
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pharmacol. , 04 April 2024
Sec. Experimental Pharmacology and Drug Discovery
Volume 15 - 2024 | https://doi.org/10.3389/fphar.2024.1366889
 Umme Habiba Juhi1
Umme Habiba Juhi1 Heba A. S. El-Nashar2*
Heba A. S. El-Nashar2* Abdullah Al Faruq1
Abdullah Al Faruq1 Md. Shimul Bhuia3,4
Md. Shimul Bhuia3,4 Irin Sultana1
Irin Sultana1 Syedul Alam5
Syedul Alam5 Farah Abuyousef6
Farah Abuyousef6 Na’il Saleh6*
Na’il Saleh6* Mohamed El-Shazly2*
Mohamed El-Shazly2* Muhammad Torequl Islam3,4,7*
Muhammad Torequl Islam3,4,7*Introduction: Cheilanthes tenuifolia is an evergreen ornamental small fern, belonging to the family Pteridaceae, that grows in warm and rocky regions worldwide. Many species of Cheilanthes genus are evidently endowed with important phytochemicals and bioactivities. This study aimed to perform a preliminary phytochemical analysis of Cheilanthes tenuifolia leaves alongside an evaluation of free radical scavenging, anti-inflammatory, antimicrobial, and clot lysis activities of extract fractions.
Materials and methods: A preliminary phytochemical analysis was done after fractionation of ethanolic extract (ECT) with n-hexane (HCT) and chloroform (CCT). Then, 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging, egg albumin and RBC membrane stabilization tests, disc diffusion, and human blood clot lysis assays were performed.
Results: Phytochemical investigations suggested that the plant is rich in alkaloids, glycosides, tannins, and flavonoids. All obtained fractions exhibited concentration-dependent radical scavenging, inhibition of egg protein denaturation and RBC membrane lysis capacities. Except for antifungal tests, ECT exhibited better DPPH radical scavenging, anti-inflammatory, antibacterial, and clot lysis capacities than HCT and CCT fractions. However, all fractions exhibited a mild anti-inflammatory activity.
Conclusion: C. tenuifolia might be a good source of antioxidant, anti-microbial, and anti-atherothrombotic agents. Further studies are required to isolate and characterize the active principles liable for each bioactivity, along with possible molecular interactions.
Recently, the herbal remedies have been recognized as alternative medical treatments for managing primary healthcare among 80% of the world’s populations, especially in low- and medium-income countries (El-Nashar et al., 2021; El-Shawi et al., 2023). It is due to the fact that most often synthetic drugs result in many unavoidable adverse events, grow resistance (e.g., antibiotics) and tolerance (e.g., narcotic drugs) due to repeated uses, and thereby reduce safety and efficacy (Ishaq et al., 2022; El-Nashar et al., 2023b; Younis et al., 2023).
The use of pteridophytes (e.g., aka, ferns and fern allies) is ancient (>2000 years) due to their many beneficial properties. Pteridaceae is recognized as an important family among the pteridophytes. Many species in this family are rich with alkaloids, glycosides, and flavonoids and have promising medicinal features, such as antioxidant, anti-cancer, anti-inflammatory, antimicrobial, antidiabetic, and neurobiological effects (Bhuia et al., 2023a). Cheilanthes tenuifolia (Burm.f.) Swartz, which belongs to the family Pteridaceae, is a petite, evergreen fern that has the ability to reach a height of 70 cm. The delicate lip fern is a species of fern loacal to North America that is considered an ornamental plant (Mahfuz et al., 2019). It grows properly in open, warm, moist, shady, rocky regions and is often found in small crevices high up on cliffs (Sen and Mukhopadhyay, 2014; Patel and Reddy, 2018). It is found in many regions of the world, including Australia, China, Malaysia, Bangladesh, Nepal, Cambodia, Laos, New Zealand, the Philippines, Polynesia, Sri Lanka, Taiwan, Uruguay, Thailand, Vietnam, Tasmania, and India. September to November is considered the growing season for the plants. In India, there are over 30 species of this plant. The fronds are 63 cm long and 17 cm wide, with a dark red-brown stipe and rachis that are smooth or have sparse hairs consisting of 2–13 cells and very few slender scales. The lamina is pentagonal, triangular, or ovate, with 3-4 pinnates at the base and 3 pinnates for most of its length. The larger pinnae are triangular-ovate, the pinnules are lanceolate or ovate, and the ultimate pinnules may have a slightly caudate shape. The margins are either lobed or entire. The upper and lower surfaces of the lamina have very few, short (less than 0.5 mm), pointed hairs consisting of 2 or 3 cells, and are occasionally almost hairless. The spores are tetrahedral or rounded-tetrahedral, granulose, and trilete, with a varying degree of reticulate-echinate ornamentation, and have a diameter of 38–53 µm with 32 per sporangium.
Ancient people (prehistoric times) used the rhizome juice of ferns for gastrointestinal disorders (including peptic ulcer), cuts, and wounds. Traditionally, C. tenuifolia leaf juice is mixed with hot water and honey to cure throat pain (Augustin and Thomas, 2015). Its leaf and stem decoctions are used for healthy hair (Hanum and Hamzah, 1999). The tribes of Northeast India use its rhizomes and root extracts as general tonics (Benniamin, 2011; Singh and Upadhyay, 2014). One study reports that the plant contains important phytochemical groups, like steroids, alkaloids, flavonoids, triterpenoids, phenolic compounds, and tannins (Ghorpade et al., 2015). To date, two important flavonoids such as quercetin and rutin have been extracted from an ethyl acetate-soluble extract of C. tenuifolia (Jarial et al., 2018). The plant-derived compound quercetin is a natural aglycone of rutin. It is frequently used in dietary supplements due to its many important and promising bioactivities, including antioxidant, anti-inflammatory, immunomodulatory, antimicrobial, anti-cancer, antidiabetic, antiallergy, antihypertensive, and organ-protective (e.g., brain, heart, liver, kidney, and GIT tract) effects (Ahmed et al., 2022). A recent investigation proposes that methanolic whole plant extract possesses a significant amount of phenolics and flavonoids and has demonstrated strong antioxidant, cytotoxic, membrane-stabilizing, and thrombolytic activity (Mahfuz et al., 2019). This study also confirmed that the plant contains stigmasterol. Therefore, there is a lack of adequate scientific studies on this hopeful medicinal plant and there is no detailed information on isolated phytochemicals, anti-inflammatory activity and its underlying mechanisms, antifungal properties, antibacterial effects against huge number of pathogenic bacteria and the molecular mechanisms of each activity.
Knowing the overall facts, this study aimed to do a preliminary phytochemical analysis along with the assessment of the radical scavenging, anti-inflammatory, antimicrobial, and clot lysis capacities of Cheilanthes tenuifolia leaf extracts and this study also evaluated the rectitude of the previous findings of the therapeutic activity of the plant.
Fresh leaves were gathered from the Bayazid hill tracts in Chittagong during July and August, which is the period when the plant grows the most. Before mass collection, the plant is identified by a taxonomist at the Bangladesh Forest Research Institute Herbarium (BFRIH), Chittagong [Voucher No. BFRIH-SA (577)]. The decayed leaves, stems, dust, and other parts of the plant were removed with great care. Next, the plant components were rinsed using a continuous flow of tap water and dried in the shade at a temperature lower than 40°C. After drying, the materials were crushed into a rough powder and placed in an airtight container that is amber in color. This container was then kept in a cool and dry place until the extraction process began.
A total of 200 g of leaf powder was extracted with 1,000 mL of absolute ethanol at solvent ratio of 1:5 using a Soxhlet extractor a temperature of 70°C for 8 h. Then the solvent was dried using a rotary evaporator under diminished pressure. Finally, a gummy ethanolic leaf extract (ECT) was collated. The percentage yield value was determined as follows:
To perform solvent-solvent partitioning of ECT, 10 mL of ECT was dissolved in double-distilled water (DDW), and then fractionated with the aid of a fractionating column with n-hexane (HCT) and subsequently with chloroform (CCT). The fractionation process involved utilizing 50 mL of each solvent, for a total of 150 mL of n-hexane and chloroform. After vigorous shaking of the mixture, each fraction was allowed to stand, and the solvent layers were then separated and decanted. The remaining extract was considered a fraction of ethanol. The extracts obtained were collected, filtered, and the solvent was evaporated below 50°C temperature. As a result of the evaporation process, a sticky concentrate was obtained. The gummy concentrates were weighed and placed in an appropriately labeled and cleaned airtight container and stored at 4°C. Percentage yield values for each fraction were determined according to the above-mentioned equation. A general scheme for fractionation has been shown in Figure 1.
Streptokinase (Altepase®) was purchased from Beacon Pharmaceuticals Ltd., Bangladesh, while ethanol, chloroform, n-hexane, tween 80, acetyl salicylic acid, ascorbic acid, DPPH, nutrient culture media, and other necessary reagents and chemicals were purchased from Merck India.
The study utilized young male Swiss albino mice, which were procured from the animal research branch of the Bangladesh Council of Scientific and Industrial Research (BCSIR) in Chattogram, Bangladesh. These mice had an average weight of 24–30 g and were maintained in a laboratory setting under standard conditions: a 12-h light/dark cycle, a room temperature of 25°C ± 2°C, and a relative humidity of 55%–60%. They were provided with a standard diet and had access to water ad libitum. To ensure their suitability for the study, the mice were acclimatized to the laboratory environment for 7 days and fasted overnight for 12 h before the experiments. All ethical considerations were taken into account, and the experimental animals were treated in accordance with the Swiss Academy of Medical Sciences and the Swiss Academy of Sciences Ethical Principles and Guidelines for Scientific Experiments with Animals (1995). Additionally, the Institutional Ethics Committee (SUB/IAEC/12.01) approved all experimental protocols.
The test dose for this study of crude extracts was selected by the acute toxicity study following the OECD guidelines using Swiss albino mice. Briefly, the crude ECT was given at doses of 500, 1,000, 2,000, and 3,000 mg/kg orally. The animals were then frequently observed for behavioral changes, toxicological symptoms, and death for 2 days (Thangjam et al., 2020).
The phytochemical screening was done according to the method described by (Batool et al., 2020).
The study assessed the ability of the extracts to scavenge free radicals using the 2,2-diphenyl-1-picrylhydrazyl (DPPH) scavenging assay, as stated by Chowdhury et al. (2010), with minor modifications. To prepare the DPPH solution, 0.004% w/v DPPH was solubilized in ethanol, and its absorbance was immediately measured. Next, 1 mL of the extract solution at a number of concentrations (20, 40, 60, 80, and 100 μg/mL) was added to 2 mL of the DPPH solution, mixed thoroughly, and allowed to stand in the dark for 30 min to complete the reaction. The same concentrations of ascorbic acid (AA) were utilized as a reference radical scavenger, while ethanol served as a blank. After the reaction time, the absorbance was measured at 517 nm using a UV spectrophotometer, and the formula given below was utilized to determine the percentage of inhibition:
The half-minimal inhibitory concentration (IC50) was measured utilizing non-linear regression analysis with the aid of Graph Pad Prism software.
This test was performed by checking inhibitory effects on egg albumin using the method stated by (Dharmadeva et al., 2018). Briefly, 0.2 mL of egg albumin (from a fresh hen’s egg) was mixed with 2.8 mL of isosaline (0.9% NaCl, pH 6.4) and 2 mL of the test sample or standard drug. Distilled water and acetyl salicylic acid (ASA) were served as control and positive controls, respectively. The sample and standard were tested at 125, 250, and 500 μg/mL. The reaction mixtures were incubated at a temperature of 37°C ± 2°C for 15 min, after which they were heated in a water bath at a temperature of 70°C for 5 min. Following this, the mixtures were allowed to cool and were filtered through Whatmann filter paper no. 1. Using a colorimeter, the absorbance of every sample was gauged at a 660 nm wavelength. The percentage of protein denaturation inhibition was computed utilizing the following equation:
where, Vt and Vc stand for absorbance of test sample and control, respectively. The IC50 values were determined as mentioned above.
This study was conducted using the model developed by (Shinde et al., 1999) with some minor adjustments. Initially, 5 mL of fresh blood was gathered from a healthy donor and mixed with di-potassium salt of EDTA (2.2 mg/mL). The blood cells were then accumulated by centrifugation and washed thrice with an isotonic solution (154 mM NaCl) in 10 mM sodium phosphate buffer (pH 7.4). The resulting cell suspension was re-centrifuged at 3,000 g for 10 min and finally re-suspended in an equal volume of isotonic buffer solution. Next, 0.5 mL of the cell suspension was added to a mixture of 5 mL of hypotonic solution (50 mM NaCl) and 0.5 mL of test or standard solution (125, 250, and 500 μg/mL) in 10 mM sodium phosphate buffered saline (pH 7.4), as specified. The control tube contained only 0.5 mL of cell suspension and 5 mL of hypotonic solution in the above-mentioned buffer. The reaction mixture was incubated for 10 min at room temperature and centrifuged at 3,000 g for 10 min. Finally, the optical density (OD) of the supernatant was quantified at 540 nm using a UV-visible spectrophotometer. The percentage inhibition of hemolysis was calculated using the following equation:
The IC50 values were determined as mentioned above.
This test was done according to the model stated by (Mbaveng et al., 2008). The investigation samples were made by solubilizing the extracts of the samples in ethanol (ECT), chloroform (CCT), and n-hexane (HCT). All the samples were tested at 500 μg/disc. Ciprofloxacin (CFN) and fluconazole (FCZ) were taken as reference drugs for anti-bacterial and anti-fungal tests, respectively, at 30 μg/disc. For this study, we used 4 G (+) and 7 G (−) bacteria and 7 fungi (Table 1). For the anti-bacterial assay, we used nutrient agar media, while for the anti-fungal assay, we used potato dextrose agar media. After the inoculation of the test pathogen and after allowing the plates to solidify, respective paper discs containing the test sample or standard drug were subsequently impregnated centrally into the agar gel separately with the aid of sterile forceps to achieve complete contact with the previously cultured medium surface. Finally, all the plates were then incubated at 37°C for 24 h for the anti-bacterial test and at 25°C for 72 h for the anti-fungal test.
This in vitro study was done according to the model developed by (Prasad et al., 2006). In this case, we distributed 0.5 mL of fresh blood in pre-weighed microcentrifuge tubes from the non-contraceptive or anti-coagulant receiving humans. After incubating the blood sample at 37°C for 45 min, the serum was cautiously excluded without disquieting the clot, and tubes were weighed. 100 μL of extract at 500 μg was added in each tube. 100 μL of streptokinase (equiv. 30,000 IU) and 100 μL of DW were added to the positive control and control marked tubes, respectively. After incubation of the tubes at 37°C for 90 min, the discharged fluid from each tube was carefully removed, and the tubes were reweighed. The percentage of clot lysis was calculated as follows:
Values are expressed as mean ± standard error of mean (SEM). One-way analysis of variance (ANOVA) was followed by Newman Keuls post host t-students test using the Graph Pad Prism software (version 6.5) considering p < 0.05 at 95% confidence of intervals.
The percentage yield of crude ECT was 5%; that of fractionated ECT, CCT, and HCT was 25, 35, and 25%, respectively. Table 2 suggests that ECT possesses alkaloids, tannins, glycosides, flavonoids, and saponins, while CCT contains alkaloids, glycosides, steroids, tannins, and flavonoids. HCT contains alkaloids, tannins, glycosides, flavonoids, saponins, and reducing sugars. All the extracts contain alkaloids, glycosides, tannins, and flavonoids. None of these extracts contain gums or amides.
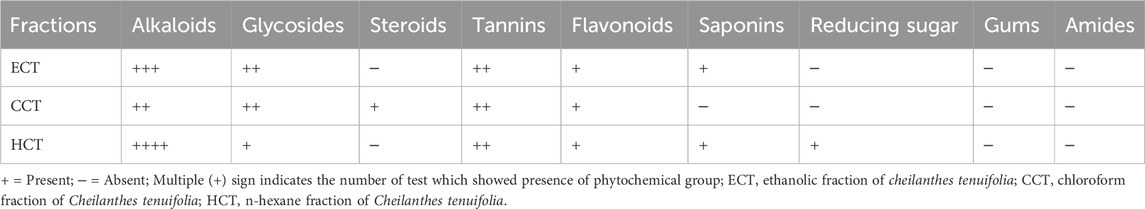
Table 2. Phytochemical groups observed in different fractions of Cheilanthes tenuifolia leaf extract.
The crude ECT up to a 3,000 mg/kg oral dose did not cause behavioral changes or toxicological symptoms, or even death, in Swiss mice. Therefore, we used the maximum test concentration of 500 μg/mL (equivalent to 500 mg/kg) as a test concentration for antimicrobial and clot lysis studies. For radical scavenging, we used the highest concentration, 100 μg/mL, while in the anti-inflammatory study, we used 500 μg/mL as a high concentration and 125 as a low concentration.
The control showed negligible DPPH radical scavenging capacity (1.54% ± 0.11%). All the extracts demonstrated a concentration-dependent radical scavenging capacity in comparison to the control group. The highest inhibition (IC50 = 22.17 ± 1.90 μg/mL) was seen with ECT at all concentrations compared to CCT and HCT. The radical scavenging capacity of all the extracts was significant (p < 0.05) as compared to the control group. However, the reference drug AA revealed significant (p < 0.05), strong, and better inhibition at 20–100 μg/mL than all the test extracts (Table 3). The IC50 values calculated for the CCT, HCT, and AA are 39.79 ± 1.02, 78.13 ± 2.08, and 18.77 ± 1.03 μg/mL, respectively. The CI and r2 value of all the treatment groups are demonstrated in Table 3.
The control showed a negligible egg protein denaturation inhibitory effect (1.23% ± 0.01%). All the extracts showed concentration-dependent protein denaturation inhibitory effects in comparison to the control group. The highest inhibition (49.84% ± 0.01%) was seen by ECT at 500 μg/mL. However, the standard drug ASA exhibited significant (p < 0.05), strong, and better inhibition at 125–500 μg/mL than all the test extracts (Table 4). The IC50 values calculated for the ECT, CCT, HCT, and ASA are 511.10 ± 2.93, 1,001.07 ± 3.09, 993.03 ± 3.57, and 122.71 ± 2.09 μg/mL, respectively.
The control showed negligible membrane lysing inhibitory effect (1.73% ± 0.01%) on HRBCs. All the extracts showed concentration-dependent membrane lysis inhibitory effects in comparison to the control group. The highest inhibition (45.85% ± 0.01%) was seen by ECT at 500 μg/mL. However, the standard drug ASA exhibited significant (p < 0.05), strong, and better inhibition at 250 and 500 μg/mL than all the test extracts (Table 5). The IC50 values calculated for the ECT, CCT, HCT, and ASA are 587.19 ± 1.33, 2021.03 ± 3.79, 1,524.09 ± 2.96, and 209.79 ± 2.13 μg/mL, respectively.
Table 6 suggests that ECT (ZI range: 8.70 ± 1.00 to 15.00 ± 1.00 mm), CCT (ZI range: 8.30 ± 0.60 to 15.30 ± 1.20 mm), and HCT (ZI range: 8.30 ± 1.50 to 12.70 ± 0.60 mm) showed sensitivity towards all the test bacteria except V. cholerae at 500 µg/disc. However, the extracts were more active against the Gram (+) species. The standard drug CFN inhibited the growth of all the investigated bacteria within the range of ZI 11.30 ± 1.00 to 16.00 ± 1.00 mm at 30 µg/disc.
Table 7 suggests that ECT (ZI range: 9.00 ± 0.20 to 11.00 ± 0.50 mm), CCT (ZI range: 8.70 ± 0.20 to 12.70 ± 0.50 mm), and HCT (ZI range: 10.00 ± 0.50 to 11.70 ± 0.20 mm) showed sensitivity towards A. niger, B. dermatitidis, C. albicans and P. ovale. However, all the extracts remain insensitive at 500 µg/disc against the other test fungi. The standard drug FCZ inhibited the growth of all the test fungi within the range of ZI 11.00 ± 0.40 to 15.30 ± 0.50 mm at 30 µg/disc.
All the selected extracts at 500 µg/100 µL exhibited significant (p < 0.05) clot lysis capacity as compared to the control (vehicle) group. Among the extracts, ECT exhibited better clot lysis capacity (61.71 ± 0.03) than the CCT and HCT. However, the standard streptokinase (equiv. 30,000 IU)/100 µL exhibited more clot lysis capacity than the test samples (Table 8).
Among the natural products, plants are common sources of traditional healing agents worldwide, especially in the poor and less developed countries (Ashmawy et al., 2023; Mia et al., 2023). On the other hand, people in developed countries are now conscious of the adverse events of modern drugs, thus there is a developing enthusiasm for research into and utilization of plant-mediated products or preparations (Abdelazim et al., 2024; Shill et al., 2024). Plant extracts contain numerous groups and components, including alkaloids, glycosides, phenolics and flavonoids, vitamins, and minerals (Gunathilake et al., 2018). Certain groups of compounds have diverse bioactivities, for example, phenolics and flavonoids have promising antioxidant and anti-inflammatory activities (Tlili et al., 2013) and play many important roles in biological systems such as anti-inflammatory (Ginwala et al., 2019), antibacterial, anti-cancer (Khalil et al., 2020), and anti-atherosclerotic effect (Salvamani et al., 2014). A certain study reported that certain organic polar solvents, such as ethanol and methanol, are the best solvents for the extraction of phenolics from natural sources (Ballard et al., 2009). Alkaloids, on the contrary, play vital roles in plants and humans. It is because these are considered defensive compounds. Alkaloids also regulate the growth of plants (Chik et al., 2013). Furthermore, glycosides have many significant therapeutic potentials, including antifungal (Khan et al., 2017) and anticancer (Khan et al., 2019) activities. Current drug development methodologies and modern medicine do not use complete plant extracts and instead rely on specific compounds. Taking the whole plant or extracts without isolating components as practiced in traditional medicine has a stronger therapeutic impact than specific substances (Wen et al., 2005; Thomford et al., 2018). Therefore, the isolated chemical constituents are important for developing novel drugs.
The plant is evidently composed of steroids, alkaloids, flavonoids, triterpenoids, phenolic compounds, and tannins (Ghorpade et al., 2015). Another study reports that the plant is rich in phenolics and flavonoids. It contains quercetin, rutin, and stigmasterol (Mahfuz et al., 2019). Our study also confirmed that the plant contains stigmasterol. In this study, we have seen that ethanolic leaf fractions of C. tenuifolia contain flavonoids. Additionally, we have also found that the plant leaf contains alkaloids, steroids, saponins, glycosides, and reducing sugars. Thus, the compounds of these phytochemical groups may be linked to the observed bioactivities. However, safety pharmacology is critical throughout the drug discovery and development process. Prior to first-in-human investigations, safety pharmacology assays, tests, and models anticipate the clinical risk profile of a possible new medicine (Morimoto et al., 2015). According to the findings of our study the crude ECT up to a 3,000 mg/kg oral dose did not show any behavioral changes or toxicological symptoms, or even death, in Swiss mice. Previous study on the plant extract showed that the LC50 values of the chloroform, n-hexane, methanol soluble and ethyl acetate extracts were 34.493 μg/mL, 205.984 μg/mL, 751.169 μg/mL and 66.235 μg/mL respectively on shrimp nauplii (Mahfuz et al., 2019).
Oxidative stress from various sources due to an overabundance of reactive species production (e.g., ROS, RNS) is considered a precursor of diseases and disorders in humans. This can lead to neurological and cardiovascular diseases, cancer, diabetes, etc. (Rekatsina et al., 2020; Forman and Zhang, 2021). Antioxidants are the substances that act against oxidative stress. External antioxidants are required once our body’s defensive mechanisms, including physiological antioxidants, fail to manage an overabundance of free radicals. Plant-based natural antioxidants play significant roles in managing this situation (Jamshidi-Kia et al., 2020). It is due to their being readily available, economic, and biocompatible. Moreover, we can readily access these compounds through our daily diets (Landete, 2013). In a recent experiment, Mahfuz et al. (2019) demonstrated that methanolic whole plant extract exhibited strong (IC50 = 9.926 μg/mL) DPPH free radical scavenging capacity. Another investigation of the plant’s methanolic extract found that the two flavonoids (rutin and quercetin) present in the extract showed considerable in vitro anti-oxidant activity; in this regard, the DPPH scavenging capability of quercetin (86.1%) was higher than that of rutin (73.2%) (Jarial et al., 2018; Daryono and Rhomawati, 2020; Daryono and Rhomawati, 2020). Another bioactive triterpenoid isolated from the n-hexane extract of the plant also has potent antioxidant capacity such as stigmasterol (Mahfuz et al., 2019), which significantly reduced the ROS generation in different in vitro investigation (Agatonovic-Kustrin et al., 2018; Bakrim et al., 2022).
In this investigation, we have seen that all the fractions of C. tenuifolia revealed significant DPPH free radical scavenging capacity in a concentration-dependent manner, with the IC50 values estimated for the ECT, HCT, and CCT within the range of 22.17 ± 1.90 to 78.13 ± 2.08 μg/mL, respectively. ECT exhibited a better DPPH radical scavenging effect than the other two fractions. It is because of the large amount of phenolics and flavonoids in this fraction such as rutin and quercetin as well as terpenoids including stigmasterol (Jarial et al., 2018; Mahfuz et al., 2019). The promising DPPH radical scavenging effect of other species of Cheilanthes such as C. anceps also reported by (Chowdhary et al., 2010).
Oxidative stress can provoke inflammatory cascades. Thus, antioxidants have protective effects in a biological system. Certain antioxidants, such as polyphenols, have an anti-ageing effect (Moliner et al., 2020), and these can prevent or delay many disease conditions, including neurological diseases and disorders, cardiovascular diseases, diabetes, and so on (Rodríguez-Yoldi, 2021; Bhuia et al., 2023b). A study performed by Mahfuz and his coworkers in 2019 suggests that the methanol, n-hexane, ethyl acetate, and chloroform fractions of C. tenuifolia showed significant membrane stabilizing capacity in the HRBC assay, where the percent inhibition of hemolysis was determined within the range of 2.97 and 73.97 (Mahfuz et al., 2019). The n-hexane fraction showed the highest percent inhibition against hypnotic solution-induced hemolysis (73.97%), while the ethyl acetate fraction showed the highest percent inhibition of heat-induced hemolysis (67.27%). Our study demonstrated that the organic fractions of C. tenuifolia, especially its ECT, exhibited significant egg protein and HRBC protection capabilities. Another study by Akbor et al. (2023) also stated that methanolic extract of the plant has a notable membrane lysing inhibitory activity (1.42% ± 0.02%) on HRBCs (Akbor et al., 2023). One report suggested that the species C. farinose is traditionally used to manage inflammation (Yonathan et al., 2006). Thus, our present findings are in agreement with the traditional values and scientific reports observed in the databases. According to the findings of different studies the bioactive substances (rutin, and quercetin) of the plant has potent anti-inflammatory activity and liable for diminishing different inflammatory markers such as cytokines (Abd Nikfarjam et al., 2017; Bhuia et al., 2023c).
Infectious diseases due to microbial attacks (e.g., bacteria, fungi, viruses, and parasites) in humans are a common consequence in the world. Among the wide variety of natural products, plants are considered major sources of antimicrobial agents (Touati et al., 2018). The species of the Pteridaceae family are known for their diverse bioactivities, including antioxidant, anti-inflammatory, antimicrobial, anti-cancer, antidiabetic, and neurobiological properties (Baskaran et al., 2018). To date, a number of bioactive compounds have been introduced from the Cheilanthes genus, for example, glycosides of apigenin, chrysoeriol, luteolin, kaempferol, and quercetin from C. concolor, C. flexuosa and C. goyazensis (Salatino and Prado, 1998). In this study, we have seen that all the organic fractions of C. tenuifolia acted against both gram (+) and gram (−) species as well as many pathogenic fungi, suggesting its broad-spectrum anti-microbial effects. Conventional antimicrobial agents have some potential adverse effects, for example, allergic reactions and enhanced resistance (Mohsen et al., 2020). For example, chloramphenicol is widely used in meningitis, but it increases the risk of aplastic anemia. Another example is sulfonamides, which were considered “wonder drugs,” but these are evidently contributing to severe skin reactions (King et al., 2018). Therefore, it is crucial to inhibit microbial pathogenesis using appropriate and safe antimicrobials (French, 2005). Previous study by Jarial et al. (2018) also manifested the antimicrobial properties of the plant and the study also revealed that isolated flavonoids such as quercetin and rutin from C. tenuifolia showed inhibitory effects against Staphylococcus aureus and Enterobacter sp. with MIC values of (2.25 and 0.45 μg/mL), respectively (Jarial et al., 2018).
Atherothrombosis is one of the major causes of morbidity and mortality in the world. Chronic pathology is responsible for vascular remodeling through various pathways, including oxidative stress (Martin-Ventura et al., 2017). ROS and RNS play important pathophysiological roles in vascular diseases, such as atherothrombosis (Chen et al., 2018). It is also evident that microorganism-mediated infections result in the production of potentially reactive molecules in our body through oxygen metabolism (Brown et al., 2014), which is responsible for many pathological conditions, including inflammation, atherosclerosis, carcinogenesis, and so on (Aruoma, 1998). The methanolic whole plant extract is evidently able to exert significant clot lysis capacity at 100 µg/tube (Mahfuz et al., 2019). The authors demonstrated that the ethyl acetate, n-hexane, methanol, and chloroform fractions exhibited 31.59, 12.10, 17.01, and 41.26% clot lysis capacities, respectively. Another study by Akbor et al. (2023) also reported that the methanolic extract of the plant hindered hemolysis in a concentration-dependent manner and inhibited 78.93% ± 0.01% hemolysis (IC50 = 46 ± 2.11 μg/mL) at the higher concentration (160 μg/mL) (Akbor et al., 2023). Findings of our study suggest that ECT, CCT, and HCT showed clot lysis capacities of 61.71 ± 0.03, 37.80 ± 0.02, and 15.19% ± 0.04% at 500 µg/tube. ECT showed better clot lysis capacity than the other two fractions. Due to the presence of different phytochemicals such as alkaloids, flavonoids, and steroids (Uddin et al., 2019; Tabassum et al., 2022), the bioactive phytoconstituents of the plant such as rutin, quercetin, and stigmasterol also have remarkable hemolytic activity (Zaragoza et al., 2021; Bari et al., 2022; Chen et al., 2022). We suppose that the observed clot lysis capacity, especially by the ECT, might be linked to the existence of phytochemical groups such as alkaloids, glycosides, and flavonoids and bioactivity such as radical scavenging, anti-inflammatory, and anti-microbial activities. Natural products and their structural counterparts have historically made significant contributions to pharmacology, particularly in cancer and infectious disorders (El-Nashar et al., 2024). Nonetheless, natural products pose hurdles for drug development, such as technical barriers to screening, isolation, characterization, and optimization, which have contributed to a drop in their pursuit by the pharmaceutical industry since the 1990s (Atanasov et al., 2021; El-Nashar et al., 2023a).
As a conclusion, the organic fractions of C. tenuifolia leaf extract remain rich in many valuable phytochemical groups, including alkaloids, tannins, glycosides, flavonoids, and saponins. All the fractions exhibited concentration-dependent and significant radical scavenging, anti-inflammatory, and anti-microbial effects against different gram (+) and gram (−) bacteria, as well as a number of pathogenic fungi, and clot lysis capacities. ECT exhibited better anti-radical (IC50 = 22.17 ± 1.90), anti-inflammatory, anti-bacterial, and clot-lysis capacity than the CCT and HCT. We suppose that all the activities, because of the presence of alkaloids, glycosides, and flavonoids within the organic fractions of C. tenuifolia leaf extract and the bioactive compound rutin, quercetin and stigmasterol play an important role in exerting therapeutic activities against oxidative stress and inflammation related diseases and disorders, pathogenic infections and a good source of thrombolytic agents. However, additional research is deemed necessary to conduct the isolation, identification, and clarification of the underlying molecular mechanisms responsible for each individual bioactivity as well as clinical investigation of the plant’s extract and exploited phytochemicals which would be useful in producing plant-based pharmaceuticals to treat numerous complicated human diseases.
The raw data supporting the conclusion of this article will be made available by the authors, without undue reservation.
The animal study was approved by the Institutional Ethics Committee (SUB/IAEC/12.01) approved all experimental protocols. The study was conducted in accordance with the local legislation and institutional requirements.
UJ: Data curation, Writing–original draft. HE-N: Supervision, Writing–review and editing. AA: Formal Analysis, Writing–original draft. MB: Formal Analysis, Writing–original draft. IS: Formal Analysis, Writing–original draft. SA: Formal Analysis, Writing–original draft. FA: Visualization, Writing–review and editing. NS: Funding acquisition, Project administration, Writing–original draft. ME-S: Supervision, Writing–review and editing. MI: Project administration, Supervision, Writing–review and editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was financially supported by United Arab Emirates University (Grant # 12S106 and SURE+2023).
The authors are thankful to the Department of Pharmacy, Southern University Bangladesh for providing laboratory facilities and hosting this project.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abdelazim, E. B., Abed, T., Goher, S. S., Alya, S. H., El-Nashar, H. A., El-Moslamy, S. H., et al. (2024). In vitro and in vivo studies of Syzygium cumini-loaded electrospun PLGA/PMMA/collagen nanofibers for accelerating topical wound healing. RSC Adv. 14, 101–117. doi:10.1039/d3ra06355k
Abd Nikfarjam, B., Adineh, M., Hajiali, F., and Nassiri-Asl, M. (2017). Treatment with rutin-a therapeutic strategy for neutrophil-mediated inflammatory and autoimmune diseases:-anti-inflammatory effects of rutin on neutrophils. J. pharmacopuncture 20, 52–56. doi:10.3831/KPI.2017.20.003
Agatonovic-Kustrin, S., Morton, D., Mizaton, H., and Zakaria, H. (2018). The relationship between major polyphenolic acids and stigmasterol to antioxidant activity in different extracts of Myrmecodia platytyrea. South Afr. J. Bot. 115, 94–99. doi:10.1016/j.sajb.2017.12.011
Ahmed, O. M., Elkomy, M. H., Fahim, H. I., Ashour, M. B., Naguib, I. A., Alghamdi, B. S., et al. (2022). Rutin and quercetin counter doxorubicin-induced liver toxicity in wistar rats via their modulatory effects on inflammation, oxidative stress, apoptosis, and Nrf2. Oxid. Med. Cell Longev. 2022, 2710607. doi:10.1155/2022/2710607
Aruoma, O. I. (1998). Free radicals, oxidative stress, and antioxidants in human health and disease. J. Am. Oil Chem. Soc. 75, 199–212. doi:10.1007/s11746-998-0032-9
Ashmawy, N. S., Gad, H. A., and El-Nashar, H. A. (2023). Comparative study of essential oils from different organs of syzygium cumini (pamposia) based on GC/MS chemical profiling and in vitro antiaging activity. Molecules 28, 7861. doi:10.3390/molecules28237861
Atanasov, A. G., Zotchev, S. B., Dirsch, V. M., and Supuran, C. T. (2021). Natural products in drug discovery: advances and opportunities. Nat. Rev. Drug Discov. 20, 200–216. doi:10.1038/s41573-020-00114-z
Augustin, N., and Thomas, B. (2015). Medico potential ferns of angamaly region, ernakulam district Kerala, India. Int. J. Curr. Pharm. Clin. Res. 5, 207–211. doi:10.1590/1519-6984.250256
Bakrim, S., Benkhaira, N., Bourais, I., Benali, T., Lee, L.-H., El Omari, N., et al. (2022). Health benefits and pharmacological properties of stigmasterol. Antioxidants 11, 1912. doi:10.3390/antiox11101912
Ballard, T. S., Mallikarjunan, P., Zhou, K., and O'Keefe, S. F. (2009). Optimizing the extraction of phenolic antioxidants from peanut skins using response surface methodology. J. Agric. Food Chem. 57, 3064–3072. doi:10.1021/jf8030925
Bari, M. S., Das, A. C., Bhuiyan, M. M. U., Khatun, S., Mitu, A., and Khandokar, L. (2022). Antioxidant, anti-inflammatory, thrombolytic, and cytotoxic activities of Gynura nepalensis DC. and isolation of its steroid constituents. Indian J. Nat. Prod. Resour. (IJNPR)[Formerly Nat. Prod. Radiance (NPR)] 13, 329–338. doi:10.56042/ijnpr.v13i3.37833
Baskaran, X. R., Geo Vigila, A. V., Zhang, S. Z., Feng, S. X., and Liao, W. B. (2018). A review of the use of pteridophytes for treating human ailments. J. Zhejiang Univ. Sci. B 19, 85–119. doi:10.1631/jzus.B1600344
Batool, R., Aziz, E., Iqbal, J., Salahuddin, H., Tan, B. K.-H., Tabassum, S., et al. (2020). In vitro antioxidant and anti-cancer activities and phytochemical analysis of Commelina benghalensis L. root extracts. Asian Pac. J. Trop. Biomed. 10, 417. doi:10.4103/2221-1691.290133
Benniamin, A. (2011). Medicinal ferns of North Eastern India with special reference to Arunachal Pradesh. Indian J. Traditional Knowl., 516–522.
Bhuia, M. S., Chowdhury, R., Sonia, F. A., Kamli, H., Shaikh, A., El-Nashar, H. A. S., et al. (2023a). Anticancer potential of the plant-derived saponin gracillin: a comprehensive review of mechanistic approaches. Chem. Biodivers. 20, e202300847. doi:10.1002/cbdv.202300847
Bhuia, M. S., Rahaman, M. M., Islam, T., Bappi, M. H., Sikder, M. I., Hossain, K. N., et al. (2023b). Neurobiological effects of gallic acid: current perspectives. Chin. Med. 18, 27. doi:10.1186/s13020-023-00735-7
Bhuia, M. S., Wilairatana, P., Ferdous, J., Chowdhury, R., Bappi, M. H., Rahman, M. A., et al. (2023c). Hirsutine, an emerging natural product with promising therapeutic benefits: a systematic review. Molecules 28, 6141. doi:10.3390/molecules28166141
Brown, A. J., Budge, S., Kaloriti, D., Tillmann, A., Jacobsen, M. D., Yin, Z., et al. (2014). Stress adaptation in a pathogenic fungus. J. Exp. Biol. 217, 144–155. doi:10.1242/jeb.088930
Chen, D., Liu, Y., Liu, P., Zhou, Y., Jiang, L., Yuan, C., et al. (2022). Orally delivered rutin in lipid-based nano-formulation exerts strong antithrombotic effects by protein disulfide isomerase inhibition. Drug Deliv. 29, 1824–1835. doi:10.1080/10717544.2022.2083726
Chen, Q., Wang, Q., Zhu, J., Xiao, Q., and Zhang, L. (2018). Reactive oxygen species: key regulators in vascular health and diseases. Br. J. Pharmacol. 175, 1279–1292. doi:10.1111/bph.13828
Chik, S. C., Or, T. C., Luo, D., Yang, C. L., and Lau, A. S. (2013). Pharmacological effects of active compounds on neurodegenerative disease with gastrodia and uncaria decoction, a commonly used poststroke decoction. ScientificWorldJournal 2013, 896873. doi:10.1155/2013/896873
Chowdhary, S., Verma, D., Pande, R., and Kumar, H. (2010). Antioxidative properties of flavonoids from Cheilanthes anceps Swartz. J. Am. Sci. 6, 203–207. doi:10.1016/j.sjbs.2020.06.006
Daryono, R., and Rhomawati, M. (2020). “An examination of medicinal potential of Pneumatopteris callosa: phytochemical screening, antibacterial, and antioxidant activity,” in IOP Conference Series: Earth and Environmental Science (Malang, Indonesia: IOP Publishing).456. 012068.
Dharmadeva, S., Galgamuwa, L. S., Prasadinie, C., and Kumarasinghe, N. (2018). In vitro anti-inflammatory activity of Ficus racemosa L. bark using albumin denaturation method. Ayu 39, 239–242. doi:10.4103/ayu.AYU_27_18
El-Nashar, H. A. S., Ali, A. M., and Salem, Y. H. (2023a). Genus pimenta: an updated comprehensive review on botany, distribution, ethnopharmacology, phytochemistry and biological approaches. Chem. Biodivers. 20, e202300855. doi:10.1002/cbdv.202300855
El-Nashar, H. A. S., El-Din, M. I. G., Hritcu, L., and Eldahshan, O. A. (2021). Insights on the inhibitory power of flavonoids on tyrosinase activity: a survey from 2016 to 2021. Molecules 26, 7546. doi:10.3390/molecules26247546
El-Nashar, H. A. S., Sayed, A. M., El-Sherief, H. A. M., Rateb, M. E., Akil, L., Khadra, I., et al. (2023b). Metabolomic profile, anti-trypanosomal potential and molecular docking studies of Thunbergia grandifolia. J. Enzyme Inhib. Med. Chem. 38, 2199950. doi:10.1080/14756366.2023.2199950
El-Nashar, H. A. S., Taleb, M., El-Shazly, M., Zhao, C., and Farag, M. A. (2024). Polysaccharides (pectin, mucilage, and fructan inulin) and their fermented products: a critical analysis of their biochemical, gut interactions, and biological functions as antidiabetic agents. Phytother. Res. 38, 662–693. doi:10.1002/ptr.8067
El-Shawi, O. E., El-Nashar, H. A. S., Abd El-Rahman, S. S., Eldahshan, O. A., and Singab, A. N. B. (2023). Protective effect of acrocarpus fraxinifolius extract against hepatic fibrosis induced by Gamma irradiation and carbon tetrachloride in albino rats. Int. J. Radiat. Biol. 99, 270–280. doi:10.1080/09553002.2022.2087926
Forman, H. J., and Zhang, H. (2021). Targeting oxidative stress in disease: promise and limitations of antioxidant therapy. Nat. Rev. Drug Discov. 20, 689–709. doi:10.1038/s41573-021-00233-1
French, G. L. (2005). Clinical impact and relevance of antibiotic resistance. Adv. Drug Deliv. Rev. 57, 1514–1527. doi:10.1016/j.addr.2005.04.005
Ghorpade, P. N., Thakar, S., Dongare, M., and Kale, M. (2015). Phytochemical analysis of four Cheilanthes species from northern western ghats of India. RJLBPCS 1, 92. doi:10.26479/2015.0102.04
Ginwala, R., Bhavsar, R., Chigbu, D. I., Jain, P., and Khan, Z. K. (2019). Potential role of flavonoids in treating chronic inflammatory diseases with a special focus on the anti-inflammatory activity of apigenin. Antioxidants (Basel) 8, 35. doi:10.3390/antiox8020035
Gunathilake, K., Ranaweera, K., and Rupasinghe, H. P. V. (2018). Change of phenolics, carotenoids, and antioxidant capacity following simulated gastrointestinal digestion and dialysis of selected edible green leaves. Food Chem. 245, 371–379. doi:10.1016/j.foodchem.2017.10.096
Hanum, F., and Hamzah, N. (1999). The use of medicinal plant species by the temuan tribe of ayer hitam forest, selangor, peninsular Malaysia. Pertanika J. Trop. Agric. Sci. 22, 85–94. doi:10.1080/09735070.2011.11886406
Ishaq, A. R., El-Nashar, H. A. S., Younis, T., Mangat, M. A., Shahzadi, M., Ul Haq, A. S., et al. (2022). Genus Lupinus (Fabaceae): a review of ethnobotanical, phytochemical and biological studies. J. Pharm. Pharmacol. 74, 1700–1717. doi:10.1093/jpp/rgac058
Jamshidi-Kia, F., Wibowo, J. P., Elachouri, M., Masumi, R., Salehifard-Jouneghani, A., Abolhasanzadeh, Z., et al. (2020). Battle between plants as antioxidants with free radicals in human body. J. Herbmed Pharmacol. 9, 191–199. doi:10.34172/jhp.2020.25
Jarial, R., Shard, A., Thakur, S., Sakinah, M., Zularisam, A., Rezania, S., et al. (2018). Characterization of flavonoids from fern Cheilanthes tenuifolia and evaluation of antioxidant, antimicrobial and anticancer activities. J. King Saud University-Science 30, 425–432. doi:10.1016/j.jksus.2017.04.007
Khalil, R., Ali, Q., Hafeez, M., and Malik, A. (2020). Phenolic acid profiling by RP-HPLC: evaluation of antibacterial and anticancer activities of Conocarpus erectus plant extracts. Biol. Clin. Sci. Res. J. 2020. doi:10.54112/bcsrj.v2020i1.10
Khan, H., Khan, Z., Amin, S., Mabkhot, Y. N., Mubarak, M. S., Hadda, T. B., et al. (2017). Plant bioactive molecules bearing glycosides as lead compounds for the treatment of fungal infection: a review. Biomed. Pharmacother. 93, 498–509. doi:10.1016/j.biopha.2017.06.077
Khan, H., Saeedi, M., Nabavi, S. M., Mubarak, M. S., and Bishayee, A. (2019). Glycosides from medicinal plants as potential anticancer agents: emerging trends towards future drugs. Curr. Med. Chem. 26, 2389–2406. doi:10.2174/0929867325666180403145137
King, L. M., Fleming-Dutra, K. E., and Hicks, L. A. (2018). Advances in optimizing the prescription of antibiotics in outpatient settings. Bmj 363, k3047. doi:10.1136/bmj.k3047
Landete, J. M. (2013). Dietary intake of natural antioxidants: vitamins and polyphenols. Crit. Rev. Food Sci. Nutr. 53, 706–721. doi:10.1080/10408398.2011.555018
Mahfuz, A., Salam, F. B. A., Deepa, K. N., and Hasan, A. N. (2019). Characterization of in-vitro antioxidant, cytotoxic, thrombolytic and membrane stabilizing potential of different extracts of Cheilanthes tenuifolia and Stigmasterol isolation from n-hexane extract. Clin. Phytoscience 5, 39–10. doi:10.1186/s40816-019-0135-x
Martin-Ventura, J. L., Rodrigues-Diez, R., Martinez-Lopez, D., Salaices, M., Blanco-Colio, L. M., and Briones, A. M. (2017). Oxidative stress in human atherothrombosis: sources, markers and therapeutic targets. Int. J. Mol. Sci. 18, 2315. doi:10.3390/ijms18112315
Mbaveng, A. T., Ngameni, B., Kuete, V., Simo, I. K., Ambassa, P., Roy, R., et al. (2008). Antimicrobial activity of the crude extracts and five flavonoids from the twigs of Dorstenia barteri (Moraceae). J. Ethnopharmacol. 116, 483–489. doi:10.1016/j.jep.2007.12.017
Mia, M. A. R., Dey, D., Sakib, M. R., Biswas, M. Y., Prottay, A. A. S., Paul, N., et al. (2023). The efficacy of natural bioactive compounds against prostate cancer: molecular targets and synergistic activities. Phytotherapy Res. 37, 5724–5754. doi:10.1002/ptr.8017
Mohsen, S., Dickinson, J. A., and Somayaji, R. (2020). Update on the adverse effects of antimicrobial therapies in community practice. Can. Fam. Physician 66, 651–659.
Moliner, C., López, V., Barros, L., Dias, M. I., Ferreira, I., Langa, E., et al. (2020). Rosemary flowers as edible plant foods: phenolic composition and antioxidant properties in Caenorhabditis elegans. Antioxidants (Basel) 9, 811. doi:10.3390/antiox9090811
Morimoto, B. H., Castelloe, E., and Fox, A. W. (2015). Safety pharmacology in drug discovery and development. Princ. Saf. Pharmacol. 229, 65–80. doi:10.1007/978-3-662-46943-9_3
Patel, M., and Reddy, M. (2018). Cheilanthes tenuifolia (burm. F.) sw.(pteridaceae): a new record of fern for Gujarat, India. Adv. Bioresearch 9, 48–51. doi:10.1080/23818107.2019.1636405
Prasad, S., Kashyap, R. S., Deopujari, J. Y., Purohit, H. J., Taori, G. M., and Daginawala, H. F. (2006). Development of an in vitro model to study clot lysis activity of thrombolytic drugs. Thromb. J. 4, 14. doi:10.1186/1477-9560-4-14
Rekatsina, M., Paladini, A., Piroli, A., Zis, P., Pergolizzi, J. V., and Varrassi, G. (2020). Pathophysiology and therapeutic perspectives of oxidative stress and neurodegenerative diseases: a narrative review. Adv. Ther. 37, 113–139. doi:10.1007/s12325-019-01148-5
Rodríguez-Yoldi, M. J. (2021). Anti-inflammatory and antioxidant properties of plant extracts. Antioxidants (Basel) 10, 921. doi:10.3390/antiox10060921
Salatino, M. L. F., and Prado, J. (1998). Flavonoid glycosides of Pteridaceae from Brazil. Biochem. Syst. Ecol. 26, 761–769. doi:10.1016/s0305-1978(98)00032-5
Salvamani, S., Gunasekaran, B., Shaharuddin, N. A., Ahmad, S. A., and Shukor, M. Y. (2014). Antiartherosclerotic effects of plant flavonoids. Biomed. Res. Int. 2014, 480258. doi:10.1155/2014/480258
Sen, K., and Mukhopadhyay, R. (2014). New report of vessel elements in Aleuritopteris and Cheilanthes. Taiwania 59, 231–239. doi:10.6165/tai.2014.59.231
Shill, M. C., El-Nashar, H. A., Mollick, P. P., Acharyya, R. N., Afrin, S., Hossain, H., et al. (2024). Longevity spinach (Gynura procumbens) ameliorated oxidative stress and inflammatory mediators in cisplatin-induced organ dysfunction in rats: comprehensive in vivo and in silico studies. Chem. Biodivers., e202301719. doi:10.1002/cbdv.202301719
Shinde, U., Phadke, A., Nair, A., Mungantiwar, A., Dikshit, V., and Saraf, M. (1999). Membrane stabilizing activity—a possible mechanism of action for the anti-inflammatory activity of Cedrus deodara wood oil. Fitoterapia 70, 251–257. doi:10.1016/s0367-326x(99)00030-1
Singh, B. P., and Upadhyay, R. (2014). Medicinal pteridophytes of Madhya Pradesh. J. Pharmacogn. Phytochemistry 3, 173–176.
Tabassum, S., Ahmad, S., Rehman Khan, K. u., Tabassum, F., Khursheed, A., Zaman, Q. U., et al. (2022). Phytochemical profiling, antioxidant, anti-inflammatory, thrombolytic, hemolytic activity in vitro and in silico potential of Portulacaria afra. Molecules 27, 2377. doi:10.3390/molecules27082377
Thangjam, N. M., Taijong, J., and Kumar, A. (2020). Phytochemical and pharmacological activities of methanol extract of Artemisia vulgaris L. leaves. Clin. Phytoscience 6, 72–78. doi:10.1186/s40816-020-00214-8
Thomford, N. E., Senthebane, D. A., Rowe, A., Munro, D., Seele, P., Maroyi, A., et al. (2018). Natural products for drug discovery in the 21st century: innovations for novel drug discovery. Int. J. Mol. Sci. 19, 1578. doi:10.3390/ijms19061578
Tlili, N., Elfalleh, W., Hannachi, H., Yahia, Y., Khaldi, A., Ferchichi, A., et al. (2013). Screening of natural antioxidants from selected medicinal plants. Int. J. food Prop. 16, 1117–1126. doi:10.1080/10942912.2011.576360
Touati, N., Saidani, K., Boudries, H., Hammiche, H., Ouazene, N., and Bedjou, F. (2018). Antibacterial activity of phenolic compounds of Pulicaria odora, wild plant in northern Algeria. Int. Food Res. J. 25, 2021–2030.
Uddin, M. Z., Rana, M. S., Hossain, S., Ferdous, S., Dutta, E., Dutta, M., et al. (2019). In vivo neuroprotective, antinociceptive, anti-inflammatory potential in Swiss albino mice and in vitro antioxidant and clot lysis activities of fractionated Holigarna longifolia Roxb. bark extract. J. Complementary Integr. Med. 17, 20190102. doi:10.1515/jcim-2019-0102
Wen, M.-C., Wei, C.-H., Hu, Z.-Q., Srivastava, K., Ko, J., Xi, S.-T., et al. (2005). Efficacy and tolerability of antiasthma herbal medicine intervention in adult patients with moderate-severe allergic asthma. J. allergy Clin. Immunol. 116, 517–524. doi:10.1016/j.jaci.2005.05.029
Yonathan, M., Asres, K., Assefa, A., and Bucar, F. (2006). In vivo anti-inflammatory and anti-nociceptive activities of Cheilanthes farinosa. J. Ethnopharmacol. 108, 462–470. doi:10.1016/j.jep.2006.06.006
Younis, T., Jabeen, F., Hussain, A., Rasool, B., Raza Ishaq, A., Nawaz, A., et al. (2023). Antioxidant and pulmonary protective potential of fraxinus xanthoxyloides bark extract against CCl(4) -induced toxicity in rats. Chem. Biodivers. 20, e202200755. doi:10.1002/cbdv.202200755
Keywords: anti-inflammatory, Cheilanthes tenuifolia, secondary metabolites, antimicrobial, membrane stabilization, anti-atherothrombotic, radical scavenging
Citation: Juhi UH, El-Nashar HAS, Al Faruq A, Bhuia MS, Sultana I, Alam S, Abuyousef F, Saleh N, El-Shazly M and Islam MT (2024) Phytochemical analysis and biological investigation of Cheilanthes tenuifolia (Burm.f.) Swartz. Front. Pharmacol. 15:1366889. doi: 10.3389/fphar.2024.1366889
Received: 22 January 2024; Accepted: 07 March 2024;
Published: 04 April 2024.
Edited by:
Elena Lucarini, University of Florence, ItalyReviewed by:
Rudi Hendra, Riau University, IndonesiaCopyright © 2024 Juhi, El-Nashar, Al Faruq, Bhuia, Sultana, Alam, Abuyousef, Saleh, El-Shazly and Islam. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Heba A. S. El-Nashar, aGViYV9waGFybWFAcGhhcm1hLmFzdS5lZHUuZWc=; Mohamed El-Shazly, bW9oYW1lZC5lbHNoYXpseUBwaGFybWEuYXN1LmVkdS5lZw==; Na’il Saleh, bi5zYWxlaEB1YWV1LmFjLmFl; Muhammad Torequl Islam, ZG10LmlzbGFtQGJzbXJzdHUuZWR1LmJk
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.