction, although further research is necessary to assess its efficacy in humans. This study highlights the importance of investigating potential pharmacological interventions for drug addiction treatment and offers valuable insights into the neurobiological mechanisms of tramadol addiction.
Data availability statement
The raw data supporting the conclusion of this article will be made available by the authors, without undue reservation.
Ethics statement
The animal study was approved by the Biomedical Ethics Research Committee (Reference 405-20) at King Abdulaziz University approved the experiments following the ethical guidelines and research protocols for living organisms prepared by the King Abdulaziz City for Science and Technology (KACST), which was authorized by Royal Decree No. M/59 on 24 August 2010. The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
AH: Conceptualization, Data curation, Formal Analysis, Investigation, Resources, Supervision, Validation, Visualization, Writing–original draft, Writing–review and editing. BA: Conceptualization, Data curation, Resources, Supervision, Writing–review and editing. FA: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Project administration, Resources, Validation, Visualization, Writing–original draft.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abdel-Ghany, R., Nabil, M., Abdel-Aal, M., and Barakat, W. (2015). Nalbuphine could decrease the rewarding effect induced by tramadol in mice while enhancing its antinociceptive activity. Eur. J. Pharmacol. 758, 11–15. doi:10.1016/j.ejphar.2015.03.062
Abdullah, E. A., Moussa, F. A. E., Amin, M. E., Basheer, M. A., and Saleh, A. A. (2020). Sleep characteristics in patients with tramadol dependence. Int. J. Health Sci. (I), 10206–10214. doi:10.53730/ijhs.v6ns1.7227
Abilio, V. C., Vera, J. A. R., Ferreira, L. S. M., Duarte, C. R. M., Martins, C. R., Torres-Leite, D., et al. (2003). Effects of melatonin on behavioral dopaminergic supersensitivity. Life Sci. 72 (26), 3003–3015. doi:10.1016/s0024-3205(03)00231-5
Alghamdi, B. S., and Alshehri, F. S. (2021). Melatonin blocks morphine-induced place preference: involvement of GLT-1, NF-κB, BDNF, and CREB in the nucleus accumbens. Front. Behav. Neurosci. 15, 762297. doi:10.3389/fnbeh.2021.762297
Alshehri, F. S., Alghamdi, B. S., Hakami, A. Y., Alshehri, A. A., and Althobaiti, Y. S. (2021). Melatonin attenuates morphine-induced conditioned place preference in Wistar rats. Brain Behav. 11 (12), e2397. doi:10.1002/brb3.2397
Ambriz-Tututi, M., Rocha-González, H. I., Cruz, S. L., and Granados-Soto, V. (2009). Melatonin: a hormone that modulates pain. Life Sci. 84 (15-16), 489–498. doi:10.1016/j.lfs.2009.01.024
Apaydin, S., Uyar, M., Karabay, N. U., Erhan, E., Yegul, I., and Tuglular, I. (2000). The antinociceptive effect of tramadol on a model of neuropathic pain in rats. Life Sci. 66 (17), 1627–1637. doi:10.1016/s0024-3205(00)00482-3
Asari, Y., Ikeda, Y., Tateno, A., Okubo, Y., Iijima, T., and Suzuki, H. (2018). Acute tramadol enhances brain activity associated with reward anticipation in the nucleus accumbens. Psychopharmacol. Berl. 235 (9), 2631–2642. doi:10.1007/s00213-018-4955-z
Bamigbade, T. A., Davidson, C., Langford, R. M., and Stamford, J. A. (1997). Actions of tramadol, its enantiomers and principal metabolite, O-desmethyltramadol, on serotonin (5-HT) efflux and uptake in the rat dorsal raphe nucleus. Br. J. Anaesth. 79 (3), 352–356. doi:10.1093/bja/79.3.352
Bantounou, M., Plascevic, J., and Galley, H. F. (2022). Melatonin and related compounds: antioxidant and anti-inflammatory actions. Antioxidants (Basel) 11, 532. doi:10.3390/antiox11030532
Barbosa, J., Leal, S., Pereira, F. C., Dinis-Oliveira, R. J., and Faria, J. (2023). Tramadol and tapentadol induce conditioned place preference with a differential impact on rewarding memory and incubation of craving. Pharm. (Basel) 16 (1), 86. doi:10.3390/ph16010086
Bardo, M., and Bevins, R. A. (2000). Conditioned place preference: what does it add to our preclinical understanding of drug reward? Psychopharmacology 153, 31–43. doi:10.1007/s002130000569
Bassiony, M. M., Youssef, U. M., Hassan, M. S., Salah El-Deen, G. M., El-Gohari, H., Abdelghani, M., et al. (2017). Cognitive impairment and tramadol dependence. J. Clin. Psychopharmacol. 37 (1), 61–66. doi:10.1097/JCP.0000000000000617
Berterame, S., Erthal, J., Thomas, J., Fellner, S., Vosse, B., Clare, P., et al. (2016). Use of and barriers to access to opioid analgesics: a worldwide, regional, and national study. Lancet 387 (10028), 1644–1656. doi:10.1016/S0140-6736(16)00161-6
Bocheva, G., Slominski, R. M., Janjetovic, Z., Kim, T. K., Böhm, M., Steinbrink, K., et al. (2022). Protective role of melatonin and its metabolites in skin aging. Int. J. Mol. Sci. 23 (3), 1238. doi:10.3390/ijms23031238
Borsani, E., Buffoli, B., Bonazza, V., Reiter, R. J., Rezzani, R., and Rodella, L. F. (2017). Single administration of melatonin modulates the nitroxidergic system at the peripheral level and reduces thermal nociceptive hypersensitivity in neuropathic rats. Int. J. Mol. Sci. 18 (10), 2143. doi:10.3390/ijms18102143
Burchakov, D., and Uspenskaya, Y. B. (2019). The antioxidant, anti-inflammatory, and sedative effects of melatonin: results of clinical trials. Neurosci. Behav. Physiology 49, 54–59. doi:10.1007/s11055-018-0691-4
Cardinali, D. P., Srinivasan, V., Brzezinski, A., and Brown, G. M. (2012). Melatonin and its analogs in insomnia and depression. J. Pineal Res. 52 (4), 365–375. doi:10.1111/j.1600-079X.2011.00962.x
Carroll, C. P., Walsh, S. L., Bigelow, G. E., Strain, E. C., and Preston, K. L. (2006). Assessment of agonist and antagonist effects of tramadol in opioid-dependent humans. Exp. Clin. Psychopharmacol. 14 (2), 109–120. doi:10.1037/1064-1297.14.2.109
Chetan, P. S., Sangeetha, R., Mohan, P. M., and Rajendra, W. (2015). Alterations in glutamate metabolism in rat brain by tramadol analgesia during non-induction of pain. Saudi J. Med. Pharm. Sci. 1, 26–36. doi:10.36348/sjmps.2015.v01i01.006
Claustrat, B., and Leston, J. (2015). Melatonin: physiological effects in humans. Neurochirurgie 61 (2-3), 77–84. doi:10.1016/j.neuchi.2015.03.002
Clough, S. J., Hutchinson, A. J., and Dubocovich, M. L. (2016). “Melatonin receptors as modulators of methamphetamine-mediated behaviors,” in Neuropathology of drug addictions and substance misuse (Elsevier), 169–180.
Conroy, D. A., Hairston, I. S., Arnedt, J. T., Hoffmann, R. F., Armitage, R., and Brower, K. J. (2012). Dim light melatonin onset in alcohol-dependent men and women compared with healthy controls. Chronobiol Int. 29 (1), 35–42. doi:10.3109/07420528.2011.636852
Desmeules, J. A., Piguet, V., Collart, L., and Dayer, P. (1996). Contribution of monoaminergic modulation to the analgesic effect of tramadol. Br. J. Clin. Pharmacol. 41 (1), 7–12. doi:10.1111/j.1365-2125.1996.tb00152.x
Du, K., Shi, Q., Zhou, X., Zhang, L., Su, H., Zhang, C., et al. (2024). Melatonin attenuates fentanyl - induced behavioral sensitization and circadian rhythm disorders in mice. Physiol. Behav. 279, 114523. doi:10.1016/j.physbeh.2024.114523
Dubocovich, M. L. (2007). Melatonin receptors: role on sleep and circadian rhythm regulation. Sleep. Med. 8 (Suppl. 3), 34–42. doi:10.1016/j.sleep.2007.10.007
Dunn, K. E., Bergeria, C. L., Huhn, A. S., and Strain, E. C. (2019). A systematic review of laboratory evidence for the abuse potential of tramadol in humans. Front. Psychiatry 10, 704. doi:10.3389/fpsyt.2019.00704
Epstein, D. H., Preston, K. L., and Jasinski, D. R. (2006). Abuse liability, behavioral pharmacology, and physical-dependence potential of opioids in humans and laboratory animals: lessons from tramadol. Biol. Psychol. 73 (1), 90–99. doi:10.1016/j.biopsycho.2006.01.010
Esposito, E., and Cuzzocrea, S. (2010). Antiinflammatory activity of melatonin in central nervous system. Curr. Neuropharmacol. 8 (3), 228–242. doi:10.2174/157015910792246155
Gillen, C., Haurand, M., Kobelt, D. J., and Wnendt, S. (2000). Affinity, potency and efficacy of tramadol and its metabolites at the cloned human mu-opioid receptor. Naunyn-Schmiedeberg's archives Pharmacol. 362, 116–121. doi:10.1007/s002100000266
Golombek, D. A., Pevet, P., and Cardinali, D. P. (1996). Melatonin effects on behavior: possible mediation by the central GABAergic system. Neurosci. Biobehav Rev. 20 (3), 403–412. doi:10.1016/0149-7634(95)00052-6
Grond, S., and Sablotzki, A. (2004). Clinical pharmacology of tramadol. Clin. Pharmacokinet. 43 (13), 879–923. doi:10.2165/00003088-200443130-00004
Hemati, K., Pourhanifeh, M. H., Dehdashtian, E., Fatemi, I., Mehrzadi, S., Reiter, R. J., et al. (2021). Melatonin and morphine: potential beneficial effects of co-use. Fundam. Clin. Pharmacol. 35 (1), 25–39. doi:10.1111/fcp.12566
Huston, J. P., Silva, M. A. d. S., Topic, B., and Müller, C. P. (2013). What's conditioned in conditioned place preference? Trends Pharmacol. Sci. 34 (3), 162–166. doi:10.1016/j.tips.2013.01.004
Jesse, C. R., Wilhelm, E. A., Bortolatto, C. F., and Nogueira, C. W. (2010). Evidence for the involvement of the noradrenergic system, dopaminergic and imidazoline receptors in the antidepressant-like effect of tramadol in mice. Pharmacol. Biochem. Behav. 95 (3), 344–350. doi:10.1016/j.pbb.2010.02.011
Kovanen, L., Saarikoski, S. T., Haukka, J., Pirkola, S., Aromaa, A., Lönnqvist, J., et al. (2010). Circadian clock gene polymorphisms in alcohol use disorders and alcohol consumption. Alcohol Alcohol 45 (4), 303–311. doi:10.1093/alcalc/agq035
Kraychete, D. C., Sakata, R. K., Issy, A. M., Bacellar, O., Jesus, R. S., and Carvalho, E. M. (2009). Proinflammatory cytokines in patients with neuropathic pain treated with Tramadol. Rev. Bras. Anestesiol. 59 (3), 297–303. doi:10.1590/s0034-70942009000300004
Kuthati, Y., Lin, S. H., Chen, I. J., and Wong, C. S. (2019). Melatonin and their analogs as a potential use in the management of Neuropathic pain. J. Formos. Med. Assoc. 118 (8), 1177–1186. doi:10.1016/j.jfma.2018.09.017
Labban, S., Alshehri, F. S., Kurdi, M., Alatawi, Y., and Alghamdi, B. S. (2021). Melatonin improves short-term spatial memory in a mouse model of Alzheimer's disease. Degener. Neurol. Neuromuscul. Dis. 11, 15–27. doi:10.2147/DNND.S291172
Lagard, C., Malissin, I., Indja, W., Risède, P., Chevillard, L., and Mégarbane, B. (2018). Is naloxone the best antidote to reverse tramadol-induced neuro-respiratory toxicity in overdose? An experimental investigation in the rat. Clin. Toxicol. (Phila) 56 (8), 737–743. doi:10.1080/15563650.2017.1401080
Lanier, R. K., Lofwall, M. R., Mintzer, M. Z., Bigelow, G. E., and Strain, E. C. (2010). Physical dependence potential of daily tramadol dosing in humans. Psychopharmacol. Berl. 211 (4), 457–466. doi:10.1007/s00213-010-1919-3
Laurido, C., Pelissie, T., Soto-Moyano, R., Valladares, L., Flores, F., and Hernández, A. (2002). Effect of melatonin on rat spinal cord nociceptive transmission. Neuroreport 13 (1), 89–91. doi:10.1097/00001756-200201210-00021
Li, Y., Li, S., Zhou, Y., Meng, X., Zhang, J. J., Xu, D. P., et al. (2017). Melatonin for the prevention and treatment of cancer. Oncotarget 8 (24), 39896–39921. doi:10.18632/oncotarget.16379
Lofwall, M. R., Walsh, S. L., Bigelow, G. E., and Strain, E. C. (2007). Modest opioid withdrawal suppression efficacy of oral tramadol in humans. Psychopharmacol. Berl. 194 (3), 381–393. doi:10.1007/s00213-007-0847-3
Lopez-Canul, M., Palazzo, E., Dominguez-Lopez, S., Luongo, L., Lacoste, B., Comai, S., et al. (2015). Selective melatonin MT2 receptor ligands relieve neuropathic pain through modulation of brainstem descending antinociceptive pathways. Pain 156 (2), 305–317. doi:10.1097/01.j.pain.0000460311.71572.5f
Mavrikaki, M., Pravetoni, M., Page, S., Potter, D., and Chartoff, E. (2017). Oxycodone self-administration in male and female rats. Psychopharmacol. Berl. 234 (6), 977–987. doi:10.1007/s00213-017-4536-6
Nabavi, S. M., Sureda, A., Xiao, J., Dehpour, A. R., Shirooie, S., Silva, A. S., et al. (2019). Anti-inflammatory effects of Melatonin: a mechanistic review. Crit. Rev. Food Sci. Nutr. 59 (Suppl. 1), S4–S16. doi:10.1080/10408398.2018.1487927
Onaolapo, O. J., and Onaolapo, A. Y. (2018). Melatonin in drug addiction and addiction management: exploring an evolving multidimensional relationship. World J. Psychiatry 8 (2), 64–74. doi:10.5498/wjp.v8.i2.64
Papp, M., Litwa, E., Gruca, P., and Mocaër, E. (2006). Anxiolytic-like activity of agomelatine and melatonin in three animal models of anxiety. Behav. Pharmacol. 17 (1), 9–18. doi:10.1097/01.fbp.0000181601.72535.9d
Pereira, N., Naufel, M. F., Ribeiro, E. B., Tufik, S., and Hachul, H. (2020). Influence of dietary sources of melatonin on sleep quality: a review. J. Food Sci. 85 (1), 5–13. doi:10.1111/1750-3841.14952
Posa, L., De Gregorio, D., Gobbi, G., and Comai, S. (2018). Targeting melatonin MT2 receptors: a novel pharmacological avenue for inflammatory and neuropathic pain. Curr. Med. Chem. 25 (32), 3866–3882. doi:10.2174/0929867324666170209104926
Reeves, K. C., Kube, M. J., Grecco, G. G., Fritz, B. M., Muñoz, B., Yin, F., et al. (2021). Mu opioid receptors on vGluT2-expressing glutamatergic neurons modulate opioid reward. Addict. Biol. 26 (3), e12942. doi:10.1111/adb.12942
Reines, S. A., Goldmann, B., Harnett, M., and Lu, L. (2020). Misuse of tramadol in the United States: an analysis of the national survey of drug use and health 2002-2017. Subst. Abuse 14, 1178221820930006. doi:10.1177/1178221820930006
Reiter, R., Tan, D. x., Terron, M. P., Flores, L. J., and Czarnocki, Z. (2007). Melatonin and its metabolites: new findings regarding their production and their radical scavenging actions. Acta Biochim. Pol. 54 (1), 1–9. doi:10.18388/abp.2007_3264
Roussin, A., Doazan-d'Ouince, O., Géniaux, H., and Halberer, C.French Network of Centre for Evaluation and Information on Pharmacodependence Addictovigilance Centres (2015). Evaluation of abuse and dependence in addiction monitoring systems: tramadol as an example. Therapies 70 (2), 203–221. doi:10.2515/therapie/2015014
Sadeghi-Adl, M., Sadat-Shirazi, M. S., Shahini, F., Akbarabadi, A., Khalifeh, S., Borzabadi, S., et al. (2020). The role of cannabinoid 1 receptor in the nucleus accumbens on tramadol induced conditioning and reinstatement. Life Sci. 260, 118430. doi:10.1016/j.lfs.2020.118430
Sánchez, S., Paredes, S. D., Martín, M. I., Barriga, C., and Rodríguez, A. B. (2004). Effect of tryptophan administration on circulating levels of melatonin and phagocytic activity. J. Appl. Biomed. 2, 169–177. doi:10.32725/jab.2004.020
Sánchez, S., Sánchez, C. L., Paredes, S. D., Rodriguez, A. B., and Barriga, C. (2008). The effect of tryptophan administration on the circadian rhythms of melatonin in plasma and the pineal gland of rats. J. Appl. Biomed. 6 (4), 177–186. doi:10.32725/jab.2008.021
Shokri, M., Sajedi, F., Mohammadi, Y., and Mehrpooya, M. (2021). Adjuvant use of melatonin for relieving symptoms of painful diabetic neuropathy: results of a randomized, double-blinded, controlled trial. Eur. J. Clin. Pharmacol. 77 (11), 1649–1663. doi:10.1007/s00228-021-03170-5
Sim-Selley, L. J., Selley, D. E., Vogt, L. J., Childers, S. R., and Martin, T. J. (2000). Chronic heroin self-administration desensitizes mu opioid receptor-activated G-proteins in specific regions of rat brain. J. Neurosci. 20 (12), 4555–4562. doi:10.1523/JNEUROSCI.20-12-04555.2000
Sprague, J. E., Leifheit, M., Selken, J., Milks, M. M., Kinder, D. H., and Nichols, D. E. (2002). In vivo microdialysis and conditioned place preference studies in rats are consistent with abuse potential of tramadol. Synapse 43 (2), 118–121. doi:10.1002/syn.10025
Srinivasan, V., Lauterbach, E. C., Ho, K. Y., Acuña-Castroviejo, D., Zakaria, R., and Brzezinski, A. (2012). Melatonin in antinociception: its therapeutic applications. Curr. Neuropharmacol. 10 (2), 167–178. doi:10.2174/157015912800604489
Subedi, M., Bajaj, S., Kumar, M. S., and Yc, M. (2019). An overview of tramadol and its usage in pain management and future perspective. Biomed. Pharmacother. 111, 443–451. doi:10.1016/j.biopha.2018.12.085
Sun, H., Gusdon, A. M., and Qu, S. (2016). Effects of melatonin on cardiovascular diseases: progress in the past year. Curr. Opin. Lipidol. 27 (4), 408–413. doi:10.1097/MOL.0000000000000314
Takahashi, T. T., Vengeliene, V., and Spanagel, R. (2017). Melatonin reduces motivation for cocaine self-administration and prevents relapse-like behavior in rats. Psychopharmacol. Berl. 234 (11), 1741–1748. doi:10.1007/s00213-017-4576-y
Tsia, P. L., and Hu, M. K. (2003). Free radical scavenging and antioxidative activity of melatonin derivatives. J. Pharm. Pharmacol. 55 (12), 1655–1660. doi:10.1211/0022357022250
Turek, F. W., and Gillette, M. U. (2004). Melatonin, sleep, and circadian rhythms: rationale for development of specific melatonin agonists. Sleep. Med. 5 (6), 523–532. doi:10.1016/j.sleep.2004.07.009
Tzschentke, T. M., Bruckmann, W., and Friderichs, E. (2002). Lack of sensitization during place conditioning in rats is consistent with the low abuse potential of tramadol. Neurosci. Lett. 329 (1), 25–28. doi:10.1016/s0304-3940(02)00571-2
Ulugol, A., Dokmeci, D., Guray, G., Sapolyo, N., Ozyigit, F., and Tamer, M. (2006). Antihyperalgesic, but not antiallodynic, effect of melatonin in nerve-injured neuropathic mice: possible involvements of the L-arginine-NO pathway and opioid system. Life Sci. 78 (14), 1592–1597. doi:10.1016/j.lfs.2005.07.002
Valdes-Tovar, M., Estrada-Reyes, R., Solís-Chagoyán, H., Argueta, J., Dorantes-Barrón, A. M., Quero-Chávez, D., et al. (2018). Circadian modulation of neuroplasticity by melatonin: a target in the treatment of depression. Br. J. Pharmacol. 175 (16), 3200–3208. doi:10.1111/bph.14197
Vanecek, J. (1995). Melatonin inhibits increase of intracellular calcium and cyclic AMP in neonatal rat pituitary via independent pathways. Mol. Cell Endocrinol. 107 (2), 149–153. doi:10.1016/0303-7207(94)03437-x
Vanecek, J., and Vollrath, L. (1989). Melatonin inhibits cyclic AMP and cyclic GMP accumulation in the rat pituitary. Brain Res. 505 (1), 157–159. doi:10.1016/0006-8993(89)90129-7
Vanecek, J., and Watanabe, K. (1998). Melatonin inhibits the increase of cyclic AMP in rat suprachiasmatic neurons induced by vasoactive intestinal peptide. Neurosci. Lett. 252 (1), 21–24. doi:10.1016/s0304-3940(98)00530-8
Vengeliene, V., Noori, H. R., and Spanagel, R. (2015). Activation of melatonin receptors reduces relapse-like alcohol consumption. Neuropsychopharmacology 40 (13), 2897–2906. doi:10.1038/npp.2015.143
Veschsanit, N., Yang, J. L., Ngampramuan, S., Viwatpinyo, K., Pinyomahakul, J., Lwin, T., et al. (2021). Melatonin reverts methamphetamine-induced learning and memory impairments and hippocampal alterations in mice. Life Sci. 265, 118844. doi:10.1016/j.lfs.2020.118844
Wood, D. M., and Dargan, P. I. (2021). Regional, National and international datasets: how they improve our understanding of the acute harms associated with prescription medicine misuse. Br. J. Clin. Pharmacol. 87 (4), 1654–1659. doi:10.1111/bcp.14592
Yu, C. X., Zhu, C. B., Xu, S. F., Cao, X. D., and Wu, G. C. (2000). Selective MT(2) melatonin receptor antagonist blocks melatonin-induced antinociception in rats. Neurosci. Lett. 282 (3), 161–164. doi:10.1016/s0304-3940(00)00883-1
Zhang, M., Jing, L., Liu, Q., Wen, R. T., Li, J. X., Li, Y. L., et al. (2012). Tramadol induces conditioned place preference in rats: interactions with morphine and buprenorphine. Neurosci. Lett. 520 (1), 87–91. doi:10.1016/j.neulet.2012.05.037
Zisapel, N. (2001). Melatonin-dopamine interactions: from basic neurochemistry to a clinical setting. Cell Mol. Neurobiol. 21 (6), 605–616. doi:10.1023/a:1015187601628
Keywords: melatonin, tramadol, conditioned place preference, opioids, addiction
Citation: Hakami AY, Alghamdi BS and Alshehri FS (2024) Exploring the potential use of melatonin as a modulator of tramadol-induced rewarding effects in rats. Front. Pharmacol. 15:1373746. doi: 10.3389/fphar.2024.1373746
Received: 20 January 2024; Accepted: 08 April 2024;
Published: 26 April 2024.
Edited by:
Dasiel Oscar Borroto-Escuela, Karolinska Institutet (KI), SwedenReviewed by:
Patricia De Gortari, National Institute of Psychiatry Ramon de la Fuente Muñiz (INPRFM), MexicoSantiago J. Ballaz, Yachay Tech University, Ecuador
Copyright © 2024 Hakami, Alghamdi and Alshehri. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fahad S. Alshehri, ZnNzaGVocmlAdXF1LmVkdS5zYQ==
†ORCID: Badrah S. Alghamdi, orcid.org/0000-0002-9411-3609; Fahad S. Alshehri, orcid.org/0000-0001-6966-0128
 Alqassem Y. Hakami
Alqassem Y. Hakami Badrah S. Alghamdi
Badrah S. Alghamdi Fahad S. Alshehri
Fahad S. Alshehri