- 1Department of Chinese Pharmaceutical Sciences and Chinese Medicine Resources, College of Chinese Medicine, China Medical University, Taichung, Taiwan
- 2Department of Nursing, Hungkuang University, Taichung, Taiwan
Introduction: Rhubarb is a traditional Chinese medicine (TCM) used to release heat and has cathartic effects. Official rhubarb in Taiwan Herbal Pharmacopeias 4th edition (THP 4th) and China Pharmacopeia 2020 (CP 2020) are the roots and rhizomes of Rheum palmatum L., Rheum tanguticum Maxim. ex Balf., and Rheum officinale Baill. However, the Rheum genus is a large genus with many different species, and owing to the similarity in appearance and taste with official rhubarb, there needs to be more clarity in the distinction between the species of rhubarb and their applications. Given the time-consuming and complicated extraction and chromatography methods outlined in pharmacopeias, we improved the qualitative analysis and quantitative analysis methods for rhubarb in the market. Hence, we applied our method to identify the species and quality of official and unofficial rhubarb.
Method: We analyzed 21 rhubarb samples from the Taiwanese market using a proposed HPLC-based extraction and qualitative analysis employing eight markers: aloe-emodin, rhein, emodin, chrysophanol, physcion, rhapontigenin, rhaponticin, and resveratrol. Additionally, we developed a TLC method for the analysis of rhubarb. KEGG pathway analysis was used to clarify the phytochemical and pharmacological knowledge of official and unofficial rhubarb.
Results: Rhein and rhapontigenin emerged as key markers to differentiate official and unofficial rhubarb. Rhapontigenin is abundant in unofficial rhubarb; however, rhein content was low. In contrast, their contents in official rhubarb were opposite to their contents in unofficial rhubarb. The TLC analysis used rhein and rhapontigenin to identify rhubarb in Taiwan’s markets, whereas the KEGG pathway analysis revealed that anthraquinones and stilbenes affected different pathways.
Discussion: Eight reference standards were used in this study to propose a quality control method for rhubarb in Taiwanese markets. We propose a rapid extraction method and quantitative analysis of rhubarb to differentiate between official and unofficial rhubarb.
1 Introduction
Rhubarb consists of the dried roots and rhizomes of Rheum palmatum L., Rheum tanguticum Maxim. ex Balf., and Rheum officinale Baill according to Taiwan Herbal Pharmacopeia 4th edition (THP 4th) or the underground parts of R. palmatum L. and R. officinale Baill as per the European Pharmacopoeia 10.0 (EP) (European Pharmacopoeia, 2020; Taiwan Herbal Pharmacopeia, 2024). As a traditional Chinese medicine (TCM), rhubarb was first recorded in Shen-Nong-Ben-Cao-Jing as a laxative, which releases heat and induces catharsis. Rhubarb has been used in numerous decoctions, such as Da-Cheng-Qi-Tang and Xiao-Cheng-Qi-Tang, among others, and in Shang-Han-Lun of Zhang-Zhong-Jing at the end of the Han Dynasty—one of the four classic Chinese medicinal literature works.
Currently, rhubarb is gaining global recognition beyond traditional medicine, with documented pharmacological effects such as bacterial resistance (Wang et al., 2010), anti-inflammatory (Choi et al., 2013), anti-cancer effects (Huang et al., 2007), and enhancement of intestinal barrier function (Xiong et al., 2018).
Rhubarb has been recorded in many countries’ pharmacopeias, including Hong Kong Chinese Materia Medica Standards (Hong Kong Chinese Materia Medica Standards, 2008), THP 4th (Taiwan Herbal Pharmacopeia, 2024), and Pharmacopeia of the People’s Republic of China (Pharmacopoeia of the People’s Republic of China, 2020), with three official species, including R. palmatum L., R. tanguticum Maxim. Ex Balf., and R. officinale Baill. These official rhubarbs contain anthraquinones, notably aloe-emodin, rhein, emodin, chrysophanol, and physcion. Anthraquinones are recognized as the most crucial chemical components in the official rhubarbs with several bioactivities such as laxative (Lombardi et al., 2022), anti-inflammation (Zhang et al., 2020), anti-bacterial (Friedman et al., 2020), anti-diabetic (Arvindekar et al., 2015), and anti-cancer properties (Adnan et al., 2021). Most anthraquinones in TCM or herbs exist in the form of glycosides (Zhao et al., 2014; Jintao et al., 2018) and plant parts like senna leaves (Vilanova-Sanchez et al., 2018), and Aloe vera (Hong et al., 2018); such plant parts are used to treat constipation (de Witte, 1993). Free anthraquinones (aglycone anthraquinones) can be absorbed through the small intestine, whereas anthraquinone glycosides reach the large intestine (Feng et al., 2013). Consequently, except for the laxative effect, the active components that traverse the intestinal barrier are free anthraquinone derivatives, rather than anthraquinone glycosides (Fairbairn, 2011; Lin et al., 2017). By contrast, aglycones contain multiple glycosides corresponding to the sugar-binding sites. Therefore, using glycosides as markers is inappropriate because of the limitations in the reference standards for a single high-pressure liquid chromatography photodiode array (HPLC-PDA). Thus, in our study, we focused on quantifying five free anthraquinones, namely, aloe-emodin, emodin, rhein, chrysophanol, and physcion, after hydrolysis, and we optimized their extraction parameters.
In addition, stilbenes are a significant group of chemical components in unofficial rhubarb that are not cataloged in national pharmacopeias. Resveratrol is well known for its strong antioxidant properties (Xia et al., 2017), and it is found in grapes and wine. In addition to resveratrol, rhapontigenin or rhaponticin exhibits anti-inflammatory (Li et al., 2021), cardio-protective (Fan, 2019), anti-hyperlipidemic (Jo et al., 2014), anti-cancer (Kim et al., 2014), and cancer prevention effects (Vervandier-Fasseur and Latruffe, 2019). The most notable stilbene compound in rhubarb is rhaponticin—a glycosidic stilbene not found in official rhubarb (Komatsu et al., 2006; Ye et al., 2007). In EP 10.0, R. rhaponticum, an unofficial rhubarb, was examined by thin-layer chromatography (TLC) using rhaponticin as a marker (European Pharmacopoeia, 2020). Thus, rhaponticin serves as an essential stilbene to differentiate official rhubarbs from unofficial ones. Rhapontigenin—aglycone metabolite of rhaponticin (Chen et al., 2020)—exerts extensive anti-allergic (Jo et al., 2014), anti-bacterial (Li et al., 2022), and anti-cancer activities through TGF-β (Yeh et al., 2016). Therefore, rhapontigenin is also an important marker that can be used to distinguish rhubarb from the market.
In recent years, with the discovery of new drugs with pharmacological effects, the identification and quality control of herbal medicines in the market has become increasingly important. Rheum is a large genus belonging to the family Polygonaceae, comprising approximately 56 accepted species distributed worldwide (Plants of the World Online, 2023). The genus Rheum has many species with similar morphology and taste but comparatively different chemical substances. Moreover, rhubarb in the market is mainly available in a dry form, causing confusion and ambiguity in the distinction between rhubarb species and their applications (Mohtashami et al., 2021). Because it is challenging to be distinguished simply with the naked eyes, adulteration and misrepresentation are prevalent issues.
In Taiwan’s herbal market, most rhubarb is imported from China in the dry form. Through our investigation utilizing TLC with rhaponticin as a marker, we found that most rhubarb in Taiwanese markets is not the official species according to THP 4th standards. Given the limitations of the reference standard, all extraction methods and chromatography in pharmacopeias are based on the hydrolysis principle to detect the aglycones of anthraquinones and stilbenes. These methods are time-consuming and complicated; based on our understanding of the phytochemical properties of rhubarb, we improved qualitative and quantitative analysis methods for rhubarb on the market using TLC and HPLC with eight reference markers, namely, aloe-emodin, rhein, emodin, chrysophanol, physcion, rhaponticin, rhapontigenin (the aglycone of rhaponticin), and resveratrol. This enhanced methodology allows for better clarity regarding the species and quality of both official and unofficial rhubarb.
2 Materials and methods
2.1 Samples’ preparation and reagents
Twenty-one dried samples of rhubarb root and rhizome were collected from different herbal stores in Taiwan and labeled as RH-1 to RH-9 and SP-1 to SP-12. All samples were identified using TLC according to the method described in CP 2020. Samples containing rhaponticin were divided into the unofficial group including RH-5 to RH-8, SP-1, SP-2, SP-3, and SP-5 to SP-12, whereas those that did not contain rhaponticin were classified into the official rhubarb group, including RH-1 to RH-4, RH-9, and SP-4 (Supplementary Figure S1). The photos of samples SP-1 and SP-4, which were used for method validation, are shown in Figure 1. The voucher specimens of all samples were deposited at the Department of Chinese Pharmaceutical Sciences and Chinese Medicine Resources, College of Chinese Medine, China Medical University, Taichung, Taiwan.
Figure 1. Rhubarb samples from Taiwan market. (A) Official rhubarb SP-4 and (B) unofficial rhubarb SP-1.
Eight reference markers including five anthraquinones and three stilbenes were purchased from different companies as follows: aloe-emodin (purity >95%) and emodin (purity >90%) from Sigma-Aldrich Chemie Gmbh., Taufkirchen, Germany; rhein (purity >98%), chrysophanol (purity >98%), physcion (purity >98%), rhapontigenin (purity >95%), and resveratrol (purity >98%) from Chengdu Purify Phytochemicals Ltd., Chengdu, China; and rhaponticin (purity >98%) from Cayman Chemical Company, Ann Arbor, Michigan, United States of America. Structures of the markers are shown in Supplementary Figure S2. Acetonitrile, methanol, phosphoric acid, hydrochloric acid, acetone, and formic acid were purchased from Merck KGaA, St. Louis, Missouri, United States of America; pentane was procured from ITW Reagents, Monza, Italy; and ethyl acetate was acquired from Honeywell International Inc., Charlotte, North Carolina, United States of America.
2.2 High-pressure liquid chromatography
2.2.1 Sample extraction method
Two samples (unofficial SP-1 and official rhubarb SP-4) were used for extraction method validation, including extraction solvent selection, acid concentration selection, extraction duration, and comparison with the extraction method outlined in THP 4th. Accordingly, 0.1 g powder of the sample was extracted in 20 mL of 1%–3% HCl in 70%–100% MeOH, refluxed for 1 h, and filtered. This extraction process was repeated once, the filtrate was combined, and the total volume made up to 100 mL. The resultant mixture was filtered through a 0.45-µm filter, and it was used as the sample solution.
2.2.2 HPLC condition
We tested the method with different columns, including Waters Symmetry C18 column, Xbridge C18 column, Cosmosil C18 column, Dikma C18 column, and Grace Alltima C18 column, all with dimensions 4.6 × 250 mm, 5 μm, under different temperatures, including 25°C, 30°C, and 35°C.
The following conditions were set: solvent A: acetonitrile, solvent B: 0.1% (v/v), 0.05% (v/v), and 0.2% (v/v) phosphoric acid; injection volume: 10 µL; flow rate: 1 mL/min; detector: photodiode array detector (PDA); mobile phase: 0–12 min, 75%–65% solvent B; 12–50 min, 65%–40% solvent B; 50–60 min, 40%–20% solvent B; 60–65 min, 20%–10% solvent B.
2.3 Thin-layer chromatography
Six reference standards (rhapontigenin, rhein, aloe-emodin, emodin, chrysophanol, and physcion) were dissolved in methanol to obtain 50 mg/L solution. For the rhubarb extraction method, 0.5 g powder of the sample was extracted in 20 mL of 2% HCl in 70% MeOH, refluxed for 45 min, and filtered to obtain the sample solution (self-development). The development solvent was pentane-ethyl acetate–acetone–formic acid in 15–5–1–0.7 (v/v) (self-development). In the TLC analysis, we used TLC silica gel F254 plate, with 3 µL application as 6 mm bands using a TLC sample semi-automatic applicator Linomat 5 (CAMAG, Muttenz, Switzerland) with Wincat program and Linomat syringes (3 mL; CAMAG, Muttenz, Switzerland). The plate was developed at 80 mm from the lower edge and visualized under 366 nm UV light.
2.4 KEGG pathway analysis
For network pharmacology analysis and KEGG analysis, we collected data from different databases as follows: TCMSP for bioactive components, screened the components with OB >20%, DL > 0.1; PubChem and BindingDB for bioactive components—target; UniProt for target—UniProt genes; DisGeNET for UniProt genes—diseases; and KEGG: Kyoto Encyclopedia of Genes and Genomes for genes—pathways.
In this section, we used Cytoscape 3.9.1 and RStudio 4.2.2 software to analyze our results.
2.5 Statistical method
To compare the content of eight markers between two groups of samples, we used the t-test method using R 4.3.2 and RStudio 4.2.2 to generate boxplots of the data.
3 Results
3.1 High-pressure liquid chromatography analysis of rhubarb with eight chemical markers
3.1.1 Chemical markers and wavelength absorption of markers
The highest absorption wavelengths of five anthraquinones were closely clustered: aloe-emodin at 225.1 nm, emodin at 221.6 nm, rhein at 229.8 nm, chrysophanol at 223.9 nm, and physcion 222.8 nm. Among the three stilbenes, the highest absorption wavelengths were 325.0, 305.9, and 323.8 nm for rhaponticin, resveratrol, and rhapontigenin, respectively. Thus, we chose 225 and 310 nm wavelengths to observe and measure the anthraquinones and stilbenes, respectively (Supplementary Figure S3).
3.1.2 Sample extraction method
Two samples (unofficial SP-1 and official rhubarb SP-4) were used to validate the extraction method. For extraction solvent selection, we compared the extraction efficiency of various solvent combinations: MeOH, 2% HCl/MeOH, 2% HCl/70% MeOH, 1% HCl/70% MeOH, and 3% HCl/70% MeOH. The results in Supplementary Figure S4 show that an acidic solvent yielded higher peak areas/weights of the eight markers than MeOH. To extract and protect the column better, we selected 2% of HCl over 1% or 3% of HCl as the extraction solvent, partly due to the limitation of the acid hydrolysis method at high temperatures and the high rhaponticin content in the sample hindered complete hydrolysis in unofficial rhubarb with 2% HCl/70% MeOH. However, when compared with the extraction method in THP 4th, satisfactory amounts of free anthraquinones were obtained, whereas all three stilbenes were highly abundant in the unofficial rhubarb SP-1 (Supplementary Figure S4). For extraction times, refluxing for 1 h of reflux and extracting twice was sufficient to extract at least 98% of components (Supplementary Figure S5). Hence, 2% HCl/70% MeOH, reflux of 1 h, and two time extraction were adopted as the extraction methods in this study.
3.1.3 Method validation
After evaluating HPLC condition with five different columns, namely, Waters, Symmetry C18 column (4.6 × 250 mm, 5 µm); Dikma C18 column (4.6 × 250 mm, 5 µm); Cosmosil C18 column (4.6 × 250 mm, 5 µm); Waters, Xbridge C18 column (4.6 × 250 mm, 5 µm); and Grace, Alltima C18 column (4.6 × 250 mm, 5 µm), we chose the Grace, Alltima C18 column, 4.6 × 250 mm, 5 μm, for its optimal separation efficiency and allowable limits of tailing factor for the markers (0.9–1.2). Linearity and calibration studies of rhaponticin, resveratrol, aloe-emodin, rhein, emodin, chrysophanol, and physcion were conducted intraday with a correlation coefficient of r2 > 0.999. The details are shown in Supplementary Tables S1, S2.
Relative standard deviation (RSD, %) of precision for the eight markers, rhaponticin, rhapontigenin, resveratrol, aloe-emodin, rhein, emodin, chrysophanol, and physcion, were 0.72%, 0.15%, 0.75%, 0.91%, 0.57%, 0.38%, 0.47%, and 0.66%, respectively. RSD precision was less than 2%. The RSD (%) of the repeatability test for aloe-emodin, rhein, emodin, chrysophanol, and physcion were 3.32%, 2.85%, 2.29%, 4.34%, and 2.70%, respectively, and that of the recovery test were 2.31%, 1.60%, 3.96%, 1.48%, and 1.63%, respectively (Supplementary Table S3). Limits of quantification (LOQ) of rhaponticin, rhapontigenin, resveratrol, aloe-emodin, rhein, emodin, chrysophanol, and physcion were 0.39, 0.97, 0.25, 0.27, 0.29, 0.29, 0.2, and 0.31 mg/L, respectively. Furthermore, limits of detection (LOD) of rhaponticin, rhapontigenin, resveratrol, aloe-emodin, rhein, emodin, chrysophanol, and physcion were 0.2, 0.12, 0.13, 0.15, 0.15, 0.15, 0.1, 0.1, and 0.16 mg/L, respectively (Supplementary Table S3).
3.1.4 Marker content in 13 market rhubarb samples with HPLC analysis
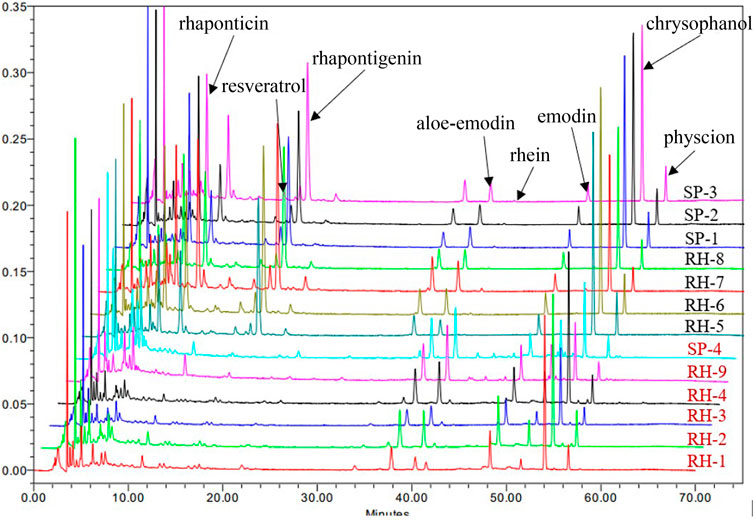
In typical chromatography of the reference standards at 225 nm, the retention times of rhaponticin, resveratrol, and rhapontigenin were 8.411, 16.572, and 18.572 min, respectively. Five peaks of aloe-emodin, rhein, emodin, chrysophanol, and physcion appeared at 38.432, 40.747, 48.440, 54.125, and 56.597 min, respectively (Figure 2). The HPLC chromatograms of the 13 market rhubarb samples are depicted in Figure 3.
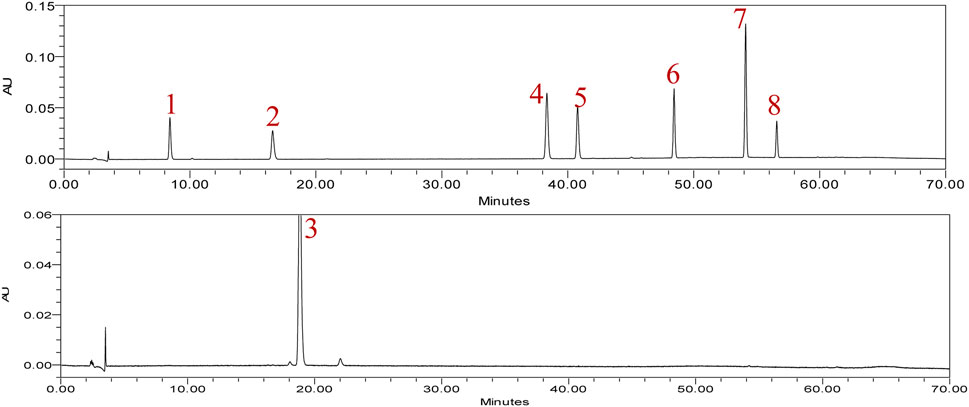
Figure 2. Typical chromatogram of eight reference standards at 225 nm. 1: rhaponticin, 2: resveratrol, 3: rhapontigenin, 4: aloe-emodin, 5: rhein, 6: emodin, 7: chrysophanol, 8: physcion.
In the HPLC analysis result, there were 13 rhubarb samples (including seven unofficial rhubarbs and six official rhubarbs); both groups of rhubarb contained anthraquinones: six official rhubarbs contained aloe-emodin, rhein, emodin, chrysophanol, physcion, and no rhapontigenin and rhaponticin were detected; all seven unofficial rhubarb contain aloe-emodin, emodin, chrysophanol, physcion, high content of rhaponticin, and rhapontigenin, but no rhein was detected. Based on our results, the total anthraquinones content in the official rhubarb and unofficial rhubarb groups were 1.45%–2.99% (average 2.28%) and 1.78%–2.66% (average 2.18%), respectively. Thus, the difference in total anthraquinones content was insignificant (p-value >0.05). However, there was a very large gap in the content of total stilbenes between two groups of rhubarb, no stilbenes or traces (0%–0.06%, average 0.03%) was detected in the official rhubarb group, whereas a very high total stilbenes (4.33%–5.92%, average 4.90%) was found in the unofficial rhubarb group. The difference in total stilbenes content was significant (p-value <0.0001) between two groups of sample. Specifically, in comparing the content of five anthraquinones, chrysophanol and rhein were the two substances with the highest concentration in all samples (RH-9 and SP-4 have the highest rhein content among the five anthraquinones), the content of rhein and emodin in the official rhubarb group were significantly higher than the content of these two substances in the unofficial rhubarb group, but the chrysophanol and physcion content in the official group rhubarb were significantly lower than those in the unofficial rhubarb group. The remaining substance, that is, aloe-emodin, had no significant difference in both groups of the sample (Figure 4). Generally, chrysophanol was the active ingredient with the highest content in all samples (except RH-9 and SP-4), but the differences between unofficial rhubarb and official rhubarb were rhein and rhapontigenin; unofficial rhubarb did not contain rhein or had very low levels of rhein (trace or under LOD), whereas the content of rhapontigenin and rhaponticin in this sample group were very high (Supplementary Table S4). This showed that rhapontigenin and rhein were two active ingredients that played an important role in distinguishing official rhubarb from unofficial rhubarb.
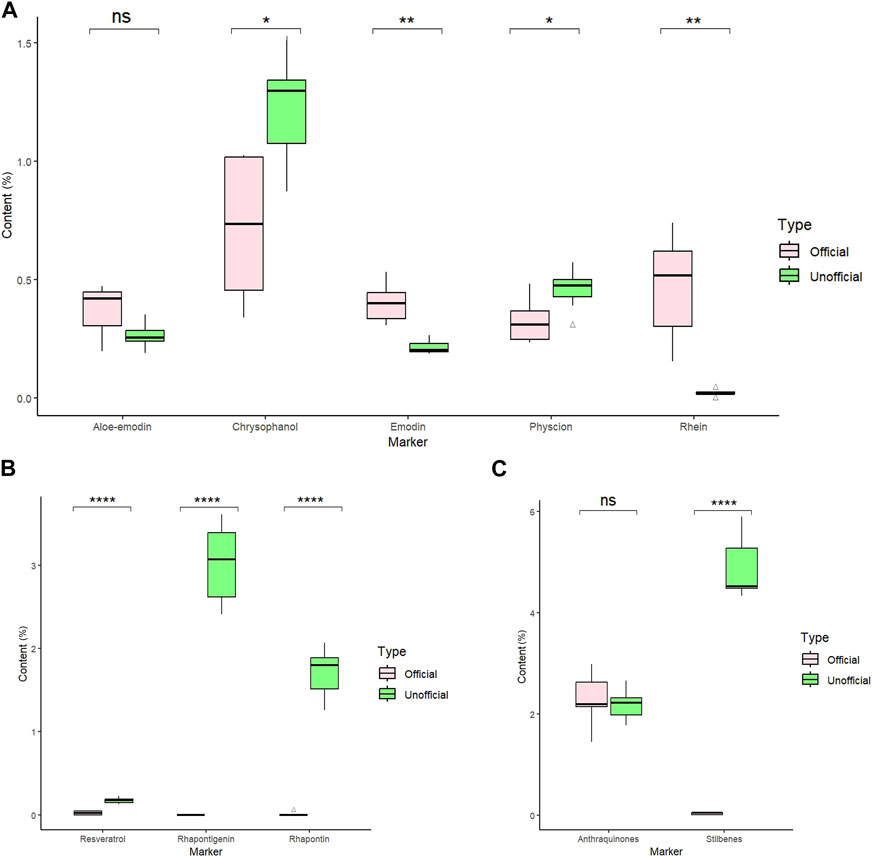
Figure 4. Eight marker content in six official and seven unofficial rhubarb samples. (A) Comparison of five anthraquinones content (%) between the two groups of rhubarb. (B) Comparison of three stilbenes content (%) between the two groups of rhubarb. (C) Comparison of total stilbenes and total anthraquinones content (%) between the two groups. Statistic method: pair t-test, ns p-value >0.05; *p-value ≦ 0.05; **p-value ≦ 0.01; ***p-value ≦ 0.001; ****p-value ≦ 0.0001.
3.2 Suggested thin-layer chromatography for qualitative analysis of rhubarb
3.2.1 TLC visualization condition
We visualized TLC under 254 nm UV light, 366 nm UV light, and white light. The results revealed that all six reference standards, namely, aloe-emodin, emodin, physcion, chrysophanol, rhein, and rhapontigenin, were clearly observed under UV light at 366 nm. Therefore, 366 nm was used for visualization in this study (Figure 5).
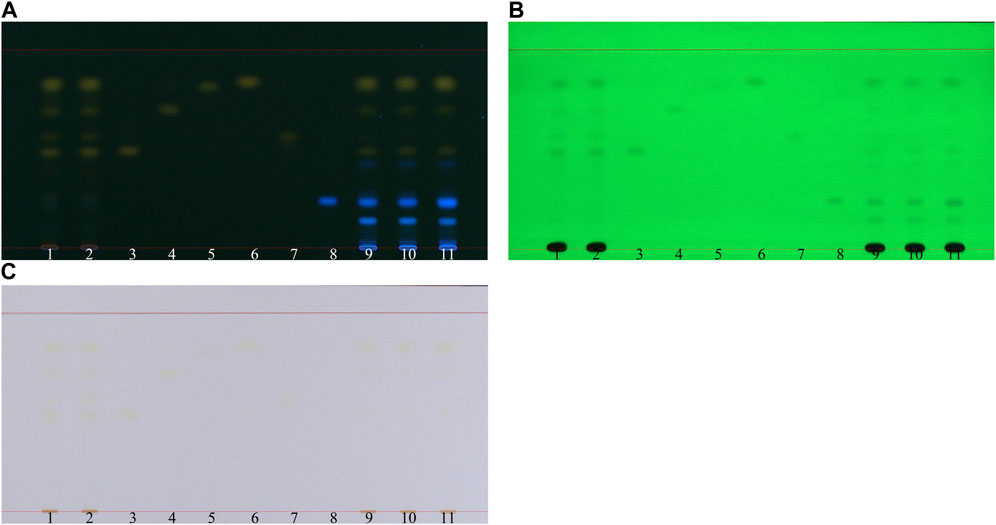
Figure 5. TLC visualization condition for rhubarb: TLC analysis of SP-1 and SP-4 with six reference markers. (A) 366 nm UV light, (B) 254 nm UV light, and (C) white light. 1–2: SP-4, 3: aloe-emodin, 4: emodin, 5: physcion, 6: chrysophanol, 7: rhein, 8: rhapontigenin, 9–10: SP-1, and 11: spike (RH-6 + rhapontigenin).
3.2.2 TLC mobile phase
We compared TLC efficiency with two solvent systems: pentane-ethyl acetate–acetone–formic acid (system 1) at 15–5–1–0.7 (v/v) and pentane-ethyl acetate–formic acid (system 2) at 15–5–0.5 (v/v). In both solvent systems, we observed yellow bands: from top to bottom, chrysophanol was closely followed by physcion, emodin, rhein, and aloe-emodin. The main fluorescent band was rhapontigenin. Both solvent systems yielded good separation efficiency; however, higher the level, more the chrysophanol and physcion bands tended to overlap. However, according to the HPLC results, chrysophanol and physcion were present in high concentrations in both groups of rhubarb; therefore, the separation of these two substances was rendered unnecessary to distinguish unofficial rhubarb. By contrast, system 1 provided better separation efficiency for rhein and rhapontigenin. Hence, we chose the solvent pentane-ethyl acetate–acetone–formic acid at 15–5–1–0.7 (v/v) for the TLC analysis (Figure 6). In the TLC analysis with this solvent, the retention factor (Rf) values for rhapontigenin, aloe-emodin, rhein, emodin, physcion, and chrysophanol were 0.24, 0.49, 0.56, 0.70, 0.82, and 0.85, respectively. Because of humidity and air temperature fluctuations, Rf may have changed slightly, but the difference was not more than 0.02, and the order of the markers was also fixed in all TLC plates for the 21 rhubarb samples.
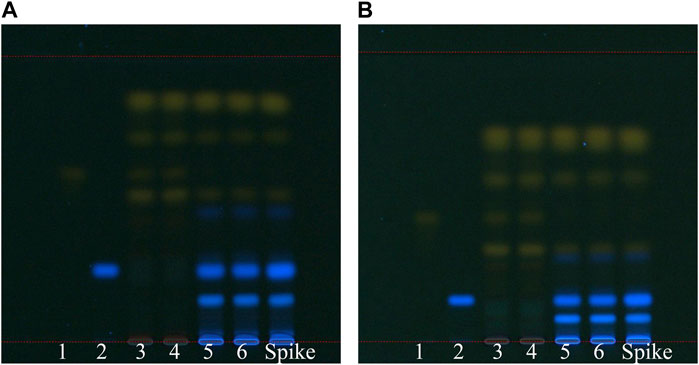
Figure 6. TLC analysis of SP-1 and SP-4 under two development solvents: (A) pentane-ethyl acetate–acetone–formic acid 15–5–1–0.7 (v/v) and (B) pentane-ethyl acetate–formic acid 15–5–0.5 (v/v). 1: rhein, 2: rhapontigenin, 3–4: SP-4, 5–6: SP-1, 7: spike (SP-1 + rhapontigenin).
3.2.3 TLC analysis of 21 rhubarb samples using six reference markers
In our TLC analysis results, six reference markers, namely, aloe-emodin, emodin, rhein, chrysophanol, physcion, and rhapontigenin, were observed under 366 nm UV light as described earlier. All 21 samples contained aloe-emodin, emodin, chrysophanol, and physcion, whereas samples RH-1, RH-2, RH-3, RH-4, RH-9, and SP- 4 contained rhein but not rhapontigenin. By contrast, unofficial rhubarb samples contained no rhein but rhapontigenin, which was consistent with the HPLC results. Using rhapontigenin as a marker instead of rhaponticin allowed simultaneous TLC with anthraquinones, shortening the experimental process for identifying market rhubarb (Figure 7).
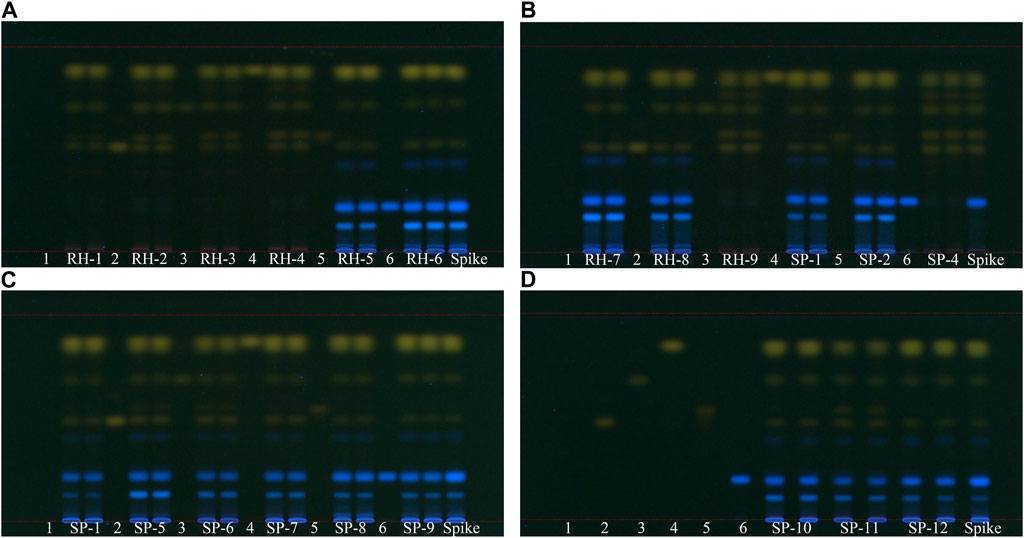
Figure 7. TLC analysis of 21 market rhubarb samples under 366 nm UV light, development solvent A pentane-ethyl acetate–acetone–formic acid 15–5–1–0.7 (v/v). 1: blank (methanol), 2: aloe-emodin, 3: emodin, 4: chrysophanol, 5: rhein, and 6: rhapontigenin. (A) Sample RH-1 to RH-6 (B) sample RH-7 to RH-9, SP-1, SP-2, and SP-6 (C) sample SP-1, SP-5 to SP-9 (D) sample SP-10 to SP-12.
3.3 Comparison of stilbenes and anthraquinones using network pharmacology and KEGG analyses
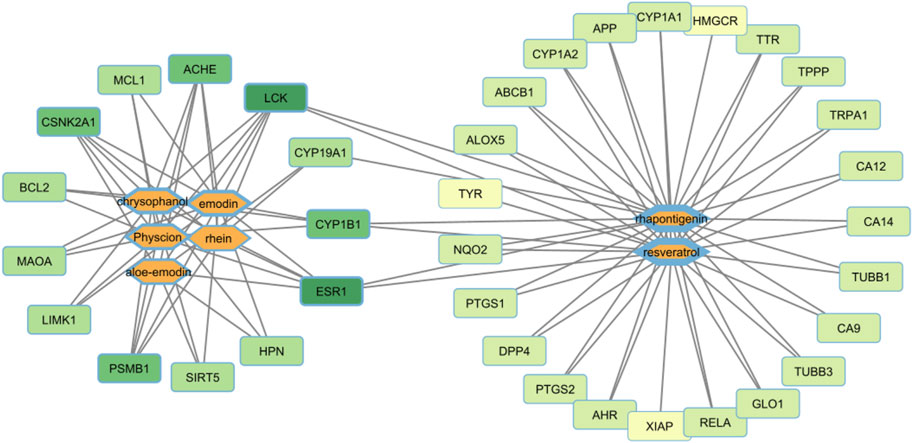
Network pharmacology and KEGG analyses revealed that anthraquinones, including aloe-emodin, emodin, rhein, chrysophanol, physcion, and their glycosides, targeted 13 proteins similarly, and stilbenes, including rhapontigenin and resveratrol, targeted 29 proteins. Protein target nomenclature followed that of BindingDB. Both groups of active components had four targets in common. In Figure 8, the target nodes with a darker green color are nodes with a greater degree, indicating that the number of compounds acting on them was higher. The anthraquinone group targets that had the largest degrees were ESR1 (estrogen receptor 1), LCK (human lymphocyte-specific protein tyrosine kinase), and ACHE (acetylcholinesterase). Similarly, for compound nodes, the thicker the border, the more targets that the compound node has. In the anthraquinones group, chrysophanol, physcion, and emodin had the majority of targets. Stilbenes also had a huge target number. Stilbenes had a wide effect, whereas anthraquinones displayed a relatively concentrated effect on a few targets. These results showed that the effects of the two groups were different. The component–target network highlighted the pharmacological differences between stilbenes and anthraquinones (Figure 8).
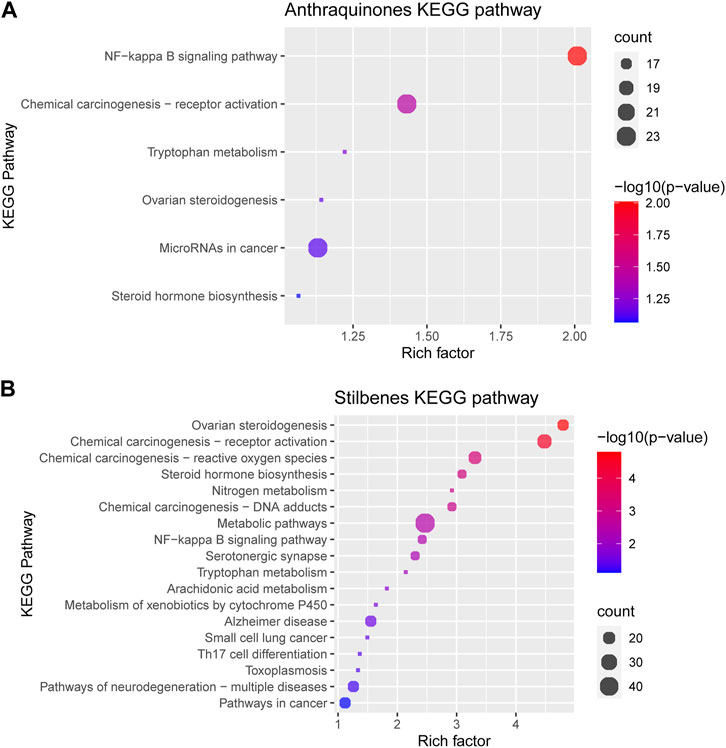
From the above network, the KEGG pathway of anthraquinones and stilbenes was conducted to clarify the difference between the two groups of active components. The greater the rich factor, the greater the intensiveness, and the −log10 (p-values) represents the level of significance of the pathway. Anthraquinones mainly had anti-inflammatory and anti-cancer effects, whereas stilbenes had ovarian regulatory and anti-cancer effects (Figure 9).
4 Discussion
4.1 Rhubarb and its adulterant in Taiwan’s market
Many Rheum species have already been identified, including R. rhabarbarum, R. emodi, R. hotaoense, and R. wittrochii (Wei et al., 2013). Comparing the pharmacological effects of R. palmatum and R. rhabarbarum L., it was found that R. rhabarbarum L. has a stronger small intestinal propulsion-promoting effect but exhibits higher cytotoxicity and acute toxicity in mice (Tianshi et al., 2012). The EP cites R. rhaponticum as a species easily confused with the official rhubarb; however, this species is primarily found in Europe and America, whereas R. rhabarbarum and others are widely distributed throughout mainland China, Tibet, Malaysia, and other countries. Given the geographical proximity and close cultural history between Taiwan and mainland China, many species, such as R. rhabarbarum, R. emodi, and R. australe, are more likely to surface in the Taiwanese market. In this study, we collected 21 rhubarb samples from the market. Using testing methods based on the pharmacopeia, we discovered that 15 rhubarb samples contained rhaponticin—an active ingredient with anti-bacterial properties, which does not exist in the three official rhubarb varieties listed in the pharmacopeias. Through many previous minor investigations, we found that over 70% of rhubarbs in the market are not official. However, determining the species of these unofficial rhubarbs is difficult because of the diversity of species in genus Rheum. Moreover, rhubarb is imported in dried form, making them appear similar, and many genes in the material are damaged during transportation and preservation, rendering gene identification challenging. To date, quantifying eight markers remains the viable method for differentiating unofficial rhubarb in the Taiwanese market. The unofficial rhubarb samples have great similarities, which may be attributed to their homogeneity in the Taiwanese market or that these samples are of the same species. Owing to the economic value of rhubarb in the market, further research is imperative to determine and identify official and unofficial rhubarb.
4.2 Qualitative and quantitative analysis methods for rhubarb in Taiwan’s markets
Our research was based on the understanding of market rhubarb, resulting in a quantitative determination method using eight essential markers, including stilbenes and anthraquinones. Although the quantitative HPLC method has yet to be established, changing market conditions necessitates a faster and simpler method with broad applications. According to many pharmacopeias, traditional rhubarb extraction procedure includes reflux, concentration, hydrolysis with HCl, heating, and separation. This procedure provides a cleaner sample with less contamination but eliminates several important active components, especially stilbenes, owing to their high polarity. Eight markers we want to detect are aglycones, and it is necessary to extract them in an acidic solvent at high temperatures to hydrolyze the glycosides. Based on the principles of glycoside hydrolysis, we devised a more straightforward method and validated through HPLC, which demonstrated minimal reduction in anthraquinones content while enabling the detection of stilbenes. This could help in differentiating official rhubarb from unofficial rhubarb.
Additionally, the HPLC conditions used acetonitrile (solvent A) and 0.1% phosphoric acid (solvent B) instead of methanol: 0.1% phosphoric acid. Acetonitrile is considered to have less absorbance, lower pressure requirement, and higher elution capacity than methanol. Therefore, it is generally safe and suitable for HPLC with a UV detector. In addition, the calibration parameters demonstrated the accuracy of our proposed HPLC method.
Based on the results of the HPLC quantitative determination, 13 samples of rhubarb, including six official rhubarb samples and seven unofficial rhubarb samples, showed no significant difference in the total anthraquinones content. Compared with the standard for total anthraquinones in pharmacopeia (NLT 1.5%), only RH-3 was found to be within the limit. However, the anthraquinones content obtained using our extraction method was slightly lower than that obtained using the method described in the THP 4th, so adhering to the extraction method from THP 4th may enable compliance with standards. Notably, all samples exhibited higher concentrations of chrysophanol than other active ingredients, especially in the unofficial rhubarb. Official rhubarb samples contained significant amounts of rhein, whereas the unofficial rhubarb samples contained exceptionally high levels of stilbenes. This result is consistent with the conclusion of a previous study by Xu Jing from China (Jing, 2001), which showed the importance of stilbenes and rhein in distinguishing the two groups of rhubarb. Stilbenes are commonly found in various species, including R. rhaponticum, R. rhabarbarum, and R. emodi (Mohtashami et al., 2021). EP 10.0 indicates rhaponticin as one of the markers for R. rhaponticum (European Pharmacopoeia, 2020). In our study, in addition to quantitative HPLC, to compare the two groups of rhubarb, we developed a TLC method using rhapontigenin, the aglycone of rhaponticin with lower polarity, together with five anthraquinones as markers for official and unofficial rhubarb. Based on the TLC results, we successfully differentiated 21 batches into two groups in the rhubarb market. This result demonstrates the applicability of this method to rhubarb in the market.
4.3 Comparison of chemical markers in official and unofficial rhubarb through KEGG pathway analysis
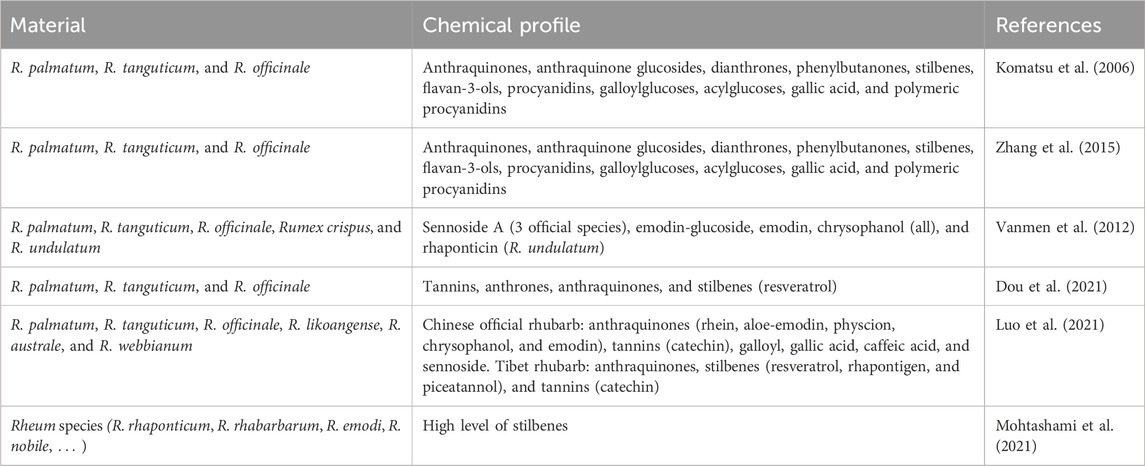
Rhubarb contains many active components, in both official and unofficial rhubarb, encompassing anthraquinones, stilbenes, and flavonoids such as myricetin, epicatechin, catechin, epicatechin-3-O-gallate, procyanidin B-2-3,3′-di-O-gallate, procyanidin B1, gallic acid, 1-o-galloyl-b-D-glucose, resveratrol, rhapontigenin, piceatannol, and methoxy resveratrol (Table 1). Anthraquinones is a class of phenolic compounds comprising the 9,10-anthraquinone skeleton and can be found in various species in the Rheum genus (Komatsu et al., 2006; Smolarz et al., 2013; Ahmad et al., 2014; Mohtashami et al., 2021). They also exist in many natural herbs, such as Aloe vera with main ingredients such as aloin, aloe-emodin, and rhein (Sadiq et al., 2022); Fallopia multiflora containing emodin, emodin-8-O-beta-D-glucoside, physcion, and physcion-8-O-beta-D-glucoside (Yan et al., 2010); Semen cassiae consisting of eight major anthraquinones (obtusifolin-2-glucoside, aurantio-obtusin, aloe-emodin, rhein, obtusifolin, emodin, chrysophanol, and physcion) (Xu et al., 2012); and Cassia obtusifolia L. and Cassia tora L. with sennoside A and sennoside B (Franz, 1993; Chen et al., 2023). Anthraquinones include monomers, bianthraquinones, and anthraquinone glycosides, which exhibit several pharmacological effects such as anti-inflammatory (De Oliveira et al., 2021) and anti-obesity properties (Zhang et al., 2018; Lee et al., 2019; Liudvytska and Kolodziejczyk-Czepas, 2022). Most anthraquinones in TCM exist in the form of glycosides (Zhao et al., 2014; Jintao et al., 2018) and the anti-constipation effect is mainly due to the action of anthraquinone glycosides (de Witte, 1993; Le et al., 2021). Sennosides induce the effect of a laxative by boosting intestinal motility, which may be connected with promoting the release of motilin in the intestines, lowering the level of enteric somatostatin, and inhibiting Na+–K + -ATPase activity in the intestinal mucosa (Wu et al., 2020). Free anthraquinones can be absorbed by the small intestine (Feng et al., 2013). As previously mentioned, the efficacy of rhubarb’s active components stems from free anthraquinone derivatives rather than anthraquinone glycosides (Fairbairn, 2011; Lin et al., 2017). Hydroxyl substitution can enhance most of the bioactivities of anthraquinones, such as anti-tumor, anti-pathogenic microorganism activities, antioxidant, and anti-osteoporosis effects (Li and Li, 2018).
KEGG pathway is a database system used to clarify the pharmacological pathways of pharmaceutical substances. To expand our understanding and compare the two groups of rhubarb in the scope of pharmacology in humans, we analyzed the KEGG pathway, in which the rich factor is the % of genes enriched in the total number of genes in the category. According to our result in the KEGG pathway, anthraquinones in rhubarb, including aloe-emodin, emodin, rhein, chrysophanol, and physcion, significantly affected the NF-kappa B signaling pathway—a prototypical pro-inflammatory signaling pathway implicated in chemical carcinogenesis. Emodin, an important anthraquinone with strong anti-inflammatory properties, protects human nucleus pulposus cells against IL-1 beta-induced apoptosis and inflammation via inhibiting ROS-mediated activation of NF-kappa B (Zhu et al., 2023). Emodin also suppresses tumor necrosis factor-alpha (TNF-alpha), interleukin-6 (IL-6), iNOS, and COX-2 expression, and inhibits LPS-induced NF-kappa B activation, IKB alpha degradation, phosphorylation of ERK, JNK, and P38 (Yang et al., 2014). In addition, aloe-emodin and its derivatives might be involved in inhibiting Akt, NF-kappa B, and JNK signaling pathways (Shang et al., 2022). Chrysophanol has an anti-inflammatory effect on osteoarthritis in mice in vitro by regulating the SIRT6/NF-kappa B and Nrf2/NF-kappa B signaling pathways (Lu et al., 2023). Rhein exerts anti-inflammatory effects by regulating the PPAR-gamma-NF-kappa B-HDAC3 axis (Wen et al., 2020). Physcion glucoside may decrease the expression of pro-inflammatory cytokines and mediators by inhibiting the TGF-beta/NF-kappa B/mitogen-activated protein kinase pathways (Geng et al., 2018).
In addition to anthraquinones, stilbenes can be found in other species of the Rheum genus (unofficial rhubarb). Stilbenes, including rhaponticin, resveratrol, and piceatannol, are a class of phenolic compounds with a basic skeleton containing 14 carbons (C6–C2–C6), in which a double-bonded ethylene bridge links the two phenyl moieties (Teka et al., 2022). Stilbenes exhibit several effects like antioxidant (Dou et al., 2017), anti-inflammatory (Tan et al., 2022), and anti-viral properties (Mattio et al., 2020). The three stilbenes discussed in this study were rhaponticin, rhapontigenin, and resveratrol. Resveratrol reduces oxidative stress and improves ovarian function in a rat model (Li and Li, 2018), plays a crucial role in preventing and treating ovarian cancer (Xu et al., 2021), exhibits antioxidant activity with the potential to induce apoptosis, and plays a chemopreventive role in UVR-induced skin carcinogenesis (Aziz and Aziz, 2018). Rhaponticin and desoxyrhaponticin can inhibit fatty acid synthase and are considered potential therapeutic agents to treat cancer (Li et al., 2014). Rhaponticin treatment improves lung cancer in vitro (Wang et al., 2021), and it has a protective effect on ovariectomy-induced osteoporosis in rats (Yang et al., 2021).
Generally, anthraquinones exhibit a strong anti-inflammatory effect, followed by anti-cancer effects. However, stilbenes have broader spectrum of effects, such as anti-cancer activities related to ovaries and ovarian hormones. The high stilbenes content in unofficial rhubarb indicates its potential value in the Taiwanese market. With more than 50% of rhubarb on the market being unofficial rhubarb, leveraging this resource can maximize the potential of unofficial source of rhubarb. Figure 10 illustrates the phytochemical differences between the three Rheum species mentioned in the pharmacopeias (R. palmatum, R. tanguticum, and R. officinale) and other Rheum species. This provides a more comprehensive overview of the application of the two groups of rhubarbs and the potential value of unofficial rhubarbs.
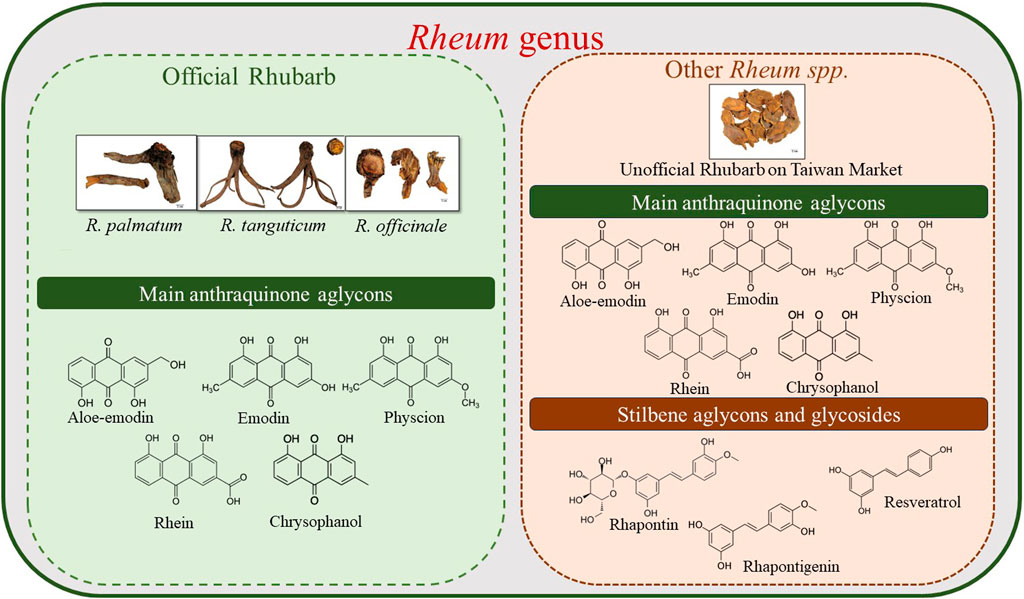
Figure 10. Phytochemical differences between the three Rheum species mentioned in Pharmacopeias (R. palmatum, R. tanguticum, and R. officinale) and unofficial rhubarb in Taiwan’s market.
4.4 The relationship between KEGG pathway analysis and qualitative and quantitative analysis of rhubarb
The relationship between KEGG pathway analysis and qualitative and quantitative analysis can be discussed in several aspects: the presence of active ingredients, the number of active ingredients, the content of active ingredients, and their specific effects.
First, regarding the presence and the number of active ingredients, it can be understood that the herb can act on specific KEGG pathways when there is an active ingredient. When there are many active ingredients, the number of KEGG pathways might differ, and the effect may be more potent. One example is the unofficial rhubarb group, which includes stilbenes and anthraquinones; under examining the KEGG pathway, the unofficial rhubarb group can act on more KEGG pathways than the official rhubarb group, which only contains anthraquinones (Figure 9). However, there is a limitation in determining how strong the effects are. Second, quantitative analysis would provide more information, as many studies have found the drug’s effects are through a dose-dependent pharmacological pathway, with the higher the dose, the stronger the activity. For example, anti-inflammatory research shows that the effect depends on the dose of free anthraquinones (Xiong et al., 2018) or the higher rhaponticin also showed a dose-dependent effect on anti-inflammatory (Zhou et al., 2021). These conclusions suggest that the content of active ingredients and their extraction are also two factors affecting the pharmacological activity of TCM herbs through many pharmacological pathways. Third, through the p-value and count index in the KEGG pathway, stilbenes and anthraquinones have strong associations with several pathways, such as anthraquinones with NF-kappa B signaling pathway and stilbenes with ovarian steroidogenesis pathway; thus, with a high concentration of active ingredients, the herb may have some special outstanding medicinal effects rather than the pathway with insignificant p-value, such as NF-kappa B signaling pathway and ovarian steroidogenesis pathway.
Qualitative and quantitative analysis methods are used to determine the presence of active ingredients and simultaneously determine the content of that active ingredient in the plant. Meanwhile, the KEGG pathway is a database through which we can know the function and pathway of that active ingredient. Hence, both have a complementary relationship to improve our understanding of active ingredients and drugs.
5 Conclusion
Our research analyzed rhubarb in Taiwan’s markets using eight reference standards: official rhubarb contains five anthraquinones, including aloe-emodin, emodin, rhein, chrysophanol, and physcion. In contrast, unofficial rhubarb exhibits high levels of rhapontigenin and anthraquinones, especially chrysophanol, but no rhein was detected. To distinguish between official and unofficial rhubarbs efficiently, we suggested a rapid extraction method and quantitative TLC analysis using rhein and rhapontigenin as pivotal markers. Furthermore, using the KEGG pathway analysis, we elucidated the differences between the pharmacological effects of official and unofficial rhubarbs. This study proposes a quantitative approach to enhance quality control of rhubarb in the market, thereby strengthening our understanding of rhubarb as an alternative.
Data availability statement
The raw data supporting the conclusion of this article will be made available by the authors, without undue reservation.
Author contributions
T-T-DA: writing–original draft, conceptualization, investigation, and methodology. Y-LH: supervision, visualization, writing–review and editing, conceptualization, project administration, and resources. Y-SC: writing–review and editing, funding acquisition, resources, supervision, and conceptualization.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Ministry of Health and Welfare, Taiwan (grant number M1007061).
Acknowledgments
The authors would like to thank the Ministry of Health and Welfare, Taiwan, for grant support, and the authors are also grateful to laboratory members Yi-Wei Lin and Hao-Ze Huang for their scientific comments and discussions regarding the experiments.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2024.1364460/full#supplementary-material
References
Adnan, M., Rasul, A., Hussain, G., Shah, M. A., Sarfraz, I., Nageen, B. e. a., et al. (2021). Physcion and physcion 8-O-β-D-glucopyranoside: natural anthraquinones with potential anti-cancer activities. Curr. Drug Targets 22, 488–504. doi:10.2174/1389450121999201013154542
Ahmad, W., Zaidi, S. M. A., Mujeeb, M., Ansari, S. H., and Ahmad, S. (2014). HPLC and HPTLC methods by design for quantitative characterization and in vitro anti-oxidant activity of polyherbal formulation containing Rheum emodi. J. Chromatogr. Sci. 52, 911–918. doi:10.1093/chromsci/bmt123
Arvindekar, A., More, T., Payghan, P. V., Laddha, K., Ghoshal, N. a., and Arvindekar, A. (2015). Evaluation of anti-diabetic and alpha glucosidase inhibitory action of anthraquinones from Rheum emodi. Food Funct. 6, 2693–2700. doi:10.1039/c5fo00519a
Aziz, S. W., and Aziz, M. H. (2018). Protective molecular mechanisms of resveratrol in UVR-induced Skin carcinogenesis. Photodermatol. Photoimmunol. Photomed. 34, 35–41. doi:10.1111/phpp.12336
Chen, D., Liu, J. R., Cheng, Y., Cheng, H., He, P., and Sun, Y. (2020). Metabolism of rhaponticin and activities of its metabolite, rhapontigenin: a review. Curr. Med. Chem. 27, 3168–3186. doi:10.2174/0929867326666190121143252
Chen, Y., Chen, X., Yang, X., Gao, P., Yue, C., Wang, L., et al. (2023). Cassiae Semen: a comprehensive review of botany, traditional use, phytochemistry, pharmacology, toxicity, and quality control. J. Ethnopharmacol. 306, 116199. doi:10.1016/j.jep.2023.116199
Choi, R. J., Ngoc, T. M., Bae, K., Cho, H. J., Kim, D. D., Chun, J., et al. (2013). Anti-inflammatory properties of anthraquinones and their relationship with the regulation of p-glycoprotein function and expression. Eur. J. Pharm. Sci. 48, 272–281. doi:10.1016/j.ejps.2012.10.027
De Oliveira, M. R., De Souza, I. C. C., and Brasil, F. B. (2021). Mitochondrial protection and anti-inflammatory effects induced by emodin in the human neuroblastoma SH-SY5Y cells exposed to hydrogen peroxide: involvement of the AMPK/Nrf2 signaling pathway. Neurochem. Res. 46 (3), 482–493. doi:10.1007/s11064-020-03181-1
de Witte, P. (1993). Metabolism and pharmacokinetics of anthranoids. Pharmacology 47 (1), 86–97. doi:10.1159/000139847
Dou, Z., Dai, Y., Zhou, Y., and Wang, S. (2021). Quality evaluation of rhubarb based on qualitative analysis of the HPLC fingerprint and UFLC–Q-TOF–MS/MS combined with quantitative analysis of eight anthraquinone glycosides by QAMS. Biomed. Chromatogr. 35, e5074. doi:10.1002/bmc.5074
Dou, Z., Dai, Y., Zhou, Y., Wang, S., and Vander Jagt, D. L. (2017). Activation of anti-oxidant Nrf2 signaling by substituted trans stilbenes. Bioorg. Med. Chem. 25, 1423–1430. doi:10.1016/j.bmc.2017.01.005
European Pharmacopoeia (2020). (10.0 ed., vol. I). European directorate for the quality of medicines and HealthCare (EDQM) of the council of Europe.
Fairbairn, J. W. (2011). The active constituents of the vegetable purgatives containing anthracene derivatives. J. Pharm. Pharmacol. 1, 683–692. doi:10.1111/j.2042-7158.1949.tb12481.x
Fan, Y. (2019). Cardioprotective effect of rhapontigenin in isoproterenol-induced myocardial infarction in a rat model. Pharmacology 103, 291–302. doi:10.1159/000496800
Feng, T.-S., Yuan, Z.-Y., Yang, R.-Q., Zhao, S., Lei, F., Xiao, X.-Y., et al. (2013). Purgative components in Rhubarbs: adrenergic receptor inhibitors linked with glucose carriers. Fitoterapia 91, 236–246. doi:10.1016/j.fitote.2013.09.020
Friedman, M., Xu, A., Lee, R., Nguyen, D. N., Phan, T. A., Hamada, S. M., et al. (2020). The inhibitory activity of anthraquinones against Pathogenic Protozoa, Bacteria, and Fungi and the relationship to structure. Molecules 25, 3101. doi:10.3390/molecules25133101
Geng, Q., Wei, Q. F., Wang, S. J., Qi, H. L., Zhu, Q., Liu, X., et al. (2018). Physcion 8-O-β-glucopyranoside extracted from Polygonum cuspidatum exhibits anti-proliferative and anti-inflammatory effects on MH7A rheumatoid arthritis-derived fibroblast-like synoviocytes through the TGF-β/MAPK pathway. Int. J. Mol. Med. 42, 745–754. doi:10.3892/ijmm.2018.3649
Hong, S. W., Chun, J., Park, S., Lee, H. J., Im, J. P., and Kim, J. S. (2018). Aloe vera is effective and safe in short-term treatment of irritable bowel syndrome: a systematic review and meta-analysis. J. Neurogastroenterol. Motil. 24, 528–535. doi:10.5056/jnm18077
Hong Kong Chinese Materia Medica Standards (2008). The government of the Hong Kong special administrative region. 2.
Huang, Q., Lu, G., Sben, H. M., Cbung, M. C. M., and Ong, C. N. (2007). Anti-cancer properties of anthraquinones from Rhubarb. Medi. Res. Rev. 27, 609–630. doi:10.1002/med.20094
Jing, X. (2001). Identification of authenticity of rhubarb. Tianjin Pharm. 06, 51–52. Available from: https://caod.oriprobe.com/articles/3875805/da_huang_zhen_wei_jian_bie_.htm
Jintao, X., Yongli, S., Liming, Y., Quanwei, Y., Chunyan, L., Xingyi, C., et al. (2018). Near-infrared spectroscopy for rapid and simultaneous determination of five main active components in Rhubarb of different geographical origins and processing. Spectrochim. Acta A Mol. Biomol. Spectrosc. 205, 419–427. doi:10.1016/j.saa.2018.07.055
Jo, S. P., Kim, J. K., and Lim, Y. H. (2014). Antihyperlipidemic effects of rhapontin and rhapontigenin from Rheum undulatum in rats fed a high-cholesterol diet. Planta Med. 80, 1067–1071. doi:10.1055/s-0034-1382999
Kim, J. S., Kang, C. G., Kim, S. H., and Lee, E. O. (2014). Rhapontigenin suppresses cell migration and invasion by inhibiting the PI3K-dependent Rac1 signaling pathway in MDA-MB-231 human breast cancer cells. J. Nat. Prod. 77, 1135–1139. doi:10.1021/np401078g
Komatsu, K., Nagayama, Y., Tanaka, K., Ling, Y., Cai, S. Q., Omote, T., et al. (2006). Comparative study of chemical constituents of Rhubarb from different origins. Chem. Pharm. Bull. (Tokyo) 54, 1491–1499. doi:10.1248/cpb.54.1491
Le, J., Ji, H., Zhou, X., Wei, X., Chen, Y., Fu, Y., et al. (2021). Pharmacology, toxicology, and metabolism of sennoside A, A medicinal plant-derived natural compound. Front. Pharmacol. 12, 714586. doi:10.3389/fphar.2021.714586
Lee, S. J., Cho, S. J., Kwon, E. Y., and Choi, M. S. (2019). Physcion reduces lipid accumulation and prevents the obesity in mice. Nutr. Metab. 16, 31. doi:10.1186/s12986-019-0362-7
Li, B., Huang, J., Yi, Y., Liu, S., Liu, R., Xiao, Z., et al. (2022). Effects of rhapontigenin as a novel quorum-sensing inhibitor on exoenzymes and biofilm formation of Pectobacterium carotovorum subsp. carotovorum and its application in vegetables. Molecules 27, 8878. doi:10.3390/molecules27248878
Li, N., and Li, L. (2018). Mechanism of resveratrol in improving ovarian function in a rat model of premature ovarian insufficiency. J. Obstet. Gynaecol. Res. 44, 1431–1438. doi:10.1111/jog.13680
Li, P., Tian, W. X., Wang, X. Y., and Ma, X. F. (2014). Inhibitory effect of desoxyrhaponticin and rhaponticin, two natural stilbene glycosides from the Tibetan nutritional food Rheum tanguticum Maxim. ex Balt, on fatty acid synthase and human breast cancer cells. Food Funct. 5, 251–256. doi:10.1039/c3fo60484e
Li, R., Chinnathambi, A., Alharbi, S. A., Shair, O. H. M., Veeraraghavan, V. P., Surapaneni, K. M., et al. (2021). Anti-inflammatory effects of rhaponticin on LPS-induced human endothelial cells through inhibition of MAPK/NF-κβ signaling pathways. J. Biochem. Mol. Toxicol. 35, e22733. doi:10.1002/jbt.22733
Lin, B. C., Harris, D. R., Kirkman, L. M. D., Perez, A. M., Qian, Y., Schermerhorn, J. T., et al. (2017). FIKK kinase, a Ser/Thr kinase important to Malaria parasites, is inhibited by tyrosine kinase inhibitors. ACS Omega 2, 6605–6612. doi:10.1021/acsomega.7b00997
Liudvytska, O., and Kolodziejczyk-Czepas, J. (2022). A review on Rhubarb-derived substances as modulators of cardiovascular risk factors-a special emphasis on anti-obesity action. Nutrients 14, 2053. doi:10.3390/nu14102053
Lombardi, N., Crescioli, G., Maggini, V., Bellezza, R., Landi, I., Bettiol, A., et al. (2022). Anthraquinone laxatives use and colorectal cancer: a systematic review and meta-analysis of observational studies. Phytother. Res. 36, 1093–1102. doi:10.1002/ptr.7373
Lu, J. J., Miao, Z. M., Jiang, Y. H., Xia, W. Y., Wang, X., Shi, Y. F. N., et al. (2023). Chrysophanol prevents IL-1β-Induced inflammation and ECM degradation in osteoarthritis via the Sirt6/NF-κB and Nrf2/NF-κB axis. Biochem. Pharmacol. 208, 115402. doi:10.1016/j.bcp.2022.115402
Luo, D., He, M., Li, J., Du, H., Mao, Q., Pei, N., et al. (2021). Integrating the rapid constituent profiling strategy and multivariate statistical analysis for herb ingredients research, with Chinese official Rhubarb and Tibetan Rhubarb as an example. Arab. J. Chem. 14, 103269. doi:10.1016/j.arabjc.2021.103269
Mattio, L. M., Catinella, G., Pinto, A., and Dallavalle, S. (2020). Natural and nature-inspired stilbenoids as antiviral agents. Eur. J. Med. Chem. 202, 112541. doi:10.1016/j.ejmech.2020.112541
Mohtashami, L., Amiri, M. S., Ayati, Z., Ramezani, M., Jamialahmadi, T., Emami, S. A., et al. (2021). Ethnobotanical uses, phytochemistry and pharmacology of different Rheum Species (Polygonaceae): a review. Adv. Exp. Med. Biol. 1308, 309–352. doi:10.1007/978-3-030-64872-5_22
Plants of the World Online (2023). Kew royal botanic garderns. Available at: https://powo.science.kew.org/taxon/urn:lsid:ipni.org:names:32581-1#children.
Sadiq, U., Gill, H., and Chandrapala, J. (2022). Temperature and pH stability of anthraquinones from native Aloe vera gel, spray-dried and freeze-dried Aloe vera powders during storage. Foods 11, 1613. doi:10.3390/foods11111613
Shang, H., Guo, J., Wang, P. T., Li, L. Y., Tian, Y., Li, X. X., et al. (2022). Design, synthesis and anti-inflammatory evaluation of aloe-emodin derivatives as potential modulators of Akt, NF-κB and JNK signaling pathways. Eur. J. Medi. Chem. 238, 114511. doi:10.1016/j.ejmech.2022.114511
Smolarz, H. D., Swatko-Ossor, M., Ginalska, G., and Medyńska, E. (2013). Antimycobacterial effect of extract and its components from Rheum rhaponticum. J. AOAC Int. 96, 155–160. doi:10.5740/jaoacint.12-010
Taiwan Herbal Pharmacopeia (2024). Taiwan herbal Pharmacopeia. 4th edition. Taipei: Ministry of Health and Welfare.
Tan, L. X., Xia, T. Q., He, Q. F., Tang, W., Huang, X. J., Song, Q. Y., et al. (2022). Stilbenes from the leaves of Cajanus cajan and their in vitro anti-inflammatory activities. Fitoterapia 160, 105229. doi:10.1016/j.fitote.2022.105229
Teka, T., Zhang, L., Ge, X., Li, Y., Han, L., and Yan, X. (2022). Stilbenes: source plants, chemistry, biosynthesis, pharmacology, application and problems related to their clinical application-a comprehensive review. Phytochemistry 197, 113128. doi:10.1016/j.phytochem.2022.113128
Tianshi, F., Yugang, W., Yushuang, C., Xinyue, X., Hua, Y., Feng, W., et al. (2012). Comparison between Rheum palmatum L. and Rheum franzenbachii Munt. on pharmacological effect and toxicity. World Sci. Technol. Traditional Chin. Med. Materia Medica 14, 1863–1870. Available from: https://xueshu.baidu.com/usercenter/paper/show?paperid=e199ffacfa569e189cba0f2d28ce7d62
Vanmen, C., Jang, Y. S., Zhu, H. M., Lee, J. H., Trung, T. N., Ngoc, T. M., et al. (2012). Chemical-based species classification of Rhubarb using simultaneous determination of five bioactive substances by HPLC and LDA analysis. Phytochem. Anal. 23, 359–364. doi:10.1002/pca.1365
Vervandier-Fasseur, D., and Latruffe, N. (2019). The potential use of resveratrol for cancer prevention. Molecules 24, 4506. doi:10.3390/molecules24244506
Vilanova-Sanchez, A., Gasior, A. C., Toocheck, N., Weaver, L., Wood, R. J., Reck, C. A., et al. (2018). Are Senna based laxatives safe when used as long term treatment for constipation in children? J. Pediatr. Surg. 53, 722–727. doi:10.1016/j.jpedsurg.2018.01.002
Wang, J., Zhao, H., Kong, W., Jin, C., Zhao, Y., Qu, Y., et al. (2010). Microcalorimetric assay on the antimicrobial property of five hydroxyanthraquinone derivatives in Rhubarb (Rheum palmatum L.) to Bifidobacterium adolescentis. Phytomedicine 17, 684–689. doi:10.1016/j.phymed.2009.10.009
Wang, X. D., Veeraraghavan, V. P., Mohan, S. K., and Lv, F. (2021). Anticancer and immunomodulatory effect of rhaponticin on Benzo(a) Pyrene-induced lung carcinogenesis and induction of apoptosis in A549 cells. Saudi J. Biol. Sci. 28, 4522–4531. doi:10.1016/j.sjbs.2021.04.052
Wei, Y. G., Li, J., and Cheng, P. X. (2013). Identification of Rhubarb and its counterfeit species. Guide China Med. 19, 483–484. Available from https://caod.oriprobe.com/articles/39150410/IdentificationofRhubarbandItsCounterfeitSpecies.htm
Wen, Q., Miao, J. F., Lau, N. K., Zhang, C. Y., Ye, P., Du, S. H., et al. (2020). Rhein attenuates lipopolysaccharide-primed inflammation through NF-κB inhibition in RAW264.7 cells: targeting the PPAR-γ signal pathway. Can. J. Physiol. Pharmacol. 98, 357–365. doi:10.1139/cjpp-2019-0389
Wu, Y. Q., Wang, J. X., Zhou, C. H., and Zhang, H. Q. (2020). Effect of sennoside on intestinal movement of mice and study of relevant mechanism. Chin. J. Clin. Pharmacol. Ther. 9 (2), 162–165. Available from: http://manu41.magtech.com.cn/Jweb_clyl/EN/Y2004/V9/I2/162
Xia, N., Daiber, A., Förstermann, U., and Li, H. (2017). Antioxidant effects of resveratrol in the cardiovascular system. Br. J. Pharmacol. 174, 1633–1646. doi:10.1111/bph.13492
Xiong, Y. X., Chen, L., Fan, L., Wang, L. L., Zhou, Y. J., Qin, D. L., et al. (2018). Free total Rhubarb anthraquinones protect intestinal injury via regulation of the intestinal immune response in a rat model of severe acute pancreatitis. Front. Pharmacol. 9, 75. doi:10.3389/fphar.2018.00075
Xu, L., Chan, C. O., Lau, C. C., Yu, Z., Mok, D. K., and Chen, S. (2012). Simultaneous determination of eight anthraquinones in Semen Cassiae by HPLC-DAD. Phytochem. Anal. 23, 110–116. doi:10.1002/pca.1331
Xu, X. L., Deng, S. L., Lian, Z. X., and Yu, K. (2021). Resveratrol targets a variety of oncogenic and oncosuppressive signaling for ovarian cancer prevention and treatment. Antioxidants 10, 1718. doi:10.3390/antiox10111718
Yan, H. J., Fang, Z. J., Fu, J., and Yu, S. X. (2010). The correlation between bioactive components of Fallopia multiflora root and environmental factors. Am. J. Chin. Med. 38, 473–483. doi:10.1142/s0192415x10007993
Yang, T. S., Wang, Q. Y., Qu, Y. Y., Feng, C. W., Li, C. R., Yang, Y., et al. (2021). Protective effect of rhaponticin on ovariectomy-induced osteoporosis in rats. J. Biochem. Mol. Toxicol. 35, e22837. doi:10.1002/jbt.22837
Yang, Z. T., Zhou, E. S., Wei, D., Li, D. P., Wei, Z. K., Zhang, W., et al. (2014). Emodin inhibits LPS-induced inflammatory response by activating PPAR-γ in mouse mammary epithelial cells. Int. Immunopharmacol. 21, 354–360. doi:10.1016/j.intimp.2014.05.019
Ye, M., Han, J., Chen, H., Zheng, J., and Guo, D. (2007). Analysis of phenolic compounds in Rhubarbs using liquid chromatography coupled with electrospray ionization mass spectrometry. J. Am. Soc. Mass Spectrom. 18, 82–91. doi:10.1016/j.jasms.2006.08.009
Yeh, Y.-H., Wang, S.-W., Yeh, Y.-C., Hsiao, H.-F., and Li, T.-K. (2016). Rhapontigenin inhibits TGF-β-mediated epithelial-mesenchymal transition via the PI3K/AKT/mTOR pathway and is not associated with HIF-1α degradation. Oncol. Rep. 35, 2887–2895. doi:10.3892/or.2016.4664
Zhang, J., Kang, H., Wang, L. F., Zhao, X. Y., and He, L. (2018). Chrysophanol ameliorates high-fat diet-induced obesity and inflammation in neonatal rats. Pharmazie 73, 228–233. doi:10.1691/ph.2018.7980
Zhang, L., Liu, H., Qin, L., Zhang, Z., Wang, Q., Zhang, Q., et al. (2015). Global chemical profiling based quality evaluation approach of Rhubarb using ultra performance liquid chromatography with tandem quadrupole time-of-flight mass spectrometry. J. Sep. Sci. 38, 511–522. doi:10.1002/jssc.201400971
Zhang, Y., Pu, W., Bousquenaud, M., Cattin, S., Zaric, J., Sun, L. K., et al. (2020). Emodin inhibits inflammation, carcinogenesis, and cancer progression in the AOM/DSS model of Colitis-associated intestinal tumorigenesis. Front. Oncol. 10, 564674. doi:10.3389/fonc.2020.564674
Zhao, N., Zhang, X. Z., Hu, C. J., Jia, T. Z., and Xiao, H. B. (2014). Metabolomics analysis revealing multiple compounds changed in Rhubarb after processing. Zhongguo Zhong Yao Za Zhi 39(9), 1607–1613. [Chinese]
Zhou, D. D., Luo, M., Huang, S. Y., Saimaiti, A., Shang, A., Gan, R. Y., et al. (2021). Effects and mechanisms of resveratrol on aging and age-related diseases. Oxid. Med. Cell. Longev. 2021, 9932218. doi:10.1155/2021/9932218
Zhu, X. J., Guo, S. Q., Zhang, M. Y., and Bai, X. L. (2023). Emodin protects against apoptosis and inflammation by regulating reactive oxygen species-mediated NF-κB signaling in interleukin-1β-stimulated human nucleus pulposus cells. Hum. Exp. Toxicol. 42, 9603271221138552. doi:10.1177/09603271221138552
Keywords: rhubarb, unofficial rhubarb, quality control, anthraquinones, stilbenes
Citation: Au T-T-D, Ho Y-L and Chang Y-S (2024) Qualitative and quantitative analysis methods for quality control of rhubarb in Taiwan’s markets. Front. Pharmacol. 15:1364460. doi: 10.3389/fphar.2024.1364460
Received: 02 January 2024; Accepted: 26 March 2024;
Published: 30 April 2024.
Edited by:
Anna Karolina Kiss, Medical University of Warsaw, PolandReviewed by:
Ding Li, Northwest A&F University, ChinaDiana Simona Antal, Victor Babes University of Medicine and Pharmacy, Romania
Copyright © 2024 Au, Ho and Chang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yuan-Shiun Chang, eXNjaGFuZ0BtYWlsLmNtdS5lZHUudHc=
†These authors have contributed equally to this work and share first authorship
 Thanh-Thuy-Dung Au
Thanh-Thuy-Dung Au Yu-Ling Ho
Yu-Ling Ho Yuan-Shiun Chang
Yuan-Shiun Chang