- 1Pharmacoeconomics Department, University of Medicine and Pharmacy of Craiova, Craiova, Romania
- 2Psychiatry Department, University of Medicine and Pharmacy of Craiova, Craiova, Romania
- 3Doctoral School, Carol Davila University of Medicine and Pharmacy, Bucharest, Romania
- 4Department of Medical Oncology, University of Medicine and Pharmacy Grigore T. Popa Iasi, Iasi, Romania
- 5Gynecologic Oncology Department, Filantropia Clinical Hospital Bucharest, Bucharest, Romania
- 6Oncology Department, University of Medicine and Pharmacy of Craiova, Craiova, Romania
Introduction: It is imperative for patients to respect the prescribed treatments to achieve the anticipated clinical outcomes, including the outpatients receiving oral anti-cancer drugs such as selective cyclin-dependent kinase 4/6 inhibitors (CDK 4/6i). With the introduction of three CDK 4/6i drugs in the Romanian pharmaceutical market in 2018, our study aimed to evaluate medication adherence and the influencing factors among patients undergoing treatment with palbociclib, ribociclib, or abemaciclib for advanced or metastatic breast cancer.
Methods: Medication adherence was assessed using the Proportion of Days Covered (PDC) method, and Spearman correlation analysis was conducted to explore the relationships between adherence, age, gender, and follow-up duration.
Results: The study enrolled 330 breast cancer patients, with an average follow-up period of 14.6 ± 12.5 months for palbociclib, 10.6 ± 7.1 months for ribociclib, and 8.6 ± 6.4 months for abemaciclib-treated patients. A small proportion of patients demonstrated non-adherence: 12.8% for palbociclib, 14.6% for ribociclib, and 14.7% for abemaciclib. Among patients receiving palbociclib, there was no significant correlation between adherence, age (rho = 0.07, p = 0.35), or gender (rho = −0.144, p = 0.054). However, a significant correlation was found with the duration of follow-up (rho = −0.304, p < 0.0001). Similar results were observed for patients receiving ribociclib or abemaciclib. Most patients received combination therapy with letrozole (46%) and exemestane (13%) for palbociclib, letrozole (48%) and fulvestrant (19%) for ribociclib, and fulvestrant (39%) and letrozole (27%) for abemaciclib,
Discussion: High adherence rates were observed among patients treated with CDK 4/6i drugs, with no significant differences noted among the three drugs in this class. However, the collected patient data was limited, lacking information on adverse reactions that could potentially lead to treatment discontinuation, as determined by the oncologist’s decision not to prescribe. Consequently, a comprehensive understanding of all factors contributing to the low adherence levels is hindered.
Introduction
Breast cancer is the second most common cancer in women after skin cancer with a percentage of 15.2% from all new cancer cases and 7.1% from all cancer deaths in 2023 (National Cancer Institute, 2023. https://www.cancer.gov/types/breast). The identification of cyclin-dependent kinases (CDK) and their regulatory mechanisms in cell cycle processes marked a pivotal advancement in cancer therapy. Among these, cyclin-dependent kinase 4 and 6 (CDK4/6) are enzymes crucially involved in cell cycle regulation. They exert significant control over the transition from the G1 (gap 1) phase to the S (synthesis) phase, where DNA replication occurs (Suryadinata et al., 2010). Maintaining a delicate equilibrium between CDK4/6 activation by cyclin D and their inhibition by cyclin-dependent kinase inhibitors (CDKi) is essential for the orderly progression of the cell cycle. Any disruption in this balance can result in uncontrolled cell division, contributing to various diseases, notably cancer (Barnum et al., 2014). In the realm of cancer treatment, CDK 4/6 inhibitors (CDK 4/6i) are employed to target overactive CDK4/6-cyclin D complexes. This is particularly pertinent in cancers like breast cancer, where this pathway often plays a central role in unregulated cell proliferation (Mariotto et al., 2017).
Palbociclib, ribociclib, and abemaciclib stand as prominent examples of CDK 4/6i widely employed in the treatment of specific forms of advanced/metastatic breast cancer (A/mBC) (Roskoski et al., 2019). Although these inhibitors demonstrate efficacy in impeding cancer cell proliferation, they are not devoid of adverse reactions and side effects (Jin et al., 2019). Previous research has indicated that abemaciclib is associated with a lower preference weight in comparison to other CDK4/6i due to adverse events, including diarrhea, abdominal pain, grade 3/4 neutropenia, tromboembolitic disease (Maculaitis et al., 2020), or acute liver injury (Beachler et al., 2021). Additionally, findings from a singular study (Cejuela et al., 2023) underscored diarrhea as a significant adverse reaction experienced by all patients, highlighting its clinical importance (Arbuckle et al., 2000). A meta-analysis regarding the risk of other side effects, such as stomatitis, demonstrated that especially palbociclib, among all CDK4/6i, could increase this risk impacting on patient adherence to the treatment (Long et al., 2021).
The global market for CDK 4/6i drugs is segmented across various categories, including drug types such as palbociclib (@Ibrance), ribociclib (@Kisqali), and abemaciclib (@Verzenio) (Finn et al., 2015). The first CDK4/6 inhibitor drug approved by the FDA was palbociclib in February 2015 (Dhillon et al., 2015; Fin et al., 2016). Subsequent approvals were granted for its utilization in combination with other hormonal therapies, rendering it a pivotal treatment option for specific breast cancer patients. Ribociclib received FDA approval in March 2017 (Hortobagyi et al., 2016). Similar to palbociclib, it was sanctioned for the treatment of hormone receptor-positive (HR+) and human epidermal growth factor receptor 2-negative (HER2-negative) advanced or metastatic breast cancer in conjunction with an aromatase inhibitor. Its scope has been broadened since then, with additional approvals for diverse hormonal therapies (Salmon et al., 2020). Abemaciclib obtained FDA approval in September 2017. It was endorsed as a standalone agent for HR+, HER2-negative advanced or metastatic breast cancer in patients who had previously undergone endocrine therapy (Dickler et al., 2017). According to the submission of its dossier to EMA, abemaciclib was approved in combination with an aromatase inhibitor (AI, as letrozole, anastrozole, or examestan) as initial endocrine-based therapy or in combination with fulvestrant as initial endocrine-based therapy or following endocrine therapy.
These CDK4/6i have substantially enhanced treatment options for patients with HR+ breast cancer by targeting the cell cycle regulation process, which plays a pivotal role in cancer growth (Wells 2020). Typically, they are utilized in combination with endocrine therapies, significantly prolonging progression-free survival (PFS) and overall survival (OS) for numerous patients (Cristofanilli et al., 2016; Finn et al., 2016; Sledge et al., 2017; Slamon et al., 2018). CDK 4/6i are also utilized together with endocrine therapy for male patients diagnosed with HR+/HER2-metastatic breast cancer (Kraus et al., 2022). It is crucial to note that approval dates and availability can vary by country, and new applications and indications for these drugs may have emerged (Bandiera et al., 2023).
In Romania, approximately 12,000 new cases of breast cancer are diagnosed annually, rendering it the second leading cause of cancer-related deaths, following lung cancer (Furtunescu et al., 2021). According to research on the effects of COVID-19 pandemic in Romania on the breast cancer patients, even if the number of patients remained the same, the cancer treatment costs have risen exponentially from 2018 to 2021 (Turcu-Stiolica et al., 2022). Following Health Technology Assessment (HTA), the National Agency for Medicines and Medical Devices (NAMMD) in Romania unconditionally approved the inclusion of palbociclib in the Positive Drug List in November 2017 (Ministry of Health of Romania, 2017). Ribociclib was unconditionally included in the Positive Drug List in August 2022 (Inclusion of ribociclib in Romania, 2022), while abemaciclib was included in April 2022 (Inclusion of abemaciclib in Romania, 2022). All three medications were recommended for the treatment of women with locally advanced or metastatic breast cancer (a/mBC), who are HR+/HER2-, in combination with an AI or fulvestrant, as initial hormonal therapy, or in women who have received prior hormonal therapy. In premenopausal or perimenopausal women, hormonal therapy should be combined with a luteinizing hormone-releasing hormone (LHRH) agonist.
Medication adherence is a hey enabler of best health outcomes and some medication adherence supporting activities were reported in order to guide research and practice on enhancing medication adherence (Kardas et al., 2023). Treatment nonadherence is associated with disease progression and mortality among patients with breast cancer (Chirgwin et al., 2016). The existing research on adherence to CDK4/6i anticancer agents is limited. Consequently, the primary aim of our research was to assess the adherence levels of CDK 4/6i and to explore potential correlations with variables such as age, gender, and the duration of patient follow-up. In addition to this primary objective, our study also sought to investigate potential disparities in medication adherence among the three distinct CDK 4/6i currently available within the pharmaceutical market in Romania. Through this research, we aimed to contribute valuable insights into the patterns of medication adherence and its associations with demographic factors, thereby enhancing our understanding of the real-world usage of these CDK 4/6i in clinical practice from Romania.
Methods
In the context of our study conducted in Romania, electronic information pertaining to reimbursed medications is exclusively accessible through the database maintained by the Romanian Health Insurance House. Ethical approval for our research endeavor, granted under Ethics Council approval number 175/29.10.2021, allowed us access to anonymized patient data sourced from community pharmacies in Dolj County, Romania, which were reported to the Health Insurance House of Dolj. The study focused on data spanning the past 5 years, from 2018 to 2022, corresponding to the period during which the first CDK 4/6 inhibitor, palbociclib, was approved for entry into the Romanian pharmaceutical market.
Specifically, our study inquired about patient records identified by the ICD-10 code C50, denoting breast cancer, with a subsequent focus on individuals receiving treatment with palbociclib, ribociclib, and abemaciclib. The data obtained for analysis encompassed essential demographic information, namely, age and gender, as well as details concerning prescription refills, including the quantity of medicines dispensed and the dates of prescription release from community pharmacies. Notably, our access to information was limited to these parameters, and we did not have access to additional patient-specific data such as comorbidities or other health covariates. This approach was undertaken within the confines of ethical guidelines and regulations, ensuring the confidentiality and privacy of patient information while enabling us to analyze patterns of CDK 4/6i usage in the studied population. The utilization of this restricted dataset was essential for our investigation into medication adherence and its potential correlations with demographic factors within the Romanian context.
Study population
All patients with breast cancer (code of disease = 124) who raised their reimbursed prescriptions from a community pharmacy from Dolj County, Romania, in the period 1 January 2018 -31 December 2022. The first patient received the first palbociclib prescription from the community pharmacy in July 2018, and she was a female of 75 years old, whereas for abemaciclib, the first patient was a female of 73 years old, in February 2021. We included all patients who had at least two fills of CDK 4/6i because it is required to compute medication adherence.
CDK 4/6i cycle dates were determined based on the electronic records from the Dolj Health Insurance House for the reimbursed prescriptions written by the oncologist.
Outcomes
The duration of follow-up was defined as the time in months from the first prescription issuing by the pharmacist in the community pharmacy to the last prescription reimbursed by the Dolj Health Insurance House according to the analyzed period (1 January 2018-31 December 2022). We considered it as the time elapsed from the medication’s starting date to the last treatment’s discontinuation date, which could be death or treatment modification.
There is no universally standardized method for measuring medication adherence. An ISPOR Report authored by Pednekar et al. highlighted the most frequently employed techniques found in the literature, which include self-reported questionnaires, proportion of days covered (PDC), and medication possession ratio (MPR). The PDC is the leading method used to calculate medication adherence using prescription refill data from electronic records at the population level. PDC was defined as the number of days that drugs were available to the patient over a time interval, but it has many formulas (Pednekar et al., 2019). We calculated the adherence using the formula as the report between Σ cycles/months of supply for medication and Σ months between last month of prescription and the first month of prescription. By definition, PDC ranges from 0 to 1. We used the conventional cutoff point of 0.8 to classify the patients into adherent (0.8 ≤ PDC ≤1) and non-adherent (0 ≤ PDC <0.8) patients (Dima et al., 2017).
Statistical analysis
We conducted descriptive analysis of continuous variables (age, adherence) using means±standard deviations (SD), median and interquartile range (IQR) and range (minimum-maximum) and of categorical variables (gender, categories of age) using frequencies and percentages. Additionally, to demonstrate the potential correlation between medication adherence and age, gender of patients, we calculated the Spearman’s coefficients and visually presented with heatmaps. To evaluate the differences between the characteristics and medication adherence of patients with different treatment, we used Kruskal–Wallis H test for continuous variables and Chi-square test for categorical variables. We visually presented the differences of medication adherence among patients with different treatments using violin graphs. We conducted statistical analysis using GraphPad Prism 10.1 (GraphPad Software Boston, USA), with the statistical significance level set at p less than 0.05, two-tailed.
Results
During the study period from 1 January 2018, to 31 December 2022, a total of 330 patients were prescribed CDK 4/6i. Among these, 180 patients (55%) were administered palbociclib, 82 (25%) received ribociclib, and 68 (20%) were prescribed abemaciclib.
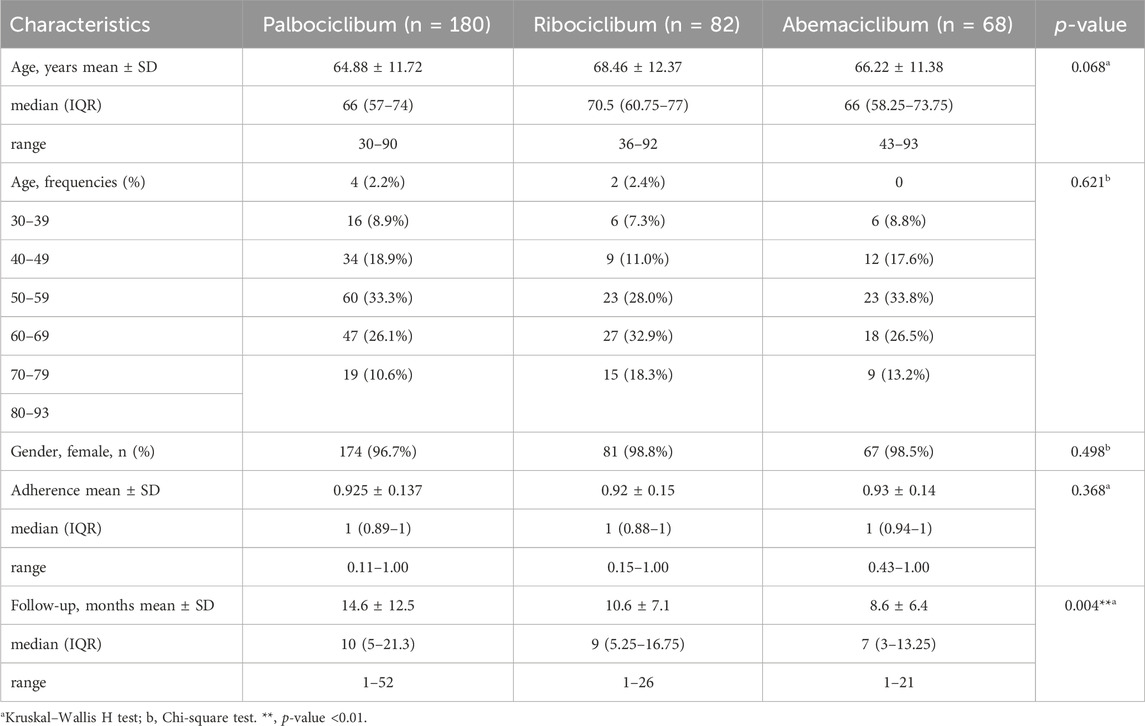
Table 1 summarizes descriptive statistics of patient characteristics, and adherence, for each group of patients, as well as the p-value after performing the comparison between them. The median (range) age was 66 (30–90) years for the palbociclib group, 71 (36–92) years for the ribociclib group, and 66 (43–93) years for the abemaciclib group of patients. Most of the patients were more than 60 years old: 70% in palbociclib patients, 79.3% in ribociclib patients and 73.5% in abemaciclib patients. Most of the patients were female, but more male patients were treated with palbociclib (3.3%) than with ribociclib (1.2%) or abemaciclib (1.5%). The follow-up varies significantly between the three groups of patients (p-value = 0.004), with higher follow-up for patients treated with palbociclib, because it was earlier introduced on the Romanian pharmaceutical market.
The eligible patients were included in our study with an average follow-up period of 14.6 ± 12.5 months for the patients treated with palbociclib, 10.6 ± 7.1 months for the patients treated with ribociclib, and 8.6 ± 6.4 months for the patients treated with abemaciclib, respectively. CDK 4/6i were generally combined with either letrozole, fulvestrant, exemestane, anastrozole, goserelin or tamoxifen as in Figure 1. No ribociclibum or abemaciclib were combined with tamoxifen in our database. Most of the patients had treatment in combination with letrozole (45.9%) and exemestan (13.4%), in case of palbociclib, letrozole (45.9%) and fulvestrant (19%), in case of ribociclib, and fulvestrant (39.1%) and letrozole (27.4%), in case of abemaciclib, as shown in Table 2. Gosereline was more combined with ribociclibum (5.4%).

FIGURE 1. Combination of CDK 4/6i with endocrine therapy by month over the study period. (A). Combination of palbociclib (PALBO) with endocrine therapy. (B). Combination of ribociclib (RIBO) with endocrine therapy. (C). Combination of abemaciclib (ABEMA) with endocrine therapy. AI (aromatase inhibitor). The percentages were computed based on the total number of patients undergoing treatment with both CDK 4/6i and endocrine therapy.
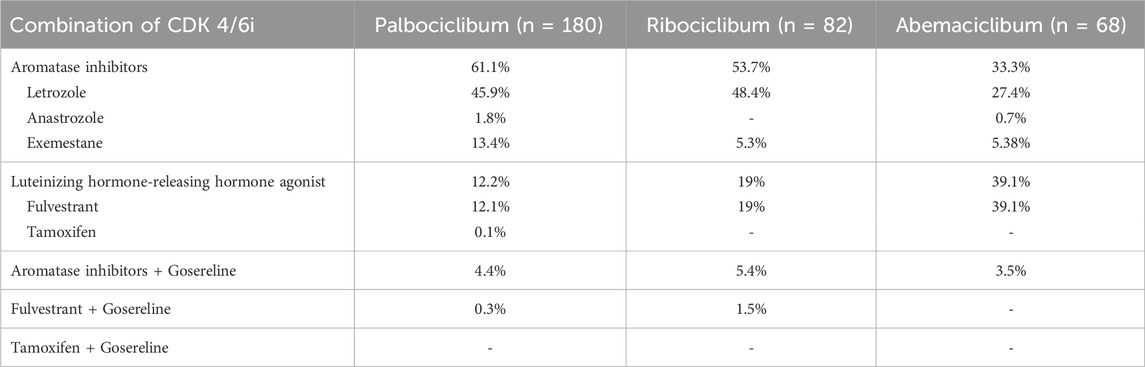
TABLE 2. Combinations of the CDK 4/6 inhibitors with aromatase inhibitors or/and luteinizing hormone-releasing hormone agonists.
The proportion of non-adherent patients taking CDK 4/6i with PDC <0.8 was 13.6%, splitting into 12.8% for palbociclib, 14.6% for ribociclib, 14.7% for abemaciclib, respectively. For a cut-off equal to 0.85, the proportion of non-adherent patients taking CDK 4/6i was 16.1%, splitting into 16% for palbociclib, 17% for ribociclib, and 16.2% for abemaciclib. For a cut-off equal to 0.90, the proportion of non-adherent patients taking CDK 4/6i was 24.8%, splitting into 25% for palbociclib, 27% for ribociclib, and 22.1% for abemaciclib. No significant difference was obtained for adherence levels among patients treated with the three CDK 4/6i, as shown in Figure 2. We observed the peaks in the CDK 4/6i and the most patients had 100% adherence for all three groups of patients. Better adherence, but not significantly higher, was observed among patients treated with abemaciclib (mean ± SD, 0.93 ± 0.14) than among patients treated with palbociclib (mean ± SD, 0.92 ± 0.14) or ribociclib (mean ± SD, 0.92 ± 0.15). The smallest adherence was observed for a patient treated with palbociclib (0.11), while the smallest adherence observed for a patient treated with ribociclib was 0.15 and the smallest adherence observed for a patient treated with abemaciclib was 0.43.
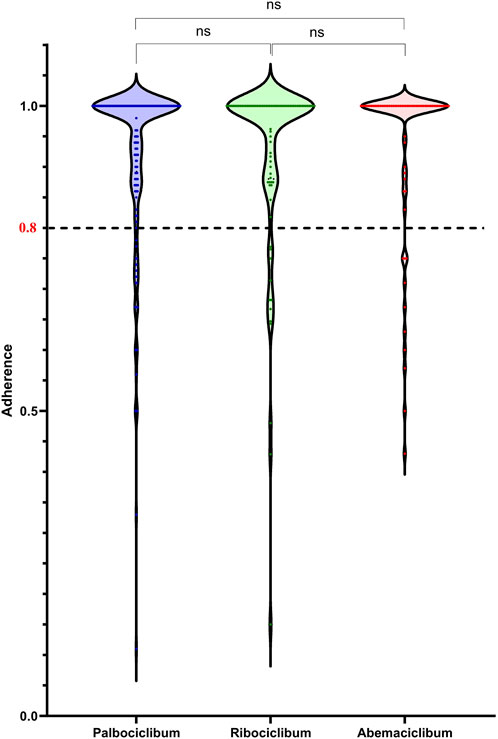
FIGURE 2. Adherence as proportion of days covered (PDC) in patients treated with either CDK 4/6i, palbociclib, ribociclib, and abemaciclib.
As in Figure 3A, in patients treated with palbociclib, there was no significant correlation between the level of adherence, age (rho = 0.07, p = 0.35) or gender (rho = −0.144, p = 0.054), but a significant correlation was observed with the duration of follow-up (rho = −0.304, p < 0.0001). Similarly, in patients receiving ribociclib, no significant correlation was found between adherence levels and age (rho = −0.097, p = 0.388) or gender (rho = −0.082, p = 0.466), but a significant correlation was identified with the follow-up duration (rho = −0.394, p < 0.0001), as is shown in Figure 3B. The same results were obtained for patients treated with abemaciclib, where no significant correlation was found between adherence levels and age (rho = 0.007, p = 0.955) or gender (rho = −0.072, p = 0.559), but a significant correlation was observed with the duration of follow-up (rho = −0.25, p = 0.04), as is shown in Figure 3C.
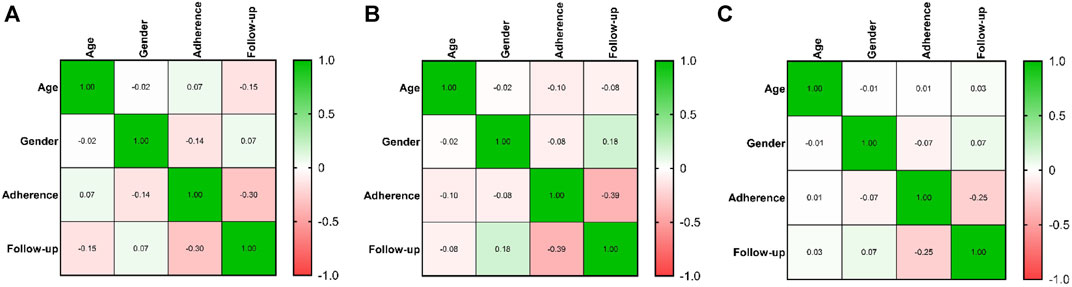
FIGURE 3. Correlation between adherence of CDK 4/6i treatment, age, gender and follow-up of treatment. The colors from heatmaps correspond to the Spearman coefficient from negative values (light orange color) to positive values (green color). (A). Heatmap of correlations in the case of palbociclib therapy. (B). Heatmap of correlations in the case of ribociclib therapy. (C). Heatmap of correlations in the case of abemaciclib therapy.
Discussion
Maintaining adherence to CDK 4/6i is a mandatory step towards reaching treatment goals for patients with HR+/HER2-a/mBC. We found a proportion of 14% of non-adherent patients taking CDK 4/6i for an 80% adherence cut-off, 16% using an 85% adherence cut-off and 25% using a 90% cut-off, without significant differences between non-adherence for palbociclib, ribociclib and abemaciclib. We obtained an average PDC values of 92.6%, which is comparable with the PDC values of 89.6% obtained by another retrospective study from Canada that included patients receiving either palbociclib or abemaciclib (Marineau et al., 2023). Marineau et al. similar values for mean PDC for palbociclib (90%) and abemaciclib (88.1%), in the same way we obtained for abemaciclib (93%) and palbociclib (92%). Using another method to measure palbociclib adherence, medication possession ratio (MPR), the same results were obtained in a real-world assessment of palbociclib adherence in USA, 88% (Engel-Nitz et al., 2023).
The ribociclib adherence was found to be 92%, similar to the adherence rates measured using patient self-reported questionnaires (87.9%, 91.6%, and 91.6% for EORTC QLQ-C30, QLQ-BR23, and HADS-D, respectively) in RIBANNA trial (Fasching et al., 2022). An ongoing clinical trial LEADER monitored ribociclib adherence by review of patients’ diaries and pill count, without still reported the results (https://clinicaltrials.gov/study/NCT03285412).
Lasala et al. reviewed the studies assessing the association between adherence to oral therapies in cancer patients and clinical outcome and found studies that used different adherence cut-offs that could be associated with different clinical outcomes (Lasala, 2021). None of these studies evaluated CDK 4/6i adherence, but we could compare with studies which included patients with breast cancer under endocrine treatment (tamoxifen, anastrozole, letrozole and exemestane) (Ma et al., 2008; Partridge et al., 2010; Xu et al., 2012; Weaver et al., 2013; Seneviratne et al., 2014; Rodrigues Guedes et al., 2017; Le Saux et al., 2018; Font et al., 2019). Twenty-five percent of non-adherence breast cancer patients were observed in a study that recorded capecitabine adherence by microelectronic monitoring system (MEMS) with a cut-off of 0.80 (Partridge et al., 2010).
The routine of frequent medication intake was proved to be one of the important barriers of adherence to oral anticancer medications among patients with breast cancer (Onwusah et al., 2023). It is important to emphasize that, despite the distinct administration schedules of CDK 4/6 inhibitors (ribociclib and palbociclib are administered once daily for 21 consecutive days followed by 7 days without treatment, while abemaciclib is administered continuously), medication adherence did not differ among the three patient groups.
Seneviratne and Xu showed a statistically significant correlation between medication adherence and OS in breast cancer patients (Xu et al., 2012; Seneviratne et al., 2015). Rodrigues Guedes did not find any correlation (Rodrigues Guedes et al., 2017). Waever et al. did not found significant correlation between adherence and cancer recurrence (Waever et al., 2013). No significant correlation was found between adherence and response according to RECIST (response evaluation criteria in solid tumours) (Le Saux et al., 2018) or relapse-free survival and toxicity (Partridge et al., 2010). Dezentjee et al. demonstrated that tamoxifen adherence was significantly associated with breast cancer event-free time (EFT) for both 80% and 90% adherence cut-offs (Dezentjee et al., 2010).
Few studies were published regarding CDK 4/6i non-adherence negatively effects. Regarding palbociclib adherence, it was measured its impact on pharmacokinetic and pharmacodynamic profiles and proved that catching up on a missed dose at the end of the cycle increases the risk of severe neutropenia in the next cycle (Bandiera et al., 2023).
In our study, we found no significant association between gender and adherence to CDK4/6i, a finding that contrasts with some research indicating gender-specific differences in medication adherence, especially in the context of experiencing adverse effects. For example, a significant difference has been noted in the occurrence of side effects in tamoxifen treatments (Xu et al., 2012). This distinction is important to take into account because the likelihood of side effects is a major factor affecting patients’ compliance with their prescription regimens.
The lack of a gender-based difference in adherence to CDK4/6i in our study is particularly intriguing when juxtaposed with these observations. It prompts further inquiry into the distinctive characteristics of CDK4/6i and their reception and tolerance by different genders and it is important to consider the variety of treatments used for male breast cancer patients.
A study published in Breast in 2022 (Yıldırım et al., 2022) highlights that most male patients were treated with CDK4/6i in combination with fulvestrant or AI rather than tamoxifen. This diverges from the general perception and findings in some interviews (Chalasani, 2023), which suggest that tamoxifen is a more commonly used treatment in male breast cancer patients. This discrepancy in treatment choices is noteworthy because it suggests variability in the clinical management of male breast cancer and potentially different side effect profiles and adherence challenges associated with each treatment.
In our study, among the patients who received palbociclib, 61% patients received a combination with AI and 12.2% a combination with LHRH, in almost the same proportions a US real-world study obtained, 76.1%, palbociclib + AI, and 23.9%, palbociclib + fulvestrant (Engel-Nitz et al., 2022). A study assessing the treatment satisfaction in women receiving palbociclib combination for a/mBC in six countries (USA, Canada, Germany, Netherlands, Argentina, and Denmark) included more patients taking palbociclib plus fulvestrant combination (58.6%), but with a smaller median age than our study–41 years old (Darden et al., 2018). In our study, among the patients who received ribociclib, 53.7% patients received ribociclib + AI and 19% patients received ribociclib + LHRH. Regarding the patients who received abemaciclib, more patients were treated in combination with LHRH (39%) than with AI (33%).
The choice of treatment - whether tamoxifen, fulvestrant, AI + GNRH inhibitors, or CDK4/6i - can have significant implications for adherence. Each medication comes with its own set of potential side effects and impacts on quality of life, which can influence a patient’s willingness and ability to remain adherent. The fact that different treatments are being chosen for male patients in various studies and clinical settings underlines the need for a deeper understanding of how treatment decisions are made and how these decisions affect adherence. This understanding is crucial in developing strategies to improve adherence, especially considering the unique challenges male breast cancer patients may face.
The finding in our study that adherence to CDK4/6i was not significantly associated with age, with most older women showing adherence, is a notable observation in the context of breast cancer treatment.
This outcome aligns interestingly with other publications as the adherence of older women to CDK4/6i in our study is encouraging, especially considering the potential survival benefits highlighted by Petrelli et al. The high adherence rate among older women in our study may reflect the effectiveness of these medications on quality of life, their tolerability, or possibly a good understanding and acceptance of treatment regimens among older patients. This observation is important as it suggests that age alone may not be a significant barrier to adherence in the context of CDK4/6i therapy, emphasizing the need for personalized treatment approaches that account for individual patient profiles rather than solely age-based strategies.
Adherence to CDK 4/6i was significantly associated with the follow-up. This aligns with the findings from other studies (Eliassen et al., 2023) which highlights that adherence and persistence to endocrine treatment are critical for improving event-free and overall survival in non-metastatic breast cancer patients. Therefore, it is plausible that the patients in our study who demonstrated better adherence over extended treatment periods might have experienced improved health outcomes, including longer survival. This potential link between sustained adherence and survival emphasizes the importance of strategies to enhance and maintain adherence in breast cancer treatment. Moreover, on the other side, with extended treatment, patients may begin to see the benefits or stabilization of their condition, reinforcing their trust in the effectiveness of the therapy and motivating them to adhere to the regimen.
Based on these results, different interventions could be developed to enhance CDK4/6i adherence. A mobile health intervention was tested integrating a connected electronic adherence monitoring smartbox and automated texting alerts, resulting a palbociclib adherence of 95.8% ± 7.6% (Sadigh et al., 2023). Baseline, before the intervention, the reported barriers were inconvenience to get prescription filled, forgetfulness, cost, and side effects. Our results regarding the adherence to palbociclib were 92.5% ± 13.7%, but without any interventions and costs could not be among the barriers because the drugs are free, with no out-of-pockets costs. The Romanian National Oncology Program covers these medicines for people diagnosed with cancer, being fully reimbursed by the National Health Insurance House in Romania.
Inherent limitations of real-world analyses using data collected during providing reimbursed drugs include the lack of important information (the stage of the disease), incomplete capture of comorbid conditions, and variations in follow-up/short duration of follow-up.
PDC, as a proxy measure of medication adherence based only on community pharmacy claims data, fails to capture the legitimate reasons for not taking CDK 4/6i drugs and does not measure the patient’s actual medication-taking behavior as self-reported like questionnaires do. Limitations of this study include the unknown reasons for prescribing treatment transient interruptions or cycle start deferrals. Toxicity or adverse effects could be the main reasons. Some adherence barriers were observed in assessing palbociclib adherence: inconvenience to get prescription filled, forgetfulness, cost, and side effects (Sadigh et al., 2023). Despite these limitations from the information extracted from our data sources, our results are the beginning of future research in measuring CDK 4/6i adherence.
Another limitation of our study is associated with the small sample size, as the investigation was conducted exclusively within one of Romania’s counties. Romania lacks patient registries and easily accessible databases. The count of patients in Dolj utilizing CDK4/6i, as reported by the Romanian National Health Insurance House, remained relatively consistent throughout the analyzed years: 8.12% in 2018, 4.72% in 2019, 4.02% in 2020, 4.82% in 2021, and 4.85% in 2022 (calculated as a percentage of the total number of patients using CDK4/6i in Romania). A meta-analysis performing an adjusted indirect comparison among the three CDK 4/6i efficacy and toxicity revealed they are equally effective in either first- or second-line therapy for estrogen receptor-positive advanced breast cancer (Petrelli et al., 2019). Choice of treatment depends on several factors, including patients’ adherence, comorbidities, and disease burden. Despite the limitations of our study, the results do not demonstrate a clear superiority of one of the three CDK 4/6i adherence, further studies are needed to understand the adherence influencing factors and the correlations of clinical outcomes with CDK 4/6i adherence (Huang et al., 2016; Murphy et al., 2012; Rugo et al., 2021).
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by the Ethics Committee of the University of Medicine and Pharmacy of Craiova, Romania. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
AT-S: Conceptualization, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Validation, Writing–original draft. IU: Investigation, Methodology, Writing–review and editing, Conceptualization. M-SS: Investigation, Data curation, Writing–original draft. VG: Funding acquisition, Investigation, Validation, Writing–review and editing. MA: Validation, Writing–review and editing, Funding acquisition, Investigation. ED: Validation, Writing–review and editing. SV: Validation, Writing–review and editing. DM: Validation, Writing–review and editing. CL: Conceptualization, Investigation, Project administration, Resources, Supervision, Validation, Visualization, Writing–review and editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. The Article Processing Charges were funded by the University of Medicine and Pharmacy of Craiova, Romania.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Arbuckle, R. B., Huber, S. L., and Zacker, C. (2000). The consequences of diarrhea occurring during chemotherapy for colorectal cancer: a retrospective study. Oncologist 5 (3), 250–259. doi:10.1634/theoncologist.5-3-250
Bandiera, C., Locatelli, I., Courlet, P., Cardoso, E., Zaman, K., Stravodimou, A., et al. (2023). Adherence to the CDK 4/6 inhibitor palbociclib and omission of dose management supported by pharmacometric modelling as part of the OpTAT study. Cancers 15 (1), 316. doi:10.3390/cancers15010316
Barnum, K. J., and O'Connell, M. J. (2014). Cell cycle regulation by checkpoints. Methods Mol. Biol. 1170, 29–40. doi:10.1007/978-1-4939-0888-2_2
Beachler, D. C., de Luise, C., Jamal-Allial, A., Yin, R., Taylor, D. C., Suzuki, A., et al. (2021). Real-world safety of palbociclib in breast cancer patients in the United States: a new user cohort study. BMC Cancer 21, 97. doi:10.1186/s12885-021-07790-z
Cejuela, M., Gil-Torralvo, A., Castilla, M. Á., Domínguez-Cejudo, M. Á., Falcón, A., Benavent, M., et al. (2023). Abemaciclib, palbociclib, and ribociclib in real-world data: a direct comparison of first- line treatment for endocrine-receptor-positive metastatic breast cancer. Int. J. Mol. Sci. 24 (10), 8488. doi:10.3390/ijms24108488
Chalasani, P. (2023). Use of CDK inhibitors in male (2019). CDK inhibitors, endocrine therapy for male patients with breast cancer. [Accessed 25th November 2023]. Available from: https://dailynews.ascopubs.org/do/cdk-inhibitors-endocrine-therapy-male-patients-breast-cancer.
Chirgwin, J. H., Giobbie-Hurder, A., Coates, A. S., Price, K. N., Ejlertsen, B., Debled, M., et al. (2016). Treatment adherence and its impact on disease-free survival in the breast international group 1-98 trial of tamoxifen and letrozole, alone and in sequence. J. Clin. Oncol. 34 (21), 2452–2459. doi:10.1200/JCO.2015.63.8619
Cristofanilli, M., Turner, N. C., Bondarenko, I., Jungsil, R., Im, S. A., Masuda, N., et al. (2016). Fulvestrant plus palbociclib versus fulvestrant plus placebo for treatment of hormone-receptor-positive, HER2–negative metastatic breast cancer that progressed on previous endocrine therapy (PALOMA-3): final analysis of the multicentre, double-blind, phase 3 randomised controlled trial. Lancet Oncol. 17, 425–439. doi:10.1016/S1470-2045(15)00613-0
Darden, C., Mitra, D., McSorley, D., Davis, K., Band, J., and Iyer, S. (2018). Treatment satisfaction in women receiving palbociclib combination therapies for advanced/metastatic breast cancer. Future Oncol. 15 (2), 141–150. doi:10.2217/fon-2018-0531
Dezentjé, V. O., van Blijderveen, N. J., Gelderblom, H., Putter, H., van Herk-Sukel, M. P., Casparie, M. K., et al. (2010). Effect of concomitant CYP2D6 inhibitor use and tamoxifen adherence on breast cancer recurrence in early-stage breast cancer. J. Clin. Oncol. 28 (14), 2423–2429. doi:10.1200/JCO.2009.25.0894
Dhillon, S. (2015). Palbociclib: first global approval. Drugs 75, 543–551. doi:10.1007/s40265-015-0379-9
Dickler, M. N., Tolaney, S. M., Rugo, H. S., Cortés, J., Diéras, V., Patt, D., et al. (2017). MONARCH 1, A phase II study of abemaciclib, a CDK4 and CDK6 inhibitor, as a single agent, in patients with refractory hr+/her2− metastatic breast cancer. Clin. Cancer Res. 23, 5218–5224. doi:10.1158/1078-0432.CCR-17-0754
Dima, A. L., and Dediu, D. (2017). Computation of adherence to medication and visualization of medication histories in R with AdhereR: towards transparent and reproducible use of electronic healthcare data. PLoS One 12, doi:10.1371/journal.pone.0174426
Eliassen, F. M., Blåfjelldal, V., Helland, T., Fonnesbech Hjorth, C., Holland, K., Lode, L., et al. (2023). Importance of endocrine treatment adherence and persistence in breast cancer survivorship: a systematic review. BMC Cancer 23, 625. doi:10.1186/s12885-023-11122-8
Fasching, P. A., Brucker, C., Decker, T., Engel, A., Gohler, T., Jackisch, C., et al. (2023). Abstract P4-01-03: progression-free survival and patient-reported outcomes in HR+, HER2– ABC patients treated with first-line ribociclib + endocrine therapy (ET) or ET monotherapy or chemotherapy in real world setting: 5th interim analysis of RIBANNA. Cancer Res. 83, P4–doi:10.1158/1538-7445.SABCS22-P4-01-03
Finn, R. S., Crown, J. P., Lang, I., Boer, K., Bondarenko, I. M., Kulyk, S. O., et al. (2015). The cyclin-dependent kinase 4/6 inhibitor palbociclib in combination with letrozole versus letrozole alone as first-line treatment of oestrogen receptor-positive, HER2-negative, advanced breast cancer (PALOMA-1/TRIO-18): a randomised phase 2 study. Lancet Oncol. 16, 25–35. doi:10.1016/S1470-2045(14)71159-3
Finn, R. S., Martin, M., Rugo, H. S., Jones, S., Im, S.-A., Gelmon, K., et al. (2016). Palbociclib and letrozole in advanced breast cancer. N. Engl. J. Med. 375, 1925–1936. doi:10.1056/NEJMoa1607303
Font, R., Espinas, J. A., Barnadas, A., Izquierdo, A., Galceran, J., Saladie, F., et al. (2019). Influence of adherence to adjuvant endocrine therapy on disease-free and overall survival: a population-based study in Catalonia, Spain. Breast Cancer Res. Treat. 175 (3), 733–740. doi:10.1007/s10549-019-05201-3
Furtunescu, F., Bohiltea, R. E., Voinea, S., Georgescu, T. A., Munteanu, O., Neacsu, A., et al. (2021). Breast cancer mortality gaps in Romanian women compared to the EU after 10 years of accession: is breast cancer screening a priority for action in Romania? (Review of the Statistics). Exp. Ther. Med. 21, 268. doi:10.3892/etm.2021.9699
Hortobagyi, G. N., Stemmer, S. M., Burris, H. A., Yap, Y.-S., Sonke, G. S., Paluch-Shimon, S., et al. (2016). Ribociclib as first-line therapy for HR-positive, advanced breast cancer. N. Engl. J. Med. 375, 1738–1748. doi:10.1056/NEJMoa1609709
Huang, W. C., Chen, C. Y., Lin, S. J., and Chang, C. S. (2016). Medication adherence to oral anticancer drugs: systematic review. Expert Rev. Anticancer Ther. 16, 423–432. doi:10.1586/14737140.2016.1159515
Inclusion of abemaciclib in Romania, (2022b). Inclusion of abemaciclib in the Positive Drug List in Romania in April 2022. (Accessed November 20, 2023). Available from: [ https://www.anm.ro/_/EVALUARE%20TEHNOLOGII%20MEDICALE/16243_Verzenios_Abemaciclibum.pdf.
Inclusion of ribociclib in Romania (2022a). Inclusion of ribociclib in the positive drug list in Romania in August 2022. Accessed November 20, 2023). Available from: https://www.anm.ro/_/EVALUARE%20TEHNOLOGII%20MEDICALE/CONTESTATII/11408_2021_Kisqali_Contestatie_Ribociclibum.pdf.
Jin, D., Tran, N., Thomas, N., and Tran, D. D. (2019). Combining CDK4/6 inhibitors ribociclib and palbociclib with cytotoxic agents does not enhance cytotoxicity. PloS ONE 14 (10), doi:10.1371/journal.pone.0223555
Kardas, P., Aarnio, E., Agh, T., van Boven, J. F. M., Dima, A. L., Ghiciuc, C. M., et al. (2023). New terminology of medication adherence enabling and supporting activities: ENABLE terminology. Front. Pharmacol. 14, 1254291. doi:10.3389/fphar.2023.1254291
Kraus, A. L., Yu-Kite, M., Mardekian, J., Cotter, M. J., Kim, S., Decembrino, J., et al. (2022). Real-world data of palbociclib in combination with endocrine therapy for the treatment of metastatic breast cancer in men. Clin. Pharmacol. Ther. 111 (1), 302–309. doi:10.1002/cpt.2454
Lasala, R., and Santoleri, F. (2022). Association between adherence to oral therapies in cancer patients and clinical outcome: a systematic review of the literature. Br. J. Clin. Pharm. 88 (5), 1999–2018. doi:10.1111/bcp.15147
Le Saux, O., Bourmaud, A., Rioufol, C., Colomban, O., Guitton, J., Schwiertz, V., et al. (2018). Over-adherence to capecitabine: a potential safety issue in breast and colorectal cancer patients. Cancer Chemother. Pharmacol. 82 (2), 319–327. doi:10.1007/s00280-018-3612-x
Long, Q., Li, X., Wu, G., Zhang, J., and Li, H. (2021). Oral adverse effects of CDK4/6 inhibitors among breast cancer patients: a systematic review and meta-analysis. Ann. Palliat. Med. 10 (6), 6556–6563. doi:10.21037/apm-21-1156
Ma, A. M., Barone, J., Wallis, A. E., Wu, N. J., Garcia, L. B., Estabrook, A., et al. (2008). Noncompliance with adjuvant radiation, chemotherapy, or hormonal therapy in breast cancer patients. Am. J. Surg. 196 (4), 500–504. doi:10.1016/j.amjsurg.2008.06.027
Maculaitis, M. C., Liu, X., Will, O., Hanson, M., McRoy, L., Berk, A., et al. (2020). Oncologist and patient preferences for attributes of CDK4/6 inhibitor regimens forthe treatment of advanced/metastatic HR positive/HER2 negative breast cancer: discrete choice experiment and best-worst scaling. Patient Prefer. adherence 14, 2201–2214. doi:10.2147/PPA.S254934
Marineau, A., St-Pierre, C., Lessard-Hurtubise, R., David, M. È., Adam, J. P., and Chabot, I. (2023). Cyclin-dependent kinase 4/6 inhibitor treatment use in women treated for advanced breast cancer: integrating ASCO/NCODA patient-centered standards in a community pharmacy. J. Oncol. Pharm. Pract. 29 (5), 1144–1153. doi:10.1177/10781552221102884
Mariotto, A. B., Etzioni, R., Hurlbert, M., Penberthy, L., and Mayer, M. (2017). Estimation of the number of women living with metastatic breast cancer in the United States. Cancer Epidemiol. Biomarkers Prev. 26 (6), 809–815. doi:10.1158/1055-9965.EPI-16-0889
Ministry of Health of Romania (2017). Inclusion of palbociclib in Romania. Inclusion of palbociclib in the Positive Drug List in Romania in November 2017. (Accessed November 20, 2023). Available from: https://www.anm.ro/_/EVALUARE%20TEHNOLOGII%20MEDICALE/6300_23.11.2017_Palbociclib_Ibrance.pdf.
Murphy, C. C., Bartholomew, L. K., Carpentier, M. Y., Bluethmann, S. M., and Vernon, S. W. (2012). Adherence to adjuvant hormonal therapy among breast cancer survivors in clinical practice: a systematic review. Breast Cancer Res. Treat. 134, 459–478. doi:10.1007/s10549-012-2114-5
National Cancer Institute (USA) (2023). Cancer statistics in US. [Accessed 23rd November 22023]. Available from: https://www.cancer.gov/types/breast.
Onwusah, D. O., Ojewole, E. B., Manyangadze, T., and Chimbari, M. J. (2023). Barriers and facilitators of adherence to oral anticancer medications among women with breast cancer: a qualitative study. Patient Prefer Adherence 17, 2821–2839. doi:10.2147/PPA.S416843
Partridge, A. H., Archer, L., Kornblith, A. B., Gralow, J., Grenier, D., Perez, E., et al. (2010). Adherence and persistence with oral adjuvant chemotherapy in older women with early-stage breast cancer in CALGB 49907: adherence companion study 60104. J. Clin. Oncol. 28 (14), 2418–2422. doi:10.1200/JCO.2009.26.4671
Pednekar, P. P., Ágh, T., Malmenäs, M., Raval, A. D., Bennett, B. M., Borah, B. J., et al. (2019). Methods for measuring multiple medication adherence: a systematic review-report of the ISPOR medication adherence and persistence special interest group. Value Health 22 (2), 139–156. doi:10.1016/j.jval.2018.08.006
Petrelli, F., Ghidini, A., Pedersini, R., Cabiddu, M., Borgonovo, K., Parati, M. C., et al. (2019). Comparative efficacy of palbociclib, ribociclib and abemaciclib for ER+ metastatic breast cancer: an adjusted indirect analysis of randomized controlled trials. Breast Cancer Res. Treat. 174 (3), 597–604. doi:10.1007/s10549-019-05133-y
Rodrigues Guedes, J. B., Guerra, M. R., Alvim, M. M., and Leite, I. C. G. (2017). Factors associated with adherence and persistence to hormonal therapy in women with breast cancer. Rev. Bras. Epidemiol. 20 (4), 636–649. doi:10.1590/1980-5497201700040007
Roskoski, R. (2019). Cyclin-dependent protein serine/threonine kinase inhibitors as anticancer drugs. Pharmacol. Res. 139, 471–488. doi:10.1016/j.phrs.2018.11.035
Rugo, H. S., Balu, S., Li, Y., Chen, G., Li, X., Turner, S., et al. (2021). Abstract PS10-09: real-world analysis of concomitant medication use with potential drug-drug interactions (DDI) in patients with metastatic breast cancer (MBC) treated with cylin dependent kinase (CDK) 4/6 inhibitors. Cancer Res. 81, PS10-09. doi:10.1158/1538-7445.SABCS20-PS10-09
Sadigh, G., Meisel, J. L., Byers, K., Robles, A., Serrano, L., Jung, O. S., et al. (2023). Improving palbociclib adherence among women with metastatic breast cancer using a CONnected CUstomized Treatment Platform: a pilot study. J. Oncol. Pharm. Pract. 7, 1957–1964. doi:10.1177/10781552231161823
Salmon, D. J., Neven, P., Chia, S., Fasching, P. A., De Laurentiis, M., Im, S.-A., et al. (2020). Overall survival with ribociclib plus fulvestrant in advanced breast cancer. N. Engl. J. Med. 382, 514–524. doi:10.1056/NEJMoa1911149
Seneviratne, S., Campbell, I., Scott, N., Kuper-Hommel, M., Kim, B., Pillai, A., et al. (2015). Adherence to adjuvant endocrine therapy: is it a factor for ethnic differences in breast cancer outcomes in New Zealand? Breast 24 (1), 62–67. doi:10.1016/j.breast.2014.11.011
Slamon, D. J., Neven, P., Chia, S., Fasching, P.Aa., De Laurentiis, M., Im, S.-A., et al. (2018). Phase III randomized study of ribociclib and fulvestrant in hormone receptorpositive, human epidermal growth factor receptor 2–negative advanced breast cancer: MONALEESA-3. J. Clin. Oncol. 36, 2465–2472. doi:10.1200/JCO.2018.78.9909
Sledge, G. W., Toi, M., Neven, P., Sohn, J., Inoue, K., Pivot, X., et al. (2017). MONARCH 2: abemaciclib in combination with fulvestrant in women with HR+/HER2-advanced breast cancer who had progressed while receiving endocrine therapy. J. Clin. Oncol. 35, 2875–2884. doi:10.1200/JCO.2017.73.7585
Suryadinata, R., Sadowski, M., and Sarcevic, B. (2010). Control of cell cycle progression by phosphorylation of cyclin-dependent kinase (CDK) substrates. Biosci. Rep. 30 (4), 243–255. doi:10.1042/BSR20090171
Turcu-Stiolica, A., Subtirelu, M. S., Bogdan, M., Dinescu, V. C., Gheorman, V., Aldea, M., et al. (2022). RWD117 impact of COVID-19 pandemic on the Romanian breast cancer burden: a county population-based study. Value Health 25 (7), S598. doi:10.1016/j.jval.2022.04.1641
Weaver, K. E., Camacho, F., Hwang, W., Anderson, R., and Kimmick, G. (2013). Adherence to adjuvant hormonal therapy and its relationship to breast cancer recurrence and survival among low-income women. Am. J. Clin. Oncol. 36 (2), 181–187. doi:10.1097/COC.0b013e3182436ec1
Wells, C. I., Vasta, J. D., Corona, C. R., Wilkinson, J., Zimprich, C. A., Ingold, M. R., et al. (2020). Quantifying CDK inhibitor selectivity in live cells. Nat. Commun. 11, 2743. doi:10.1038/s41467-020-16559-0
Xu, S., Yang, Y., Tao, W., Song, Y., Chen, Y., Ren, Y., et al. (2012). Tamoxifen adherence and its relationship to mortality in 116 men with breast cancer. Breast Cancer Res. Treat. 136 (2), 495–502. doi:10.1007/s10549-012-2286-z
Keywords: palbociclib, abemaciclib, ribociclib, CDK 4/6 inhibitors, breast cancer, adherence, proportion of days covered (PDC)
Citation: Turcu-Stiolica A, Udristoiu I, Subtirelu M-S, Gheorman V, Aldea M, Dumitrescu EA, Volovat SR, Median DM and Lungulescu CV (2024) Digging in real-word electronic database for assessing CDK 4/6 inhibitors adherence in breast cancer patients from Romania. Front. Pharmacol. 15:1345482. doi: 10.3389/fphar.2024.1345482
Received: 27 November 2023; Accepted: 12 February 2024;
Published: 23 February 2024.
Edited by:
Ines Potočnjak, University Hospital Centre Sisters of Charity, CroatiaReviewed by:
Kamal Al-Shami, German Cancer Research Center (DKFZ), GermanyMartin Wawruch, Comenius University, Slovakia
Copyright © 2024 Turcu-Stiolica, Udristoiu, Subtirelu, Gheorman, Aldea, Dumitrescu, Volovat, Median and Lungulescu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ion Udristoiu, aW9uLnVkcmlzdG9pdUB1bWZjdi5ybw==; Adina Turcu-Stiolica, YWRpbmEudHVyY3VAdW1mY3Yucm8=
†These authors have contributed equally to this work
 Adina Turcu-Stiolica
Adina Turcu-Stiolica Ion Udristoiu2*
Ion Udristoiu2* Mihaela-Simona Subtirelu
Mihaela-Simona Subtirelu Elena Adriana Dumitrescu
Elena Adriana Dumitrescu Simona Ruxandra Volovat
Simona Ruxandra Volovat Cristian Virgil Lungulescu
Cristian Virgil Lungulescu