- 1Department of Pharmacy, Nanjing Drum Tower Hospital, Affiliated Hospital of Medical School, Nanjing University, Nanjing, Jiangsu, China
- 2School of Basic Medicine and Clinical Pharmacy, China Pharmaceutical University, Nanjing, Jiangsu, China
- 3Department of Pharmacy, Nanjing Medical University, Nanjing, Jiangsu, China
- 4Women’s Hospital of Nanjing Medical University, Nanjing Women and Children’s Healthcare Hospital, Nanjing, China
- 5Department of Emergency Medicine, Nanjing Drum Tower Hospital, Affiliated Hospital of Medical School, Nanjing University, Nanjing, Jiangsu, China
Despite the availability of effective vaccines and treatments for SARS-CoV-2, managing COVID-19 in patients with systemic lupus erythematosus (SLE) remains challenging, particularly considering drug-drug interactions (DDIs). Here, we present a case of DDIs between Tacrolimus (Tac) and nirmatrelvir/ritonavir (NMV/r) in a 32-year-old male with SLE. Following self-administration of NMV/r and resumption of Tac after 5 days, the patient experienced acute nephrotoxicity and neurotoxicity, accompanied by supratherapeutic Tac levels, despite Tac being withheld during NMV/r. The primary cause of this acute toxicity is attributed to ritonavir’s inhibitory effect on both CYP3A4 enzymes and P-glycoprotein. Upon admission, Tac was discontinued, and supportive therapies were initiated. Phenytoin, a CYP3A4 inducer, was administered to lower Tac levels under the guidance of clinical pharmacists, effectively alleviating the patient’s acute toxic symptoms. The half-life of Tac during the treatment of phenytoin was calculated to be 55.87 h. And no adverse reactions to phenytoin were observed. This case underscores the persistence of enzyme inhibition effects and demonstrates the effectiveness and safety of utilizing CYP3A4 enzyme inducers to mitigate Tac concentrations. Furthermore, it emphasizes the importance of healthcare providers and patients being vigilant about DDIs in Tac recipients. Lastly, it highlights the indispensable role of pharmacist involvement in clinical decision-making and close monitoring in complex clinical scenarios. Although our findings are based on a single case, they align with current knowledge and suggest the potential of individualized combination therapy in managing challenging COVID-19 cases in immunocompromised patients.
1 Introduction
The COVID-19 pandemic, caused by the novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has had a profound global impact. In response, the Federal Drug Administration (FDA) granted emergency use authorization (EUA) to nirmatrelvir/ritonavir (Paxlovid, NMV/r) to mitigate the significant morbidity and mortality associated with COVID-19. Systemic lupus erythematosus (SLE), an autoimmune disorder affecting various body systems, is typically managed with calcineurin inhibitors (CNIs), such as Tacrolimus (Tac, or FK506), to suppress abnormal immune responses (Fanouriakis et al., 2019). NMV/r is advocated for treating COVID-19 in SLE patients, who are particularly susceptible to severe complications post-infection (Murphy, 2022). A critical consideration is the pronounced Tac toxicity arising from the ritonavir-Tac interaction.
This case report addresses the reversal of Tac toxicity induced by concurrent NMV/r use through the activation of cytochrome P450 (CYP) 3A4 with phenytoin in an SLE patient. To our knowledge, this is the first reported case of managing phenytoin to mitigate acute Tac toxicity induced by NMV/r in an SLE patient. This case stands out for its novel identification of ritonavir-induced Tac toxicity and is among the few reported instances demonstrating phenytoin’s efficacy and safety in resolving acute Tac toxicity.
2 Methods
2.1 Case report
The patient data required for the case report were retrieved from the electronic patient information system of Nanjing Drum Tower Hospital. Patient consent for publication was obtained.
2.2 Structured review
We searched MEDLINE literature of PubMed by using the search term “Tacrolimus” AND “Nirmatrelvir/ritonavir OR Paxlovid” to identify the relevant reports up to 11 May 2024. Additionally, other databases including Web of Science, Cochrane Database, and ClinicalTrials.gov were searched using the same search term. All the reports obtained were screened for exclusion in the review according to the following criteria: 1) comment, correspondence, and response; 2) meeting abstract, editorial material; 3) unrelated to topic; 4) insufficient information on therapy. After a thorough analysis of the full-text articles, a secondary search was performed to exclude the guidelines, dosing suggestions reviews, data from the Fears database, case reports or case series without acute toxicities, and retrospective studies.
The extracted data included reported country and year, patient demographics, primary indications for Tac, previous Tac and NMV/r doses, NMV/r duration, Tac dose adjustment during NMV/r administration, toxic manifestation, initial maximum Tac level after NMV/r administration, CYP inducers and other treatments, restart Tac doses after treatment, Tac levels after Tac resumption, and patient prognosis.
3 Case presentation
3.1 Case report
A 32-year-old male, weighing 70 kg, and with a 6-year history of SLE, was admitted to the hospital on 20 August 2023. He had been receiving a long-term regimen of oral Tac 1 mg twice daily and prednisone 5 mg daily. Over the past 3 years, his baseline serum creatinine levels had stabilized at 0.74–0.94 mg/dL, while his Tac blood levels remain untested. Notably, he had never been vaccinated against COVID-19, and his parents had no history of immune-related diseases.
Approximately 2 weeks prior to admission, he presented with symptoms including a fever of 39°C, muscle aches, cough with sputum production, shortness of breath, a widespread rash, diarrhea, and a positive nucleic acid test for COVID-19. He initiated with NMV/r (300 mg/100 mg twice daily) himself 8 days before admission (on August 12), during which his Tac and prednisone were withheld. After 5 days of NMV/r treatment, he noticed improvement in COVID-19 symptoms and resumed his Tac and prednisone. However, the next day, he experienced chest tightness, shortness of breath, and profusely sweating. On August 20, his condition deteriorated, marked by aggravated chest tightness, breathlessness, brownish sputum, muscle pain, nervousness, disorganized speech, involuntary muscle spasms, and tremors, prompting his admission to our hospital’s emergency department.
Upon admission, his laboratory results showed a creatinine of 3.9 mg/dL, hyponatremia (sodium level at 122.6 mmol/L), and mild hyperkalemia (potassium level at 5.23 mmol/L). Electrocardiography (ECG) indicated sinus tachycardia (157 bpm) with ST-T changes. Remarkably, his Tac concentration was exceptionally high at 57.6 ng/mL. He was managed with tracheal intubation, rehydration, sedation and analgesia, anti-infective treatment, continuous venovenous hemodiafiltration (CVVHDF), and additional supportive therapies.
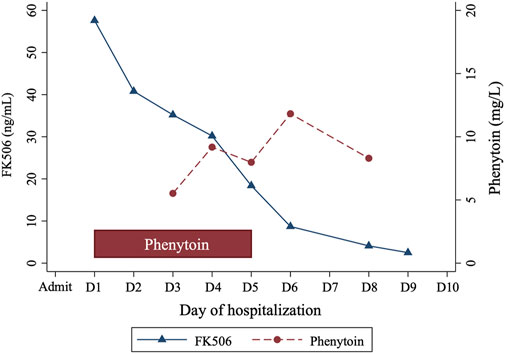
Clinicians sought consultation from the clinical pharmacists on effectively reducing Tac levels. Considering Tac’s high protein binding and limited clearance by continuous renal replacement therapy (CRRT), the pharmacist recommended a cytochrome P450 (CYP) enzyme inducer (either phenytoin or rifampicin) to lower Tac concentrations. The patient received oral phenytoin (100 mg thrice daily) for 5 days, resulting in a gradual decrease in Tac levels from 57.6 ng/mL to 2.5 ng/mL over 8 days (Figure 1). Phenytoin trough level, measured 2 days post-administration, ranged from 5.52 mg/L to 11.82 mg/L. His creatinine levels improved to 1.21 mg/dL. The Tac elimination rate constant (ke) and the half-life (t1/2) were calculated as follows (Shiohira et al., 2024):
The calculated t1/2 were days 1–5: 55.87 h (during the treatment with phenytoin), days 6–9: 37.50 h (after treatment with phenytoin).
By the 11th day, the patient regained consciousness with normal cerebrospinal fluid tests. He was extubated on day 17, showing no respiratory symptoms or muscle tremors but experiencing dysphagia. Brain magnetic resonance imaging showed no abnormalities. Upon discharge, his condition had improved significantly, with creatinine at 0.32 mg/dL, sodium at 136.1 mmol/L, and potassium at 3.34 mmol/L. He was discharged without Tac, and prescribed hydroxychloroquine (200 mg twice daily), methylprednisolone (20 mg daily), and potassium chloride (1 g thrice daily). No adverse reactions to phenytoin were observed, and there was no evidence of SLE recurrence or exacerbation. A follow-up after 1 month showed symptomatic improvement, albeit with an increased heart rate, necessitating maintenance therapy with prednisolone (8 mg daily) and metoprolol (47.5 mg daily).
3.2 Review
We encompassed 31 publications (Figure 2), comprising 27 case reports and 4 case series, involving a collective of 42 patients, among whom 38 experienced Tac toxicities. Table 1 summarizes the demographics, clinical manifestations, treatments, and outcomes. Patients’ age ranged from 13 to 85 years, with 21 (50.0%) being male. The primary indications for Tac use included: 36 (85.7%) solid organ transplants, 2 (4.8%) systemic lupus erythematosus, 1 (2.4%) myasthenia gravis, 1 (2.4%) hematopoietic stem cell transplant, 1 (2.4%) ulcerative colitis, and 1 (2.4%) interstitial pneumonia associated with dermatomyositis.
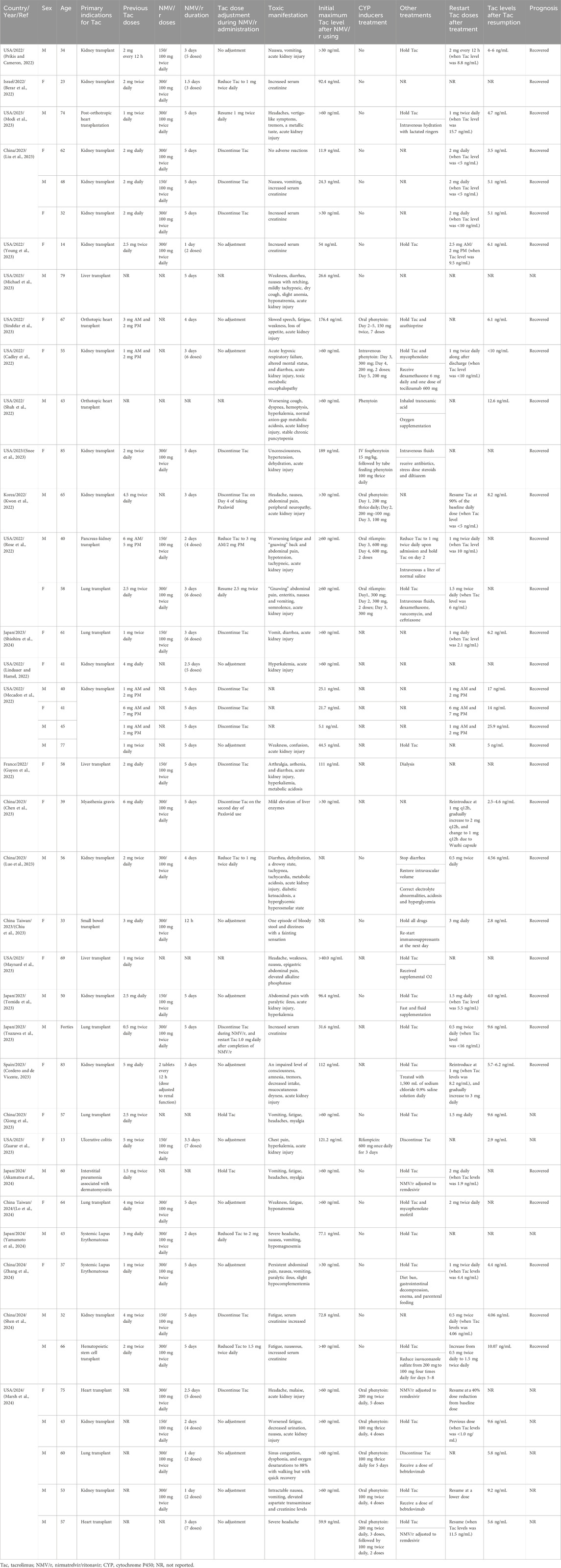
Table 1. Characteristics, clinical presentation, and outcomes of patients with acute tacrolimus toxicities after Paxlovid administration from case reports and case series.
The reported toxicity manifestations were diverse, encompassing nephrotoxicity, gastrointestinal toxicity, neurotoxicity, metabolic disturbances, cardiovascular toxicity, hematological toxicity, musculoskeletal toxicity, and hepatotoxicity. These toxic effects generally manifested during NMV/r treatment and could persist post-treatment.
Treatment regimens for acute Tac toxicities varied. Of the 38 patients, 17 received the full prescribed dose of NMV/r, while 10 had their NMV/r doses adjusted based on renal function. The dosing details for the remaining 11 patients were not explicitly reported. Tac management during NMV/r treatment also differed: 20 patients maintained their original dosage, 5 opted for reduced doses, 8 discontinued Tac immediately, 1 stopped Tac on the 4th day post-NMV/r, and another stopped on the second day post-NMV/r. In 3 cases, specific management strategies were not documented.
Observed Tac concentrations varied significantly, with the maximum recorded level reaching 189 ng/mL. The minimum concentration associated with toxic symptoms was 24.3 ng/mL, surpassing the therapeutic levels. Of the 38 patients, only 14 patients were treated with CYP inducers, including 10 with phenytoin at doses ranging from 200 mg/d to 600 mg/d for 2–5 days, and 4 with rifampin at doses ranging from 300 mg/d to 600 mg/d for 2–3 days. Following hospitalization and treatment, 26 patients resumed Tac therapy: 8 patients reverted to their initial dose, 17 proceeded with a reduced dose, and in one case, the details were not specified. Notably, 14 of these patients resumed Tac therapy at concentrations ≤10 ng/mL and 3 patients at concentrations ≤16 ng/mL. 29 patients showed clear improvement in symptoms, while the discharge status of 9 patients was not mentioned.
4 Discussion
Tac, a calcineurin inhibitor, is widely utilized for immunosuppression in solid organ transplant recipients. It has garnered attention for its efficacy in treating SLE, leading to expanded usage in this context (Watanabe et al., 2016). Despite its effectiveness in managing SLE, Tac poses challenges due to its narrow therapeutic window, typically targeting concentrations of 4–6 ng/mL in SLE patients (Fanouriakis et al., 2019). Previous investigations have suggested that patients with various immune-mediated diseases commonly experienced varying degrees of elevation in Tac levels during or following NMV/r use, potentially leading to acute toxicity. The primary toxicities observed in current cases were nephrotoxicity and neurotoxicity. Acute Tac-induced nephrotoxicity, characterized by a moderate increase in serum creatine levels due to acute afferent arteriolar vasoconstriction, primarily affects tubular epithelial cells, vascular endothelial cells, arteriolar myocytes, and interstitial fibroblasts. These cellular damages result from high concentrations of Tac-binding proteins that inhibit calcineurin activity, leading to functional and structural renal impairment (Braithwaite et al., 2021). Unlike nephrotoxicity, Tac-induced neurotoxicity, typically diagnosed based on neurological symptoms and severity, may stem from inhibited calcineurin activity, essential for neurological function (Bechstein, 2000). Neurotoxicity is also associated with posterior reversible encephalopathy syndrome (PRES), often triggered by hypertension, sepsis, or renal failure, characterized by vasogenic edema due to blood-brain barrier dysregulation and impaired cerebral vasoconstriction (Dhar, 2017; Farouk and Rein, 2020). The incidence and severity of acute nephrotoxicity and neurotoxicity correlate with supratherapeutic Tac trough concentrations, (Sikma et al., 2018; Miano et al., 2020), often observed in transplant patients with levels exceeding 15 ng/mL (Braithwaite et al., 2021). However, the precise threshold for acute toxicities in SLE patients remains unclear.
Tac, primarily metabolized by CYP3A4 and serving as a substrate for P-glycoprotein, undergoes first-pass intestinal metabolism, with an intestinal availability of 0.14 and a hepatic availability of 0.96 (Tomida et al., 2023). Ritonavir inhibits P-glycoprotein, thereby diminishing Tac absorption and subsequently elevating Tac levels. Moreover, ritonavir tightly binds to the active site of CYP3A4, forming an irreversible bond with the heme iron via the thiazole nitrogen. This action decreases the redox potential of the CYP protein and impedes its reduction by CYP450 reductase (Sevrioukova and Poulos, 2010). Consequently, ritonavir can disrupt Tac metabolism by irreversibly inhibiting CYP3A4 and P-glycoprotein activity, resulting in increased systemic exposure and reduced metabolic clearance of Tac (Fishbane et al., 2022). A pharmacokinetic evaluation revealed that co-administration with NMV/r resulted in an 18.7-fold increase in Tac bioavailability and a 35% reduction in clearance (Tomida et al., 2023). In healthy volunteers, steady-state concentrations of ritonavir (100 mg/day) led to a 17-fold and 57-fold elevation in the Tac concentration at 24 h (C24) and the area under the plasma concentration-time curve (AUC0-inf), respectively, with Tac half-life extending from 32 h to 232 h (Badri et al., 2015). Another prospective pharmacokinetic study demonstrated that ritonavir could sustain elevated Tac levels even after discontinuation of NMV/r, possibly due to continued inhibition of CYP3A metabolism (Xu et al., 2024). The half-life of Tac is approximately 35 h, the metabolic inhibition of Tac persisted up to 110 h after ritonavir discontinuation (Yamamoto et al., 2024). In our case, despite Tac resumption following NMV/r cessation, a significant surge in Tac levels, accompanied by nephrotoxicity and neurotoxicity, was observed in the patient. This underscores the sustained inhibitory effect of NMV/r on Tac, persisting even after discontinuation.
Currently, treatment of Tac toxicity relies mainly on supportive care, as there is no specific antidote available. Ceschi et al. proposed early interventions such as gastrointestinal decontamination using activated charcoal or nasogastric aspiration to potentially reduce Tac absorption in cases of acute overdose (Ceschi et al., 2013). However, the efficacy of these measures is limited by Tac’s high protein binding and minimal biliary excretion (Quirós-Tejeira et al., 2005; Jantz et al., 2013). Due to Tac’s lipophilic nature and extensive erythrocyte binding (99%), extracorporeal removal is ineffective in Tac toxicity cases. Nonetheless, renal replacement therapy may be necessary to manage volume overload or electrolyte disturbances in cases of acute Tac-induced nephrotoxicity (Naccarato et al., 2021). Given the close relationship between Tac toxicity and enzyme inhibition, inducing Tac metabolism via CYP activation is considered a potential therapeutic approach. Literature suggests that the half-life of the CYP3A4 enzyme is approximately 2–3 days, with full recovery of enzyme activity requiring several days for the regeneration of new enzymes (Chen and Raymond, 2006; Magnusson et al., 2008). CYP3A4 inducers, such as phenytoin or rifampin, offer alternative therapeutic approaches to enhance CYP3A4 enzyme activity. Phenytoin, in particular, presents potential advantages in managing severe neurological symptoms induced by Tac toxicity (e.g., seizures or convulsions) (Hoppe et al., 2022). Hence, phenytoin was administered in our case to decrease Tac blood concentration. Phenytoin exerts a robust induction effect on CYP3A by activating the constitutive androstane receptor (CAR), which binds to the promoter region of CYP3A genes. Moreover, the induction effect of phenytoin on enzymes is dose-dependent (Brodie et al., 2013). However, phenytoin’s narrow therapeutic window and relatively long half-life (approximately 42 h orally) may affect the metabolism of other drugs due to its hepatic enzyme induction, increasing the risk of adverse reactions (Xiong et al., 2023). Therefore, it is necessary to monitor the blood concentration of phenytoin. Detailed pharmacokinetic data on the interaction between phenytoin and Tac in SLE patients is lacking. A reported case noted that the elimination half-life of Tac was <85.5 h within 1–6 days after discontinuation of NMV/r and Tac (Shiohira et al., 2024). In our case, the patient had discontinued NMV/r 4 days before admission and was concurrently administered phenytoin for 5 days upon admission, resulting in a Tac elimination half-life of 55.87 h. However, due to differences in medication regimens between the two cases, a direct comparison of Tac elimination half-lives is not feasible. Larger-scale studies are required to determine whether phenytoin can shorten Tac’s elimination half-life. Once Tac concentrations approach the therapeutic target, discontinuation of phenytoin is recommended (Lange et al., 2017).
Given the narrow therapeutic range of Tac and its significant drug-drug interactions (DDIs) with NMV/r, leading to serious toxicity, effective management strategies are essential. However, current literature on managing this DDI, especially in organ transplant recipients, is limited to a few small-scale retrospective studies. For patients on NMV/r, strategies such as temporarily holding or reducing Tac dosage and closely monitoring Tac levels may help prevent toxicity. The French Society of Pharmacology and Therapeutics recommends suspending Tac 12 h before starting NMV/r and resuming the usual daily dose (DD) 24 h after the last NMV/r dose (Lemaitre et al., 2022). Devresse et al. outlined a protocol involving discontinuing Tac 12 h before NMV/r initiation and administrating 20% of the cyclosporine dose. Ten of these patients resumed Tac on the second day after NMV/r discontinuation (Devresse et al., 2022). A similar approach was suggested by Lange et al. (2022) Salerno et al. shared their experiences with 25 solid organ transplant recipients, adjusting the regimen by either withholding Tac/mTOR inhibitors or reducing the cyclosporine dose to 20% of the baseline daily dose during NMV/r treatment, and restarting Tac 2–5 days post-treatment (Salerno et al., 2022). Dewey et al. described 12 lung transplant recipients who started NMV/r 10–14 h after the last Tac dose, with most reintroducing Tac within 4 days post-NMV/r (Dewey et al., 2023). Another retrospective study recommended withholding Tac for 24 h and resuming it 72 h after the last NMV/r dose (Giguère et al., 2023). Despite noting supratherapeutic Tac levels post-NMV/r, these studies reported minimal severe complications, suggesting the efficacy and safety of a well-timed NMV/r initiation and Tac resumption strategy in long-term Tac patients.
Estimating the overall change in Tac exposure poses a challenge, underscoring the importance of measuring Tac concentrations on days 3, 6, and 7 following NMV/r initiation to inform Tac reintroduction (Wang et al., 2022). A retrospective study, acknowledging the variance in Tac formulations, proposed differing reintroduction timelines: 24 h after the last Paxlovid dose for immediate-release Tac, and 48 h for sustained-release or long-acting formulations (Belden et al., 2023). These findings indicate that NMV/r does not necessarily preclude Tac usage, and standardized management can be mitigate toxicity arising from drug interactions. However, the majority of evidence for Tac dose adjustment arises from organ transplant cases, with a noticeable dearth of data for SLE. At our institution, clinical pharmacists play a vital role in transplant and emergency pharmacotherapy. They collaborate with clinical physicians to optimize drug treatment management for patients with acute toxicity. Unfortunately, in this instance, the patient self-administered NMV/r without physician or pharmacist consultation. Therefore, healthcare providers should prioritize vigilance toward potential drug interactions when prescribing medications, while patients should be educated about such interactions before initiating medication. Additionally, for Tac users, alternative COVID-19 antivirals with fewer interactions like oral molnupiravir or intravenous remdesivir, should be considered. However, the parenteral nature of remdesivir may limit its practically for outpatient administration.
5 Conclusion
This case underscores the critical importance of remaining vigilant regarding the drug-drug interactions (DDIs) between Tac and NMV/r in SLE patients, emphasizing the persistent nature of enzyme inhibition. It also highlights the indispensable role of therapeutic drug monitoring in Tac therapy management. Furthermore, the efficacy and safety of phenytoin as a pharmacokinetic inducer in mitigating Tac toxicity are underscored by this case. These findings underscore the complexity of managing DDIs in Tac recipients and emphasize the essential involvement of pharmacists in clinical decision-making and close monitoring in such scenarios.
6 The patient’s perspective
We reported the following about the patient’s experience: “I am a long-term SLE patients who has been taking Tac. This experience has made me deeply aware of the dangers of drug-drug interactions. During this visit and hospitalization, I received comprehensive treatment from hospital doctors, pharmacists, and nurses. After the treatment, my swallowing function and kidney function have significantly improved. Although I experienced symptoms of increased heart rate, I am satisfied with the treatment outcomes.”
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Ethics statement
This study was approved by the Ethics Committee of Nanjing Drum Tower Hospital. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
CJ: Conceptualization, Data curation, Funding acquisition, Investigation, Methodology, Writing–original draft, Writing–review and editing. XY: Methodology, Writing–original draft, Writing–review and editing. PXi: Investigation, Methodology, Writing–review and editing. XL: Data curation, Writing–review and editing. HaZ: Investigation, Data curation, Writing–review and editing. HT: Methodology, Writing–review and editing. YL: Methodology, Writing–review and editing. HuZ: Data curation, Writing–review and editing. PXu: Supervision, Writing–review and editing. JW: Supervision, Writing–review and editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Clinical Trials from the Affiliated Drum Tower Hospital, Medical School of Nanjing University, Nanjing, Jiangsu, China (grant number 2023-LCYJ-PY-24); Project of China Hospital Reform and Development Research Institute, Nanjing University, Nanjing, Jiangsu, China (grant number NDYGN2023007); Aid project of Nanjing Drum Tower Hospital Health, Education and Research Foundation, Nanjing, Jiangsu, China. The funders had no involvement in the preparation or writing up of this work.
Acknowledgments
The authors express sincere gratitude to the patient and the participants.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Akamatsu, H., Kohno, Y., Hashizume, J., Nakagawa, H., Kodama, Y., Kawano, H., et al. (2024). Effect of rifampicin administration on CYP induction in a dermatomyositis patient with vasospastic angina attributable to nilmatrelvir/ritonavir-induced blood tacrolimus elevation: a case report. J. Infect. Chemother., doi:10.1016/j.jiac.2024.02.006
Badri, P., Dutta, S., Coakley, E., Cohen, D., Ding, B., Podsadecki, T., et al. (2015). Pharmacokinetics and dose recommendations for cyclosporine and tacrolimus when coadministered with ABT-450, ombitasvir, and dasabuvir. Am. J. Transpl. 15, 1313–1322. doi:10.1111/ajt.13111
Bechstein, W. O. (2000). Neurotoxicity of calcineurin inhibitors: impact and clinical management. Transpl. Int. 13, 313–326. doi:10.1007/s001470050708
Belden, K. A., Yeager, S., Schulte, J., Cantarin, M. P. M., Moss, S., Royer, T., et al. (2023). Saving lives with nirmatrelvir/ritonavir one transplant patient at a time. Transpl. Infect. Dis. 25, e14037. doi:10.1111/tid.14037
Berar, Y. N., Bogner, I., Saker, K., and Tannous, E. (2022). Paxlovid-tacrolimus drug-drug interaction in a 23-year-old female kidney transplant patient with COVID-19. Clin. Drug. Investig. 42, 693–695. doi:10.1007/s40261-022-01180-4
Braithwaite, H. E., Darley, D. R., Brett, J., Day, R. O., and Carland, J. E. (2021). Identifying the association between tacrolimus exposure and toxicity in heart and lung transplant recipients: a systematic review. Transpl. Rev. Orl. 35, 100610. doi:10.1016/j.trre.2021.100610
Brodie, M. J., Mintzer, S., Pack, A. M., Gidal, B. E., Vecht, C. J., and Schmidt, D. (2013). Enzyme induction with antiepileptic drugs: cause for concern? Epilepsia 54, 11–27. doi:10.1111/j.1528-1167.2012.03671.x
Cadley, S. M., Sethi, A., and Knorr, J. P. (2022). CYP induction to reverse tacrolimus toxicity resulting from concomitant Paxlovid use. Transpl. Infect. Dis. 24, e13982. doi:10.1111/tid.13982
Ceschi, A., Rauber-Luthy, C., Kupferschmidt, H., Banner, N. R., Ansari, M., Krahenbuhl, S., et al. (2013). Acute calcineurin inhibitor overdose: analysis of cases reported to a national poison center between 1995 and 2011. Am. J. Transpl. 13, 786–795. doi:10.1111/j.1600-6143.2012.04347.x
Chen, J., and Raymond, K. (2006). Roles of rifampicin in drug-drug interactions: underlying molecular mechanisms involving the nuclear pregnane X receptor. Ann. Clin. Microbiol. Antimicrob. 5, 3. doi:10.1186/1476-0711-5-3
Chen, Y., Wan, W., Yao, X., and Guan, Y. (2023). Drug-drug interaction between paxlovid and tacrolimus in a patient with myasthenia gravis and SARS-CoV-2 infection. J. Neuroimmunol. 385, 578245. doi:10.1016/j.jneuroim.2023.578245
Chiu, T. Y., Weng, C. C., Ha, S. C., Tsai, H. W., Koh, C. C., and Chen, Y. (2023). Management of COVID-19 infection in a small bowel transplant recipient: a case report. Proc. 55, 1873–1876. doi:10.1016/j.transproceed.2023.05.008
Cordero, C. G., and de Vicente, M. S. (2023). Elevated tacrolimus blood concentration due to the interaction with nirmatrelvir/ritonavir during COVID-19 treatment: a case report. Proc. 55, 1826–1828. doi:10.1016/j.transproceed.2023.03.001
Devresse, A., Sébastien, B., De Greef, J., Lemaitre, F., Boland, L., Haufroid, V., et al. (2022). Safety, efficacy, and relapse of nirmatrelvir-ritonavir in kidney transplant recipients infected with SARS-CoV-2. Int. Rep. 7, 2356–2363. doi:10.1016/j.ekir.2022.08.026
Dewey, K. W., Yen, B., Lazo, J., Seijo, L., Jariwala, R., Shah, R. J., et al. (2023). Nirmatrelvir/ritonavir use with tacrolimus in lung transplant recipients: a single-center case series. Transplantation 107, 1200–1205. doi:10.1097/tp.0000000000004394
Dhar, R. (2017). Neurologic complications of transplantation. Handb. Clin. Neurol. 141, 545–572. doi:10.1016/B978-0-444-63599-0.00030-2
Fanouriakis, A., Kostopoulou, M., Alunno, A., Aringer, M., Bajema, I., Boletis, J. N., et al. (2019). 2019 update of the EULAR recommendations for the management of systemic lupus erythematosus. Ann. Rheum. Dis. 78, 736–745. doi:10.1136/annrheumdis-2019-215089
Farouk, S. S., and Rein, J. L. (2020). The many faces of calcineurin inhibitor toxicity-what the FK? Adv. Chronic. Kidney. Dis. 27, 56–66. doi:10.1053/j.ackd.2019.08.006
Fishbane, S., Hirsch, J. S., and Nair, V. (2022). Special considerations for paxlovid treatment among transplant recipients with SARS-CoV-2 infection. Am. J. Kidney. Dis. 79, 480–482. doi:10.1053/j.ajkd.2022.01.001
Giguère, P., Deschenes, M. J., Loon, M. V., Hoar, S., Fairhead, T., Pazhekattu, R., et al. (2023). Management and outcome of COVID-19 infection using nirmatrelvir/ritonavir in kidney transplant patients. Clin. J. Am. Soc. Nephrol. 18, 913–919. doi:10.2215/cjn.0000000000000186
Guyon, J., Novion, M., Fulda, V., Ducint, D., Molimard, M., Couzi, L., et al. (2022). A UPLC-MS/MS method for plasma biological monitoring of nirmatrelvir and ritonavir in the context of SARS-CoV-2 infection and application to a case. J. Am. Soc. Mass. Spectrom. 33, 1975–1981. doi:10.1021/jasms.2c00204
Hoppe, J. M., Holderied, A., Schonermarck, U., Vielhauer, V., Anders, H. J., and Fischereder, M. (2022). Drug-induced CYP induction as therapy for tacrolimus intoxication. Clin. Nephrol. Case. Stud. 10, 42–46. doi:10.5414/CNCS110744
Jantz, A. S., Patel, S. J., Suki, W. N., Knight, R. J., Bhimaraj, A., and Gaber, A. O. (2013). Treatment of acute tacrolimus toxicity with phenytoin in solid organ transplant recipients. Rep. Transpl. 2013, 375263. doi:10.1155/2013/375263
Kwon, E. J., Yun, G. A., Park, S., Kim, S., Chae, D. W., Park, H. S., et al. (2022). Treatment of acute tacrolimus toxicity with phenytoin after Paxlovid (nirmatrelvir/ritonavir) administration in a kidney transplant recipient. Kidney. Res. Clin. Pract. 41, 768–770. doi:10.23876/j.krcp.22.218
Lange, N. W., Salerno, D. M., Berger, K., and Tsapepas, D. S. (2017). Using known drug interactions to manage supratherapeutic calcineurin inhibitor concentrations. Clin. Transpl. 31. doi:10.1111/ctr.13098
Lange, N. W., Salerno, D. M., Jennings, D. L., Choe, J., Hedvat, J., Kovac, D. B., et al. (2022). Nirmatrelvir/ritonavir use: managing clinically significant drug-drug interactions with transplant immunosuppressants. Am. J. Transpl. 22, 1925–1926. doi:10.1111/ajt.16955
Lemaitre, F., Grégoire, M., Monchaud, C., Bouchet, S., Saint-Salvi, B., Polard, E., et al. (2022). Management of drug-drug interactions with nirmatrelvir/ritonavir in patients treated for Covid-19: guidelines from the French Society of Pharmacology and Therapeutics (SFPT). Therapie 77, 509–521. doi:10.1016/j.therap.2022.03.005
Lindauer, K. E., and Hamel, A. G. (2022). Case report: nirmatrelvir/ritonavir and tacrolimus in a kidney transplant recipient with COVID-19. Am. Fam. Physician 105, 569–570.
Liu, Y., Liu, Y., Cai, C., Hui, F., Zhang, Y., Wang, B., et al. (2023). Effect of paxlovid on tacrolimus concentration in perioperative kidney transplant patients infected with COVID-19: a case report. Proc. 55, 1822–1825. doi:10.1016/j.transproceed.2023.07.007
Lo, C. M., Chen, W. H., Tsai, M. Y., Lu, H. I., Hsiao, Y. H., Chuang, K. H., et al. (2024). A case report of drug interaction between co-packaged nirmatrelvir-ritonavir and tacrolimus causing hyponatremia in a lung transplant recipient. J. Cardiothorac. Surg. 19, 132. doi:10.1186/s13019-024-02599-w
Luo, W., He, Y., Wei, M. G., Lu, G. B., and Yi, Q. (2023). Paxlovid-tacrolimus drug-drug interaction caused severe diarrhea that induced combined diabetic ketoacidosis and a hyperglycemic hyperosmolar state in a kidney transplant patient: a case report. J. Med. Case. Rep. 17, 406. doi:10.1186/s13256-023-04135-1
Magnusson, M. O., Dahl, M. L., Cederberg, J., Karlsson, M. O., and Sandström, R. (2008). Pharmacodynamics of carbamazepine-mediated induction of CYP3A4, CYP1A2, and Pgp as assessed by probe substrates midazolam, caffeine, and digoxin. Clin. Pharmacol. Ther. 84, 52–62. doi:10.1038/sj.clpt.6100431
Marsh, J., Logan, A. T., Bilgili, E. P., Bowman, L. J., and Webb, A. R. (2024). Phenytoin enzyme induction for management of supratherapeutic tacrolimus levels due to drug-drug interaction with nirmatrelvir/ritonavir: case series and discussion. Am. J. Health. Syst. Pharm., zxae032. doi:10.1093/ajhp/zxae032
Maynard, R. D., Bates, P., and Korpi-Steiner, N. (2023). Monitoring tacrolimus toxicity following Paxlovid administration in a liver transplant patient. Pract. Lab. Med. 36, e00322. doi:10.1016/j.plabm.2023.e00322
Mecadon, K., Arvanitis, P., Farmakiotis, D., and Rogers, R. (2022). Single-center experience with nirmatrelvir/ritonavir in kidney transplant recipients on tacrolimus maintenance immunosuppression. Clin. Transpl. 36, e14752. doi:10.1111/ctr.14752
Miano, T. A., Flesch, J. D., Feng, R., Forker, C. M., Brown, M., Oyster, M., et al. (2020). Early tacrolimus concentrations after lung transplant are predicted by combined clinical and genetic factors and associated with acute kidney injury. Clin. Pharmacol. Ther. 107, 462–470. doi:10.1002/cpt.1629
Michael, S., Heilbronner, R., Lloyd, C. M., and Levitin, H. W. (2023). Paxlovid-induced tacrolimus toxicity in the treatment of COVID-19: a case report. Cureus 15, e35489. doi:10.7759/cureus.35489
Modi, S., Kahwash, R., and Kissling, K. (2023). Case Report: tacrolimus toxicity in the setting of concurrent Paxlovid use in a heart-transplant recipient. Eur. Heart. J. Case. Rep. 7, ytad193. doi:10.1093/ehjcr/ytad193
Murphy, L. (2022). Systemic lupus erythematosus: overview, management and COVID-19. Br. J. Nurs. 31, 348–355. doi:10.12968/bjon.2022.31.7.348
Naccarato, M., Kwee, F., Zaltzman, J., and Fong, I. W. (2021). Ritonavir-boosted antiretroviral therapy precipitating tacrolimus toxicity in a renal transplant patient: is it time for a priori tacrolimus dosage reduction? AIDS 35, 2065–2068. doi:10.1097/QAD.0000000000003002
Prikis, M., and Cameron, A. (2022). Paxlovid (Nirmatelvir/Ritonavir) and tacrolimus drug-drug interaction in a kidney transplant patient with SARS-2-CoV infection: a case report. Proc. 54, 1557–1560. doi:10.1016/j.transproceed.2022.04.015
Quirós-Tejeira, R. E., Chang, I. F., Bristow, L. J., Karpen, S. J., and Goss, J. A. (2005). Treatment of acute tacrolimus whole-blood elevation with phenobarbital in the pediatric liver transplant recipient. Pediatr. Transpl. 9, 792–796. doi:10.1111/j.1399-3046.2005.00368.x
Rose, D. T., Gandhi, S. M., Bedard, R. A., Mondy, K. E., Chu, A. L., Gamble, K. C., et al. (2022). Supratherapeutic tacrolimus concentrations with nirmatrelvir/ritonavir in solid organ transplant recipients requiring hospitalization: a case series using rifampin for reversal. Open. Forum. Infect. Dis. 9, ofac238. doi:10.1093/ofid/ofac238
Salerno, D. M., Jennings, D. L., Lange, N. W., Kovac, D. B., Shertel, T., Chen, J. K., et al. (2022). Early clinical experience with nirmatrelvir/ritonavir for the treatment of COVID-19 in solid organ transplant recipients. Am. J. Transpl. 22, 2083–2088. doi:10.1111/ajt.17027
Sevrioukova, I. F., and Poulos, T. L. (2010). Structure and mechanism of the complex between cytochrome P4503A4 and ritonavir. Proc. Natl. Acad. Sci. U. S. A. 107, 18422–18427. doi:10.1073/pnas.1010693107
Shah, A., Nasrullah, A., Butt, M. A., and Young, M. (2022). Paxlovid with caution: novel case of paxlovid-induced tacrolimus toxicity in a cardiac transplant patient. Eur. J. Case Rep. Intern Med. 9, 003528. doi:10.12890/2022_003528
Shen, D., Gong, Y., Qian, Y., Zhu, J., and Gao, J. (2024). Nirmatrelvir/ritonavir treatment of patients with COVID-19 taking tacrolimus: case series describing the results of drug-drug interactions. J. Int. Med. Res. 52, 3000605241247705. doi:10.1177/03000605241247705
Shiohira, H., Arakaki, S., Uehara, W., Uehara, H., Yamamoto, K., and Nakamura, K. (2024). Nirmatrelvir/ritonavir-induced elevation of blood tacrolimus levels in a patient in the maintenance phase post liver transplantation. J. Infect. Chemother. 30, 77–80. doi:10.1016/j.jiac.2023.09.006
Sikma, M. A., Hunault, C. C., Kirkels, J. H., Verhaar, M. C., Kesecioglu, J., and de Lange, D. W. (2018). Association of whole blood tacrolimus concentrations with kidney injury in heart transplantation patients. Eur. J. Drug. Metab. Pharmacokinet. 43, 311–320. doi:10.1007/s13318-017-0453-7
Sindelar, M., McCabe, D., and Carroll, E. (2023). Tacrolimus drug-drug interaction with nirmatrelvir/ritonavir (Paxlovid™) managed with phenytoin. J. Med. Toxicol. 19, 45–48. doi:10.1007/s13181-022-00922-2
Snee, I., Drobina, J., and Mazer-Amirshahi, M. (2023). Tacrolimus toxicity due to enzyme inhibition from ritonavir. Am. J. Emerg. Med. 69, 218.e5–218.e7. doi:10.1016/j.ajem.2023.04.045
Tomida, T., Itohara, K., Yamamoto, K., Kimura, T., Fujita, K., Uda, A., et al. (2023). A model-based pharmacokinetic assessment of drug-drug interaction between tacrolimus and nirmatrelvir/ritonavir in a kidney transplant patient with COVID-19. Metab. Pharmacokinet. 53, 100529. doi:10.1016/j.dmpk.2023.100529
Tsuzawa, A., Katada, Y., Umemura, K., Sugimoto, M., Nishikawa, A., Sato, Y. K., et al. (2023). A case report of a prolonged decrease in tacrolimus clearance due to co-administration of nirmatrelvir/ritonavir in a lung transplant recipient receiving itraconazole prophylaxis. J. Pharm. Health. Care. Sci. 9, 12. doi:10.1186/s40780-023-00280-3
Wang, A. X., Koff, A., Hao, D., Tuznik, N. M., and Huang, Y. (2022). Effect of nirmatrelvir/ritonavir on calcineurin inhibitor levels: early experience in four SARS-CoV-2 infected kidney transplant recipients. Am. J. Transpl. 22, 2117–2119. doi:10.1111/ajt.16997
Watanabe, H., Yamanaka, R., Sada, K. E., Zeggar, S., Katsuyama, E., Katsuyama, T., et al. (2016). The efficacy of add-on tacrolimus for minor flare in patients with systemic lupus erythematosus: a retrospective study. Lupus. 25, 54–60. doi:10.1177/0961203315600538
Xiong, Y., Wang, X., Li, S., Zhang, Q., Guo, L., Chen, W., et al. (2023). Case report: supratherapeutic tacrolimus concentrations with nirmatrelvir/ritonavir in a lung transplant patient: a case report using Rifampin for reversal. Front. Pharmacol. 14, 1285078. doi:10.3389/fphar.2023.1285078
Xu, X., Zhang, H., Liu, L., Fu, Q., Wu, C., Lin, X., et al. (2024). Pharmacokinetics of nirmatrelvir/ritonavir and the drug-drug interaction with calcineurin inhibitor in renal transplant recipients. Eur. J. Clin. Pharmacol., doi:10.1007/s00228-024-03691-9
Yamamoto, N., Tsuchiya, Y., Fukuda, M., Niiro, H., and Hirota, T. (2024). A case report of drug interactions between nirmatrelvir/ritonavir and tacrolimus in a patient with systemic lupus erythematosus. Cureus 16, e52506. doi:10.7759/cureus.52506
Young, C., Papiro, T., and Greenberg, J. H. (2023). Elevated tacrolimus levels after treatment with nirmatrelvir/ritonavir (Paxlovid) for COVID-19 infection in a child with a kidney transplant. Pediatr. Nephrol. 38, 1387–1388. doi:10.1007/s00467-022-05712-0
Zaarur, L., Patel, A., and Pasternak, B. (2023). Drug interaction between tacrolimus and paxlovid (Nirmatrelvir/Ritonavir) in an adolescent with inflammatory bowel disease. JPGN. Rep. 4, e352. doi:10.1097/PG9.0000000000000352
Keywords: systemic lupus erythematosus, tacrolimus, nirmatrelvir/ritonavir, CYP induction, case report
Citation: Jiang C, Yan X, Xia P, Luo X, Zheng H, Tong H, Liu Y, Zhu H, Xu P and Wang J (2024) Case report and literature review: management of Paxlovid (nirmatrelvir/ritonavir)-induced acute tacrolimus toxicity in a patient with systemic lupus erythematosus. Front. Pharmacol. 15:1364121. doi: 10.3389/fphar.2024.1364121
Received: 04 January 2024; Accepted: 20 May 2024;
Published: 19 June 2024.
Edited by:
Francesc J. Moreso, Vall d’Hebron University Hospital, SpainReviewed by:
Ancuta Lupu, Grigore T. Popa University of Medicine and Pharmacy, RomaniaAnant D. Patil, DY Patil Deemed to be University, India
Copyright © 2024 Jiang, Yan, Xia, Luo, Zheng, Tong, Liu, Zhu, Xu and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Peng Xu, exu_peng@163.com; Jun Wang, wjgaogou@aliyun.com
†These authors have contributed equally to this work and share first authorship
 Chenxiao Jiang
Chenxiao Jiang Xiaodi Yan2†
Xiaodi Yan2† Peng Xia
Peng Xia Xuemei Luo
Xuemei Luo Hanwen Tong
Hanwen Tong Jun Wang
Jun Wang