
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pharmacol. , 01 May 2024
Sec. Pharmacogenetics and Pharmacogenomics
Volume 15 - 2024 | https://doi.org/10.3389/fphar.2024.1360352
This article is part of the Research Topic State-of-the-art hypothesis-driven systems pharmacology and artificial intelligence approaches to decipher disease complexity View all 5 articles
Background: Prostate cancer is a leading cause of cancer-related deaths among men, marked by heterogeneous clinical and molecular characteristics. The complexity of the molecular landscape necessitates tools for identifying multi-gene co-alteration patterns that are associated with aggressive disease. The identification of such gene sets will allow for deeper characterization of the processes underlying prostate cancer progression and potentially lead to novel strategies for treatment.
Methods: We developed ProstaMine to systematically identify co-alterations associated with aggressiveness in prostate cancer molecular subtypes defined by high-fidelity alterations in primary prostate cancer. ProstaMine integrates genomic, transcriptomic, and clinical data from five primary and one metastatic prostate cancer cohorts to prioritize co-alterations enriched in metastatic disease and associated with disease progression.
Results: Integrated analysis of primary tumors defined a set of 17 prostate cancer alterations associated with aggressive characteristics. We applied ProstaMine to NKX3-1-loss and RB1-loss tumors and identified subtype-specific co-alterations associated with metastasis and biochemical relapse in these molecular subtypes. In NKX3-1-loss prostate cancer, ProstaMine identified novel subtype-specific co-alterations known to regulate prostate cancer signaling pathways including MAPK, NF-kB, p53, PI3K, and Sonic hedgehog. In RB1-loss prostate cancer, ProstaMine identified novel subtype-specific co-alterations involved in p53, STAT6, and MHC class I antigen presentation. Co-alterations impacting autophagy were noted in both molecular subtypes.
Conclusion: ProstaMine is a method to systematically identify novel subtype-specific co-alterations associated with aggressive characteristics in prostate cancer. The results from ProstaMine provide insights into potential subtype-specific mechanisms of prostate cancer progression which can be formed into testable experimental hypotheses. ProstaMine is publicly available at: https://bioinformatics.cuanschutz.edu/prostamine.
Prostate cancer (PCa) is the second most common cancer in men and the fourth most common cancer overall. In terms of mortality, PCa accounts for the fifth-most deadly cancer in men worldwide (Sung et al., 2021). Prostate cancer is a highly heterogeneous disease in both its clinical presentation and molecular features (Boyd et al., 2012). This heterogeneity makes it difficult to anticipate outcomes of newly diagnosed patients, where the vast majority of patients with localized disease will have little impact on overall survival; however, an unknown subset of 10%–15% of patients will progress with aggressive disease. The genomic landscape of PCa has been well-defined, with alterations, such as the TMPRSS2:ERG fusion (Tomlins et al., 2005; Taylor et al., 2010); SPOP mutations (Barbieri et al., 2012; Abeshouse et al., 2015); and the losses of NKX3-1 (Emmert-Buck et al., 1995; Abdulkadir et al., 2002), CHD1 (Augello et al., 2019), MAP3K7 (Wu et al., 2012), and PTEN (Cairns et al., 1997; Wang et al., 2003), playing a role in disease development but none sufficient to drive PCa in men to become metastatic. With the exception of AR amplifications in castration-resistant and metastatic tumors (Abeshouse et al., 2015; Abida et al., 2019), few alterations are enriched or consistently associated with metastatic disease alone.
Recent efforts have focused on understanding how multiple alterations interact to drive aggressive PCa. In genetically engineered mice, co-loss of RB1 and PTEN facilitates lineage plasticity and metastasis, and additional deletion of TP53 promotes therapeutic resistance (Ku et al., 2017; Mu et al., 2017). In tumor xenograft studies, a combined alteration of RB1 and TP53 drives increased tumor growth, stem-like features, and therapeutic resistance to multiple antiandrogens (Nyquist et al., 2020). Co-occurring deletion of MAP3K7 and CHD1 is another example of coordinating interactions; this dual loss drives aggressive phenotypes both in vitro and in vivo, contributes to increased ARv7 expression, and is highly enriched in brain metastases (Rodrigues et al., 2015; Ormond et al., 2019; Jillson et al., 2021). These results indicate that aggressiveness in PCa is driven by the combination of multiple genomic loci, predominantly the loss of multiple tumor suppressors. These findings also suggest that the specific combination of alterations is important for disease development. There is a need to identify the genetic interactions that contribute to the progression of primary prostate cancer into metastatic disease in a molecular subtype-specific manner.
To systematically address this need, we developed a tool, ProstaMine, that integrates molecular and clinical data from multiple, independent PCa cohorts to identify co-alterations associated with molecular subtypes defined in primary disease that are enriched in metastasis and promote biochemical relapse. We leverage five primary PCa cohorts to identify putative molecular features, which we then use to find enriched co-alterations in a cohort of metastatic disease. We demonstrate our approach in PCa defined by the loss of NKX3-1 or RB1 and make ProstaMine publicly accessible to evaluate user-defined subtypes through a user-friendly R Shiny application (https://bioinformatics.cuanschutz.edu/prostamine).
Genomic, transcriptomic, and clinical data analyzed in this study were from primary and metastatic tumors profiled in the literature (Taylor et al., 2010; Barbieri et al., 2012; Baca et al., 2013; Hieronymus et al., 2014; Abeshouse et al., 2015; Abida et al., 2019). We selected these studies based on the availability of copy number alteration (CNA), gene expression, and clinicopathologic data (Supplementary Figure S1). The curatedPCaData R package (v.0.99.4), which provides harmonized data and common, updated gene annotation across 19 independent PCa cohorts, was used to access all genomic, transcriptomic, and clinicopathologic data. CuratedPCaData allowed us to perform consistent and robust downstream analysis with details on methods and curation available in Laajala et al. (2023). Copy number data comprise discretized GISTIC2 calls (Mermel et al., 2011). Gene expression data are normalized counts transformed into z-scores relative to other tumor samples from the same study. Tumor grade data are based on the Gleason scoring system, and tumor stage data are based on the TNM staging system.
Copy number alteration gains (Gains) were defined as genes with a GISTIC value >0. Copy number alteration losses (Losses) were defined as genes with a GISTIC value <0 and/or genes with single-base substitutions having a predicted damaging effect on protein function, as computed by SIFT or PolyPhen-2 (Ng and Henikoff, 2003; Adzhubei et al., 2010). Gene alteration frequency was computed as the ratio of tumors with the alteration of the gene to the total number of tumors. The ratio of tumors covered was determined for each set of genes captured by alteration frequency cut-offs between 0 and 1. At each cut-off, coverage was calculated by the number of tumors with the alteration of at least one gene in the set to the total number of tumors. Alteration hotspots were defined as the contiguous loci containing five or more genes above a 10% alteration frequency cut-off. Visualization of the alteration landscape and alteration hotspots was done using the karyoploteR R package (v.1.22.0).
A total of 17 alteration hotspot regions were selected based on the criteria from Section 2.2. Through manual inspection, we noted the alteration hotspots harbored genes with known involvement in prostate cancer or cancers of other tissues and that these genes were located either directly at or near the peak of the alteration hotspot. We selected a single cancer-associated gene to represent each of the PCa17 hotspots and then consensus clustering spanning k = 2 to k = 6 to identify the four subgroups (A-high, CG-1, CG-2, and A-low; Supplementary Figure S2). Between k = 4 and k = 5, we noted a marginal change in the area under the CDF curve. Cluster memberships at k = 4 indicated four stable clusters each holding a robust number of patients (Supplementary Figure S2). We selected k = 4 as the distinct number of molecularly defined primary tumor subgroups. Clustering analysis was performed using the ConsensusClusterPlus R package (v.1.64.0), and the alteration heatmap was generated using the ComplexHeatmap R package (v.2.12.0).
Mutual exclusivity was calculated using Fisher’s exact test for all pairwise combinations of CHD1, MAP3K7, LRP1B, ERG, SHQ1, TP53, HDAC5, and PTEN alterations. The ComplexHeatmap R package (v.2.12.0) was used to visualize mutual exclusivity analysis across all primary tumors.
The Kaplan–Meier analysis was performed using progression-free survival data from TCGA and Taylor studies (Taylor et al., 2010; Abeshouse et al., 2015), as reported in the curatedPCaData R package. The survival R package (v.3.5–7) was used to fit Cox proportional hazards models and compute statistics for progression-free survival times between groups using the logrank test. The survminer R package (v0.4.9) was used to visualize survival curves.
ProstaMine first subsets the alteration data into two groups: patient tumors that are wild-type, or diploid, for the selected alteration (WT tumors) and patient tumors harboring the selected alteration, or the selected subtype (ST tumors). This results in four tumor groups for downstream analysis including the following: WT primary tumors, ST primary tumors, WT metastatic tumors, and ST metastatic tumors. After defining groups, the algorithm proceeds in three sequential steps: 1) genomic analysis, 2) transcriptomic analysis, and 3) clinical analysis.
For genomic analysis, primary and metastatic alteration data were used to calculate alteration frequencies for Gains and Losses. Fisher’s exact test was used to compute the statistical enrichment of alterations between the four tumor groups. Alterations present at a frequency of 2% or greater in ST primary tumors compared to WT primary tumors were captured and defined as primary tumor subtype co-alterations. Primary tumor subtype co-alterations that were present at a frequency of 2% or greater in ST metastatic tumors compared to ST primary tumors were selected for further analysis. Hits from the genomic analysis can be filtered above the 2% baseline by adjusting the primary co-alteration frequency difference and metastatic co-alteration frequency difference filtering parameters in the ProstaMine application.
For transcriptomic analysis, differential gene expression was computed by comparing the mean expression of primary ST tumors with and without the alteration and metastatic ST tumors with and without the alteration. Gains with a negative fold change in gene expression and Losses with a positive fold change in gene expression in primary and metastatic tumors were removed from the analysis. Statistical significance for the difference in gene expression was computed using a Student’s t-test. Hits from the transcriptomic analysis can be filtered by adjusting the concordant DGE FDR filtering parameter in the ProstaMine application.
For clinical data analysis, ProstaMine computed the association of each alteration’s gene expression with the Gleason grade group and progression-free survival. For each alteration, ST and WT primary tumors were median-stratified by gene expression into upper and lower groups. A Fisher’s exact test was used to compute the enrichment of the Gleason grade group ≥ 8 in the upper versus lower groups. The logrank test was used to compute statistical differences in progression-free survival times between these same groups. Alterations lacking concordant Gleason grade group enrichment and progression-free survival differences were removed. Alterations lacking concordant metastasis and progression-free survival associations were also removed. Alterations with a survival difference of p ≤ 0.2 in ST tumors and p ≥ 0.3 in WT tumors were captured as hits. Hits from the clinical analysis can be filtered below the p = 0.2 baseline by adjusting the survival p-value filtering parameter in the ProstaMine application.
We developed a prioritization scheme for ProstaMine hits. The effect size for each hit in primary co-alteration, metastasis, and progression-free survival was ranked and then normalized by the total number of hits (Eq. 1). The final score of subtype-specific aggressiveness was calculated by weighting the normalized rank for the co-alteration frequency difference in ST primary tumors, co-alteration frequency difference in ST metastatic tumors, and association with progression-free survival (Eq. 2):
For NKX3-1-loss and RB1-loss prostate cancer, we set ProstaMine filtering parameters as follows: primary and metastatic Co–alteration rate difference = 0.05, primary and metastatic co-alteration FDR = 0.05, primary and metastatic DGE FDR = 0.2, and survival p-val = 0.05. For enrichment analysis in Metascape, we used the default settings for the Metascape’s Express Analysis option (Zhou et al., 2019).
All genomic, transcriptomic, and clinicopathologic data used in this study are accessible through the curatedPCaData R package (Laajala et al., 2023). The corresponding code generated for performing all of the analyses in this study, creating ProstaMine and the Shiny application, is available at: github. com/MikeOrman/ProstaMine-Publication.git. ProstaMine is made available at: https://bioinformatics.cuanschutz.edu/prostamine.
We first verified that the molecular data published across independent molecular profiling studies consistently identified bona fide PCa gene alterations using the Taylor et al. (2010), Baca et al. (2013), Barbieri et al. (2012), Hieronymus et al. (2014), and TCGA cohorts (Supplementary Figure S1) (Taylor et al., 2010; Barbieri et al., 2012; Baca et al., 2013; Hieronymus et al., 2014; Abeshouse et al., 2015). Alteration data from these five profiling studies were harmonized into a singular alteration matrix containing 15,869 genes across 921 primary PCa tumors. We calculated the somatic alteration frequency for each gene to define the PCa alteration landscape (Figure 1A).
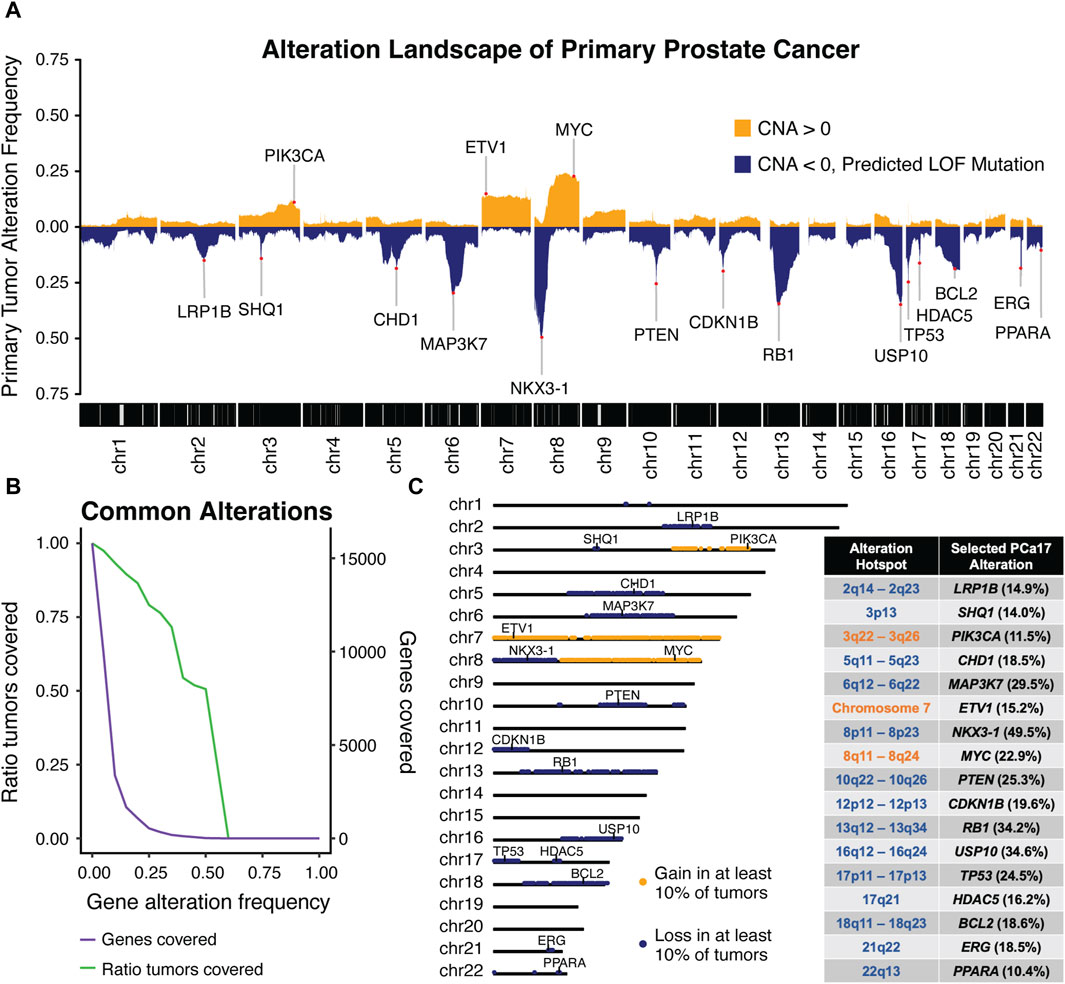
Figure 1. Integrated analysis of five independent molecular profiling studies in primary prostate was performed to generate (A) a combined alteration landscape of primary prostate cancer tumors and determine (B) primary tumor coverage over a range of alteration frequency cut-offs. An alteration frequency cut-off of 10% captured (C) 17 characteristic regions of alteration with each region harboring a canonical cancer-associated gene used to define the prostate cancer-17, or PCa17.
The peaks observed in the genomic landscape span distinct genomic intervals (Figure 1A). To systematically identify these alteration hotspots, we computed the ratio of tumors covered by alterations meeting a given alteration frequency cut-off (Figure 1B). We observed a sharp decline in the number of genes captured at an alteration frequency cut-off of 10% and found that 93% of tumors were included at this cut-off. Using this threshold, we defined 17 alteration hotspots corresponding with the peaks depicted in the Figure 1A alteration landscape. Alteration hotspots captured 3,373 genes covering the following 17 chromosomal locations: 2q14-2q23, 3p13, 3q22-3q26, 5q11-5q23, 6q12-6q22, 7, 8p11-8p23, 8q11-8q24, 10q22-10q26, 12p12-12p13, 13q12-13q34, 16q12-16q24, 17p11-17p13, 17q21, 18q11-18q23, 21q22, and 22q13 (Figure 1C). These alteration hotspots were in agreement with previously reported regions of loss and gain measured in PCa tumors (Carter et al., 1990; Gao et al., 1995; Latil et al., 1997; Kibel et al., 1998; Ozen et al., 1998; Erbersdobler et al., 1999; Alers et al., 2000; Sattler et al., 2000; Dai et al., 2001; Verhagen et al., 2002; van Dekken et al., 2003; Paris et al., 2004; Ueda et al., 2005; Perner et al., 2006; Saramäki et al., 2006; Camp et al., 2007; Liu et al., 2007; Scheble et al., 2010; Kluth et al., 2018; 2015; Hieronymus et al., 2017). We found that each alteration hotspot harbored at least one cancer-associated gene that was present either directly at or very near the peak alteration frequency of the hotspot. We reasoned that grouping primary PCa tumors by these high-fidelity, cancer-associated alterations could be a powerful approach to stratify primary tumor aggressiveness in PCa. Thus, we selected a single cancer-associated gene to define each of the 17 alteration hotspots; we named this set of genes prostate cancer-17, or PCa17 (Figure 1C).
We used the PCa17 alteration profiles and consensus clustering to define four distinct tumor subgroups (Figure 2A). One subgroup had a reduced number of overall alterations (A-Low, blue). Conversely, a second subgroup was characterized by a high number of alterations (A-High, orange) (Figure 2A). Certain alterations were enriched in one of the two remaining groups, co-alteration group 1 (CG-1, red) and co-alteration group 2 (CG-2, green) (Figure 2A). Alterations in ERG, PTEN, SHQ1, TP53, and HDAC5 were depleted in CG-1 tumors at a frequency of 4%–13% and enriched in CG-2 tumors at a frequency of 48%–51%. Alterations in MAP3K7, CHD1, and LRP1B were enriched in CG-1 tumors at a frequency of 54%–63% and depleted in CG-2 tumors at a frequency of 9%–11% (Figure 2A). Given the patterns of enrichment and depletion observed in subgroups CG-1 and CG-2, we also tested for the co-alteration of MAP3K7/CHD1/LRP1B and ERG/PTEN/SHQ1/TP53/HDAC5 across all primary tumors. We found that MAP3K7, CHD1, and LRP1B alterations formed a set of significantly co-occurring alterations (Figure 2B), while ERG, TP53, HDAC5, PTEN, and SHQ1 alterations constituted a second set of significantly co-occurring alterations (Figure 2B). NKX3-1 and RB1 were the most commonly altered genes in A-Low tumors at a 12% frequency. In CG-1, CG-2, and A-high tumors, the NKX3-1 alteration frequency increased to 61%–84%, and the RB1 alteration frequency increased to 36%–75% (Figure 2A).
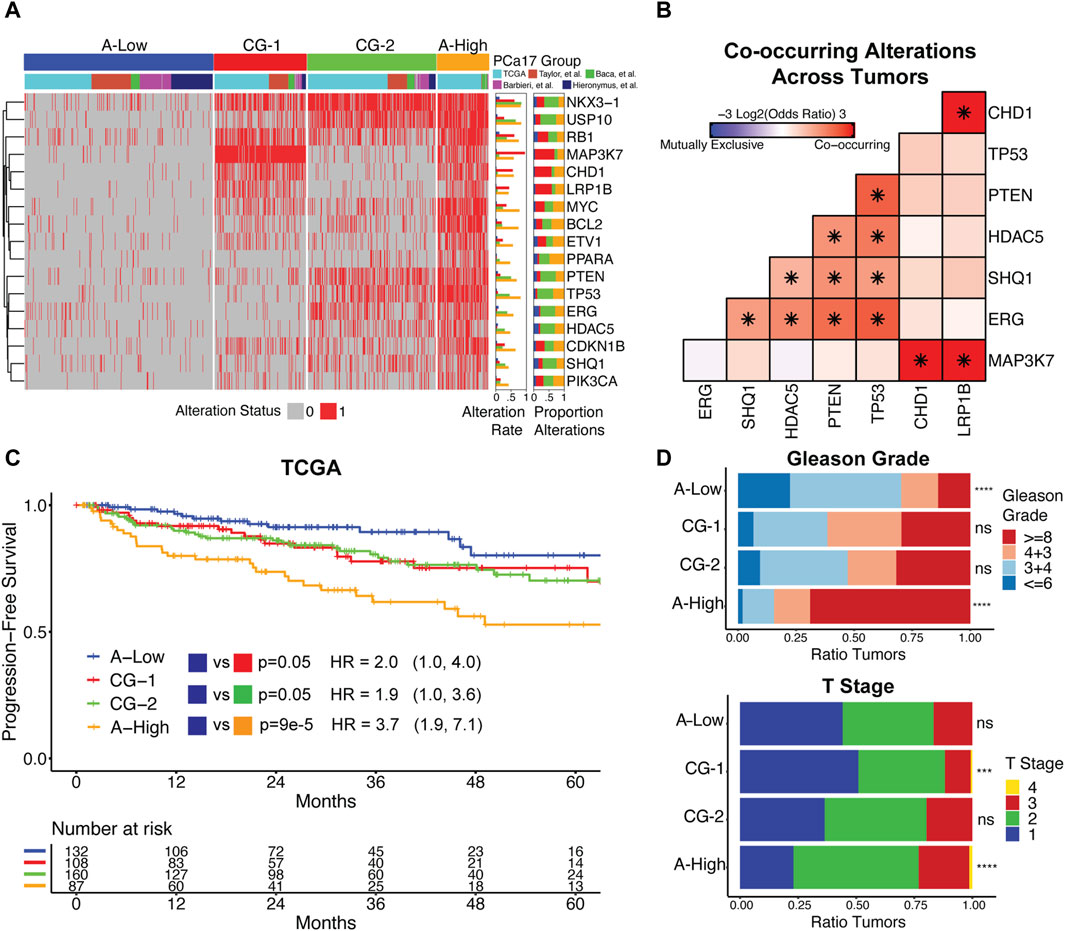
Figure 2. (A) Clustering primary prostate cancer tumors by PCa17 alterations defines four tumor subgroups with low overall alteration (A-Low), high overall alteration (A-High), and two distinct patterns of co-alteration (CG-1 and CG-2). (B) Mutual exclusivity analysis of TP53, PTEN, ERG, HDAC5, SHQ1, MAP3K7, CHD1, and LRP1B alterations across all primary tumors using Fisher’s exact test (*FDR<0.05). (C) Progression-free survival and proportional hazard ratios with 95% confidence interval comparing the tumor subgroups (A-High, CG-1, and CG-2) to A-Low in TCGA dataset. The logrank test was used to test for differences in progression-free survival times between groups. (D) Tumor grade and stage data for the four tumor subgroups. Fisher’s exact test was used to test for the enrichment of Gleason grade ≥8 and T2+ tumors for each tumor subgroup versus all other tumors (***FDR<0.001, ****FDR<0.0001).
We analyzed associations between tumor subgroups and clinicopathologic features used in PCa staging. Survival analysis in TCGA dataset indicated that A-High tumors were the most likely to experience biochemical relapse over a 5-year period, followed by CG-1 and CG-2 tumors relative to A-Low tumors (Figure 2C). In the Taylor et al. dataset, we also found that CG-1 and CG-2 tumors were more likely to experience biochemical relapse over a 5-year period relative to A-Low tumors, although at modest significance (Supplementary Figure S3). Analysis of tumor grade and stage revealed that A-Low tumors were depleted of high-grade tumors (Gleason grade group ≥8), while A-High tumors were enriched with high-grade and T2+ tumors. CG-1 and CG-2 tumors displayed intermediate levels of tumor grade (Figure 2D). Clinical data on lymphatic spread (N1) showed enrichment in A-High tumors (Supplementary Figure S4). We also assessed the genomic instability of each group by the total number of CNAs. A-Low tumors had the least amount of CNAs relative to the other subgroups, while A-High tumors harbored the most CNAs (Supplementary Figure S4). Overall, these results show that grouping primary prostate cancer tumors by PCa17 alterations can stratify them into groups of less aggressive (A-Low) to intermediate (CG-1 and CG-2) to more aggressive characteristics (A-High).
Analysis of primary tumors confirmed many high-abundance molecularly defined subtypes in PCa. Considering the high abundance of these subtypes in primary PCa tumors, our goal was to define additional co-occurring alterations that may cooperate with these common single-genomic alterations to promote aggressive disease. We developed ProstaMine to be a publicly accessible bioinformatics tool that prioritizes subtype-specific co-alterations associated with metastasis and biochemical relapse in PCa. ProstaMine integrates tumor data on somatic CNAs, single-base substitutions, gene expression, biochemical relapse, and Gleason grade group across six independent PCa cohorts. ProstaMine leverages these data to identify co-alterations associated with aggressive tumors from molecularly defined Loss and Gain subtypes (Figure 3).
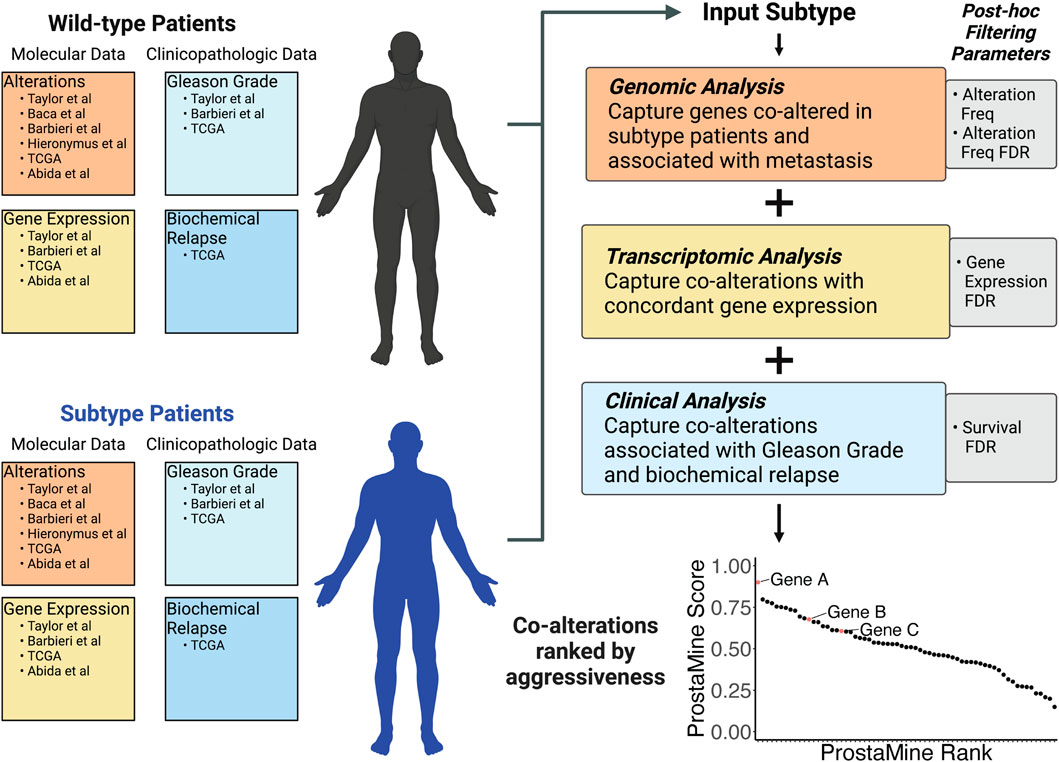
Figure 3. ProstaMine is a bioinformatics tool that integrates molecular and clinical data across six independent molecular profiling studies to determine a ranked list of subtype-specific co-alterations associated with aggressive features in prostate cancer.
ProstaMine has the capability to mine any user-selected molecular subtype. Molecular alterations occurring early in tumor evolution are particularly good for ProstaMine because these subtypes are likely to contain many co-alterations that push the subtype into an aggressive state over the course of tumor development and progression. Recent work has shown that NKX3-1 and RB1 copy number losses are among the first alterations to occur in the evolution of prostate cancer (Espiritu et al., 2018). We reasoned that there are co-alterations that coordinate with NKX3-1 and RB1 losses to drive tumor progression, and we can use ProstaMine to find these co-alterations.
In NKX3-1-loss tumors, ProstaMine identified 73 Loss co-alterations distributed across 16 chromosomal locations: 1p13-1p34, 2q37, 4p15-4p16, 5q12-5q23, 6q13-6q27, 9p24, 10q11-10q26, 11q22, 12p11-12p13, 13q12-13q33, 15q21-15q25, 16q13-16q24, 17p13, 18q12-18q23, 20p11, and 22q11-22q12 (Figure 4A). The third ranked single gene hit identified by ProstaMine was GSTO2, followed by SMAD4 and MT1M, which were ranked sixth and eighth, respectively. GSTO2 and MT1M have been described as tumor suppressors in cancer, and SMAD4 is listed as a tumor suppressor gene in the COSMIC Cancer Gene Census (Mao et al., 2012; Sondka et al., 2018; Terayama et al., 2020; Xu et al., 2020; Li et al., 2021, 2023; Sumiya et al., 2022). GSTO2 and MT1M functions have not been reported as factors in PCa progression, whereas loss of SMAD4 function has been shown to drive tumor growth and metastasis (Ding et al., 2011). Losses of GSTO2, SMAD4, and MT1M were significantly enriched in NKX3-1-loss primary tumors at a frequency of 10%–19% above primary WT tumors (Figure 4B). These alterations were enriched in metastatic NKX3-1-loss tumors at a frequency of 18%–29% above primary NKX3-1-loss tumors (Figure 4B). GSTO2, SMAD4, and MT1M co-alterations also had concordant and significantly reduced gene expression in primary and metastatic NKX3-1-loss tumors (Supplementary Figure S5B). Reduced gene expression of GSTO2, SMAD4, and MT1M was significantly associated with shorter time to biochemical relapse in NKX3-1-loss tumors but not in NKX3-1-WT tumors (Figure 4C). Reduced gene expression of GSTO2, SMAD4, and MT1M was also significantly associated with high-grade tumor histology (Gleason grade group ≥ 8) in NKX3-1-loss tumors (Supplementary Figure S5C).
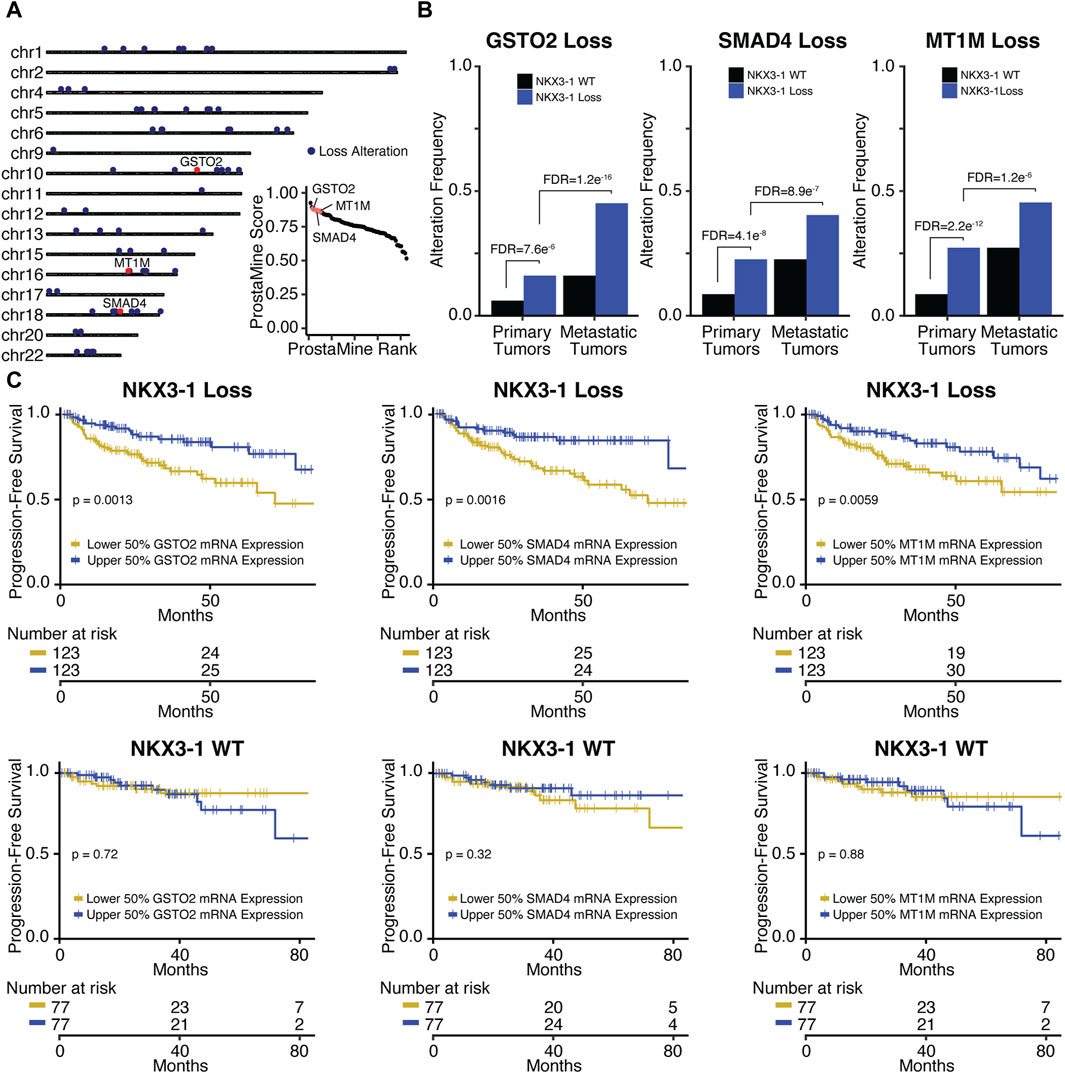
Figure 4. Application of ProstaMine to NKX3-1-loss prostate cancer (A) identified 73 loss co-alterations associated with aggressiveness with GSTO2, SMAD4, and MT1M loss alterations being top hits. Association of GSTO2, SMAD4, and MT1M hits with (B) NKX3-1-loss and metastasis and (C) progression-free survival. Fisher’s exact test was used to test for differences in alteration enrichment in (B) and the logrank test was used to test for differences in progression-free survival times in (C).
GSTO2 encodes a glutathione transferase involved in cellular detoxification. GSTO2 functions as a tumor suppressor through p38-mediated MAPK signaling in esophageal and squamous skin cell carcinoma (Terayama et al., 2020; Sumiya et al., 2022). SMAD4 encodes a transcription factor serving as the central regulator of the TGFb-activated and BMP4-activated SMAD signaling pathways. SMAD4 provides a barrier to metastatic progression in PTEN-null mouse prostates, and when deleted, it drives highly aggressive prostate cancer that metastasizes to the lymph node and lung (Ding et al., 2011). MT1M encodes a metallothionein protein that functions as a tumor suppressor by downregulating the NF-kB pathway activity and subsequent proliferation in hepatocellular carcinoma (Mao et al., 2012). In lung carcinoma, MT1M overexpression inhibits cell viability and migration through MDM2/p53 signaling, and in esophageal carcinoma, it inhibits the epithelial–mesenchymal transition (EMT) through the SOD1/PI3K signaling axis (Xu et al., 2020; Li et al., 2021). Taken together, the tumor suppressive mechanisms of GSTO2 and MT1M in these contexts suggest that p38, NF-kB, MDM2/p53, and SOD1/PI3K signaling may be contributing to aggressiveness in NKX3-1-loss tumors.
Pathway analysis of ProstaMine hits for NKX3-1-loss tumors identified several enriched signatures that are related to fatty acid metabolism, metabolism of lipids, and autophagy (Supplementary Figure S5A) (Zhou et al., 2019). In prostate cancer, altered fatty acid metabolism provides additional substrates for growth and signaling molecules that promote cancer cell proliferation, invasion, metastasis, and immune evasion. These processes are mediated by a number of molecular players including AR, PTEN/PI3K/AKT, c-Myc, and AMPK (Sena and Denmeade, 2021). In healthy tissues, autophagy controls the recycling of cellular material to maintain homeostasis; however, in PCa, the role of autophagy is contextual, and it can have both tumor suppressive and promotional effects (Loizzo et al., 2022). Taken together, pathway analysis of ProstaMine hits in NKX3-1-loss tumors suggests that dysregulated fatty acid metabolism and autophagy are important processes contributing to aggressiveness in NKX3-1-loss PCa.
In RB1-loss tumors, ProstaMine identified 42 co-alterations distributed across 14 chromosomal locations: 1p13-1p34, 2p22, 4p16, 5q22, 8p21-8p23, 9p24, 10p13, 10q25-10q26, 11q23-11q24, 15q21-15q25, 16q11-16q24, 18q21, 19q13, and 22q11-22q12 (Figure 5A). The top ranked hit was CHMP1A, followed by B2M and RSU1 which were ranked 11th and 23rd, respectively (Figure 5A). B2M, CHMP1A, and RSU1 functions have not been linked to PCa progression. These genes have all been reported to be tumor suppressors (Li et al., 2009; 2008; Gkretsi et al., 2019; Louca et al., 2019; Wang et al., 2021). CHMP1A, B2M, and RSU1 loss alterations were significantly enriched in RB1-loss primary tumors at a frequency of 7%–18% above RB1-WT primary tumors and were significantly enriched in RB1-loss metastatic tumors at a frequency of 16%–23% above RB1-loss primary tumors (Figure 5B). CHMP1A and RSU1 loss alterations had significantly reduced concordant gene expression in primary and metastatic RB1-loss tumors (Supplementary Figure S6B). B2M loss alterations also showed a concordant reduction in gene expression but at moderate significance (Supplementary Figure S6B). Reduced gene expression of CHMP1A, B2M, and RSU1 was significantly associated with decreased time to biochemical relapse in RB1-loss tumors but not in RB1-WT tumors (Figure 5C). Low B2M gene expression was significantly associated with high-grade tumor histology in RB1-loss tumors (Supplementary Figure S6C). RB1-loss tumors with low CHMP1A and RSU1 gene expression also had more high-risk Gleason scores compared to those with high expression, although not statistically significant (Supplementary Figure S6C).
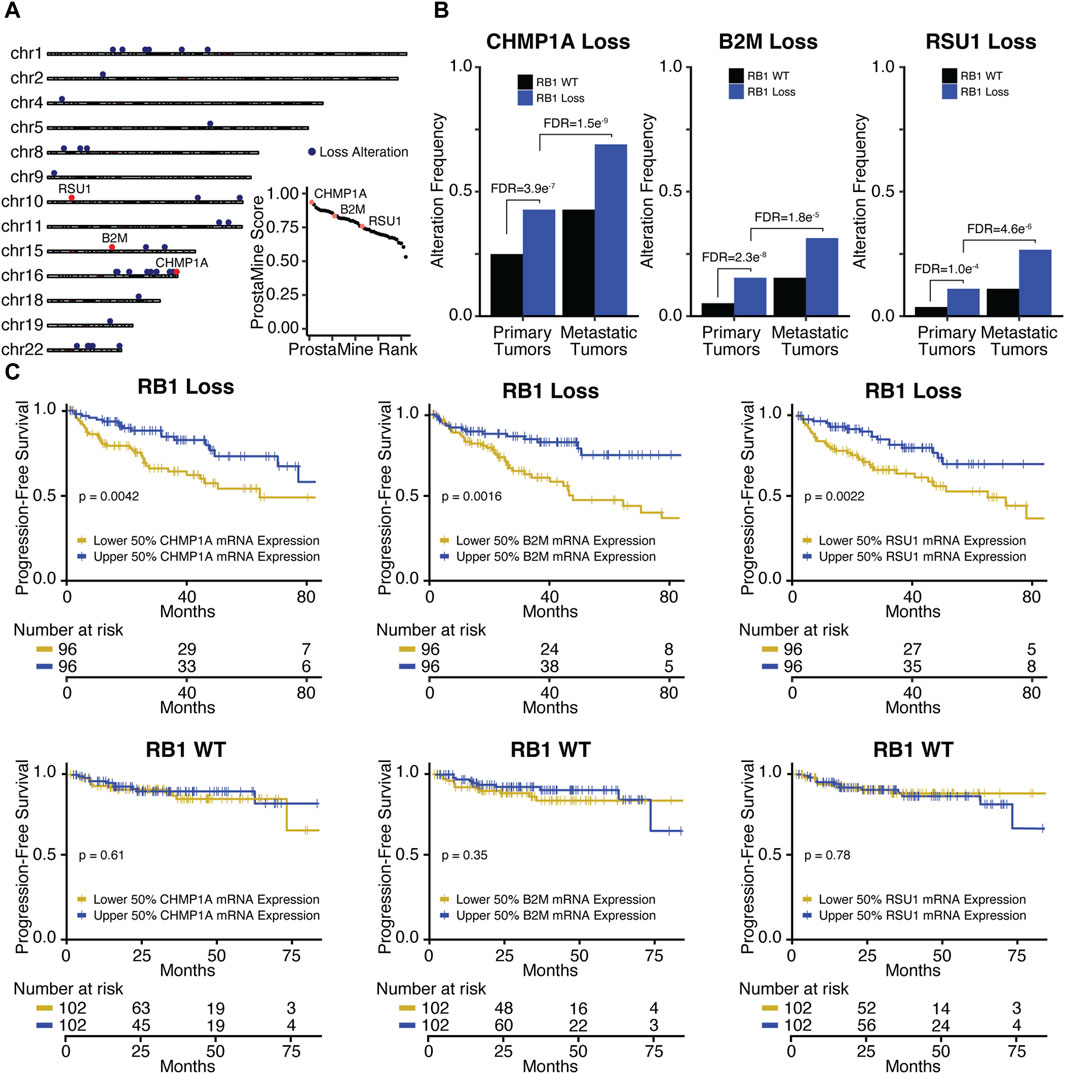
Figure 5. Application of ProstaMine to RB1-loss prostate cancer (A) identified 42 loss co-alterations associated with aggressiveness with CHMP1A, B2M, and RSU1 loss alterations being top hits. Association of CHMP1A, B2M, and RSU1 hits with (B) RB1-loss and metastasis, and (C) progression-free survival. Fisher’s exact test was used to test for differences in alteration enrichment in (B), and the logrank test was used to test for differences in progression-free survival times in (C).
CHMP1A encodes a chromatin remodeling protein that functions as a tumor suppressor gene in pancreatic cancer cells through the activation of p53 and retinoic acid signaling (Li et al., 2008; Li et al., 2009). B2M encodes a component of the MHC class I antigen presentation machinery and functions in immune surveillance. Alteration of B2M is common across different cancer types, and evidence suggests that B2M loss drives poor response to immunotherapy through disruption of MHC class I protein expression at the cell surface (Wang et al., 2021). RSU1 encodes a focal adhesion protein that suppresses v-Ras-dependent oncogenic transformation (Cutler et al., 1992) and has recently been shown that suppression of RSU1 increases cell invasion through increased MMP13 expression and STAT6 phosphorylation (Louca et al., 2019).
We observed an overlap in several ProstaMine hits between RB1-loss and NKX3-1-loss tumors. Most notably, GSTO2 and MT1M alterations were also among the top hits in RB1-loss tumors and pathway analysis of RB1-loss hits via Metascape once again identified autophagy among the significantly enriched pathways. Identification of GSTO2- and MT1M-loss alterations by ProstaMine in NKX3-1-loss and RB1-loss contexts suggests these co-alterations may be general regulators of aggressiveness in PCa. Five hits were found to regulate autophagy in NKX3-1-loss and RB1-loss tumors with three of these five hits overlapping between the two tumor subtypes. These findings provide evidence for dysregulated autophagy as a factor involved in the aggressiveness of RB1-loss PCa and further suggest a role for dysregulated autophagy as a general contributor to PCa aggressiveness.
By combining five independent molecular profiling studies in primary PCa, we systematically identified high-confidence alteration hotspots across the primary PCa genome and found associations with clinicopathologic features related to aggressiveness. The majority of genes present in the PCa17 alteration signature have well-characterized tumor-suppressive or oncogenic roles that drive PCa. Grouping patients by these PCa17 alterations revealed a positive relationship between total alterations and aggressive features in primary PCa and is consistent with prior work, showing that a high copy number alteration burden predicts prostate cancer relapse (Hieronymus et al., 2014). We showed the most commonly altered genes in A-Low tumors are NKX3-1 and RB1 and that their alteration frequencies increase in CG-1, CG-2, and A-High tumors. Thus, the loss of NKX3-1 and RB1 are found across the entire spectrum of disease aggressiveness and is consistent with their role as initiating alterations in the evolution of PCa (Espiritu et al., 2018).
Analysis of molecular and clinical progression in PCa has revealed two distinct evolutionary trajectories including SPOP mutation → CHD1 loss and ERG fusion → PTEN loss (Liu et al., 2021). We confirmed the co-alteration between ERG/PTEN and further captured SHQ1, HDAC5, and TP53 loss alterations as significantly co-altered with ERG and PTEN. These findings suggest SHQ1, HDAC5, and TP53 loss alterations may also be molecular features that help promote progression in primary PCa. Although SPOP was not included in the PCa17 alteration signature due to an alteration frequency below the 10% cutoff, we still found that CHD1- and MAP3K7-loss alterations were significantly co-occurring, as reported previously (Rodrigues et al., 2015). Interestingly, LRP1B loss was tightly associated with CHD1 and MAP3K7 loss, suggesting that LRP1B loss alterations may have functional significance in MAP3K7-loss and CHD1-loss PCa.
The co-alterations identified by ProstaMine may also inform potential therapeutic targeting strategies for specific PCa molecular subtypes. Co-alterations defined by Gains may be directly targetable for genes with corresponding pharmacological inhibitor(s). Alternatively, co-alterations defined by Losses will point toward subtype-specific pathway dysregulation, and the dysregulated pathways can potentially be therapeutically targeted. In NKX3-1-loss and RB1-loss PCa, ProstaMine identified exclusively Loss co-alterations, following filtering. MT1M loss was a top ProstaMine hit in both NKX3-1-loss and RB1-loss prostate cancer and has not been reported as a factor involved in PCa progression. MT1M suppression is known to promote cell growth and stemness properties in gastric cancer cell lines through increased GLI1 expression (Li et al., 2023). Interestingly, SHH-GLI1 pathway components often show enhanced expression in tumor versus normal prostatic epithelia, and suppressing GLI1 expression in primary prostate tumor cell cultures inhibits cell proliferation (Sanchez et al., 2004). Taken together, the ProstaMine results and results from other cancer types suggest that targeted inhibition of GLI1 or the SHH-GLI1 pathway may reduce the aggressiveness of NKX3-1-loss and RB1-loss PCa. Additionally, our pathway analysis of ProstaMine hits showed enrichment of fatty acid metabolism in NKX3-1-loss PCa and enrichment of autophagy in both NKX3-1-loss and RB1-loss PCa. Identification of positive regulators of fatty acid metabolism that are overexpressed in NKX3-1-loss prostate cancer may provide attractive targets for inhibiting the aggressiveness of this subtype. Likewise, identification of positive regulators of autophagy overexpressed in NKX3-1-loss and RB1-loss prostate cancer may be effective targets for slowing the aggressiveness of both subtypes.
The development of ProstaMine was possible through the integration of different data types including CNAs, single-base substitutions, gene expression, and clinicopathologic features. When integrating these data types from the available independent PCa studies, we noted four of the six studies contained matched genomic, transcriptomic, and clinical data. Inclusion of additional independent PCa profiling studies with these matched data types would improve the statistical power of ProstaMine, particularly for low-frequency subtypes. We also considered the representation of primary versus metastatic tumors used in ProstaMine. We obtained 919 primary tumors and 484 metastatic tumors, with 446 of these metastatic tumors sourced from Abida et al. (2019) and the remaining 38 coming from Taylor et al. (2010) (Taylor et al., 2010; Abida et al., 2019). Addition of more metastatic tumor data from independent profiling studies would balance the representation of metastatic tumors and improve the performance of ProstaMine. Our approach did not consider any treatment information for primary and metastatic tumor samples. Including treatment information would allow ProstaMine to interrogate the role of treatments in PCa subtypes.
The analysis of co-occurring alterations in cancer is a powerful approach for identifying subtype-specific mechanisms, driving disease development, progression, and metastasis. ProstaMine builds on this approach through integration of molecular and clinical data across multiple independent cancer profiling studies and is the first tool for the identification of molecular subtype-specific drivers of aggressive phenotypes in PCa.
We developed ProstaMine to identify co-alterations associated with metastasis and biochemical relapse in molecular subtypes of PCa. ProstaMine was applied to NKX3-1-loss and RB1-loss tumors and identified co-altered genes that function in canonical PCa signaling pathways including MAPK, NF-kB, p53, SMAD, and PI3K. These co-alterations also function in fatty acid metabolism and autophagy processes. ProstaMine is available to the larger research community to identify candidate genes and generate hypotheses on the mechanisms that drive aggressiveness in molecularly defined subtypes of PCa.
The original contributions presented in the study are included in the article/Supplementary Material; further inquiries can be directed to the corresponding author.
All data used for this study were taken from publicly available and de-identified patient tumors. The studies were conducted in accordance with the local legislation and institutional requirements.
MO: writing–review and editing, writing–original draft, visualization, validation, software, methodology, investigation, formal analysis, and conceptualization. VS: writing–review and editing, visualization, software, resources, investigation, and data curation. TL: writing–review and editing, software, resources, and data curation. SC: writing–review and editing, supervision, and conceptualization. JC: writing–review and editing, writing–original draft, visualization, supervision, methodology, funding acquisition, formal analysis, and conceptualization.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was generously supported by the Anschutz Foundation and CA241647 to JC, CA231978 to JC and SC, CA262279 to SC, and FICAN Cancer Researcher by the Finnish Cancer Institute and Finnish Cultural Foundation to TL. This work used resources from the Biostatistics and Bioinformatics Shared Resource supported by the University of Colorado Cancer Center, an NCI designated Comprehensive Cancer Center (CA046934).
JC is the co-founder of OncoRx Insight.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2024.1360352/full#supplementary-material
Abdulkadir, S. A., Magee, J. A., Peters, T. J., Kaleem, Z., Naughton, C. K., Humphrey, P. A., et al. (2002). Conditional loss of Nkx3.1 in adult mice induces prostatic intraepithelial neoplasia. Mol. Cell. Biol. 22, 1495–1503. doi:10.1128/MCB.22.5.1495-1503.2002
Abeshouse, A., Ahn, J., Akbani, R., Ally, A., Amin, S., and Andry, C. D. (2015). The molecular taxonomy of primary prostate cancer. Cell. 163, 1011–1025. doi:10.1016/j.cell.2015.10.025
Abida, W., Cyrta, J., Heller, G., Prandi, D., Armenia, J., Coleman, I., et al. (2019). Genomic correlates of clinical outcome in advanced prostate cancer. Proc. Natl. Acad. Sci. 116, 11428–11436. doi:10.1073/pnas.1902651116
Adzhubei, I. A., Schmidt, S., Peshkin, L., Ramensky, V. E., Gerasimova, A., Bork, P., et al. (2010). A method and server for predicting damaging missense mutations. Nat. Methods 7, 248–249. doi:10.1038/nmeth0410-248
Alers, J. C., Rochat, J., Krijtenburg, P.-J., Hop, W. C., Kranse, R., Rosenberg, C., et al. (2000). Identification of genetic markers for prostatic cancer progression. Lab. Investig. 80, 931–942. doi:10.1038/labinvest.3780096
Augello, M. A., Liu, D., Deonarine, L. D., Robinson, B. D., Huang, D., Stelloo, S., et al. (2019). CHD1 loss alters AR binding at lineage-specific enhancers and modulates distinct transcriptional programs to drive prostate tumorigenesis. Cancer Cell. 35, 603–617. doi:10.1016/j.ccell.2019.03.001
Baca, S. C., Prandi, D., Lawrence, M. S., Mosquera, J. M., Romanel, A., Drier, Y., et al. (2013). Punctuated evolution of prostate cancer genomes. Cell. 153, 666–677. doi:10.1016/j.cell.2013.03.021
Barbieri, C. E., Baca, S. C., Lawrence, M. S., Demichelis, F., Blattner, M., Theurillat, J. P., et al. (2012). Exome sequencing identifies recurrent SPOP, FOXA1 and MED12 mutations in prostate cancer. Nat. Genet. 44, 685–689. doi:10.1038/ng.2279
Boyd, L. K., Mao, X., and Lu, Y.-J. (2012). The complexity of prostate cancer: genomic alterations and heterogeneity. Nat. Rev. Urol. 9, 652–664. doi:10.1038/nrurol.2012.185
Cairns, P., Okami, K., Halachmi, S., Halachmi, N., Esteller, M., Herman, J. G., et al. (1997). Frequent inactivation of PTEN/MMAC1 in primary prostate cancer. Cancer Res. 57, 4997–5000.
Camp, N. J., Cannon-Albright, L. A., Farnham, J. M., Baffoe-Bonnie, A. B., George, A., Powell, I., et al. (2007). Compelling evidence for a prostate cancer gene at 22q12.3 by the international consortium for prostate cancer genetics. Hum. Mol. Genet. 16, 1271–1278. doi:10.1093/hmg/ddm075
Carter, B. S., Ewing, C. M., Ward, W. S., Treiger, B. F., Aalders, T. W., Schalken, J. A., et al. (1990). Allelic loss of chromosomes 16q and 10q in human prostate cancer. Proc. Natl. Acad. Sci. U. S. A. 87, 8751–8755. doi:10.1073/pnas.87.22.8751
Cutler, M. L., Bassin, R. H., Zanoni, L., and Talbot, N. (1992). Isolation of rsp-1, a novel cDNA capable of suppressing v-Ras transformation. Mol. Cell. Biol. 12, 3750–3756. doi:10.1128/mcb.12.9.3750
Dai, Q., Deubler, D. A., Maxwell, T. M., Zhu, X. L., Cui, J., Rohr, L. R., et al. (2001). A common deletion at chromosomal region 17q21 in sporadic prostate tumors distal to BRCA1. Genomics 71, 324–329. doi:10.1006/geno.2000.6436
Ding, Z., Wu, C., Chu, G. C., Xiao, Y., Ho, D., Zhang, J., et al. (2011). SMAD4–dependent barrier constrains prostate cancer growth and metastatic progression. Nature 470, 269–273. doi:10.1038/nature09677
Emmert-Buck, M. R., Vocke, C. D., Pozzatti, R. O., Duray, P. H., Jennings, S. B., Florence, C. D., et al. (1995). Allelic loss on chromosome 8p12-21 in microdissected prostatic intraepithelial neoplasia. Cancer Res. 55, 2959–2962.
Erbersdobler, A., Graefen, M., Wullbrand, A., Hammerer, P., and Henke, R.-P. (1999). Allelic losses at 8p, 10q, 11p, 13q, 16q, 17p, and 18q in prostatic carcinomas: the impact of zonal location, Gleason grade, and tumour multifocality. Prostate Cancer Prostatic Dis. 2, 204–210. doi:10.1038/sj.pcan.4500324
Espiritu, S. M. G., Liu, L. Y., Rubanova, Y., Bhandari, V., Holgersen, E. M., Szyca, L. M., et al. (2018). The evolutionary landscape of localized prostate cancers drives clinical aggression. Cell. 173, 1003–1013. doi:10.1016/j.cell.2018.03.029
Gao, X., Zacharek, A., Salkowski, A., Grignon, D. J., Sakr, W., Porter, A. T., et al. (1995). Loss of heterozygosity of the BRCA1 and other loci on chromosome 17q in human prostate cancer. Cancer Res. 55, 1002–1005.
Gkretsi, V., Kalli, M., Efstathiades, C., Papageorgis, P., Papanikolaou, V., Zacharia, L. C., et al. (2019). Depletion of Ras Suppressor-1 (RSU-1) promotes cell invasion of breast cancer cells through a compensatory upregulation of a truncated isoform. Sci. Rep. 9, 10050. doi:10.1038/s41598-019-46575-0
Hieronymus, H., Iaquinta, P. J., Wongvipat, J., Gopalan, A., Murali, R., Mao, N., et al. (2017). Deletion of 3p13-14 locus spanning FOXP1 to SHQ1 cooperates with PTEN loss in prostate oncogenesis. Nat. Commun. 8, 1081. doi:10.1038/s41467-017-01198-9
Hieronymus, H., Schultz, N., Gopalan, A., Carver, B. S., Chang, M. T., Xiao, Y., et al. (2014). Copy number alteration burden predicts prostate cancer relapse. Proc. Natl. Acad. Sci. 111, 11139–11144. doi:10.1073/pnas.1411446111
Jillson, L. K., Rider, L. C., Rodrigues, L. U., Romero, L., Karimpour-Fard, A., Nieto, C., et al. (2021). MAP3K7 loss drives enhanced androgen signaling and independently confers risk of recurrence in prostate cancer with joint loss of CHD1. Mol. Cancer Res. 19, 1123–1136. doi:10.1158/1541-7786.MCR-20-0913
Kibel, A. S., Schutte, M., Kern, S. E., Isaacs, W. B., and Bova, G. S. (1998). Identification of 12p as a region of frequent deletion in advanced prostate cancer. Cancer Res. 58, 5652–5655.
Kluth, M., Ahrary, R., Hube-Magg, C., Ahmed, M., Volta, H., Schwemin, C., et al. (2015). Genomic deletion of chromosome 12p is an independent prognostic marker in prostate cancer. Oncotarget 6, 27966–27979. doi:10.18632/oncotarget.4626
Kluth, M., Volta, H., Hussein, M., Taskin, B., Frogh, S., Möller-Koop, C., et al. (2018). Deletion of 3p13 is a late event linked to progression of TMPRSS2:ERG fusion prostate cancer. Cancer Manag. Res. 10, 5909–5917. doi:10.2147/CMAR.S172637
Ku, S. Y., Rosario, S., Wang, Y., Mu, P., Seshadri, M., Goodrich, Z. W., et al. (2017). Rb1 and Trp53 cooperate to suppress prostate cancer lineage plasticity, metastasis, and antiandrogen resistance. Science 355, 78–83. doi:10.1126/science.aah4199
Laajala, T. D., Sreekanth, V., Soupir, A. C., Creed, J. H., Halkola, A. S., Calboli, F. C. F., et al. (2023). A harmonized resource of integrated prostate cancer clinical, -omic, and signature features. Sci. Data 10, 430. doi:10.1038/s41597-023-02335-4
Latil, A., Cussenot, O., Fournier, G., Driouch, K., and Lidereau, R. (1997). Loss of heterozygosity at chromosome 16q in prostate adenocarcinoma: identification of three independent regions. Cancer Res. 57, 1058–1062.
Li, D., Peng, W., Wu, B., Liu, H., Zhang, R., Zhou, R., et al. (2021). Metallothionein MT1M suppresses carcinogenesis of esophageal carcinoma cells through inhibition of the epithelial-mesenchymal transition and the SOD1/PI3K Axis. Mol. Cells 44, 267–278. doi:10.14348/molcells.2021.2179
Li, J., Belogortseva, N., Porter, D., and Park, M., 2008. Chmp1A functions as a novel tumor suppressor gene in human embryonic kidney and ductal pancreatic tumor cells. Cell. Cycle Georget. Tex 7, 2886–2893. doi:10.4161/cc.7.18.6677
Li, J., Orr, B., White, K., Belogortseva, N., Niles, R., Boskovic, G., et al. (2009). Chmp 1A is a mediator of the anti-proliferative effects of All-trans Retinoic Acid in human pancreatic cancer cells. Mol. Cancer 8, 7. doi:10.1186/1476-4598-8-7
Li, K., Sun, S., Lu, Y., Liang, W., Xu, X., Zhang, H., et al. (2023). MT1M regulates gastric cancer progression and stemness by modulating the Hedgehog pathway protein GLI1. Biochem. Biophys. Res. Commun. 670, 63–72. doi:10.1016/j.bbrc.2023.05.121
Liu, D., Augello, M. A., Grbesa, I., Prandi, D., Liu, Y., Shoag, J. E., et al. 2021. Tumor subtype defines distinct pathways of molecular and clinical progression in primary prostate cancer. J. Clin. Investig. 131, e147878. doi:10.1172/JCI147878
Liu, W., Ewing, C. M., Chang, B.-L., Li, T., Sun, J., Turner, A. R., et al. (2007). Multiple genomic alterations on 21q22 predict various TMPRSS2/ERG fusion transcripts in human prostate cancers. Genes. Chromosom. Cancer 46, 972–980. doi:10.1002/gcc.20482
Loizzo, D., Pandolfo, S. D., Rogers, D., Cerrato, C., di Meo, N. A., Autorino, R., et al. (2022). Novel insights into autophagy and prostate cancer: a comprehensive review. Int. J. Mol. Sci. 23, 3826. doi:10.3390/ijms23073826
Louca, M., Stylianou, A., Minia, A., Pliaka, V., Alexopoulos, L. G., Gkretsi, V., et al. (2019). Ras suppressor-1 (RSU-1) promotes cell invasion in aggressive glioma cells and inhibits it in non-aggressive cells through STAT6 phospho-regulation. Sci. Rep. 9, 7782. doi:10.1038/s41598-019-44200-8
Mao, J., Yu, H., Wang, C., Sun, L., Jiang, W., Zhang, P., et al. (2012). Metallothionein MT1M is a tumor suppressor of human hepatocellular carcinomas. Carcinogenesis 33, 2568–2577. doi:10.1093/carcin/bgs287
Mermel, C. H., Schumacher, S. E., Hill, B., Meyerson, M. L., Beroukhim, R., and Getz, G. (2011). GISTIC2.0 facilitates sensitive and confident localization of the targets of focal somatic copy-number alteration in human cancers. Genome Biol. 12, R41. doi:10.1186/gb-2011-12-4-r41
Mu, P., Zhang, Z., Benelli, M., Karthaus, W. R., Hoover, E., Chen, C. C., et al. (2017). SOX2 promotes lineage plasticity and antiandrogen resistance in TP53- and RB1-deficient prostate cancer. Science 355, 84–88. doi:10.1126/science.aah4307
Ng, P. C., and Henikoff, S. (2003). SIFT: predicting amino acid changes that affect protein function. Nucleic Acids Res. 31, 3812–3814. doi:10.1093/nar/gkg509
Nyquist, M. D., Corella, A., Coleman, I., De Sarkar, N., Kaipainen, A., Ha, G., et al. (2020). Combined TP53 and RB1 loss promotes prostate cancer resistance to a spectrum of therapeutics and confers vulnerability to replication stress. Cell. Rep. 31, 107669. doi:10.1016/j.celrep.2020.107669
Ormond, D. R., Kleinschmidt-DeMasters, B. K., Cavalcante, D., Smith, E. E., Cramer, S. D., and Lucia, M. S. (2019). Prostatic adenocarcinoma CNS parenchymal and dural metastases: alterations in ERG, CHD1 and MAP3K7 expression. J. Neurooncol. 142, 319–325. doi:10.1007/s11060-019-03099-x
Ozen, M., Navone, N. M., Multani, A. S., Troncoso, P., Logothetis, C. J., Chung, L. W., et al. (1998). Structural alterations of chromosome 5 in twelve human prostate cancer cell lines. Cancer Genet. cytogenet. 106, 105–109. doi:10.1016/S0165-4608(98)00051-X
Paris, P. L., Andaya, A., Fridlyand, J., Jain, A. N., Weinberg, V., Kowbel, D., et al. (2004). Whole genome scanning identifies genotypes associated with recurrence and metastasis in prostate tumors. Hum. Mol. Genet. 13, 1303–1313. doi:10.1093/hmg/ddh155
Perner, S., Demichelis, F., Beroukhim, R., Schmidt, F. H., Mosquera, J. M., Setlur, S., et al. (2006). TMPRSS2:ERG fusion-associated deletions provide insight into the heterogeneity of prostate cancer. Cancer Res. 66, 8337–8341. doi:10.1158/0008-5472.CAN-06-1482
Rodrigues, L. U., Rider, L., Nieto, C., Romero, L., Karimpour-Fard, A., Loda, M., et al. 2015. Coordinate loss of MAP3K7 and CHD1 promotes aggressive prostate cancer, Cancer Res. 75, 1021–1034. doi:10.1158/0008-5472.CAN-14-1596
Sanchez, P., Hernández, A. M., Stecca, B., Kahler, A. J., DeGueme, A. M., Barrett, A., et al. (2004). Inhibition of prostate cancer proliferation by interference with SONIC HEDGEHOG-GLI1 signaling. Proc. Natl. Acad. Sci. 101, 12561–12566. doi:10.1073/pnas.0404956101
Saramäki, O. R., Porkka, K. P., Vessella, R. L., and Visakorpi, T. (2006). Genetic aberrations in prostate cancer by microarray analysis. Int. J. Cancer 119, 1322–1329. doi:10.1002/ijc.21976
Sattler, H. P., Lensch, R., Rohde, V., Zimmer, E., Meese, E., Bonkhoff, H., et al. (2000). Novel amplification unit at chromosome 3q25-q27 in human prostate cancer. Prostate 45, 207–215. doi:10.1002/1097-0045(20001101)45:3<207::aid-pros2>3.0.co;2-h
Scheble, V. J., Braun, M., Beroukhim, R., Mermel, C. H., Ruiz, C., Wilbertz, T., et al. (2010). ERG rearrangement is specific to prostate cancer and does not occur in any other common tumor. Mod. Pathol. 23, 1061–1067. doi:10.1038/modpathol.2010.87
Sena, L. A., and Denmeade, S. R. (2021). Fatty acid synthesis in prostate cancer: vulnerability or epiphenomenon? Cancer Res. 81, 4385–4393. doi:10.1158/0008-5472.CAN-21-1392
Sondka, Z., Bamford, S., Cole, C. G., Ward, S. A., Dunham, I., and Forbes, S. A. (2018). The COSMIC Cancer Gene Census: describing genetic dysfunction across all human cancers. Nat. Rev. Cancer 18, 696–705. doi:10.1038/s41568-018-0060-1
Sumiya, R., Terayama, M., Hagiwara, T., Nakata, K., Sekihara, K., Nagasaka, S., et al. (2022). Loss of GSTO2 contributes to cell growth and mitochondria function via the p38 signaling in lung squamous cell carcinoma. Cancer Sci. 113, 195–204. doi:10.1111/cas.15189
Sung, H., Ferlay, J., Siegel, R. L., Laversanne, M., Soerjomataram, I., Jemal, A., et al. (2021). Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Ca. Cancer J. Clin. 71, 209–249. doi:10.3322/caac.21660
Taylor, B. S., Schultz, N., Hieronymus, H., Gopalan, A., Xiao, Y., Carver, B. S., et al. (2010). Integrative genomic profiling of human prostate cancer. Cancer Cell. 18, 11–22. doi:10.1016/j.ccr.2010.05.026
Terayama, M., Yamada, K., Hagiwara, T., Inazuka, F., Sezaki, T., Igari, T., et al. (2020). Glutathione S-transferase omega 2 regulates cell growth and the expression of E-cadherin via post-transcriptional down-regulation of β-catenin in human esophageal squamous cells. Carcinogenesis 41, 875–886. doi:10.1093/carcin/bgz189
Tomlins, S. A., Rhodes, D. R., Perner, S., Dhanasekaran, S. M., Mehra, R., Sun, X. W., et al. (2005). Recurrent fusion of TMPRSS2 and ETS transcription factor genes in prostate cancer. Science 310, 644–648. doi:10.1126/science.1117679
Ueda, T., Komiya, A., Suzuki, H., Shimbo, M., Sakamoto, S., Imamoto, T., et al. (2005). Loss of heterozygosity on chromosome 2 in Japanese patients with prostate cancer. Prostate 64, 265–271. doi:10.1002/pros.20228
van Dekken, H., Alers, J. C., Damen, I. A. A. J., Vissers, K. J., Krijtenburg, P. J., Hoedemaeker, R. F., et al. (2003). Genetic evaluation of localized prostate cancer in a cohort of forty patients: gain of distal 8q discriminates between progressors and nonprogressors. Lab. Investig. 83, 789–796. doi:10.1097/01.LAB.0000074889.76221.49
Verhagen, P. C. M. S., Hermans, K. G. L., Brok, M. O., van Weerden, W. M., Tilanus, M. G. J., de Weger, R. A., et al. (2002). Deletion of chromosomal region 6q14-16 in prostate cancer. Int. J. Cancer 102, 142–147. doi:10.1002/ijc.10677
Wang, H., Liu, B., and Wei, J. (2021). Beta2-microglobulin(B2M) in cancer immunotherapies: biological function, resistance and remedy. Cancer Lett. 517, 96–104. doi:10.1016/j.canlet.2021.06.008
Wang, S., Gao, J., Lei, Q., Rozengurt, N., Pritchard, C., Jiao, J., et al. (2003). Prostate-specific deletion of the murine Pten tumor suppressor gene leads to metastatic prostate cancer. Cancer Cell. 4, 209–221. doi:10.1016/S1535-6108(03)00215-0
Wu, M., Shi, L., Cimic, A., Romero, L., Sui, G., Lees, C. J., et al. (2012). Suppression of Tak1 promotes prostate tumorigenesis. Cancer Res. 72, 2833–2843. doi:10.1158/0008-5472.CAN-11-2724
Xu, W., Jiang, G.-J., Shi, G.-Z., Chen, M. Z., Ma, T. L., and Tan, Y. F. (2020). Metallothionein 1M (MT1M) inhibits lung adenocarcinoma cell viability, migration, and expression of cell mobility-related proteins through MDM2/p53/MT1M signaling. Transl. Cancer Res. 9, 2710–2720. doi:10.21037/tcr.2020.02.61
Keywords: prostate cancer, molecular subtypes, NKX3-1, RB1, data mining algorithm, bioinformatics analysis
Citation: Orman MV, Sreekanth V, Laajala TD, Cramer SD and Costello JC (2024) ProstaMine: a bioinformatics tool for identifying subtype-specific co-alterations associated with aggressiveness in prostate cancer. Front. Pharmacol. 15:1360352. doi: 10.3389/fphar.2024.1360352
Received: 22 December 2023; Accepted: 13 March 2024;
Published: 01 May 2024.
Edited by:
Justin Hwang, University of Minnesota Twin Cities, United StatesReviewed by:
Moray Campbell, The Ohio State University, United StatesCopyright © 2024 Orman, Sreekanth, Laajala, Cramer and Costello. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: James C. Costello, SmFtZXMuQ29zdGVsbG9AY3VhbnNjaHV0ei5lZHU=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.