
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Pharmacol. , 25 June 2024
Sec. Renal Pharmacology
Volume 15 - 2024 | https://doi.org/10.3389/fphar.2024.1372421
 Qian Wang1†
Qian Wang1† Jianmin Yu2†
Jianmin Yu2† Weizhu Deng1†
Weizhu Deng1† Chao Liu3
Chao Liu3 Jian Yang1
Jian Yang1 Yaqing Li1
Yaqing Li1 Guangyan Cai1
Guangyan Cai1 Xiangmei Chen1
Xiangmei Chen1 Zheyi Dong1*
Zheyi Dong1*Background: Sodium/glucose cotransporter-2 inhibitors (SGLT2i) are associated with cardiovascular benefits. The aim of this systematic review and meta-analysis is to summarize the influence of SGLT2i on the incidence of acute kidney injury (AKI), and to ascertain whether it is affected by confounding variables such as age, baseline renal function and concurrent use of renin-angiotensin-aldosterone system inhibitors (RAASi) or mineralocorticoid receptor antagonists (MRA).
Methods: PubMed, Embase, and Cochrane Library databases were searched for randomized controlled trials comparing the influence of SGLT2i versus placebo/blank treatment on AKI in the adult population. A fixed-effect model was used if the heterogeneity was not significant; otherwise, a randomized-effect model was used.
Results: Eighteen studies comprising 98,989 patients were included. Compared with placebo/blank treatment, treatment with SGLT2i significantly reduced the risk of AKI (risk ratio [RR]: 0.78, 95% confidence interval [CI]: 0.71 to 0.84, p < 0.001; I2 = 0%). Subgroup analysis suggested consistent results in patients with diabetes, chronic kidney disease, and heart failure (for subgroup difference, p = 0.32). Finally, univariate meta-regression suggested that the influence of SGLT2i on the risk of AKI was not significantly modified by variables such as age (coefficient: 0.011, p = 0.39), baseline estimated glomerular filtration rate (coefficient: −0.0042, p = 0.13) or concomitant use of RAASi (coefficient: 0.0041, p = 0.49) or MRA (coefficient: −0.0020, p = 0.34).
Conclusion: SGLT2i may be effective in reducing the risk of AKI, and the effect might not be modified by age, baseline renal function and concurrent use of RAASi or MRA.
Sodium/glucose cotransporter-2 inhibitors (SGLT2i) represent a novel class of oral antidiabetic medications that have demonstrated additional advantageous effects on cardiac and renal function (Frak et al., 2023; Klen and Dolzan, 2023; Lam-Chung, 2023). From a pharmacological standpoint, SGLT2i functions by inhibiting the reabsorption of glucose in the initial proximal tubule of the kidney, thereby augmenting the excretion of glucose in the urine and reducing the overall glucose burden on the body (Vallon and Verma, 2021). In individuals diagnosed with type 2 diabetes mellitus (T2DM), an initial meta-analysis of three extensive clinical trials revealed that the utilization of SGLT2i was associated with an 11% decrease in the risk of major adverse cardiovascular events, a 23% decrease in the risk of cardiovascular death or hospitalization for heart failure (HF), and a 45% decrease in the risk of progression of renal disease (Zelniker et al., 2019). In a study involving patients with HF, it was demonstrated that SGLT2i effectively reduced the likelihood of cardiovascular death and hospitalizations for HF across a diverse range of patients, thus establishing their significance as a fundamental therapy for HF, regardless of ejection fraction or care setting (Vaduganathan et al., 2022). Furthermore, a recent meta-analysis encompassing 13 clinical trials revealed that SGLT2i exhibited efficacy in altering the risk of kidney disease progression, not only in patients with T2DM at high cardiovascular risk, but also in patients with chronic kidney disease (CKD) or HF regardless of diabetic status (2022). Consequently, the indications for SGLT2i have expanded beyond T2DM to include HF and CKD, supported by accumulating evidence. Nevertheless, conflicting findings have emerged regarding the potential occurrence of acute kidney injury (AKI) when utilizing SGLT2i (Copur et al., 2023). One case report documented a dialysis-dependent AKI following the initiation of SGLT2i, with a suggested association to osmotic nephropathy (Phadke et al., 2020). Furthermore, a recent investigation utilizing the most up-to-date records from the United States Food and Drug Administration’s Adverse Event Reporting System has indicated a potential link between SGLT2i and the development of AKI, although this association may be mitigated in instances where renin-angiotensin-aldosterone system inhibitors (RAASi), such as angiotensin converting enzyme inhibitors (ACEI) or angiotensin II receptor blockers (ARB), are concurrently administered (Katsuhara and Ikeda, 2021). Nevertheless, it is worth noting that several observational studies have failed to demonstrate an elevated risk of AKI associated with the use of SGLT2 inhibitors (Rampersad et al., 2020; Zhuo et al., 2022). In order to conduct a comprehensive assessment of the impact of SGLT2i on the occurrence of AKI, we conducted a systematic review and meta-analysis of eligible randomized controlled trials (RCTs). Furthermore, we investigated whether the effect of SGLT2i on the risk of AKI could be influenced by study-specific factors such as age, baseline renal function, and concurrent use of RAASi at the study level.
This study was designed and implemented according to the Cochrane Handbook guidelines (Higgins et al., 2021) and the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) statement (Page et al., 2021a; Page et al., 2021b).
A combination of strategies was used to search PubMed, Embase, and Cochrane Library for relevant studies with: (1) “SGLT 2 inhibitor” OR “SGLT-2 inhibitor” OR “SGLT2” OR “sodium glucose transporter 2 inhibitor” OR “sodium glucose transporter ii inhibitor” OR “sodium glucose cotransporter 2 inhibitors” OR “dapagliflozin” OR “canagliflozin” OR “tofogliflozin” OR “bexagliflozin” OR “empagliflozin” OR “luseogliflozin” OR “remogliflozin” OR “ertugliflozin” OR “henagliflozin” OR “ipragliflozin” OR “licogliflozin” OR “sergliflozin” OR “sotagliflozin”; (2) “acute” OR “abrupt”; (3) “kidney” OR “renal”; and (4) “random” OR “randomly” OR “randomized” OR “control” OR “placebo”. Relevant clinical studies have been limited to humans. We also manually searched for reference lists to review and original articles that were related to the topic. Database searches were conducted on 14 May 2024.
Studies were included if they fulfilled the following criteria according to the PICOS principles.
P (patients): adult patient population without limitations of the diagnosis, which could be patients with T2DM, CKD, or HF.
I (intervention): SGLT2i.
C (control): placebo or blank treatment.
O (outcome): incidence of AKI compared between patients with SGLT2i and controls during follow-up. The diagnosis of AKI was in accordance with the criteria used among the original studies
S (study design): parallel-group RCTs, published as full-length articles in peer-reviewed journals.
Non-RCTs, studies that did not include an intervention group of SGLT2i, those comparing the effects of different doses of SGLT2i, single-arm studies without controls, or studies not evaluating the outcome of AKI were excluded. For studies with overlapping patients, the study with the largest sample size was included.
The process of data extraction, mining, and quality evaluations was carried out by two authors working independently. In the event of any disagreement, the corresponding author was consulted to address and resolve such inconsistencies. Information regarding publication details (author, year of publication, and study country), study design (blind or open-label), patient characteristics (diagnosis, demographic information, baseline renal function as evaluated by estimated glomerular filtration rate (eGFR), proportions of patients with concurrent use of any RAASi, and proportions of patients with concurrent use of mineralocorticoid receptor antagonists [MRA]), details of interventions and controls, follow-up durations, and diagnostic criteria for AKI was extracted. The quality of RCTs was assessed utilizing the Cochrane Risk of Bias Tool (Higgins et al., 2021), adhering to the subsequent criteria: (1) random sequence generation; (2) allocation concealment; (3) participant and staff blinding; (4) outcome assessor blinding; (5) presentation of incomplete outcome data; (6) reporting of selective results; and (7) identification of other potential biases.
The numbers of patients with AKI events and total numbers of patients allocated to the SGLT2i and control groups were extracted from the original reports. The influence of SGLT2i on the incidence of AKI in adult patients compared to control was summarized as risk ratio (RR) and corresponding 95% confidence intervals (CIs). The Cochrane Q test was performed (Higgins and Thompson, 2002). Heterogeneity was also estimated by calculating I2 and I2 > 50% suggested significant heterogeneity (Higgins et al., 2003). In the pooled analyses, a random-effects model was employed when significant heterogeneity was identified; alternatively, a fixed-effects model was utilized (Higgins et al., 2021). A sensitivity analysis was performed by only including high-quality studies (all seven domains of Cochrane Risk of Bias Tool judged as low risk). Additionally, a predefined subgroup analysis was conducted based on the patients’ diagnosis and the specific SGLT2i drugs administered. Furthermore, a univariate meta-regression analysis was conducted to investigate whether the study characteristics of continuous variables could significantly alter the impact of SGLT2i on AKI, such as mean age of the patients, proportion of men, mean eGFR at baseline, proportion of patients using any RAASi, proportion of patients using MRA, and mean follow-up duration of the study. Publication bias was assessed using funnel plots and Egger’s regression asymmetry test (Egger et al., 1997). Statistical significance was defined as p < 0.05. The statistical analysis was performed using Stata software (version 12.0; Stata Corporation) and RevMan (version 5.1; Cochrane, Oxford, United Kingdom).
A diagram illustrating the process of database searching and study identification is presented in Figure 1. The search of the databases yielded a total of 798 articles, of which 599 were identified as unique after removing duplicates. Subsequently, 557 articles were excluded based on their title and abstract, primarily due to their lack of relevance to the research objectives. A thorough examination of the full text was conducted on 42 articles, resulting in the exclusion of 24 articles for the reasons depicted in Figure 1. Ultimately, the final analysis encompassed a total of 18 RCTs (Zinman et al., 2015; Neal et al., 2017; McMurray et al., 2019; Perkovic et al., 2019; Wiviott et al., 2019; Cannon et al., 2020; Heerspink et al., 2020; Bhatt et al., 2021a; Anker et al., 2021; Bhatt et al., 2021b; Kosiborod et al., 2021; Zannad et al., 2021; Solomon et al., 2022; Voors et al., 2022; Feitosa et al., 2023; Herrington et al., 2023; Butler et al., 2024; Cox et al., 2024).
An overview of the included studies is presented in Table 1. Since one of the included studies reported the outcome according to different doses of SGLT2i, and the other one reported the outcome according to whether the patients were with CKD, these datasets were included independently in the meta-analysis. Overall, 20 datasets from 18 RCTs (Zinman et al., 2015; Neal et al., 2017; McMurray et al., 2019; Perkovic et al., 2019; Wiviott et al., 2019; Cannon et al., 2020; Heerspink et al., 2020; Bhatt et al., 2021a; Anker et al., 2021; Bhatt et al., 2021b; Kosiborod et al., 2021; Zannad et al., 2021; Solomon et al., 2022; Voors et al., 2022; Feitosa et al., 2023; Herrington et al., 2023; Butler et al., 2024; Cox et al., 2024) involving 98,989 patients were included. Generally, patients with T2DM, CKD, HF, acute myocardial infarction, and hospitalized patients with COVID-19 were included. The mean ages of the patients were 61–72 years, with the baseline mean eGFR varying from 37 to 85 mL/min/1.73 m2. In the intervention group, SGLT2i including empagliflozin, canagliflozin, dapagliflozin, ertugliflozin, and sotagliflozin were used. The follow-up durations were from 1 to 50 months. As for the diagnosis for AKI, the Medical Dictionary for Regulatory Activities (MDRA) preferred term for AKI was used for most of the included studies (Zinman et al., 2015; Neal et al., 2017; McMurray et al., 2019; Perkovic et al., 2019; Wiviott et al., 2019; Cannon et al., 2020; Bhatt et al., 2021a; Anker et al., 2021; Bhatt et al., 2021b; Kosiborod et al., 2021; Zannad et al., 2021; Solomon et al., 2022; Voors et al., 2022; Butler et al., 2024; Cox et al., 2024), while for the other studies, a doubling (Heerspink et al., 2020) or a 1.5-times increment of serum creatinine (Herrington et al., 2023) or the Kidney Disease Improving Global Outcomes criteria (Feitosa et al., 2023) were used. According to Table 2, the quality of each included RCTs was assessed according to the Cochrane Risk of Bias Tool. Most of the included studies were double-blind placebo controlled studies (Zinman et al., 2015; Neal et al., 2017; McMurray et al., 2019; Perkovic et al., 2019; Wiviott et al., 2019; Cannon et al., 2020; Heerspink et al., 2020; Bhatt et al., 2021a; Anker et al., 2021; Bhatt et al., 2021b; Kosiborod et al., 2021; Zannad et al., 2021; Solomon et al., 2022; Voors et al., 2022; Herrington et al., 2023; Butler et al., 2024) with adequate report of details of random sequence generation and allocation concealment. Only two studies were open-label studies (Feitosa et al., 2023; Cox et al., 2024), with no detailed report of random sequence generation or allocation concealment.
Overall, 20 datasets from 18 RCTs, involving 52,773 patients receiving SGLT2i and 46,216 patients receiving placebo/blank treatment, were included in the meta-analysis. All the RRs and 95% CI were extracted from the original studies except data for one study (Anker et al., 2021), which was extracted from a previous meta-analysis after being provided directly to the authors (2022). Compared with placebo/blank treatment, treatment with SGLT2i significantly reduced the risk of AKI (RR: 0.78, 95% CI: 0.71 to 0.84, p < 0.001; Figure 2A) with no significant heterogeneity (for Cochrane Q test, p = 0.49; I2 = 0%). The sensitivity analysis limited to high-quality studies (Zinman et al., 2015; Neal et al., 2017; McMurray et al., 2019; Perkovic et al., 2019; Wiviott et al., 2019; Cannon et al., 2020; Heerspink et al., 2020; Bhatt et al., 2021a; Anker et al., 2021; Bhatt et al., 2021b; Kosiborod et al., 2021; Zannad et al., 2021; Solomon et al., 2022; Voors et al., 2022; Herrington et al., 2023; Butler et al., 2024) showed similar results (RR: 0.77, 95% CI: 0.71 to 0.84, p < 0.001; Figure 2B). Subgroup analysis suggested consistent results in patients with T2DM (RR: 0.82, 95% CI: 0.73 to 0.92, p < 0.001; I2 = 4%), CKD (RR: 0.87, 95% CI: 0.76 to 0.99, p = 0.04; I2 = 0%), and HF (RR: 0.74, 95% CI: 0.63 to 0.87, p < 0.001; I2 = 11%; p for subgroup difference = 0.32; Figure 3). In addition, subgroup analysis also did not suggest that the results were significantly affected by individual SGLT2i drugs used (p for subgroup difference = 0.09; Figure 4). Finally, univariate meta-regression with a random-effects model suggested that the influence of SGLT2i on the risk of AKI was not significantly modified by study characteristics such as mean age of the patients, proportion of men, baseline mean eGFR, proportion of patients with concomitant use of RAASi, proportion of patients with concomitant use of MRA, or mean follow-up duration (p all > 0.05; Table 3).
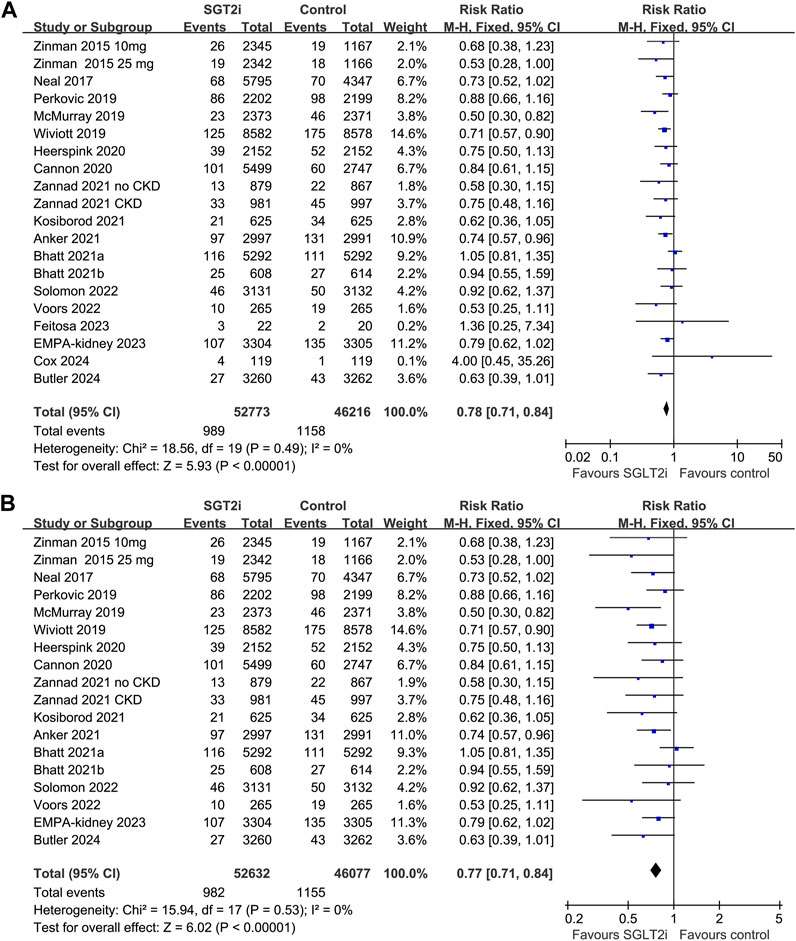
Figure 2. Forest plots for the meta-analysis of the influence of SGLT2i on the risk of AKI in adult patients; (A) the overall meta-analysis; and (B) the sensitivity analysis limited to high-quality studies.
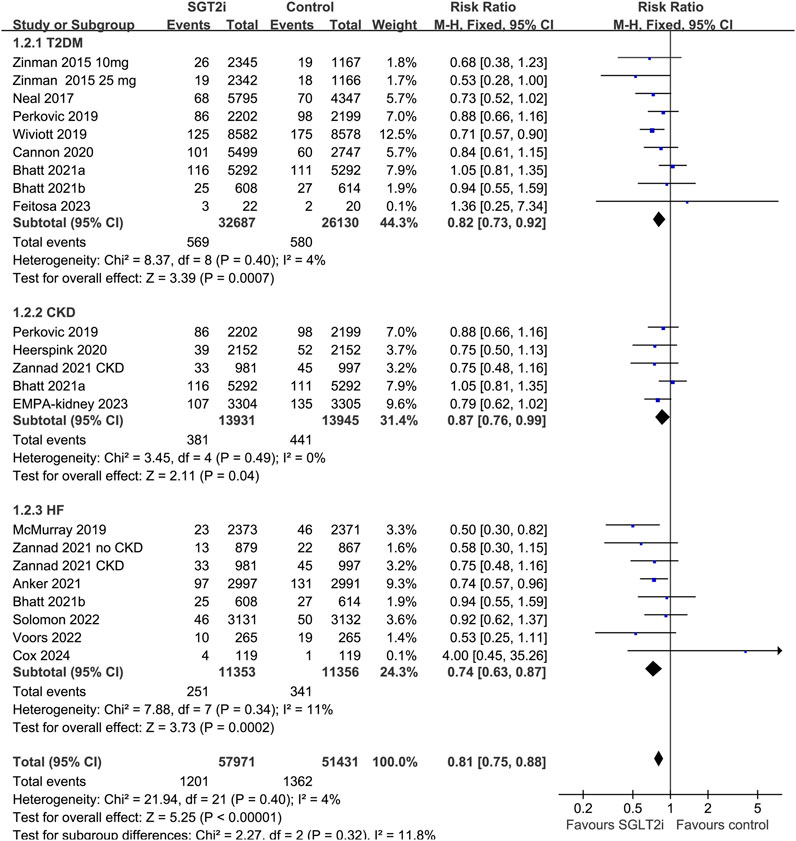
Figure 3. Forest plots for the subgroup analysis of the influence of SGLT2i on the risk of AKI according to the diagnosis of the patients.
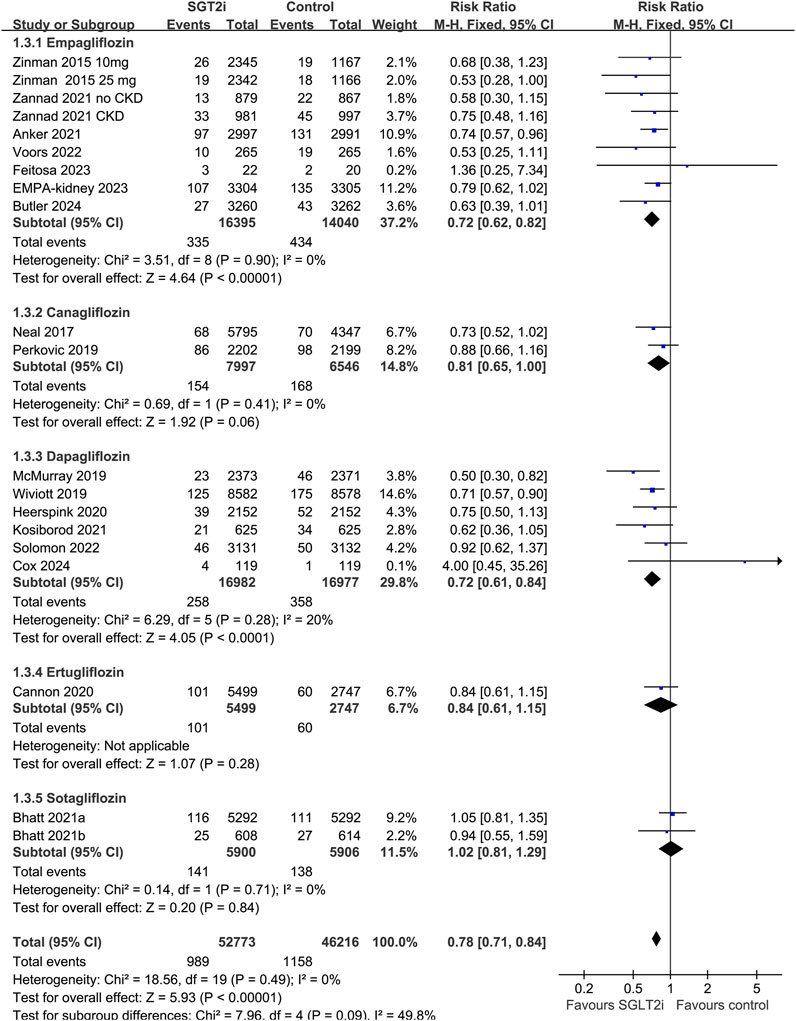
Figure 4. Forest plots for the subgroup analysis of the influence of SGLT2i on the risk of AKI according to individual SGLT2i drugs used.
The symmetrical funnel plots observed in the meta-analyses of the impact of SGLT2i on AKI in adult patients indicate a minimal likelihood of publication bias (Figure 5). Furthermore, the results of Egger’s regression test support this notion, as it yielded a p-value of 0.32, indicating a low risk of publication bias.
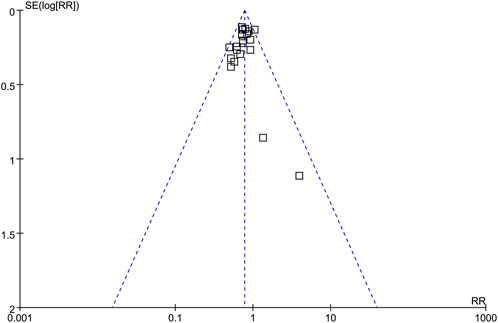
Figure 5. Funnel plots for the publication bias underlying the meta-analysis of the influence of SGLT2i on the risk of AKI in adult patients.
In this meta-analysis, we conducted a comprehensive synthesis of data from 18 RCTs, comprising 20 datasets. The findings of our study indicate a significant reduction in the risk of AKI among adult patients when comparing the use of SGLT2i to placebo or blank treatment. The sensitivity analysis limited to high-quality RCTs showed similar results. Subgroup analyses further demonstrated consistent results in patients with T2DM, CKD, and HF. Additionally, our subgroup analysis suggests that the impact of SGLT2i on AKI does not appear to be influenced by the specific type of SGLT2i utilized. Finally, meta-regression analysis suggested that the influence of SGLT2i on AKI was also not likely to be modified by difference of study characteristics, such as mean age of the patients, proportion of men, mean baseline eGFR, proportions of patients with concomitant use of RAASi and MRA, or follow-up durations. Taken together, these results indicate that SGLT2i may be effective in reducing the risk of AKI, and the effect of SGLT2i on AKI may not be influenced by the baseline renal function or concurrent use of RAASi.
Some meta-analyses were published before or during the preparation this manuscript, which generally showed that SGLT2 inhibitors can exert the benefit in reducing AKI in patients with T2D, heart failure, or CKD; and this benefit does not vary with various characteristics, such as the diagnosis of the patients and type of SGLT2 inhibitors (Menne et al., 2019; Neuen et al., 2019; Zhao et al., 2020; Qiu et al., 2021; Baigent et al., 2022; Gong et al., 2022; Rigato et al., 2023). Compared to the previous meta-analyses, our study has several strengths. First, an extensive literature search was performed which retrieved 18 relevant up-to-date RCTs. Second, only RCTs were included, which minimized the biases related to the design of observational studies. In addition, although the results of the overall and subgroup analyses were generally consistent with the findings of the previous meta-analyses, we for the first time performed meta-regression analyses to investigate the potential influence of study characteristics such as age, baseline renal function, and concurrent use of RAASi at the study level. This is clinically important, because these factors have been related to the risk of AKI. Overall, results of the meta-analysis provided further evidence that SGLT2i may be effective in reducing the risk of AKI, and the effect might not be modified by age, baseline renal function and concurrent use of RAASi or MRA.
Although concerns have been raised reading AKI related to SGLT2i use in some case reports, subsequent investigations in high quality clinical trials and meta-analysis showed that SGLT2i may confer renal proactive efficacy and delay the deterioration of renal function (Lin et al., 2023). The current meta-analysis, by integrating the evidence from RCTs, further expanded the renal benefits of SGLT2i by showing that SGLT2i are effective in reducing the risk of AKI as compared to placebo/blank treatment. The potential mechanisms underlying the renal protective effect of SGLT2i may be multifactorial. An initial investigation conducted on non-diabetic mice using a renal ischemia/reperfusion injury model demonstrated that Luseogliflozin effectively mitigated peritubular capillary congestion/hemorrhage, alleviated hypoxia, and enhanced the expression of vascular endothelial growth factor (VEGF)-A, thereby exhibiting a protective effect on the kidneys during acute situations (Zhang et al., 2018). Furthermore, another study conducted on diabetic rats with myocardial infarction-associated AKI revealed that pretreatment with empagliflozin for 2 weeks resulted in improved hyperglycemia, elevated blood β-hydroxybutyrate levels, suppressed expression of NGAL and KIM-1 induced by MI, and ultimately prevented the pathogenesis of AKI (Kuno et al., 2020). Furthermore, previous research has demonstrated the significant reduction of both systemic and renal inflammation by empagliflozin, which has contributed to the observed survival benefits in an LPS-model of acute septic renal injury (Maayah et al., 2021). Additionally, a more recent study has indicated that dapagliflozin may mitigate contrast-induced acute kidney injury through the suppression of the hypoxia-inducible factor-1α pathway (Huang et al., 2022). Consequently, there is a need for further investigation into the key molecular pathways that underlie the preventive effectiveness of SGLT2i on AKI.
Results of subgroup analysis suggested that although no significant difference was observed for the influence of each individual SGLT2i drugs on AKI, the positive results were mainly driven by studies involving empagliflozin, canagliflozin, and dapagliflozin, but not for studies with ertugliflozin or sotagliflozin. However, these results should be interpreted with caution because only two datasets were available for the subgroups of ertugliflozin and sotagliflozin, and more studies are needed for further evaluation. Interestingly, results of meta-regression analysis suggested that the effect of SGLT2i on AKI did not seem to be significantly affected by eGFR at baseline, suggesting that potential renal protective efficacy of SGLT2i may also be consistent in patients with renal dysfunction before treatment (eGFR as low as 20 mL/min/1.73 m2). In addition, it has been suggested that excessive decline by SGLT2i combined with the excessive decline in trans-glomerular pressure induced by concomitant use of RAASi may further increase the risk of AKI (Szalat et al., 2018). Accordingly, we explored the influence of proportions of patients with concurrent use of RAASi and MRA on the effect of SGLT2i on AKI. Results suggested that the potential renal protective efficacy of SGLT2i may not be significantly modified by concurrent use of RAASi or MRA. This is consistent with a recently published post hoc analysis which showed that dapagliflozin consistently reduced the risk of kidney outcomes in T2DM patients irrespective of background use of various cardiovascular medications (Oyama et al., 2022). However, our results of meta-regression analysis according to baseline renal function and concurrent use of RAASi should be considered as exploring study because these results were based on the analysis of study-level data rather than individual-patient data.
This meta-analysis also has limitations. First, different SGLT2i drugs with different dosages were used among the included studies. Further studies are needed to determine if the influence of SGLT2i on AKI is consistent among individual SGLT2i drugs, and if there is a dose-effect relationship. Second, key aspects such as diabetes severity and duration, CKD, or HF, which have potential implications on SGLT2i effectiveness, may affect the influence of SGLT2i on AKI. Although, our meta-analysis is based on data at the study level rather than individual patient level; therefore, we were unable to determine the influence of these factors on the results. In addition, there are other medications which may also affect the risk of AKI besides RAASi and MRA, such as nonsteroidal anti-inflammatory drugs (NSAIDs). However, the status of NSAIDs use was generally not reported among the included studies, and we were therefore unable to determine its influence on the results of the meta-analysis. Moreover, we only included studies published in English as full-length article in peer-reviewed journals. Grey literature, such as conference abstracts and unpublished data were not included. Although excluding grey literature may improve the reliability of the finding because most grey literature are not strictly peer-reviewed, excluding these data may also increase the risk of publication bias. Finally, for most of the included studies, AKI was diagnosed based on MDRA preferred term for AKI. The influence of different diagnostic criteria for AKI, particularly those applicable in real-world clinical practice needs to be further evaluated.
As a summary, results of the meta-analysis suggest that SGLT2i may be effective in reducing the risk of AKI as compared to placebo/blank treatment in adult patients, and the influence of SGLT2i on AKI may not be affected by baseline renal function and concurrent use of RAASi.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
QW: Conceptualization, Formal Analysis, Writing–original draft. JY: Conceptualization, Formal Analysis, Writing–review and editing. WD: Conceptualization, Writing–review and editing. CL: Writing–review and editing, Resources. JY: Resources, Writing–review and editing. YL: Writing–review and editing, Data curation. GC: Data curation, Writing–review and editing. XC: Data curation, Writing–review and editing. ZD: Writing–review and editing, Conceptualization.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study was supported by Beijing Natural Science Foundation (Nos. L232122 and L222133), National Natural Science Foundation of China (No. 62250001), and Science and Technology Project of Beijing (No. Z221100007422121).
We thank Medjaden Inc. for its assistance in the preparation of this manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Anker, S. D., Butler, J., Filippatos, G., Ferreira, J. P., Bocchi, E., Bohm, M., et al. (2021). Empagliflozin in heart failure with a preserved ejection fraction. N. Engl. J. Med. 385 (16), 1451–1461. doi:10.1056/NEJMoa2107038
Baigent, C., Emberson, J. R., Haynes, R., Herrington, W. G., Judge, P., Landray, M. J., and et al., (2022). Impact of diabetes on the effects of sodium glucose co-transporter-2 inhibitors on kidney outcomes: collaborative meta-analysis of large placebo-controlled trials. Lancet 400 (10365), 1788–1801. doi:10.1016/S0140-6736(22)02074-8
Bhatt, D. L., Szarek, M., Pitt, B., Cannon, C. P., Leiter, L. A., McGuire, D. K., et al. (2021a). Sotagliflozin in patients with diabetes and chronic kidney disease. N. Engl. J. Med. 384 (2), 129–139. doi:10.1056/NEJMoa2030186
Bhatt, D. L., Szarek, M., Steg, P. G., Cannon, C. P., Leiter, L. A., McGuire, D. K., et al. (2021b). Sotagliflozin in patients with diabetes and recent worsening heart failure. N. Engl. J. Med. 384 (2), 117–128. doi:10.1056/NEJMoa2030183
Butler, J., Jones, W. S., Udell, J. A., Anker, S. D., Petrie, M. C., Harrington, J., et al. (2024). Empagliflozin after acute myocardial infarction. N. Engl. J. Med. 390 (16), 1455–1466. doi:10.1056/NEJMoa2314051
Cannon, C. P., Pratley, R., Dagogo-Jack, S., Mancuso, J., Huyck, S., Masiukiewicz, U., et al. (2020). Cardiovascular outcomes with ertugliflozin in type 2 diabetes. N. Engl. J. Med. 383 (15), 1425–1435. doi:10.1056/NEJMoa2004967
Copur, S., Yildiz, A., Basile, C., Tuttle, K. R., and Kanbay, M. (2023). Is there any robust evidence showing that SGLT2 inhibitor use predisposes to acute kidney injury? J. Nephrol. 36 (1), 31–43. doi:10.1007/s40620-022-01422-w
Cox, Z. L., Collins, S. P., Hernandez, G. A., McRae, A. T., Davidson, B. T., Adams, K., et al. (2024). Efficacy and safety of dapagliflozin in patients with acute heart failure. J. Am. Coll. Cardiol. 83 (14), 1295–1306. doi:10.1016/j.jacc.2024.02.009
Egger, M., Davey Smith, G., Schneider, M., and Minder, C. (1997). Bias in meta-analysis detected by a simple, graphical test. BMJ 315 (7109), 629–634. doi:10.1136/bmj.315.7109.629
Feitosa, M. P. M., Lima, E. G., Abizaid, A. A. C., Mehran, R., Lopes, N. H. M., de Assis Fischer Ramos, T., et al. (2023). The safety of SGLT-2 inhibitors in diabetic patients submitted to elective percutaneous coronary intervention regarding kidney function: SAFE-PCI pilot study. Diabetol. Metab. Syndr. 15 (1), 138. doi:10.1186/s13098-023-01107-9
Frak, W., Hajdys, J., Radzioch, E., Szlagor, M., Mlynarska, E., Rysz, J., et al. (2023). Cardiovascular diseases: therapeutic potential of SGLT-2 inhibitors. Biomedicines 11 (7), 2085. doi:10.3390/biomedicines11072085
Gong, C., Shen, S. C., Zhang, K., Zhou, L., Shen, J. J., Zhao, J. Y., et al. (2022). Association of sodium-glucose cotransporter 2 inhibitors with cardiovascular outcome and safety events: a meta-analysis of randomized controlled clinical trials. Front. Cardiovasc Med. 9, 926979. doi:10.3389/fcvm.2022.926979
Heerspink, H. J. L., Stefansson, B. V., Correa-Rotter, R., Chertow, G. M., Greene, T., Hou, F. F., et al. (2020). Dapagliflozin in patients with chronic kidney disease. N. Engl. J. Med. 383 (15), 1436–1446. doi:10.1056/NEJMoa2024816
Herrington, W. G., Staplin, N., Wanner, C., Green, J. B., Hauske, S. J., Emberson, J. R., et al. (2023). Empagliflozin in patients with chronic kidney disease. N. Engl. J. Med. 388 (2), 117–127. doi:10.1056/NEJMoa2204233
Higgins, J., Thomas, J., Chandler, J., Cumpston, M., Li, T., Page, M., et al. (2021). Cochrane Handbook for systematic reviews of interventions version 6.2 (London, United Kingdom: The Cochrane Collaboration). Available at: www.training.cochrane.org/handbook.
Higgins, J. P., and Thompson, S. G. (2002). Quantifying heterogeneity in a meta-analysis. Stat. Med. 21 (11), 1539–1558. doi:10.1002/sim.1186
Higgins, J. P., Thompson, S. G., Deeks, J. J., and Altman, D. G. (2003). Measuring inconsistency in meta-analyses. BMJ 327 (7414), 557–560. doi:10.1136/bmj.327.7414.557
Huang, X., Guo, X., Yan, G., Zhang, Y., Yao, Y., Qiao, Y., et al. (2022). Dapagliflozin attenuates contrast-induced acute kidney injury by regulating the HIF-1α/HE4/NF-κB pathway. J. Cardiovasc Pharmacol. 79 (6), 904–913. doi:10.1097/FJC.0000000000001268
Katsuhara, Y., and Ikeda, S. (2021). Correlations between SGLT-2 inhibitors and acute renal failure by signal detection using FAERS: stratified analysis for reporting country and concomitant drugs. Clin. Drug Investig. 41 (3), 235–243. doi:10.1007/s40261-021-01006-9
Klen, J., and Dolzan, V. (2023). SGLT2 inhibitors in the treatment of diabetic kidney disease: more than just glucose regulation. Pharmaceutics 15 (7), 1995. doi:10.3390/pharmaceutics15071995
Kosiborod, M. N., Esterline, R., Furtado, R. H. M., Oscarsson, J., Gasparyan, S. B., Koch, G. G., et al. (2021). Dapagliflozin in patients with cardiometabolic risk factors hospitalised with COVID-19 (DARE-19): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Diabetes Endocrinol. 9 (9), 586–594. doi:10.1016/S2213-8587(21)00180-7
Kuno, A., Kimura, Y., Mizuno, M., Oshima, H., Sato, T., Moniwa, N., et al. (2020). Empagliflozin attenuates acute kidney injury after myocardial infarction in diabetic rats. Sci. Rep. 10 (1), 7238. doi:10.1038/s41598-020-64380-y
Lam-Chung, C. E. (2023). Comprehensive review of SGLT2 inhibitors’ efficacy through their diuretic mode of action in diabetic patients. Front. Endocrinol. (Lausanne) 14, 1174692. doi:10.3389/fendo.2023.1174692
Lin, D. S., Yu, A. L., Lo, H. Y., Lien, C. W., Lee, J. K., Chiang, F. T., et al. (2023). Differential effects of sodium-glucose cotransporter 2 inhibitors on cardiovascular and renal outcomes according to renal function: a dose-response meta-analysis involving 10 randomized clinical trials and 71 553 individuals. Eur. J. Endocrinol. 189 (1), S17–S25. doi:10.1093/ejendo/lvad078
Maayah, Z. H., Ferdaoussi, M., Takahara, S., Soni, S., and Dyck, J. R. B. (2021). Empagliflozin suppresses inflammation and protects against acute septic renal injury. Inflammopharmacology 29 (1), 269–279. doi:10.1007/s10787-020-00732-4
McMurray, J. J. V., Solomon, S. D., Inzucchi, S. E., Kober, L., Kosiborod, M. N., Martinez, F. A., et al. (2019). Dapagliflozin in patients with heart failure and reduced ejection fraction. N. Engl. J. Med. 381 (21), 1995–2008. doi:10.1056/NEJMoa1911303
Menne, J., Dumann, E., Haller, H., and Schmidt, B. M. W. (2019). Acute kidney injury and adverse renal events in patients receiving SGLT2-inhibitors: a systematic review and meta-analysis. PLoS Med. 16 (12), e1002983. doi:10.1371/journal.pmed.1002983
Neal, B., Perkovic, V., Mahaffey, K. W., de Zeeuw, D., Fulcher, G., Erondu, N., et al. (2017). Canagliflozin and cardiovascular and renal events in type 2 diabetes. N. Engl. J. Med. 377 (7), 644–657. doi:10.1056/NEJMoa1611925
Neuen, B. L., Young, T., Heerspink, H. J. L., Neal, B., Perkovic, V., Billot, L., et al. (2019). SGLT2 inhibitors for the prevention of kidney failure in patients with type 2 diabetes: a systematic review and meta-analysis. Lancet Diabetes Endocrinol. 7 (11), 845–854. doi:10.1016/S2213-8587(19)30256-6
Oyama, K., Raz, I., Cahn, A., Goodrich, E. L., Bhatt, D. L., Leiter, L. A., et al. (2022). Efficacy and safety of dapagliflozin according to background use of cardiovascular medications in patients with type 2 diabetes: a prespecified secondary analysis of a randomized clinical trial. JAMA Cardiol. 7 (9), 914–923. doi:10.1001/jamacardio.2022.2006
Page, M. J., McKenzie, J. E., Bossuyt, P. M., Boutron, I., Hoffmann, T. C., Mulrow, C. D., et al. (2021a). The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 372, n71. doi:10.1136/bmj.n71
Page, M. J., Moher, D., Bossuyt, P. M., Boutron, I., Hoffmann, T. C., Mulrow, C. D., et al. (2021b). PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. BMJ 372, n160. doi:10.1136/bmj.n160
Perkovic, V., Jardine, M. J., Neal, B., Bompoint, S., Heerspink, H. J. L., Charytan, D. M., et al. (2019). Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N. Engl. J. Med. 380 (24), 2295–2306. doi:10.1056/NEJMoa1811744
Phadke, G., Kaushal, A., Tolan, D. R., Hahn, K., Jensen, T., Bjornstad, P., et al. (2020). Osmotic nephrosis and acute kidney injury associated with SGLT2 inhibitor use: a case report. Am. J. Kidney Dis. 76 (1), 144–147. doi:10.1053/j.ajkd.2020.01.015
Qiu, M., Ding, L. L., Zhang, M., and Zhou, H. R. (2021). Safety of four SGLT2 inhibitors in three chronic diseases: a meta-analysis of large randomized trials of SGLT2 inhibitors. Diab Vasc. Dis. Res. 18 (2), 14791641211011016. doi:10.1177/14791641211011016
Rampersad, C., Kraut, E., Whitlock, R. H., Komenda, P., Woo, V., Rigatto, C., et al. (2020). Acute kidney injury events in patients with type 2 diabetes using SGLT2 inhibitors versus other glucose-lowering drugs: a retrospective cohort study. Am. J. Kidney Dis. 76 (4), 471–479. doi:10.1053/j.ajkd.2020.03.019
Rigato, M., Fadini, G. P., and Avogaro, A. (2023). Safety of sodium-glucose cotransporter 2 inhibitors in elderly patients with type 2 diabetes: a meta-analysis of randomized controlled trials. Diabetes Obes. Metab. 25 (10), 2963–2969. doi:10.1111/dom.15193
Solomon, S. D., McMurray, J. J. V., Claggett, B., de Boer, R. A., DeMets, D., Hernandez, A. F., et al. (2022). Dapagliflozin in heart failure with mildly reduced or preserved ejection fraction. N. Engl. J. Med. 387 (12), 1089–1098. doi:10.1056/NEJMoa2206286
Szalat, A., Perlman, A., Muszkat, M., Khamaisi, M., Abassi, Z., and Heyman, S. N. (2018). Can SGLT2 inhibitors cause acute renal failure? Plausible role for altered glomerular hemodynamics and medullary hypoxia. Drug Saf. 41 (3), 239–252. doi:10.1007/s40264-017-0602-6
Vaduganathan, M., Docherty, K. F., Claggett, B. L., Jhund, P. S., de Boer, R. A., Hernandez, A. F., et al. (2022). SGLT-2 inhibitors in patients with heart failure: a comprehensive meta-analysis of five randomised controlled trials. Lancet 400 (10354), 757–767. doi:10.1016/S0140-6736(22)01429-5
Vallon, V., and Verma, S. (2021). Effects of SGLT2 inhibitors on kidney and cardiovascular function. Annu. Rev. Physiol. 83, 503–528. doi:10.1146/annurev-physiol-031620-095920
Voors, A. A., Angermann, C. E., Teerlink, J. R., Collins, S. P., Kosiborod, M., Biegus, J., et al. (2022). The SGLT2 inhibitor empagliflozin in patients hospitalized for acute heart failure: a multinational randomized trial. Nat. Med. 28 (3), 568–574. doi:10.1038/s41591-021-01659-1
Wiviott, S. D., Raz, I., Bonaca, M. P., Mosenzon, O., Kato, E. T., Cahn, A., et al. (2019). Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N. Engl. J. Med. 380 (4), 347–357. doi:10.1056/NEJMoa1812389
Zannad, F., Ferreira, J. P., Pocock, S. J., Zeller, C., Anker, S. D., Butler, J., et al. (2021). Cardiac and kidney benefits of empagliflozin in heart failure across the spectrum of kidney function: insights from EMPEROR-reduced. Circulation 143 (4), 310–321. doi:10.1161/CIRCULATIONAHA.120.051685
Zelniker, T. A., Wiviott, S. D., Raz, I., Im, K., Goodrich, E. L., Bonaca, M. P., et al. (2019). SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet 393 (10166), 31–39. doi:10.1016/S0140-6736(18)32590-X
Zhang, Y., Nakano, D., Guan, Y., Hitomi, H., Uemura, A., Masaki, T., et al. (2018). A sodium-glucose cotransporter 2 inhibitor attenuates renal capillary injury and fibrosis by a vascular endothelial growth factor-dependent pathway after renal injury in mice. Kidney Int. 94 (3), 524–535. doi:10.1016/j.kint.2018.05.002
Zhao, M., Sun, S., Huang, Z., Wang, T., and Tang, H. (2020). Network meta-analysis of novel glucose-lowering drugs on risk of acute kidney injury. Clin. J. Am. Soc. Nephrol. 16 (1), 70–78. doi:10.2215/CJN.11220720
Zhuo, M., Paik, J. M., Wexler, D. J., Bonventre, J. V., Kim, S. C., and Patorno, E. (2022). SGLT2 inhibitors and the risk of acute kidney injury in older adults with type 2 diabetes. Am. J. Kidney Dis. 79 (6), 858–867.e1. doi:10.1053/j.ajkd.2021.09.015
Keywords: acute kidney injury, meta-analysis, randomized controlled trials, sodium/glucose cotransporter-2 inhibitors, systematic review
Citation: Wang Q, Yu J, Deng W, Liu C, Yang J, Li Y, Cai G, Chen X and Dong Z (2024) Influence of sodium/glucose cotransporter-2 inhibitors on the incidence of acute kidney injury: a meta-analysis. Front. Pharmacol. 15:1372421. doi: 10.3389/fphar.2024.1372421
Received: 18 January 2024; Accepted: 05 June 2024;
Published: 25 June 2024.
Edited by:
Edgar Jaimes, Memorial Sloan Kettering Cancer Center, United StatesCopyright © 2024 Wang, Yu, Deng, Liu, Yang, Li, Cai, Chen and Dong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zheyi Dong, c2hlbmdkYWkyNkAxNjMuY29t
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.