- 1Department of Pharmacy, Renmin Hospital, Wuhan University, Wuhan, China
- 2School of Pharmaceutical Sciences, Wuhan University, Wuhan, China
- 3Department of Pharmacy, Changzhou Jintan District Hospital of Traditional Chinese Medicine, Changzhou, China
Background and objective: Cancer-associated venous thromboembolism (CAVTE) is a preventable, life-threatening complication with a considerable morbidity and mortality. Primary venous thromboembolism (VTE) prophylaxis is currently recommended; however, the health and economic benefits have not been evaluated and compared in China. This study aimed to assess and compare the cost-effectiveness of anticoagulants in primary CAVTE prevention among cancer patients in China.
Methods: A Markov model with a 5-year horizon was established to evaluate the costs and effectiveness of direct oral anticoagulants (DOACs) compared to low-molecular-weight heparins (LMWHs) and no prevention in primary prophylaxis of CAVTE in China. Key clinical outcomes were obtained from the available clinical trials, comparing DOACs (rivaroxaban and apixaban) with LMWHs or with no thromboprophylaxis. Utility and the cost inputs were all obtained from the published literature or local data with public sources. The total costs, quality-adjusted life-years (QALYs), and incremental cost-effectiveness ratios (ICERs) were estimated as the main endpoints of the modal for each strategy. The assessment of uncertainty was performed involving deterministic sensitivity analysis and probabilistic sensitivity analysis (PSA). Impact of time horizon, generic drug price, and individual DOACs were assessed in scenario and subgroup analyses.
Results: Primary prophylaxis using DOACs were projected to yield 1.866 QALYs at a cost of $3,287.893, resulting in the ICERs of $12,895.851 (DOACs vs. no-thromboprophylaxis) and $43,613.184/QALYs (LMWHs vs. DOACs). Sensitivity analysis revealed that ICER was sensitive to the VTE and bleeding risk, drug cost of anticoagulants, self-payment ratio, and overall death rate of cancer. Probabilistic sensitivity analysis showed that DOACs and LMWHs had a 48% and 45% probability of being cost-effective at a 5-year time horizon, respectively. When the time horizon extended to 10 years, DOACs achieved a cost-effective probability of 43%. Among individual DOACs, apixaban was found to be the preferred strategy in VTE prevention due to its incremental health gain with an acceptable cost increase.
Conclusion: Primary thromboprophylaxis with DOACs was cost-effective in cancer patients at a willing-to-pay (WTP) threshold of $37,125.24/QALY in China. Cancer death rate, risk of VTE and major bleeding, and the drug cost assumed greater relevance and importance in the decision-making process for primary thromboprophylaxis in cancer.
1 Introduction
Cancer-associated venous thromboembolism (CAVTE) is a prevalent and severe complication observed in the clinical course of malignant tumors (Girardi et al., 2023). In recent years, with the significant improvement in prognosis due to targeted treatment and immunotherapy for tumors, the harm of serious complications on cancer patients has become more prominent (Mulder et al., 2021). The hypercoagulable state, induced by malignance (Ay et al., 2017) and further exacerbated by therapeutic interventions such as chemotherapy, hormonal drugs, or surgical procedures, significantly escalates the incidence of venous thromboembolism (VTE) and causes the overall mortality rate of malignant tumors to increase 2–6 times in the cancer population (Timp et al., 2013). Given the high incidence and mortality rate of CAVTE and its serious impact on patient survival quality, active anticoagulant prophylaxis, especially primary prevention, recently gained prominence worldwide for tumor patients at a high risk of embolism to reduce the occurrence of VTE and improve quality of life (Streiff et al., 2023).
Traditionally, heparin anticoagulants, especially low-molecular-weight heparins (LMWHs), are preferred in VTE prevention due to the controllable anticoagulant strength, the appropriate initiation time, and the low bleeding risk, which is critical for cancer patients (Geerts et al., 2008). Owing to the accumulating safety and efficacy evidence revealed in several large, randomized controlled trials (RCTs) (Rutjes et al., 2020; Baloch et al., 2023), direct oral anticoagulants (DOACs), such as rivaroxaban and apixaban, gradually turned into competition, especially for out-and-ambulatory cancer patients that are referred drugs with good feasibility (Akin et al., 2015). Nevertheless, it still remains unclear whether the clinical benefits offered by DOACs in primary prophylaxis are worth the extra expense, particularly in developing countries like China, which bear a heavier financial burden of cancer than western nations. In this study, we perform a comprehensive analysis to assess the cost-effectiveness of DOACs versus LMWHs and no prophylaxis from the perspective of a Chinese payer to provide a reference for the rational drug use of anticoagulants in the cancer population.
2 Methods
2.1 Model design
We used TreeAge Pro 2022 software to build a Markov model with a cycle period of 1 month and a run period of 5 years based on the clinical and economic impact of cancer survival. According to previously published domestic and international literature (Kimpton et al., 2019; Li et al., 2020), the model was composed of several distinct health states, including no complications, pulmonary embolism (PE), deep vein thrombosis (DVT), intracranial hemorrhage (ICH), chronic thromboembolic pulmonary hypertension (CTEPH), post-thrombotic syndrome (PTS), and death, as shown in Figure 1. The following assumptions were made to reflect the approximate progression of thromboprophylaxis of VTE in patients with cancer, according to the previous literature (Du and Wu, 2020; Li et al., 2020). At the onset of the simulation, all patients were presumed to be in a state free of complications. As each cycle progresses, patients have the potential to either maintain their current health state or transition to a subsequent state due to a clinical event. Recurrence of VTE was allowed in the model in the form of PE or DVT. By a certain chance, CTEPH and PTS would develop after PE and DVT, respectively. A bleeding event was categorized into clinically relevant non-major bleeding (CRNMB) and major bleeding (MB). In our model, MB specifically referred to gastrointestinal hemorrhage (GIB) and ICH as these types of bleeding are associated with significant health loss and substantial clinical resource consumption. A study on the long-term use of antithrombotic treatment in patients with ICH (Ottosen et al., 2016) revealed that approximately 65.7% of patients did not continue with antithrombotic treatment. Consequently, our model simulates a cessation of treatment following an ICH event. In accordance with the clinical guideline of VTE treatment, a transition to therapeutic doses of anticoagulants was also presumed following any occurrence of VTE.
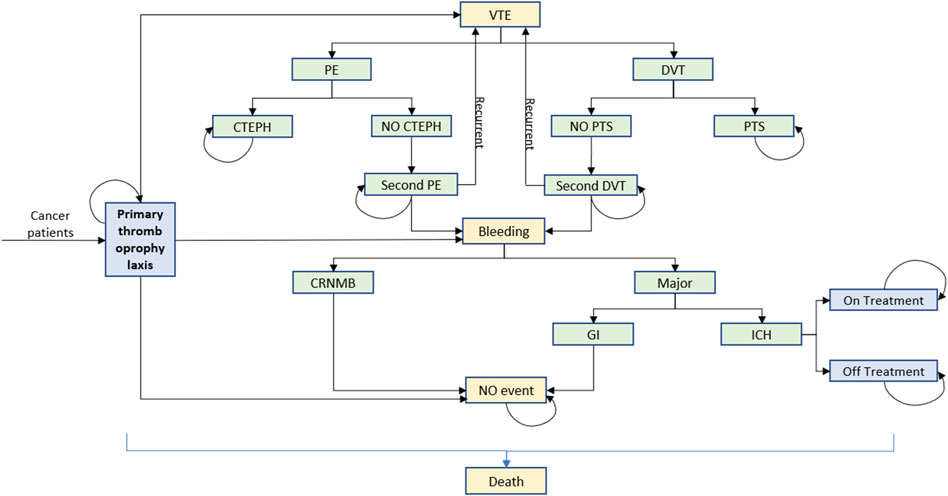
Figure 1. Markov model build with TreeAge Pro 2022. Seven distinct health states were no complications, PE, DVT, ICH, CTEPH, PTS, and death. VTE, venous thromboembolism; PE, pulmonary thromboembolism; DVT, deep vein thrombosis; CTEPH, chronic thromboembolic pulmonary hypertension; PTS, post-thrombotic syndrome; CRNMB, clinically relevant non-major bleeding; GI, gastrointestinal; ICH, intracranial hemorrhage.
2.2 Date and sources
A network meta-analysis was conducted to assess the clinical advantages and bleeding hazards of DOACs and LMWHs in the prevention of CAVTE in comparison to a regimen without thrombotic prophylaxis. Search strategies, study endpoints, and inclusion and exclusion criteria are described in detail in Supplementary Tables S1, S2. A total of 9,024 patients from 22 RCTs were enrolled (Altinbas et al., 2004; Kakkar et al., 2004; Klerk et al., 2005; Wang et al., 2005; Sideras et al., 2006; Agnelli et al., 2009; Perry et al., 2010; van Doormaal et al., 2011; Haas et al., 2012; Levine et al., 2012; Lecumberri et al., 2013; Vadhanraj et al., 2013; Zhang et al., 2013; Zwicker et al., 2013; Zhu et al., 2014; Pelzer et al., 2015; Macbeth et al., 2016; Khorana et al., 2017; Ek et al., 2018; Meyer et al., 2018; Carrier et al., 2019; Khorana et al., 2019). The anticoagulants under investigation in our study included nadroparin, certoparin, dalteparin, enoxaparin, bemiparin, tinzaparin, apixaban, and rivaroxaban (Supplementary Tables S3, S4). The Cochrane bias risk assessment and funnel plots were performed to evaluate the bias among the included RCTs (Supplementary Tables S5–S9). Clinical event rates for patients with no prophylaxis were extracted from published RCTs and combined by a random-effects model (Supplementary Tables S13, S14). The comparative rates of outcomes in the DOACs and LMWHs groups were then calculated by a classic method of applying the corresponding rates of no prophylaxis to the relative risks (RRs) (Supplementary Tables S10, S11) obtained in above meta-analysis. The overall mortality rate was sourced from a 5-year cancer survival survey conducted by the National Health Commission of China (Zeng et al., 2024) and was applied to each group. The long-term death rates of PE, DVT, CTEPH, and PTS were obtained from previous published works (Schulman et al., 2006; Martinez et al., 2018), which are described in detail in Table 1. The transfer probability of recurrent VTE comes from two retrospective studies (Nakano et al., 2021; Ogino et al., 2021). The proportions of major bleeding, ICH, and PE were also extracted and combined from published clinical trials (Kakkar et al., 2004; Klerk et al., 2005; Sideras et al., 2006; Agnelli et al., 2009; Perry et al., 2010; van Doormaal et al., 2011; Haas et al., 2012; Levine et al., 2012; Lecumberri et al., 2013; Vadhanraj et al., 2013; Zhang et al., 2013; Zwicker et al., 2013; Pelzer et al., 2015; Macbeth et al., 2016; Khorana et al., 2017; Ek et al., 2018; Meyer et al., 2018; Carrier et al., 2019; Khorana et al., 2019). In the absence of dynamic data on the occurrence of events, all clinical event rates were converted into monthly probabilities and assumed to remain constant throughout each Markov cycle.
2.3 Costs and utility inputs
The cost of this study is aligned with the current Chinese healthcare system. From the perspective of patients, we considered only the direct medical costs, including the costs of drug, expenses related to the management of clinical events, and local self-copay ratio in Chinese Medicare. All costs are expressed in United States dollars, using the average exchange rate of 2023 (¥ = $0.144). The cost of the drug is calculated by multiplying the unit price by the dosage. The unit price of drugs was obtained from the average price listed in the public database (yaozh.com). The dosage for anticoagulant prevention or treatment was incorporated in the model in consistence with the NCCN guidelines (Streiff et al., 2023) or the drug package insert. In accordance with the guidelines for the management of cancer-associated thrombosis, it was assumed that prophylactic medication would be administered lifelong, while three-month therapeutic doses were supposed in symptomatic DVT and PE before transitioning to prophylactic dosing. The treatment costs of ICH and gastrointestinal bleeding were both derived from “China Health Statistical Yearbook 2022.” Patients with PTS and CTEPH require long-term treatment, and the annual cost is based on clinical data from six hospitals in China (Chen et al., 2011).
Utility value is a widely used parameter for assessing the impact of an intervention on the quality of life. For cancer patients without complications, a baseline utility of 0.650 was adopted in accordance with the previous literature (Du and Wu, 2020). The permanent dis-utilities of 0.250, 0.190, 0.470, 0.360, and 0.050 were used for PE, DVT, ICH, CTEPH, and PTS, respectively, according to the previous literature studies (Table 1) to calculate the long-term impact of these events on health. One-time dis-utilities of 0.270 and 0.013 were assigned for BIG and CRNMB due to their transient impact on health (Table 1). All costs and utilities were discounted at an annual rate of 5%, according to the recommendation of China Guidelines for pharmacoeconomic evaluations.
3 Analyses
The key metrics assessed in base-case analysis included incremental costs, incremental quality-adjusted life-years (QALYs), and the incremental cost-effectiveness ratio (ICER). In light of the current lack of a recommended willingness-to-pay threshold (WTP) in China, we employed three times the per capita gross national product (GDP) of 2022, which amounts to $37,125.240, as a reference point for assessing the cost-effectiveness of various treatment options. To explore the influence of parameter uncertainty on the final results, scenario analysis, one-way sensitivity analysis, and probabilistic sensitivity analysis (PSA) were also performed in this study. In one-way sensitivity analysis, the parameter inputs were assumed to vary over their 95% confidence intervals. If a confidence interval was not provided, a variation of ±20% from the mean values was estimated for the costs and ±10% for the transfer probabilities. In the scenario analysis, we explored variations in time horizon and the cost reduction associated with the introduction of generic drugs after patent expiry. In PSA, appropriate distribution functions were assigned to each parameter according to the type of data. Beta distributions were applied for clinical outcomes and health utilities, while log-normal distributions were used for the costs. Relative risks of clinical events in DOACs and LMWHs, in comparison to no prophylaxis, were assigned to beta distributions. We conducted a 10,000-subject Monte Carlo simulation based on these variable distributions, allowing all parameter inputs to vary stochastically in the PSA. The PSA results are visually presented as scatterplots.
4 Results
4.1 Base-case analysis
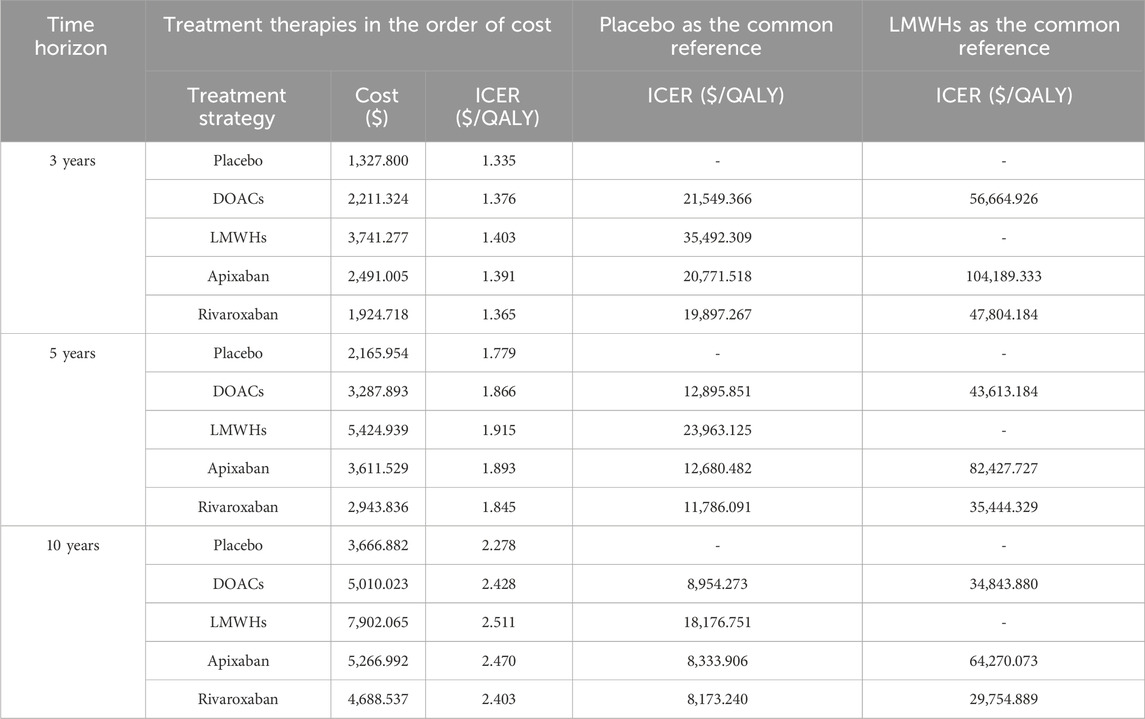
In base-case analysis, with a 5-year projected time, the estimated outcomes for primary prophylaxis using DOACs were projected to yield 1.866 QALYs at a cost of $ 3,287.893. In comparison, prophylaxis with LMWHs resulted in 1.915 QALYs at a cost of $ 5,424.939, while opting for no thromboprophylaxis achieved 1.779 QALYs at a cost of $2,165.954. Compared with no prophylaxis, DOACs and LMWHs were associated with a gain of 0.087 and 0.136 QALYs at additional costs of $ 1,121.939 and $3,258.985, respectively. The ICERs were $12,895.851 and 23,963.125 per QALY, respectively (Table 2). These ICERs were less than WTP, indicating that primary thrombosis prophylaxis was cost-effective in the prevention of CAVTE in the cancer population. When DOACs were set as a competitor drug, prophylaxis with LMWHs was associated with a gain of 0.049 QALYs at the incremental cost of $2,137.046. The estimated ICER was $43,613.184/QALY. This value exceeded three times the per capita GDP of China, indicating that DOACs were the preferable anticoagulants over the traditional LMWHs for VTE primary prophylaxis in malignancy.
In addition, the QALYs and the costs of individual DOACs, including apixaban and rivaroxaban, were also calculated. Compared with no prophylaxis, thromboprophylaxis with either apixaban or rivaroxaban resulted in higher overall costs and improved health outcomes. The predicted costs were $3,611.529 for apixaban and $2,943.836 for rivaroxaban, while the health gained were 1.893 QALYs and 1.845 QALYs, respectively. The ICERs were estimated to be $13910.27/QALY when apixaban was compared to rivaroxaban, suggesting that apixaban was a more cost-effective drug for thromboprophylaxis (Table 2).
4.2 Scenario analysis
In the scenario analysis, the impacts of the time horizons and market access of generic drugs after patent expiry were examined. With the extension of the time horizon, incremental QALYs and treatment costs were estimated but with a gradual decline in ICER in DOACs compared with no prophylaxis, which was from $21,549.366/QALY for a 3-year period to $8,954.273/QALY for a 10-year period. This suggested that long-term primary prophylaxis with anticoagulants leads to improved benefits in health and economic aspects (Table 2). When compared with LMWHs, DOACs exhibited decreased ICERs in 10-year simulation ($43,613.184//QALY vs. $34,843.880//QALY) and shifted from a dominating to dominated status. This finding might be related to the relative higher death risk in DOACs, leading to the lower health gain over a longer time simulation. When original anticoagulants and their generic counterparts were incorporated, it was observed that prophylaxis with both original and generic anticoagulants were cost-effective. However, generic DOACs produced the lowest ICER, falling below the GDP per capita threshold (Table 3). It suggested that generic DOACs were the preferred prophylaxis option for VTE prevention in China.
4.3 Sensitivity analyses
Figure 2 displays the univariate sensitivity analyses of the individual parameter inputs that exerted the greatest influence on the ICERs, arranged according to their respective levels of impact. When DOACs were compared with no prophylaxis, the relative risk of VTE in DOACs was found to have a great impact on the ICER, followed by the proportion of drug reimbursement and the cost of DOACs. Furthermore, it is observed that changing all the inputs within their reasonable range only resulted in the changes in ICER values but not in the reversion of the final result, indicating that DOACs are robustly cost-effective compared to no prophylaxis (Figure 2A).
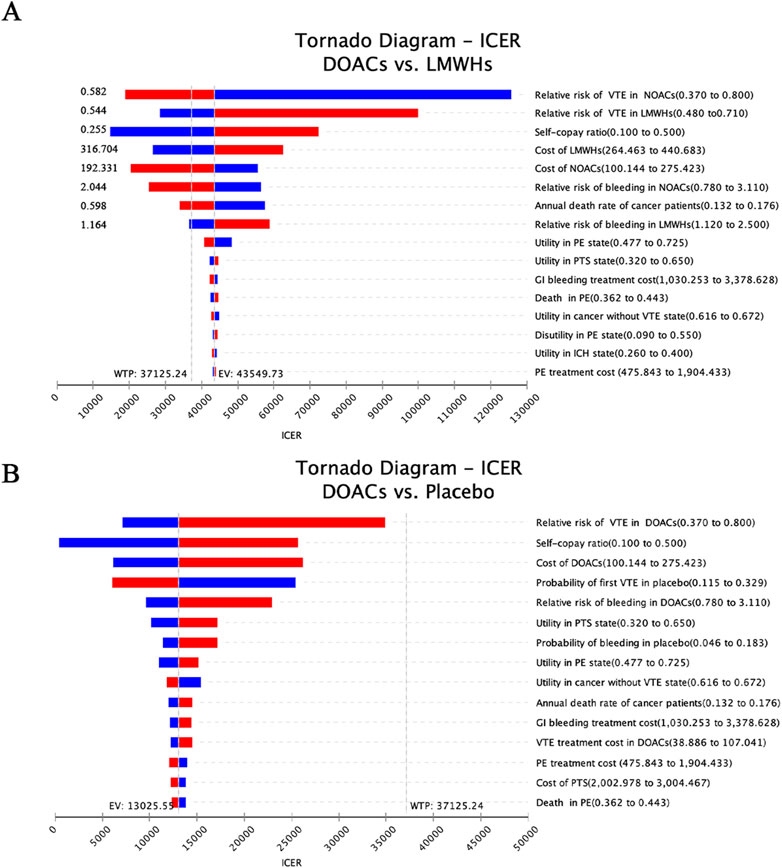
Figure 2. Tornado diagram illustrating the results of the one-way sensitivity analysis (A) DOACs vs LMWHs and (B) DOACs vs Placebo. Each bar represents the range of variation within the set intervals, ordered from top to bottom by the magnitude of their impact.
When LMWHs were employed as counterparts to DOACs, it was discovered that the result is sensitive to several factors: the VTE risks, self-copay ratio, cost of DOACs and LMWHs, bleeding risks of thromboprophylaxis, and the annual death rate of cancer (Figure 2B). Specifically, when the VTE risk reached 0.582, the bleeding risk exceeded 2.044, or the drug cost surpassed $192.331, the ICERs for DOACs fell below the willingness-to-pay threshold of $37,125.24. This indicated a shift in strategy for DOACs from being dominant to being dominated. Conversely, LMWHs demonstrated a cost-effective advantage over DOACs in VTE prevention in cancer patients when a low risk of VTE and bleeding (less than 0.544 and 1.164, respectively) or a price decrease (lower than $316.704) was applied. Moreover, an increased self-copay ratio and a smaller death rate were found to enhance the cost-effective advantage of DOACs in the primary prophylaxis of CAVTE.
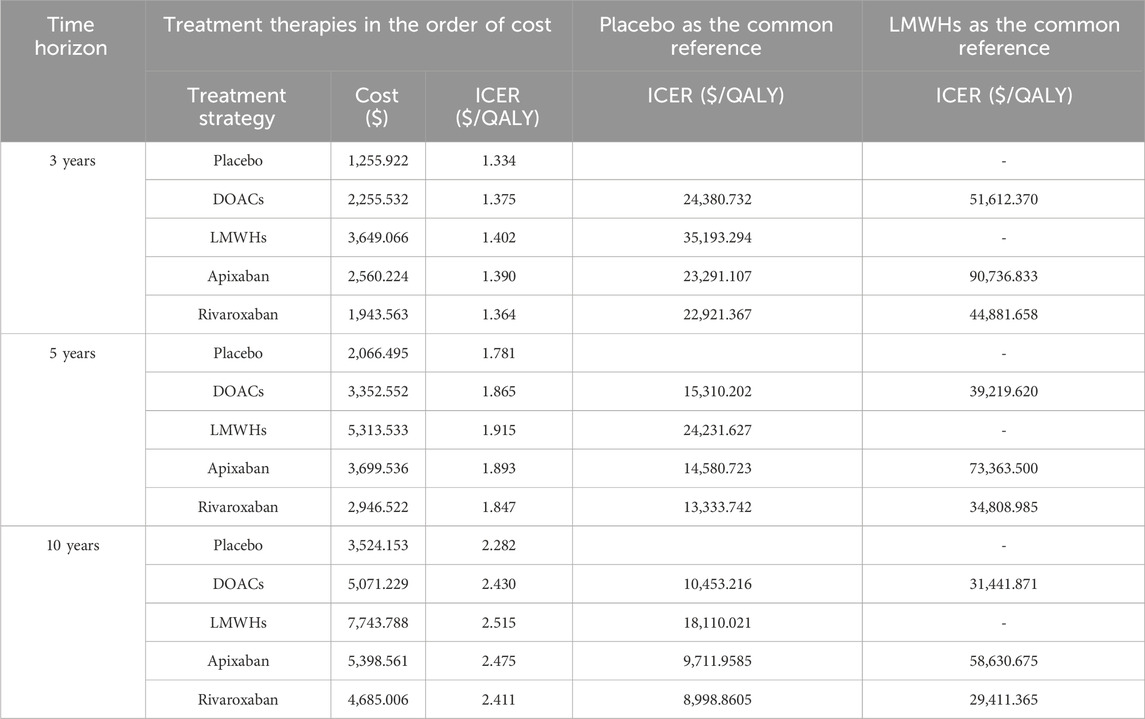
Figure 3 represents the results of PSA of DOACs, LMWHs, and no prophylaxis over a time horizon of 5-year, and detailed results are listed in Table 4. Primary prophylaxis with DOACs and LMWHs resulted in the average costs of $ 3,352.552 and $ 5,313.533, respectively, and the total cost of no prophylaxis was estimated to be $ 2,066.495. Meanwhile, the corresponding health gain was 1.865, 1.915, and 1.781 QALY, respectively. A cost-effectiveness acceptability curve was plotted to illustrate the proportion of simulations that were cost-effective at WTP values (Figure 4). Using a WTP threshold of $37,125.240, the probability of acceptance was 48% for DOACs, 45% for LMWHs, and 7% for no prophylaxis. When a 10-year time horizon was applied, these probabilities changed into 43% and 55% (Supplementary Material). For individual DOACs, the average costs, QALYs, and ICERs were also calculated in PSA. Although minimal additional costs and health gain were required, apixaban was found to be more cost-effective than rivaroxaban. These findings were closely aligned with those of the base-case analysis. Acceptable probabilities of apixaban were estimated to be 66% and 68% for the 5-year and 10-year time periods, while rivaroxaban had acceptable probabilities of 29% and 31%, respectively (Supplementary Tables S15, S16).
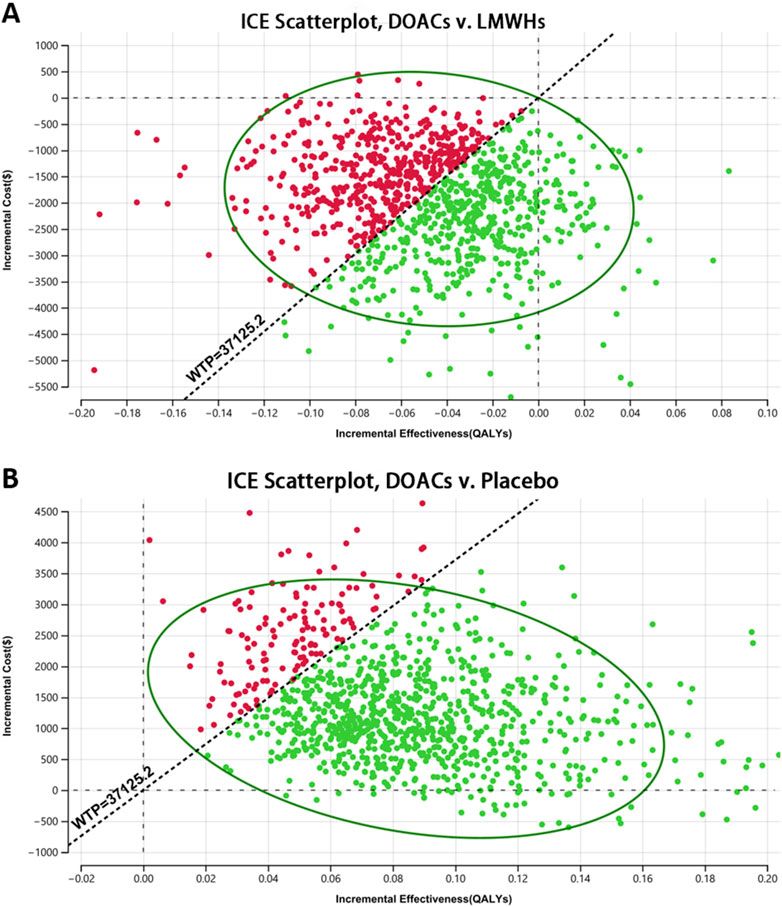
Figure 3. Scatter plot of the probabilistic sensitivity analysis (PSA) (A) DOACs vs. LMWHs and (B) DOACs vs. placebo. Scatter plot diagram illustrated the results of a 10,000-subject Monte Carlo simulation, estimating the probability of acceptance for each treatment strategy.

Figure 4. Willingness-to-pay curve estimated from a 10,000-subject Monte Carlo simulation representing the change in the probability of acceptance for three drugs as the WTP increases.
5 Discussion
VTE is a burdensome but preventable complication that frequently occurs in patients with active cancer. Given the dramatic improvement of cancer survival from targeted treatment and immunotherapy, the health and potential economic benefits of preventing serious complications have become increasingly important in the management of cancer. In this research, we focused on the primary thromboprophylaxis of VTE in cancer patients to perform a cost-effectiveness analysis comparing DOACs with traditional LMWHs and no prophylaxis comprehensively. This is the first study to achieve an indirect comparison of DOACs with a network meta-analysis approach to assess their cost-effectiveness in primary prevention based on medical costs and resources used in healthcare decisions in China.
The administration of anticoagulant prophylaxis in cancer patients is a complex issue due to the increased bleeding risk associated with tumors. For a long time, only the prevention of recurrent VTE was emphasized (de Jong et al., 2020), and the available anticoagulant drugs was limited to low-molecular-weight heparin, which has a relatively controllable risk of bleeding (Streiff et al., 2018). There is a scarcity of research on the economic evaluation of primary VTE prevention in cancer patients, particularly when comparing different types of anticoagulants. In this research, incorporating the latest RCT results and the evidence of a comprehensive systematic literature review, thromboprophylaxis with DOACs was studied in cancer patients compared with LMWHs from a pharmacoeconomic perspective. The results revealed that DOACs offered clinical benefits over those of LMWHs but at a lower cost. The estimated ICER was $43,613.184/QALYs (LMWHs vs. DOACs), indicating that DOACs are a more cost-effective option for primary VTE prevention in cancer patients in the current Chinese social environment. This result is consistent with several previous works (Lanitis et al., 2016; Heisen et al., 2017; Shin et al., 2022) that compared DOACs with LMWHs in the secondary prevention of cancer-associated thrombosis in the US healthcare system. However, another study conducted in China suggested that the cost-effectiveness of DOACs for thromboprophylaxis in patients initiating chemotherapy is unlikely (Du and Wu, 2020). The inconsistency in conclusions may arise from various factors. First, both the previous report and our research identified the price of DOACs as one of the most significant parameter inputs that have a substantial impact on the result. In our research, we applied lower prices after the patent expired in accordance with the marketing entry of generic drugs in China. Despite a slight increase in bleeding and additional costs compared to no-thrombosis prophylaxis, DOACs ultimately demonstrated their current pharmacoeconomic advantage in VTE prevention in China. Second, our model considered detailed bleeding events, such as ICH, GIB, and CRNMB. This approach aims to reflect the natural process and incorporate the best available evidence regarding the performance of thromboprophylaxis in patients with cancer. Additionally, a comprehensive analysis of 22 randomized controlled trails, including DOACs, LMWHs, and no thromboprophylaxis, was conducted in this work to assess the clinical benefits of thromboprophylaxis in the prevention of cancer-associated VTE, while evidence of LMWHs was excluded in the previous paper. We think that all the above changes might lead to a shift toward DOACs becoming the dominating strategy.
Clinical practice guidelines and data from numerous clinical trials have established that appropriate VTE prophylaxis is both safe and effective. However, practice surveys indicate that VTE prophylaxis remains under-used in cancer patients (Streiff et al., 2023). Our study highlights the cost-effectiveness of DOACs in the primary prevention of VTE in cancer patients within the Chinese healthcare context. The comparative health benefits of DOACs, combined with reduced medical costs and the convenience of oral administration, make them a viable option for widespread clinical use, potentially leading to more efficient allocation of healthcare resources. In scenario analyses simulating real therapeutic settings, long-term prophylaxis and the use of generic DOACs were found to offer additional pharmacoeconomic advantages. Subgroup analyses revealed that apixaban, while slightly increasing both health benefits and medical costs, yielded an ICER below the willingness-to-pay threshold, indicating that apixaban is the preferred drug for VTE prevention among individual DOACs. These findings provide valuable information for the management and practice of VTE prophylaxis in clinical settings.
In base-case and PSA, DOACs have been demonstrated to be more cost-effective than LMWHs. However, the results were found to be sensitive to the relative risks of VTE and bleeding, which reflect the clinical benefits and harms associated with thromboprophylaxis in the cancer population. When poor protection or high bleeding risks were estimated in DOACs, the ICERs would become favorable for LMWHs. This indicates that the additional benefits of DOACs in primary thromboprophylaxis for cancer patients may be reduced in certain tumor types, such as gastrointestinal cancer, due to the higher associated risk of bleeding. Consistent with several clinical studies (Di Nisio et al., 2016; Agnelli, 2019), our research also observed a slight improvement in mortality with DOAC thromboprophylaxis compared to LMWH. However, the increased mortality highlights the pharmacoeconomic advantage of DOACs over LMWHs in primary thrombosis prevention. Therefore, it is recommended that the optimal thrombosis prevention strategy be tailored to the specific cancer type and stage. Moreover, drug–drug interactions (DDIs) can affect the safety and efficacy profiles of drug therapy. LMWHs have fewer DDIs due to their unique metabolic pathway and mechanism of action, whereas the liver metabolism of DOACs leads to more potential DDIs during their use (Tsoukalas et al., 2022). This could lead to intricate variations in the pharmacoeconomic findings of our study, which require further exploration.
The primary strength of this study lies in its comparison of the cost-effectiveness of available DOACs (rivaroxaban and apixaban) with LMWHs and no thromboprophylaxis in VTE prevention among the cancer population in China. However, there are several intrinsic limitations. First, a comprehensive analysis of 22 randomized controlled trials was conducted to assess the clinical outcomes of thromboprophylaxis on the prevention of cancer-associated VTE. Nevertheless, the inherent heterogeneity of different studies encompassing variations in patient cohorts, types of anticoagulants, dosages, and improvements in treatment regimens, nursing, and medical management over time may impact vital clinical outcomes and introduce bias into the final results. Second, the probabilities of clinical events were estimated based on the available RCTs with follow-up periods up to 1.5 years, which may introduce a potential bias when extrapolating these inputs to a longer time horizon. Third, it is important to note that the clinical outcomes obtained from the indirect network analysis, which included the Asia–Pacific cohort, may still be affected if real-world evidence based on the Chinese population is involved. This may result in systematic bias and limit the validity of our findings. Fourth, our model only considers the discontinuation of thromboprophylaxis following a major bleeding event. Other factors such as dosage variations, patient compliance, and switching between anticoagulants were not incorporated into this study due to the lack of specific data. Additionally, it is worth noting that LMWHs have fewer DDIs than DOACs. The disparity in DDIs may result in more intricate alterations in critical clinical outcomes, potentially compromising the validity of DOACs, particularly when dealing with populations taking multiple medications in real-world settings. Furthermore, DOACs’ benefits in primary thromboprophylaxis were currently obtained from three available RCTs, involving only apixaban and rivaroxaban. Variations in safety profiles in individual DOACs have been noticed (Attard et al., 2022; Ning et al., 2023), along with differences in bleeding risk based on cancer types (Farge et al., 2022). This highlights the importance of considering cancer types and updating of evidence of more individual anticoagulants when making the decision of thromboprophylaxis.
6 Conclusion
From the perspective of the Chinese payer, primary prophylaxis with DOACs is more cost-effective than LMWHs and no thromboprophylaxis, and apixaban was the preferred drug in VTE prevention. The results were sensitive to factors such as VTE and bleeding risk, the cost of anticoagulants, the self-payment ratio in Chinese Medicare, and the overall cancer death rate.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material; further inquiries can be directed to the corresponding authors.
Author contributions
YW: writing–review and editing, writing–original draft, project administration, methodology, and investigation. TY: writing–review and editing, writing–original draft, validation, investigation, and formal analysis. GJ: writing–original draft, methodology, and data curation. TW: writing–original draft and data curation. BZ: writing–review and editing and supervision.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. The work was supported by the Scientific Research Project of Hubei Health Commission (Grant No. WJ2023F026) and the Pharmaceutical Research Capacity Building Project of Bethune Charitable Foundation (Grant No. Z04JKM2021005).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2024.1373333/full#supplementary-material
References
Agnelli, G. (2019). Direct oral anticoagulants for thromboprophylaxis in ambulatory patients with cancer. N. Engl. J. Med. 380 (8), 781–783. doi:10.1056/NEJMe1816060
Agnelli, G., Gussoni, G., Bianchini, C., Verso, M., Mandalà, M., Cavanna, L., et al. (2009). Nadroparin for the prevention of thromboembolic events in ambulatory patients with metastatic or locally advanced solid cancer receiving chemotherapy: a randomised, placebo-controlled, double-blind study. Lancet Oncol. 10 (10), 943–949. doi:10.1016/s1470-2045(09)70232-3
Akin, M., Schäfer, A., Akin, I., Widder, J., and Brehm, M. (2015). Use of new oral anticoagulants in the treatment of venous thromboembolism and thrombotic prophylaxis. Cardiovasc Hematol. Disord. Drug Targets 15 (2), 92–96. doi:10.2174/1871529x1502151209110620
Altinbas, M., Coskun, H. S., Er, O., Ozkan, M., Eser, B., Unal, A., et al. (2004). A randomized clinical trial of combination chemotherapy with and without low-molecular-weight heparin in small cell lung cancer. J. Thromb. Haemost. 2 (8), 1266–1271. doi:10.1111/j.1538-7836.2004.00871.x
Attard, L. M., Gatt, A., Bertoletti, L., Delluc, A., and Riva, N. (2022). Direct oral anticoagulants for the prevention and acute treatment of cancer-associated thrombosis. Vasc. Health Risk Manag. 18, 793–807. doi:10.2147/vhrm.S271411
Ay, C., Pabinger, I., and Cohen, A. T. (2017). Cancer-associated venous thromboembolism: burden, mechanisms, and management. Thromb. Haemost. 117 (2), 219–230. doi:10.1160/th16-08-0615
Baloch, M. F., Adepoju, A. V., Falki, V., Hajjaj, M., Habet, T., Habet, K., et al. (2023). Comparative efficacy of oral apixaban and subcutaneous low molecular weight heparins in the treatment of cancer-associated thromboembolism: a meta-analysis. Cureus 15 (8), e43447. doi:10.7759/cureus.43447
Büller, H. R., Prins, M. H., Lensin, A. W., Decousus, H., Jacobson, B. F., Minar, E., et al. (2012). Oral rivaroxaban for the treatment of symptomatic pulmonary embolism. N. Engl. J. Med. 366 (14), 1287–1297. doi:10.1056/NEJMoa1113572
Carrier, M., Abou-Nassar, K., Mallick, R., Tagalakis, V., Shivakumar, S., Schattner, A., et al. (2019). Apixaban to prevent venous thromboembolism in patients with cancer. N. Engl. J. Med. 380 (8), 711–719. doi:10.1056/NEJMoa1814468
Chen, X., Eggington, S., Wang, C., and Zhu, M. (2011). Cost-effectiveness study of rivaroxaban in the prevention of venous thromboembolism after total knee replacement. China Pharm. 22 (30), 2787–2790.
de Jong, L. A., van der Velden, A. W. G., Hulst, M. V., and Postma, M. J. (2020). Cost-effectiveness analysis and budget impact of rivaroxaban compared with dalteparin in patients with cancer at risk of recurrent venous thromboembolism. BMJ Open 10 (11), e039057. doi:10.1136/bmjopen-2020-039057
Di Nisio, M., Porreca, E., Candeloro, M., De Tursi, M., Russi, I., and Rutjes, A. W. (2016). Primary prophylaxis for venous thromboembolism in ambulatory cancer patients receiving chemotherapy. Cochrane Database Syst. Rev. 12 (12), Cd008500. doi:10.1002/14651858.CD008500.pub4
Du, J., and Wu, B. (2020). New oral anticoagulants for thromboprophylaxis in patients with cancer receiving chemotherapy: an economic evaluation in a Chinese setting. Clin. Drug Investig. 40 (7), 653–663. doi:10.1007/s40261-020-00926-2
Ek, L., Gezelius, E., Bergman, B., Bendahl, P. O., Anderson, H., Sundberg, J., et al. (2018). Randomized phase III trial of low-molecular-weight heparin enoxaparin in addition to standard treatment in small-cell lung cancer: the RASTEN trial. Ann. Oncol. 29 (2), 398–404. doi:10.1093/annonc/mdx716
Farge, D., Frere, C., Connors, J. M., Khorana, A. A., Kakkar, A., Ay, C., et al. (2022). 2022 international clinical practice guidelines for the treatment and prophylaxis of venous thromboembolism in patients with cancer, including patients with COVID-19. Lancet Oncol. 23 (7), e334–e347. doi:10.1016/s1470-2045(22)00160-7
Fox, K. A., Piccini, J. P., Wojdyla, D., Becker, R. C., Halperin, J. L., Nessel, C. C., et al. (2011). Prevention of stroke and systemic embolism with rivaroxaban compared with warfarin in patients with non-valvular atrial fibrillation and moderate renal impairment. Eur. Heart J. 32 (19), 2387–2394. doi:10.1093/eurheartj/ehr342
Geerts, W. H., Bergqvist, D., Pineo, G. F., Heit, J. A., Samama, C. M., Lassen, M. R., et al. (2008). Prevention of venous thromboembolism: American college of chest physicians evidence-based clinical practice guidelines (8th edition). Chest 133 (6 Suppl. l), 381S–453S. doi:10.1378/chest.08-0656
Girardi, L., Wang, T. F., Ageno, W., and Carrier, M. (2023). Updates in the incidence, pathogenesis, and management of cancer and venous thromboembolism. Arteriosclerosis Thrombosis Vasc. Biol. 43 (6), 824–831. doi:10.1161/atvbaha.123.318779
Haas, S. K., Freund, M., Heigener, D., Heilmann, L., Kemkes-Matthes, B., von Tempelhoff, G.-F., et al. (2012). Low-molecular-weight heparin versus placebo for the prevention of venous thromboembolism in metastatic breast cancer or stage III/IV lung cancer. Clin. Appl. Thromb. Hemost. 18 (2), 159–165. doi:10.1177/1076029611433769
Heisen, M., Treur, M. J., Heemstra, H. E., Giesen, E. B. W., and Postma, M. J. (2017). Cost-effectiveness analysis of rivaroxaban for treatment and secondary prevention of venous thromboembolism in The Netherlands. J. Med. Econ. 20 (8), 813–824. doi:10.1080/13696998.2017.1331912
Kakkar, A. K., Levine, M. N., Kadziola, Z., Lemoine, N. R., Low, V., Patel, H. K., et al. (2004). Low molecular weight heparin, therapy with dalteparin, and survival in advanced cancer: the fragmin advanced malignancy outcome study (FAMOUS). J. Clin. Oncol. 22 (10), 1944–1948. doi:10.1200/jco.2004.10.002
Khorana, A. A., Francis, C. W., Kuderer, N. M., Carrier, M., Ortel, T. L., Wun, T., et al. (2017). Dalteparin thromboprophylaxis in cancer patients at high risk for venous thromboembolism: a randomized trial. Thromb. Res. 151, 89–95. doi:10.1016/j.thromres.2017.01.009
Khorana, A. A., Soff, G. A., Kakkar, A. K., Vadhan-Raj, S., Riess, H., Wun, T., et al. (2019). Rivaroxaban for thromboprophylaxis in high-risk ambulatory patients with cancer. N. Engl. J. Med. 380 (8), 720–728. doi:10.1056/NEJMoa1814630
Kim, K., Lee, Y. J., Kim, T. H., Uhm, J. S., Pak, H. N., Lee, M. H., et al. (2018). Effect of non-vitamin K antagonist oral anticoagulants in atrial fibrillation patients with newly diagnosed cancer. Korean Circ. J. 48 (5), 406–417. doi:10.4070/kcj.2017.0328
Kimpton, M., Kumar, S., Wells, P. S., Carrier, M., and Thavorn, K. (2019). Cost-utility analysis of apixaban compared to usual care for the primary thromboprophylaxis of ambulatory cancer patients initiating chemotherapy. Blood 134 (Supplement_1), 329. doi:10.1182/blood-2019-127286
Klerk, C. P., Smorenburg, S. M., Otten, H. M., Lensing, A. W., Prins, M. H., Piovella, F., et al. (2005). The effect of low molecular weight heparin on survival in patients with advanced malignancy. J. Clin. Oncol. 23 (10), 2130–2135. doi:10.1200/jco.2005.03.134
Lanitis, T., Leipold, R., Hamilton, M., Rublee, D., Quon, P., Browne, C., et al. (2016). Cost-effectiveness of apixaban versus other oral anticoagulants for the initial treatment of venous thromboembolism and prevention of recurrence. Clin. Ther. 38 (3), 478–493. doi:10.1016/j.clinthera.2016.01.020
Lecumberri, R., López Vivanco, G., Font, A., González Billalabeitia, E., Gúrpide, A., Gómez Codina, J., et al. (2013). Adjuvant therapy with bemiparin in patients with limited-stage small cell lung cancer: results from the ABEL study. Thromb. Res. 132 (6), 666–670. doi:10.1016/j.thromres.2013.09.026
Levine, M. N., Gu, C., Liebman, H. A., Escalante, C. P., Solymoss, S., Deitchman, D., et al. (2012). A randomized phase II trial of apixaban for the prevention of thromboembolism in patients with metastatic cancer. J. Thromb. Haemost. 10 (5), 807–814. doi:10.1111/j.1538-7836.2012.04693.x
Li, A., Carlson, J. J., Kuderer, N. M., Schaefer, J. K., Li, S., Garcia, D. A., et al. (2020). Cost-effectiveness analysis of low-dose direct oral anticoagulant (DOAC) for the prevention of cancer-associated thrombosis in the United States. Cancer 126 (8), 1736–1748. doi:10.1002/cncr.32724
Macbeth, F., Noble, S., Evans, J., Ahmed, S., Cohen, D., Hood, K., et al. (2016). Randomized phase III trial of standard therapy plus low molecular weight heparin in patients with lung cancer: FRAGMATIC trial. J. Clin. Oncol. 34 (5), 488–494. doi:10.1200/jco.2015.64.0268
Martinez, C., Wallenhorst, C., Teal, S., Cohen, A. T., and Peacock, A. J. (2018). Incidence and risk factors of chronic thromboembolic pulmonary hypertension following venous thromboembolism, a population-based cohort study in England. Pulm. Circ. 8 (3), 2045894018791358. doi:10.1177/2045894018791358
Meyer, G., Besse, B., Doubre, H., Charles-Nelson, A., Aquilanti, S., Izadifar, A., et al. (2018). Anti-tumour effect of low molecular weight heparin in localised lung cancer: a phase III clinical trial. Eur. Respir. J. 52 (4), 1801220. doi:10.1183/13993003.01220-2018
Mulder, F. I., Horváth-Puhó, E., van Es, N., van Laarhoven, H. W. M., Pedersen, L., Moik, F., et al. (2021). Venous thromboembolism in cancer patients: a population-based cohort study. Blood 137 (14), 1959–1969. doi:10.1182/blood.2020007338
Nakano, Y., Adachi, S., Imai, R., Yoshida, M., Shimokata, S., Murohara, T., et al. (2021). Mortality, recurrent thromboembolism and major bleeding in cancer-associated and non-cancer pulmonary embolism patients treated with direct oral anticoagulants. Circ. J. 88, 243–250. doi:10.1253/circj.CJ-20-1247
Nielsen, P. B., Larsen, T. B., Skjøth, F., Gorst-Rasmussen, A., Rasmussen, L. H., and Lip, G. Y. (2015). Restarting anticoagulant treatment after intracranial hemorrhage in patients with atrial fibrillation and the impact on recurrent stroke, mortality, and bleeding: a nationwide cohort study. Circulation 132 (6), 517–525. doi:10.1161/circulationaha.115.015735
Ning, H., Yang, N., Ding, Y., Chen, H., Wang, L., Han, Y., et al. (2023). Efficacy and safety of direct oral anticoagulants for the treatment of cancer-associated venous thromboembolism: a systematic review and Bayesian network meta-analysis. Med. Clin. Barc. 160 (6), 245–252. doi:10.1016/j.medcli.2022.06.022
Ogino, Y., Ishigami, T., Sato, R., Nakahashi, H., Minamimoto, Y., Kimura, Y., et al. (2021). Direct oral anticoagulant therapy for isolated distal deep vein thrombosis associated with cancer in routine clinical practice. J. Clin. Med. 10 (20), 4648. doi:10.3390/jcm10204648
Ottosen, T. P., Grijota, M., Hansen, M. L., Brandes, A., Damgaard, D., Husted, S. E., et al. (2016). Use of antithrombotic therapy and long-term clinical outcome among patients surviving intracerebral hemorrhage. Stroke 47 (7), 1837–1843. doi:10.1161/strokeaha.116.012945
Pelzer, U., Opitz, B., Deutschinoff, G., Stauch, M., Reitzig, P. C., Hahnfeld, S., et al. (2015). Efficacy of prophylactic low-molecular weight heparin for ambulatory patients with advanced pancreatic cancer: outcomes from the CONKO-004 trial. J. Clin. Oncol. 33 (18), 2028–2034. doi:10.1200/jco.2014.55.1481
Perry, J. R., Julian, J. A., Laperriere, N. J., Geerts, W., Agnelli, G., Rogers, L. R., et al. (2010). PRODIGE: a randomized placebo-controlled trial of dalteparin low-molecular-weight heparin thromboprophylaxis in patients with newly diagnosed malignant glioma. J. Thromb. Haemost. 8 (9), 1959–1965. doi:10.1111/j.1538-7836.2010.03973.x
Rutjes, A. W., Porreca, E., Candeloro, M., Valeriani, E., and Di Nisio, M. (2020). Primary prophylaxis for venous thromboembolism in ambulatory cancer patients receiving chemotherapy. Cochrane Database Syst. Rev. 12 (12), Cd008500. doi:10.1002/14651858.CD008500.pub4
Schulman, S., Lindmarker, P., Holmström, M., Lärfars, G., Carlsson, A., Nicol, P., et al. (2006). Post-thrombotic syndrome, recurrence, and death 10 years after the first episode of venous thromboembolism treated with warfarin for 6 weeks or 6 months. J. Thromb. Haemost. 4 (4), 734–742. doi:10.1111/j.1538-7836.2006.01795.x
Shin, Y. E., Kumar, A., Hwang, M., Mackey, M., and Wu, W. K. (2022). Cost-utility analysis comparing direct oral anticoagulant and low molecular weight heparin therapies for secondary prevention of cancer-associated thrombosis. Clin. Drug Investig. 42 (12), 1075–1083. doi:10.1007/s40261-022-01217-8
Sideras, K., Schaefer, P. L., Okuno, S. H., Sloan, J. A., Kutteh, L., Fitch, T. R., et al. (2006). Low-molecular-weight heparin in patients with advanced cancer: a phase 3 clinical trial. Mayo Clin. Proc. 81 (6), 758–767. doi:10.4065/81.6.758
Streiff, M. B., Holmstrom, B., Angelini, D., Ashrani, A., Bockenstedt, P. L., Chesney, C., et al. (2018). NCCN guidelines insights: cancer-associated venous thromboembolic disease, version 2.2018. J. Natl. Compr. Canc Netw. 16 (11), 1289–1303. doi:10.6004/jnccn.2018.0084
Streiff, M. B., Holmstrom, B., Angelini, D., Ashrani, A., Buckner, T., Fanikos, J., et al. (2023). NCCN guidelines insights: cancer-associated venous thromboembolic disease, version 2.2018. J. Natl. Compr. Canc Netw. 16, 1289–1303. doi:10.6004/jnccn.2018.0084
Timp, J. F., Braekkan, S. K., Versteeg, H. H., and Cannegieter, S. C. (2013). Epidemiology of cancer-associated venous thrombosis. Blood 122 (10), 1712–1723. doi:10.1182/blood-2013-04-460121
Tsoukalas, N., Brito-Dellan, N., Font, C., Butler, T., Rojas-Hernandez, C. M., Butler, T., et al. (2022). Complexity and clinical significance of drug-drug interactions (DDIs) in oncology: challenging issues in the care of patients regarding cancer-associated thrombosis (CAT). Support Care Cancer 30 (10), 8559–8573. doi:10.1007/s00520-022-07235-8
Vadhanraj, S., Zhou, X., Varadhachary, G. R., Milind, J., Fogelman, D., Shroff, R., et al. (2013). Randomized controlled trial of dalteparin for primary thromboprophylaxis for venous thromboembolism (VTE) in patients with advanced pancreatic cancer (APC): risk factors predictive of VTE. Blood 122, 580. doi:10.1182/blood.v122.21.580.580
van Doormaal, F. F., Di Nisio, M., Otten, H. M., Richel, D. J., Prins, M., and Buller, H. R. (2011). Randomized trial of the effect of the low molecular weight heparin nadroparin on survival in patients with cancer. J. Clin. Oncol. 29 (15), 2071–2076. doi:10.1200/jco.2010.31.9293
Wang, H., Liao, M., Gu, A., Chen, Y., Chen, Z., and Han, B. (2005). Clinical study of anticoagulant combination with chemotherapy in the treatment of advanced non-small cell lung cancer. Tumor 25 (03), 250–253. doi:10.3781/j.issn.1000-7431.2005.03.015
Wumaier, K., Li, W., Chen, N., and Cui, J. (2021). Direct oral anticoagulants versus low molecular weight heparins for the treatment of cancer-associated thrombosis: a cost-effectiveness analysis. Thromb. J. 19 (1), 68. doi:10.1186/s12959-021-00319-1
Yang, L., and Wu, J. (2020). Cost-effectiveness of rivaroxaban compared with enoxaparin plus warfarin for the treatment of hospitalised acute deep vein thrombosis in China. BMJ Open 10 (7), e038433. doi:10.1136/bmjopen-2020-038433
Zeng, H., Zheng, R., Sun, K., Zhou, M., Wang, S., Li, L., et al. (2024). Cancer survival statistics in China 2019–2021: a multicenter, population-based study. J. Natl. Cancer Cent 4 (3), 203–213. doi:10.1016/j.jncc.2024.06.005
Zhang, Y., Jia, X., Zhao, J., and Ma, L. (2013). Clinical research on low molecular heparin in the prevention of venous thromboembolism of patients with lung cancer received chemotherapy. J. Clin. Pulm. Med. 18 (11), 2082–2084. doi:10.3969/j.issn.1009-6663.2013.11.067
Zhu, M., Zhang, H., Wang, J., Feng, F., and Zhu, H. (2014). Clinical study of low molecular weight heparin combined with chemotherapy in the treatment of advanced gastric cancer. Mod. J. Integr. Traditional Chin. West. Med. 23 (04), 378–380.
Zwicker, J. I., Liebman, H. A., Bauer, K. A., Caughey, T., Campigotto, F., Rosovsky, R., et al. (2013). Prediction and prevention of thromboembolic events with enoxaparin in cancer patients with elevated tissue factor-bearing microparticles: a randomized-controlled phase II trial (the Microtec study). Br. J. Haematol. 160 (4), 530–537. doi:10.1111/bjh.12163
Keywords: primary thromboprophylaxis, direct oral anticoagulants, cost-effectiveness, cancer-associated venous thromboembolism, low-molecular-weight heparin
Citation: Wu Y, Yin T, Jian G, Wan T and Zhou B (2024) Cost-effectiveness analysis of direct oral anticoagulants versus low-molecular-weight heparin and no thromboprophylaxis in primary prevention of cancer-associated venous thromboembolism in China. Front. Pharmacol. 15:1373333. doi: 10.3389/fphar.2024.1373333
Received: 19 January 2024; Accepted: 19 August 2024;
Published: 23 September 2024.
Edited by:
Heike Wulff, University of California, Davis, United StatesReviewed by:
Zhenguo Zhai, China-Japan Friendship Hospital, ChinaHanno Riess, Charité University Medicine Berlin, Germany
Copyright © 2024 Wu, Yin, Jian, Wan and Zhou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yue Wu, bWF5bW9vbkB3aHUuZWR1LmNu; Benhong Zhou, YmVuaG9uZ3poQHdodS5lZHUuY24=
†These authors have contributed equally to this work
 Yue Wu
Yue Wu TianChen Yin
TianChen Yin GuiLin Jian1,2
GuiLin Jian1,2 Tao Wan
Tao Wan