
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Pharmacol., 20 February 2024
Sec. Neuropharmacology
Volume 15 - 2024 | https://doi.org/10.3389/fphar.2024.1348297
This article is part of the Research TopicReviews In Neuropharmacology 2023: Microbiota Gut-Brain Axis, Therapeutic Insights for Neurodegenerative DiseasesView all 6 articles
 Akash Kumar1
Akash Kumar1 Bhagavathi Sundaram Sivamaruthi2,3
Bhagavathi Sundaram Sivamaruthi2,3 Swarnima Dey1,4
Swarnima Dey1,4 Yogesh Kumar1
Yogesh Kumar1 Rishabha Malviya5
Rishabha Malviya5 Bhupendra G. Prajapati6*
Bhupendra G. Prajapati6* Chaiyavat Chaiyasut3*
Chaiyavat Chaiyasut3*Various microbial communities reside in the gastrointestinal tract of humans and play an important role in immunity, digestion, drug metabolism, intestinal integrity, and protection from pathogens. Recent studies have revealed that the gut microbiota (GM) is involved in communication with the brain, through a bidirectional communication network known as the gut-brain axis. This communication involves humoral, immunological, endocrine, and neural pathways. Gut dysbiosis negatively impacts these communication pathways, leading to neurological complications and cognitive deficits. Both pre-clinical and clinical studies have demonstrated that probiotics can restore healthy GM, reduce intestinal pH, and reduce inflammation and pathogenic microbes in the gut. Additionally, probiotics improve cell-to-cell signaling and increase blood-brain-derived neurotrophic factors. Probiotics emerge as a potential approach for preventing and managing neurological complications and cognitive deficits. Despite these promising findings, the safety concerns and possible risks of probiotic usage must be closely monitored and addressed. This review article provides a brief overview of the role and significance of probiotics in cognitive health.
Cognition was once assumed to be controlled only by the central nervous system (CNS) (Zhu et al., 2020), but recent studies have revealed that a diverse range of factors plays a role in its regulation and influence. The gastrointestinal tract of humans is a habitat of various microbial communities, referred to as gut microbiota (GM) (Patel et al., 2023). GM may impact host health and is regulated by various factors such as stress, age, food, and host genetics (Long-Smith et al., 2020). The gut and brain communicate through a bidirectional communication network, known as the gut-brain axis (GBA) (Kumar et al., 2023). GM dysbiosis may alter brain structure, brain vascular physiology, and blood-brain barrier (BBB) permeability which may cause neurological disorders and cognitive impairment (Zhu et al., 2020). Therefore, GM may be a potential target for preventing and treating cognitive impairment (Sun et al., 2020; Eastwood et al., 2021).
Probiotics are “live microorganisms that, when administrated in sufficient amounts, confer a health benefit to the host” (Martín and Langella_2019). Probiotic administration results in the restoration of GM, changes in microbiota-derived metabolites, reduction in inflammation, and maintains hypothalamic pituitary adrenal (HPA) axis function and gut barrier integrity (Plaza-Diaz et al., 2019). In recent years, pre-clinical and clinical studies have described the role of probiotics in cognitive health (Sivamaruthi et al., 2019; Thangaleela et al., 2022). This review aims to provide a concise summary of the probiotic potential for preventing and treating cognitive impairments.
The gut and brain communicate through a complex network which includes various pathways. The neurological pathway includes the enteric nervous system (ENS), vagus nerves (VN), and gastrointestinal neurotransmitters. Modulation of afferent sensory nerves produces biologically active catecholamines and local neurotransmitters such as histamine. GM may impact the availability of nutrients, resulting in the alteration of peptide release from enteroendocrine cells and affecting the GBA. The dysbiosis of GM may cause inflammation and release of cytokines, which influence the GBA. Bacterial metabolites may affect the humoral system, cross from the BBB, and regulate microglia for cognitive development (Appleton, 2018; Gwak and Chang, 2021). The communication pathways of the microbiota-GBA have been represented in Figure 1 (Lin et al., 2020).
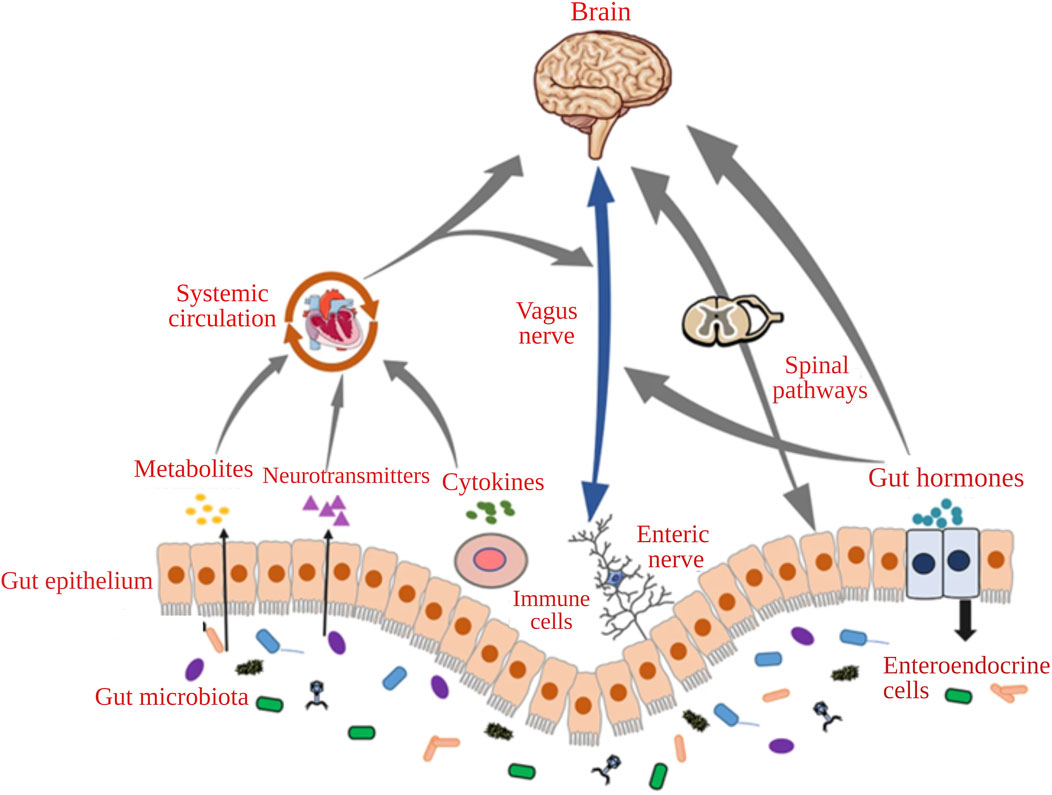
FIGURE 1. The communication pathways of the microbiota-gut-brain axis (Adapted with permission from Lin et al., 2020).
Studies have demonstrated that gut bacteria can produce neurotransmitters and other signaling chemicals that affect brain function and development (Morais et al., 2020; Chen et al., 2021). Recent studies detailed the associations between GM, mental health, and psychological diseases and disorders including anxiety and depression (Kumar et al., 2023). Genetics, nutrition, environment, and early life experiences play a role in mental health and development via GM. However, to completely understand the influence of GBA on human health and wellbeing, further studies are required (Kelsey et al., 2019). According to a meta-analysis, infants born via cesarean section had a slightly higher chance of developing attention deficit hyperactivity disorder (ADHD) and autism spectrum disorder (ASD) than those born vaginally (Curran et al., 2015). A study revealed that children with ASD specifically exhibit different patterns of bacterial classes, for example, ASD children have an abundance of toxin-producing bacteria, like Clostridia (Kelsey et al., 2019).
The association of GM with the cognitive function of the infant has been reported. Three different microbial clusters have been identified, based on the variations in the prevalence of three important bacterial species, Faecalibacterium, Bacterioides, and Ruminococcaceae. The study showed the differences between the three groups in the Early Learning Composite Score (measures an infant’s overall performance in activities involving cognitive and motor development). The newborns with a comparatively high abundance of Bacteroides had the greatest scores. In contrast, newborns with a reasonably high abundance of Faecalibacterium had the lowest cognitive and motor development scores. Also, the research findings revealed structural variations in the brain; the newborns with a substantial abundance of Bacteroides showing a bigger right superior occipital gyrus at age one compared to infants in the other two groups (Carlson et al., 2018). Kelsey et al. reported that gut microbial diversity at first year of life reflected cognitive development at second year of age. So, the result was predicted that the microbiome affects the functions of cognitive development differently during infancy than in later stages in the developing phases of life (Kelsey et al., 2019).
The microbiome’s initial formation and the nervous system’s growth and maturation co-occur from the very earliest stages of fetal development. Recent studies reveal the role of microbiomes in brain development and neurological diseases and disorders (Dash et al., 2022; Nandwana et al., 2022). Any issue impairing brain function might also potentially result in several neurodevelopmental disorders (NDDs) (Dash et al., 2022; Wang et al., 2022). Several NDDs have been reported, including intellectual development disorder, ADHD, ASD, specific learning disabilities like dyslexia or dyscalculia, conduct disorders, motor disorders (tic disorders, Tourette’s syndrome), and a congenital disorder-cerebral palsy (Antolini and Colizzi, 2023). The people with NDD struggle to operate in social, intellectual, professional, and personal spheres of life. Impaired brain activities affect emotions, self-control, learning capacity, memory, intelligence quotient, and social skills. The first sign and behavioral difficulties may appear in early infancy, but the entire spectrum of NDDs becomes visible when the child grows. The spectrum of developmental impairments frequently persists throughout the person’s lifetime (Thapar et al., 2017). The symptoms of NDDs might be alleviated by interventions that target GM (Vendrik et al., 2020).
A linkage was found between the brain’s functions or dysfunction and the host’s GM (Iliodromiti et al., 2023). A meta-analysis study compared GM of kids with and without ASD. The gut of children with ASD has significantly higher concentrations of Bacteroides, Parabacteroides, and Clostridium species, while Bifidobacterium species are lower. This dysbiosis could contribute to the development of ASD (Iglesias-Vázquez et al., 2020). Also, another study showed that autistic kids had high concentrations of specific bacteria such as Clostridium, Desulfovibrio, Sutterella, and Lactobacillus. Still, the results are inconsistent (Bezawada et al., 2020), indicating that further studies are needed to know whether the altered microbiome is the cause or consequence of the condition.
Bundgaard-Nielsen et al. reported that the people with ADHD and ASD share GM sign (both alpha and beta diversity), which was different from the control. A high abundance of Eggerthella, Hungatelle and Ruminococcus gnavus, and a lower relative abundance of Coprobacter and Howardella were found among the ADHD and ASD subjects (Bundgaard-Nielsen et al., 2023). Meta-analysis reported no significant differences in ADHD patients, in terms of their alpha diversity indexes. Especially, genus Blautia abundance was higher in ADHD subjects than controls (Wang et al., 2022). The meta-analysis showed that only heterogenic data is available on the GM of ADHD patients. So, further studies are required using more study subjects, which may explain the relationship between microbial dysbiosis and ADHD.
Probiotics are living, non-pathogenic bacteria and yeast that are beneficial for the human body when administrated and improve and promote microbial balance, particularly in the digestive system (Martín and Langella, 2019). They primarily comprise Lactobacillus and Bifidobacterium species or Saccharomyces boulardii (Mishra and Acharya, 2021). The probiotic strains are involved in several physiological events, including reducing the pH of the intestine, cell-to-cell signaling, lower and preventing the colonization of pathogenic microbes and regulating the immune response of the host (Iacob et al., 2019). A separate probiotic group is called “psychobiotics”, which could improve psychological and mental health and influence mood, anxiety, focus, memory, and cognition (Sharma and Kaur, 2020; Thangaleela et al., 2022).
The collection of microbes, their genomes, and their products that inhabit the human intestine is referred to as the gut “microbiome” as opposed to the microbes themselves, which are referred to as “microbiota” (De Vos et al., 2022). The most common strain of GM belongs to the phyla like Bacteroides, Proteobacteria, and Actinobacteria while the most prevalent genera are Streptococcus, Pseudomonas, Bacteroides, Fusobacteria, Clostridium, and Lactobacillus (Iliodromiti et al., 2023). The gut bacteria associated with the host chronic inflammation and defense system (Ferreira et al., 2014), preserve the underlying framework of the mucosal barrier (Paone and Cani, 2020), and aid in the host’s metabolism (Lindsay et al., 2020). GM is associated with the production of several gastrointestinal hormones, short-chain fatty acids and vitamins, and medication uptake (Mendoza-León et al., 2023). It is known that the disruption in healthy GM results in inflammation (Huang et al., 2019). Additionally, it has been claimed that inflammatory states are linked to diseases like depression and anxiety. The GBA is thought to be responsible for this phenomenon (Kumar et al., 2023).
GM may influence various brain growth and operations elements, such as microglia and astrocyte polarisation, maturation, and control of neurotransmission, neurogenesis, and myelination (Ghezzi et al., 2021). Probiotics could influence the composition of GM and restore the gut ecosystem and be used as a potential approach for preventing and treating cognitive deficits (Sivamaruthi et al., 2019; Thangaleela et al., 2022). For example, the supplementation of a probiotic mixture (Lactobacillus acidophilus, L. rhamnosus and Bifidobacteria longum) for 3 months improved the abundance of Bifidobacteria and Lactobacilli levels and symptoms of autism assessed by the Autism Treatment Evaluation Checklist (Shaaban et al., 2018).
Probiotics maintain a healthy environment in the intestine and reduce the various risk factors (Varela-Trinidad et al., 2022). Ishii et al. reported the facilitation of hippocampus learning and memory in mice model of Parkinson’s disease after administering Bifidobacterium breve (B. breve) strain A1. B. breve A1 supplementation recovered the transcriptional level expression of synaptophysin (SYP) and postsynaptic density protein-95 (PSD95) synaptic protein, which is involved in synaptic formation and stability (Ishii et al., 2021). Likely, the supplementation of a probiotic mixture containing B. bifidum, B. longum, L. rhamnosus, L. rhamnosus GG, L. plantarum LP28, and Lactococcus lactis subsp. lactis has improved the motor neuron function in mice models of Parkinson’s disease. Probiotic interventions could provide neuroprotection and reduce the exerts, improving dopaminergic neuronal degeneration (Hsieh et al., 2020). B. animalis subsp. Lactis and arginine administration improved cognitive flexibility in C57BL/6 mice, and the study proposed that maintaining a controlled intestinal environment with functional foods like probiotics could improve cognitive flexibility (Ikuta et al., 2023).
The administration of L. plantarum and B. bifidum in Wistar rats for 8 weeks increased the concentration of choline acetyltransferase and brain-derived neurotrophic factor in the hippocampus (Ağagündüz et al., 2022). L. plantarum DP189 strain helps in the regulation of the P13K/AKE/GSK-3β pathway in Alzheimer’s disease mice, resulting in the improvement of dysbiosis and prevented tau hyperphosphorylation (Song et al., 2022). It has been reported that L. plantarum PS128 aids in regulating glycogen synthase kinase 3β activity in streptozotocin-accelerated cognitive dysfunction mice (Huang et al., 2021).
There were several probiotic strains like B. fragilis, Prevotella histicola, Lactobacillus sp., B. animalis, L. plantarum, L. paracasei administered to the animal model, which showed the essential impact on the level of anti-inflammatory markers and delay the onset of multiple sclerosis. There would be enhancement in the level of Treg’s (CD4+ CD25+ Fox P3+) and regulate the balance of Th1/Th17 and Th2 cytokines by probiotics in multiple sclerosis (Dziedzic and Saluk, 2022).
Gut microbial disruption can negatively impact mental health. Therefore, restoration of gut microbiota could be used as an intervention to improve mental health. Modifying the GM could affect the functioning of the hippocampus. Bacterial toxins (Lipopolysaccharides; typical consequences of dysbiosis) and amyloid beta (Aβ) may interact with certain pathways like the vagus nerve pathway, the immune pathway related to the cytokines, and the systemic pathway, which is related to hormones and neurotransmitters to enhance the permeability of blood-brain barrier, mucosal-intestine barrier and finally results in the malfunctioning of the hippocampus (Tang et al., 2021).
Probiotics play a crucial role in reducing oxidative stress by producing various antioxidant enzymes (catalase and superoxide dismutase), antioxidants (butyrate, folate, and glutathione), and chelating metal ions. Probiotics may prevent immune actions like inflammatory responses by inhibiting TLR activation (Dobielska et al., 2022). The reduced inflammatory state could enhance the blood-brain barrier integrity and improve neurological functions (Wang et al., 2023). In addition to probiotic’s antioxidant and anti-inflammatory properties, they may improve cognitive function in depression by reducing hypothalamic-pituitary-adrenal (HPA)-axis dysfunction, and by increasing monoamine levels and neuroplasticity (Dobielska et al., 2022).
Studies have demonstrated that probiotics are associated with increased expression of BDNF and may be responsible for better cognitive performance (Huang et al., 2019). Prolonged supplementation of probiotics increases the concentration of tryptophan in the peripheral system and improves mental health. Probiotics exert their antidepressant effects by upregulating enzymes involved in serotonin synthesis (Lukić et al., 2022). Probiotics can change 5-hydroxytryptamine receptors, dopamine, and protein c-Fos levels; they may be responsible for modulation in the biochemistry of the CNS (Wang et al., 2016). Probiotics (Rouxiella badensis subsp. acadiensis) also increase the expression of certain mRNA, serotonin-1A (5-HT1A), and serotonin (5-HT)-2C receptors in the hippocampus (Yahfoufi et al., 2021), which could improve cognition.
Probiotics could modify the levels of neurotransmitters and neuromodulators, including serotonin, gamma-aminobutyric acid, acetylcholine, norepinephrine, N-acetyl aspartate, dopamine, and glutamate, which regulates the brain’s activity via metabolic pathways (Chudzik et al., 2021; Dicks, 2022). Though the previous studies provide established knowledge of the possible mechanisms of probiotics-mediated cognitive improvement, further studies are needed to improve the probiotic-based treatment opportunities for cognitive declines.
Both clinical trials and observational studies are crucial for assessing the effectiveness of probiotics in cognitive health. During aging, the chances of dementia and changes in their behavior also increase. Several studies reported the impact of supplementation probiotics on cognition and GM (Table 1). The supplementation of Lactiplantibacillus plantarum OLL 2712 for 12 weeks reduces inflammation by lowering the abundance of certain genera such as Oscillibacter, Monoglobus, and Lachnoclostridium in elderly adults (aged >65 years). The OLL2712 supplementation improved visual and composite memory (Sakurai et al., 2022). Tang et al. reported that probiotics (Lactobacilli and Bifidobacteria) act as neuroprotective agents in cognitively impaired elderly individuals and AD patients (Tang et al., 2021). Patients with Parkinson’s disease was supplemented with probiotics containing L. casei Shirota (6.5 × 109 colony forming units) daily for 5 weeks, which increases the bowel opening and reduces constipation, bloating, and abdominal pain. This study suggested that probiotics can effectively alleviate constipation symptoms in Parkinsons patients (Cassani et al., 2011).

TABLE 1. The representative studies on the influences of probiotic intervention on cognitive impairment.
Hu et al. reported that the supplementation of Lactobacillus acidophilus, Bifidobacterium longum and Enterococcus faecalis (>107 CFU of each strain × 4; twice/day) improved the verbal memory domain of performance in elderly subjects. The intervention group had a lower incidence of decline in specific verbal memory tests like the Hopkins Verbal Learning Test-Revised. Hospital stay duration, mortality rates, inflammatory markers, and white blood cell levels did not change significantly in both groups (Hu et al., 2023). Bifidobacterium bifidum BGN4 and Bifidobacterium longum BORI intervention for 12 weeks reduced the inflammation-causing gut bacteria, increased blood brain-derived neurotrophic factor (BDNF), and reduced stress levels in healthy elders. The study revealed that probiotic intake had no significant impact on cognitive domains such as language, memory, visuo-spatial processing, or other executive functions. However, after 12 weeks of intervention, mental flexibility significantly improved in the probiotic group. Additionally, probiotics intake decreased stress levels and the abundance of specific bacterial genera, including Eubacterium, Allisonella, Clostridiales, and Prevotellaceae (Kim et al., 2021).
A meta-analysis by Zhu et al. (2021) reported that probiotics significantly improved cognitive functions, particularly in mild cognitive impairment. At the same time, some factors can influence these benefits, such as probiotic strains, dosage, duration of intervention, and disease severity. In another meta-analysis, the impact of probiotics on cognitive function in patients with AD, mild cognitive impairment, and PD was analyzed. The study suggested probiotics could improve insulin resistance, lipid metabolisms, and cognitive and gastrointestinal health (Xiang et al., 2022). Not all the randomized clinical trials showed the positive impacts of probiotics on the subject’s cognitive health (Table 1).
Though probiotics have health benefits, they have some limitations (Kothari et al., 2019; Sotoudegan et al., 2019; Congur and Dalgic, 2023). Probiotics may cause systemic infection by bacterial translocation, gastrointestinal side effects, transfer of antibiotic resistance genes, toxic metabolic effects, and immune stimulation, The bacterial translocation is responsible for sepsis, fungemia, bacteremia, endocarditis (Congur and Dalgic, 2023). Premature infants, people with weak immunity, are more susceptible to infections. Immune stimulation may occur through bacterial toxins, including lipoteichoic acid, peptidoglycan, and lipopolysaccharides. Also, the risk of immune activation depends upon the strain of microorganisms and the dose administered (Kothari et al., 2019). Patients with short bowel syndrome risk developing probiotic-induced d-lactic acidosis due to unwarranted bacterial growth in the small intestine (Rao et al., 2018). d-lactic acidosis encephalopathy has neurologic symptoms such as memory loss, delirium, ataxia, and dysarthria (Kowlgi and Chhabra, 2015). Generally, infants, critically ill patients, patients with compromised immune systems, and cancers are considered risky subjects (Sotoudegan et al., 2019).
Certain key safety points must be adopted to recommend probiotics for safer use. Microbiome profiling is recommended because it can help identify factors affecting how individuals respond to probiotics differently and test various theories and processes. Manufacturers of older strains who might not have used modern techniques of assessing the risk of antibiotic resistance should re-evaluate their strains to ensure compliance. Manufacturers ought to disclose each probiotic strain’s antibiogram and, if necessary, offer an empirical course of therapy. Research into animal models is encouraged to improve our knowledge of detecting possible long-term impacts of probiotics, particularly regarding next-generation strains. Companies must monitor and report adverse occurrences in compliance with regulatory regulations for foods, dietary supplements, and medications (Merenstein et al., 2023).
Preclinical studies have highlighted the effects of probiotics on neurotransmitters, brain structure, and cognitive function in animal models. At the same time, clinical trials have demonstrated potential human benefits, including improved cognitive outcomes and reduced anxiety and depression. However, the safety concerns and possible risks of probiotic usage must be closely monitored and addressed. The next-generation of probiotics, for example, Akkermansia muciniphilia, has protein Amuc-1100 and extracellular vesicles that help regulate the metabolic system and gut barrier integrity and reduce lipopolysaccharides leakage and inflammation. However, further investigations and microbiome characterization are required to understand individual reactions and optimize the therapeutic potential of probiotics in preventing and managing cognitive deficits.
AK: Formal Analysis, Methodology, Writing–original draft. BS: Conceptualization, Formal Analysis, Project administration, Supervision, Writing–original draft, Writing–review and editing. SD: Data curation, Formal Analysis, Writing–original draft. YK: Data curation, Formal Analysis, Writing–original draft. RM: Data curation, Formal Analysis, Writing–original draft. BP: Conceptualization, Project administration, Supervision, Validation, Writing–original draft. CC: Funding acquisition, Project administration, Resources, Supervision, Writing–original draft, Writing–review and editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This research was funded by Fundamental Fund 2024, Chiang Mai University, Chiang Mai, Thailand.
The authors (BS and CC) gratefully acknowledge Chiang Mai University, Chiang Mai, for its support.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Ağagündüz, D., Kocaadam-Bozkurt, B., Bozkurt, O., Sharma, H., Esposito, R., Özoğul, F., et al. (2022). Microbiota alteration and modulation in Alzheimer's disease by gerobiotics: the gut-health axis for a good mind. Biomed. Pharmacother. 153, 113430. doi:10.1016/j.biopha.2022.113430
Aljumaah, M. R., Bhatia, U., Roach, J., Gunstad, J., and Azcarate Peril, M. A. (2022). The gut microbiome, mild cognitive impairment, and probiotics: a randomized clinical trial in middle-aged and older adults. Clin. Nutr. 41 (11), 2565–2576. doi:10.1016/j.clnu.2022.09.012
Antolini, G., and Colizzi, M. (2023). Where do neurodevelopmental disorders go? casting the eye away from childhood towards adulthood. Healthc. (Basel) 11 (7), 1015. doi:10.3390/healthcare11071015
Appleton, J. (2018). The gut-brain axis: influence of microbiota on mood and mental health. Integr. Med. (Encinitas) 17 (4), 28–32.
Asaoka, D., Xiao, J., Takeda, T., Yanagisawa, N., Yamazaki, T., Matsubara, Y., et al. (2022). Effect of probiotic Bifidobacterium breve in improving cognitive function and preventing brain atrophy in older patients with suspected mild cognitive impairment: results of a 24-week randomized, double-blind, placebo-controlled trial. J. Alzheimers Dis. 88 (1), 75–95. doi:10.3233/JAD-220148
Bernier, F., Ohno, K., Katsumata, N., Shimizu, T., and Xiao, J. (2021). Association of plasma hemoglobin A1c with improvement of cognitive functions by probiotic Bifidobacterium breve supplementation in healthy adults with mild cognitive impairment. J. Alzheimers Dis. 81 (2), 493–497. doi:10.3233/JAD-201488
Bezawada, N., Phang, T. H., Hold, G. L., and Hansen, R. (2020). Autism spectrum disorder and the gut microbiota in children: a systematic review. Ann. Nutr. Metab. 76 (1), 16–29. doi:10.1159/000505363
Bundgaard-Nielsen, C., Lauritsen, M. B., Knudsen, J. K., Rold, L. S., Larsen, M. H., Hindersson, P., et al. (2023). Children and adolescents with attention deficit hyperactivity disorder and autism spectrum disorder share distinct microbiota compositions. Gut Microbes 15 (1), 2211923. doi:10.1080/19490976.2023.2211923
Carlson, A. L., Xia, K., Azcarate-Peril, M. A., Goldman, B. D., Ahn, M., Styner, M. A., et al. (2018). Infant gut microbiome associated with cognitive development. Biol. Psychiatry 83 (2), 148–159. doi:10.1016/j.biopsych.2017.06.021
Cassani, E., Privitera, G., Pezzoli, G., Pusani, C., Madio, C., Iorio, L., et al. (2011). Use of probiotics for the treatment of constipation in Parkinson’s disease patients. Minerva Gastroenterol. Dietol. 57, 117–121.
Chen, Y., Xu, J., and Chen, Y. (2021). Regulation of neurotransmitters by the gut microbiota and effects on cognition in neurological disorders. Nutrients 13 (6), 2099. doi:10.3390/nu13062099
Chudzik, A., Orzyłowska, A., Rola, R., and Stanisz, G. J. (2021). Probiotics, prebiotics and postbiotics on mitigation of depression symptoms: modulation of the brain-gut-microbiome axis. Biomolecules 11 (7), 1000. doi:10.3390/biom11071000
Congur, E. C., and Dalgic, N. (2023). A revision of probiotic safety in children. J. Pediatr. Inf. 17 (2), e73–e78. doi:10.5578/ced.20239801
Curran, E. A., O’Neill, S. M., Cryan, J. F., Kenny, L. C., Dinan, T. G., Khashan, A. S., et al. (2015). Research review: birth by caesarean section and development of autism spectrum disorder and attention-deficit/hyperactivity disorder: a systematic review and meta-analysis. J. Child. Psychol. Psychiatry 56 (5), 500–508. doi:10.1111/jcpp.12351
Czajeczny, D., Kabzinska, K., and Wojciak, R. W. (2023). Effects of Bifidobacterium lactis BS01 and Lactobacillus acidophilus LA02 on cognitive functioning in healthy women. Appl. Neuropsychol. Adult 30 (5), 552–560. doi:10.1080/23279095.2021.1967155
Dash, S., Syed, Y. A., and Khan, M. R. (2022). Understanding the role of the gut microbiome in brain development and its association with neurodevelopmental psychiatric disorders. Front. Cell. Dev. Biol. 10, 880544. doi:10.3389/fcell.2022.880544
De Vos, W. M., Tilg, H., Van Hul, M., and Cani, P. D. (2022). Gut microbiome and health: mechanistic insights. Gut 71 (5), 1020–1032. doi:10.1136/gutjnl-2021-326789
Dicks, L. M. T. (2022). Gut bacteria and neurotransmitters. Microorganisms 10 (9), 1838. doi:10.3390/microorganisms10091838
Dobielska, M., Bartosik, N. K., Zyzik, K. A., Kowalczyk, E., and Karbownik, M. S. (2022). Mechanisms of cognitive impairment in depression. May probiotics help? Front. Psychiatry 13, 904426. doi:10.3389/fpsyt.2022.904426
Dziedzic, A., and Saluk, J. (2022). Probiotics and commensal gut microbiota as the effective alternative therapy for multiple sclerosis patients’ treatment. Int. J. Mol. Sci. 23 (22), 14478. doi:10.3390/ijms232214478
Eastwood, J., Walton, G., Van Hemert, S., Williams, C., and Lamport, D. (2021). The effect of probiotics on cognitive function across the human lifespan: a systematic review. Neurosci. Biobehav. Rev. 128, 311–327. doi:10.1016/j.neubiorev.2021.06.032
Fei, Y., Wang, R., Lu, J., Peng, S., Yang, S., Wang, Y., et al. (2023). Probiotic intervention benefits multiple neural behaviors in older adults with mild cognitive impairment. Geriatr. Nurs. 51, 167–175. doi:10.1016/j.gerinurse.2023.03.006
Ferreira, C. M., Vieira, A. T., Vinolo, M. A. R., Oliveira, F. A., Curi, R., and Martins, F. D. S. (2014). The central role of the gut microbiota in chronic inflammatory diseases. J. Immunol. Res. 2014, 689492. doi:10.1155/2014/689492
Ghezzi, L., Cantoni, C., Pinget, G. V., Zhou, Y., and Piccio, L. (2021). Targeting the gut to treat multiple sclerosis. J. Clin. Invest. 131 (13), e143774. doi:10.1172/JCI143774
Gwak, M. G., and Chang, S. Y. (2021). Gut-brain connection: microbiome, gut barrier, and environmental sensors. Immune Netw. 21 (3), e20. doi:10.4110/in.2021.21.e20
Handajani, Y. S., Turana, Y., Yogiara, Y., Sugiyono, S. P., Lamadong, V., Widjaja, N. T., et al. (2022). Effects of tempeh probiotics on elderly with cognitive impairment. Front. Aging Neurosci. 14, 891773. doi:10.3389/fnagi.2022.891773
Hsieh, T. H., Kuo, C. W., Hsieh, K. H., Shieh, M. J., Peng, C. W., Chen, Y. C., et al. (2020). Probiotics alleviate the progressive deterioration of motor functions in a mouse model of Parkinson’s disease. Brain Sci. 10, 206. doi:10.3390/brainsci10040206
Hu, L., Luo, M., Huang, H., Wu, L., Ouyang, W., Tong, J., et al. (2023). Perioperative probiotics attenuates postoperative cognitive dysfunction in elderly patients undergoing hip or knee arthroplasty: a randomized, double-blind, and placebo-controlled trial. Front. Aging Neurosci. 14, 1037904. doi:10.3389/fnagi.2022.1037904
Huang, H. J., Chen, J. L., Liao, J. F., Chen, Y. H., Chieu, M. W., Ke, Y. Y., et al. (2021). Lactobacillus plantarum PS128 prevents cognitive dysfunction in Alzheimer's disease mice by modulating propionic acid levels, glycogen synthase kinase 3 beta activity, and gliosis. BMC Complement. Med. Ther. 21 (1), 259. doi:10.1186/s12906-021-03426-8
Huang, T. T., Lai, J. B., Du, Y. L., Xu, Y., Ruan, L. M., and Hu, S. H. (2019). Current understanding of gut microbiota in mood disorders: an update of human studies. Front. Genet. 10, 98. doi:10.3389/fgene.2019.00098
Iacob, S., Iacob, D. G., and Luminos, L. M. (2019). Intestinal microbiota as a host defense mechanism to infectious threats. Front. Microbiol. 9, 3328. doi:10.3389/fmicb.2018.03328
Iglesias-Vázquez, L., Van Ginkel Riba, G., Arija, V., and Canals, J. (2020). Composition of gut microbiota in children with autism spectrum disorder: a systematic review and meta-analysis. Nutrients 12 (3), 792. doi:10.3390/nu12030792
Ikuta, K., Joho, D., Kakeyama, M., and Matsumoto, M. (2023). Bifidobacterium animalis subsp. lactis and arginine mixture intake improves cognitive flexibility in mice. Front. Nutr. 10, 1164809. doi:10.3389/fnut.2023.1164809
Iliodromiti, Z., Triantafyllou, A. R., Tsaousi, M., Pouliakis, A., Petropoulou, C., Sokou, R., et al. (2023). Gut microbiome and neurodevelopmental disorders: a link yet to be disclosed. Microorganisms 11 (2), 487. doi:10.3390/microorganisms11020487
Ishii, T., Furuoka, H., Kaya, M., and Kuhara, T. (2021). Oral administration of probiotic Bifidobacterium breve improves facilitation of hippocampal memory extinction via restoration of aberrant higher induction of neuropsin in an MPTP-induced mouse model of Parkinson’s disease. Biomedicines 9 (2), 167. doi:10.3390/biomedicines9020167
Kelsey, C., Dreisbach, C., Alhusen, J., and Grossmann, T. (2019). A primer on investigating the role of the microbiome in brain and cognitive development. Dev. Psychobiol. 61 (3), 341–349. doi:10.1002/dev.21778
Kim, C. S., Cha, L., Sim, M., Jung, S., Chun, W. Y., Baik, H. W., et al. (2021). Probiotic supplementation improves cognitive function and mood with changes in gut microbiota in community-dwelling older adults: a randomized, double-blind, placebo-controlled, multicenter trial. J. Gerontol. A Biol. Sci. Med. Sci. 76 (1), 32–40. doi:10.1093/gerona/glaa090
Kothari, D., Patel, S., and Kim, S. K. (2019). Probiotic supplements might not be universally-effective and safe: a review. Biomed. Pharmacother. 111, 537–547. doi:10.1016/j.biopha.2018.12.104
Kowlgi, N. G., and Chhabra, L. (2015). d-lactic acidosis: an underrecognized complication of short bowel syndrome. Gastroenterol. Res. Pract. 2015, 476215. doi:10.1155/2015/476215
Kumar, A., Pramanik, J., Goyal, N., Chauhan, D., Sivamaruthi, B. S., Prajapati, B. G., et al. (2023). Gut microbiota in anxiety and depression: unveiling the relationships and management options. Pharmaceuticals 16 (4), 565. doi:10.3390/ph16040565
Lin, B., Wang, Y., Zhang, P., Yuan, Y., Zhang, Y., and Chen, G. (2020). Gut microbiota regulates neuropathic pain: potential mechanisms and therapeutic strategy. J. Headache Pain 21 (1), 103. doi:10.1186/s10194-020-01170-x
Lindsay, E. C., Metcalfe, N. B., and Llewellyn, M. S. (2020). The potential role of the gut microbiota in shaping host energetics and metabolic rate. J. Anim. Ecol. 89 (11), 2415–2426. doi:10.1111/1365-2656.13327
Long-Smith, C., O'Riordan, K. J., Clarke, G., Stanton, C., Dinan, T. G., and Cryan, J. F. (2020). Microbiota-gut-brain axis: new therapeutic opportunities. Annu. Rev. Pharmacol. Toxicol. 60, 477–502. doi:10.1146/annurev-pharmtox-010919-023628
Lukić, I., Ivković, S., Mitić, M., and Adžić, M. (2022). Tryptophan metabolites in depression: modulation by gut microbiota. Front. Behav. Neurosci. 16, 987697. doi:10.3389/fnbeh.2022.987697
Martín, R., and Langella, P. (2019). Emerging health concepts in the probiotics field: streamlining the definitions. Front. Microbiol. 10, 1047. doi:10.3389/fmicb.2019.01047
Mendoza-León, M. J., Mangalam, A. K., Regaldiz, A., González-Madrid, E., Rangel-Ramírez, A. M., Álvarez-Mardonez, O., et al. (2023). Gut microbiota short-chain fatty acids and their impact on the host thyroid function and diseases. Front. Endocrinol. (Lausanne) 14, 1192216. doi:10.3389/fendo.2023.1192216
Merenstein, D., Pot, B., Leyer, G., Ouwehand, A. C., Preidis, G. A., Elkins, C. A., et al. (2023). Emerging issues in probiotic safety: 2023 perspectives. Gut microbes 15 (1), 2185034. doi:10.1080/19490976.2023.2185034
Mishra, S., and Acharya, S. (2021). A brief overview on probiotics: the health friendly microbes. Biomed. Pharmacol. J. 14 (4), 1869–1880. doi:10.13005/bpj/2285
Morais, L. H., Schreiber, H. L., and Mazmanian, S. K. (2020). The gut microbiota–brain axis in behaviour and brain disorders. Nat. Rev. Microbiol. 19 (4), 241–255. doi:10.1038/s41579-020-00460-0
Nandwana, V., Nandwana, N. K., Das, Y., Saito, M., Panda, T., Das, S., et al. (2022). The role of microbiome in brain development and neurodegenerative diseases. Molecules 27 (11), 3402. doi:10.3390/molecules27113402
Paone, P., and Cani, P. D. (2020). Mucus barrier, mucins and gut microbiota: the expected slimy partners? Gut 69 (12), 2232–2243. doi:10.1136/gutjnl-2020-322260
Patel, P., Butani, K., Kumar, A., Singh, S., and Prajapati, B. G. (2023). Effects of fermented food consumption on non-communicable diseases. Foods 12 (4), 687. doi:10.3390/foods12040687
Plaza-Diaz, J., Ruiz-Ojeda, F. J., Gil-Campos, M., and Gil, A. (2019). Mechanisms of action of probiotics. Adv. Nutr. 10 (1), S49–S66. doi:10.1093/advances/nmy063
Rao, S. S. C., Rehman, A., Yu, S., and Andino, N. M. (2018). Brain fogginess, gas and bloating: a link between SIBO, probiotics and metabolic acidosis. Clin. Transl. Gastroenterol. 9 (6), 162. doi:10.1038/s41424-018-0030-7
Sakurai, K., Toshimitsu, T., Okada, E., Anzai, S., Shiraishi, I., Inamura, N., et al. (2022). Effects of Lactiplantibacillus plantarum OLL2712 on memory function in older adults with declining memory: a randomized placebo-controlled trial. Nutrients 14 (20), 4300. doi:10.3390/nu14204300
Sanborn, V., Aljumaah, M., Azcarate-Peril, M. A., and Gunstad, J. (2022). Examining the cognitive benefits of probiotic supplementation in physically active older adults: a randomized clinical trial. Appl. Physiol. Nutr. Metab. 47 (8), 871–882. doi:10.1139/apnm-2021-0557
Shaaban, S. Y., El Gendy, Y. G., Mehanna, N. S., El-Senousy, W. M., El-Feki, H. S., Saad, K., et al. (2018). The role of probiotics in children with autism spectrum disorder: a prospective, open-label study. Nutr. Neurosci. 21 (9), 676–681. doi:10.1080/1028415X.2017.1347746
Sharma, V., and Kaur, S. (2020). The effect of probiotic intervention in ameliorating the altered central nervous system functions in neurological disorders: a review. Open Microbiol. J. 14, 18–29. doi:10.2174/1874285802014010018
Shi, S., Zhang, Q., Sang, Y., Ge, S., Wang, Q., Wang, R., et al. (2022). Probiotic Bifidobacterium longum BB68S improves cognitive functions in healthy older adults: a randomized, double-blind, placebo-controlled trial. Nutrients 15 (1), 51. doi:10.3390/nu15010051
Sivamaruthi, B. S., Prasanth, M. I., Kesika, P., and Chaiyasut, C. (2019). Probiotics in human mental health and diseases-A mini review. Trop. J. Pharm. Res. 18 (4), 889–895. doi:10.4314/tjpr.v18i4.29
Song, X., Zhao, Z., Zhao, Y., Wang, Z., Wang, C., Yang, G., et al. (2022). Lactobacillus plantarum DP189 prevents cognitive dysfunction in D-galactose/AlCl3 induced mouse model of Alzheimer's disease via modulating gut microbiota and PI3K/Akt/GSK-3β signaling pathway. Nutr. Neurosci. 25 (12), 2588–2600. doi:10.1080/1028415X.2021.1991556
Sotoudegan, F., Daniali, M., Hassani, S., Nikfar, S., and Abdollahi, M. (2019). Reappraisal of probiotics’ safety in human. Food Chem. Toxicol. 29, 22–29. doi:10.1016/j.fct.2019.04.032
Sun, Y., Baptista, L. C., Roberts, L. M., Jumbo-Lucioni, P., McMahon, L. L., Buford, T. W., et al. (2020). The gut microbiome as a therapeutic target for cognitive impairment. J. Gerontol. A Biol. Sci. Med. Sci. 75 (7), 1242–1250. doi:10.1093/gerona/glz281
Tang, W., Meng, Z., Li, N., Liu, Y., Li, L., Chen, D., et al. (2021). Roles of gut microbiota in the regulation of hippocampal plasticity, inflammation, and hippocampus-dependent behaviors. Front. Cell. Infect. Microbiol. 10, 611014. doi:10.3389/fcimb.2020.611014
Thangaleela, S., Sivamaruthi, B. S., Kesika, P., and Chaiyasut, C. (2022). Role of probiotics and diet in the management of neurological diseases and mood states: a review. Microorganisms 10 (11), 2268. doi:10.3390/microorganisms10112268
Thapar, A., Cooper, M., and Rutter, M. (2017). Neurodevelopmental disorders. Lancet Psychiatry 4 (4), 339–346. doi:10.1016/S2215-0366(16)30376-5
Varela-Trinidad, G. U., Domínguez-Díaz, C., Solórzano-Castanedo, K., Íñiguez-Gutiérrez, L., Hernández-Flores, Td. J., and Fafutis-Morris, M. (2022). Probiotics: protecting our health from the gut. Microorganisms 10 (7), 1428. doi:10.3390/microorganisms10071428
Vendrik, K. E. W., Ooijevaar, R. E., de Jong, P. R. C., Laman, J. D., van Oosten, B. W., van Hilten, J. J., et al. (2020). Fecal microbiota transplantation in neurological disorders. Front. Cell. Infect. Microbiol. 10, 98. doi:10.3389/fcimb.2020.00098
Wang, H., Lee, I. S., Braun, C., and Enck, P. (2016). Effect of probiotics on central nervous system functions in animals and humans: a systematic review. J. Neurogastroenterol. Motil. 22 (4), 589–605. doi:10.5056/jnm16018
Wang, N., Gao, X., Zhang, Z., and Yang, L. (2022). Composition of the gut microbiota in attention deficit hyperactivity disorder: a systematic review and meta-analysis. Front. Endocrinol. (Lausanne) 13, 838941. doi:10.3389/fendo.2022.838941
Wang, P., Yin, X., Chen, G., Li, L., Le, Y., Xie, Z., et al. (2021). Perioperative probiotic treatment decreased the incidence of postoperative cognitive impairment in elderly patients following non-cardiac surgery: a randomised double-blind and placebo-controlled trial. Clin. Nutr. 40 (1), 64–71. doi:10.1016/j.clnu.2020.05.001
Wang, Y., Du, W., Hu, X., Yu, X., Guo, C., Jin, X., et al. (2023). Targeting the blood–brain barrier to delay aging-accompanied neurological diseases by modulating gut microbiota, circadian rhythms, and their interplays. Acta Pharm. Sin. B 13, 4667–4687. doi:10.1016/j.apsb.2023.08.009
Xiang, S., Ji, J.-L., Li, S., Cao, X.-P., Xu, W., Tan, L., et al. (2022). Efficacy and safety of probiotics for the treatment of alzheimer's disease, mild cognitive impairment, and Parkinson's disease: a systematic review and meta-analysis. Front. Aging Neurosci. 14, 730036. doi:10.3389/fnagi.2022.730036
Yahfoufi, N., Ah-Yen, E. G., Chandrasegaram, R., Aly, S., Murack, M., Kadamani, A. K., et al. (2021). Adolescent use of potential novel probiotic Rouxiella badensis subsp. acadiensis (Canan SV-53) mitigates pubertal LPS-Induced behavioral changes in adulthood in a sex-specific manner by modulating 5HT1A receptors expression in specific brain areas. Compr. Psychoneuroendocrinol. 7, 100063. doi:10.1016/j.cpnec.2021.100063
Zhu, G., Zhao, J., Zhang, H., Chen, W., and Wang, G. (2021). Probiotics for mild cognitive impairment and Alzheimer’s disease: a systematic review and meta-analysis. Foods 10 (7), 1672. doi:10.3390/foods10071672
Keywords: cognition, gut-brain axis, Lactobacillus, Bifidobacterium, inflammation
Citation: Kumar A, Sivamaruthi BS, Dey S, Kumar Y, Malviya R, Prajapati BG and Chaiyasut C (2024) Probiotics as modulators of gut-brain axis for cognitive development. Front. Pharmacol. 15:1348297. doi: 10.3389/fphar.2024.1348297
Received: 02 December 2023; Accepted: 05 February 2024;
Published: 20 February 2024.
Edited by:
Budheswar Dehury, Regional Medical Research Center (ICMR), IndiaReviewed by:
Attayeb Mohsen, National Institutes of Biomedical Innovation, Health and Nutrition, JapanCopyright © 2024 Kumar, Sivamaruthi, Dey, Kumar, Malviya, Prajapati and Chaiyasut. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bhupendra G. Prajapati, Ymh1cGVuMjdAZ21haWwuY29t; Chaiyavat Chaiyasut, Y2hhaXlhdmF0QGdtYWlsLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.