- 1Chongqing Changshou Traditional Cinese Medicine Hospital, Chongqing, China
- 2Traditional Chinese Medicine Hospital Dianjiang Chongqing, Chongqing, China
Background: Chronic heart failure (CHF) is a prevalent and highly challenging cardiovascular disease associated with high mortality rates. The occurrence and progression of CHF are closely linked to left ventricular remodeling (LVR) and inflammation. Addressing LVR and reducing inflammation can significantly slow down the progression of CHF and improve patient prognosis.
Objective: To evaluate the effects of Xinmailong injection (XMLI) on LVR and inflammatory mediators in CHF patients.
Method: The randomized controlled trials investigating the effectiveness of XMLI treatment for CHF were retrieved from eight databases up until 31 December 2023. To evaluate the methodological quality of included studies, the Cochrane bias risk tool was employed. Furthermore, statistical analysis, sensitivity analysis, and publication bias assessment were conducted using Stata 17.0 software.
Result: Compared with conventional treatment (CT), the combination therapy of XMLI and CT significantly improved LVR and reduced inflammatory mediators, mainly manifested by an increase in LVEF (MD = 6.40, 95% CI: 5.25 to 7.55, p = 0.000), a decrease in LVEDD (MD = −4.63, 95% CI: −5.69 to −3.57, p = 0.000) and LVESD (MD = −4.00, 95% CI: −5.50 to −2.50, p = 0.000), as well as a decrease in TNF-α (MD = −7.93, 95% CI: −9.86 to −6.00, p = 0.000), IL-6 (MD = −5.25, 95% CI: −6.59 to −3.92, p = 0.000), IL-18 (MD = −36.07, 95% CI: −46.76 to −25.38, p = 0.000), CRP (MD = −4.41, 95% CI: −6.40 to −2.42, p = 0.000), hs-CRP (MD = −4.90, 95% CI: −5.71 to −4.08, p = 0.000), and an increase in IL-10 (MD = 20.19, 95% CI: 10.42 to 29.97, p = 0.000). In addition, the combination therapy showed enhanced clinical efficacy (OR = 4.08, 95% CI: 3.10 to 5.37, p = 0.000), decreased expression levels of BNP (MD = −138.48, 95% CI: −155.48 to −121.48, p = 0.000), and NT-pro BNP (MD = −315.63, 95% CI: −359.25 to −272.00, p = 0.000), and increased the 6-MWD (MD = 71.02, 95% CI: 57.23 to 84.81, p = 0.000). It is noteworthy that the combination therapy did not lead to an increase in the incidence of adverse reactions (OR = 1.01, 95% CI: 0.68 to 1.50, p = 0.97).
Conclusion: This systematic review and meta-analysis demonstrated the superiority of combining XMLI and CT therapies over CT alone in improving LVR and reducing inflammatory mediators in patients with CHF. Importantly, this combination therapy does not increase adverse reactions. However, it is crucial to exercise caution while interpreting the survey results due to the limited quality of the included studies.
Systematic Review Registration: https://www.crd.york.ac.uk/PROSPERO/display_record.php?RecordID=492715, Identifier CRD42023492715.
1 Introduction
Chronic heart failure (CHF) is a major public health problem, affecting 26 million people worldwide and leading to a high incidence rate and mortality. This condition brings a huge burden to both patients and society due to its complex clinical syndrome caused by multiple etiologies (Conrad et al., 2018; Mensah et al., 2023). CHF can be classified into two distinct subtypes, namely, heart failure with reduced ejection fraction (HFrEF, LVEF less than 40%) and heart failure with mid-range ejection fraction (HFmrEF, LVEF ranging from 40% to 49%). While the pathological and physiological mechanisms of CHF are not fully understood, left ventricular remodeling (LVR) and increased inflammation are known characteristics of the condition (Dick and Epelman, 2016; Aimo et al., 2019). There is a close relationship between LVR, inflammatory response, and the occurrence and progression of CHF (Hartupee and Mann, 2013; Dick and Epelman, 2016; Tong et al., 2018). Elevated levels of pro-inflammatory factors have been found to be positively correlated with the severity and adverse outcomes of CHF (Smart and Steele, 2011). Therefore, an important strategy to alleviate symptoms and improve prognosis in CHF patients is to enhance LVR and reduce inflammation.
LVR, which refers to the structural and functional changes in the left ventricle of the heart, is a consequence of various etiologies that contribute to the development of CHF (Aimo et al., 2019). These changes in ventricular structure and function can significantly impair cardiac performance, leading to worsened symptoms and outcomes for patients. Numerous studies have demonstrated the potential of interventions aimed at improving LVR to delay or even reverse the progression of CHF (Biering-Sørensen et al., 2020; Lee et al., 2021). In addition to LVR, inflammation has been recognized as a key pathophysiological factor in CHF (Hartupee and Mann, 2013). Elevated levels of pro-inflammatory factors, including tumor necrosis factor α (TNF-a), interleukin-6 (IL-6), IL-18, C-reactive protein (CRP), and hypersensitive C-reactive protein (hs-CRP), have been closely associated with the severity and adverse consequences of the disease in CHF patients (Arvunescu et al., 2023). Promising results have been reported in targeting or regulating the activity of these inflammatory mediators (Murphy et al., 2020). Consequently, targeting both LVR and inflammatory mediators has emerged as a significant therapeutic strategy for alleviating symptoms and improving prognosis in patients with CHF.
Xinmailong injection (XMLI) is a composite peptide injection extracted from Periplaneta americana L, containing adenosine, inosine, protocatechuic acid, and pyroglutamyl dipeptide as its main active ingredients (Qi et al., 2017). Modern pharmacological studies have elucidated the cardioprotective properties of XMLI, notably in inhibiting oxidative stress (Jiang et al., 2021) and inflammatory response (Jin et al., 2022), regulating cell autophagy (Li et al., 2016), and modulating cytokine expression (Liu et al., 2017). Jiang et al. (Jiang et al., 2021) observed that XMLI modulates HO-1 mediated lysosomal function and autophagy in H9C2 cells, reduces oxidative stress and mitigates DOX-induced cardiac toxicity. Jin et al. (Jin et al., 2022) revealed that XMLI can reduce ROS production, minimize inflammatory response, and decrease cell apoptosis by improving PKC and PLA2 protein-mediated myocardial ischemia. Li et al. (Li et al., 2016) demonstrated that XMLI targets autophagy by activating the PI3K/Akt pathway and inhibiting Erk1/2 and P38 MAPK pathways, effectively alleviating epirubicin-induced cardiomyopathy. Additionally, Liu et al. (Liu et al., 2017) highlighted that XMLI inhibits connective tissue growth factor (CTGF), enhancement of heart function, and reduction of alcoholic myocardial fibrosis in rat models. As a result, XMLI is widely utilized as an adjuvant medication for CHF in China. However, there is a limited comprehensive evaluation of XMLI’s impact on LVR and inflammatory mediators in patients with CHF. Given the crucial role of LVR and inflammation in the development and progression of CHF, this study aims to bridge this knowledge gap through a meta-analysis of clinical randomized controlled trials (RCTs).
2 Methods
2.1 Study registration
This meta-analysis followed the PRISMA (preferred reporting item for systematic evaluation and meta-analysis) guidelines (Hutton et al., 2015) and was registered with PROSPERO (NO. CRD42023492715).
2.2 Database and search strategy
To investigate the treatment of CHF with XMLI, the two reviewers conducted an extensive literature search. Relevant studies were searched and retrieved from various databases, including PubMed, Embase, Web of Science, Cochrane Library, Wanfang Data, China Knowledge Infrastructure Database (CNKI), China Biomedical Database (CBM), and China Science and Technology Journal Database (VIP). The search employed a combination of MeSH terminology and textual terminology. The search terms include “Xinmailong injection”, “Xinmailong ", “heart failure”, and “chronic heart failure”. The search was conducted from their establishment to 31 December 2023. In addition, the reviewers manually searched the reference lists of published literature to ensure comprehensive coverage. Detailed search strategies can be found in the Supplementary Material.
2.3 Inclusion and exclusion criteria
According to the PICOS principle, the following conditions must be met for inclusion in the study: 1) RCTs without any language restrictions on publication. 2) Participants diagnosed with CHF, aged 18 and above. 3) The intervention group received a combination of XMLI and conventional treatment (CT), while the control group received CT based on heart failure (HF) guidelines. 4) The primary outcome measures primarily focus on LVR (LVEF, LVEDD, LVESD) and inflammatory mediators (TNF-α, IL-6, IL-10, IL-18, CRP, hs-CRP).
The exclusion criteria are as follows: 1) Non-RCTs. 2) Unstable heart failure. 3) Repeated publication, retaining only complete data for research. 4) Research without primary outcome measures. 5) The full study cannot be obtained through databases or other means.
2.4 Data extraction
Two reviewers (XH and XC) independently evaluated the included studies and extracted data. If any discrepancies or disagreements arose during the evaluation process, a third reviewer (MY) was available for discussion and resolution. The data extraction was conducted by the two researchers (XH and XC) using a pre-established table that included several important parameters. These parameters encompassed the article title, first author, publication year, sample size, intervention drugs, dosage and course of treatment, outcome measures, and adverse reactions.
2.5 Quality assessment
Two reviewers (YL and JY) independently evaluated the methodological quality of the included studies using the Cochrane Collaboration risk of bias tool (Sterne et al., 2019). The evaluation encompassed various aspects, including randomization methods, allocation concealment, blinding, completeness of outcome data, selective reporting, and other sources of bias. The results of the evaluations were then cross-checked to ensure accuracy and consistency. The risk of bias for each study was classified as low, unclear, or high. Any disagreements that arose during the methodological quality assessment process were resolved through discussions involving third reviewer (XM).
2.6 Data analysis
All meta-analyses were conducted using RevMan5.4 and Stata 17.0 software. For dichotomous data, a 95% confidence interval (CI) risk ratio (RR) was calculated, while continuous data utilized a mean difference (MD) with a 95% CI. Heterogeneity among the included studies was evaluated using I2. I2 ≤ 50% was considered as low heterogeneity, and a fixed-effects model was applied. Conversely, a random-effects model was applied. Furthermore, subgroup analysis was performed based on differences in LVEF to investigate possible factors influencing the results. Sensitivity analysis was performed on the primary outcome measures to evaluate the impact of individual studies on the combined effect size. The Egge’s tests were employed to test for potential publication bias.
3 Results
3.1 Search results and study characteristics
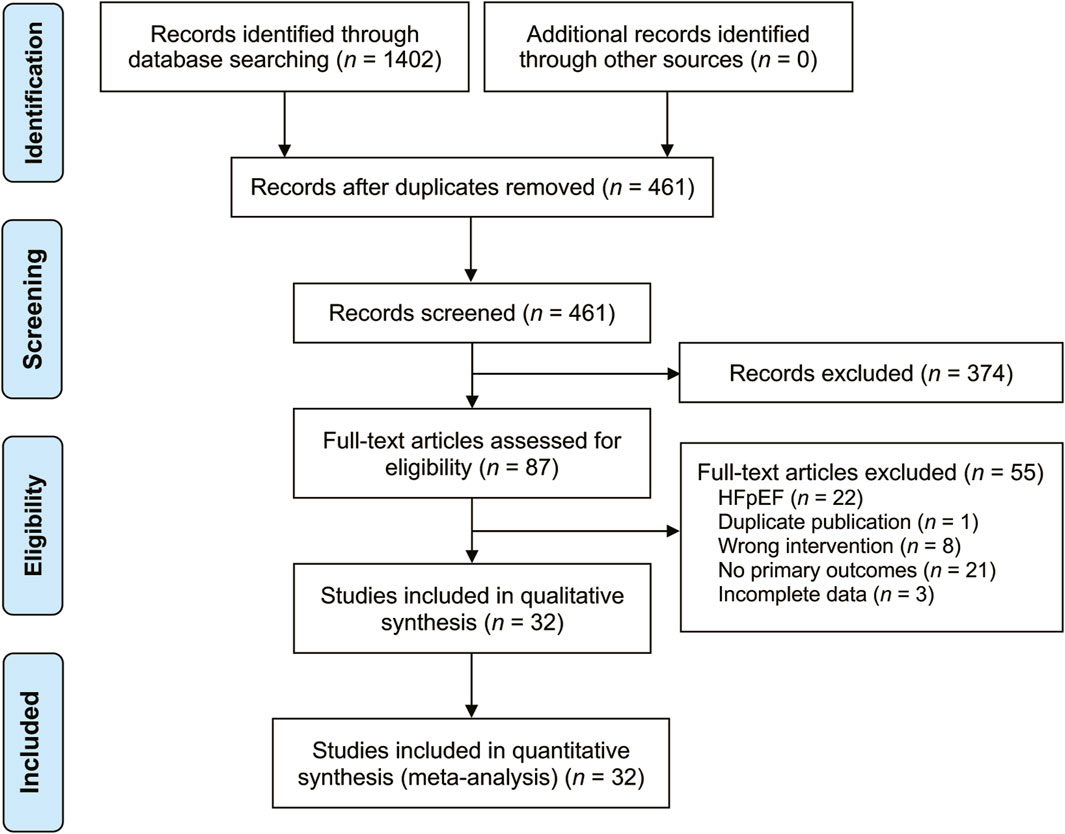
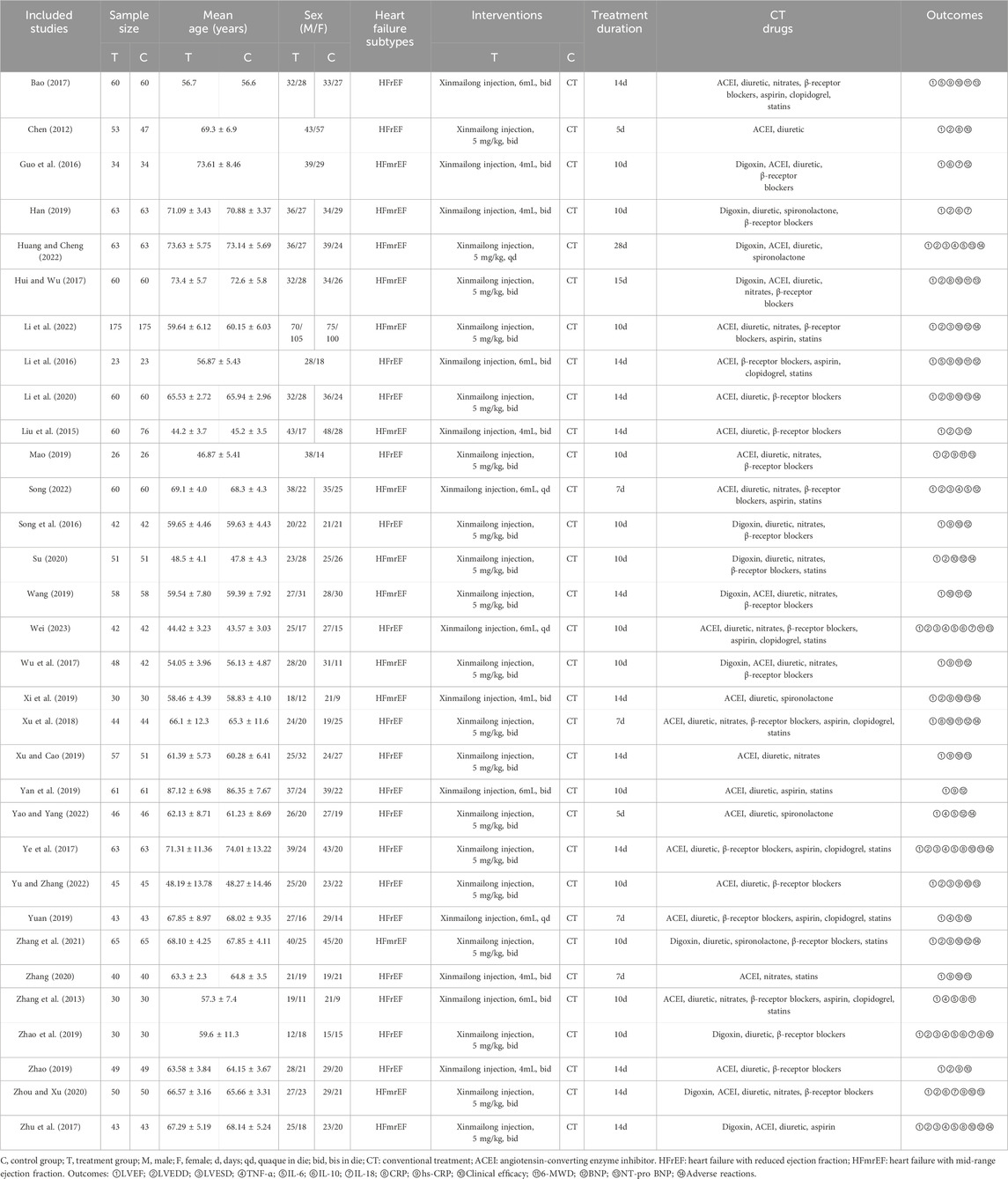
A total of 1,402 related studies were retrieved through a systematic search. After deduplication and screening, 32 studies (Bao, 2017; Chen, 2012; Guo et al., 2016; Han, 2019; Huang and Cheng, 2022; Hui and Wu, 2017; Li et al., 2022; Li et al., 2016; Li et al., 2020; Liu et al., 2015; Mao, 2019; Song, 2022; Song et al., 2016; Su, 2020; Wang, 2019; Wei, 2023; Wu et al., 2017; Xi et al., 2019; Xu et al., 2018; Xu and Cao, 2019; Yan et al., 2019; Yao and Yang, 2022; Ye et al., 2017; Yu and Zhang, 2023; Yuan, 2019; Zhang et al., 2021; Zhang, 2020; Zhang et al., 2013; Zhao et al., 2019; Zhao, 2019; Zhou and Xu, 2020; Zhu et al., 2017) published from 2012 to 2023 were selected for the final analysis. The literature search results are displayed in Figure 1. These studies were conducted in China and involved 3,346 patients (1855 males and 1,491 females) with varying sample sizes (ranging from 23 to 175) and treatment courses (ranging from 5 to 28 days). The control group received CT recommended by the HF guidelines, while the treatment group received XMLI combined with CT. No statistically significant differences in general information were found between the two groups. The included studies provided results on various parameters, including LVEF (Chen, 2012; Zhang et al., 2013; Liu et al., 2015; Guo et al., 2016; Li et al., 2016; Song et al., 2016; Bao, 2017; Hui and Wu, 2017; Wu et al., 2017; Ye et al., 2017; Zhu et al., 2017; Xu et al., 2018; Han, 2019; Mao, 2019; Song, 2022; Wang, 2019; Xi et al., 2019; Xu and Cao, 2019; Yan et al., 2019; Yuan, 2019; Zhao, 2019; Zhao et al., 2019; Li et al., 2020; Su, 2020; Zhang, 2020; Zhou and Xu, 2020; Zhang et al., 2021; Huang and Cheng, 2022; Li et al., 2022; Yao and Yang, 2022; Wei, 2023; Yu and Zhang, 2023), LVEDD (Chen, 2012; Liu et al., 2015; Hui and Wu, 2017; Ye et al., 2017; Zhu et al., 2017; Han, 2019; Mao, 2019; Song, 2022; Xi et al., 2019; Zhao, 2019; Zhao et al., 2019; Li et al., 2020; Su, 2020; Zhou and Xu, 2020; Zhang et al., 2021; Huang and Cheng, 2022; Li et al., 2022; Wei, 2023; Yu and Zhang, 2023), LVESD (Liu et al., 2015; Song, 2022; Ye et al., 2017; Zhu et al., 2017; Zhao, 2019; Huang and Cheng, 2022; Li et al., 2022; Wei, 2023; Yu and Zhang, 2023), TNF-α (Zhang et al., 2013; Ye et al., 2017; Zhu et al., 2017; Yuan, 2019; Zhao et al., 2019; Huang and Cheng, 2022; Song, 2022; Yao and Yang, 2022; Wei, 2023), IL-6 (Zhang et al., 2013; Li et al., 2016; Song, 2022; Bao, 2017; Ye et al., 2017; Zhu et al., 2017; Yuan, 2019; Zhao et al., 2019; Huang and Cheng, 2022; Yao and Yang, 2022; Wei, 2023), IL-10 (Guo et al., 2016; Han, 2019; Zhao et al., 2019; Zhou and Xu, 2020; Wei, 2023), IL-18 (Guo et al., 2016; Han, 2019; Zhao et al., 2019; Zhou and Xu, 2020; Wei, 2023), CRP (Chen, 2012; Zhang et al., 2013; Hui and Wu, 2017; Ye et al., 2017; Zhu et al., 2017; Xu et al., 2018; Zhao, 2019), hs-CRP (Li et al., 2016; Song et al., 2016; Bao, 2017; Wu et al., 2017; Mao, 2019; Xi et al., 2019; Xu and Cao, 2019; Yan et al., 2019; Zhao, 2019; Zhao et al., 2019; Li et al., 2020; Zhang, 2020; Zhou and Xu, 2020; Zhang et al., 2021; Yu and Zhang, 2023), clinical efficacy (Chen, 2012; Li et al., 2016; Song et al., 2016; Bao, 2017; Hui and Wu, 2017; Ye et al., 2017; Zhu et al., 2017; Xu et al., 2018; Wang, 2019; Xi et al., 2019; Xu and Cao, 2019; Yuan, 2019; Zhao, 2019; Zhao et al., 2019; Li et al., 2020; Su, 2020; Zhang, 2020; Zhou and Xu, 2020; Zhang et al., 2021; Li et al., 2022; Yu and Zhang, 2023), 6-MWD (Zhang et al., 2013; Li et al., 2016; Bao, 2017; Hui and Wu, 2017; Wu et al., 2017; Xu et al., 2018; Mao, 2019; Wang, 2019; Wei, 2023), BNP (Liu et al., 2015; Song, 2022; Guo et al., 2016; Li et al., 2016; Song et al., 2016; Wu et al., 2017; Zhu et al., 2017; Xu et al., 2018; Wang, 2019; Yan et al., 2019; Zhao, 2019; Su, 2020; Zhang et al., 2021; Li et al., 2022; Yao and Yang, 2022), and NT-pro BNP (Bao, 2017; Hui and Wu, 2017; Ye et al., 2017; Mao, 2019; Xi et al., 2019; Xu and Cao, 2019; Li et al., 2020; Zhang, 2020; Zhou and Xu, 2020; Huang and Cheng, 2022; Wei, 2023; Yu and Zhang, 2023). Among these studies, only 10 studies (Ye et al., 2017; Zhu et al., 2017; Xu et al., 2018; Xi et al., 2019; Li et al., 2020; Su, 2020; Zhang et al., 2021; Huang and Cheng, 2022; Li et al., 2022; Yao and Yang, 2022) reported adverse reactions. The basic characteristics of the included studies are present in Table 1.
3.2 Risk of bias assessment
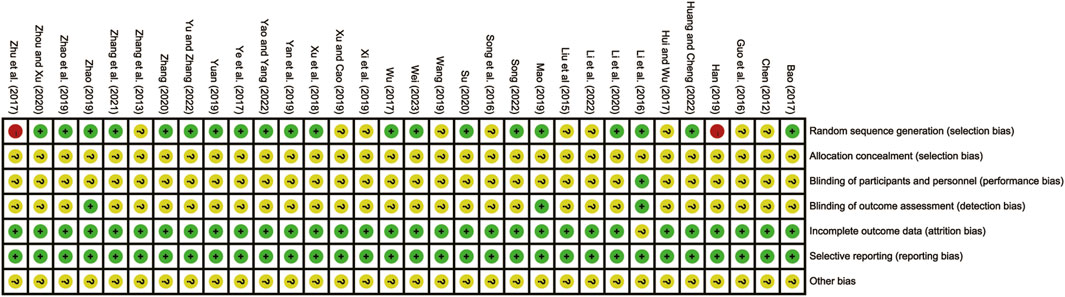
The overall quality of the included studies varied. 20 studies (Bao, 2017; Huang and Cheng, 2022; T. T; Li et al., 2016; Li et al., 2020; Mao, 2019; Song, 2022; Su, 2020; Wei, 2023; Wu et al., 2017; Xu et al., 2018; Yan et al., 2019; Yao and Yang, 2022; Ye et al., 2017; Yu and Zhang, 2023; Yuan, 2019; Zhang et al., 2021; Zhang, 2020; Zhao et al., 2019; Zhao, 2019; Zhou and Xu, 2020) utilized the low-risk random number table method. Conversely, 10 studies (Chen, 2012; Zhang et al., 2013; Liu et al., 2015; Guo et al., 2016; Song et al., 2016; Hui and Wu, 2017; Wang, 2019; Xi et al., 2019; Xu and Cao, 2019; Li et al., 2022) lacked clear descriptions of randomization, resulting in an unclear risk assessment. Two studies (Zhu et al., 2017; Han, 2019) were considered high-risk as they grouped patients based on admission order. None of the studies reported hidden allocation, leading to an unclear risk assessment for all of them. In terms of design, four studies (T. T. Li et al., 2016; Mao, 2019; Yan et al., 2019; Zhao, 2019) were multicenter double-blind tests, which were considered to be low-risk. Additionally, all included studies were published during a period of low risk of selective reporting and were given priority based on their locality. However, none of the studies clearly indicated the presence of other biases, resulting in an overall unclear risk assessment. The risk of bias assessment is detailed in Figure 2.
3.3 LVR parameters
3.3.1 LVEF
32 studies (Bao, 2017; Chen, 2012; Guo et al., 2016; Han, 2019; Huang and Cheng, 2022; Hui and Wu, 2017; Li et al., 2022; T. T; Li et al., 2016; Li et al., 2020; Liu et al., 2015; Mao, 2019; Song, 2022; Song et al., 2016; Su, 2020; Wang, 2019; Wei, 2023; Wu et al., 2017; Xi et al., 2019; Xu et al., 2018; Xu and Cao, 2019; Yan et al., 2019; Yao and Yang, 2022; Ye et al., 2017; Yu and Zhang, 2023; Yuan, 2019; Zhang et al., 2021; Zhang, 2020; Zhang et al., 2013; Zhao et al., 2019; Zhao, 2019; Zhou and Xu, 2020; Zhu et al., 2017) evaluated LVEF with high heterogeneity (I2 = 90.0%, p = 0.000) and merged it with a random-effects model. The combination therapy of XMLI and CT significantly improved LVEF compared to CT alone (MD = 6.40, 95% CI: 5.25 to 7.55, p = 0.000, Figure 3). Subgroup analysis based on different subtypes of HF revealed a noteworthy enhancement in LVEF for patients with HFrEF (MD = 7.22, 95% CI: 5.63 to 8.82, p = 0.000, Figure 3) and HFmrEF (MD = 5.35, 95% CI: 3.68 to 7.01, p = 0.000, Figure 3) when the combination therapy was administered. Interestingly, the improvement was particularly prominent among patients with HFrEF.
3.3.2 LVEDD
19 studies (Chen, 2012; Liu et al., 2015; Hui and Wu, 2017; Ye et al., 2017; Zhu et al., 2017; Han, 2019; Mao, 2019; Song, 2022; Xi et al., 2019; Zhao, 2019; Zhao et al., 2019; Li et al., 2020; Su, 2020; Zhou and Xu, 2020; Zhang et al., 2021; Huang and Cheng, 2022; Li et al., 2022; Wei, 2023; Yu and Zhang, 2023) evaluated LVEDD with high heterogeneity (I2 = 90.0%, p = 0.000) and merged it with a random-effects model. The combination therapy of XMLI and CT significantly reduced LVEDD compared to CT alone (MD = −4.63, 95% CI: −5.69 to −3.57, p = 0.000, Figure 4A). Subgroup analysis based on different subtypes of HF revealed a noteworthy reduction in LVEDD for patients with HFrEF (MD = −5.48, 95% CI: −8.19 to −2.77, p = 0.000, Figure 4A) and HFmrEF (MD = −3.98, 95% CI: −4.78 to −3.18, p = 0.000, Figure 4A) when the combination therapy was administered. Interestingly, the reduction was particularly prominent among patients with HFrEF.
3.3.3 LVESD
Nine studies (Liu et al., 2015; Song, 2022; Ye et al., 2017; Zhu et al., 2017; Zhao, 2019; Huang and Cheng, 2022; Li et al., 2022; Wei, 2023; Yu and Zhang, 2023) evaluated LVESD with high heterogeneity (I2 = 91.1%, p = 0.000) and merged it with a random-effects model. The combination therapy of XMLI and CT significantly reduced LVESD compared to CT alone (MD = −4.00, 95% CI: −5.50 to −2.50, p = 0.000, Figure 4B). Subgroup analysis based on different subtypes of HF revealed a noteworthy reduction in LVESD for patients with HFrEF (MD = −5.62, 95% CI: −7.99 to −3.24, p = 0.000, Figure 4B) and HFmrEF (MD = −2.96, 95% CI: −4.31 to −1.61, p = 0.000, Figure 4B) when the combination therapy was administered. Interestingly, the reduction was particularly prominent among patients with HFrEF.
3.4 Inflammatory mediators
3.4.1 TNF-α
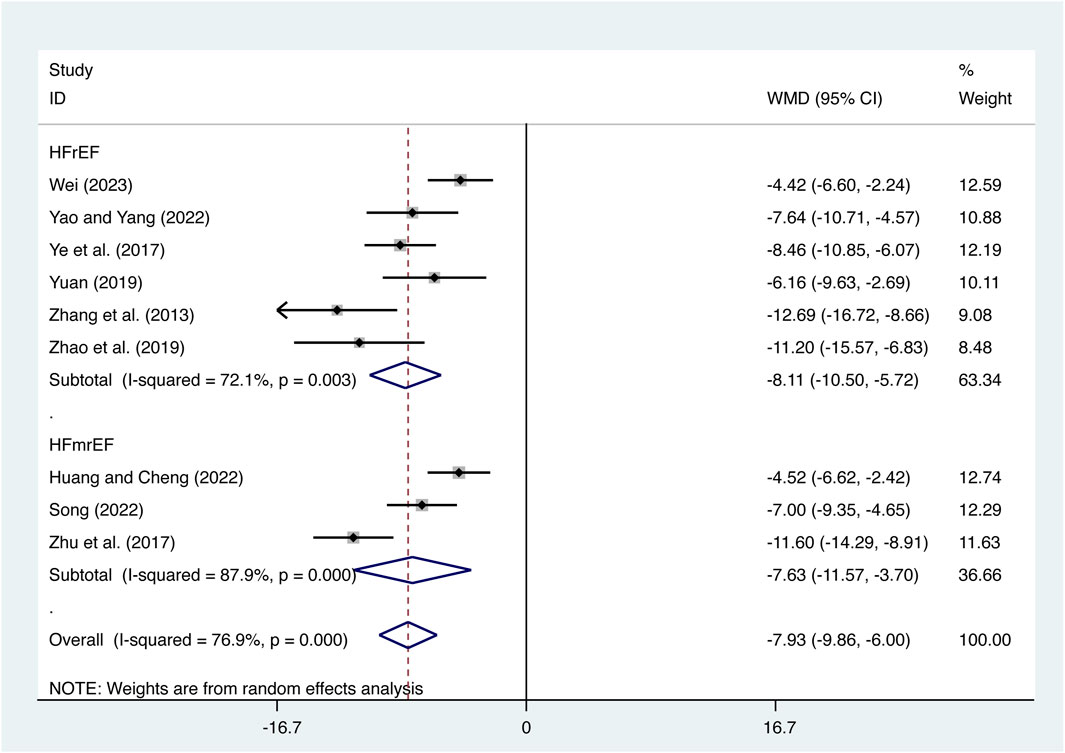
Nine studies (Zhang et al., 2013; Ye et al., 2017; Zhu et al., 2017; Yuan, 2019; Zhao et al., 2019; Huang and Cheng, 2022; Song, 2022; Yao and Yang, 2022; Wei, 2023) evaluated TNF-α expression levels with high heterogeneity (I2 = 76.9%, p = 0.000) and merged it with a random-effects model. The combination therapy of XMLI and CT significantly reduced TNF-α expression levels compared to CT alone (MD = −7.93, 95% CI: −9.86 to −6.00, p = 0.000, Figure 5). Subgroup analysis based on different subtypes of HF revealed a noteworthy reduction in TNF-α expression levels for patients with HFrEF (MD = −8.11, 95% CI: −10.50 to −5.72, p = 0.000, Figure 5) and HFmrEF (MD = −7.63, 95% CI: −9.86 to −6.00, p = 0.000, Figure 5) when the combination therapy was administered. Interestingly, the reduction was particularly prominent among patients with HFrEF.
3.4.2 IL-6
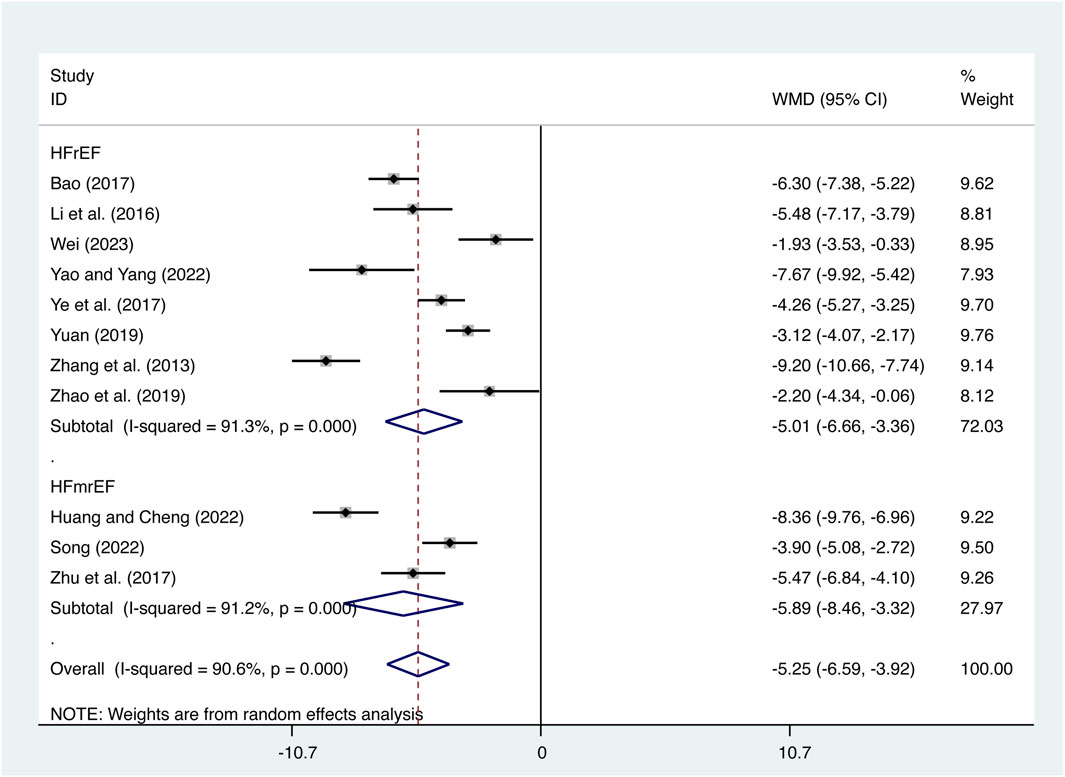
11 studies (Bao, 2017; Huang and Cheng, 2022; T. T; Li et al., 2016; Song, 2022; Wei, 2023; Yao and Yang, 2022; Ye et al., 2017; Yuan, 2019; Zhang et al., 2013; Zhao et al., 2019; Zhu et al., 2017) evaluated IL-6 expression levels with high heterogeneity (I2 = 90.6%, p = 0.000) and merged it with a random-effects model. The combination therapy of XMLI and CT significantly reduced IL-6 expression levels compared to CT alone (MD = −5.25, 95% CI: −6.59 to −3.92, p = 0.000, Figure 6). Subgroup analysis based on different subtypes of HF revealed a noteworthy reduction in IL-6 expression levels for patients with HFrEF (MD = −5.01, 95% CI: −6.66 to −3.36, p = 0.000, Figure 6) and HFmrEF (MD = −5.89, 95% CI: −8.46 to −3.32, p = 0.000, Figure 6) when the combination therapy was administered. Interestingly, the reduction was particularly prominent among patients with HFmrEF.
3.4.3 IL-10
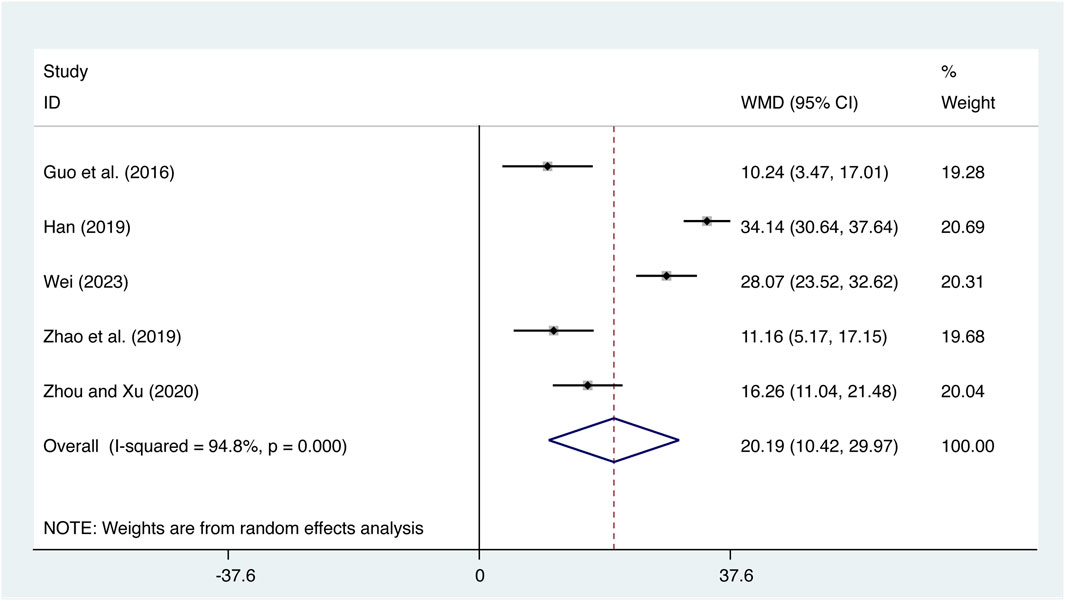
Five studies (Guo et al., 2016; Han, 2019; Zhao et al., 2019; Zhou and Xu, 2020; Wei, 2023) evaluated IL-10 expression levels with high heterogeneity (I2 = 94.8%, p = 0.000) and merged it with a random-effects model. The combination therapy of XMLI and CT significantly increased IL-10 expression levels compared to CT alone (MD = 20.19, 95% CI: 10.42 to 29.97, p = 0.000, Figure 7).
3.4.4 IL-18
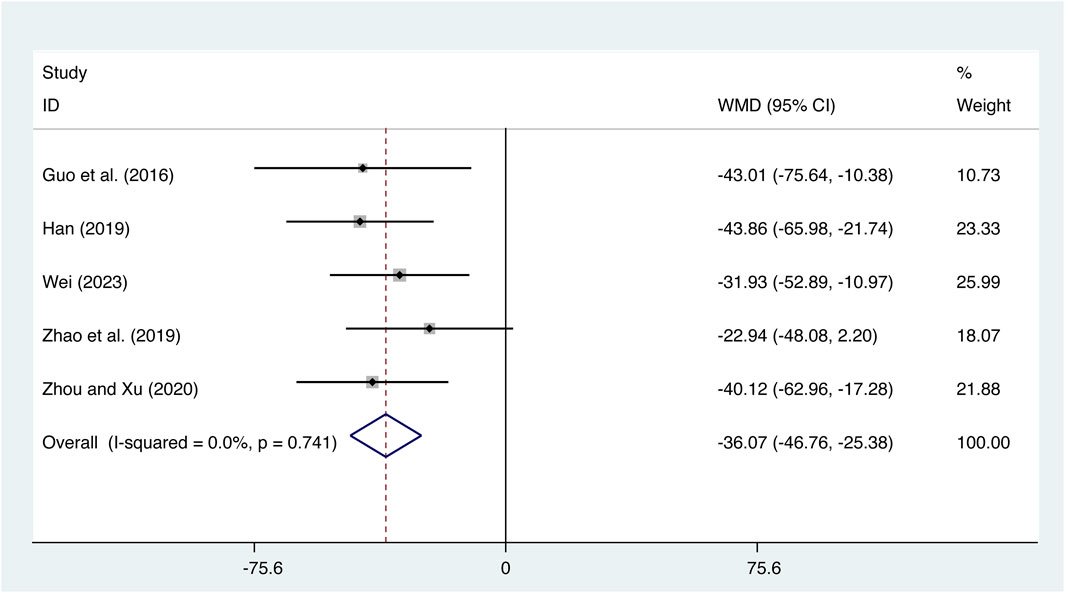
Five studies (Guo et al., 2016; Han, 2019; Zhao et al., 2019; Zhou and Xu, 2020; Wei, 2023) evaluated IL-18 expression levels with low heterogeneity (I2 = 0.0%, p = 0.741) and merged it with a fixed-effects model. The combination therapy of XMLI and CT significantly reduced IL-18 expression levels compared to CT alone (MD = −36.07, 95% CI: −46.76 to −25.38, p = 0.000, Figure 8).
3.4.5 CRP
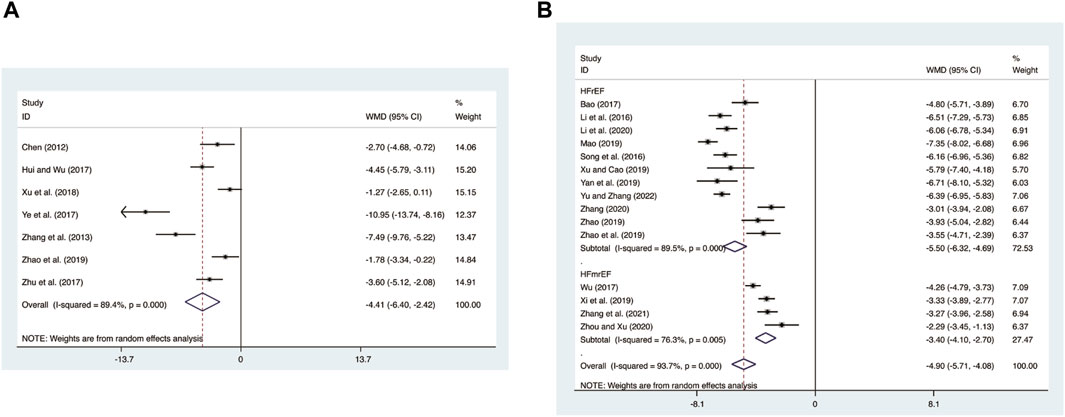
Seven studies (Chen, 2012; Zhang et al., 2013; Hui and Wu, 2017; Ye et al., 2017; Zhu et al., 2017; Xu et al., 2018; Zhao, 2019) evaluated CRP expression levels with high heterogeneity (I2 = 89.4%, p = 0.000) and merged it with a random-effects model. The combination therapy of XMLI and CT significantly reduced CRP expression levels compared to CT alone (MD = −4.41, 95% CI: −6.40 to −2.42, p = 0.000, Figure 9A).
3.4.6 hs-CRP
15 studies (Bao, 2017; T. T; Li et al., 2016; Li et al., 2020; Mao, 2019; Song et al., 2016; Wu et al., 2017; Xi et al., 2019; Xu and Cao, 2019; Yan et al., 2019; Yu and Zhang, 2023; Zhang et al., 2021; Zhang, 2020; Zhao et al., 2019; Zhao, 2019; Zhou and Xu, 2020) evaluated hs-CRP expression levels with high heterogeneity (I2 = 93.7%, p = 0.000) and merged it with a random-effects model. The combination therapy of XMLI and CT significantly reduced hs-CRP expression levels compared to CT alone (MD = −4.90, 95% CI: −5.71 to −4.08, p = 0.000, Figure 9B). Subgroup analysis based on different subtypes of HF revealed a noteworthy reduction in hs-CRP expression levels for patients with HFrEF (MD = −5.50, 95% CI: −6.32 to −4.69, p = 0.000, Figure 9B) and HFmrEF (MD = −3.40, 95% CI: −4.10 to −2.70, p = 0.000, Figure 9B) when the combination therapy was administered. Interestingly, the reduction was particularly prominent among patients with HFrEF.
3.5 Secondary outcomes
3.5.1 Clinical efficacy
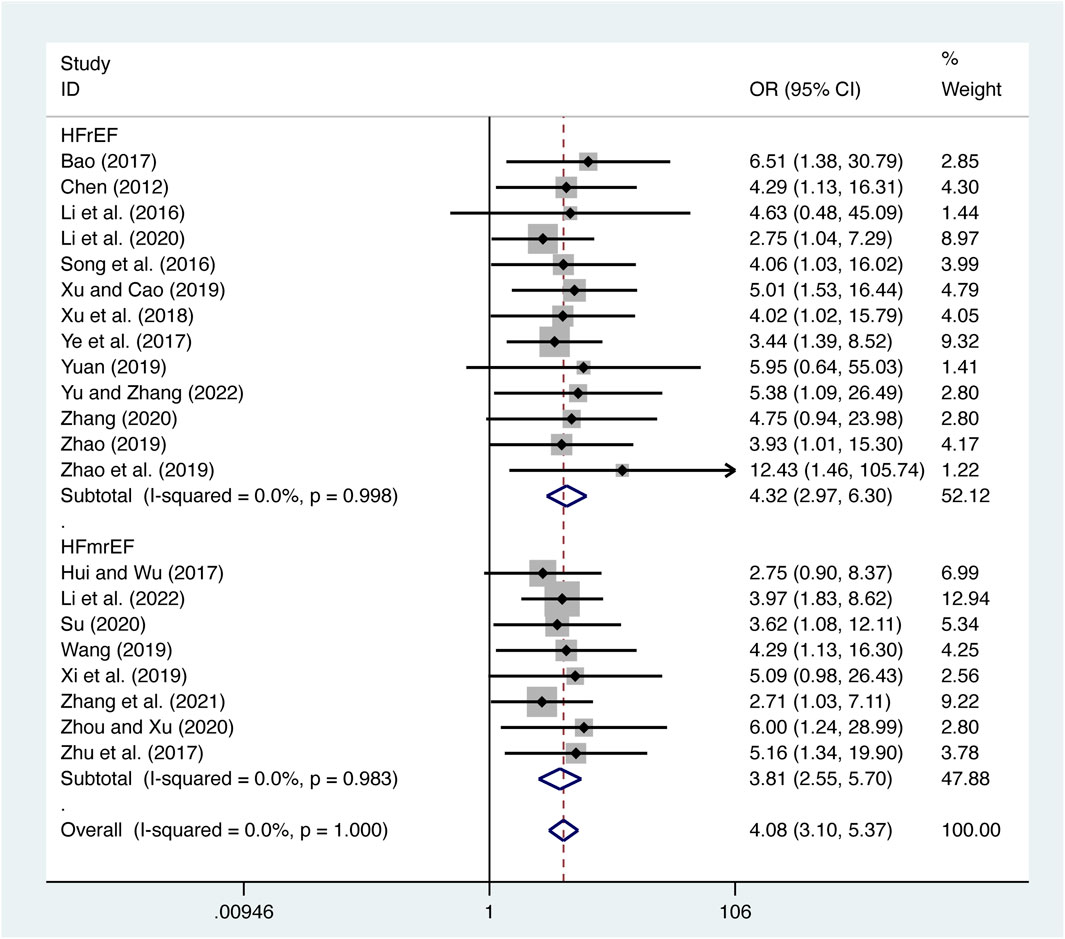
21 studies (Bao, 2017; Chen, 2012; Hui and Wu, 2017; Li et al., 2022; T. T; Li et al., 2016; Li et al., 2020; Song et al., 2016; Su, 2020; Wang, 2019; Xi et al., 2019; Xu et al., 2018; Xu and Cao, 2019; Ye et al., 2017; Yu and Zhang, 2023; Yuan, 2019; Zhang et al., 2021; Zhang, 2020; Zhao et al., 2019; Zhao, 2019; Zhou and Xu, 2020; Zhu et al., 2017) evaluated clinical efficacy with low heterogeneity (I2 = 0.0%, p = 1.000) and merged it with a fixed-effects model. The combination therapy of XMLI and CT significantly improved clinical efficacy compared to CT alone (OR = 4.08, 95% CI: 3.10 to 5.37, p = 0.000, Figure 10). Subgroup analysis based on different subtypes of HF revealed a noteworthy enhancement in clinical efficacy for patients with HFrEF (OR = 4.32, 95% CI: 2.97 to 6.30, p = 0.000, Figure 10) and HFmrEF (OR = 3.81, 95% CI: 2.55 to 5.70, p = 0.000, Figure 10) when the combination therapy was administered. Interestingly, the improvement was particularly prominent among patients with HFrEF.
3.5.2 6-MWD
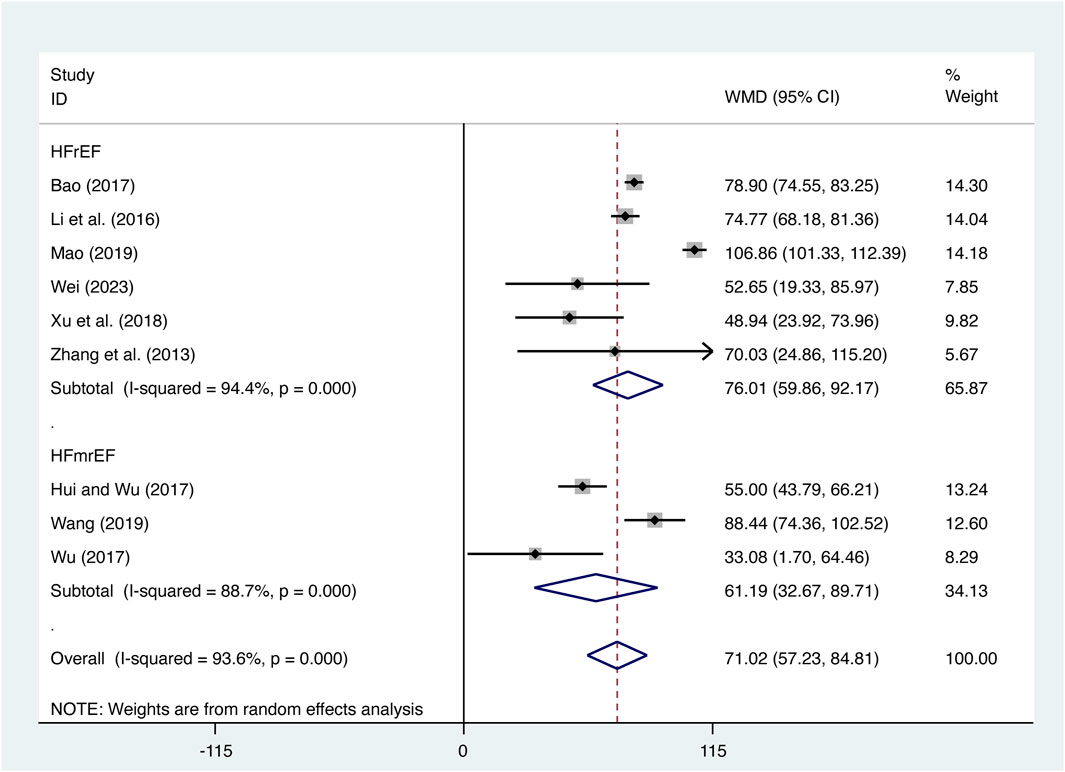
Nine studies (Bao, 2017; Hui and Wu, 2017; T. T; Li et al., 2016; Mao, 2019; Wang, 2019; Wei, 2023; Wu et al., 2017; Xu et al., 2018; Zhang et al., 2013) evaluated 6-MWD with high heterogeneity (I2 = 93.6%, p = 0.000) and merged it with a random-effects model. The combination therapy of XMLI and CT significantly increased 6-MWD compared to CT alone (MD = 71.02, 95% CI: 57.23 to 84.81, p = 0.000, Figure 11). Subgroup analysis based on different subtypes of HF revealed a noteworthy enhancement in 6-MWD for patients with HFrEF (MD = 76.01, 95% CI: 59.86 to 92.17, p = 0.000, Figure 11) and HFmrEF (MD = 61.19, 95% CI: 32.67 to 89.71, p = 0.000, Figure 11) when the combination therapy was administered. Interestingly, the improvement was particularly prominent among patients with HFrEF.
3.5.3 BNP
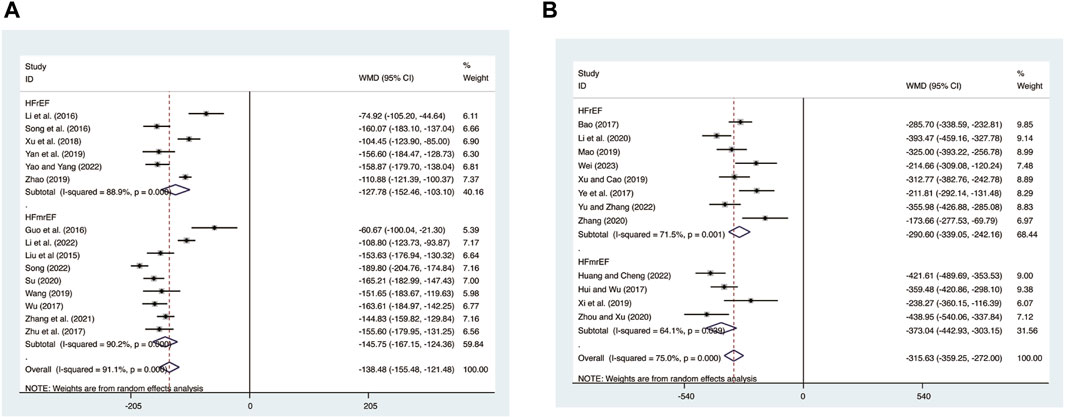
15 studies (Guo et al., 2016; Li et al., 2022; T. T; Li et al., 2016; Liu et al., 2015; Song, 2022; Song et al., 2016; Su, 2020; Wang, 2019; Wu et al., 2017; Xu et al., 2018; Yan et al., 2019; Yao and Yang, 2022; Zhang et al., 2021; Zhao, 2019; Zhu et al., 2017) evaluated BNP expression levels with high heterogeneity (I2 = 91.1%, p = 0.000) and merged it with a random-effects model. The combination therapy of XMLI and CT significantly reduced BNP expression levels compared to CT alone (MD = −138.48, 95% CI: −155.48 to −121.48, p = 0.000, Figure 12A). Subgroup analysis based on different subtypes of HF revealed a noteworthy reduction in BNP expression levels for patients with HFrEF (MD = −127.78, 95% CI: −152.46 to −103.10, p = 0.000, Figure 12A) and HFmrEF (MD = −145.75, 95% CI: −167.15 to −124.36, p = 0.000, Figure 12A) when the combination therapy was administered. Interestingly, the reduction was particularly prominent among patients with HFmrEF.
3.5.4 NT-pro BNP
12 studies (Bao, 2017; Hui and Wu, 2017; Ye et al., 2017; Mao, 2019; Xi et al., 2019; Xu and Cao, 2019; Li et al., 2020; Zhang, 2020; Zhou and Xu, 2020; Huang and Cheng, 2022; Wei, 2023; Yu and Zhang, 2023) evaluated NT-pro BNP expression levels with high heterogeneity (I2 = 75.0%, p = 0.000) and merged it with a random-effects model. The combination therapy of XMLI and CT significantly reduced NT-pro BNP expression levels compared to CT alone (MD = −315.63, 95% CI: −359.25 to −272.00, p = 0.000, Figure 12B). Subgroup analysis based on different subtypes of HF revealed a noteworthy reduction in NT-pro BNP expression levels for patients with HFrEF (MD = −290.60, 95% CI: −339.05 to −242.16, p = 0.000, Figure 12B) and HFmrEF (MD = −373.04, 95% CI: −442.93 to −303.15, p = 0.000, Figure 12B) when the combination therapy was administered. Interestingly, the reduction was particularly prominent among patients with HFmrEF.
3.5.5 Adverse reactions
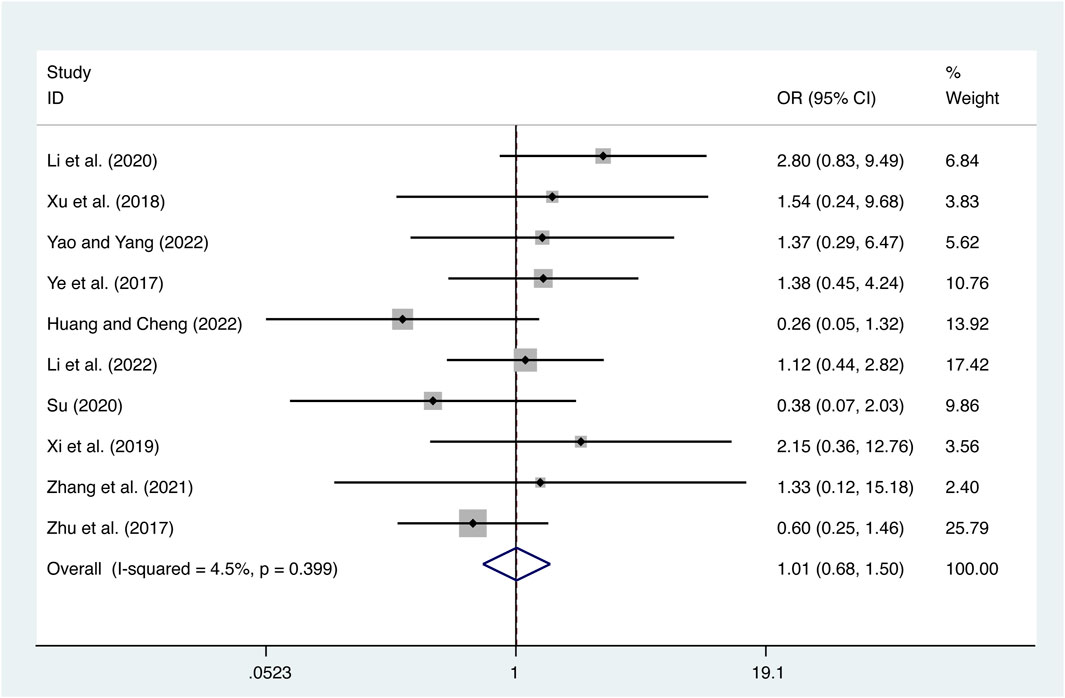
10 studies (Ye et al., 2017; Zhu et al., 2017; Xu et al., 2018; Xi et al., 2019; Li et al., 2020; Su, 2020; Zhang et al., 2021; Huang and Cheng, 2022; Li et al., 2022; Yao and Yang, 2022) reported on adverse reactions with low heterogeneity (I2 = 4.5%, p = 0.399) and merged it with a fixed-effects model. The combination therapy of XMLI and CT revealed no significant difference in adverse reactions compared to CT alone (OR = 1.01, 95% CI: 0.68 to 1.50, p = 0.97, Figure 13). The study drug is associated with several common adverse reactions, including dizziness, headache, nausea, vomiting, diarrhea, palpitations, fatigue, rash, hypokalemia, dyspnea, hypotension, tachycardia, and liver dysfunction. It is important to note that these adverse reactions typically subside with appropriate symptomatic treatment. Notably, none of the participants in the study discontinued the use of the drug as a result of experiencing adverse reactions. For more comprehensive information, please consult the Supplementary Material.
3.6 Sensitivity analysis
To assess the reliability and robustness of the consolidation results, a sensitivity analysis was conducted. This analysis involved sequentially excluding individual studies and examining their impact on various variables, including LVEF (Figure 14A), LVEDD (Figure 14B), LVESD (Figure 14C), TNF-α (Figure 14D), IL-6 (Figure 14E), IL-10 (Figure 14F), IL-18 (Figure 14G), CRP (Figure 14H), and hs-CRP (Figure 14I). Interestingly, the exclusion of any of these studies had no significant effect on the combined results. This finding suggests that the merged results are both robust and reliable, as clearly shown in Figure 14.
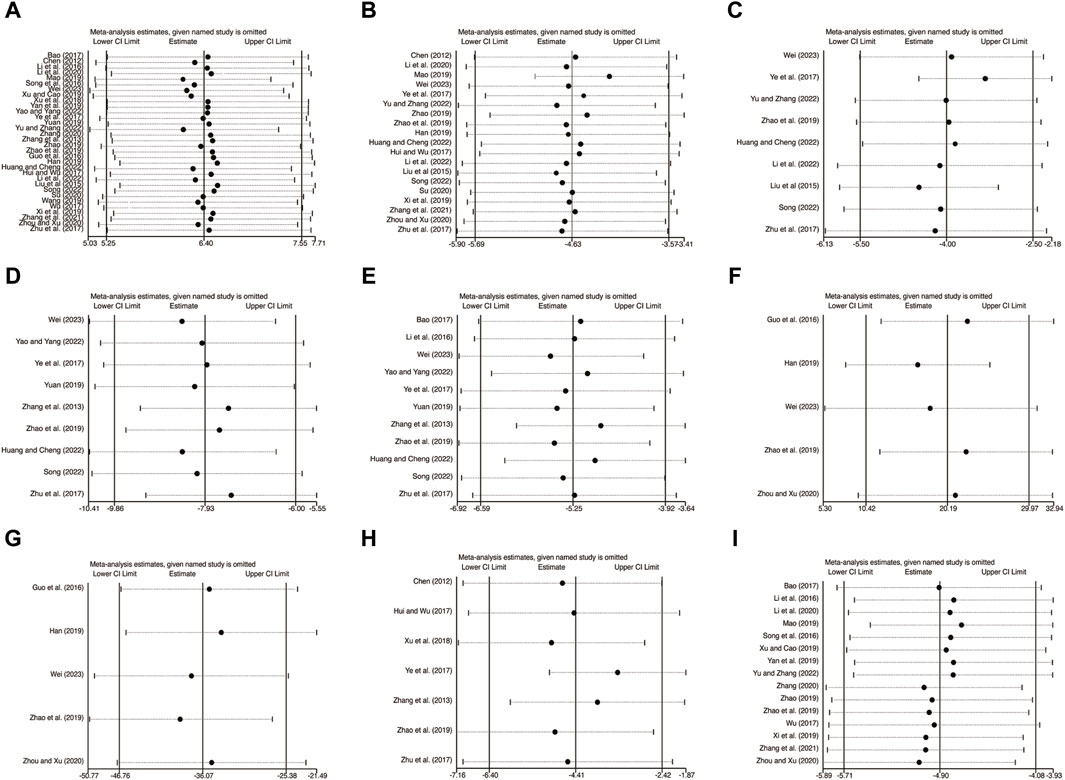
Figure 14. The results of sensitivity analysis. (A) LVEF. (B) LVEDD. (C) LVESD. (D) TNF-α. (E) IL-6. (F) IL-10. (G) IL-18. (H) CRP. (I) hs-CRP.
3.7 Publication bias
In order to evaluate publication bias, the Egger’s test was utilized specifically for LVEF, LVEDD, IL-6, hs-CRP, BNP, and NT-pro BNP, as depicted in Figure 15. Remarkably, the results of the analysis revealed that there was no significant publication bias for LVEF (Figure 15A, p = 0.667), LVEDD (Figure 15B, p = 0.188), IL-6 (Figure 15C, p = 0.500), hs-CRP (Figure 15D, p = 0.836), BNP (Figure 15E, p = 0.767), and NT-pro BNP (Figure 15F, p = 0.298).
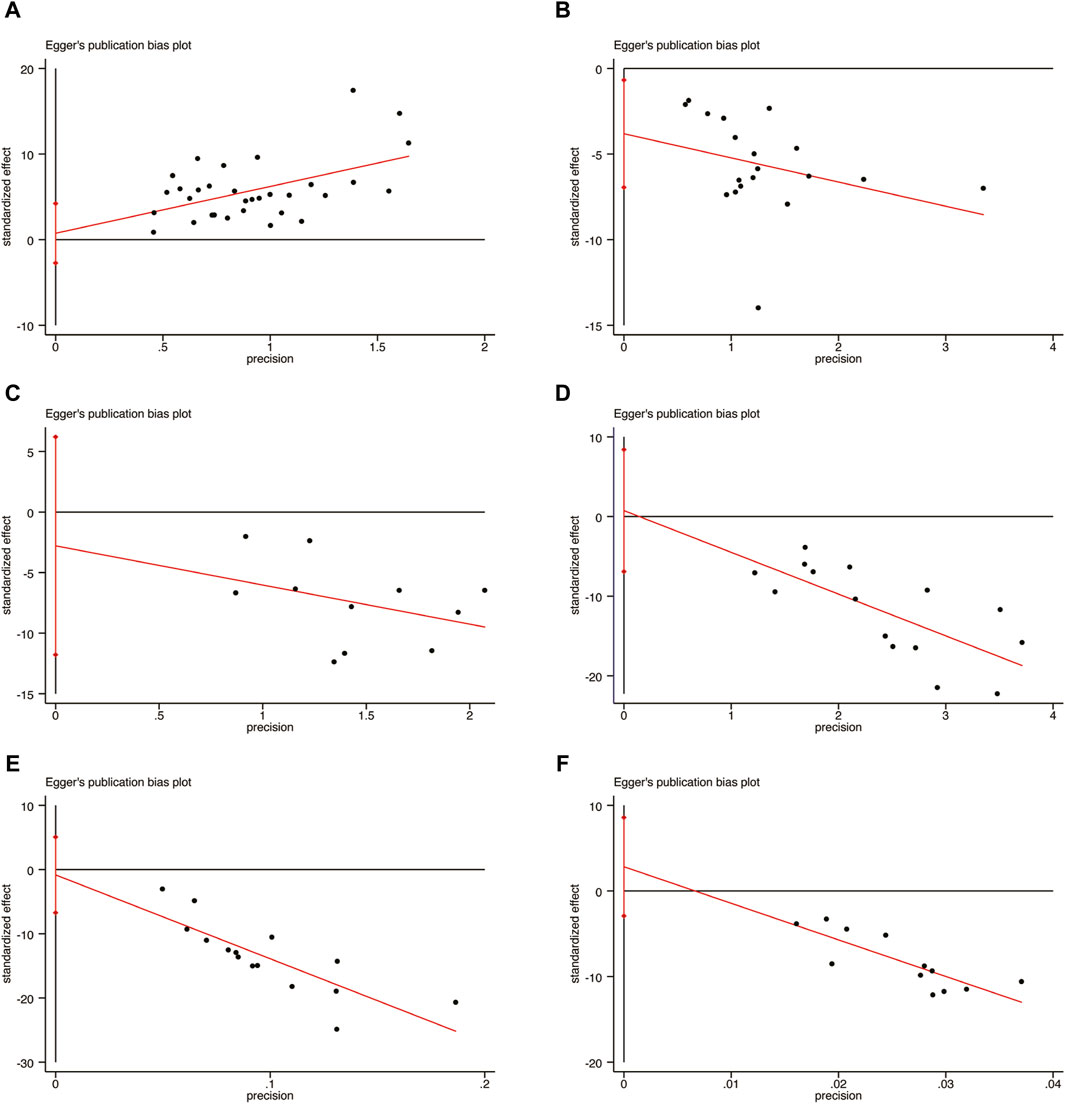
Figure 15. Egger’s publication funnel plot. (A) LVEF. (B) LVEDD. (C) IL-6. (D) hs-CRP. (E) BNP. (F) NT-pro BNP.
4 Discussion
4.1 Summary of findings
This meta-analysis is the first to investigate the effects of XMLI on LVR and inflammatory mediators in patients with CHF. A total of 32 RCTs were included in this analysis, revealing several important findings. Firstly, the combination of XMLI and CT significantly improved LVR in HF patients. This improvement was supported by an increase in LVEF, as well as a decrease in LVEDD and LVESD. As well as levels of BNP, and NT-pro BNP were decreased. Furthermore, the combination therapy also resulted in a significant reduction in inflammatory mediators. Specifically, there was a decrease in the expression levels of pro-inflammatory cytokines TNF-a, IL-6, IL-18, CRP, and hs-CRP. Conversely, there was an increase in the expression levels of the anti-inflammatory cytokine IL-10. In addition to improving LVR and reducing inflammatory mediators, the combination therapy showed higher clinical efficacy and improvement of the 6-MWD. Importantly, the combination therapy demonstrated good safety, with only minor adverse events reported. These events were manageable in terms of symptoms and had no impact on treatment outcomes. Based on these compelling results, our meta-analysis suggests that XMLI effectively improves LVR in HF patients, reduces inflammatory mediators, and enhances overall clinical efficacy.
To ensure the robustness and reliability of our findings, sensitivity analysis was conducted. Individual studies were sequentially deleted, and sensitivity analysis was performed on key indicators of LVR and inflammatory mediators, further confirming the validity of our results. Additionally, Egger’s test was conducted to evaluate publication bias, with the results showing no significant publication bias. This further strengthens the validity and reliability of our findings.
4.2 Comparison with previous studies
Although Lu et al.'s (Lu et al., 2018) previous meta-analysis evaluated the clinical efficacy of XMLI in treating CHF, our study specifically focuses on its effects on LVR and inflammatory mediators. It should be noted that there are several shortcomings in previous studies: Firstly, HF is classified into different subtypes based on ejection fraction according to the HF management guidelines. However, previous studies did not consider these subtypes or conduct subgroup analysis based on ejection fraction during meta-analysis, which may introduce heterogeneity and bias. Secondly, sensitivity analysis was not performed, and only funnel plots were used to evaluate publication bias in previous studies, which affects the robustness and reliability of the research findings. Finally, previous studies mainly focused on clinical indicators such as symptom improvement, exercise tolerance, and quality of life in CHF patients treated with XMLI. The impact of XMLI on LVR and inflammatory mediators has not been extensively explored. Understanding these specific effects is crucial for a comprehensive assessment of XMLI’s therapeutic potential in CHF patients.
4.3 Strengths and limitations
This meta-analysis of RCTs is the first to specifically investigate the effects of XMLI on LVR and inflammatory mediators in patients with CHF. To enhance the reliability of our findings, we will conduct subgroup analyses based on different types of HF, eliminating potential confounding factors associated with disease types. Additionally, our study addresses a crucial aspect of CHF by evaluating the impact of XMLI on LVR, a key pathological and physiological mechanism contributing to high hospitalization and mortality rates in CHF patients. Furthermore, we comprehensively evaluate the role of inflammatory response in LVR, highlighting its significance in the progression of HF, an aspect that previous studies have yet to fully address.
Several limitations should be considered when interpreting the results of this study. Firstly, the included studies in this meta-analysis exhibit relatively low overall quality, with limited reporting of allocation concealment and blinding, which poses a serious risk of bias. Secondly, significant heterogeneity is observed among the RCTs, although subgroup and sensitivity analyses are conducted without identifying the sources of heterogeneity. This variation may be associated with the lack of standardized dosage and intervention duration. Thirdly, the small size of the included studies highlights the need for larger scale research to ensure result reliability. Therefore, caution should be exercised when interpreting the findings. Fourthly, it is important to note that all studies included in this analysis were conducted in China and exclusively involved Chinese participants. This limited geographical scope may introduce sources of heterogeneity. To ensure the applicability of these findings to different races, future studies should incorporate with more diverse samples from various geographical regions. Lastly, the limited number of studies examining TNF-α, IL-10, IL-8, and CRP results in low supporting evidence. Future research should prioritize larger scale and more rigorous studies to verify the stability of our findings.
4.4 Implication
To strengthen the evidence regarding the efficacy of XMLI treatment for CHF, future clinical research should address the following areas. Firstly, studies should be conducted on different types of HF to comprehensively assess the efficacy of XMLI in treating HF. By doing so, bias can be minimized, and more accurate conclusions can be generated. Secondly, rigorous adherence to clinical research standards, including strict randomization, allocation concealment, and blinding techniques, should be ensured. Encouraging placebo-controlled randomized trials can provide more precise results. Thirdly, it is crucial to report RCTs in a complete and comprehensive manner by employing standardized reporting trial statements. Alongside primary outcome measures, additional information such as comorbidities, disease duration, medication usage, readmission rates, follow-up duration, and endpoint time should be reported, facilitating the analysis of heterogeneity and prognosis clarification. Finally, given the significant role of LVR and inflammation in the progression of HF, future research should focus on targeted and high-quality studies in these areas.
5 Conclusion
The results of the systematic review and meta-analysis suggest that the combination of XML and CT can effectively improve LVR and reduce inflammatory mediators in CHF patients, with a good safety profile. However, it is crucial to approach these findings with caution due to the low level of evidence and high heterogeneity observed in the included studies, particularly in regard to the evaluation of inflammatory mediators. To strengthen these conclusions, future research should prioritize high-quality RCTs that can provide more substantive evidence.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Author contributions
XH: Conceptualization, Investigation, Methodology, Software, Visualization, Writing–original draft, Writing–review and editing. XC: Data curation, Investigation, Visualization, Writing–original draft. YL: Investigation, Validation, Writing–original draft. JY: Formal Analysis, Methodology, Validation, Writing–original draft. WN: Formal Analysis, Software, Validation, Writing–original draft. MY: Conceptualization, Methodology, Supervision, Writing–original draft, Writing–review and editing. XM: Conceptualization, Supervision, Writing–original draft, Writing–review and editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2024.1370448/full#supplementary-material
References
Aimo, A., Gaggin, H. K., Barison, A., Emdin, M., and Januzzi, J. L. (2019). Imaging, biomarker, and clinical predictors of cardiac remodeling in heart failure with reduced ejection fraction. JACC Heart Fail 7 (9), 782–794. doi:10.1016/j.jchf.2019.06.004
Arvunescu, A. M., Ionescu, R. F., Cretoiu, S. M., Dumitrescu, S. I., Zaharia, O., and Nanea, I. T. (2023). Inflammation in heart failure-future perspectives. J. Clin. Med. 12 (24), 7738. doi:10.3390/jcm12247738
Bao, Y. Y. (2017). Clinical observation of Xinmailong injection combined with Shenfu injection in the treatment of heart failure after coronary intervention in AMI. J. Pract. Tradit. Chin. Med. 33 (09), 1022–1023.
Biering-Sørensen, T., Minamisawa, M., Claggett, B., Liu, J., Felker, G. M., McMurray, J. J. V., et al. (2020). Cardiac myosin activator omecamtiv mecarbil improves left ventricular myocardial deformation in chronic heart failure: the COSMIC-HF trial. Circ. Heart Fail 13 (12), e008007. doi:10.1161/circheartfailure.120.008007
Chen, C. W. (2012). Therapeutic effect of Xinmailong injection on chronic congestive heart failure. J. Qiqihar Univ. Med. 33 (18), 2461–2462.
Conrad, N., Judge, A., Tran, J., Mohseni, H., Hedgecott, D., Crespillo, A. P., et al. (2018). Temporal trends and patterns in heart failure incidence: a population-based study of 4 million individuals. Lancet 391 (10120), 572–580. doi:10.1016/s0140-6736(17)32520-5
Dick, S. A., and Epelman, S. (2016). Chronic heart failure and inflammation: what do we really know? Circ. Res. 119 (1), 159–176. doi:10.1161/circresaha.116.308030
Guo, L., Zhang, L., and Qu, H. Y. (2016). Effect of Xinmailong injection on cardiac function and inflammatory mediators in patients with chronic heart failure. China J. Integr. Med. Cardio-/Cerebrovasc. Dis. 14 (15), 1762–1764.
Han, B. (2019). Effect of Xinmailong injection on cardiac function and inflammatory response in patients with chronic heart failure. Clin. Med. 39 (05), 95–97. doi:10.19528/j.issn.1003-3548.2019.05.040
Hartupee, J., and Mann, D. L. (2013). Positioning of inflammatory biomarkers in the heart failure landscape. J. Cardiovasc Transl. Res. 6 (4), 485–492. doi:10.1007/s12265-013-9467-y
Huang, L., and Cheng, X. L. (2022). Effect of sacubitril valsartan combined with Xinmailong injection in the treatment of elderly heart failure and its impact on inflammatory mediators. Chin. J. Clin. Ration. Drug U. S. 15 (12), 40–42.
Hui, H., and Wu, G. P. (2017). Clinical observation of Xinmailong injection combined with Qiliqiangxin capsule in the treatment of chronic heart failure. China J. Integr. Med. Cardio-/Cerebrovasc. Dis. 15 (22), 2869–2872.
Hutton, B., Salanti, G., Caldwell, D. M., Chaimani, A., Schmid, C. H., Cameron, C., et al. (2015). The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann. Intern Med. 162 (11), 777–784. doi:10.7326/m14-2385
Jiang, Y., Liu, Y., Xiao, W., Zhang, D., Liu, X., Xiao, H., et al. (2021). Xinmailong attenuates doxorubicin-induced lysosomal dysfunction and oxidative stress in H9c2 cells via HO-1. Oxid. Med. Cell Longev. 2021, 5896931. doi:10.1155/2021/5896931
Jin, L., Yin, Q., Mao, Y., Gao, Y., Han, Q., Mei, R., et al. (2022). Putative prevention of XML injection against myocardial ischemia is mediated by PKC and PLA2 proteins. Front. Cell Dev. Biol. 10, 827691. doi:10.3389/fcell.2022.827691
Lee, M. M. Y., Brooksbank, K. J. M., Wetherall, K., Mangion, K., Roditi, G., Campbell, R. T., et al. (2021). Effect of empagliflozin on left ventricular volumes in patients with type 2 diabetes, or prediabetes, and heart failure with reduced ejection fraction (SUGAR-DM-HF). Circulation 143 (6), 516–525. doi:10.1161/circulationaha.120.052186
Li, H., Mao, Y., Zhang, Q., Han, Q., Man, Z., Zhang, J., et al. (2016). Xinmailong mitigated epirubicin-induced cardiotoxicity via inhibiting autophagy. J. Ethnopharmacol. 192, 459–470. doi:10.1016/j.jep.2016.08.031
Li, P. Z., Zhu, J. X., Wang, Y. J., Fan, L. L., Zhou, Y., Sun, F. R., et al. (2022). Effect of Xinmailong injection on heart failure with reduced left ventricular ejection fraction. Northwest Pharm. J. 37 (05), 106–109.
Li, Z. H., Li, Y., and Li, Y. (2020). Effects of Xinmailong injection combined with Sodium Nitroprusside on levels of hs-CRP, CysC and NT-pro BNP in patients with chronic heart failure of coronary heart disease. Chin. Arch. Tradit. Chin. Med. 38 (05), 182–185. doi:10.13193/j.issn.1673-7717.2020.05.043
Liu, G. H., Huang, J., and Liu, Y. S. (2017). Effect of Xinmailong injection on myocardial fibrosis in alcoholic cardiomyopathy rats. China J. Integr. Med. Cardio-/Cerebrovasc. Dis. 15 (10), 1177–1180.
Liu, W. Q., Ao, Q., Wang, X. W., and Yin, X. H. (2015). Effect of Xinmailong injection on BNP and hs-CRP in patients with dilated heart disease. Contemp. Med. 21 (18), 134–135.
Lu, X. H., Zhang, L., Wang, J. B., Liu, H. H., Li, H. T., Zhou, H. Q., et al. (2018). Clinical efficacy and safety of Xinmailong injection for the treatment of chronic heart failure: a meta-analysis. Front. Pharmacol. 9, 810. Article 810. doi:10.3389/fphar.2018.00810
Mao, H. Y. (2019). Clinical efficacy of Xinmailong injection in the treatment of dilated cardiomyopathy and heart failure patients. China J. Integr. Med. Cardio-/Cerebrovasc. Dis. 17 (19), 2996–2997.
Mensah, G. A., Fuster, V., Murray, C. J. L., and Roth, G. A.Global Burden of Cardiovascular Diseases and Risks Collaborators (2023). Global burden of cardiovascular diseases and risks, 1990-2022. J. Am. Coll. Cardiol. 82 (25), 2350–2473. doi:10.1016/j.jacc.2023.11.007
Murphy, S. P., Kakkar, R., McCarthy, C. P., and Januzzi, J. L. (2020). Inflammation in heart failure: JACC state-of-the-art review. J. Am. Coll. Cardiol. 75 (11), 1324–1340. doi:10.1016/j.jacc.2020.01.014
Qi, J., Yu, J., Tan, Y., Chen, R., Xu, W., Chen, Y., et al. (2017). Mechanisms of Chinese medicine Xinmailong's protection against heart failure in pressure-overloaded mice and cultured cardiomyocytes. Sci. Rep. 7, 42843. doi:10.1038/srep42843
Smart, N. A., and Steele, M. (2011). The effect of physical training on systemic proinflammatory cytokine expression in heart failure patients: a systematic review. Congest. Heart Fail 17 (3), 110–114. doi:10.1111/j.1751-7133.2011.00217.x
Song, F. F. (2022). Effect of Xinmailong injection on heart function and levels of inflammatory factors and miR⁃23a in elderly patients with heart failure after acute myocardial infarction. Acta Univ. Tradit. Med. Sin. Pharmacol. Shanghai 36 (02), 20–25. doi:10.16306/j.1008-861x.2022.02.004
Song, X. X., Yan, C. H., Zhang, X. T., and Liu, A. M. (2016). Clinical study on Xinmailong injection combined with Bisoprolol in treatment of coronary heart disease patients with heart failure. Drugs Clin 31 (05), 623–627.
Sterne, J. A. C., Savović, J., Page, M. J., Elbers, R. G., Blencowe, N. S., Boutron, I., et al. (2019). RoB 2: a revised tool for assessing risk of bias in randomised trials. Bmj 366, l4898. doi:10.1136/bmj.l4898
Su, R. Y. (2020). Effects of Xinmailong combined with Bisoprolol on serum TGF-β and ICAM-1 expression in coronary heart disease patients with heart failure. Chin. J. Mod. Appl. Pharm. 37 (07), 863–867.
Tong, S., Zhang, L., Joseph, J., and Jiang, X. (2018). A potential and novel therapeutic approach to ischemic heart failure: algisyl-LVR. Int. J. Cardiol. 271, 209. doi:10.1016/j.ijcard.2018.05.052
Wang, N. (2019). Effect of Xinmailong injection combined with Western medicine on cardiac function and myocardial damage in patients with chronic heart failure. China J. Integr. Med. Cardio-/Cerebrovasc. Dis. 17 (16), 2483–2486.
Wei, J. J. (2023). Effect of Xinmailong injection on the levels of inflammatory factors in patients with heart failure after acute myocardial infarction. Med. Health Res. 7 (06), 92–94.
Wu, Z. F., Chen, W. W., and Liu, X. X. (2017). Effect of Xinmailong injection on heart failure of coronary heart disease patients’ serum BNP, hs-CRP and VEGF. Chin. Arch. Tradit. Chin. Med. 35 (09), 2433–2435. doi:10.13193/j.issn.1673-7717.2017.09.065
Xi, Y. J., Li, Z. R., Ma, Z. R., and Chang, Y. M. (2019). Effect of Xinmailong injection combined with low-dose dopamine in the treatment of dilated cardiomyopathy. Chin. J. Gerontol. 39 (16), 3870–3873.
Xu, E. W., Fang, Y., Zhang, Q. B., Fan, X. J., and Sun, L. N. (2018). Clinical study on Xinmailong injection combined with Milrinone in treatment of chronic congestive heart failure. Drugs Clin 33 (09), 2276–2280.
Xu, W., and Cao, J. (2019). Clinical efficacy of Xinmailong injection in patients with coronary heart disease and heart failure. Chin. Tradit. Pat. Med. 41 (08), 2012–2014.
Yan, R. Y., Sun, Q. Y., and Liu, Q. M. (2019). Therapeutic efect of Xinmailong injection on advanced aged patients with acute myocardial infarction heart failure. Chin. J. Cardiovasc Rehabil. Med. 28 (04), 473–477.
Yao, X., and Yang, X. X. (2022). Clinical efficacy of Xinmailong injection combined with Perindopril in the treatment of congestive heart failure. Shanghai Med. Pharm. J. 43 (21), 34–37.
Ye, K. F., P, S., Zhang, W., Fan, P., Hua, C. E., and Xia, Q. (2017). Observation on the therapeutic effect of Xinmailong injection in the treatment of chronic heart failure in the elderly. China J. Integr. Med. Cardio-/Cerebrovasc. Dis. 15 (19). Article 2018110529.
Yu, X., and Zhang, L. (2023). Effect of Xinmailong injection combined with Metoprolol succinate sustained release tablets on patients with chronic heart failure. Chin. J. Drug Abuse P. R. Treat. 29 (10), 1830–1833.
Yuan, X. Y. (2019). Effect of Xinmailong injection on inflammatory mediators in patients with heart failure after acute myocardial infarction. Jiangxi J. Tradit. Chin. Med. 50 (01), 40–41.
Zhang, J., Zhou, H., Shen, J., and He, F. L. (2021). The effect of Xinmailong injection in the treatment of chronic heart failure and its influence on cardiac function and the level of tumor marker CA125. J. Chin. Prescr. Drug 19 (12), 105–107.
Zhang, X. (2020). Effect of Xinmailong injection combined with western medicine on LVEDV and LVESV in patients with severe coronary heart disease and heart failure. Chin. J. Disaster Med. 8 (06), 310–312+315. doi:10.13919/j.issn.2095-6274.2020.06.004
Zhang, X. H., Fan, J. X., Qin, L. Q., Liu, H. B., Liu, Z. H., Ji, Z. G., et al. (2013). Effect of Xinmailong injection on inflammatory mediators in patients with heart failure after acute myocardial infarction. China J. Integr. Med. Cardio-/Cerebrovasc. Dis. 11 (06), 667–668.
Zhao, G. Y. J. G., Yuan, J. W., Sun, Z., Qu, H. Y., and Gao, S. (2019). Effects of Metoprolol combined with Xinmailong injection on cardiac function and inflammatory mediators in patients with chronic heart failure. Chin. Mod. Doct 57 (20), 51–53.
Zhao, Y. (2019). Effect of Xinmailong injection on BNP, hs-CRP levels and cardiac function in patients with chronic heart failure. Strait Pharm. J. 31 (04), 151–152.
Zhou, X. C., and Xu, L. (2020). Effect of Xinmailong injection on cardiac function and inflammatory mediators in patients with chronic heart failure. Clin. J. Tradit. Chin. Med. 32 (02), 344–346. doi:10.16448/j.cjtcm.2020.0238
Keywords: Xinmailong injection, chronic heart failure, left ventricular remodeling, inflammation mediators, randomized controlled trials, systematic review, meta-analysis
Citation: Han X, Chen X, Liu Y, Yang J, Nie W, Yang M and Mou X (2024) Xinmailong injection on left ventricular remodeling and inflammatory mediators in patients with CHF: a systematic review and meta-analysis. Front. Pharmacol. 15:1370448. doi: 10.3389/fphar.2024.1370448
Received: 14 January 2024; Accepted: 25 March 2024;
Published: 09 April 2024.
Edited by:
Luca Rastrelli, University of Salerno, ItalyReviewed by:
Li-Da Wu, Nanjing Medical University, ChinaMarcellino Monda, University of Campania Luigi Vanvitelli, Italy
Copyright © 2024 Han, Chen, Liu, Yang, Nie, Yang and Mou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mingjiu Yang, MTM4OTYzNDYzNzFAMTYzLmNvbQ==; Xinglang Mou, ZGp4enl5bXhsQDE2My5jb20=
 Xu Han
Xu Han Xi Chen1
Xi Chen1