
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Pharmacol. , 03 April 2024
Sec. Ethnopharmacology
Volume 15 - 2024 | https://doi.org/10.3389/fphar.2024.1352657
Bai Hua Qian Hu (Qianhu; Peucedanum praeruptorum Dunn) is a classical medicinal plant traditionally prescribed for respiratory ailments, including cough, pulmonary hypertension, and asthma. In this review, we summarize the research progress of the toxicology, pharmacokinetics, pharmacology, phytochemistry, botany, quality control, and traditional uses of P. praeruptorum in order to support future investigations into the scientific and therapeutic promise of this important medicinal plant. Information pertaining to P. praeruptorum was collected from scientific databases (ScienceDirect, Springer, SciFinder, PubMed, Baidu Scholar, Google Scholar, Web of Science), as well as toxicology papers from local conferences, M. Sc. and Ph.D. theses and dissertations, local magazines, classic texts on Chinese botanical drugs, and peer-reviewed journals. The Plant List (www.theplantlist.org) was utilized to verify the taxonomy of P. praeruptorum. P. praeruptorum was found to contain more than 119 distinct phytochemicals, including simple coumarins, pyranocoumarins, furanocoumarins, flavonoids, ketones, organic acids, and sterols, among others (e.g., praeruptorins A and B). Both crude plant extracts and purified metabolites of P. praeruptorum have been reported as treatments for hypertension, osteoporosis, Huntington’s disease, and cancer. In addition, extracts of P. praeruptorum are reported to exhibit diverse pharmacological activities, including osteogenic, anti-osteoclastogenic, antidepressant, neuroprotective, antitumor, and anti-inflammatory effects. Research into the pharmacology and phytochemistry of P. praeruptorum partially support both traditional uses and extraction methods. However, further research is required to elucidate the relationships between these metabolites, their molecular mechanisms, their structure-function roles, and their antagonistic and synergistic effects.
Peucedanum L. (Umbelliferae) consists of 120 species of herbaceous perennial plants which are distributed widely across the globe (Editorial Committee of Flora of China, 1992). One member of the genus, Peucedanum praeruptorum Dunn, is cultivated in montane habitats at an altitude between 250 and 2000 m. In traditional Chinese medicine (TCM), the root tissues of P. praeruptorum (Qianhu) have been utilized for hundreds of years to address diverse respiratory ailments, including cough, asthma, and pulmonary hypertension (Zhou et al., 2013). The roots of P. praeruptorum demonstrate diverse pharmacological activities, including anti-inflammatory, neuroprotective, antitumor, anti-osteoclastogenic, antidepressant, and osteogenic effects (Song et al., 2022). In addition, P. praeruptorum has been found to contain an array of useful phytochemicals, including simple coumarins, pyranocoumarins, furanocoumarins, flavonoids, ketones, sterols, and organic acids, and others (Song et al., 2022).
However, to date, there has been no comprehensive and systematic evaluation of the bioactivities, pharmacology, structures, functions, and toxicities of these phytochemicals, or of P. praeruptorum crude extracts. Moreover, the traditional uses of P. praeruptorum and their pharmacological evidence have not been critically evaluated. Here, we systematically summarized the toxicology, molecular mechanisms, pharmacology, phytochemistry, botany, quality control, and traditional uses of P. praeruptorum to validate the medicinal use of this species. To further clarify the material basis of P. praeruptorum’s medicinal effect, identifying the structures of metabolites will provide a certain theoretical basis for the further development and utilization of Qianhu. The information presented here can aid the planning of clinical trials and the development of novel medicines containing P. praeruptorum or its active constituents.
Information pertaining to P. praeruptorum was sourced from scientific databases (ScienceDirect, Web of Science, Springer, Google Scholar, SciFinder, PubMed, Baidu Scholar), as well as toxicology papers from local conferences, M. Sc. and Ph.D. theses and dissertations, local magazines, classic texts on Chinese botanical drugs, and peer-reviewed journals. We utilized the following, as well as related, keywords to perform the literature review: P. praeruptorum Dunn, secondary metabolites, toxicology, safety, ethnobotanical survey, quality control, pharmacology, medicinal uses, phytochemistry, and biological activity. The Plant List (www.theplantlist.org) was utilized to verify the taxonomy of P. praeruptorum and verify subspecies and cultivars. The chemical structures were drawn using ChemDraw.
P. praeruptorum Dunn (Figure 1) is an herbaceous perennial in the Umbelliferae family. P. praeruptorum Dunn is the only accepted name for the species (www.theplantlist.org), although it has two other synonyms: P. praeruptorum var. grande K.T. Fu and P. praeruptorum subsp. hirsutiusculum Ma. P. praeruptorum is found in the wild in south China, including in Zhejiang, Anhui, Jiangxi, Hubei, Hunan, Guizhou, Sichuan, and Yunnan provinces (Figure 2). The traditional production areas are northwest Zhejiang, southeast Anhui, and northeast Jiangxi, where the plant is called “Zhe Qianhu,” “Ning Qianhu,” and “Xin Qianhu,” respectively (Zhou et al., 2021).
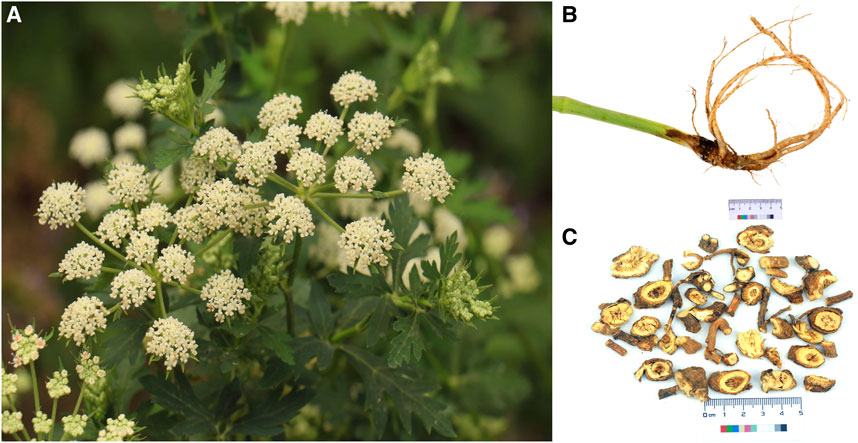
Figure 1. The aerial tissues (A), medicinal root tissues (B), and commercial presentation (C) of Peucedanum praeruptorum Dunn.
According to the Flora of China (Editorial Committee of Flora of China, 1992), P. praeruptorum grows along forest edges, near roadsides, and in semi-open grassy areas within montane habitats at an altitude of 250–2000 m (Liu et al., 2021). P. praeruptorum can reach a height of 60–100 cm. The plant has a cylindrical stem, with a glabrous lower part and piliferous branches on the upper part. The medulla is solid. Leaves are wide ovate or triangular ovate. The compound umbel is terminal or lateral, and 3.5–9 cm in diameter. The fruits are oval, 4 mm in length and 3 mm in width. The back of the fruit is flat. The rhizomes are strong, brown, and 1–1.5 cm in diameter. The roots are conical and branched, with thin root ends. For medicinal use, roots are collected from winter to spring. Abluent, fresh, thin slices are dried prior to medicinal use.
The List of Mingyi Bielu (《名医别录》), which dates to the Wei-Jin and South-North Dynasties (A.D. 220-450), was the first to record the roots of P. praeruptorum as TCM. Many ancient texts, such as the Rihuazi Bencao (《日华子本草》) (Five Dynasties, A.D. 908–923), the Compendium of Materia Medica (《本草纲目》) (Ming Dynasty, A.D. 1578), and the Illustrated Classics of Materia Medica (《本草图经》) (Song Dynasty, A.D. 1061) also record that P. praeruptorum was widely used to treat colds, headaches, coughs, asthma, and chest congestion (He et al., 2007). In the Chinese Pharmacopoeia 2000 (Chinese Pharmacopoeia Committee of People’s Repulic of China, 2000), Qianhu is defined as the roots of either P. decursivum (Miq.) Maxim (Zihuaqianhu) or P. praeruptorum Dunn. However, P. decursivum is not traditionally used as a source of Qianhu, and thus, was removed from the Chinese Pharmacopoeia 2005 (Chinese Pharmacopoeia Committee of People’s Repulic of China, 2005). More recently, the Chinese Pharmacopoeia 2010, 2015, and 2020 (Chinese Pharmacopoeia Committee of People’s Repulic of China, 2010; Chinese Pharmacopoeia Committee of People’s Repulic of China, 2015; Chinese Pharmacopoeia Committee of People’s Repulic of China, 2020) define Qianhu as the roots of P. praeruptorum exclusively, while Zihuaqianhu is defined as the roots of P. decursivum.
P. praeruptorum has many folk names, including yimacai, luoguicai, shuiqianhu, shuifangfeng, shanyuansui, guanqianhu, and shanduhuo. In TCM, Qianhu is used to treat chronic respiratory failure, acute bronchitis, and iridocyclitis after cataract surgery (Wang, 2016). The roots of P. praeruptorum have been utilized in a variety of traditional preparations, and are often used in combination with Mentha haplocalyx Briq., Arctium lappa L., and Platycodon grandiflorum (Jacq.) A. DC. to treat external wind-heat, body heat, headache, cough, and phlegm. In addition, P. praeruptorum is used in combination with Citrus reticulata Blanco, Pinellia ternata (Thunb.) Breit., and Prunus armeniaca L. var. ansu Maxim. to treat cough, chest congestion, vomiting, and nausea. The roots of P. praeruptorum have been used in more than 189 TCM preparations and 525 classical prescriptions (https://db.yaozh.com, accessed 7th December, 2023). Examples of TCM prescriptions containing P. praeruptorum are listed in Table 1. “Bai He Qian Hu Tang,” “Da Qian Hu Tang,” “Fu Ling Qian Hu Tang,” “Jiu Wei Qian Hu Tang,” and “Xing Su San” are Chinese classical prescriptions recorded in many ancient books. The botanical drugs “Tong Xuan Li Fei Ke Li” and “Tong Xuan Li Fei Pian” (Chinese Pharmacopoeia Committee of People’s Repulic of China, 2020), which are accredited by the National Medical Products Administration, are produced and marketed in China to treat cough. However, further research is required to clarify any potential synergisms or interactions between the bioactive phytochemicals in P. praeruptorum and those of other medicinal plants, as well as to elucidate their mechanisms of action. According to the Chinese Pharmacopoeia 2020 (Chinese Pharmacopoeia Committee of People’s Repulic of China, 2020), although Qianhu can disperse wind-heat, reduce cough and phlegm, and dissipate adverse Qi, comprehensive studies of its constitutive bioactive monomers should be conducted.
P. praeruptorum is reported to contain a diverse array of phytochemicals, including simple coumarins (1–13), pyranocoumarins (14–66), furanocoumarins (67–94), ketones (95, 96), sterols (97, 98), and organic acids (99–105), and others (106–119) (Table 2). The majority of these phytochemicals were isolated from root tissues, which are the traditional medicinal material. Among these isolated metabolites, angular pyranocoumarins (e.g., praeruptorins A and B) are the most abundant bioactive metabolites in P. praeruptorum tissues (Song et al., 2015).
Thirteen simple coumarins (Figure 3) have been isolated from P. praeruptorum root tissues, including umbelliferone 1, scopoletin 2, isoscopoletin 3, isofraxidin 4, 8-carboxy-7-hydroxy coumarin 5, skimmin 6, scopolin 7, osthenol 8, praeroside VI 9, apiosylskimmin 10, hymexelsin 11, eleutheroside B1 12, and (−)-peucedanol 13. However, the pharmacological activities of these simple coumarins have rarely been reported.
Fifty-five pyranocoumarins (Figure 4) have been isolated from P. praeruptorum root tissues, including praeruptorin C 14, praeruptorin E 15, qianhucoumarin D 16, qianhucoumarin A 17, khellactone 18, qianhucoumarin B 19, (9R,10R)-9-acetoxy-8,8-dimethyl-9,10-dihydro-2H, 8H-benzo [1,2-b:3,4-b′]dipyran-2-one-10-yl-ester 20, (±)-cis-4′-acetyl-3′-crotonoykhellactone 21, qianhucoumarin E 22, hyuganin D 23, qianhucoumarin I 24, hyuganin C 25, qianhucoumarin J 26, praeruptorin B 27, (−)-praeruptorin A 28, cis-3′-isovaleryl-4′-senecioylkhellactone 29, decursinol angelate 30, 3′(S),4′(S)-3′,4′-disenecioyl-3′,4′-dihydroseselin 31, 3′-O-acetyl-4′(S)-O-angeloyl-khellact 32, 3′,4′-disenecioyl-cis-khellactone 33, pteryxin 34, selinidin 35, isobocconin 36, aegelinol 37, suksdorfin 38, D-laserpitin 39, (−)-trans-khellactone 40, (+)-cis-khellactone 41, neopeucedalactone 42, decursitin D 43, praeroside V 44, cis-3′,4′-diisovalerylkhellactone 45, praeroside III 46, praeroside II 47, (±)-peuformosin 48, praeruptorin D 49, peucedanocoumarin II 50, isoepoxypteryxin 51, qianhucoumarin H 52, praeroside IV 53, (+)-praeruptorin A 54, (±)-cis-4′-ethy-3′-tigloylkhellactone 55, (3S′,4S′)-3-angeloyloxy-4-hydroxy-3,4-dihydroseselin 56, hyuganin B 57, corymbocoumarin 58, Pd-C-II 59, peucedanocoumarin I 60, (+)-samidin 61, (3′S, 4′S)-3′-O-isobutyroyl-4′-O-isovaleroylkhellactone 62, Pd-Ib 63, qianhucoumarin C 64, Pd-C-I 65, and peucedanocoumarin III 66. Research suggests that praeruptorin B possesses antitumor activity, and praeruptorin E possesses anti-inflammatory activity (Yu et al., 2012; Lin et al., 2020).
Furanocoumarins possess neuroprotective, anti-inflammatory, and anticancer activities in animals, and serve as phytotoxins and allelochemicals in plants (Jian et al., 2020). Twenty-nine furanocoumarins (Figure 5) have been isolated from P. praeruptorum root tissues, including psoralen 67, angelicin 68, xanthotoxin 69, bergapten 70, imperatorin (IMP) 71, deltoin 72, isopimpinellin 73, rutaretin 74, arnocoumarin 75, qianhucoumarin G 76, nodakenetin 77, nodakenetin tiglate 78, marmesinin 79, oxypeucedanin 80, marmesin-11-O-β-D-glucopyranosyl (1→6)-β-D-glucopyranoside 81, rutarin 82, oxypeucedanin hydrate 83, marmesin 84, sphondin 85, oroselol 86, peucedanoside A 87, peucedanoside B 88, apterin 89, praeroside VII 90, isorutarin 91, nodakenin 92, praeroside I 93, and (2′S)-rutaretin-4′-O-(6-p-hydroxybenzoyl-β-D-glucopyranoside) 94.
Two ketones (tanshinone I 95 and tanshinone IIA 96) were confirmed in the roots of P. praeruptorum. Two sterols (β-sitosterol 97 and daucosterol 98) and seven organic acids (vanillic acid 99, gallic acid 100, butyric acid 101, palmitic acid 102, 4H-1-benzopyran-4-one,5-hydroxy-6-methoxy-2-phenyl-7-O-α-D-glucuronyl acid 103, tetracosanoic acid 104, and 9,10-dihydrophenanthrinic acid 105) were confirmed in the stem and leaves of P. praeruptorum. However, the pharmacological activities of these ketones, sterols, and organic acids were not found in the available studies. Figure 6 shows the chemical structures of these phytochemicals.
Other metabolites (Figure 7), such as 2,6-dimethyl quinoline 106, 3-(4′-formylphenoxy)-4-methoxybenzaldehyde 107, 3-(4′-formylphenoxy)-4-methoxybenzaldehyde 108, bis(2-ethylhexyl) phthalate 109, 4-[β-D-apiofuranosyl-(1→6)-β-D-glucopyranosyloxy]-3-methoxypropiophenone 110, baihuaqianhuoside 111, galactitol 112, (−)-sclerodin 113, adenoside 114, acetylatractylodinol 115, 4H-1-benzopyran-4-one,5-hydroxy-6-methoxy-2-phenyl-7-O-α-D-glucuronyl methyl ester 116, polyacetylene 117, D-mannitol monohexadecanoate 118, and α-D-glucopyranose-1-hexadecanoate 119, have been isolated from P. praeruptorum root tissues. However, the pharmacological activities of these phytochemicals were not found in the available studies.
P. praeruptorum exhibits diverse pharmacological activities (Table 3), including anti-inflammatory, expectorant, antitussive, antitumor, neuroprotective, anti-osteoclastogenic, and antidepressant effects. The antitumor, immunoregulatory, and anti-inflammatory activities are the most notable, and putative molecular mechanisms are shown in Figure 8.
Praeruptorin C (pyranocoumarins) was traditionally used as an antibechic and antibronchitic drug. In one study, praeruptorin C treatment was found to regulate excitatory synaptic proteins in the anterior cingulate cortex, attenuate the release of proinflammatory cytokines, and inhibit the activation of microglia (Su et al., 2021). However, further studies need to assess the effects of praeruptorin C in other pain models. In another study, administration of (±)-praeruptorin A (30, 60, 120 mg/kg) to ovalbumin-sensitized BALB/c mice for 56 days increased the level of INF-γ and reduced the expression of IL-13/-4 in bronchoalveolar lavage fluid (BALF); decreased the level of immunoglobulin (Ig) E in serum; and suppressed airway inflammation, hyperresponsiveness, and remodeling. In the study, levels of cytokines in BALF, immunoglobulin (Ig) E in serum as well as expression of TGF-β1 and Smad proteins in lung tissue were measured by enzyme-linked immunosorbent assay, immunohistochemistry or Western blot analysis. (Xiong et al., 2012). Treatment of inflamed human periodontal membrane cells with praeruptorin D (10, 20, 30, 40 μg/mL) inhibited TNF-α and IL-1β expression. According to experimental requirements, the negative control group was healthy cells group and positive control group was minocycline hydrochloride group (Yu, 2022). Administration of praeruptorins C, D, and E (2, 4, 8, 16 μg/mL) to RAW264.7 macrophages stimulated with lipopolysaccharide (LPS) for 24 h inhibited NF-κB and STAT3 activation. Although all three had anti-inflammatory activities, praeruptorins D and E exhibited greater anti-inflammatory activities than praeruptorin C and in vivo pharmacological potencies need to be further evaluated. (Yu et al., 2012). One study demonstrated that the administration of IMP (furanocoumarins) at doses of 5, 10, and 15 μmol/L for 1 h in the RBL-2H3 allergic inflammatory cell model promoted IFN-γ expression; decreased the expression of TNF-α, COX-2, IL-6, IL-4, and IL-3; and inhibited RBL-2H3 cell degranulation. These results indicate that IMP is effective for inhibiting the inflammatory response in the RBL-2H3 allergic inflammatory cell model mediated by IgE immunoregulation (Long et al., 2019). However, the authors did not evaluate the effectiveness of IMP using animal models. Moreover, P. praeruptorum polysaccharide treatments ranging from 25 to 200 μg/mL increased the expression of costimulatory and accessory factors, increased the secretion of chemokines and inflammatory factors, and enhanced phagocytosis and pinocytosis. In this way, P. praeruptorum polysaccharides modulated the inflammatory response of macrophages via the NF-κB and TLR2/TLR4-dependent MAPK pathways (Zhao et al., 2022). Another study demonstrated that Dl-praeruptorin A reduced inflammation in an LPS-induced acute lung injury mouse model, specifically inhibiting endothelial inflammation (Wang et al., 2012; Zhou et al., 2016). Nonetheless, the mechanism of Dl-praeruptorin A against acute lung injury needs further study prior to its use in clinical treatment.
P. praeruptorum water extract treatment (45 g/kg) was found to resolve phlegm (Liu et al., 1997; Meng et al., 1997). The administration of praeruptorin C and nodakenin (10 mg/kg) to mice, with phenolsulfonphthalein as an expectorant indicator, increased phenolsulfonphthalein excretion in tracheal tissues. Both praeruptorin C and nodakenin showed expectorant effects. And ammonium chloride served as a positive control (Liu et al., 2009). However, phenolsulfonphthalein is mainly excreted from the urine by the kidneys, and drugs that affect renal function are prone to false positive results. Similarly, treating BALB/c mice for 5 days with nodakenin (10 mg/kg) promoted the DNA binding activity of NF-κB, increased the levels of IκBα and P65 in the cytoplasm, decreased the expression of p-P65 and P65 in the nucleus, reduced the level of IgE in serum and IL-13/-5/-4 in BALF, and suppressed airway hyperreactivity and inflammation (Xiong et al., 2014). Notably, the pharmacological properties of P. praeruptorum before and after honey roasting were found to be different. The 5.0 and 10.0 g/kg doses of honey-roasted products showed stronger expectorant and antitussive effects than raw products. However, the 2.5 g/kg dose of honey-roasted and raw products was more effective as relieving asthma (Zhang et al., 2010b). Although P. praeruptorum has long been used to resolve phlegm, descend Qi, clear heat, and dissipate wind in TCM (Zhao et al., 2022), comprehensive studies of its constitutive bioactive monomers and their molecular mechanisms, as well as clinical trials, are necessary to improve its expectorant and antitussive activities with minimal side effects.
More than 85% of all kidney cancers worldwide are characterized as renal cell carcinoma (RCC). In one study, treating RCC cells for 24 h with praeruptorin B (0–30 μmol/L) inhibited both migrability and invasibility, as well as downregulated the expression of cathepsins V and C in ACHN and 786-O cells (Lin et al., 2020). Similarly, praeruptorin B (20, 40, 60 μmol/L) was reported to inhibit both the proliferation and migration of SK-OV-3 ovarian cancer cells, as well as downregulate the expression of FASN, c-Myc, SREBP-1c, and cyclin D1. The likely mechanism is that the SREBP-1c/FASN signaling pathway regulates the energy metabolism of SK-OV-3 ovarian cancer cells, thus inhibiting their proliferation. Real-time fluorescence quantitative polymerase chain reaction (RT-qPCR) was used to detect the mRNA expressions of proliferating genes such as c-Myc and CyclinD1 and the mRNA expressions of key genes of energy metabolism such as SREBP-1c and FASN in tumor cells and Western blot was used to detect the expressions of SREBP-1c, FASN proteins (Xue et al., 2021).
A 48 h treatment with praeruptorin A (25 μmol/L) combined with taxol (125 nmol/L) induced apoptosis in A2780/TAX ovarian cancer cells, and inhibited their migration by downregulating MMP9 and MMP2 expression. However, it remains to be seen whether praeruptorin A can enhance the chemotherapeutic efficacy of taxol, or whether it retains its curative effect against ovarian cancer in vivo (Chen et al., 2022). Another study on HeLa and SiHa cell lines reported that praeruptorin A could increase the levels of tissue inhibitors of metalloproteinase-2 and decrease the expression of matrix metalloproteinase-2; downregulate S-phase kinase-associated protein 2 and cyclin D1; upregulate p27, p21, p16, and Rb; and induce G0/G1 phase cell cycle arrest. However, praeruptorin A could not inhibit cell viability in IgG-treated cells, the effect of IgG interference with praeruptorin A in HeLa cells (Wu et al., 2017). Additionally, praeruptorin A has been found effective at inhibiting the migrability and invasibility of hepatocellular carcinoma (HCC) cells by activating extracellular signal-regulated kinase signaling and inhibiting matrix metalloproteinase-1 expression. However, in vivo metastasis animal model is even worthier for further investigation to examine the antimetastatic effect and safety evaluation of praeruptorin A (Yu et al., 2021). In LS174T cells, praeruptorin A (2.5, 10, 40 μmol/L) could significantly upregulate cytochrome P450 3A4 levels and activity via a pregnane X receptor-mediated pathway. However, siRNA knockdown of the pregnane X receptor resulted in suppressed expression of cytochrome P450 3a11 in mouse primary hepatocytes (Huang et al., 2013).
Administration of praeruptorins A and B to SGC7901 human gastric cancer cells for 24 h produced cytotoxic effects in SGC7901 cells, resulting in antiproliferation. praeruptorin A could also enhance the action of doxorubicin on SGC7901 cells (Liang et al., 2010). Administration of praeruptorins A and C (10, 25, 50 μmol/L) to HepG2 cells in vitro for 24 or 48 h increased the expression of multidrug resistance-associated protein 2 by way of the constitutive androstane receptor-mediated pathway (Zhou et al., 2013). In H1299, PC9, H1975, and PC9/ER human non-small-cell lung cancer (NSCLC) cell lines, praeruptorin A and pteryxin restricted the HGF-induced phosphorylation of MET in PC9/ER and H1975 cells, increased PARP cleavage in H1975 cells and the proportion of annexin V-positive cells, and overall induced apoptosis and reduced cell viability. However, praeruptorin A and pteryxin could not inhibit HGF-induced AKT phosphorylation and prompted apoptosis in NSCLC cells regardless of EGFR TKI resistance or epidermal growth factor receptor (EGFR) mutation status (Park et al., 2022).
In another study, 24 h of treatment with the angular pyranocoumarin (±)-4′-O-acetyl-3′-O-angeloyl-cis-khellactone (0, 10, 20, 30, 40 μg/mL) was found to promote apoptosis in U266 cells, thereby constraining proliferation. The most likely mechanism involved the upregulation of caspase-3/-8 expression and the downregulation of hTERT, p-AKT, and pERK (Yu et al., 2015). Neopeucedalactone, a pyranocoumarin isolated from P. praeruptorum roots, was found to inhibit the growth of human leukemic HL-60, prostate cancer PC-3, and THP-1 cell lines in vitro (Li et al., 2020).
One study demonstrated that 24 h of treatment with praeruptorin C (0, 1, and 10 μmol/L) could partially reverse the upregulated expression of GluN2B-containing N-methyl-D-aspartate receptors, inhibit neuronal apoptosis, and balance Bax and Bcl-2 expression. Although it was suggested that praeruptorin C exerted its neuroprotective effects by reversing intracellular Ca2+ overload, it is possible other pathways or mechanisms were involved (Yang et al., 2013). In another study, praeruptorin C (1.5, 3.0 mg/kg) was found to alleviate depressive behavior, motor deficit, and neuronal excitotoxicity in 3-nitropropionic acid (3-NP)-treated mice via upregulating the expression of HTT, DARPP32, and BDNF in striatum tissue. Motor behavior was tested using the open field test and rotarod test, while psychiatric symptoms were tested using the forced swimming test and tail suspension test. We suggest that, based on these findings, praeruptorin C may prove therapeutic for cognitive, psychiatric, and movement disorders associated with Huntington’s disease (Wang et al., 2017).
Osteoporosis results in an elevated risk of fracture, compromised bone strength due to low bone density, and metabolic defects. Bone homeostasis depends on the resorption of bone by osteoclasts and formation of bone by osteoblasts. Imbalance of this tightly coupled process can cause diseases such as osteoporosis (Chen et al., 2018). In one study, exposure of RAW264.7 cells for 4 h to praeruptorin C (0, 20 μmol/L) reduced osteoclast formation by obstructing the JNK and NF-κB pathways, without disturbing the p38 and ERK pathways. In ovariectomized (OVX) mice, a model for post-menopausal bone loss, praeruptorin C was found to increase bone mass and decrease osteoclast activity. The antiresorptive properties of praeruptorin C suggest that it may be an effective treatment for osteoporosis, although further research is required (Liu et al., 2017). In bone marrow-derived macrophages, praeruptorin A (10 μmol/L) treatment for 30 min inhibited RANKL-stimulated osteoclast differentiation and p38 and Akt signaling (Yeon, Jeong-Tae, et al., 2014). Praeruptorin D could promote the osteogenic differentiation and proliferation of inflammatory periodontal membrane cells, and upregulate osteogenic gene (RUNX-2, ALP, and OCN) expression at doses of 10, 20, 30, and 40 μg/mL by using RT-qPCR and Alizarin red S staining (Yu, 2022).
Clinical depression is characterized by sustained depressive mood and cognitive dysfunction, including restlessness, anhedonia, sleep disorders, guilt, and repeated thoughts of suicide (Liu et al., 2011). The occurrence of depression is closely related to damage to hippocampal neurons. Chronic stress can damage the hippocampus, resulting in atrophy, apoptosis, or reduced regeneration of hippocampal neurons (Wang and Xu, 2014). Dl-praeruptorin A can protect the nervous and cardio-cerebrovascular systems. For example, 28 days of treatment with Dl-praeruptorin A (10, 30, 60 mg/kg) improved the synaptic ultrastructure of hippocampal CA1 region and increased neurotrophic factors and nerve growth factors in the hippocampus (Wang and Xu, 2014). In addition, 28 days of treatment with IMP (15, 30 mg/kg) significantly enhanced 5-HT1AR expression, 5-HT level, and sucrose preference; increased the incidence of grooming, rearing, and crossing behaviors; reduced immobility; and decreased 5-HTT expression. These results indicate that IMP exhibits antidepressant effects in rats, likely due to changes in the concentration of 5-HT and 5-HTT, and in the expression of 5-HT1AR, in the hippocampus and prefrontal cortex (Zheng et al., 2019). However, the mechanism responsible for the antidepressant activity of P. praeruptorum extracts remains unknown, and clinical pharmacological experiments are lacking.
P. praeruptorum has been shown to exhibit other therapeutic activities, including ventricular remodeling, inhibiting hepatic microsomal drug-metabolizing enzymes, ameliorating memory disruption, and alleviating ischemia/reperfusion injury. Administration of P. praeruptorum alcohol extracts to rats for 28 days affected ventricular remodeling and apoptosis-related proteins in different ways, and had a positive influence on ventricular remodeling (Tu et al., 2004). Treatment of mice with total coumarins (50, 100, 200 mg/kg) prolonged the hypnotic duration of pentobarbital sodium in a dose-dependent manner, inhibited the activities of aniline hydroxylase and aminopyrine N-demethylase, minimally influenced the hypnotic effect of barbital sodium, and inhibited the activity of hepatic microsomal drug-metabolizing enzymes (Wang et al., 2004). In addition, administration of nodakenin (10 mg/kg) reduced scopolamine-induced cognitive impairments associated with the Y-maze test and passive avoidance test, as well as minimized escape latency in the Morris water maze test. Nodakenin has been shown to block acetylcholinesterase activity in a dose-dependent manner in vitro (Kim et al., 2007). Administration of Dl-praeruptorin A (0.5, 1.0, 2.0 mg/kg) reduced the levels of bcl-2, bax, Fas, and IL-6, and raised the bcl-2/bax ratio, under hypotension without bradycardia. A positive, linear correlation has been demonstrated between bax, Fas, bcl-2, and IL-6, where neutrophil infiltration was minimal. Dl-praeruptorin A was also found to prevent postischemic cell death in rat heart, likely due to the automodulation of immediate-early gene expression of bax, Fas, bcl-2, and IL-6 during myocardial ischemia/reperfusion (Chang et al., 2003). In rats, praeruptorin C (5, 15, 30 mg/kg) was found to reduce ischemia/reperfusion injury induced by coronary ligation, most likely by reducing oxygen free radicals (Liu and Wang, 2009).
Praeruptorins A, B, and C, the primary metabolites of P. praeruptorum, exhibit diverse biological activities, including neuroprotective, antitumor, anti-inflammatory, immunoregulatory, anti-osteoclastogenic, and antidepressant effects. Recently, researchers developed a liquid chromatography–selected ion monitoring–mass spectrometry (LC–SIM–MS) method to conduct a pharmacokinetic study of WaiGan KeSou Formula decoction administered to rats at a dose of 20 mL. The monarch drug of WaiGan KeSou Formula was Qianhu, and its index compound was Praeruptorin A. Within 24 h, the peak concentration (Cmax) of praeruptorin A in plasma was 172.697 ± 17.254 ng/mL, the peak time was 1.50 h, the elimination half-life (t1/2) was 1.02 h, the mean retention time was 3.42 h, the AUC0–τ (area under the curve) was 504.866 ± 50.317 h ng/mL, and the AUC0–∞ was 514.401 ± 36.950 h ng/mL (He and Wu, 2021). In addition, LC-MS/MS was utilized to evaluate the plasma concentrations of praeruptorin A in rats after a single intragastric dose of 8 g/kg body weight. The researchers found that praeruptorin A was detectable up to 24 h after administration, with an AUC0–t of 311.80 ± 42.38 ng h/mL, a Cmax of 31.09 ± 4.84 ng/mL, and a t1/2 of 7.52 ± 1.00 h (Zhou et al., 2015).
A sensitive, selective, and rapid online solid phase extraction-chiral LC–MS/MS method was developed to conduct a pharmacokinetic study of praeruptorins B and C after orally administering P. praeruptorum extract to rats. Praeruptorins B and C were detectable in rat plasma up to 24 h after administration, with AUC0–t values of 187.29 ± 15.02 (B) and 91.64 ± 9.37 h ng/mL (C), Cmax values of 19.66 ± 4.25 (B) and 7.59 ± 1.98 ng/mL (C), and t1/2 values of 8.20 ± 1.21 h (B) and 14.97 ± 3.66 h (C) (Zhou et al., 2015). Nonetheless, additional pharmacokinetic studies should be conducted on the other bioactive metabolites present in P. praeruptorum, including praeruptorin D, praeruptorin E, qianhucoumarin B, and praeroside I.
Qianhu is typically processed by washing and immediately drying at low temperature, which must be kept below 60°C. Fresh slices should be thicker than 6 mm (Ren et al., 2021). To maintain medicinal quality, the Chinese Pharmacopoeia dictates the use of microscopic, morphological, HPLC, and TLC detection and identification, as well as ethanol extraction and cold-dipping. By utilizing cold-dipping, the ethanol extract must be more than 20.0% for P. praeruptorum. According to the requirements of the Chinese Pharmacopoeia (Chinese Pharmacopoeia Committee of People’s Repulic of China, 2020), the moisture content (after drying) must not exceed 12.0% and the ash content must not exceed 8.0%. Moreover, different extraction methods have different effects on the index metabolites of P. praeruptorum (praeruptorins A and B). The reflux method is favored for the extraction of praeruptorin A, producing a much higher praeruptorin A content than ultrasonic methods. Conversely, the ultrasonic method is favored for the extraction of praeruptorin B, producing a much higher praeruptorin B content than the reflux method (Xu et al., 2022). However, it is inadvisable to utilize only one crude, quantitative marker when assessing the quality of P. praeruptorum extracts. An array of bioactive metabolites has been detected in P. praeruptorum by HPLC, UV, gas chromatography (GC), NMR, high-speed counter-current chromatography coupled with electrospray ionization multi-stage MS (prep-HSCCC/ESI-MS(n)) (Zhou et al., 2015).
The medicinal quality of P. praeruptorum is affected by the altitude at which it is produced. According to reports by Luo et al. (2022), an altitude of 900 m improved the praeruptorin A content, while an altitude of 650 m improved the praeruptorin B content. Moreover, the influence of altitude was greater on praeruptorin B content than on praeruptorin A content (Luo et al., 2022). In another study, praeruptorin A and B contents were higher in plants cultivated at high altitudes than in plants cultivated at low altitudes by using HPLC-DAD method to determine the contents of praeruptorin A and B in the 24 batches of Qianhu from different producing areas. According to the experimental results, cluster analysis and principal component analysis were carried out (Yang et al., 2021). In addition, the key climatic factors affecting the praeruptorin content are average relative humidity, average maximum temperature in July, average annual temperature, and average temperature in July (Xu et al., 2021). However, the relationships between Qianhu quality and climatic factors have not been widely investigated, and warrant further study.
Praeruptorin A and B contents are also influenced by plant organ, harvesting time, cultivation environment, and fertilization strategy. According to reports by Li et al. (2022), the HPLC method was used to determine the content of praeruptorin A and B in the cultured P. praeruptorum at different harvesting periods, as well as to analyze the fluctuation of praeruptorin A and B at different harvesting periods. Praeruptorin A and B contents are highest in P. praeruptorum roots, followed by stems, and are lowest in leaves. In the 14 samples, the praeruptorin A content was found to be much higher in 2-year-old P. praeruptorum than in 1-year-old P. praeruptorum. Moreover, plants cultivated on southern slopes exhibited an approximately 80% higher content of praeruptorin A than plants grown on slopes of other orientations. However, no significant differences in the contents of praeruptorin A or B were observed in P. praeruptorum harvested before or after bolting (Li et al., 2022). Finally, the reasonable application of P and K fertilizers has been found to improve both the yield and medicinal quality P. praeruptorum, although the application of N fertilizer should be controlled (Zhou et al., 2022).
Praeruptorin C exerted no toxicity on primary cultures of mouse neurons at doses of 0 and 10 μg/mol (Yang et al., 2013). Moreover, an emulsion of praeruptorin C did not exert any toxicity or induce any behavioral changes at doses of 5 and 40 μg/mol in OVX mice by performing a CCK-8 assay (Liu et al., 2017). In BMMs, praeruptorin A was found to be atoxic at doses under 10 mmol/L, but significantly cytotoxic at doses over 20 mmol/L (Yeon et al., 2014). In HepG2 cells, praeruptorins A and C were found to be atoxic at doses of 10 and 100 μg/mol, respectively (Zhou et al., 2013). However, a maximum dose of 200 μg/mol dramatically increased cell toxicity. The toxicity of praeruptorin B on SK-OV-3 ovarian cancer cells was not obvious at doses of 20 and 60 μmol/L (Xue et al., 2021). HCC cell line and normal liver THLE-2 cells were treated with different concentrations of praeruptorin A (0, 10, 20, 30, 40 μg/mL) for 24 h. The cell viability was assessed through 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide assay. The results implied that praeruptorin A did not induce cytotoxicity in an HCC cell line at doses of 10 and 40 μg/mL (Yu et al., 2021). In addition, praeruptorin A was atoxic to normal liver THLE-2 cells under at concentrations of 10–30 μg/mol. The Chinese Pharmacopoeia 2020 (Chinese Pharmacopoeia Committee of People’s Repulic of China, 2020) recommends a Qianhu dosage of 3–10 g/d. In addition, Qianhu can safely be used with Pinelliae Rhizoma, but not with Gleditsia sinensis Lam. or Veratrum nigrum L. (“Bencaojing Jizhu” 《本草经集注》). Finally, Qianhu is not considered suitable for people with Yin deficiency syndrome (a series of symptoms caused by the deficiency of yin essence or fluid in the human body. Yin: the dark, not active, female principle of the Universe in Chinese philosophy) (Wen, 2023), cough, cold, or cold fluid syndrome. According to cell studies, P. praeruptorum appears to be atoxic at a low dose but cytotoxic at higher doses (Zhou et al., 2013; Yeon et al., 2014).
P. praeruptorum is a classical medicinal plant commonly used in TCM preparations. Here, we systematically evaluated the toxicology, molecular mechanisms, pharmacology, phytochemistry, botany, quality control, and traditional uses of P. praeruptorum in order to validate the medicinal use of this species. According to the Chinese Pharmacopoeia and classical Chinese botanical drugs, P. praeruptorum has been historically prescribed to treat a wide spectrum of diseases including cough, asthma, and pulmonary hypertension. Pharmacological studies suggest that P. praeruptorum exhibits anti-inflammatory, expectorant, antitussive, antitumor, neuroprotective, anti-osteoclastogenic, and antidepressant effects, and largely support the traditional uses of this plant. To date, more than 119 distinct phytochemicals have been identified in P. praeruptorum extracts, the most common of which are pyranocoumarins and furanocoumarins.
Although there has been considerable progress in evaluating the phytochemistry and pharmacology of P. praeruptorum, there are still gaps in our knowledge. First, according to the Chinese Pharmacopoeia 2020 (Chinese Pharmacopoeia Committee of People’s Repulic of China, 2020), Qianhu can disperse wind-heat, reduce cough and phlegm, and dissipate adverse Qi, indicating that Qianhu may alleviate the effects of wind-heat on the lungs (Song et al., 2015). However, most of the active metabolites of P. praeruptorum effective against cough and wind-heat are currently administered as crude extracts. Therefore, comprehensive investigations should be conducted to identify the effective metabolites and elucidate their modes of action in order to facilitate clinical trials. Second, we found few reports on the toxicity of P. praeruptorum extracts or potential botanical drugs interactions. The potential adverse effects, contraindications, and toxicities of P. praeruptorum extracts and their bioactive metabolites should therefore be studied in vitro, in vivo, and in clinical trials. Third, the majority of the reviewed research was conducted in cell cultures or animal models. Clinical trials in humans will be required to truly evaluate the efficacy P. praeruptorum in addressing depression, osteoporosis, cancer, and inflammation, among other diseases. Forth, most of the P. praeruptorum containing health products are mainly derived from its root rich in chemical compounds, while non-medicinal parts are rarely exploited. Therefore, it may be interesting to extend the research to the non-medicinal parts of the inexpensive flowers, leaves, and stems of P. praeruptorum to ensure the fully utilization of its edible and medicinal values. Finally, new and updated analytical and quality control methods will be required to identify novel markers of quality for the assessment of TCM preparations.
In conclusion, P. praeruptorum is rich in medicinal materials, and its pharmacological effects are extensive. With the advantages of modern instruments and data analysis technology in identifying chemical components and separation, Qianhu medicinal materials can be better developed and new drug discovery (Liu, 2020). Future research should be conducted to investigate the mode of action responsible for the pharmacological activities of P. praeruptorum extracts, as well as to comprehensively evaluate the potential toxicities, adverse effects, and contraindications of this botanical drugs. Alongside updated quality control measures, these investigations will facilitate clinical trials. Updated in vivo pharmacological studies must be performed to validate the traditional uses of P. praeruptorum. Finally, the clinical safety and efficacy of P. praeruptorum-derived phytochemicals in the treatment of depression, osteoporosis, cancer, and other diseases, require validation.
QW: Writing–review and editing, Writing–original draft, Visualization, Investigation, Data curation. QS: Writing–review and editing. QH: Writing–review and editing. LQ: Writing–review and editing. BZ: Writing–review and editing, Supervision.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study was supported by the Talent Projects of Zhejiang Chinese Medical University (2021ZR09), Young Innovative Talents Project of Zhejiang Medical Health Science and Technology (2022RC052), Natural Science Foundation of Zhejiang Province (LQ21H280003), National Natural Science Foundation of China (82003896), Ningbo Natural Science Foundation (202003N4334).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
BALF, bronchoalveolar lavage fluid; LPS, lipopolysaccharide; CFA, complete Freund’s adjuvant; ER, erlotinib-resistant; OVX, ovariectomized; HCC, hepatocellular carcinoma; HR-ESI-MS, high-resolution-electrospray ionization-mass spectrometry; Ig, immunoglobulin; IL, interleukin; IMP, imperatorin; IR, infrared spectroscopy; LC–MS/MS, liquid chromatography-mass spectrometry/-mass spectrometry; LC–SIM–MS, liquid chromatography-selected ion monitoring-mass spectrometry; MS, mass spectrometry; NMR, nuclear magnetic resonance; NO, nitric oxide; NSCLC, epidermal growth factor receptor; NSCLC, non-small-cell lung cancer; RCC, renal cell carcinoma; RT-qPCR, real-time fluorescence quantitative polymerase chain reaction; SIMS, secondary ion mass spectroscopy; TCM, traditional Chinese medicine; TNF-α, tumor necrosis factor αtumor necrosis factor α; UV, ultraviolet.
Asahara, T., Sakakibara, I., Okuyama, T., and Shibata, S. (1984). Studies on coumarins of a Chinese drug “Qian-Hu” V. Coumarin-Glycosides from “Zi-Hua qian-hu”1. Planta Med. 50 (6), 488–492. doi:10.1055/s-2007-969780
Chang, H., Okada, Y., Okuyama, T., and Tu, P. (2007). 1H and 13C NMR assignments for two new angular furanocoumarin glycosides from Peucedanum praeruptorum. Magn. Reson. Chem. 45 (7), 611–614. doi:10.1002/mrc.2005
Chang, H. T. (1998). Studies on the components from the root of Peucedanum praeruptorum in reducing pulmonary hypertension. Shenyang: Master, Shenyang Pharmaceutical University.
Chang, H. T., and Li, X. (1999a). Coumarins from peucedanum praeruptorum dunn. J. Shenyang Pharm. Univ. 16 (02), 28–31. doi:10.3969/j.issn.1006-2858.1999.02.007
Chang, H. T., and Li, X. (1999b). Studies on chemical constituents from roots of Peucedanum praeruptorum Ⅴ. Chin. Tradit. Herb. Drugs 30 (06), 414–416. doi:10.3321/j.issn:0253-2670.1999.06.007
Chang, H. T., Okada, Y., Ma, T. J., Okuyama, T., and Tu, P. F. (2008). Two new coumarin glycosides from Peucedanum praeruptorum. J. Asian Nat. Prod. Res. 10 (5-6), 577–581. doi:10.1080/10286020801966740
Chang, T. H., Liu, X. Y., Zhang, X. H., Xing, J., and Wang, H. L. (2003). Effects of Peucedanum Praeruptorum Dunn and dl-praeruptorin A on IL-6 level and Fas, bax, bcl-2 protein expressions in ischemia-reperfusion myocardium of rats. J. China Med. Univ. 32 (01), 5–7+10. doi:10.3969/j.issn.0258-4646.2003.01.001
Chen, F.-Y., Tu, L.-F., Liu, D.-P., Ma, J., and Luo, Y.-M. (2021). Chemical constituents from the roots of Peucedanum praeruptorum Dunn and their chemotaxonomic significance. Biochem. Syst. Ecol. 99 (2021), 104355. doi:10.1016/j.bse.2021.104355
Chen, L. L., Chu, S. S., Zhang, L., Xie, J., Dai, M., Wu, X., et al. (2019). Tissue-specific metabolite profiling on the different parts of bolting and unbolting peucedanum praeruptorum dunn (Qianhu) by laser microdissection combined with UPLC-Q/TOF(-)MS and HPLC(-)DAD. Molecules 24 (7), 1439. doi:10.3390/molecules24071439
Chen, Q. H., Liu, X. L., Pan, L. X., and OuYang, J. M. (2022). Study on the effects of praeruptorin A combined with low concentration of taxol on taxol-resistant ovarian cancer cell line A2780/TAX. Chin. J. Hosp. Pharm. 42 (17), 1755–1759+1765. doi:10.13286/j.1001-5213.2022.17.03
Chen, X., Wang, Z., Duan, N., Zhu, G., Schwarz, E. M., and Xie, C. (2018). Osteoblast-osteoclast interactions. Connect. Tissue Res. 59 (2), 99–107. doi:10.1080/03008207.2017.1290085
Chen, Z. X., Huang, B. S., She, Q. L., and Zeng, G. F. (1979). The chemical constituents of Bai-Hua-Qian-Hu, the root of Peucedanum praeruptorum Dunn. (Umbelliferae)--four new coumarins (author's transl). Acta Pharm. Sin. 14 (08), 486–496. doi:10.16438/j.0513-4870.1979.08.007
Cheong, C. T., Kim, W. C., Jin, M. H., Kim, H. J., Kang, S. J., Kang, S. H., et al. (2002). Inhibitors of melanogenesis from the roots of peucedanum praeruptorum. Kor. J. Pharmacogn. 33 (4), 395–398.
Chinese Pharmacopoeia Committee of People's Repulic of China (2000). Chinese Pharmacopoeia Ⅰ. Beijing: China Medical Science Press.
Chinese Pharmacopoeia Committee of People's Repulic of China (2005). Chinese Pharmacopoeia Ⅰ. Beijing: China Medical Science Press.
Chinese Pharmacopoeia Committee of People's Repulic of China (2010). Chinese Pharmacopoeia Ⅰ. Beijing: China Medical Science Press.
Chinese Pharmacopoeia Committee of People's Repulic of China (2015). Chinese Pharmacopoeia Ⅰ. Beijing: China Medical Science Press.
Chinese Pharmacopoeia Committee of People's Repulic of China (2020). Chinese Pharmacopoeia Ⅰ. Beijing: China Medical Science Press.
Editorial Committee of Flora of China (1992). Flora of China. Beijing: Chinese Academy of Sciences, Science Press.
He, D. M., Wu, F. H., and Kong, L. Y. (2007). Review on pharmacological action of Peucedanum praeruptorum. Pharm. Clin. Res. 15 (03), 167–170. doi:10.13664/j.cnki.pcr.2007.03.001
He, G. X., and Wu, S. H. (2021). Rat plasma pharmacokinetic research of praeruptorin A in waigan kesou formula. Strait Pharm. J. 33 (08), 19–22. doi:10.3969/j.issn.1006-3765.2021.08.008
Huang, L., Bi, H. C., Li, Y. H., Zhang, J. Q., Kuang, S. Y., Zhang, L., et al. (2013). Regulation of human pregnane X receptor and its target gene cytochrome P450 3A by praeruptorin A isolated from the herbal medicine Peucedanum praeruptorum. Planta Med. 79 (16), 1509–1515. doi:10.1055/s-0033-1350795
Ishii, H., Okada, Y., Baba, M., and Okuyama, T. (2008). Studies of coumarins from the Chinese drug Qianhu, XXVII: structure of a new simple coumarin glycoside from Bai-Hua Qianhu, Peucedanum praeruptorum. Chem. Pharm. Bull. (Tokyo) 56 (9), 1349–1351. doi:10.1248/cpb.56.1349
Jian, X., Zhao, Y., Wang, Z., Li, S., Li, L., Luo, J., et al. (2020). Two CYP71AJ enzymes function as psoralen synthase and angelicin synthase in the biosynthesis of furanocoumarins in Peucedanum praeruptorum Dunn. Plant Mol. Biol. 104 (3), 327–337. doi:10.1007/s11103-020-01045-4
Jong, T. T., Hwang, H. C., Jean, M. Y., Wu, T. S., and Teng, C. M. (1992). An antiplatelet aggregation principle and X-ray structural analysis of cis-khellactone diester from Peucedanum japonicum. J. Nat. Prod. 55 (10), 1396–1401. doi:10.1021/np50088a002
Kim, D. H., Kim, D. Y., Kim, Y. C., Jung, J. W., Lee, S., Yoon, B. H., et al. (2007). Nodakenin, a coumarin compound, ameliorates scopolamine-induced memory disruption in mice. Life Sci. 80 (21), 1944–1950. doi:10.1016/j.lfs.2007.02.023
Kong, L. Y., Li, X., Pei, Y. H., Yu, R. M., Min, Z. D., and Zhu, T. R. (1994a). Isolation and structure elucidation of Baihuaqianhuside and Pd-C-I from Peucedanum praeruptorum. Acta Pharm. Sin. 29 (04), 276–280. doi:10.16438/j.0513-4870.1994.04.007
Kong, L. Y., Li, X., Pei, Y. H., and Zhu, T. R. (1994b). Isolation and structural elucidation of qianhucoumarin D and qianhucoumarin E from peucedanum praeruptorum. Acta Pharm. Sin. 29 (01), 49–54. doi:10.16438/j.0513-4870.1994.01.010
Kong, L. Y., Li, Y., Min, Z. D., Li, X., and Zhu, T. R. (1996). Coumarins from Peucedanum praeruptorum. Phytochemistry 41 (5), 1423–1426. doi:10.1016/0031-9422(95)00783-0
Kong, L. Y., Pei, Y. H., Li, X., and Zhu, T. R. (1993a). On the chemical constituents of the root of Peucedanum praeruptorum. Chin.Tradit. Herb. Drugs 24 (08), 401–404+446.
Kong, L. Y., Pei, Y. H., Li, X., Zhu, T. R., and Okuyama, T. (1993b). Isolation and structure elucidation of qianhucoumarin A. Acta Pharm. Sin. 28 (06), 432–436. doi:10.16438/j.0513-4870.1993.06.007
Lee, J., Lee, Y. J., Kim, J., and Bang, O. S. (2015). Pyranocoumarins from root extracts of peucedanum praeruptorum dunn with multidrug resistance reversal and anti-inflammatory activities. Molecules 20 (12), 20967–20978. doi:10.3390/molecules201219738
Li, X. L., Song, C., Jia, B., Liu, L., Ou, J. M., and Han, B. X. (2022). Analysis of factors affecting the content of praeruptorin A and B in Peucedanum praeruptorum Dunn. Chin. Wild Plant Resour. 41 (01), 26–32. doi:10.3969/j.issn.1006-9690.2022.01.005
Li, X. Y., Zu, Y. Y., Ning, W., Tang, M. X., Gong, C., Niu, S. L., et al. (2020). A new xanthyletin-type coumarin from the roots of Peucedanum praeruptorum. J. Asian Nat. Prod. Res. 22 (3), 287–294. doi:10.1080/10286020.2018.1551887
Liang, T., Yue, W., and Li, Q. (2010). Chemopreventive effects of Peucedanum praeruptorum DUNN and its major constituents on SGC7901 gastric cancer cells. Molecules 15 (11), 8060–8071. doi:10.3390/molecules15118060
Lin, C. L., Hung, T. W., Ying, T. H., Lin, C. J., Hsieh, Y. H., and Chen, C. M. (2020). Praeruptorin B mitigates the metastatic ability of human renal carcinoma cells through targeting CTSC and CTSV expression. Int. J. Mol. Sci. 21 (8), 2919. doi:10.3390/ijms21082919
Liu, D. P. (2020). Studies on the chemical constituents of Peucedanum praeruptorum Dunn. Master. Nanchang: Jiangxi University of Traditional Chinese Medicine.
Liu, J. L., Wang, S. S., Li, X. F., Lu, X. Y., Li, H., and Hong, C. X. (2021). Investigation and analysis on the present situation of Peucedanum praeruptorum resources in its producing area. Mod. Agric. Sci. Technol. 2021 (01), 89–92+99. doi:10.3969/j.issn.1007-5739.2021.01.035
Liu, J. L., Wang, X. Y., Zhang, L. L., Fang, M. J., Wu, Y. L., Wu, Z., et al. (2014). Two-dimensional countercurrent chromatography×high performance liquid chromatography with heart-cutting and stop-and-go techniques for preparative isolation of coumarin derivatives from Peucedanum praeruptorum Dunn. J. Chromatogr. A 1374 (2014), 156–163. doi:10.1016/j.chroma.2014.11.053
Liu, J. L., Yuan, Y. H., and Chen, N. H. (2011). Research progress in treatment of depression. Chin. Pharmacol. Bull. 27 (09), 1193–1196. doi:10.3969/j.issn.1001-1978.2011.09.003
Liu, X., Chin, J. F., Qu, X., Bi, H., Liu, Y., Yu, Z., et al. (2017). The beneficial effect of praeruptorin C on osteoporotic bone in ovariectomized mice via suppression of osteoclast formation and bone resorption. Front. Pharmacol. 8, 627. doi:10.3389/fphar.2017.00627
Liu, X. Y., and Wang, G. X. (2009). Effects of praeruptorin C in rats with myocardial ischemia reperfusion injury. Chin. J. Cardiovasc. Res. 7 (2), 146–148. doi:10.3969/j.issn.1672-5301.2009.02.024
Liu, Y., Li, X. Y., Song, Z. Z., and Wei, H. Y. (2009). Expectorant action of Praeruptofin C and nodakenin. Lishizhen Med. Mater. Med. Res. 20 (05), 1049. doi:10.3969/j.issn.1008-0805.2009.05.012
Liu, Y., Wei, H. Y., Yao, S. H., and Zheng, X. Z. (1997). Comparison of the expectorant pharmacological effects of Peucedanum. Guid. J. Tradit. Chin. Med. 3 (01), 41–43.
Long, T., Song, P., Liang, P. Y., Ou, S. J., and Wang, S. X. (2019). Immunomodulatory effect of imperatorin on RBL-2H3 allergic inflammatory cell model induced by IgE. J. Guangzhou Univ. Tradit. Chin. Med. 36 (12), 2001–2006. doi:10.13359/j.cnki.gzxbtcm.2019.12.026
Lou, H. X., Sun, L. R., Yu, W. T., Fan, P. H., Cui, L., Gao, Y. H., et al. (2004). Absolute configuration determination of angular dihydrocoumarins from Peucedanum praeruptorum. J. Asian Nat. Prod. Res. 6 (3), 177–184. doi:10.1080/10286020310001653255
Luo, M., Xu, G., Tang, Q. S., Guo, Y., Deng, C. F., Luo, S., et al. (2022). Response of medicinal quality to altitude gradient in Peucedanum praeruptorum Dunn. Chin. Wild Plant Resour. 41 (11), 20–24. doi:10.3969/j.issn.1006-9690.2022.11.004
Meng, X. L., Jia, M. R., Zhang, Y., Wu, P., Tang, M. C., Tang, S. W., et al. (1997). Pharmacological studies of variet of Qian-hu. Pharmacol. Clin. Chin. Mater. Med. 13 (01), 36–39.
Okuyama, T., and Shibata, S. (1981). Studies on coumarins of a Chinese drug “Qian-Hu”. Planta Med. 42 (1), 89–96. doi:10.1055/s-2007-971551
Okuyama, T., Takata, M., and Shibata, S. (1989). Structures of linear furano- and simple-coumarin glycosides of Bai-Hua Qian-Hu. Planta Med. 55 (1), 64–67. doi:10.1055/s-2006-961828
Park, H. J., Jeong, J. H., and Park, S. H. (2022). The root extract of peucedanum praeruptorum dunn exerts anticancer effects in human non-small-cell lung cancer cells with different EGFR mutation statuses by suppressing MET activity. Molecules 27 (7), 2360. doi:10.3390/molecules27072360
Ren, J. J., Sun, J., Yu, X. P., Shen, X. X., and Wang, Z. A. (2021). Effects of different habitat processing methods on quality of Peucedani Radix. Mod. Chin. Med. 23 (05), 844–848. doi:10.13313/j.issn.1673-4890.20200526009
Song, Y., Jing, W., Yan, R., and Wang, Y. (2015). Research progress of the studies on the roots of Peucedanum praeruptorum dunn (Peucedani radix). Pak. J. Pharm. Sci. 28 (1), 71–81.
Song, Z. Q., Li, B., Tian, K. Y., Hong, L., Wu, W., and Zhang, H. Y. (2022). Research progress on chemical constituents and pharmacological activities of peucedani radix and peucedani decursivi radix. Chin. Tradit. Herb. Drugs 53 (03), 948–964. doi:10.7501/j.issn.0253-2670.2022.03.035
Su, D. J., Li, L. F., Wang, S. Y., Yang, Q., Wu, Y. J., Zhao, M. G., et al. (2021). Pra-C exerts analgesic effect through inhibiting microglial activation in anterior cingulate cortex in complete Freund's adjuvant-induced mouse model. Mol. Pain 17, 1744806921990934. doi:10.1177/1744806921990934
Takata, M., Okuyama, T., and Shibata, S. (1988). Studies on coumarins of a Chinese drug, “qian-hu”; VIII. Structures of new coumarin-glycosides of “Bai-hua qian-hu”. Planta Med. 54 (4), 323–327. doi:10.1055/s-2006-962446
Takata, M., Shibata, S., and Okuyama, T. (1990). Structures of angular pyranocoumarins of Bai-hua qian-hu, the root of peucedanum praeruptorum1. Planta Med. 56 (3), 307–311. doi:10.1055/s-2006-960966
Tu, X., Wang, J. M., Tu, Q., and Tu, J. W. (2004). Effects of Peucedanum praeruptorum Dunn extract on left ventricular remodeling and Bcl-2, Bax protein expression in aorta coarctation rats. Chin. J. Clin. Pharmacol. Ther. 9 (04), 394–398. doi:10.3969/j.issn.1009-2501.2004.04.009
Wang, D. C., Zhao, X. M., Li, T. D., Ma, J., Kong, Z. F., Li, K., et al. (2004). Effect of total coumarins from Peucedanum Praeruptorum Dunn on the activity hepatic drug-metabolizing enzymes in mice. Her. Med. 23 (08), 522–524. doi:10.3870/j.issn.1004-0781.2004.08.002
Wang, K., Nie, Z. X., Sun, Y. P., Liu, S. Z., and Wang, G. K. (2018). Current surgical strategies and techniques of aortic valve diseases in children. J. Anhui Univ. Chin. Med. 37 (05), 83–90. doi:10.21037/tp.2018.02.03
Wang, L., Wang, J., Yang, L., Zhou, S. M., Guan, S. Y., Yang, L. K., et al. (2017). Effect of Praeruptorin C on 3-nitropropionic acid induced Huntington's disease-like symptoms in mice. Biomed. Pharmacother. 86 (2017), 81–87. doi:10.1016/j.biopha.2016.11.111
Wang, M. (2016). Pharmacological analysis and clinical application of Qianhu. Asia-Pacific Tradit. Med. 12 (18), 75–76. doi:10.11954/ytctyy.201618032
Wang, X. M., and Xu, H. H. (2014). Studies on the antidepressant effect and mechanism of Praeruptorin A on CUMS rats. J. Chin. Med. Mater. 37 (12), 2259–2262. doi:10.13863/j.issn1001-4454.2014.12.035
Wang, Y., Yang, C., Chang, H., Li, G., Jin, X., and Zou, J. (2012). Pd-Ia inhibited inflammation in LPS-induced HUVECs via PPARα. Chin. Pharmacol. Bull. 28 (11), 1594–1597. doi:10.3969/j.issn.1001-1978.2012.11.027
Wen, H. (2023). Research on intelligent diagnosis method of Yin deficiency syndrome based on multimodal information fusion. Beijing: Beijing University of Chemical Technology. Master.
Wu, M. H., Lin, C. L., Chiou, H. L., Yang, S. F., Lin, C. Y., Liu, C. J., et al. (2017). Praeruptorin A inhibits human cervical cancer cell growth and invasion by suppressing MMP-2 expression and ERK1/2 signaling. Int. J. Mol. Sci. 19 (1), 10. doi:10.3390/ijms19010010
Xiong, Y. Y., Shi, W. J., Yu, H., and Zhang, X. L. (2014). Inhibitory effects of nodakenin on the airway inflammation and NF-κB signaling pathway in a murine asthmatic model. Basic Clin. Med. 34 (05), 690–694. doi:10.16352/j.issn.1001-6325.2014.05.012
Xiong, Y. Y., Wang, J. S., Wu, F. H., Li, J., and Kong, L. Y. (2012). The effects of (±)-Praeruptorin A on airway inflammation, remodeling and transforming growth factor-β1/Smad signaling pathway in a murine model of allergic asthma. Int. Immunopharmacol. 14 (4), 392–400. doi:10.1016/j.intimp.2012.08.019
Xu, G., Tan, Q. S., Yang, D., Fu, Y., Guo, Y., Luo, C., et al. (2022). Effect of index components in Peucedanum Praerupterum by different extraction methods. Asia-Pacific Tradit. Med. 18 (03), 42–44. doi:10.11954/ytctyy.202203010
Xu, P., Liang, W. Q., Zhang, H. J., Hu, Y. J., Chen, R. B., and Pu, J. B. (2021). Correlation analysis between active ingredients and climate factors in Radix Peucedanum. China J. Tradit. Chin. Med. Pharm. 36 (09), 5614–5618.
Xue, N. M., Chai, M. Y., Yang, W. S., and Zhou, X. C. (2021). Inhibitory effect of praeruptorin B on SK-OV-3 cell proliferation in ovarian cancer by SREBP-1c/FASN signaling pathway. Chin. J. Gerontol. 41 (13), 2773–2777. doi:10.3969/j.issn.1005-9202.2021.13.026
Yang, L., Kang, X. J., Du, W. F., Hong, H., Ge, W. H., Wang, L. G., et al. (2021). Effect of altitude of producing area on contents of praeruptorin A and praeruptorin B in Qianhu. Chin. Arch. Tradit. Chin. Med. 39 (08), 14–18. doi:10.13193/j.issn.1673-7717.2021.08.004
Yang, L., Li, X. B., Yang, Q., Zhang, K., Zhang, N., Guo, Y. Y., et al. (2013). The neuroprotective effect of praeruptorin C against NMDA-induced apoptosis through down-regulating of GluN2B-containing NMDA receptors. Toxicol. Vitro 27 (2), 908–914. doi:10.1016/j.tiv.2013.01.001
Yeon, J. T., Kim, K. J., Choi, S. W., Moon, S. H., Park, Y. S., Ryu, B. J., et al. (2014). Anti-osteoclastogenic activity of praeruptorin A via inhibition of p38/Akt-c-Fos-NFATc1 signaling and PLCγ-independent Ca2+ oscillation. PLoS One 9 (2), e88974. doi:10.1371/journal.pone.0088974
Yu, C. L., Yu, Y. L., Yang, S. F., Hsu, C. E., Lin, C. L., Hsieh, Y. H., et al. (2021). Praeruptorin A reduces metastasis of human hepatocellular carcinoma cells by targeting ERK/MMP1 signaling pathway. Environ. Toxicol. 36 (4), 540–549. doi:10.1002/tox.23059
Yu, C. Y. (2022). Study on the effect of PD on the proliferation and osteogenic activity of human inflammatory periodontal membrane cells. Lanzhou: LanZhou University. Master.
Yu, P. J., Jin, H., Zhang, J. Y., Wang, G. F., Li, J. R., Zhu, Z. G., et al. (2012). Pyranocoumarins isolated from Peucedanum praeruptorum Dunn suppress lipopolysaccharide-induced inflammatory response in murine macrophages through inhibition of NF-κB and STAT3 activation. Inflammation 35 (3), 967–977. doi:10.1007/s10753-011-9400-y
Yu, Q. H., Ma, L., Shen, Y. P., Zhai, W., and Zhou, Y. H. (2015). Effect of angular pyranocoumarin isolated from peucedanum praeruptorum on the proliferation and apoptosis of U266 cells. Chin. J. Hematol. 36 (11), 937–941. doi:10.3760/cma.j.issn.0253-2727.2015.10.010
Zhang, C., Li, L., Xiao, Y. Q., Li, W., Yin, X. J., Tian, G. F., et al. (2010a). A new phenanthraquinone from the roots of Peucedanum praeruptorum. Chin. Chem. Lett. 21 (7), 816–817. doi:10.1016/j.cclet.2010.03.020
Zhang, C., Li, L., Xiao, Y. Q., Massahiko, T., and Kimiye, B. (2011). Coumarins from the roots of Peucedanum praeruptorum. Chin. J. Tradit. Chin. Med. 26 (09), 1995–1997.
Zhang, C., Xiao, Y. Q., Li, L., Massahiko, T., and Kimiye, B. (2012). Chemical constituents from roots of Peucedanum praeruptorum (Ⅴ). China J. Chin. Materia Medica 37 (23), 3573–3576. doi:10.4268/cjcmm20122314
Zhang, C., Xiao, Y. Q., Massahiko, T., and Kimiye, B. (2005). Studies on chemical constituents in roots of Peucedanum praeruptorum (Ⅰ). China J. Chin. Mater. Med. 30 (09), 675–677. doi:10.3321/j.issn:1001-5302.2005.09.009
Zhang, C., Xiao, Y. Q., Massahiko, T., and Kimiye, B. (2006). Studies on chemical constituents from roots of Peucedanum praeruptorum II. China J. Chin. Mater. Med. 31 (16), 1333–1335. doi:10.3321/j.issn:1001-5302.2006.16.008
Zhang, C., Xiao, Y. Q., Massahiko, T., and Kimiye, B. (2009). Studies on chemical constituents from roots of Peucedanum praeruptorum Ⅲ. China J. Chin. Mater. Med. 34 (08), 1005–1006. doi:10.3321/j.issn:1001-5302.2009.08.019
Zhang, C., Yin, X. J., Li, L., Li, W., Xiao, Y. Q., Yu, D. R., et al. (2010b). Comparative studies on pharmacological effects for processed pieces from peucedanum praeruptorum. Chin. J. Exp. Tradit. Med. Formulae 16 (15), 146–148. doi:10.13422/j.cnki.syfjx.2010.15.053
Zhao, M., Hou, J., Zheng, S., Ma, X., Fu, X., Hu, S., et al. (2022). Peucedanum praeruptorum Dunn polysaccharides regulate macrophage inflammatory response through TLR2/TLR4-mediated MAPK and NF-κB pathways. Biomed. Pharmacother. 152 (2022), 113258. doi:10.1016/j.biopha.2022.113258
Zheng, X. X., Liu, J. H., Liu, M. H., Cheng, Y., and Cao, Y. J. (2019). Antidepressant effect and mechanism of imperatorin. Chin. Pharmacol. Bull. 35 (01), 101–105. doi:10.3969/j.issn.1001-1978.2019.01.020
Zhou, G., Chen, G., and Liu, H. (2015). Simultaneous quantification of three pyranocoumarins of Peucedanum praeruptorum in rat plasma by liquid chromatography-tandem mass spectrometry: application to pharmacokinetic study. J. Chromatogr. Sci. 53 (4), 511–518. doi:10.1093/chromsci/bmu077
Zhou, J. R., Sun, J., Ren, J. J., Shao, Q. S., and Wang, Z. A. (2022). Effects of combined application of N, P, and K in autumn on yield and quality of Peucedanum praeruptorum. Mod. Chin. Med. 24 (12), 2443–2449. doi:10.13313/j.issn.1673-4890.20220222003
Zhou, J. R., Sun, J., Shen, X. X., Shao, Q. S., Wang, L. Y., and Wang, Z. A. (2021). Study on genetic diversity of peucedanum praeruptorum germplasm resources in traditional production areas. J. Chin. Med. Mater. 44 (11), 2543–2548. doi:10.13863/j.issn1001-4454.2021.11.009
Zhou, X., Bi, H., Jin, J., Niu, L., Cai, D., Deng, R., et al. (2013). Effects of praeruptorin A and praeruptorin C, a racemate isolated from Peucedanum praeruptorum, on MRP2 through the CAR pathway. Planta Med. 79 (17), 1641–1647. doi:10.1055/s-0033-1350955
Keywords: Peucedanum praeruptorum, pharmacology, phytochemistry, traditional uses, quality control
Citation: Wang Q, Sun Q, Huang Q, Qin L and Zhu B (2024) The traditional uses, pharmacology, and phytochemistry of Peucedanum praeruptorum Dunn. Front. Pharmacol. 15:1352657. doi: 10.3389/fphar.2024.1352657
Received: 08 December 2023; Accepted: 04 March 2024;
Published: 03 April 2024.
Edited by:
Mohammad Reza Khazdair, Birjand University of Medical Sciences, IranReviewed by:
Noor Zulfiqar, University of Agriculture, Faisalabad, PakistanCopyright © 2024 Wang, Sun, Huang, Qin and Zhu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Luping Qin, bHBxaW5AemNtdS5lZHUuY24=; Bo Zhu, emh1Ym9AemNtdS5lZHUuY24=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.