
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pharmacol. , 22 February 2024
Sec. Pharmacology of Anti-Cancer Drugs
Volume 15 - 2024 | https://doi.org/10.3389/fphar.2024.1355081
This article is part of the Research Topic Genomic Discoveries and Pharmaceutical Development in Urologic Tumors View all 17 articles
 Kejia Zhu1,2,3,4†
Kejia Zhu1,2,3,4† Yao Chang1,5†
Yao Chang1,5† Delong Zhao1
Delong Zhao1 Andong Guo1
Andong Guo1 Jishuang Cao6
Jishuang Cao6 Chenrui Wu6
Chenrui Wu6 Yong Guan1,2,3
Yong Guan1,2,3 Sentai Ding1,4,6*
Sentai Ding1,4,6*Background: A vast number of researchers have discovered high levels of human epidermal growth factor receptor-2 (HER2) expression in urothelial carcinoma (UC), but they do not use a uniform scoring system. Based on the 2021 edition of clinical pathological expert consensus on HER-2 testing in UC in China, we investigated the expression level and clinical significance of HER2 in high-grade UC. Furthermore, we looked at the prognosis of patients with locally advanced/metastatic UC after combining HER2 targeting antibody-drug conjugates (ADC) medication disitamab vedotin (DV) with programmed cell death protein 1 (PD-1) inhibitor tislelizumab.
Patients and methods: From 2019 to 2022, we collected paraffin specimens of UC from the Department of Urology at the Provincial Hospital Affiliated to Shandong First Medical University. HER2 expression-related factors were investigated. Patients with advanced UC who have failed systemic chemotherapy at least once and had received immune checkpoint inhibitor (ICI) medication during second-line treatment were selected and treated with DV in combination with tislelizumab. We assessed the therapy’s efficacy and safety.
Results: 185 patients with high-grade UC were included in this investigation. 127 patients (68.7%) were HER2 positive (IHC 2+/3+) according to the 2021 Clinical pathological expert consensus on HER2 testing in UC in China. The clinical stage of UC differed statistically significantly between the HER2-and HER2+ groups (p = 0.019). Sixteen advanced UC patients were treated with DV and tislelizumab for a median of 14 months. The disease control rate was 87.5%, while the objective response rate (ORR) was 62.5%. The ORR of HER2+ individuals was higher than that of HER2-individuals (70.0% vs. 50.0%). The median progression-free survival or overall survival was not reached. In this study, the incidence of treatment-related adverse events was 68.8% (11/16), with all of them being grade 1 or 2 adverse reactions.
Conclusion: HER2 protein expressed at a high percentage in UC, and 68.7% patients expressed HER2 positive (IHC 2+/3+). HER2+ expression is positively correlated with higher clinical stage of UC. HER2 targeted ADC drug disitamab vedotin combining with PD-1 inhibitor tislelizumab has shown efficacy, safety and controllable adverse reactions in the treatment of advanced UC.
The urothelial carcinoma (UC) is one of the most prevalent cancers worldwide, with primary locations including the bladder, ureter, and renal pelvis. According to the Global Cancer Statistics in 2020, there were 573,278 new cases of bladder cancer globally and 85,649 in China (Sung et al., 2021). UC can be classified into low-grade UC and high-grade UC. High-grade UC is often associated with stromal invasion and has a poor prognosis. Locally advanced or metastatic (la/mUC) cases account for approximately 5%–11% of all UC cases (Hepp et al., 2021; He et al., 2023). Patients with la/mUC face a bleak prognosis, as the 5-year survival rate ranges from a mere 4.6%–34%. Therefore, there is an urgent need for an improved non-surgical treatment approach.
Currently, adjuvant therapy for advanced UC includes chemotherapy, immunotherapy, and targeted therapy. Chemotherapy is the recommended first-line treatment, while immunotherapy (particularly programmed cell death protein 1 (PD-1)/programmed cell death-ligand 1 (PD-L1) inhibitors) is a second-line option. Enfortumab vedotin antibody-drug conjugates (ADC) after prior platinum chemotherapy and checkpoint inhibitor immunotherapy (ICI) have demonstrated significant survival benefits in la/mUC patients compared to chemotherapy (Rosenberg et al., 2019; Chang et al., 2021). The efficacy and safety of the combination therapy comprising enfortumab vedotin and pembrolizumab as a first-line treatment in cisplatin-ineligible patients with la/mUC were also confirmed (Hoimes et al., 2023).
In recent years, an additional ADC targeting the human epidermal growth factor receptor 2 (HER2, also known as ERBB2) has been developed and implemented in clinical practice. It has been established that HER2 plays a crucial role in the pathogenesis and progression of various malignant tumors, including urothelial carcinoma. Consequently, it is imperative to elucidate the expression of HER2 protein in urothelial carcinoma and its clinicopathological correlation to facilitate the clinical application of anti-HER2 targeted therapy for this disease (Olt et al., 1990; Press et al., 1990; Mendelsohn and Baselga, 2003). Currently, the existing detection methods for HER2 expression primarily rely on breast cancer evaluation standards that lack a standardized scoring system; thus further validation is warranted. Notably, several HER2-targeted ADCs have been approved in recent years, such as trastuzumab emtansine and trastuzumab deruxtecan. Additionally, a novel HER2-targeted ADC named disitamab vedotin (DV) was granted approval by the National Medical Products Administration in January 2022. However, more comprehensive clinical data are required to evaluate the efficacy of this medication.
In this article, we detected the expression of HER2 based on a 2021 edition of Clinical pathological expert consensus on HER-2 testing in UC in China. Besides, we analyzed its clinicopathological relationship with high-grade UC and explored the efficacy of HER2 targeted ADC drug disitamab vedotin, also known as RC48. As well as the efficacy of DV and PD-1 inhibitor tislelizumab combination therapy.
All clinical cases of high-grade UC with pathological diagnosis from 2019 to 2022 were included in this study, conducted at the Department of Urology and Pathology, Shandong Provincial Hospital Affiliated to Shandong First Medical University. The pathological diagnosis was based on the 2016 edition of the World Health Organization’s diagnostic criteria for urological pathology and genetics. UC specimens were obtained through various surgical procedures including transurethral resection of bladder tumor, partial cystectomy, radical cystectomy, segmental ureterectomy, or total ureteropelvic resection.
For evaluating the efficacy of DV-tislelizumab combination therapy, patients treated between 2020 and 2022 were included in our analysis. The inclusion criteria consisted of: 1) age ≥18 years; histological or cytological confirmation of la/mUC with at least one measurable tumor lesion; 2) Eastern Cooperative Oncology Group (ECOG) performance status ≤1; 3) previous failure with systemic chemotherapy allowed; and 4) prior treatment with PD-1/PD-L1 inhibitors permitted. Exclusion criteria encompassed: 1) insufficient availability of critical clinical data; 2) inability to detect HER2 expression or lack of pathological sections; 3) presence of concurrent malignancies; and finally; 4) history of tyrosine kinase inhibitor therapy.
The collected high-grade UC specimens were fixed within 1 h after isolation. Prior to fixation, the specimen was incised at intervals of 0.5–1 cm, and gauze or filter paper was inserted between the tissues for optimal fixation. In cases where the tumor tissue was fragmented, packing fixation was employed. A solution of 10% formalin, with a volume ten times that of the specimen, was utilized for fixation, with biopsy specimens being fixed for a duration ranging from 6 to 24 h. For larger specimens, fixation extended from 12 to 48 h. Subsequently, the paraffin-embedded specimens were subjected to HER2 immunohistochemistry (IHC) analysis following the scoring criteria outlined in accordance with the Clinical Pathological Expert Consensus on HER2 Testing in UC in China (2021 edition): no staining or <10% of invasive cancer cells exhibiting incomplete and weakly stained membranes (scored as 0); ≥10% of invasive cancer cells displaying incomplete and weakly stained membranes (scored as 1+); ≥10% of invasive cancer cells showing weak-moderate full membrane staining or <10% of invasive cancer cells demonstrating strong staining of intact cell membrane (scored as 2+); ≥10% of invasive cancer cells exhibiting strong staining of intact cell membrane (scored as 3+) (HERExpert Committee on Urothelial Carcinoma of Chinese Society of Clinical Oncology, 2021). Additionally, patient demographics including gender and age along with clinical parameters such as maximum tumor size, smoking history, primary sites, histopathological diagnosis, clinical stage classification, muscle invasion status, regional lymph node metastasis presence as well as some laboratory results were also recorded.
Locally-advanced/metastatic UC patients meeting the inclusion criteria were enrolled in this study to receive combination therapy with DV-tislelizumab. The treatment regimen consisted of DV administered at a dose of 2.0 mg/kg every 2 weeks, in combination with tislelizumab, a PD-1 inhibitor, administered at a dose of 200 mg every 3 weeks. Treatment was continued until patients discontinued due to disease progression (PD), intolerable side effects (SE), death, or withdrawal of informed consent. The patients underwent baseline physical examinations. Efficacy assessments were conducted every 4 treatment cycles, following the 1.1 version of the Solid Tumor Response Evaluation Standard and the International Standard for Common Terminology of Adverse Events (RECIST) (Eisenhauer et al., 2009). Short-term evaluation included objective response rate (ORR) and disease control rate (DCR) determination. Median progression-free survival (PFS) and overall survival (OS) served as long-term evaluation endpoints. During treatment, patients were monitored biweekly for blood routine tests (RT), biochemical parameters, liver functions, thyroid functions, stool and urine RT. Side effects were evaluated according to Common Terminology Criteria for Adverse Events (CTCAE) 5.0. Grade 1 or 2 side effects were managed based on symptoms, while grade 3 or higher side effects led to discontinuation from the study protocol. Informed consent was obtained from all participants, and ethical approval was granted by our institution’s ethics committee.
Chi-square test was applied to analyze the characteristics of different primary sites, and relationship between HER2 and UC muscle invasion, clinical stage, regional lymph node metastasis, smoke history, primary sites and gender. ANOVA test was used to analyze the difference among three primary sites. t-test and Mann-Whitney test were used to analyze the differences between HER2+/−groups. Logistic analysis was used for the correlation between HER2 and some blood indicators analysis. p < 0.05 was set as statistically significant. All analyses were performed using SPSS version 27.0 software.
In our study, a total of 185 patients with high-grade UC were included, comprising 139 males and 46 females, with a median age of 68 years (range: 41–93). Among them, bladder was the primary site for UC in 127 cases (68.7%), followed by ureter in 36 cases (19.5%) and renal pelvis in 22 cases (11.9%) (Table 1). According to the diagnostic criteria outlined in the World Health Organization’s (WHO) latest edition from 2016, muscle invasive UC was observed in 103 cases (primary site: bladder-54; ureter-31; renal pelvis-18), while non-muscle invasive UC was found in 82 cases (primary site: bladder-73; ureter-5; renal pelvis-4) (Figure 1).
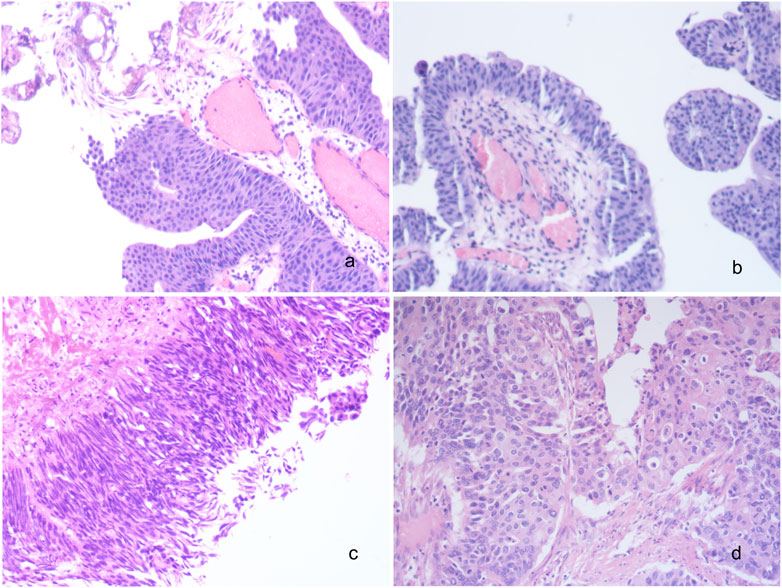
FIGURE 1. Histomorphological spectrum of UC. (A) Low grade non-invasive urothelial bladder carcinoma; (B) Low grade invasive urothelial bladder carcinoma; (C) High grade non-invasive urothelial bladder carcinoma; (D) High grade invasive urothelial bladder carcinoma; ×200. UC, urothelial carcinoma.
Among the patients with primary tumor located in the bladder (n = 127), transurethral resection of bladder tumor was performed on 61 patients with a HER2 positive rate of 80.3%. Radical cystectomy was conducted on 50 patients with a HER2 positive rate of 46.0%, partial cystectomy on 14 patients with a HER2 positive rate of 64.3%, and no surgical treatment or bladder instillation but only immunotherapy using PD-1 inhibitor was administered to 2 patients who had a HER2 positive rate of 50.0%; For 36 patients with a primary tumor located in the ureter, radical nephroureterectomy was performed on 26 patients (HER2 positive rate: 50.0%), while segmental ureterectomy was performed on 10 patients (HER2 positive rate: 70.0%). Among the cohort of 22 patients with primary tumors located in the renal pelvis, a total of 19 patients underwent radical nephroureterectomy combined with partial cystectomy (with a HER2 positive rate of 57.9%). For the remaining 3 patients who did not undergo surgical intervention, PD-1 inhibitor immunotherapy was administered instead (with a HER2 positive rate of 33.3%).
Among the 185 high-grade UC tissues, HER2 protein expression was observed in 159 cases (86.0%) (Figures 2A–D). Of these, 32 cases (17.3%) showed HER2 1+ expression, while 110 cases (59.5%) exhibited HER2 2+ expression and only 17 cases (7.6%) displayed HER2 3+ expression. 127 of the analyzed samples were high-grade bladder tumor tissues, with a majority of them showing HER2 protein expression in 115 cases (90.6%), 21 (16.5%) expressed HER2 1+, 79 (62.2%) expressed HER2 2+ and 16 (12.6%) expressed HER2 3+; Additionally, 36 cases were high-grade ureteral tumor tissues, and among them 25 cases (69.4%) expressed HER2 protein. Of these, 5 cases (13.9%) had HER2 score of 1+, 19 cases (52.8%) had HER2 score of 2+ and only 1 case (2.8%) had HER2 3+. Among the 22 high-grade renal pelvis tumor tissues, HER2 protein was expressed in 19 cases (86.4%), with 6 cases (27.3%) showing HER2 1+ expression and 13 cases (59.1%) exhibiting HER2 2+ expression (Figure 2E).
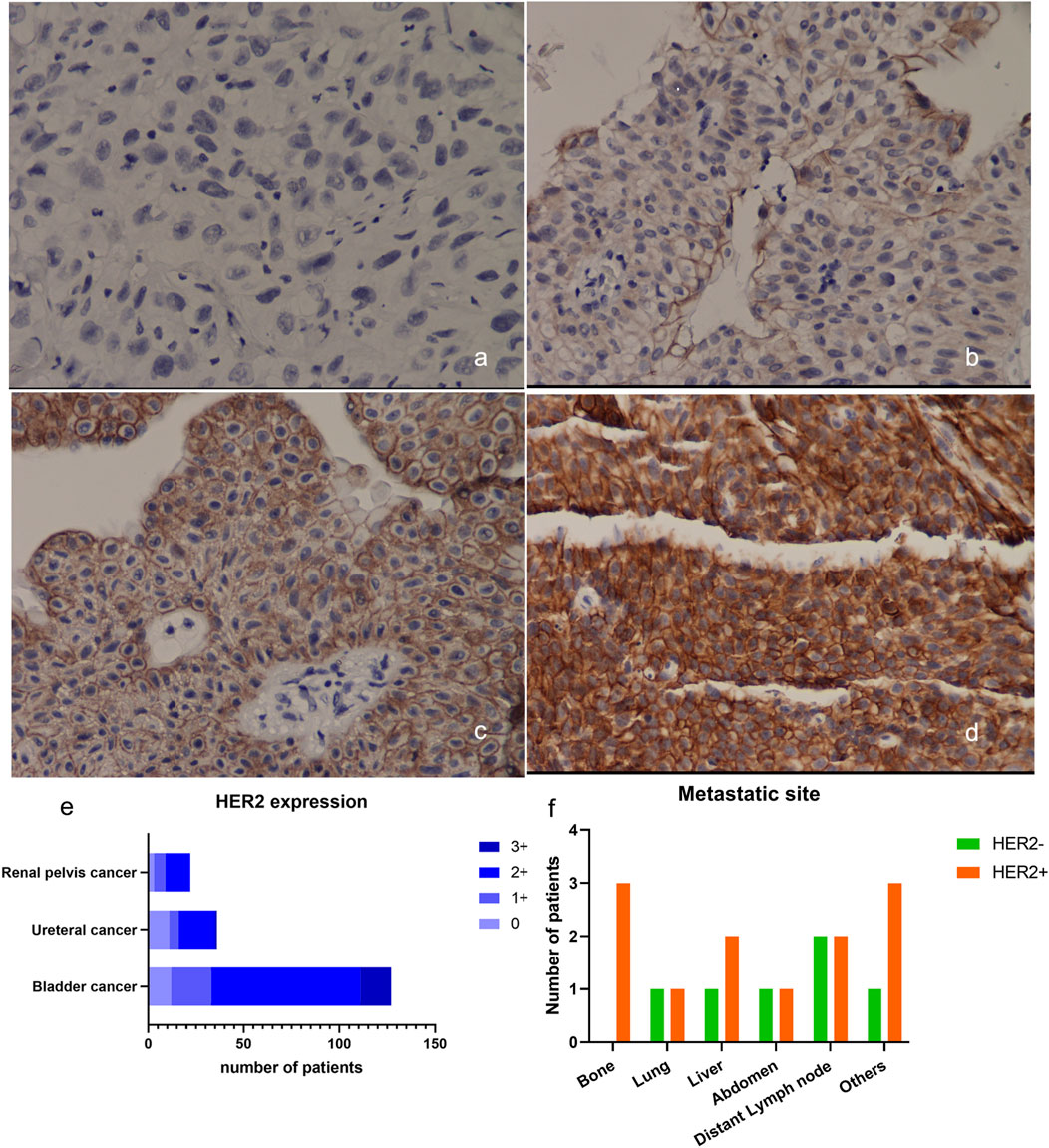
FIGURE 2. Expression of HER2 in UC. (A–D) Example of HER2 expression in urothelial bladder carcinoma. (A) HER2 IHC scored 0+; (B) HER2 IHC scored 1+; (C) HER2 IHC scored 2+; (D) HER2 IHC scored 3+, ×200; (E) Expression of HER2 in UC from different primary sites; (F) The metastatic sites of patients included in the DV treatment therapy at baseline. HER2, human epidermal growth factor receptor 2; IHC, immunohistochemistry.
The HER2 positive rate (IHC 2+ and 3+) in 185 high-grade UC patients was found to be 68.65% (127/185). We conducted an analysis to identify potential factors associated with HER2 positivity. Notably, clinical stage exhibited a statistically significant correlation with HER2 positivity in UC (p = 0.019). However, no significant associations were observed between HER2 positivity and gender (p = 0.345), age (p = 0.289), tumor size (p = 0.107), smoking status (p = 0.175), muscle invasion (p = 0.133), regional lymph node metastasis (p = 0.143) or primary site of the tumor (p = 0.066) as shown in Table 2. Furthermore, we investigated whether any blood indicators could predict the expression of HER2+. Logistic regression analysis revealed that none of the blood indicators examined showed predictive value for HER2+ expression (Figure 3).
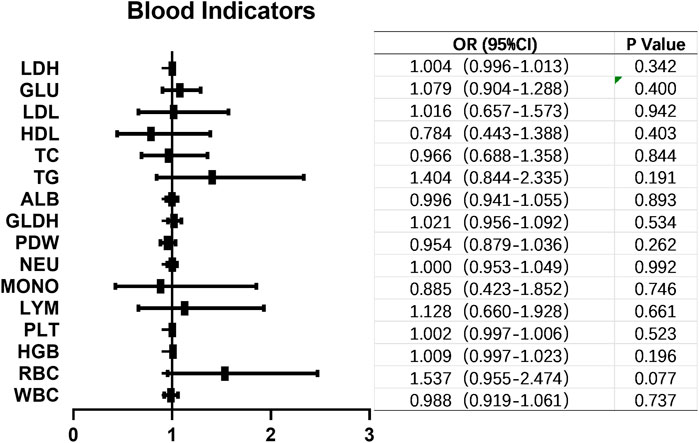
FIGURE 3. Blood indicators that might correlated with HER2+ in UC. No blood indicators showed correlation with HER2+ expression in UC. OR, odds ratio; CI, confidence interval; LDH, lactatedehydrogenase; GLU, glucose; LDL, low density lipoprotein; HDL, high density lipoprotein; TC, total cholesterol; TG, triglyceride; ALB, albumin; GLDH, glutamic dehydrogenase; PDW, platelet distribution width; NEU, neutrophil; MONO, monocyte; LYM, lymphocyte; PLT, platelet; HGB, hemoglobin; WBC, white blood cell.
Sixteen patients with locally-advanced/metastatic UC, who had failed first-line treatment, were enrolled in this study. They received DV 240 mg every 2 weeks in combination with PD-1 inhibitors tislelizumab 200 mg every 3 weeks. The cohort consisted of an equal distribution of male and female patients, with a median age of 66 (range: 51–81) years old. Metastatic sites included the liver (n = 3), lung (n = 2), and bone (n = 3). Table 3; Figure 2F present the baseline characteristics of these patients.
After a median follow-up duration of 14 (1.0–19.0) months, we presented the treatment cycle and prognosis of each patient in Figure 4. According to RECIST 1.1 criteria, one patient achieved complete response (CR), while nine patients showed partial response (PR). Four patients exhibited stable disease (SD), and two patients experienced progressive disease (PD) as shown in Figures 4A, B. The ORR was found to be 62.5%, with tumor size reduction observed in twelve out of sixteen patients compared to baseline measurements, indicating a decrease rate of 75% as depicted in Figure 4E. The DCR was determined to be 87.5%. Among the ten HER2-positive patients expressing HER2 at level 2+, the ORR reached up to 70%. For six HER2-negative patients expressing HER2 at level 1+, the ORR was recorded as being at a rate of 50%. Median progression-free survival or overall survival has not been reached yet, as illustrated in Figures 4C, D.
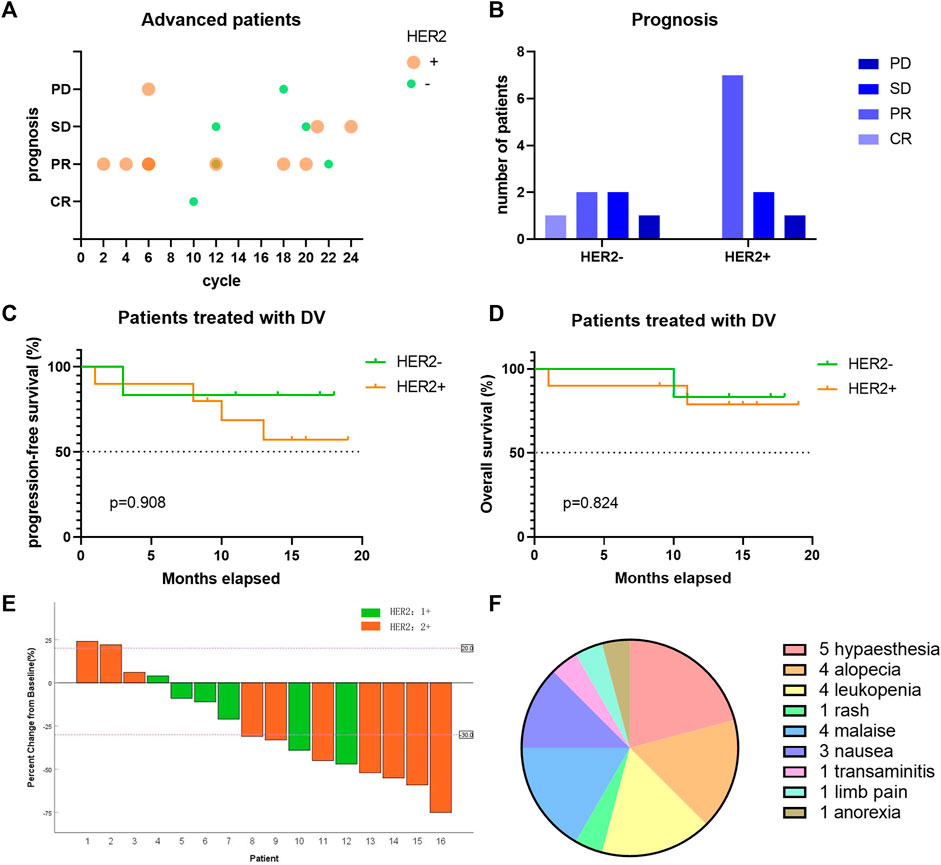
FIGURE 4. The efficacy of DV on the treatment of locally-advanced/metastatic UC patients. (A) Each bubble represents one patient, with different treatment cycle, HER2 expression and different prognosis. Bubbles with darker color reflected more than one patient. (B) The short-term prognosis of advanced UC patients divided by HER2 expression. (C–D) The long-term prognosis of advanced UC patients divided by HER2 expression. (E) Changes of patients’ tumor size compared to baseline. (F) Side effects of DV treatment in locally-advanced/metastatic UC. DV, disitamab vedotin; CR, complete response; PR, partial response; SD, stable disease; PD, progression disease.
Eleven patients reported side effects following DV treatment, with the most frequently observed being hypaesthesia (5/16), alopecia (4/16), leukopenia (4/16), debilitation (4/16), and digestive tract symptoms such as nausea and anorexia (4/16) (Figure 4F). These adverse events were all classified as grade 1 or 2, with no occurrences of grade 3 or higher. Symptomatic treatment was administered to all patients.
Urothelial carcinoma is the third most common cancer with HER2 overexpression, following breast and stomach cancer. Recent clinical trials have demonstrated the efficacy of HER2 targeted therapy in urothelial carcinoma. However, there is variability in the reported rates of HER2 positivity. A European study found that 4%–20% of urothelial carcinoma patients exhibited HER2 expression (Bellmunt et al., 2015). Cheetham and Petrylak (2016) reported a range of 5%–89% for bladder UCs overexpressing HER2 protein. In Chinese bladder UC patients, the expression of HER2 differs from other countries and also varies among provinces. Studies conducted in Beijing showed a HER2 positive rate ranging from 36.1% to 44% in UCs (Fan et al., 2022; Zhou et al., 2023). In our study conducted in Shandong Province, China, we observed that 159 out of 185 cases (86%) of high-grade UC expressed HER2 protein, with 127 cases exhibiting positive staining (IHC score 2+ and 3+), accounting for 68.6%. Furthermore, we found variations in HER2 expression among different primary sites within high-grade UCs; bladder UCs had a higher rate of positivity (74.8%) compared to ureters (55.6%) or renal pelvises (59.1%). The majority of cases exhibited moderate levels of HER2 expression at an IHC score of 2+ (110/185; 59%), while only a small percentage showed strong staining at an IHC score of 3+ (17/185; 9%). This was significantly lower than those with weak staining at an IHC score of 1+ (32/185; 17%) or moderate staining at an IHC score of 2+. Wide ranges of HER2+ reappearances have been observed in several studies, which can be attributed to suboptimal staining processes and the lack of standardized criteria specific for UC (Scherrer et al., 2022). The frequency of HER2 protein overexpression is influenced by multiple factors, including ERBB2 mutation and amplification. In muscle invasive bladder cancer, the expression rate of HERR2 amplified or single nucleotide variation (SNV) was found to be less than 20% (Kiss et al., 2017). Samples with ERBB2 amplification exhibited higher mRNA and protein expression levels. However, gene amplification alone does not solely drive high HER2 expression in bladder cancer; SNVs occur prior to ERBB2 amplification. SNVs occurring in the extracellular region of ERBB2 appear to result in lower protein expression detection. The practicality and high sensitivity of IHC-based HER2 detection still remain significant.
The detection of HER2 expression in urothelial carcinoma is not currently incorporated into routine clinical practice, thus the understanding of HER2 expression in urothelial carcinoma remains unclear. Despite the known overexpression of HER2 in various tumors, there are still conflicting data regarding its role as a carcinogenic driver or prognostic marker for urothelial carcinoma (Krüger et al., 2002; Bellmunt et al., 2015), Notably, treatment with ADC targeted HER2 has significantly improved survival rates for patients with HER2+ breast cancer and gastric cancer. Previous studies on the detection criteria for urothelial cancer HER2 mostly referred to breast cancer or gastric cancer, resulting in substantial variations among research findings. In 2021, China released the Expert Consensus on Clinical Pathology of Human Epidermal Growth Factor Receptor 2 Detection in Urinary Cancer which highlighted key differences between detection criteria for urothelial cancer and breast cancer. For urothelial cancer, scoring is defined as follows: 0 indicates non-staining or <10% incomplete and weak staining of infiltrating cancer cell membranes; 1+ denotes ≥10% incomplete and weak staining; 2+ signifies ≥10% weak to moderate staining of intact cell membrane or <10% strongly stained intact cell membrane; and finally, 3+ represents ≥10% strong staining of intact cell membranes.
A higher percentage of HER2+ expression is observed in high-grade UC. Bai et al. (2022) conducted immunohistochemical staining to evaluate HER2 expression in 108 patients with bladder UC who underwent radical cystectomy. They discovered that 57.4% of patients exhibited HER2 overexpression, which was significantly correlated with elevated tumor grade (p = 0.006) and staging (p < 0.001) (Bai et al., 2022). A meta-analysis by Zhao et al. (2015) also revealed a positive association between HER2 expression and high tumor grade. Similarly, Krüger et al. (2002) found that HER2 overexpression was more frequently detected in the high-grade cancer group compared to the low-grade cancer group among 138 bladder cancer cases. It should be noted that within the high-grade category, there are histological types associated with both better and poorer prognosis. Additionally, areas of high-grade UC specimens often exhibit negative tissue for HER2, indicating significant heterogeneity within UC, particularly in poorly differentiated tumors with a higher grade; thus suggesting that differences in HER2 overexpression may be linked to tumor heterogeneity.
According to our study, a significant difference was observed in the percentage of HER2+ among different clinical stages of UC (p = 0.019) (Table 2). This finding is consistent with another research study, which demonstrated a strong association between elevated levels of HER2 and the stage of UC at both mRNA and protein levels (p < 0.001) (Hussein et al., 2021). It is worth noting that HER2 overexpression is considered an early event in urothelial tumor development and rarely occurs during subsequent tumor progression. Therefore, there may be limited correlation with depth of myometrial invasion or lymph node metastasis (Goodman and Osunkoya, 2016). According to our current knowledge, there is limited literature discussing the association between blood indicators and HER2 expression in UC. However, relevant studies have been conducted in breast cancer. Specifically, red cell distribution width (RDW), RDW to platelet ratio (RPR), and platelet cell distribution width (PDW) have shown correlations with HER-2 expression in breast cancer tissues (Takeuchi et al., 2019).
Disitamab vedotin served as a second-line therapy for patients with locally-advanced or metastatic UC expressing HER2. In a phase II study involving 43 HER2-positive UC patients, the ORR was determined to be 51.2% after a follow-up period of 20.3 months, while the median PFS and OS were found to be 6.9 months and 13.9 months, respectively (Sheng et al., 2021a). Another clinical study reported an ORR of 46.9% and a median PFS of 4.3 months, with a median OS of 14.8 months (Sheng et al., 2021b). Apart from DV, there are several other HER2 ADCs that have also been applied in UC. Trastuzumab emtansine (TDM1), an ADC comprising the anti-HER2 antibody rastuzumab, has received FDA approval for treating HER2-positive bladder cancer patients who have previously undergone paclitaxel/rastuzumab therapy. In the phase II KAMELEON study (NCT02999672), patients with advanced urothelial bladder cancer positive for HER2 were included (de Vries et al., 2023). Following a median follow-up duration of 7.39 months (4.11–10.02) and a median exposure duration of 7.14 weeks for the metastatic UBC cohort, an overall response rate (90% CI) of 38.5% (16.57%–64.52%) to TDM1 was achieved. In total, 84.6% (11/13) of patients in the UC cohort experienced ≥1 adverse event, all considered treatment-related. Trastuzumab deruxtecan (DS-8201a) is another HER2 ADC compound composed of a spliceable linker connecting trastuzumab and an exatecan derivative acting as a topoisomerase I inhibitor. Notably, DS-8201a exhibits a higher drug-antibody ratio compared to TDM1, enabling its efficacy even in tumors with low HER2 expression. The ORR of DS-8201a was reported as 25% (4/16) in a phase I dose-escalation and dose-expansion study (Banerji et al., 2019). Considering the remarkable efficacy of disitamab vedotin, DV received approval from the National Medical Products Administration in January 2022. The extracellular domain of RC48 is a humanized anti-HER2 antibody that conjugates with microtubule protein inhibitors (MMAE) through a cleavable linker. MMAE released via enzymatic hydrolysis exhibits high membrane permeability and exerts therapeutic effects on tumor cells exhibiting low or no expression of HER2 (Padua et al., 2022). Accordingly, an ongoing follow-up study (NCT04073602) investigating RC48-ADC in patients with low HER2 expression enrolled a total of nineteen participants, revealing an ORR of 26.3%, median PFS of 5.5 months, and median OS of 16.4 months (Xu et al., 2022a). Our findings align closely with those observed in our study.
ICIs targeting PD-1 have demonstrated promising results in the treatment of bladder cancer, particularly in cases of metastatic UC that have progressed after chemotherapy. Recent studies have reported on the combination therapy of PD-1 and HER2 targeting ADCs. In a breast cancer model study, the combination of disitamab vedotin and PD-1 antibody exhibited remarkable efficacy in mice, surpassing the effects observed with either disitamab vedotin or PD-1 antibody alone. Furthermore, this combined treatment facilitated the formation of immunological memory, providing protection against tumor rechallenge (Huang et al., 2022). The observed infiltration of immune cells in mouse tumors following disitamab vedotin therapy suggests the potential for synergistic therapeutic effects by combining an immune checkpoint inhibitor (PD-1 inhibitor). Clinical data also supports this, with a report on the combination of RC48 and pembrolizumab in advanced UC (Xu et al., 2022b). The patient achieved CR and long-term PFS (>12 months). In a phase Ib/II study (RC48-C014), preliminary results demonstrated promising synergistic efficacy of RC48 in combination with toripalimab for advanced UC patients (Zhou et al., 2022a). The recommended dosage was RC48-ADC 2 mg/kg + toripalimab 3 mg/kg administered every 2 weeks. After a median follow-up of 8.0 months for 36 patients, the confirmed ORR was 76.7%. The median PFS at that time was 9.2 months, while the median OS had not been reached (Sheng et al., 2022). Another clinical study conducted in Fujian Province, China enrolled nine locally advanced or metastatic UC patients who were treated with DV combined with tislelizumab/toripalimab. After a median follow-up of 12 months, the ORR was found to be 88.9% (Wei et al., 2023). Additionally, ongoing clinical trials are investigating vedotin-tislelizumab as neoadjuvant treatment for HER2-positive locally advanced bladder urothelial carcinoma patients (Wen et al., 2024; Wen et al., 2022). In our study, the addition of DV was implemented following the failure of tislelizumab monotherapy. Notably, positive PD-L1 expression was observed in 20% of cases, while the efficacy of PD-1 alone exhibited limitations (Ma et al., 2023). Encouragingly, a clinical trial (RC48-C014) demonstrated that combining RC48-ADC with toripalimab yielded promising efficacy (ORR of 75% in all patients) for individuals with metastatic urothelial carcinoma (Zhou et al., 2022b), surpassing the outcomes achieved by DV monotherapy. Consequently, we opted for combination therapy involving disitamab vedotin and tislelizumab. In our study involving sixteen locally-advanced/metastatic UC patients, after a median follow-up of 14 months, the ORR among HER2+ patients was observed to be 70%, whereas it was found to be 50% among HER2-patients. Overall, the ORR reached up to 62.5% across all sixteen patients studied. These findings highlight the efficacy of RC48 not only in HER2+ but also in HER2- UC patients.
The RC48 exhibited manageable adverse effects in patients with UC. In our study, 68.8% (11/16) of the patients reported side effects, all of which were classified as grade 1 or 2. The most frequently reported side effects included hypaesthesia, hair loss, and leukopenia. In other studies on UC, patients experienced grade 1 or 2 side effects such as loss of appetite, rash, and fatigue (Wei et al., 2023), as well as grade 3 side effects including hypoesthesia and neutropenia (Sheng et al., 2021a). Other reported grade 3 side effects comprised anemia, hypoalbuminemia, urinary tract infection, and autoimmune encephalitis (Chen et al., 2023). All these side effects were effectively managed through appropriate treatments.
There are several limitations in our study. Firstly, the duration of follow-up was insufficient to obtain data on PFS or OS. In this paper, we solely investigated HER2 expression in UC based on the 2021 edition of the clinical pathological expert consensus on HER2 testing in UC in China. The association between HER2 positivity and long-term prognosis in UC patients remains unknown. Secondly, advanced patients received a combination therapy of DV and PD-1 inhibitors rather than DV alone, as our aim was to achieve improved patient outcomes. Further research is warranted to gain a better understanding of the long-term efficacy of DV monotherapy in UC patients. Although preclinical studies have shown promising results with RC48 used alongside PD-1/L1 inhibitors, additional evidence is required from clinical practice.
HER2 is a promising therapeutic target for UC, and its expression level holds critical significance in treatment response. Currently, HER2-targeting ADCs have demonstrated remarkable efficacy in select clinical trials (Zhou et al., 2023). However, the existing evaluation criteria for HER2 are inadequate for UC, leading to substantial discrepancies among research findings. Therefore, the establishment of a standardized scoring system is imperative to accurately identify individuals suitable for anti-HER2 ADC therapy and holds significant clinical implications. In this study, we adopted a novel Chinese standard to assess the expression rate of HER2 in high-grade UC patients with the aim of promoting uniformity in evaluating HER2 expression across UC studies. Our results indicate widespread protein expression of HER2 in urothelial carcinoma and reveal its close association with advanced stages of high-grade urothelial carcinoma. Targeting HER2 presents a potential therapeutic pathway for tumor management in UC patients. Combination therapy involving DV inhibitors and PD-1 blockade demonstrates both efficacy and acceptable side effects when treating advanced UC patients with either positive or negative HER2 status.
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
The studies involving humans were approved by the ethics committee of Shandong Provincial Hospital Affiliated to Shandong First Medical University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
KZ: Data curation, Funding acquisition, Software, Writing–original draft, Writing–review and editing. YC: Data curation, Investigation, Methodology, Writing–original draft. DZ: Data curation, Investigation, Writing–original draft. AG: Data curation, Writing–original draft. JC: Methodology, Writing–original draft. CW: Data curation, Writing–original draft. YG: Data curation, Formal Analysis, Software, Writing–review and editing. SD: Conceptualization, Project administration, Supervision, Writing–review and editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Shandong Provincial Natural Science Foundation (Grant No. ZR2021QH313, ZR202306200045, and ZR2023LZL005); and the Academic Promotion Programme by Shandong First Medical University (Grant No. 2020LI001).
The authors thank the study patients for their participation and trust.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Bai, X., He, W., Yin, H., Li, X., Zhou, X., Wei, Z., et al. (2022). Prognostic significance of HER2 status evaluation using immunohistochemistry in patients with urothelial carcinoma of the bladder: a retrospective single-center experience. Exp. Ther. Med. 24 (5), 704. doi:10.3892/etm.2022.11640
Banerji, U., van Herpen, C. M. L., Saura, C., Thistlethwaite, F., Lord, S., Moreno, V., et al. (2019). Trastuzumab duocarmazine in locally advanced and metastatic solid tumours and HER2-expressing breast cancer: a phase 1 dose-escalation and dose-expansion study. Lancet Oncol. 20 (8), 1124–1135. doi:10.1016/S1470-2045(19)30328-6
Bellmunt, J., Werner, L., Bamias, A., Fay, A. P., Park, R. S., Riester, M., et al. (2015). HER2 as a target in invasive urothelial carcinoma. Cancer Med. 4 (6), 844–852. doi:10.1002/cam4.432
Chang, E., Weinstock, C., Zhang, L., Charlab, R., Dorff, S. E., Gong, Y., et al. (2021). FDA approval summary: enfortumab vedotin for locally advanced or metastatic urothelial carcinoma. Clin. Cancer Res. 27 (4), 922–927. doi:10.1158/1078-0432.CCR-20-2275
Cheetham, P. J., and Petrylak, D. P. (2016). New agents for the treatment of advanced bladder cancer. Oncol. Willist. Park 30 (6), 571–588.
Chen, M., Yao, K., Cao, M., Liu, H., Xue, C., Qin, T., et al. (2023). HER2-targeting antibody-drug conjugate RC48 alone or in combination with immunotherapy for locally advanced or metastatic urothelial carcinoma: a multicenter, real-world study. Cancer Immunol. Immunother. 72 (7), 2309–2318. doi:10.1007/s00262-023-03419-1
de Vries, E. G. E., Ruschoff, J., Lolkema, M., Tabernero, J., Gianni, L., Voest, E., et al. (2023). Phase II study (KAMELEON) of single-agent T-DM1 in patients with HER2-positive advanced urothelial bladder cancer or pancreatic cancer/cholangiocarcinoma. Cancer Med. 12 (11), 12071–12083. doi:10.1002/cam4.5893
Eisenhauer, E. A., Therasse, P., Bogaerts, J., Schwartz, L. H., Sargent, D., Ford, R., et al. (2009). New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur. J. Cancer 45 (2), 228–247. doi:10.1016/j.ejca.2008.10.026
Fan, Y., Li, Q., Shen, Q., Liu, Z., Zhang, Z., Hu, S., et al. (2022). Head-to-Head comparison of the expression differences of NECTIN-4, TROP-2, and HER2 in urothelial carcinoma and its histologic variants. Front. Oncol. 12, 858865. doi:10.3389/fonc.2022.858865
Goodman, A. L., and Osunkoya, A. O. (2016). Human epidermal growth factor receptor 2 expression in micropapillary urothelial carcinoma of the bladder: an analysis of 27 cases. Hum. Pathol. 57, 160–164. doi:10.1016/j.humpath.2016.07.014
He, W., Chen, C., Lin, T., Xu, Q., Ye, C., Du, J., et al. (2023). Epidemiology, treatments, and related biomarkers of locally advanced or metastatic urothelial carcinoma in Chinese population: a scoping review. Cancer Med. 12 (14), 15384–15403. doi:10.1002/cam4.6112
Hepp, Z., Shah, S. N., Smoyer, K., and Vadagam, P. (2021). Epidemiology and treatment patterns for locally advanced or metastatic urothelial carcinoma: a systematic literature review and gap analysis. J. Manag. Care Spec. Pharm. 27 (2), 240–255. doi:10.18553/jmcp.2020.20285
HERExpert Committee on Urothelial Carcinoma of Chinese Society of Clinical Oncology (2021). Clinical pathological expert consensus on HER-2 testing in urothelial carcinoma in China. Zhonghua Zhong Liu Za Zhi 43 (10), 1001–1006. doi:10.3760/cma.j.cn112152-20210809-00597
Hoimes, C. J., Flaig, T. W., Milowsky, M. I., Friedlander, T. W., Bilen, M. A., Gupta, S., et al. (2023). Enfortumab vedotin plus pembrolizumab in previously untreated advanced urothelial cancer. J. Clin. Oncol. 41 (1), 22–31. doi:10.1200/JCO.22.01643
Huang, L., Wang, R., Xie, K., Zhang, J., Tao, F., Pi, C., et al. (2022). A HER2 target antibody drug conjugate combined with anti-PD-(L)1 treatment eliminates hHER2+ tumors in hPD-1 transgenic mouse model and contributes immune memory formation. Breast Cancer Res. Treat. 191 (1), 51–61. doi:10.1007/s10549-021-06384-4
Hussein, S., Fathi, A., Abouhashem, N. S., Amer, S., Hemeda, M., and Mosaad, H. (2021). SATB-1 and Her2 as predictive molecular and immunohistochemical markers for urothelial cell carcinoma of the bladder. Cancer Biomark. 30 (2), 249–259. doi:10.3233/CBM-200072
Kiss, B., Wyatt, A. W., Douglas, J., Skuginna, V., Mo, F., Anderson, S., et al. (2017). Her2 alterations in muscle-invasive bladder cancer: patient selection beyond protein expression for targeted therapy. Sci. Rep. 7, 42713. doi:10.1038/srep42713
Krüger, S., Weitsch, G., Büttner, H., Matthiensen, A., Böhmer, T., Marquardt, T., et al. (2002). HER2 overexpression in muscle-invasive urothelial carcinoma of the bladder: prognostic implications. Int. J. cancer 102 (5), 514–518. doi:10.1002/ijc.10731
Ma, Y. T., Hua, F., Zhong, X. M., Xue, Y. J., Li, J., Nie, Y. C., et al. (2023). Clinicopathological characteristics, molecular landscape, and biomarker landscape for predicting the efficacy of PD-1/PD-L1 inhibitors in Chinese population with mismatch repair deficient urothelial carcinoma: a real-world study. Front. Immunol. 14, 1269097. doi:10.3389/fimmu.2023.1269097
Mendelsohn, J., and Baselga, J. (2003). Status of epidermal growth factor receptor antagonists in the biology and treatment of cancer. J. Clin. Oncol. 21 (14), 2787–2799. doi:10.1200/JCO.2003.01.504
Olt, G., Berchuck, A., and Bast, R. C. (1990). The role of tumor markers in gynecologic oncology. Obstet. Gynecol. Surv. 45 (9), 570–577. doi:10.1097/00006254-199009000-00002
Padua, T. C., Moschini, M., Martini, A., Pederzoli, F., Nocera, L., Marandino, L., et al. (2022). Efficacy and toxicity of antibody-drug conjugates in the treatment of metastatic urothelial cancer: a scoping review. Urol. Oncol. 40 (10), 413–423. doi:10.1016/j.urolonc.2022.07.006
Press, M. F., Jones, L. A., Godolphin, W., Edwards, C. L., and Slamon, D. J. (1990). HER-2/neu oncogene amplification and expression in breast and ovarian cancers. Prog. Clin. Biol. Res. 354A, 209–221.
Rosenberg, J. E., O'Donnell, P. H., Balar, A. V., McGregor, B. A., Heath, E. I., Yu, E. Y., et al. (2019). Pivotal trial of enfortumab vedotin in urothelial carcinoma after platinum and anti-programmed death 1/programmed death ligand 1 therapy. J. Clin. Oncol. 37 (29), 2592–2600. doi:10.1200/JCO.19.01140
Scherrer, E., Kang, A., Bloudek, L. M., and Koshkin, V. S. (2022). HER2 expression in urothelial carcinoma, a systematic literature review. Front. Oncol. 12, 1011885. doi:10.3389/fonc.2022.1011885
Sheng, X., He, Z., Han, W., Zhou, A.-P., Luo, H., Shi, Y., et al. (2021b). An open-label, single-arm, multicenter, phase II study of RC48-ADC to evaluate the efficacy and safety of subjects with HER2 overexpressing locally advanced or metastatic urothelial cancer (RC48-C009). 39(15_Suppl. l):4584. doi:10.1200/jco.2021.39.15_suppl.4584
Sheng, X., Yan, X., Wang, L., Shi, Y., Yao, X., Luo, H., et al. (2021a). Open-label, multicenter, phase II study of RC48-ADC, a HER2-targeting antibody-drug conjugate, in patients with locally advanced or metastatic urothelial carcinoma. Clin. Cancer Res. 27 (1), 43–51. doi:10.1158/1078-0432.CCR-20-2488
Sheng, X., Zhou, L., He, Z., Guo, H., Yan, X., Li, S., et al. (2022). Preliminary results of a phase Ib/II combination study of RC48-ADC, a novel humanized anti-HER2 antibody-drug conjugate (ADC) with toripalimab, a humanized IgG4 mAb against programmed death-1 (PD-1) in patients with locally advanced or metastatic urothelial carcinoma. 40(16_Suppl. l):4518. doi:10.1200/jco.2022.40.16_suppl.4518
Sung, H., Ferlay, J., Siegel, R. L., Laversanne, M., Soerjomataram, I., Jemal, A., et al. (2021). Global cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA a cancer J. Clin. 71 (3), 209–249. doi:10.3322/caac.21660
Takeuchi, H., Abe, M., Takumi, Y., Hashimoto, T., Miyawaki, M., Okamoto, T., et al. (2019). Elevated red cell distribution width to platelet count ratio predicts poor prognosis in patients with breast cancer. Sci. Rep. 9 (1), 3033. doi:10.1038/s41598-019-40024-8
Wei, Y., Zhang, R., Yu, C., Hong, Z., Lin, L., Li, T., et al. (2023). Disitamab vedotin in combination with immune checkpoint inhibitors for locally and locally advanced bladder urothelial carcinoma: a two-center's real-world study. Front. Pharmacol. 14, 1230395. doi:10.3389/fphar.2023.1230395
Wen, F., Lin, T., Tan, P., Zheng, X., Liu, J., Zhang, P., et al. (2022). 135O A multi-center phase Ib/II study of RC48-ADC combined with tislelizumab as neoadjuvant treatment in patients with HER2 positive locally advanced MIBC. Ann. Oncol. 33, S1485. doi:10.1016/j.annonc.2022.10.170
Wen, F., Lin, T., Zhang, P., and Shen, Y. (2024). RC48-ADC combined with tislelizumab as neoadjuvant treatment in patients with HER2-positive locally advanced muscle-invasive urothelial bladder cancer: a multi-center phase Ib/II study (HOPE-03). Front. Oncol. 13, 1233196. doi:10.3389/fonc.2023.1233196
Xu, H., Sheng, X., Zhou, L., Yan, X., Li, S., Chi, Z., et al. (2022a). A phase II study of RC48-ADC in HER2-negative patients with locally advanced or metastatic urothelial carcinoma. J. Clin. Oncol. 40 (16_Suppl. l), 4519. doi:10.1200/jco.2022.40.16_suppl.4519
Xu, Z., Ma, J., Chen, T., and Yang, Y. (2022b). Case report: the remarkable response of pembrolizumab combined with RC48 in the third-line treatment of metastatic urothelial carcinoma. Front. Immunol. 13, 978266. doi:10.3389/fimmu.2022.978266
Zhao, J., Xu, W., Zhang, Z., Song, R., Zeng, S., Sun, Y., et al. (2015). Prognostic role of HER2 expression in bladder cancer: a systematic review and meta-analysis. Int. urology Nephrol. 47 (1), 87–94. doi:10.1007/s11255-014-0866-z
Zhou, L., Shao, Z., Liu, Y., Yan, X., Li, J., Wu, X., et al. (2023). HER2 expression associated with clinical characteristics and prognosis of urothelial carcinoma in a Chinese population. Oncologist 28 (8), e617–e624. doi:10.1093/oncolo/oyad070
Zhou, L., Xu, H., Li, S., Yan, X., Li, J., Wu, X., et al. (2022a). Study RC48-C014: preliminary results of RC48-ADC combined with toripalimab in patients with locally advanced or metastatic urothelial carcinoma. 40(6_Suppl. l):515. doi:10.1200/jco.2022.40.6_suppl.515
Keywords: urothelial carcinoma, high-grade, HER2, antibody-drug conjugate, clinical significance, prognosis, pathology
Citation: Zhu K, Chang Y, Zhao D, Guo A, Cao J, Wu C, Guan Y and Ding S (2024) Expression of HER2 in high-grade urothelial carcinoma based on Chinese expert consensus and the clinical effects of disitamab vedotin-tislelizumab combination therapy in the treatment of advanced patients. Front. Pharmacol. 15:1355081. doi: 10.3389/fphar.2024.1355081
Received: 13 December 2023; Accepted: 07 February 2024;
Published: 22 February 2024.
Edited by:
Lei Yin, Shanghai Jiao Tong University, ChinaReviewed by:
Wang Yidi, First Affiliated Hospital of Zhengzhou University, ChinaCopyright © 2024 Zhu, Chang, Zhao, Guo, Cao, Wu, Guan and Ding. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sentai Ding, ZGluZ3NlbnRhaUAxMjYuY29t
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.