- 1Department of Neonatology, Shandong Provincial Hospital Affiliated to Shandong First Medical University, Jinan, China
- 2Department of Anesthesiology, Shandong Provincial Hospital Affiliated to Shandong First Medical University, Jinan, China
Background: Recent advancements in China’s perinatal and neonatal intensive care have significantly reduced neonatal mortality, yet preterm births before 32 weeks remain the primary cause of neonatal fatalities and contribute to long-term disabilities. The prognosis of very preterm infants (VPIs) is significantly affected by factors including the intrauterine environment, delivery method and neonatal intensive care. Cesarean section which often used for preterm births has implications that are not fully understood, particularly concerning the type of anesthesia used. This study examines the impact of general anesthesia (GA) during cesarean delivery on VPI outcomes, aiming to identify strategies for mitigating GA-associated risks.
Methods: This cohort study analyzed 1,029 VPIs born via cesarean section under 32 weeks’ gestation at our single-center from 1 January 2018, to 31 December 2022. Detailed medical records, encompassing perioperative information, maternal data and neonatal outcomes were meticulously examined. The primary aim of this investigation was to compare maternal characteristics and neonatal outcomes between VPIs delivered under GA and neuraxial anesthesia (NA). A significance level of p < 0.05 was established.
Results: Of the 1,029 VPIs analyzed, 87.95% (n = 905) were delivered via NA and 12.05% (n = 124) via GA. Mothers with hypertensive pregnancy diseases and emergency operations were more inclined to choose GA. VPIs delivered under GA showed a lower Apgar score at one and 5 minutes (p < 0.01), increased need for tracheal intubation resuscitation (32.2% vs. 12.2%, p < 0.01) and a greater incidence of severe neurological injury (SNI) (14.5% vs. 5%, p < 0.01). Multivariable analysis revealed GA was significantly associated with lower Apgar scores at one (OR 6.321, 95% CI 3.729–10.714; p < 0.01) and 5 minutes (OR 4.535, 95% CI 2.975–6.913; p < 0.01), higher risk of tracheal intubation resuscitation (OR = 3.133, 95% CI = 1.939–5.061; p < 0.01) and SNI (OR = 3.019, 95% CI = 1.615–5.643; p < 0.01). Furthermore, for VPIs delivered under GA, a prolonged interval from skin incision to fetus delivery was associated with a lower 5-min Apgar score (p < 0.01).
Conclusion: This study revealed the significant impact of GA on adverse outcomes among VPIs. In cases when GA is required, proactive measures should be instituted for the care of VPIs such as expediting the interval from skin incision to fetal delivery.
Introduction
Various factors exert influence on the prognostic outcomes of preterm infants, among which the mode of delivery holds particular significance. Recent studies have demonstrated that cesarean sections performed for standard obstetrical indications are linked to improved morbidity and mortality among preterm infants. (Holzer et al., 2016; Karayel Eroglu et al., 2022). Hence, it is noteworthy that preterm infants, particularly very preterm infants (VPIs), are frequently delivered via cesarean section. There exists a well-established global consensus favoring the use of neuraxial anesthesia (NA) techniques (such as spinal anesthesia, epidural anesthesia, or combined spinal and epidural anesthesia) for cesarean sections. This preference is underpinned by compelling evidence demonstrating improved maternal outcomes (Mushambi et al., 2015; American Society of Anesthesiologists Committee on Standards and Practice Parameters., 2016), including reduced risks of short-term maternal morbidity or mortality, decreased incidence of wound infections, and alleviated postoperative pain (Chattopadhyay et al., 2014; Sayed et al., 2015).
General anesthesia (GA) stands out for its ability to provide the shortest onset time, making it a viable choice in cases of urgent medical or obstetric indications necessitating cesarean section (Palanisamy et al., 2011; Butwick et al., 2015). However, for preterm infants delivered via cesarean section, the criteria determining the selection of GA assume critical clinical significance. Anesthesiologists must carefully weigh these determinants while prioritizing the safety of both the maternal and neonates. Several studies have demonstrated that the GA group exhibits lower newborn Apgar scores and higher rates of neonatal intensive care unit (NICU) admissions compared with the NA group (Bao et al., 2022; Shi et al., 2024). While some research has explored maternal and neonatal outcomes associated with GA administration during cesarean sections (Nwafor et al., 2014; Butwick et al., 2015), there remains a notable lack of studies specifically addressing the impact of GA on the outcomes of VPIs delivered by cesarean section.
Due to the rising incidence of preterm births, SNI has emerged as a progressively concerning complication that significantly influences the prognoses of preterm infants. Correctly managing the processes of delivery and neonatal resuscitation assumes paramount importance as crucial steps in mitigating the adverse outcomes associated with SNI in preterm infants. Our research endeavors have encompassed a series of cohort studies, leveraging comprehensive databases to assess the myriad factors influencing the outcomes of VPIs. However, one critical facet of our investigations has hitherto remained unexplored: the impact of anesthesia modes on the outcomes of VPIs delivered via cesarean section. In the current study, we have undertaken an investigation to ascertain whether distinct anesthesia modalities (GA or NA) exhibit associations with neonatal adverse outcomes in VPIs.
Methods
Study design and setting
The study cohort utilized in our research was derived from a dataset established through the Sino-northern Neonatal Network (SNN), a network characterized by ongoing prospective data collection. A comprehensive description of this network and the methodologies employed for the assessment of post-natal conditions and the quality control of resuscitation procedures in preterm infants has been previously documented in other publication (Dong et al., 2023). We conducted data analysis on a cohort comprising 1,058 preterm infants with a gestational age of less than 32 weeks who were delivered via cesarean section. This cohort was drawn from cases occurring between 1 January 2018, and 31 December 2022, at our singular tertiary care institution (Shandong Provincial Hospital Affiliated to Shandong First Medical University, China). The analysis encompassed maternal demographic information and the subsequent comparison of neonatal outcomes. The choice of anesthesia method was determined by the attending anesthesiologist, taking into consideration the urgency of the surgical procedure and maternal complications. All aspects of maternal anesthesia management adhered to established practice guidelines for obstetric anesthesia, as outlined by the American Society of Anesthesiologists Committee on Standards and Practice Parameters (American Society of Anesthesiologists Task Force on Obstetric Anesthesia and the Society for Obstetric Anesthesia and Perinatology, 2016). Infants with documented severe congenital malformations, those with partial data availability, or those delivered under compound anesthesia were excluded from the study. Ultimately, a total of 1,029 eligible VPIs were included in this investigation (Figure 1).
Definition of variables
Severe neurological injury (SNI) was defined as the presence of intraventricular hemorrhage (IVH) of grade 3 or 4 and/or porencephalic ventricular leukomalacia (PVL) (Egesa et al., 2021). To detect cerebral lesions, the standard protocol involved conducting one or two cranial ultrasound scans within the first 48 h post-birth, followed by weekly scans for the subsequent 2 weeks. In instances where cranial ultrasound is unfeasible, magnetic resonance imaging (MRI) is utilized, either at 36 weeks’ postmenstrual age (PMA) or upon discharge. We categorized bronchopulmonary dysplasia (BPD) as necessitating respiratory support at 36 weeks’ PMA (Strueby and Thébaud, 2018). Severe retinopathy of prematurity (ROP) was identified as ROP stage 3, 4, or 5, and/or cases receiving ROP treatment (laser or intraocular injection) (Chiang et al., 2021). Severe necrotizing enterocolitis of newborn (NEC) was defined as NEC stage 2 or 3, following Bell’s criteria (Kim, 2014). The Score for Neonatal Acute Physiology, Version II (SNAP-II), a standardized and clinically validated tool, is employed to assess the severity of newborn illnesses and mortality risk, with scoring criteria based on prior studies (Richardson et al., 2001).
Statistical analysis
SPSS 26.0 statistical software was used for analysis. Normally distributed continuous variables are expressed as means ± standard deviation, abnormally distributed continuous variables are expressed as median (interquartile range) and categorical variables are expressed as number (percentage). Quantitative indicators are compared by t-test or Wilcoxon rank-sum test according to the data distribution, while categorical indicators are compared by chi-square test or Fisher’s exact probability method (if chi-square test is not applicable). Regarding neonatal outcomes, variables significantly correlated with the mode of anesthesia in univariate analyses were retained as covariates in a multivariable logistic regression model. The model’s goodness of fit was evaluated using the Hosmer–Lemeshow statistic, and a p-value < 0.05 was considered statistically significant while confidence intervals (CIs) were set at 95%.
Results
Medical and obstetrical indications for parturients receiving general anesthesia
Our study included 1,029 VPIs delivered via cesarean section before 32 weeks of gestational age. Of these, 124 were delivered under GA and 905 under NA. Due to multiple birth, 124 VPIs in GA group originated from 112 parturients. The indications for GA included: maternal thrombocytopenia or coagulation disorders (47%), taking antiplatelet/anticoagulation drugs (21%), bleeding risk (14%), cardiac related diseases (5%), and other reasons (7%). The rationale was unclear in 6% of cases (Figure 2).
Other reasons include: 2 cases of acute pancreatitis,1 case of osteogenesis imperfecta, 2 cases of umbilical cord prolapse, 1 case of maternal obesity, 1 case of cerebral palsy, 1 case of maternal rejection of spinal anesthesia.
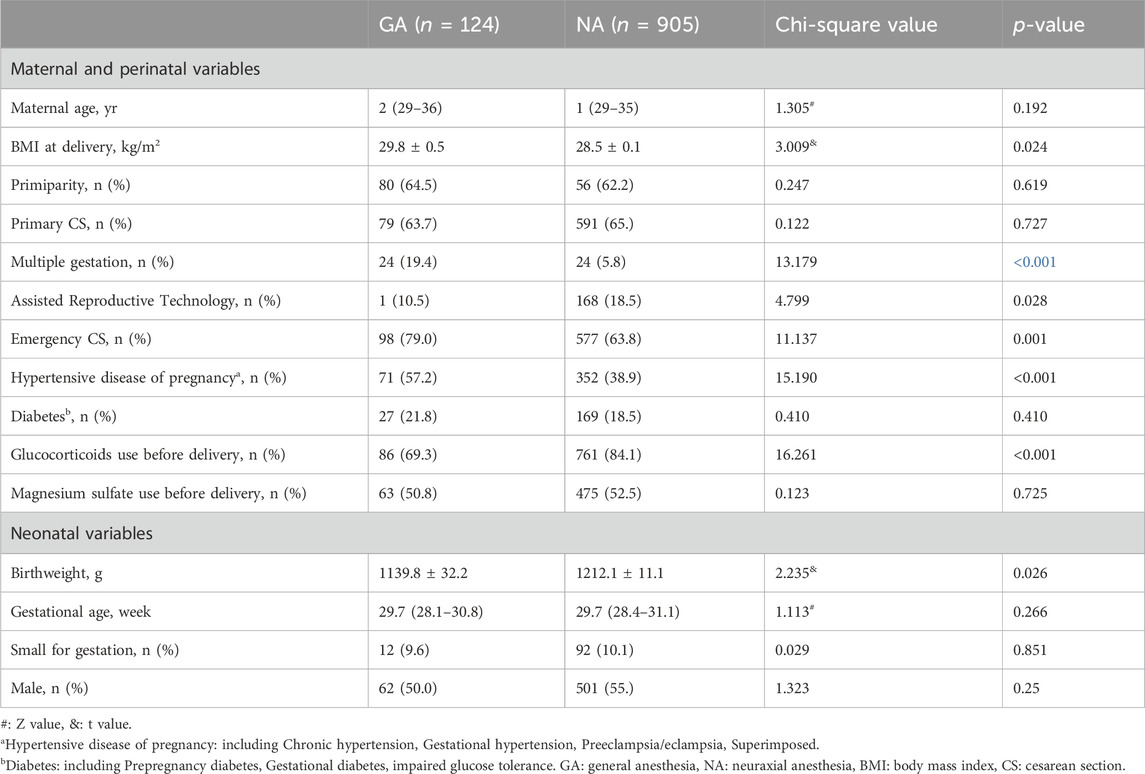
Baseline maternal and neonatal characteristics
No significant differences were observed between the two groups regarding maternal age, primiparity status, incidence of primary cesarean section, gestational diabetes, and prenatal magnesium sulfate use. However, the GA group exhibited significantly higher maternal BMI (29.8 ± 0.5vs 28.5 ± 0.1, p = 0.024), prevalence of hypertensive disorders of pregnancy (71/124 [57.2%] vs. 352/905 [38.9%], p < 0.001), and frequency of emergency cesarean sections (98/124 [79%] vs. 577/905 [63.8%], p = 0.001) compared to the NA group. Conversely, prenatal glucocorticoid use (86/124 [69.3%] vs. 761/905 [84.1%], p < 0.001), utilization of assisted fertility techniques (13/124 [10.5%] vs. 168/905 [18.5%], p = 0.028), and incidence of multiple births (24/124 [19.4%] vs. 324/905 [35.8%], p < 0.001) were significantly higher in the NA group compared to the GA group (Table 1).
Regarding neonatal baseline characteristics (Table 1), no significant differences were found in gestational age at birth, sex distribution, and the incidence of being small for gestational age between the two groups. However, birth weight(g) was significantly lower in the GA group compared to the NA group (1139.8 ± 32.2vs 1212.1 ± 11.1, p = 0.026).
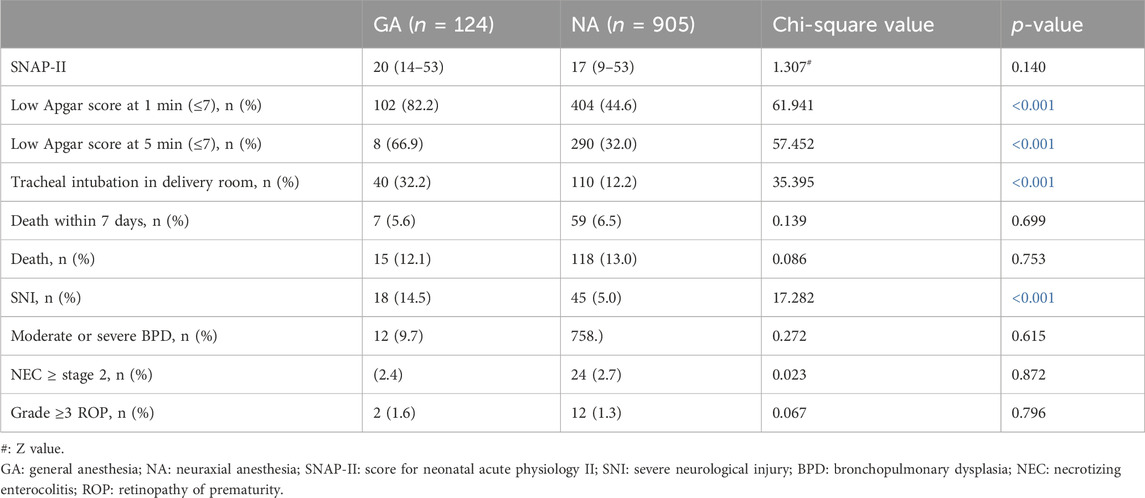
Adverse outcomes of neonates delivered under GA and NA
In our analysis of neonatal outcomes between the two groups, VPIs in the GA group demonstrated a higher incidence of low Apgar scores (≤7) at 1 minute (102/124 [82.2%] vs. 404/905 [44.6%], p < 0.001) and 5 minutes post-delivery (83/124 [66.9%] vs. 290/905 [32.0%], p < 0.001) compared to the NA group. Additionally, the necessity for resuscitation through tracheal intubation in the delivery room was more prevalent in the GA group (40/124 [32.2%] vs. 110/905 [12.2%], p < 0.001). SNI was higher in the GA group compared to the NA group (18/124 [14.5%] vs. 45/905 [5.0%], p < 0.001). No significant differences were observed between the two groups in other outcomes, including SNAP-II, mortality within 7 days after birth and ultimately, and the complications of moderate or severe BPD, NEC of stage 2 or higher, and ROP of Grade 3 or higher, as detailed in Table 2.
Association between general anesthesia and adverse outcomes of neonates
The odds of adverse outcomes in VPIs delivered under GA were higher than those under NA, including low Apgar score (≤7) at one and 5 minutes after delivery, as well as SNI and resuscitation by tracheal intubation in delivery room. Adjusted for maternal BMI, multiple gestation, assisted reproductive technology, emergency cesarean, hypertensive disease of pregnancy, glucocorticoids use before delivery and neonatal birthweight, multivariate logistic regression analysis showed that exposure to general anesthesia during cesarean section significantly added the odds of low Apgar score (≤7) at 1 minute (OR adj 6.321; 95% CI 3.729–10.714)and 5 minute (OR adj 4.535; 95% CI 2.975–6.913), SNI (OR adj 3.019; 95% CI 1.615–5.643), and tracheal intubation in delivery room (OR adj 3.133; 95%CI 1.939–5.061) (Table 3).
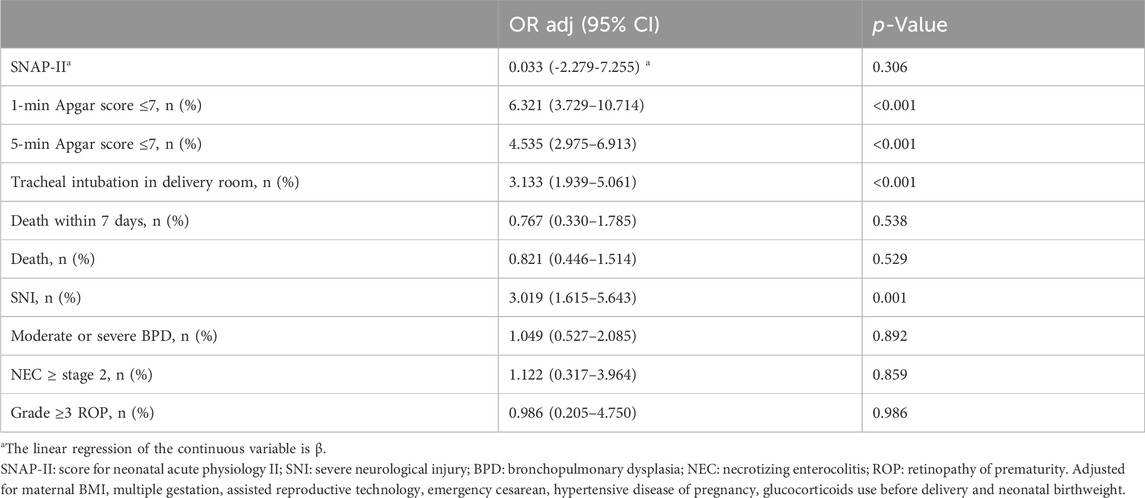
Table 3. Multivariate analysis on associations between general anesthesia and adverse outcomes of neonates.
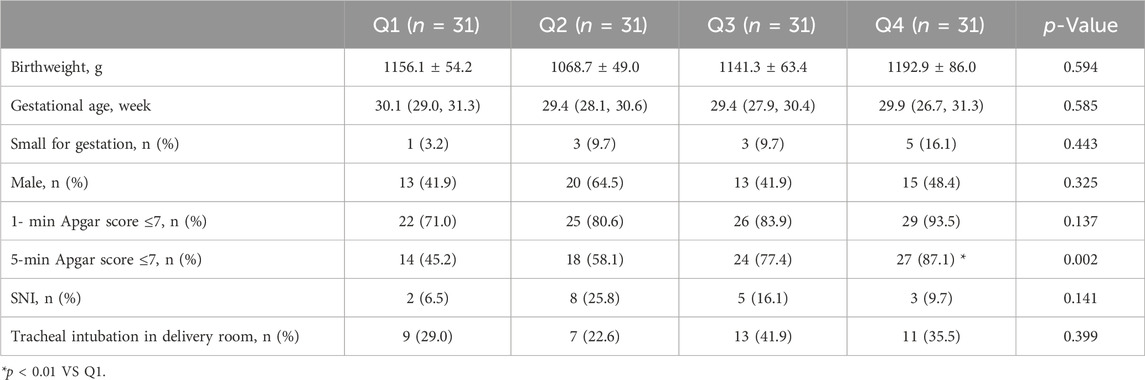
Association between exposure time to GA and adverse outcomes
124 neonates delivered under GA were divided into four subgroups according to the interval time from skin incision to fetal delivery in ascending order, with 31 cases in each subgroup (Q1∼Q4). The medians and interquartile ranges of the four subgroups are Q1 [2 (2–3)], Q2 [5 (4–5)], Q3 [6 (6–7.5) and Q4 [13 (10–16.5)] respectively (Unit: minute). Chi-square test and Boferroni method were used to determine whether there were statistical differences in variables between multi-subgroups. As shown in Table 4, on the basis of no statistically significant difference in baseline data for neonatal data (birthweight, gestational age, small for gestation, sex), the proportion of neonates with 5-min Apgar score less than or equal to 7 points increased with the extension of the interval time, and the difference in Q4 subgroup was statistically significant (p < 0.01). While incidence of low 1-min Apgar score (≦7), SNI and tracheal intubation in delivery room did not differ from every group (Table 4).
Discussion
In this observational study, we observed an association between adverse neonatal outcomes and the use of GA in VPIs delivered by cesarean section. Specifically, GA was linked to a higher frequency of perinatal adverse outcomes, including low Apgar scores at one and 5 minutes, the need for tracheal intubation at birth, and SNI, compared to neonates delivered under NA. This association remained significant even after adjusting for potential confounding clinical factors. Additionally, a prolonged duration of GA exposure was correlated with low 5-min Apgar score in neonates.
Data from a 2015 multi-center United States survey revealed that 17.6% of women underwent cesarean sections between 24 + 0 and 36+6 weeks of gestation via GA (Butwick et al., 2015). The proportion of GA in our study was marginally lower, potentially attributable to variations in the gestational ages of the preterm infants examined and advancements in anesthesia techniques and perinatal management in recent years (Bollag et al., 2021).
Cesarean sections can be executed under NA, encompassing spinal anesthesia and epidural anesthesia, or under GA. The selection of anesthesia mode is typically influenced by clinical indications, the anesthesiologist’s experience, and maternal preferences. NA provides the advantage of maternal consciousness during cesarean delivery and minimizes anesthetic exposure to the neonate. Additionally, NA reduces the risks of maternal aspiration and difficult airway management, commonly associated with GA (Mushambi et al., 2015). NA remains the preferred and gold standard mode of anesthesia for cesarean sections. However, specific conditions necessitate considering GA as a secondary option. These include infection at the needle insertion site, significant coagulopathy, hypovolemic shock, increased intracranial pressure due to space-occupying lesions, and scenarios where provider expertise in NA is inadequate or other critical conditions that endanger maternal-fetal life and require immediate termination (Afolabi and Lesi, 2012). Our findings indicate that maternal thrombocytopenia or coagulation disorders, and the use of antiplatelet/anticoagulation medications, are primary factors influencing the choice of GA. Pre-eclampsia continues to be a leading cause of maternal and neonatal morbidities, particularly in developing countries. The definitive treatment for pre-eclampsia involves timely termination of pregnancy, which consequently leads to a higher incidence of cesarean sections among VPIs in pre-eclamptic women (Liu et al., 2008; Nankali et al., 2012). Thrombocytopenia and coagulation disturbances, key characteristics of HELLP syndrome, often contraindicate the use of NA (Adorno et al., 2022; del-Rio-Vellosillo and Garcia-Medina, 2015). This may elucidate our finding that mothers with HELLP syndrome exhibited a higher likelihood of undergoing GA in our study. Additionally, we observed a significantly higher proportion of VPIs from emergency cesarean sections in the GA group. In scenarios such as placental abruption, placenta previa with massive bleeding, umbilical cord prolapse, or imminent fetal heart rate loss, both obstetricians and patients often favor GA due to its rapid induction and reduced cardiovascular instability (Guglielminotti et al., 2019; Ikeda et al., 2020). Previous research identified obesity as a risk factor for the selection of GA during cesarean sections (Butwick et al., 2015). Our current study corroborates this finding, noting a significantly higher maternal BMI in the GA group compared to the NA group, aligning with their results.
The choice of anesthesia during cesarean section can significantly impact neonatal outcomes (Saygı et al., 2015). Research exploring the relationship between anesthesia mode during cesarean delivery and outcomes in preterm infants has yielded varying results. One study indicated that mortality rates in preterm infants (<33 weeks’ gestational age) born to women receiving SA were higher compared to those born under GA. Conversely, another cohort study focusing on preterm infants ≤32 weeks’ gestational age found that neonates born to women receiving EA had higher Apgar scores than those from mothers who received GA (Rolbin et al., 1994; Laudenbach et al., 2009). The Apgar scoring system, a traditional and practical method for immediate neonatal assessment post-delivery, is crucial for identifying infants in need of resuscitation and predicting perinatal survival (Casey et al., 2001). In our study, the incidence of low 1-min and 5-min Apgar scores among VPIs was significantly higher, and a greater number of VPIs in the GA group required resuscitation with tracheal intubation in the delivery room. These findings indirectly reaffirm the importance of this traditional evaluation method.
Research indicated that the impact of drugs related to GA on the developing brain was mediated through both morphological alterations and molecular mechanisms (Vutskits and Xie, 2016). Groundbreaking studies, particularly within the scope of fetal alcohol syndrome, had elucidated that exposure to substances functioning as pharmacological antagonists of the NMDA-type glutamate receptor (NMDAR) or as positive allosteric modulators of the type A GABA receptor (GABAAR)-mediated neurotransmission, precipitated rapid cellular demise during specific developmental periods in the immature rodent brain, contingent on the brain region (Ikonomidou, C. et al., 1999; Ikonomidou, C. et al., 2000). Preliminary investigations had uncovered GA prompt region-specific temporal alterations in the expression of brain-derived neurotrophic factor (BDNF), with the subsequent modifications in the activity of downstream signaling pathways posited to trigger the initiation of apoptotic sequences (Yon, J. H., et al., 2005; Popic, J. et al., 2012). In recent years, the potential neurotoxic effects of GA on the developing brain have garnered increased attention. Animal studies suggest that exposure to GA agents in utero can induce apoptosis and developmental neural degeneration in the fetal brain (Palanisamy, 2012). Notably, the most critical period for anesthetic-induced neurodegeneration is approximated to be around the 26th week of gestation in humans (Clancy et al., 2007). Given the heightened susceptibility of VPIs to maternally administered drugs, due to immature enzyme systems, an incomplete blood-brain barrier, and limited serum protein availability for drug binding, it is crucial to assess both early neonatal outcomes and the long-term effects of anesthetic exposure. Various anesthetic agents can have a broad spectrum of impacts on newborns. Consequently, our study also examined the well-defined adverse outcomes associated with long-term survival and quality of life in VPIs, including SNI, BPD, NEC, and ROP. IVH is a prevalent type of brain injury in preterm infants, often leading to complications such as PVL (Ballabh, 2010; Su et al., 2016). Studies indicate an increased incidence of severe IVH with decreasing gestational age and birth weight (Zhao et al., 2022). Additionally, low Apgar scores are well-established risk factors for IVH (Coskun et al., 2017; Zea-Vera et al., 2019). This could explain the higher prevalence of SNI observed in VPIs from the GA group compared to those from the NA group in our study.
Few studies have explored the association between the interval from anesthesia induction to birth and perinatal outcomes during GA, yielding mixed results. Kate Swanson’s research indicated that a longer interval time (exceeding 4 min) from the initiation of general endotracheal anesthesia to cesarean delivery correlates with a higher frequency of perinatal complications. In bivariable analysis, a 5-min Apgar score of less than 7 was more prevalent in groups with an interval time exceeding 4 min. However, this association did not remain significant in multivariable analysis (Swanson et al., 2021). Hu L and colleagues reported that extending the interval from anesthesia induction to delivery, within certain limits, does not significantly effect fetal outcomes in full-term cesarean deliveries (Hu et al., 2017). However, these studies focused solely on full-term deliveries. In contrast, our current study observed that a longer interval from skin incision to delivery during GA was associated with lower 5-min Apgar scores in preterm deliveries. This finding suggests that, in addition to large molecule drugs like non-depolarizing muscle relaxants, other GA-related drugs may also cross the placental barrier and effect the fetus. Common obstetric general anesthesia agents, such as propofol and ketamine, are associated with increased risks of tachycardia, hypertension, and respiratory adverse events in infants, including cough and laryngospasm (Hayes et al., 2020). Recent studies suggest the feasibility of using opioids at the time of maternal anesthesia induction (Caissie et al., 2022). However, concerns about neonatal respiratory depression risk often leads anesthesiologists to avoid these drugs during induction. Prolonged skin incision-to-delivery intervals under GA may result in increased fetal exposure to anesthetic drugs, potentially elevating the risk of fetal respiratory depression. Minimizing exposure duration to GA could therefore offer clinical benefits for VPIs.
This study has several limitations. First, we were unable to evaluate certain factors, notably the interval from the decision to perform a cesarean section to the delivery of the neonate, a critical determinant of neonatal outcomes. This is partly because prolonged delivery times in emergency situations often involve logistical challenges, such as transporting the patient to the operating room (Wong et al., 2017; Temesgen et al., 2020). Second, despite our efforts to adjust for relevant confounders, residual confounding biases may still exist. This limitation stems from the retrospective nature of our study, wherein data were occasionally recorded inconsistently or inaccurately in medical records.
In conclusion, our study suggests that for VPIs delivered via cesarean section, GA is associated with lower Apgar scores, increased need for tracheal intubation resuscitation, and adverse neurological outcomes. Prolonged exposure to GA may contribute to neonatal postnatal depression. While these findings do not negate the utility of GA in cesarean sections for VPIs, they underscore the importance of specific precautions. These include minimizing the duration of fetal delivery and ensuring adequate preparation for neonatal resuscitation when GA is selected for cesarean sections in VPIs.
Data availability statement
The datasets generated and analyzed in the current study are not readily publicly available because the data is collected from Shandong Neonatal Network (SNN), and our relevant research has not been published. Requests to access the datasets could be directed to the corresponding author (YY, YWxpY2UyMDQwMkAxMjYuY29t).
Ethics statement
The studies involving humans were approved by the Ethics Committee of Shandong Provincial Hospital affiliated to Shandong First Medical University and Shandong University. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants' legal guardians/next of kin.
Author contributions
LW: Data curation, Writing–original draft. CL: Formal Analysis, Writing–original draft. XW: Data curation, Software, Writing–review and editing. SZ: Data curation, Software, Writing–review and editing. LZ: Formal Analysis, Writing–review and editing. BW: Conceptualization, Writing–review and editing. YY: Conceptualization, Supervision, Writing–review and editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by Clinical Research Fund of Shandong Medical Association–Qilu Special Project (Project No. YXH2022DZX02001) and Project of Maternal and child Health Hospital of Shandong Province (2021SFF007).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Adorno, M., Maher-Griffiths, C., and Grush Abadie, H. R. (2022). HELLP syndrome. Crit. Care Nurs. Clin. N. Am. 34, 277–288. doi:10.1016/j.cnc.2022.04.009
Afolabi, B. B., and Lesi, F. E. A. (2012). Regional versus general anaesthesia for caesarean section. Cochrane Database Syst. Rev. 17 (10), CD004350.
American Society of Anesthesiologists Committee on Standards and Practice Parameters (2016). Practice guidelines for obstetric anesthesia: an updated report by the American society of anesthesiologists Task Force on obstetric anesthesia and the society for obstetric anesthesia and Perinatology. Anesthesiology 124, 270–300. doi:10.1097/ALN.0000000000000935
Ballabh, P. (2010). Intraventricular hemorrhage in premature infants: mechanism of disease. Pediatr. Res. 67, 1–8. doi:10.1203/PDR.0b013e3181c1b176
Bao, Y., Zhang, T., Li, L., Zhou, C., Liang, M., Zhou, J., et al. (2022). A retrospective analysis of maternal complications and newborn outcomes of general anesthesia for cesarean delivery in a single tertiary hospital in China. BMC Anesthesiol. 22, 208–220. doi:10.1186/s12871-022-01753-y
Bollag, L., Lim, G., Sultan, P., Habib, A. S., Landau, R., Zakowski, M., et al. (2021). Society for obstetric anesthesia and Perinatology: consensus statement and recommendations for enhanced recovery after cesarean. Anesth. Analgesia 132, 1362–1377. doi:10.1213/ANE.0000000000005257
Butwick, A. J., El-Sayed, Y. Y., Blumenfeld, Y. J., Osmundson, S. S., and Weiniger, C. F. (2015). Mode of anaesthesia for preterm caesarean delivery: secondary analysis from the maternal–fetal medicine units network caesarean registry. Br. J. Anaesth. 115, 267–274. doi:10.1093/bja/aev108
Caissie, N., Héroux, J., Lefebvre, M., Lamarche, D., Dubois, M.-C., Rivard, G., et al. (2022). Opioids for Cesarean delivery under general anesthesia and neonatal outcome: a historical cohort study. Can. J. Anesthesia/Journal Can. d'anesthésie 69, 1017–1024. doi:10.1007/s12630-022-02222-3
Casey, B. M., McIntire, D. D., and Leveno, K. J. (2001). The continuing value of the Apgar score for the assessment of newborn infants. N. Engl. J. Med. 344, 467–471. doi:10.1056/NEJM200102153440701
Chattopadhyay, S., Das, A., and Pahari, S. (2014). Fetomaternal outcome in severe preeclamptic women undergoing emergency cesarean section under either general or spinal anesthesia. J. Pregnancy 2014, 325098–325110. doi:10.1155/2014/325098
Chiang, M. F., Quinn, G. E., Fielder, A. R., Ostmo, S. R., Paul Chan, R. V., Berrocal, A., et al. (2021). “International classification of retinopathy of prematurity,” in Ophthalmology, 128, e51–e68. Third Edition.
Clancy, B., Finlay, B. L., Darlington, R. B., and Anand, K. J. S. (2007). Extrapolating brain development from experimental species to humans. NeuroToxicology 28, 931–937. doi:10.1016/j.neuro.2007.01.014
Coskun, Y., Isik, S., Bayram, T., Urgun, K., Sakarya, S., and Akman, I. (2017). A clinical scoring system to predict the development of intraventricular hemorrhage (IVH) in premature infants. Child's Nerv. Syst. 34, 129–136. doi:10.1007/s00381-017-3610-z
del-Rio-Vellosillo, M., and Garcia-Medina, J. J. (2015). Anesthetic considerations in HELLP syndrome. Acta Anaesthesiol. Scand. 60, 144–157. doi:10.1111/aas.12639
Dong, X.-y., Zhang, W.-w., Han, J.-m., Bi, D., Yang, Z.-y., Wang, X.-l., et al. (2023). Determining resuscitation threshold for extremely preterm infants based on the survival rates without severe neurological injury. J. Glob. Health 13, 04059–04071. doi:10.7189/jogh.13.04059
Egesa, W. I., Odoch, S., Odong, R. J., Nakalema, G., Asiimwe, D., Ekuk, E., et al. (2021). Germinal matrix-intraventricular hemorrhage: a tale of preterm infants. Int. J. Pediatr. 2021, 6622598–6622614. doi:10.1155/2021/6622598
Guglielminotti, J., Landau, R., and Li, G. (2019). Adverse events and factors associated with potentially avoidable use of general anesthesia in cesarean deliveries. Anesthesiology 130, 912–922. doi:10.1097/ALN.0000000000002629
Hayes, J. A., Aljuhani, T., De Oliveira, K., and Johnston, B. C. (2020). Safety and efficacy of the combination of propofol and ketamine for procedural sedation/anesthesia in the pediatric population: a systematic review and meta-analysis. Anesth. Analgesia 132, 979–992. doi:10.1213/ANE.0000000000004967
Holzer, I., Lehner, R., Ristl, R., Husslein, P. W., Berger, A., and Farr, A. (2016). Effect of delivery mode on neonatal outcome among preterm infants: an observational study. Wien. Klin. Wochenschr. 129, 612–617. doi:10.1007/s00508-016-1150-2
Hu, L., Pan, J., Zhang, S., Yu, J., He, K., Shu, S., et al. (2017). Propofol in combination with remifentanil for cesarean section: placental transfer and effect on mothers and newborns at different induction to delivery intervals. Taiwan. J. Obstetrics Gynecol. 56, 521–526. doi:10.1016/j.tjog.2016.09.010
Ikeda, T., Kato, A., Bougaki, M., Araki, Y., Ohata, T., Kawashima, S., et al. (2020). A retrospective review of 10-year trends in general anesthesia for cesarean delivery at a university hospital: the impact of a newly launched team on obstetric anesthesia practice. BMC Health Serv. Res. 20, 421–432. doi:10.1186/s12913-020-05314-2
Ikonomidou, C., Bittigau, P., Ishimaru, M. J., Wozniak, D. F., Koch, C., Genz, K., et al. (2000). Ethanol-induced apoptotic neurodegeneration and fetal alcohol syndrome. Science 287, 1056–1060. doi:10.1126/science.287.5455.1056
Ikonomidou, C., Bosch, F., Miksa, M., Bittigau, P., Vöckler, J., Dikranian, K., et al. (1999). Blockade of NMDA receptors and apoptotic neurodegeneration in the developing brain. Science 283, 70–74. doi:10.1126/science.283.5398.70
Karayel Eroglu, H., Gulasi, S., Mert, M. K., and Cekinmez, E. K. (2022). Relationship between the mode of delivery, morbidity and mortality in preterm infants. J. Trop. Pediatr. 68, fmac074–6. doi:10.1093/tropej/fmac074
Kim, J. H. (2014). Necrotizing enterocolitis: the road to zero. Seminars Fetal Neonatal Med. 19, 39–44. doi:10.1016/j.siny.2013.10.001
Laudenbach, V., Mercier, F. J., Rozé, J. C., Larroque, B., Ancel, P. Y., Kaminski, M., et al. (2009). Anaesthesia mode for caesarean section and mortality in very preterm infants: an epidemiologic study in the EPIPAGE cohort. Int. J. Obstetric Anesth. 18, 142–149. doi:10.1016/j.ijoa.2008.11.005
Liu, C.-M., Cheng, P.-J., and Chang, S.-D. (2008). Maternal complications and perinatal outcomes associated with gestational hypertension and severe Preeclampsia in Taiwanese women. J. Formos. Med. Assoc. 107, 129–138. doi:10.1016/S0929-6646(08)60126-6
Mushambi, M. C., Kinsella, S. M., Popat, M., Swales, H., Ramaswamy, K. K., Winton, A. L., et al. (2015). Obstetric Anaesthetists' Association and Difficult Airway Society guidelines for the management of difficult and failed tracheal intubation in obstetrics. Anaesthesia 70, 1286–1306. doi:10.1111/anae.13260
Nankali, A., Malek-khosravi, S., Zangeneh, M., Rezaei, M., Hemati, Z., and Kohzadi, M. (2012). Maternal complications associated with severe Preeclampsia. J. Obstetrics Gynecol. India 63, 112–115. doi:10.1007/s13224-012-0283-0
Nwafor, M. I., Aniebue, U. U., Nwankwo, T. O., Onyeka, T. C., and Okafor, V. U. (2014). Perinatal outcome of preterm cesarean section in a resource-limited centre: a comparison between general anaesthesia and subarachnoid block. Niger. J. Clin. Pract. 17, 613–618. doi:10.4103/1119-3077.141428
Palanisamy, A. (2012). Maternal anesthesia and fetal neurodevelopment. Int. J. Obstetric Anesth. 21, 152–162. doi:10.1016/j.ijoa.2012.01.005
Palanisamy, A., Mitani, A. A., and Tsen, L. C. (2011). General anesthesia for cesarean delivery at a tertiary care hospital from 2000 to 2005: a retrospective analysis and 10-year update. Int. J. Obstetric Anesth. 20, 10–16. doi:10.1016/j.ijoa.2010.07.002
Popic, J., Pesic, V., Milanovic, D., Todorovic, S., Kanazir, S., Jevtovic-Todorovic, V., et al. (2012). Propofol-induced changes in neurotrophic signaling in the developing nervous system in vivo. PLoS ONE 7, e34396. doi:10.1371/journal.pone.0034396
Richardson, D. K., Corcoran, J. D., Escobar, G. J., and Lee, S. K. (2001). SNAP-II and SNAPPE-II: simplified newborn illness severity and mortality risk scores. J. Pediatr. 138, 92–100. doi:10.1067/mpd.2001.109608
Rolbin, S. H., Cohen, M. M., Levinton, C. M., Kelly, E.Nn., and Farine, D. (1994). The premature infant: anesthesia for cesarean delivery. Anesth. Analg. 78, 912–917. doi:10.1213/00000539-199405000-00013
Saygı, A. İ., Özdamar, Ö., Gün, İ., Emirkadı, H., Müngen, E., and Akpak, Y. K. (2015). Comparison of maternal and fetal outcomes among patients undergoing cesarean section under general and spinal anesthesia: a randomized clinical trial. Sao Paulo Med. J. 133, 227–234. doi:10.1590/1516-3180.2014.8901012
Shi, X., Xu, C., Wen, Y., Jiang, M., Yu, H., Wang, X., et al. (2024). Perinatal outcome of emergency cesarean section under neuraxial anesthesia versus general anesthesia: a seven-year retrospective analysis. BMC Anesthesiol. 24, 33–42. doi:10.1186/s12871-024-02412-0
Strueby, L., and Thébaud, B. (2018). Novel therapeutics for bronchopulmonary dysplasia. Curr. Opin. Pediatr. 30, 378–383. doi:10.1097/MOP.0000000000000613
Su, B.-H., Lin, H.-Y., Huang, F.-K., Tsai, M.-L., and Huang, Y.-T. (2016). Circulatory management focusing on preventing intraventricular hemorrhage and pulmonary hemorrhage in preterm infants. Pediatr. Neonatol. 57, 453–462. doi:10.1016/j.pedneo.2016.01.001
Swanson, K., Liang, L., Grobman, W. A., Higgins, N., Roy, A., and Son, M. (2021). Duration of exposure to general endotracheal anesthesia during cesarean deliveries at term and perinatal complications. Am. J. Perinatology 39, 232–237. doi:10.1055/s-0041-1739355
Temesgen, M. M., Gebregzi, A. H., Kasahun, H. G., Ahmed, S. A., and Woldegerima, Y. B. (2020). Evaluation of decision to delivery time interval and its effect on feto-maternal outcomes and associated factors in category-1 emergency caesarean section deliveries: prospective cohort study. BMC Pregnancy Childbirth 20, 164–174. doi:10.1186/s12884-020-2828-z
Vutskits, L., and Xie, Z. (2016). Lasting impact of general anaesthesia on the brain: mechanisms and relevance. Nat. Rev. Neurosci. 17 (11), 705–717. doi:10.1038/nrn.2016.128
Wong, T. C., Lau, C. Q., Tan, E. L., and Kanagalingam, D. (2017). Decision-to-delivery intervals and total duration of surgery for Caesarean sections in a tertiary general hospital. Singap. Med. J. 58, 332–337. doi:10.11622/smedj.2016098
Yon, J. H., Daniel-Johnson, J., Carter, L. B., and Jevtovic-Todorovic, V. (2005). Anesthesia induces neuronal cell death inthe developing rat brain via the intrinsic and extrinsic apoptotic pathways. Neuroscience 135, 815–827. doi:10.1016/j.neuroscience.2005.03.064
Zea-Vera, A., Turin, C. G., Rueda, M. S., Guillén-Pinto, D., Medina-Alva, P., Tori, A., et al. (2019). Intraventricular hemorrhage and periventricular leukomalacia in low birth-weight neonates in three hospitals in Lima, Peru. Rev. Peru. Med. Exp. Salud Pública 36, 448–453. doi:10.17843/rpmesp.2019.363.3922
Keywords: cesarean section, very preterm infants, general anesthesia, neuraxial anesthesia, neonatal outcomes
Citation: Wang L, Liu C, Wang X, Zhu S, Zhang L, Wang B and Yu Y (2024) The impact of general anesthesia on the outcomes of preterm infants with gestational age less than 32 weeks delivered via cesarean section. Front. Pharmacol. 15:1360691. doi: 10.3389/fphar.2024.1360691
Received: 23 December 2023; Accepted: 07 March 2024;
Published: 20 March 2024.
Edited by:
Wei Zhao, Shandong University, ChinaReviewed by:
Jessian Munoz, Texas Children’s Hospital, United StatesEnfu Tao, Wenling Maternal and Child Health Care Hospital, China
Copyright © 2024 Wang, Liu, Wang, Zhu, Zhang, Wang and Yu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yonghui Yu, YWxpY2UyMDQwMkAxMjYuY29t; Bo Wang, d2FuZ2IxNjg4QHNpbmEuY29t
†These authors have contributed equally to this work
 Lijun Wang
Lijun Wang