- 1Department of Neurosurgery, Second Affiliated Hospital, School of Medicine, Zhejiang University, Hangzhou, Zhejiang, China
- 2Key Laboratory of Precise Treatment and Clinical Translational Research of Neurological Diseases, Hangzhou, Zhejiang, China
- 3Department of Neurosurgery, Affiliated Tumor Hospital of Guangxi Medical University, Nanning, China
- 4Department of Medical Science Education, College of Health Sciences, Western University of Health Sciences, Pomona, CA, United States
- 5Burrell College of Osteopathic Medicine, New Mexico State University, Las Cruces, NM, United States
The apelin/APJ system has garnered increasing attention in recent years. In this review, we comprehensively discuss the physiological and pathological mechanisms of the apelin/APJ system in stroke. The apelin/APJ system is widely expressed in the central nervous system (CNS). However, the distribution of the apelin/APJ system varies across different regions and subcellular organelles of the brain. Additionally, the neuroprotective effects of the apelin/APJ system have been reported to inhibit oxidative and nitrative stresses via various signaling pathways. Despite this, the clinical application of the apelin/APJ system remains distant, as apelin has numerous active forms and signaling pathways. The development of a range of drugs targeting the apelin/APJ system holds promise for treating stroke.

GRAPHICAL ABSTRACT | The Apelin/APJ system exerted neuroprotection after stroke via decreasing ROS levels and increasing antioxidant protein expression.
1 Introduction
Cerebrovascular diseases rank as the second leading cause of death, following heart diseases (Heron, 2007; Zhang et al., 2021a). Stroke is characterized by a sudden disturbance in blood flow, leading to mild or severe neurological dysfunction (The World Health Organization, 1988; World Health Organization, 2004). According to reports, 15 million people suffer from stroke annually, with 5.5 million succumbing to the disease (Banerjee et al., 2005). Stroke rates are increasing, particularly in developing countries, posing a significant societal burden (Zhang et al., 2021b; Shen et al., 2020).
Strokes can be classified as ischemic or hemorrhagic (Xu et al., 2018a; Wang et al., 2022; Xu and Zhong, 2013). They result from an interruption in the blood supply to brain tissues, leading to reduced oxygen and nutrients. Extensive research has been conducted on the pathological mechanisms and various therapeutic drugs for stroke, including studies on cellular apoptosis, oxidative stress, inflammation, brain edema, and cell death (Yi et al., 2013; Han et al., 2004). Currently, no drugs are specifically effective in treating stroke.
The apelin protein and its receptor APJ are extensively distributed in the brain, primarily located in oligodendrocytes and neurons (Gregory et al., 2004; Lee et al., 1993). Growing evidence suggests that apelin and its receptor APJ play crucial roles in protecting neural cells post-stroke (Bhalala et al., 2013; Tatemoto et al., 1998). Therefore, targeting the apelin/APJ system offers neuroprotection for stroke patients. This review aims to highlight the latest developments in the study of the apelin/APJ system’s functions and therapeutic potential in stroke patients.
2 Introduction of the apelin/APJ system
2.1 Apelin
The apelin gene expresses a 77-amino acid preproprotein, which is cleaved into several active peptides (Habata et al., 1999; Hosoya et al., 2000; Reaux et al., 2001). The full-length apelin, originating from bovine stomach extracts, consists of 36 amino acids. The presence of apelin-36 was further confirmed in bovine colostrum, and a 13-amino acid peptide (apelin-13) was also identified.
In the peptide, the N-terminus is modified by pyroglutamate, a post-translational modification that renders the protein resistant to enzymatic cleavage. Several potential proteolytic sites on apelin-36 suggest the existence of other endogenous apelin isoforms, such as apelin-19, apelin-17, apelin-16, and apelin-12, all of which can activate the APJ receptor (Kawamata et al., 2001; Tatemoto et al., 2001; Lee et al., 2005; Szokodi et al., 2002; Ronkainen et al., 2007). However, peptides containing fewer than 12 amino acids are inactive. In contrast to preproprotein, shorter forms of apelin exhibit higher binding affinity and greater activity, with pyroglutamated apelin-13 being the most potent.
Under hypoxic conditions, hypoxia-inducible factor-1 (HIF-1) upregulates apelin protein levels (Wang et al., 2006). During lactation, apelin synthesis is upregulated in breast tissue via upstream stimulatory factor-1 (Boucher et al., 2005). In fasting states, adipocyte apelin gene expression decreases, while refeeding stimulates its expression, possibly through changes in insulin and counter-regulatory hormone concentrations (Wei et al., 2005; Reaux-Le Goazigo et al., 2004). Furthermore, apelin expression in hypothalamic neurons is increased through the regulation of arginine vasopressin (Vickers et al., 2002). Post-translational processing of apelin is less understood, but angiotensin-converting enzyme 2 (ACE2) may be involved (O'Dowd et al., 1993). ACE2 efficiently hydrolyzes apelin-13 and apelin-36, although its physiological significance remains unknown.
2.2 The APJ receptor
The human APJ receptor, first reported by O'Dowd et al., is a G protein-coupled receptor with a 377-amino acid sequence located on chromosome 11 (Chng et al., 2013). In 1998, Tatemoto et al. identified apelin as the endogenous ligand of APJ (Habata et al., 1999). It wasn’t until 2013 that two groups independently discovered Elabela as another APJ ligand (Pauli et al., 2014; O'Carroll et al., 2006). The genetic regulation of APJ is complex, with a TATA-less promoter region playing a role in APJ gene expression. Physiological stimuli such as stress, salt loading, and water deprivation induce APJ synthesis (Pugh and Tjian, 1991). Several genes with TATA-less promoters are activated by Sp1 (Japp and Newby, 2008), which is also crucial for APJ promoter activation. Other factors influencing promoter activity include estrogen, CCAAT/enhancer-binding protein, and glucocorticoids (Pugh and Tjian, 1991).
2.3 Distribution of the apelin/APJ system in the CNS
The apelin/APJ system is extensively distributed in the CNS and other tissues (Lee et al., 2000) (Figure 1). In detail, the apelin/APJ system is expressed in neurons of the cerebral cortex, pituitary gland cells, hippocampus, hypothalamus, T-lymphocytes, and pancreatic islet cells. Apelin expression levels are associated with APJ expression.
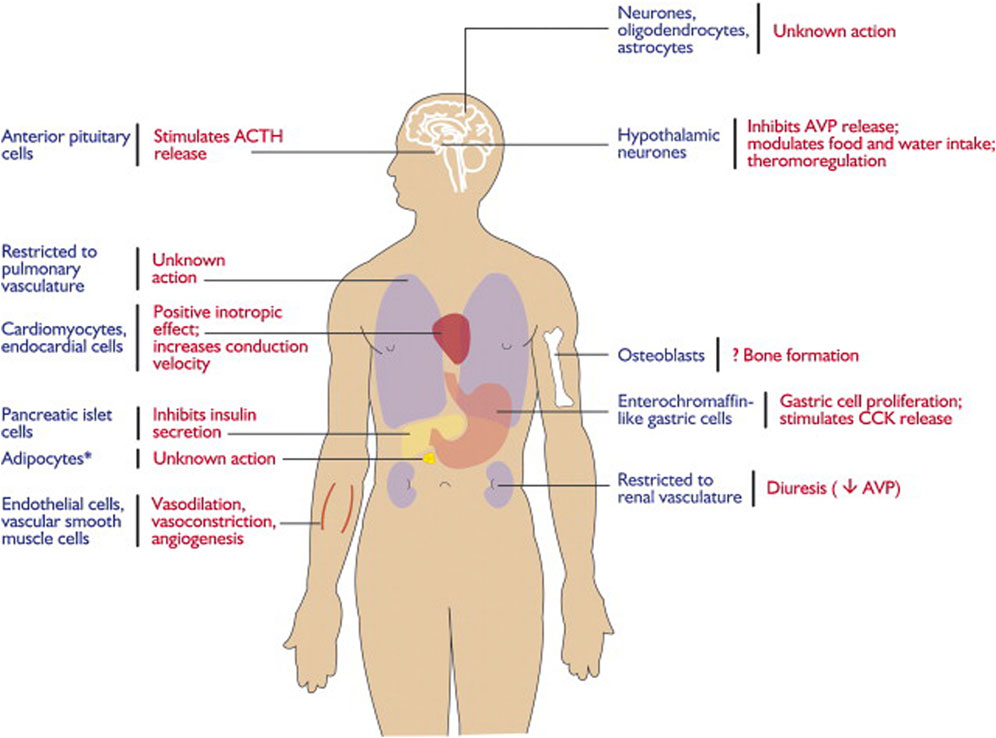
Figure 1. Expression and physiological functions of the apelin–APJ system. Reproduced with permission from ref. (Chng et al., 2013).
In the CNS, apelin is expressed both centrally and peripherally. Northern blot analysis detected apelin mRNA in the CNS of rats (O'Carroll et al., 2000; Medhurst et al., 2003). The highest mRNA expression of apelin was observed in the cerebral cortex, hippocampus, pineal gland, spinal cord, and olfactory tubercle of rats (O'Carroll et al., 2000; Reaux et al., 2002) (Figure 2). Immunocytochemical techniques identified apelin-LI in other neural cells, including neurons of the hypothalamus, pons, and medulla oblongata (Kawamata et al., 2001; Kleinz et al., 2005). In humans, apelin expression was first reported in the hippocampus, thalamus, basal ganglion, hypothalamus, frontal cortex, and basal forebrain (O'Carroll et al., 2000). Another study localized apelin mRNA in the corpus callosum, spinal cord, substantia nigra, amygdala, and pituitary (Reaux et al., 2002). Apelin-LI is expressed in the endoplasmic reticulum, secretory vesicles, and Golgi complex of endothelial cells, but not in cell organelles of inducible endothelial peptide secretion (Lee et al., 2004).
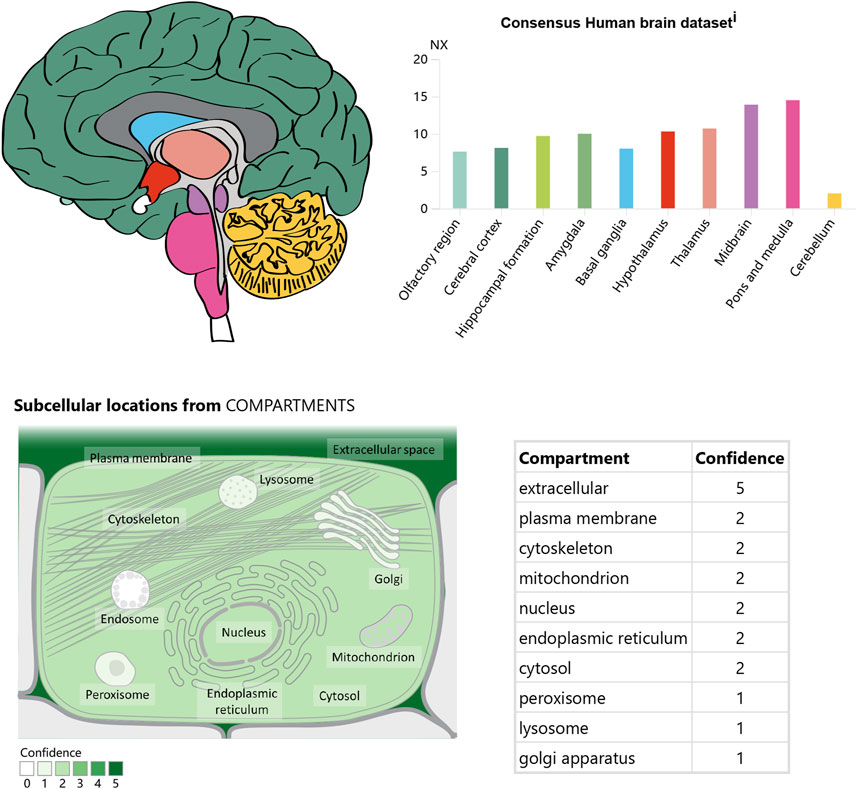
Figure 2. The expression and location of Apelin in the brain. Referenced from the human protein atlas: [Apelin-APJ system] (https://www.proteinatlas.org/ENSG00000134817 - APLNR/brain).
In the CNS, APJ can be detected in the hippocampus, cerebral cortex, pituitary gland, and hypothalamus, with the highest levels in the hypothalamus (O'Carroll et al., 2000; Medhurst et al., 2003) (Figure 3). Immunocytochemical detection showed that apelin receptors are also expressed in the nuclei of several neuron types, including hypothalamic and thalamic nuclei (Reaux et al., 2002). APJ expressed in human embryonic kidney cells shares similar characteristics with that expressed in rat hypothalamic and cerebellar nuclei (Choe et al., 2000).
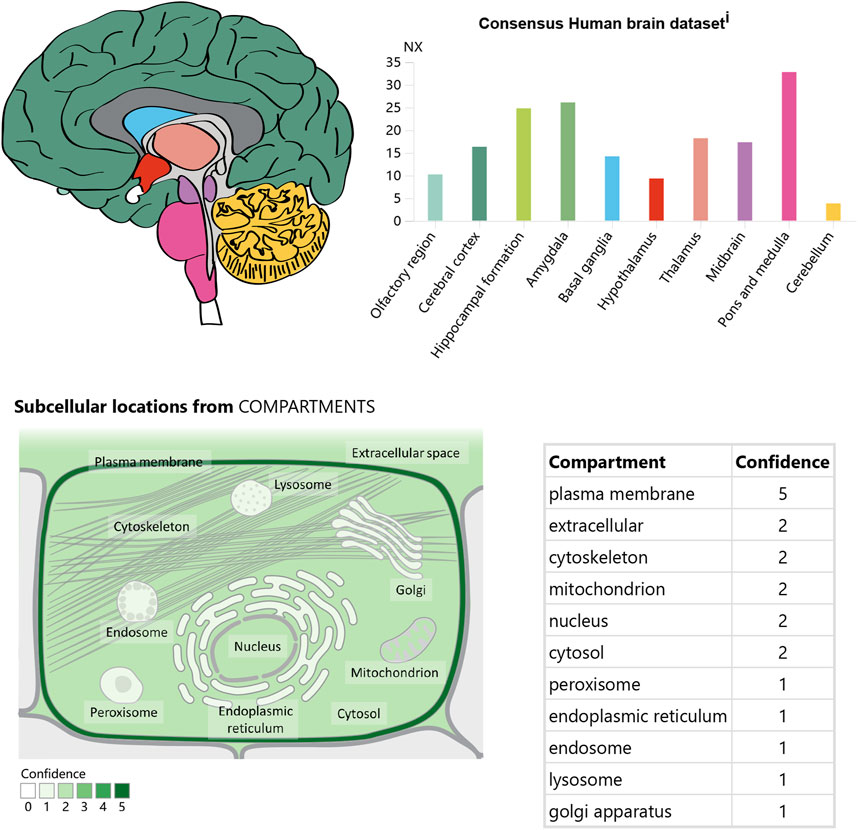
Figure 3. The expression and location of APJ in the brain. Referenced from the human protein atlas: [APJ System] (https://www.proteinatlas.org/ENSG00000171388 -APLN/brain).
Studies on cells expressing APJ in the CNS revealed its presence in neurons, astrocytes, and oligodendrocytes, but not in macrophages or microglia. Immunocytochemical analysis using an antibody against the apelin receptor confirmed its distribution in the human brain (Masri et al., 2006). APJ-LI was detected only in human pyramidal and cerebellar neurons in culture.
2.4 Biochemistry: intracellular signaling mechanisms
Studies have shown that apelin inhibits the production of forskolin-stimulated cyclic AMP, suggesting that APJ is coupled with inhibitory G proteins (Gi) (Habata et al., 1999). Additionally, apelin can activate extracellular-regulated kinases (ERKs) in a Ras-independent manner (Masri et al., 2002) and activate p70S6 kinase in an ERK- and Akt-dependent manner (Neves et al., 2002). Pertussis toxin inhibits these signaling cascades, indicating that they are supported by APJ-Gi coupling. However, pertussis toxin does not completely suppress apelin’s inotropic effect (Ronkainen et al., 2007) but can activate phospholipase C and protein kinase C, which are activated by Gq proteins (Zhou et al., 2003). Therefore, it is possible that APJ receptors couple with both Gq and Gi proteins. Phosphatidylinositol-4,5-bisphosphate (PIP2) can be hydrolyzed by phospholipase C to produce inositol-1,4,5-triphosphate (IP3) (Zhou et al., 2003), demonstrating that apelin can increase intracellular Ca2+ concentrations (Masri et al., 2006). Notably, several apelin-mediated signaling cascades are reduced in sensitization following activation (Masri et al., 2002), likely due to the internal positioning of APJ receptors (Eggena et al., 1979). Different sizes of apelin fragments lead to different durations of receptor internalization, correlated with varying patterns of desensitization (Masri et al., 2002; Eggena et al., 1979). Finally, APJ receptors are also localized to the nucleus (Choe et al., 2000), suggesting they can regulate transcription in addition to activating intracellular cascades (Tang et al., 2021).
3 Oxidative stress in the CNS
3.1 Characteristics of oxidative stress in the CNS
Oxidative stress causes significant damage to organs under ischemic conditions, including the heart, liver, kidneys, and especially the brain (Andrianova et al., 2020; Yang et al., 2016; Ikeda and Miyahara, 2003; Frank, 2006). Anatomical, physiological, and functional factors make the brain particularly susceptible to oxidative injury. Human brains consume 20% of the body’s oxygen due to their high metabolic rate, although they account for only 2% of body weight. This higher oxygen availability results in increased ROS production (Gu et al., 2011). The brain’s dependence on glymphatic waste disposal, modest antioxidant defenses, excitotoxic and auto-oxidizable neurotransmitters, polyunsaturated fatty acids prone to peroxidation, limited regenerative capacity, redox-active metal burden, and calcium load make it sensitive to ROS.
Oxidative stress-related neurofunctional damage may result from various cellular pathophysiological processes within neural cells. The oxidative stress vulnerability of neurons differs biochemically. Psychological stress may compromise antioxidant enzyme function by disrupting the oxidant-antioxidant balance in the brain, depleting glutathione and increasing oxidative stress. When glutamate toxicity occurs simultaneously with mitochondrial dysfunction, oxidative stress, and calcium overload, it leads to brain damage, impaired neural cell communication, and ultimately neuro-dysfunction. Controlling ROS levels, either by quenching pro-oxidants or enhancing antioxidant defense, is crucial for the CNS. This review provides a biologically plausible explanation of how oxidative damage might contribute to psychiatric symptoms [Figure 4].
3.2 Signal pathways of ROS in the brain
The mechanisms by which ROS induce brain injuries remain unclear. However, ROS have been demonstrated to cause various cellular pathologies, including blood-brain barrier disruption, neuronal apoptosis, and neuroinflammation (Makino et al., 1996). Evidence suggests that N-methyl-D-aspartate receptor glutamate toxicity and glucocorticoid receptor signaling are involved (Okamoto et al., 1999; Tanaka et al., 1999; Albrecht et al., 2010; Nguyen et al., 2011; Sorce and Krause, 2009). Neurodegenerative diseases such as cerebrovascular disorders, Parkinson’s disease, and Alzheimer’s disease have been linked to increased brain oxidative damage (Kamata et al., 2005).
Oxidative stress is believed to damage macromolecules by activating particular signaling pathways in cells, altering gene expression, and leading to cell death (Finkel, 2000; Meng et al., 2002; Hu and Wieloch, 1994). The mechanisms linking oxidative stress signals to cellular responses remain unclear, but several proteins play important roles, including mitogen-activated protein kinases (MAPKs: JNK, ERK1/2, ERK5, p38MAPK) (Kuan et al., 2003; Suzaki et al., 2002; Dunah et al., 2002), Sp1 (Susin et al., 1999), oxidoreductase apoptosis-inducing factor (AIF) (Vahsen et al., 2004; Chalovich et al., 2006), transcription factors such as cAMP response element-binding protein (CREB) (Zaman et al., 1999; Van Der Heide et al., 2004), and Forkhead (FOXO) (Brunet et al., 2004; Essers et al., 2004; Orellana-Urzúa et al., 2020).
4 The functions of the apelin/APJ system in stroke
4.1 Pathological mechanisms of stroke
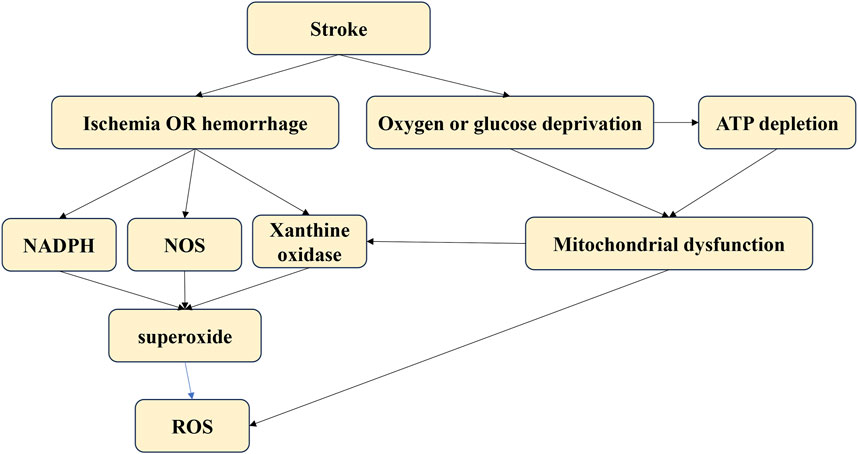
Strokes result from an interrupted blood supply to brain tissues, leading to reduced oxygen and nutrients. The lack of blood supply causes a rapid increase in ROS production immediately after an acute stroke. These ROS can damage neural cells, leading to further pathological processes such as neuroinflammation, autophagy, neuro-apoptosis, and blood-brain barrier disruption (Rodrigo et al., 2013). The main sources of ROS after a stroke include mitochondria, nitric oxide synthases, NADPH oxidase, and xanthine oxidase (Wu et al., 2017). Targeting oxidative stress post-stroke is critical to preventing oxidative damage and subsequent pathological processes in stroke patients.
4.2 Expression of apelin/APJ after stroke
Apelin and APJ expression levels vary at different time points during a stroke (Lv et al., 2013). In one of my previous studies, we found that the endogenous level of apelin-13 increased at 12 h and peaked at 24 h after SAH, while the levels of APJ started to increase at 6 h and peaked at 24 h after SAH (Xu et al., 2019). Various transcription factors, such as ATF4, Sp1, STAT3, and HIF-1α, play crucial roles in regulating the apelin/APJ system (Pugh and Tjian, 1991; He et al., 2015; Han et al., 2008; Zhang et al., 2009; Yeh et al., 2011). Cerebral ischemia results in oxygen and glucose deprivation, which is associated with abnormal apelin/APJ signaling (Zhang et al., 2009; Yeh et al., 2011). HIF-1α and Sp1 induce the expression of apelin/APJ after a stroke (Pugh and Tjian, 1991; He et al., 2015; Han et al., 2008). Under ischemic conditions, HIF-1α translocates to the nucleus and activates the transcription and expression of apelin and APJ proteins (Wang et al., 2006; Han et al., 2008). Apelin and APJ expression is induced in neurons during the early stages of ischemia by increased Sp1 via HIF-1α (Woo et al., 2012; Fan et al., 2017). Reperfusion, however, results in the downregulation of the apelin/APJ system. In mice subjected to chronic normobaric hypoxia, APJ expression in the hippocampus is significantly reduced, and apelin-13 can reverse this reduction (Sheng et al., 2012). The apelin/APJ system is also affected by ER stress, inflammation, and oxidative stress (Mei et al., 2015; Morimoto et al., 2007). For example, cerebral I/R injury is mediated by the ER stress response, which is activated during reperfusion but not ischemia (Nakka et al., 2010; Xin et al., 2014; Jeong et al., 2014). Reperfusion may induce apelin expression due to ER stress regulation by ATF4 via the p38 MAPK pathway (Liu et al., 2018). Apelin-12 has been shown to inhibit the JNK and p38MAPK signaling pathways, leading to cell apoptosis in MCAO-induced ischemic mice (Arani Hessari et al., 2022).
Due to the crucial roles of the apelin/APJ system in stroke, targeting it could provide novel treatments for stroke.
4.3 Modulation of oxidative stress by the apelin/APJ system after stroke
The apelin/APJ system is widely studied for its neuroprotective properties, including anti-neuroinflammation, anti-apoptosis, and antioxidative stress. Several studies have reported the neuroprotective effects of the apelin/APJ system in the CNS. However, research on stroke has largely focused on its anti-apoptosis and anti-inflammatory effects, overlooking its antioxidative stress effects (Gholamzadeh et al., 2021; Xu et al., 2018b; Xu et al., 2019). This study focuses solely on the antioxidative stress effects of the apelin/APJ system, providing guidance for future research.
The apelin/APJ system inhibits oxidative and nitrative stresses, producing neuroprotective effects. Our previous study showed that the apelin/APJ system exerts significant antioxidative effects by suppressing endoplasmic reticulum stress-associated oxidative stress via AMPK/TXNIP/NLRP3 signaling pathways (Wu et al., 2015). It also increases superoxide dismutase activity and decreases malondialdehyde (MDA) levels to reduce oxidative stress induced by I/R injury (Duan et al., 2019). Apelin-13 has been shown to significantly decrease ROS and MDA levels while increasing antioxidant protein expression in a dose-dependent manner via the AMPK/GSK-3β/Nrf2 pathway (Khoshnam et al., 2017). The apelin/APJ system may protect cells from oxidative stress-induced death by decreasing ROS production and promoting ROS clearance.
Nitric oxide (NO) exhibits different effects in ischemic stroke: it is neuroprotective when produced by endothelial NOS (eNOS) but mediates oxidative/nitrosative injuries when generated by neuronal NOS (nNOS) (Iadecola et al., 1997; Salcedo et al., 2007). Similarly, apelin has dual effects on vascular function. Activation of the apelin/APJ axis induces peripheral arterial relaxation in a NO- and endothelium-dependent manner (Japp et al., 2008; Modgil et al., 2013). However, in male rats, the apelin/APJ system inhibits NO-induced cerebral artery relaxation by blocking calcium-activated K (BKCa) channels via the PI3K/Akt pathway (Mughal et al., 2018; McKinnie et al., 2017). Further research is needed to determine how apelin affects oxidative/nitrosative stress in ischemic stroke.
5 Potential targets of the apelin/APJ system
As indicated above, the apelin/APJ system plays a major role in the occurrence and development of several diseases, including strokes. Therefore, targeting the apelin/APJ system can be a promising approach to treating neurological diseases (Brame et al., 2015). Recent studies have identified small molecule agonists and antagonists targeting the apelin/APJ system. For example, an antidiuretic hormone-inducing non-peptide agonist E339-3D6 has been reported to induced vasorelaxation of rat aorta precontracted with noradrenaline and potently inhibited systemic vasopressin release by activating with apelin receptor, which can be a potential target to allow development of a new generation of vasodilator and aquaretic agents (Murza et al., 2014). [20040517]. Additionally, ML233 selectively inhibits AT1 receptors, inducing vasoconstriction via phospholipase C by binding to APJ (Gerbier et al., 2017). These molecules can decrease renin levels generated via the cAMP pathway (Murza et al., 2012). Research on antagonists of APJ receptors is also progressing rapidly. ML221 was the first such antagonist to be developed (Jia et al., 2012); another antagonist, ALX40-4C, has also been identified. The ALX40-4C receptor antagonist, consisting of nine arginine residues, is effective for both APJ and CXCR4 receptors and inhibits intracellular calcium mobilization and receptor internalization in response to ligands, and it can be the potential utility for further elucidation of HIV-1 neuropathogenesis and therapy of HIV-1-induced encephalopathy (Jia et al., 2012). PMID: 12890632 Additionally, some apelin-13-based molecules showed more potent and stable analogs targeted at APJ (Juhl et al., 2016). Cao et al. showed that apelin analogs directly reduced blood pressure by activating the Akt-eNOS/NO pathway. Drugs targeting apelin might help treat inflammation-related diseases associated with oxidative stress. Puerarin has been shown to reduce apelin expression and protect against renal hypertension (Juhl et al., 2016; Centers for Disease Control and Prevention, 2001), suggesting its potential in treating oxidative stress-linked blood pressure. To conclude, Apelin/APJ-targeting drugs contribute to pharmacological research and understanding the mechanism of oxidative stress-mediated diseases.
6 Conclusion
The apelin/APJ system is extensively distributed in the brain and plays vital roles in regulating neurological diseases, including stroke. It shows significant neuroprotective effects by suppressing oxidative and nitrative stresses via different signaling pathways. Recent years have seen the discovery of potential drugs targeting APJ and apelin (E339-3D6, ML233, ML221, and ALX40-4C), and pharmacological interactions with Apelin/APJ have become reliable tools for exploring this system’s role in oxidative stress-mediated diseases.
Further in-depth studies on the physiological and pathological effects of the apelin/APJ system and its potential mechanisms will greatly aid clinical prevention and intervention in strokes. The development of drugs targeting the apelin/APJ system will benefit patients and alleviate the pressures on families and society.
This study extensively shows the apelin/APJ system and its antioxidative roles in stroke. However, some limitations should be addressed: 1. This study focuses solely on the antioxidative effects of the apelin/APJ system in stroke, overlooking other physiological roles such as anti-apoptosis and anti-neuroinflammation. Application of this results should be more carefully. 2. Although some clinical trials have been registered (Num. ChiCTR2200060945, ChiCTR2100054712, ChiCTR-OOC-15006043, ChiCTR-ODT-13004019), clinical application of apelin/APJ has not yet been reported. The clinical values of apelin/APJ system should be further explored in future studies. 3. Currently, limited studies focus on the antioxidative effects of the apelin/APJ system in stroke, which remains largely unexplored. Future studies of the apelin/APJ system should include different cellular signaling pathways of oxidative stress, various sources of ROS, and different clinical drugs that can be further explored. 4. The connection of different signaling pathways mediated by apelin/APJ system should be further explored, which can greatly increase the readability and application of apelin/APJ system.
Author contributions
WX: Conceptualization, Writing–original draft. JYa: Supervision, Writing–review and editing. ZT: Writing–review and editing. CL: Writing–review and editing. LG: Conceptualization, Data curation, Formal Analysis, Writing–review and editing. HW: Conceptualization, Formal Analysis, Investigation, Writing–review and editing. JinZ: Conceptualization, Investigation, Supervision, Writing–review and editing. JiaZ: Supervision, Writing–review and editing. AS: Investigation, Supervision, Writing–review and editing. JYu: Data curation, Funding acquisition, Validation, Visualization, Writing–review and editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by the National Natural Science Foundation of China (82001231, 82001299, 82060225, and 82260239); Zhejiang Provincial Natural Science Foundation of China (LQ21H090009); Guangxi Natural Science Foundation (no. 2020GXNSFAA297154); and Guangxi Medical University Education and Teaching Reform Project (2020XJGA24).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
CNS, central nervous system; WHO, World Health Organization; HIF-1, hypoxia-inducible factor-1; ACE2, angiotensin-converting enzyme 2; ERKs, extracellular-regulated kinases; PIP2, phosphatidylinositol-4,5-bisphosphate; IP3, inositol-1,4,5-triphosphate; DG, dentate gyrus; ROS, radical oxidative stress; AIF, apoptosis-inducing factor; CREB, cAMP response element-binding protein; ATF4, activating transcription factor 4; STAT3, signal transducer and activator of transcription 3; HIF-1α, hypoxia-inducible factor 1 alpha; MAPK, mitogen-activated protein kinase; JNK, C-Jun N-terminal kinase; MDA, malondialdehyde; SOD, superoxide dismutase; NO, nitric oxide; BKCa, blocking calcium-activated K.
References
Albrecht, P., Lewerenz, J., Dittmer, S., Noack, R., Maher, P., and Methner, A. (2010). Mechanisms of oxidative glutamate toxicity: the glutamate/cystine antiporter system xc-as a neuroprotective drug target. CNS and neurological Disord. drug targets 9 (3), 373–382. doi:10.2174/187152710791292567
Andrianova, N. V., Zorov, D. B., and Plotnikov, E. Y. (2020). Targeting inflammation and oxidative stress as a therapy for ischemic kidney injury. Biochem. Biokhimiia 85 (12), 1591–1602. doi:10.1134/S0006297920120111
Arani Hessari, F., Sharifi, M., Yousefifard, M., Gholamzadeh, R., Nazarinia, D., and Aboutaleb, N. (2022). Apelin-13 attenuates cerebral ischemia/reperfusion injury through regulating inflammation and targeting the JAK2/STAT3 signaling pathway. J. Chem. Neuroanat. 126, 102171. doi:10.1016/j.jchemneu.2022.102171
Banerjee, T. K., Roy, M. K., and Bhoi, K. K. (2005). Is stroke increasing in India--preventive measures that need to be implemented. J. Indian Med. Assoc. 103 (3), 162.
Bhalala, O. G., Srikanth, M., and Kessler, J. A. (2013). The emerging roles of microRNAs in CNS injuries. Nat. Rev. Neurol. 9 (6), 328–339. doi:10.1038/nrneurol.2013.67
Boucher, J., Masri, B., Daviaud, D., Gesta, S., Guigné, C., Mazzucotelli, A., et al. (2005). Apelin, a newly identified adipokine up-regulated by insulin and obesity. Endocrinology 146 (4), 1764–1771. doi:10.1210/en.2004-1427
Brame, A. L., Maguire, J. J., Yang, P., Dyson, A., Torella, R., Cheriyan, J., et al. (2015). Design, characterization, and first-in-human study of the vascular actions of a novel biased apelin receptor agonist. Hypertens. (Dallas Tex 1979) 65 (4), 834–840. doi:10.1161/HYPERTENSIONAHA.114.05099
Brunet, A., Sweeney, L. B., Sturgill, J. F., Chua, K. F., Greer, P. L., Lin, Y., et al. (2004). Stress-dependent regulation of FOXO transcription factors by the SIRT1 deacetylase. Sci. (New York NY) 303 (5666), 2011–2015. doi:10.1126/science.1094637
Centers for Disease Control and Prevention (2001). Prevalence of disabilities and associated health conditions among adults--United States, 1999. MMWR Morb. Mortal. Wkly. Rep. 50 (7), 120–125.
Chalovich, E. M., Zhu, J. H., Caltagarone, J., Bowser, R., and Chu, C. T. (2006). Functional repression of cAMP response element in 6-hydroxydopamine-treated neuronal cells. J. Biol. Chem. 281 (26), 17870–17881. doi:10.1074/jbc.M602632200
Chng, S. C., Ho, L., Tian, J., and Reversade, B. (2013). ELABELA: a hormone essential for heart development signals via the apelin receptor. Dev. cell 27 (6), 672–680. doi:10.1016/j.devcel.2013.11.002
Choe, W., Albright, A., Sulcove, J., Jaffer, S., Hesselgesser, J., Lavi, E., et al. (2000). Functional expression of the seven-transmembrane HIV-1 co-receptor APJ in neural cells. J. Neurovirol 6 (Suppl. 1), S61–S69.
Duan, J., Cui, J., Yang, Z., Guo, C., Cao, J., Xi, M., et al. (2019). Neuroprotective effect of Apelin 13 on ischemic stroke by activating AMPK/GSK-3β/Nrf2 signaling. J. neuroinflammation 16 (1), 24. doi:10.1186/s12974-019-1406-7
Dunah, A. W., Jeong, H., Griffin, A., Kim, Y. M., Standaert, D. G., Hersch, S. M., et al. (2002). Sp1 and TAFII130 transcriptional activity disrupted in early Huntington's disease. Sci. (New York NY) 296 (5576), 2238–2243. doi:10.1126/science.1072613
Eggena, P., Zhu, J. H., Clegg, K., and Barrett, J. D. (1979)1993). Nuclear angiotensin receptors induce transcription of renin and angiotensinogen mRNA. Hypertens. Dallas Tex 22 (4), 496–501. doi:10.1161/01.hyp.22.4.496
Essers, M. A., Weijzen, S., de Vries-Smits, A. M., Saarloos, I., de Ruiter, N. D., Bos, J. L., et al. (2004). FOXO transcription factor activation by oxidative stress mediated by the small GTPase Ral and JNK. EMBO J. 23 (24), 4802–4812. doi:10.1038/sj.emboj.7600476
Fan, J., Ding, L., Xia, D., Chen, D., Jiang, P., Ge, W., et al. (2017). Amelioration of apelin-13 in chronic normobaric hypoxia-induced anxiety-like behavior is associated with an inhibition of NF-κB in the hippocampus. Brain Res. Bull. 130, 67–74. doi:10.1016/j.brainresbull.2017.01.005
Finkel, T. (2000). Redox-dependent signal transduction. FEBS Lett. 476 (1-2), 52–54. doi:10.1016/s0014-5793(00)01669-0
Frank, M. G. (2006). The mystery of sleep function: current perspectives and future directions. Rev. Neurosci. 17 (4), 375–392. doi:10.1515/revneuro.2006.17.4.375
Gerbier, R., Alvear-Perez, R., Margathe, J. F., Flahault, A., Couvineau, P., Gao, J., et al. (2017). Development of original metabolically stable apelin-17 analogs with diuretic and cardiovascular effects. FASEB J. official Publ. Fed. Am. Soc. Exp. Biol. 31 (2), 687–700. doi:10.1096/fj.201600784R
Gholamzadeh, R., Ramezani, F., Tehrani, P. M., and Aboutaleb, N. (2021). Apelin-13 attenuates injury following ischemic stroke by targeting matrix metalloproteinases (MMP), endothelin-B receptor, occludin/claudin-5, and oxidative stress. J. Chem. Neuroanat. 118, 102015. doi:10.1016/j.jchemneu.2021.102015
Gregory, R. I., Yan, K. P., Amuthan, G., Chendrimada, T., Doratotaj, B., Cooch, N., et al. (2004). The Microprocessor complex mediates the genesis of microRNAs. Nature 432 (7014), 235–240. doi:10.1038/nature03120
Gu, Y., Dee, C. M., and Shen, J. (2011). Interaction of free radicals, matrix metalloproteinases, and caveolin-1 impacts blood-brain barrier permeability. Front. Biosci. Sch. Ed. 3 (4), 1216–1231. doi:10.2741/222
Habata, Y., Fujii, R., Hosoya, M., Fukusumi, S., Kawamata, Y., Hinuma, S., et al. (1999). Apelin, the natural ligand of the orphan receptor APJ, is abundantly secreted in the colostrum. Biochimica biophysica acta 1452 (1), 25–35. doi:10.1016/s0167-4889(99)00114-7
Han, J., Lee, Y., Yeom, K. H., Kim, Y. K., Jin, H., and Kim, V. N. (2004). The Drosha-DGCR8 complex in primary microRNA processing. Genes and Dev. 18 (24), 3016–3027. doi:10.1101/gad.1262504
Han, S., Wang, G., Qi, X., Englander, E. W., and Greeley, G. H. (2008). Involvement of a Stat3 binding site in inflammation-induced enteric apelin expression. Am. J. physiology Gastrointest. liver physiology 295 (5), G1068–G1078. doi:10.1152/ajpgi.90493.2008
He, L., Xu, J., Chen, L., and Li, L. (2015). Apelin/APJ signaling in hypoxia-related diseases. Clin. chimica acta; Int. J. Clin. Chem. 451 (Pt B), 191–198. doi:10.1016/j.cca.2015.09.029
Hosoya, M., Kawamata, Y., Fukusumi, S., Fujii, R., Habata, Y., Hinuma, S., et al. (2000). Molecular and functional characteristics of APJ. Tissue distribution of mRNA and interaction with the endogenous ligand apelin. J. Biol. Chem. 275 (28), 21061–21067. doi:10.1074/jbc.M908417199
Hu, B. R., and Wieloch, T. (1994). Tyrosine phosphorylation and activation of mitogen-activated protein kinase in the rat brain following transient cerebral ischemia. J. Neurochem. 62 (4), 1357–1367. doi:10.1046/j.1471-4159.1994.62041357.x
Iadecola, C., Zhang, F., Casey, R., Nagayama, M., and Ross, M. E. (1997). Delayed reduction of ischemic brain injury and neurological deficits in mice lacking the inducible nitric oxide synthase gene. J. Neurosci. official J. Soc. Neurosci. 17 (23), 9157–9164. doi:10.1523/JNEUROSCI.17-23-09157.1997
Ikeda, S., and Miyahara, Y. (2003). Ischemic heart disease and oxidative stress. Nihon rinsho Jpn. J. Clin. Med. 61 (Suppl. 5), 831–836.
Japp, A. G., Cruden, N. L., Amer, D. A., Li, V. K., Goudie, E. B., Johnston, N. R., et al. (2008). Vascular effects of apelin in vivo in man. J. Am. Coll. Cardiol. 52 (11), 908–913. doi:10.1016/j.jacc.2008.06.013
Japp, A. G., and Newby, D. E. (2008). The apelin-APJ system in heart failure: pathophysiologic relevance and therapeutic potential. Biochem. Pharmacol. 75 (10), 1882–1892. doi:10.1016/j.bcp.2007.12.015
Jeong, K., Oh, Y., Kim, S. J., Kim, H., Park, K. C., Kim, S. S., et al. (2014). Apelin is transcriptionally regulated by ER stress-induced ATF4 expression via a p38 MAPK-dependent pathway. Apoptosis Int. J. Program. cell death. 19 (9), 1399–1410. doi:10.1007/s10495-014-1013-0
Jia, Z. Q., Hou, L., Leger, A., Wu, I., Kudej, A. B., Stefano, J., et al. (2012). Cardiovascular effects of a PEGylated apelin. Peptides 38 (1), 181–188. doi:10.1016/j.peptides.2012.09.003
Juhl, C., Els-Heindl, S., Schönauer, R., Redlich, G., Haaf, E., Wunder, F., et al. (2016). Development of potent and metabolically stable APJ ligands with high therapeutic potential. ChemMedChem 11 (21), 2378–2384. doi:10.1002/cmdc.201600307
Kamata, H., Honda, S., Maeda, S., Chang, L., Hirata, H., and Karin, M. (2005). Reactive oxygen species promote TNFalpha-induced death and sustained JNK activation by inhibiting MAP kinase phosphatases. Cell 120 (5), 649–661. doi:10.1016/j.cell.2004.12.041
Kawamata, Y., Habata, Y., Fukusumi, S., Hosoya, M., Fujii, R., Hinuma, S., et al. (2001). Molecular properties of apelin: tissue distribution and receptor binding. Biochimica biophysica acta 1538 (2-3), 162–171. doi:10.1016/s0167-4889(00)00143-9
Khoshnam, S. E., Winlow, W., Farzaneh, M., Farbood, Y., and Moghaddam, H. F. (2017). Pathogenic mechanisms following ischemic stroke. Neurological Sci. official J. Italian Neurological Soc. Italian Soc. Clin. Neurophysiology 38 (7), 1167–1186. doi:10.1007/s10072-017-2938-1
Kleinz, M. J., Skepper, J. N., and Davenport, A. P. (2005). Immunocytochemical localisation of the apelin receptor, APJ, to human cardiomyocytes, vascular smooth muscle and endothelial cells. Regul. Pept. 126 (3), 233–240. doi:10.1016/j.regpep.2004.10.019
Kuan, C. Y., Whitmarsh, A. J., Yang, D. D., Liao, G., Schloemer, A. J., Dong, C., et al. (2003). A critical role of neural-specific JNK3 for ischemic apoptosis. Proc. Natl. Acad. Sci. U. S. A. 100 (25), 15184–15189. doi:10.1073/pnas.2336254100
Lee, D. K., Cheng, R., Nguyen, T., Fan, T., Kariyawasam, A. P., Liu, Y., et al. (2000). Characterization of apelin, the ligand for the APJ receptor. J. Neurochem. 74 (1), 34–41. doi:10.1046/j.1471-4159.2000.0740034.x
Lee, D. K., Lanca, A. J., Cheng, R., Nguyen, T., Ji, X. D., Gobeil, F., et al. (2004). Agonist-independent nuclear localization of the Apelin, angiotensin AT1, and bradykinin B2 receptors. J. Biol. Chem. 279 (9), 7901–7908. doi:10.1074/jbc.M306377200
Lee, D. K., Saldivia, V. R., Nguyen, T., Cheng, R., George, S. R., and O'Dowd, B. F. (2005). Modification of the terminal residue of apelin-13 antagonizes its hypotensive action. Endocrinology 146 (1), 231–236. doi:10.1210/en.2004-0359
Lee, R. C., Feinbaum, R. L., and Ambros, V. (1993). The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell 75 (5), 843–854. doi:10.1016/0092-8674(93)90529-y
Liu, D. R., Hu, W., and Chen, G. Z. (2018). Apelin-12 exerts neuroprotective effect against ischemia-reperfusion injury by inhibiting JNK and P38MAPK signaling pathway in mouse. Eur. Rev. Med. Pharmacol. Sci. 22 (12), 3888–3895. doi:10.26355/eurrev_201806_15273
Lv, X. R., Zheng, B., Li, S. Y., Han, A. L., Wang, C., Shi, J. H., et al. (2013). Synthetic retinoid Am80 up-regulates apelin expression by promoting interaction of RARα with KLF5 and Sp1 in vascular smooth muscle cells. Biochem. J. 456 (1), 35–46. doi:10.1042/BJ20130418
Makino, Y., Tanaka, H., Dahlman-Wright, K., and Makino, I. (1996). Modulation of glucocorticoid-inducible gene expression by metal ions. Mol. Pharmacol. 49 (4), 612–620.
Masri, B., Lahlou, H., Mazarguil, H., Knibiehler, B., and Audigier, Y. (2002). Apelin (65-77) activates extracellular signal-regulated kinases via a PTX-sensitive G protein. Biochem. biophysical Res. Commun. 290 (1), 539–545. doi:10.1006/bbrc.2001.6230
Masri, B., Morin, N., Pedebernade, L., Knibiehler, B., and Audigier, Y. (2006). The apelin receptor is coupled to Gi1 or Gi2 protein and is differentially desensitized by apelin fragments. J. Biol. Chem. 281 (27), 18317–18326. doi:10.1074/jbc.M600606200
McKinnie, S. M. K., Wang, W., Fischer, C., McDonald, T., Kalin, K. R., Iturrioz, X., et al. (2017). Synthetic modification within the RPRL region of apelin peptides: impact on cardiovascular activity and stability to neprilysin and plasma degradation. J. Med. Chem. 60 (14), 6408–6427. doi:10.1021/acs.jmedchem.7b00723
Medhurst, A. D., Jennings, C. A., Robbins, M. J., Davis, R. P., Ellis, C., Winborn, K. Y., et al. (2003). Pharmacological and immunohistochemical characterization of the APJ receptor and its endogenous ligand apelin. J. Neurochem. 84 (5), 1162–1172. doi:10.1046/j.1471-4159.2003.01587.x
Mei, Y., Thompson, M. D., Cohen, R. A., and Tong, X. (2015). Autophagy and oxidative stress in cardiovascular diseases. Biochimica biophysica acta 1852 (2), 243–251. doi:10.1016/j.bbadis.2014.05.005
Meng, T. C., Fukada, T., and Tonks, N. K. (2002). Reversible oxidation and inactivation of protein tyrosine phosphatases in vivo. Mol. cell 9 (2), 387–399. doi:10.1016/s1097-2765(02)00445-8
Modgil, A., Guo, L., O'Rourke, S. T., and Sun, C. (2013). Apelin-13 inhibits large-conductance Ca2+-activated K+ channels in cerebral artery smooth muscle cells via a PI3-kinase dependent mechanism. PloS one 8 (12), e83051. doi:10.1371/journal.pone.0083051
Morimoto, N., Oida, Y., Shimazawa, M., Miura, M., Kudo, T., Imaizumi, K., et al. (2007). Involvement of endoplasmic reticulum stress after middle cerebral artery occlusion in mice. Neuroscience 147 (4), 957–967. doi:10.1016/j.neuroscience.2007.04.017
Mughal, A., Sun, C., and O'Rourke, S. T. (2018). Apelin reduces nitric oxide-induced relaxation of cerebral arteries by inhibiting activation of large-conductance calcium-activated K channels. J. Cardiovasc. Pharmacol. 71 (4), 223–232. doi:10.1097/FJC.0000000000000563
Murza, A., Belleville, K., Longpré, J. M., Sarret, P., and Marsault, É. (2014). Stability and degradation patterns of chemically modified analogs of apelin-13 in plasma and cerebrospinal fluid. Biopolymers 102 (4), 297–303. doi:10.1002/bip.22498
Murza, A., Parent, A., Besserer-Offroy, É., Tremblay, H., Karadereye, F., Beaudet, N., et al. (2012). Elucidation of the structure-activity relationships of apelin: influence of unnatural amino acids on binding, signaling, and plasma stability. ChemMedChem 7 (2), 318–325. doi:10.1002/cmdc.201100492
Nakka, V. P., Gusain, A., and Raghubir, R. (2010). Endoplasmic reticulum stress plays critical role in brain damage after cerebral ischemia/reperfusion in rats. Neurotox. Res. 17 (2), 189–202. doi:10.1007/s12640-009-9110-5
Neves, S. R., Ram, P. T., and Iyengar, R. (2002). G protein pathways. Sci. (New York NY) 296 (5573), 1636–1639. doi:10.1126/science.1071550
Nguyen, D., Alavi, M. V., Kim, K. Y., Kang, T., Scott, R. T., Noh, Y. H., et al. (2011). A new vicious cycle involving glutamate excitotoxicity, oxidative stress, and mitochondrial dynamics. Cell death and Dis. 2, e240. doi:10.1038/cddis.2011.117
O'Carroll, A. M., Lolait, S. J., and Howell, G. M. (2006). Transcriptional regulation of the rat apelin receptor gene: promoter cloning and identification of an Sp1 site necessary for promoter activity. J. Mol. Endocrinol. 36 (1), 221–235. doi:10.1677/jme.1.01927
O'Carroll, A. M., Selby, T. L., Palkovits, M., and Lolait, S. J. (2000). Distribution of mRNA encoding B78/apj, the rat homologue of the human APJ receptor, and its endogenous ligand apelin in brain and peripheral tissues. Biochimica biophysica acta 1492 (1), 72–80. doi:10.1016/s0167-4781(00)00072-5
O'Dowd, B. F., Heiber, M., Chan, A., Heng, H. H., Tsui, L. C., Kennedy, J. L., et al. (1993). A human gene that shows identity with the gene encoding the angiotensin receptor is located on chromosome 11. Gene 136 (1-2), 355–360. doi:10.1016/0378-1119(93)90495-o
Okamoto, K., Tanaka, H., Ogawa, H., Makino, Y., Eguchi, H., Hayashi, S., et al. (1999). Redox-dependent regulation of nuclear import of the glucocorticoid receptor. J. Biol. Chem. 274 (15), 10363–10371. doi:10.1074/jbc.274.15.10363
Orellana-Urzúa, S., Rojas, I., Líbano, L., and Rodrigo, R. (2020). Pathophysiology of ischemic stroke: role of oxidative stress. Curr. Pharm. Des. 26 (34), 4246–4260. doi:10.2174/1381612826666200708133912
Pauli, A., Norris, M. L., Valen, E., Chew, G. L., Gagnon, J. A., Zimmerman, S., et al. (2014). Toddler: an embryonic signal that promotes cell movement via Apelin receptors. Sci. (New York NY) 343 (6172), 1248636. doi:10.1126/science.1248636
Pugh, B. F., and Tjian, R. (1991). Transcription from a TATA-less promoter requires a multisubunit TFIID complex. Genes and Dev. 5 (11), 1935–1945. doi:10.1101/gad.5.11.1935
Reaux, A., De Mota, N., Skultetyova, I., Lenkei, Z., El Messari, S., Gallatz, K., et al. (2001). Physiological role of a novel neuropeptide, apelin, and its receptor in the rat brain. J. Neurochem. 77 (4), 1085–1096. doi:10.1046/j.1471-4159.2001.00320.x
Reaux, A., Gallatz, K., Palkovits, M., and Llorens-Cortes, C. (2002). Distribution of apelin-synthesizing neurons in the adult rat brain. Neuroscience 113 (3), 653–662. doi:10.1016/s0306-4522(02)00192-6
Reaux-Le Goazigo, A., Morinville, A., Burlet, A., Llorens-Cortès, C., and Beaudet, A. (2004). Dehydration-induced cross-regulation of apelin and vasopressin immunoreactivity levels in magnocellular hypothalamic neurons. Endocrinology 145 (9), 4392–4400. doi:10.1210/en.2004-0384
Rodrigo, R., Fernández-Gajardo, R., Gutiérrez, R., Matamala, J. M., Carrasco, R., Miranda-Merchak, A., et al. (2013). Oxidative stress and pathophysiology of ischemic stroke: novel therapeutic opportunities. CNS and neurological Disord. drug targets 12 (5), 698–714. doi:10.2174/1871527311312050015
Ronkainen, V. P., Ronkainen, J. J., Hänninen, S. L., Leskinen, H., Ruas, J. L., Pereira, T., et al. (2007). Hypoxia inducible factor regulates the cardiac expression and secretion of apelin. FASEB J. official Publ. Fed. Am. Soc. Exp. Biol. 21 (8), 1821–1830. doi:10.1096/fj.06-7294com
Salcedo, A., Garijo, J., Monge, L., Fernandez, N., Luis Garcia-Villalon, A., Sanchez Turrion, V., et al. (2007). Apelin effects in human splanchnic arteries: role of nitric oxide and prostanoids. Regul. Pept. 144 (1-3), 50–55. doi:10.1016/j.regpep.2007.06.005
Shen, Y., Li, Y., Chen, C., Wang, W., and Li, T. (2020). D-dimer and diffusion-weighted imaging pattern as two diagnostic indicators for cancer-related stroke: a case-control study based on the STROBE guidelines. Med. Baltim. 99 (4), e18779. doi:10.1097/MD.0000000000018779
Sheng, R., Liu, X. Q., Zhang, L. S., Gao, B., Han, R., Wu, Y. Q., et al. (2012). Autophagy regulates endoplasmic reticulum stress in ischemic preconditioning. Autophagy 8 (3), 310–325. doi:10.4161/auto.18673
Sorce, S., and Krause, K. H. (2009). NOX enzymes in the central nervous system: from signaling to disease. Antioxidants and redox Signal. 11 (10), 2481–2504. doi:10.1089/ars.2009.2578
Susin, S. A., Lorenzo, H. K., Zamzami, N., Marzo, I., Snow, B. E., Brothers, G. M., et al. (1999). Molecular characterization of mitochondrial apoptosis-inducing factor. Nature 397 (6718), 441–446. doi:10.1038/17135
Suzaki, Y., Yoshizumi, M., Kagami, S., Koyama, A. H., Taketani, Y., Houchi, H., et al. (2002). Hydrogen peroxide stimulates c-Src-mediated big mitogen-activated protein kinase 1 (BMK1) and the MEF2C signaling pathway in PC12 cells: potential role in cell survival following oxidative insults. J. Biol. Chem. 277 (11), 9614–9621. doi:10.1074/jbc.M111790200
Szokodi, I., Tavi, P., Foldes, G., Voutilainen-Myllyla, S., Ilves, M., Tokola, H., et al. (2002). Apelin, the novel endogenous ligand of the orphan receptor APJ, regulates cardiac contractility. Circulation Res. 91 (5), 434–440. doi:10.1161/01.res.0000033522.37861.69
Tanaka, H., Makino, Y., Okamoto, K., Iida, T., Yan, K., and Yoshikawa, N. (1999). Redox regulation of the glucocorticoid receptor. Antioxidants and redox Signal. 1 (4), 403–423. doi:10.1089/ars.1999.1.4-403
Tang, Z., Dong, H., Li, T., Wang, N., Wei, X., Wu, H., et al. (2021). The synergistic reducing drug resistance effect of cisplatin and ursolic acid on osteosarcoma through a multistep mechanism involving ferritinophagy. Oxidative Med. Cell. Longev. 2021, 5192271. doi:10.1155/2021/5192271
Tatemoto, K., Hosoya, M., Habata, Y., Fujii, R., Kakegawa, T., Zou, M. X., et al. (1998). Isolation and characterization of a novel endogenous peptide ligand for the human APJ receptor. Biochem. biophysical Res. Commun. 251 (2), 471–476. doi:10.1006/bbrc.1998.9489
Tatemoto, K., Takayama, K., Zou, M. X., Kumaki, I., Zhang, W., Kumano, K., et al. (2001). The novel peptide apelin lowers blood pressure via a nitric oxide-dependent mechanism. Regul. Pept. 99 (2-3), 87–92. doi:10.1016/s0167-0115(01)00236-1
The World Health Organization (1988). The World Health Organization MONICA Project (monitoring trends and determinants in cardiovascular disease): a major international collaboration. WHO MONICA Project Principal Investigators. J. Clin. Epidemiol. 41 (2), 105–114. doi:10.1016/0895-4356(88)90084-4
Vahsen, N., Cande, C., Brière, J. J., Bénit, P., Joza, N., Larochette, N., et al. (2004). AIF deficiency compromises oxidative phosphorylation. EMBO J. 23 (23), 4679–4689. doi:10.1038/sj.emboj.7600461
Van Der Heide, L. P., Hoekman, M. F., and Smidt, M. P. (2004). The ins and outs of FoxO shuttling: mechanisms of FoxO translocation and transcriptional regulation. Biochem. J. 380 (Pt 2), 297–309. doi:10.1042/BJ20040167
Vickers, C., Hales, P., Kaushik, V., Dick, L., Gavin, J., Tang, J., et al. (2002). Hydrolysis of biological peptides by human angiotensin-converting enzyme-related carboxypeptidase. J. Biol. Chem. 277 (17), 14838–14843. doi:10.1074/jbc.M200581200
Wang, G., Qi, X., Wei, W., Englander, E. W., and Greeley, G. H. (2006). Characterization of the 5'-regulatory regions of the rat and human apelin genes and regulation of breast apelin by USF. FASEB J. official Publ. Fed. Am. Soc. Exp. Biol. 20 (14), 2639–2641. doi:10.1096/fj.06-6315fje
Wang, J., Gao, S., Lenahan, C., Gu, Y., Wang, X., Fang, Y., et al. (2022). Melatonin as an antioxidant agent in stroke: an updated review. Aging Dis. 13 (6), 1823–1844. doi:10.14336/AD.2022.0405
Wei, L., Hou, X., and Tatemoto, K. (2005). Regulation of apelin mRNA expression by insulin and glucocorticoids in mouse 3T3-L1 adipocytes. Regul. Pept. 132 (1-3), 27–32. doi:10.1016/j.regpep.2005.08.003
Woo, S. K., Kwon, M. S., Geng, Z., Chen, Z., Ivanov, A., Bhatta, S., et al. (2012). Sequential activation of hypoxia-inducible factor 1 and specificity protein 1 is required for hypoxia-induced transcriptional stimulation of Abcc8. J. Cereb. blood flow metabolism official J. Int. Soc. Cereb. Blood Flow Metabolism 32 (3), 525–536. doi:10.1038/jcbfm.2011.159
World Health Organization (2004). The World Health report 2004: changing history. Geneva: World Health Organization.
Wu, G., Li, L., Liao, D., and Wang, Z. (2015). Protective effect of Apelin-13 on focal cerebral ischemia-reperfusion injury in rats. Nan fang yi ke da xue xue bao = J. South. Med. Univ. 35 (9), 1335–1339.
Wu, Y., Wang, X., Zhou, X., Cheng, B., Li, G., and Bai, B. (2017). Temporal expression of Apelin/Apelin receptor in ischemic stroke and its therapeutic potential. Front. Mol. Neurosci. 10, 1. doi:10.3389/fnmol.2017.00001
Xin, Q., Ji, B., Cheng, B., Wang, C., Liu, H., Chen, X., et al. (2014). Endoplasmic reticulum stress in cerebral ischemia. Neurochem. Int. 68, 18–27. doi:10.1016/j.neuint.2014.02.001
Xu, W., Gao, L., Li, T., Zheng, J., Shao, A., and Zhang, J. (2018b). Apelin-13 alleviates early brain injury after subarachnoid hemorrhage via suppression of endoplasmic reticulum stress-mediated apoptosis and blood-brain barrier disruption: possible involvement of ATF6/CHOP pathway. Neuroscience 388, 284–296. doi:10.1016/j.neuroscience.2018.07.023
Xu, W., Gao, L., Zheng, J., Li, T., Shao, A., Reis, C., et al. (2018a). The roles of microRNAs in stroke: possible therapeutic targets. Cell Transplant. 27 (12), 1778–1788. doi:10.1177/0963689718773361
Xu, W., Li, T., Gao, L., Zheng, J., Yan, J., Zhang, J., et al. (2019). Apelin-13/APJ system attenuates early brain injury via suppression of endoplasmic reticulum stress-associated TXNIP/NLRP3 inflammasome activation and oxidative stress in a AMPK-dependent manner after subarachnoid hemorrhage in rats. J. neuroinflammation 16 (1), 247. doi:10.1186/s12974-019-1620-3
Xu, X. H., and Zhong, Z. (2013). Disease modeling and drug screening for neurological diseases using human induced pluripotent stem cells. Acta Pharmacol. Sin. 34 (6), 755–764. doi:10.1038/aps.2013.63
Yang, Y., Zhang, S., Fan, C., Yi, W., Jiang, S., Di, S., et al. (2016). Protective role of silent information regulator 1 against hepatic ischemia: effects on oxidative stress injury, inflammatory response, and MAPKs. Expert Opin. Ther. targets 20 (5), 519–531. doi:10.1517/14728222.2016.1153067
Yeh, S. H., Yang, W. B., Gean, P. W., Hsu, C. Y., Tseng, J. T., Su, T. P., et al. (2011). Translational and transcriptional control of Sp1 against ischaemia through a hydrogen peroxide-activated internal ribosomal entry site pathway. Nucleic acids Res. 39 (13), 5412–5423. doi:10.1093/nar/gkr161
Yi, B. R., Kim, S. U., and Choi, K. C. (2013). Development and application of neural stem cells for treating various human neurological diseases in animal models. Lab. Anim. Res. 29 (3), 131–137. doi:10.5625/lar.2013.29.3.131
Zaman, K., Ryu, H., Hall, D., O'Donovan, K., Lin, K. I., Miller, M. P., et al. (1999). Protection from oxidative stress-induced apoptosis in cortical neuronal cultures by iron chelators is associated with enhanced DNA binding of hypoxia-inducible factor-1 and ATF-1/CREB and increased expression of glycolytic enzymes, p21(waf1/cip1), and erythropoietin. J. Neurosci. official J. Soc. Neurosci. 19 (22), 9821–9830. doi:10.1523/JNEUROSCI.19-22-09821.1999
Zhang, F., Liu, L., Zhang, C., Ji, S., Mei, Z., and Li, T. (2021b). Association of metabolic syndrome and its components with risk of stroke recurrence and mortality: a meta-analysis. Neurology 97 (7), e695–e705. doi:10.1212/WNL.0000000000012415
Zhang, F., Wang, K., Du, P., Yang, W., He, Y., Li, T., et al. (2021a). Risk of stroke in cancer survivors: a meta-analysis of population-based cohort studies. Neurology 96 (4), e513–e526. doi:10.1212/WNL.0000000000011264
Zhang, Z., Yu, B., and Tao, G. Z. (2009). Apelin protects against cardiomyocyte apoptosis induced by glucose deprivation. Chin. Med. J. 122 (19), 2360–2365.
Keywords: apelin, APJ, stroke, oxidative stress, neuroprotection
Citation: Xu W, Yan J, Travis ZD, Lenahan C, Gao L, Wu H, Zheng J, Zhang J, Shao A and Yu J (2025) Apelin/APJ system: a novel promising target for anti-oxidative stress in stroke. Front. Pharmacol. 15:1352927. doi: 10.3389/fphar.2024.1352927
Received: 09 December 2023; Accepted: 10 December 2024;
Published: 15 January 2025.
Edited by:
Rui Liu, Chinese Academy of Medical Sciences and Peking Union Medical College, ChinaReviewed by:
Santiago J. Ballaz, Yachay Tech University, EcuadorTian Li, Air Force Medical University, China
Wei Liu, Central South University, China
Katarzyna Czarzasta, Medical University of Warsaw, Poland
Copyright © 2025 Xu, Yan, Travis, Lenahan, Gao, Wu, Zheng, Zhang, Shao and Yu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jun Yu, MjUwNTAyMEB6anUuZWR1LmNu; Anwen Shao, MjExMTgxMTZAemp1LmVkdS5jbg==
†These authors have contributed equally to this work and share first authorship
 Weilin Xu
Weilin Xu Jun Yan
Jun Yan Zachary D. Travis4
Zachary D. Travis4 Liansheng Gao
Liansheng Gao Haijian Wu
Haijian Wu Jianmin Zhang
Jianmin Zhang Anwen Shao
Anwen Shao Jun Yu
Jun Yu