- 1Assisted Reproduction Unit, Department of Obstetrics and Gynecology, Sir Run Run Shaw Hospital, School of Medicine, Zhejiang University, Hangzhou, China
- 2Institute for Reproductive Health, School of Pharmacy, Hangzhou Medical College, Hangzhou, China
Extracellular vesicles (EVs) are nanometer-sized lipid bilayer vesicles released by cells, playing a crucial role in mediating cellular communication. This review evaluates the effect of EVs on early embryonic development in vitro by systematically searching the literature across three databases, Embase, PubMed, and Scopus, from inception (Embase, 1947; PubMed, 1996; and Scopus, 2004) to 30 June 2024. A total of 28 studies were considered relevant and included in this review. The EVs included in these investigations have been recovered from a range of sources, including oviduct fluid, follicular fluid, uterine fluid, seminal plasma, embryos, oviduct epithelial cells, endometrial epithelial cells, amniotic cells, and endometrial-derived mesenchymal stem cells collected primarily from mice, rabbits, cattle and pigs. This diversity in EV sources highlights the broad interest and potential applications of EVs in embryo culture systems. These studies have demonstrated that supplementation with EVs derived from physiologically normal biofluids and cells to the embryo culture medium system has positive effects on embryonic development. Conversely, EVs derived from cells under pathological conditions have shown a negative impact. This finding underscores the importance of the source and condition of EVs used in culture media. Further, the addition of EVs as a culture medium supplement holds significant therapeutic potential for optimizing in vitro embryo culture systems. In conclusion, this evaluation offers a thorough assessment of the available data on the role of EVs in embryo culture media and highlights the potential and challenges of using EVs in vitro embryo production.
1 Introduction
Assisted reproductive technology (ART) has provided hope to thousands of infertile couples over recent decades. A critical step in assisted reproduction involves culturing the preimplantation embryo in vitro. During this process, from the fertilization of mature oocytes to the blastocyst stage, three critical events occur, including the transition from maternal to zygotic transcripts, compaction, and the first distinct lineage differentiation into inner cell mass (ICM) and trophectoderm (TE) (Hamatani et al., 2006). Embryo development in vitro depends on various factors, including embryo density, the concentration of embryo-secreted factors linked with the culture volume, oviductal or endometrial cells provided by co-culture systems, the stiffness of the substrate in contact with the embryo; and fluid mechanical stimulation, such as vibration and tilting, mimicked by the dynamic culture system (Gualtieri et al., 2024). Furthermore, the composition of the culture medium plays a crucial role in embryonic development, directly affecting these key developmental events and the implantation potential of embryos (Morbeck et al., 2017; Koscinski et al., 2018; Menezo and Elder, 2020). Current research and industry trends are towards using chemically defined media due to their suitability for manufacturing and monitoring. Convincing evidence has suggested that the current culture medium used in vitro embryo culture is suboptimal and needs improvement (Stone et al., 2014; Chronopoulou and Harper, 2015; Ducreux et al., 2023).
Recent studies have identified extracellular vesicles (EVs) as key factors influencing embryo development (Machtinger et al., 2016; Bastos et al., 2022; Chen et al., 2022; Gualtieri et al., 2024). EVs are a heterogeneous population of membranous nano to micro-sized particles secreted by almost all cell types. They contain a range of cargo, including proteins, lipids, nucleic acids, and metabolites, which can be taken up by other cells, and elicit a broad variety of biological effects (Alminana et al., 2017; Alminana and Bauersachs, 2020; Aguilera et al., 2024; Zhang et al., 2024). EVs play a crucial role in mediating communication between the female reproductive tract and gametes/embryos. For example, EVs in the maternal oviduct communicate with both male and female gametes, whereas those in the uterus communicate with male gametes and embryos. The ciliated and secretory cells of the oviduct epithelial lining that can produce the EVs, along with the oviduct fluid, establish the culture system for early embryonic development in vivo (Coy et al., 2012; Alminana and Bauersachs, 2020). This interaction, facilitated by endocrine and paracrine signaling factors, provides an optimal environment for the normal development of early embryos (Ezzati et al., 2014). It is further hypothesized that EVs may be beneficial for the adequate developmental competence of embryos in vitro culture (Machtinger et al., 2016; Gualtieri et al., 2024).
Therefore, EV supplementation in culture media may offer a viable option to optimize the embryo culture medium system and enhance IVF efficiency. This review aims to discuss the influence of EVs from various origins on embryo development and to summarize the currently available evidence on the effect of EVs as a potential supplementing component of culture medium on embryo development in vitro.
2 Search methods
To identify relevant studies, we performed a systematic search across three major databases: Embase (Elsevier, Amsterdam, Netherlands), PubMed (NCBI, Bethesda, MD), and Scopus (Elsevier, Amsterdam, Netherlands). This search was restricted to studies published in English from the inception of Embase (1947), PubMed (1996), and Scopus (2004) to 30 June 2024. We used a combination of search terms (“extracellular vesicles” OR “microvesicles” OR “microparticles” OR “exosomes” OR “epididymosomes” OR “prostasomes” OR “oviductosomes” OR “uterosomes”) AND (“embryo*” OR “development”) AND (“add*” OR “supplement*”). The detailed search approach is supplied in Supplementary Table S1.
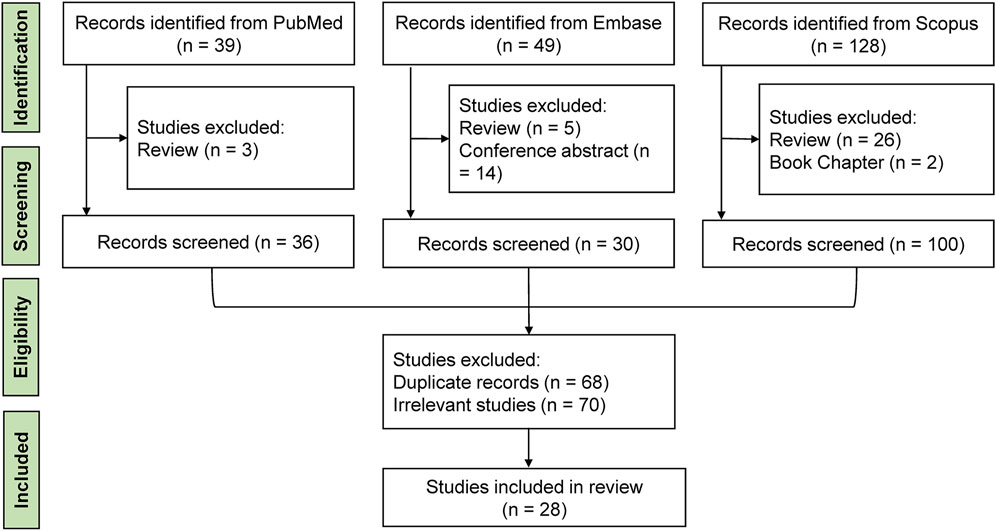
Initially, two reviewers (YMX and HXZ) independently screened publications based on titles and abstracts, excluding irrelevant and duplicate studies. Any discrepancies were resolved through discussion with a third reviewer (KL). Full-length articles that focused on the supplementation of culture medium with EVs and their impact on embryo development in vitro were retrieved for further evaluation. Studies examining the effects of EVs on sperm function, egg development, or specific components like microRNAs were excluded. We also excluded meta-analyses, letters, reviews, conference proceedings, and theses. Selected articles’ references were examined to find further relevant studies. Ultimately, 28 records were deemed relevant and included in this review. The workflow of the literature review is illustrated in Figure 1. From each selected study, we extracted data on the year of publication, authors, species, origins of EVs, centrifugal force, EVs diameter range, EVs markers, the added concentration of EVs, embryo stage, embryo assessment, and main findings.
3 Effects of EVs derived from different origins on embryonic development in vitro
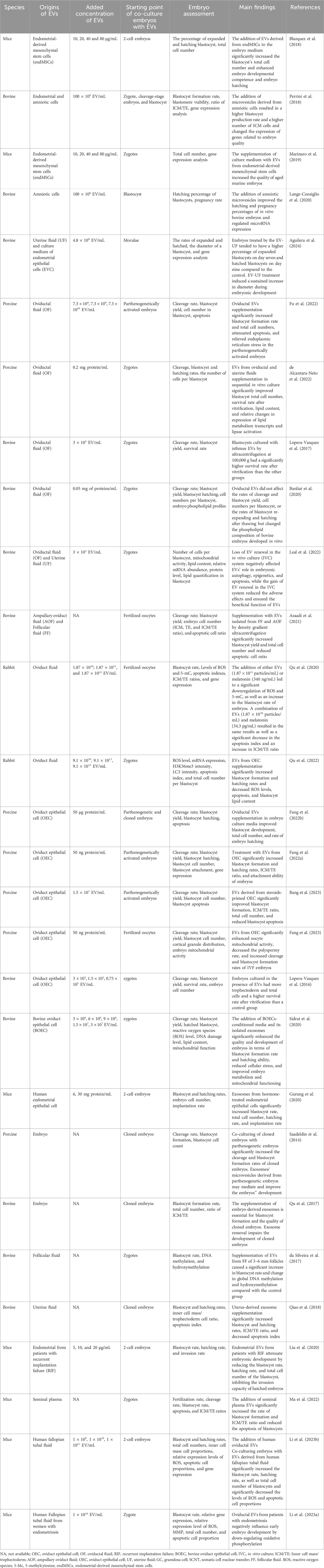
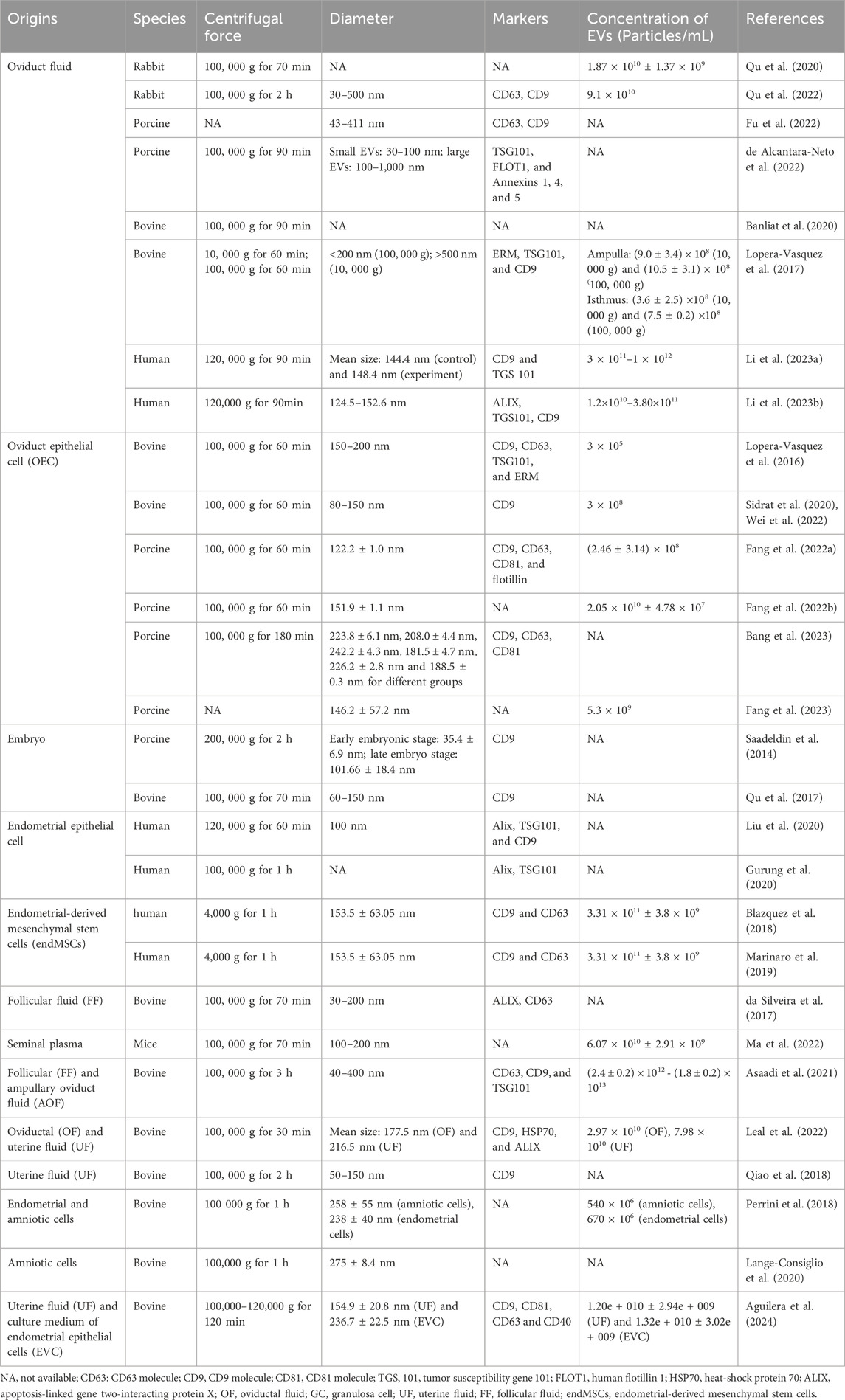
The effects of EVs from different origins on embryonic development in vitro are summarized in Table 1, with their characteristics detailed in Table 2.
3.1 EVs derived from oviductal fluid
Oviductal fluid containing EVs, known as oviductosomes (Al-Dossary et al., 2013), is the first environment encountered by a mammalian embryo. The oviduct, comprising the infundibulum, ampulla, and isthmus, is where fertilization and early embryo development occur. Numerous studies have evaluated the effects of oviductal fluid EVs on the development and quality of in vitro-cultured embryos (Lopera-Vasquez et al., 2017; Banliat et al., 2020; Qu et al., 2020; Asaadi et al., 2021; de Alcantara-Neto et al., 2022; Fu et al., 2022; Leal et al., 2022; Qu et al., 2022; Li et al., 2023a; Li et al., 2023b). Research has spanned to various mammals, including humans (Li et al., 2023a; Li et al., 2023b), rabbits (Qu et al., 2020; Qu et al., 2022), pigs (de Alcantara-Neto et al., 2022; Fu et al., 2022), and cattle (Lopera-Vasquez et al., 2017; Banliat et al., 2020; Asaadi et al., 2021; Leal et al., 2022). For instance, Lopera-Vásquez et al. demonstrated that adding bovine oviduct EVs to the in vitro culture of bovine embryos improved blastocyst quality compared to serum-supplemented media (Lopera-Vasquez et al., 2017). These EVs also upregulated genes related to metabolism, epigenetics, and water channels (Lopera-Vasquez et al., 2017). Asaadi and colleagues found that oviduct fluid EVs derived by OptiPrep™ density gradient ultracentrifugation (ODG UC) positively impacted embryo quality compared to EVs isolated by single-step size exclusion chromatography (Asaadi et al., 2021). Leal et al. showed that EV supplementation in a sequential culture system improved cell number, reduced lipid content, and increased post-vitrification survival of bovine embryos (Leal et al., 2022). Banliat et al. discovered that oviductal EVs induced significant changes in the phospholipid composition of embryos, suggesting a role in embryonic lipid metabolism (Banliat et al., 2020). Qu et al. found that melatonin in oviductal fluid and EVs increased the blastocyst rate and reduced ROS and 5-methylcytosine levels. The authors also demonstrated that the beneficial effect of oviductal fluid and its EVs was inhibited by luzindole, a melatonin receptor agonist (Qu et al., 2020). Fu et al. confirmed that oviductal EVs alleviate endoplasmic reticulum stress, benefiting early embryonic development (Fu et al., 2022). However, EVs added to the culture medium may degrade, leading to decreased embryonic development competence and increased oxidative stress, autophagy imbalance, and abnormal epigenetic modification (Qu et al., 2022). Renewing EVs in the culture system significantly reduced these adverse effects (Qu et al., 2022). De Alcântara-Neto et al. found that 2 days of EV treatment enhanced cleavage and blastocyst rates (de Alcantara-Neto et al., 2022). These EVs, released from oviductal epithelial cells and blood transudation, are internalized by embryos and influence their development. Co-culturing murine embryos with EVs derived from human fallopian tubal fluid significantly increased the blastocyst rate, hatching rate, and total cell number of blastocysts while decreasing the ROS levels and apoptotic cell proportions (Li et al., 2023b). However, oviductal EVs from patients with endometriosis negatively influence early murine embryo development by down-regulating oxidative phosphorylation (Li et al., 2023a).
3.2 EVs derived from oviduct epithelial cells (OEC) cultured in vitro
The oviduct epithelium, consisting of ciliated and secretory cells, produces the oviduct fluid that supports fertilization and early embryonic development. EVs secreted by OEC cultured in vitro have been isolated and characterized through various techniques, including transmission electron microscopy and proteomics. Adding EVs from OECs to in vitro culture media positively affected embryo development in mammals such as pigs and cattle (Lopera-Vasquez et al., 2016; Sidrat et al., 2020; Fang et al., 2022a; Fang et al., 2022b; Wei et al., 2022; Bang et al., 2023; Fang et al., 2023). Lopera-Vásquez et al. first reported that EVs from bovine OECs improved the quality of embryos cultured in vitro, suggesting that EVs mediated oviduct-embryo communication (Lopera-Vasquez et al., 2016). Sidrat et al. revealed that exosomes from bovine oviduct epithelial cells (BOEC) re-establish pyruvate flux, improving mitochondrial function during preimplantation development (Sidrat et al., 2020). Wei et al. found that exosomes from BOECs significantly improved oocyte maturation, early embryo development, and implantation potential (Wei et al., 2022). Fang et al. demonstrated that EVs from OEC increased the rate of embryo breakage and the ICM/TE ratio of porcine embryos, enhancing reprogramming and implantation-related genes (Fang et al., 2022a). Another study by Fang et al. showed that OEC EVs improved the quality and developmental ability of porcine parthenogenetic and cloned embryos (Fang et al., 2022b). Additionally, Fang et al. demonstrated that EVs isolated from OEC improved the cortical granules concentration and mitochondrial activity of porcine oocytes (Fang et al., 2023). Furthermore, the secretion of EVs may be associated with the hormonal environment in cell culture. Bang and colleagues mimicked in vivo hormonal conditions and treated porcine OECs with estradiol and progesterone, finding that EVs from conditioned OECs decreased apoptotic rate that, in turn, contributed to improved blastocyst development (Bang et al., 2023). EV characteristics of OEC linked with the hormonal condition are confirmed by our previous study, showing that the size and cargoes of EVs from mouse oviduct fluids are influenced by the estrous cycle (Yi et al., 2022). These results imply that hormones may regulate the effect of EVs from OEC on embryo development.
3.3 EVs derived from endometrial epithelial cells cultured in vitro
The complex process of embryo implantation calls for effective communication between the endometrium and the embryo (Kurian and Modi, 2019). EVs have been identified as crucial mediators in early pregnancy, particularly during implantation. Co-culture systems using embryos and EVs derived from endometrial epithelial cells have been developed to explore this interaction (Gurung et al., 2020). Gurung et al. investigated the role of different components of the secretome, including total secretome (TS), soluble secretome (SS), and crude exosomes from hormonally primed human endometrial epithelial cell culture medium, in mediating mouse embryo development and implantation (Gurung et al., 2020). They found that exosomes significantly enhanced the total cell number of in vitro embryos and the blastocyst hatching rate, suggesting that endometrial exosomes can enter embryonic cytoplasm and regulate embryo growth and implantation (Gurung et al., 2020). Liu and colleagues further explored this by isolating endometrial EVs from patients with recurrent implantation failure and fertile women, using a co-culture model to compare their effects on murine embryos (Liu et al., 2020). They found that endometrial EVs from patients with recurrent implantation failure attenuate embryonic development and decreased invasion capacity, highlighting the potentially harmful role of EVs in pathological conditions (Liu et al., 2020). Authors speculated that the molecules upregulated in the endometrial of patients with recurrent implantation failure, such as miR-145 (Revel et al., 2011; Wen et al., 2018), may transmitted to embryonic cells via EVs, thereby impeding the migrating and invasion capacity of trophoblasts (Liu et al., 2020). These findings underscore the potential of EVs from endometrial epithelial cells in improving in vitro culture conditions and emphasize the importance of adequate endometrium-embryo communication for successful implantation.
3.4 EVs from Co-Cultured embryos
Preimplantation embryo culture in groups can produce a supportive microenvironment through the secretion of various autocrine and paracrine factors, significantly enhancing embryo development (Saadeldin et al., 2014; Qu et al., 2017; Wydooghe et al., 2017). These factors are secured through active secretion, passive flow, or cargo loading into EVs (Wydooghe et al., 2017). Saadeldin et al. found that co-culturing porcine embryos derived from somatic cell nuclear transfer (SCNT) with parthenogenetic embryos improved cleavage rates and blastocyst formation, mediated by exosomes and microvesicles secreted by parthenogenetic embryos (Saadeldin et al., 2014). Similarly, Qu et al. demonstrated that supplementing the culture medium with exosomes derived from bovine SCNT embryos benefits their subsequent development, while the removal of exosomes during medium replacement impairs the development potential of SCNT embryos (Qu et al., 2017). These studies highlight the importance of EVs in creating a conducive environment for embryo development.
3.5 EVs from amniotic cells
Amniotic cells have been shown to create a more suitable microenvironment for embryo growth, enhancing in vitro embryo development. Studies have found that co-culturing embryos with amniotic cells significantly increases the blastocyst formation rate compared to co-culture with bone marrow-derived cells and cumulus cells (Lange-Consiglio et al., 2012). Perrini et al. reported that the addition of microvesicles derived from amniotic cells resulted in a higher blastocyst production rate and a higher number of ICM cells (Perrini et al., 2018). Additionally, the expression of the proapoptotic Bax gene was downregulated (Melka et al., 2010), while the expression of antioxidant selenoprotein GPX1 was significantly upregulated in the group cultured with amniotic cells (Perrini et al., 2018). Further research by the same team indicated that amniotic microvesicles improved the hatching and pregnancy percentages of in vitro bovine embryos and modulated the expression of specific microRNAs associated with embryo implantation (Lange-Consiglio et al., 2020). These findings suggest that amniotic cells and their derived EVs play a vital role in supporting embryo development and implantation.
3.6 EVs from uterine fluid
EVs released by the endometrial epithelial cells into the uterine cavity are essential for embryo development and implantation. Qiao et al. reported that exosomes from bovine uterine fluid significantly improved blastocyst yield, hatching rate, and inner cell mass/trophectoderm cell ratio while decreasing apoptosis in cloned embryos (Qiao et al., 2018). Leal et al. established a sequential in vitro culture system using EVs from the bovine oviduct and uterine fluids, which improved embryo quality post-thaw survival rate, total cell number and reduced lipid content (Leal et al., 2022). Aguilera et al. demonstrated that culturing embryos in a medium supplemented with EVs from uterine fluid tended to have a higher percentage of expanded blastocysts on day seven and hatched blastocysts on day nine compared to the control, with a sustained increase in diameter during embryo development (Aguilera et al., 2024). The expression of the IFNT gene related to embryo implantation was highly expressed in the group treated with EVs from uterine fluid (Aguilera et al., 2024). Collectively, the data obtained from these studies suggest that uterine fluid EVs influence embryo development and implantation potential.
3.7 EVs from follicular fluid
Follicular fluid EVs play a crucial role in oocyte maturation and embryo development. These EVs have been detected in various species, including equine, cats, cattle, and humans (da Silveira et al., 2012; Sohel et al., 2013; de Almeida Monteiro Melo Ferraz et al., 2020; Neyroud et al., 2022; Soares et al., 2023; Luis-Calero et al., 2024). Studies have shown that follicular fluid EVs improve blastocyst formation rates, as well as alter transcript levels and DNA methylation patterns (da Silveira et al., 2017). These impacts are thought to be mediated by particular miRNAs within the EVs that regulate DNA-protein interactions in embryos (da Silveira et al., 2017). Likewise, Asaadi et al. found that bovine embryos cultured with follicular fluid EVs had higher blastocyst yield and quality compared to control groups (Asaadi et al., 2021). These findings indicate that follicular fluid EVs enhance preimplantation embryo development and implantation potential.
3.8 EVs from endometrial-derived mesenchymal stem cells (endMSCs)
The cross-talk between the endometrium and embryo is essential for supporting embryo implantation, and this intercellular communication is partially mediated by EV release (Homer et al., 2017). The endMSCs are a subgroup of stem cells in endometrial tissue that produce various paracrine factors involved in cell growth and proliferation (Zuo et al., 2018). Blázquez et al. first reported that the addition of EVs derived from endMSCs to embryo medium significantly increased the blastocyst’s total cell number and enhanced embryo developmental competence and hatching (Blazquez et al., 2018). Further research by the same team demonstrated a beneficial effect on the development of embryos from aged female mice with supplementation of EVs derived from endMSCs (Marinaro et al., 2019). Gene expression analysis in the resulting blastocysts showed significant changes in key genes involved in metabolism, placentation, the oxidative stress response in cells, and the development of trophectoderm and inner cell masses (Marinaro et al., 2019). These findings highlight the potential of endMSC-derived EVs in supporting embryo development and implantation.
3.9 Other origins of EVs
Seminal plasma, originating from the male accessory glands and the epididymal duct, plays a significant role in modulating the maternal environment during natural pregnancy (Druart and de Graaf, 2018; Schjenken and Robertson, 2020). EVs from seminal plasma are believed to contribute to early embryo development by mediating signal communication between seminal plasma and the embryo. Ma et al. found that adding EVs from mouse seminal plasma to the embryo culture medium decreased blastocyst apoptosis and increased blastocyst formation rates and inner cell mass/trophectoderm cell ratios, suggesting improved embryo development (Ma et al., 2022). These investigations underscore the importance of seminal plasma EVs in supporting early embryonic growth.
In summary, supplementing culture media with healthy EVs derived from various origins, including oviduct fluid, follicular fluid, uterine fluid, seminal plasma, embryo, oviduct epithelial cells, endometrial epithelial cells, amniotic cells, and endometrial-derived mesenchymal stem cells, appears to enhance the developmental capacity and implantation potential of embryos. This is evidenced by increased total cell numbers, higher blastocyst yield and hatching rates, decreased ROS levels and fewer apoptotic cells. These findings emphasize the positive influence of EVs on in vitro embryo development, suggesting that EVs would serve as the supplement of in vitro culture media.
4 Mechanisms of EVs functioning in embryonic development
EVs can be internalized by embryonic cells in vitro. They can pass through the zona pellucida and subsequently be internalized by embryonic cells, positively influencing development and significantly enhancing blastocyst formation, hatching rates, and total cell numbers (da Silveira et al., 2017; Qu et al., 2020; Asaadi et al., 2021). The process of EV-mediated intercellular signaling process involves three main steps: EV release from donor cells, EV uptake by recipient cells, and targeted delivery of cargo to recipient cells (Wan et al., 2022). The mechanisms by which cells uptake EVs depending on their source and the cell type, including clathrin-mediated endocytosis, macropinocytosis, lipid raft-mediated endocytosis, receptor-mediated endocytosis, and direct membrane fusion (Mulcahy et al., 2014; Esmaeili et al., 2022).
EVs carry a variety of cargoes. The two main types of cargo are proteins and nucleic acids, which are crucial during the peri-implantation stage (Kurian and Modi, 2019; Fu et al., 2020; Nakamura et al., 2020). Protein cargoes in EVs are associated with many biological processes related to fertilization and embryonic development. Proteins associated with fertilization include oviduct glycoprotein (OVGP1), heat-shock protein family HSP90, HSPA8, HSP70, and myosin 9 (MYH) (Kadam et al., 2006; Elliott et al., 2009; Alminana et al., 2017; Zhao and Kan, 2019; Braganca et al., 2021; Lee et al., 2021; Zhao et al., 2022). Proteins involved in embryonic development include those regulating: (1) gene expression as potential biomarkers, PAG1, IFN-T, and PLAC8; (2) energy and metabolism, PLIN2, ACACA, LDHA, LDLR, PPARGC1B, FASN, and PNPLA2; (3) epigenetic regulation, DNMT3A, H3K36me3, TFAM, SNRPN; (4) oxidative stress, GPX1, MnSOD, GLUT1, GAPDH, GPX3, SOD2, GSTM2, miR-126, miR-21, and miR-128; and (5) autophagy, ATG5, BECN1, and MAP1LC3C (Lopera-Vasquez et al., 2016; Lopera-Vasquez et al., 2017; Leal et al., 2022; Qu et al., 2022). Nucleic acid cargoes in EVs that have been studied can be divided into two types of RNA: miRNA (de Alcantara-Neto et al., 2022; Leal et al., 2022) and transfer RNA-derived small RNAs (tsRNAs) (Fan et al., 2024). Li et al. identified 79 miRNAs commonly present in all EV samples out of 617 known miRNAs, with target genes involved in several signaling pathways, including the p53, Ras, PI3K/AKT, and Hippo pathways, which are essential for embryonic cell division, differentiation, and development. Examination of the transcript levels of the genes connected to embryo development showed significant upregulation in levels of Actr3, Eomes, and Wnt3a (Li et al., 2023b). Fan et al. (2024) revealed the abundance of tsRNAs in EVs secreted by blastocysts, and inhibition of tDR-14:32-Glu-CTC-1 promotes embryo hatching while influencing embryo implantation-related genes and pathways (Fan et al., 2024).
EVs regulate diverse critical functions during embryo development through multilevel intercellular signaling. The signaling encompasses cell division, growth, metabolism, and apoptosis during early embryo development (Alminana and Bauersachs, 2020; de Alcantara-Neto et al., 2022; Qu et al., 2022). EVs regulate crucial cellular activities such as adhesion, migration, and proliferation (Ng et al., 2013; Zdravkovic et al., 2015). EVs interact with target cells to promote embryonic cell growth, division, and implantation (Bastos et al., 2022; Chen et al., 2022). They significantly promote embryo implantation by up-regulating reprogramming genes related to embryonic cell proliferation and differentiation, such as POU5F1, NANOG, SOX2, c-MYC, and Klf4 (Jaber et al., 2017; Hassani et al., 2019; Fang et al., 2022a). Comprehensive quantitative proteomic profiling has revealed changes in human trophectodermal spheroids, including adhesion proteome and function, regulated by the transfer of endometrial EV cargo proteins to the trophectoderm (Evans et al., 2019). Similar to proteins in EVs, miRNAs encapsulated in EVs also modulate target-cell function by affecting the expression of transcripts and proteins during early embryo development, influencing embryo growth, morphology, and implantation, and emerging as crucial mediators of cross-talk during peri-implantation (Ng et al., 2013; Vilella et al., 2015; Balaguer et al., 2018).
Taken together, EVs, in the unique microenvironment of early embryonic development, are internalized by embryonic cells, carrying a variety of cargoes essential for development. Once internalized, EVs regulate a range of critical functions within embryos. The process of EV-mediated intercellular signaling facilitates communication and signaling that are essential for successful growth and enhances trophectoderm adhesion and invasion, thereby increasing implantation potential.
5 Future directions on clinical implications: optimizing the in vitro culture media
The success of IVF treatments depends on the quality of embryos, especially as the number of embryos transferred in clinics decreases. Unfortunately, embryos cultured in vitro often exhibit lower blastocyst formation rates, reduced pregnancy rates, and higher fetal loss compared to those developed in vivo. Clinical observations have identified the transfer of low-quality embryos as a major cause of implantation failure in IVF treatments (Abel et al., 2019; Bori et al., 2022). Some patients even lack the opportunity for embryo transfer due to the absence of high-quality embryos. Therefore, optimizing the conditions affecting embryo development in IVF labs is essential.
The supplementation of EVs in the in vitro culture media may be one promising strategy for enhancing embryo development. Despite the use of various culture media (Sepulveda et al., 2009; Paternot et al., 2010; Hardarson et al., 2015), the optimal culture medium remains unclear (Utsunomiya et al., 2022). The hypothesis that the lack of communication between female reproductive tracts and gamete/embryo contributes to the suboptimal competence of embryos cultured in vitro has led to the exploration of EV supplementation. EVs from sources such as oviduct fluid, oviduct/endometrium epithelial cells, uterine fluid, follicular fluid, embryo, and seminal plasma have shown positive effects on embryo development when added to IVF culture systems. Conversely, EVs from pathological conditions, such as endometriosis or recurrent implantation failure, may negatively impact early embryonic development (Wang et al., 2019; Qu et al., 2022; Li et al., 2023a; Makieva et al., 2024). Thus, replacing these with normal EVs or those from different sources could improve embryonic development, making EV supplementation a prospective approach to enhance embryo quality.
EV supplementation may prepare for precise and personalized treatment in IVF. Embryonic development failure in different patients may result from pathological EVs that either lack specific cargo, such as functional proteins or have impaired functions of these cargoes. Tailoring treatments or interventions could involve using a medium supplemented with exogenous EVs that carry the necessary normal and functional cargo. These EVs can be sourced from the patient’s healthy tissues, from other healthy individuals, or through engineering strategies to customize extracellular vesicles (Esmaeili et al., 2022). Recent research has focused on the components within EVs that influence embryonic development. For instance, Benedetti et al. studied the effects of microRNA-34c from follicular fluid-derived EVs on blastocyst quality (Benedetti et al., 2024). Similarly, Cañón-Beltrán et al. investigated the impact of miR-148b from bovine oviductal EVs on early embryo development (Canon-Beltran et al., 2024). Understanding these specific components can lay the foundation for developing precise interventions using EVs with targeted molecular cargo. Additionally, studies using bovine models offer valuable insights into the potential applications of EVs in personalized medicine. These studies inform the development of tailored EV-based therapies for treating infertility in humans.
To fully harness the potential of EVs in embryo culture, standardization of EV characteristics and treatment protocols is crucial. EVs have garnered significant attention for optimizing human embryo culture media, especially for patients with no high-quality embryos across multiple treatment cycles. However, the beneficial effects of EVs depend on several factors, including their origin, concentration, size, molecular signatures, and encapsulated contents, as well as the treatment protocols of embryos, such as embryo stage, cell numbers, and treatment time. Therefore, standardized reporting guidelines for EV characteristics and embryo treatment procedures are necessary.
Despite the promise of EV supplementation, several challenges remain. The molecular mechanisms underlying the formation and release of EVs in both physiological and pathological conditions are not fully understood. It is unclear which specific molecular cargoes in EVs play a role in embryonic development and what their targets are. Most insights are derived from animal studies, and research on human embryos is ethically controversial and tightly controlled in many countries. Additionally, technological challenges limit research in this field.
6 Conclusion
This review summarizes the state of knowledge regarding the effects of EVs from various origins on embryo development, including oviduct fluid, oviduct/endometrium epithelial cells, uterine fluid, follicular fluid, embryos, seminal plasma, amniotic cells, and endometrial-derived mesenchymal stem cells. It provides evidence that EVs may serve as a component supplementation of in vitro culture media and explains the mechanism of EVs functioning in embryo development. Moreover, the future direction of EV supplementation is discussed, including potential applications and challenges. In conclusion, EV supplementation in vitro culture media may provide a strategy and pave the way for innovative therapies to improve human embryo development and advance clinical outcomes in ART treatment.
Author contributions
YMX: Conceptualization, Investigation, Visualization, Writing–original draft, Writing–review and editing. HZ: Writing–original draft, Writing–review and editing. YX: Writing–original draft, Writing–review and editing. KL: Conceptualization, Funding acquisition, Investigation, Supervision, Visualization, Writing–original draft, Writing–review and editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was funded by the Zhejiang Provincial Natural Science Foundation (LY22H040011), the Zhejiang Provincial Program for the Cultivation of High-level Innovative Health Talents (The year 2018) and the Clinical and Basic Joint Research Project of ZJPPH (C-2023-YXLH11).
Acknowledgments
The authors would like to thank the authors whose work was cited in this present paper.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2024.1366992/full#supplementary-material
References
Abel, K., Healey, M., Finch, S., Osianlis, T., and Vollenhoven, B. (2019). Associations between embryo grading and congenital malformations in IVF/ICSI pregnancies. Reprod. Biomed. Online 39 (6), 981–989. doi:10.1016/j.rbmo.2019.07.035
Aguilera, C., Wong, Y. S., Gutierrez-Reinoso, M. A., Velasquez, A. E., Melo-Baez, B., Cabezas, J., et al. (2024). Embryo-maternal communication mediated by extracellular vesicles in the early stages of embryonic development is modified by in vitro conditions. Theriogenology 214, 43–56. doi:10.1016/j.theriogenology.2023.10.005
Al-Dossary, A. A., Strehler, E. E., and Martin-Deleon, P. A. (2013). Expression and secretion of plasma membrane Ca2+-ATPase 4a (PMCA4a) during murine estrus: association with oviductal exosomes and uptake in sperm. PLoS One 8 (11), e80181. doi:10.1371/journal.pone.0080181
Alminana, C., and Bauersachs, S. (2020). Extracellular vesicles: multi-signal messengers in the gametes/embryo-oviduct cross-talk. Theriogenology 150, 59–69. doi:10.1016/j.theriogenology.2020.01.077
Alminana, C., Corbin, E., Tsikis, G., Alcantara-Neto, A. S., Labas, V., Reynaud, K., et al. (2017). Oviduct extracellular vesicles protein content and their role during oviduct-embryo cross-talk. Reproduction 154 (3), 153–168. doi:10.1530/REP-17-0054
Asaadi, A., Dolatabad, N. A., Atashi, H., Raes, A., Van Damme, P., Hoelker, M., et al. (2021). Extracellular vesicles from follicular and ampullary fluid isolated by density gradient ultracentrifugation improve bovine embryo development and quality. Int. J. Mol. Sci. 22 (2), 578. doi:10.3390/ijms22020578
Balaguer, N., Moreno, I., Herrero, M., Gonzalez, M., Simon, C., and Vilella, F. (2018). Heterogeneous nuclear ribonucleoprotein C1 may control miR-30d levels in endometrial exosomes affecting early embryo implantation. Mol. Hum. Reprod. 24 (8), 411–425. doi:10.1093/molehr/gay026
Bang, S., Qamar, A. Y., Fang, X., Kim, H., Han, A., Kang, H., et al. (2023). Effects of extracellular vesicles derived from steroids-primed oviductal epithelial cells on porcine in vitro embryonic development. Theriogenology 209, 213–223. doi:10.1016/j.theriogenology.2023.07.006
Banliat, C., Le Bourhis, D., Bernardi, O., Tomas, D., Labas, V., Salvetti, P., et al. (2020). Oviduct fluid extracellular vesicles change the phospholipid composition of bovine embryos developed in vitro. Int. J. Mol. Sci. 21 (15), 5326. doi:10.3390/ijms21155326
Bastos, N. M., Ferst, J. G., Goulart, R. S., and Coelho da Silveira, J. (2022). The role of the oviduct and extracellular vesicles during early embryo development in bovine. Anim. Reprod. 19 (1), e20220015. doi:10.1590/1984-3143-AR2022-0015
Benedetti, C., Pavani, K. C., Gansemans, Y., Azari-Dolatabad, N., Pascottini, O. B., Peelman, L., et al. (2024). From follicle to blastocyst: microRNA-34c from follicular fluid-derived extracellular vesicles modulates blastocyst quality. J. Anim. Sci. Biotechnol. 15 (1), 104. doi:10.1186/s40104-024-01059-8
Blazquez, R., Sanchez-Margallo, F. M., Alvarez, V., Matilla, E., Hernandez, N., Marinaro, F., et al. (2018). Murine embryos exposed to human endometrial MSCs-derived extracellular vesicles exhibit higher VEGF/PDGF AA release, increased blastomere count and hatching rates. PLoS One 13 (4), e0196080. doi:10.1371/journal.pone.0196080
Bori, L., Meseguer, F., Valera, M. A., Galan, A., Remohi, J., and Meseguer, M. (2022). The higher the score, the better the clinical outcome: retrospective evaluation of automatic embryo grading as a support tool for embryo selection in IVF laboratories. Hum. Reprod. 37 (6), 1148–1160. doi:10.1093/humrep/deac066
Braganca, G. M., Alcantara-Neto, A. S., Batista, R., Brandao, F. Z., Freitas, V. J. F., Mermillod, P., et al. (2021). Oviduct fluid during IVF moderately modulates polyspermy in in vitro-produced goat embryos during the non-breeding season. Theriogenology 168, 59–65. doi:10.1016/j.theriogenology.2021.03.022
Canon-Beltran, K., Cajas, Y. N., Almpanis, V., Egido, S. G., Gutierrez-Adan, A., Gonzalez, E. M., et al. (2024). MicroRNA-148b secreted by bovine oviductal extracellular vesicles enhance embryo quality through BPM/TGF-beta pathway. Biol. Res. 57 (1), 11. doi:10.1186/s40659-024-00488-z
Chen, K., Liang, J., Qin, T., Zhang, Y., Chen, X., and Wang, Z. (2022). The role of extracellular vesicles in embryo implantation. Front. Endocrinol. (Lausanne) 13, 809596. doi:10.3389/fendo.2022.809596
Chronopoulou, E., and Harper, J. C. (2015). IVF culture media: past, present and future. Hum. Reprod. Update 21 (1), 39–55. doi:10.1093/humupd/dmu040
Coy, P., Garcia-Vazquez, F. A., Visconti, P. E., and Aviles, M. (2012). Roles of the oviduct in mammalian fertilization. Reproduction 144 (6), 649–660. doi:10.1530/REP-12-0279
da Silveira, J. C., Andrade, G. M., Del Collado, M., Sampaio, R. V., Sangalli, J. R., Silva, L. A., et al. (2017). Supplementation with small-extracellular vesicles from ovarian follicular fluid during in vitro production modulates bovine embryo development. PLoS One 12 (6), e0179451. doi:10.1371/journal.pone.0179451
da Silveira, J. C., Veeramachaneni, D. N., Winger, Q. A., Carnevale, E. M., and Bouma, G. J. (2012). Cell-secreted vesicles in equine ovarian follicular fluid contain miRNAs and proteins: a possible new form of cell communication within the ovarian follicle. Biol. Reprod. 86 (3), 71. doi:10.1095/biolreprod.111.093252
de Alcantara-Neto, A. S., Cuello, C., Uzbekov, R., Bauersachs, S., Mermillod, P., and Alminana, C. (2022). Oviductal extracellular vesicles enhance porcine in vitro embryo development by modulating the embryonic transcriptome. Biomolecules 12 (9), 1300. doi:10.3390/biom12091300
de Almeida Monteiro Melo Ferraz, M., Fujihara, M., Nagashima, J. B., Noonan, M. J., Inoue-Murayama, M., and Songsasen, N. (2020). Follicular extracellular vesicles enhance meiotic resumption of domestic cat vitrified oocytes. Sci. Rep. 10 (1), 8619. doi:10.1038/s41598-020-65497-w
Druart, X., and de Graaf, S. (2018). Seminal plasma proteomes and sperm fertility. Anim. Reprod. Sci. 194, 33–40. doi:10.1016/j.anireprosci.2018.04.061
Ducreux, B., Barberet, J., Guilleman, M., Perez-Palacios, R., Teissandier, A., Bourc'his, D., et al. (2023). Assessing the influence of distinct culture media on human pre-implantation development using single-embryo transcriptomics. Front. Cell Dev. Biol. 11, 1155634. doi:10.3389/fcell.2023.1155634
Elliott, R. M., Lloyd, R. E., Fazeli, A., Sostaric, E., Georgiou, A. S., Satake, N., et al. (2009). Effects of HSPA8, an evolutionarily conserved oviductal protein, on boar and bull spermatozoa. Reproduction 137 (2), 191–203. doi:10.1530/REP-08-0298
Esmaeili, A., Alini, M., Baghaban Eslaminejad, M., and Hosseini, S. (2022). Engineering strategies for customizing extracellular vesicle uptake in a therapeutic context. Stem Cell Res. Ther. 13 (1), 129. doi:10.1186/s13287-022-02806-2
Evans, J., Rai, A., Nguyen, H. P. T., Poh, Q. H., Elglass, K., Simpson, R. J., et al. (2019). Human endometrial extracellular vesicles functionally prepare human trophectoderm model for implantation: understanding bidirectional maternal-embryo communication. Proteomics 19 (23), e1800423. doi:10.1002/pmic.201800423
Ezzati, M., Djahanbakhch, O., Arian, S., and Carr, B. R. (2014). Tubal transport of gametes and embryos: a review of physiology and pathophysiology. J. Assist. Reprod. Genet. 31 (10), 1337–1347. doi:10.1007/s10815-014-0309-x
Fan, Y., Pavani, K. C., Smits, K., Van Soom, A., and Peelman, L. (2024). tRNA(Glu)-derived fragments from embryonic extracellular vesicles modulate bovine embryo hatching. J. Anim. Sci. Biotechnol. 15 (1), 23. doi:10.1186/s40104-024-00997-7
Fang, X., Bang, S., Tanga, B. M., Seo, C., Zhou, D., Seong, G., et al. (2023). Oviduct epithelial cell-derived extracellular vesicles promote the developmental competence of IVF porcine embryos. Mol. Med. Rep. 27 (6), 122. doi:10.3892/mmr.2023.13009
Fang, X., Tanga, B. M., Bang, S., Seo, C., Kim, H., Saadeldin, I. M., et al. (2022a). Oviduct epithelial cell-derived extracellular vesicles improve porcine trophoblast outgrowth. Vet. Sci. 9 (11), 609. doi:10.3390/vetsci9110609
Fang, X., Tanga, B. M., Bang, S., Seong, G., Saadeldin, I. M., Lee, S., et al. (2022b). Oviduct epithelial cells-derived extracellular vesicles improve preimplantation developmental competence of in vitro produced porcine parthenogenetic and cloned embryos. Mol. Reprod. Dev. 89 (1), 54–65. doi:10.1002/mrd.23550
Fu, B., Ma, H., and Liu, D. (2020). Extracellular vesicles function as bioactive molecular transmitters in the mammalian oviduct: an inspiration for optimizing in vitro culture systems and improving delivery of exogenous nucleic acids during preimplantation embryonic development. Int. J. Mol. Sci. 21 (6), 2189. doi:10.3390/ijms21062189
Fu, B., Ma, H., Zhang, D. J., Wang, L., Li, Z. Q., Guo, Z. H., et al. (2022). Porcine oviductal extracellular vesicles facilitate early embryonic development via relief of endoplasmic reticulum stress. Cell Biol. Int. 46 (2), 300–310. doi:10.1002/cbin.11730
Gualtieri, R., De Gregorio, V., Candela, A., Travaglione, A., Genovese, V., Barbato, V., et al. (2024). In vitro culture of mammalian embryos: is there room for improvement? Cells 13 (12), 996. doi:10.3390/cells13120996
Gurung, S., Greening, D. W., Catt, S., Salamonsen, L., and Evans, J. (2020). Exosomes and soluble secretome from hormone-treated endometrial epithelial cells direct embryo implantation. Mol. Hum. Reprod. 26 (7), 510–520. doi:10.1093/molehr/gaaa034
Hamatani, T., Ko, M., Yamada, M., Kuji, N., Mizusawa, Y., Shoji, M., et al. (2006). Global gene expression profiling of preimplantation embryos. Hum. Cell 19 (3), 98–117. doi:10.1111/j.1749-0774.2006.00018.x
Hardarson, T., Bungum, M., Conaghan, J., Meintjes, M., Chantilis, S. J., Molnar, L., et al. (2015). Noninferiority, randomized, controlled trial comparing embryo development using media developed for sequential or undisturbed culture in a time-lapse setup. Fertil. Steril. 104 (6), 1452–1459. doi:10.1016/j.fertnstert.2015.08.037
Hassani, S. N., Moradi, S., Taleahmad, S., Braun, T., and Baharvand, H. (2019). Transition of inner cell mass to embryonic stem cells: mechanisms, facts, and hypotheses. Cell Mol. Life Sci. 76 (5), 873–892. doi:10.1007/s00018-018-2965-y
Homer, H., Rice, G. E., and Salomon, C. (2017). Review: embryo- and endometrium-derived exosomes and their potential role in assisted reproductive treatments-liquid biopsies for endometrial receptivity. Placenta 54, 89–94. doi:10.1016/j.placenta.2016.12.011
Jaber, M., Sebban, S., and Buganim, Y. (2017). Acquisition of the pluripotent and trophectoderm states in the embryo and during somatic nuclear reprogramming. Curr. Opin. Genet. Dev. 46, 37–43. doi:10.1016/j.gde.2017.06.012
Kadam, K. M., D'Souza, S. J., Bandivdekar, A. H., and Natraj, U. (2006). Identification and characterization of oviductal glycoprotein-binding protein partner on gametes: epitopic similarity to non-muscle myosin IIA, MYH 9. Mol. Hum. Reprod. 12 (4), 275–282. doi:10.1093/molehr/gal028
Koscinski, I., Merten, M., Kazdar, N., and Gueant, J. L. (2018). Culture conditions for gametes and embryos: which culture medium? Which impact on newborn? Gynecol. Obstet. Fertil. Senol. 46 (5), 474–480. doi:10.1016/j.gofs.2018.03.010
Kurian, N. K., and Modi, D. (2019). Extracellular vesicle mediated embryo-endometrial cross talk during implantation and in pregnancy. J. Assist. Reprod. Genet. 36 (2), 189–198. doi:10.1007/s10815-018-1343-x
Lange-Consiglio, A., Lazzari, B., Pizzi, F., Idda, A., Cremonesi, F., and Capra, E. (2020). Amniotic microvesicles impact hatching and pregnancy percentages of in vitro bovine embryos and blastocyst microRNA expression versus in vivo controls. Sci. Rep. 10 (1), 501. doi:10.1038/s41598-019-57060-z
Lange-Consiglio, A., Maggio, V., Pellegrino, L., and Cremonesi, F. (2012). Equine bone marrow mesenchymal or amniotic epithelial stem cells as feeder in a model for the in vitro culture of bovine embryos. Zygote 20 (1), 45–51. doi:10.1017/S0967199410000493
Leal, C. L. V., Canon-Beltran, K., Cajas, Y. N., Hamdi, M., Yaryes, A., Millan de la Blanca, M. G., et al. (2022). Extracellular vesicles from oviductal and uterine fluids supplementation in sequential in vitro culture improves bovine embryo quality. J. Anim. Sci. Biotechnol. 13 (1), 116. doi:10.1186/s40104-022-00763-7
Lee, S. H., Lira-Albarran, S., and Saadeldin, I. M. (2021). Comprehensive proteomics analysis of in vitro canine oviductal cell-derived extracellular vesicles. Anim. (Basel) 11 (2), 573. doi:10.3390/ani11020573
Li, Y., Cai, L., Guo, N., Liu, C., Wang, M., Zhu, L., et al. (2023a). Oviductal extracellular vesicles from women with endometriosis impair embryo development. Front. Endocrinol. (Lausanne) 14, 1171778. doi:10.3389/fendo.2023.1171778
Li, Y., Liu, C., Guo, N., Cai, L., Wang, M., Zhu, L., et al. (2023b). Extracellular vesicles from human Fallopian tubal fluid benefit embryo development in vitro. Hum. Reprod. Open 2023 (2), hoad006. doi:10.1093/hropen/hoad006
Liu, C., Yao, W., Yao, J., Li, L., Yang, L., Zhang, H., et al. (2020). Endometrial extracellular vesicles from women with recurrent implantation failure attenuate the growth and invasion of embryos. Fertil. Steril. 114 (2), 416–425. doi:10.1016/j.fertnstert.2020.04.005
Lopera-Vasquez, R., Hamdi, M., Fernandez-Fuertes, B., Maillo, V., Beltran-Brena, P., Calle, A., et al. (2016). Extracellular vesicles from BOEC in in vitro embryo development and quality. PLoS One 11 (2), e0148083. doi:10.1371/journal.pone.0148083
Lopera-Vasquez, R., Hamdi, M., Maillo, V., Gutierrez-Adan, A., Bermejo-Alvarez, P., Ramirez, M. A., et al. (2017). Effect of bovine oviductal extracellular vesicles on embryo development and quality in vitro. Reproduction 153 (4), 461–470. doi:10.1530/REP-16-0384
Luis-Calero, M., Marinaro, F., Fernandez-Hernandez, P., Ortiz-Rodriguez, J. M., J, G. C., Pericuesta, E., et al. (2024). Characterization of preovulatory follicular fluid secretome and its effects on equine oocytes during in vitro maturation. Res. Vet. Sci. 171, 105222. doi:10.1016/j.rvsc.2024.105222
Ma, Y., Wang, J., Qiao, F., and Wang, Y. (2022). Extracellular vesicles from seminal plasma improved development of in vitro-fertilized mouse embryos. Zygote 30 (5), 619–624. doi:10.1017/S0967199422000041
Machtinger, R., Laurent, L. C., and Baccarelli, A. A. (2016). Extracellular vesicles: roles in gamete maturation, fertilization and embryo implantation. Hum. Reprod. Update 22 (2), 182–193. doi:10.1093/humupd/dmv055
Makieva, S., Giacomini, E., Scotti, G. M., Lazarevic, D., Pavone, V., Ottolina, J., et al. (2024). Extracellular vesicles secreted by human aneuploid embryos present a distinct transcriptomic profile and upregulate MUC1 transcription in decidualised endometrial stromal cells. Hum. Reprod. Open 2024 (2), hoae014. doi:10.1093/hropen/hoae014
Marinaro, F., Macias-Garcia, B., Sanchez-Margallo, F. M., Blazquez, R., Alvarez, V., Matilla, E., et al. (2019). Extracellular vesicles derived from endometrial human mesenchymal stem cells enhance embryo yield and quality in an aged murine model†. Biol. Reprod. 100 (5), 1180–1192. doi:10.1093/biolre/ioy263
Melka, M. G., Rings, F., Holker, M., Tholen, E., Havlicek, V., Besenfelder, U., et al. (2010). Expression of apoptosis regulatory genes and incidence of apoptosis in different morphological quality groups of in vitro-produced bovine pre-implantation embryos. Reprod. Domest. Anim. 45 (5), 915–921. doi:10.1111/j.1439-0531.2009.01463.x
Menezo, Y., and Elder, K. (2020). Epigenetic remodeling of chromatin in human ART: addressing deficiencies in culture media. J. Assist. Reprod. Genet. 37 (8), 1781–1788. doi:10.1007/s10815-020-01884-6
Morbeck, D. E., Baumann, N. A., and Oglesbee, D. (2017). Composition of single-step media used for human embryo culture. Fertil. Steril. 107 (4), 1055–1060. doi:10.1016/j.fertnstert.2017.01.007
Mulcahy, L. A., Pink, R. C., and Carter, D. R. (2014). Routes and mechanisms of extracellular vesicle uptake. J. Extracell. Vesicles 3. doi:10.3402/jev.v3.24641
Nakamura, K., Kusama, K., Suda, Y., Fujiwara, H., Hori, M., and Imakawa, K. (2020). Emerging role of extracellular vesicles in embryo-maternal communication throughout implantation processes. Int. J. Mol. Sci. 21 (15), 5523. doi:10.3390/ijms21155523
Neyroud, A. S., Chiechio, R. M., Moulin, G., Ducarre, S., Heichette, C., Dupont, A., et al. (2022). Diversity of extracellular vesicles in human follicular fluid: morphological analysis and quantification. Int. J. Mol. Sci. 23 (19), 11676. doi:10.3390/ijms231911676
Ng, Y. H., Rome, S., Jalabert, A., Forterre, A., Singh, H., Hincks, C. L., et al. (2013). Endometrial exosomes/microvesicles in the uterine microenvironment: a new paradigm for embryo-endometrial cross talk at implantation. PLoS One 8 (3), e58502. doi:10.1371/journal.pone.0058502
Paternot, G., Debrock, S., D'Hooghe, T. M., and Spiessens, C. (2010). Early embryo development in a sequential versus single medium: a randomized study. Reprod. Biol. Endocrinol. 8, 83. doi:10.1186/1477-7827-8-83
Perrini, C., Esposti, P., Cremonesi, F., and Consiglio, A. L. (2018). Secretome derived from different cell lines in bovine embryo production in vitro. Reprod. Fertil. Dev. 30 (4), 658–671. doi:10.1071/RD17356
Qiao, F., Ge, H., Ma, X., Zhang, Y., Zuo, Z., Wang, M., et al. (2018). Bovine uterus-derived exosomes improve developmental competence of somatic cell nuclear transfer embryos. Theriogenology 114, 199–205. doi:10.1016/j.theriogenology.2018.03.027
Qu, P., Luo, S., Du, Y., Zhang, Y., Song, X., Yuan, X., et al. (2020). Extracellular vesicles and melatonin benefit embryonic develop by regulating reactive oxygen species and 5-methylcytosine. J. Pineal Res. 68 (3), e12635. doi:10.1111/jpi.12635
Qu, P., Qing, S., Liu, R., Qin, H., Wang, W., Qiao, F., et al. (2017). Effects of embryo-derived exosomes on the development of bovine cloned embryos. PLoS One 12 (3), e0174535. doi:10.1371/journal.pone.0174535
Qu, P., Zhao, J., Hu, H., Cao, W., Zhang, Y., Qi, J., et al. (2022). Loss of renewal of extracellular vesicles: harmful effects on embryo development in vitro. Int. J. Nanomedicine 17, 2301–2318. doi:10.2147/IJN.S354003
Revel, A., Achache, H., Stevens, J., Smith, Y., and Reich, R. (2011). MicroRNAs are associated with human embryo implantation defects. Hum. Reprod. 26 (10), 2830–2840. doi:10.1093/humrep/der255
Saadeldin, I. M., Kim, S. J., Choi, Y. B., and Lee, B. C. (2014). Improvement of cloned embryos development by co-culturing with parthenotes: a possible role of exosomes/microvesicles for embryos paracrine communication. Cell Reprogr. 16 (3), 223–234. doi:10.1089/cell.2014.0003
Schjenken, J. E., and Robertson, S. A. (2020). The female response to seminal fluid. Physiol. Rev. 100 (3), 1077–1117. doi:10.1152/physrev.00013.2018
Sepulveda, S., Garcia, J., Arriaga, E., Diaz, J., Noriega-Portella, L., and Noriega-Hoces, L. (2009). In vitro development and pregnancy outcomes for human embryos cultured in either a single medium or in a sequential media system. Fertil. Steril. 91 (5), 1765–1770. doi:10.1016/j.fertnstert.2008.02.169
Sidrat, T., Khan, A. A., Joo, M. D., Wei, Y., Lee, K. L., Xu, L., et al. (2020). Bovine oviduct epithelial cell-derived culture media and exosomes improve mitochondrial Health by restoring metabolic flux during pre-implantation development. Int. J. Mol. Sci. 21 (20), 7589. doi:10.3390/ijms21207589
Soares, M., Pinto, M. M., Nobre, R. J., de Almeida, L. P., da Graca Rasteiro, M., Almeida-Santos, T., et al. (2023). Isolation of extracellular vesicles from human follicular fluid: size-exclusion chromatography versus ultracentrifugation. Biomolecules 13 (2), 278. doi:10.3390/biom13020278
Sohel, M. M., Hoelker, M., Noferesti, S. S., Salilew-Wondim, D., Tholen, E., Looft, C., et al. (2013). Exosomal and non-exosomal transport of extra-cellular microRNAs in follicular fluid: implications for bovine oocyte developmental competence. PLoS One 8 (11), e78505. doi:10.1371/journal.pone.0078505
Stone, B. A., March, C. M., Ringler, G. E., Baek, K. J., and Marrs, R. P. (2014). Casting for determinants of blastocyst yield and of rates of implantation and of pregnancy after blastocyst transfers. Fertil. Steril. 102 (4), 1055–1064. doi:10.1016/j.fertnstert.2014.06.049
Utsunomiya, T., Yao, T., Itoh, H., Kai, Y., Kumasako, Y., Setoguchi, M., et al. (2022). Creation, effects on embryo quality, and clinical outcomes of a new embryo culture medium with 31 optimized components derived from human oviduct fluid: a prospective multicenter randomized trial. Reprod. Med. Biol. 21 (1), e12459. doi:10.1002/rmb2.12459
Vilella, F., Moreno-Moya, J. M., Balaguer, N., Grasso, A., Herrero, M., Martinez, S., et al. (2015). Hsa-miR-30d, secreted by the human endometrium, is taken up by the pre-implantation embryo and might modify its transcriptome. Development 142 (18), 3210–3221. doi:10.1242/dev.124289
Wan, C., Stowell, M. H. B., and Shen, J. (2022). Progress and gaps of extracellular vesicle-mediated intercellular cargo transfer in the central nervous system. Commun. Biol. 5 (1), 1223. doi:10.1038/s42003-022-04050-z
Wang, X., Tian, F., Chen, C., Feng, Y., Sheng, X., Guo, Y., et al. (2019). Exosome-derived uterine microRNAs isolated from cows with endometritis impede blastocyst development. Reprod. Biol. 19 (2), 204–209. doi:10.1016/j.repbio.2019.06.003
Wei, Y., Idrees, M., Sidrat, T., Joo, M., Xu, L., Ko, J., et al. (2022). BOEC-exo addition promotes in vitro maturation of bovine oocyte and enhances the developmental competence of early embryos. Anim. (Basel) 12 (4), 424. doi:10.3390/ani12040424
Wen, Z., Chen, Y., Long, Y., Yu, J., and Li, M. (2018). Tumor necrosis factor-alpha suppresses the invasion of HTR-8/SVneo trophoblast cells through microRNA-145-5p-mediated downregulation of Cyr61. Life Sci. 209, 132–139. doi:10.1016/j.lfs.2018.08.005
Wydooghe, E., Vandaele, L., Heras, S., De Sutter, P., Deforce, D., Peelman, L., et al. (2017). Autocrine embryotropins revisited: how do embryos communicate with each other in vitro when cultured in groups? Biol. Rev. Camb Philos. Soc. 92 (1), 505–520. doi:10.1111/brv.12241
Yi, C., Ni, Y., Sun, P., Gao, T., and Li, K. (2022). Differential size distribution and estrogen receptor cargo of oviductal extracellular vesicles at various stages of estrous cycle in mice. Reprod. Sci. 29 (10), 2847–2858. doi:10.1007/s43032-022-00862-w
Zdravkovic, T., Nazor, K. L., Larocque, N., Gormley, M., Donne, M., Hunkapillar, N., et al. (2015). Human stem cells from single blastomeres reveal pathways of embryonic or trophoblast fate specification. Development 142 (23), 4010–4025. doi:10.1242/dev.122846
Zhang, J., Su, R., Wang, Y., Wang, H., Li, S., Yang, X., et al. (2024). Protective effect of small extracellular vesicles (EVs) derived from ACE2-modified human umbilical cord mesenchymal stem cells against renal ischemia-reperfusion injury. Nephrol. Carlt. 29 (1), 5–17. doi:10.1111/nep.14237
Zhao, Y., and Kan, F. W. K. (2019). Human OVGP1 enhances tyrosine phosphorylation of proteins in the fibrous sheath involving AKAP3 and increases sperm-zona binding. J. Assist. Reprod. Genet. 36 (7), 1363–1377. doi:10.1007/s10815-019-01502-0
Zhao, Y., Vanderkooi, S., and Kan, F. W. K. (2022). The role of oviduct-specific glycoprotein (OVGP1) in modulating biological functions of gametes and embryos. Histochem Cell Biol. 157 (3), 371–388. doi:10.1007/s00418-021-02065-x
Keywords: extracellular vesicles, exosomes, cellular communication, culture media, embryo development, mechanism
Citation: Xue Y, Zheng H, Xiong Y and Li K (2024) Extracellular vesicles affecting embryo development in vitro: a potential culture medium supplement. Front. Pharmacol. 15:1366992. doi: 10.3389/fphar.2024.1366992
Received: 08 January 2024; Accepted: 05 September 2024;
Published: 18 September 2024.
Edited by:
Nucharin Songsasen, Smithsonian Conservation Biology Institute (SI), United StatesReviewed by:
Jessian Munoz, Texas Children’s Hospital, United StatesMaría Gracia Gervasi, University of Connecticut, United States
Mulyoto Pangestu, Monash University, Australia
David Martin-Hidalgo, Universidad de Extremadura, Spain
Kathryn Storey, Smithsonian Conservation Biology Institute (SI), United States
Copyright © 2024 Xue, Zheng, Xiong and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kun Li, bGlrdW5AaG1jLmVkdS5jbg==
 Yamei Xue
Yamei Xue Haixia Zheng
Haixia Zheng Yuping Xiong
Yuping Xiong Kun Li
Kun Li