- Hunan Provincial People’s Hospital, The First Affiliated Hospital of Hunan Normal University, Changsha, China
Background: PCSK9 inhibitors are a novel class of lipid-lowering medications, and numerous clinical studies have confirmed their significant role in improving the progression of chronic kidney disease. However, recent case reports have indicated new evidence regarding their association with acute kidney injury (AKI), with some patients experiencing acute tubular injury after PCSK9 inhibitors use.
Objectives: To clarify the relationship between PCSK9 inhibitors and AKI, we conducted a pharmacovigilance study.
Methods: Using the Food and Drug Administration Adverse Event Reporting System (FAERS) database from the third quarter of 2015 to the fourth quarter of 2022, a disproportionality analysis was employed to identify adverse events suggestive of AKI after PCSK9 inhibitors use. The drugs of interest included evolocumab and alirocumab.
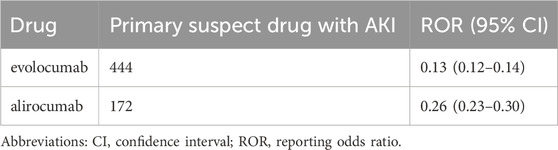
Results: A total of 144,341 adverse event reports related to PCSK9 inhibitors were analyzed, among which 444 cases were suspected of AKI for evolocumab, and 172 cases for alirocumab. Evolocumab had a greater impact on AKI in males (ROR 1.4, 95% CI 1.54–1.69). The ROR and 95% CI for evolocumab and Alirocumab were 0.13 (0.12–0.14) and 0.26 (0.23–0.30) respectively. Further analysis of AKI associated with the concomitant use of PCSK9 inhibitors with cephalosporins, furosemide, torsemide, pantoprazole, omeprazole, and esomeprazole revealed ROR and 95% CI of 0.38 (0.23–0.62), 0.38 (0.31–0.48), 0.18 (0.08–0.38), 0.23 (0.17–0.29), 0.20 (0.16–0.26), and 0.14 (0.10–0.20) respectively.
Conclusion: Through the FAERS database, we analyzed the clinical characteristics of AKI associated with PCSK9 inhibitors, exploring its risks. Our findings suggest that PCSK9 inhibitors might have a potential protective effect against AKI and exhibit similar effects when co-administered with other nephrotoxic drugs.
Highlights
• PCSK9 inhibitors may be potentially protective against AKI to some extent and show similar effects when combined with other nephrotoxic drugs.
1 Background
Proprotein Convertase Subtilisin/kexin Type 9 (PCSK9) is a serine protease synthesized by liver cells, which circulates in the bloodstream and forms complexes with Low-Density Lipoprotein Receptors (LDL-R). These complexes are eventually degraded in lysosomes within liver cells, leading to decreased surface LDL-R levels. LDL-R is a crucial factor for liver cell uptake and metabolism of LDL cholesterol (LDL-C). Consequently, PCSK9 elevates LDL-C levels in the body (Rosenson et al., 2018). Research indicates that elevated serum LDL-C levels are an independent risk factor for Atherosclerotic Cardiovascular Disease (ASCVD). Clinical trial data have demonstrated a correlation between lowering LDL-C levels and reducing cardiovascular risk (Silverman et al., 2016). Therefore, reducing LDL-C is a key strategy for primary and secondary prevention of ASCVD (Stone et al., 2014). PCSK9 inhibitors significantly reduce LDL-C levels in the human body through two main pathways: first, by inhibiting the binding of PCSK9 to LDL-C receptors and second, by intervening in the synthesis and processing of PCSK9 (Lagace, 2014). In a Phase I clinical trial, the anti-PCSK9 siRNA ALN-PCS reduced free PCSK9 levels by 70% and lowered LDL-C levels by 40% (Fitzgerald et al., 2014).
PCSK9 in circulation primarily originates from the liver. Additionally, it is expressed in the pancreas, kidneys, intestines, and central nervous system. While PCSK9 regulates cholesterol metabolism by modulating LDL receptor expression in the liver, in vitro and in vivo studies suggest that PCSK9 is involved in various other physiological processes (Stoekenbroek et al., 2018). Studies (Toth et al., 2018) have demonstrated the lipid-lowering effects of PCSK9 inhibitors in patients with chronic kidney disease (CKD), showing good safety and efficacy. At week 24, LDL-C reduction ranged from 46.1% to 62.2% in patients with or without renal impairment, respectively. The overall incidence of adverse reactions was similar between the treatment and control groups (82.1% vs. 82.8% in CKD patients; 78.4% vs. 78.2% in non-CKD patients). Furthermore, the more severe the CKD, the greater the absolute reduction in cardiovascular deaths, myocardial infarctions, or strokes associated with PCSK9 inhibitors use (Charytan et al., 2019). Patients with CKD stage 3 or higher, when treated with PCSK9 inhibitors for 30 months, experienced a significantly greater absolute risk reduction compared to patients with normal renal function, with reductions of −2.5% (95% confidence interval (CI) −4.7% to −0.4%) and −1.7% (95% CI: -2.8%–0.5%) respectively. Studies (Haas et al., 2016; Jatem et al., 2021) also revealed that knockout mice with nephrotic syndrome lacking liver Pcsk9 exhibited a 40%–50% reduction in plasma cholesterol and triglycerides. Patients with primary refractory nephrotic syndrome showed an average LDL-C reduction of 36.8% ± 4.9% mmol/L after 4 weeks of PCSK9 inhibitors therapy, which remained stable throughout the follow-up period. In contrast, total cholesterol or LDL-C levels showed no significant change in the statin-treated group, suggesting that PCSK9 inhibitors might be effective and safe alternatives for treating hypercholesterolemia associated with refractory nephrotic syndrome. Recent research (Skeby et al., 2023) has reported interactions between PCSK9 and Megalin in proximal tubular cells. By affecting megalin-driven protein reabsorption, PCSK9 influences urinary protein excretion. PCSK9 inhibitors increase renal megalin in mice with kidney disease, simultaneously reducing urinary albumin excretion. This discovery provides new strategies for treating CKD.
However, there are also case reports (Jhaveri et al., 2017; Pickett et al., 2020) suggesting that PCSK9 inhibitors can induce AKI. One case involved a 62-year-old female patient (Jhaveri et al., 2017) with comorbidities such as heart disease, hyperlipidemia, Stage 5 CKD, and hypertension. The patient denied using any non-steroidal anti-inflammatory drugs, antibiotics, or herbal supplements. After using alirocumab, her serum creatinine increased from a baseline of 2.3 mg/dL to 5.0 mg/dL. Kidney biopsy revealed acute tubular injury and necrosis. The authors speculated that this might be due to the overexpression of renal PCSK9 during the inhibition of the inflammatory process, which is actually a protective response to cellular damage. Another case involved a 72-year-old male patient (Pickett et al., 2020) with coronary artery disease, statin intolerance, and Stage 3 CKD. After using alirocumab, the patient also experienced acute tubular injury detected in kidney biopsy. Upon discontinuation of the medication, serum creatinine levels returned to baseline.
Currently, there are no specific research reports on the correlation between PCSK9 inhibitors and AKI. Whether they can reduce the risk of AKI remains unknown. AKI is characterized by a rapid decline in kidney function, accompanied by the accumulation of metabolic products such as creatinine and urea, constituting a clinical syndrome (Bellomo et al., 2012). It is typically transient and often overlooked. However, AKI is associated with increased risk of mortality. A prospective study (Kaddourah et al., 2017) involving 4,683 patients from multiple countries showed that by day 28, 543 patients developed severe AKI, leading to an increased risk of death (adjusted odds ratio 1.77; 95% CI, 1.17–2.68). The SEA-AKI study (Kulvichit et al., 2022) also reported an in-hospital mortality rate of up to 14.6% for AKI. Moreover, as the severity of AKI increases, the risk of death also rises (Hoste and Kellum, 2006; Hoste et al., 2015). The adjusted odds ratios for in-hospital death in AKI stages 1, 2, and 3 were 1.679 (95% CI 0.890–3.169; p = 0.109), 2.945 (95% CI 1.382–6.276; p = 0.005), and 6.884 (95% CI 3.876–12.228; p < 0.001), respectively (Hoste et al., 2015).
To further elucidate the correlation between PCSK9 inhibitors and AKI, we conducted a pharmacovigilance study using FAERS database. This spontaneously reported adverse reaction database is freely accessible and includes a diverse population and various medications. It is useful for capturing adverse events occurring shortly after drug exposure and can detect adverse events not found in clinical trials, especially for rare events with low background rates.
2 Methods
2.1 Data source
The data for this study were obtained from FAERS database, a large, publicly accessible database consisting of adverse reaction cases reported by various populations, including healthcare professionals, consumers, and lawyers. AERSMine, a website developed based on the FAERS database, provides convenient and precise search services. It has become a mature tool for mining and analyzing drug adverse reactions (Sarangdhar et al., 2016; Xia et al., 2022; Xia et al., 2023). We conducted an observational, retrospective, cross-sectional pharmacovigilance study using post-marketing data from the FAERS database, spanning from the third quarter of 2015 to the fourth quarter of 2022. Ethical approval was not required for this study as it utilized de-identified data.
2.2 Drug selection and adverse reaction definition
The drugs of interest in this study were evolocumab and alirocumab, both approved by the FDA and EMA in 2015. We used the preferred terms (PTs) under the category of acute kidney failure based on the standard MedDRA query (SMQ) as keywords to identify target adverse reactions. Selected PTs are showed in Supplementary Material. For a better description of the characteristics and further analysis of PCSK9 inhibitors’ association with AKI, we specified the drug role as “primary suspect.”
2.3 Drug interaction analysis
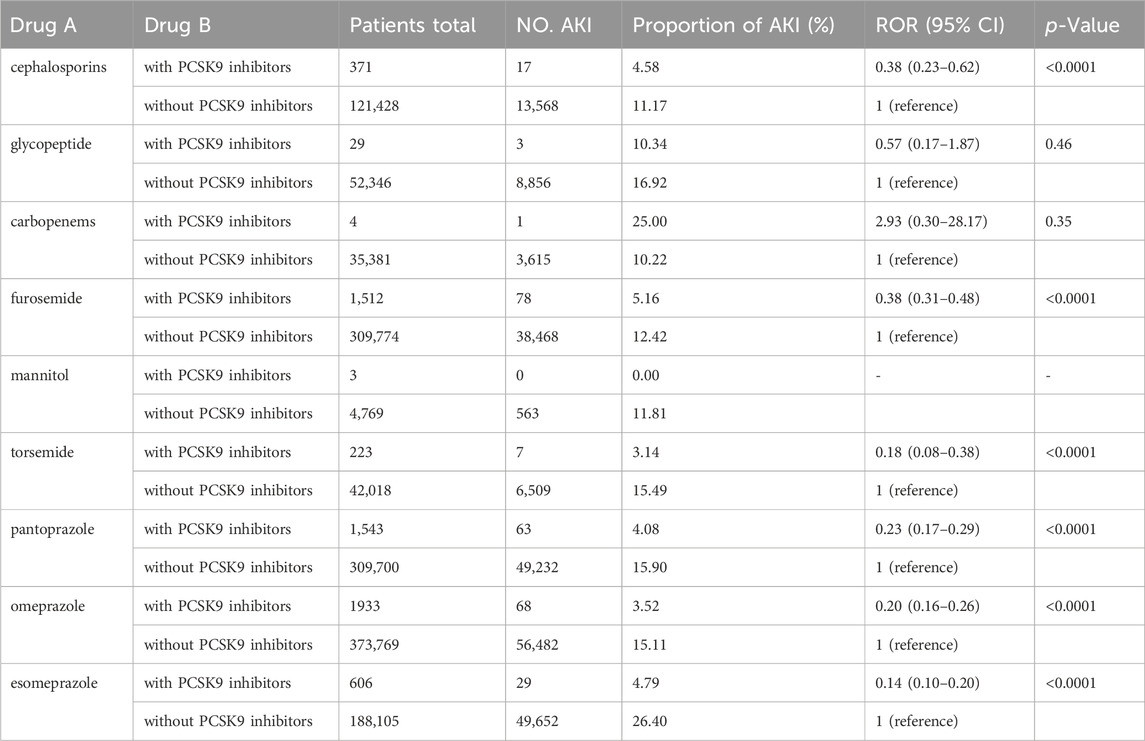
In clinical practice, combination therapy is common. However, the impact of concomitant use of PCSK9 inhibitors with other medications on AKI is not well understood. Therefore, we analyzed commonly used nephrotoxic drugs to explore the effect of their co-administration with PCSK9 inhibitors on AKI. A nationwide cross-sectional survey (Liu et al., 2021) involving 23 academic hospitals in 17 provinces of China revealed that the top three categories of drugs causing drug-induced AKI were antimicrobial drugs, diuretics, and proton pump inhibitors (PPIs). Within these categories, the most common drugs were cephalosporins, glycopeptides, and carbapenems for antimicrobial drugs; furosemide, mannitol, and torsemide for diuretics; and pantoprazole, omeprazole, and esomeprazole for PPIs. We analyzed the occurrence of AKI when PCSK9 inhibitors were co-administered with these nine drugs.
2.4 Data mining and statistical methods
In this study, we employed the Reporting Odds Ratio (ROR) method, a disproportionality analysis, for risk analysis and mining. This method is also the most commonly used approach in current pharmacovigilance studies (Anand et al., 2019; Zhou et al., 2023). ROR with positive signal detection criteria was defined as having a report count ≥3 and a lower limit of the 95% CI of ROR >1. The method for calculating the ROR is provided in the Supplementary Material. Additionally, based on existing literature reports, we analyzed the risk of AKI when PCSK9 inhibitors were co-administered with common nephrotoxic drugs. Subgroup analyses were performed in different gender, age groups and underlying diseases to enhance the reliability and stability of the research results. Pearson’s chi-squared test or Fisher’s exact test was used to compare the reporting of PCSK9 inhibitors-related AKI, with statistical significance determined by a 95% CI, and p < 0.05 was considered significant. Statistical analysis was performed using SPSS 25.0 and Microsoft Excel 2019.
3 Results
3.1 Basic characteristics
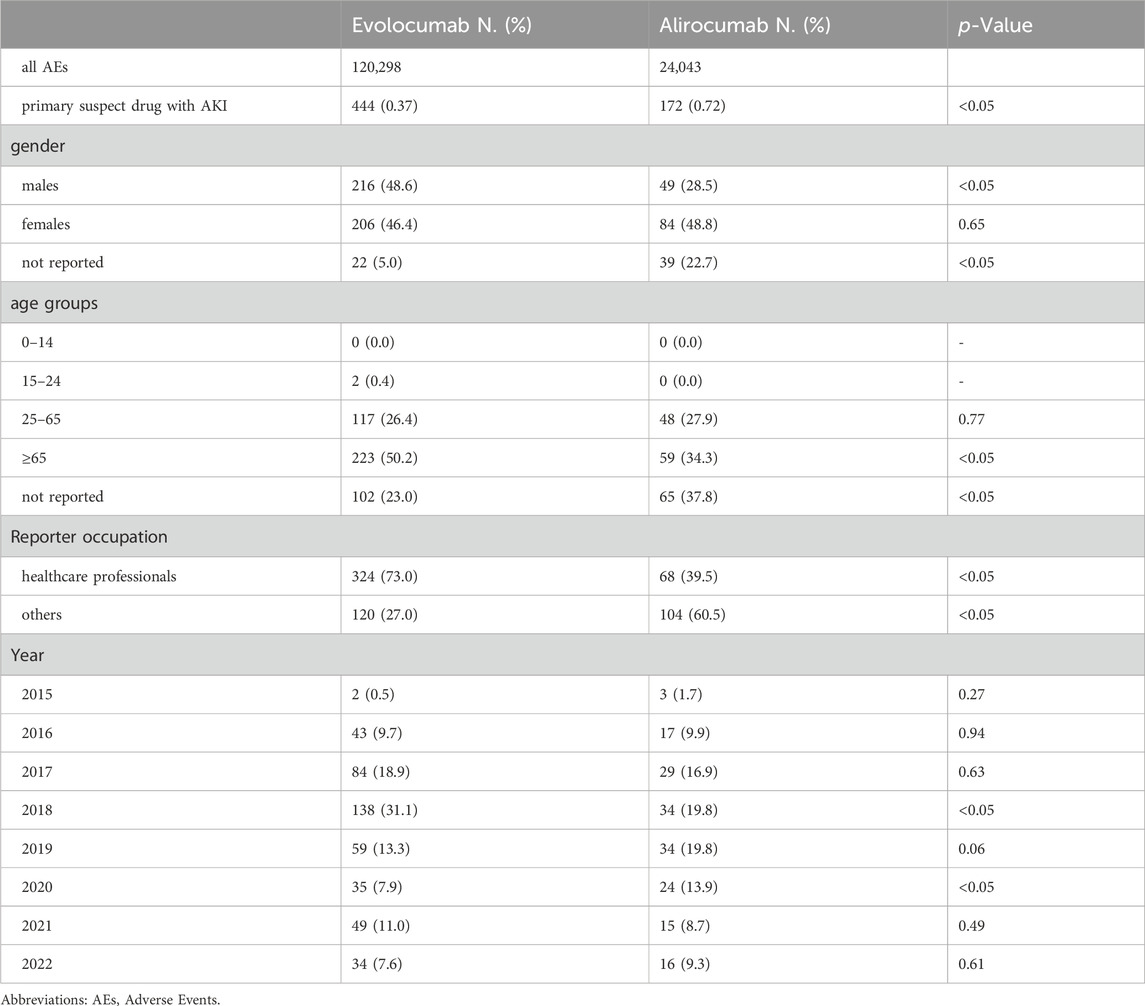
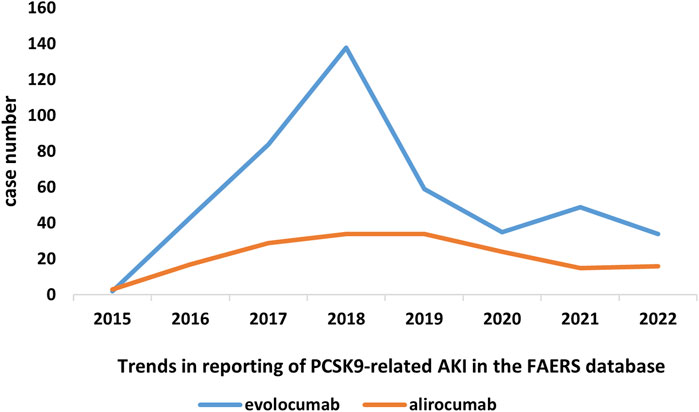
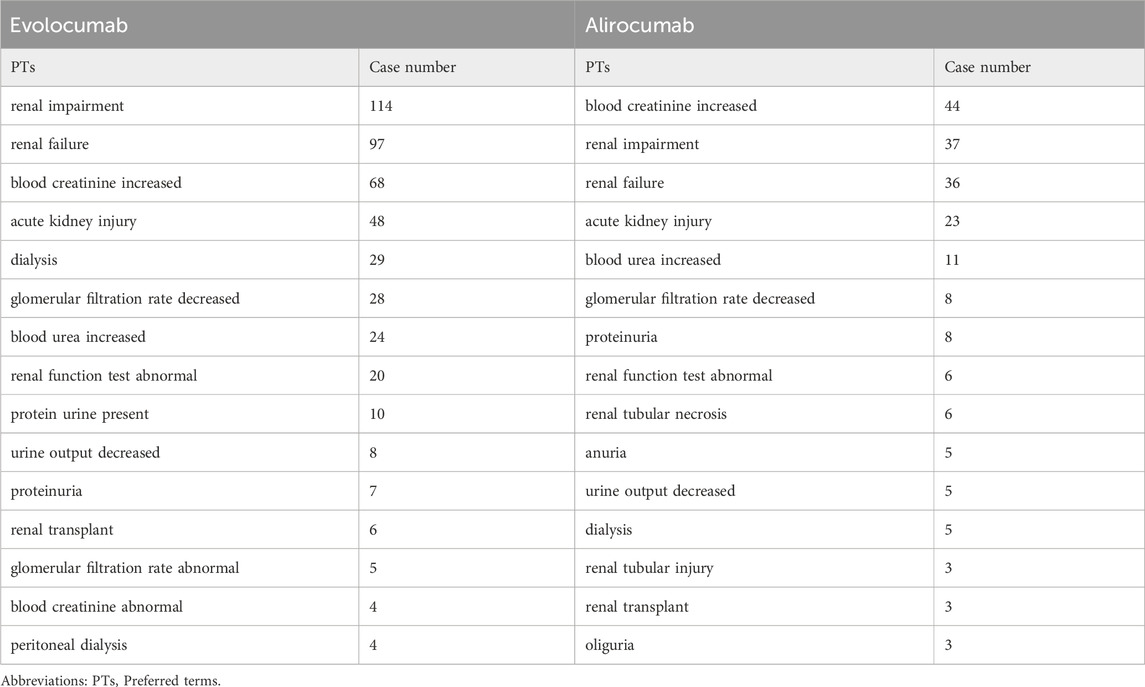
In this study, we ultimately included 444 cases of evolocumab and 172 cases of alirocumab, as shown in Figure 1. All of which were primary suspect cases associated with AKI, reported in the FAERS database from the third quarter of 2015 to the fourth quarter of 2022. Table 1 summarizes the clinical characteristics of these cases. The reporting proportions of evolocumab-related AKI were similar between males (48.6%) and females (46.4%), p = 0.55, while alirocumab-related AKI was notably higher in females, p < 0.05. Evolocumab-related AKI cases were primarily reported in individuals aged 65 and above, with the rest distributed mainly between 25–65 years and in age-unreported cases. In contrast, alirocumab-related AKI cases were relatively evenly distributed among individuals aged 25–65, 65 and above, and those with unknown ages, accounting for 27.9%, 34.3%, and 37.8%, respectively. Notably, AKI related to both drugs almost did not occur in individuals below 24 years old, likely due to the specific demographic of users. Evolocumab-related cases were predominantly reported by healthcare professionals, while the pattern was reversed for alirocumab, p < 0.05. The number of reported AKI adverse reactions for both drugs gradually increased after market introduction, reaching a peak in 2018 and subsequently declining, as shown in Table 1 and Figure 2. Specifically, evolocumab-related AKI cases reached 138 in 2018, accounting for 31.1% of the total cases. The most frequently reported PTs for evolocumab and alirocumab-related AKI were “blood creatinine increased,” “renal failure,” “renal impairment,” and “acute kidney injury,” as listed in Table 2. The patient outcomes for AKI cases associated with evolocumab and alirocumab are presented in the Supplementary Material. Overall, the number of hospitalizations and other serious outcomes is higher, while instances of death, disability, and life-threatening conditions are relatively fewer.
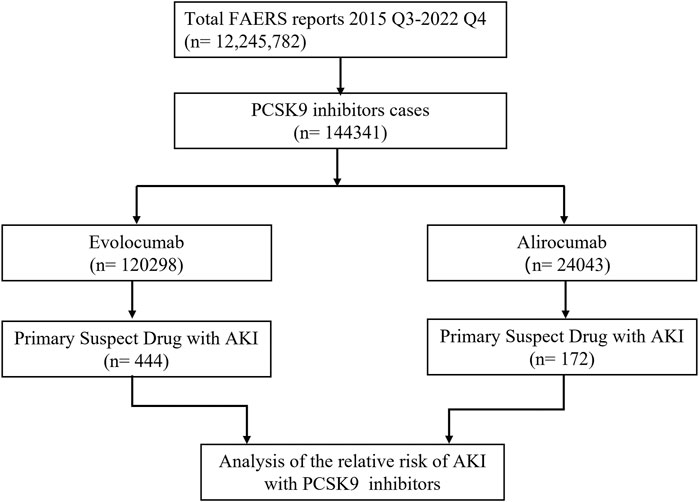
Figure 1. Flow chart of study design. In this study, 12, 245, 782 reports were retrieved from the FAERS database during the third quarter of 2015 to the fourth quarter of 2022. Among these reports, 144,341 cases were linked to adverse reactions related to PCSK9 inhibitors. To investigate the correlation between PCSK9 inhibitors and AKI, we selected the primary suspect drug for further analysis.
3.2 Disproportionality analysis
In this study, we used ROR algorithm in disproportionality analysis to detect the association between AKI and the use of evolocumab and alirocumab. The results are presented in Table 3. We found a negative correlation between the use of these two drugs and the reporting of AKI, indicating a potential protective effect against AKI. Furthermore, evolocumab demonstrated a stronger protective effect compared to alirocumab, with ROR and 95% CI for evolocumab-related and alirocumab-related AKI being 0.13 (0.12–0.14) and 0.26 (0.23–0.30), respectively.
3.3 Interaction analysis
In our analysis, we investigated the occurrence of AKI when PCSK9 inhibitors were combined with nine clinically common nephrotoxic drugs previously reported in studies (Liu et al., 2021). The results demonstrated that PCSK9 inhibitors could mitigate the nephrotoxic effects of cephalosporins, furosemide, torsemide, pantoprazole, omeprazole, and esomeprazole, with significant differences observed (ROR and 95% CI: 0.38 [0.23–0.62], 0.38 [0.31–0.48], 0.18 [0.08–0.38], 0.23 [0.17–0.29], 0.20 [0.16–0.26], and 0.14 [0.10–0.20], respectively). The results are presented in Table 4. However, conclusive results could not be drawn for the other three drugs due to insufficient case numbers.
3.4 Subgroup analysis
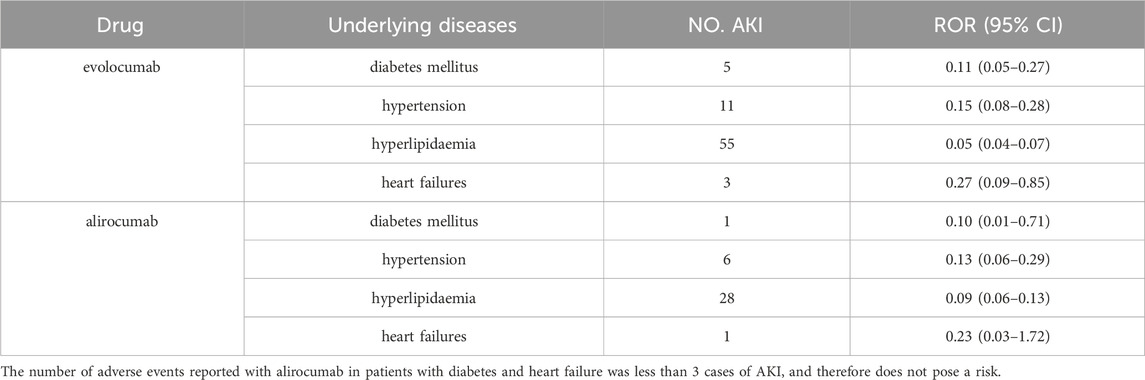
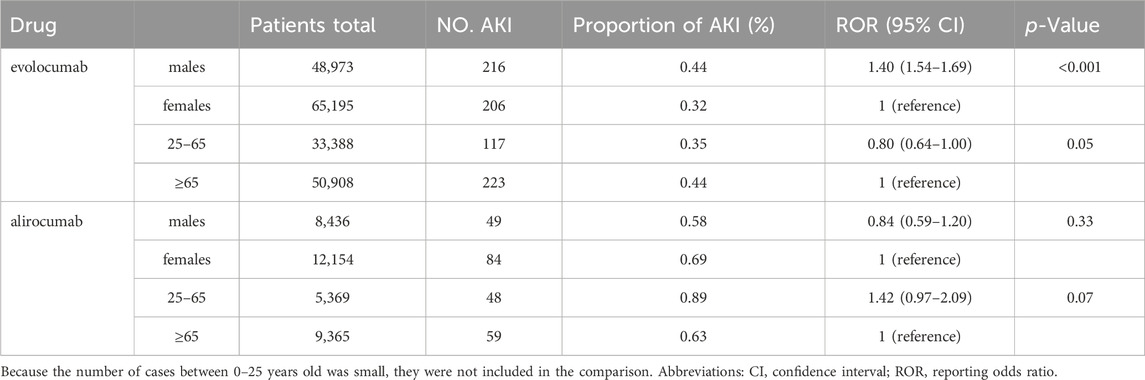
Table 5 and Table 6 presents the results of subgroup analysis. In patients with underlying disease, PCSK9 inhibitors still showed potential AKI protection, see Table 5. Evolocumab-related AKI was more likely to occur in males (ROR = 1.40, 95% CI = 1.54–1.69). In comparison with the population aged 65 and above, individuals between the ages of 25–65 appeared to have a slightly lower risk of AKI after using evolocumab, although this difference was not statistically significant. On the other hand, alirocumab showed a higher risk of AKI in patients aged 25–65, but again, this difference was not statistically significant. It is important to note that due to the limited number of reported cases in patients under 25 years old, a direct comparison for this age group could not be made. Additionally, the differences observed in this analysis require further investigation with larger sample sizes and more comprehensive prospective studies to draw definitive conclusions.
4 Discussion
Despite numerous clinical studies confirming the lipid-lowering effects of PCSK9 inhibitors in CKD patients and recent research indicating their potential to improve CKD, their protective role in AKI remains unclear. Most cases of AKI are transient and challenging to intervene in practical clinical trials. Additionally, case reports have suggested that PCSK9 inhibitors may cause acute renal tubular injury. Therefore, investigating the correlation between PCSK9 inhibitors and AKI in a large-scale pharmacovigilance database study is crucial.
This study, based on the FAERS database, analyzed the association between PCSK9 inhibitors evolocumab and alirocumab and AKI. The results revealed a protective effect of PCSK9 inhibitors against AKI. Moreover, the study identified the main characteristics of AKI cases related to PCSK9 inhibitors and explored the impact of PCSK9 inhibitors in combination with common nephrotoxic drugs on AKI. To our knowledge, this is the largest real-world study investigating the risk of AKI associated with PCSK9 inhibitors.
While some case reports have linked PCSK9 inhibitors to AKI, this retrospective large-scale pharmacovigilance analysis showed a reduced risk of AKI in patients using PCSK9 inhibitors compared to those who did not. Both target drugs, evolocumab and elirocumab, exhibited similar effects with ROR of 0.13 (95% CI 0.12–0.14) and 0.26 (95% CI 0.23–0.30), respectively. Subgroup analysis also reached consistent conclusions. Studies have indicated that PCSK9 inhibitors are associated with anti-inflammatory (Giunzioni et al., 2016; Liu and Frostegård, 2018), autophagic (Ding et al., 2018; Huang et al., 2022), and oxidative stress responses (Huang et al., 2022), which are independent of low-density lipoprotein reduction. Recent research has revealed that these effects are mediated by SIRT3 (D'Onofrio et al., 2023), a highly expressed mitochondrial deacetylase, which plays a vital role in preventing AKI by regulating energy metabolism, inhibiting oxidative stress, suppressing inflammation, improving apoptosis, inhibiting early fibrosis, and maintaining mitochondrial homeostasis (Yuan et al., 2023). Activation of TFEB-mediated autophagy can also alleviate mitochondrial dysfunction in cisplatin-induced AKI (Zhu et al., 2020).
A large multicenter cross-sectional study summarized common nephrotoxic drugs in medical institutions (Liu et al., 2021). We conducted a disproportionate analysis of these drugs in combination with PCSK9 inhibitors and found that PCSK9 inhibitors can reduce the occurrence of AKI caused by common nephrotoxic drugs such as furosemide, pantoprazole, omeprazole, and esomeprazole. Therefore, considering PCSK9 inhibitors in patients with a high risk of AKI or those using nephrotoxic drugs seems wise. Additionally, existing real-world data studies have also shown the protective effect of PCSK9 inhibitors on AKI caused by medications. For example, the relative risk (RR) between the use of evolocumab and the occurrence of contrast-induced acute kidney injury (CI-AKI) was 0.34 (95% CI 0.17–0.66,p < 0.01) (Ma et al., 2022). It is worth mentioning that the common nephrotoxic drugs we selected may not be applicable to all countries and regions, although these drugs were derived from a nationwide cross-sectional survey that included 23 academic hospitals in 17 provinces in China. For example, cephalosporins and carbopenems may be uncommon as common nephrotoxic drugs. However, a pharmacovigilance study of FAERS found AKI ROR of cephalosporins is 6.07 (5.23–7.05) (Patek et al., 2020). Other studies believe that ceftriaxone calcium crystals induce AKI by NLRP3-mediated inflammation and oxidative stress injury (Yifan et al., 2020). Further investigation is warranted to accurately determine the nephrotoxicity of carbapenems. Nevertheless, current evidence suggests that, when co-administered with vancomycin, carbapenems present a reduced risk of AKI compared to piperacillin-tazobactam (Rutter and Burgess, 2018; Chen et al., 2023). However, risk of AKI after piperacillin-tazobactam is comparable to meropenem without concurrent use of vancomycin (Su et al., 2023).
We conducted a risk analysis of PCSK9 inhibitors-related AKI in different genders and age groups. We found that evolocumab is more likely to induce AKI in males (ROR = 1.40, 95% CI = 1.54–1.69), while alirocumab showed the opposite trend (ROR = 0.84, 95% CI = 0.59–1.20), although the latter did not reach statistical significance. Due to limited case numbers for patients under 25 years old, we only compared the reporting differences related to PCSK9 inhibitor-induced AKI between the age groups of 25–65 and over 65. We found a slightly lower risk of AKI in the 25–65 age group with evolocumab use compared to those over 65, and a higher risk with alirocumab use in patients aged 25–65, although these differences were not statistically significant. We observed that males might be a risk factor for evolocumab-related AKI, consistent with previous research results (Siew et al., 2016). However, in another study (Hilmi et al., 2015) on risk prediction models for AKI in liver transplant patients, females were identified as a risk factor (OR = 1.8, 95% CI 1.18–2.88). In fact, studies indicate that alirocumab exhibits a more pronounced effect in reducing LDL-C levels in males compared to females (Vallejo-Vaz et al., 2018; Paquette et al., 2023). Upon performing an analysis based on each 50% reduction in LDL-C levels, there was observed a 24% lower risk of major adverse cardiac events (MACE) in females (p = 0.1094), and a 29% lower risk in males (p = 0.0125), respectively (Vallejo-Vaz et al., 2018). Consequently, it can be hypothesized that males may derive greater benefit from alirocumab therapy. Further large-scale clinical trials are necessary to substantiate this speculation. Since this study is based on publicly available spontaneously reported drug surveillance databases, the reporting of patient gender is not mandatory and may lead to missing data, potentially introducing bias to the study results. Additionally, the sample size in this study was limited, and the characteristics of the study subjects were not entirely consistent; hence, caution is needed when extrapolating the conclusions.
Our research results showed an increasing trend in PCSK9 inhibitors-related AKI reports since the drug’s market launch, reaching its peak in 2018, followed by a gradual decline, especially for evolocumab. Evolocumab-related AKI cases were more common in people over 65 years old. Unfortunately, adverse event reporting in the FAERS database is spontaneous and influenced by various factors, such as the duration of drug marketing, media attention, types of adverse reactions, drug categories, indications, and related regulatory policies. Moreover, we do not have access to all clinical information related to the reported AKI cases, including gender, age, underlying diseases, concomitant medications, surgical procedures, and other AKI risk factors, which may confound the results. Drug surveillance studies based on the FAERS database cannot establish a causal relationship or determine the incidence rate of PCSK9 inhibitor-related AKI. They can only provide preliminary evidence of the potential correlation between the drug and adverse events. Regarding the selection of target populations, our study included a broad population, which might introduce bias into the results.
5 Conclusion
This study, based on the FAERS database, identified signals related to AKI associated with two PCSK9 inhibitors, Evolocumab and Alirocumab, revealing the protective effect of PCSK9 inhibitors against AKI. Furthermore, when used in combination with common nephrotoxic drugs, these inhibitors can reduce the risk of AKI caused by these medications. This provides clinicians with more comprehensive grounds and reasons for selecting PCSK9 inhibitors, especially for patients with a higher risk of AKI or those concurrently using nephrotoxic drugs due to hyperlipidemia. However, further large-scale randomized controlled trials are still necessary to validate these findings.
Data availability statement
Publicly available datasets were analyzed in this study. This data can be found here: https://research.cchmc.org/aers/home.
Ethics statement
Written informed consent was not obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article because the data in the article uses de-identified data and does not involve patient privacy.
Author contributions
HL: Data curation, Formal Analysis, Investigation, Methodology, Software, Supervision, Writing–original draft, Writing–review and editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2024.1353848/full#supplementary-material
References
Anand, K., Ensor, J., Trachtenberg, B., and Bernicker, E. H. (2019). Osimertinib-induced cardiotoxicity: a retrospective review of the FDA adverse events reporting System (FAERS). JACC CardioOncol 1, 172–178. doi:10.1016/j.jaccao.2019.10.006
Bellomo, R., Kellum, J. A., and Ronco, C. (2012). Acute kidney injury. Lancet 380, 756–766. doi:10.1016/S0140-6736(11)61454-2
Charytan, D. M., Sabatine, M. S., Pedersen, T. R., Im, K., Park, J. G., Pineda, A. L., et al. (2019). Efficacy and safety of evolocumab in chronic kidney disease in the FOURIER trial. J. Am. Coll. Cardiol. 73, 2961–2970. doi:10.1016/j.jacc.2019.03.513
Chen, A. Y., Deng, C. Y., Calvachi-Prieto, P., Armengol de la Hoz, M. Á., Khazi-Syed, A., Chen, C., et al. (2023). A large-scale multicenter retrospective study on nephrotoxicity associated with empiric broad-spectrum antibiotics in critically ill patients. Chest 164, 355–368. doi:10.1016/j.chest.2023.03.046
Ding, Z., Wang, X., Liu, S., Shahanawaz, J., Theus, S., Fan, Y., et al. (2018). PCSK9 expression in the ischaemic heart and its relationship to infarct size, cardiac function, and development of autophagy. Cardiovasc Res. 114, 1738–1751. doi:10.1093/cvr/cvy128
D'Onofrio, N., Prattichizzo, F., Marfella, R., Sardu, C., Martino, E., Scisciola, L., et al. (2023). SIRT3 mediates the effects of PCSK9 inhibitors on inflammation, autophagy, and oxidative stress in endothelial cells. Theranostics 13, 531–542. doi:10.7150/thno.80289
Fitzgerald, K., Frank-Kamenetsky, M., Shulga-Morskaya, S., Liebow, A., Bettencourt, B. R., Sutherland, J. E., et al. (2014). Effect of an RNA interference drug on the synthesis of proprotein convertase subtilisin/kexin type 9 (PCSK9) and the concentration of serum LDL cholesterol in healthy volunteers: a randomised, single-blind, placebo-controlled, phase 1 trial. Lancet 383, 60–68. doi:10.1016/S0140-6736(13)61914-5
Giunzioni, I., Tavori, H., Covarrubias, R., Major, A. S., Ding, L., Zhang, Y., et al. (2016). Local effects of human PCSK9 on the atherosclerotic lesion. J. Pathol. 238, 52–62. doi:10.1002/path.4630
Haas, M. E., Levenson, A. E., Sun, X., Liao, W. H., Rutkowski, J. M., de Ferranti, S. D., et al. (2016). The role of proprotein convertase subtilisin/kexin type 9 in nephrotic syndrome-associated hypercholesterolemia. Circulation 134, 61–72. doi:10.1161/CIRCULATIONAHA.115.020912
Hilmi, I. A., Damian, D., Al-Khafaji, A., Planinsic, R., Boucek, C., Sakai, T., et al. (2015). Acute kidney injury following orthotopic liver transplantation: incidence, risk factors, and effects on patient and graft outcomes. Br. J. Anaesth. 114, 919–926. doi:10.1093/bja/aeu556
Hoste, E. A., Bagshaw, S. M., Bellomo, R., Cely, C. M., Colman, R., Cruz, D. N., et al. (2015). Epidemiology of acute kidney injury in critically ill patients: the multinational AKI-EPI study. Intensive Care Med. 41, 1411–1423. doi:10.1007/s00134-015-3934-7
Hoste, E. A., and Kellum, J. A. (2006). RIFLE criteria provide robust assessment of kidney dysfunction and correlate with hospital mortality. Crit. Care Med. 34, 2016–2017. doi:10.1097/01.CCM.0000219374.43963.B5
Huang, G., Lu, X., Zhou, H., Li, R., Huang, Q., Xiong, X., et al. (2022). PCSK9 inhibition protects against myocardial ischemia-reperfusion injury via suppressing autophagy. Microvasc. Res. 142, 104371. doi:10.1016/j.mvr.2022.104371
Jatem, E., Lima, J., Montoro, B., Torres-Bondia, F., and Segarra, A. (2021). Efficacy and safety of PCSK9 inhibitors in hypercholesterolemia associated with refractory nephrotic syndrome. Kidney Int. Rep. 6, 101–109. doi:10.1016/j.ekir.2020.09.046
Jhaveri, K. D., Barta, V. S., and Pullman, J. (2017). Praluent (Alirocumab)-Induced renal injury. J. Pharm. Pract. 30, 7–8. doi:10.1177/0897190016683304
Kaddourah, A., Basu, R. K., Bagshaw, S. M., Goldstein, S. L., and Investigators, A. (2017). Epidemiology of acute kidney injury in critically ill children and young adults. N. Engl. J. Med. 376, 11–20. doi:10.1056/NEJMoa1611391
Kulvichit, W., Sarnvanichpitak, K., Peerapornratana, S., Tungsanga, S., Lumlertgul, N., Praditpornsilpa, K., et al. (2022). In-hospital mortality of critically Ill patients with interactions of acute kidney injury and acute respiratory failure in the resource-limited settings: results from SEA-AKI study. J. Crit. Care 71, 154103. doi:10.1016/j.jcrc.2022.154103
Lagace, T. A. (2014). PCSK9 and LDLR degradation: regulatory mechanisms in circulation and in cells. Curr. Opin. Lipidol. 25, 387–393. doi:10.1097/MOL.0000000000000114
Lameire, N. H., Bagga, A., Cruz, D., De Maeseneer, J., Endre, Z., Kellum, J. A., et al. (2013). Acute kidney injury: an increasing global concern. Lancet 382, 170–179. doi:10.1016/S0140-6736(13)60647-9
Liu, A., and Frostegård, J. (2018). PCSK9 plays a novel immunological role in oxidized LDL-induced dendritic cell maturation and activation of T cells from human blood and atherosclerotic plaque. J. Intern Med. 284, 193–210. doi:10.1111/joim.12758
Liu, C., Yan, S., Wang, Y., Wang, J., Fu, X., Song, H., et al. (2021). Drug-induced hospital-acquired acute kidney injury in China: a multicenter cross-sectional survey. Kidney Dis. (Basel) 7, 143–155. doi:10.1159/000510455
Ma, Y., Zha, L., Zhang, Q., Cao, L., Zhao, R., Ma, J., et al. (2022). Effect of PCSK9 inhibitor on contrast-induced acute kidney injury in patients with acute myocardial infarction undergoing intervention therapy. Cardiol. Res. Pract. 2022, 1638209. doi:10.1155/2022/1638209
Paquette, M., Faubert, S., Saint-Pierre, N., Baass, A., and Bernard, S. (2023). Sex differences in LDL-C response to PCSK9 inhibitors: a real world experience. J. Clin. Lipidol. 17, 142–149. doi:10.1016/j.jacl.2022.12.002
Patek, T. M., Teng, C., Kennedy, K. E., Alvarez, C. A., and Frei, C. R. (2020). Comparing acute kidney injury reports among antibiotics: a pharmacovigilance study of the FDA adverse event reporting System (FAERS). Drug Saf. 43, 17–22. doi:10.1007/s40264-019-00873-8
Pickett, J. K., Shah, M., Gillette, M., Jones, P., Virani, S., Ballantyne, C., et al. (2020). Acute tubular injury in a patient on a proprotein convertase subtilisin/kexin type 9 inhibitor. JACC Case Rep. 2, 1042–1045. doi:10.1016/j.jaccas.2020.04.039
Rosenson, R. S., Hegele, R. A., Fazio, S., and Cannon, C. P. (2018). The evolving future of PCSK9 inhibitors. J. Am. Coll. Cardiol. 72, 314–329. doi:10.1016/j.jacc.2018.04.054
Rutter, W. C., and Burgess, D. S. (2018). Incidence of acute kidney injury among patients treated with piperacillin-tazobactam or meropenem in combination with vancomycin. Antimicrob. Agents Chemother. 62, 002644–e318. doi:10.1128/AAC.00264-18
Sarangdhar, M., Tabar, S., Schmidt, C., Kushwaha, A., Shah, K., Dahlquist, J. E., et al. (2016). Data mining differential clinical outcomes associated with drug regimens using adverse event reporting data. Nat. Biotechnol. 34, 697–700. doi:10.1038/nbt.3623
Siew, E. D., Parr, S. K., Abdel-Kader, K., Eden, S. K., Peterson, J. F., Bansal, N., et al. (2016). Predictors of recurrent AKI. J. Am. Soc. Nephrol. 27, 1190–1200. doi:10.1681/ASN.2014121218
Silver, S. A., Long, J., Zheng, Y., and Chertow, G. M. (2017). Cost of acute kidney injury in hospitalized patients. J. Hosp. Med. 12, 70–76. doi:10.12788/jhm.2683
Silverman, M. G., Ference, B. A., Im, K., Wiviott, S. D., Giugliano, R. P., Grundy, S. M., et al. (2016). Association between lowering LDL-C and cardiovascular risk reduction among different therapeutic interventions: a systematic review and meta-analysis. Jama 316, 1289–1297. doi:10.1001/jama.2016.13985
Skeby, C. K., Hummelgaard, S., Gustafsen, C., Petrillo, F., Frederiksen, K. P., Olsen, D., et al. (2023). Proprotein convertase subtilisin/kexin type 9 targets megalin in the kidney proximal tubule and aggravates proteinuria in nephrotic syndrome. Kidney Int. 104, 754–768. doi:10.1016/j.kint.2023.06.024
Stoekenbroek, R. M., Lambert, G., Cariou, B., and Hovingh, G. K. (2018). Inhibiting PCSK9 - biology beyond LDL control. Nat. Rev. Endocrinol. 15, 52–62. doi:10.1038/s41574-018-0110-5
Stone, N. J., Robinson, J. G., Lichtenstein, A. H., Bairey Merz, C. N., Blum, C. B., Eckel, R. H., et al. (2014). 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J. Am. Coll. Cardiol. 63, 2889–2934. doi:10.1016/j.jacc.2013.11.002
Su, G., Xiao, C., Cao, Y., Gao, P., Xie, D., Cai, Q., et al. (2023). Piperacillin/tazobactam and risk of acute kidney injury in adults hospitalized with infection without vancomycin: a multi-centre real-world data analysis. Int. J. Antimicrob. Agents 61, 106691. doi:10.1016/j.ijantimicag.2022.106691
Toth, P. P., Dwyer, J. P., Cannon, C. P., Colhoun, H. M., Rader, D. J., Upadhyay, A., et al. (2018). Efficacy and safety of lipid lowering by alirocumab in chronic kidney disease. Kidney Int. 93, 1397–1408. doi:10.1016/j.kint.2017.12.011
Uchino, S., Kellum, J. A., Bellomo, R., Doig, G. S., Morimatsu, H., Morgera, S., et al. (2005). Acute renal failure in critically ill patients: a multinational, multicenter study. JAMA 294, 813–818. doi:10.1001/jama.294.7.813
Vallejo-Vaz, A. J., Ginsberg, H. N., Davidson, M. H., Eckel, R. H., Cannon, C. P., Lee, L. V., et al. (2018). Lower on-treatment low-density lipoprotein cholesterol and major adverse cardiovascular events in women and men: pooled analysis of 10 ODYSSEY phase 3 alirocumab trials. J. Am. Heart Assoc. 7, e009221. doi:10.1161/JAHA.118.009221
Xia, S., Gong, H., Zhao, Y., Guo, L., Wang, Y., et al. (2023). Tumor lysis syndrome associated with monoclonal antibodies in patients with multiple myeloma: a pharmacovigilance study based on the FAERS database. Clin. Pharmacol. Ther. 114, 211–219. doi:10.1002/cpt.2920
Xia, S., Zhao, Y. C., Guo, L., Gong, H., Wang, Y. K., et al. (2022). Do antibody-drug conjugates increase the risk of sepsis in cancer patients? A pharmacovigilance study. Front. Pharmacol. 13, 967017. doi:10.3389/fphar.2022.967017
Yifan, Z., Benxiang, N., Zheng, X., Luwei, X., Liuhua, Z., Yuzheng, G., et al. (2020). Ceftriaxone calcium crystals induce acute kidney injury by NLRP3-mediated inflammation and oxidative stress injury. Oxid. Med. Cell Longev. 2020, 6428498. doi:10.1155/2020/6428498
Yuan, J., Zhao, J., Qin, Y., Zhang, Y., Wang, A., Ma, R., et al. (2023). The protective mechanism of SIRT3 and potential therapy in acute kidney injury. Qjm 117, 247–255. doi:10.1093/qjmed/hcad152
Zhou, C., Peng, S., Lin, A., Jiang, A., Peng, Y., Gu, T., et al. (2023). Psychiatric disorders associated with immune checkpoint inhibitors: a pharmacovigilance analysis of the FDA Adverse Event Reporting System (FAERS) database. EClinicalMedicine 59, 101967. doi:10.1016/j.eclinm.2023.101967
Keywords: PCSK9 inhibitors, acute kidney injury, evolocumabEvolocumab, aAlirocumab, pharmacovigilance
Citation: Liu H (2024) Association between PCSK9 inhibitors and acute kidney injury: a pharmacovigilance study. Front. Pharmacol. 15:1353848. doi: 10.3389/fphar.2024.1353848
Received: 11 December 2023; Accepted: 27 May 2024;
Published: 01 August 2024.
Edited by:
Ya-Long Feng, Xianyang Normal University, ChinaReviewed by:
Alexandre O. Gérard, Centre Hospitalier Universitaire de Nice, FranceLi Li, Southern Medical University, China
Copyright © 2024 Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hailing Liu, a3h5ajIwMjNAMTYzLmNvbQ==
 Hailing Liu
Hailing Liu