
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pharmacol. , 07 March 2024
Sec. Predictive Toxicology
Volume 15 - 2024 | https://doi.org/10.3389/fphar.2024.1345992
 Johanna Ebmeyer1*†
Johanna Ebmeyer1*† Abdulkarim Najjar1*†
Abdulkarim Najjar1*† Daniela Lange1*†
Daniela Lange1*† Mareike Boettcher1
Mareike Boettcher1 Silja Voß1
Silja Voß1 Katrin Brandmair1
Katrin Brandmair1 Jaqueline Meinhardt1
Jaqueline Meinhardt1 Jochen Kuehnl1
Jochen Kuehnl1 Nicola J. Hewitt2
Nicola J. Hewitt2 Christopher-Tilman Krueger1
Christopher-Tilman Krueger1 Andreas Schepky1
Andreas Schepky1We performed an ab initio next-generation risk assessment (NGRA) for a fragrance ingredient, benzyl salicylate (BSal), to demonstrate how cosmetic ingredients can be evaluated for systemic toxicity endpoints based on non-animal approaches. New approach methodologies (NAMs) used to predict the internal exposure included skin absorption assays, hepatocyte metabolism, and physiologically based pharmacokinetic (PBPK) modeling, and potential toxicodynamic effects were assessed using pharmacology profiling, ToxProfiler cell stress assay, transcriptomics in HepG2 and MCF-7 cells, ReproTracker developmental and reproductive toxicology (DART) assays, and cytotoxicity assays in human kidney cells. The outcome of the NGRA was compared to that of the traditional risk assessment approach based on animal data. The identification of the toxicologically critical entity was a critical step that directed the workflow and the selection of chemicals for PBPK modeling and testing in bioassays. The traditional risk assessment and NGRA identified salicylic acid (SA) as the “toxdriver.” A deterministic PBPK model for a single-day application of 1.54 g face cream containing 0.5% BSal estimated the Cmax for BSal (1 nM) to be much lower than that of its major in vitro metabolite, SA (93.2 nM). Therefore, SA was tested using toxicodynamics bioassays. The lowest points of departure (PoDs) were obtained from the toxicogenomics assays. The interpretation of these results by two companies and methods were similar (SA only results in significant gene deregulation in HepG2 cells), but PoD differed (213 μM and 10.6 µM). A probabilistic PBPK model for repeated applications of the face cream estimated the highest Cmax of SA to be 630 nM. The resulting margins of internal exposure (MoIE) using the PoDs were 338 and 16, which were more conservative than those derived from external exposure and in vivo PoDs (margin of safety values were 9,705). In conclusion, both traditional and ab initio NGRA approaches concluded that the daily application of BSal in a cosmetic leave-on face cream at 0.5% is safe for humans. The processing and interpretation of toxicogenomics data can lead to different PoDs, which can subsequently affect the calculation of the MoIE. This case study supports the use of NAMs in a tiered NGRA ab initio approach.
All cosmetic ingredients undergo a safety assessment process to ensure consumer safety. Since the seventh revision (EU, 2003) of the European Directive 76/768/EEC (EU, 2009) was published 20 years ago banning the use of animal studies due to ethical reasons, the cosmetics industry has been a major contributor for the development of new approach methodologies (NAMs). Therefore, next-generation risk assessments (NGRAs) of cosmetic ingredients are based on the strategic use of non-animal methods only, i.e., in vitro, in silico, and in chemico data, in an exposure-led and hypothesis-driven approach to deliver human-relevant safety decisions (Najjar et al., 2023). The acceptance of this requires the demonstration of its consumer-protective application in the form of hypothetical case studies using NGRA principles (Dent et al., 2018). To address this, Cosmetics Europe´s Long Range Science Strategy (LRSS) program included case studies for the NAM-based NGRA to evaluate their practical application for cosmetic ingredients. This approach has been successfully demonstrated in read-across case studies for caffeine (Bury et al., 2021), parabens (Ouedraogo et al., 2022), and phenoxyethanol (OECD, 2021), which have been published in the OECD IATA Toolbox (OECD, 2019a; OECD, 2019b; OECD, 2020). These case studies were based on the SEURAT-1 safety assessment workflow described by Berggren et al. (2017) and Desprez et al. (2018), and according to the International Cooperation on Cosmetics Regulation (ICCR) (Dent et al., 2018).
The aim of the current study was to use an NAM-only workflow in an ab initio case study as a proof-of-concept to evaluate the readiness of these methods for assessing the systemic safety of the cosmetic ingredients without the use of read-across and exposure-based waiving. Genotoxicity and local effects, e.g., skin irritation and sensitization, were not considered in this assessment since validated NAMs are already available for these endpoints and they were considered by the Scientific Committee for Consumer Safety (SCCS) to not be of concern for the safety assessment for the ingredient evaluated here (SCCS, 2023a; SCCS, 2023c). The cosmetic ingredient used for this case study was benzyl salicylate (BSal), a natural product found in Desmos chinensis, Nicotiana cavicola, and other organisms (PubChem database). BSal is used in cosmetics as a fragrance ingredient and light stabilizer, with a maximum concentration of 0.5% as a light stabilizer and 0.15% in other leave-on cosmetic products (CIR, 2018). The structure of BSal is shown in Figure 1. This case study set aims to test the current ability to make a safety decision for a cosmetic ingredient without using any in vivo data. The safety of this ingredient has been evaluated using traditional risk assessment methods (CIR, 2018; SCCS, 2023a); therefore, in vivo legacy information from sources such as the CIR review and SCCS opinion was excluded from the NAM-only ab initio workflow and only used to compare the outcome and make a final assessment of the protective value of the NGRA. The selection of a well-established cosmetic ingredient as the subject of the case study enabled the comparison of the NGRA-based safety decision to the traditional (in vivo-based) safety assessment outcome.
The overall goal of the case study was to use and evaluate the suitability of NAMs to protect the consumer using a risk-based approach rather than predicting the adverse effects that may be expressed at irrelevant exposure levels to the ingredient. The strategy applied, therefore, involved two main activities. First, collecting or generating a broad suite of bioactivity data in a variety of human-derived ligands and cell lines. Second, employing physiologically based pharmacokinetic (PBPK) modeling to determine whether bioactivity occurs at consumer-relevant concentrations (WHO, 2010). The margins of internal exposure (MoIE) were estimated for BSal using a model to predict blood concentrations following BSal exposure in humans and comparing the results with concentrations that do elicit cellular responses in vitro. A similar ab initio approach was used by Baltazar et al. (2020) using coumarin as a case study chemical. The MoIE differs from a traditional margin of exposure or margin of safety (MoE/MoS), in that it is calculated as the ratio of a measure of internal exposure, such as blood or target-tissue concentration, rather than comparing external exposure doses (Bessems et al., 2017). The use of a model increases confidence in the risk assessment by incorporating chemical-specific information on the uptake, distribution, metabolism, and excretion of the chemical (Clewell et al., 2008). The overall strategy used was aligned with the United States Environmental Protection Agency’s (US EPA) next-generation blueprint for computational toxicology, which seeks to characterize whether a chemical acts via defined biological pathways/targets or if it may induce cellular changes by a non-specific mechanism (Thomas et al., 2019). Ultimately, the aim of the ab initio case study was to support the concept that this approach is “Protection not Prediction” (Thomas et al., 2019), whereby NAMs are used in this case study not to predict a specific toxicity endpoint but to ensure a safe level of exposure which does not result in biologically relevant adverse effects.
The safety assessment was conducted in a tiered fashion according to the workflow described by Berggren et al. (2017) and by Desprez et al. (2018). An overview of the in silico and in vitro methods used in the tiered workflow is shown in Figure 2, and details of the protocols are described in Supplementary Material S2. The key physicochemical properties of BSal and its metabolites that could affect the bioavailability of BSal are listed in Supplementary Table S1.

FIGURE 2. Overview of the in silico and in vitro methods used in the tiered workflow for the ab initio NGRA for BSal.
The use scenario selected for the ab initio case study was a twice daily topical application of a face cream (a total of 1.54 g/day) containing 0.5% BSal in a human adult population. The calculated relative daily exposure of a face cream is estimated to be 25.6 mg/kg bw/day [based on the application of 1.54 g/day to a 60-kg (default value) adult human (SCCS, 2023c)]. The application of this amount to face skin at 0.5% equates to 128 μg/kg bw/day, which is above the Thresholds of Toxicological Concern (TTC) for non-genotoxic chemicals. The TTC values for systemic toxicity supported by the SCCS for non-genotoxic Cramer Classes I and III compounds are 46 and 2.3 μg/kg bw/day, respectively. The workflow and inclusion criteria for the TTC are described in Supplementary Material S2.2. BSal is not genotoxic, based on in vitro assays (Ames test, HPRT test, chromosome aberration in vitro, and the in vitro micronucleus test) as well as in silico predictions (Supplementary Table S2); therefore, the lower TTC of 0.0025 μg/kg bw/day for genotoxic chemicals does not apply. The value of 128 μg/kg bw/day for BSal is, however, greater than the TTC value for systemic toxicity for non-genotoxic Cramer Classes III (2.3 μg/kg bw/day). In conclusion, the calculation of the daily exposure indicated that the TTC could not be applied, and more investigations are required.
In studies using fresh pig skin (see Supplementary Material S2.3 for the method), 24 h after the application of a formulation containing 0.5% BSal, there were several analytes identified in the epidermis, dermis, and receptor fluid. These included the parent compound, BSal, as well as salicylic acid (SA), benzoic acid (BA), benzyl alcohol (BAlc), and hippuric acid (Table 1), as well as traces of Phase II conjugates of BSal. After 24 h, the majority of the BSal was recovered during the skin wash as the unchanged parent compound and very little of it penetrated the epidermis or dermis as the parent compound (dermal penetration was 2.2% as BSal only). Of note, only two analytes reached the receptor fluid, namely, SA and BAlc, with the levels of the parent chemical below the LOQ. This indicates that the extensive first-pass metabolism of BSal occurs in the skin. BSal was present in the epidermis and dermis; therefore, both BSal and its metabolites can be assumed to enter the systemic circulation after topical application.
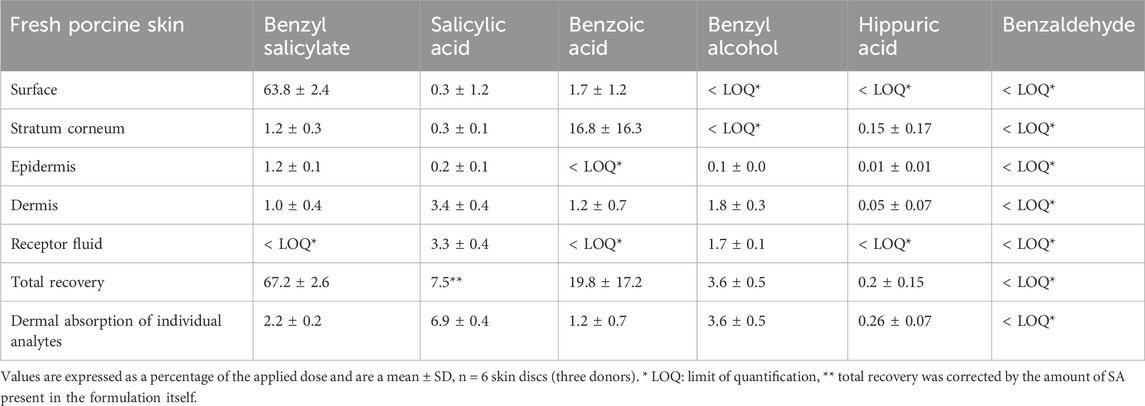
TABLE 1. Absorption and metabolism of BSal 24 h after the application of 0.5% BSal in formulation to the viable pig skin.
The total dermal penetration of BSal and SA was 9.1% of the applied dose. The mass balance was 74.7% when BSal and SA were included and increased to 90.8% with integration of additional metabolites of BSal. This result is in line with the OECD 428 test guideline, “Skin Absorption: in vitro Method.” Additionally, the measured dermal penetration and recovery data for BSal are in accordance with data derived from studies on frozen human skin using radioactive BSal [9.01% of the applied dose (SCCS, 2023a)].
Meteor and GLORYx (Supplementary Material S2.4) were used to predict the potential metabolites formed from BSal (Figure 3). The software predicted multiple metabolites via different pathways, which included the following: (1) hydrolysis of BSal to SA and BAlc, with further metabolism of BAlc to benzaldehyde, BA and, finally, hippuric acid, plus further Phase II conjugation or hydroxylation of SA; (2) Phase II conjugation of BSal; and (3) hydroxylation of BSal. The latter was assessed to be of minor relevance since hydrolysis (i.e., pathway A) takes place very rapidly and thus is much more likely. The Extended Clearance Classification System (ECCS) was implemented to predict the predominant clearance mechanism based on the physicochemical properties and passive membrane permeability of the parent and the metabolites (Varma et al., 2015). The ECCS predicts BSal and BAlc as class 2 [metabolism as the primary clearance mechanism (high-permeability neutrals)] and SA and BA as class 1A [metabolism as the primary systemic clearance mechanism (high-permeability acids with molecular weight (MW) ≤400 Da).
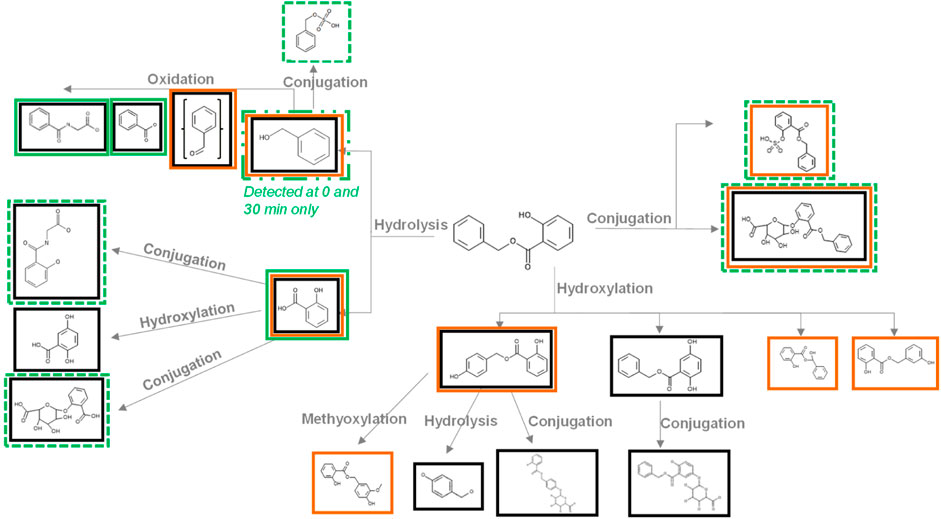
FIGURE 3. Metabolites predicted by Meteor and GLORYx and detected during the incubations of BSal with PHH. Metabolites in black boxes were predicted by Meteor, metabolites in orange boxes were predicted by GLORYx, and metabolites in green boxes were detected in incubations with PHH (a dashed green line denotes metabolites identified by accurate mass only and are probably only present in trace amounts).
The metabolism of BSal by primary human hepatocytes (PHHs) was very rapid, with only 10% of the initial concentration remaining after 30 min (Figure 4, see Supplementary Material S2.5 for the method). Despite the rapid metabolism, 0.1%–0.2% of the initial concentration was detected in the PHH after 90, 120, and 240 min. The half-life of BSal in the PHH was 9.2 min, with an in vitro intrinsic clearance (CLint, in vitro) of 94.2 μL/min/million cells. Based on the in vitro results, the estimated half-life of SA was >950 min (∼16 h), with a CLint, in vitro of ∼0.9 μL/min/million cells.
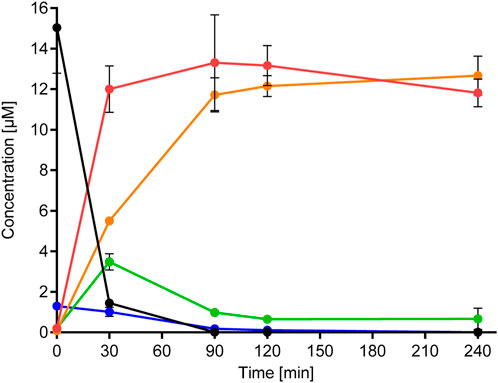
FIGURE 4. Metabolism of BSal and formation of metabolites in PHH. Values are indicated as mean ± SD of triplicate samples. Black symbols/lines denote BSal, red symbols/lines denote SA, orange symbols/lines denote hippuric acid, green symbols/lines denote BA, and blue symbols/lines denote BAlc.
Figure 3 shows the results of the prediction models Meteor and GLORYx and compares these with those of the metabolites detected in hepatic incubations with BSal. In vitro results correlated with the predicted hydrolysis pathway, with the exception that SA metabolites were not observed in vitro. The main pathway in the PHH was hydrolysis to SA and benzylic metabolites (BA, BAlc, and hippuric acid, shown in Figure 4), as well as trace amounts of Phase II conjugates. SA was the main metabolite, the formation of which mirrored the depletion of the parent compound (Figure 4). BAlc was detected in the first timepoint, following which it decreased and was not detected after 240 min. This is in accordance with the results from additional incubations of BAlc with the PHH, which indicate its rapid metabolism within 30 min. BA was formed from BSal, with a peak concentration at 30 min, but BA was quickly further metabolized to hippuric acid (the final detoxified metabolite which is expected to be excreted). Notably, SA was not metabolized further under these conditions, which was also confirmed in separate incubations of SA with the PHH and liver S9 assay (see Supplementary Material S2.6 for the liver S9 method and Supplementary Material S2.7 for the analytical methods and calculations).
The conclusions from the in vitro hepatic incubations are that (a) BSal that reaches the systemic circulation is rapidly metabolized to SA and benzylic metabolites; (b) benzylic metabolites do not reach high concentrations and are rapidly further metabolized to hippuric acid, which is regarded as an excretion metabolite; (c) SA is stable under these conditions and is, thus, a relevant metabolite; (d) there was a good comparison between the predicted metabolites using in silico models (Figure 3) and the major metabolites formed in in vitro incubations with the PHH (Figure 4), providing confidence that relevant metabolites were accounted for in the safety assessment.
Since SA is a stable main metabolite, its excretion was investigated using different uptake and efflux transporter studies (see Supplementary Material S2.8 for the method). These assays revealed that SA was not a substrate of the human ABC (efflux) transporters: MDR1, MRP2, and MRP4 or the human SLC (uptake) transporters: OAT1 and OAT2v1. SA may be a borderline substrate of the tOAT3 SLC transporter since, although the fold accumulation was above the 2-fold cut-off at 10 µM and 2 min, the fold accumulation was below the 2-fold cut-off at the rest of the test conditions (data not shown).
Incubations with fresh primary human proximal tubule cells (hPTCs) indicated that there was an increase in the movement of SA across the monolayers over time with increasing concentrations (1–100 µM), suggesting transcellular transport (Supplementary Figure S1A). However, SA did not appear to move predominantly in either direction or preferentially accumulate in the cells, and the concentration had no impact on the direction of the flux (Supplementary Figure S1D). Coincubation with probenecid, an inhibitor of OATs and MRP2, tended to increase permeation in the absorptive direction (A–B). This effect was marginal but statistically significant and consistent at 10 and 100 µM and may in part be due to inhibition of OATs at the basolateral membrane of the hPTCs. This effect was transient at the lower concentration but was sustained at a higher concentration, with no net movement at 10 µM after 120 min with probenecid and sustained absorptive movement at 100 μM, suggesting that this is a dose-dependent and transporter “drug–drug interaction.” The intracellular concentration was not influenced by inhibition of the transporters.
In a deterministic PBPK model for the human adult and the physicochemical properties listed in Supplementary Table S1, an approximate estimation of the plasma concentrations of the most relevant parent/metabolites after a single-day application of 1.54 g/day face cream containing 0.5% BSal to an adult human was made. This resulted in total Cmax values of 1 nM for BSal, 93.2 nM for SA, 0.5 nM for BAlc, and 4.6 nM for BA (Figure 5A). These values were used to conclude that (a) BSal should not be tested further since the exposure to this was negligible compared to that of SA and (b) that SA should be tested in bioassays and the concentration of 93.2 nM expected in vivo should be included in the testing to ensure that relevant concentrations were used. The total Cmax for SA was ∼90-fold higher than that of BSal; in addition, the fraction unbound in the plasma (Fup) of BSal and SA is 0.66% and 2.9%–18%, respectively. This results in higher exposure of unbound SA compared to unbound BSal by at least ∼5-fold. Therefore, SA was considered to be the most relevant metabolite of BSal from a systemic exposure point of view. Hence, SA was selected for testing in toxicodynamics bioassays.
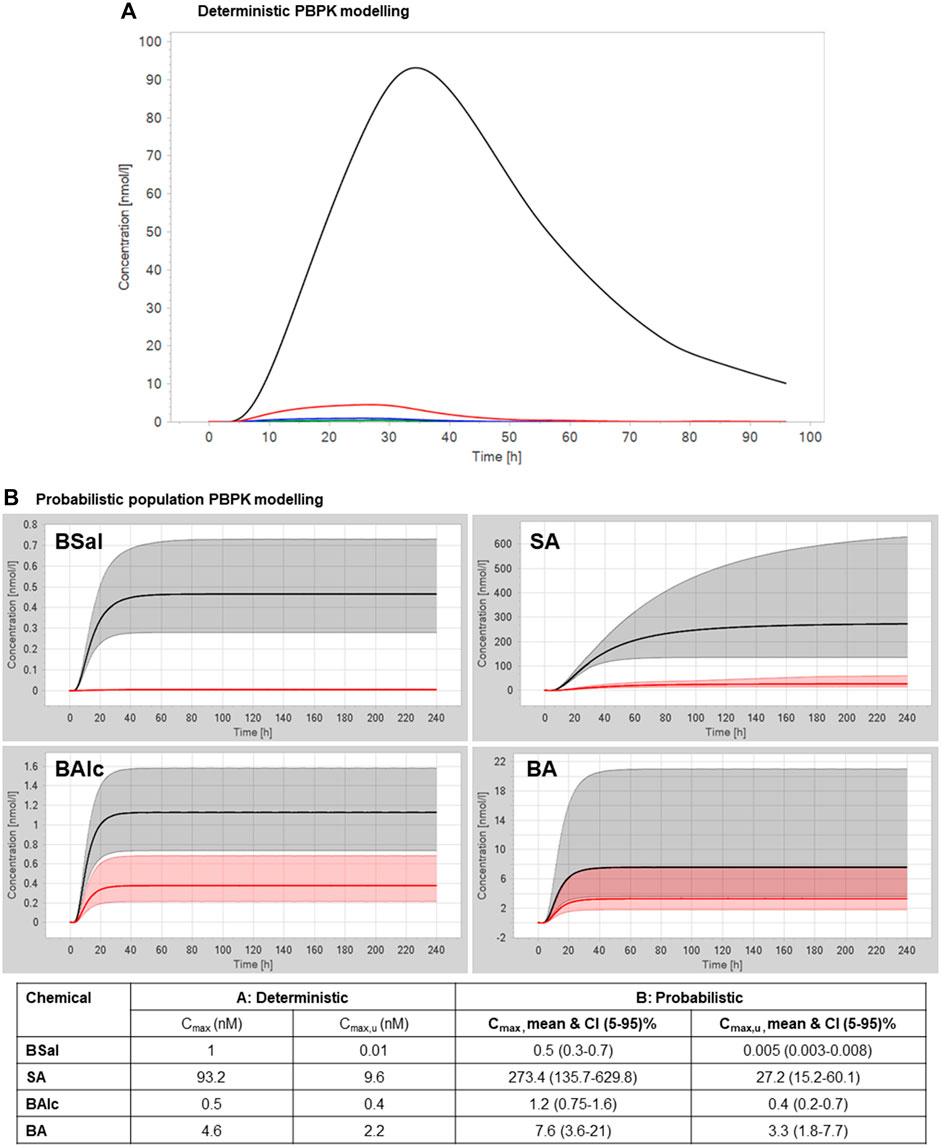
FIGURE 5. (A) Deterministic PBPK modeling for plasma concentrations of BSal and its metabolites after a single-day application of 1.54 g face cream containing 0.5% BSal. The green line represents BSal, the black line represents SA, the blue line represents BAlc, and the red line represents BA. (B) Probabilistic PBPK modeling for plasma concentrations of BSal and its metabolites after repeated application of 1.54 g/day face cream containing 0.5% BSal. The black line represents the total geometric mean, the red line represents the unbound geometric mean, the gray shaded areas represent the 5%–95% total plasma concentration range, and the red shaded areas represent the 5%–95% unbound plasma concentration range.
BSal is metabolized in the skin to SA, BAlc, BA, and hippuric acid. However, some BSal reaches the systemic circulation. Systemically bioavailable BSal is rapidly metabolized in the liver to SA and benzylic metabolites. Benzylic metabolites do not reach high concentrations and are rapidly further metabolized to hippuric acid, which is regarded as an excretion metabolite. The deterministic PBPK model resulted in SA concentrations approx. 90 times higher than those of BSal. Thus, SA is considered the most relevant metabolite for safety assessment. Therefore, toxicodynamics assays were performed with SA.
BSal and its metabolites detected in incubations with the PHH were evaluated using in silico and in vitro tools to identify potential toxic effects and the mode of action (MoA). In silico alerts [using Derek Nexus, QSAR toolbox, Case Ultra, and ADMET predictor (see Supplementary Material S2.9 for information on the model versions)] and in in vitro test results for BSal and its metabolites, as well as estrogen-, androgen-, and thyroid signaling and steroidogenesis (EATS) assay results from the ToxCast database, are summarized in Supplementary Tables S2 and S3, respectively. Both in silico alerts and in vitro tests indicated that BSal was non-genotoxic but that it has the potential to interact with the endocrine system via the estrogen pathway [it was positive in several in vitro ToxCast assays for the estrogen receptor (ER), androgen receptor (AR), and steroidogenesis but not for the thyroid pathway]. However, BSal was positive in 9 out of 18 ER assays and was positive in 1 out of 14 AR assays, where the lowest reported AC50 was 36.5 µM in a binding assay for ERα (assay name: OT_ER_ERaERa_0480). Based on in silico alerts only, BSal and its metabolite, SA, were indicated to cause reproductive and developmental toxicity. SA, however, was indicated to be non-genotoxic and not an endocrine disruptor based on in silico alerts and in vitro tests. Additional in silico alerts indicated that SA causes nephrotoxicity and hepatotoxicity. The potential for nephrotoxicity was followed up in a targeted test (see Section 3.3.2, targeted testing: nephrotoxicity). Hippuric acid was negative in all 259 ToxCast assays. The potential for hepatoxicity was addressed by the cell stress panel, which uses HepG2 cells, a liver tissue-derived cancer cell line (Section 3.2.8) and by toxicogenomics testing in HepG2 cells (Section 3.2.10). The developmental and reproductive toxicology (DART) alert was followed up by performing the ReproTracker assay (Section 3.3.1).
SA was considered to be the most important molecule to investigate based on the in silico alerts for toxicity and on its predominant exposure levels compared to BSal and its other metabolites.
Saturation of cellular stress pathways can result in a host of adverse health effects including embryotoxicity, neurotoxicity, and hepatotoxicity (Simmons et al., 2009). SA was tested over a concentration range of 10 μM to 10 mM in the ToxProfiler cell stress assay (Supplementary Material S2.10). The ToxProfiler assay is based on a panel of seven HepG2 reporter cell lines, analyzing chemically induced activation of seven distinct stress pathways by GFP-tagged biomarkers of these pathways. The following pathways were studied using the respective biomarkers: oxidative stress (SRXN1-GFP), genetic stress (p21-GFP), ER stress (CHOP-GFP), autophagy (LC3-GFP), ion stress (MT1X-GFP), protein stress (HSPA1B-GFP), and inflammation (ICAM1-GFP).
SA was not cytotoxic up to the highest tested concentration and activated only ICAM1, a stress pathway reporter linked to inflammation (Supplementary Figure S2). The observed response, although significant, was marginal (a ratio of <0.1 when normalized to GFP responses) compared to the responses to the assay’s reference compounds. Based on these observations, the PoD for SA was derived to be 4,000 μM; however, there was only a slight increase followed by a decrease in the signal at the next higher incubation dosage; therefore, the effect may not be biologically relevant. Furthermore, these results indicate that the in silico alert for hepatotoxicity (Section 3.2.7) was not supported by in vitro data.
SA was subjected to a panel of 83 target ligand-binding assays (see Supplementary Material S2.11 for details). The panel based on the safety pharmacological profiling panel as published by Bowes et al., used 44 targets associated with in vivo adverse drug reactions pertaining to the central nervous system, cardiovascular, pulmonary, and gastrointestinal toxicities (Bowes et al., 2012). The set of targets was extended to broaden the biological coverage. The experiments used an SA concentration of 10 µM and encompassed two technical replicates. Results showing an inhibition or stimulation higher than 50% were considered to represent the effects of the test compound, and responses between 25% and 50% indicated some but less certain effects, both results requiring follow-up testing in this NGRA-based, ab initio case study. Acetylcholinesterase is the most sensitive target, albeit the inhibition was only 25.4%. In a follow-up study, concentrations of 10 μM to 1 mM SA inhibited acetylcholinesterase by even less (only up to 5%). A literature search indicated that SA is a very weak COX inhibitor (Patrignani and Patrono, 2015); therefore, despite not being a hit in the screening assay (10 µM did not inhibit COX1 or COX2), concentrations up to 10 mM were tested for their ability to inhibit COX1 and COX2. This assay showed that SA did not inhibit COX1 up to 10 mM and inhibited COX2 by 29% at 10 mM. It was not possible to calculate an IC50 for COX2 (which was set at > 10 mM).
HepG2 and MCF-7 cells were treated with SA up to 1 mM with 0.1% DMSO used as a vehicle control. As observed in the cell stress assay using HepG2 reporter cell lines (Section 3.2.8), SA was not cytotoxic up to the highest tested concentration in the HepG2 and MCF-7 cells used in this assay. Whole-genome transcriptomics dose response analysis was performed to derive a pathway-based no-observed-transcriptional-effect level (NOTEL, referred to as PoD) (according to (Lobenhofer et al., 2004)) for SA in HepG2 and MCF-7 cells (see Supplementary Material S2.12 for details). The raw data were evaluated by two analysis methods with slightly different statistical approaches. In both cases, 1.5-fold deregulation was used as a cutoff for identifying the number of up- and downregulated differentially expressed genes (DEGs). Analysis Method 1 considered Benjamini–Hochberg-corrected p-values as the false discovery rate (FDR) with a cutoff of FDR <0.05. Analysis Method 2 identified 1.5-fold deregulated genes with a global permutation-based p-value ≤0.005. For more details of the analysis methods, see Supplementary Material S2.12. Figure 6 shows the comparison of the number of statistically significant DEGs according to the above criteria in HepG2 and MCF-7 cells after 6- and 24-h treatment and analyzed using both methods. Cells were treated with SA or the positive control, trichostatin A. Trichostatin A is an antibiotic antifungal known to impact gene expression by inhibiting mammalian histone deacetylases (Vanhaecke et al., 2004). Thus, strong gene expression changes were expected.
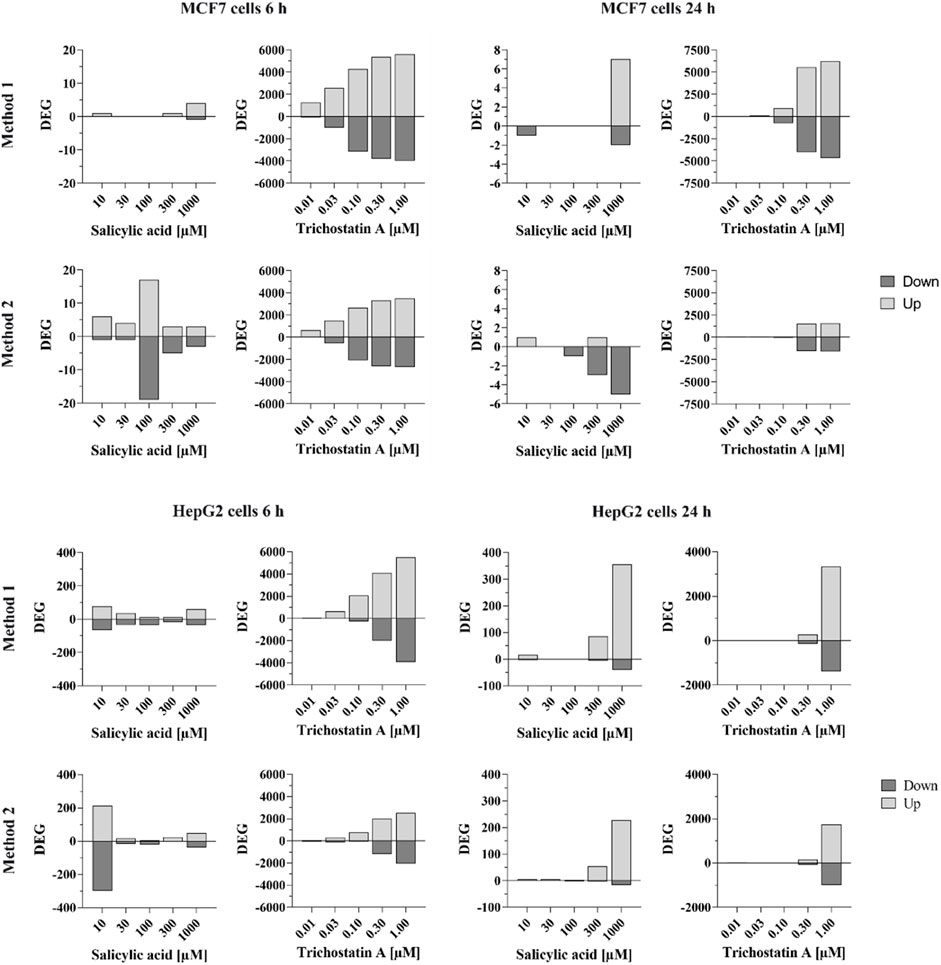
FIGURE 6. Number of significantly differentially expressed genes in MCF-7 and HepG2 cells treated with SA or trichostatin A for 6 or 24 h according to analysis method 1 (top) or analysis method 2 (bottom). Differentially expressed genes (up- or down-regulated) are shown with absolute fold-change ≥ ±1.5 and Benjamini–Hochberg-corrected p-value (false discovery rate, FDR) FDR <0.05 (analysis method 1) or Global Permutation-based p-value ≤0.005 (analysis method 2).
Trichostatin A resulted in clear concentration-dependent increases in the number of DEGs at both timepoints in both cell lines. The responses were similar using both analysis methods, although more genes were affected, with a gradual increase across the concentrations, when the data were analyzed with Method 1.
Analysis Method 1 resulted in very few genes in MCF-7 cells that were statistically significantly altered by SA. For example, only five and nine genes were altered by > 1.5-fold or < -1.5-fold in MCF-7 cells after treatment with 1,000 µM SA after 6 and 24 h, respectively, compared to ∼10,000 altered by trichostatin A at 1 μM at both timepoints (analysis Method 1). There was no concentration-dependent effect of SA on MCF-7 cells observed using analysis Method 1; therefore, data from this cell line were excluded from the derivation of the PoD.
Relatively more genes were statistically significantly altered by SA in HepG2 cells, the number of which was higher after 24 h. After 6 h, the number of DEGs increased with increasing concentrations, apart from the 10 µM concentration. However, almost none of the identified significantly DEGs were deregulated by more than one concentration (each concentration affected different individual genes) as shown in Venn diagrams analyzing the overlap of similar deregulated genes at different concentrations (analysis Method 1, Figure 7). In contrast to the 6 h timepoint, at 24 h, almost all the genes that were differentially deregulated by 300 µM SA were also differentially deregulated by 1,000 µM (analysis Method 1, comparing Venn diagrams analyzing the overlap of similar deregulated genes at the different concentrations in Figure 7). This indicated that the response using HepG2 cells incubated with SA for 24 h was concentration-dependent and could be used to derive the PoD. The positive control trichostatin A, which is expected to cause a concentration-dependent effect, clearly showed similar DEGs.
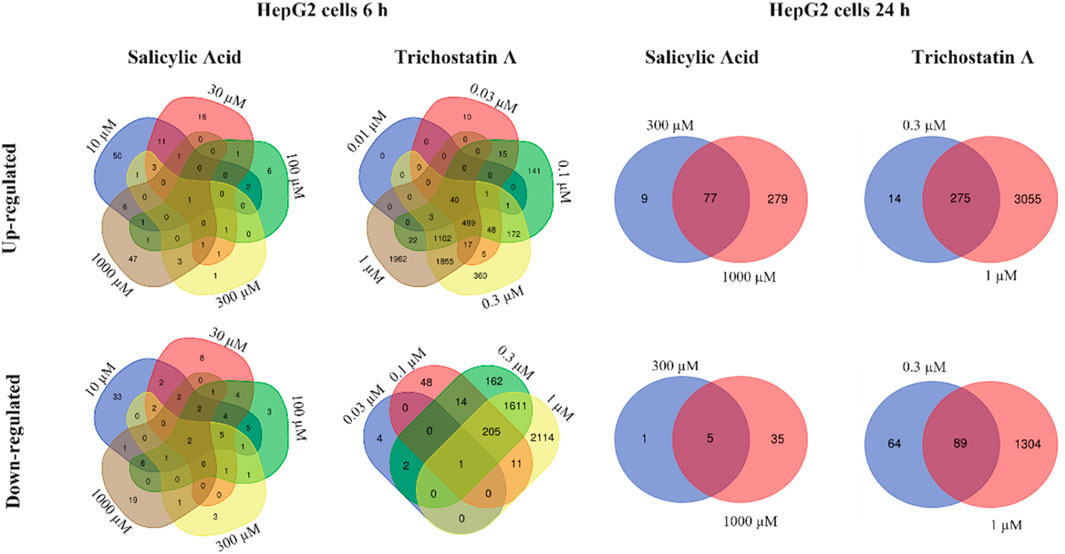
FIGURE 7. Venn diagrams showing the overlap of similar up- and down-regulated genes for different concentrations of SA and trichostatin A in HepG2 cells incubated for 6 and 24 h.
Analysis using Method 2 showed similar trends compared to analyses using Method 1, but with different absolute numbers. Again, only very few DEGs were observed in MCF-7 cells, both after 6-h and 24-h treatment. In HepG2 cells, there were more DEGs observed. As with the analysis using Method 1, there was no concentration-dependent effect observed after 6-h treatment but after 24-h treatment. Thus, benchmark dose analysis was performed for both analysis methods based on the HepG2 24-h dataset.
In order to derive a potential PoD, benchmark dose modeling was performed. BMD modeling was performed with HepG2 incubated for the 24-h dataset since only this dataset showed a concentration-dependent effect of SA using analysis Method 1 (see above). In both, analyses Method 1 and Method 2, base line +1 SD was considered as a benchmark response. For considering a conservative approach, the PoD was derived from the lowest pathway affected using the lower 95% confidence limit of the benchmark dose. In analysis Method 1, any ontology category using Gene Ontology terms and Reactome with at least five or more best model elements found among the defined ontology category elements and with Fisher’s exact test p-value <0.05 was considered enriched. The genes associated with each enriched pathway were then used to estimate the pathway or category BMD1SD and BMDL. Analysis Method 2 considered GO terms and Reactome pathways with the following criteria: Fisher’s exact right tail p-value ≤0.05, pathway coverage ≥10%, and the number of significant dose responsive genes in pathway ≥3. Base line +1 SD was considered to be the BMD. Based on these criteria, PoDs of 213 µM (analysis Method 1) and 10.6 µM (analysis Method 2) were derived. The marked difference in the two analysis methods is most likely based on the different inclusion criteria (five genes vs. three genes +10% coverage).
The ReproTracker assay was performed due to the DART alert for SA (see Supplementary Material S2.13 for the method). ReproTracker is a human-induced pluripotent stem cell (hiPSC)-based biomarker assay that assesses various key events during early embryonic development. A potential impairment of key developmental stages is analyzed by morphological changes and the expression of specific marker genes in hiPSCs differentiated into cardiomyocytes, hepatocytes, or neural rosettes. SA had no significant effect on the expression pattern of the biomarker genes in all three cell types up to the concentrations of 1 mM (Supplementary Figure S3). SA was, therefore, classified as not teratogenic up to 1 mM. The highest dose tested (1 mM) resulted in morphological changes in hepatocytes and neural rosettes, as well as a slight, but not significant decrease of one cardiomyocyte marker (BMP4). Based on these effects, a PoD of 500 µM was set for this endpoint.
The nephrotoxicity of SA was evaluated due to the nephrotoxicity alert from in silico predictions (using Derek). A comprehensive evaluation of the nephrotoxicity was conducted using hPTCs cultured in a transwell format (see Supplementary Material S2.14 for the method). SA was not toxic with respect to TEER, LDH release, or ATP content at any of the concentrations tested up to 300 μM (data not shown). Clinical biomarkers were also used to determine the nephrotoxicity, none of which were markedly or concentration-dependently affected (data not shown).
To account for the uncertainty of the in vitro measurements and the variation in anatomy and physiology, a probabilistic PBPK model was used to estimate the plasma concentration considering in a virtual population of 100 individuals (Najjar et al., manuscript in preparation). The virtual population was built based on a range of body weights and ages. The uncertainty of the in vitro parameters was with respect to the standard deviations to define the range of these parameters rather than using the mean values. The relevant PK parameters were reported, considering the mean and confidence intervals 5%–95% that were used later for the MoIE calculations. The resulting population PBPK modeling (together with the values from deterministic modeling) of plasma concentrations of BSal and its metabolites after a repeated application of 1.54 g/day face cream containing 0.5% BSal is shown in Figure 5B. The higher spread of data is due to the large difference in plasma protein-binding values (which ranged from 3 to ∼20% for SA), which has a big impact on the renal filtration of SA (as the only route of elimination). The highest mean plasma concentration was for SA (273.4 nM), which was over 500-fold higher than the predicted mean Cmax for BSal. This value of 273.4 nM SA was used in the calculation of the MoIE. The model already has uncertainties built into it; therefore, this value includes several assumptions that were discussed (data not shown, manuscript is in preparation).
Figure 8 provides an overview of the tiered workflow and the related in silico and in vitro NAMs used to calculate the MoIE. Several MoIE values were derived from PoDs from the in vitro toxicodynamics assays and plasma concentrations predicted using PBPK modeling (Table 2 and Table 3). Since this assessment is conducted using plasma concentrations rather than external doses, the comparison is an MoIE. We selected the two lowest PoDs from the in vitro bioassays, which were based on the toxicogenomics results evaluated by Analysis Method 1 (213 µM) and Analysis Method 2 (10.6 µM) since it is not yet known as to which of the two methods is more appropriate.
FIGURE 8. Overview of the tiered workflow and the related in silico and in vitro NAMs used in the NGRA.
In this assessment, the total plasma concentrations were used, rather than unbound concentrations, because they are compared with nominal doses used in the in vitro assays (biokinetics in the toxicogenomics incubations were not measured). When the mean total Cmax concentration of 0.273 µM was compared to the PoDs, the MoIEs were 780 and 38 for PoDs of 213 and 10.6 µM, respectively. When the more conservative CI95 value of 0.630 µM was used for the calculation of the MoIE, the values decreased further, with the lowest value of 16 derived using the Analysis Method 2 PoD of 10.6 µM.
For comparison with the MoIE values derived using an NGRA, MoS values were also calculated using the traditional approach with legacy in vivo data to determine the PoD and a calculation of the exposure according to the external dose and dermal absorption data (Table 4). The explanation of the selection of the values used in the calculation is detailed in the SCCS opinion on BSal (SCCS, 2023a). For BSal present at 0.5% in a face cream, the SCCS based the PoD on SA since BSal is rapidly metabolized to this primary stable metabolite. Despite the results from in vitro metabolism studies and PBPK population modeling indicating that BSal does enter the systemic circulation, the maximum concentration (CI95) of 0.7 nM BSal was much lower than that for SA (630 nM), indicating that SA is the driver of systemic effects. Therefore, the lowest PoD for SA was taken from a reproductive toxicity study on rats in which an NOAEL of 75 mg/kg bw was derived based on teratogenic and embryotoxic effects (SCCS, 2023b). Correction factors for the molecular weight (converting SA and other metabolites to BSal), skin first-pass metabolism (assuming 100% conversion), and the route-to-route exposure (which is 1 since the route is oral for BSal and SA) were applied to the PoD. The systemic exposure dose (SED) was calculated by adjusting the amount of the product applied with the amount absorbed after topical application. As shown in Table 3, the MoS value for BSal was 9,705.
1) The NOAELs for the safety comparator were obtained from the literature: SA from SCCS (2023b); 2) adjustment of metabolites to the parent by accounting for the molecular weight; 3) complete metabolism of BSal to SA; 4) dermal absorption: metabolite plus BSal plus + 1SD each; 5) rounded to 123 mg/kg/day by SCCS.
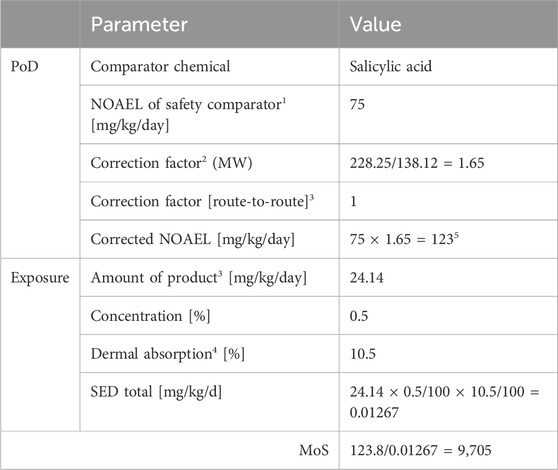
TABLE 4. Calculation of the MoS using the traditional approach using in vivo PoDs and external exposure.
Since the European ban on the use of animal testing to evaluate the safety of cosmetic ingredients, there have been significant advances in the development and strategic use of NAMs to achieve the same goal. While validated NAMs are available to evaluate the hazard potential of cosmetic ingredients to cause local effects, e.g., skin and eye irritation and skin sensitization, the potential of chemicals to cause systemic and long-term toxicity requires a more complex strategy, i.e., an NGRA (SCCS, 2023c). This takes into account human-relevant effects and relates them to exposure to derive a safe concentration of a cosmetic product component which is protective to human health. We followed the tiered NGRA testing strategy described by Berggren et al. (2017) and Desprez et al. (2018) and recommended in the SCCS Notes of Guidance. While a read-across strategy was possible for BSal, we investigated the level of protection to human health when an ab initio assessment was conducted because future cosmetic ingredients may not have the luxury of structural or biological analogs with which to conduct a read-across assessment. The levels of protection according to the NGRA approach (i.e., MoIE) using NAMs and using a traditional MoS calculation using animal data were evaluated. The results of the tiered workflow are summarized in Table 5.
A critical aspect of the safety assessment was the identification of the toxicologically relevant entity since this dictates which chemical(s) (parent and/or metabolites) should be tested in the toxicodynamics bioassays. Tier 1 skin absorption and metabolism experiments using ex vivo pig skin indicated that there was extensive first-pass metabolism in the skin after application of the intended dose of BSal in a formulation and that almost all of BSal was metabolized to several metabolites. The dermal bioavailability was determined (in vitro) to be 9.1% by summing up the amount of BSal and salicylic acid reaching the living skin layers and receptor fluid. These results using fresh pig skin correlated well with the results from a study using frozen human skin (and 14C-radio-labelled BSal present in a formulation at 0.5%), in which the skin absorption was 9.01% of the applied dose (SCCS, 2023a). Pig skin is accepted as a suitable surrogate for human skin (OECD, 2004; SCCS, 2023c) and has been shown by others to generate comparable skin absorption and metabolism results with those in human skin (Gerstel et al., 2016; Géniès et al., 2019). This was the first evidence in the current case study that subsequent in vitro bioassays should not focus on BSal, but rather its metabolites.
Metabolite prediction models help confirm results obtained in in vitro metabolism studies, or they can provide an indication of potential metabolites before the assays are run (which helps with searching for specific masses in the MS analysis). In this study, Meteor and GLORYx software were used to predict BSal metabolites. Both models predicted three possible pathways (hydrolysis, conjugation, and hydroxylation), with one or more metabolites representing these pathways flagged by both models. Considering the high capacity of the skin and liver for hydrolyzing esters (Jewell et al., 2007), it was concluded that this would be the main pathway involved in BSal metabolism. Indeed, there was a good concordance between the results obtained using Meteor and GLORYx and those from in vitro incubations of BSal with PHH, including the direct conjugation of BSal, as well as its hydrolysis to SA and benzyl derivatives. One exception was that further metabolites of SA predicted by Meteor and GLORYx were not observed in the in vitro incubations with the PHH, possibly due to the short duration of the incubation (4 h). Therefore, for PBPK modeling, it was assumed that SA is excreted via glomerular filtration despite the knowledge that, in humans, SA is partly excreted as conjugates (glucuronide and glycine conjugates) and as an oxidized metabolite (gentisic acid) (Needs and Brooks, 1985). Since this is an ab initio study, the literature information was not used in the PBPK model. In terms of the safety assessment, the assumption that SA is not metabolized is a conservative strategy since further metabolism of SA would decrease systemic concentrations and thus increase the MoIE. Future studies could investigate the use of long-term incubation models for hepatic metabolism, e.g., sandwich cultures and liver spheroids, if no metabolism of test chemicals is observed after 4-h incubation with PHH suspensions.
A second major metabolite formed in incubations of BSal with PHH was BA. The formation of this metabolite peaked at 30 min but was quickly further metabolized to hippuric acid, which continued to be formed until all the BSal had been consumed (by ∼2 h). BAlc and virtually all benzyl derivatives are readily oxidized to BA, which is then conjugated with glycine to produce hippuric acid, a detoxified metabolite (Irwin et al., 2016). In theory, according to the ab initio concept, the detoxification of BA occurring in vivo would not be known; therefore, it was necessary to predict the plasma concentrations of BA as well as SA in the next stage of the case study.
Since NGRAs are exposure-driven, PBPK modeling now represents a key aspect of the quantitative exposure assessment to investigate the kinetics of parent chemical and metabolite ADME parameters. PBPK modeling allows ADME parameters to be followed over time, in contrast to traditional risk assessments, in which only static parameters are used (exposure is calculated by considering the NOEAL and % absorbed). Therefore, PBPK modeling provides a more precise overview of the fate of the parent test chemical and its metabolites, leading to a more reliable safety assessment. In the first step, the PBPK model incorporated mean values in a deterministic approach. This supported the conclusion that BSal would be present in the systemic circulation at very low concentrations (1 nM). The hydrolysis product, BAlc, and its oxidized metabolite, BA, were also predicted to be present at low concentrations (0.5 and 4.6 nM, respectively). In contrast, SA was predicted to be present in plasma at a much higher concentration of 93.2 nM. Based on this simulation, only SA was tested in the in vitro bioassays. Of note, a concentration of 93.2 nM is much lower than the interim iTTC of 1 µM recommended by the SCCS (SCCS, 2023c) and, therefore, could have been used to conclude that neither the parent test chemical nor its major metabolite, SA, exceeded the iTTC, indicating there was no toxicological concern of BSal under the use condition being evaluated. However, the ToxCast and in silico toxicity prediction models indicated that SA has potential for developmental and reproductive toxicity, which excludes this from the iTTC approach.
Probabilistic PBPK modeling was considered to estimate the range of Cmax by considering the probable distribution of the input parameters, which reflect the uncertainty in the in vitro measurements and variation in human physiology and anatomy. This resulted in similar plasma concentrations for BSal, BAlc, and BA, but higher values for SA: a mean of 273.4 nM. PBPK models can also be refined with respect to the contribution of uptake and efflux transporters to excretion, and since SA was identified as being metabolically stable, additional studies were conducted to determine whether it is a substrate for transporters known to be expressed in the kidney. Transporter studies using hPTCs suggested that transport was mainly transcellular. This meant that renal clearance was not adjusted according to transporters but was based on the glomerular filtration rate and Fup. This is considered to be protective of human health because lack of transporter-mediated uptake into the kidney results in lower excretion of SA and a higher concentration in plasma. Of note, uncertainty factors for PBPK modeling were already incorporated in the model, e.g., the high range of values for Fup for the analytes and the assumption that SA is excreted unchanged in the urine.
In the Tier 1 hypothesis generation, in silico alerts and in vitro tests indicated that BSal has potential endocrine disruption effects via the estrogen pathway. By contrast, the in silico alerts and in vitro tests indicated that the major metabolite and comparator chemical, SA, is not an endocrine disruptor. The lowest AC50 for BSal was 36.5 µM in a binding assay for ERα, which is > 52,000-fold higher than the CI95 Cmax for BSal. Therefore, it was concluded that BSal would not cause endocrine disruption at the concentrations predicted in the plasma after the application of BSal in a face cream.
In the initial stage of Tier 1 hypothesis generation, ToxCast data and the in silico models indicated that SA was linked to nephrotoxicity and hepatotoxicity. Therefore, targeted testing explored whether SA was toxic to primary human kidney cells (hPTCs) according to standard viability parameters and clinically relevant biomarkers. This was also addressed in Tier 1, using the ToxProfiler cell stress assay, which was conducted as a general marker of systemic toxicity, rather than a targeted test in Tier 2 targeted testing [but did provide an indication of the potential for both nephrotoxicity and hepatotoxicity (Simmons et al., 2009)]. These assays ruled out the liver and kidney as target organs for SA effects, with PoDs in the millimolar range. Even if hepatotoxic effects are caused by SA, they are covered within the assessment by the assays conducted (the ToxProfiler assay indicated only a very slight effect in HepG2 cells) and the final PoD, which was based on toxicogenomics in HepG2 cells.
Pharmacology profiling and toxicogenomics assay were run to investigate a potential MoA for SA. The targets used in the pharmacology profiling assay panel were designed to provide a broad biological space (Burbank et al., 2022). The original panel comprised 44 targets and was designed for pharmaceuticals. This panel was extended to include additional targets, including kinases, as well as targets related to DART (ERα/β, PPARα/γ/δ), hepatotoxicity (e.g., BSEP), and cardiotoxicity (e.g., ion channels). This approach that helps identify targets has been advocated by the SCCS in the most recent Notes of Guidance, with the aim of increasing confidence in an NGRA (SCCS, 2023c). In the current study, none of the targets tested were significantly impacted by SA at a concentration up to millimolar concentrations.
The only assay in which PoDs were in the micromolar range was from non-targeted testing toxicogenomics assays using HepG2 cells. In contrast, the effects of SA on MCF-7 cells were minimal (very few genes deregulated) and not concentration-dependent and were thus attributed to non-specific transcriptome changes. An interesting aspect of this case study was the interpretation of the same set of toxicogenomics raw data by two biostatisticians from two companies. This is important because the way in which data are processed and interpreted can result in differences in the overall conclusion of an NGRA, as was demonstrated in this study whereby the MoIE differed by 20-fold, depending on the PoD used. The PoD derived from HepG2 cells incubated with SA for 24 h was 213 µM according to Analysis Method 1 and 10.6 µM according to Analysis Method 2. The difference between the two evaluations was due to the different number of genes per pathway used (Method 1: ≥5 genes deregulated; Method 2: ≥3 genes deregulated and covering at least 10% of the genes described of the respective pathway, as well as an additional test as a correction for multiple testing). Neither approach is incorrect; they simply differ in the criteria used to derive the PoD. This highlights the need to standardize data processing or at least provide transparent processes to be able to track how the data were handled. Regarding the transparent tracking of processes used, the US EPA has developed a so-called generic “Transcriptomics Reporting Framework” (TRF) to address this issue (Gant et al., 2017). The TRF describes raw data selection, data normalization, recognition of outliers, and statistical analysis.
This case study originated from the idea of comparing a traditional safety assessment with an NGRA assessment. Within this investigation, transcriptional changes have proven to be the most sensitive parameter and were used as the PoD, with the caveat that the interpretation of these transcriptional changes with respect to their links to toxicity remains uncertain. However, this approach was adopted to comply with the concept of protectiveness. When the CI95 was used to predict the internal exposure, the MoIE was 338 calculated using the PoD of 213 µM from BMDL Method 1 and 16 using the PoD of 10.6 µM from BMDL Method 2. There is no current agreement on the required minimum MoIE in NGRAs to assess a compound as safe and, while some have reported MoIEs greater than 100 using a similar approach to that in the current case study (Baltazar et al., 2020), this might not be necessary, considering the conservativeness of NAM-based approaches. Others have suggested the use of a so-called “Bioactivity/Exposure Ratio” (BER), which is equivalent to the MoIE and indicates whether the use of an ingredient is safe or not—the higher the BER, the less likely the systemic concentration will be bioactive and potentially cause adverse effects (SCCS, 2023c). The use of the BER is being increasingly used by others, e.g., the US EPA (Harrill, 2018), Health Canada (Health Canada, 2021), and ICCR principles (Dent et al., 2018; Carmichael et al., 2022). Regardless of which nomenclature is used, it is still to be determined what value constitutes a suitable threshold above which a chemical can be considered to be unlikely to cause adverse effects to human health. It is hoped that case studies like this will help refine and/or add weight to the values already implemented by others.
Since the PoD used here was based on a change in gene expression rather than an apical adverse effect, it could be assumed that the ab initio NGRA for BSal is sufficiently conservative to assure consumer safety. In addition, PBPK modeling is evolving to such an extent that there is more confidence in the predictions, which means that the use of the upper CI95 concentration as an estimation of the exposure may be an over-conservative prediction of the internal concentration or that a minimum MoIE needed to reach a safety decision should be re-defined. This is in accordance with a recent report in which high-throughput safety assessments based on in vitro PoDs covering a broad biological space and exposure predictions were sufficiently protective in most cases and were at least as protective as using in vivo PoDs (Paul Friedman et al., 2020). When a traditional approach used in the BSal opinion of the SCCS (SCCS, 2023a) using in vivo-derived PoDs and exposure calculated from the external dose and percentage absorbed, the MoS for the same consumer use (i.e., application of 0.5% BSal in a face cream) was 9,705. This indicates that while both assessments identified SA as the toxdriver, the ab initio NGRA approach, based on the comparison of internal exposure and in vitro-derived PoDs, was more conservative compared with the traditional method to ensure consumer safety.
In conclusion, we have demonstrated that both traditional and ab initio NGRA approaches concluded that the use of BSal in a cosmetic leave-on face cream at 0.5% is safe for humans. While we used default safety assessment factors for the traditional approach, we purposefully chose higher levels of conservatism in applying the NGRA approach. This pertains especially to parameters chosen for the PBPK model as the outcome from this model is critical to estimate the internal exposure concentrations for the MoIE calculations. On the other hand, the identification of a suitable margin to depict human safety requires additional insights. For this case study, the identification of the toxicologically critical entity was a critical step that directed the workflow and the selection of chemicals that were considered in PBPK modeling and tested in bioassays. Both the traditional risk assessment and NGRA identified SA as the “toxdriver.”
The overall conclusions from toxicogenomics assays by two companies were similar in terms of identifying that SA only results in significant gene deregulation in HepG2 cells at 24 h. Importantly, the raw data processing and interpretations highlighted how these can lead to different PoDs, which can subsequently affect the calculation of the MoIE. Thus, there is an urgent need to define clear rules and approaches for PoD derivation from toxicogenomics data.
This case study based on BSal supports the use of NAMs in a tiered NGRA ab initio approach.
The original contributions presented in the study are included in the article/Supplementary Material; further inquiries can be directed to the corresponding author.
Ethical approval was not required for the studies on humans in accordance with the local legislation and institutional requirements because only commercially available established cell lines were used.
JE: conceptualization, data curation, formal analysis, investigation, methodology, visualization, and writing–review and editing. AN: conceptualization, data curation, formal analysis, investigation, methodology, software, supervision, visualization, and writing–review and editing. DL: conceptualization, data curation, formal analysis, investigation, methodology, supervision, visualization, and writing–review and editing. MB: conceptualization, data curation, formal analysis, investigation, methodology, visualization, and writing–review and editing. SV: conceptualization, data curation, formal analysis, investigation, methodology, software, visualization, and writing–review and editing. KB: data curation, formal analysis, methodology, and writing–review and editing. JM: data curation, formal analysis, methodology, visualization, and writing–review and editing. JK: conceptualization, data curation, formal analysis, investigation, methodology, visualization, and writing–review and editing. NH: conceptualization, data curation, investigation, methodology, and writing–original draft. C-TK: conceptualization, formal analysis, methodology, visualization, and writing–review and editing. AS: conceptualization, project administration, supervision, and writing–review and editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was funded by the European cosmetics industry through the Cosmetics Europe Long Range Science Strategy (https://www.lrsscosmeticseurope.eu/).
Authors JE, AN, DL, MB, SV, KB, JM, JK, C-TK, and AS were employed by Beiersdorf AG.
The remaining author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2024.1345992/full#supplementary-material
Baltazar, M. T., Cable, S., Carmichael, P. L., Cubberley, R., Cull, T., Delagrange, M., et al. (2020). A next-generation risk assessment case study for coumarin in cosmetic products. Toxicol. Sci. 176, 236–252. doi:10.1093/toxsci/kfaa048
Berggren, E., White, A., Ouedraogo, G., Paini, A., Richarz, A. N., Bois, F. Y., et al. (2017). Ab initio chemical safety assessment: a workflow based on exposure considerations and non-animal methods. Comput. Toxicol. 4, 31–44. doi:10.1016/j.comtox.2017.10.001
Burbank, M., Hewitt, N. J., Kenna, G., Kukic, P., Boettcher, M., Ebmeyer, J., et al. (2022). LP-16 in vitro pharmacologic profiling for cosmetic chemical systemic toxicity safety testing: preliminary data. Toxicol. Lett. 368, S289. doi:10.1016/j.toxlet.2022.07.758
Bury, D., Alexander-White, C., Clewell, H. J., Cronin, M., Desprez, B., Detroyer, A., et al. (2021). New framework for a non-animal approach adequately assures the safety of cosmetic ingredients - a case study on caffeine. Regul. Toxicol. Pharmacol. 123, 104931. doi:10.1016/j.yrtph.2021.104931
Carmichael, P. L., Baltazar, M. T., Cable, S., Cochrane, S., Dent, M., Li, H., et al. (2022). Ready for regulatory use: NAMs and NGRA for chemical safety assurance. Altex 39, 359–366–366. doi:10.14573/altex.2204281
Cir (2018). Safety assessment of benzyl salicylate as used in cosmetics. Draft report for panel review. Retrieved from [website] https://www.cir-safety.org/sites/default/files/Benzyl%20Salicylate.pdf.
Dent, M., Amaral, R. T., Da Silva, P. A., Ansell, J., Boisleve, F., Hatao, M., et al. (2018). Principles underpinning the use of new methodologies in the risk assessment of cosmetic ingredients. Comput. Toxicol. 7, 20–26. doi:10.1016/j.comtox.2018.06.001
Desprez, B., Dent, M., Keller, D., Klaric, M., Ouédraogo, G., Cubberley, R., et al. (2018). A strategy for systemic toxicity assessment based on non-animal approaches: the Cosmetics Europe Long Range Science Strategy programme. Toxicol Vitro 50, 137–146. doi:10.1016/j.tiv.2018.02.017
Eu (2003). Directive 2003/15/EC of the European parliament and of the council of 27. February 2003 amending Council Directive 76/768/EEC on the approximation of the laws of the Member States relating to cosmetic products (Text with EEA relevance) http://data.europa.eu/eli/dir/2003/15/oj.
Eu (2009). Regulation (EC) No 1223/2009 of the European parliament and of the council of 30 november 2009 on cosmetic products. Accessed http://data.europa.eu/eli/reg/2009/1223/oj.
Gant, T. W., Sauer, U. G., Zhang, S. D., Chorley, B. N., Hackermüller, J., Perdichizzi, S., et al. (2017). A generic Transcriptomics Reporting Framework (TRF) for 'omics data processing and analysis. Regul. Toxicol. Pharmacol. 91 (Suppl. 1), S36-S45–s45. doi:10.1016/j.yrtph.2017.11.001
Géniès, C., Jamin, E. L., Debrauwer, L., Zalko, D., Person, E. N., Eilstein, J., et al. (2019). Comparison of the metabolism of 10 chemicals in human and pig skin explants. J. Appl. Toxicol. 39, 385–397. doi:10.1002/jat.3730
Gerstel, D., Jacques-Jamin, C., Schepky, A., Cubberley, R., Eilstein, J., Grégoire, S., et al. (2016). Comparison of protocols for measuring cosmetic ingredient distribution in human and pig skin. Toxicol Vitro 34, 153–160. doi:10.1016/j.tiv.2016.03.012
Harrill, J. (2018). “Bioactivity exposure ratio (BER) analysis using high throughput transcriptomics (HTTr) chemical screening data,” in Presented at national centre for the replacement refinement and reduction of animals in research (NC3R) workshop (London, N/A, UK, December), 10–11. doi:10.23645/epacomptox.7754927
Health canada (2021). Science approach document - bioactivity exposure ratio: application in priority setting and risk assessment. https://www.canada.ca/content/dam/eccc/documents/pdf/pded/bioactivity-exposure-ratio/Science-approach-document-bioactivity-exposure-ratio.pdf.
Irwin, C., Van Reenen, M., Mason, S., Mienie, L. J., Westerhuis, J. A., and Reinecke, C. J. (2016). Contribution towards a metabolite profile of the detoxification of benzoic acid through Glycine conjugation: an intervention study. PLoS One 11, e0167309. doi:10.1371/journal.pone.0167309
Jewell, C., Ackermann, C., Payne, N. A., Fate, G., Voorman, R., and Williams, F. M. (2007). Specificity of procaine and ester hydrolysis by human, minipig, and rat skin and liver. Drug Metab. Dispos. 35, 2015–2022. doi:10.1124/dmd.107.015727
lotion Series on Testing and Assessment, 2021 Lotion series on testing and assessment. No. 349. ENV/CBC/MONO(2021)35. Retrieved from [website]: https://www.oecd.org/officialdocuments/publicdisplaydocumentpdf/?cote=ENV/CBC/MONO(2021)35&docLanguage=En.
Najjar, A., Kramer, N., Gardner, I., Hartung, T., and Steger-Hartmann, T. (2023). Editorial: advances in and applications of predictive toxicology: 2022. Front. Pharmacol. 14, 1257423. doi:10.3389/fphar.2023.1257423
Needs, C. J., and Brooks, P. M. (1985). Clinical pharmacokinetics of the salicylates. Clin. Pharmacokinet. 10, 164–177. doi:10.2165/00003088-198510020-00004
Oecd (2004). OECD guideline for the testing of chemicals. Skin absorption: in vitro method. 428 (Adopted April 13, 2004).
Oecd (2019a). Case study on the use of an integrated approach to testing and assessment (IATA) and New approach methods to inform a theoretical read-across for dermal exposure to propylparaben from cosmetics. Retrieved from [website]: https://www.oecd.org/officialdocuments/publicdisplaydocumentpdf/?cote=ENV/JM/MONO(2020)16&docLanguage=en.
Oecd (2019b). Case study on the use of integrated approaches for testing and assessment for systemic toxicity arising from cosmetic exposure to caffeine. Retrieved from [website]: https://www.oecd.org/officialdocuments/publicdisplaydocumentpdf/?cote=ENV/JM/MONO(2020)17&docLanguage=en.
Oecd (2020). Case study on the use of integrated approaches for testing and assessment for the systemic toxicity of phenoxyethanol when included at 1% in a body lotion. Retrieved from [website] https://www.oecd.org/officialdocuments/publicdisplaydocumentpdf/?cote=ENV/CBC/MONO(2021)35&docLanguage=En.
Oecd (2021). Case study on use of an integrated approach for testing and assessment (IATA) for systemic toxicity of phenoxyethanol when included at 1% in a body.
Ouedraogo, G., Alexander-White, C., Bury, D., Clewell, H. J., Cronin, M., Cull, T., et al. (2022). Read-across and new approach methodologies applied in a 10-step framework for cosmetics safety assessment - a case study with parabens. Regul. Toxicol. Pharmacol. 132, 105161. doi:10.1016/j.yrtph.2022.105161
Patrignani, P., and Patrono, C. (2015). Cyclooxygenase inhibitors: from pharmacology to clinical read-outs. Biochim. Biophys. Acta 1851, 422–432. doi:10.1016/j.bbalip.2014.09.016
Paul Friedman, K., Gagne, M., Loo, L. H., Karamertzanis, P., Netzeva, T., Sobanski, T., et al. (2020). Utility of in vitro bioactivity as a lower bound estimate of in vivo adverse effect levels and in risk-based prioritization. Toxicol. Sci. 173, 202–225. doi:10.1093/toxsci/kfz201
Pubchem database (2023). Benzyl salicylate. Rerieved from [website]: https://pubchem.ncbi.nlm.nih.gov/compound/8363.
Sccs (2023a). Scientific committee on consumer safety, opinion on benzyl salicylate (CAS No. 118-58-1, EC No. 204-262-9), preliminary version of 6-7 june 2023, 37. SCCS/1656/23.
Sccs (2023b). Scientific committee on consumer safety, opinion on salicylic acid (CAS No. 69-72-7, EC No. 200-712-3), preliminary version of 14 december 2022, final version of 6-7 june 2023. SCCS/1646/22.
Sccs (2023c). Scientific committee on consumer safety, SCCS notes of guidance for the testing of cosmetic ingredients and their safety evaluation 12th revision. 15 May 2023, SCCS/1647/22.
Simmons, S. O., Fan, C. Y., and Ramabhadran, R. (2009). Cellular stress response pathway system as a sentinel ensemble in toxicological screening. Toxicol. Sci. 111, 202–225. doi:10.1093/toxsci/kfp140
Thomas, R. S., Bahadori, T., Buckley, T. J., Cowden, J., Deisenroth, C., Dionisio, K. L., et al. (2019). The next generation blueprint of computational toxicology at the U.S. Environmental protection agency. Toxicol. Sci. 169, 317–332. doi:10.1093/toxsci/kfz058
Vanhaecke, T., Papeleu, P., Elaut, G., and Rogiers, V. (2004). Trichostatin A - like hydroxamate histone deacetylase inhibitors as therapeutic agents: toxicological point of view. Curr. Med. Chem. 11, 1629–1643. doi:10.2174/0929867043365099
Varma, M. V., Steyn, S. J., Allerton, C., and El-Kattan, A. F. (2015). Predicting clearance mechanism in drug discovery: extended clearance classification system (ECCS). Pharm. Res. 32, 3785–3802. doi:10.1007/s11095-015-1749-4
Keywords: cosmetic ingredients, new approach methodologies, benzyl salicylate, physiologically based pharmacokinetic modeling, ab initio safety assessment
Citation: Ebmeyer J, Najjar A, Lange D, Boettcher M, Voß S, Brandmair K, Meinhardt J, Kuehnl J, Hewitt NJ, Krueger C-T and Schepky A (2024) Next generation risk assessment: an ab initio case study to assess the systemic safety of the cosmetic ingredient, benzyl salicylate, after dermal exposure. Front. Pharmacol. 15:1345992. doi: 10.3389/fphar.2024.1345992
Received: 28 November 2023; Accepted: 12 February 2024;
Published: 07 March 2024.
Edited by:
Stefan Schildknecht, Hochschule Albstadt-Sigmaringen, GermanyReviewed by:
Giulia Callegaro, Leiden University, NetherlandsCopyright © 2024 Ebmeyer, Najjar, Lange, Boettcher, Voß, Brandmair, Meinhardt, Kuehnl, Hewitt, Krueger and Schepky. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Abdulkarim Najjar, YWJkdWxrYXJpbS5uYWpqYXJAYmVpZXJzZG9yZi5jb20=; Johanna Ebmeyer, am9oYW5uYS5lYm1leWVyQGJlaWVyc2RvcmYuY29t; Daniela Lange, ZGFuaWVsYWRyLmxhbmdlQGJlaWVyc2RvcmYuY29t
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.