- 1Department of Biochemistry, Faculty of Veterinary Medicine, Damanhour University, Damanhour, Egypt
- 2Department of Pathology, Faculty of Veterinary Medicine, Alexandria University, Alexandria, Egypt
- 3Department of Cytology and Histology, Faculty of Veterinary Medicine, Damanhour University, Damanhour, Egypt
- 4Department of Pharmacology and Therapeutics, Faculty of Veterinary Medicine, Damanhour University, Damanhour, Egypt
- 5Department of Toxicology and Forensic Medicine, Faculty of Veterinary Medicine, Damanhour University, Damanhour, Egypt
- 6Department of Physiology, Faculty of Veterinary Medicine, Damanhour University, Damanhour, Egypt
- 7Department of Anatomy and Embryology, Faculty of Veterinary Medicine, Alexandria University, Alexandria, Egypt
- 8Department of Biology, College of Science, Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia
- 9Department of Medical Laboratory Technology, Faculty of Applied Medical Sciences, University of Tabuk, Tabuk, Saudi Arabia
Introduction: 7,12-dimethylbenz (a) anthracene (DMBA) is a harmful polycyclic aromatic hydrocarbon derivative known for its cytotoxic, carcinogenic, and mutagenic effects in mammals and other species. Annona muricata, L. (Graviola; GRV) is a tropical fruit tree traditionally well-documented for its various medicinal benefits. This investigation is the first report on the potential antioxidant and antinfammatory reno-protective impact of GRV against DMBA-induced nephrotoxicity in rats.
Methods: Forty male albino rats were allocated into four equal groups (n = 10). The 1st group served as the control, the 2nd group (GRV) was gastro-gavaged with GRV (200 mg/kg b.wt), the 3rd group (DMBA) was treated with a single dose of DMBA (15 mg/kg body weight), and the 4th group (DMBA + GRV) was gastro-gavaged with a single dose of DMBA, followed by GRV (200 mg/kg b.wt). The GRV administration was continued for 8 weeks.
Results and Discussion: Results revealed a significant improvement in renal function, represented by a decrease in urea, creatinine, and uric acid (UA) in the DMBA + GRV group. The antioxidant potential of GRV was confirmed in the DMBA + GRV group by a significant decline in malondialdehyde (MDA) and a significant increase in catalase (CAT), superoxide dismutase (SOD), glutathione S transferase (GST), and reduced glutathione (GSH) compared to DMBA-intoxicated rats; however, it was not identical to the control. Additionally, the antiinflammatory role of GRV was suggested by a significant decline in mRNA expression of cytochrome P450, family 2, subfamily e, polypeptide 1 (CYP2E1), tumor necrosis factor-alpha (TNF-α), and interleukin 1 beta (IL-1β) in the DMBA + GRV group. Moreover, GRV improved the histopathologic and immunohistochemical expression of TNF-α, CYP450, and IL1β in DMBA-intoxicated kidney tissue. Conclusively, GRV is a natural medicinal product that can alleviate the renal injury resulting from environmental exposure to DMBA. The reno-protective effects of GRV may involve its anti-inflammatory and/or antioxidant properties, which are based on the presence of phytochemical compounds such as acetogenins, alkaloids, and flavonoids.
1 Introduction
Although modern industrial development has significantly improved human life, it has also raised various environmental concerns. Industrial pollutants pose a concern for health since they are present in the soil, water, air, and food (Chen et al., 2022). Incomplete combustion of fossil fuels in internal combustion engines, coke manufacturing, household heating, and incineration, as well as natural occurrences like forest fires and volcanoes, all result in the creation of polycyclic aromatic hydrocarbons (PAHs) (Chen et al., 2022). PAHs are a group of organic pollutants released into the environment in large quantities, mostly due to human activities (Barrett et al., 2022). 7,12-dimethylbenz (a) anthracene (DMBA) is a harmful PAH derivative that has well-documented cytotoxic, carcinogenic, mutagenic, and immunosuppressive effects on mammals and other animals (Fidianingsih et al., 2022). In previous studies, DMBA led to the potential growth of malignant tumors in the bladder, kidney, liver, and brain (John et al., 2022). Moreover, it has altered liver metabolic enzymes and induced harmful renal disorders (Molnar et al., 2022). The adverse initiator and promoter properties of DMBA are attributed to its produced metabolites, which can alkylate DNA or other cellular macromolecules (Mathiyazhagan et al., 2022), trigger the formation of reactive oxygen species (ROS) (Hosny et al., 2021), alter behavior and cellular dysfunction (Li et al., 2016), and initiate carcinogenicity, particularly in animal research and cancer models (Zhang et al., 2022).
Generally, environmental pollutants raise the risk of renal, cardiac, and pulmonary disorders (Lu et al., 2022). Various environmental xenobiotic toxicants attack the kidney, resulting in nephrotoxicity (Ren et al., 2022). Currently, nephrotoxicity and chronic renal disease have been recognized as public health problems (Sanchez et al., 2022). The development of glomerular and tubular epithelial cell damage could be attributed to the disruption of cell membrane integrity and normal cellular processes in mitochondria (Dakrory et al., 2015b). DMBA has been reported to induce severe nephrotoxicity, which is characterized by renal tubular necrosis, nuclear chromatin condensation, injured mitochondria, and increased lysosome number (Ozturk et al., 2006).
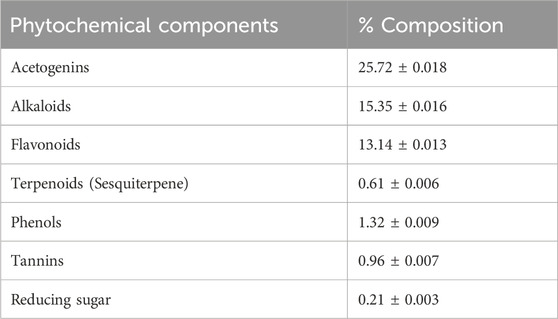
Traditionally, several disorders have been treated with natural medicines. Therefore, it is important to find natural substances with potent renopreventive properties with minimum or no side effects. The Annona muricata L. fruit tree, often known as the soursop or graviola (GRV), is mainly grown in tropical regions of Central and South America (Moghadamtousi et al., 2014). GRV is a natural antioxidant supplement that improves body performance under stressful conditions (Samarghandian et al., 2013). In the tropics, this plant’s bark, fruit seeds, leaves, and root are all used in herbal medicine (Sabra and Ahmed, 2018). According to available research, A. muricata extract helps prevent kidney damage because it contains tannins, glycosides, flavonoids, and saponins. These compounds are used to treat a variety of illnesses, including renal impairment (Arthur et al., 2011; Hassan et al., 2019). The graviola extract’s phytochemical analysis revealed many valuable secondary metabolites, such as tannins and steroids (El-Shahat et al., 2021). In numerous studies, GRV has been shown to protect against oxidative stress, enhance antioxidant enzymes such as catalase, superoxide dismutase, glutathione peroxidase, and glutathione, improve blood lipid levels, decrease LDL, total cholesterol, and triglycerides, increase HDL, and exert a wide range of beneficial effects, including anticonvulsive, anticancer, anti-arthritic, anti-malarial, hepatoprotective, and antidiabetic ones (Rady et al., 2018; Shukry et al., 2020; El-Shahat et al., 2021). Recent researches support the usefulness of GRV in the prevention of cancer and degenerative diseases, including renal disease, due to the presence of potent antioxidants such as polyphenols and flavonoids, which provide defense against the accumulation of reactive oxygen species (ROS) (Alam et al., 2014). These ROS are key signaling molecules that play a crucial role in the development of pro-inflammatory disorders (Mittal et al., 2014). Findings from recent studies confirmed that the presence of anti-inflammatory agents in GRV extracts, such as alkaloids, saponins, flavonoids, and tannins, which inhibit COX-2 and prostaglandin synthesis, can normalize the level of proinflammatory mediators (Serafini et al., 2010; Foong and Hamid, 2012; Laksmitawati et al., 2016; Shukry et al., 2020).
To date, few studies have investigated the anti-inflammatory effect of GRV extract against inflammatory renal damage. No studies are available concerning its impact on the cytochrome P450 family 2 subfamily E member 1 (CYP2E1). Thus, the current study aims to evaluate the alterations in renal function, oxidative/antioxidative status, gene expression of NF-α, CYP2E1, and IL-β1, as well as immunohistochemical expression of TNF-α, IL1β, and CYP450 in renal tissues of DMBA-intoxicated rats, and to assess, for the first time, the possible therapeutic impacts of GRV in DMBA-induced nephrotoxicity.
2 Materials and methods
2.1 Chemicals
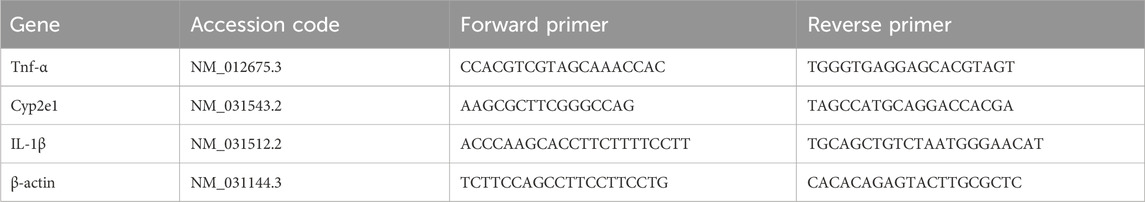
DMBA was supplied by Sigma Chemical Co. (St. Louis, MO, United States). Forward and reverse primers for cytochrome P450, family 2, subfamily e, polypeptide 1 (CYP2E1), tumor necrosis factor alpha (TNF-α; 24835), interleukin 1 beta (IL-1β; I2393), and β-actin were purchased from Sigma Chemical Co. (St. Louis, MO, United States). Graviola (GRV) capsules were purchased from Inkanatural (Lima, Peru) (Tables 1, 2). Kits for catalase (CAT) (Cat. No. CAT 25 17), superoxide dismutase (SOD) (Cat. No. SD 25 21), glutathione-S-transferase (GST) (Cat. No. GT 2519), reduced glutathione (GSH) (Cat. No. RG 2523), and malondialdehyde (MDA) (Cat. No. MD 2529) were purchased from the Bio-Diagnostics Co. (Cairo, Egypt). Biochemical test kits for urea (10505), creatinine (creat; 10053), and uric acid (UA; 10694) were provided by Human Diagnostic Worldwide Co. (Wiesbaden, Germany).
2.2 Characterization of phenolic compounds: HPLC analysis
The phenolic reference standard for HPLC was used to identify phenolic components in the methanolic and aqueous extract of A. muricata (Sakakibara et al., 2003). The following gradient elution program was used, with solution A (50 mM sodium phosphate in 10% methanol; pH 3.3) and solution B (70% methanol) being eluted at 35°C: A solution’s percentages are as follows: 0–15 min–100%; 15–45 min–70%; 45–65 min–65%; 65–70 min–60%; 70–95 min–50% of Solution A; 95–100 min–0% of Solution A. 20 μL was the injection volume, and the flow rate was 1 mL min−1. Table 3 shows the different wavelengths (around λ max) at which detection were tested for different phenolic compounds.
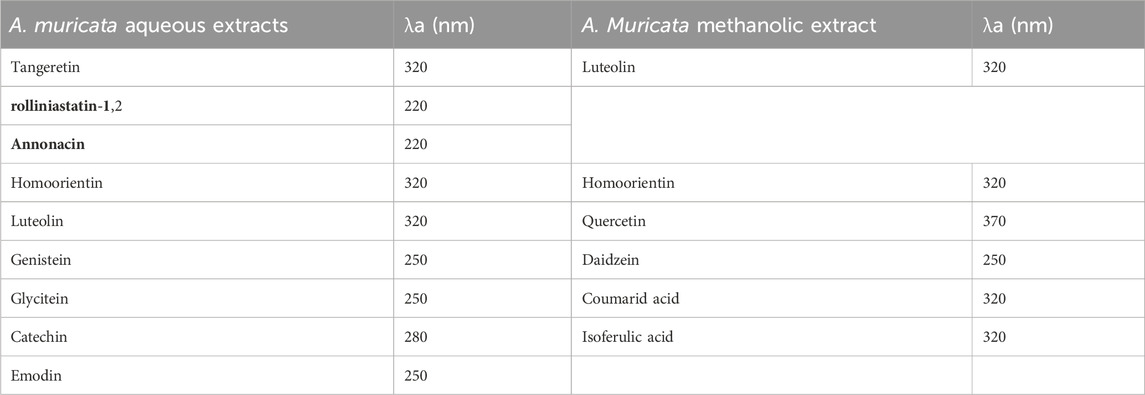
TABLE 3. Major phenolic compounds identified in methanol and aqueous extracts of A. Muricata by HPLC.
2.3 Animals and ethical statement
Forty male Wistar albino rats (average weight 130 ± 10 gm, average age of 6 weeks old) were obtained from the Animal House Colony, National Research Centre, Cairo, Egypt, were kept for 2 weeks for adaptation. They kept under a 12-h light-and-darkness cycle and controlled temperature of 21°C ± 2°C. Throughout the experiment, animals received standard rodent diets (23% crude protein, 7% crude fiber, 3.0% crude fat, 8% acid insoluble ash, 1%–2.5% calcium, 0.5%–1% phosphorus, and 0.9% sodium) and free access to water. The animal studies were ethically approved (DMU/VetMed-2023/020) by the Hygiene and Preventive Medicine research committee, Faculty of Veterinary Medicine, Damanhur University, Egypt.
2.4 Experimental design
After acclimation, rats were randomly assigned to four groups (10 rats each). The 1st group was served as a control and administered a single dose of sesame oil (1 mL; as a vehicle for DMBA), followed by a daily dose of distilled water (1 mL; as a solvent for GRV), which started on the 2nd day and continued for 8 weeks via gastric gavage. The 2nd group (GRV) was gastro-gavage with a single dose of sesame oil (1 mL), followed by GRV (200 mg/kg b.wt.) according to Florence et al. (2014) from the second day, and continued for 8 weeks. The 3rd group (DMBA) was treated with a single dose of DMBA (15 mg/kg body weight) dissolved in 1 mL of sesame oil, according to Dakrory et al. (2015a). Rats in the 4th group (DMBA + GRV) were gastro-gavaged with a single dose of DMBA (15 mg/kg body weight), followed by GRV (200 mg/kg body weight), which started from the second and continued for 8 weeks.
At the end of the experiment, rats were anesthetized given 87 mg ketamine/kg of body weight and 13 mg xylazine/kg, beginning 10 to 15 min after simultaneous injection and lasting 15 to 30 min. Rats also were sacrificed by cervical dislocation (Kumar and Clover, 2015). The blood samples were collected by heart puncture before the abdominal incision. Serum samples were separated by centrifugation at 1008 G-force and stored at −20°C for further biochemical analysis of renal function markers. Following euthanasia, the kidney was immediately collected, weighed, and divided into three parts in a saline phosphate buffer (PBS) solution. The first part was kept at −80 for gene expression studies. The second part was kept in a buffered formalin solution (10%) for histopathological examination and immunohistochemical analysis, while the third part was homogenized and kept at −20°C for further evaluation of malondialdehyde (MDA) and antioxidant parameters.
2.5 Assessment of renal function in serum
Markers for renal functions (urea, creatinine, and uric acid) were spectrophotometrically assessed using commercially available diagnostic kits (Human Scient Co., Germany), according to Chescheir, (2019), Chromy et al. (2008), and Kessler and Siekmann, (1999).
2.6 Assessment of oxidative/antioxidative markers in homogenized renal tissues
Following euthanasia, renal tissues were immediately collected, weighed, homogenized, and centrifuged at 4,000 rpm for 15 min (137 mM NaCl, 2,7 mM KCl, ten mM Na2HPO4, two mM KH2PO4, pH 7,4). The supernatants were used to assess catalase (Aebi, 1984), SOD (Wang et al., 2016), GST (Habig et al., 1974), GSH (Beutler et al., 1963), and MDA (Ohkawa et al., 1979) according to the manufacturer’s instructions using the commercially available kits (Bio-diagnostic Co., Cairo, Egypt).
2.7 Assessment of mRNA expression of TNF-α, CYP2E1, and IL-β1
The mRNA has been isolated, according to Boom et al. (1990). Following this, cDNA is synthesized using the RevertAidTM Synthesis Kit, according to Wiame et al. (2000). Gene expression of CYP2E1, TNF-α, and IL1β was performed in the presence of β-actin as a housekeeping gene (Longo et al., 1990) (Table 4). The master mix was prepared, and the reaction was performed in a thermal cycler (MyCycler, Bio-Rad, Germany). The whole volume of the reaction was 25 μL for each gene of interest. The length of the PCR primers was 18-22 bp. The target gene’s relative quantification to the reference was determined using the ΔΔCT method (Livak and Schmittgen, 2001).
2.8 Histopathological examination
The rats' kidneys were dissected after an incision in the abdominal wall. The collected specimens were thoroughly washed and fixed in a neutral buffered formalin solution (10%) for 48 h. The fixed specimens were then processed via the conventional paraffin embedding technique. Briefly, samples were washed in distilled water for 30 min, dehydrated in an ascending grade of ethyl alcohol, cleared in several changes of xylene, and then embedded and blocked out in paraffin wax. Four µm thick sections were microtomed and stained with hematoxylin and eosin (H & E), periodic acid Schiff reaction (PAS), and a combination of periodic acid Schiff reaction (PAS) with Alcian Blue (AB) according to Bancroft and Gamble (2008) and Dosumu et al. (2021). The PAS technique was used for the demonstration of glycoproteins and mucins (neutral and acid mucin) and for the visualization of basement membranes. Alcian Blue/PAS is used to differentiate between neutral mucins and acid mucins, where the expression patterns of neutral and acid mucins may indicate certain pathological lesions if present. A blind pathologic examination was performed by two expert pathologists. A digital camera (Leica EC3; Leica, Germany) connected to a light microscope (Leica DM500) captured several representative photomicrographs. The extent of kidney injury in H&E sections was assessed by a semi-quantitative scoring system; ten rats from each group had their kidneys inspected and graded for various pathologic lesions in the glomerulus, renal tubules, and interstitium. Five random fields were evaluated for each rat. The degree of severity of the detected histopathologic lesions was estimated according to Khafaga et al. (2021). Four levels of severity were used in the scoring system: no histologic change (−), mild (+), moderate (+), and severe (+++) pathologic changes. The individual score for each rat was estimated, and then the grading decision was determined by calculating the median score for each group. For the quantitative histomorphometric analysis of PAS and the Alcian Blue/PAS staining technique, original representative micrographs (×100) were captured from five randomly selected fields in each section for the quantitative histomorphometric analysis of staining optical denisty. ImageJ software (v1.46r, NIH, Bethesda, MD, United States) was used to estimate the differences in optical densities, which were represented by the difference in distribution of positively reacted cells. Optical densities were evaluated as the percentage of the mean number of pixels versus the correlated value at which the pixel of the respective intensity was present (Onouchi et al., 2013; Varghese et al., 2014).
2.9 Immunohistochemical analysis
Immunohistochemical analysis was performed in accordance with Tawfik et al. (2021). In brief, the prepared sections were dewaxed, rehydrated in a graded series of ethyl alcohol, exposed to antigen retrieval for 20 min via citrate buffer, deactivated for endogenous peroxidase by H2O2 (3%) for 5 min, and blocked for the non-specific reaction by a 60-min incubation with normal goat serum (10%). After that, sections were incubated overnight with anti-TNF-α antibody (Abcam, Cat. Ab220210, Cambridge, United Kingdom), anti-CYP450 antibody (Abcam, Cat. Ab197053, Cambridge, United Kingdom), and anti-IL1β antibody (Abcam, Cat. Ab 9722, Cambridge, United Kingdom) at 4°C. Sections were then incubated with biotin-conjugated goat anti-rabbit IgG antiserum (Histofine kit, Nichirei Corporation, Japan) for 60 min, followed by 30 min of incubation with streptavidin-peroxidase conjugate (Histofine kit, Nichirei Corporation, Japan). The streptavidin-biotin complex was visualized by a 3,3′-diaminobenzidine tetrahydrochloride (DAB)-H2O2 solution. Counterstaining with Mayer’s hematoxylin solution was done. Finally, representative photomicrographs were captured from each group by a digital camera (EC3, Leica, Germany) connected to a light microscope (Leica DM500). Original representative photomicrographs (×100) were taken from five randomly selected fields in each section for the quantitative histomorphometric analysis of immunostaining intensity. ImageJ software (v1.46r, NIH, Bethesda, MD, United States) was used to estimate the differences in optical densities, which were represented by the difference in distribution of positively immunoreacted cells. Optical densities were evaluated as the percentage of the mean number of pixels versus the correlated value at which the pixel of the respective intensity was present (Onouchi et al., 2013; Varghese et al., 2014).
2.10 Statistical analysis
The statistical analysis was carried out using SPSS program version 20 (SPSS, Richmond, VA, United States), with the data presented as means ± SE. Data were analyzed using one-way ANOVA, and the significant differences between treatments were assessed by a post hoc review of the Duncan test at p < 0.01. The assumptions of observation independence as well as the normality and homogeneity of variances were assessed by Shapiro-Wilk test before conducting the ANOVA test.
3 Results
During the experiment, rats in the DAMP group showed symptoms of decreased body weight, loss of appetite, and ruffled hair. Only one rat died on the tenth day of the experiment. On the contrary, the control group and other treated groups did not suffer from any apparent symptoms or deaths. The therapeutic effect of GRV on various parameters will be presented below.
3.1 Phytochemicals identified and HPLC analysis
The GRV phytochemical screening data, detailing the chemical constituents in plant extracts, are presented in Tables 1, 2. This screening is essential for identifying specific phytochemicals in the extracts, particularly in the methanol and aqueous extracts of Annona muricata leaves. These compounds were identified using High-Performance Liquid Chromatography (HPLC) analysis, a technique that separates, identifies, and quantifies compounds in a mixture. For accurate identification, we referenced a library with analytical properties of over 100 phenolic standards, established by Sakakibara et al. 2003. This library includes crucial data like maximum absorption wavelength (λ max), retention time, and calibration limits. Our HPLC results were then compared to these standard values, ensuring precise compound identification. The identified phenolic compounds in the extracts, along with their corresponding wavelengths in nanometers (nm), are listed in Table 3.
3.2 The therapeutic impact of GRV on renal functions of DMBA-intoxicated rats
As shown in Table 5, serum urea and creatinine levels were significantly increased in the DMBA-treated group compared to the control group. At the same time, oral administration of GRV to DMBA-intoxicated rats caused a significant decrease (p < 0.0001) in their concentration compared to the corresponding DMBA groups; however, it still significantly increased (p < 0.0001) compared to the control group. A similar result was reported for uric acid (p < 0.001). There was no discernible difference between the control group and the GRV administration alone.
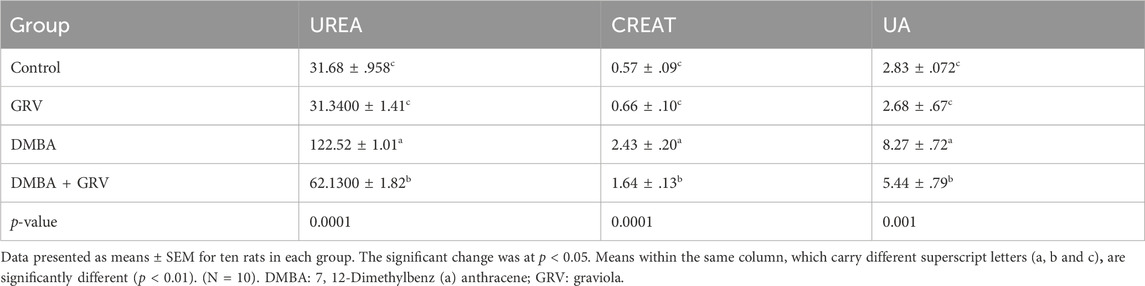
TABLE 5. The therapeutic impact of graviola (GRV) on some renal markers (urea, creatinine, and uric acids) of rats intoxicated with DMBA and treated with graviola compared to control rats.
3.3 The therapeutic impact of GRV on antioxidant markers in renal tissues of DMBA -intoxicated rats
As shown in Table 6, CAT and SOD enzymatic activities and GSH non-enzymatic levels were significantly decreased (p < 0.0001) in the DMBA group as compared to the corresponding control groups. Whereas, oral administration of GRV in DMBA-intoxicated rats caused a significant increase (p < 0.0001) in the activities of CAT and SOD, besides GSH levels, when compared to the DMBA group. However, it still significantly decreased (p < 0.0001) compared to control rats. The same results were obtained for GST enzymatic activities (p < 0.001). Furthermore, a significant increase (p < 0.0001) in the MDA concentration was observed in DMBA-intoxicated rats compared to their counterparts in the control group. While MDA concentrations were significantly decreased in DMBA-intoxicated rats treated with GRV compared to the DMBA group, they still significantly increased (p < 0.0001) compared to the control group. In contrast, there was no discernible difference between the control group and the GRV-treated rats.
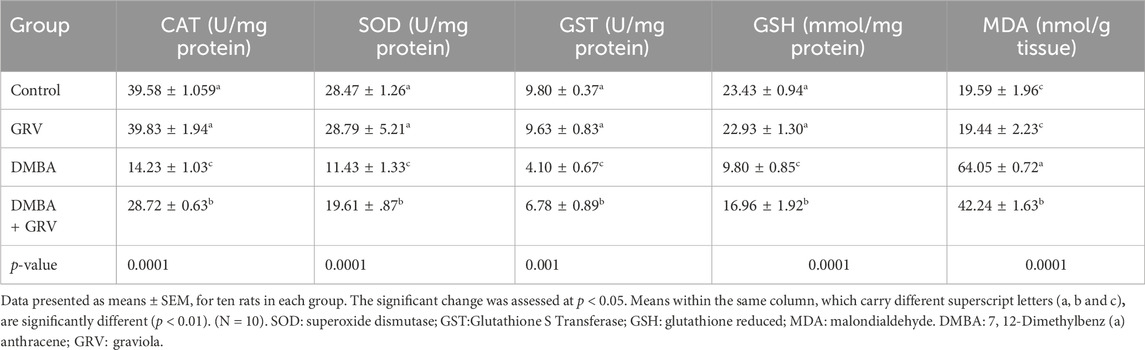
TABLE 6. The therapeutic impact of graviola (GRV) on lipid peroxidation represented as malondialdehyde (MDA) and antioxidant’s activities of CAT, SOD, GST, and GSH of rats intoxicated with DMBA and treated with GRV compared to control rats.
3.4 The therapeutic impact of GRV on CYP2E1 and pro-inflammatory cytokines (TNF-α and IL-β1) mRNA expression
Data shown in Figure 1 revealed a significant increase (p < 0.0001) in mRNA expression levels of CYP2E1, TNF-α, and IL-1β in the DMBA groups compared to the control rats. DMBA-intoxicated rats treated with oral administration of GRV showed a significant reduction (p < 0.0001) in the mRNA expression of CYP2E1, TNF-α, and IL-1β compared to the corresponding DMBA groups. However, they still significantly increased (p < 0.0001) in comparison to the control group.
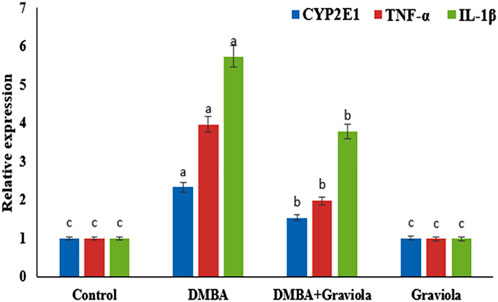
FIGURE 1. Relative gene expression of CYP2E1, TNF-α and IL-1β mRNA in renal tissue of control and treated groups. Means within the same figure, which carry different superscript letters, are significantly different (p < 0.01). CYP2E1: Cytochrome P450 Family 2 Subfamily E Member 1; TNF-α: tumour necrosis factor alpha; IL-1β:interleukin-1-beta.
3.5 Histopathological assessment
As shown in Figure 2, the histopathological examination of rats’ kidneys in the control and GRV groups revealed normal glomeruli, renal tubules, renal epithelium, and interstitium (Figure 2 a,b). While DMBA-intoxicated rats showed dilatation and congestion of intertubular and glomerular blood vasculature, attenuation and degeneration of renal epithelium, nuclear pyknosis, and cytoplasmic eosinophilia of several renal epithelial cells (Figure 2C), In addition, several sections showed extravasation of erythrocytes into the surrounding interstitium and intertubular lymphocytic infiltration. On the contrary, rats in the DMBA + GRV group showed marked improvement in the histological structure of renal tubules and renal epithelium with mild glomerular congestion (Figure 2D). Semiquantitative scoring for different pathologic lesions revealed a significant increase in the DMBA-intoxicated group compared to the control group. However, a significant decrease in the means of these scored lesions was reported in the DMBA + GRV group compared to the DMBA-intoxicated group, although it was not identical for the control group (Table 7).
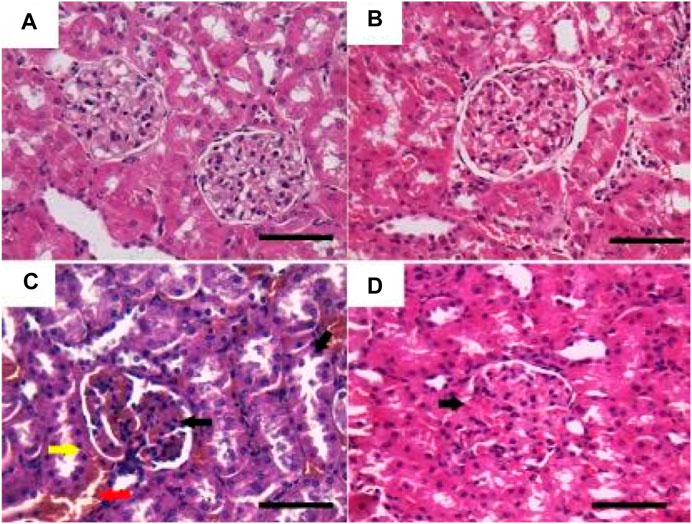
FIGURE 2. Representative photomicrographs showing the histopathological lesions of renal tissues of (a) control, (b) GRV-treated, (c) DMBA -treated, and (f) DMBA + GRV-treated rats stained with H&E; scale bar = 50 µm: (A, B) Normal glomeruli, renal tubules, renal epithelium, and interstitium. (C) Dilatation and congestion of intertubular (red arrow) and glomerular (black arrow) blood vasculature, attenuation and degeneration of renal epithelium, nuclear pyknosis, and cytoplasmic eosinophilia of renal epithelial cells (yellow arrow). (D) Marked improvement in the histological structure of renal tubules and renal epithelium with mild glomerular congestion (black arrow).
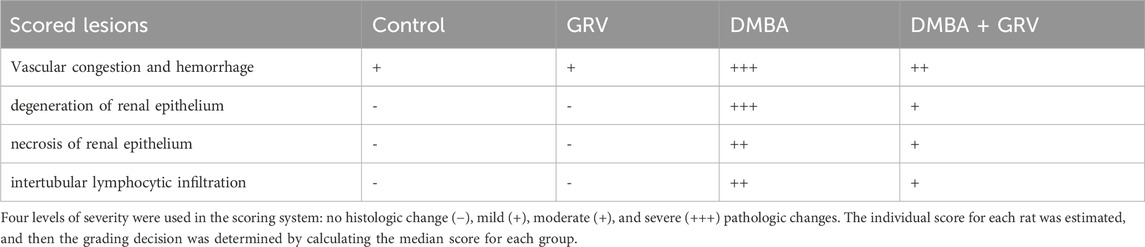
TABLE 7. The semiquantitative lesion scores of rats’ kidney treated with graviola (GRV), DMBA: 7, 12-Dimethylbenz (a) anthracene, or its combination.
On the other hand, sections stained with PAS showed a positive reaction in the glomerular basement membrane and apical brush borders of renal tubules in the control and GRV groups (Figures 3A, B). However, PAS-stained sections from DMBA-treated rats showed positive stained, thickened, and wrinkly glomerular and tubular basement membranes, with partial destruction of brush borders (Figure 3C). A nearly normal shape and thickening of the glomerular and tubular basement membranes and tubular brush borders were observed in DMBA + GRV rats (Figure 3D). The quantitative scoring for PAS staining density revealed a significant increase in the DMBA-intoxicated group due to the thickening of basement membranes compared to control rats. However, a significant decrease in the optical density of PAS-positive tissues was reported in the DMBA + GRV group compared to the DMBA-intoxicated group, albeit it was not identical for the control group (Figure 8A).
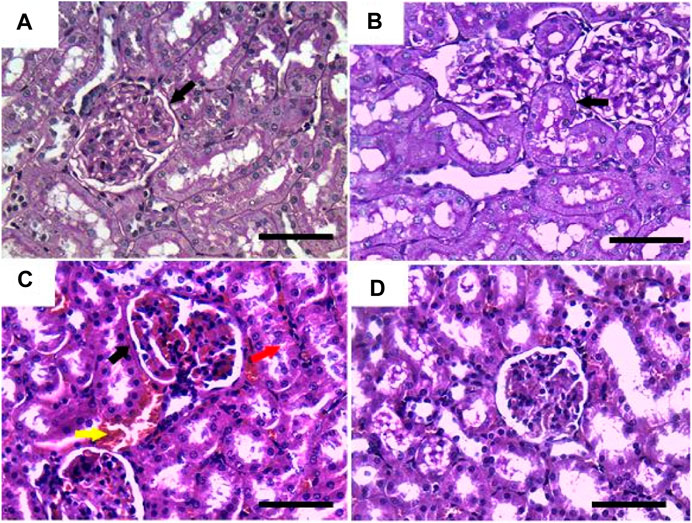
FIGURE 3. Representative photomicrographs showing the histopathological lesions of renal tissues of (a) control, (b) GRV-treated, (c) DMBA -treated, and (f) DMBA + GRV-treated rats stained with PAS; scale bar = 50 µm: (A, B) Positive reaction in the glomerular basement membrane and apical brush borders of renal tubules (black arrows). (C) Thickened and wrinkly glomerular and tubular basement membranes (black arrow), with partial distruction of brush borders (red arrow) and intertubular congestion (yellow arrow). (D) Nearly normal shape and thickening of the glomerular and tubular basement membranes, as well as tubular brush borders.
In Figure 4, Albican Blue/PAS staining for control, intoxicated, and treated rats showed positive reactions in the renal glomeruli and renal tubules without marked or noticeable changes in distribution and expression pattern of mucins between tissues from different groups. The quantitative scoring for Albican Blue/PAS staining density revealed non-significant changes between control and treated rats in different groups (Figure 8B).

FIGURE 4. Representative photomicrographs showing the histopathological lesions of renal tissues of (a) control, (b) GRV-treated, (c) DMBA -treated, and (f) DMBA + GRV-treated rats stained with alcian blue/PAS; scale bar = 50 µm: (A, B, C, D) Positive reactions in renal glomeruli and renal tubules without marked or noticeable changes between tissues from different groups.
3.6 Immunohistochemical findings
As illustrated in Figure 5, negative immunostaining against TNF-α was reported in the control group (Figure 5A). However, the immunohistochemical examination of the kidneys of rats treated with GRV showed mild glomerular and tubular immunoreactivity (Figure 5B). On the contrary, moderate to intense glomerular and tubular immune reactions were noticed in the DMBA-treated group (Figure 5C). Rats in the DMBA + GRV-treated group revealed decreased glomerular and tubular immune reactivity compared to their counterparts in the DMBA-treated group (Figure 5C). The quantitative scoring for TNF-α- immunoreacted cells revealed a significant increase in kidneys in the DMBA-intoxicated group compared to control rats. However, a significant decrease in the optical density of TNF-α immunopositive cells was reported in the DMBA + GRV group compared to the DMBA-intoxicated group, although it was not identical for the control group (Figure 8C).
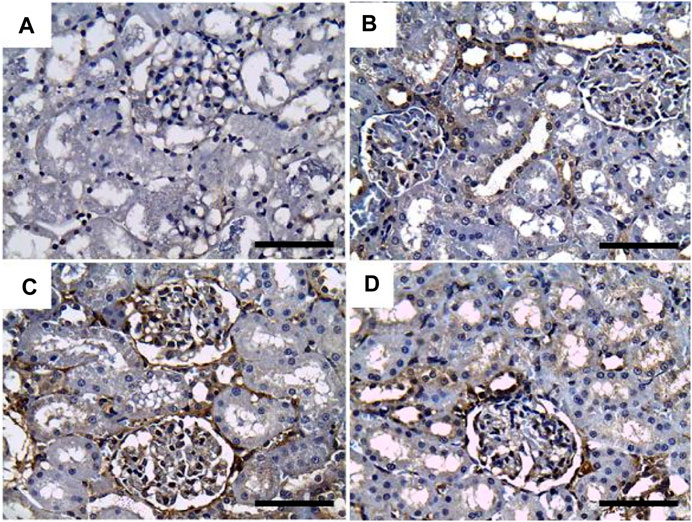
FIGURE 5. Representative photomicrographs showing the immunohistochemical expression of TNF-α in renal tissues of (a) control, (b) GRV-treated, (c) DMBA -treated, and (f) DMBA + GRV-treated rats; scale bar = 50 µm: (A) Negative immunostaining against TNF-α. (B) Mild glomerular and tubular immunoreactivity. (C) Moderate to intense glomerular and tubular immune reactions. (D) Decreased glomerular and tubular immune reactivity compared to their counterparts in the DMBA-treated group.
Additionally, Figure 6 showed that the immunoexpression of IL-1β in the control group revealed negative glomerular and tubular immunoreactivity (Figure 6A). However, mild glomerular immunoreactivity against IL-1β was reported in the GRV-treated group (Figure 6B). On the contrary, intense immunostaining of the glomerulus and renal tubules was noticed in DMBA-intoxicated rats (Figure 6C). While rats in DMBA + GRV-treated rats showed moderate glomerular immunoreactivity (Figure 6D), The quantitative scoring for IL-1β-immune-reacted cells revealed a significant increase in kidneys in the DMBA-intoxicated group compared to control rats. However, a significant decrease in the optical density of IL-1β-immunopositive cells was reported in the DMBA + GRV group compared to the DMBA-intoxicated group, albeit it was not identical for the control group (Figure 8D).
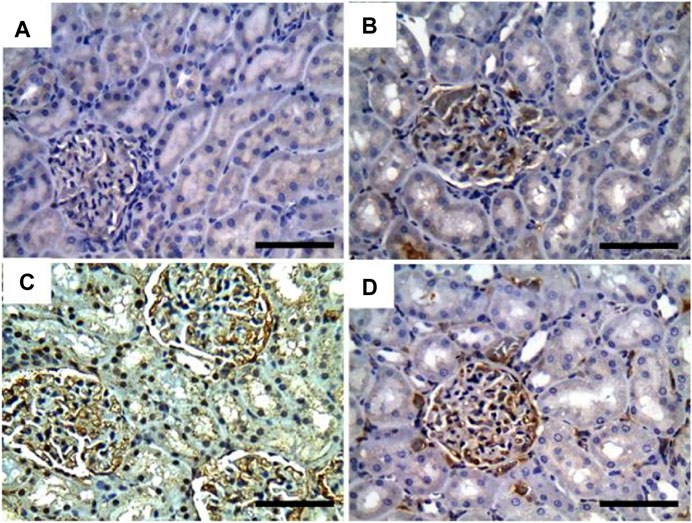
FIGURE 6. Representative photomicrographs showing the immunohistochemical expression of IL1-β in renal tissues of (a) control, (b) GRV-treated, (c) DMBA -treated, and (f) DMBA + GRV-treated rats; scale bar = 50 µm: (A) Negative glomerular and tubular immunoreactivity. (B) Mild glomerular immunoreactivity against IL-1β. (C) Intense immunostaining of the glomerulus and renal tubules. (D) Moderate glomerular immunoreactivity.
Regarding CYP2E1 immune expression, control and GRV rats showed negative immunoreactivity against CYP2E1 (Figures 7A, B). On the contrary, moderate to intense glomerular and tubular immunoexpression was noted in DMBA-intoxicated rats (Figure 7C). However, mild to moderate glomerular immunoreactivity against CYP2E1 was observed in DMBA + GRV-treated rats (Figure 7D). The quantitative scoring for CYP2E1-immune-expressed cells revealed a significant increase in kidneys in the DMBA-intoxicated group compared to control rats. However, a significant decrease in the optical density of CYP2E1-immunopositive cells was reported in the DMBA + GRV group compared to the DMBA-intoxicated group, although it was not identical for the control group (Figure 8E).
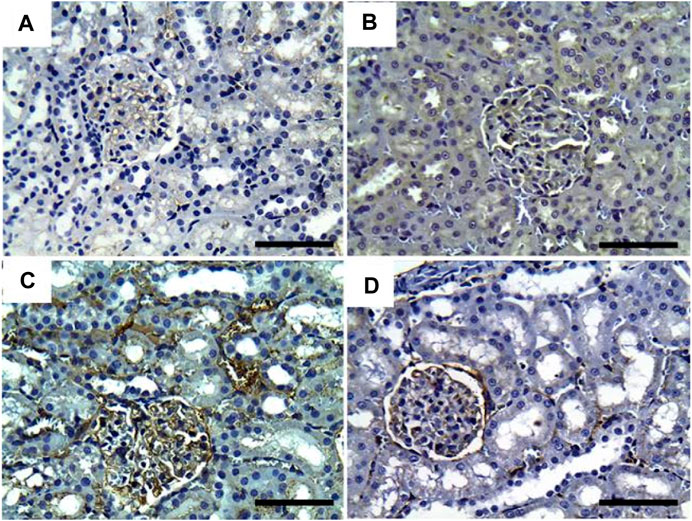
FIGURE 7. Representative photomicrographs showing the immunohistochemical expression of CYP450 in renal tissues of (a) control, (b) GRV-treated, (c) DMBA-treated, and (f) DMBA + GRV-treated rats; scale bar = 50 µm: (A, B) Negative immunoreactivity against CYP2E1. (C) Moderate to intense glomerular and tubular immunoexpression. (D) Mild to moderate glomerular immunoreactivity against CYP450.
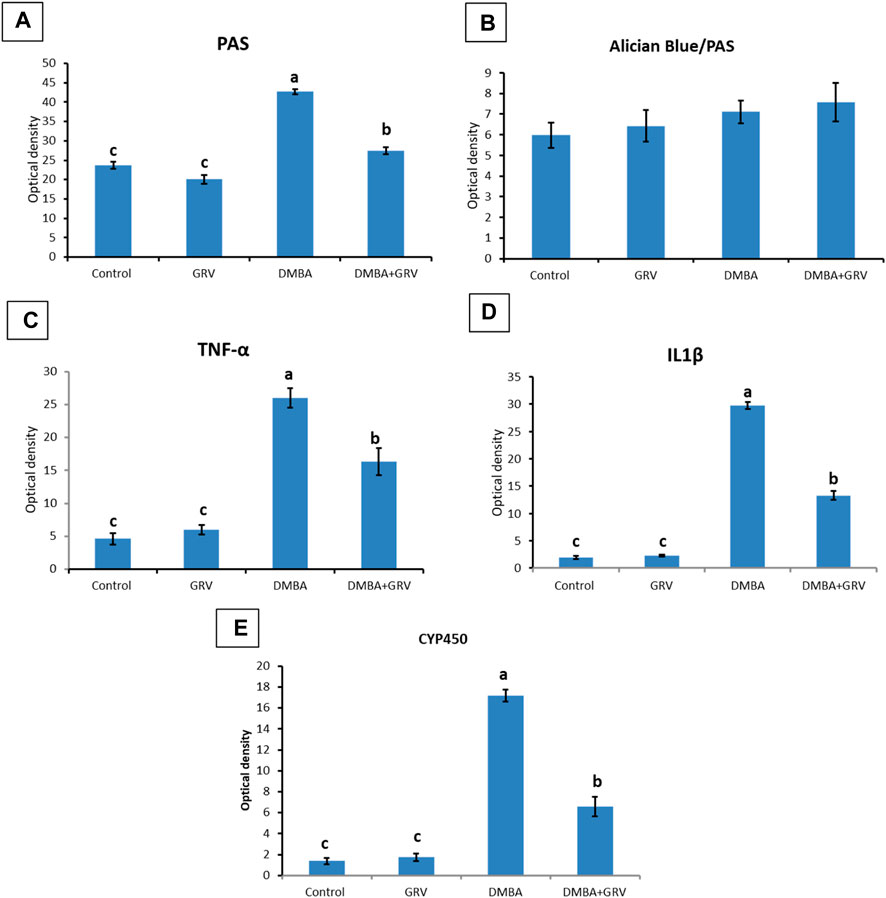
FIGURE 8. Quantitative histomorphometric analysis for staining optical density in renal tissues stained with: (A) PAS, (B) Alician Blue/PAS, (C) TNF- α, (D) IL-1β, and (E) CYP450. Means within the same figure, which carry different superscript letters, are significantly different (p < 0.01). CYP 450: Cytochrome P450 F; TNF-α: tumour necrosis factor alpha; IL-1β:interleukin-1-beta.
4 Discussion
Annona muricata L. (soursop or graviola) is a widely known tropical plant consumed by the community. It is also traditionally used to treat diverse ailments such as fever, pain, respiratory illness, infection, diabetes, cancer, and hypertension (Mutakin et al., 2022). The family Annonaceae has a long history of being recognized and used as a natural herbal treatment all throughout the world (Moghadamtousi et al., 2015a). Consuming graviola significantly improves human health (Saleem et al., 2017). It is therapeutically used for anticancer, antibacterial, antiviral, anti-fungal, anti-malarial, anti-tumor, anxiolytic, anti-stress, analgesic, hypoglycemic, hypotensive, hepatoprotective, gastroprotective, anti-inflammatory, and immunomodulatory properties (Chaparro et al., 2014; Gavamukulya et al., 2017). However, few reports are available concerning their beneficial impacts against renal injury. Polycyclic aromatic hydrocarbons (PAHs), one type of atmospheric pollutant, are widely dispersed in the environment and enter human bodies through food, drink, and the air. DMBA, an PAH environmental pollutant, has several harmful and carcinogenic consequences (Kleiner et al., 2002). Previously, DMBA treatment increased urea and creatinine serum concentrations due to an upregulation of protein catabolism (Burchiel et al., 1990). Meanwhile, urea represents the nitrogenous waste produced by protein degradation (Akhouri et al., 2020). As a result, elevated urea and creatinine levels in the serum indicate renal disease (Krishnamoorthy and Sankaran, 2016). While the increased serum uric acid level indicates nephrotoxicity (Burchiel et al., 1990), which can indicate kidney impairment (Akhouri et al., 2020). The current study reported a significant increase in the concentration of urea, creatinine, and uric acid in the serum of the DMBA-treated group. On the other side, the DMBA + GRV-treated group showed enhanced renal functions. The potential efficacy of graviola is due to extensive phytochemical evaluations on different parts of the A. muricata plant, which have shown the presence of various phytoconstituents and compounds, including alkaloids (Yang et al., 2015), megastigmanes (Nawwar et al., 2012), flavonol triglycerides (Nawwar et al., 2012), phenolics (Jimenez et al., 2014), cyclopeptides, and essential oils (Pélissier et al., 1994; Kossouoh et al., 2007). However, Annona species, including A. muricata, are a generally rich source of annonaceous acetogenin compounds (Rupprecht et al., 1990) and major minerals such as K, Ca, Na, Cu, Fe, and Mg, suggesting that regular consumption of A. muricata fruit can help provide essential nutrients and elements to the human body (Gyamfi et al., 2011).
Oxidative stress is the main cause of nephrotoxicity (Kasprzak, 2023), where the reactive oxygen metabolites are formed by the mitochondria of the renal cortex (Padmini and Kumar, 2012). However, the toxicity of the glomerular and tubular epithelial cells may be induced by mechanisms compromising the mitochondria’s regular cellular functions and/or the integrity of their membranes (Dakrory et al., 2015b), which induces renal injury (Du and Yang, 1994). Early research suggests that DMBA stimulates the generation of reactive oxygen species (ROS), which damages DNA, causes lipid peroxidation, and depletes the antioxidant defense mechanisms in cells (Dakrory et al., 2015b). ROS affect mesangial and endothelial cells, which can result in oxidative stress and alter the glomerulus' appearance and function (Kandeel et al., 2011; Sharma et al., 2012). When the amount of reactive oxygen species (O2, H2O2, and -OH) generated exceeds the antioxidant capacity of the cells, oxidative damage results (Kesba and El-Beltagi, 2012). The damage caused by the ROS generated during DMBA metabolism may spread from the site of formation to other locations inside or even outside the cells (Koul et al., 2010). Reactive carbonyl compounds, the most common form of malondialdehyde, are a complicated series of molecules that can be formed when lipid peroxides produced from the disintegration of polyunsaturated fatty acids (Akande and Akinyinka, 2005; Gandhi et al., 2017). According to the current study, MDA levels significantly increased in the DMBA-treated group compared to the control groups; these results are consistent with Sharma and Paliwal (2014). However, the MDA concentration significantly dropped in the GRV-treated group. This decrease demonstrated the extract’s capacity to counteract the oxidative damage caused by DMBA. Graviola’s antioxidant capability clarified the normalization effect in the DMBA + GRV treatment group. Few studies have described the polyphenols found in A. muricata leaves. The leaves of A. muricata were shown to contain kaempferol, quercetin-glucoside, quercetin, and rutin in studies by Yang et al. (2015) and Oliveira et al. (2017). Nevertheless, the majority of research examined crude extracts; the greater antioxidant potential of aquas and methanolic extracts of A. muricata may be explained by the high concentration of phenolic components in these extracts. Numerous studies have demonstrated that the chemicals discovered by HPLC analysis, including rutin, quercetin, procyanidins B2 and C1, (epi) catechin, and kaempferol, are strong antioxidants. These compounds possess the ability to produce stable antioxidant-derived radicals, as well as the ability to scavenge free radicals and reduce hydrogen or donate electrons (Cos et al., 2003; Gu et al., 2006; Vellosa et al., 2011; Ganeshpurkar and Saluja, 2017). The antioxidant potential of A. muricata leaves has also been documented in earlier in vitro and in vivo investigations (Florence et al., 2014; Moghadamtousi et al., 2015b; Olas, 2023), which supports our findings. Moreover, Baskar et al. (2007) reported that GRV has a defensive ability against free radicals (OH) and H2O2. Consequently, it blocks the elevation of LPO (Spitz et al., 2004) and converts the ROS into harmless properties (Ekaluo et al., 2016).
Enzymatic and non-enzymatic antioxidants are endogenous antioxidants that can combat ROS and lessen oxidative stress. A number of metabolic pathways changed, along with alterations in lipid peroxidation-generating processes and antioxidant defense mechanisms (Paliwal et al., 2011). Catalase, glutathione-S-transferase, superoxide`e dismutase (SOD), and other enzymes have been suggested as indicators for oxidative stress (Bedi and Priyanka, 2012; Sullivan et al., 2020). In the current study, the oxidative stress induced by DMBA is indicated by a significant reduction in CAT, SOD, and GST, where these enzymes were blocked by the excessive ROS production. This adverse oxidative impact is countered by the increased levels of antioxidant enzymes, which were related to GRV treatment. These enzymes stop the production of hydroxyl radicals and guard the components of the cell against oxidative damage (Gill and Tuteja, 2010; Wu et al., 2011). Flavonoids and other phenolic acids included in GRV are known to inhibit the oxidation of cellular macromolecules via their radical scavenging properties or by activating ROS-reducing efficacy, which is attributed to the upregulation of the antioxidant genes (Jiménez-Osorio et al., 2015). Moreover, Baskar et al. (2007) concluded that GRV possesses potent antioxidant activities due to the presence of acetogenins, which can play a crucial role in ROS scavenging. These findings are consistent with Sharma and Paliwal (2013) and Mohamed et al. (2021).
In previous research, it has been proposed that the progression of renal injury depends on pro-inflammatory cytokines produced in renal tissues (Navarro and Mora-Fernández, 2006). In the present study, DMBA increased the gene expression of TNF-α, IL1β, and CYP2E1 as compared to the control group. The enhancement of cytokines secretion from various cells enhances the expression of COX2, which is known as an enzyme to produce a variety of inflammatory mediators like leukotrienes (LTs), prostaglandins, and others (Sarbishegi et al., 2016; Hong et al., 2021). Recently, it was concluded that treatment with GRV extract reduced inflammatory cytokines (IL-6, IL8) (Hong et al., 2021) and inhibited both COX-1 and COX-2 (Rady et al., 2018). These findings suggested the crucial role of GRV in the alleviation of inflammatory responses in renal tissues via inhibiting COX-2 as well as proinflammatory cytokines (Helal and Abd Elhameed, 2021). Data from the current study support these findings, where rats from the DMBA + GRV group showed a significant decrease in levels of TNF-α, IL1β, and CYP2E1 compared to the group that received DMBA alone. The presence of anti-inflammatory substances (alkaloids, saponins, flavonoids, and tannins) in graviola extracts may prevent the formation of prostaglandins, which retain the expression levels of TNF-α, CYP2E1, and IL1β to the normal level (Serafini et al., 2010). Several studies are still needed to explore the anti-inflammatory power of the main individual constituents of GRV.
DMBA-induced oxidative stress induces damage to lipids, proteins, and nucleic acids, leading to interconnected disruptions of cellular metabolism (Krishnamoorthy and Sankaran, 2016). Therefore, proteinuria, renal tubular necrosis, and increased specific signals such as TNF-α, chemokines, and cytokines are all signs of DMBA-induced nephrotoxicity (Sharma and Paliwal, 2012). The histopathological and immunohistochemical examinations of the kidneys of the different experimental groups supported the results of antioxidant status and oxidative stress markers. DMBA produced marked histopathological alterations in the kidney, including dilation of tubules and sloughing of the epithelium, which indicates advanced disintegration of tubules (Sharma et al., 2012). Treatment with GRV leaf extract normalizes nephrotoxicity’s histolopathological abnormalities (Sabra and Ahmed, 2018). In contrast, GRV administered to DMBA-induced rats protected renal tissue from degenerative changes that are mediated by oxidative stress. This protective effect of GRV leaves may be due to its anti-inflammatory action and its ability to neutralize free radicals due to the presence of flavonoids and other polyphenols (Sabra and Ahmed, 2018).
Results from PAS and Ablican Blue/PAS-stained sections revealed interstitial lymphocytic infiltration, hemorrhage, thickened and wrinkled tubular basement membranes, and partial disruption of the brush border in DMBA-intoxicated rats. However, the DMBA + GRV-treated group showed marked attenuation of DMBA-related adverse impacts, in addition to positive reactions in the glomerular basement membrane and apical microvilli of renal tubules. Similar results were previously reported by Yen-Nien et al. (2019). In addition, the immunohistochemical examination of TNF-α, CYP2E1, and IL-β1 in DMBA-intoxicated groups showed a significant increase in the immunohistochemical expression of the examined inflammatory cytokines. However, concurrent treatment with GRV revealed a reduction of these inflammatory cytokines, which revealed its efficient anti-inflammatory effect. These findings was supported by Mitsui et al. (2015), which demonstrated that cytochrome P450 1B1 (CYP1B1) has been upregulated in many types of cancer, including renal cell carcinoma. Also, Sonoda et al. (2015) studied the levels of TNF receptors 1 and 2 (TNFR1 and TNFR2) in serum and urine, which were associated with other markers of kidney injury and renal histological findings. Further studies are still needed to explain the anti-inflammatory impact of GRV and its related mechanisms.
5 Conclusion
Based on the findings mentioned above, it could be concluded that GRV is a naturally beneficial medicinal product that can alleviate the renal toxicity resulting from environmental exposure to DMBA. The reno-protective effects offered by the GRV may involve its anti-inflammatory, antioxidant, and/or oxidative free radical scavenging properties. The antioxidant activities of GRV are based on the presence of phytochemical compounds such as kaempferol, quercetin-glucoside, quercetin, and rutin, which were proved to be strong antioxidants due to their abilities to scavenge ROS, reduce hydrogen or donate electrons, and produce stable antioxidant-derived radicals. Additionally, the crucial anti-inflammatory role of GRV was proved by their abilities to inhibit COX-2 and proinflammatory cytokines due to the presence of anti-inflammatory substances (alkaloids, saponins, flavonoids, and tannins) in GRV extracts, which prevent the formation of prostaglandins and retain the expression levels of TNF-α, CYP2E1, and IL1β to the normal levels. Therefore, the useful effect of GRV appeared when consumed for long periods and was preferred as a protective agent against polycyclic aromatic hydrocarbons, particularly 7,12-dimethylbenz[a]anthracene (DMBA).
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
The animal study was approved by the ethically approved (DMU/VetMed-2023/020) by the Hygiene and Preventive Medicine research committee, Faculty of Veterinary Medicine, Damanhur University, Egypt. The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
MZ: Conceptualization, Data curation, Formal Analysis, Investigation, Resources, Writing–original draft. AK: Data curation, Formal Analysis, Investigation, Project administration, Resources, Writing–review and editing. SM: Investigation, Methodology, Project administration, Resources, Software, Supervision, Writing–review and editing. LW: Conceptualization, Data curation, Funding acquisition, Investigation, Supervision, Validation, Visualization, Writing–review and editing. HS: Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Resources, Software, Writing–review and editing. AE: Conceptualization, Formal Analysis, Funding acquisition, Methodology, Resources, Writing–original draft, Writing–review and editing. NB: Investigation, Methodology, Project administration, Resources, Validation, Writing–review and editing. MA: Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Visualization, Writing–review and editing. NS: Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Software, Validation, Visualization, Writing–review and editing. HG: Conceptualization, Data curation, Formal Analysis, Resources, Supervision, Writing–original draft, Writing–review and editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
We want to acknowledge Princess Nourah bint Abdulrahman University Researchers Supporting Project number (PNURSP2024 R227), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Akande, A. A., and Akinyinka, A. O. (2005). Serum malondialdehyde levels during menstral cycle. Afr. J. Biotechnol. 4, 11.
Akhouri, V., Kumari, M., and Kumar, A. J. S. R. (2020). Therapeutic effect of Aegle marmelos fruit extract against DMBA induced breast cancer in rats. Sci. Rep. 10, 18016–18112. doi:10.1038/s41598-020-72935-2
Alam, M. A., Subhan, N., Rahman, M. M., Uddin, S. J., Reza, H. M., and Sarker, S. D. (2014). Effect of citrus flavonoids, naringin and naringenin, on metabolic syndrome and their mechanisms of action. Adv. Nutr. 5 (4), 404–417. doi:10.3945/an.113.005603
Arthur, F. K. N., Woode, E., Terlabi, E. O., and Larbie, C. (2011). Evaluation of acute and subchronic toxicity of Annona muricata (Linn.) aqueous extract in animals. Eur. J. Exp. Biol. 1, 4.
Bancroft, J. D., and Gamble, M. (2008). Theory and practice of histological techniques. Elsevier health sciences.
Barrett, E. S., Workman, T., Hazlehurst, M. F., Kauderer, S., Loftus, C., Kannan, K., et al. (2022). Prenatal polycyclic aromatic hydrocarbon (PAH) exposure in relation to placental corticotropin releasing hormone (pCRH) in the CANDLE pregnancy cohort. Front. Endocrinol. 13, 1011689. doi:10.3389/fendo.2022.1011689
Baskar, R., Rajeswari, V., and Kumar, T. S. (2007). In vitro antioxidant studies in leaves of Annona species. Indian J. Exp. Biol. 45, 480–485.
Bedi, P., and Priyanka, S. (2012). Effects of garlic against 7-12, Dimethyl benzanthracene induced toxicity in Wistar albino rats. Asian J. Pharm. Clin. Res. 5, 170–173.
Beutler, E., Duron, O., and Kelly, B. M. (1963). Improved method for the determination of blood glutathione. J. laboratory Clin. Med. 61, 882–888.
Boom, R., Sol, C. J., Salimans, M. M., Jansen, C. L., Wertheim-van Dillen, P. M., and van der Noordaa, J. (1990). Rapid and simple method for purification of nucleic acids. J. Clin. Microbiol. 28, 495–503. doi:10.1128/JCM.28.3.495-503.1990
Burchiel, S. W., De Ann, P. D., Gomez, M. P., Montano, R. M., Barton, S. L., and Seamer, L. C. (1990). Inhibition of lymphocyte activation in splenic and gut-associated lymphoid tissues following oral exposure of mice to 7, 12-dimethylbenz [a] anthracene. Toxicol. Appl. Pharmacol. 105 (3), 434–442. doi:10.1016/0041-008x(90)90147-m
Chaparro, S. P., Tavera, M. L., Martínez, J. J., and Gil, J. H. (2014). Propiedades funcionales de la harina y de los aislados proteicos de la semilla de guanábana (Annona muricata). Revista UDCA Actual. Divulgación Científica 17 (1), 151–159. doi:10.31910/rudca.v17.n1.2014.950
Chen, Y. P., Zeng, Y., Guan, Y. F., Huang, Y. Q., Liu, Z., Xiang, K., et al. (2022a). Particle size-resolved emission characteristics of complex polycyclic aromatic hydrocarbon (PAH) mixtures from various combustion sources. Environ. Res. 214, 113840. doi:10.1016/j.envres.2022.113840
Chen, Z., Tian, Z., Liu, X., and Sun, W. (2022b). The potential risks and exposure of Qinling giant pandas to polycyclic aromatic hydrocarbon (PAH) pollution. Environ. Pollut. 292, 118294. doi:10.1016/j.envpol.2021.118294
Chescheir, N. C. (2019). Serum uric acid measurement in women with hypertensive disorders of pregnancy. Obstetrics Gynecol. 134, 636–638. doi:10.1097/AOG.0000000000003408
Chromy, V., Rozkosna, K., and Sedlak, P. (2008). Determination of serum creatinine by Jaffe method and how to calibrate to eliminate matrix interference problems. Clin. Chem. laboratory Med. 46, 1127–1133. doi:10.1515/CCLM.2008.224
Cos, P., Hermans, N., Calomme, M., Maes, L., De Bruyne, T., Pieters, L., et al. (2003). Comparative study of eight well-known polyphenolic antioxidants. J. Pharm. Pharmacol. 55 (9), 1291–1297. doi:10.1211/0022357021693
Dakrory, A. I., Al Harbi, M. S., and Mohamed, A. S. (2015b). Antioxidant role of Holothuria atra extract against nephrotoxicity induced by 7, 12-dimethylbenz (a) anthracene in male albino rats. Int. J. Adv. Res. 3 (2), 275–287.
Dakrory, A. I., Fahmy, S. R., Soliman, A. M., Mohamed, A. S., and Amer, S. A. (2015a). Protective and curative effects of the sea cucumber Holothuria atra extract against DMBA-induced hepatorenal diseases in rats. BioMed Res. Int. 2015, 563652. doi:10.1155/2015/563652
Dosumu, O. A., Bababode, A. I., Rotimi, S. O., Akamo, A. J., Omotosho, O. O., Sani, L. O., et al. (2021). DMBA-induced kidney dysfunction: effects of supplementary dietary vitamin K in rats. Trop. J. Nat. Prod. Res. (TJNPR) 5 (5), 917–923. doi:10.26538/tjnpr/v5i5.19
Du, X. H., and Yang, C. L. (1994). Mechanism of gentamicin nephrotoxicity in rats and the protective effect of zinc-induced metallothionein synthesis. Nephrol. Dial. Transplant. 9, 135–140.
Ekaluo, U. B., Uno, U. U., Edu, N. E., Ekpo, P. B., and Etta, S. E. (2016). Effect of Trevo dietary supplement on caffeine induced oxidative stress in albino rat models. Pharm. Chem. J. 3 (2), 92–97.
El-Shahat, A. N., Hamza, R. G., Mounir, A. M., and Al-Seeni, M. N. (2021). Ameliorative effect of graviola fruit juice on the damaged tissues of gamma-irradiated male rats. Pak. J. Zool. 54, 1–8. doi:10.17582/journal.pjz/20201113181133
Fidianingsih, I., Aryandono, T., Widyarini, S., Herwiyanti, S., and Sunarti, S. (2022). Chemopreventive effect of dietary maranta arundinacea L. Against DMBA-induced mammary cancer in sprague dawley rats through the regulation of autophagy expression. Asian Pac. J. cancer Prev. 23, 985–993. doi:10.31557/APJCP.2022.23.3.985
Florence, N. T., Benoit, M. Z., Jonas, K., Alexandra, T., Desire, D. D., Pierre, K., et al. (2014). Antidiabetic and antioxidant effects of Annona muricata (Annonaceae), aqueous extract on streptozotocin-induced diabetic rats. J. Ethnopharmacol. 151, 784–790. doi:10.1016/j.jep.2013.09.021
Foong, C. P., and Hamid, R. A. (2012). Evaluation of anti-inflammatory activities of ethanolic extract of Annona muricata leaves. Rev. Bras. Farm. 22, 1301–1307. doi:10.1590/s0102-695x2012005000096
Gandhi, S., Shariff, S. Z., Al-Jaishi, A., Reiss, J. P., Mamdani, M. M., Hackam, D. G., et al. (2017). Second-generation antidepressants and hyponatremia risk: a population-based cohort study of older adults. Am. J. kidney Dis. 69, 87–96. doi:10.1053/j.ajkd.2016.08.020
Ganeshpurkar, A., and Saluja, A. K. (2017). The pharmacological potential of rutin. Saudi Pharm. J. 25 (2), 149–164. doi:10.1016/j.jsps.2016.04.025
Gavamukulya, Y., Wamunyokoli, F., and El-Shemy, H. A. (2017). Annona muricata: is the natural therapy to most disease conditions including cancer growing in our backyard? A systematic review of its research history and future prospects. Asian Pac. J. Trop. Med. 10 (9), 835–848. doi:10.1016/j.apjtm.2017.08.009
Gill, S. S., and Tuteja, N. (2010). Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant physiology Biochem. 48, 909–930. doi:10.1016/j.plaphy.2010.08.016
Gu, L., House, S. E., Wu, X., Ou, B., and Prior, R. L. (2006). Procyanidin and catechin contents and antioxidant capacity of cocoa and chocolate products. J. Agric. food Chem. 54 (11), 4057–4061. doi:10.1021/jf060360r
Gyamfi, K., Sarfo, D., Nyarko, B., Akaho, E., Serfor-Armah, Y., and Ampomah-Amoako, E. (2011). Assessment of elemental content in the fruit of graviola plant, Annona muricata, from some selected communities in Ghana by instrumental neutron activation analysis. Elixir Food Sci. 41, 5671–5675.
Habig, W. H., Pabst, M. J., and Jakoby, W. B. (1974). Glutathione S-transferases: the first enzymatic step in mercapturic acid formation. J. Biol. Chem. 249 (22), 7130–7139. doi:10.1016/s0021-9258(19)42083-8
Hassan, A., Mohammed, L., Abd ElMoneim, M., and Abd ElBaky, A. (2019). Hepatic and renal protective effects of Annona muricata leaf and fruit extracts on Ehrlich ascites carcinoma in mice. Zagazig Veterinary J. 47 (3), 234–247. doi:10.21608/zvjz.2019.12593.1040
Helal, M. G., and Abd Elhameed, A. G. (2021). Graviola mitigates acetic acid–induced ulcerative colitis in rats: insight on apoptosis and Wnt/Hh signaling crosstalk. Environ. Sci. Pollut. Res. 28, 29615–29628. doi:10.1007/s11356-021-12716-0
Hong, G. U., Choi, M., Chung, M. H., and Ro, J. Y. (2021). Anti-inflammatory effects of graviola stem bark extracts on the testosterone-induced benign prostatic hyperplasia model in rats. Food Suppl. Biomaterials Health 1 (2). doi:10.52361/fsbh.2021.1.e18
Hosny, S., Sahyon, H., Youssef, M., and Negm, A. (2021). Oleanolic acid suppressed dmba-induced liver carcinogenesis through induction of mitochondrial-mediated apoptosis and autophagy. Nutr. cancer 73 (6), 968–982. doi:10.1080/01635581.2020.1776887
Jimenez, V. M., Gruschwitz, M., Schweiggert, R. M., Carle, R., and Esquivel, P. (2014). Identification of phenolic compounds in soursop (Annona muricata) pulp by high-performance liquid chromatography with diode array and electrospray ionization mass spectrometric detection. Food Res. Int. 65, 42–46. doi:10.1016/j.foodres.2014.05.051
Jiménez-Osorio, A. S., Gonzalez-Reyes, S., and Pedraza-Chaverri, J. (2015). Natural Nrf2 activators in diabetes. Clin. Chim. acta 448, 182–192. doi:10.1016/j.cca.2015.07.009
John, E. M., Keegan, T. H., Terry, M. B., Koo, J., Ingles, S. A., Nguyen, J. T., et al. (2022). Urinary biomarkers of polycyclic aromatic hydrocarbons and timing of pubertal development: the California PAH study. Epidemiology 33, 777–787. doi:10.1097/EDE.0000000000001535
Kandeel, M., Abdelaziz, I., Elhabashy, N., Hegazy, H., and Tolba, Y. (2011). Nephrotoxicity and oxidative stress of single large dose or two divided doses of gentamicin in rats. Pak. J. Biol. Sci. PJBS 14 (11), 627–633. doi:10.3923/pjbs.2011.627.633
Kasprzak, K. S. (2023). Oxidative DNA damage in metal-induced carcinogenesis. Toxicol. Metals I, 299–320.
Kesba, H. H., and El-Beltagi, H. S. (2012). Biochemical changes in grape rootstocks resulted from humic acid treatments in relation to nematode infection. Asian Pac. J. Trop. Biomed. 2 (4), 287–293. doi:10.1016/S2221-1691(12)60024-0
Kessler, A., and Siekmann, L. (1999). Measurement of urea in human serum by isotope dilution mass spectrometry: a reference procedure. Clin. Chem. 45, 1523–1529. doi:10.1093/clinchem/45.9.1523
Khafaga, A. F., Elewa, Y. H., Atta, M. S., and Noreldin, A. E. (2021). Aging-related functional and structural changes in renal tissues: lesson from a camel model. Microsc. Microanal. 27 (3), 566–578. doi:10.1017/s1431927621000210
Kleiner, H. E., Vulimiri, S. V., Starost, M. F., Reed, M. J., and DiGiovanni, J. (2002). Oral administration of the citrus coumarin, isopimpinellin, blocks DNA adduct formation and skin tumor initiation by 7, 12-dimethylbenz [a] anthracene in SENCAR mice. Carcinogenesis 23 (10), 1667–1675. doi:10.1093/carcin/23.10.1667
Kossouoh, C., Moudachirou, M., Adjakidje, V., Chalchat, J. C., and Figuérédo, G. (2007). Essential oil chemical composition of Annona muricata L. leaves from Benin. J. Essent. oil Res. JEOR 19 (4), 307–309. doi:10.1080/10412905.2007.9699288
Koul, A., Arora, N., and Tanwar, L. J. N. H. (2010). Lycopene mediated modulation of 7, 12 dimethlybenz (A) anthracene induced hepatic clastogenicity in male Balb/c mice. Nutr. Hosp. 25, 304–310. doi:10.3305/nh.2010.25.2.4607
Krishnamoorthy, D., and Sankaran, M. (2016). Modulatory effect of Pleurotus ostreatus on oxidant/antioxidant status in 7, 12-dimethylbenz (a) anthracene induced mammary carcinoma in experimental rats-A dose-response study. J. cancer Res. Ther. 12, 386–394. doi:10.4103/0973-1482.148691
Kumar, A. H., and Clover, A. J. (2015). Intraperitoneal co-administration of low dose urethane with xylazine and ketamine for extended duration of surgical anesthesia in rats. Laboratory animal Res. 31, 174–179. doi:10.5625/lar.2015.31.4.174
Laksmitawati, D. R., Prasanti, A. P., Larasinta, N., Syauta, G. A., Hilda, R., Ramadaniati, H. U., et al. (2016). Anti-inflammatory potential of Gandarusa (Gendarussa vulgaris Nees) and Soursoup (Annona muricata L) extracts in LPS stimulated-macrophage cell (RAW264.7). J. Nat. Remedies 16, 73–81. doi:10.18311/jnr/2016/5367
Li, R., Jia, Z., and Trush, M. A. (2016). Defining ROS in biology and medicine. React. Oxyg. species (Apex, NC) 1 (1), 9–21. doi:10.20455/ros.2016.803
Livak, K. J., and Schmittgen, T. D. (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25, 402–408. doi:10.1006/meth.2001.1262
Longo, M. C., Berninger, M. S., and Hartley, J. L. (1990). Use of uracil DNA glycosylase to control carry-over contamination in polymerase chain reactions. Gene 93, 125–128. doi:10.1016/0378-1119(90)90145-h
Lu, C. L., Liao, C. H., Wu, W. B., Zheng, C. M., Lu, K. C., and Ma, M. C. (2022). Uremic toxin indoxyl sulfate impairs hydrogen sulfide formation in renal tubular cells. Antioxidants 11, 361. doi:10.3390/antiox11020361
Mathiyazhagan, J., Siva, R., Jayaraj, R., Madhyastha, H., and Kodiveri Muthukaliannan, G. (2022). Preventive effect of combined zingiber officinale and Terminalia chebula against DMBA-induced breast cancer rats via mTOR inhibition. Nutr. cancer 74, 687–696. doi:10.1080/01635581.2021.1903948
Mitsui, Y., Chang, I., Fukuhara, S., Hiraki, M., Arichi, N., Yasumoto, H., et al. (2015). CYP1B1 promotes tumorigenesis via altered expression of CDC20 and DAPK1 genes in renal cell carcinoma. BMC cancer 15, 942. doi:10.1186/s12885-015-1951-0
Mittal, M., Siddiqui, M. R., Tran, K., Reddy, S. P., and Malik, A. B. (2014). Reactive oxygen species in inflammation and tissue injury. Antioxidants redox Signal. 20, 1126–1167. doi:10.1089/ars.2012.5149
Moghadamtousi, S. Z., Fadaeinasab, M., Nikzad, S., Mohan, G., Ali, H. M., and Kadir, H. A. (2015a). Annona muricata (Annonaceae): a review of its traditional uses, isolated acetogenins and biological activities. Int. J. Mol. Sci. 16, 15625–15658. doi:10.3390/ijms160715625
Moghadamtousi, S. Z., Kadir, H. A., Paydar, M., Rouhollahi, E., and Karimian, H. (2014). Annona muricata leaves induced apoptosis in A549 cells through mitochondrial-mediated pathway and involvement of NF-κB. BMC complementary Altern. Med. 14 (1), 299–313. doi:10.1186/1472-6882-14-299
Moghadamtousi, S. Z., Rouhollahi, E., Hajrezaie, M., Karimian, H., Abdulla, M. A., and Kadir, H. A. (2015b). Annona muricata leaves accelerate wound healing in rats via involvement of Hsp70 and antioxidant defence. Int. J. Surg. 18, 110–117. doi:10.1016/j.ijsu.2015.03.026
Mohamed, S. A., Mohamed, A. S., El-Zayat, E., and Shehata, M. R. (2021). Protective and curative mechanisms of echinochrome against 7, 12-Dimethylbenz [a] anthracene-induced renal toxicity in rats. GSC Adv. Res. Rev. 6 (1), 047–055. doi:10.30574/gscarr.2021.6.1.0007
Molnar, R., Szabo, L., Tomesz, A., Deutsch, A., Darago, R., Raposa, B. L., et al. (2022). The chemopreventive effects of polyphenols and coffee, based upon a DMBA mouse model with microRNA and mTOR gene expression biomarkers. Cells 11, 1300. doi:10.3390/cells11081300
Mutakin, M., Fauziati, R., Fadhilah, F. N., Zuhrotun, A., Amalia, R., and Hadisaputri, Y. E. (2022). Pharmacological activities of soursop (Annona muricata Lin.). Molecules 27, 1201. doi:10.3390/molecules27041201
Navarro, J. F., and Mora-Fernández, C. (2006). The role of TNF-alpha in diabetic nephropathy: pathogenic and therapeutic implications. Cytokine & growth factor Rev. 17, 441–450. doi:10.1016/j.cytogfr.2006.09.011
Nawwar, M., Ayoub, N., Hussein, S., Hashim, A., El-Sharawy, R., Wende, K., et al. (2012). A flavonol triglycoside and investigation of the antioxidant and cell stimulating activities of Annona muricata Linn. Archives pharmacal Res. 35, 761–767. doi:10.1007/s12272-012-0501-4
Ohkawa, H., Ohishi, N., and Yagi, K. (1979). Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 95 (2), 351–358. doi:10.1016/0003-2697(79)90738-3
Olas, B. (2023). The antioxidant potential of graviola and its potential medicinal application. Nutrients 15 (2), 402. doi:10.3390/nu15020402
Oliveira, A. P., Sa, I., Pereira, D. M., Goncalves, R. F., Andrade, P. B., and Valentao, P. (2017). Exploratory studies on the in vitro anti-inflammatory potential of two herbal teas (Annona muricata L. And jasminum grandiflorum L.), and relation with their phenolic composition. Chem. Biodivers. 14 (6), e1700002. doi:10.1002/cbdv.201700002
Onouchi, S., Ichii, O., Otsuka, S., Hashimoto, Y., and Kon, Y. (2013). Analysis of duodenojejunal flexure formation in mice: implications for understanding the genetic basis for gastrointestinal morphology in mammals. J. Anat. 223 (4), 385–398. doi:10.1111/joa.12093
Ozturk, F., Ozturk, I. C., Batcioglu, K., and Vardi, N. (2006). The effect of melatonin on 7,12-dimethyl-benz[a]anthracene injury in comparison with vitamin E + selenium in mouse kidneys. Fundam. Clin. Pharmacol. 20, 359–364. doi:10.1111/j.1472-8206.2006.00419.x
Padmini, M. P., and Kumar, J. V. (2012). A histopathological study on gentamycin induced nephrotoxicity in experimental albino rats. IOSR J. Dent. Med. Sci. 1 (1), 14–17. doi:10.9790/0853-0111417
Paliwal, R., Sharma, V., and Pracheta, S. S. (2011). Hepatoprotective and antioxidant potential of Moringa oleifera pods against DMBA-induced hepatocarcinogenesis in male mice. Int. J. Drug Dev. Res. 3 (2), 128–138.
Pélissier, Y., Marion, C., Kone, D., Lamaty, G., Menut, C., and Bessière, J.-M. (1994). Volatile components of Annona muricata L. J. Essent. Oil Res. 6, 411–414. doi:10.1080/10412905.1994.9698410
Rady, I., Bloch, M. B., Chamcheu, R.-C. N., Banang Mbeumi, S., Anwar, M. R., Mohamed, H., et al. (2018). Anticancer properties of graviola (Annona muricata): a comprehensive mechanistic review. Oxidative Med. Cell. Longev. 2018, 1826170. doi:10.1155/2018/1826170
Ren, Y., Wang, Z., and Xue, J. (2022). Gut-derived uremic toxin trimethylamine-N-oxide in cardiovascular disease under end-stage renal disease: an injury mechanism and therapeutic target. Sheng Wu Yi Xue Gong Cheng Xue Za Zhi 39, 848–852. doi:10.7507/1001-5515.202110017
Rupprecht, J. K., Hui, Y.-H., and McLaughlin, J. L. (1990). Annonaceous acetogenins: a review. J. Nat. Prod. 53, 237–278. doi:10.1021/np50068a001
Sabra, H. A., and Ahmed, N. M. (2018). Reno-protective effect of graviola (Annona muricata) leaves against lead acetate toxicity on experimental albino rats. Biochem. Lett. 14 (1), 1–13. doi:10.21608/blj.2018.47203
Sakakibara, H., Honda, Y., Nakagawa, S., Ashida, H., and Kanazawa, K. (2003). Simultaneous determination of all polyphenols in vegetables, fruits, and teas. J. Agric. food Chem. 51 (3), 571–581. doi:10.1021/jf020926l
Saleem, U., Ejaz-ul-Haq, M., Chudary, Z., and Ahmad, B. (2017). Pharmacological screening of Annona muricata: a review. Asian J Agri Biol. 5 (1), 38–46.
Samarghandian, S., Borji, A., Farahmand, S. K., Afshari, R., and Davoodi, S. (2013). Crocus sativus L.(saffron) stigma aqueous extract induces apoptosis in alveolar human lung cancer cells through caspase-dependent pathways activation. BioMed Res. Int. 2013, 417928. doi:10.1155/2013/417928
Sanchez, D. S., Fischer Sigel, L. K., Balestracci, A., Ibarra, C., Amaral, M. M., and Silberstein, C. (2022). Eliglustat prevents Shiga toxin 2 cytotoxic effects in human renal tubular epithelial cells. Pediatr. Res. 91, 1121–1129. doi:10.1038/s41390-021-01622-3
Sarbishegi, M., Khajavi, O., and Arab, M. R. (2016). Withania coagulans extract induces cell apoptosis and inhibits COX-2 expression in a rat model of benign prostatic hyperplasia. Nephro-Urology Mon. 8 (5), e39284. doi:10.5812/numonthly.39284
Serafini, M., Peluso, I., and Raguzzini, A. (2010). Flavonoids as anti-inflammatory agents. Proc. Nutr. Soc. 69 (3), 273–278. doi:10.1017/S002966511000162X
Sharma, V., and Paliwal, R. (2012). Chemo protective role of Moringa oleifera and its isolated saponin against DMBA induced tissue damage in male mice: a histopathological analysis. Int. J. Drug Dev. Res. 4 (4), 215–228.
Sharma, V., and Paliwal, R. (2013). Isolation and characterization of saponins from Moringa oleifera (moringaceae) pods. Int. J. Pharm. Pharm. Sci. 5, 179–183.
Sharma, V., and Paliwal, R. (2014). Potential chemoprevention of 7, 12-dimethylbenz [a] anthracene induced renal carcinogenesis by Moringa oleifera pods and its isolated saponin. Indian J. Clin. Biochem. 29, 202–209. doi:10.1007/s12291-013-0335-y
Sharma, V., Paliwal, R., Janmeda, P., and Sharma, S. (2012). Chemopreventive efficacy of Moringa oleifera pods against 7, 12-dimethylbenz [a] anthracene induced hepatic carcinogenesis in mice. Asian Pac. J. Cancer Prev. 13 (6), 2563–2569. doi:10.7314/apjcp.2012.13.6.2563
Shukry, M., El-Shehawi, A. M., El-Kholy, W. M., Elsisy, R. A., Hamoda, H. S., Tohamy, H. G., et al. (2020). Ameliorative effect of graviola (Annona muricata) on mono sodium glutamate-induced hepatic injury in rats: antioxidant, apoptotic, anti-inflammatory, lipogenesis markers, and histopathological studies. Animals 10 (11), 1996. doi:10.3390/ani10111996
Sonoda, Y., Gohda, T., Suzuki, Y., Omote, K., Ishizaka, M., Matsuoka, J., et al. (2015). Circulating TNF receptors 1 and 2 are associated with the severity of renal interstitial fibrosis in IgA nephropathy. PloS one 10, e0122212. doi:10.1371/journal.pone.0122212
Spitz, D. R., Azzam, E. I., Jian Li, J., and Gius, D. (2004). Metabolic oxidation/reduction reactions and cellular responses to ionizing radiation: a unifying concept in stress response biology. Cancer Metastasis Rev. 23, 311–322. doi:10.1023/B:CANC.0000031769.14728.bc
Sullivan, L. K., Livingston, E. W., Lau, A. G., Rao-Dayton, S., and Bateman, T. A. (2020). A mouse model for skeletal structure and function changes caused by radiation therapy and estrogen deficiency. Calcif. tissue Int. 106, 180–193. doi:10.1007/s00223-019-00617-x
Tawfik, M. F., Oda, S. S., and Khafaga, A. F. (2021). Pathological and immunohistochemical microscopy of natural cases of canine and feline neoplastic mammary lesions. Microsc. Microanal. 27 (4), 910–922. doi:10.1017/S143192762101196X
Varghese, F., Bukhari, A. B., Malhotra, R., and De, A. (2014). IHC Profiler: an open source plugin for the quantitative evaluation and automated scoring of immunohistochemistry images of human tissue samples. PloS one 9 (5), e96801. doi:10.1371/journal.pone.0096801
Vellosa, J. C. R., Regasini, L. O., Khalil, N. M., Bolzani, V. D. S., Khalil, O. A., Manente, F. A., et al. (2011). Antioxidant and cytotoxic studies for kaempferol, quercetin and isoquercitrin. Eclética quimica 36, 07–20. doi:10.1590/s0100-46702011000200001
Wang, C. C., Chang, C. H., Chang, S. C., Fan, G. J., Lin, M. J., Yu, B., et al. (2016). In vitro free radicals scavenging activity and antioxidant capacity of solid-state fermented wheat bran and its potential modulation of antioxidative molecular targets in chicken PBMC. Rev. Bras. Zootec. 45, 451–457. doi:10.1590/s1806-92902016000800005
Wiame, I., Remy, S., Swennen, R., and Sagi, L. (2000). Irreversible heat inactivation of DNase I without RNA degradation. BioTechniques 29, 252–254. doi:10.2144/00292bm11
Wu, M., Xu, H., Shen, Y., Qiu, W., and Yang, M. (2011). Oxidative stress in zebrafish embryos induced by short-term exposure to bisphenol A, nonylphenol, and their mixture. Environ. Toxicol. Chem. 30, 2335–2341. doi:10.1002/etc.634
Yang, C., Gundala, S. R., Mukkavilli, R., Vangala, S., Reid, M. D., and Aneja, R. (2015). Synergistic interactions among flavonoids and acetogenins in Graviola (Annona muricata) leaves confer protection against prostate cancer. Carcinogenesis 36, 656–665. doi:10.1093/carcin/bgv046
Yen-Nien, H., Deng, G., and Mao, J. J. (2019). Practical application of “ABOUT HERBS” website: herbs and dietary supplement use in oncology settings. Cancer J. (Sudbury, Mass.) 25, 357–366. doi:10.1097/PPO.0000000000000403
Keywords: graviola, nephrotoxicity, MDA, DMBA, TNF-α, IL-1β, CYP2E1, antioxidant enzymes
Citation: Zeweil MM, Khafaga AF, Mahmoud SF, Wasef L, Saleh H, Elrehim AMA, Bassuoni NF, Alwaili MA, Saeedi NH and Ghoneim HA (2024) Annona Muricata L. extract restores renal function, oxidative stress, immunohistochemical structure, and gene expression of TNF-α, IL-β1, and CYP2E1 in the kidney of DMBA-intoxicated rats. Front. Pharmacol. 15:1348145. doi: 10.3389/fphar.2024.1348145
Received: 01 December 2023; Accepted: 16 January 2024;
Published: 01 February 2024.
Edited by:
Ahmed Esmat Abdel Moneim, Helwan University, EgyptReviewed by:
Taghred M. Saber, Zagazig University, EgyptHoda Ibrahim Bahr, Suez Canal University, Egypt
Amriamamdouh Mousa, National Research Centre, Egypt
Ravindra M. Samartha, Bhopal Memorial Hospital and Research Centre, India
Copyright © 2024 Zeweil, Khafaga, Mahmoud, Wasef, Saleh, Elrehim, Bassuoni, Alwaili, Saeedi and Ghoneim. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mohamed M. Zeweil, bW9oYW1lZC56ZXdlaWxAdmV0bWVkLmRtdS5lZHUuZWc=
 Mohamed M. Zeweil
Mohamed M. Zeweil Asmaa F. Khafaga
Asmaa F. Khafaga Sahar F. Mahmoud3
Sahar F. Mahmoud3 Nizar H. Saeedi
Nizar H. Saeedi Hanan A. Ghoneim
Hanan A. Ghoneim