
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
BRIEF RESEARCH REPORT article
Front. Pharmacol. , 12 July 2024
Sec. Drugs Outcomes Research and Policies
Volume 15 - 2024 | https://doi.org/10.3389/fphar.2024.1370040
This article is part of the Research Topic Women in Drugs Outcomes Research and Policies: 2023 View all 11 articles
 Jieqiong Liu1,2†
Jieqiong Liu1,2† Xuan Zhang3†
Xuan Zhang3† Gang Liang1†
Gang Liang1† Jianping Zhu1
Jianping Zhu1 Yi Yang1
Yi Yang1 Ying Zheng2
Ying Zheng2 Yun Han1,4,5
Yun Han1,4,5 Lingyan Yu5,6
Lingyan Yu5,6 Yuhua Zhao7*
Yuhua Zhao7* Zhenwei Yu1,5*
Zhenwei Yu1,5*Background: The latest published therapeutic drug monitoring (TDM) guidelines for vancomycin recommend changing trough-based monitoring to area under the concentration-to-time curve (AUC)-based monitoring. This study aimed to evaluate the implementation status and perceptions of vancomycin AUC-based TDM in China and to determine the challenges in performing AUC-based TDM.
Methods: A nationwide cross-sectional survey was conducted in China using an online questionnaire. The questionnaire comprised a total of 25 questions with open- and closed-ended answers to collect information about the current implementation of vancomycin TDM and the participants’ perceptions of these practices. The questionnaire responses were collected via the Questionnaire Star platform and analyzed.
Results: A total of 161 questionnaires were completed by 131 hospitals and were included. Approximately 59.5% (78/131) of the surveyed hospitals conducted vancomycin TDM; however, only 10.7% (14/131) of these hospitals performed AUC-based vancomycin TDM. Of the eligible participants, 58.4% (94/161) had experience with vancomycin TDM, and only 37 participants (37/161, 23.0%) had the ability to estimate the AUC, primarily through Bayesian simulation (33/161, 20.5%). The participants considered the following challenges to implementing AUC-based monitoring: (1) the high cost of AUC-based monitoring; (2) inadequate knowledge among pharmacists and/or physicians; (3) the complexity of AUC calculations; (4) difficulty obtaining AUC software; and (5) unclear benefit of AUC-based monitoring.
Conclusion: The majority of surveyed hospitals have not yet implemented AUC-based vancomycin TDM. Multiple challenges should be addressed before wide implementation of AUC-based monitoring, and guidance for trough-based monitoring is still needed.
Vancomycin is a commonly used glycopeptide antibiotic in clinical practice for the treatment of serious infections caused by gram-positive bacteria, including methicillin-resistant Staphylococcus aureus (MRSA) (Tong et al., 2015; Burns and Goldman, 2020). Vancomycin has a narrow therapeutic window and large interindividual pharmacokinetic variability; thus, therapeutic drug monitoring (TDM) has been a key approach for maximizing its therapeutic efficacy and minimizing the risk of nephrotoxicity (Perin et al., 2020). The optimal TDM practice for vancomycin is evolving but still controversial (Jorgensen et al., 2021; Lodise and Drusano, 2021). The 2009 American guideline recommends monitoring vancomycin trough concentrations in routine clinical practice, which can be used as a surrogate marker for the 24-hour area under the curve (AUC) because of the historical difficulty in estimating the AUC for vancomycin (Rybak et al., 2009). This guideline recommended a target trough concentration of 15–20 mg/L to increase the likelihood of attaining an AUC of ≥400 mg h/L (Rybak et al., 2009). However, there is increasing evidence of limitations in vancomycin trough monitoring, such as a poor linear relationship between trough concentrations and the AUC, and that trough-guided TDM possibly leads to overexposure, thereby increasing the risk of nephrotoxicity (Patel et al., 2011; Clark et al., 2019; Yamada et al., 2023). In light of these findings and the increasing accessibility of AUC estimation software, the 2020 American guideline and 2022 Japanese guideline recommended a pivotal change in vancomycin TDM target from trough to 24-hour area under the curve/minimum inhibitory concentration (AUC/MIC) or AUC (with a surrogate MIC of 1 mg/L), which is in accordance with its pharmacokinetics and pharmacodynamics profile and no longer recommended the trough guided doing (Rybak et al., 2020; Matsumoto et al., 2022). As it would be a challenge for pharmacists and physicians to estimate the AUC based on limited samples in routine clinical practice, the Chinese guideline recommended the AUC and trough concentration both for vancomycin TDM (He et al., 2020).
Currently, there is limited knowledge regarding the implementation status of AUC-based vancomycin TDM in Chinese hospitals, as well as a lack of understanding about the perceptions of pharmacists and physicians regarding AUC-guided vancomycin monitoring. Thus, we conducted this nationwide cross-sectional survey to determine the overall implementation status, perception and knowledge of AUC-based vancomycin monitoring and to identify the main difficulties in performing AUC-based TDM. The findings of this study will provide valuable evidence for determining the current extent and approach to implementing vancomycin AUC-based monitoring and provide guidance on how to further implement vancomycin monitoring in the future.
This nationwide cross-sectional survey was conducted in China using an online questionnaire. A convenient sampling approach was applied to enroll participants throughout mainland China in August 2023. The participants were invited to answer the questions through a link to the questionnaire via social media (WeChat group). Participation was voluntary, confidential, and anonymous.
The ethics committee of Sir Run Shaw Hospital, School of Medicine, Zhejiang University, reviewed the protocol and decided that ethical approval was not needed.
The questionnaire comprised a total of 25 questions with open- and closed-ended answers to collect information about the current implementation status of vancomycin TDM and the participants’ perceptions of these practices. The English version of the questionnaire is available in Supplementary Table S1. This survey was created by investigators, and the questionnaire piloting was conducted by several anti-infective clinical pharmacists to assess its relevance, clarity, validity, reliability and completeness. The data collected in the survey included the participants’ demographic information, the implementation status of vancomycin TDM in the participants’ hospitals, the pattern of vancomycin TDM (e.g., trough-based TDM or AUC-based TDM), the participant’s ability to estimate the AUC of vancomycin, the method of estimating the AUC of vancomycin (e.g., Bayesian estimation or first-order PK equations), and the participants’ perceptions about changing the vancomycin TDM strategy from trough-based to AUC-based and challenges or barriers to implementing AUC-based vancomycin TDM. This questionnaire was designed with skip logic to reduce the completion time and minimize survey fatigue.
The questionnaire responses were collected via the Questionnaire Star platform (https://www.wjx.cn/), which is the largest online survey platform in China, and analyzed via Microsoft Excel 2019 (Yin et al., 2022). When “other” answers were selected for certain questions, the investigators independently reviewed the free-text responses and assessed the intent of their responses. Based on the investigators’ assessments, responses with similar intent were classified together. All the results are presented descriptively as numbers and percentages.
A total of 162 questionnaire responses were obtained from 131 hospitals in 20 provinces in China. One questionnaire was excluded from the final analysis because of an incomplete response. Therefore, 161 participants with complete responses were eligible and included in the analysis. The demographic characteristics of the participants and hospitals are shown in Tables 1, 2. The main participants were pharmacists from tertiary hospitals.
We investigated the overall implementation status of performing AUC-based TDM. Surprisingly, routine vancomycin TDM was administered in only 59.5% (78/131) of the surveyed hospitals. Moreover, only 10.7% (14/131) of these hospitals used AUC-based vancomycin TDM (Table 2). Of the eligible participants, 58.4% (94/161) had experience with vancomycin TDM, and only 37 participants (37/161, 23.0%) had the ability to estimate the AUC (Table 1). The hospitals surveyed preferred a combination of the two methods of monitoring (100/161, 62.1%), and more than half of the respondents indicated that they expected to conduct or transition to AUC-based monitoring within 1 year (92/161, 57.1%), although a significant number of respondents indicated that they were not sure about the need to transition (59/161, 36.6%).
The perceptions and knowledge of AUC-based monitoring in participants who had experience with vancomycin TDM are shown in Table 3. Participants identified patients at high risk of nephrotoxicity (74/94, 78.7%) as the preferred indications for vancomycin TDM, followed by critically ill patients (70/94, 74.5%). The most commonly accepted AUC/MIC target value for vancomycin was 400–600 (33/94, 35.1%), which was also recommended by American and Japanese guidelines. However, the appropriate AUC for vancomycin was still unclear for many people (45/94, 47.9%). In addition, participants considered the most appropriate vancomycin trough concentration targets to be 10–15 mg/L for adult patients (66/94, 70.2%) and 15–20 mg/L for adult patients with severe MRSA infections (64/94, 68.1%), which were recommended by the Chinese guidelines.
For the guidelines to change the monitoring index of vancomycin from the trough concentration to the AUC, pharmacists and physicians have varying perspectives. Of the 161 respondents, 35 pharmacists and physicians expressed their views on the current vancomycin TDM guidelines. Most of the respondents (24/35, 68.6%) supported that AUC monitoring is a more accurate and meaningful approach, which is highly conducive to individualized use in the clinic to improve therapeutic efficacy. However, a portion of the respondents (6/35, 17.1%) held a less optimistic view due to perceived complexities associated with AUC calculation and the current lack of sufficient high-quality evidence on benefits of AUC-based monitoring, thereby posing challenges for its routine implementation.
The challenges and barriers to implementing AUC-based monitoring as perceived by the participants are shown in Figure 1. Unsurprisingly, the highest barrier to implementing vancomycin TDM was the cost of AUC-based monitoring (113/161, 70.2%), which included but was not limited to Bayesian software costs, and staff training costs. Inadequate knowledge about AUC-based monitoring (105/161, 65.2%) was the second challenge. The complexity of the AUC calculations and the difficulty of obtaining AUC software were also identified as important challenges by approximately half of the participants. Furthermore, the unclear benefit of AUC-based monitoring is also an important barrier that should be considered.
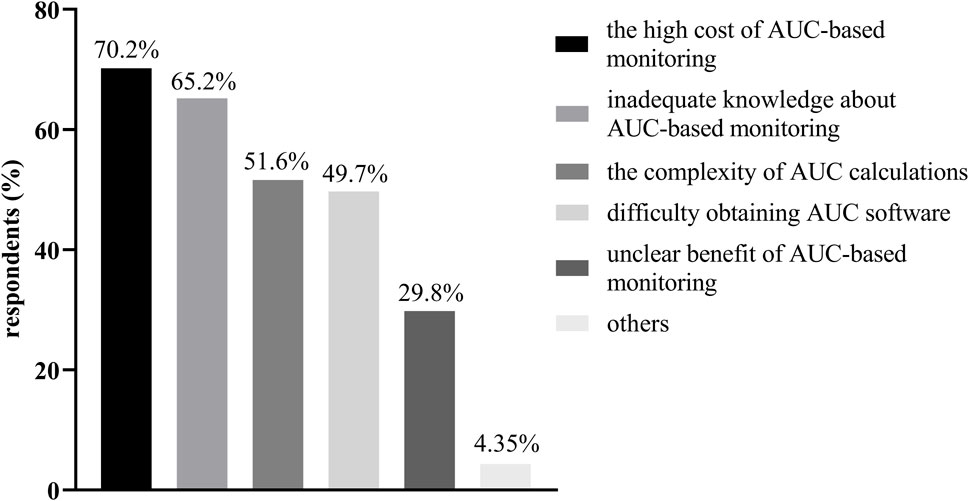
Figure 1. The challenges and barriers to implementing AUC-based monitoring as perceived by the participants.
To the best of our knowledge, this is the first survey to evaluate the implementation status and perception of vancomycin AUC-based TDM in China. Our study included 131 hospitals from 20 provinces in China and could adequately reflect the status of vancomycin TDM. Based on the results of this study, vancomycin AUC-based TDM has not yet been widely implemented in clinical practice, and most hospitals still use trough-based TDM. The perceptions of pharmacists and physicians about vancomycin TDM were inconsistent with the current guidelines. Difficulties in AUC estimation and high cost were the main issues that needed to be accounted for before the implementation of AUC-based monitoring. It is too early to recommend AUC-based monitoring only in China, as well as other resource limited areas. This survey also demonstrated the dilemmas and doubts of vancomycin AUC monitoring, which may be helpful in its further implementation.
The revised vancomycin TDM guidelines, which recommend AUC-based monitoring, were published more than 3 years ago (He et al., 2020; Rybak et al., 2020; Matsumoto et al., 2022). However, this study revealed that AUC-based monitoring was still not commonly used among Chinese hospitals. Only 10.7% (14/131) of the responding hospitals adopted AUC-based monitoring, whereas 51.9% (68/131) used conventional peak and/or trough-based monitoring. Similar situations in other countries have also been reported. A cross-sectional survey of a national health consortium performed in 2019 showed that 23.1% of responding academic medical centers performed AUC-based TDM (Kufel et al., 2019). Another survey performed in 2022, 2 years after the publication of American updated guideline, revealed that only 29.7% of the institutions had implemented an AUC dosing program in hospitals across America (Bradley et al., 2021). It can be estimated that AUC-based monitoring is uncommon in developing countries. Thus, we can see that AUC-based monitoring only, as recommended by some guidelines, seems to be unsuitable for resource limited areas.
We also investigated respondents’ perceptions and knowledge of AUC-based vancomycin monitoring. It is concerning that guideline-recommended populations and TDM targets were inconsistent, which confused physicians and pharmacists. The Japanese guidelines recommend that AUC-guided TDM should be routinely used for all MRSA infections, irrespective of the severity or complexity of the infection (Matsumoto et al., 2022). Even in institutions where calculating the AUC using Bayesian methods is difficult, the use of AUC-guided dosing should be considered for patients at high risk of acute kidney injury (Matsumoto et al., 2022). Similarly, the guidelines published by the Anti-infectives Committee of the International Association of Therapeutic Drug Monitoring and Clinical Toxicology (IATDMCT) recommend that TDM should be indicated for all patients who are expected to receive vancomycin for longer than 48 h (Reuter et al., 2022). On the other hand, the guidelines published by the Chinese and American authors did not recommend vancomycin TDM for all patients but rather for patients at high risk of nephrotoxicity, patients with severe infections, neonates/children, and so on (He et al., 2020; Rybak et al., 2020). From the results we can see that respondents’ perceptions and knowledge of vancomycin were not fully consistent with any guidelines. Pharmacists and physicians were not able to timely track the updates of guidelines and deeper understand the changing of TDM targets. Therefore, it is paramount important to establish a more precise and clearer guidance for better clinical practice.
There is uncertainty in the academic community regarding whether AUC monitoring is required for all patients. In our previous study, we found that a trough concentration of 15–20 mg/mL had a good relationship with an AUC of 400–600 mg·h/L in critically ill patients not receiving renal replacement therapy, and trough-guide TDM may be sufficient in these populations (Yu et al., 2023). The other two studies proposed a similar idea. Huang et al. developed a hybrid model of trough and AUC monitoring through plan‒do‒study‒act (PDSA) cycles and reported that trough-based TDM was a pragmatic strategy for short-term anticipated dosing, while AUC-based TDM was the most impactful and cost-effective for patients at high risk of nephrotoxicity (Huang et al., 2021). The value of universal AUC-based monitoring was also questioned by Dilworth and Wright, who suggested that an easier and more effective way to reduce toxicity may be to focus on effective antibiotic stewardship to reduce overall prescribing rather than optimizing dosing based on limited hypothetical data (Dilworth et al., 2020; Wright et al., 2021). This evidence seems to indicate that AUC monitoring is not necessary for all patients. Therefore, high quality evidences are urgently needed for clinical decision making.
In addition, it is important to provide education or staff training to increase awareness of vancomycin TDM among pharmacists, physicians, nurses and laboratory staff, especially those using Bayesian software, to implement vancomycin TDM successfully. This education should provide personalized multimodal strategies with profession-specific content (Reuter et al., 2022). For example, physician education should focus on evidence or problem-based learning, while nurse education should include receiving clear instructions and protocols through in-service training (Van Dort et al., 2020). In contrast, for those who need to interpret the data to make dose recommendations, education based on the background and rationale for pharmacokinetics and pharmacodynamics should be provided to aid in understanding dosing decisions (Reuter et al., 2022). Furthermore, convincing studies about vancomycin TDM are needed to resolve these inconsistencies and achieve a consensus. We investigated the factors that impede the implementation of vancomycin AUC-based TDM. Unsurprisingly, participants generally identified monitoring costs as the most significant barrier. The annual cost of purchasing software, as well as subsequent software maintenance and staff training, may be enormous. However, a previous report showed that AUC monitoring was cost-neutral and could significantly reduce patient costs (Lee et al., 2020). However, this cost‒benefit study did not consider the impact of empirical therapies that are common in clinical practice or the implementation fees of EMRs and staff training; thus, the overall costs may have been underestimated (Lee et al., 2020). It is not surprising that the guidelines are more supportive of AUC-based dosing strategies than troughs are; this change would be an enormous task for hospitals, requiring significant time, effort, cost, and training (Bland et al., 2021). Therefore, we wanted to find a safe and feasible way to reduce costs and to accommodate the needs of medical institutions that are not equipped to conduct monitoring, for example, by establishing regional medical centers to centralize testing. Moreover, given that the majority of current models rely on sparsely sampled or limited datasets, a Bayesian-based vancomycin calculation website utilizing intensive sampling or a larger number of samples would significantly enhance AUC calculations. Additionally, implementing a decision tree model could effectively reduce unnecessary resource consumption.
Difficulties in the estimation of the AUC were one of the main barriers to the implementation of AUC-based monitoring. The guidelines recommend Bayesian estimation as the preferred method for calculating the AUC of vancomycin (He et al., 2020; Rybak et al., 2020; Matsumoto et al., 2022). Other methods, such as first-order PK equations, require two steady-state vancomycin concentrations, which may result in additional sampling and testing (Meng et al., 2019). Moreover, the calculation is complex. The advantage of Bayesian estimation is that the AUC of vancomycin can be estimated using trough-only data or plasma concentration data at any random time within the first 24–48 h (Rybak et al., 2020). Notably, the use of Bayesian software to calculate the vancomycin AUC and optimize the dose presupposes the use of a well-developed vancomycin population PK model as a Bayesian prior. Obviously, Bayesian programs adopting such priors are extremely rare, and most of them were developed based on sparse sampling (Aljutayli et al., 2022). On the other hand, there are differences in the clinical settings for which different software programs are applicable, so a combination of multiple software programs may be required to meet clinical needs (He et al., 2022). Furthermore, due to the heterogeneity among vancomycin population pharmacokinetic models, selecting an appropriate model for clinical use is not trivial. Models developed in a specific patient population may perform poorly when applied to more general inpatient populations or other patient populations, making them highly susceptible to bias in dosing decisions (Greppmair et al., 2023). Even for the same patient population, different models may lead to different results, which may be related to the sample size, heterogeneous study designs or assay methodology. Broeker et al. compared thirty-one published population pharmacokinetic models of vancomycin and elucidated that the relative bias and relative root mean squared error of the a priori predictions varied substantially (−122.7%–67.96% and 44.3%–136.8%, respectively) (Broeker et al., 2019). Therefore, some scholars recommend that extensive evaluation is required before applying any model to clinical patients (Guo et al., 2019).
Moreover, there is still uncertainty regarding whether the implementation of vancomycin AUC-based monitoring increases the likelihood of clinical cure. Systematic evaluation and meta-analysis revealed considerable heterogeneity in the pooled sensitivity and specificity of the vancomycin AUC/MIC ratio for predicting clinical outcomes, and the majority of these studies failed to demonstrate a relationship between the AUC/MIC and positive clinical outcomes (Dalton et al., 2020). Another retrospective study in patients with enterococcal infections showed that an AUC/MIC ≥400 was associated with significant differences in clinical and microbiological responses, as well as a higher rate of nephrotoxicity compared to an AUC/MIC <400 (Katip and Oberdorfer, 2021).
This study has several limitations. First, this electronic survey was widely distributed through social media (WeChat group), and we could not measure the true response rate because of the inability to know how many questionnaires were actually distributed; thus, it may introduce a non-response bias. Second, most of the hospitals surveyed in this study were tertiary care hospitals in Eastern China, and sampling bias may exist. In addition, some participants selected “other” for some questions and entered free text for clarification. The inclusion of these textual responses may still introduce bias, despite an independent review of these texts by our investigators. Furthermore, despite the considerable cost being the primary limiting factor for implementing AUC-based monitoring, we did not collect expenditure data comparing AUC-based and trough-based TDM. This aspect merits further investigation in future studies to enhance our comprehension of the feasibility of promoting AUC-guided TDM. Finally, we omitted collecting information regarding hospitals’ selection of software for calculating the AUC and evaluating its reliability. Such data could serve as a reference for other hospitals intending to conduct AUC TDM in the future.
The majority of surveyed hospitals have not yet implemented AUC-based vancomycin TDM, especially in economically underdeveloped areas. The ability of physicians and pharmacists to estimate the AUC is also generally inadequate and requires further training. The highest ranked barrier to implementing vancomycin TDM was the cost of AUC-based monitoring, followed by the unfamiliarity of pharmacists and/or physicians. Given the low implementation rate and the lack of standardization of methods for estimating the AUC of vancomycin, it may be too early to recommend AUC-based TDM only, and trough-based monitoring is still needed. We look forward to more comprehensive analyses of vancomycin monitoring across diverse populations, and to developing a decision-tree model that will provide practical implementation strategies.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
JL: Data curation, Formal Analysis, Investigation, Methodology, Visualization, Writing–original draft. XZ: Data curation, Writing–review and editing. GL: Data curation, Software, Writing–review and editing. JZ: Data curation, Writing–review and editing. YY: Data curation, Writing–review and editing. YiZ: Data curation, Writing–review and editing. YH: Data curation, Writing–review and editing. LY: Data curation, Writing–review and editing. YuZ: Project administration, Supervision, Writing–review and editing. ZY: Conceptualization, Investigation, Project administration, Validation, Writing–original draft, Writing–review and editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2024.1370040/full#supplementary-material
Aljutayli, A., Thirion, D. J. G., and Nekka, F. (2022). Critical assessment of the revised guidelines for vancomycin therapeutic drug monitoring. Biomed. Pharmacother. 155, 113777. doi:10.1016/j.biopha.2022.113777
Bland, C. M., Crosby, C. M., Orvin, D. L., Smith, S. E., and Jones, B. M. (2021). Transitioning from guideline approval to practical implementation of AUC-based monitoring of vancomycin. Biomed. Pharmacother. 78, 1270–1272. doi:10.1093/ajhp/zxab132
Bradley, N., Lee, Y., and Sadeia, M. (2021). Assessment of the implementation of AUC dosing and monitoring practices with vancomycin at hospitals across the United States. J. Pharm. Pract. 35, 864–869. doi:10.1177/08971900211012395
Broeker, A., Nardecchia, M., Klinker, K. P., Derendorf, H., Day, R. O., Marriott, D. J., et al. (2019). Towards precision dosing of vancomycin: a systematic evaluation of pharmacometric models for Bayesian forecasting. Clin. Microbiol. Infect. 25, 1286.e1–1286. doi:10.1016/j.cmi.2019.02.029
Burns, A. N., and Goldman, J. L. (2020). A moving target—vancomycin therapeutic monitoring. J. Pediatr. Infect. Dis. Soc. 9, 474–478. doi:10.1093/jpids/piaa078
Clark, L., Skrupky, L. P., Servais, R., Brummitt, C. F., and Dilworth, T. J. (2019). Examining the relationship between vancomycin area under the concentration time curve and serum trough levels in adults with presumed or documented staphylococcal infections. Ther. Drug Monit. 41, 483–488. doi:10.1097/FTD.0000000000000622
Dalton, B. R., Rajakumar, I., Langevin, A., Ondro, C., Sabuda, D., Griener, T. P., et al. (2020). Vancomycin area under the curve to minimum inhibitory concentration ratio predicting clinical outcome: a systematic review and meta-analysis with pooled sensitivity and specificity. Clin. Microbiol. Infect. 26, 436–446. doi:10.1016/j.cmi.2019.10.029
Dilworth, T. J., Schulz, L. T., and Rose, W. E. (2020). Vancomycin advanced therapeutic drug monitoring: exercise in futility or virtuous endeavor to improve drug efficacy and safety?. Clin. Infect. Dis. 72, e675–e681. doi:10.1093/cid/ciaa1354
Greppmair, S., Brinkmann, A., Roehr, A., Frey, O., Hagel, S., Dorn, C., et al. (2023). Towards model-informed precision dosing of piperacillin: multicenter systematic external evaluation of pharmacokinetic models in critically ill adults with a focus on Bayesian forecasting. Intensive Care Med. 49, 966–976. doi:10.1007/s00134-023-07154-0
Guo, T., Van Hest, R. M., Roggeveen, L. F., Fleuren, L. M., Thoral, P. J., Bosman, R. J., et al. (2019). External evaluation of population pharmacokinetic models of vancomycin in large cohorts of intensive care unit patients. Antimicrob. Agents Chemother. 63, 025433. doi:10.1128/AAC.02543-18
He, N., Su, S., Ye, Z., Du, G., He, B., Li, D., et al. (2020). Evidence-based guideline for therapeutic drug monitoring of vancomycin: 2020 update by the division of therapeutic drug monitoring, Chinese pharmacological society. Clin. Infect. Dis. 71, 363–371. doi:10.1093/cid/ciaa1536
He, N., Yan, Y., Liu, B., Zhang, Y., Su, S., and Zhai, S. (2022). Investigation of the applicability and predication accuracy of different pharmacokinetic-guided individualized vancomycin dosing tools. Chin. J. Clin. Pharmacol. 38, 2884–2888.
Huang, J., Chan, J. D., Nguyen, T., Jain, R., and Escobar, Z. K. (2021). Doing more with less: pragmatic implementation of vancomycin area-under-the-curve (AUC) monitoring. J. Pharm. Pract. 36, 10–14. doi:10.1177/08971900211027271
Jorgensen, S., Spellberg, B., Shorr, A., and Wright, W. (2021). Should therapeutic drug monitoring based on the vancomycin area under the concentration-time curve Be standard for serious methicillin-resistant Staphylococcus aureus infections? No. Clin. Infect. Dis. 72, 1502–1506. doi:10.1093/cid/ciaa1743
Katip, W., and Oberdorfer, P. (2021). A monocentric retrospective study of AUC/MIC ratio of vancomycin associated with clinical outcomes and nephrotoxicity in patients with enterococcal infections. Pharmaceutics 13, 1378. doi:10.3390/pharmaceutics13091378
Kufel, W. D., Seabury, R. W., Mogle, B. T., Beccari, M. V., Probst, L. A., and Steele, J. M. (2019). Readiness to implement vancomycin monitoring based on area under the concentration–time curve: a cross-sectional survey of a national health consortium. Am. J. Health. Syst. Pharm. 76, 889–894. doi:10.1093/ajhp/zxz070
Lee, B. V., Fong, G., Bolaris, M., Neely, M., Minejima, E., Kang, A., et al. (2020). Cost–benefit analysis comparing trough, two-level AUC and Bayesian AUC dosing for vancomycin. Clin. Microbiol. Infect. 27, 1346.e1–1346.e7. doi:10.1016/j.cmi.2020.11.008
Lodise, T., and Drusano, G. (2021). Vancomycin area under the curve–guided dosing and monitoring for adult and pediatric patients with suspected or documented serious methicillin-resistant Staphylococcus aureus infections: putting the safety of our patients first. Clin. Infect. Dis. 72, 1497–1501. doi:10.1093/cid/ciaa1744
Matsumoto, K., Oda, K., Shoji, K., Hanai, Y., Takahashi, Y., Fujii, S., et al. (2022). Clinical practice guidelines for therapeutic drug monitoring of vancomycin in the framework of model-informed precision dosing: a consensus review by the Japanese society of chemotherapy and the Japanese society of therapeutic drug monitoring. Pharmaceutics 14, 489. doi:10.3390/pharmaceutics14030489
Meng, L., Wong, T., Huang, S., Mui, E., Nguyen, V., Espinosa, G., et al. (2019). Conversion from vancomycin trough concentration-guided dosing to area under the curve-guided dosing using two sample measurements in adults: implementation at an academic medical center. Pharmacotherapy 39, 433–442. doi:10.1002/phar.2234
Patel, N., Pai, M. P., Rodvold, K. A., Lomaestro, B., Drusano, G. L., and Lodise, T. P. (2011). Vancomycin: we can’t get there from here. Clin. Infect. Dis. 52, 969–974. doi:10.1093/cid/cir078
Perin, N., Roger, C., Marin, G., Molinari, N., Evrard, A., Lavigne, J.-P., et al. (2020). Vancomycin serum concentration after 48 h of administration: a 3-years survey in an intensive care unit. Antibiotics 9, 793. doi:10.3390/antibiotics9110793
Reuter, S. E., Stocker, S. L., Alffenaar, J.-W. C., Baldelli, S., Cattaneo, D., Jones, G., et al. (2022). Optimal practice for vancomycin therapeutic drug monitoring: position statement from the anti-infectives committee of the international association of therapeutic drug monitoring and clinical Toxicology. Ther. Drug Monit. 44, 121–132. doi:10.1097/FTD.0000000000000944
Rybak, M., Lomaestro, B., Rotschafer, J. C., Moellering, R., Craig, W., Billeter, M., et al. (2009). Therapeutic monitoring of vancomycin in adult patients: a consensus review of the American society of health-system pharmacists, the infectious diseases society of America, and the society of infectious diseases pharmacists. Am. J. Health. Syst. Pharm. 66, 82–98. doi:10.2146/ajhp080434
Rybak, M. J., Le, J., Lodise, T. P., Levine, D. P., Bradley, J. S., Liu, C., et al. (2020). Therapeutic monitoring of vancomycin for serious methicillin-resistant Staphylococcus aureus infections: a revised consensus guideline and review by the American society of health-system pharmacists, the infectious diseases society of America, the pediatric infectious diseases society, and the society of infectious diseases pharmacists. Am. J. Health. Syst. Pharm. 77, 835–864. doi:10.1093/ajhp/zxaa036
Tong, S. Y. C., Davis, J. S., Eichenberger, E., Holland, T. L., and Fowler, V. G. (2015). Staphylococcus aureus infections: epidemiology, pathophysiology, clinical manifestations, and management. Clin. Microbiol. Rev. 28, 603–661. doi:10.1128/CMR.00134-14
Van Dort, B. A., Baysari, M. T., Carland, J. E., Stocker, S. L., Braithwaite, H. E., Fernon, A. R., et al. (2020). Education to improve vancomycin use: the perspectives of educators and education recipients. Intern. Med. J. 50, 565–572. doi:10.1111/imj.14408
Wright, W. F., Jorgensen, S. C. J., and Spellberg, B. (2021). Heaping the pelion of vancomycin on the ossa of methicillin-resistant Staphylococcus aureus: back to basics in clinical care and guidelines. Clin. Infect. Dis. 72, e682–e684. doi:10.1093/cid/ciaa1360
Yamada, Y., Niwa, T., Ono, Y., Yamada, S., Niwa, K., Yasue, M., et al. (2023). Comparison of the incidence of vancomycin-associated nephrotoxicity following the change from trough-guided dosing to AUC-guided doing using trough-only data. J. Antimicrob. Chemother. 78, 2933–2937. doi:10.1093/jac/dkad333
Yin, X., Gong, Y., Sun, N., Li, D., Wu, J., Wang, J., et al. (2022). Prevalence of inappropriate use behaviors of antibiotics and related factors among Chinese antibiotic users: an online cross-sectional survey. BMC Infect. Dis. 22, 689. doi:10.1186/s12879-022-07671-1
Yu, Z., Liu, J., Yu, H., Zhou, L., Zhao, Y., Zhong, L., et al. (2023). Should the trough concentration of vancomycin be abandoned in therapeutic drug monitoring? A multicenter retrospective study in critically ill patients without any form of dialysis. Int. J. Antimicrob. Agents 61, 106812. doi:10.1016/j.ijantimicag.2023.106812
Keywords: vancomycin, survey, therapeutic drug monitoring, trough concentration, area under the concentration-time curve
Citation: Liu J, Zhang X, Liang G, Zhu J, Yang Y, Zheng Y, Han Y, Yu L, Zhao Y and Yu Z (2024) Is it time to recommend AUC-based vancomycin therapeutic drug monitoring only? A cross-sectional survey in China. Front. Pharmacol. 15:1370040. doi: 10.3389/fphar.2024.1370040
Received: 13 January 2024; Accepted: 01 July 2024;
Published: 12 July 2024.
Edited by:
Ceu Mateus, Lancaster University, United KingdomReviewed by:
Xiao Li, Shandong Provincial Qianfoshan Hospital, ChinaCopyright © 2024 Liu, Zhang, Liang, Zhu, Yang, Zheng, Han, Yu, Zhao and Yu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yuhua Zhao, emhhb3l1aHVhMTk4N0AxMjYuY29t; Zhenwei Yu, eXp3X3NycnNoQHpqdS5lZHUuY24=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.